Abstract
Influenza virus induces apoptosis in cultured cell lines as well as in animal tissues. HeLa cells were infected with influenza virus A/Udon/72 (H3N2) under conditions resulting in almost 100% infection. Such cells underwent typical caspase-dependent apoptosis and were efficiently phagocytosed by macrophages prepared from peritoneal fluids of thioglycolate-treated mice. The membrane phospholipid phosphatidylserine appeared on the surfaces of virus-infected cells at around the time efficient phagocytosis became detectable. In fact, the phagocytosis was almost completely inhibited in the presence of liposomes containing phosphatidylserine, which did not influence the antibody-dependent uptake of zymosan particles by the same macrophages. These results indicate that macrophages phagocytose influenza virus-infected HeLa cells in a manner mediated by phosphatidylserine that appears on the surfaces of infected cells during the process of apoptosis.
Many viruses induce apoptotic death in host cells, but the physiological meaning of this phenomenon is not yet understood (9, 27). HeLa cells infected with influenza A virus undergo Fas/Fas ligand-mediated and caspase-dependent apoptosis (7, 25, 28), but the virus appears to replicate normally and virus progeny are released into the culture medium (26). Cells undergoing apoptosis are in general rapidly and selectively engulfed by phagocytes (3, 29), and this presumably prevents inflammation that would otherwise be caused by the noxious contents of dead cells (14, 16, 17). It could thus be reasonably expected that influenza virus-infected cells would be susceptible to apoptosis-dependent phagocytosis.
Recently, it was reported that phagocytosis of apoptotic cells leads to antigen presentation to lymphocytes; dendritic cells that phagocytosed influenza virus-infected cells undergoing apoptosis stimulated CD8+ T cells (1, 2). It was shown, on the other hand, that phagocytosis of influenza virus-infected cells by macrophages led to elimination of the virus (8). In both cases, virus-infected cells were phagocytosed depending on the occurrence of apoptosis (2, 8). These results suggest that apoptosis of influenza virus-infected cells protects the organism from viral invasion in a dual manner. Apoptosing cells expose a phagocytic marker(s) on their surfaces, and phagocytes recognize this marker and use a specific receptor(s) to engulf the cells presenting this marker (14, 16, 17). As a first step toward an understanding of the molecular basis of the phagocytosis of influenza virus-infected cells, we searched for the marker molecule(s) responsible for the recognition of influenza virus-infected cells by macrophages.
MATERIALS AND METHODS
Infection and growth of influenza virus in HeLa cells.
HeLa S3 cells were maintained in Eagle's minimal essential medium containing 10% fetal bovine serum at 37°C in 5% CO2. HeLa cells were infected with a wild-type strain of influenza A/Udon/72 (H3N2) virus, SP626, at a multiplicity of infection of two, as described previously (7, 28). Virus growth was monitored by either an immunohistochemical analysis or a plaque assay (24). For immunohistochemistry, influenza virus-infected HeLa cells were maintained in poly-d-Lys-coated culture containers, fixed, and permeabilized, as described previously (8). The cells were treated with an anti-influenza virus antiserum which recognizes NP, M1, HA, and NA (19) and then with a fluorescein isothiocyanate (FITC)-conjugated anti-rabbit immunoglobulin G (IgG) antibody (Immunotech, Marseilles, France) and examined by fluorescence and phase-contrast microscopy (BX50 microscope; Olympus, Tokyo, Japan). The amount of virus released into the culture medium was determined by a plaque assay using MDCK cells and expressed as PFU as described previously (25).
Apoptosis analysis.
