Abstract
Background:
Frailty is a prevalent state associated with several aging-related traits and conditions. The relationship between frailty and stroke remains understudied. Here we aim to investigate whether the Hospital Frailty Risk Score (HFRS) is associated with the risk of stroke and determine whether a significant association between genetically determined frailty and stroke exists.
Design:
Observational study using data from All of Us research program and Mendelian Randomization (MR) analyses.
Methods:
Participants from All of Us with available electronic health records were selected for analysis. All of Us began national enrollment in 2018 and is expected to continue for at least 10 years. All of Us is recruiting members of groups that have traditionally been underrepresented in research. All participants provided informed consent at the time of enrollment, and the date of consent was recorded for each participant. Incident stroke was defined as stroke event happening on or after the date of consent to the All of Us study HFRS was measured with a 3-year look-back period before the date of consent for stroke risk. The HFRS was stratified into four categories: No-frailty (HFRS = 0), Low (HFRS ≥1 and < 5), Intermediate (≥5 and <15) and High (HFRS ≥ 15). Lastly, we implemented MR analyses to evaluate whether genetically determined frailty is associated with stroke risk.
Results:
253,226 participants were at risk of stroke. In multivariable analyses frailty status was significantly associated with risk of any (ischemic or hemorrhagic) stroke following a dose-response way: not-frail versus low HFRS (HR 4.9; CI 3.5–6.8; p< 0.001, not-frail versus intermediate HFRS (HR 11.4; CI 8.3–15.7; p< 0.001) and not-frail versus high HFRS (HR 42.8; CI 31.2–58.6; p< 0.001). We found similar associations when evaluating ischemic and hemorrhagic stroke separately (p-value for all comparisons <0.05). MR confirmed this association by indicating that genetically determined frailty was independently associated with risk of any stroke (OR, 1.45; 95% CI, 1.15–1.84; p=0.002).
Conclusions:
Frailty, based on the HFRS was associated with higher risk of any stroke. MR analyses confirmed this association providing evidence to support a causal relationship.
Keywords: Hospital Frailty Risk Score, Stroke, All of Us, Mendelian randomization
Graphical Abstract

INTRODUCTION
Frailty is a state characterized by progressive decline in normal physiology of several systems.1 Frailty constitutes an emerging global health problem as its prevalence continues to rise alongside an aging population.2 The prevalence of frailty is estimated to increase in the following decades associated with changing demographics making it an important issue for stroke research.3–5
Mounting evidence indicates that frailty is an important predictor of higher risk and worse outcomes for different conditions, 6,7,8,9 including stroke.10,11,12,13,14
A frailty-based consideration of how the multisystem decline in physiological reserves modifies the natural history of stroke is important for future stroke research. Identification of frailty as a causal factor for higher risk of ischemic and hemorrhagic stroke would lay the foundation for important follow-up research focused on using frailty scores to identify high-risk individuals, selecting patients for clinical trials, and discovering new pathophysiological insights.
The most well validated and widely accepted tools to measure frailty are the phenotypic definition of frailty and the accumulation of deficits definition of frailty. The accumulation of deficits definition of frailty, often termed as the frailty index (FI) is based on the cumulative effect of medical, functional, and psychosocial age-related deficits. The greater the number of deficits one has, the higher the likelihood of adverse health outcomes. The FI, developed by Rockwood and colleagues, is a count of 70 clinical deficits from the Canadian Study of Health and Aging. Each deficit is mapped to the interval 0–1 to represent the severity of the problem.15
The Hospital Frailty Risk Score (HFRS) is an increasingly used and a well-validated risk score developed to measure frailty utilizing the International Classification of Diseases, Tenth Revision (ICD-10) diagnostic codes.16 This score is based on the cumulative deficit definition of frailty and is based entirely on ICD-10, which facilitates the ascertainment of frailty in national and international registries that primarily use ICD-10 codes. The HFRS was developed from a cluster analysis assessing ICD-10 diagnoses, bed-days, and hospital costs. For this analysis they previously identified a small set of ICD-10 codes as candidate markers of frailty. In the initial cluster analysis, they found a group that had a higher incidence of ICD-10 codes indicative of frailty. From this group, HFRS was derived using over-represented ICD-10 codes and further validated in different cohorts. 16
HFRS has been showed to predict adverse events across difference conditions. 8,17–19 In recent studies from Kilkenny et, al. and Pinho et, al. frailty ascertained using HFRS was associated with worse outcomes following stroke/transient ischemic attack.10,20
Given that these previous studies demonstrated an important role of frailty in stroke we aimed to investigate whether HFRS-calculated frailty is associated with an increased risk of stroke in a large and diverse population-based study, the All of Us Research program (All of Us). In addition, we sought to determine whether this association was partially independent of classical cardiovascular risk factors and age. We determined the average mediated effects of the HFRS going through known risk factors for stroke including hypertension, hyperlipidemia, diabetes and smoking status. We subsequently pursued Mendelian Randomization (MR) analyses to investigate a causal relationship between frailty and stroke.
Although observational studies have shown a strong association between frailty and stroke, it is challenging to establish a causal relationship due to potential confounding and reverse causation. Mendelian Randomization (MR) is a statistical approach that can help to address these limitations by using genetic variants as instrumental variables to test for causal relationships. In the current study, we use MR to investigate the causal relationship between frailty and stroke, providing a more robust understanding of the potential causal relationship between these two variables.
METHODS
Data availability
All of Us, supported by the National Institutes of Health, aims to enroll 1 million Americans aged 18 or older from demographically, geographically, and medically diverse groups across the United States, with open enrollment to all who choose to participate. The program began national enrollment in 2018 and is expected to continue for at least 10 years.21 All of Us is recruiting members of groups that have traditionally been underrepresented in research. All participants provided informed consent at the time of enrollment, and the date of consent was recorded for each participant. Exclusion criteria include inability to provide informed consent, inability to speak and understand English or Spanish, and residency outside the United States. Data from the All of are available at allofus.nih.gov. Data were extracted using standardized procedures and were de-identified to protect participant privacy.
At baseline, participants undergo a comprehensive assessment that includes collection of demographic information, medical history, medication use, lifestyle factors, and physical measurements. Participants also provide blood and urine samples for biobanking and undergo genomic testing. Follow-up assessments are conducted at regular intervals, with participants providing updates on their medical history and health outcomes. All of U includes more than 340 recruitment sites across the United States, including academic medical centers, community health centers, and other healthcare organizations.
Study Design and Participants
We conducted a two-stage study that combined observational and genetic analyses.
