Abstract
Nuclear pore complexes (NPCs) mediate the exchange of materials between the nucleoplasm and cytoplasm, playing a key role in the separation of nucleic acids and proteins into their required compartments. The static structure of the NPC is relatively well defined by recent cryo-EM and other studies. The functional roles of dynamic components in the pore of the NPC, phenylalanyl-glycyl (FG) repeat rich nucleoporins, is less clear because of our limited understanding of highly dynamic protein systems. These proteins form a ‘restrained concentrate’ which interacts with and concentrates nuclear transport factors (NTRs) to provide facilitated nucleocytoplasmic transport of cargoes. Very rapid on- and off-rates among FG repeats and NTRs supports extremely fast facilitated transport, close to the rate of macromolecular diffusion in cytoplasm, while complexes without specific interactions are entropically excluded, though details on several aspects of the transport mechanism and FG repeat behaviors remain to be resolved. However, as discussed here, new technical approaches combined with more advanced modeling methods will likely provide an improved dynamic description of NPC transport, potentially at the atomic level in the near future. Such advances are likely to be of major benefit in comprehending the roles the malfunctioning NPC plays in cancer, ageing, viral diseases, and neurodegeneration.
Keywords: cell nucleus, nuclear pores, nuclear protein transportport proteins, nucleic acids
Introduction: structure of a behemoth
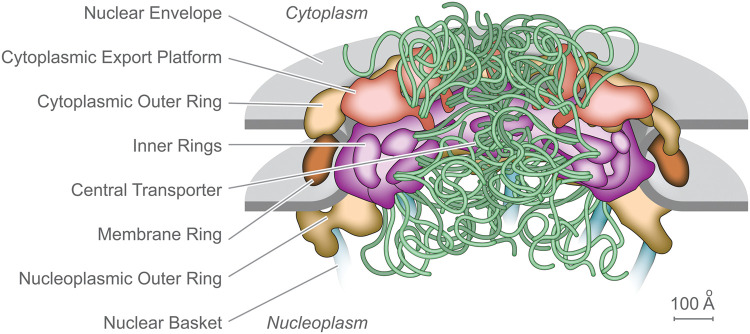
Nuclear pore complexes (NPCs) are among the largest macromolecular assemblies in a eukaryotic cell. NPCs sit in the double-layered nuclear envelope (NE), which provides a barrier separating the nucleoplasm from the cytoplasm. There, NPCs form a platform for the organization of numerous nuclear functions, and critically, act as the sole mediators of macromolecular trafficking into and out of the nucleus. Malfunction of the NPC or its components are linked to many disease states [1–3]. Steady progress has been made in discerning the fine structure of the NPC in yeast and vertebrates, resulting in recent relatively good consensus maps of the NPC's architectural principle features (Figure 1); each NPC is an octagonally near symmetric cylindrical assembly some 100 nm across and 50–100 MDa in mass (depending on species), comprised of ∼500 proteins (termed Nups) representing ∼30 different types that have been fully cataloged for both yeast and vertebrates (reviewed in e.g. [4–6]).
Figure 1. Static representation of an NPC, based on a yeast (S. cerevisiae) structure, with the major features labeled [9].
In contrast with this structural understanding, significant areas concerning the dynamic mechanism of the transporting NPC remain undefined. Overall, the dynamic interactions that mediate and regulate transport span about twelve orders of magnitude in time, from nanoseconds to 10 s of seconds. As we need to comprehend this entire scale, no one approach is fully sufficient to give us a complete and unbiased view [7,8]. Here, we discuss why much of the NPC's transport mechanism has proven so refractory to mainstream structural approaches, and how this has led to significant confusion both inside and outside the field, as well as contradictory models representing the full complexity of the structure, dynamics, and biology of the NPC. On the positive side, recent ingenious and orthogonal investigations from multiple groups have begun to overcome prior limitations, and development of exciting new methods is likely to provide major new insights, that already hint that the transport mechanism is perhaps more complex and surprising than previously anticipated.
The road from static representation to functional understanding
In the nuclear envelope (NE), NPCs are the stationary component of nuclear transport, mediating the mobile phase, comprising the bi-directional traffic of import of proteins to and export of RNAs from the nucleus [9–11]. Much of transport across the NPC is mediated by multiple members of the karyopherin (Kap) family of nuclear transport receptors (NTRs), at rates approaching 1000 molecules/NPC/sec ([12,13] and references therein). Import-Kaps (importins) transport cargos into the nucleus while export-Kaps (exportins) ferry cargos out of the nucleus. Protein cargos are targeted for transport by having a nuclear localization signal (NLS) or export signal (NES). NLSs/NESs bind Kaps, which, in turn, translocate through the NPC, after which the Kap–cargo complex dissociates in its target compartment; their transport directionality is controlled by the nucleotide state of the GTPase Ran, shuttled across the NPC by its dedicated transporter NTF2/p10, a representative of the other major NTR family whose other members, Mex67/NXF1 — Mtr2/NXT1, mediate the export of mRNAs. Other RNAs are exported by cognate Kaps, either directly or via adaptor proteins (and in the case of the 60S pre-ribosomal subunit, also utilizing Mex67/Mtr2) (reviewed in [14]). While small molecules such as metabolites and ions can freely diffuse across the NPC, macromolecules not associated with NTRs cannot pass as efficiently through the NPC, which thus functions as a selective barrier in the CT, leading to the distinction between fast facilitated diffusion (i.e. NTRs/cargoes) and slow or negligible passive diffusion of other macromolecules. While it was previously thought that there was something of a hard upper limit of ∼40 kDa or ∼9 nm radius for this passive diffusion, we now know that there is a power relationship between macromolecular size and the efficiency of its exclusion from the CT [15,16]; which means that few macromolecules access the nucleus through passive diffusion [17]
The transport mechanism is driven by dynamics and disorder
It was originally proposed that the NPC's selective barrier might utilize mechanoenzymes, either by an iris-like gate or motor-driven translocation at the NPC through a permeability barrier of some kind, or that the translocation of NTRs across the NPC was propelled directly by cycles of Ran GTP hydrolysis [19–21]. However, it has been shown that nucleotide hydrolysis is not required for the translocation step across the NPC. Instead, diffusion seemed to be key to trafficking, involving a restriction of passive diffusion and promotion of selective, facilitated diffusion within the CT. An important clue to the transport mechanism came when it was shown that the CT is lined with proteins termed FG Nups [22]. FG Nups are so-called because they contain large intrinsically disordered regions (IDRs) that carry many Phe–Gly (FG) repeats, each separated by ∼20 residues of predominantly hydrophilic linkers (Figures 2 and 3) [11].
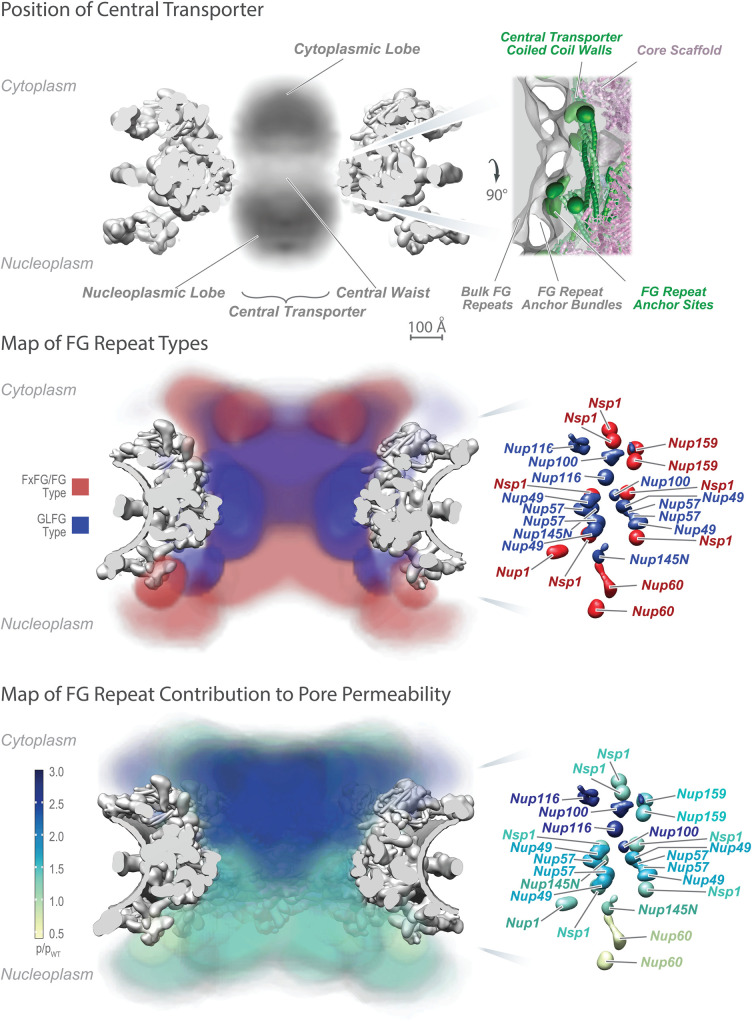
Figure 2. Positions of the FG Nups in the yeast NPC.