Cell viability and chromatin condensation were analyzed under a microscope after staining cells with trypan blue and Hoechst 33342, respectively. Translocation of the membrane phospholipid phosphatidylserine (PS) from the cytoplasmic to the exoplasmic leaflet of the plasma membrane was determined by flow cytometry using annexin V, which specifically binds to PS, as described previously (10, 12). In brief, cells were treated with FITC-labeled annexin V (Bender MedSystems, Vienna, Austria) and propidium iodide, a membrane-impermeative fluorochrome, and analyzed in a flow cytometer (EPICS-XL; Coulter, Hialeah, Fla.). The cells that were less intensely stained with propidium iodide and thus impermeable by annexin V were gated and analyzed for the amount of bound FITC-annexin V. To inhibit apoptosis, z-VAD-fmk (23) (Peptide Institute, Osaka, Japan), an inhibitor of caspases, was added to the medium when virus-infected cells were put into the culture dishes.
Macrophage preparation and phagocytosis assay.
Macrophages were isolated from the peritoneal cavities of thioglycolate-treated BDF1 mice and maintained in RPMI 1640 containing 10% fetal bovine serum at 37°C until use, as described previously (4, 21). The phagocytosis assay was performed essentially as described previously (20). Briefly, HeLa cells were labeled with biotin (NHS-LS-Biotin; Pierce, Rockford, Ill.), mixed with macrophages (at a ratio of five target cells to one macrophage), and incubated at 37°C for 2 h. The mixture was washed by pipetting with phosphate-buffered saline (PBS) and then with trypsin (0.5 μg/ml) to remove HeLa cells free from or lightly attached to macrophages. The remaining cells were further fixed with PBS containing 2% paraformaldehyde, 0.5% glutaraldehyde, and 0.05% Triton X-100 and then supplemented with FITC-conjugated avidin (fluorescein-avidin D; Vector, Burlingame, Calif.). The number of macrophages containing engulfed cells was determined using fluorescence and phase-contrast microscopy and expressed relative to the total number of macrophages; this ratio was termed the phagocytic index. Zymosan particles (Sigma, St. Louis, Mo.) were swollen in water at 100°C for 1 h and washed with PBS. The particles were then labeled with 5-carboxyfluorescein, succinimidyl ester (Molecular Probes, Eugene, Oreg.), and incubated with mouse IgG (Zymed, San Francisco, Calif.). The fluorescein-labeled and opsonized zymosan particles were added to macrophages that had been plated on coverslips with serum-free medium. The mixture was kept on ice for 10 min and then incubated at 37°C for 1 h. Unincorporated particles were washed out by pipetting with PBS, and the macrophages were fixed. The phagocytic index was determined as described above. The means and standard deviations (SDs) for typical examples from at least three independent experiments are presented.
Liposome preparation.
Phospholipids (Avanti Polar Lipids, Alabaster, Ala.) were dried as films, suspended in PBS, and sonicated (20). Liposomes were formed using either phosphatidylcholine only (PC liposomes) or a combination of phosphatidylcholine and PS at a molar ratio of 7:3 (PS liposomes).
RESULTS
Virus growth and apoptosis in influenza virus-infected HeLa cells.
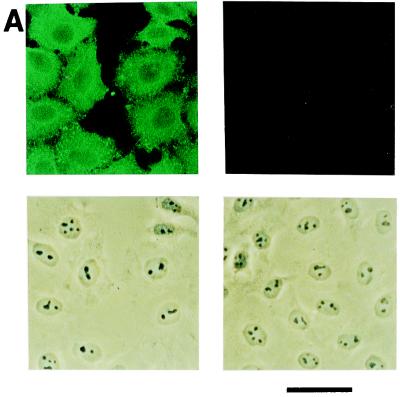
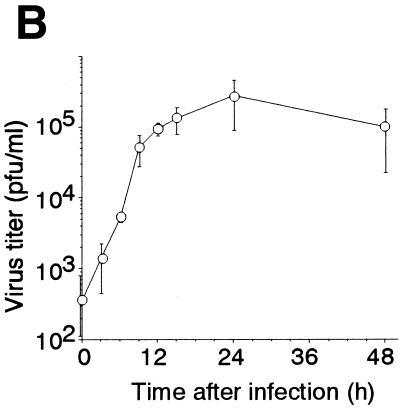
When HeLa cells infected with a wild-type strain of influenza virus for various periods were subjected to an immunohistochemical analysis using an antiserum against virions, the cells were almost 100% positive at 6 h after infection (Fig. 1A, left panels), and the positivity continued for the next 18 h (data not shown). The same antibody did not react with uninfected cells (Fig. 1A, right panels). The virus titer in the culture medium, which was determined by a plaque assay using MDCK cells, increased as the time of culturing of the infected cells increased (Fig. 1B). The titer started to increase at 9 h and reached a plateau at 12 h. These results indicate that near 100% infection was achieved and that virus release began at approximately 9 h postinfection under these conditions.
