Abstract
Background:
To date, no studies have directly assessed potential cannabis use disorder (CUD) in medical cannabis (MC) patients pre- vs post-MC treatment. Given that MC patients use cannabis for symptom alleviation rather than intoxication, we hypothesized that MC patients would exhibit few symptoms of CUD after initiating MC treatment.
Methods:
As part of an ongoing observational, longitudinal study, 54 MC patients completed baseline assessments prior to initiating MC use and returned for at least one follow-up assessment after three, six, and/or twelve months of a self-selected MC treatment regimen; detailed MC treatment information was collected and quantified. All patients completed the Cannabis Use Disorder Identification Test - Revised (CUDIT-R) at each visit. Changes in individual items scores and total scores were assessed over time, and we examined whether total CUDIT-R scores correlated with frequency of MC use, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) exposure. Further, Cronbach's alpha analyses were conducted to provide preliminary data regarding the psychometric properties of the CUDIT-R when used among MC patients.
Results:
Although total CUDIT-R scores increased relative to baseline, on average, ratings fell below the ‘hazardous use’ threshold at each visit. Analyses of individual items revealed that increases in total scores were primarily attributable to increases in frequency of use and not necessarily other aspects of problematic use. Total CUDIT-R scores were not associated with number of MC uses or CBD exposure, but a significant relationship was detected between increased THC exposure and higher CUDIT-R scores. Importantly however, analyses revealed that the CUDIT-R does not appear to be an appropriate tool for identifying CUD in MC patients.
Conclusions:
Screening tools specifically designed to assess CUD in MC patients are needed and should distinguish between frequent use and problematic use; exposure to individual cannabinoids must also be considered.
Keywords: medical cannabis, cannabis use disorder, CUDIT-R, longitudinal, THC, CBD
Although there is general consensus that most individuals who use cannabis for recreational purposes (i.e., using cannabis to feel high or alter one's current state of being) do not develop cannabis use disorder (CUD), estimated rates are highly variable. For example, data from two large epidemiological survey studies indicate that CUD rates range from 11-15% (Compton et al., 2016) to approximately 30% (Hasin et al., 2015) in those who use cannabis recreationally. In addition, few studies have assessed CUD in those who use cannabis specifically for medical purposes. Interestingly however, one study found that increased access to medical cannabis (MC) programs was associated with higher rates of cannabis use among adults age 26 or older, but nothigher rates of CUD (Williams et al., 2017), raising the question of whether MC use could be associated with a lower risk for CUD than recreational cannabis use.
As a full diagnostic assessment is time-consuming and often not feasible in many clinical and research settings, several tools have been developed to screen for CUD, including the Severity of Dependence Scale (SDS; Gossop et al., 1995), Cannabis Abuse Screening Test (CAST; Legleye et al., 2012), Alcohol, Smoking and Substance Involvement Screening Test (ASSIST; WHO Assist Working Group, 2002), Cannabis Use Problems Identification Test (CUPIT; Bashford et al., 2010), Cannabis Use Disorders Identification Test (CUDIT; Adamson & Sellman, 2003) and its revised version (CUDIT-R; Adamson et al., 2010). While each has strengths, the CUDIT-R was selected for the current study as it is very commonly used in clinical and research settings as a self-report screening tool, designed based on Diagnostic and Statistical Manual (DSM) criteria, and offers sound psychometric properties when used to assess those who use cannabis for recreational purposes (Adamson et al., 2010). In addition, we have utilized the CUDIT-R in previous research studies assessing recreational cannabis use.
To date, no studies have directly addressed whether MC patients develop symptoms or behaviors associated with problematic cannabis use. Given the growing number of MC patients, it is important to accurately assess potential CUD in this population. Accordingly, using the CUDIT-R, we examined symptoms and behaviors related to problematic cannabis use over 12 months of MC treatment. We hypothesized that MC patients would exhibit increased frequency of use relative to baseline, but endorse few problems associated with MC use given their primary motivation for use is symptom alleviation. Further, we predicted that increased exposure to cannabidiol (CBD), a primary non-intoxicating constituent of cannabis, would not be associated with higher CUDIT-R scores in MC patients, whereas increased exposure to delta-9-tetrahydrocannabinol (THC), the primary intoxicating constituent of cannabis, may be related to higher CUDIT-R scores. In addition, as results raised the possibility that CUDIT-R scores may not accurately reflect problematic use in MC patients, we conducted a secondary set of analyses to assess internal consistency and reliability of the CUDIT-R in the current sample of MC patients; these analyses were designed to generate preliminary data regarding the psychometric properties of the CUDIT-R when used in MC patients.
METHODS
All participants completed an informed consent process in which study procedures, risks, benefits, and the voluntary nature of the study were explained. This study was approved by the Partners Healthcare Institutional Review Board.
Participants
As part of an ongoing, longitudinal study, we recruited individuals interested in using cannabis or cannabinoids specifically to treat one or more medical/psychiatric conditions (e.g., pain, mood, anxiety/PTSD, sleep-related symptoms) but who had not yet begun MC treatment. Study participants were recruited from a variety of sources, including social media advertisements, MC certification centers, and through our institution's online recruitment platform (Rally with Mass General Brigham). Individuals were considered eligible if they were 18 or older and planned to use cannabinoid-based products to treat medical/psychiatric conditions. All were required to have a certification for MC or plan to use products not requiring certification (i.e., hemp-derived products). At baseline, MC patients were required to be cannabis naïve (≤15 lifetime uses) or, if they reported a history of previous recreational cannabis use, were required to be abstinent from regular use (>1x/month) for one year or more to minimize the effects of previous cannabis exposure. All patients also had to test negative for urinary THC metabolites at baseline. In addition, as part of this in-depth study involving face-to-face assessments and cognitive testing (Gruber et al., 2018; Gruber et al., 2016; Gruber et al., 2021; Sagar et al., 2021), patients were required to have an estimated IQ of at least 75 which was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). All patients also completed medical and clinical history questionnaires and interviews, cognitive assessments, other self-report ratings, and multimodal neuroimaging prior to initiation of MC treatment; however, only data from the CUDIT-R self-report scale are reported here.
