Abstract
Objectives
Our research aimed to evaluate the effectiveness of first‐line immune checkpoint inhibitors (ICIs) with etoposide and platinum (EP) for extensive‐stage small cell lung cancer (ES‐SCLC) and identify prognostic factors, as real‐world outcomes and the inconsistency of PD‐1 and PD‐L1 inhibitors are uncertain.
Methods
We selected ES‐SCLC patients in three centers and conducted a propensity score‐matched analysis. The Kaplan–Meier method and Cox proportional hazards regression were conducted to compare the survival outcomes. We also performed univariate and multivariate Cox regression analyses to investigate predictors.
Results
Among 236 patients included, 83 pairs of cases were matched. The EP plus ICIs cohort had a longer median overall survival (OS) (17.3 months) than the EP cohort (13.4 months) (hazard ratio [HR], 0.61 [0.45, 0.83]; p = 0.001). The median progression‐free survival (PFS) was also longer in the EP plus ICIs cohort (8.3 months) than in the EP cohort (5.9 months) (HR, 0.44 [0.32, 0.60]; p < 0.001). The EP plus ICIs group had a higher objective response rate (ORR) (EP: 62.3%, EP + ICIs: 84.3%, p < 0.001). Multivariate analysis presented that liver metastases (HR, 2.08; p = 0.018) and lymphocyte–monocyte ratio (LMR) (HR, 0.54; p = 0.049) were independent prognostic factors for OS, and performance status (PS) (HR, 2.11; p = 0.015), liver metastases (HR, 2.64; p = 0.002), and neutrophil‐lymphocyte ratio (NLR) (HR, 0.45; p = 0.028) were for PFS in patients with chemo‐immunotherapy.
Conclusion
Our real‐world data demonstrated that ICIs with chemotherapy as the first‐line setting for ES‐SCLC are effective and safe. PS, liver metastases, and inflammatory markers could serve as valuable risk factors.
Keywords: extensive‐stage SCLC, PD‐1 inhibitors, PD‐L1 inhibitors, propensity score‐matched analysis, risk factors
Our real‐world study demonstrated that immune checkpoint inhibitors combined with chemotherapy as first‐line treatment for extensive‐stage small cell lung cancer have longer median overall survival (OS) and progression‐free survival than chemotherapy alone, whether before or after propensity score matching. Liver metastases and lymphocyte–monocyte ratio could serve as independent prognostic factors for OS in patients with chemo‐immunotherapy.
INTRODUCTION
Small cell lung cancer (SCLC), neuroendocrine cancer that accounts for ~ 15% of lung cancer patients worldwide, is known for its aggressive progression. 1 Unfortunately, the prognosis for cases with SCLC is poor, with a 5‐year survival rate of only 5%, largely because of the rapid growth of the tumor and early development of metastases. 2 , 3 The majority of SCLC cases are classified as extensive‐stage (ES) SCLC, accounting for approximately two‐thirds of all diagnoses. 3 The traditional first‐line setting for ES‐SCLC in the past two decades has been chemotherapy consisting of etoposide combined with platinum (EP). 4 Although chemotherapy could elicit a response in 60% to 80% of ES‐SCLC patients, complete remission was observed in only 15% to 20%, and most patients relapsed soon after initial treatment. 4 , 5
With the advancement of immunotherapy and an improved understanding of tumor immunity, its application in clinical settings has been on the rise. As some studies reported, chemotherapy could modulate the tumor microenvironment by inducing immunogenic cell death, the majority of chemotherapeutic drugs have demonstrated immunostimulatory effects, such as increasing immunogenicity and T‐cell infiltration, activating effector cells, and suppressing immunosuppressive cells. 6 , 7 This preclinical evidence provides the rationale for combining chemotherapy and immune checkpoint inhibitors (ICIs). Two recent randomized phase three trials (CASPIAN and IMpower133) showed that the addition of programmed death ligand 1 (PD‐L1) inhibitor to EP has been shown to significantly improve both overall survival (OS) and progression‐free survival (PFS) when in comparison with chemotherapy alone. 8 , 9 Such benefit is relatively small and was not even observed in the KEYNOTE‐604 study that used the programmed death 1 (PD‐1) inhibitor pembrolizumab, this study confirmed that the addition of pembrolizumab to chemotherapy prolonged OS, but the significance threshold was not met. 10 However, ASTRUM‐005 study published in 2022 that used Serplulimab (another PD‐1 inhibitor) combined with chemotherapy reached a significant OS benefit. 11 The inconsistent results raised the question about the optimal chemotherapy and immunotherapy combination in the first‐line setting for ES‐SCLC cases, with particular attention given to the choice between anti‐PD‐1 agents and anti‐PD‐L1 agents. Therefore, it is essential to carefully weigh the benefits and threats of ICIs combined with chemotherapy for patients with ES‐SCLC. Moreover, to exactly stratify ES‐SCLC patients who could take advantage of chemotherapy with ICIs, it is essential to find reliable prognostic factors. Systemic inflammatory responses have been confirmed to be associated with the development and spread of malignancies. 12 As a result, some inflammatory and nutritional markers have an intriguing role in clinical practice as prognostic factors, for example, systemic inflammation response index (SIRI) and prognostic nutrition index (PNI). 13 , 14 , 15 , 16 However, the predictive value of these markers for ES‐SCLC cases who received chemotherapy with ICIs remains uncertain.
In this multicenter and retrospective study, we analyzed the therapeutic effect of immunotherapy (PD‐L1 inhibitors or PD‐1 inhibitors) combined with EP and EP alone in real‐world clinical cases of ES‐SCLC to offer a reference for clinical treatment. At the same time, we carried out a propensity score‐matched analysis to minimalize the bias brought on by the patients’ baseline characteristics. Besides the comparison of survival outcomes, we conducted a multivariate analysis based on the clinical characteristics and serum inflammatory and tumor markers of patients to identify the independent prognostic factors of ES‐SCLC cases who received ICIs in combination with chemotherapy.
MATERIALS AND METHODS
Study design and patients
Our multicenter and retrospective study was carried out in compliance with the amended Declaration of Helsinki and got approval from the appropriate ethical committees of Jinling Hospital (Ethics number: 202103275). Because this study was retrospective in design, informed consent from the patients who were enrolled in it was not required, and patients’ information was secured. This study selected SCLC patients who were treated at three institutions (Jinling Hospital, Jiangsu Cancer Hospital, and The First Affiliated Hospital of Guangzhou Medical University) between June 2019 and March 2021. Histologically or cytologically confirmed SCLC, first‐line standard chemotherapy consisting of etoposide plus platinum with or without immunotherapy, and systematic classification into extensive disease according to Veterans Administration Lung Study Group before treatment were required for participation in this study. 17 Additionally, the patients who only received 1 to 2 cycles of therapy or had a history of another tumor were excluded.
Data collection and evaluation
The Kaplan–Meier (K–M) method was carried out to estimate PFS and OS to assess the time from first‐line therapy to relapse or death. On the ground of Response Evaluation Criteria in Solid Tumors, the response to chemotherapy or chemo‐immunotherapy was evaluated. The calculation of objective response rate (ORR) was based on the proportion of cases that attained complete response (CR) and partial response (PR), whereas the disease control rate (DCR) was based on the proportion of cases that attained complete or PR as well as stable disease (SD).
