Abstract
Background
This study aimed to examine the treatment and prognosis of patients with type B2 + B3 thymoma and compare it with those patients with type B2 and B3 thymoma.
Methods
We conducted a retrospective analysis of the results of 39 patients with type B2 + B3 thymoma, 133 patients with type B2 thymoma, and 64 patients with type B3 thymoma. The Kaplan–Meier technique was used to generate survival curves. For multivariate analysis, the Cox proportional hazard model was applied.
Results
With a median follow‐up of 60 months (range: 1–128 months), the percentage of patients with tumor, node, metastasis (TNM) stage III and IV disease gradually increased from 19.5% to 25.6% to 35.9% among those with histological subtypes B2, B2 + B3, and B3, respectively, p = 0.045. Twenty‐three patients experienced recurrence or metastasis. The total 10‐year progression‐free survival (PFS) rates were 86.0% overall (85.0% in type B2, 87.2% in type B2 + B3, and 87.5% in type B3). Age, R0 resection, and Masaoka–Koga stage were found to have a significant on PFS in all patients. There was no statistically significant difference in PFS between different histotypes of thymoma, p = 0.650. PFS was predicted by R0 resection in all histotypes and by the Masaoka–Koga stage in the type B2 subgroup.
Conclusion
Combining the two staging methods to guide the diagnosis and treatment of patients with B2 + B3 thymoma is recommended. R0 resection is recommended to reduce recurrence. Patients with B2 + B3 thymoma have a prognosis similar to those with a B2 thymoma or a B3 thymoma alone.
Keywords: prognosis, real‐world study, thymoma, treatment, type B2 + B3
Key question: This research aimed to examine the treatment and prognosis of patients with type B2 + B3 thymoma. Key findings: Patients with type B2 + B3 thymoma have a good prognosis, which is similar to that of type B2 or B3 thymoma. Take‐home message: R0 resection is the best treatment and the main prognostic factor for type B2 + B3 thymoma.
INTRODUCTION
The World Health Organization (WHO) updated its histological classification of thymomas, a rare malignancy and the most common mediastinal tumors, 1 in 2021. 2 , 3 Based on the morphological features of epithelial tumor cells, the percentage of the nontumoral lymphocytic component, and the degree to which the tumor resembles normal thymic architecture, thymomas are further classified into subtypes (A, AB, B1, B2, and B3). 2 , 4
Given the prevalence of thymomas with various histological features, a subtype of thymoma known as combined thymomas was previously recognized but later abandoned in 2014. 2 Similar to the Gleason scale, International Thymic Malignancy Interest Group recommended that all tumor subtypes be listed in order of predominance when making a diagnosis; minor components should be reported in 10% increments, and thymoma components of 0% to 10% can be neglected. 2 Some borderline cases can be mixed in any proportion of type B2 and B3 thymoma, which should be reported as type B2 + B3 thymoma (i.e., more than 10% of both components) rather than as type B2 or B3 thymoma due to a morphological continuum between the two subtypes. 2 , 4
In clinical practice, we found that many patients have been diagnosed with type B2 + B3 thymoma. However, the clinical characteristics, treatment outcomes, and prognosis of these patients have rarely discussed in previously published studies. 5 For this reason, we aimed to summarize the management experience of patients with type B2 + B3 thymoma by reviewing the information of those who were diagnosed with this tumor at our hospital over the past decade and comparing it to that of patients with type B2 and B3 thymoma.
METHODS
Ethics statement
This publication was approved by the Institutional Review Board (IRB) of Peking Union Medical College Hospital (PUMCH) on December 8, 2022. ID number of the IRB is K22C2462. The patient's informed consent was waived by the IRB.
Patient characteristics
A total of 245 patients were diagnosed with type B2 thymoma, type B3 thymoma, or type B2 + B3 thymoma between January 2012 and December 2021 at the PUMCH, Chinese Academy of Medical Science and Peking Union Medical College. During this period, 41 patients were diagnosed with type B2 + B3, 137 patients with type B2, and 67 with type B3. A total of nine patients (2 cases of type B2 + B3, 3 cases of type B3, and 4 cases of type B2) were excluded from the study because of intraoperative death or no definite stage recorded. Finally, the data from 236 patients were retrospectively analyzed.
Diagnosis and treatment
All patients were pathologically confirmed via surgery or needle biopsy. The pathological diagnosis of all patients (including biopsy samples) was reviewed by professional pathologists and meets the 2021 WHO diagnostic criteria. The stage of thymoma was classified based on the tumor, node, metastasis (TNM) staging system and with reference to the Masaoka–Koga staging system. 6 , 7 The lymph nodes of all patients undergoing surgery were evaluated pathologically to determine the TNM stage. The patients included in the study received at least one treatment at our hospital, including surgery, chemotherapy, radiotherapy, immunotherapy, or ablation. Recurrence or metastases was confirmed via chest and abdominal computed tomography as well as positron emission tomography.
