Background.
Traditionally, antibody-mediated rejection (AMR) has been suspected mainly by a rise in serum creatinine (Scr) and confirmed by allograft biopsy. There is limited literature describing the trend of Scr after treatment, and how that trend might differ between patients with histological response and with no response to treatment.
Methods.
We included all cases of AMR at our program between March 2016 and July 2020 who had a follow-up biopsy after the index biopsy, with initial diagnosis of AMR. We trended the Scr and change in Scr (delta Scr) and its association with being a responder (microvascular inflammation, MVI ≤1) or nonresponder (MVI >1), as well as graft failure.
Results.
A total of 183 kidney transplant recipients were included, 66 in the responder group and 177 in the nonresponder group. The MVI scores and sum chronicity scores, along with transplant glomerulopathy scores, were higher in the nonresponder group. However, Scr at index biopsy was similar in responders (1.74 ± 0.70) versus nonresponders (1.83 ± 0.65; P = 0.39), as were the delta Scr at various time points. After adjustment for multiple variables, delta Scr was not associated with being a nonresponder. Also, delta Scr value at follow-up biopsy compared with index biopsy among responders was 0 ± 0.67 (P = 0.99) and among nonresponders was –0.01 ± 0.61 (P = 0.89). Being a nonresponder was significantly associated with an increased risk of graft failure at the last follow-up in univariate analysis but was not in multivariate analysis (hazard ratio 1.35; 95% confidence interval, 0.58-3.17; P = 0.49).
Conclusions.
We found that Scr is not a good predictor of the resolution of MVI, supporting the utility of follow-up biopsies after treatment of AMR.
Antibody-mediated rejection (AMR) is the most common cause of kidney allograft failure.1 Serum creatinine is a commonly used screening test for changes in kidney allograft function.2 The serum creatinine level is the most valuable prognostic marker of subsequent graft function.2 However, various studies have demonstrated that the serum creatinine level is not a sensitive marker for detecting changes in renal function.3,4 By the time there is a significant rise in serum creatinine, there could have been various acute and chronic changes in the kidney allograft suggestive of AMR, including peritubular capillaritis (ptc), glomerulitis (g), and transplant glomerulopathy (cg), which could have been missed if patients had not undergone an allograft biopsy.5
Diagnostic criteria for AMR are evolving. The most commonly used guideline for the diagnosis of AMR is the Banff criteria. This is an international consensus guideline for reporting the pathology of biopsies for solid organ transplants and is reviewed and updated every 2 y.6 The 3 main features for the diagnosis of AMR according to the Banff guidelines are the presence of histological evidence of tissue injury that is evident by microvascular inflammation (MVI, g, or ptc); evidence of current or recent antibody interaction with vascular endothelium evident by linear C4d staining in peritubular capillaries, moderate MVI ≥2, or increased gene transcripts strongly associated with AMR; and serological evidence of donor-specific antibodies (DSAs).7 With the latest Banff guidelines, the need for antibody interaction and the presence of DSA is less imperative.8,9 However, MVI is maintained as critical for diagnosing AMR. In a recent joint XVIth Banff Allograft Pathology and Canadian Society of Transplantation Meeting, there was a proposal to use the term MVI with or without DSA instead of AMR.10
The management of AMR is also evolving, but current outcomes after AMR are disappointing. In one study, median graft survival after chronic active AMR was <2 y.11 However, outcomes of treated subclinical AMR are similar to that of patients without rejection.12 At our program, all patients with the diagnosis and treatment of AMR undergo follow-up biopsy after approximately 12 wk of treatment. It is not known whether the serum creatinine trend in between these biopsies predicts histological findings on the follow-up biopsy. In this study, we describe our experience with kidney transplant recipients diagnosed with biopsy-proven AMR who underwent treatment and follow-up biopsies to determine how well the serum creatinine trend between the 2 biopsies predicted the resolution of MVI.
PATIENTS AND METHODS
Patients
We evaluated all patients at the University of Wisconsin who underwent a kidney allograft biopsy between March 1, 2016, and July 31, 2020. All patients with biopsy-proven active or chronic active AMR with sum MVI (sum of g+ptc) scores of ≥2 and who had received treatment along with follow-up biopsy after treatment were included in the study and divided into 2 groups based on the follow-up biopsy findings of MVI. Patients were considered to be responders if MVI score was ≤1 and to be nonresponders if MVI score was persistently ≥2 in the follow-up biopsy. We trended the serum creatinine between the index biopsy and the follow-up biopsy to see the association between the trend and being a responder versus a nonresponder. We hypothesized that responders would have significant improvement in the serum creatinine from index biopsy to follow-up biopsy, whereas nonresponders would not have significant improvement. Factors associated with being responders and risk factors for death-censored graft failure (DCGF) were outcomes of interest.
