Abstract
Background:
Extracorporeal membrane oxygenation (ECMO), a rescue therapy for pulmonary failure, has traditionally been limited by anticoagulation requirements. Recent practice has challenged the absolute need for anticoagulation, expanding the role of ECMO to patients with higher bleeding risk. We hypothesize that mortality, bleeding, thrombotic events, and transfusions do not differ between heparin-sparing and full therapeutic anticoagulation strategies in veno-venous (VV) ECMO management.
Materials and methods:
Adult VV ECMO patients between October 2011 and May 2018 at a single center were reviewed. A heparin-sparing strategy was implemented in October 2014; we compared outcomes in an as-treated fashion. The primary end point was survival. Secondary end points included bleeding, thrombotic complications, and transfusion requirements.
Results:
Forty VV ECMO patients were included: 17 (147 circuit-days) before and 23 (214 circuit-days) after implementation of a heparin-sparing protocol. Patients treated with heparin-sparing anticoagulation had a lower body mass index (28.5 ± 7.1 versus 38.1 ± 12.4, P = 0.01), more often required inotropic support before ECMO (82 versus 50%, P = 0.05), and had a lower mean activated clotting time (167 ± 15 versus 189 ± 15 s, P < 0.01). There were no significant differences in survival to decannulation (59 versus 83%, P = 0.16) or discharge (50 versus 72%, P = 0.20), bleeding (32 versus 33%, P = 1.0), thromboembolic events (18 versus 39%, P = 0.17), or transfusion requirements (median 1.1 versus 0.9 unit per circuit-day, P = 0.48).
Conclusions:
Survival, bleeding, thrombotic complications, and transfusion requirements did not differ between heparin-sparing and full therapeutic heparin strategies for management of VV ECMO. VV ECMO can be a safe option in patients with traditional contraindications to anticoagulation.
Keywords: Extracorporeal membrane oxygenation (ECMO), Severe respiratory failure, Hypoxia, Anticoagulation, Heparin
Introductionss
Extracorporeal membrane oxygenation (ECMO) is increasingly being utilized as a rescue modality for patients with severe reversible pulmonary failure, such as acute respiratory distress syndrome.1–7 Although it can be a life-saving intervention in this patient population, it still carries an in-hospital mortality ranging from 30% to 55% in the literature.4,7–10 Anticoagulation has traditionally been used to prevent thrombosis of the ECMO circuit; however, it has also been shown to contribute to the incidence of bleeding.11 Serious bleeding is reported in 39.1% of patients on veno-venous (VV) ECMO and can result in the need for large volume blood transfusion.12 Gastrointestinal bleeding is one of the most common bleeding complications during ECMO support, but bleeding at other sites including the nares (epistaxis), thoracic cavity, surgical sites, cannulation sites, and brain (intracranial hemorrhage [ICH]) has been reported.13–17
ECMO has been shown to be a viable option in a select subset of polytrauma patients with associated acute respiratory distress syndrome and/or severe pulmonary trauma, and its use is increasing in this population.18–22 However, trauma patients often have contraindications to therapeutic anticoagulation related to their associated injuries, and traditionally, significant trauma has been considered a relative contraindication to ECMO.23 A few small studies have looked at the viability of managing VV ECMO using novel anticoagulation-sparing strategies compared to traditional full anticoagulation and have shown comparable outcomes.24–31 We hypothesize that, among VV ECMO patients treated at a single tertiary center, there is no difference in mortality, bleeding, thrombotic events, or transfusion requirements in heparin-sparing versus full anticoagulation strategies.
Materials and methods
After institutional review board approval, a retrospective chart review was conducted for all adult patients (age > 18 y) treated consecutively with VV ECMO at a single institution between October 2011 and May 2018. Data collection was performed under a waiver of consent.
ECMO circuit componentss
During the entirety of the study, ECMO systems consisted of ROTAFLOW centrifugal pumps (Maquet/Getinge: Rastatt, Germany) in line with a standard membrane oxygenator and heat exchanger, linked with P.h.i.s.i.o-coated polyvinylchloride tubing (LivaNova: London, UK). All oxygenators and pump heads were noneheparin-coated (Quadrox-i, Maquet/Getinge: Rastatt, Germany), with the exception of one heparin-bonded oxygenator (Quadrox-i) used before the October 2014 protocol change. A Medos hilite 7000 LT oxygenator (GISH Biomedical: Rancho Santa Margarita, CA) was used in one patient after October 2014 due to limited availability of the Quadrox-i oxygenator.
The standard practice of evaluation of the oxygenator consisted of inspection at shift change by the ECMO clinicians (respiratory therapists and/or registered nurses with specific institutional ECMO training) with intensivist notification if there was an issue. The intensivist also evaluated the circuit daily. The oxygenator was changed if there was significant visible clot burden contributing to loss of oxygenator performance based on intensivist and/or ECMO clinician assessment.
Before October 2014, cannulation was achieved with Avalon Elite bicaval dual-lumen catheters (Maquet/Getinge: Rastatt, Germany) placed preferentially in the right internal jugular vein via a percutaneous Seldinger technique. In the preprotocol change group, 100% (17) of patients were cannulated in this fashion. After October 2014, cannulation was achieved with noncoated Bio-Medicus single-lumen catheters (Medtronic: Minneapolis, MN) placed in the common femoral vein for drainage, and either the internal jugular vein or contralateral common femoral vein for return via a percutaneous Seldinger technique. In the postprotocol group, 96% (22) were cannulated using the aforementioned method, and 4% (1) were cannulated percutaneously using an Avalon Elite bicaval dual-lumen catheter, due to severe bilateral lower extremity fractures.
ECMO management
In October 2014, the institution transitioned to a single surgical service managing patients on ECMO to standardize care. Once patients were placed on ECMO, they were transferred to the cardiothoracic intensive care unit and managed using a multidisciplinary approach between the cardiovascular intensivist and the cardiac surgery team. Routine circuit maintenance was managed by the same group of dedicated ECMO clinicians throughout the study.