MC patients completed follow-up visits after three, six, and twelve months of MC use. At the time of analyses, 54 patients completed a baseline visit and had returned for at least one of the follow-up visits at 3, 6, and/or 12 months. Of these 54 patients, 28 completed all four visits. Six patients missed an interim visit(s), 10 patients are in-progress (currently enrolled and awaiting their next follow-up), and 10 discontinued from the study (n=3 stopped MC use; n=7 were lost to follow-up). Of those lost to follow-up, three reported discontinuing for reasons unrelated to MC treatment (e.g., moved out of state, diagnosed with cancer); four stopped responding, and the reason for discontinuation could not be ascertained.
As not all patients included in the current analyses completed all four study visits, those with missing data were divided into two discrete groups: 1) data missing at random (MAR; n=47) and 2) unknown whether data were MAR (n=7). Data were considered MAR for MC patients who completed the study but had missed a visit(s), enrolled individuals who are still considered “in progress,” and those who reported withdrawing from the study due to reasons unrelated to MC use. For those who stopped MC treatment or were lost to follow-up for unknown reasons, analyses were conducted to determine whether missing data could be considered MAR. Specifically, changes in ratings related to MC treatment (e.g., mood, anxiety, sleep, quality of life) between baseline and 3 months were compared between 1) those who remained enrolled throughout the 12-month timepoint, are “in progress,” or withdrew from the study for reasons unrelated to MC use and 2) those who stopped using MC or were lost to follow-up for unknown reasons. No significant between-group differences emerged for any of these variables; therefore, it was determined that missing data could be treated as MAR.
CUDIT-R
The CUDIT-R is an 8-item self-report screening tool for CUD, which assesses frequency of use; hours stoned during days of use; inability to stop using once started; failure to meet expectations; time spent getting, using, or recovering from cannabis; memory or concentration problems after cannabis use; using in hazardous situations (e.g., driving, caring for children); and desire to stop/reduce cannabis use. Seven items are scored on a scale ranging from 0-4 (higher frequency/severity of symptoms is reflected by higher ratings), while the final question regarding thoughts about cutting down use is scored as 0 (no), 2 (yes, but not in the past 6 months), and 4 (yes, during the past 6 months). Scores are summed to generate a total score, ranging from 0 to 32. A total score of 8 or more reflects “hazardous cannabis use,” while scores of 13 or more indicate “possible CUD” (Adamson et al., 2010; Adamson & Sellman, 2003). Although the CUDIT-R has been validated in recreational cannabis consumers, little data exists regarding the psychometric properties of the CUDIT-R when used to assess those who use cannabis for medical purposes.
Cannabinoid Exposure
As an observational study, patients chose their own MC treatment regimens, which were closely tracked using a number of metrics. All were asked to record MC treatment regimen information in MC diaries once they established a regular MC use regimen. Further, study participants completed monthly phone check-ins to corroborate MC diary information using a modified timeline followback procedure (TLFB) optimized to collect recent cannabis use data (Robinson et al., 2014; Sobell et al., 1988). Through these methods, patients were asked to provide qualitative information regarding product type and mode of use (i.e., joint, vaporizer, solution/tincture, edibles, capsules, topicals, etc.) and quantitative information regarding episodes of MC use/week and amount of product used. Data were reviewed and clarified at in-person visits. Additionally, patients sent samples of their most frequently used MC products to an outside laboratory for cannabinoid constituent profiling (ProVerde Laboratories, Inc.), which was used to quantify THC and CBD levels for each product. For products not analyzed, MC patients provided constituent information based on product labels and/or certificates of analyses from dispensaries or product websites. These data, in combination with MC diary information, were used to calculate standard metrics of cannabinoid exposure (THC mg/week and CBD mg/week) for each interval between study visits.
Statistical Analyses
Over the course of MC treatment, MC patients’ CUDIT-R scores (total scores and individual item scores) were examined at baseline and after three, six, and twelve months of MC treatment. Given the primary goal of assessing MC patients’ post-treatment changes compared to their own baseline, individual repeated measures analyses of variance (rmANOVAs) were selected as the most parsimonious model to assess CUDIT-R ratings at each follow-up visit relative to baseline ratings (i.e., baseline vs 3 months [n=51], baseline vs 6 months [n=43], and baseline vs 12 months [n=30]). These methods maximized sample sizes for each contrast and increased statistical power.
To examine the impact of MC use variables on symptoms of CUD, Pearson's r (2-tailed) correlation analyses were utilized to explore the relationship between total CUDIT-R scores and MC use variables, including number of MC uses/week, THC exposure (mg/week), and CBD exposure (mg/week).
Lastly, Cronbach's alpha was calculated for the CUDIT-R to assess its internal consistency, a specific aspect of reliability, in MC patients. Further, item-deletion analyses were completed to assess the internal consistency of each CUDIT-R item. Together, these analyses provided preliminary data regarding the psychometric properties of the CUDIT-R when used in MC patients. For these analyses, data from MC patients’ first follow-up visit after 3 months of treatment were utilized to maintain the largest sample size and greatest statistical power.
RESULTS
Demographics
MC patients (20 men, 34 women) were between the ages of 23-78, mostly White (88.89%), and exhibited above average IQ. All individuals in this sample identified as cisgender. Reported duration of abstinence from recreational cannabis use ranged from 3-47 years.