First, we tested for associations between frailty and stroke risk. Second, we implemented summary statistics-based MR analyses to evaluate whether genetically-determined frailty is associated with stroke risk.
Exposure ascertainment
We calculated the HFRS using the 109 ICD-10 diagnostic codes over-represented in frail individuals, described elsewhere.16 We ascertained conditions that happened within a 3-year look-back period before the date of consent to the All of Us study. Specific values were applied to each condition and an aggregated score per patient was determined. The frailty score was stratified into four categories: No-frailty (HFRS= 0), Low (HFRS ≥1 and < 5), Intermediate (≥5 and <15) and High (HFRS ≥ 15), as previously described by Gilbert et, al.16
Stroke ascertainment
We identified stroke patients using validated ICD-9 and ICD-10 codes from participants with available EHR (Supplementary Table 1). Due to the longitudinal nature of All of Us, we used the date of consent (previously described) to identify patients with prevalent stroke (stroke event happening before the date of consent to the All of Us study) and with incident stroke (stroke event happening on or after the date of consent to the All of Us study).
Assembly of Analytic Sample
After excluding participants without EHR data (113,692), 258,688 participants were available for complete-case analysis. In our study sample (253,226 participants), we excluded participants with prevalent stroke to evaluate the relationship between HFRS and stroke risk. (Figure 1).
Figure 1. Assembly of Analytic Sample.
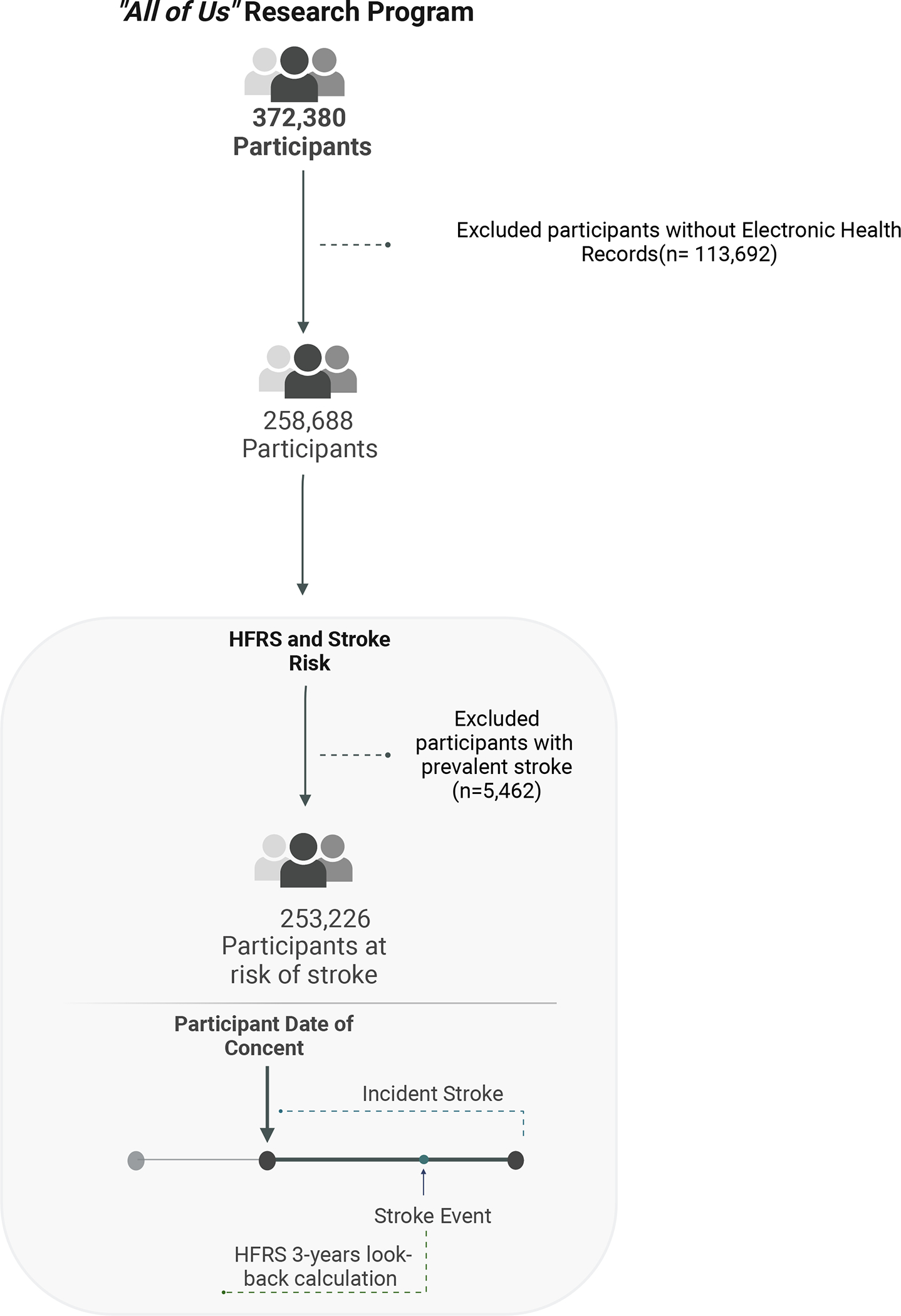
• HFRS = Hospital Frailty Risk Score
Genetic instruments and summary statistics
We used publicly available summary statistics from published genome-wide association studies (GWASs) of frailty22 and stroke.23,24 In the GWAS for frailty, Atkins et. al, identified 14 genetic loci associated with frailty (as measured by the FI) in 164,610 UK community-based individuals aged 60 to 70 years of European ancestry, with meta-analysis of data from similarly aged Swedish individuals in TwinGene.22 As the genetic instrument for frailty, we used independent (R2 <0.1) single nucleotide polymorphisms (SNPs) associated with the frailty index25 at genome-wide levels (p < 5e−8). (Supplementary Table 2) All selected SNPs were aligned to the GRCh37 assembly of the human genome. Palindromic SNPs were excluded and the effect alleles were aligned to model increases in frailty. We used the MEGASTROKE GWAS, for all types of stroke, all ischemic stroke, cardioembolic stroke, small vessel stroke and large artery stroke. MEGASTROKE GWAS is a large-scale international collaboration launched by the International Stroke Genetics Consortium, that releases summary statistics from the 2018 meta-analysis of Genome-wide Association (GWA) data in stroke and stroke subtypes.24 For ICH, we used the latest ICH GWAS for all ICH, lobar ICH, and non-lobar ICH.23,24 Finally for subarachnoid hemorrhage (SAH) we used the intracranial aneurysmal and subarachnoid hemorrhage (aSAH) latest GWAS. 26
We adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) and STROBE Mendelian Randomization statement to ensure transparent and complete reporting of our study. To this end, we used the checklists to guide the reporting of our study design, methods, results, and conclusions.27,28
Statistical Analysis
Discrete variables are presented as counts (percentages [%]) and continuous variables as means (standard deviation [SD]).