Before October 2014, patients were started on full therapeutic anticoagulation with intravenous (IV) unfractionated heparin (activated clotting time [ACT] goal: 180–200 s(s)), unless there were bleeding risks (e.g., trauma, gastrointestinal bleeding, etc.) that required altering the anticoagulation strategy. In October 2014, there was an institutional paradigm shift to a heparin-sparing protocol. At the time of cannulation, the decision to administer a heparin bolus was at the discretion of the cannulating surgeon. The circuit was then managed either without heparin or with low-dose continuous IV heparin (ACT goal: 140–180 s). Only two patients were initiated on full therapeutic IV heparin due to clots noted at the time of cannulation. This heparin-sparing strategy was continued unless the patient developed visual fibrin on the oxygenator with evidence of flow impediment at which time they were converted to full therapeutic anticoagulation. In both eras, point of care ACTs were checked every hour until stabilization, at which point the interval was increased to every 2 h. One patient in the heparin-sparing group had severe pulmonary failure from viral pneumonia and concomitant hepatic congestion from post–heart transplant rejection (transplant 1 mo before respiratory failure) that resulted in coagulopathy and increased ACTs, which given the nature of the increased ACTs, were excluded from the ACT analysis.
In both cohorts, evaluation for ECMO was requested at the discretion of the attending intensivist, and the decision for cannulation was at the discretion of the cannulating attending surgeon. All decisions were made on a case-by-case basis; no formal institutional protocol listing specific indications or contraindications for ECMO exists.
Outcomes
The following clinical variables were examined for all patients: demographics, indication for ECMO, duration of ECMO, intensive care unit length of stay, hospital length of stay, inotropic support both before and during ECMO, renal failure requiring renal replacement therapy (continuous renal replacement or intermittent hemodialysis), inhaled pulmonary vasodilator use, transfusion requirements, Glasgow Coma Scale on admission, the Extracorporeal Life Support Organization (ELSO) RESP score,9 the Simplified Acute Physiology II Score,32 aspirin/clopidogrel administration within 5 d of initiation of ECMO and during ECMO, pre-ECMO laboratory values, cannulation site, and use of systemic anticoagulation on initiation and during the ECMO course. Additional variables examined for trauma patients included the following: mechanism of traumatic injury, injury severity score (ISS), the presence of ICH on admission computed tomography scan, the presence of a spinal cord injury, and the presence of orthopedic injuries resulting in non–weight-bearing status of the lower extremities.
Given the small case series, we focused primarily on an astreated analysis defined as whether patients spent >50% of circuit-days on a heparin-sparing strategy as this was felt to be the most clinically relevant. Sensitivity analyses grouping patients instead as preprotocol versus postprotocol change, and as intention-to-treat (based on whether continuous IV heparin was initiated immediately after cannulation or not), were performed and did not substantively differ from the astreated results presented. A circuit day was defined as a 24-hour period from 7 AM until 7 AM the following day. Primary outcomes were survival to decannulation and survival to discharge. Secondary outcomes were bleeding complications, thromboembolic events, and transfusion requirements. Bleeding complications were categorized into minor and major complications. Minor complications included the following: bleeding from the cannula sites that required intervention, either at the bedside or in the operating room. Major complications included the following: recent surgical site hemorrhage, gastrointestinal bleeding (GIB) and pulmonary hemorrhage that required transfusion of blood products, and new or worsened ICH. Thromboembolic complications included the following: the need for oxygenator change, unexpected cessation of the ECMO circuit due to clot, and thrombotic events (defined as a new diagnosis of pulmonary embolism [PE], deep vein thrombosis [DVT], stroke, or other major vessel thrombosis based on diagnostic imaging). Of note, our institution does not routinely screen for PEs, DVTs, or other major thrombi; imaging is only obtained for symptoms or signs of thromboembolic complications.
Statistical analysis
Unless otherwise noted, data are presented as mean ± standard deviation for normally distributed data, median [interquartile range] for nonnormally distributed data, or percentage for binary data. Univariate differences between groups were assessed using Student’s t-test for normally distributed data, Mann-Whitney U-test for nonnormal data, and Fisher’s exact test for binary data. Multivariable logistic regression models were developed for survival to decannulation, bleeding complications, and thrombotic complications. Models began by including parameters which differed significantly between groups with P < 0.05 and subjected to forward selection with additional candidate parameters only included if Akaike information criteria were reduced. Standard errors were adjusted by robust clustering by ECMO indication (medical versus trauma). Goodness of fit was assessed using the Hosmer-Lemeshow test, and significant predictor nonlinearity modeled using cubic splines. All analyses were performed by the authors using Stata v14 (StataCorp LP, College Station, TX).
Results
Forty patients were treated with VV ECMO (Figure). Overall, bleeding complications occurred in 32.5% of patients (7% of trauma patients), and thromboembolic events occurred in 27.5% of patients (29% of trauma patients). Overall survival was 60% (71.4% in trauma patients). Seventeen patients (147 circuit days) were managed before implementation of a standardized heparin-sparing protocol, and 23 patients (214 circuit days) were managed after protocol implementation. In the preprotocol group, 39 (27%) of circuit days were managed with low- or no-heparin (trauma patients and those felt to have an increased risk of bleeding), versus 108 (73%) circuit days managed with full therapeutic heparin anticoagulation. By contrast, in the postprotocol group, 190 (89%) circuit days were heparin-sparing versus 24 (11%) circuit days with full therapeutic heparin anticoagulation (3 patients were converted to a full anticoagulation strategy after fibrin clots developed on the oxygenator, one patient was started initially on a full anticoagulation strategy per the surgeon preference, and one patient was transitioned to argatroban after developing heparin-induced thrombocytopenia). In addition, in the postprotocol group, 35% (75) of all circuit days were completely heparin-free (including no administration of DVT prophylaxis), with nine patients undergoing completely heparin-free courses of ECMO (a total of 33 circuit days).