Upper: Representation of observed electron density of the central transporter within the rigid frame of the NPC. Upper Right: detailed observed density local to the central waist illustrating the FG repeat anchor site. Center: distribution of types of FG repeats within the central transporter based on their mapped anchor sites and inferred by assuming random coil behavior of the FG segments and the absence of NTR/cargoes [15]. Lower: heat map of the contribution of the FG repeat region of each FG Nup to maintaining the passive permeability barrier limiting the passage of nonspecific macromolecules, which appears to be largely maintained by FG Nups enriched in GLFG repeats forming a cap at the cytoplasmic entrance of the NPC [15]. The severity of the permeability defect measured as permeability relative to permeability in wild type, is indicated in increasing shades from minor (light green) to severe (dark blue). Adapted from [18]. The figure is modified form one copyrighted by MPR.
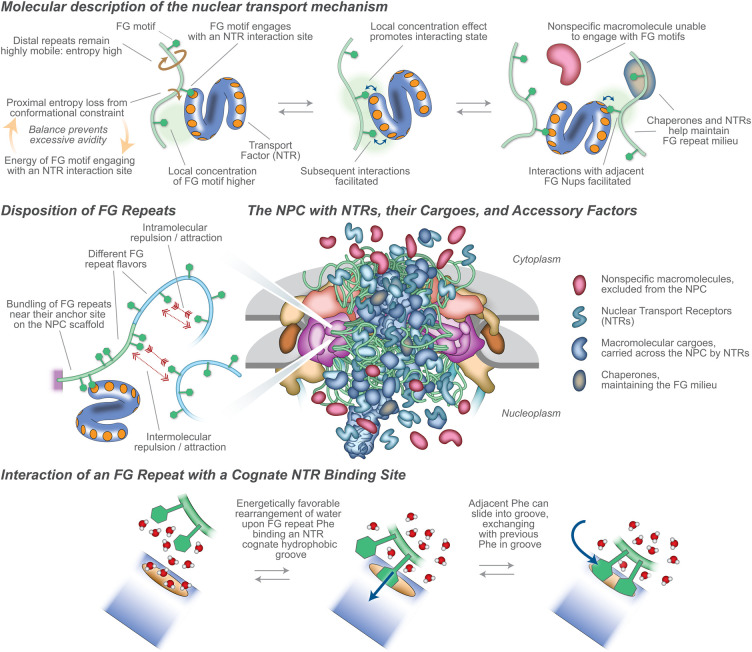
Figure 3. The NPC during nucleocytoplasmic transport.
Upper: Molecular representation of the nuclear transport mechanism, at the scale of NTRs and non-specific macromolecules in the proximity of FG repeats. Middle Left: Disposition of FG repeats, showing different potential behaviors. Middle Right: Representation of an NPC with transiting macromolecules, to approximate scale and stoichiometry. Lower: Diagram of two adjacent FG repeats (green) interacting in solvent water (red/white) with a cognate interaction site (orange) on an NTR (blue).
Approximately one-third of all Nups contain these regions, which are in the volume of the CT. Changing the dynamic and disordered states of such IDRs is entropically unfavorable, such that any macromolecule attempting to enter their space or push them aside experiences an ‘entropic repulsion’ effect [8,23]. Crucially, it is these FG repeats that were also shown to interact with multiple cognate sites on each NTR and so specifically facilitate its passage across the NPC [24–26]. Based on this information, a ‘virtual gating’ model was proposed in which dynamic multivalent interactions of NTRs with these FG repeats would provide just sufficient avidity to allow their rapid passage across the CT by overcoming entropic repulsion effects of the same IDR regions that otherwise exclude the passage of non-binding, non-specific macromolecules [22,27]. Indeed, it now seems evident that the mechanism for facilitated transport in the CT must include three features: first, the rate of facilitated transport across the NPC is similar to free diffusion within cells [28], so the internal mechanism of facilitated selection must be extraordinarily rapid; second, to maintain facilitated selection, the ratio of concentration of NTRs/cargoes to passive molecules (non-NTRs) within the CT must exceed that external to the CT, i, e, NTRs/cargoes must be relatively concentrated in the CT by interaction with it [9,29–31]; and third, to be consistent with the first and second points, a simple mechanism of inhibition of non-NTR transport is entropic exclusion [10,27,32,33], although exactly how that mechanism plays out in the NPC is still unclear (below). We will now address how these features are produced by the NPC.
Near to the madding crowd: complexity and crowding within the central transporter generates specificity in transport
Frustratingly, the fact that the CT's component materials are either intrinsically disordered (FG repeats) or extremely heterogeneous (NTRs and their cargoes) has made structure-function studies of it extraordinarily challenging, such that it has been described as ‘structurally elusive and mechanistically controversial’ [7]. Collectively, the anchor sites for the FG repeats in the walls of the CT direct them towards the CT's central axis to generate a highly concentrated and dynamic FG repeat milieu; the anchor sites for most FG repeats are clustered, so that they emanate as bundles near the walls of the CT which can be visualized by electron microscopy, and then merge into a cloud near the CT's axis [9,18] (Figure 2). This generates two organizational features: firstly, the regions of FGs in the bundles near the CT's wall are more diffusionally restricted, as has been indicated in vivo [34,35]; and secondly, different kinds of FG repeat (termed ‘flavors’) are at specific positions in the CT's volume (Figure 2). At least in yeast, these flavors can be divided into two broad classes based on an approximate consensus of their Phe-containing repeats and the amino acid composition of the repeat spacers: one of Phe–X–Phe–Gly (FXFG) — like repeats (where X is usually a small hydrophilic amino acid) with hydrophilic spacers often carrying some charged amino acids (Asp, Glu or Lys), and the other of Gly–Leu–Phe–Gly (GLFG) or Phe–Gly (FG) — like consensus repeats spaced by hydrophilic segments of low charge [36]; however, there is considerable variation between FG Nup flavors and even between the same FG Nup homologs of different species [11,37]. One possible reason for such flavor varieties is that they confer different biophysical properties to specific positions in the CT. The role of differences in charged residues in the spacer regions has been suggested to be of significance in the CT by repulsion between like-charged sequences [38] and partitioning of charged and less charged sequences resulting in a permeability barrier [39], but experimental support is lacking. Compelling in vivo data also point to the idea that these different flavors and their locations in the CT delineate specific pathways for subsets of NTRs through the NPC [40–48], e.g. it is clear membrane proteins can be actively transported through the NPC in a route distinct from those of soluble cargoes [49,50].
Surprisingly, despite their high concentration the FG repeats do not comprise the majority of the CT — rather, well over three-quarters of the CT at any moment is made of a constant flux of NTRs and their cargoes (see below) [9,18,29,31]. Their sizes vary widely up to many tens of megadaltons, these large cargoes consisting of ribonucleoprotein/mRNA (termed mRNP) cargoes and ribosomal subunit precursors [18,51]. Recently experiments on large cargoes indicated that the increased free energy cost of inserting a large cargo into the dense FG Nup barrier is compensated by the binding to FG Nups via more NTRs per cargo [52], and the very largest cargoes may require expansion of the NPC in some fashion [53,54] consistent with the heterogenous diameters of observed pores [9,55]. This enormous preponderance of NTRs and their cargoes in the CT is the elephant in the room; earlier models have concentrated solely on the roles and states of the FG Nups (below), but in nature, FG repeats in the NPC always exist in the presence of a considerable molar excess of NTRs, meaning that experiments that reconstitute FG repeats in the absence of NTRs could well be examining unnatural states.
We are thus faced with accounting for how these three quarters or more of the CT's mass contributes to its transport behavior, and this realization has led to a recent shift in focus, onto the interplay between FG repeats and NTRs, rather than just the FG repeats alone [56,57]. Crucially, this high concentration of NTRs and cargoes in the NPC, all specifically enriching around the FG repeats, can outcompete and so inhibit nonspecific macromolecular exchange which cannot interact with FG repeats [31,58–60]. Moreover, there is mounting evidence for a slowly exchanging pool of NTRs being maintained at the CT [31,61,62]. Such observations have led to a ‘Kap-Centric’ model, wherein there is a slower exchanging pool of NTRs that are key players in modulating and maintaining the NPC's barrier to non-specific macromolecular exchange [31,63,64]. In summary, ‘the FG Nups are necessary but insufficient for NPC barrier function. NTRs constitute integral constituents of the NPC whose barrier, transport, and cargo release functionalities establish a continuum under a mechanism of Kap-centric control’ [31].
Fast and furious: active macromolecular transport across the NPC through NTR–FG repeat interactions
A defining characteristic of nuclear transport is the tremendous rates at which each NPC can bidirectionally transport an astonishing variety of cargoes. The family of NTRs is quite large and still may not be fully defined. Remarkably, dozens of different NTRs each mediate separate but often overlapping transport pathways for specific classes of cargoes across the NPC. This multitude of FG interaction sites on each NTR (above; see also Figure 3) again presents the question of how rapid transport avoids slowing by avidity. Generally, the time scales and energies of interactions between FG repeats and NTRs appear very rapid in vitro [65,66], and are difficult to measure precisely either in vivo or in vitro [8]. The specificity of the interactions is clearly linked strongly to the phenylalanyl side chain of FG Nups as revealed by crystallography, NMR, MD simulations, and other methods [67–69]. From solution methods [65,66], and consistent with in situ high speed AFM [70] and MD simulation [69], the time scale of each single site NTR–FG interactions is likely of the order of microseconds, so that e.g. ‘weak and ultrafast multivalent Kap–FG interactions allow the Kap–cargo complexes to translocate in a fast and selective manner’ [7]. Atom scale molecular dynamics, supported by NMR data, indicated that the fast exchange between NTRs and individual FG motifs may rely on a sliding-and-exchange mechanism [69], indicating that FG motifs slide on the ample grooves that form NTRs’ binding pockets. This anisotropic sliding may in turn enable fast exchange and rapid facilitated diffusion, such that interacting FG repeats and NTRs exchange particularly rapidly compared with other protein–protein interactions of similar affinity, allowing for the remarkable transport rates observed experimentally (Figure 3).