Stage 1. Longitudinal association between frailty and stroke risk. A) Primary analysis.
Our primary hypothesis was that frail persons, identified by a high HFRS, have a higher risk of stroke. To test this hypothesis, we constructed univariable and multivariable Cox proportional hazard regression models adjusted by sex, race/ethnicity, and cardiovascular risk factors. We adjusted for cardiovascular conditions to evaluate the proportion of the association between frailty and stroke risk that is not mediated by cardiovascular conditions. We evaluated associations between HFRS categories (no-frailty, low HFRS, intermediate and high HFRS) and risk of all strokes. Because the underlying biology differs according to the specific type of stroke, our secondary analyses evaluated ischemic and hemorrhagic stroke separately. B) Effect modification. We tested for interactions between HFRS and potential effect modifiers including age, sex, and race/ethnicity by introducing a product term in the regression models. Additionally, we performed stratified analyses across subgroups defined by these variables to determine the point estimates among each of these subgroups. C) Mediation analysis. We utilized the R package Mediation to determine the average mediated effects of the HFRS going through known risk factors for stroke, including hypertension, hyperlipidemia, diabetes and smoking status.29
Stage 2. Summary statistics based Mendelian Randomization.
MR constitutes a special case of instrumental variable analysis, a widely used analytical framework for causal inference. When the MR assumptions are met, it is possible to identify and quantify causal relationships between exposures and outcomes of interest.30,31
We conducted a 2-sample MR study, where the genetic information for the exposure (frailty) and the outcome (risk of stroke) come from various studies.22–25 Our primary analysis was the inverse variance weighted (IVW) method. In sensitivity analyses, we used the Weighted median (WM), MR-Egger, and MR-PRESSO methods. Additionally, we tested for the presence of pleiotropy (effect of the genetic instruments going through other pathways not related to frailty) using the MR-Egger intercept and MR-PRESSO global test.
Software
To analyzed data from All of Us we used The Researcher Workbench, a cloud-based platform where registered researchers can access Registered and Controlled Tier data. Jupiter notebooks with both Python for data cleaning and R for statistical analysis were employed. 32
For MR analysis we used MendelianRandomization package and R (version 4.1.3) for association testing, and MR analysis. 33
RESULTS
The Controlled Tier Dataset V6 of All of Us included data from 372,380 participants, including 258,688 with electronic health records (EHR) information that was harmonized using the Observational Medical Outcomes Partnership Common Data Model (OMOP CDM). 34 After excluding 113,692 participants without electronic health record (EHR) data and 5,462 with prevalent stroke, 253,226 were included in the first stage of this study. (Figure 1, Supplementary Table 3) Mean age was 51.83 years (SD 16.91) and 152,139 participants were female (60%). Race/ethnic groups included 51,641 Black (20.4%), 47,559 Hispanic (18.8%), 130,099 White (51.4%) and 6,995 Asian (2.8%) participants. The number of participants with high, intermediate, low HFRS and no-frailty were 34,581 (13.7%), 68,341 (27.0%) 69,083 (27.3%) and 81,221 (32.1%) respectively (Table 1).
Table 1.
Baseline of participants at risk of stroke.
| Variable | Overall | High HFRS | Intermediate HFRS | Low HFRS | No-Frailty |
|---|---|---|---|---|---|
| 253,226 | 34,581 | 68,341 | 69,083 | 81,221 | |
| Age, y; mean (SD) | 51.83 (16.91) | 60.11 (15.37) | 55.09 (16.03) | 48.81 (16.79) | 48.12 (16.58) |
| Sex | |||||
| Female | 152,139 (60.1%) | 21,413 (61.9%) | 43,010 (62.9%) | 41,926 (60.7%) | 45,790 (56.4%) |
| Male | 93,941 (37.1%) | 12,163 (35.2%) | 23,461 (34.3%) | 25,293 (36.6%) | 33,024 (40.7%) |
| Non-binary/Not answered | 7,093 (2.8%) | 996 (2.9%) | 1,858 (2.7%) | 1,854 (2.7%) | 2,385 (2.9%) |
| Race/Ethnicity | |||||
| Asian | 6,995 (2.8%) | 402 (1.2%) | 1,350 (2.0%) | 2,207 (3.2%) | 3,036 (3.7%) |
| Black or African American | 51,641 (20.4%) | 5,658 (16.4%) | 11,150 (16.3%) | 11,919 (17.3%) | 22,914 (28.2%) |
| Hispanic or Latino | 47,559 (18.8%) | 5,457 (15.8%) | 11,707 (17.1%) | 13,978 (20.2%) | 16,417 (20.2%) |
| Other | 16,932 (6.7%) | 2,369 (6.9%) | 4,423 (6.5%) | 4,614 (6.7%) | 5,526 (6.8%) |
| White | 130,099 (51.4%) | 20,695 (59.8%) | 39,711 (58.1%) | 36,365 (52.6%) | 33,328 (41.0%) |
| Cardiovascular Risk Factors | |||||
| Hypertension | 106,870 (42.2%) | 27,414 (79.3%) | 38,499 (56.3%) | 24,985 (36.2%) | 15,972 (19.7%) |
| Hyperlipidemia | 101,146 (39.9%) | 25,263 (73.1%) | 38,072 (55.7%) | 24,150 (35.0%) | 13,661 (16.8%) |
| Diabetes | 44,904 (17.7%) | 14,573 (42.1%) | 16,047 (23.5%) | 9,052 (13.1%) | 5,232 (6.4%) |
| Ever smoked | 101,055 (39.9%) | 16,469 (47.6%) | 28,147 (41.2%) | 24,842 (36.0%) | 31,597 (38.9%) |
• HFRS = Hospital Frailty Risk Score
• SD = Standard deviation
HFRS and risk of any stroke
Higher frailty, modeled via HFRS, was significantly associated with increased risk of stroke. When looking at participants with no-frailty, low, intermediate and high HFRS we found 49 (0.1%), 236 (0.3%), 743 (1.1%) and 1,963 (5.7%) participants with any stroke respectively; 42 (0.1%), 212 (0.3%), 659 (1.0 %) and 1,620 (4.7%) participants with ischemic stroke respectively; and 7 (0.0%), 24 (0.0%), 84 (0.1%) and 343 (1.0%) participants with hemorrhagic stroke respectively (unadjusted p<0.001) (Table 2).