FIG. 1.
Growth of influenza virus in HeLa cells. (A) Immunohistochemical analysis of virus-infected HeLa cells with an antibody raised against virions. HeLa cells at 6 h postinfection (left) and mock-infected cells (right) were treated with the primary antibody followed by the addition of a fluorescence-labeled secondary antibody and examined under a fluorescence and a phase-contrast microscope. Fluorescence (top) and phase-contrast (bottom) views are shown. Bar = 50 μm. (B) Time course of virus growth. The virus titer in the culture medium was determined by a plaque assay. A typical example from three independent experiments is shown with the means and SDs.
We then determined the time course of apoptosis in influenza virus-infected cells in terms of increases in plasma membrane permeability and in the number of cells with condensed chromatin. These changes became evident almost simultaneously after 9 h of infection (Fig. 2).
FIG. 2.
Influenza virus-induced apoptosis of HeLa cells. HeLa cells infected with influenza virus for the indicated periods were analyzed for cell viability and chromatin condensation. The percentages of cells with impermeable plasma membranes and condensed chromatin are presented.
Phagocytosis of influenza virus-infected HeLa cells by mouse peritoneal macrophages.
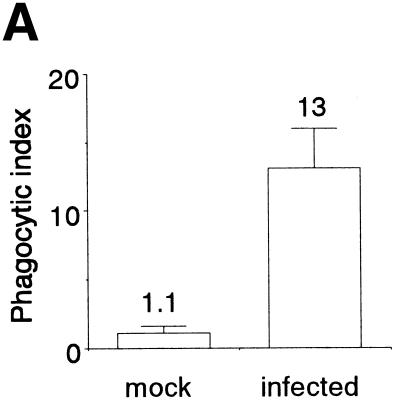
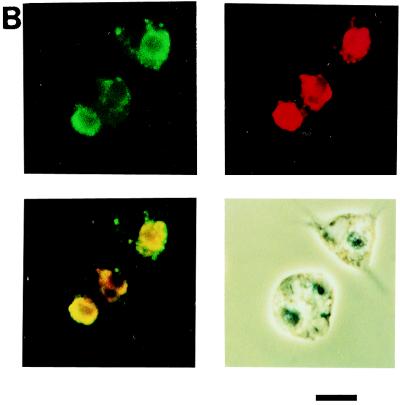
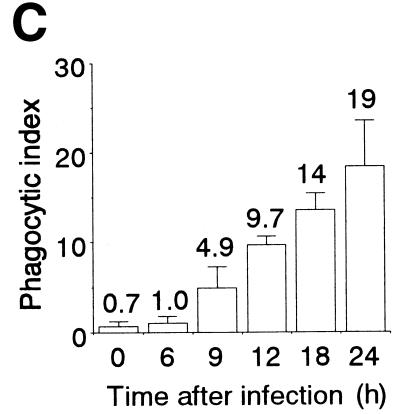
HeLa cells infected with influenza virus for 24 h were labeled with biotin and mixed with mouse peritoneal macrophages. The mixture was further cultured for 2 h, washed, fixed, permeabilized, and supplemented with FITC-avidin. When the reacted cells were examined by fluorescence and phase-contrast microscopy, many fluorescent particles were detected in the cytoplasms of macrophages, but control mock-infected cells were not significantly engulfed by macrophages (data not shown). Quantitative analysis of the phagocytosis reaction revealed that HeLa cells became susceptible to phagocytosis by macrophages upon infection with influenza virus (Fig. 3A). In order to more directly examine whether phagocytosed cells were infected with influenza virus, after the phagocytosis reaction macrophages were simultaneously treated with Texas red-labeled avidin and an anti-influenza virus antibody followed by the addition of an FITC-labeled secondary antibody and examined by fluorescence and phase-contrast microscopy. Most of the engulfed HeLa cells, stained in red, were positive for the antibody (Fig. 3B), indicating that influenza virus-infected cells were selectively phagocytosed by macrophages. We next determined the extent of phagocytosis, using HeLa cells that had been infected with influenza virus for various periods. Phagocytosis became evident at significant levels at 9 h, and the phagocytic index continued to increase thereafter (Fig. 3C). This indicates that virus release, apoptosis induction, and phagocytosis by macrophages occurred with similar time courses during infection.