Patients reported using MC to treat a variety of symptoms/conditions. The majority used MC to treat pain-related conditions (n=33), such as musculoskeletal pain (e.g., arthritis, joint or muscle pain), nerve-related pain or fibromyalgia, and headaches. In addition, patients reported using MC to treat symptoms of anxiety or PTSD (n=31), sleep (n=22), mood/depression (n=14), attention (n=4), and other general medical conditions (n=4), including chronic Lyme disease, psoriasis, restless legs syndrome, and fatigue related to multiple sclerosis. Thirty-six patients reported using MC for more than one indication. Over the course of the study, patients reported using MC 9-11 times/week on average, and THC exposure was notably lower than CBD exposure at each visit. Table 1 includes additional demographic information as well as information regarding cannabis use patterns, including episodes of MC use/week, THC and CBD exposure, types of cannabis products used and modes of administration.
Table 1.
Demographics & Medical Cannabis/Cannabinoid Use
| Demographics (n =54) | Frequency (%) |
|---|---|
| Sex Assigned at Birth | 20 Male (37.04%) |
| 34 Female (62.96%) | |
| Race | 48 White (88.89%) |
| 2 Asian (3.70%) | |
| 2 Black/African American (3.70%) | |
| 1 Other (1.85%) | |
| 1 Prefer Not to Answer (1.85%) | |
| Ethnicity | Hispanic (1.85%) |
| Non-Hispanic (98.15%) | |
| Mean (SD) | |
| Age | 49.17 (16.45) |
| IQ | 121.02 (7.54) |
| Medical Cannabis/Cannabinoid Use | Mean (SD) |
| Baseline cannabis abstinence (years)a | 23.57 (14.20) |
| Average MC Uses/Week | |
| Baseline to 3 Monthsb | 9.26 (6.33) |
| 3 Months to 6 Monthsc | 10.81 (8.35) |
| 6 Months to 12 Monthsd | 11.28 (8.60) |
| Average THC mg/week | |
| Baseline to 3 Monthse | 64.48 (186.69) |
| 3 Months to 6 Monthsf | 43.14 (79.76) |
| 6 Months to 12 Monthsg | 38.37 (50.43) |
| Average CBD mg/week | |
| Baseline to 3 Monthse | 158.04 (290.61) |
| 3 Months to 6 Monthsf | 204.97 (326.13) |
| 6 Months to 12 Monthsg | 97.76 (257.81) |
| Routes of Administrationh | |
| Smoke | 13 (55.56%) |
| Vape | 27 (50.00%) |
| Oromucosal (Oil, Tincture, Solution) | 33 (61.11%) |
| Oral (Edible, Tablet, Capsule) | 22 (40.74%) |
| Cutaneous (Lotion, Salve) | 4 (7.41%) |
| Transdermal (Patches) | 0 (0.0%) |
| Transmucosal (Suppository) | 0 (0.0%) |
CBD=cannabidiol; MC = medical cannabis; THC=delta-9-tetrahydrocannabinol
Average abstinence is reported for n=28 with a previous history of regular cannabis use; n=26 reported no previous regular cannabis use or were cannabis naïve;
n=49;
n=43;
n=29;
n=37;
n=31;
n=22;
n=54, participants could report multiple modes of use
CUDIT-R Scores
Individual rmANOVAs demonstrated that total CUDIT-R scores significantly increased in MC patients at all follow-up visits relative to baseline (ps<.01; see Table 2; Figure 1). Importantly, although ratings significantly increased, average total CUDIT-R scores were ≤6.73 across all follow-up visits, which is below the threshold for “hazardous use” (score of 8) and well below the cutoff for “possible CUD” (score of 13). To determine which specific symptoms or behaviors contributed to total CUDIT-R score increases, changes in individual item scores were also examined (Table 2; Figure 2). “Frequency of use” had the largest effect sizes and was the only item demonstrating a significant increase at all follow-up visits relative to baseline other than “thought about cutting down use” which had much smaller effect sizes. At some, but not all, follow-up visits, statistically significant increases were also intermittently noted for “failure to meet expectations”, “time spent getting cannabis or recovering from use”, “memory/attention problems”, and “use in hazardous situations”. Observed power for all comparisons is provided in Supplemental Table 1. Of the 54 patients included in the study, the number of MC patients who surpassed the threshold for possible CUD at each visit was as follows: baseline = 0 (0.00%), three months = 2 (3.70%), six months = 2 (3.70%), twelve months = 3 (5.56%).
Table 2.
Changes in CUDIT-R Score Over Time
| Baseline n=51 | 3 Months n=51 | 3 Months - Baseline | rmANOVAa | Baselinen=43 | 6 Monthsn=43 | 6 Months - Baseline | rmANOVAb | Baselinen=30 | 12 Monthsn=30 | 12 Months - Baseline | rmANOVAc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | Mean difference [95% CI] | F | p (η2) | Mean (SD) | Mean (SD) | Mean difference [95% CI] | F | p (η2) | Mean (SD) | Mean (SD) | Mean difference [95% CI] | F | p (η2) |