The emerging picture is that NTRs (with or without cargoes) can transiently skip between FG sites on FG Nups with a low interaction enthalpy dependent on the local concentration of FG Nups and limited in avidity [71] by the entropic motions of the FG Nups’ IDP character [72], while passive diffusion is limited by entropic exclusion, with minimal benefit from interactions with the FG sites. Where the energy for interaction of an F with an NTR pocket comes from is still unclear, but as well as direct amino acid interactions, it seems likely that rearrangement of water molecules from around the hydrophobic F residue and pocket may play a part (Figure 3). A key concept is that the chain mobility of the FG Nups provides entropic exclusion limiting additional enthalpic gains from additional sites. In a detailed specific case, the modest enthalpy of a single interaction of an FG with a given cognate NTR binding pocket (∼−7 kCal/M) is offset by the entropic cost of constraining the FG repeat plus spacer to the NTR's vicinity (∼3 kCal/M). More interactions yield non-linearly more interaction enthalpy, but that is balanced by a similar increase in entropic cost The local concentration effect on enthalpy increasing avidity is limited to 4–6 additional FG sites [72] (Figure 3). One analogy is that the FG Nups form a ‘cloud’ of rapidly diffusing phenylalanine ligands that are constrained to the vicinity of the CT, acting like a ‘solvent’ for the NTR/cargoes with superfast, transient, and weak interactions, although unlike normal solvents the interaction sites are linked (Figure 3). Additional complexity is added by the specificity of interactions between classes of FG Nups and specific NTRs, and by potential interactions (‘cohesion’) among the components.
FG repeat biophysical behaviors: the quick and the dead?
What kind of ‘solvent’ might the FG repeats form in the CT, and in addition to the NTRs’ role, how might its physical states contribute to exclusion of non-specific materials? Biologically, solvents are normally liquids, but the FG repeats cannot form a liquid, because the FG repeats are not freely mobile but rather tethered. As the nature of the barrier to diffusion is hard to discern in NPCs in a cell, various approaches to mimicking the roles of FG Nups have been used. Significantly, selective transport can be mimicked by nanopores of defined composition with FG Nups incorporated using synthetic membranes [58,64,77–80], which demonstrates that the essential phenomenon of selectivity can be reconstructed ex vivo, although providing little information about the state in the CT. However, when isolated as free proteins in vitro, FG repeats can assume a range of polymorphisms, from simple intrinsically disordered proteins (IDPs) in solution, to polymer brushes tethered to a surface, to different classes of condensate: liquid–liquid phase separations [73], hydrogels [81], prions [82,83], or rigid amyloid-like gels [75] (Figure 4 and its legend; See Definitions below). These states span a substantial range of viscoelastic properties (see Fig. 4 legend; See Definitions below) [84]. Moreover, all display in vitro at least some of the characteristics also seen in situ, though none have the performance of native NPCs [80]. This range of FG repeat regions’ states and behaviors is reflected in different proposed models for their function in the CT in vivo (reviewed in [39]). But this is putting the cart before the horse — all these states can be manoeuvred to display certain transport characteristics while not addressing what state the FG repeats form in the NPC. So which is the actual state, and speed of FG repeat conformational fluctuations, in vivo, remain a subject of investigation and debate.
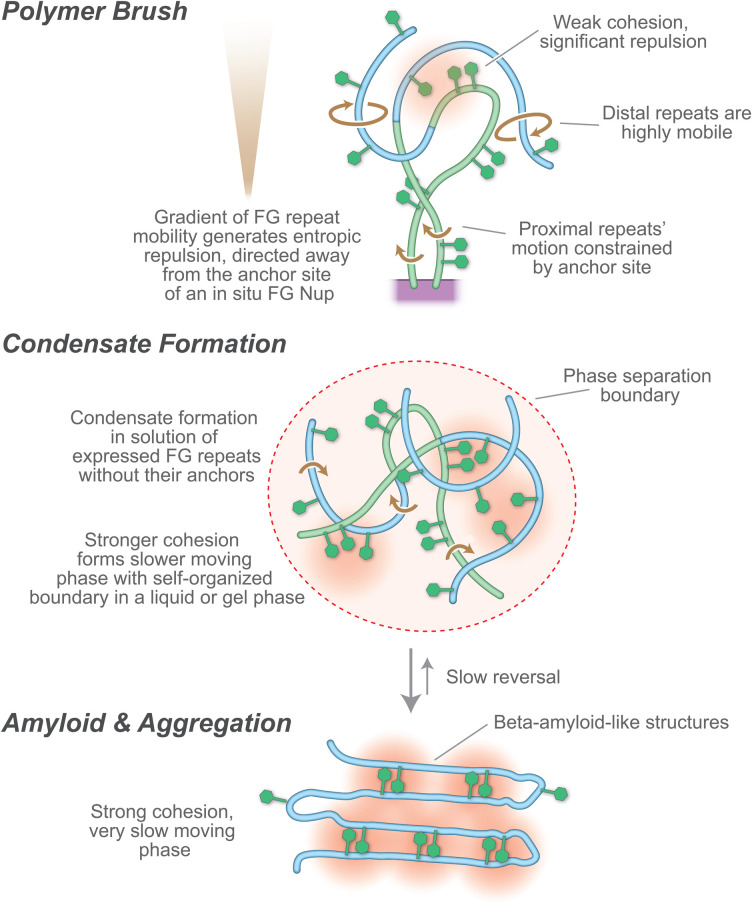
Figure 4. Illustration of the polymorphism associated with FG Nups in vitro.
Because of their tethering, and lack of tertiary protein structure, the FG Nups are in a polymer brush formation. Controversy centers on whether the brush is entirely intrinsically disordered [65,66], with the degree and speed of motion varying with distance from the tether, and the grafting density (upper) or whether cohesion of the FG repeats results in varying degrees of condensation leading to gels [73,74] (middle) or amyloids [75,76] (lower).
When IDPs are tethered to a surface, as they are in the CT, they will form a polymer brush (see definitions below), evidence for which is seen for FG repeats in vivo [85] and in isolated NPCs [9,18] (Figures 3 and 4). Inclusion of any other macromolecule in the brush is entropically unfavorable as it restricts the entropic thermal motion of the chains in the brush, resulting in an force pushing away from the brush; in the CT, this would result in a kinetic barrier excluding non-specific macromolecules from the pore, these forces scaling with the size of the macromolecule [15,22,27,86]. FG repeats grafted to surfaces (reviewed in [47] [8]) and pores carrying walls grafted with disordered polymers generate such an effective kinetic barrier [87]. While NTRs would also experience (and provide; see above) entropic exclusion, their avidity to the same FG repeats would offset this entropic exclusion and so allow their rapid passage across the NPC [22,27], as quantitatively demonstrated for FG repeats and NTRs in vitro [72].
Increasing condensation from freely soluble to rigid gels in these materials is referred to in the field as the result of ‘cohesiveness’ (Figure 4). Note that cohesiveness is used as an omnibus term covering all weak interactions associated with compactness within a single chain, interactions between chains of the same type (homotypic) and interactions with other FG Nups in the vicinity (heterotypic). Based on observations of apparent radius of gyration [36], the GLFG flavor of FG repeats (above) has generally been assigned a higher degree of cohesiveness. In solution this is reflected by GLFG repeats being able to display the complete range of polymorphisms (above) [73,75,76,88]. There is no direct evidence testing which of these states or combination of states is present in the NPC, even though the suggestion that a high degree of condensation (or cohesion) plays a functional role has been widely propagated [74,75,81,88–91]. The nature of the stabilizing cross-links in such gels is also unclear, as is how NTRs reversibly dissolve these links on interactions with F sites. While the suggestion that Phe–Phe interactions may provide such links to form a ‘hydrophobic gel’ [28,74], there is no evidence for significant Phe–Phe interactions in solution or in gel states by NMR [65,73,75,93] or in solution by SANS [94], although such weak interactions are inferred in amyloids of Nup98 by cryo-EM [76].
With the advent of liquid–liquid phase separation (LLPS) in cell biology [95,96], the idea was raised that FG repeats, too, form an LLPS. Indeed, under various conditions, soluble FG repeats can be induced to form LLPSs in vitro [90,97–99]. The debate now surrounds: which properties of LLPS the central transporter's FG repeats actually possess? Certainly, LLPSs have two characteristics that FG repeats in the NPC lack, namely, the polymers comprising LLPSs are untethered, and they are defined by their self-organized surface tension [100–102] (See Fig. 4 legend.).