FIG. 3.
Phagocytosis of influenza virus-infected HeLa cells by mouse peritoneal macrophages. (A) Cells infected with virus for 18 h and mock-infected cells were subjected to a phagocytosis assay. The means and SDs are shown. (B) Immunohistochemical analysis of macrophages after the phagocytosis reaction with the anti-influenza virus antibody. All HeLa cells (18 h postinfection) used were marked by Texas red (red), and virus-infected cells were detected with an FITC-conjugated secondary antibody (green). Top left, FITC signal from virus-infected cells; top right, Texas red signal from all HeLa cells; bottom left, merged view of Texas red and FITC signals; bottom right, phase-contrast view. Bar = 10 μm. (C) Time course of phagocytosis reaction. HeLa cells infected with influenza virus for the indicated periods were subjected to a phagocytosis assay.
Involvement of PS in phagocytosis of virus-infected cells.
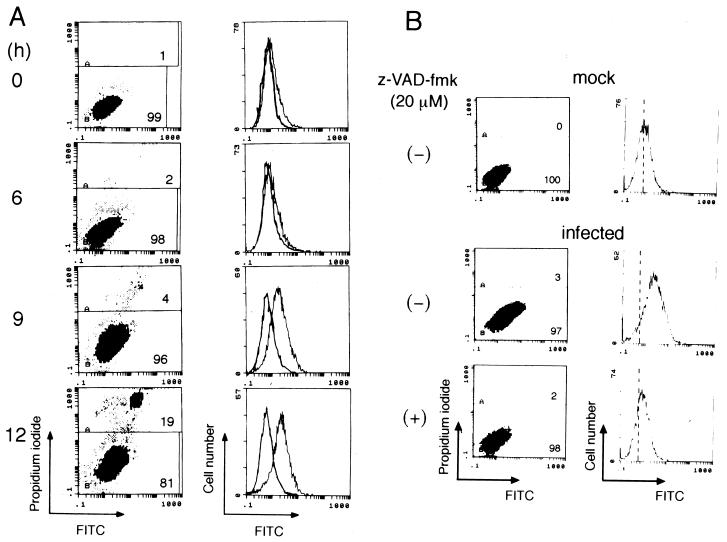
Since phagocytosis of influenza virus-infected HeLa cells depends on the occurrence of apoptosis (8), the infected cells are likely to possess a phagocytosis marker(s) that makes apoptotic cells recognizable by phagocytes. Previous experiments showed that the phagocytosis marker PS, a phospholipid which is normally restricted to the inner leaflet of the membrane bilayer, translocates to the outer leaflet and is exposed to the surfaces of influenza virus-infected HeLa cells (7). Such PS externalization occurred about 9 h after virus infection (Fig. 4A), at which time the extent of phagocytosis increased greatly (Fig. 3C). We thus hypothesized that PS serves as a phagocytosis marker for influenza virus-infected HeLa cells.
FIG. 4.
