Abstract
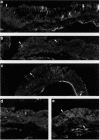
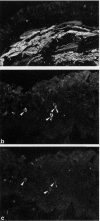
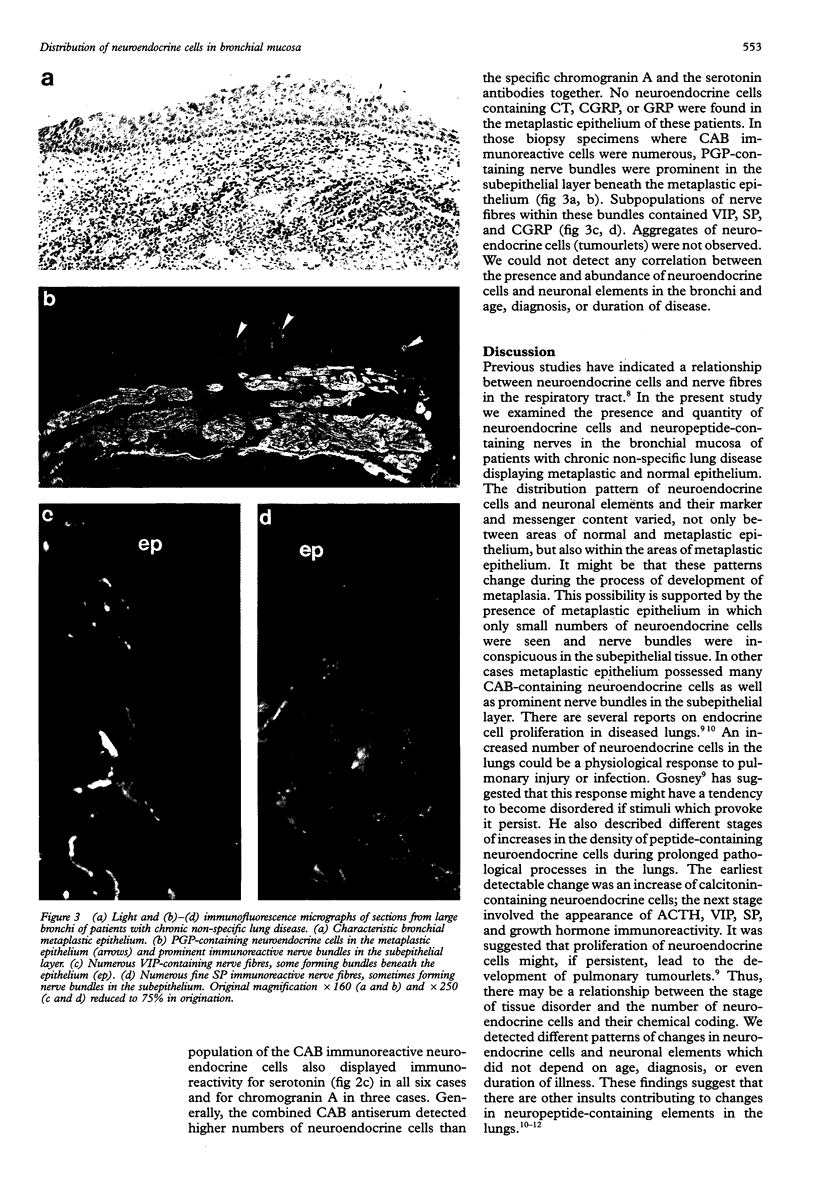
BACKGROUND--It is not clear whether there is any association between metaplasia of the bronchial epithelium and changes in the distribution of neuroendocrine cells. This study examined, by immunohistological techniques, the distribution of neuroendocrine cells and juxtamucoscal nerve fibres in bronchial biopsies showing metaplastic changes. METHODS--Bronchial biopsies from 12 subjects with epithelial metaplasia associated with bronchiectasis and diffuse pulmonary fibrosis were examined by conventional light microscopy and immunohistological techniques for protein gene product 9.5 (PGP), chromogranin A and B (CAB), serotonin, vasoactive intestinal peptide (VIP), substance P (SP), calcitonin gene-related peptide (CGRP), calcitonin (CT), and gastrin releasing peptide (GRP). RESULTS--Regions of non-metaplastic epithelium contained numerous PGP and serotonin immunoreactive cells. Sub-populations of these cells displayed CAB, CGRP, CT, and GRP immunoreactivity. Metaplastic epithelium contained only a few weakly stained PGP, serotonin, CAB, GRP, CT and CGRP immunoreactive cells in six cases. Metaplastic epithelium was characterised by a high number of CAB-containing cells in six cases and in these biopsies prominent PGP-containing nerve bundles were seen in the subepithelial layer beneath the metaplastic epithelium. CONCLUSIONS--The distribution patterns of neuroendocrine cells and neuronal elements vary between areas of normal and metaplastic epithelium and within areas of metaplastic epithelium. Neuronal hyperplasia was associated with an increase in the number of CAB-containing cells within the metaplastic epithelium.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adriaensen D., Scheuermann D. W. Neuroendocrine cells and nerves of the lung. Anat Rec. 1993 May;236(1):70–86. doi: 10.1002/ar.1092360111. [DOI] [PubMed] [Google Scholar]
- Aguayo S. M., Miller Y. E., Waldron J. A., Jr, Bogin R. M., Sunday M. E., Staton G. W., Jr, Beam W. R., King T. E., Jr Brief report: idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease. N Engl J Med. 1992 Oct 29;327(18):1285–1288. doi: 10.1056/NEJM199210293271806. [DOI] [PubMed] [Google Scholar]