Definitions of different potential FG repeat states
Polymer brush
‘Polymer brushes are long-chain polymer molecules attached by one end to a surface or interface by some means, with a density of attachment points high enough so that the chains are obliged to stretch away from the interface, sometimes much farther than the typical unstretched size of a chain.’ [103]. Therefore, the CT by definition contains polymer brushes, though additional properties of state may be displayed.
Condensate
Dynamic and reversible assemblies of molecules that can dissolve and be reused to perform their function [104]. The usual implication is that the density of a condensate is the result of weak intermolecular forces between the components, and the condensate has significant displacement of solvent from itself. A condensate may be a component of a liquid–liquid separated phase or may be part of a complex structure with components not able to form liquid phases e.g. chromatin [105].
Gel
Material with elastic properties, usually with significant permeability to solvent though the gel itself exhibits no steady-state flow and is usually cross-linked. Gels are typically the result of condensates at high concentration [106]. For the NPC, it usually describes in vitro formation from GLFG rich FG repeats, and is ascribed without direct evidence to possible function in the CT.
Liquid–liquid phase separation
‘Phase separation describes the process by which a well-mixed solution of components de-mixes into two or more coexisting phases with uniform properties. In the simple case of liquid–liquid phase separation, a liquid solution de-mixes into two liquid phases, one dense phase and one dilute phase.’ [104]. In LLPSs, it is solely the surface tension from selective cohesion of its freely mobile components that defines a concentrated compartment [101].
Viscoelastic complex
As a result of the formation of some of the above items, the properties of the complexes may be highly variable from essentially fluid, responding to changes of pressure by diffusion, to more gel-like or solid properties, which are elastic, deforming to changes of pressure [107].
But what is in the central transporter?
Overall, the functional role in vivo for changes of state of FG repeats involving LLPS, gels or amyloids is entirely unknown. This issue of structural pleomorphism and its functional implications is a major challenge generally to the structural biology of amyloid and gel-like systems, complicating in vitro reconstitution e.g. [108]. Moreover, CT assembly is likely tightly regulated, to balance functional activities against the ageing and aggregation that has been implicated in leading to amyloid like disease states [104]. Indeed, there appear to be active balance and maintenance systems that limit FG repeat condensation in vivo. Thus, the observation that removal of highly condensed FG Nups may be facilitated by chaperones [109,110] interacting with FG repeats, suggests that chaperone-like activity may play a role in assembling and maintaining the NPC. Moreover, other work indicates that NTRs contribute actively to preventing aggregation of FG repeats [111], and FG Nup condensate puncta outside the NPC appear to be transient non-essential and even toxic condensates that are absent from healthy cells [112] (also reviewed in [113]), and the more aggregated forms have been associated with disease states e.g. in neurodegeneration [114–116].
Taking all this together, it seems reasonable to suggest that the polymer brush FG repeats of the CT form a ‘restrained concentrate’, where a concentrating effect of FG repeats is achieved by the anchoring of FG Nups in the CT walls, and the density and selective transport behavior of the CT contents results mainly from this constraint plus the recruitment of NTRs/cargoes (above), without necessarily having a specific requirement for extensive cohesion to form a gel. This view is most consistent with solution studies of different isolated FG repeat flavors which show a picture of highly mobile, minimally cohesive IDPs [65,66,72,117] with fast, low affinity interactions with NTRs and with very high mobility of tagged FG repeat regions in vivo [34,54,85,118]. Notable in this regard, the Lim lab has also contributed key observations that the central portion of the in situ CT is dynamic using high speed atomic force microscopy, definitively establishing the movement of the center of the NPC in the 100 ms and faster range and showing that intermingling FG Nups there do not appear to cohere into a highly cross-linked meshwork like an amyloid [35,70]. Some form of weak cohesive forces must exist to some (currently ill-defined) degree; for example, the density and packing of the FG Nups and NTRs/cargoes are subject to the usual dynamic interactions including van der Waals attractive and repulsive forces, complementing specific FG/NTR interactions in which NTRs may bridge between different FG repeats, and other potential specific interactions [39,119,120]. These forces may have some ‘tuning’ role to play in adjusting the permeability and selectivity parameters of the CT.
Pores in action: a new view in vivo
Recent innovative approaches have begun to move the field away from the drawbacks of reliance on only in vitro data, to now garnering detailed nanoscale dynamic data on NTRs and FG repeats in the CTs of in situ or living NPCs. In particular, the examination of functional FG Nups by measuring fluorescence energy transfer between two neutral fluorophores placed at different positions along the length of FG repeats in the in vivo NPC provides a significant advance in our understanding of their dynamic structure [118]. Pioneering advances in design of small amino acid fluorophores, insertion of multiple labeling sites in the appropriate genes, and measurement of the distance distribution of 18 NUP98 segments is consistent with the in-NPC state being close to that of a random polymer in a ‘good solvent’, and is significantly different from the value observed in solution at low concentration that is consistent with a compacted state (as also seen by other methods) [73]. More detailed analysis of the fluorescence lifetime decay using simulations is consistent with extremely rapid polymer motion, and suggestive of some shuffled packing of the FG repeats towards the periphery with a concentration of NTR/cargoes towards the center [118]. Similar new approaches in situ and in vivo are on the horizon, promising direct observation of transport in action at the molecular level.
Current research reviewed has also concentrated on the function and mechanism of the CT. However, other recent work has pointed to additional factors that may play critical roles in the transport mechanism. These include: a potential role of numerous transport factors in forming the nucleocytoplasmic Ran gradient [57]; accounting for the dilation of the NPC in diffusion control and in response to environmental changes [9,55,56,121,122]; and the existence of multiple and very distinct NPC isoforms, even within the same cell, that may have different transport roles [9,55]. The diversity of NTRs, FG repeats and even NPC isoforms also raises the question as to whether only one general mechanism is employed by the NPC in selective transport and it even seems possible that different NTRs may employ distinct mechanisms, perhaps in concert with specialized NPC isoforms.
New tools on the block: data integration, modeling and simulation
On their own, these multiple biophysical and cell biological observations are not readily merged into a simple hypothesis of how the CT permits rapid and selective diffusion. Recent modeling papers have focused on the underlying thermodynamic issues of enthalpic/entropic balance in FG Nup/NTR interactions, on the dynamics associated with diffusion, on the roles of cohesion in FG Nup self-interactions, and on the dynamic architecture of the CT and its role in providing specific pathways for diffusion. These both integrate the prior observations and suggest new hypotheses testable by experiment.
While their role in vivo is unclear, FG repeat gels are significant as a model for phase separation studies, and an underlying thermodynamic model was recently developed [88] rationalizing the observed increased stability of gels with temperature [73]. The critical limit for gel formation was also calculated for different amino acid compositions using coarse grained modeling at the bead-per-residue level [123]. The effects of cohesiveness on the selective permeability of in vitro FG repeat assemblies were simulated over a wide range of cohesiveness, showing that an increase in cohesiveness leads to decreasing permeability but that permeability may be enhanced with weak cohesiveness [124]. Modeling of the FG Nups in the CT in the absence of transport factors suggested a heterogeneous diffusion barrier of several condensates formed by electrostatic pairing rather than FG–FG interactions [39]. The role of the dynamic architecture of the CT was modeled and compared with experimental fluorescence anisotropy data by use of a bead equivalent of 4 nm resolution, and it was proposed that FG repeats are highly mobile and can reptate throughout the CT on timescales similar to experiment [125]. The role of transient formation of voids permitting a size dependent permeation was analyzed [126] using the Onck force field [127]. Regarding modeling passive transport, a Brownian dynamics simulation, with FG repeats represented as spring-like polymer beads and passive diffusing macromolecules as rigid spheres, suggested that the barrier to non-specific diffusion resulted largely from the highly dynamic FG repeats and entropic exclusion [15].
Several recent papers address more directly the modeling of FG repeat's interactions with NTRs. Based on experimental data for FG repeats, NTF2, and non-specific components’ interactions, agent-based modeling [128] discriminated between binding models to discriminate multivalent cases [59]. Crowding by different NTRs affecting interactions with FG Nups were proposed from coarse-grained classical density functional theory application with two residues per bead model for FG Nups, and spheres for NTRs, with the significant conclusion that at high NTR concentrations, there is increased flux [47] consistent with experimental data [129]. Complementing two experimental works on biomimetic nanopores with separate FG Nups [64,77] coarse grained modeling suggested that the NTR Kap95 forms a stable population bound to the CT periphery with fast transport proceeding in the FG-rich central channel.
A fair judgment is that these models are based on a wide range of assumptions/parameters, and that currently their results, in terms of describing details of the transport mechanism, are rather divergent. As the field integrates more data and refines models in the areas of siting of CT components, of parametrization of interactions energies of FG repeats and NTR/cargoes, of increased simulation times comparable to transit times in the NPC, and of comparison to more detailed tracking of individual NTR transits (e.g. [40]), there will hopefully be some convergence towards a realistic representation of the mechanisms of transport. While our knowledge of how FG repeats and NTR/cargoes interact in vivo is limited, there is however consensus that ‘stronger interactions and higher concentrations can block the transport. Importantly, accumulation of the transport proteins in the pore can also impede the translocation of inert molecules’ [8]. Similarly, there is agreement that FG repeat/NTR interactions are key to selective diffusion, while there remains controversy in the models about the nature and role of FG repeat cohesive interaction and their role in limiting passive diffusion. A key missing ingredient of current simulations is lack of incorporation of the ‘elephant’ — our updated knowledge of the high density of NTR/cargoes within the CT, and perhaps also limited appreciation of the difficulty of understanding the whole NPC — whose size and detail may require a so-called paradigm shift of approach rather than the cumulative accretion of data [130]
Perspectives
Every year, more and more connections are discovered between NPC dysfunction and a range of serious, widespread and challenging human diseases; these include many cancers, neurodegenerative diseases, and a host of viral diseases including most recently SARS-CoV-2 [131,132]. Designing effective therapeutics for such dysregulations depends upon the biomedical community gaining a detailed and comprehensive understanding of all the functionalities associated with NPCs, foremost among these being the mechanisms underlying nucleocytoplasmic transport.