Most of the allograft biopsies were performed for cause, mainly in the setting of unexplained acute kidney injury (AKI) or unexplained proteinuria. Although there was no protocol to perform a biopsy based on an absolute rise in serum creatinine values, most of the recipients underwent kidney biopsy for an unexplained rise in serum creatinine >0.3 mg/dL and based on clinical judgment, taking into account factors such as noncompliance and immunological risk. During AKI, the estimated glomerular filtration rate (eGFR) may not be appropriate to estimate allograft function.13 Also, most of the criteria for the diagnosis of AKI, including the Kidney Disease: Improving Global Outcomes,14 Acute Kidney Injury Network,15 and RIFLE criteria (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease),16 are based on serum creatinine rather than eGFR. Therefore, in this study, we report our outcomes based on the serum creatinine rather than the eGFR.
Study Protocol and Data Collection
This study was approved by the University of Wisconsin School of Medicine and Public Health Institutional Review Board (protocol number: 2014-1072). Data collection included basic demographic information, date of kidney transplantation, age, race, gender, induction immunosuppression, and type of transplant. We collected the histology of kidney biopsies, DSA information, as well as uncensored and DCGF. DCGF was defined as a return to dialysis or retransplantation by end of the data analysis in September 2022. The patient’s last follow-up was censored at death or DCGF for those who experienced it or at last serum creatinine among those with a functioning graft. Delta serum creatinine was calculated as the difference between serum creatinine at index biopsy and serum creatinine at various time points after that.
Anti-HLA Antibody Screening by Solid-Phase Fluorescent Beads
Donor-specific HLA class I and II antibodies were detected pretransplant and posttransplant using Luminex single antigen beads (One Lambda, Canoga Park, CA) and performed according to the manufacturer’s instructions with the single modification in which a reduced volume of beads (3 versus 5 µL) was used, as reported previously.17 Briefly, antibodies were identified using multiple criteria including patterns of epitope reactivity, mean fluorescent intensity value, specific bead behaviors, and assay background, as described previously.18
The strength of DSA was represented as the sum of the mean fluorescence intensity value of all DSAs. Patients diagnosed with de novo DSA (dnDSA) had no detectable DSA pretransplant or posttransplant when tested before the diagnosis of dnDSA. Routine posttransplant monitoring of DSA was performed on all transplant recipients at 6 and 12 mo and annually thereafter. Patients with a pretransplant calculated panel-reactive antibody >0 were tested at an additional 3-wk time point, and patients with pretransplant DSA were tested at additional 3-wk, 6-wk, and 3-mo time points. All patients undergoing transplant biopsies for any reason had DSA testing as a part of the biopsy visit.19
Immunosuppression
Patients undergoing kidney transplant received induction immunosuppression with a depleting agent (antithymocyte globulin or alemtuzumab) or nondepleting agent (basiliximab) based on immunological risk factors. Patients with pretransplant DSA, end-stage kidney disease because of glomerulonephritis, and those planned for early steroid withdrawal were more likely to receive depleting agents for induction. Patients were typically maintained on a triple immunosuppressive regimen with a calcineurin inhibitor (tacrolimus), antiproliferative agent (mycophenolate mofetil or mycophenolic acid), and steroids. Some patients had early steroid withdrawal, based on clinical judgment and the patient’s request. Doses and drug levels were individually adjusted at the physician’s discretion based on the patient’s clinical condition, including infection, malignancy, and rejection, as described before.20
Laboratory Follow-Up and Kidney Allograft Biopsy
All transplant recipients were followed at either the University Hospital or various outreach regional clinics at least once a y until graft failure or until the patient decides to transfer their care to a different transplantation center as described before.21 All recipients had routine blood tests, including serum creatinine, more frequently in the beginning and every 1 to 3 mo indefinitely. Most biopsies were done for the cause, mainly related to an unexplained rise in serum creatinine or proteinuria. Protocol biopsies were performed at mo 3 and 12 for all patients with pretransplant DSAs and those who developed dnDSAs as previously described.17
AMR Treatment
AMR treatment protocols at our institution are based on both the severity of rejection and the time after transplant at which AMR is diagnosed as previously described.17 Briefly, for early rejection (within 3 mo posttransplant), treatment includes dexamethasone 100 mg bolus and taper, plasmapheresis 4-6 sessions, and IVIG 100 mg/kg after each plasmapheresis followed by IVIG 500 mg/kg every wk 4×. Late rejection (>3 mo posttransplant) is treated with dexamethasone 100 mg bolus and taper and IVIG 500 mg/kg every wk 4×. Rituximab 375 mg/m2 single dose is added on the basis of clinician judgment of clinical and laboratory findings.
Recipients undergo a follow-up kidney biopsy after approximately 12 wk posttreatment of AMR. During this period, patients are recommended to get laboratory tests, including serum creatinine, every 1 to 2 wk. Also, all patients would have serum creatinine checked on the d of biopsies (both index and follow-up). Recipients with persistent MVI >1 on the follow-up biopsy are usually retreated with the same regimen but are less likely to receive rituximab or other treatment, based on the provider’s discretion.