Fig –
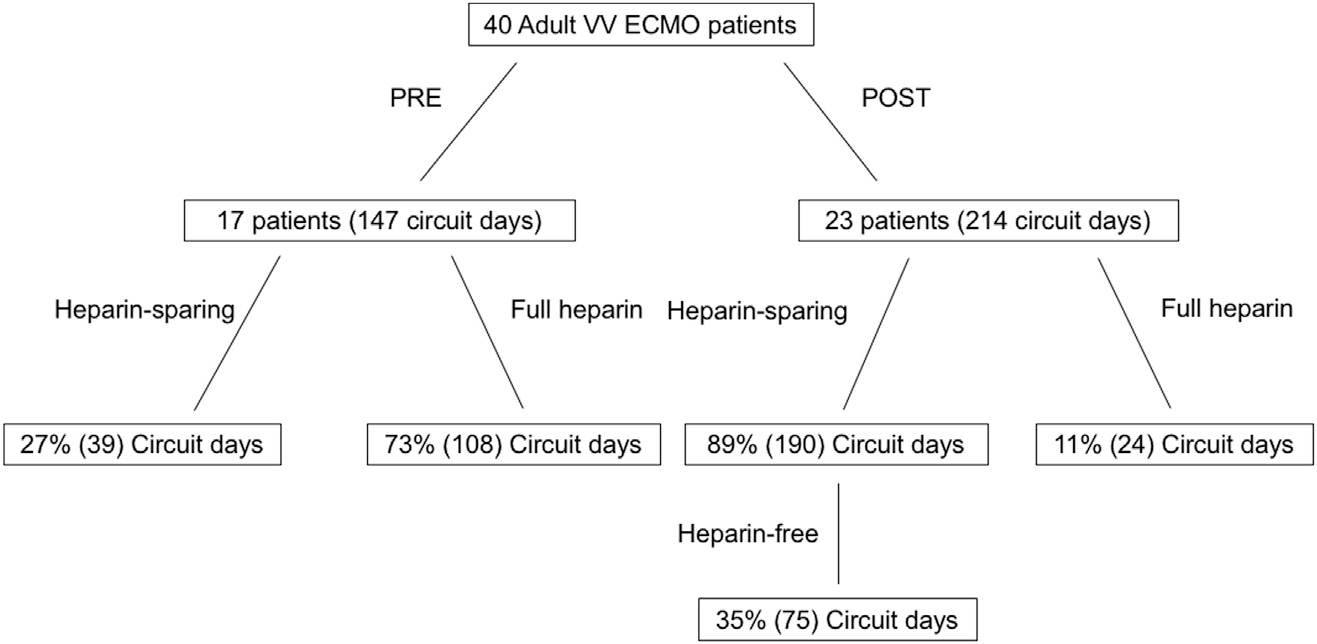
Study population with breakdown into final cohorts.
To investigate the effect of the heparin treatment strategy, patients were categorized into the following groups: those who spent the majority of circuit days (>50%) on a heparin-sparing strategy versus those who spent the majority of circuit days (>50%) on a full-heparin strategy (Table 1). The only significant differences between the two as-treated groups were lower body mass index (28.5 ± 7.1 versus 38.1 ± 12.4 kg/m2, P = 0.01) and lower mean ACT in the heparin-sparing group (167 ± 15 versus 189 ± 15 s, P < 0.01). Notably, there were no significant differences in survival to either decannulation (59 versus 83%, P = 0.16) or discharge (50% versus 72%, P = 0.20). Using multivariable analysis to adjust for the effects of age and ELSO RESP9 score, a heparin-sparing strategy was not an adjusted predictor of survival to decannulation (odds ratio 0.34 [0.11–1.03], P = 0.06). Clinical data in all patients who died are presented in Table 2. We also evaluated the specific subset of nine patients from the postprotocol group who received entirely heparin-free ECMO courses and found that there were no differences in survival, complications, or transfusions in this subgroup (all P > 0.10). Heparin was the only anticoagulant administered in this study, other than the case of a single patient in the heparin-sparing group who developed heparin-induced thrombocytopenia and was managed with an argatroban infusion. No patients in either group received either aspirin or clopidogrel in the 5 d before ECMO cannulation or during their ECMO course. No patients crossed into a no-heparin strategy from a full-heparin strategy due to bleeding during the course of the study. Nine patients crossed over from a no-heparin strategy to a heparin-sparing strategy; one patient crossed-over into full therapeutic anticoagulation for 3 d before being transitioned back down to a heparin-sparing strategy due to increased clot on the oxygenator during daily visual inspection.
Table 1 –
Demographics, patient characteristics, and outcomes for an as-treated analysis between the two cohorts (majority of circuit days on heparin-sparing versus full heparin).
| Demographics, patient characteristics, and outcomes | Heparin-sparing |
Full heparin |
P-value |
|---|---|---|---|
| (n = 22) | (n = 18) | ||
|
| |||
| Age (y) | 39.6 ± 16.2 | 36.2 ± 11.3 | 0.44 |
| Body mass index (kg/m2) | 28.5 ± 7.1 | 38.1 ± 12.4 | 0.01 |
| Trauma | 32% (7) | 38% (7) | 1.00 |
| Injury severity score | 38.6 ± 10.3 | 26.0 ± 12.2 | 0.06 |
| RESP score | 3.5 ± 4.2 | 4.3 ± 2.5 | 0.44 |
| SAPS2 score | 64.6 ± 15.8 | 62.1 ± 14.0 | 0.66 |
| Time from intubation to cannulation (d) | 1.5 (0–6) | 4.5 (1–7) | 0.39 |
| Renal replacement | 45% (10) | 50% (9) | 1.00 |
| Inotrope requirement | 82% (18) | 50% (9) | 0.05 |
| Bleeding complication | 32% (7) | 33% (6) | 1.00 |
| Thrombotic complication | 18% (4) | 39% (7) | 0.17 |
| Mean activated clotting time (s) | 167 ± 15 | 189 ± 15 | <0.01 |
| Mean unit(s) RBC per day | 1.1 (0.6–1.8) | 0.9 (0.6–1.4) | 0.48 |
| ICU length of stay (d) | 22 (15–33) | 26 (12–45) | 0.41 |
| Ventilator days | 22 (13–28) | 20 (11–37) | 0.74 |
| Hospital length of stay (d) | 28 (15–38) | 33 (20–45) | 0.56 |
| Total circuit days | 8 (4–12) | 7.5 (5–9) | 0.57 |
| Survival to decannulation | 59% (13) | 83% (15) | 0.16 |
| Survival to discharge | 50% (11) | 72% (13) | 0.20 |
Table 2 –
Mortality data.