There is now consensus on many aspects of the mechanisms underlying nucleocytoplasmic transport. FG Nups anchored in the walls of CT generate a brush of intrinsically disordered FG repeat regions that form a high local concentration of FG motifs. NTRs, often carrying cargoes, cross by binding these FG motifs. The Interaction between NTRs and FG repeats leads to a high local concentration of NTRs in the CT, further strongly contributing to competitive exclusion of non-specific macromolecules. Much of the remaining controversy revolves around how non-specific macromolecules are further prevented from crossing the NPC, with possibilities ranging from FG repeats forming slow moving highly cross-linked gels, to weakly (or essentially non-) cohesive FG repeats being highly mobile and entropically excluding only non-interacting macromolecules while facilitating rapid transit of NTRs with cargoes.
Resolving the remaining controversies, discussed above, is obviously a major priority. We must also come to grips with the astonishing diversity and pliability now being revealed in the NPC's architecture and mechanisms; that different NPCs have different compositions and so may specialize for different transport pathways, that NPCs can change shape in such a way that may modulate transport, and that different cargo types may take different paths across the NPC with different mechanistic details and at different times. Finally, as more mechanistic links between NPCs and diseases are understood, the potential for therapeutic intervention in nucleocytoplasmic transport will likely greatly increase.
Acknowledgements
We are grateful to the Cowburn and Rout labs for discussions, and a reviewer for suggestions.
Abbreviations
- GLFG
Gly–Leu–Phe–Gly
- IDPs
intrinsically disordered proteins
- IDRs
intrinsically disordered regions
- LLPS
liquid–liquid phase separation
- NE
nuclear envelope
- NPCs
nuclear pore complexes
- NTRs
nuclear transport factors
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
Supported by NIH grants R01 GM117212 (D.C.), P41 GM109824 and R01 GM112108 (M.P.R.).
References
- 1.Mettenleiter, T.C. (2016) Breaching the barrier-the nuclear envelope in virus infection. J. Mol. Biol. 428, 1949–1961 10.1016/j.jmb.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 2.Chandra, B., Michmerhuizen, N.L., Shirnekhi, H.K., Tripathi, S., Pioso, B.J., Baggett, D.W.et al. (2022) Phase separation mediates NUP98 fusion oncoprotein leukemic transformation. Cancer Discov. 12, 1152–1169 10.1158/2159-8290.CD-21-0674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spead, O., Zaepfel, B.L. and Rothstein, J.D. (2022) Nuclear pore dysfunction in neurodegeneration. Neurotherapeutics 19, 1050–1060 10.1007/s13311-022-01293-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hampoelz, B., Andres-Pons, A., Kastritis, P. and Beck, M. (2019) Structure and assembly of the nuclear pore complex. Annu. Rev. Biophys. 48, 515–536 10.1146/annurev-biophys-052118-115308 [DOI] [PubMed] [Google Scholar]
- 5.Petrovic, S., Mobbs, G.W., Bley, C.J., Nie, S., Patke, A. and Hoelz, A. (2022) Structure and function of the nuclear pore complex. Cold Spring Harb. Perspect. Biol. 14, a041264 10.1101/cshperspect.a041264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Martinez, J. and Rout, M.P. (2021) One ring to rule them all? Structural and functional diversity in the nuclear pore complex. Trends Biochem. Sci. 46, 595–607 10.1016/j.tibs.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang, K. and Szleifer, I. (2020) Modeling the nucleoporins that form the hairy pores. Biochem. Soc. Trans. 48, 1447–1461 10.1042/BST20190941 [DOI] [PubMed] [Google Scholar]
- 8.Hoogenboom, B.W., Hough, L.E., Lemke, E.A., Lim, R.Y.H., Onck, P.R. and Zilman, A. (2021) Physics of the nuclear pore complex: theory, modeling and experiment. Phys. Rep. 921, 1–53 10.1016/j.physrep.2021.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akey, C.W., Singh, D., Ouch, C., Echeverria, I., Nudelman, I., Varberg, J.M.et al. (2022) Comprehensive structure and functional adaptations of the yeast nuclear pore complex. Cell 185, 361–78.e25 10.1016/j.cell.2021.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rout, M.P. and Aitchison, J.D. (2001) The nuclear pore complex as a transport machine. J. Biol. Chem. 276, 16593–6 10.1074/jbc.R100015200 [DOI] [PubMed] [Google Scholar]
- 11.Heinss, N., Sushkin, M., Yu, M. and Lemke, E.A. (2020) Multifunctionality of F-rich nucleoporins. Biochem. Soc. Trans. 48, 2603–2614 10.1042/BST20200357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wing, C.E., Fung, H.Y.J. and Chook, Y.M. (2022) Karyopherin-mediated nucleocytoplasmic transport. Nat. Rev. Mol. Cell Biol. 23, 307–328 10.1038/s41580-021-00446-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalita, J., Kapinos, L.E. and Lim, R.Y.H. (2021) On the asymmetric partitioning of nucleocytoplasmic transport: recent insights and open questions. J. Cell Sci. 134, jcs240382 10.1242/jcs.240382 [DOI] [PubMed] [Google Scholar]
- 14.Ashkenazy-Titelman, A., Shav-Tal, Y. and Kehlenbach, R.H. (2020) Into the basket and beyond: the journey of mRNA through the nuclear pore complex. Biochem. J. 477, 23–44 10.1042/BCJ20190132 [DOI] [PubMed] [Google Scholar]
- 15.Timney, B.L., Raveh, B., Mironska, R., Trivedi, J.M., Kim, S.J., Russel, D.et al. (2016) Simple rules for passive diffusion through the nuclear pore complex. J. Cell Biol. 215, 57–76 10.1083/jcb.201601004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popken, P., Ghavami, A., Onck, P.R., Poolman, B. and Veenhoff, L.M. (2015) Size-dependent leak of soluble and membrane proteins through the yeast nuclear pore complex. Mol. Biol. Cell 26, 1386–1394 10.1091/mbc.E14-07-1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wuhr, M., Guttler, T., Peshkin, L., McAlister, G.C., Sonnett, M., Ishihara, K.et al. (2015) The nuclear proteome of a vertebrate. Curr. Biol. 25, 2663–2671 10.1016/j.cub.2015.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, S.J., Fernandez-Martinez, J., Nudelman, I., Shi, Y., Zhang, W., Raveh, B.et al. (2018) Integrative structure and functional anatomy of a nuclear pore complex. Nature 555, 475–482 10.1038/nature26003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akey, C.W. (1990) Visualization of transport-related configurations of the nuclear pore transporter. Biophys. J. 58, 341–355 10.1016/S0006-3495(90)82381-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart, M. (1992) Nuclear pore structure and function. Semin. Cell Biol. 3, 267–277 10.1016/1043-4682(92)90028-t [DOI] [PubMed] [Google Scholar]
- 21.Ryan, K.J. and Wente, S.R. (2000) The nuclear pore complex: a protein machine bridging the nucleus and cytoplasm. Curr. Opin. Cell Biol. 12, 361–371 10.1016/s0955-0674(00)00101-0 [DOI] [PubMed] [Google Scholar]
- 22.Rout, M.P., Aitchison, J.D., Suprapto, A., Hjertaas, K., Zhao, Y. and Chait, B.T. (2000) The yeast nuclear pore complex: composition, architecture, and transport mechanism. J. Cell Biol. 148, 635–651 10.1083/jcb.148.4.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolthausen, E., Deuschel, J.D. and Zeitouni, O. (1995) Entropic repulsion of the lattice free-field. Commun. Math. Phys. 170, 417–443 10.1007/Bf02108336 [DOI] [Google Scholar]
- 24.Clarkson, W.D., Kent, H.M. and Stewart, M. (1996) Separate binding sites on nuclear transport factor 2 (NTF2) for GDP-Ran and the phenylalanine-rich repeat regions of nucleoporins p62 and Nsp1p. J. Mol. Biol. 263, 517–524 10.1006/jmbi.1996.0594 [DOI] [PubMed] [Google Scholar]
- 25.Bayliss, R., Ribbeck, K., Akin, D., Kent, H.M., Feldherr, C.M., Gorlich, D.et al. (1999) Interaction between NTF2 and xFxFG-containing nucleoporins is required to mediate nuclear import of RanGDP. J. Mol. Biol. 293, 579–593 10.1006/jmbi.1999.3166 [DOI] [PubMed] [Google Scholar]
- 26.Bayliss, R., Corbett, A.H. and Stewart, M. (2000) The molecular mechanism of transport of macromolecules through nuclear pore complexes. Traffic 1, 448–456 10.1034/j.1600-0854.2000.010602.x [DOI] [PubMed] [Google Scholar]
- 27.Rout, M.P., Aitchison, J.D., Magnasco, M.O. and Chait, B.T. (2003) Virtual gating and nuclear transport: the hole picture. Trends Cell Biol. 13, 622–628 10.1016/j.tcb.2003.10.007 [DOI] [PubMed] [Google Scholar]