Patient information before treatment, including age, sex, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status (PS), baseline information of tumor, count of leukocyte, neutrophil, lymphocyte, monocyte, and platelet, lactate dehydrogenase (LDH), serum albumin, tumor markers, and weight and height, was collected. Several indexes have been used to assess inflammation and nutritional status in our study, including the following: neutrophil–lymphocyte ratio (NLR) = neutrophil/lymphocyte; platelet–lymphocyte ratio (PLR) = platelet/lymphocyte; lymphocyte–monocyte ratio (LMR) = lymphocyte/monocyte; systemic immune‐inflammation index (SII) = platelet × NLR 16 ; SIRI = neutrophil × monocyte/lymphocyte 16 ; PNI = serum albumin (g/L) + 5 × total lymphocyte count (109/L) 13 ; body mass index (BMI) = weight(kg)/height(m)2; and advanced lung cancer inflammation index (ALI) = BMI × (serum albumin/NLR). 14 The baseline LDH level and the dNLR (derived neutrophil‐lymphocyte ratio: neutrophil count/[white blood cell count–neutrophil count]) level were used to calculate the lung immune prognostic index (LIPI). 15 NLR, PLR, LMR, SII, SIRI, PNI, and ALI are transformed into classification variables by the optimal cutoff value of the receiver operating curve (ROC). LDH and tumor markers are transformed into classification variables by the upper limit of normal (ULN).
Statistical analysis
The baseline characters of the included patients were analyzed by using descriptive statistics, including frequencies and percentages. Categorical variables were assessed using the χ2‐square test and the Kruskal–Wallis test.
Propensity score‐matching was conducted using a 1:1 matching design with a tolerance of 0.05. A logistic regression model was conducted to estimate the propensity score, which included the following covariates: age, sex, PS, smoking status, primary site, presence of distant metastases, and lymph node metastases, presence of pleural effusion and pericardial effusion, and the history of radiation therapy. Survival analyses were performed using the Kaplan–Meier (K–M) method with the log‐rank test, as well as Cox proportional hazards regression analysis was carried out to compare the two groups.
To investigate predictors for OS and PFS, univariate and multivariate Cox regression analyses were carried out on all patients who received chemotherapy combined with immunotherapy before matching. In the univariate analysis, factors that presented a significant association with the risk of OS or PFS (p < 0.05) were included in the subsequent multivariate Cox regression analysis.
All the analyses were conducted by R software (version 4.2.2) and SPSS 25.0 software. A statistically significant difference was defined as a p‐value of <0.05.
RESULTS
Patient characteristics
Of the 382 patients in the initial set, 236 were eligible for inclusion, including 120 patients who received first‐line EP alone and 116 patients who received first‐line EP plus ICIs (Figure S1). The baseline characters of all eligible 236 patients are presented in Table 1. At baseline, 45 (19.1%) patients had brain metastases and 61 (25.8%) patients had liver metastases. The number of cases treated with anti‐PD‐1 agents and anti‐PD‐L1 agents was 72 (30.5%) and 44 (18.6%), respectively. Propensity score‐matching resulted in two groups of 83 patients each, with comparable baseline characters (p > 0.05) (Table 1).
TABLE 1.
Baseline characteristics of patients before and after propensity score matching according to first‐line therapy.
| Characteristic | n (%) | Before matching, n (%) | After matching, n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| All patients (n = 236) | EP (n = 120) | EP + ICIs (n = 116) | p | EP (n = 83) | EP + ICIs (n = 83) | p | ||
| Age | <65 | 113 (47.9) | 63 (52.50) | 50 (43.10) | 0.149 | 43 (51.81) | 41 (49.40) | 0.756 |
| ≥65 | 123 (52.1) | 57 (47.50) | 66 (56.90) | 40 (48.19) | 42 (50.60) | |||
| Sex | Male | 206 (87.3) | 100 (83.33) | 106 (91.38) | 0.064 | 73 (87.95) | 74 (89.16) | 0.807 |
| Female | 30 (12.7) | 20 (16.67) | 10 (8.62) | 10 (12.05) | 9 (10.84) | |||
| Smoking history | Never | 66 (28.0) | 27 (22.50) | 39 (33.62) | 0.057 | 22 (26.51) | 19 (22.89) | 0.589 |
| Former/current | 170 (72.0) | 93 (77.50) | 77 (66.38) | 61 (73.49) | 64 (77.11) | |||
| PS | 0 | 106 (44.9) | 53 (44.17) | 53 (45.69) | 0.814 | 44 (53.01) | 37 (44.58) | 0.277 |
| 1 | 130 (55.1) | 67 (55.83) | 63 (54.31) | 39 (46.99) | 46 (55.42) | |||
| Primary Site | Left | 108 (45.8) | 54 (45.00) | 54 (46.55) | 0.811 | 39 (46.99) | 37 (44.58) | 0.755 |
| Right | 128 (54.2) | 66 (55.00) | 62 (53.45) | 44 (53.01) | 46 (55.42) | |||
| Extrathoracic metastases | No | 66 (28.0) | 36 (30.00) | 30 (25.86) | 0.479 | 27 (32.53) | 25 (30.12) | 0.738 |
| Yes | 170 (72.0) | 84 (70.00) | 86 (74.14) | 56 (67.47) | 58 (69.88) | |||
| Brain metastases | No | 191 (80.9) | 96 (80.00) | 95 (81.90) | 0.711 | 66 (79.52) | 68 (81.93) | 0.694 |
| Yes | 45 (19.1) | 24 (20.00) | 21 (18.10) | 17 (20.48) | 15 (18.07) | |||
| Liver metastases | No | 175 (74.2) | 92 (76.67) | 83 (71.55) | 0.370 | 61 (73.49) | 61 (73.49) | 1.000 |
| Yes | 61 (25.8) | 28 (23.33) | 33 (28.45) | 22 (26.51) | 22 (26.51) | |||
| Bone metastases | No | 165 (69.9) | 84 (70.00) | 81 (69.83) | 0.977 | 62 (74.70) | 60 (72.29) | 0.725 |
| Yes | 71 (30.1) | 36 (30.00) | 35 (30.17) | 21 (25.30) | 23 (27.71) | |||
| Lymph node metastases | No/local | 107 (45.3) | 68 (56.67) | 39 (33.62) | <0.001 | 40 (48.19) | 36 (43.37) | 0.533 |
| Distant | 129 (54.7) | 52 (43.33) | 77 (66.38) | 43 (51.81) | 47 (56.63) | |||
| Pleural effusion | No | 118 (50.0) | 64 (53.33) | 54 (46.55) | 0.298 | 46 (55.42) | 42 (50.60) | 0.534 |
| Yes | 118 (50.0) | 56 (46.67) | 62 (53.45) | 37 (44.58) | 41 (49.40) | |||
| Pericardial effusion | No | 195 (82.6) | 100 (83.33) | 95 (81.90) | 0.771 | 72 (86.75) | 68 (81.93) | 0.393 |
| Yes | 41 (17.4) | 20 (16.67) | 21 (18.10) | 11 (13.25) | 15 (18.07) | |||
| Radiotherapy a | No | 152 (64.4) | 75 (62.50) | 77 (66.38) | 0.534 | 56 (67.47) | 56 (67.47) | 1.000 |
| Yes | 84 (35.6) | 45 (37.50) | 39 (33.62) | 27 (32.53) | 27 (32.53) | |||
Radiotherapy included first‐line thoracic radiotherapy (EP: n = 33; EP + ICIs: n = 35), radiotherapy for brain metastases (EP: n = 17; EP + ICIs: n = 8) and radiotherapy for bone metastases (EP: n = 3; EP + ICIs: n = 3).