| Patient data | Heparin-sparing | Survival to decannulation | Time from decannulation to death (d) | Cause of death |
|---|---|---|---|---|
|
| ||||
| Preprotocol | ||||
| 35 WF with ARDS due to pulmonary edema after surgery for closed loop obstruction | Y | N | 0 | Withdrawal by family after diagnosis of diffuse bilateral SAH |
| 40 AAF with bacterial pneumonia | N | N | 0 | Maximum medical support despite ECMO, withdrawal by family |
| 52 AAM with multiple KSW to the chest and abdomen | N | N | 0 | Worsening acidosis leading to cardiopulmonary arrest despite ECMO |
| 50 WF with bacterial pneumonia | N | Y | 6 | Multisystem organ failure after decannulation |
| 26 HM with bacterial pneumonia | N | Y | 11 | Pulseless electrical arrest |
| Postprotocol | ||||
| 54 AAF with bacterial pneumonia and pulmonary edema | Y | N | 0 | Voluminous pulmonary edema and biventricular failure, futile care |
| 58 WF with asthma and superimposed pneumonia | Y | Y | 8 | Pupillary asymmetry and maximum medical therapy, family chose to withdraw |
| 46 WM with ARDS from MVC | Y | N | 0 | Brain herniation |
| 27 AAM with multiple GSW to the flank, neck, and face (underwent exploratory thoracotomy) | Y | Y | 7 | Cardiopulmonary arrest |
| 65 AAF with bacterial pneumonia | Y | N | 0 | No improvement despite ECMO, deemed futile care |
| 26 WF with post-influenza pneumonia | Y | N | 0 | Multisystem organ failure despite ECMO |
| 63 WM with bacterial pneumonia (previous heart transplantation 1 mo prior) |
Y | N | 0 | Worsening clinical status despite ECMO and maximal medical therapy, family chose to withdrawal |
| 54 AAM with ARDS from pulmonary contusions after MVC rollover | Y | N | 0 | Worsening clinical status despite ECMO and maximal medical therapy, family chose to withdrawal |
| 60 WM with post-influenza pneumonia | Y | N | 0 | Worsening clinical status despite ECMO and maximal medical therapy, family chose to withdrawal |
| 63 WM with post-influenza pneumonia | Y | N | 0 | Worsening clinical status despite ECMO and maximal medical therapy, family chose to withdrawal |
| 35 WM with post-influenza pneumonia | Y | N | 0 | Worsening clinical status despite ECMO and maximal medical therapy, family chose to withdrawal |
AA = African American; ARDS = acute respiratory distress syndrome; ECMO = extracorporeal membrane oxygenation; F = female; GSW = gunshotwound; H = Hispanic; KSW = knife stab wound; M = male; MVC = motor vehicle collision; SAH = subarachnoid hemorrhage; W = white.
There were 14 trauma patients placed on VV ECMO during the study period: seven were managed with full-heparin anticoagulation (46 circuit days, 0% heparin-sparing) and 7 with a heparin-sparing strategy (45 circuit days, 91% heparin-sparing). Patient information, injuries, and ISS for this subset of trauma patients are shown in Table 3. Of these injured patients, three had intracranial bleeding on arrival: of these, 1 had evolution of a subarachnoid hemorrhage leading to herniation and brain death. Ten of the 14 trauma patients had contraindications to anticoagulation (including ICH, spinal cord injuries, splenic or liver lacerations, or active bleeding; see Table 3); however, none had bleeding complications, even in the case of four patients who received full therapeutic IV heparin.
Table 3 –
Patient data, injuries, and ISS of all trauma patients.
| Full-heparin cohort | ||
|---|---|---|
|
| ||
| Patient data | Injuries | ISS |
|
| ||
| 34 M s/p GSW L flank | Injury to the small intestine (x2), stomach (x2), retroperitoneum, mesentery | 13 |
| 52 WM s/p MVC w/ejection | Bilateral PTX, rib fx, C7 fx, retroperitoneal hematoma, bilateral fibula fx, left malleolar fx, T6 chance fx | 43 |
| 22 WM s/p fall from deer stand | T12 chance fx, fx superior endplate T12, bilateral tibia fx, bilateral open calcaneus fx, bilateral pulmonary contusions | 18 |
| 24 AAM s/p GSW L chest | Injury to diaphragm, stomach, transverse colon, liver laceration, left PTX | 18 |
| 21 WF s/p unrestrained MVC | Liver laceration, spleen laceration, right ankle fx, left femur fx | 38 |
| 52 AAM s/p KSW chest and abdomen | Injury to the liver, stomach, small intestine, R radial artery injury | 17 |
| 41 WM s/p MVC rollover | Left PTX, left femur fx, left rib fx w/pulmonary contusions | 35 |
|
| ||
| Heparin-sparing cohort | ||
| Patient data | Injuries | ISS |
|
| ||
| 23 AAM s/p pedestrian versus car | Left ulna/radius fx, left fibula fx, left rib fx, left scapula fx, spleen laceration | 17 |
| 46 WM s/p unrestrained MVC | SAH, spleen laceration, right adrenal hematoma, L2 comp fx, temporal bone fx, bilateral rib fx, left femur fx, left tibia fx, left fibula fx, right clavicle fx, sacral fx | 43 |
| 27 AAM s/p multiple GSWs | Right ICA injury, pulmonary lacerations, spleen laceration, multiple facial fx, left rib fx, multiple spine fx | 38 |
| 19 WM s/p MVC rollover | Liver laceration, spleen laceration, active extravasation from right kidney, multiple pelvic fx, right rib fx | 41 |
| 18 AAM s/p restrained MVC | Retroperitoneal hematoma, left femur fx, occipital condyle fx, right HTX, bilateral fibula fx, right forearm fx, left pelvic fx | 50 |
| 54 AAM s/p MVC rollover w/ejection | SDH, bilateral PTX, right HTX, T12 compression fx, bilateral rib fx, manubrial fx, left clavicle fx | 38 |
| 25 WM s/p MVC w/ejection | Liver laceration and hematoma, right malleolus fx, bilateral pulmonary contusions, perisplenic hematoma, bilateral rib fx, punctate intracranial contusion, right occipital condyle fx | 43 |
AAM = African American male; Fx = fracture; GSW = gunshot wound; HTX = hemothorax; ICA = internal carotid artery; KSW = knife stab wound; M = male; MVC = motor vehicle collision; PTX = pneumothorax; SAH = subarachnoid hemorrhage; SDH = subdural hematoma; WM = white male; WF = white female.