| Total Score | 1.35 (1.98) | 6.06 (2.78) | 4.71 [3.98, 5.43] | 168.78 (.77) | <.01 (2.18) | 1.63 (3.13) | 6.35 [3.68, 5.76] | 4.72 | 84.09 | <.01 (.67) | 1.20 (1.42) | 6.73 (3.85) | 5.53 [4.19, 6.88] | 70.57 | <.01 (.71) |
| Frequency | 0.55(0.64) | 3.55 (0.73) | 3.00 [2.72, 3.28] | 459.00 | <.01 (.90) | 0.63 (0.69) | 3.51 (0.88) | 2.88 [2.52, 3.25] | 257.08 | <.01 (.86) | 0.60 (0.62) | 3.67 (0.71) | 3.07 [2.69, 3.45] | 273.95 | <.01 (.90) |
| Hours Stoned | 0.43 (0.76) | 0.69 (0.99) | 0.26 [-0.03, 0.54] | 3.34 | .07 (.06) | 0.49 (0.77) | 0.72 (0.93) | 0.23 [-0.07, 0.53] | 2.46 | .12 (.06) | 0.40 (0.67) | 0.67 (0.92) | 0.27 [-0.13, 0.66] | 1.94 | .17 (.06) |
| Can’t Stop | 0.00 (0.00) | 0.10 (0.46) | 0.10 [-0.03, 0.23] | 2.33 | .13 (.04) | 0.00 (0.00) | 0.07 (0.34) | 0.07 [-0.03, 0.17] | 1.83 | .18 (.04) | 0.00 (0.00) | 0.20 (0.76) | 0.20 [-0.08, 0.48] | 2.07 | .16(.07) |
| Failure to Meet Expectations | 0.02 (0.14) | 0.22 (0.61) | 0.20 [0.03, 0.37] | 5.44 | .02 (.10) | 0.02 (0.15) | 0.12 (0.32) | 0.09 [-0.02, 0.21] | 2.78 | .10 (.06) | 0.00 (0.00) | 0.13 (0.35) | 0.13 [<0.01, 0.26] | 4.46 | .04 (.13) |
| Time Spent Getting Cannabis or Recovering | 0.04 (0.29) | 0.14 (0.35) | 0.10 [<-0.01, 0.20] | 3.77 | .06 (.07) | 0.05 (0.31) | 0.33 (0.78) | 0.28 [0.02, 0.54] | 4.59 | .04 (.10) | 0.00 (0.00) | 0.33 (0.80) | 0.33 [0.03, 0.63] | 5.18 | .03 (.15) |
| Memory/ Attention Problems | 0.06 (0.24) | 0.45 (.92) | 0.39 [0.12, 0.66] | 8.50 | .01 (.15) | 0.09 (0.29) | 0.35 (0.72) | 0.26 [0.01, 0.50] | 4.51 | .04 (.10) | 0.03 (0.18) | 0.30 (0.70) | 0.27 [-0.01, 0.54] | 3.90 | .06 (.12) |
| Use in Hazardous Situation | 0.06 (0.24) | 0.22 (0.64) | 0.16 [-0.02, 0.34] | 3.03 | .09 (.06) | 0.07 (0.26) | 0.47 (1.03) | 0.40 [0.07, 0.72] | 6.10 | .02 (.13) | 0.10 (0.31) | 0.10 (0.31) | 0.00 [-0.10, 0.10] | 0.00 | 1.00 (.00) |
| Thought about Cutting Down Use | 0.20 (0.83) | 0.71 (1.49) | 0.51 [0.12, 0.90] | 7.00 | .01 (.12) | 0.28 (0.93) | .79 (1.52) | 0.51 [0.03, 1.00] | 4.51 | .04 (.10) | 0.07 (0.37) | 1.33 (1.77) | 1.26 [0.63, 1.90] | 16.64 | <.01 (.37) |
Note. Three of the 54 total MC patients missed the 3-month follow-up but completed later follow-up visits, resulting in n=51 for the baseline vs 3-month comparison. CI = confidence interval, rmANOVA = repeated measures analysis of variance, SD = standard deviation. Significant values (p<.05) are bolded.
Degrees of freedom (df)=1,50.
Degrees of freedom (df)=1,42.
Degrees of freedom (df)=1,29
Figure 1.
CUDIT-R Total Scores
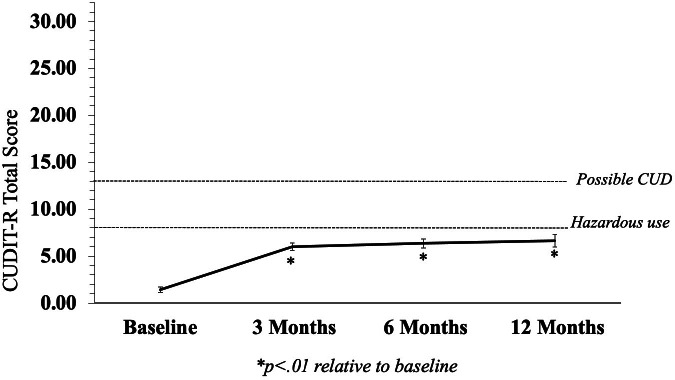
Although total CUDIT-R scores increased in MC patients relative to baseline, on average ratings fell below the threshold for hazardous use or possible CUD at each visit.
Figure 2.
Individual CUDIT-R Item Scores
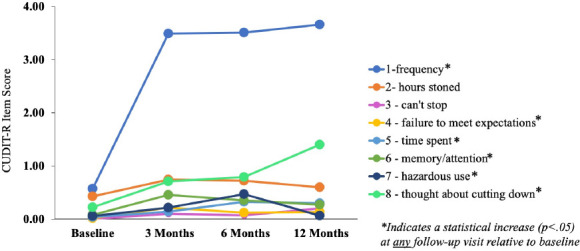
Frequency of MC use increased significantly, and although statistically significant differences were noted for several other items, these scores do not appear to be clinically meaningful given that average scores do not reflect positive endorsement of these symptoms.
Correlation Analyses: CUDIT-R and MC Use Variables
Correlations assessing the relationship between total CUDIT-R scores and MC use variables revealed that number of MC uses per week was not related to total CUDIT-R scores (r(47)=.12, p=.42). When the relationships between total CUDIT-R scores and exposure to individual cannabinoids (THC and CBD) were examined, results revealed higher exposure to THC (mg/week) was significantly associated with higher total CUDIT-R scores (r(35)=.35, p=.03). No significant relationship was detected between CBD mg/week and total CUDIT-R scores (r(35)=.01, p=.95).
CUDIT-R Internal Consistency and Reliability
Given that increases in total CUDIT-R scores appeared largely attributable to frequency of use, we conducted further analyses to examine internal consistency and reliability of the CUDIT-R in our sample of MC patients. Cronbach's alpha analyses revealed that in the current sample of patients using MC for 3 months (n=51), the CUDIT-R had an unacceptable level of internal consistency (alpha=.30).