Abbreviations: EP, etoposide and platinum; ICIs, immune checkpoint inhibitors; n, number; PS, performance satus.
Survival outcomes before and after propensity score matching
The survival outcomes were analyzed by K–M curves. The median OS (mOS) was 17.3 months (95% confidence interval [CI] [14.5–20.1]) in the EP plus ICIs cohort and 13.4 months (95% CI [11.8–15.1]) in the EP cohort (HR, 0.61 [0.45, 0.83]; p = 0.001) (Figure 1(a)). Similarly, the median progression‐free survival (mPFS) was also longer in the EP plus ICIs cohort (8.3 months; 95% CI [7.0–9.7]) than in the EP cohort (5.9 months; 95% CI [5.2–6.5]) (HR, 0.44 [0.32, 0.60]; p < 0.001) (Figure 1(b)).
FIGURE 1.
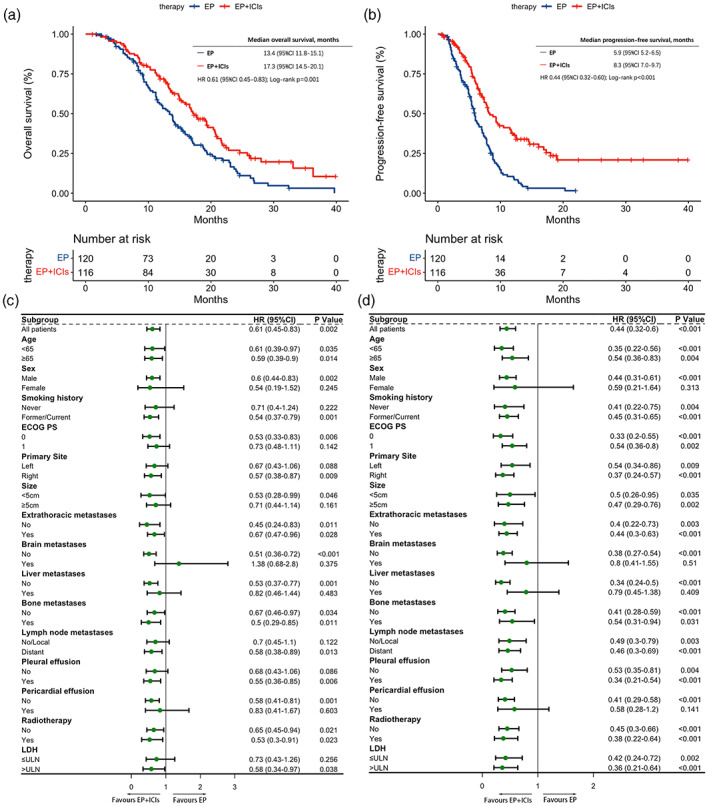
Survival outcomes. (a) Kaplan–Meier curves of overall survival stratified by the first‐line therapy. (b) Kaplan–Meier curves of progression‐free survival stratified by the first‐line therapy. (c) Forest plot of subgroup analysis of overall survival. (d) Forest plot of subgroup analysis of progression‐free survival. CI, confidence interval; EP, etoposide and platinum; ICIs, immune checkpoint inhibitors; HR, hazard ratio; LDH, lactate dehydrogenase; ECOG PS, Eastern Cooperative Oncology Group performance status; ULN, upper limit of normal.
The benefit of anti‐PD‐1/PD‐L1 agents in terms of OS and PFS was consistently evident in the majority of the predetermined subgroups of patients, which were categorized based on baseline clinical and demographic characteristics (Figure 1(c),(d)). In addition, it is worth mentioning that females (OS: p = 0.245; PFS: p = 0.313), and patients with brain metastases (OS: p = 0.375; PFS: p = 0.510), liver metastases (OS: p = 0.483; PFS: p = 0.409), and pericardial effusion (OS: p = 0.603; PFS: p = 0.141) at initial diagnosis failed to benefit from immunotherapy combined with chemotherapy regardless of OS or PFS (Figure 1(c),(d)).
As described in the statistical methods, propensity score matching was carried out. The mOS of cases treated with chemotherapy and immunotherapy was 17.7 months (95% CI [14.6–20.8]), which was longer than the cases treated with chemotherapy alone (12.8 months; 95% CI [10.4–15.2]) (HR, 0.59 [0.41, 0.85]; p = 0.005) (Figure 2(a)). At the same time, the mPFS was longer in the EP plus ICIs cohort (8.0 months; 95% CI [6.3–9.8]) than in the EP cohort (6.0 months; 95% CI [4.8–7.2]) (HR, 0.52 [0.36, 0.75]; p < 0.001) (Figure 2(b)). The results are consistent before and after the matching.
FIGURE 2.
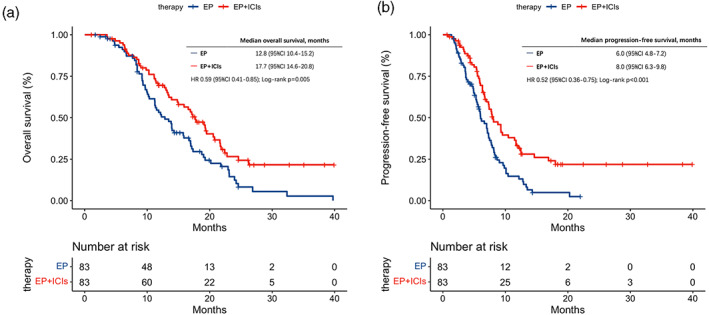
Kaplan–Meier curves in the propensity score‐matched cohort. (a) Kaplan–Meier curves of overall survival stratified by the first‐line therapy in the propensity score‐matched cohort. (b) Kaplan–Meier curves of progression‐free survival stratified by the first‐line therapy in the propensity score‐matched cohort. CI, confidence interval; EP, etoposide and platinum; ICIs, immune checkpoint inhibitors; HR, hazard ratio.
Treatment response and treatment‐related adverse events
The response was assessed in patients who had complete information. The ORR and DCR of all patients included were 73.0% and 96.8%. The ORR of EP alone, EP plus PD‐1 inhibitors, and EP plus PD‐L1 inhibitors were 62.3%, 87.9%, and 78.6%, respectively. The DCR of them were 94.7%, 100%, and 97.6%, respectively. A significant difference in the ORR between the EP plus ICIs cohort and the EP cohort was found, but no significant difference in the DCR was observed (ORR: EP: 62.3%, EP + ICIs: 84.3%, p < 0.001; DCR: EP: 94.7%, EP + ICIs: 99.1%, p = 0.143) (Figure 3(a)).
FIGURE 3.