Thirteen patients had bleeding complications while on ECMO during the study period. There were six patients with bleeding complications in the full-heparin anticoagulation group: six minor complications and two major complications (2 new GIBs). One of the six patients was a trauma patient, with no contraindication to heparin. There were seven patients with bleeding complications in the heparin-sparing group: seven minor complications and four major complications (1 GIB, 1 new ICH, 1 surgical site hemorrhage, and 1 pulmonary hemorrhage). None of the bleeding complications in the heparin-sparing group occurred in a trauma patient. All bleeding complications recorded occurred during the patient’s ECMO course; there were no clear trends as to the timing of complications relative to the day since ECMO start. Multivariable analysis showed that each day of ECMO duration was associated with a 30% increase in the odds of a bleeding complication (P < 0.01). When adjusted for ECMO duration, heparin strategy was not a significant predictor of bleeding complication (odds ratio 0.49 [0.13–1.83], P = 0.289).
Eleven patients had thrombotic complications during the study period. There were seven patients with thrombotic complications in the full-heparin anticoagulation group: 4 DVTs, 4 oxygenator changes, 2 nonfatal pump malfunctions, and 1 PE. The DVTs and PE occurred after ECMO decannulation in all but one patient; a DVT occurred while on ECMO in one patient. There were two trauma patients in this full-heparin subgroup with thrombotic complications, accounting for one of the diagnoses of DVT as well as an oxygenator change and unexpected cessation of the pump. There were four patients with thrombotic complications in the heparin-sparing group: 2 DVTs, 2 oxygenator changes, 1 unexpected cessation of pump, and 1 PE. The DVTs and PE all occurred after the patients were decannulated from ECMO. There were no trauma patients in this heparin-sparing subgroup with thrombotic complications. All recorded PEs occurred after the patients were decannulated from the ECMO circuit. With regards to DVTs, one patient in the full-heparin cohort developed a DVT while on ECMO; the remainder of the patients in the full-heparin cohort and all the patients diagnosed with DVTs in the heparin-sparing cohort were diagnosed after decannulation from ECMO. When adjusted for ECMO duration, heparin strategy was not a significant predictor of thrombotic complication (odds ratio 0.405 [0.01–17.8], P = 0.322).
Of note, there were five patients, all in the full-heparin cohort, that experienced both a bleeding and thrombotic complication. The breakdown of these complications was as follows: patient 1–bleeding from the cannula site and both an oxygenator exchange and unexpected cessation of the ECMO circuit; patient 2–bleeding from the cannula site and unexpected cessation of the ECMO circuit; patient 3–bleeding from the cannula site, GIB, an oxygenator exchange, and DVT (diagnosed while on ECMO); patient 4–bleeding from the cannula site and oxygenator exchange; and patient 5–bleeding from the cannula site, GIB, and DVTs (diagnosed after decannulation from ECMO).
Discussion
The use of ECMO, both VV and veno-arterial (VA), increased 433% from 2006 to 2011.33 As ECMO has become more commonly used, questions have arisen as to whether it is superior, or at least noninferior, to conventional mechanical ventilation alone. Two randomized control trials have been published recently looking at VV ECMO versus mechanical ventilation. The trial comparing conventional ventilatory support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) was published in 2009.7 It was a multicenter randomized control trial of 180 patients (2001–2006) and showed that severe disability-free survival was better in patients randomized to care at an ECMO versus non-ECMO center at the time of their enrollment. There are several criticisms of this trial. First, 24% of the patients randomized to an ECMO center were never cannulated, a significant number for this intention-to-treat analysis. Second, the authors excluded patients with contraindications to anticoagulation. Third, the mechanical ventilation arm was not standardized and included patients from 92 conventional treatment centers. Finally, there was incomplete follow-up data in nearly half of all patients. The Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress syndrome (EOLIA) trial was published in 2018.34 It was a multicenter randomized control trial that planned to enroll 331 patients; however, it was discontinued after enrollment of only 249 patients. The trial was stopped early for futility as there was no statistical difference in the 60-day mortality between the ECMO and mechanical ventilation groups. Although this trial did standardize the mechanical ventilation arm, the study was underpowered. It also allowed for significant crossover to the ECMO arm (28%) while still performing an intention-to-treat analysis, making it difficult to interpret the results. As with the CESAR trial, the EOLIA trial also excluded patients with contraindications to anticoagulation. A recent large retrospective study looking specifically at trauma patients on ECMO concluded that trauma should not be considered a contraindication to ECMO; these patients have reasonable survival (70%) compared to their nontrauma counterparts.20 However, the investigators did not address the potential effects of anticoagulation on ECMO bleeding or thrombotic complications, or survival.20 While ELSO recommends a continuous infusion of unfractionated heparin to achieve an ACT goal of 180–200, there is still significant institutional variability, and no ideal therapeutic range or specific monitoring strategy has been identified.35–37 Several case series have looked at heparin-sparing strategies with patients on VV ECMO and have shown no increased risk in morbidity or mortality, although most have been small volume or have only looked at heparin free versus heparin (summarized in Table 4).21,24–31,38–45 Krueger et al. investigated using only prophylactic subcutaneous enoxaparin in patients on VV ECMO and found that only one-third required blood transfusion with an overall survival rate of 66%; however, this still does not address patients who cannot tolerate any anticoagulation.31
Table 4 –
Literature review of anticoagulation strategies in extracorporeal membrane oxygenation.