Moreover, item deletion analyses revealed that removing “frequency of use” from the analyses increased internal consistency (alpha=.40), as did removal of “thought about cutting down use” (alpha=.48). As alpha increased after removing these items, it appears that frequency of use and thought about cutting down cannabis use are not assessing the same construct (i.e., CUD) as the remainder of CUDIT-R items in this cohort of MC patients. Even after removal of these items, alpha remained below the level of acceptable internal consistency.
DISCUSSION
Findings suggest that on the CUDIT-R, MC patients in this study generally endorse expected increases in frequency of cannabis use between baseline (pre-MC use) and follow-up visits occurring three, six, and twelve months after initiation of MC treatment. However, increases in other CUD symptoms appear minimal, and on average, MC patients in the current study do not meet the threshold for “hazardous” cannabis use over the course of twelve months of MC treatment. When examining total CUDIT-R scores in each individual MC patient, a small number of patients did surpass the threshold for possible CUD following initiation of MC treatment (two patients after both three and six months, and three patients after twelve months). However, Cronbach's alpha analyses assessing internal consistency revealed that the CUDIT-R demonstrates extremely low reliability in the study sample; such low reliability raises the question of whether this scale is a valid indicator of CUD in MC patients. In fact, when these data are considered in conjunction with results of the correlation analyses demonstrating that number of MC uses is not related to CUD in this population, as well as a qualitative examination of the CUDIT-R items (discussed below), our findings suggest that the CUDIT-R, which was validated only in recreational cannabis consumers, may not be an appropriate screening tool for assessing CUD in MC patients.
Overall, although significant increases in frequency of cannabis use were observed, this change was expected, as patients in the study were not using MC at baseline, and follow-up assessments only occurred after initiation of regular MC treatment. Importantly, correlation analyses revealed that number of MC uses/week was not significantly correlated with total CUDIT-R scores, further supporting the idea that frequency of MC use is not likely a useful indicator of problematic use in MC patients, and raising questions regarding the validity of the CUDIT-R in those who use cannabis for medical purposes. Interestingly, higher levels of THC exposure were significantly associated with higher CUDIT-R scores, suggesting that THC exposure may be a more salient marker of problematic use than the “frequency of use” item. In fact, previous studies have shown that using cannabis with higher levels of THC is related to increased severity of CUD symptoms (Freeman & Winstock, 2015). CBD exposure, however, was not related to CUDIT-R scores, which is not surprising given that CBD is non-intoxicating, has a low potential for abuse liability as it does not cause tolerance or withdrawal symptoms, and lacks rewarding effects (WHO Expert Committee on Drug Dependence, 2018)
Analyses of changes in individual CUDIT-R items also revealed that while “frequency of use” scores increased relative to baseline, other signs of problematic use were generally not endorsed after initiation of MC treatment. Notably, statistically significant increases from baseline were intermittently observed for some of the other CUDIT-R items, but results do not appear to be clinically meaningful, given near zero scores and small effect sizes. In addition, statistically significant increases for “thoughts about cutting down” use may be explained by the unique scoring criteria for this item. Average ratings remained below a score of 2, which is needed to reflect a positive endorsement of this symptom.
As previously noted, Cronbach's alpha analyses revealed unacceptable internal consistency in the current sample (alpha =.30). Although the CUDIT-R is considered a well-validated screening tool for CUD, it was developed for use in recreational cannabis consumers, and not MC patients. To date, only one other study, (published while the current longitudinal study was already underway), has assessed the reliability of the CUDIT-R in MC patients (Loflin et al., 2018). This study examined a specific subpopulation of MC patients (Veterans) using a cross-sectional approach in which they examined baseline data from a longitudinal study; however, unlike the current study, study participants were already using MC at baseline. Loflin and colleagues reported that although the CUDIT-R's internal consistency fell within the acceptable range, the calculated Cronbach's alpha was considered modest (alpha=.73). It is possible that Cronbach's alpha was higher in the Loflin et al. study than in the current study as MC patients described in Loflin et al. may have also used cannabis for recreational purposes. Although this was not explicitly reported, the study population included veterans who were members of an alliance that allowed those with a MC card to gain bimonthly access to free cannabis products. Accordingly, a significant number of veterans enrolled in the Loflin et al. study may have used cannabis for both recreational and medical purposes, while patients in the current study nearly exclusively used cannabis for medical purposes. Given that the CUDIT-R has demonstrated acceptable psychometric properties in those who use cannabis for recreational purposes, this may account for the higher alpha observed by Loflin and colleagues.
Further, in the current study, removal of “frequency of use” and “thought about cutting down use” increased the overall alpha of the CUDIT-R in MC patients, which suggests that these items do not reflect the same construct as the other items of this scale. In other words, “frequency of use” and “thought about cutting down use” do not appear to be reflective of CUD symptoms in MC patients. Qualitative examination of these items identified by our item-deletion analyses offers some insight into why these items are not appropriate indicators of problematic use in MC patients. For example, increased frequency of use could indicate that a patient is suffering from more severe medical symptoms and therefore needs to use MC more often to achieve symptom relief. It is also possible that if MC is providing adequate symptom relief, patients will be motivated to use MC frequently and regularly. Moreover, as frequent MC use is analogous to taking a conventional medication regularly, consistent use of a medication could actually be viewed as a sign of treatment adherence rather than a sign of problematic use. Therefore, it may be more helpful to differentiate frequent use from using more than needed to achieve a therapeutic benefit, as the latter is likely to be more indicative of problematic use among MC patients. In addition, “thought about cutting down use” may indicate that patients feel as though they do not need as much MC to get the same effect, or endorsement of this item may be a function of patients feeling the financial burden related to the cost of MC, especially as it is not covered by insurance. Other questions on the CUDIT-R may not be directly reflective of problematic use in MC patients either. Specifically, “time spent getting cannabis” may reflect the distance some patients travel, or the time patients wait to purchase MC products. Accordingly, although some patients in this study did surpass the threshold for potential CUD at follow-up visits, these numbers are not likely an accurate representation of the number of MC patients who develop CUD. Specifically, as qualitative examination of individual CUDIT-R items indicates that most items (with the exception of frequency of use) would be more appropriate for MC patients if caveats were issued, it appears that the CUDIT-R is more likely to result in false CUD positives among MC patients than false negatives (failures to identify cases of CUD).