Caspase-dependent externalization of PS in influenza virus-infected HeLa cells. (A) HeLa cells infected with influenza virus for the indicated periods were analyzed for PS externalization in a flow cytometer. Cells less intensely stained with propidium iodide (bottom area in the left panels) were analyzed for the binding of annexin V (right panels). Thick lines, mock-infected cells; thin lines, virus-infected cells. Numbers in the left panels indicate the percentages of cells in the corresponding areas. (B) HeLa cells infected with virus for 9 h in the absence or presence of z-VAD-fmk were analyzed for PS externalization. Vertical dotted lines indicate the mean fluorescence in the analysis of mock-infected cells.
We first examined whether PS externalization requires activated caspases. To test this, influenza virus infection was done in the presence of z-VAD-fmk, and the amount of PS on the cell surface was determined by flow cytometry. This inhibitor does not affect the growth of influenza virus (26). The results clearly showed that the increase in the number of cells with externalized PS which was observed upon virus infection was almost completely abrogated by the addition of the inhibitor (Fig. 4B). It was thus concluded that PS externalization in influenza virus-infected HeLa cells depends on apoptosis and is an event downstream of the caspase cascade.
In order to examine whether PS externalized on the surfaces of virus-infected cells is recognized by macrophages, we conducted a phagocytosis assay in the presence of PS liposomes using HeLa cells infected with virus for various periods. PS liposomes significantly inhibited phagocytosis at all times examined, whereas PC liposomes showed a minimal effect (Fig. 5A). When the liposomes were added at various concentrations, PS liposomes inhibited phagocytosis in a dose-dependent manner, while PC liposomes did not show an inhibitory effect at any concentration (Fig. 5B). In contrast, macrophage uptake of IgG-coated zymosan particles, which is presumably mediated through Fc/Fc receptor interaction, was not affected by PS liposomes (Fig. 5C). This shows that inhibition of phagocytosis of virus-infected cells does not reflect a nonspecific effect of liposomes on macrophage action. All of these results collectively indicated that phagocytosis of influenza virus-infected HeLa cells by macrophages was mediated by PS which was exposed on the surfaces of HeLa cells during the process of apoptosis.
FIG. 5.
Effect of liposomes on phagocytosis of influenza virus-infected HeLa cells. (A) HeLa cells infected with virus for the indicated periods were subjected to a phagocytosis assay in the presence of PS liposomes or PC liposomes (1 mM). (B) Liposomes were added at various concentrations in the phagocytosis reaction using HeLa cells infected for 24 h. The extent of phagocytosis is shown relative to that for a control reaction with no added liposomes, which was considered 100%. (C) Phagocytosis of opsonized zymosan particles by macrophages was conducted in the presence or absence of PS liposomes. The extent of phagocytosis is shown relative to that for a control reaction with no added liposomes, which was considered 100%. The means of the phagocytic index in control reactions were 2.5 (9 h), 6.7 (12 h), 10 (18 h), 11 (24 h [A]), 14 (24 h [B]), and 34 (C).
DISCUSSION
Influenza virus-infected cells are engulfed by phagocytes, such as dendritic cells (1, 2) and macrophages (2, 8), in an apoptosis-dependent manner. Apoptosing cells are generally recognized by phagocytes through marker molecules, which appear on cell surfaces upon apoptosis induction (14, 16, 17). Therefore, it was reasonable to expect that apoptosing influenza virus-infected cells possess such a marker. The results of this study revealed that PS, the best-characterized phagocytosis marker (6, 11), is the one that makes macrophages recognize and engulf influenza virus-infected cells. PS, which is localized at the cytoplasmic side of the membrane bilayer in normal cells (30), translocated to cell surfaces during the apoptosis pathway in influenza virus-infected cells and served as a phagocytosis marker. Macrophages are likely to recognize influenza virus-infected cells using a presumed PS receptor, and one such molecule has recently been identified (5). The presence of a phagocytosis-inducing PS receptor has also been reported for other phagocytes, such as testicular Sertoli cells (22) and vascular endothelial cells (13). Albert et al. (2) suggested that dendritic cells recognize influenza virus-infected monocytes using αVβ5 integrin and CD36, the latter of which binds to PS (15). Although the involvement of PS in the recognition between the two cell types has not been examined, CD36 could be a PS receptor that induces dendritic cell phagocytosis.