Statistical Analysis
Continuous data were compared using the Student t test or the Wilcoxon rank-sum test, as appropriate, whereas categorical data were analyzed using the Fisher exact test or chi-square test. P values of ≤0.05 were considered statistically significant. Risk factors associated with being a nonresponder and DCGF were studied using univariate and multivariate stepwise Cox regression analyses. Some of the baseline characteristics, changes in serum creatinine (delta creatinine from the index biopsy), and immunopathological features were used to assess the risk of graft failure. Variables associated with outcomes at a P value of ≤0.10 in univariate analysis were kept in the multivariate analysis. Death-censored graft survival was analyzed using Kaplan-Meier analyses.
RESULTS
A total of 183 recipients fulfilled our selection criteria; 66 (36%) were responders and 117 (64%) were nonresponders (Table 1). The majority of biopsy were for cause, mainly because of an unexplained rise in serum creatinine. However, a total of 61 patients, 20 (30%) in the responder group and 41 (35%) in the nonresponder group, underwent DSA-guided protocol biopsies (P = 0.54). All the included baseline characteristics were similar between the groups, including the interval from transplant to index biopsy and the interval between the index and follow-up biopsies. The mean serum creatine at the time of index biopsy among the responders was 1.74 ± 0.70 mg/dL compared with 1.83 ± 0.65 mg/dL among nonresponders (P = 0.39; Table 2). Although not all recipients had serum creatinine checked at all time points of 1 wk, 2 wk, 4 wk, 8 wk, and 10 wk post index biopsy, all had serum creatinine checked at the time of the index biopsy and follow-up biopsy. On comparing index biopsy serum creatinine with the various time points post index biopsy, there were no statistically significant differences found in serum creatinine either among the responders or nonresponders. The change in serum creatinine from the index biopsy to the follow-up biopsy was 0 ± 0.67 mg/dL (P = 0.99) among responders and –0.01 ± 0.61 mg/dL among nonresponders (P = 0.89).
TABLE 1.
Baseline characteristics of participants
| Characteristics | Responder (N = 66) | Nonresponder (N = 117) | P |
|---|---|---|---|
| Age at transplant (y) | 45.5 ± 14.4 | 42.8 ± 16.2 | 0.25 |
| Preemptive transplant, n (%) | 16 (24) | 25 (21) | 0.20 |
| Male, n (%) | 38 (58) | 81 (69) | 0.11 |
| Nonwhite, n (%) | 16 (24) | 36 (31) | 0.35 |
| Cause of ESKD, n (%) | 0.87 | ||
| Diabetes | 10 (15) | 16 (14) | |
| Hypertension | 6 (9) | 15 (13) | |
| Glomerular disease | 23 (35) | 42 (36) | |
| Other | 27 (41) | 44 (38) | |
| Living donor, n (%) | 26 (39) | 36 (31) | 0.24 |
| Previous transplant, n (%) | 16 (24) | 33 (28) | 0.56 |
| Mean HLA mismatch (of 6) | 3.9 ± 1.4 | 4.0 ± 1.4 | 0.88 |
| Induction agent, n (%) | 0.63 | ||
| Antithymocyte globulin | 25 (38) | 37 (32) | |
| Alemtuzumab | 5 (8) | 14 (12) | |
| IL-2 inhibitor | 30 (46) | 58 (50) | |
| Other/none/unknown | 6 (9) | 8 (7) | |
| Indication for the index biopsy, n (%) | |||
| Protocol | 20 (30) | 41 (35) | 0.54 |
| For cause | 46 (70) | 77 (65) | |
| Interval from transplant to index biopsy (mo) | 87.5 ± 93.9 | 80.9 ± 71.9 | 0.59 |
| Interval from index biopsy to follow-up biopsy (d) | 102.0 ± 31.6 | 93.5 ± 28.9 | 0.07 |
| Interval from index biopsy to last follow-up (mo) | 41.2 ± 16.3 | 38.3 ± 17.2 | 0.27 |
| DCGF within 1 y of index biopsy | 3 (5) | 5 (4) | 0.93 |
| Uncensored graft failures at last follow-up (%) | 19 (29) | 55 (47) | 0.02 |
| DCGF at last follow-up (%) | 11 (20) | 41 (39) | 0.02 |
Bold values indicate statistically significant value with P <0.05.
DCGF, death-censored graft failure; ESKD, end-stage kidney disease; IL-2, interleukin 2.
TABLE 2.