| Authors, year | Center, country | Study type | Number of patients enrolled | Initially heparin free | AC target | Comments |
|---|---|---|---|---|---|---|
|
| ||||||
| Abrams et al., 201524 | Columbia University, USA | Case series | Four patients (3 VV ECMO, 1 VA ECMO), acute hypoxemic respiratory failure from diffuse alveolar hemorrhage | Yes | aPTT 40–60 s | One patient started on heparin-free course with heparin restarted after 36 h. three patients started on heparin. |
| Ahmad et al., 201721 | University of Maryland, USA | Case series | 46 (39 VV ECMO, 7 VA ECMO), trauma | Yes | ACT 160–180 s and/or aPTT 60 to 80 s or 45 to 55 s | Use of systemic anticoagulation had no correlation with morbidity but was associated with survival (16/17 survivors versus 12/21 nonsurvivors anticoagulated) |
| Arlt et al., 201029 | University Hospital Regensburg, Germany | Case series | 10 (7 VV ECMO, 3 VA ECMO) w/severe trauma associated coagulopathy | Yes | ACT 120–140 (after resolution of coagulopathy) | All initially heparin-free. No thromboembolic events or unexpected blood clot formation |
| Biderman et al., 201338 | Rabin Medical Center, Israel | Case series | 10 (5 ECMO, 5 interventional lung assist) | Yes | - | Only 3/10 received heparin during first 48 h |
| Biscotti et al., 201528 | Columbia University Medical Center, USA | Case series | Two patients (VV ECMO) with TBI | No | aPTT 40–60s | Low-dose heparin. Both patients had complete neurologic recovery. |
| Cronin et al., 201439 | University of California San Diego, USA | Case report | One patient (VV ECMO after pulmonary endarterectomy) | Yes | Completely heparin-free course of ECMO. Expired 49 d after decannulation. | |
| Firstenberg et al., 201240 | The Ohio State University, USA | Case report | One patient (VV ECMO after traumatic injury) | Yes | Started 48 h after cannulation at 4.0 units/kg/h | Survived to discharge |
| Herbert et al., 201441 | St. Vincent’s Hospital, University of New South Wales, Garvan Institute for Medical Research, Australia | Case report | One patient with Goodpasture syndrome (VV ECMO for pulmonary hemorrhage) | Yes | aPTT peak 104 | 25/26 ECMO days heparinfree. Survived to discharge. |
| Krueger et al., 201631 | Freiburg University Medical Center, Germany | Single-center observational study | 61 patients, respiratory and trauma indications | No | 40 mg enoxaparin daily (60 mg enoxaparin for BMI > 40, 80 mg enoxaparin for BMI > 50) | Four patients with thrombotic complications. No fatal bleeding events, no intracranial hemorrhage. |
| Messing et al., 201442 | George Washington University Hospital, USA | Case report | One patient (VV ECMO after severe trauma) | Yes | ACT 180–200 s | Initially heparin-free, started on heparin after three thrombosed oxygenators, 15/20 d on heparin, survived to discharge |
| Muellenbach et al., 201126 | University of Würzburg, Germany | Case series | Three patients | Yes | aPTT 40–60 s | All initially heparin-free courses, anticoagulation started once stabilized |
| Prat et al., 201530 | French Armed Forces Institute of Biomedical Research France; University of Texas Health Science Center, USA; Fondazione IRCCS, Italy; US Army Institute of Surgical Research, USA | Animal experiment, prospective cohort (sheep) | Nine Sheep | No | Bolus versus standard heparin anticoagulation | Bolus heparin injection can maintain ECMO support for up to 10 h in a sheep model of ARDS |
| Robba et al., 201725 | Cambridge University Hospitals, UK | Case series | Four patients (VV ECMO), severe hypoxemic respiratory failure secondary to traumatic ARDS with preexisting bleeding or intracranial hemorrhage | Yes (1/4 patients) | ACT > 150 s (3/4 patients) | Bleeding complications in all three patients on heparin, clots in vena cava, and oxygenator in heparinfree patient |
| Stoll et al., 201443 | Berufsgenossenschaftliches Universitatsklinikum, Germany | Case report | One patient (VV ECMO after thoracic trauma) | Yes | Heparin 200 I/u (after 72 h heparin free) | Patient recovered |
| Yen et al., 200844 | National Taiwan University Hospital, Taiwan | Case report | One patient (VA ECMO after decompressive craniotomy) | Yes | No heparin during course of VA ECMO | |
| Yeo et al., 201545 | University Yangsan Hospital, South Korea | 71 (49 VA ECMO, 22 VV ECMO), respiratory failure | No | Conventional (ACT 180–220 s) versus lower group (ACT 140160 s) | Incidence of major bleeding and bleeding-induced death higher in conventional group | |
| Wen et al, 201527 | Changhua Christian Hospital, Taiwan | Case report | One patient (VV ECMO after severe trauma) | Yes | No heparin during course of VV ECMO | |
aPTT = activated partial thromboplastin time; ARDS = acute respiratory distress syndrome; BMI = body mass index; TBI = traumatic brain injury.
Therefore, a significant knowledge gap exists regarding the association between heparin management strategy and survival, bleeding, and thrombosis rates in ECMO patients. This study is unique in that the implementation of a protocol change to a standard heparin-sparing anticoagulation strategy for VV ECMO allowed for a comparison of two naturally occurring groups (full heparin versus heparin-sparing) specifically addressing this knowledge gap. It is also one of the largest single-institution studies to look at anticoagulation strategies in VV ECMO. Our study demonstrates no statistically significant differences in mortality, bleeding risk, or thrombotic complication risk in either univariate or multivariable analysis between a heparin-sparing and full therapeutic heparin anticoagulation strategy. This suggests that it is acceptable to initiate VV ECMO patients on a heparin-sparing strategy and reassess daily for the necessity of converting to a full anticoagulation strategy, allowing the inclusion of ECMO in the therapeutic armamentarium for severe respiratory failure in traditionally excluded patients, such as trauma patients and other populations at high risk of bleeding. In fact, this change in practice can be observed in this study by the inclusion of more, and higher average ISS, trauma patients in the postprotocol change, heparin-sparing group. Additionally highlighting the safety of this approach, while all trauma patients in the postprotocol group had a traditional contraindication to heparin, no thrombotic or bleeding complications were observed.