Taken together, current views of CUD should be amended to capture signs of problematic MC use among patients. As in the case of opioid use disorder, for example, tolerance and withdrawal criteria are not considered for individuals who are using opioids under appropriate medical supervision. With regard to cannabis, similar exclusions from DSM-5 criteria may need to be applied. It is likely that signs of problematic use manifest differently in MC patients who have a markedly different motivation for cannabis use – symptom alleviation – relative to recreational consumers who use cannabis specifically to alter their state of being or to feel “high.” As a result, MC patients and recreational consumers often seek different cannabis products resulting in different levels of exposure to specific cannabinoids. Typically, recreational cannabis consumers seek products with high levels of THC, which is generally associated with negative neurobiologic outcomes (Kowal et al., 2015; Ramaekers et al., 2006; Rigucci et al., 2016), to achieve mood-altering effects. Further, chronic exposure to THC is thought to alter excitatory and inhibitory signaling in certain brain regions that could ultimately affect reward processing (Parsons & Hurd, 2015). Altered reward processing is closely linked to addictive disorders, given that intoxication produces pleasurable feelings, leading to repeated use as an individual seeks to achieve these rewarding effects (Everitt & Robbins, 2016). As previously noted, use of products with higher levels of THC has also been associated with increased addiction severity (Freeman & Winstock, 2015). Although MC patients may use products containing THC, many seek products with varied cannabinoid constituent profiles that are often less or non-intoxicating (Sagar & Gruber, 2018). Specifically, many MC patients choose products high in CBD, known for its therapeutic benefits and potential neuroprotective properties (Blessing et al., 2015; de Mello Schier et al., 2014; Fernandez-Ruiz et al., 2013; Iseger & Bossong, 2015; Zuardi, 2008).
Calculations of overall THC and CBD exposure suggest that this preference is reflected in the current study sample, as MC patients as a group had substantially higher CBD exposure relative to THC. CBD has been shown to limit or mitigate negative effects associated with THC (Englund et al., 2013; Morgan & Curran, 2008; Morgan et al., 2012; Morgan et al., 2010; Yucel et al., 2016; Zuardi et al., 1982), and preliminary data suggest CBD may have efficacy in the treatment of substance use disorders, including nicotine (Morgan et al., 2013), cocaine (Lujan et al., 2018), and opioids (Hurd et al., 2019; Hurd et al., 2015). Further, one case study suggests positive effects of CBD in treating CUD (Shannon & Opila-Lehman, 2015), which is supported by the current findings, as higher THC, but not CBD exposure was significantly correlated with increased CUDIT-R scores.
Findings from this study indicate that most MC patients in the current sample do not reach the threshold for CUD using the CUDIT-R, a common screening tool for CUD. However, these findings must be considered in light of several limitations. First, a definitive diagnosis of CUD can only be made using a diagnostic interview such as the Structured Clinical Interview for DSM (SCID). The CUDIT-R was selected for use in this study as it is widely used screening tool to assess cannabis consumers in both clinical and research settings and sometimes serves as a proxy for diagnostic instruments given the significant overlap between CUDIT-R items and DSM-5 criteria for CUD. However, it is important to note that this instrument is based on DSM-IV criteria as it was developed before the release of the DSM-5. Although most criteria are similar between the two DSM versions, “craving or desire to use cannabis” was added to the DSM-5; this is the only criterion not reflected in the CUDIT-R. Nonetheless, until valid and reliable tools are available to screen for CUD in MC patients, future studies examining CUD in this population should utilize a diagnostic interview like the SCID-5, which allows the clinician to use their judgement to determine if an individual's behavior is actually representative of problematic use.
Currently, sample sizes are moderate. Plans for the ongoing, longitudinal study involve monitoring symptoms of CUD in larger samples and over longer durations (up to two years). However, longitudinal studies assessing patients over the course of several years may be necessary to detect potential development of CUD. In addition, MC patients who were cannabis naïve or who had limited recent cannabis exposure were specifically recruited, and all patients reported a primary goal of symptom alleviation. Given that most MC patients in the current sample report using cannabis exclusively for medical purposes, they may represent a unique group of patients. As such, results may not be generalizable to other populations such as “mixed” cannabis consumers who use cannabis both medically and recreationally.
Similarly, findings may not apply to MC patient populations who choose products with higher amounts of THC than CBD, as patients in the current sample overall reported notably higher exposure to CBD. Findings may also not be generalizable to more racial and ethnically diverse samples, as the current sample was predominantly comprised of White individuals. Despite potential limited generalizability, this study represents the first face-to-face, direct assessment of CUD in MC patients pre- vs post-MC treatment.
Although findings may appear to be in contrast with existing literature indicating that rates of CUD in MC patients are comparable to CUD rates in recreational cannabis consumers (Lin et al., 2016; Turna et al., 2020), this is likely related to recruitment criteria and assessment tools. For example, MC patients enrolled in Lin et al. were permitted to use cannabis for recreational purposes, and Turna et al. did not directly compare those who used cannabis exclusively for recreational purposes vs those who used exclusively for medical purposes. Further, Lin and colleagues’ data is based on the National Survey on Drug Use and Health, which utilizes questions similar to those asked in the CUDIT-R, while Turna et al. implemented the CUDIT-R itself; both approaches are problematic as results from the current study demonstrate that these types of queries are not appropriate for assessing CUD in MC patients, and may result in increased rates of CUD false positives and reduced sensitivity. CUD is likely a unique construct among those using cannabis medically, and existing tools developed for use in recreational consumers do not appear to be reliable, valid measures for assessing CUD in MC patients.