- COONS A. H., LEDUC E. H., CONNOLLY J. M. Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med. 1955 Jul 1;102(1):49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson B., Arnberg H., Oberg K., Hellman U., Lundqvist G., Wernstedt C., Wilander E. A polyclonal antiserum against chromogranin A and B--a new sensitive marker for neuroendocrine tumours. Acta Endocrinol (Copenh) 1990 Feb;122(2):145–155. doi: 10.1530/acta.0.1220145. [DOI] [PubMed] [Google Scholar]
- Gosney J. R. Neuroendocrine cell populations in postnatal human lungs: minimal variation from childhood to old age. Anat Rec. 1993 May;236(1):177–180. doi: 10.1002/ar.1092360121. [DOI] [PubMed] [Google Scholar]
- Gosney J. R., Sissons M. C., Allibone R. O. Neuroendocrine cell populations in normal human lungs: a quantitative study. Thorax. 1988 Nov;43(11):878–882. doi: 10.1136/thx.43.11.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. E., Wobken J. D., Landrum B. G. Changes in bombesin, calcitonin, and serotonin immunoreactive pulmonary neuroendocrine cells in cystic fibrosis and after prolonged mechanical ventilation. Am Rev Respir Dis. 1988 Jan;137(1):123–131. doi: 10.1164/ajrccm/137.1.123. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Van Lommel A. T., Dom R. J. Innervation of rabbit intrapulmonary neuroepithelial bodies. Quantitative and qualitative ultrastructural study after vagotomy. J Neurol Sci. 1985 Jan;67(1):81–92. doi: 10.1016/0022-510x(85)90024-3. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Cano M., Rosa P., Hille A., Huttner W. B. Distribution of chromogranin A and secretogranin I (chromogranin B) in neuroendocrine cells and tumors. Am J Pathol. 1988 Feb;130(2):296–304. [PMC free article] [PubMed] [Google Scholar]
- Luts A., Uddman R., Alm P., Basterra J., Sundler F. Peptide-containing nerve fibers in human airways: distribution and coexistence pattern. Int Arch Allergy Immunol. 1993;101(1):52–60. doi: 10.1159/000236498. [DOI] [PubMed] [Google Scholar]
- Persson C. G. Mucosal exudation in respiratory defence: neural or non-neural control? Int Arch Allergy Appl Immunol. 1991;94(1-4):222–226. doi: 10.1159/000235366. [DOI] [PubMed] [Google Scholar]
- Weiler R., Feichtinger H., Schmid K. W., Fischer-Colbrie R., Grimelius L., Cedermark B., Papotti M., Bussolati G., Winkler H. Chromogranin A and B and secretogranin II in bronchial and intestinal carcinoids. Virchows Arch A Pathol Anat Histopathol. 1987;412(2):103–109. doi: 10.1007/BF00716181. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Huttner W. B. Synaptophysin and chromogranins/secretogranins--widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58(2):95–121. doi: 10.1007/BF02890062. [DOI] [PubMed] [Google Scholar]