Table 2.
Distribution of stroke types and outcomes across HFRS.
| Variable | Overall | High | Intermediate | Low | No-frailty | Unadjusted p-value |
|---|---|---|---|---|---|---|
| Risk analyses | ||||||
| n | 253,226 | 34,609 | 68,322 | 69,074 | 81,221 | - |
| Any Stroke | 2,991 (1.2%) | 1,963 (5.7%) | 743 (1.1%) | 236 (0.3%) | 49 (0.1%) | < 0.001 |
| Ischemic Stroke | 2,533 (1.0%) | 1,620 (4.7%) | 659 (1.0%) | 212 (0.3%) | 42 (0.1%) | < 0.001 |
| Hemorrhagic Stroke | 458 (0.2%) | 343 (1.0%) | 84 (0.1%) | 24 (0.0%) | 7 (0.0%) | < 0.001 |
Similar statistically significant differences were observed in multivariable analyses adjusted by cardiovascular risk factors. Study participants with low, intermediate and high HFRS versus no-frailty had HR 4.7 (CI 3.4–6.4; p< 0.001), HR 11.5 (11.5; CI, 8.60– 15.5; p< 0.001) and HR 45.7 ( CI, 34.1– 61.2; p< 0.001 of any stroke, respectively (Table 3). Our primary analysis focusing exclusively on ischemic stroke indicated that study participants with low, intermediate, and high HFRS had HR 4.9 (CI 3.5– 6.8; p<0.001), HR 11.4 (CI, 8.33– 15.7; p< 0.001) and HR 42.8 (CI, 31.2– 58.6; p< 0.001) of ischemic stroke versus no-frailty study participants. Frailty was also associated with the risk of hemorrhagic stroke. Compared with participants with no-frailty, participants with low, intermediate and high HFRS had HR 4 (CI 1.74–9.47, p=0.001), HR 13.2 (CI, 6.0– 28.9; p< 0.001) and HR 98.2 (CI, 45.0–211.6; p< 0.001) of hemorrhagic stroke, respectively (Table 3). Multivariable analyses adjusted by only age, sex, and race/ethnicity remain significant. (Supplementary table 4)
Table 3.
Cox proportional hazard regression models for HFRS and stroke risk.
| Variable |
Univariate
HR (95% CI) |
Multivariate
HR (95% CI) |
| Any Stroke | ||
| HFRS | ||
| No-frailty | REFERENCE | |
| Low | 5.5 (4.1–7.6) | 4.7 (3.4–6.4) |
| Intermediate | 17.8 (13.3–23.7) | 11.5 (8.6–15.5) |
| High | 90.0 (67.7–119.5) | 45.7 (34.1–61.2) |
| Ischemic Stroke | ||
| HFRS | ||
| No-frailty | REFERENCE | |
| Low | 5.9 (4.2–8.3) | 4.9 (3.5–6.8) |
| Intermediate | 18.7 (13.7–25.6) | 11.4 (8.3–15.7) |
| High | 91.9 (67.6–124.9) | 42.8 (31.2–58.6) |
| Hemorrhagic Stroke | ||
| HFRS | ||
| No-frailty | REFERENCE | |
| Low | 4.0 (1.7–9.4) | 4.0 (1.74–9.47) |
| Intermediate | 14.2 (6.6–30.8) | 13.2 (6.0–28.9) |
| High | 114.2 (54.0–241.5) | 98.2 (45.0–211.6) |
Adjusted by age, sex, race/ethnicity, and cardiovascular risk factors (smoking, hypertension, hyperlipidemia, type 2 diabetes). All p-values were <0.001.
• HFRS = Hospital Frailty Risk Score
• HR = Hazard ratio
Effect modification
We tested for interaction by introducing product terms to the regression models. Results were different across age groups for any type of stroke (interaction p<0.001), ischemic stroke (interaction p=<0.001) and intracerebral hemorrhage (interaction p=<0.001). No significant interactions were found among sex and race/ethnicity groups for any type of stroke, ischemic and hemorrhagic stroke (all interactions p>0.05) (Supplementary Table 5).
Mediation analysis
We tested whether known vascular risk factors for stroke mediated the association between frailty and stroke. We found that part of this effect was mediated through hypertension for any stroke (23.6%), ischemic stroke (24.8%), and hemorrhagic stroke (17.8%) (p<0.001); diabetes for any stroke 2.5%, and ischemic stroke 3.1% (p<0.001); and hyperlipidemia in 14.2% for any stroke and 15.6% for ischemic stroke respectively (p<0.001). Diabetes and hyperlipidemia were not significant mediators for hemorrhagic stroke. Smoking was not a significant mediator (all p>0.05). However, the HFRS remained significant after accounting for these classical cardiovascular risk factors suggesting an independent effect of the score.
Mendelian randomization
Several different MR techniques indicated that the association between frailty and risk of stroke could be causal. Our primary MR analyses using the inverse variance weighted approach indicated that genetically-determined frailty was associated with 45% higher estimates of any stroke (OR, 1.45; 95% CI, 1.15–1.84; p=0.002), 39% higher estimates of ischemic stroke (OR, 1.39; 95% CI, 1.07–1.70; p=0.012) and 6-fold higher estimates of ICH (OR, 6.33; 95% CI, 1.59–25.27; p=0.008), without evidence of horizontal pleiotropy. (Figure 2, Table 4) In additional analyses we evaluated ischemic stroke subtypes and hemorrhagic stroke subtypes obtaining consistent results (Supplementary Table 6).
Figure 2.