The survival rate at our tertiary referral center (60% overall, 71.4% trauma patients) is on par with survival rates in the literature: 60%–70% for all patients on VV ECMO and 70% for trauma patients.4, 8, 9 Bleeding complications are also comparable and slightly lower than those reported in the literature. At our institution, 32.5% of patients in the total cohort had a bleeding complication as compared to 39% in the literature.12 When looking specifically at trauma patients, 7% had a bleeding complication versus an average of 9% in the literature.12 Thrombotic complications in the literature are often reported as oxygenator dysfunction requiring replacement (29%) and venous thrombosis (10%).10 In our population, 12.5% of patients had oxygenator dysfunction requiring replacement and 15% had a venous thrombosis. It is important to comment on the differences in survival and risks of bleeding and thrombotic events between VV and VA ECMO. VA ECMO is well known in the literature to be associated with a lower chance of survival (30%–40% versus VV 60%–70%), higher chance of bleeding (68.5% versus VV 39.1%), and more thrombotic events (16.7% versus VV 9.4%). Our study focused only on those patients placed on VV ECMO and did not address anticoagulation practices, overall survival, or complication rate for patients placed on VA ECMO at our institution.12
There are important limitations to consider in the interpretation of this study. First, there are a small number of 45 patients and an even smaller subgroup of 14 trauma patients. Second, there were changes in practice unrelated to our anticoagulation strategy that could potentially confound the results, specifically, changing both catheter types and managing services. Before October 2014, the institution utilized the Avalon Elite bicaval dual-lumen catheter placed percutaneously in the right internal jugular vein, preferentially. After October 2014, we changed to Bio-Medicus single-lumen catheters placed percutaneously in the common femoral vein for drainage, and either the internal jugular or contralateral common femoral for return. While no data exist to suggest there is a difference in the rate of bleeding or thrombotic events between the two catheters, there are other distinct advantages and disadvantages to each. The bicaval dual lumen catheters have the advantage of a single insertion site, which decreases the risks of both bleeding and infection. They also allow for patient ambulation while on ECMO, and as they are positioned more posterolateral, make surgical positioning and transport easier.46 The disadvantages of using a bicaval dual lumen catheter are as follows: 1) a more limited range of lumen sizes, which can negatively impact flow and 2) increased difficulty of insertion given the need for precise placement, necessitating fluoroscopy and/or echocardiographic guidance.46 In addition, there is increased instability of the catheter after placement and concern for cerebral venous congestion with a large catheter in the internal jugular vein.46 The bicaval dual lumen catheters may also have an advantage over single-lumen catheters in those patients with bilateral lower extremity orthopedic injuries and other procedural or logistical difficulties in accessing the femoral veins. Although this was not addressed in this study given its small size, one patient in the heparin-sparing group did have an Avalon Elite bicaval dual lumen catheter inserted into the right internal jugular vein due to severe bilateral lower extremity injuries; this patient survived to discharge and had no bleeding or thrombotic complications. We attempted to account for both differences in catheter type as well as a change in managing service by performing preprotocol versus postprotocol and intention-to-treat analyses as well as the as-treated analysis presented previously and found no meaningful differences in study findings; however, these logistical and equipment differences remain potential confounding factors. In addition, as a retrospective study, we cannot rule out that the lack of statistical significance observed is not due to inadequate power; however, this highlights the need for larger multicenter studies specifically geared toward evaluating anticoagulation strategies in patients on VV ECMO.
Conclusion
This 40-patient case series of anticoagulation management while on VV ECMO suggests that patients can be safely maintained on VV ECMO with a heparin-sparing anticoagulation strategy.
Acknowledgment
The authors would like to thank Dr. Wondwosen K. Yimer for his assistance with some of the statistical analysis. The authors would like to thank Dr. Ramola Panchal and Brady Holder for their assistance in data acquisition. One of the authors, K.T.C., is funded by an NIH T32 HL-105324 Training Grant. One of the authors, H.C., is partially supported by the National Institutes of General Medical Sciences under the National Institutes of Health under Award Number 1U54GM115428. One of the authors, M.E.K., is partially funded by a NIH 1U54 GM-115428-01 from Mississippi Center for Translational Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Disclosure
Dr. Copeland’s spouse is a consultant for SynCardia total artificial heart system.
REFERENCES
- 1.Maxwell BG, Powers AJ, Sheikh AY, et al. Resource use trends in extracorporeal membrane oxygenation in adults: an analysis of the Nationwide Inpatient Sample 1998–2009. J Thorac Cardiovasc Surg. 2014;148:416–421.e1. [DOI] [PubMed] [Google Scholar]
- 2.Munoz J, Santa-Teresa P, Tomey MJ, et al. Extracorporeal membrane oxygenation (ECMO) in adults with acute respiratory distress syndrome (ARDS): a 6-year experience and case-control study. Heart Lung. 2017;46:100–105. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy FH, McDermott KM, Kini V, et al. Trends in U.S. Extracorporeal membrane oxygenation use and outcomes: 2002–2012. Semin Thorac Cardiovasc Surg. 2015;27:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paden ML, Conrad SA, Rycus PT, Thiagarajan RR. Extracorporeal life support organization registry report 2012. ASAIO J. 2013;59:202–210. [DOI] [PubMed] [Google Scholar]
- 5.Park PK, Napolitano LM. Bartlett RH Extracorporeal membrane oxygenation in adult acute respiratory distress syndrome. Crit Care Clin. 2011;27:627–646. [DOI] [PubMed] [Google Scholar]
- 6.Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. [DOI] [PubMed] [Google Scholar]
- 7.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. [DOI] [PubMed] [Google Scholar]
- 8.Pappalardo F, Pieri M, Greco T, et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: the ECMOnet score. Intensive Care Med. 2013;39:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–1382. [DOI] [PubMed] [Google Scholar]
- 10.Zangrillo A, Landoni G, Biondi-Zoccai G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15:172–178. [PubMed] [Google Scholar]
- 11.Raiten JM, Wong ZZ, Spelde A, et al. Anticoagulation and transfusion therapy in patients requiring extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2017;31:1051–1059. [DOI] [PubMed] [Google Scholar]
- 12.Mazzeffi M, Greenwood J, Tanaka K, et al. al. Bleeding, transfusion, and mortality on extracorporeal life support: ECLS working group on thrombosis and hemostasis. Ann Thorac Surg. 2016;101:682–689. [DOI] [PubMed] [Google Scholar]