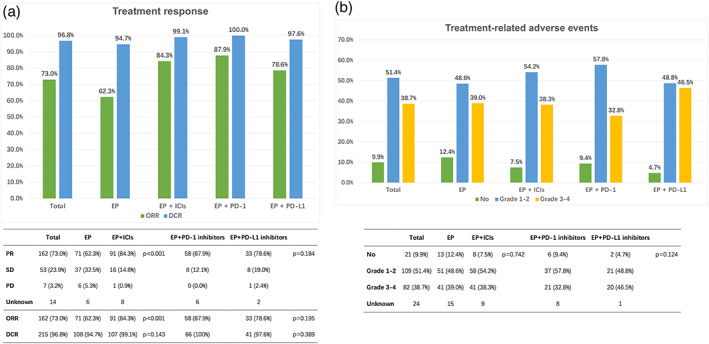
(a) Tumor response in all extensive‐stage small cell lung cancer (ES‐SCLC) patients. (b) Treatment‐related adverse events in all ES‐SCLC patients. CR, complete response; DCR, disease control rate; EP, etoposide and platinum; ICIs, immune checkpoint inhibitors; ORR, objective response rate; PR, partial response; SD, stable disease.
The treatment‐related adverse events (trAEs) are summarized in Figure 3(b). Among all eligible patients, 191 (90.1%) had encountered at least one trAE, including 109 (51.4%) and 82 (38.7%) patients with grade 1–2 and grade 3–4 trAEs, respectively. The rates of trAEs in the two groups were not statistically significant (p = 0.742).
Cox regression analysis for prognostic factors in patients who received ICIs with chemotherapy
We conducted the univariate and multivariate analysis in terms of PFS and OS to determine, which clinical characteristics or serum inflammatory and tumor markers are linked to the survival of ES‐SCLC patients who underwent first‐line chemotherapy in combination with ICIs, which was summarized in Table 2.
TABLE 2.
Univariate and multivariate analyses of progression‐free survival and overall survival in ES‐SCLC patients who received first‐line immunotherapy with chemotherapy.
| Characteristics | Progression‐free survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| Age | <65 | ||||||||
| ≥65 | 1.25 (0.78–2.01) | 0.356 | 1.26 (0.79–2.01) | 0.334 | |||||
| Sex | Male | ||||||||
| Female | 0.83 (0.33–2.08) | 0.696 | 0.65 (0.26–1.62) | 0.361 | |||||
| Smoking history | Never | ||||||||
| Former/current | 1.05 (0.64–1.74) | 0.841 | 0.58 (0.36–0.93) | 0.024 | 0.65 (0.36–1.17) | 0.149 | |||
| PS | 0 | ||||||||
| 1 | 1.64 (1.02–2.65) | 0.043 | 2.11 (1.15–3.86) | 0.015 | 1.4 (0.88–2.23) | 0.153 | |||
| Primary site | Left | ||||||||
| Right | 1.04 (0.65–1.66) | 0.863 | 0.83 (0.53–1.32) | 0.435 | |||||
| Size | <5 cm | ||||||||
| ≥5 cm | 1.35 (0.74–2.46) | 0.33 | 1.18 (0.67–2.06) | 0.569 | |||||
| Extrathoracic metastases | No | ||||||||
| Yes | 1.39 (0.81–2.4) | 0.237 | 1.34 (0.79–2.29) | 0.281 | |||||
| Brain metastases | No | ||||||||
| Yes | 1.75 (1–3.08) | 0.050 | 1.76 (1.01–3.07) | 0.048 | 1.32 (0.63–2.74) | 0.464 | |||
| Liver metastases | No | ||||||||
| Yes | 2.48 (1.52–4.05) | <0.001 | 2.64 (1.41–4.94) | 0.002 | 1.88 (1.16–3.04) | 0.010 | 2.08 (1.13–3.82) | 0.018 | |
| Bone metastases | No | ||||||||
| Yes | 1.65 (1–2.74) | 0.050 | 1.15 (0.71–1.88) | 0.572 | |||||
| Lymph node metastases | No/local | ||||||||
| Distant | 0.73 (0.45–1.19) | 0.204 | 0.82 (0.51–1.32) | 0.425 | |||||
| Pleural effusion | No | ||||||||
| Yes | 0.91 (0.57–1.45) | 0.685 | 1.13 (0.72–1.8) | 0.595 | |||||
| Pericardial effusion | No | ||||||||
| Yes | 1.47 (0.83–2.6) | 0.189 | 1.62 (0.91–2.87) | 0.098 | |||||
| Radiotherapy | No | ||||||||
| Yes | 0.61 (0.37–1) | 0.052 | 0.73 (0.44–1.21) | 0.230 | |||||
| ICIs | PD‐1 inhibitors | ||||||||
| PD‐L1 inhibitors | 0.94 (0.58–1.52) | 0.794 | 1.47 (0.92–2.34) | 0.108 | |||||
| CEA | ≤5 | ||||||||
| >5 | 1 (0.58–1.74) | 0.990 | 0.96 (0.55–1.64) | 0.869 | |||||
| CA125 | ≤35 | ||||||||
| >35 | 1.33 (0.77–2.31) | 0.312 | 1.48 (0.87–2.53) | 0.152 | |||||
| CA153 | ≤25 | ||||||||
| >25 | 1.5 (0.58–3.85) | 0.400 | 1.38 (0.49–3.9) | 0.545 | |||||
| SCC | ≤1.5 | ||||||||
| >1.5 | 0.95 (0.33–2.74) | 0.931 | 1.33 (0.55–3.22) | 0.532 | |||||
| NSE | ≤16.3 | ||||||||
| >16.3 | 1.94 (0.47–7.99) | 0.359 | 2.63 (0.64–10.83) | 0.180 | |||||
| Cyfra21 | ≤3.3 | ||||||||
| >3.3 | 1.22 (0.56–2.63) | 0.620 | 1.3 (0.62–2.71) | 0.485 | |||||
| LDH | ≤245 | ||||||||
| >245 | 1.4 (0.78–2.5) | 0.261 | 1.13 (0.65–1.97) | 0.666 | |||||
| LIPI | Good | ||||||||
| Intermediate | 1.43 (0.77–2.63) | 0.255 | 0.95 (0.53–1.71) | 0.871 | |||||
| Poor | 1.04 (0.24–4.55) | 0.955 | 1.25 (0.38–4.19) | 0.713 | |||||
| NLR | ≤3.10 | ||||||||
| >3.10 | 0.47 (0.25–0.87) | 0.017 | 0.45 (0.22–0.92) | 0.028 | 0.56 (0.31–1.01) | 0.056 | |||
| PLR | ≤277.01 | ||||||||
| >277.01 | 1.53 (0.76–3.06) | 0.233 | 2 (0.99–4.04) | 0.053 | |||||
| LMR | ≤3.26 | ||||||||
| >3.26 | 0.93 (0.52–1.64) | 0.793 | 0.51 (0.28–0.94) | 0.031 | 0.54 (0.30–0.99) | 0.049 | |||
| SII | ≤473.85 | ||||||||
| >473.85 | 0.52 (0.29–0.94) | 0.030 | 0.89 (0.45–1.74) | 0.732 | 0.78 (0.43–1.41) | 0.409 | |||
| SIRI | ≤0.89 | ||||||||
| >0.89 | 1.32 (0.66–2.64) | 0.439 | 1.76 (0.82–3.76) | 0.145 | |||||
| PNI | ≤47.75 | ||||||||
| >47.75 | 0.98 (0.53–1.81) | 0.943 | 0.61 (0.32–1.14) | 0.121 | |||||
| ALI | ≤206.46 | ||||||||
| >206.46 | 0.9 (0.26–3.04) | 0.859 | 0.46 (0.18–1.17) | 0.102 | |||||
Abbreviations: ALI, advanced lung cancer inflammation index; CI, confidence interval; ES‐SCLC, extensive‐stage small cell lung cancer; HR, hazard ratio; ICIs, immune checkpoint inhibitors; LDH, lactate dehydrogenase; LIPI, lung immune prognostic index; LMR, lymphocyte to monocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet to lymphocyte ratio; PNI, prognostic nutrition index; PS, performance status; SII, systemic immune‐inflammation index; SIRI, systemic inflammation response index.