Changes in serum creatinine levels
| Responder | Nonresponder | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time | N | Mean ± SD (mg/dL) | Delta change from the index (mg/dL) | P (vs index biopsy) | N | Mean ± SD (mg/dL) | Delta change (mg/dL) | P (vs index biopsy) | |
| Serum Cr | Index biopsy | 66 | 1.74 ± 0.70 | – | – | 117 | 1.83 ± 0.65 | – | 0.39 |
| 1 wk | 33 | 1.95 ± 0.89 | 0.20 ± 0.77 | 0.22 | 52 | 1.79 ± 0.60 | –0.04 ± 0.64 | 0.68 | |
| 2 wk | 44 | 1.85 ± 0.76 | 0.11 ± 0.73 | 0.44 | 74 | 1.83 ± 0.57 | 0 ± 0.62 | 0.95 | |
| 4 wk | 52 | 1.77 ± 0.70 | 0.03 ± 0.70 | 0.84 | 96 | 1.75 ± 0.62 | –0.08 ± 0.64 | 0.33 | |
| 8 wk | 47 | 1.78 ± 0.62 | 0.04 ± 0.67 | 0.75 | 92 | 1.86 ± 0.58 | 0.03 ± 0.62 | 0.73 | |
| 10 wk | 38 | 2.04 ± 1.83 | 0.29 ± 1.24 | 0.24 | 66 | 1.81 ± 0.67 | –0.02 ± 0.66 | 0.84 | |
| Follow-up biopsy | 66 | 1.74 ± 0.64 | 0 ± 0.67 | 0.99 | 117 | 1.82 ± 0.57 | –0.01 ± 0.61 | 0.89 | |
Cr, creatinine.
The details of Banff scores and DSA levels among responders and nonresponders are summarized in Table 3. At index biopsy, responders had a lower percentage of recipients with DSA against class II antigen, lower mean MVI scores, a lower degree of cg scores, and lower sum chronicity scores. There was no significant difference in the treatment of AMR between the groups. As expected, at the follow-up biopsy, most of the Banff scores were statistically different between the groups.
TABLE 3.
DSA and Banff scores at index biopsy
| Index biopsy | Follow-up biopsy | |||||||
|---|---|---|---|---|---|---|---|---|
| Responder | Nonresponder | P | Responder | Nonresponder | P | |||
| DSA at index biopsy | Against class I(%) | 14 (21) | 39 (33) | 0.08 | 7 (11) | 26 (22) | 0.051 | |
| Against class II (%) | 33 (50) | 78 (67) | 0.03 | 27 (41) | 60 (51) | 0.18 | ||
| Sum MFI class I | 2998 ± 7948 | 3873 ± 5184 | 0.64 | 1938 ± 4020 | 2646 ± 3699 | 0.66 | ||
| Sum MFI class II | 12 082 ± 14 109 | 12 769 ± 13 777 | 0.81 | 8821 ± 9918 | 11 777 ± 13 362 | 0.31 | ||
| Banff scores | g | 0 | 4 (6) | 5 (4) | 0.005 | 51 (77) | 3 (3) | <0.001 |
| 1 | 29 (44) | 33 (28) | 15 (23) | 38 (33) | ||||
| 2 | 26 (39) | 40 (35) | 0 | 48 (41) | ||||
| 3 | 7 (11) | 39 (34) | 0 | 28 (24) | ||||
| ptc | 0 | 14 (21) | 16 (14) | 0.04 | 58 (88) | 13 (11) | <0.001 | |
| 1 | 37 (56) | 52 (44) | 8 (12) | 68 (58) | ||||
| 2 | 15 (23) | 44 (38) | 0 | 33 (28) | ||||
| 3 | 0 | 5 (4) | 0 | 3 (93) | ||||
| Mean MVI (g+ptc) | 2.56 ± 0.89 | 3.29 ± 1.12 | <0.001 | 0.35 ± 0.48 | 3.08 ± 1.0 | <0.001 | ||
| C4d | 0 | 57 (86) | 86 (74) | 0.15 | 62 (94) | 90 (77) | 0.01 | |
| 1 | 1 (2) | 2 (2) | 2 (3) | 3 (3) | ||||
| 2 | 3 (5) | 5 (4) | 0 | 6 (5) | ||||
| 3 | 5 (8) | 24 (21) | 2 (3) | 18 (15) | ||||
| cg | 0 | 41 (65) | 48 (43) | 0.03 | 51 (77) | 52 (45) | <0.001 | |
| 1 | 11 (18) | 27 (24) | 6 (9) | 24 (21) | ||||
| 2 | 5 (8) | 21 (19) | 4 (6) | 21 (18) | ||||
| 3 | 6 (10) | 17 (15) | 5 (8) | 19 (16) | ||||
| Mean sum chronicity scores(cg+cv+ct+cv) | 3.23 ± 2.20 | 4.12 ± 2.25 | 0.009 | 3.26 ± 2.09 | 4.12 ± 2.41 | 0.02 | ||
| Treatment | IVIG | 63 (96) | 111 (95) | 0.86 | ||||
| Steroids | 63 (96) | 111(96) | 0.86 | |||||
| Rituximab | 30 (46) | 69 (59) | 0.08 | |||||
| Plasmapheresis | 4 (6) | 3 (3) | 0.24 | |||||
Bold values indicate statistically significant value with P <0.05.
cg, transplant glomerulopathy; ct, tubular atrophy; cv, fibrous intimal thickening; DSA, donor-specific antibody; g, glomerulitis; MFI, mean fluorescent intensity; MVI, microvascular inflammation; ptc, peritubular capillaritis.