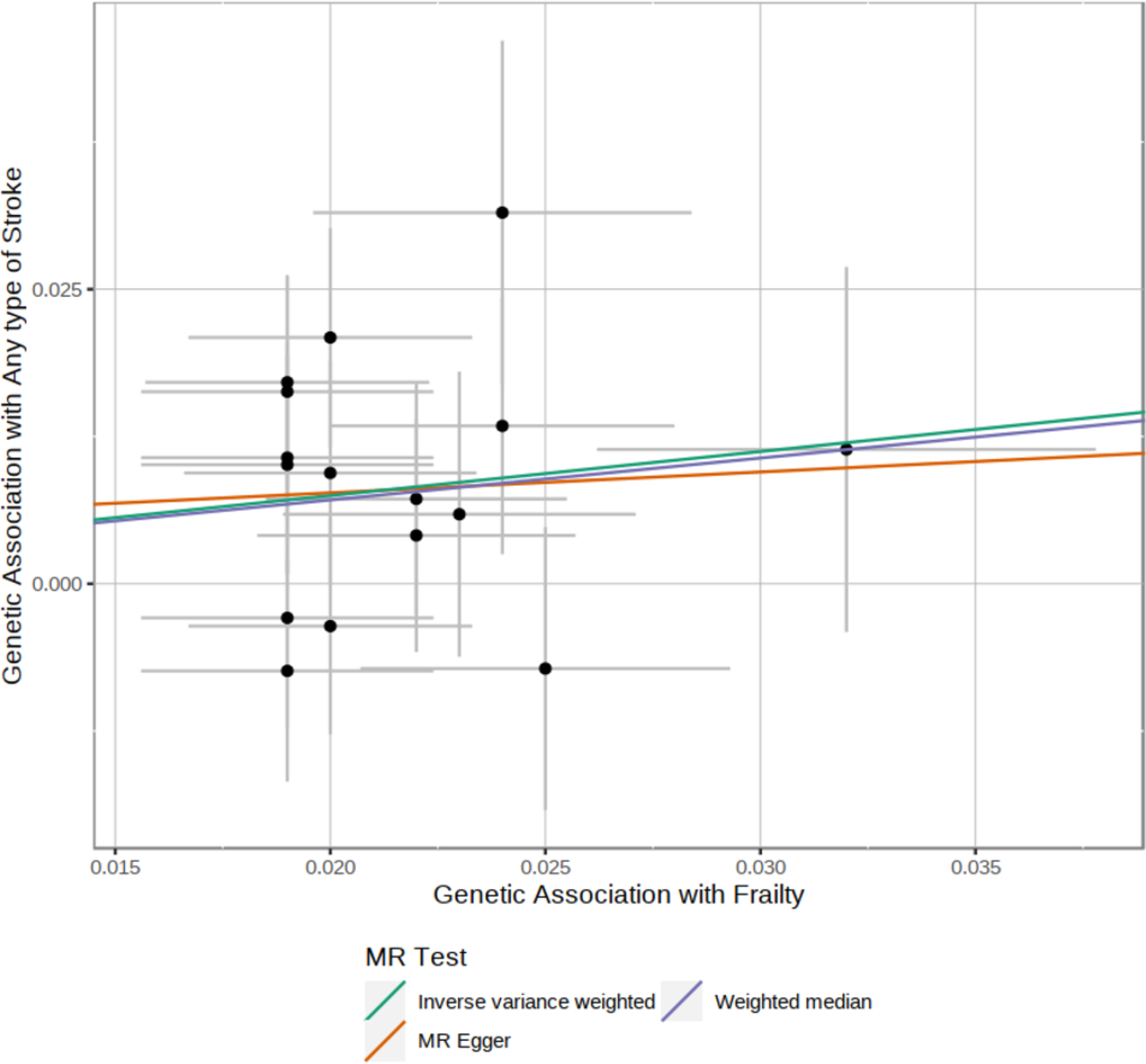
Mendelian randomization results for genetically determined frailty and risk of any type of stroke.
• IVW = Inverse-variance weighted
• WM = Weighted-median
• MR-Egger = Mendelian randomization-Egger
Table 4.
Mendelian randomization summary statistics.
| MR Method | SNIPS N | OR | CI | p-value |
|---|---|---|---|---|
| Any Stroke | ||||
| IVW | 16 | 1.45 | (95%CI 1.1–1.8) | 0.002 |
| WM | 16 | 1.43 | (95%CI 1.0–2.0) | 0.038 |
| MR-Egger | 16 | 1.19 | (95%CI 0.1–7.8) | 0.85 |
| MR-PRESSO | 16 | 1.45 | (95%CI 1.15–1.8) | 0.006 |
| MR-Egger Intercept | 16 | 1 | (95%CI 0.9–1.0) | 0.83 |
| Global Test | 16 | - | - | 0.53 |
| Any Ischemic Stroke | ||||
| IVW | 16 | 1.39 | (95%CI 1.0–1.7) | 0.012 |
| WM | 16 | 1.29 | (95%CI 0.9–1.8) | 0.13 |
| MR-Egger | 16 | 0.96 | (95%CI 0.1–7.4) | 0.97 |
| MR-PRESSO | 16 | 1.39 | (95%CI 1.1–1.6) | 0.003 |
| MR-Egger Intercept | 16 | 1.01 | (95%CI 0.9–1.0) | 0.730 |
| Global Test | 16 | - | - | 0.945 |
| All ICH | ||||
| IVW | 14 | 6.33 | (95%CI 1.5–25.2) | 0.008 |
| WM | 14 | 11.83 | (95%CI 1.7–78.3) | 0.010 |
| MR-Egger | 14 | 1782.24 | (95%CI 0.0–66882234.1) | 0.18 |
| MR-PRESSO | 14 | 6.33 | (95%CI 1.7–22.4) | 0.013 |
| MR-Egger Intercept | 14 | 0.89 | (95%CI 0.7–1.1) | 0.31 |
| Global Test | 14 | - | - | 0.65 |
• MR = Mendelian randomization
• SNP = Single-nucleotide polymorphisms
• OR = Odds ratio
• CI = Confident interval
• IVW = Inverse-variance weighted
• WM = Weighted-median
• MR-PRESSO= Mendelian randomization pleiotropy residual sum and outlier
• MR-Egger = Mendelian randomization-Egger
We explored traits associated to the GWAS of frailty, we found that SNP rs3959554 is associated with coronary artery disease (Supplementary table 2); we did a secondary MRization summary statistics excluding this SNP and we still found significant association between genetically determined frailty and stroke. (Supplementary table 7)
DISCUSSION
We report the results of a combined observational and genetic study that evaluated the role of frailty in different types of strokes. We used data from the All of Us Research Program, the largest population-based open-access study conducted in the United States.21 We found that higher frailty scores were independently associated with higher risk of any stroke, ischemic stroke, and hemorrhagic stroke, and that these associations were consistent across several race/ethnic groups. MR analyses demonstrated a significant association between genetically-determined frailty and risk of any type of stroke, any ischemic stroke and all ICH supporting a causal link between these conditions.