Follow‐up and statistical analysis
The patients were followed up every 6 months after the completion of the treatment in the initial 5 years and every 12 months thereafter. The duration of progression‐free survival (PFS) was calculated from the date of operation until the detection of disease progression for patients who had stable disease after the initial treatment. A total of six patients died during this study. Because this number was very small, we set the primary endpoint of the study as disease progression, indicated by a recurrence or metastasis of the tumors.
Descriptive statistics are reported as the mean, range, and standard deviation of continuous variables and as the frequency and proportion of categorical variables. The t‐test was applied to compare the difference between the mean values of continuous variables, and the χ 2 test and Fisher's exact test were employed to compare the difference between the proportions of categorical variables. The Kaplan–Meier method was employed to estimate the PFS for the entire cohort as well as to perform univariate analysis for the assessment of prognostic factors. Univariate and multivariate analyses were performed using the Cox regression model. Statistical analysis was performed using SPSS 26.0 and GraphPad Prism 9.
RESULTS
Characteristics
The study included a total of 236 patients, but we were unable to obtain complete information due to a loss of follow‐up in 13 patients. Table 1 and Figure 1 illustrate the most salient clinical characteristics of the patients analyzed in this study. Of all patients, 41.9% had myasthenia gravis (MG), with type B3 patients having a significantly lower prevalence of MG compared to type B2 and type B2 + B3 patients (p = 0.038 and p = 0.028, respectively). Moreover, four patients developed additional paraneoplastic diseases, including one case each of primary thrombocytosis, Sjogren's syndrome, psoriasis, and autoimmune encephalitis. TNM staging system showed a linear increase in the percentage of patients at an advanced stage across the three histotypes of B2, B2 + B3, and B3 with respective rates of 19.5%, 25.6%, and 35.9%, respectively, p = 0.045. Contrary to expectations, Masaoka–Koga staging system did not reveal any such variation.
TABLE 1.
The characteristics of the study subjects.
| Variables | Total | Type B2 | Type B2 + B3 | Type B3 |
|---|---|---|---|---|
| Gender | ||||
| Male | 137 (58.1%) | 77 (57.9%) | 23 (59.0%) | 37 (57.8%) |
| Female | 99 (41.9%) | 56 (42.1%) | 16 (41.0%) | 27 (42.2%) |
| Age (years) | ||||
| Average | 48.7 (±12.8) | 47.1 (±12.8) | 49.7 (±11.8) | 51.3 (±12.9) |
| Myasthenia gravis | 99 (41.9%) | 60 (45.1%) | 20 (51.2%) | 19 (29.6%) |
| TNM stage | ||||
| I + II | 177 (75.0%) | 107 (80.5%) | 29 (74.4%) | 41 (64.1%) |
| III + IV | 59 (25.0%) | 26 (19.5%) | 10 (25.6%) | 23 (35.9%) |
| Masaoka–Koga stage | ||||
| I + II | 150 (63.6%) | 92 (69.2%) | 22 (56.4%) | 36 (56.3%) |
| III + IV | 86 (36.4%) | 41 (30.8%) | 17 (43.6%) | 28 (43.7%) |
| Tumor size | ||||
| <6 cm | 152 (64.4%) | 85 (63.9%) | 23 (59.0%) | 44 (68.8%) |
| ≥6 cm | 84 (35.6%) | 48 (36.1%) | 16 (41.0%) | 20 (31.2%) |
| Smoking | 131.6 (±305.9) | 131.9 (±311.1) | 117.9 (±271.6) | 139.4 (±318.8) |
| Tumor invasion | ||||
| Yes | 89 (37.7%) | 45 (33.8%) | 17 (43.6%) | 27 (42.2%) |
| No | 147 (62.3%) | 88 (66.2%) | 22 (56.4%) | 37 (57.8%) |
FIGURE 1.