The outcomes of the univariate analysis revealed that ECOG PS (p = 0.043), liver metastases (p < 0.001), baseline NLR (p = 0.017), and baseline SII (p = 0.030) were associated with better PFS in ES‐SCLC cases that received first‐line ICIs treatment. After multivariate analysis, the outcomes presented that ECOG PS = 1 (HR, 2.11; 95% CI [1.15–3.86]; p = 0.015), liver metastases (HR, 2.64; 95% CI [1.41–4.94]; p = 0.002), and baseline NLR >3.10 (HR, 0.45; 95% CI [0.22–0.92]; p = 0.028) were independent prognostic factors for PFS (Table 2). Our results also indicated that smoking history (p = 0.024), brain metastases (p = 0.048), liver metastases (p = 0.010), and baseline LMR (p = 0.031) were significantly linked to better OS through univariate analysis. In the multivariate analysis, we identified liver metastases (HR, 2.08; 95% CI [1.13–3.82]; p = 0.018) and baseline LMR >3.26 (HR, 0.54; 95% CI [0.30–0.99]; p = 0.049) as independent prognostic factors for OS in cases received first‐line chemotherapy combined with ICIs (Table 2).
Comparison of PD‐L1 inhibitors and PD‐1 inhibitors
No statistically significant difference in survival outcomes (OS: HR, 1.47 95% CI [0.92–2.34], p = 0.108; PFS: HR, 0.94, 95% CI [0.58–1.52], p = 0.791) was found between cases who treated with PD‐L1 inhibitors and cases who treated with PD‐1 inhibitors (Figure 4). Moreover, ORR, DCR, and the trAEs in the EP plus PD‐1 inhibitors cohort and the EP plus PD‐L1 inhibitors cohort were similar. (ORR: p = 0.195; DCR: p = 0.389; trAEs: p = 0.124) (Figure 3).
FIGURE 4.
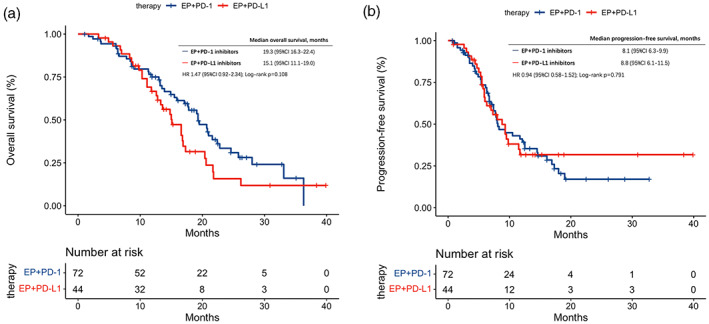
(a) Kaplan–Meier curves of overall survival stratified by the type of ICIs in patients treated with EP + ICIs. (b) Kaplan–Meier curves of progression‐free survival stratified by the type of ICIs in patients treated with EP + ICIs. CI, confidence interval; EP, etoposide and platinum; HR, hazard ratio.
Differences between our real‐world study and the clinical trials
We have summarized a table to compare the basic information from our multi‐center retrospective study with those clinical trials published before. Our survival outcomes were numerically better than previous clinical trials (IMpower133 and CASPIAN) but similar to the more recent trials CAPSTONE‐1 and ASTRUM‐005. The proportion of males and patients with ECOG PS = 0 at enrollment in our study was higher than in the clinical trials and cases with a history of smoking and liver metastases were less in our study (Table 3).
TABLE 3.
Differences between our real‐world study and the phase three clinical trials.
| Study | IMpower133 8 | CASPIAN 9 | KEYNOTE 604 10 | CAPSTONE‐1 20 | ASTRUM‐005 11 | Our study |
|---|---|---|---|---|---|---|
| No. | 403 | 537 | 453 | 462 | 585 | 236 |
| Age ≥65 years (%) | 44.8 vs. 47.5 | 38 vs. 42 | 49.6 vs. 55.1 | 33 vs. 37 | 39.6 vs. 39.3 | 56.9 vs. 47.5 |
| Male (%) | 64.2 vs. 65.3 | 71 vs. 68 | 66.7 vs. 63.1 | 80 vs. 81 | 81.5 vs. 83.7 | 91.4 vs. 83.3 |
| Smoking history (%) | 95.5 vs. 98.5 | 92 vs. 94 | 96.5 vs. 96.4 | 78 vs. 77 | 79.2 vs. 82.1 | 66.4 vs. 77.5 |
| PS = 0 (%) | 36.3 vs. 33.2 | 37 vs. 33 | 26.3 vs. 24.9 | 14 vs. 13 | 18.3 vs. 16.3 | 45.7 vs. 44.2 |
| Brain metastases (%) | 8.5 vs. 8.9 | 10 vs. 10 | 14.5 vs. 9.8 | 2 vs. 2 | 12.9 vs. 14.3 | 18.1 vs. 20.0 |
| Liver metastases (%) | 37 | 40 vs. 39 | 41.7 vs. 40.9 | 32 vs. 32 | 25.4 vs. 26.0 | 28.5 vs. 23.3 |
| mOS | 12.3 vs. 10.3 | 12.9 vs. 10.5 | 10.8 vs. 9.7 | 15.3 vs. 12.8 | 15.4 vs. 10.9 | 17.3 vs. 13.4 |
| HR = 0.76 | HR = 0.75 | HR = 0.80 | HR = 0.72 | HR = 0.63 | HR = 0.61 | |
| (0.60–0.95) | (0.62–0.91) | (0.64–0.98) | (0.58–0.9) | (0.49–0.82) | (0.45–0.83) | |
| p = 0.0154 | p = 0.0032 | p = 0.0164 | p = 0.0017 | p < 0.001 | p = 0.001 | |
| mPFS | 5.2 vs. 4.3 | 5.1 vs. 5.4 | 4.5 vs. 4.3 | 5.8 vs. 5.6 | 5.7 vs. 4.3 | 8.3 vs. 5.9 |
| HR = 0.77 | HR = 0.80 | HR = 0.75 | HR = 0.67 | HR = 0.48 | HR = 0.44 | |
| (0.62–0.96) | (0.66–0.96) | (0.61–0.91) | (0.54–0.83) | (0.38–0.59) | (0.32–0.60) | |
| p = 0.02 | p = 0.0023 | p < 0.0001 | p < 0.001 | p < 0.001 |
Note: EP + ICIs group vs. EP group.