Conclusions
In the current study, MC patients generally exhibit low risk patterns of cannabis use, as average scores indicate MC patients generally do not meet CUDIT-R criteria for hazardous use or possible CUD. Although some patients did surpass the threshold for possible CUD after initiation of MC use, these data do not likely reflect actual rates of CUD in MC patients, as analyses suggest the CUDIT-R does not have adequate psychometric properties when used to assess CUD in patients using cannabis exclusively for medical purposes. New metrics are needed to accurately assess CUD in MC patients, and future studies should validate newly developed tools in larger, more diverse samples, including those who may use cannabis for both medical and recreational purposes.
Funding and Acknowledgements:
All authors declare no conflicts of interest.
REFERENCES
- Adamson, S. J., Kay-Lambkin, F. J., Baker, A. L., Lewin, T. J., Thornton, L., Kelly, B. J., & Sellman, J. D. (2010). An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug and alcohol dependence, 110(1-2), 137–143. [DOI] [PubMed] [Google Scholar]
- Adamson, S. J., & Sellman, J. D. (2003). A prototype screening instrument for cannabis use disorder: the Cannabis Use Disorders Identification Test (CUDIT) in an alcohol-dependent clinical sample. Drug and alcohol review, 22(3), 309–315. [DOI] [PubMed] [Google Scholar]
- Bashford, J., Flett, R., & Copeland, J. (2010). The Cannabis Use Problems Identification Test (CUPIT): development, reliability, concurrent and predictive validity among adolescents and adults. Addiction, 105(4), 615–625. [DOI] [PubMed] [Google Scholar]
- Blessing, E. M., Steenkamp, M. M., Manzanares, J., & Marmar, C. R. (2015). Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics, 12(4), 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton, W. M., Han, B., Jones, C. M., Blanco, C., & Hughes, A. (2016). Marijuana use and use disorders in adults in the USA, 2002–14: analysis of annual cross-sectional surveys. The lancet. Psychiatry, 3(10), 954–964. [DOI] [PubMed] [Google Scholar]
- de Mello Schier, A. R., de Oliveira Ribeiro, N. P., Coutinho, D. S., Machado, S., Arias-Carrion, O., Crippa, J. A., Zuardi, A. W., Nardi, A. E., & Silva, A. C. (2014). Antidepressant-like and anxiolytic-like effects of cannabidiol: a chemical compound of Cannabis sativa. CNS & neurological disorders drug rargets, 13(6), 953–960. [DOI] [PubMed] [Google Scholar]
- Englund, A., Morrison, P. D., Nottage, J., Hague, D., Kane, F., Bonaccorso, S., Stone, J. M., Reichenberg, A., Brenneisen, R., Holt, D., Feilding, A., Walker, L., Murray, R. M., & Kapur, S. (2013). Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. Journal of psychopharmacology, 27(1), 19–27. [DOI] [PubMed] [Google Scholar]
- Everitt, B. J., & Robbins, T. W. (2016). Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annual review of psychology, 67, 23–50. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz, J., Sagredo, O., Pazos, M. R., Garcia, C., Pertwee, R., Mechoulam, R., & Martinez-Orgado, J. (2013). Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? British journal of clinical pharmacology, 75(2), 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, T. P., & Winstock, A. R. (2015). Examining the profile of high-potency cannabis and its association with severity of cannabis dependence. Psychological medicine, 45(15), 3181–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop, M., Darke, S., Griffiths, P., Hando, J., Powis, B., Hall, W., & Strang, J. (1995). The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction, 90(5), 607–614. [DOI] [PubMed] [Google Scholar]
- Gruber, S. A., Sagar, K. A., Dahlgren, M. K., Gonenc, A., Smith, R. T., Lambros, A. M., Cabrera, K. B., & Lukas, S. E. (2018). The Grass Might Be Greener: Medical Marijuana Patients Exhibit Altered Brain Activity and Improved Executive Function after 3 Months of Treatment. Frontiers in pharmacology, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, S. A., Sagar, K. A., Dahlgren, M. K., Racine, M. T., Smith, R. T., & Lukas, S. E. (2016). Splendor in the Grass? A Pilot Study Assessing the Impact of Medical Marijuana on Executive Function. Frontiers in pharmacology, 7, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, S. A., Smith, R. T., Dahlgren, M. K., Lambros, A. M., & Sagar, K. A. (2021). No pain, all gain? Interim analyses from a longitudinal, observational study examining the impact of medical cannabis treatment on chronic pain and related symptoms. Experimental and clinical psychopharmacology, 29(2), 147–156. [DOI] [PubMed] [Google Scholar]
- Hasin, D. S., Saha, T. D., Kerridge, B. T., Goldstein, R. B., Chou, S. P., Zhang, H., Jung, J., Pickering, R. P., Ruan, W. J., Smith, S. M., Huang, B., & Grant, B. F. (2015). Prevalence of Marijuana Use Disorders in the United States Between 2001-2002 and 2012-2013. JAMA psychiatry, 72(12), 1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd, Y. L., Spriggs, S., Alishayev, J., Winkel, G., Gurgov, K., Kudrich, C., Oprescu, A. M., & Salsitz, E. (2019). Cannabidiol for the Reduction of Cue-Induced Craving and Anxiety in Drug-Abstinent Individuals With Heroin Use Disorder: A Double-Blind Randomized Placebo-Controlled Trial. The American journal of psychiatry, 176(11), 911–922. [DOI] [PubMed] [Google Scholar]
- Hurd, Y. L., Yoon, M., Manini, A. F., Hernandez, S., Olmedo, R., Ostman, M., & Jutras-Aswad, D. (2015). Early Phase in the Development of Cannabidiol as a Treatment for Addiction: Opioid Relapse Takes Initial Center Stage. Neurotherapeutics, 12(4), 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseger, T. A., & Bossong, M. G. (2015). A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophrenia research, 162(1-3), 153–161. [DOI] [PubMed] [Google Scholar]