- 13.Shrode CW, Draper KV, Huang RJ, et al. Significantly higher rates of gastrointestinal bleeding and thromboembolic events with left ventricular assist devices. Clin Gastroenterol Hepatol. 2014;12:1461–1467. [DOI] [PubMed] [Google Scholar]
- 14.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. [DOI] [PubMed] [Google Scholar]
- 15.Genovese EA, Dew MA, Teuteberg JJ, et al. Incidence and patterns of adverse event onset during the first 60 days after ventricular assist device implantation. Ann Thorac Surg. 2009;88:1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suarez J, Patel CB, Felker GM, et al. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ Heart Fail. 2011;4:779–784. [DOI] [PubMed] [Google Scholar]
- 17.Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–321. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Ha SO, Han SJ, et al. Extracorporeal membrane oxygenation support in trauma versus nontrauma patients with noninfectious acute respiratory failure. Artif Organs. 2017;41:431–439. [DOI] [PubMed] [Google Scholar]
- 19.Guirand DM, Okoye OT, Schmidt BS, et al. Venovenous extracorporeal life support improves survival in adult trauma patients with acute hypoxemic respiratory failure: a multicenter retrospective cohort study. J Trauma Acute Care Surg. 2014;76:1275–1281. [DOI] [PubMed] [Google Scholar]
- 20.Swol J, Brodie D, Napolitano L, et al. Indications and outcomes of extracorporeal life support in trauma patients. J Trauma Acute Care Surg. 2018;84:831–837. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad SB, Menaker J, Kufera J, et al. Extracorporeal membrane oxygenation after traumatic injury. J Trauma Acute Care Surg. 2017;82:587–591. [DOI] [PubMed] [Google Scholar]
- 22.Bedeir K, Seethala R, Kelly E. Extracorporeal life support in trauma: worth the risks? A systematic review of published series. J Trauma Acute Care Surg. 2017;82:400–406. [DOI] [PubMed] [Google Scholar]
- 23.Bohman JK, Vogt MN, Hyder JA. Retrospective report of contraindications to extracorporeal membrane oxygenation (ECMO) among adults with acute respiratory distress syndrome (ARDS). Heart Lung. 2016;45:227–231. [DOI] [PubMed] [Google Scholar]
- 24.Abrams D, Agerstrand CL, Biscotti M, et al. Extracorporeal membrane oxygenation in the management of diffuse alveolar hemorrhage. ASAIO J. 2015;61:216–218. [DOI] [PubMed] [Google Scholar]
- 25.Robba C, Ortu A, Bilotta F, et al. Extracorporeal membrane oxygenation for adult respiratory distress syndrome in trauma patients: a case series and systematic literature review. J Trauma Acute Care Surg. 2017;82:165–173. [DOI] [PubMed] [Google Scholar]
- 26.Muellenbach RM, Kredel M, Kunze E, et al. Prolonged heparinfree extracorporeal membrane oxygenation in multiple injured acute respiratory distress syndrome patients with traumatic brain injury. J Trauma Acute Care Surg. 2012;72:1444–1447. [DOI] [PubMed] [Google Scholar]
- 27.Wen PH, Chan WH, Chen YC, et al. Non-heparinized ECMO serves a rescue method in a multitrauma patient combining pulmonary contusion and nonoperative internal bleeding: a case report and literature review. World J Emerg Surg. 2015;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biscotti M, Gannon WD, Abrams D, et al. Extracorporeal membrane oxygenation use in patients with traumatic brain injury. Perfusion. 2015;30:407–409. [DOI] [PubMed] [Google Scholar]
- 29.Arlt M, Philipp A, Voelkel S, et al. Extracorporeal membrane oxygenation in severe trauma patients with bleeding shock. Resuscitation. 2010;81:804–809. [DOI] [PubMed] [Google Scholar]
- 30.Prat NJ, Meyer AD, Langer T, et al. Low-dose heparin anticoagulation during extracorporeal life support for acute respiratory distress syndrome in conscious sheep. Shock. 2015;44:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krueger K, Schmutz A, Zieger B, Kalbhenn J. Venovenous extracorporeal membrane oxygenation with prophylactic subcutaneous anticoagulation only: an observational study in more than 60 patients. Artif Organs. 2017;41:186–192. [DOI] [PubMed] [Google Scholar]
- 32.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute Physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. [DOI] [PubMed] [Google Scholar]
- 33.Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015;61:31–36. [DOI] [PubMed] [Google Scholar]
- 34.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. [DOI] [PubMed] [Google Scholar]
- 35.Murphy DA, Hockings LE, Andrews RK, et al. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev. 2015;29:90–101. [DOI] [PubMed] [Google Scholar]
- 36.Esper SA, Levy JH, Waters JH, Welsby IJ. Extracorporeal membrane oxygenation in the adult: a review of anticoagulation monitoring and transfusion. Anesth Analg. 2014;118:731–743. [DOI] [PubMed] [Google Scholar]
- 37.Esper SA, Welsby IJ, Subramaniam K, et al. Adult extracorporeal membrane oxygenation: an international survey of transfusion and anticoagulation techniques. Vox Sang. 2017;112:443–452. [DOI] [PubMed] [Google Scholar]
- 38.Biderman P, Einav S, Fainblut M, et al. Extracorporeal life support in patients with multiple injuries and severe respiratory failure: a single-center experience? J Trauma Acute Care Surg. 2013;75:907–912. [DOI] [PubMed] [Google Scholar]
- 39.Cronin B, Maus T, Pretorius V, et al. Case 13–2014: management of pulmonary hemorrhage after pulmonary endarterectomy with venovenous extracorporeal membrane oxygenation without systemic anticoagulation. J Cardiothorac Vasc Anesth. 2014;28:1667–1676. [DOI] [PubMed] [Google Scholar]
- 40.Firstenberg MS, Nelson K, Abel E, McGregor J, Eiferman D. Extracorporeal membrane oxygenation for complex multiorgan system trauma. Case Rep Surg. 2012;2012:897184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herbert DG, Buscher H, Nair P. Prolonged venovenous extracorporeal membrane oxygenation without anticoagulation: a case of Goodpasture syndrome-related pulmonary haemorrhage. Critical care and resuscitation. J Australas Acad Crit Care Med. 2014;16:69–72. [PubMed] [Google Scholar]
- 42.Messing JA, Agnihothri RV, Van Dusen R, et al. Prolonged use of extracorporeal membrane oxygenation as a rescue modality following traumatic brain injury. ASAIO J. 2014;60:597–599. [DOI] [PubMed] [Google Scholar]
- 43.Stoll MC, Rademacher F, Klak K, et al. Veno-venous extracorporeal membrane oxygenation therapy of a severely injured patient after secondary survey. Am J Emerg Med. 2014;32:1300.e1–1300.e2. [DOI] [PubMed] [Google Scholar]
- 44.Yen TS, Liau CC, Chen YS, Chao A. Extracorporeal membrane oxygenation resuscitation for traumatic brain injury after decompressive craniotomy. Clin Neurol Neurosurg. 2008;110:295–297. [DOI] [PubMed] [Google Scholar]
- 45.Yeo HJ, Kim DH, Jeon D, Kim YS, Cho WH. Low-dose heparin during extracorporeal membrane oxygenation treatment in adults. Intensive Care Med. 2015;41:2020–2021. [DOI] [PubMed] [Google Scholar]
- 46.Tulman DB, Stawicki SP, Whitson BA, et al. Veno-venous ECMO: a synopsis of nine key potential challenges, considerations, and controversies. BMC Anesthesiol. 2014;14:65. [DOI] [PMC free article] [PubMed] [Google Scholar]