Abbreviations: HR, hazard ratio; mOS, median overall survival; mPFS, median progression‐free survival; PS, performance status.
DISCUSSION
The past decade has witnessed a significant shift in the clinical treatment landscape of solid tumors with the introduction of ICIs. 18 , 19 Although ES‐SCLC has traditionally had a poor prognosis, recent phase three trials have confirmed improved outcomes with the addition of ICIs to chemotherapy. 8 , 9 , 11 , 20 However, only a few retrospective studies reported the survival outcomes in the real world, some without control groups, 21 , 22 some with insufficient case numbers, 23 , 24 and some including only PD‐L1 inhibitors. 25 , 26 , 27 Concerns about the inconsistency of anti‐PD‐1 agents and anti‐PD‐L1 agents suggested by the consequences of clinical trials are inconclusive. 10 , 11 The clinical benefits obtained from ICIs therapy in real‐world ES‐SCLC patients are still worth exploring. Moreover, tumor mutational burden (TMB) and PD‐L1 expression have not demonstrated clear predictive value in ES‐SCLC patients receiving chemotherapy plus immunotherapy in the first‐line setting. 28 No broadly accepted biomarkers that predict benefits from ICIs have been identified to date. 29 As a result, identifying biomarkers to predict the optimal population to benefit from chemo‐immunotherapy has become a priority for oncologists.
Our retrospective study was conducted across three medical centers and confirmed the effectiveness and safety of combining ICIs with EP in the first‐line setting of ES‐SCLC patients. In our data, PFS and OS were longer in the cases treated with chemotherapy and immunotherapy, which is consistent with the results of the recent randomized phase three trials. 8 , 9 , 11 , 21 The ORR was ~22% points higher in cases with EP plus ICIs than in cases with EP (84.3% vs. 62.3%). Additionally, our subgroup analysis found consistent benefits in most subgroups, although females and patients with brain metastases, liver metastases, and pericardial effusion failed to benefit from the combination therapy. The published clinical trials mentioned above also found that patients with brain or liver metastases at enrollment did not have a better survival outcome even with immunotherapy. 8 , 9 , 11 , 21 This may be linked to the poorer initial physical condition of these cases. It is worth noting that our survival outcomes in patients treated with ICIs and chemotherapy were numerically better than those of the IMpower133 and CASPIAN. 8 , 9 However, the latest clinical trials (CAPSTONE‐1 and ASTRUM‐005) reached better survival outcomes, which were similar to our data, proving that our findings remain reliable. 11 , 20 The CAPSTONE‐1 study was a multicenter trial conducted in 47 hospitals in China and the ASTRUM‐005 study included two‐thirds of Asian patients. Our study also included patients from three Chinese centers. These suggest that the better survival outcomes may be because of the Asian patient cohort. Based on the comparison, we could speculate that the longer survival outcomes in our study are associated with a higher proportion of males, PS = 0, no history of smoking, and no liver metastases at enrollment. This is also consistent with our following conclusion that liver metastases and ECOG PS are independent prognostic factors according to multivariate cox regression analysis. The differences in survival outcomes also may be because of the limitation of the retrospective study, such as the frequency of evaluation of the response to chemotherapy or chemo‐immunotherapy. In addition, rates of trAEs were similar in the two cohorts, also confirming the safety of the ICIs.
According to our findings, no significant difference was observed between cases that received anti‐PD‐1 or anti‐PD‐L1 agents, although the outcomes were longer numerically in the PD‐1 group. The KEYNOTE‐604 study, which used anti‐PD‐1 agents, has not achieved consistent results as the clinical trials with PD‐L1 inhibitors. 10 One notable exception to this trend is the ASTRUM‐005 study, in which the OS reached 15.4 months with the use of Serplulimab. It is the most significant OS benefit among the trials that have been published so far. 11 Because of the absence of head‐to‐head comparisons, some meta‐analyses have been carried out to compare the clinical effectiveness of these two different ICIs indirectly. One meta‐analysis involving 23 studies published between 2013 and 2016 found that anti‐PD‐1 agents and anti‐PD‐L1 agents are likely to have comparable toxicity and effectiveness. 30 However, another meta‐analysis published in 2019 suggested that PD‐1 inhibitors were linked to favorable survival results compared with PD‐L1 inhibitors. 31 The debate on whether PD‐1 and PD‐L1 inhibitors cause different clinical outcomes remains unresolved and more investigation is required.
Multivariate analysis of our study showed that ECOG PS = 1, liver metastases, and baseline NLR ≤3.10 were independent risk factors for PFS, and liver metastases and baseline LMR ≤3.26 were for OS. Recently, increasing evidence suggests that performance status has a significant impact on tumor development 32 and our research supported this. Many previous studies and ours have reached the same result that liver metastases were associated with a poorer prognosis. 33 According to Yu et al. 34 liver metastases reduced the amount of circulating CD8+ T cells through the apoptosis of activated T cells following their interaction with macrophages, and liver‐directed radiotherapy reduced macrophages to increase the anticancer effectiveness of immunotherapy in preclinical models. If this theory is supported by additional prospective studies, careful monitoring of the liver metastases progression and application of local therapy to liver metastases would probably increase the efficacy of immunotherapy and help to improve survival rates.
At several stages of tumor growth, inflammatory responses are crucial. 35 Some indexes related to inflammation and nutritional status, such as the NLR, SIRI, and LIPI, have been identified as valuable prognostic factors for some cancers. 13 , 14 , 15 , 16 However, it remains unclear if these markers still have a similar effect in cases with ES‐SCLC. Qi et al. 36 found that several baseline factors were discovered to be significantly associated with OS outcomes for ES‐SCLC cases who received chemotherapy and atezolizumab, including NLR, PLR, LMR, SII, PNI, and LIPI. In the multivariate analysis, PLR was the only independent prognostic factor identified. Our study similarly explored the prognostic effect of the pretreatment inflammatory and nutritional markers for ES‐SCLC patients, who received first‐line chemo‐immunotherapy, and we identified NLR and LMR have potential predictive value. The underlying mechanisms of the link between decreased NLR/LMR and poor survival outcomes in SCLC cases remain unclear. Galvano et al. 14 conducted a study to find out that lung neuroendocrine carcinoma patients with elevated NLR demonstrated an augmented likelihood of poor outcomes. Additionally, a previous study discovered that increased neutrophils induced the upregulation of cytokines and chemokines, which may have facilitated the development of cancers. 37 These findings, in contrast to our results, suggested that the effect of NLR on tumor development is to a large extent unknown, particularly after the addition of ICIs that affect the tumor immune microenvironment. Moreover, in our multivariate cox regression analysis for OS, the significance between the high‐level and low‐level groups of NLR was not observed, compared to PFS. This implies that NLR could not be a suitable prognostic marker for ES‐SCLS patients who received first‐line ICIs. Zhao et al. 38 recently reported that lower LMR level displayed a significantly poorer response rate to chemotherapy in patients with NSCLC receiving chemotherapy. In addition, in a large cohort study and a meta‐analysis, a higher LMR has been suggested to be linked to favorable prognosis in cases with lung cancer. 39 , 40 All the above results were consistent with our study. The precise mechanisms of the association remain uncertain, but several hypotheses have been proposed on this subject. It is well known that lymphocytes are essential for the antitumor immune response by triggering the death of the cytotoxic cell and inhibiting tumor growth. 41 , 42 In addition, by differentiating into tumor‐associated macrophages, monocytes could trigger genetic mutations, stimulate angiogenesis, and inhibit anti‐tumor immunity. 43 , 44 The importance of inflammatory biomarkers in the therapy of SCLC cases with chemo‐immunotherapy required more evidence.