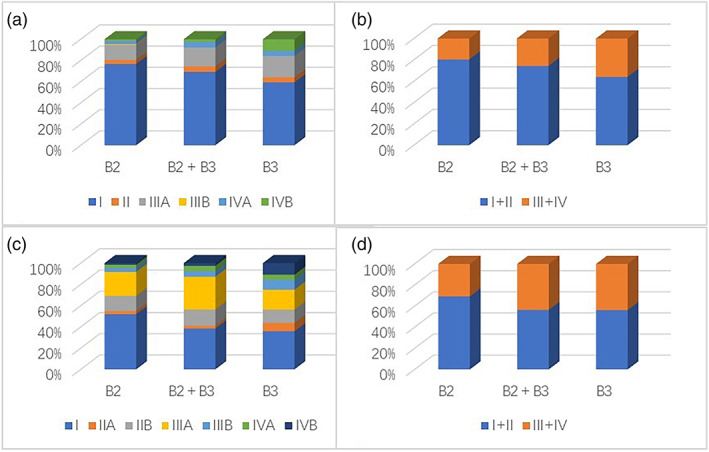
Stage distribution of thymoma histotypes. The stage classification is based on the tumor, node, metastasis (TNM) staging system (a + b) and Masaoka–Koga staging system (c + d).
Treatment and outcome
The median time patients were followed up was for 60 months (1–128 months). Table 2 provides extensive visualization of the patients’ treatment and development. A total of 77 patients (32.6%) underwent open surgery, including 69 cases (29.2%) of sternotomy. A total of 146 patients (61.9%) underwent minimally invasive surgery, including 144 cases (61.0%) video‐assisted thoracoscopic surgery and two cases of subxiphoid surgery. A total of eight patients failed to receive surgery. A total of six patients died during follow‐up. Three patients died from thymoma recurrence or metastasis of their thymoma, and three others died of unrelated causes while under follow‐up. Following initial treatment, 23 patients experienced progression, and four experienced a second progression. Treatments such as radiotherapy, 2 chemotherapy, 1 and ablation 1 were given to patients who had experienced a second progression. The median PFS of patients received chemotherapy (both pre‐ and postoperatively) was 68.0 months. The chemotherapy group has a lower recurrence rate than the nonchemotherapy group (22.9% vs. 45.0%), p = 0.087.
TABLE 2.
Treatment and progression of the study patients.
| Variables | Total | Type B2 | Type B2 + B3 | Type B3 |
|---|---|---|---|---|
| Residual disease | ||||
| R0 | 217 (91.9%) | 128 (96.2%) | 36 (92.3%) | 53 (82.8%) |
| R1/R2 | 7 (3.0%) | 1 (0.8%) | 1 (2.6%) | 5 (7.8%) |
| Progression | ||||
| Yes | 23 (9.7%) | 12 (9.0%) | 4 (10.3%) | 7 (10.9%) |
| No | 203 (86.0%) | 113 (85.0%) | 34 (87.2%) | 56 (87.5%) |
| Postoperative therapy | ||||
| Radiotherapy | 107 (45.3%) | 54 (40.6%) | 17 (43.6%) | 36 (56.3%) |
| Chemotherapy | 14 (5.9%) | 3 (2.3%) | 6 (15.4%) | 5 (7.8%) |
| Concurrent chemoradiation | 2 (0.8%) | 1 (0.8%) | 1 (2.6%) | 0 (0.0%) |
| Chemotherapy + radiotherapy | 7 (3.0%) | 0 (0.0%) | 2 (5.1%) | 5 (7.8%) |
| Radiotherapy + chemotherapy | 9 (3.8%) | 3 (2.3%) | 1 (2.6%) | 5 (7.8%) |
| None | 85 (36.0%) | 62 (46.6%) | 11 (28.2%) | 12 (18.8%) |
Prognosis and prognostic factors
Chi‐square and Fisher's exact tests were used to assess the impact of gender, age, TNM stage, Masaoka–Koga stage, R0 resection, tumor size, radiotherapy, MG, tumor invasion, smoking, and histotype on PFS (Figures 2 and 3). The prognosis of patients with early‐stage, noninvasive and R0 resected tumors was better, p < 0.001, and the prognosis of tumors larger than 6 cm was worse, p = 0.027. Table 3 shows the subgroup analysis results of each pathological type. Except for MG, the above factors were included in the multivariate analyses (Table 4). In the univariate analyses, the TNM stage, Masaoka–Koga stage, R0 resection, and tumor invasion were predictive of PFS in total and all histotypes. In addition, age and smoking were predictive of PFS in the type B2 subgroup; tumor size was predictive of PFS in all patients. In the multivariate analysis of all patients, age, R0 resection, and Masaoka–Koga stage were significantly predictive of PFS. In subgroup analyses, R0 resection was predictive of PFS in all histotypes, and the Masaoka–Koga stage was predictive of PFS in the type B2 subgroup.
FIGURE 2.