- Kowal, M. A., Hazekamp, A., Colzato, L. S., van Steenbergen, H., van der Wee, N. J., Durieux, J., Manai, M., & Hommel, B. (2015). Cannabis and creativity: highly potent cannabis impairs divergent thinking in regular cannabis users. Psychopharmacology, 232(6), 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legleye, S., Kraus, L., Piontek, D., Phan, O., & Jouanne, C. (2012). Validation of the Cannabis Abuse Screening Test in a sample of cannabis inpatients. European addiction research, 18(4), 193–200. [DOI] [PubMed] [Google Scholar]
- Lin, L. A., Ilgen, M. A., Jannausch, M., & Bohnert, K. M. (2016). Comparing adults who use cannabis medically with those who use recreationally: Results from a national sample. Addictive behaviors, 61, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loflin, M., Babson, K., Browne, K., & Bonn-Miller, M. (2018). Assessment of the validity of the CUDIT-R in a subpopulation of cannabis users. The American journal of drug and alcohol abuse, 44(1), 19–23. [DOI] [PubMed] [Google Scholar]
- Lujan, M. A., Castro-Zavala, A., Alegre-Zurano, L., & Valverde, O. (2018). Repeated Cannabidiol treatment reduces cocaine intake and modulates neural proliferation and CB1R expression in the mouse hippocampus. Neuropharmacology, 143, 163–175. [DOI] [PubMed] [Google Scholar]
- Morgan, C. J., & Curran, H. V. (2008). Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. British journal of psychiatry, 192(4), 306–307. [DOI] [PubMed] [Google Scholar]
- Morgan, C. J., Das, R. K., Joye, A., Curran, H. V., & Kamboj, S. K. (2013). Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addictive behaviors, 38(9), 2433–2436. [DOI] [PubMed] [Google Scholar]
- Morgan, C. J., Gardener, C., Schafer, G., Swan, S., Demarchi, C., Freeman, T. P., Warrington, P., Rupasinghe, I., Ramoutar, A., Tan, N., Wingham, G., Lewis, S., & Curran, H. V. (2012). Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychological medicine, 42(2), 391–400. [DOI] [PubMed] [Google Scholar]
- Morgan, C. J., Schafer, G., Freeman, T. P., & Curran, H. V. (2010). Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected]. British journal of psychiatry, 197(4), 285–290. [DOI] [PubMed] [Google Scholar]
- Parsons, L. H., & Hurd, Y. L. (2015). Endocannabinoid signalling in reward and addiction. Nature reviews. Neuroscience, 16(10), 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers, J. G., Kauert, G., van Ruitenbeek, P., Theunissen, E. L., Schneider, E., & Moeller, M. R. (2006). High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology, 31(10), 2296–2303. [DOI] [PubMed] [Google Scholar]
- Rigucci, S., Marques, T. R., Di Forti, M., Taylor, H., Dell'Acqua, F., Mondelli, V., Bonaccorso, S., Simmons, A., David, A. S., Girardi, P., Pariante, C. M., Murray, R. M., & Dazzan, P. (2016). Effect of high-potency cannabis on corpus callosum microstructure. Psychological medicine, 46(4), 841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, S. M., Sobell, L. C., Sobell, M. B., & Leo, G. I. (2014). Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychology of addictive behaviors, 28(1), 154–162. [DOI] [PubMed] [Google Scholar]
- Sagar, K. A., Dahlgren, M. K., Lambros, A. M., Smith, R. T., El-Abboud, C., & Gruber, S. A. (2021). An Observational, Longitudinal Study of Cognition in Medical Cannabis Patients over the Course of 12 Months of Treatment: Preliminary Results. Journal of the international neuropsychological society, 27, 248–660. [DOI] [PubMed] [Google Scholar]
- Sagar, K. A., & Gruber, S. A. (2018). Marijuana matters: reviewing the impact of marijuana on cognition, brain structure and function, & exploring policy implications and barriers to research. International review of psychiatry, 30(3),1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon, S., & Opila-Lehman, J. (2015). Cannabidiol Oil for Decreasing Addictive Use of Marijuana: A Case Report. Integrative medicine, 14(6), 31–35. [PMC free article] [PubMed] [Google Scholar]
- Sobell, L. C., Sobell, M. B., Leo, G. I., & Cancilla, A. (1988). Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. British journal of addiction, 83(4), 393–402. [DOI] [PubMed] [Google Scholar]
- Turna, J., Balodis, I., Munn, C., Van Ameringen, M., Busse, J., & MacKillop, J. (2020). Overlapping patterns of recreational and medical cannabis use in a large community sample of cannabis users. Comprehensive psychiatry, 102, 152188. [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (1999). Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation. [Google Scholar]
- WHO Assist Working Group. (2002). The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction, 97(9), 1183–1194. https://www.ncbi.nlm.nih.gov/pubmed/12199834 [DOI] [PubMed] [Google Scholar]
- WHO Expert Committee on Drug Dependence. (2018). Cannabidiol (CBD): Critical Review Report. Geneva, Switzerland: World Health Organization; Retrieved from https://www.who.int/medicines/access/controlled-substances/CannabidiolCriticalReview.pdf [Google Scholar]
- Williams, A. R., Santaella-Tenorio, J., Mauro, C. M., Levin, F. R., & Martins, S. S. (2017). Loose regulation of medical marijuana programs associated with higher rates of adult marijuana use but not cannabis use disorder. Addiction, 112(11), 1985–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel, M., Lorenzetti, V., Suo, C., Zalesky, A., Fornito, A., Takagi, M. J., Lubman, D. I., & Solowij, N. (2016). Hippocampal harms, protection and recovery following regular cannabis use. Translational psychiatry, 6, e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi, A. W. (2008). Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Revista brasileira de psiquiatria, 30(3), 271–280. [DOI] [PubMed] [Google Scholar]
- Zuardi, A. W., Shirakawa, I., Finkelfarb, E., & Karniol, I. G. (1982). Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology, 76(3), 245–250. [DOI] [PubMed] [Google Scholar]