Cox regression analysis was performed, looking for factors associated with being a nonresponder (Table 4). In univariate analysis, factors associated with being a nonresponder were increased delta serum creatinine at 4 wk, higher MVI scores, C4d scores, cg scores, and sum chronicity scores at index biopsy. After adjustment for multiple variables, the only factors associated with being a nonresponder were higher MVI scores (per each 1 increase; hazard ratio [HR] 1.29; 95% confidence interval [CI], 1.02-1.64; P = 0.03) and higher C4d scores (per each 1 increase; HR 1.23; 95% CI, 1.02-1.48; P = 0.03).
TABLE 4.
Factors associated to be nonresponder
| Univariate analyses | Multivariate analyses | |||||
|---|---|---|---|---|---|---|
| Covariate | HR | 95% CI | P | HR | 95% CI | P |
| Delta Scr at 1 wk (per 0.1) | 1.33 | 0.66-2.70 | 0.41 | |||
| Delta Scr at 2 wk (per 0.1) | 1.48 | 0.85-2.56 | 0.16 | |||
| Delta Scr at 4 wk (per 0.1) | 1.46 | 1.01-2.12 | 0.04 | 1.39 | 0.97-1.98 | 0.07 |
| Delta Scr at 8 wk (per 0.1) | 1.57 | 0.86-2.90 | 0.14 | |||
| Delta Scr at 10 wk (per 0.1) | 1.58 | 0.81-3.06 | 0.17 | |||
| Delta Scr at follow-up biopsy (per 0.1) | 1.38 | 0.87-2.19 | 0.16 | |||
| Indication for biopsy as for cause | 0.84 | 0.57-1.24 | 0.38 | |||
| Interval from transplant to index biopsy (per mo) | 1.0 | 0.99-1.01 | 0.99 | |||
| Age at transplant (per y) | 0.99 | 0.98-1.01 | 0.48 | |||
| HLA mismatch (per each) | 1.01 | 0.89-1.14 | 0.84 | |||
| Male | 1.28 | 0.86-1.90 | 0.21 | |||
| None white recipient | 0.99 | 0.67-1.48 | 0.99 | |||
| Cause of ESKD DM vs other | 0.79 | 0.46-1.35 | 0.38 | |||
| Living donor | 0.99 | 0.66-1.48 | 0.97 | |||
| Previous transplant | 1.03 | 0.68-1.54 | 0.88 | |||
| Depleting induction | 0.93 | 0.65-1.34 | 0.71 | |||
| MVI scores at index biopsy (per each) | 1.41 | 1.18-1.68 | <0.001 | 1.29 | 1.02-1.64 | 0.03 |
| C4d scores at index biopsy (per each) | 1.16 | 1.01-.35 | 0.04 | 1.23 | 1.02-1.48 | 0.03 |
| Cg scores at index biopsy (per each) | 1.25 | 1.05-1.48 | 0.01 | 1.07 | 0.81-1.40 | 0.62 |
| Sum chronicity scores (per each) | 1.12 | 1.04-1.21 | 0.002 | 1.04 | 0.91-1.19 | 0.57 |
| DSA class II present at index biopsy | 1.54 | 1.04-2.27 | 0.03 | 1.31 | 0.83-2.07 | 0.23 |
| Rituximab for treatment | 1.27 | 0.88-1.85 | 0.19 | |||
Bold values indicate statistically significant value with P <0.05.
cg, transplant glomerulopathy; CI, confidence interval; DM, diabetes; DSA, donor-specific antibody; ESKD, end-stage kidney disease; HR, hazard ratio; MVI, microvascular inflammation; Scr, serum creatinine.
