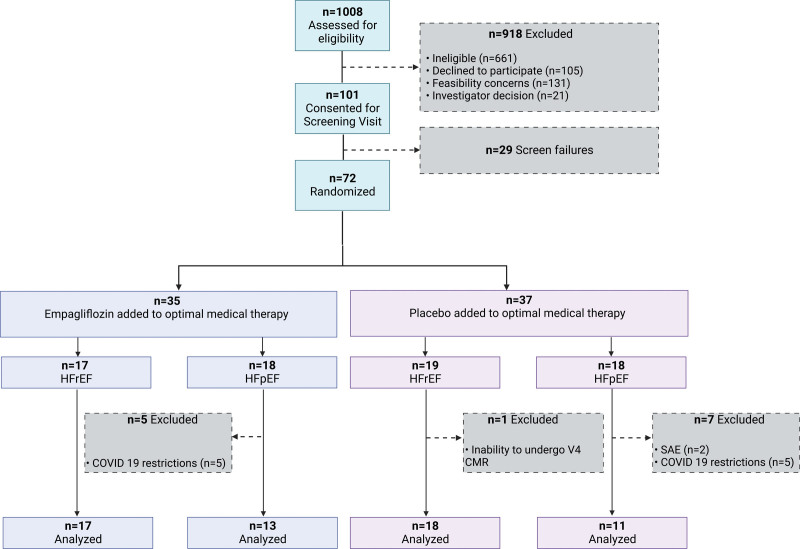
Figure 1.
Patient flow diagram for the EMPA-VISION double-blind, phase-III randomized controlled trial. Patients (n=1008) were assessed for eligibility; 101 patients were consented and underwent a screening visit. Of those, 72 were eventually eligible and randomly allocated to either 10 mg of empagliflozin (n=35) or matching placebo (n=37) once daily, stratified in their respective cohorts (HFrEF and HFpEF). One participant with HFrEF in the placebo group withdrew from further participation before the individual end of treatment (visit 4). In the HFpEF cohort, 5 patients in the empagliflozin arm and 5 in the placebo arm were excluded due to missing data because of COVID-19 lockdown restrictions. Two patients in the placebo group withdrew from treatment due to serious adverse events. CMR indicates cardiovascular magnetic resonance; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; and SAE, serious adverse event.