- 28.Ribbeck, K. and Gorlich, D. (2001) Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 20, 1320–1330 10.1093/emboj/20.6.1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapinos, L.E., Schoch, R.L., Wagner, R.S., Schleicher, K.D. and Lim, R.Y. (2014) Karyopherin-centric control of nuclear pores based on molecular occupancy and kinetic analysis of multivalent binding with FG nucleoporins. Biophys. J. 106, 1751–1762 10.1016/j.bpj.2014.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim, R.Y., Huang, B. and Kapinos, L.E. (2015) How to operate a nuclear pore complex by Kap-centric control. Nucleus 6, 366–372 10.1080/19491034.2015.1090061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapinos, L.E., Huang, B., Rencurel, C. and Lim, R.Y.H. (2017) Karyopherins regulate nuclear pore complex barrier and transport function. J. Cell Biol. 216, 3609–3624 10.1083/jcb.201702092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuda, A. and Mofrad, M.R.K. (2021) Free energy calculations shed light on the nuclear pore complex's selective barrier nature. Biophys. J. 120, 3628–3640 10.1016/j.bpj.2021.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim, R.Y., Aebi, U. and Fahrenkrog, B. (2008) Towards reconciling structure and function in the nuclear pore complex. Histochem. Cell Biol. 129, 105–116 10.1007/s00418-007-0371-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson, C.E., Mattheyses, A.L., Kampmann, M. and Simon, S.M. (2013) Conserved spatial organization of FG domains in the nuclear pore complex. Biophys. J. 104, 37–50 10.1016/j.bpj.2012.11.3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakiyama, Y., Mazur, A., Kapinos, L.E. and Lim, R.Y. (2016) Spatiotemporal dynamics of the nuclear pore complex transport barrier resolved by high-speed atomic force microscopy. Nat. Nanotechnol. 11, 719–723 10.1038/nnano.2016.62 [DOI] [PubMed] [Google Scholar]
- 36.Yamada, J., Phillips, J.L., Patel, S., Goldfien, G., Calestagne-Morelli, A., Huang, H.et al. (2010) A bimodal distribution of two distinct categories of intrinsically disordered structures with separate functions in FG nucleoporins. Mol. Cell. Proteomics 9, 2205–2224 10.1074/mcp.M000035-MCP201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denning, D.P. and Rexach, M.F. (2007) Rapid evolution exposes the boundaries of domain structure and function in natively unfolded FG nucleoporins. Mol. Cell. Proteomics 6, 272–282 10.1074/mcp.M600309-MCP200 [DOI] [PubMed] [Google Scholar]
- 38.Peyro, M., Soheilypour, M., Lee, B.L. and Mofrad, M.R. (2015) Evolutionarily conserved sequence features regulate the formation of the FG network at the center of the nuclear pore complex. Sci. Rep. 5, 15795 10.1038/srep15795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang, K., Tagliazucchi, M., Park, S.H., Rabin, Y. and Szleifer, I. (2020) Nanocompartmentalization of the nuclear pore lumen. Biophys. J. 118, 219–231 10.1016/j.bpj.2019.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowdhury, R., Sau, A. and Musser, S.M. (2022) Super-resolved 3D tracking of cargo transport through nuclear pore complexes. Nat. Cell Biol. 24, 112–122 10.1038/s41556-021-00815-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strawn, L.A., Shen, T., Shulga, N., Goldfarb, D.S. and Wente, S.R. (2004) Minimal nuclear pore complexes define FG repeat domains essential for transport. Nat. Cell Biol. 6, 197–206 10.1038/ncb1097 [DOI] [PubMed] [Google Scholar]
- 42.Bayliss, R., Littlewood, T., Strawn, L.A., Wente, S.R. and Stewart, M. (2002) GLFG and FxFG nucleoporins bind to overlapping sites on importin-beta. J. Biol. Chem. 277, 50597–50606 10.1074/jbc.M209037200 [DOI] [PubMed] [Google Scholar]
- 43.Allen, N.P., Huang, L., Burlingame, A. and Rexach, M. (2001) Proteomic analysis of nucleoporin interacting proteins. J. Biol. Chem. 276, 29268–29274 10.1074/jbc.M102629200 [DOI] [PubMed] [Google Scholar]
- 44.Fiserova, J., Richards, S.A., Wente, S.R. and Goldberg, M.W. (2010) Facilitated transport and diffusion take distinct spatial routes through the nuclear pore complex. J. Cell Sci. 123, 2773–2780 10.1242/jcs.070730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang, W. (2013) Distinct, but not completely separate spatial transport routes in the nuclear pore complex. Nucleus 4, 166–175 10.4161/nucl.24874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma, J., Goryaynov, A. and Yang, W. (2016) Super-resolution 3D tomography of interactions and competition in the nuclear pore complex. Nat. Struct. Mol. Biol. 23, 239–247 10.1038/nsmb.3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis, L.K., Ford, I.J. and Hoogenboom, B.W. (2022) Crowding-induced phase separation of nuclear transport receptors in FG nucleoporin assemblies. Elife 11, e72627 10.7554/eLife.72627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao, Y., Skowyra, M.L., Feng, P. and Rapoport, T.A. (2022) Protein import into peroxisomes occurs through a nuclear pore-like phase. Science 378, eadf3971 10.1126/science.adf3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meinema, A.C., Poolman, B. and Veenhoff, L.M. (2013) Quantitative analysis of membrane protein transport across the nuclear pore complex. Traffic 14, 487–501 10.1111/tra.12048 [DOI] [PubMed] [Google Scholar]
- 50.Mudumbi, K.C., Czapiewski, R., Ruba, A., Junod, S.L., Li, Y., Luo, W.et al. (2020) Nucleoplasmic signals promote directed transmembrane protein import simultaneously via multiple channels of nuclear pores. Nat. Commun. 11, 2184 10.1038/s41467-020-16033-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kubitscheck, U. and Siebrasse, J.P. (2017) Kinetics of transport through the nuclear pore complex. Semin. Cell Dev. Biol. 68, 18–26 10.1016/j.semcdb.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 52.Paci, G., Zheng, T., Caria, J., Zilman, A. and Lemke, E.A. (2020) Molecular determinants of large cargo transport into the nucleus. Elife 9, e55963 10.7554/eLife.55963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Visa, N., Alzhanova-Ericsson, A.T., Sun, X., Kiseleva, E., Bjorkroth, B., Wurtz, T.et al. (1996) A pre-mRNA-binding protein accompanies the RNA from the gene through the nuclear pores and into polysomes. Cell 84, 253–264 10.1016/s0092-8674(00)80980-0 [DOI] [PubMed] [Google Scholar]
- 54.Pulupa, J., Prior, H., Johnson, D.S. and Simon, S.M. (2020) Conformation of the nuclear pore in living cells is modulated by transport state. Elife 9, e60654 10.7554/eLife.60654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmerli, C.E., Allegretti, M., Rantos, V., Goetz, S.K., Obarska-Kosinska, A., Zagoriy, I.et al. (2021) Nuclear pores dilate and constrict in cellulo. Science 374, eabd9776 10.1126/science.abd9776 [DOI] [PubMed] [Google Scholar]
- 56.Zheng, T. and Zilman, A. (2023) Self-regulation of the nuclear pore complex enables clogging-free crowded transport. Proc. Natl Acad. Sci. U.S.A. 120, e2212874120 10.1073/pnas.2212874120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barbato, S., Kapinos, L.E., Rencurel, C. and Lim, R.Y.H. (2020) Karyopherin enrichment at the nuclear pore complex attenuates Ran permeability. J. Cell Sci. 133, jcs238121 10.1242/jcs.238121 [DOI] [PubMed] [Google Scholar]
- 58.Jovanovic-Talisman, T., Tetenbaum-Novatt, J., McKenney, A.S., Zilman, A., Peters, R., Rout, M.P.et al. (2009) Artificial nanopores that mimic the transport selectivity of the nuclear pore complex. Nature 457, 1023–107 10.1038/nature07600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lennon, K.M., Soheilypour, M., Peyro, M., Wakefield, D.L., Choo, G.E., Mofrad, M.R.K.et al. (2021) Characterizing binding interactions that are essential for selective transport through the nuclear pore complex. Int. J. Mol. Sci. 22, 10898 10.3390/ijms221910898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zilman, A., Di Talia, S., Jovanovic-Talisman, T., Chait, B.T., Rout, M.P. and Magnasco, M.O. (2010) Enhancement of transport selectivity through nano-channels by non-specific competition. PLoS Comput. Biol. 6, e1000804 10.1371/journal.pcbi.1000804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Derrer, C.P., Mancini, R., Vallotton, P., Huet, S., Weis, K. and Dultz, E. (2019) The RNA export factor Mex67 functions as a mobile nucleoporin. J. Cell Biol. 218, 3967–3976 10.1083/jcb.201909028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ben-Yishay, R., Mor, A., Shraga, A., Ashkenazy-Titelman, A., Kinor, N., Schwed-Gross, A.et al. (2019) Imaging within single NPCs reveals NXF1's role in mRNA export on the cytoplasmic side of the pore. J. Cell Biol. 218, 2962–2981 10.1083/jcb.201901127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalita, J., Kapinos, L.E., Zheng, T., Rencurel, C., Zilman, A. and Lim, R.Y.H. (2022) Karyopherin enrichment and compensation fortifies the nuclear pore complex against nucleocytoplasmic leakage. J. Cell Biol. 221, e202108107 10.1083/jcb.202108107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fragasso, A., de Vries, H.W., Andersson, J., van der Sluis, E.O., van der Giessen, E., Onck, P.R.et al. (2022) Transport receptor occupancy in nuclear pore complex mimics. Nano Res. 15, 9689–9703 10.1007/s12274-022-4647-1 [DOI] [Google Scholar]