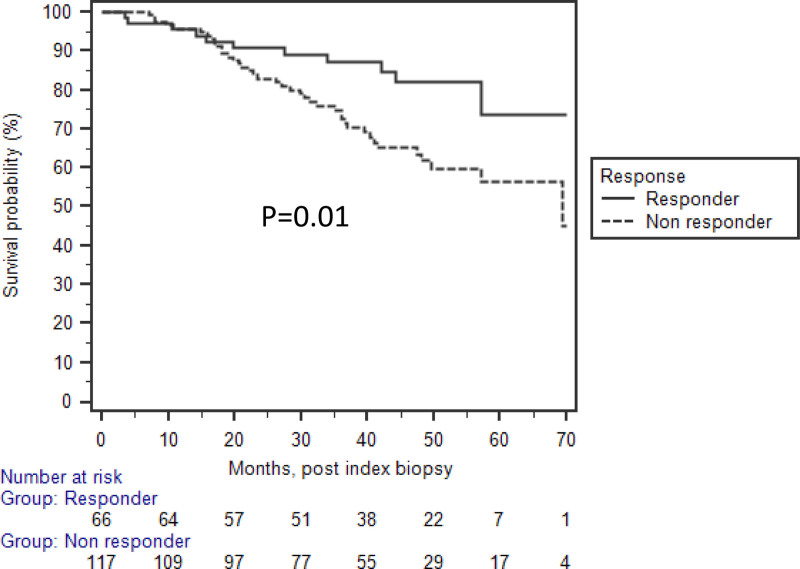
There were a total of 52 DCGF at the last follow-up, 11 (20%) in the responder group and 41 (39%) in the nonresponder group (P = 0.02). Looking for risk factors for DCGF (Table 5), in univariate analysis, delta serum creatinine values at 1 wk and at follow-up biopsy were associated with a lower risk of DCGF, along with the younger age of the recipient. Being a nonresponder, higher cg scores and higher sum chronicity scores were associated with increased risk for DCGF. In unadjusted Kaplan-Meier survival analysis, nonresponders were associated with an increased risk of DCGF (Figure 1). After adjustment of multiple significant variables from the univariate analysis, the only variable associated with decreased risk of DCGF in multivariate analysis was delta serum creatinine (HR 0.18; 95% CI, 0.04-0.78; P = 0.02) at 1 wk from the index biopsy.
TABLE 5.
Factors associated with DCGF at last follow-up
| Univariate analyses | Multivariate analyses | |||||
|---|---|---|---|---|---|---|
| Covariate | HR | 95% CI | P | HR | 95% CI | P |
| Delta Scr at 1 wk (per 0.1) | 0.16 | 0.05-0.60 | 0.006 | 0.18 | 0.04-0.78 | 0.02 |
| Delta Scr at 2 wk (per 0.1) | 0.42 | 0.32-1.63 | 0.43 | |||
| Delta Scr at 4 wk (per 0.1) | 0.99 | 0.54-1.83 | 0.99 | |||
| Delta Scr at 8 wk (per 0.1) | 0.51 | 0.18-1.40 | 0.19 | |||
| Delta Scr at 10 wk (per 0.1) | 0.97 | 0.70-1.35 | 0.97 | |||
| Delta Scr at follow-up biopsy (per 0.1) | 0.42 | 0.19-0.91 | 0.02 | 2.42 | 0.68-8.65 | 0.17 |
| Indication for biopsy: for cause | 1.48 | 0.81-2.71 | 0.20 | |||
| Nonresponder | 2.25 | 1.16-4.38 | 0.01 | 1.35 | 0.58-3.17 | 0.49 |
| Age at transplant (per y) | 0.97 | 0.96-0.99 | 0.02 | 0.97 | 0.94-1.01 | 0.08 |
| HLA mismatch (per each) | 0.93 | 0.77-1.13 | 0.51 | |||
| Male | 0.87 | 0.50-1.53 | 0.64 | |||
| None white recipient | 1.20 | 0.66-2.18 | 0.54 | |||
| Cause of ESKD: DM vs other | 1.43 | 0.69-2.94 | 0.33 | |||
| Living donor | 0.79 | 0.43-1.43 | 0.43 | |||
| Previous transplant | 1.04 | 0.57-1.90 | 0.90 | |||
| Depleting induction | 0.95 | 0.55-1.65 | 0.87 | |||
| MVI scores at index biopsy (per each) | 1.17 | 0.92-1.48 | 0.19 | |||
| C4d scores at index biopsy (per each) | 1.11 | 0.89-1.39 | 0.35 | |||
| Cg scores at index biopsy (per each) | 1.39 | 1.10-1.76 | 0.005 | 1.06 | 0.69-1.65 | 0.77 |
| Sum chronicity scores at index biopsy (per each) | 1.21 | 1.07-1.35 | 0.001 | 1.19 | 0.87-1.43 | 0.38 |
| DSA class II present at index biopsy | 1.48 | 0.82-2.67 | 0.19 | |||
| DSA class II present at follow-up biopsy | 1.63 | 0.93-2.86 | 0.11 | |||
| Rituximab for treatment | 0.88 | 0.51-1.53 | 0.88 | |||
Bold values indicate statistically significant value with P <0.05.
cg, transplant glomerulopathy; CI, confidence interval; DCGF, death-censored graft failure; DM, diabetes; DSA, donor-specific antibody; ESKD, end-stage kidney disease; HR, hazard ratio; MVI, microvascular inflammation; Scr, serum creatinine.
FIGURE 1.
Nonresponders were associated to have lower death-censored graft survival compared with the responder in unadjusted analysis (P = 0.01).
All analyses were repeated while excluding patients who underwent DSA-guided protocol biopsy with stable serum creatinine, and the results were essentially the same (data not shown).