The extent of phagocytosis continued to increase even after PS externalization was completed at 12 h postinfection, and phagocytosis at all time points was almost completely inhibited by the addition of PS liposomes. This indicates that the exposure of PS is necessary but not sufficient for efficient phagocytosis of influenza virus-infected cells. We presume the presence of another molecule which is involved in recognition of virus-infected cells by macrophages, most probably in cooperation with PS. Some viral protein(s) could be such a molecule, since an antihemagglutinin antibody inhibited the binding of virus-infected cells to macrophages (18). We previously showed that cells exposing PS independent of apoptosis are phagocytosed, but only inefficiently, in a PS-mediated manner (21). It is thus possible that a candidate molecule(s) gains its function upon apoptosis induction. Identification of such a molecule(s) is important for fully understanding the molecular basis of phagocytosis, not only of influenza virus-infected cells but also of apoptotic cells in general.
Phagocytosis of influenza virus-infected cells leads to inhibition of virus growth (9). We showed here that influenza virus-treated cells became susceptible to macrophage phagocytosis at an early stage of infection. It is thus anticipated that phagocytosis of influenza virus-infected cells plays a role in the initial defense against viral invasion. Since phagocytosis of influenza virus-infected cells by dendritic cells leads to antigen presentation to CD8+ T lymphocytes (1, 2), apoptosis-dependent phagocytosis of virus-infected cells may protect the organism from influenza virus in two different ways. Further experiments using experimental animals will be necessary to examine whether such a host defense system really functions in vivo.
ACKNOWLEDGMENTS
We thank K. Shimizu for the anti-influenza virus antiserum.
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan; by a grant from the Organized Research Combination System of the Science and Technology Agency of Japan; and by a grant from the Uehara Memorial Foundation.
REFERENCES
- 1.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 2.Albert M L, Pearce S F A, Francisco L M, Sauter B, Roy P, Silverstein R L, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αVβ5 and CD36 and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis R E, Yuan J, Horvitz H R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 4.Fadok V A, Voelker D R, Campbell P A, Cohen J J, Bratton D L, Henson P M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 5.Fadok V A, Bratton D L, Rose D M, Pearson A, Ezekewitz R A B, Henson P M. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 6.Fadok V A, Bratton D L, Frasch S C, Warner M L, Henson P M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998;5:551–562. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto I, Takizawa T, Ohba Y, Nakanishi Y. Co-expression of Fas and Fas-ligand on the surface of influenza virus-infected cells. Cell Death Differ. 1998;5:426–431. doi: 10.1038/sj.cdd.4400362. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto I, Pan J, Takizawa T, Nakanishi Y. Virus clearance through apoptosis-dependent phagocytosis of influenza A virus-infected cells by macrophages. J Virol. 2000;74:3399–3403. doi: 10.1128/jvi.74.7.3399-3403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granville D J, Carthy C M, Yang D, Hunt D W C, McManus B M. Interaction of viral proteins with host cell death machinery. Cell Death Differ. 1998;5:653–659. doi: 10.1038/sj.cdd.4400388. [DOI] [PubMed] [Google Scholar]
- 10.Koopman G, Reutelingsperger C P M, Kuijten G A M, Keehnen R M J, Pals S T, van Oers M H J. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 11.Krahling S, Callahan M K, Williamson P, Schlegel R A. Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 1999;6:183–189. doi: 10.1038/sj.cdd.4400473. [DOI] [PubMed] [Google Scholar]