Cardiovascular risk factors such as hypertension, hyperlipidemia, obesity, smoking and diabetes are the strongest contributors to stroke risk. In fact, around 87% of strokes are estimated to result from these factors.35 However, in the past few years, several non-conventional risk factors have been suggested to increase the risk of stroke.35,36 Examples of these are kidney disease, pregnancy, migraine with aura, obstructive sleep apnea and chronic inflammatory diseases.36–43 These observations highlight the complex pathophysiology underlying stroke risk, and the probable synergistic effects among risk factors. In this setting, there is increasing interest in physiological states like frailty that capture the combined contribution of several of these risk factors due to their downstream location in the causal pathway.44
Frailty is a state of increased vulnerability with impaired physiological function across multiple systems of the body. 1 The HFRS is the only score based entirely on ICD-10 codes which allows us to measure frailty in national and international registries. It constitutes a risk score with established benefit in the prediction of complications in the hospitalized elderly and in an increasing number of pathological states including stroke.8,10,16In our study, we demonstrated that, compared to patients with no-frailty, those with low, intermediate and high HFRS had 4.7, 11.5 and 45.7 times higher risk of any stroke. Importantly, while a proportion of this effect was mediated by cardiovascular risk factors, the association between HFRS and stroke risk remained significant after adjusting for these factors, suggesting that the overall vulnerability present in frail patients is independently associated with stroke risk.
Our study also provides new information on two other important matters. First, the majority of studies assessed frailty at the time of stroke. We were able to calculate it before the stoke episode thanks to the longitudinal nature of All of us. Second, we evaluated the association between HFRS and stroke risk across several subgroups represented in All of Us and showed that this relationship becomes stronger in older persons. These findings suggest that the role of frailty in stroke may grow as age increases, a finding that has been observed for other diseases.45,46 Importantly, these stratified analyses also indicated that the relationship between frailty and stroke is equally strong for women and men, and across different race/ethnic groups. Third, we performed MR analyses using known genetic risk factors for frailty. These genetic analyses confirmed the observed association and provided evidence for a causal role of frailty in stroke. Genetic variants constitute an excellent tool to assess causality due to their random assortment during meiosis that makes them relatively immune to post-partum confounders.
In combination, our findings point to a number of interesting and practical applications. First, the utilization of formal tools to quantify frailty (HFRS and other similar frailty metrics) could be helpful in the everyday clinical care of stroke patients providing a consistent way of determining the overall “functional age” of the patient, that may or may not reflect the chronological age. Medical data naturally generated by routine clinical work in inpatients or outpatient settings, like symptoms, signs, diagnoses, laboratory tests results, or even lifestyle factors indicators are stored in electronic health records (EHRs). These data could be used in frailty assessment tools, especially in frailty based cumulative deficit model methods. Using frailty assessment tools based on EHRs may bridge the gap between frailty research and clinical frailty assessment.47–49
Similarly, a formal quantification of frailty in stroke patients could be helpful in the design, execution and analysis of clinical trials evaluating novel treatments for stroke, complementing the notion of chronological age with information about the clinical status of patients.
Our study has a number of limitations. First, there are limitations inherent to all EHR-based observational studies, which may contain coding and reporting biases, and data may be misclassified or incomplete. Second, there are some limitations based on the cluster analysis methods used to define HFRS. The HFRS was validated to screen frail individuals older than 75 years in secondary care, which is much older than the population of All of Us.50 Arguably, population specific risk scores could be developed to better capture conditions that lead to frailty in this younger age group. Negative perceptions linked to the term “frail” have been previously described and are associated with administrations of treatments that do not follow standard of care. 51,52 It is therefore possible the observed risk of stroke could be due to less intensive prevention efforts in these patients. We would like to note that genetic analysis overcomes these limitations of observational studies.30,31
CONCLUSION
Higher HFRS was associated with an increased risk for any stroke as well as ischemic and hemorrhagic stroke subtypes. HFRS remained significant after accounting for vascular risk factors suggesting an independent effect of the score. MR analyses revealed a significant association between genetically-determined frailty and risk of any type of stroke, all ischemic stroke and all ICH supporting a causal link between these conditions. Further research is needed to understand the potential value of the HFRS for risk stratification with the goal of providing personalized preventive strategies.
Supplementary Material
FUNDING SOURCES
The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276. In addition, the All of Us Research Program would not be possible without the partnership of its participants.
Dr Acosta reports employment by Rad AI. Dr Rivier reports grants from Pyxis partners. Dr de Havenon reports grants from American Academy of Neurology; stock options in Certus; compensation from Novo Nordisk for consultant services; stock options in TitinKM; compensation from Integra for consultant services; and grants from National Institutes of Health. Dr Sharma reports grants from NIH Clinical Center. Dr Sheth reports compensation from Sense for data and safety monitoring services; compensation from Astrocyte for consultant services; grants from BARD; a patent pending for Stroke wearables licensed to Alva Health; grants from Biogen; grants from Hyperfine; compensation from Rhaeos for consultant services; employment by Yale School of Medicine; compensation from ZOLL Medical Corporation for data and safety monitoring services; compensation from Certus for consultant services; compensation from Cerevasc for consultant services; grants from Novartis; compensation from CSL Behring for consultant services; and service as President for Advanced Innovation in Medicine. Dr Matouk reports compensation from Silk Road Medical, Inc. for consultant services. Dr Falcone is supported by the American Heart Association. Dr. Gill is supported by the Yale Pepper Center (P30AG021342). The other authors have nothing to disclose.
Non-Standard Abbreviations and Acronyms:
- HFRS
Hospital Frailty Risk Score
- ICD-10
International Classification of Diseases, Tenth Revision
- All of Us
All of Us Research program
- MR
Mendelian Randomization
- EHR
Electronic Health Records
- GWAS
Genome-wide association Studies
Footnotes
CONFLICTS OF INTEREST
We report no conflict of interest.