DISCUSSION
In this large series of 183 kidney transplant recipients with an initial diagnosis of AMR and treatment along with follow-up biopsy, we looked at various factors associated with the resolution of MVI, with a particular focus on the serum creatinine change between the 2 biopsies. Interestingly, we did not find significant changes in serum creatinine, either among the responders or nonresponders between the 2 biopsies, despite significant improvement in histopathology on the follow-up biopsies among the responders. Higher MVI scores and C4d staining were likely not to respond to treatment. Because we usually repeat the AMR treatment after follow-up biopsies among nonresponders, it may not be surprising that in multivariate analysis (HR 1.35; 95% CI, 0.58-3.17) there was no significantly increased association between nonresponders and DCGF. These findings support the utility of follow-up biopsies after the treatment of AMR and the retreatment of persistent MVI on the follow-up biopsy, rather than solely relying on the serum creatinine.
Currently, there is no Food and Drug Administration–approved treatment for AMR.22 Components of the current standard of care for the management of AMR include steroid pulse, IVIG, depletion of B cells, removal of DSA, inhibition of plasma cells, and interleukin-6 blockade.23-27 Outcomes of the treatment of AMR, especially in the presence of chronic changes or chronic active AMR are poor.11,28,29 Furthermore, histological responses to treatment for AMR are also disappointing. In one study, among 82 kidney transplant recipients with chronic active AMR, Aziz et al reported that only 7.3% had complete resolution of MVI with scores of 0 among recipients treated with steroids and IVIG at 3 mo follow-up biopsy. From the same cohort, the resolution of MVI was up to 19.5% if rituximab was also added.30 The same study found a more encouraging response rate as assessed by eGFR, at 27%, among groups treated with steroids/IVIG and 66% when rituximab was also added.30 These findings are also consistent with the idea that serum creatinine or creatinine-based eGFR measurement may not be consistent with the histological findings after treatment of AMR. Similarly, multiple other studies have reported discrepancies between persistent MVI and serum creatinine or eGFR as a secondary analysis.17,27,30,31 Piñeiro et al31 reported that among 90 recipients with AMR, with a median interval for follow-up biopsy of 2 mo, 71% of recipients still had persistent MVI scores of ≥1.
In the present study, we were not able to find any association between changes in serum creatinine (delta serum creatinine) and the resolution of MVI. As expected, recipients with higher degrees of MVI or C4d at the index biopsy were likely to have persistent MVI on the follow-up biopsy, suggesting that these patients may need close follow-up. The role of routine monitoring with donor-derived cell-free DNA to assess response to treatment of rejection is evolving and promising and could be one of the monitoring tools.32,33
This study has the expected limitations of a single-center observational study, reflecting our specific population and clinical approach. Our findings are reflective of our practices, and this should be factored into the interpretation. However, this substantial data set with more granular data provides useful information for estimating risks and outcomes. Also, to the best of our knowledge, this study is the largest of its kind, with 183 kidney transplant recipients with AMR and follow-up biopsy. In summary, we found that serum creatinine is not a good predictor to determine the resolution of MVI and support the need for a follow-up biopsy.
Footnotes
The authors declare no conflicts of interest.
D.M. has received research funds from Virginia Lee Cook Foundation.
The data that support the findings of this study are available from the corresponding author (S.P.) upon reasonable request.
S.P. contributed in data collection, design, analysis, and article preparation. W.Z., F.A., N.G., and M.M. contributed in editing. M.P. and M.S. contributed in data collection and editing. D.M. contributed in concept, analysis, article preparation, and editing.