- 12.Martin S J, Reutelingsperger C P M, Green D R. Annexin V: a specific probe for apoptotic cells. In: Cotton Y G, Martin S J, editors. Techniques in apoptosis: a user's guide. London, England: Portland Press; 1996. pp. 107–119. [Google Scholar]
- 13.Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, Masaki T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci USA. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren Y, Savill J. Apoptosis: the importance of being eaten. Cell Death Differ. 1998;5:563–568. doi: 10.1038/sj.cdd.4400407. [DOI] [PubMed] [Google Scholar]
- 15.Rigotti A, Acton S L, Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- 16.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 17.Savill J. Recognition and phagocytosis of cells undergoing apoptosis. Br Med Bull. 1997;53:491–508. doi: 10.1093/oxfordjournals.bmb.a011626. [DOI] [PubMed] [Google Scholar]
- 18.Scott C B, Ratcliffe D R, Cramer E B. Human monocytes are unable to bind to or phagocytize IgA and IgG immune complexes formed with influenza virus in vitro. J Immunol. 1996;157:351–359. [PubMed] [Google Scholar]
- 19.Shimizu K, Mukaigawa J, Oguro M, Ono Y, Nakajima K, Kida H. Inhibition of transcriptase activity of influenza A virus in vitro by anti-haemagglutinin antibodies. Vaccine. 1985;3:207–210. doi: 10.1016/0264-410x(85)90107-0. [DOI] [PubMed] [Google Scholar]
- 20.Shiratsuchi A, Umeda M, Ohba Y, Nakanishi Y. Recognition of phosphatidylserine on the surface of apoptotic spermatogenic cells and subsequent phagocytosis by Sertoli cells of the rat. J Biol Chem. 1997;272:2354–2358. doi: 10.1074/jbc.272.4.2354. [DOI] [PubMed] [Google Scholar]
- 21.Shiratsuchi A, Osada S, Kanazawa S, Nakanishi Y. Essential role of phosphatidylserine externalization in apoptosing cell phagocytosis by macrophages. Biochem Biophys Res Commun. 1998;246:549–555. doi: 10.1006/bbrc.1998.8663. [DOI] [PubMed] [Google Scholar]
- 22.Shiratsuchi A, Kawasaki Y, Ikemoto M, Arai H, Nakanishi Y. Role of class B scavenger receptor type I in phagocytosis of apoptotic rat spermatogenic cells by Sertoli cells. J Biol Chem. 1999;274:5901–5908. doi: 10.1074/jbc.274.9.5901. [DOI] [PubMed] [Google Scholar]
- 23.Slee E A, Zhu H, Chow S C, MacFarlane M, Nicholson D W, Cohen G M. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem J. 1996;315:21–24. doi: 10.1042/bj3150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takizawa T, Matsukawa S, Higuchi Y, Nakamura S, Nakanishi Y, Fukuda R. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J Gen Virol. 1993;74:2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- 25.Takizawa T, Fukuda R, Miyawaki T, Ohashi K, Nakanishi Y. Activation of the apoptotic Fas antigen-encoding gene upon influenza virus infection involving spontaneously produced beta-interferon. Virology. 1995;209:288–296. doi: 10.1006/viro.1995.1260. [DOI] [PubMed] [Google Scholar]
- 26.Takizawa T, Tatematsu C, Ohashi K, Nakanishi Y. Recruitment of apoptotic cysteine proteases (caspases) in influenza virus-induced cell death. Microbiol Immunol. 1999;43:245–252. doi: 10.1111/j.1348-0421.1999.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 27.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wada N, Matsumura M, Ohba Y, Kobayashi N, Takizawa T, Nakanishi Y. Transcription stimulation of Fas-encoding gene by nuclear factor for interleukin-6 expression upon influenza virus infection. J Biol Chem. 1995;270:18007–18012. doi: 10.1074/jbc.270.30.18007. [DOI] [PubMed] [Google Scholar]
- 29.Wyllie A H, Kerr J F R, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 30.Zwaal R F, Schroit A J. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]