- 65.Hough, L.E., Dutta, K., Sparks, S., Temel, D.B., Kamal, A., Tetenbaum-Novatt, J.et al. (2015) The molecular mechanism of nuclear transport revealed by atomic-scale measurements. Elife 4, e10027 10.7554/eLife.10027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milles, S., Mercadante, D., Aramburu, I.V., Jensen, M.R., Banterle, N., Koehler, C.et al. (2015) Plasticity of an ultrafast interaction between nucleoporins and nuclear transport receptors. Cell 163, 734–745 10.1016/j.cell.2015.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stewart, M. (2022) Function of the nuclear transport machinery in maintaining the distinctive compositions of the nucleus and cytoplasm. Int. J. Mol. Sci. 23, 2578 10.3390/ijms23052578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stewart, M. (2006) Structural basis for the nuclear protein import cycle. Biochem. Soc. Trans. 34, 701–704 10.1042/BST0340701 [DOI] [PubMed] [Google Scholar]
- 69.Raveh, B., Karp, J.M., Sparks, S., Dutta, K., Rout, M.P., Sali, A.et al. (2016) Slide-and-exchange mechanism for rapid and selective transport through the nuclear pore complex. Proc. Natl Acad. Sci. U.S.A. 113, E2489–E2497 10.1073/pnas.1522663113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakiyama, Y., Panatala, R. and Lim, R.Y.H. (2017) Structural dynamics of the nuclear pore complex. Semin. Cell Dev. Biol. 68, 27–33 10.1016/j.semcdb.2017.05.021 [DOI] [PubMed] [Google Scholar]
- 71.Erlendsson, S. and Teilum, K. (2020) Binding revisited-avidity in cellular function and signaling. Front. Mol. Biosci. 7, 615565 10.3389/fmolb.2020.615565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hayama, R., Sparks, S., Hecht, L.M., Dutta, K., Karp, J.M., Cabana, C.M.et al. (2018) Thermodynamic characterization of the multivalent interactions underlying rapid and selective translocation through the nuclear pore complex. J. Biol. Chem. 293, 4555–4563 10.1074/jbc.AC117.001649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Najbauer, E.E., Ng, S.C., Griesinger, C., Gorlich, D. and Andreas, L.B. (2022) Atomic resolution dynamics of cohesive interactions in phase-separated Nup98 FG domains. Nat. Commun. 13, 1494 10.1038/s41467-022-28821-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frey, S., Richter, R.P. and Gorlich, D. (2006) FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 314, 815–817 10.1126/science.1132516 [DOI] [PubMed] [Google Scholar]
- 75.Ader, C., Frey, S., Maas, W., Schmidt, H.B., Gorlich, D. and Baldus, M. (2010) Amyloid-like interactions within nucleoporin FG hydrogels. Proc. Natl Acad. Sci. U.S.A. 107, 6281–6285 10.1073/pnas.0910163107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ibanez de Opakua, A., Geraets, J.A., Frieg, B., Dienemann, C., Savastano, A., Rankovic, M.et al. (2022) Molecular interactions of FG nucleoporin repeats at high resolution. Nat. Chem. 14, 1278–1285 10.1038/s41557-022-01035-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fragasso, A., de Vries, H.W., Andersson, J., van der Sluis, E.O., van der Giessen, E., Dahlin, A.et al. (2021) A designer FG-Nup that reconstitutes the selective transport barrier of the nuclear pore complex. Nat. Commun. 12, 2010 10.1038/s41467-021-22293-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ketterer, P., Ananth, A.N., Laman Trip, D.S., Mishra, A., Bertosin, E., Ganji, M.et al. (2018) DNA origami scaffold for studying intrinsically disordered proteins of the nuclear pore complex. Nat. Commun. 9, 902 10.1038/s41467-018-03313-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fisher, P.D.E., Shen, Q., Akpinar, B., Davis, L.K., Chung, K.K.H., Baddeley, D.et al. (2018) A programmable DNA origami platform for organizing intrinsically disordered nucleoporins within nanopore confinement. ACS Nano 12, 1508–1518 10.1021/acsnano.7b08044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andersson, J., Svirelis, J., Medin, J., Jarlebark, J., Hailes, R. and Dahlin, A. (2022) Pore performance: artificial nanoscale constructs that mimic the biomolecular transport of the nuclear pore complex. Nanoscale Adv. 4, 4925–4937 10.1039/d2na00389a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frey, S. and Gorlich, D. (2007) A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 130, 512–523 10.1016/j.cell.2007.06.024 [DOI] [PubMed] [Google Scholar]
- 82.Halfmann, R., Wright, J.R., Alberti, S., Lindquist, S. and Rexach, M. (2012) Prion formation by a yeast GLFG nucleoporin. Prion 6, 391–399 10.4161/pri.20199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo, L., Kim, H.J., Wang, H., Monaghan, J., Freyermuth, F., Sung, J.C.et al. (2018) Nuclear-import receptors reverse aberrant phase transitions of RNA-binding proteins with prion-like domains. Cell 173, 677–92.e20 10.1016/j.cell.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mittag, T. and Pappu, R.V. (2022) A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol. Cell 82, 2201–2214 10.1016/j.molcel.2022.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mattheyses, A.L., Kampmann, M., Atkinson, C.E. and Simon, S.M. (2010) Fluorescence anisotropy reveals order and disorder of protein domains in the nuclear pore complex. Biophys. J. 99, 1706–1717 10.1016/j.bpj.2010.06.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gu, C., Coalson, R.D., Jasnow, D. and Zilman, A. (2017) Free energy of nanoparticle binding to multivalent polymeric substrates. J. Phys. Chem. B 121, 6425–6435 10.1021/acs.jpcb.7b00868 [DOI] [PubMed] [Google Scholar]
- 87.Emilsson, G., Xiong, K., Sakiyama, Y., Malekian, B., Ahlberg Gagner, V., Schoch, R.L.et al. (2018) Polymer brushes in solid-state nanopores form an impenetrable entropic barrier for proteins. Nanoscale 10, 4663–4669 10.1039/c7nr09432a [DOI] [PubMed] [Google Scholar]
- 88.Ng, S.C. and Gorlich, D. (2022) A simple thermodynamic description of phase separation of Nup98 FG domains. Nat. Commun. 13, 6172 10.1038/s41467-022-33697-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Labokha, A.A., Gradmann, S., Frey, S., Hulsmann, B.B., Urlaub, H., Baldus, M.et al. (2013) Systematic analysis of barrier-forming FG hydrogels from Xenopus nuclear pore complexes. EMBO J. 32, 204–218 10.1038/emboj.2012.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frey, S., Rees, R., Schunemann, J., Ng, S.C., Funfgeld, K., Huyton, T.et al. (2018) Surface properties determining passage rates of proteins through nuclear pores. Cell 174, 202–17.e9 10.1016/j.cell.2018.05.045 [DOI] [PubMed] [Google Scholar]
- 91.Hulsmann, B.B., Labokha, A.A. and Gorlich, D. (2012) The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model. Cell 150, 738–751 10.1016/j.cell.2012.07.019 [DOI] [PubMed] [Google Scholar]
- 92.Tetenbaum-Novatt, J., Hough, L.E., Mironska, R., McKenney, A.S., Rout, M.P. (2012) Nucleocytoplasmic transport: a role for nonspecific competition in karyopherin-nucleoporin interactions. Mol Cell Proteomics. 11, 31–46 10.1074/mcp.M111.013656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Milles, S. and Lemke, E.A. (2014) Mapping multivalency and differential affinities within large intrinsically disordered protein complexes with segmental motion analysis. Angew. Chem. Int. Ed. Engl. 53, 7364–7367 10.1002/anie.201403694 [DOI] [PubMed] [Google Scholar]
- 94.Sparks, S., Temel, D.B., Rout, M.P. and Cowburn, D. (2018) Deciphering the “Fuzzy” interaction of FG nucleoporins and transport factors using small-angle neutron scattering. Structure 26, 477–84.e4 10.1016/j.str.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee, C.F., Brangwynne, C.P., Gharakhani, J., Hyman, A.A. and Julicher, F. (2013) Spatial organization of the cell cytoplasm by position-dependent phase separation. Phys. Rev. Lett. 111, 088101 10.1103/PhysRevLett.111.088101 [DOI] [PubMed] [Google Scholar]