REFERENCES
- 1.Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Vazquez MA, Harmon WE, et al. Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J Am Soc Nephrol. 2000;11:S1–86. [PubMed] [Google Scholar]
- 3.Ross EA, Wilkinson A, Hawkins RA, et al. The plasma creatinine concentration is not an accurate reflection of the glomerular filtration rate in stable renal transplant patients receiving cyclosporine. Am J Kid Dis. 1987;10:113–117. [DOI] [PubMed] [Google Scholar]
- 4.Tomlanovich S, Golbetz H, Perlroth M, et al. Limitations of creatinine in quantifying the severity of cyclosporine-induced chronic nephropathy. Am J Kid Dis. 1986;8:332–337. [DOI] [PubMed] [Google Scholar]
- 5.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–1167. [DOI] [PubMed] [Google Scholar]
- 6.Roufosse C, Simmonds N, Clahsen-van Groningen M, et al. A 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 2018;102:1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas M, Sis B, Racusen LC, et al. ; Banff meeting report writing committee. Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. [DOI] [PubMed] [Google Scholar]
- 8.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 kidney meeting report: revised diagnostic criteria for chronic active T cell–mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18:293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loupy A, Haas M, Roufosse C, et al. The Banff 2019 kidney meeting report (i): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banff Foundation for Allograft Pathology. XVIth Banff Meeting Allograft pathology, Banff Canada 19th-23rd September 2022 Joint meeting with the Canadian Society of Transplantation. Available at: https://banfffoundation.org/wp-content/uploads/2022/10/Banff-2022-Kidney-Summary.pdf. Accessed January 10, 2023. [Google Scholar]
- 11.Redfield RR, Ellis TM, Zhong W, et al. Current outcomes of chronic active antibody mediated rejection—a large single center retrospective review using the updated Banff 2013 criteria. Hum Immunol. 2016;77:346–352. [DOI] [PubMed] [Google Scholar]
- 12.Parajuli S, Joachim E, Alagusundaramoorthy S, et al. Subclinical antibody-mediated rejection after kidney transplantation: treatment outcomes. Transplantation. 2019;103:1722–1729. [DOI] [PubMed] [Google Scholar]
- 13.Prowle JR, Ishikawa K, May CN, et al. Renal plasma flow and glomerular filtration rate during acute kidney injury in man. Ren Fail. 2010;32:349–355. [DOI] [PubMed] [Google Scholar]
- 14.Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:649–672. [DOI] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, et al. ; Acute Kidney Injury Network. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73:538–546. [DOI] [PubMed] [Google Scholar]
- 17.Parajuli S, Mandelbrot DA, Muth B, et al. Rituximab and monitoring strategies for late antibody-mediated rejection after kidney transplantation. Transplantation direct. 2017;3:e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis TM. Interpretation of HLA single antigen bead assays. Transplant Rev (Orlando). 2013;27:108–111. [DOI] [PubMed] [Google Scholar]
- 19.Parajuli S, Reville PK, Ellis TM, et al. Utility of protocol kidney biopsies for de novo donor-specific antibodies. Am J Transplant. 2017;17:3210–3218. [DOI] [PubMed] [Google Scholar]
- 20.Muth BL, Astor BC, Turk J, et al. Outpatient management of delayed graft function is associated with reduced length of stay without an increase in adverse events. Am J Transplant. 2016;16:1604–1611. [DOI] [PubMed] [Google Scholar]
- 21.Parajuli S, Mandelbrot DA, Aziz F, et al. Characteristics and outcomes of kidney transplant recipients with a functioning graft for more than 25 years. Kidney Dis (Basel). 2018;4:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velidedeoglu E, Cavaillé-Coll MW, Bala S, et al. Summary of 2017 FDA public workshop: antibody-mediated rejection in kidney transplantation. Transplantation. 2018;102:e257–e264. [DOI] [PubMed] [Google Scholar]
- 23.Barnett AN, Hadjianastassiou VG, Mamode N. Rituximab in renal transplantation. Transplant Int. 2013;26:563–575. [DOI] [PubMed] [Google Scholar]
- 24.Moreso F, Crespo M, Ruiz JC, et al. Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: a multicenter, prospective, randomized, double-blind clinical trial. Am J Transplant. 2018;18:927–935. [DOI] [PubMed] [Google Scholar]
- 25.Eskandary F, Regele H, Baumann L, et al. A Randomized trial of bortezomib in late antibody-mediated kidney transplant rejection. J Am Soc Nephrol. 2018;29:591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi J, Aubert O, Vo A, et al. Assessment of tocilizumab (anti-interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant. 2017;17:2381–2389. [DOI] [PubMed] [Google Scholar]
- 27.Degner KR, Wilson NA, Reese SR, et al. Short-term immunopathological changes associated with pulse steroids/IVIG/rituximab therapy in late kidney allograft antibody mediated rejection. Kidney360. 2020;1:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sijpkens YW, Doxiadis IIN, Mallat MJ, et al. Early versus late acute rejection episodes in renal transplantation. Transplantation. 2003;75:204–208. [DOI] [PubMed] [Google Scholar]
- 29.Walsh RC, Brailey P, Girnita A, et al. Early and late acute antibody-mediated rejection differ immunologically and in response to proteasome inhibition. Transplantation. 2011;91:1218–1226. [DOI] [PubMed] [Google Scholar]
- 30.Aziz F, Parajuli S, Jorgenson M, et al. Chronic active antibody-mediated rejection in kidney transplant recipients: treatment response rates and value of early surveillance biopsies. Transplant Direct. 2022;8:e1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piñeiro GJ, Montagud-Marrahi E, Ríos J, et al. Influence of persistent inflammation in follow-up biopsies after antibody-mediated rejection in kidney transplantation. Front Med. 2021;8:761919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf-Doty TK, Mannon RB, Poggio ED, et al. Dynamic response of donor-derived cell-free DNA following treatment of acute rejection in kidney allografts. Kidney360. 2021;2:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinojosa RJ, Chaffin K, Gillespie M, et al. Donor-derived cell-free DNA may confirm real-time response to treatment of acute rejection in renal transplant recipients. Transplantation. 2019;103:e61. [DOI] [PubMed] [Google Scholar]