BIBLIOGRAPHY
- 1.Satake S, Arai H. Chapter 1 Frailty: Definition, diagnosis, epidemiology. Geriatrics and Gerontology International. 2020;20(S1):7–13. [DOI] [PubMed] [Google Scholar]
- 2.Dent E, Martin C, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Series Frailty 2 Management of frailty: opportunities, challenges, and future directions.; 2019. [DOI] [PubMed] [Google Scholar]
- 3.Welsh CE, Matthews FE, Jagger C. Trends in life expectancy and healthy life years at birth and age 65 in the UK, 2008–2016, and other countries of the EU28: An observational cross-sectional study. The Lancet Regional Health - Europe. 2021;2:100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gale CR, Cooper C, Sayer AA. Prevalence of frailty and disability: findings from the English Longitudinal Study of Ageing. Age and Ageing. 2015;44(1):162–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans NR, Todd OM, Minhas JS, Fearon P, Harston GW, Mant J, Mead G, Hewitt J, Quinn TJ, Warburton EA. Frailty and cerebrovascular disease: Concepts and clinical implications for stroke medicine. International Journal of Stroke. 2022;17(3):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davenport A Application of the Clinical Frailty Score and body composition and upper arm strength in haemodialysis patients. Clinical Kidney Journal. 2022;15(3):553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dicpinigaitis AJ, McIntyre MK, Al-Mufti F, Kazim SF, Li B, Schmidt MH, Gandhi CD, Cole CD, Bowers CA. Association of baseline frailty status with clinical outcome following aneurysmal subarachnoid hemorrhage. Journal of Stroke and Cerebrovascular Diseases. 2022;31(5). [DOI] [PubMed] [Google Scholar]
- 8.Koo AB, Elsamadicy AA, Renedo D, Sarkozy M, Sherman J, Reeves BC, Havlik J, Antonios J, Sujijantarat N, Hebert R, et al. Higher Hospital Frailty Risk Score is associated with increased complications and healthcare resource utilization after endovascular treatment of ruptured intracranial aneurysms. Journal of NeuroInterventional Surgery. 2022:neurintsurg-2021–018484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klara K, Leonardo B, Navani N, Vito D, Germano G, Andrea B, Giuseppe R, Fabio P. Frailty in patients with lung cancer: a systematic review and meta-analysis. Chest. 2022. [DOI] [PubMed] [Google Scholar]
- 10.Kilkenny MF, Phan HT, Lindley RI, Kim J, Lopez D, Dalli LL, Grimley R, Sundararajan V, Thrift AG, Andrew NE, et al. Utility of the Hospital Frailty Risk Score Derived from Administrative Data and the Association with Stroke Outcomes. Stroke. 2021:2874–2881. [DOI] [PubMed] [Google Scholar]
- 11.Kanai M, Noguchi M, Kubo H, Nozoe M, Kitano T, Izawa KP, Mase K, Shimada S. Pre-Stroke Frailty and Stroke Severity in Elderly Patients with Acute Stroke. Journal of Stroke and Cerebrovascular Diseases. 2020;29(12). [DOI] [PubMed] [Google Scholar]
- 12.Taylor-Rowan M, Cuthbertson G, Keir R, Shaw R, Drozdowska B, Elliott E, Stott D, Quinn TJ. The prevalence of frailty among acute stroke patients, and evaluation of method of assessment. Clinical Rehabilitation. 2019;33(10):1688–1696. [DOI] [PubMed] [Google Scholar]
- 13.Schnieder M, Bähr M, Kirsch M, Maier I, Behme D, Riedel CH, Psychogios MN, Brehm A, Liman J, von Arnim CAF. Analysis of frailty in geriatric patients as a prognostic factor in endovascular treated patients with large vessel occlusion strokes. Journal of Clinical Medicine. 2021;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winovich DiT Longstreth WT, Arnold AM, Varadhan R, Zeki Al Hazzouri A, Cushman M, Newman AB, Odden MC Factors Associated with Ischemic Stroke Survival and Recovery in Older Adults. Stroke. 2017;48(7):1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2007;62(7):722–727. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, Arora S, Street A, Parker S, Roberts HC, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. The Lancet. 2018;391(10132):1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto Y, Hori S, Ushida K, Shirai Y, Shimizu M, Kato Y, Shimizu A, Momosaki R. Impact of Frailty Risk on Adverse Outcomes after Traumatic Brain Injury: A Historical Cohort Study. Journal of Clinical Medicine. 2022;11(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ushida K, Shimizu A, Hori S, Yamamoto Y, Momosaki R. Hospital Frailty Risk Score Predicts Outcomes in Chronic Obstructive Pulmonary Disease Exacerbations: Frailty in Chronic Obstructive Pulmonary Disease. Archives of Gerontology and Geriatrics. 2022;100(November 2021):104658. [DOI] [PubMed] [Google Scholar]
- 19.Pulido LC, Meyer M, Reinhard J, Kappenschneider T, Grifka J, Weber M. Hospital frailty risk score predicts adverse events in spine surgery. European Spine Journal. 2022;31(7):1621–1629. [DOI] [PubMed] [Google Scholar]
- 20.Pinho J, Küppers C, Nikoubashman O, Wiesmann M, Schulz JB, Reich A, Werner CJ. Frailty is an outcome predictor in patients with acute ischemic stroke receiving endovascular treatment. Age and Ageing. 2021;50(5):1785–1791. [DOI] [PubMed] [Google Scholar]
- 21.All of Us Research Program Investigators, Denny JC, Rutter JL, et al. The “All of Us” Research Program. N Engl J Med. 2019;381(7):668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkins JL, Jylhävä J, Pedersen NL, Magnusson PK, Lu Y, Wang Y, Hägg S, Melzer D, Williams DM, Pilling LC. A genome-wide association study of the frailty index highlights brain pathways in ageing. Aging Cell. 2021;20(9):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L, Giese AK, Van Der Laan SW, Gretarsdottir S, Anderson CD, Chong M, Adams HHH, Ago T, Almgren P, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nature Genetics. 2018;50(4):524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo D, Falcone GJ, Devan WJ, Brown WM, Biffi A, Howard TD, Anderson CD, Brouwers HB, Valant V, Battey TWK, et al. Meta-analysis of genome-wide association studies identifies 1q22 as a susceptibility locus for intracerebral hemorrhage. American Journal of Human Genetics. 2014;94(4):511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. TheScientificWorldJournal. 2001;1:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakker MK, van der Spek RAA, van Rheenen W, Morel S, Bourcier R, Hostettler IC, Alg VS, van Eijk KR, Koido M, Akiyama M, Terao C, Matsuda K, et al. Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nature Genetics. 2020;52(12):1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. International Journal of Surgery. 2014;12(12):1495–1499.25046131 [Google Scholar]
- 28.Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, Vanderweele TJ, Higgins JPT, Timpson NJ, Dimou N, et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA - Journal of the American Medical Association. 2021;326(16):1614–1621. [DOI] [PubMed] [Google Scholar]
- 29.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Journal of Statistical Software mediation: R Package for Causal Mediation Analysis; 2014. [Google Scholar]