There were a few limitations to our research. First, as the study design is retrospective, propensity score matching could not completely exclude confounding variables and selective bias. Second, because of good performance status (PS = 0/1), the cases in our study could not represent the whole SCLC community. It is worth exploring whether patients with PS = 2 could benefit from chemo‐immunotherapy, and we look forward to future investigations. Third, the limited follow‐up period might have influenced the outcomes. Therefore, additional research including larger samples and longer follow‐ups is necessary. Last, the ideal cutoff value as a standard for these markers has not been determined; some investigators select the median value, whereas others based their decision on results from earlier studies. We use the ROC to choose the optimal cutoff.
CONCLUSION
In conclusion, our real‐world data demonstrated that ICIs plus EP as a first‐line setting for ES‐SCLC is effective and safe. Additionally, ECOG PS, liver metastases, and pretreatment inflammatory markers may serve as valuable prognostic factors for survival outcomes in ES‐SCLC cases that received first‐line ICIs plus chemotherapy, suggesting their potential prognostic value. Evaluation of biomarkers that could predict the prognosis of chemo‐immunotherapy in SCLC patients requires further research.
AUTHOR CONTRIBUTIONS
Jingyuan Xie: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, validation, visualization, writing – original draft, writing – review and editing. Mo Chen: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, writing – original draft, writing – review and editing. Hedong Han: Conceptualization, formal analysis, methodology, project administration, software, validation, visualization, writing – original draft. Ke Xu: Data curation, formal analysis, investigation, methodology. Guihuan Qiu: Investigation. Xinqing Lin: Project administration, resources, supervision. Yong Song: Conceptualization, funding acquisition, project administration, resources, supervision, validation, writing – review and editing. Jinjun Ye: Project administration, resources, supervision. Tangfeng Lv: Conceptualization, funding acquisition, project administration, supervision, writing – review and editing. Ping Zhan: Conceptualization, methodology, funding acquisition, writing – original draft, writing – review and editing.
FUNDING INFORMATION
This work was supported by grants from the 16th batch “Summit of the Six Top Talents” Program of Jiangsu Province (grant number WSN‐154); China Postdoctoral Science Foundation 12th batch Special fund (Postdoctoral number: 45786); China Postdoctoral Science Foundation 64th batch (Postdoctoral number: 45786); Jiangsu Provincial Postdoctoral Science Foundation (grant number 2018K049A); the Natural Science Foundation of Jiangsu province (grant number BK20180139); Jiangsu Provincial Health Committee Medical projects (grant number M2022110); Natural Science Foundation of Jiangsu Province (grant number BK20210146).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting information
APPENDIX S1. Supplementary Information.
Xie J, Chen M, Han H, Xu K, Qiu G, Lin X, et al. Clinical impact of first‐line PD‐1 or PD‐L1 inhibitors combined with chemotherapy in extensive‐stage small cell lung cancer patients: A real‐world multicenter propensity score‐matched study. Thorac Cancer. 2023;14(15):1327–1338. 10.1111/1759-7714.14874
Jingyuan Xie, Mo Chen, and Hedong Han are contributed equally to this paper.
Contributor Information
Yong Song, Email: yong.song@nju.edu.cn.
Jinjun Ye, Email: jjye2004@163.com.
Tangfeng Lv, Email: bairoushui@163.com.
Ping Zhan, Email: zhanping207@163.com.
REFERENCES
- 1. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small‐cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–44. 10.1200/JCO.2005.04.4859 [DOI] [PubMed] [Google Scholar]
- 2. Gazdar AF, Minna JD. Developing new, rational therapies for recalcitrant small cell lung cancer. J Natl Cancer Inst. 2016;108(10):djw119. 10.1093/jnci/djw119 [DOI] [PubMed] [Google Scholar]
- 3. Semenova EA, Nagel R, Berns A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev. 2015;29(14):1447–62. 10.1101/gad.263145.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farago AF, Keane FK. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. 2018;7(1):69–79. 10.21037/tlcr.2018.01.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Demedts IK, Vermaelen KY, van Meerbeeck JP. Treatment of extensive‐stage small cell lung carcinoma: current status and future prospects. Eur Respir J. 2010;35(1):202–15. 10.1183/09031936.00105009 [DOI] [PubMed] [Google Scholar]
- 6. Zhou L, Xu Q, Huang L, Jin J, Zuo X, Zhang Q, et al. Low‐dose carboplatin reprograms tumor immune microenvironment through STING signaling pathway and synergizes with PD‐1 inhibitors in lung cancer. Cancer Lett. 2021;500:163–71. 10.1016/j.canlet.2020.11.049 [DOI] [PubMed] [Google Scholar]
- 7. Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30(2):219–35. 10.1093/annonc/mdy551 [DOI] [PubMed] [Google Scholar]
- 8. Liu SV, Reck M, Mansfield AS, et al. Updated overall survival and PD‐L1 subgroup analysis of patients with extensive‐stage small‐cell lung cancer treated with Atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. 2021;39(6):619–30. 10.1200/JCO.20.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum‐etoposide versus platinum‐etoposide alone in first‐line treatment of extensive‐stage small‐cell lung cancer (CASPIAN): updated results from a randomised, controlled, open‐label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. 10.1016/S1470-2045(20)30539-8 [DOI] [PubMed] [Google Scholar]
- 10. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first‐line therapy for extensive‐stage small‐cell lung cancer: randomized, double‐blind, phase III KEYNOTE‐604 study. J Clin Oncol. 2020;38(21):2369–79. 10.1200/JCO.20.00793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of first‐line Serplulimab vs placebo added to chemotherapy on survival in patients with extensive‐stage small cell lung cancer: the ASTRUM‐005 randomized clinical trial. JAMA. 2022;328(12):1223–32. 10.1001/jama.2022.16464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- 13. Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, et al. Peripheral blood markers predictive of outcome and immune‐related adverse events in advanced non‐small cell lung cancer treated with PD‐1 inhibitors. Cancer Immunol Immunother. 2020;69(9):1813–22. 10.1007/s00262-020-02585-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galvano A, Peri M, Guarini AA, Castiglia M, Grassadonia A, de Tursi M, et al. Analysis of systemic inflammatory biomarkers in neuroendocrine carcinomas of the lung: prognostic and predictive significance of NLR, LDH, ALI, and LIPI score. Ther Adv Med Oncol. 2020;12:1758835920942378. 10.1177/1758835920942378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in patients with advanced non‐small cell lung cancer. JAMA Oncol. 2018;4(3):351–7. 10.1001/jamaoncol.2017.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qiu J, Ke D, Lin H, Yu Y, Zheng Q, Li H, et al. Using inflammatory indexes and clinical parameters to predict radiation esophagitis in patients with small‐cell lung cancer undergoing chemoradiotherapy. Front Oncol. 2022;12:898653. 10.3389/fonc.2022.898653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG, et al. Staging small cell lung cancer: veterans administration lung study group versus International Association for the Study of Lung Cancer–what limits limited disease? Lung Cancer. 2002;37(3):271–6. 10.1016/s0169-5002(02)00072-7 [DOI] [PubMed] [Google Scholar]