- 96.Hyman, A.A., Weber, C.A. and Julicher, F. (2014) Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58 10.1146/annurev-cellbio-100913-013325 [DOI] [PubMed] [Google Scholar]
- 97.Celetti, G., Paci, G., Caria, J., VanDelinder, V., Bachand, G. and Lemke, E.A. (2020) The liquid state of FG-nucleoporins mimics permeability barrier properties of nuclear pore complexes. J. Cell Biol. 219, e201907157 10.1083/jcb.201907157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmidt, H.B. and Gorlich, D. (2015) Nup98 FG domains from diverse species spontaneously phase-separate into particles with nuclear pore-like permselectivity. Elife 4, e04251 10.7554/eLife.04251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmidt, H.B. and Gorlich, D. (2016) Transport selectivity of nuclear pores, phase separation, and membraneless organelles. Trends Biochem. Sci. 41, 46–61 10.1016/j.tibs.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 100.Taylor, N., Elbaum-Garfinkle, S., Vaidya, N., Zhang, H., Stone, H.A. and Brangwynne, C.P. (2016) Biophysical characterization of organelle-based RNA/protein liquid phases using microfluidics. Soft Matter. 12, 9142–9150 10.1039/c6sm01087c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feric, M., Vaidya, N., Harmon, T.S., Mitrea, D.M., Zhu, L., Richardson, T.M.et al. (2016) Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697 10.1016/j.cell.2016.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamazaki, T., Yamamoto, T. and Hirose, T. (2022) Micellization: a new principle in the formation of biomolecular condensates. Front. Mol. Biosci. 9, 974772 10.3389/fmolb.2022.974772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Milner, S.T., Witten, T.A. and Cates, M.E. (2002) Theory of the grafted polymer brush. Macromolecules 21, 2610–2619 10.1021/ma00186a051 [DOI] [Google Scholar]
- 104.Alberti, S. and Hyman, A.A. (2021) Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 22, 196–213 10.1038/s41580-020-00326-6 [DOI] [PubMed] [Google Scholar]
- 105.Weisbrod, S. (1982) Active chromatin. Nature 297, 289–295 10.1038/297289a0 [DOI] [PubMed] [Google Scholar]
- 106.Jones, R.G. (2009) Compendium of Polymer Terminology and Nomenclature: IUPAC Recommendations 2008, 2nd ed, RSC Pub, Cambridge, U.K [Google Scholar]
- 107.Michieletto, D. and Marenda, M. (2022) Rheology and viscoelasticity of proteins and nucleic acids condensates. JACS Au 2, 1506–1521 10.1021/jacsau.2c00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lovestam, S., Koh, F.A., van Knippenberg, B., Kotecha, A., Murzin, A.G., Goedert, M.et al. (2022) Assembly of recombinant tau into filaments identical to those of Alzheimer's disease and chronic traumatic encephalopathy. Elife 11, e76494 10.7554/eLife.76494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuiper, E.F.E., Gallardo, P., Bergsma, T., Mari, M., Kolbe Musskopf, M., Kuipers, J.et al. (2022) The chaperone DNAJB6 surveils FG-nucleoporins and is required for interphase nuclear pore complex biogenesis. Nat. Cell Biol. 24, 1584–1594 10.1038/s41556-022-01010-x [DOI] [PubMed] [Google Scholar]
- 110.Prophet, S.M., Rampello, A.J., Niescier, R.F., Gentile, J.E., Mallik, S., Koleske, A.J.et al. (2022) Atypical nuclear envelope condensates linked to neurological disorders reveal nucleoporin-directed chaperone activities. Nat. Cell Biol. 24, 1630–1641 10.1038/s41556-022-01001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Milles, S., Huy Bui, K., Koehler, C., Eltsov, M., Beck, M. and Lemke, E.A. (2013) Facilitated aggregation of FG nucleoporins under molecular crowding conditions. EMBO Rep. 14, 178–183 10.1038/embor.2012.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thomas, L., Ismail, B.T., Askjaer, P. and Seydoux, G. (2022) Cytoplasmic nucleoporin foci are stress-sensitive, non-essential condensates in C. elegans. bioRxiv, 1–68 10.1101/2022.08.22.504855 [DOI] [Google Scholar]
- 113.Springhower, C.E., Rosen, M.K. and Chook, Y.M. (2020) Karyopherins and condensates. Curr. Opin. Cell Biol. 64, 112–123 10.1016/j.ceb.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Coyne, A.N. and Rothstein, J.D. (2022) Nuclear pore complexes: a doorway to neural injury in neurodegeneration. Nat. Rev. Neurol. 18, 348–362 10.1038/s41582-022-00653-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li, N. and Lagier-Tourenne, C. (2018) Nuclear pores: the gate to neurodegeneration. Nat. Neurosci. 21, 156–158 10.1038/s41593-017-0066-0 [DOI] [PubMed] [Google Scholar]
- 116.Spannl, S., Tereshchenko, M., Mastromarco, G.J., Ihn, S.J. and Lee, H.O. (2019) Biomolecular condensates in neurodegeneration and cancer. Traffic 20, 890–911 10.1111/tra.12704 [DOI] [PubMed] [Google Scholar]
- 117.Denning, D.P., Patel, S.S., Uversky, V., Fink, A.L. and Rexach, M. (2003) Disorder in the nuclear pore complex: the FG repeat regions of nucleoporins are natively unfolded. Proc. Natl Acad. Sci. U.S.A. 100, 2450–2455 10.1073/pnas.0437902100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu, M., Heidari, M., Mikhaleva, S., Tan, P.S., Mingu, S., Ruan, H.et al. (2022) Deciphering the conformations and dynamics of FG-nucleoporins in situ. bioArkiv, 1–17 10.1101/2022.07.07.499201 [DOI] [Google Scholar]
- 119.Davis, L.K., Ford, I.J., Saric, A. and Hoogenboom, B.W. (2020) Intrinsically disordered nuclear pore proteins show ideal-polymer morphologies and dynamics. Phys. Rev. E 101, 022420 10.1103/PhysRevE.101.022420 [DOI] [PubMed] [Google Scholar]
- 120.Davis, L.K., Saric, A., Hoogenboom, B.W. and Zilman, A. (2021) Physical modeling of multivalent interactions in the nuclear pore complex. Biophys. J. 120, 1565–1577 10.1016/j.bpj.2021.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McCarthy, M.R. and Lusk, C.P. (2022) One ring doesn't rule them all: distinct nuclear pore complexes in a single cell. Cell 185, 230–231 10.1016/j.cell.2021.12.042 [DOI] [PubMed] [Google Scholar]
- 122.Petrovic, S., Samanta, D., Perriches, T., Bley, C.J., Thierbach, K., Brown, B.et al. (2022) Architecture of the linker-scaffold in the nuclear pore. Science 376, eabm9798 10.1126/science.abm9798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ghavami, A., Van der Giessen, E. and Onck, P.R. (2018) Sol-gel transition in solutions of FG-Nups of the nuclear pore complex. Extreme Mech. Lett. 22, 36–41 10.1016/j.eml.2018.04.006 [DOI] [Google Scholar]
- 124.Gu, C., Vovk, A., Zheng, T., Coalson, R.D. and Zilman, A. (2019) The role of cohesiveness in the permeability of the spatial assemblies of FG nucleoporins. Biophys. J. 116, 1204–1215 10.1016/j.bpj.2019.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pulupa, J., Rachh, M., Tomasini, M.D., Mincer, J.S. and Simon, S.M. (2017) A coarse-grained computational model of the nuclear pore complex predicts Phe-Gly nucleoporin dynamics. J. Gen. Physiol. 149, 951–966 10.1085/jgp.201711769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Winogradoff, D., Chou, H.Y., Maffeo, C. and Aksimentiev, A. (2022) Percolation transition prescribes protein size-specific barrier to passive transport through the nuclear pore complex. Nat. Commun. 13, 5138 10.1038/s41467-022-32857-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ghavami, A., Veenhoff, L.M., van der Giessen, E. and Onck, P.R. (2014) Probing the disordered domain of the nuclear pore complex through coarse-grained molecular dynamics simulations. Biophys. J. 107, 1393–1402 10.1016/j.bpj.2014.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soheilypour, M. and Mofrad, M.R.K. (2018) Agent-based modeling in molecular systems biology. Bioessays 40, e1800020 10.1002/bies.201800020 [DOI] [PubMed] [Google Scholar]
- 129.Yang, W. and Musser, S.M. (2006) Nuclear import time and transport efficiency depend on importin beta concentration. J. Cell Biol. 174, 951–961 10.1083/jcb.200605053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kuhn, T.S. (1970) The Structure of Scientific Revolutions, Chicago University of Chicago Press, Chicago [Google Scholar]
- 131.Beyer, D.K. and Forero, A. (2022) Mechanisms of antiviral immune evasion of SARS-CoV-2. J. Mol. Biol. 434, 167265 10.1016/j.jmb.2021.167265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang, K., Miorin, L., Makio, T., Dehghan, I., Gao, S., Xie, Y.et al. (2021) Nsp1 protein of SARS-CoV-2 disrupts the mRNA export machinery to inhibit host gene expression. Sci. Adv. 7, eabe7386 10.1126/sciadv.abe7386 [DOI] [PMC free article] [PubMed] [Google Scholar]