- 30.Georgakis MK, Gill D. Mendelian Randomization Studies in Stroke: Exploration of Risk Factors and Drug Targets with Human Genetic Data. Stroke. 2021;(September):2992–3003. [DOI] [PubMed] [Google Scholar]
- 31.Acosta JN, Szejko N, Falcone GJ. Mendelian Randomization in Stroke: A Powerful Approach to Causal Inference and Drug Target Validation. Frontiers in Genetics. 2021;12(August):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramirez AH, Sulieman L, Schlueter DJ, Halvorson A, Qian J, Ratsimbazafy F, Loperena R, Mayo K, Basford M, Deflaux N, Muthuraman KN, Natarajan K, Kho A, Xu H, Wilkins C, et al. The All of Us Research Program: Data quality, utility, and diversity. Patterns. 2022;3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yavorska OO, Burgess S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. International Journal of Epidemiology. 2017;46(6):1734–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klann JG, Joss MAH, Embree K, Murphy SN. Data model harmonization for the All Of Us Research Program: Transforming i2b2 data into the OMOP common data model. PLoS ONE. 2019;14(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anon. Heart Disease and Stroke Statistics-2021 Update A Report from the American Heart Association. Circulation. 2021:E254–E743. [DOI] [PubMed] [Google Scholar]
- 36.Bang OY, Ovbiagele B, Kim JS. Nontraditional risk factors for ischemic stroke: An update. Stroke. 2015;46(12):3571–3578. [DOI] [PubMed] [Google Scholar]
- 37.Vashistha V, Lee M, Wu YL, Kaur S, Ovbiagele B. Low glomerular filtration rate and risk of myocardial infarction: A systematic review and meta-analysis. International Journal of Cardiology. 2016;223:401–409. [DOI] [PubMed] [Google Scholar]
- 38.Masson P, Webster AC, Hong M, Turner R, Lindley RI, Craig JC. Chronic kidney disease and the risk of stroke: A systematic review and meta-analysis. Nephrology Dialysis Transplantation. 2015;30(7):1162–1169. [DOI] [PubMed] [Google Scholar]
- 39.Huang R, Chen X. Increased Spot Urine Albumin-to-Creatinine Ratio and Stroke Incidence: A Systematic Review and Meta-Analysis. Journal of Stroke and Cerebrovascular Diseases. 2019;28(10). [DOI] [PubMed] [Google Scholar]
- 40.Swartz RH, Cayley ML, Foley N, Ladhani NNN, Leffert L, Bushnell C, McClure JA, Lindsay MP. The incidence of pregnancy-related stroke: A systematic review and meta-analysis. International Journal of Stroke. 2017;12(7):687–697. [DOI] [PubMed] [Google Scholar]
- 41.MacClellan LR, Giles W, Cole J, Wozniak M, Stern B, Mitchell BD, Kittner SJ. Probable migraine with visual aura and risk of ischemic stroke: The stroke prevention in young women study. Stroke. 2007;38(9):2438–2445. [DOI] [PubMed] [Google Scholar]
- 42.Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: Systematic review and meta-analysis. BMJ (Online). 2009;339(7728):1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie C, Zhu R, Tian Y, Wang K. Association of obstructive sleep apnoea with the risk of vascular outcomes and all-cause mortality: A meta-analysis. BMJ Open. 2017;7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kant IMJ, Mutsaerts HJMM, van Montfort SJT, Jaarsma-Coes MG, Witkamp TD, Winterer G, Spies CD, Hendrikse J, Slooter AJC, de Bresser J, et al. The association between frailty and MRI features of cerebral small vessel disease. Scientific Reports. 2019;9(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kojima G, Liljas AEM, Iliffe S. Frailty syndrome: Implications and challenges for health care policy. Risk Management and Healthcare Policy. 2019;12:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howlett SE, Rutenberg AD, Rockwood K. The degree of frailty as a translational measure of health in aging. Nature Aging. 2021;1(8):651–665. [DOI] [PubMed] [Google Scholar]
- 47.Lim A, Choi JY, Ji H, Lee H. Frailty assessment using routine clinical data: An integrative review. Archives of Gerontology and Geriatrics. 2022;99(September 2021):104612. [DOI] [PubMed] [Google Scholar]
- 48.Luo J, Liao X, Zou C, Zhao Q, Yao Y, Fang X, Spicer J. Identifying Frail Patients by Using Electronic Health Records in Primary Care: Current Status and Future Directions. Frontiers in Public Health. 2022;10(June):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rejeski J, Xiao T, Wheless W, Pajewski NM, Jensen E, Callahan KE. An automated electronic health-record derived frailty index is associated with adverse events after endoscopy. Journal of the American Geriatrics Society. 2022;70(2):629–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soong J, Bell D, Poots AJ. The challenges of using the Hospital Frailty Risk Score.; 2018. [DOI] [PubMed] [Google Scholar]
- 51.Schoenborn NL, Van Pilsum Rasmussen SE, Xue QL, Walston JD, McAdams-Demarco MA, Segev DL, Boyd CM. Older adults’ perceptions and informational needs regarding frailty. BMC Geriatrics. 2018;18(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diemberger I, Fumagalli S, Mazzone AM, Bakhai A, Reimitz PE, Pecen L, Manu MC, Gordillo de Souza JA, Kirchhof P, De Caterina R. Perceived vs. objective frailty in patients with atrial fibrillation and impact on anticoagulant dosing: an ETNA-AF-Europe sub-analysis. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2022;24(9):1404–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of Us, supported by the National Institutes of Health, aims to enroll 1 million Americans aged 18 or older from demographically, geographically, and medically diverse groups across the United States, with open enrollment to all who choose to participate. The program began national enrollment in 2018 and is expected to continue for at least 10 years.21 All of Us is recruiting members of groups that have traditionally been underrepresented in research. All participants provided informed consent at the time of enrollment, and the date of consent was recorded for each participant. Exclusion criteria include inability to provide informed consent, inability to speak and understand English or Spanish, and residency outside the United States. Data from the All of are available at allofus.nih.gov. Data were extracted using standardized procedures and were de-identified to protect participant privacy.
At baseline, participants undergo a comprehensive assessment that includes collection of demographic information, medical history, medication use, lifestyle factors, and physical measurements. Participants also provide blood and urine samples for biobanking and undergo genomic testing. Follow-up assessments are conducted at regular intervals, with participants providing updates on their medical history and health outcomes. All of U includes more than 340 recruitment sites across the United States, including academic medical centers, community health centers, and other healthcare organizations.