- 18. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y, Gao M, Huang Z, Yu J, Meng X. SBRT combined with PD‐1/PD‐L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges. J Hematol Oncol. 2020;13(1):105. 10.1186/s13045-020-00940-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, et al. Adebrelimab or placebo plus carboplatin and etoposide as first‐line treatment for extensive‐stage small‐cell lung cancer (CAPSTONE‐1): a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2022;23(6):739–47. 10.1016/S1470-2045(22)00224-8 [DOI] [PubMed] [Google Scholar]
- 21. Lee S, Shim HS, Ahn BC, Lim SM, Kim HR, Cho BC, et al. Efficacy and safety of atezolizumab, in combination with etoposide and carboplatin regimen, in the first‐line treatment of extensive‐stage small‐cell lung cancer: a single‐center experience. Cancer Immunol Immunother. 2022;71(5):1093–101. 10.1007/s00262-021-03052-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li L, Pi C, Yan X, Lu J, Yang X, Wang C, et al. Prognostic value of the pretreatment lung immune prognostic index in advanced small cell lung cancer patients treated with first‐line PD‐1/PD‐L1 inhibitors plus chemotherapy. Front Oncol. 2021;11:697865. 10.3389/fonc.2021.697865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim JU, Kang HS, Shin AY, Yeo CD, Kim SK, Kim JW, et al. Investigation of poor predictive factors in extensive stage small cell lung cancer under etoposide‐platinum‐atezolizumab treatment. Thorac Cancer. 2022;13(23):3384–92. 10.1111/1759-7714.14697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang Y, Ai X, Xu H, Yang G, Yang L, Hao X, et al. Treatment patterns and outcomes of immunotherapy in extensive‐stage small‐cell lung cancer based on real‐world practice. Thorac Cancer. 2022;13(23):3295–303. 10.1111/1759-7714.14684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen H, Ma X, Liu J, Yang Y, Fang Y, Wang L, et al. Clinical outcomes of atezolizumab in combination with etoposide/platinum for treatment of extensive‐stage small‐cell lung cancer: a real‐world, multicenter, retrospective, controlled study in China. Chin. J Cancer Res. 2022;34(4):353–64. 10.21147/j.issn.1000-9604.2022.04.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qu J, Kalyani FS, Shen Q, Yang G, Cheng T, Liu L, et al. Efficacy and safety of PD‐L1 inhibitors plus chemotherapy versus chemotherapy alone in first‐line treatment of extensive‐stage small‐cell lung cancer: a retrospective real‐world study. J Oncol. 2022;2022:3645489–12. 10.1155/2022/3645489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma J, Tian Y, Hao S, Zheng L, Hu W, Zhai X, et al. Outcomes of first‐line anti‐PD‐L1 blockades combined with brain radiotherapy for extensive‐stage small‐cell lung cancer with brain metastasis. J Neurooncol. 2022;159(3):685–93. 10.1007/s11060-022-04111-7 [DOI] [PubMed] [Google Scholar]
- 28. Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small‐cell lung cancer. Nat Rev Clin Oncol. 2020;17(5):300–12. 10.1038/s41571-019-0316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Plaja A, Moran T, Carcereny E, Saigi M, Hernández A, Cucurull M, et al. Small‐cell lung cancer long‐term survivor patients: how to find a needle in a haystack? Int J Mol Sci. 2021;22(24):13508. doi: 10.3390/ijms222413508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pillai RN, Behera M, Owonikoko TK, Kamphorst AO, Pakkala S, Belani CP, et al. Comparison of the toxicity profile of PD‐1 versus PD‐L1 inhibitors in non‐small cell lung cancer: a systematic analysis of the literature. Cancer. 2018;124(2):271–7. 10.1002/cncr.31043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta‐analysis. JAMA Oncol. 2020;6(3):375–84. 10.1001/jamaoncol.2019.5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jang RW, Caraiscos VB, Swami N, Banerjee S, Mak E, Kaya E, et al. Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract. 2014;10(5):e335–41. 10.1200/jop.2014.001457 [DOI] [PubMed] [Google Scholar]
- 33. Tsilimigras DI, Brodt P, Clavien PA, Muschel RJ, Angelica MI, Endo I, et al. Liver metastases. Nat Rev Dis Primers. 2021;7(1):27. 10.1038/s41572-021-00261-6 [DOI] [PubMed] [Google Scholar]
- 34. Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage‐mediated T cell elimination. Nat Med. 2021;27(1):152–64. 10.1038/s41591-020-1131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. 10.1016/j.immuni.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qi WX, Xiang Y, Zhao S, Chen J. Assessment of systematic inflammatory and nutritional indexes in extensive‐stage small‐cell lung cancer treated with first‐line chemotherapy and atezolizumab. Cancer Immunol Immunother. 2021;70(11):3199–206. 10.1007/s00262-021-02926-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, et al. Neutrophils recruit regulatory T‐cells into tumors via secretion of CCL17–a new mechanism of impaired antitumor immunity. Int J Cancer. 2014;135(5):1178–86. 10.1002/ijc.28770 [DOI] [PubMed] [Google Scholar]
- 38. Zhao K, Wang C, Shi F, Huang Y, Ma L, Li M, et al. Combined prognostic value of the SUVmax derived from FDG‐PET and the lymphocyte‐monocyte ratio in patients with stage IIIB‐IV non‐small cell lung cancer receiving chemotherapy. BMC Cancer. 2021;21(1):66. 10.1186/s12885-021-07784-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation‐based lymphocyte‐ monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One. 2014;9(9):e108062. 10.1371/journal.pone.0108062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jin J, Yang L, Liu D, Li WM. Prognostic value of pretreatment lymphocyte‐to‐monocyte ratio in lung cancer: a systematic review and meta‐analysis. Technol Cancer Res Treat. 2021;20:1533033820983085. 10.1177/1533033820983085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van der Leun AM, Thommen DS, Schumacher TN. CD8(+) T cell states in human cancer: insights from single‐cell analysis. Nat Rev Cancer. 2020;20(4):218–32. 10.1038/s41568-019-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miyoshi Y, Yoshimura Y, Saito K, Muramoto K, Sugawara M, Alexis K, et al. High absolute lymphocyte counts are associated with longer overall survival in patients with metastatic breast cancer treated with eribulin‐but not with treatment of physician's choice‐in the EMBRACE study. Breast Cancer. 2020;27(4):706–15. 10.1007/s12282-020-01067-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Devalaraja S, To TKJ , Folkert IW, Natesan R, Alam MZ, Li M. Tumor‐derived retinoic acid regulates Intratumoral monocyte differentiation to promote immune suppression. Cell. 2020;180(6):1098–1114 e16. 10.1016/j.cell.2020.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour‐associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1. Supplementary Information.