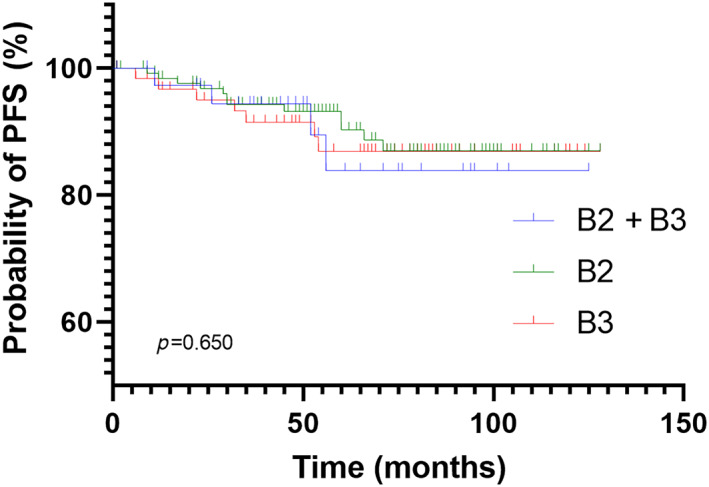
Kaplan–Meier curve for progression‐free survival (PFS) according to the histotype, p = 0.650.
FIGURE 3.
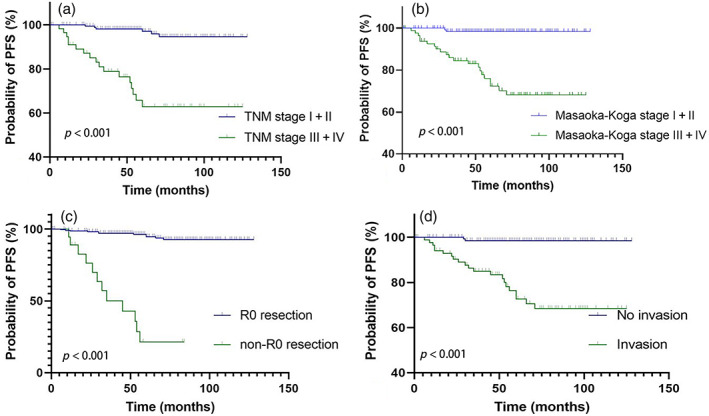
Kaplan–Meier curve for progression‐free survival (PFS) according to the TNM stage (a), p < 0.001; Kaplan–Meier curve for the PFS according to Masaoka–Koga stage (b), p< 0.001; Kaplan–Meier curve for the PFS according to R0 resection (c), p < 0.001; Kaplan–Meier curve for the PFS according to tumor invasion (d), p < 0.001.
TABLE 3.
Chi‐square test and Fisher's exact test results of the progression‐free survival (PFS) (subgroup analysis).
| Variables | B2 | p‐value | B2 + B3 | p‐value | B3 | p‐value |
|---|---|---|---|---|---|---|
| Gender | 0.239 | 0.286 | 0.223 | |||
| Male | 9 (11.7%) | 1 (4.3%) | 6 (16.2%) | |||
| Female | 3 (5.4%) | 3 (18.8%) | 1 (3.7%) | |||
| Age (years) | 0.014 | >0.999 | >0.999 | |||
| <50 | 11 (14.3%) | 2 (11.1%) | 3 (11.1%) | |||
| ≥50 | 1 (1.8%) | 2 (9.5%) | 4 (10.8%) | |||
| TNM stage | 0.005 | 0.003 | <0.001 | |||
| I + II | 6 (5.6%) | 0 (0.0%) | 0 (0.0%) | |||
| III − IV | 6 (23.1%) | 4 (40.0%) | 7 (30.4%) | |||
| M–K stage | <0.001 | 0.016 | 0.001 | |||
| I + II | 2 (2.2%) | 0 (0.0%) | 0 (0.0%) | |||
| III + IV | 10 (24.4%) | 4 (23.5%) | 7 (25.0%) | |||
| R0 resection | 0.005 | <0.001 | <0.001 | |||
| Yes | 9 (7.0%) | 1 (2.8%) | 1 (1.9%) | |||
| No | 3 (60.0%) | 3 (100.0%) | 6 (54.5%) | |||
| Tumor size | 0.093 | >0.999 | 0.191 | |||
| ≥6 cm | 7 (14.6%) | 2 (12.5%) | 4 (20.0%) | |||
| <6 cm | 5 (5.9%) | 2 (8.7%) | 3 (6.8%) | |||
| Radiotherapy | 0.231 | 0.919 | 0.678 | |||
| Yes | 8 (13.6%) | 2 (9.5%) | 6 (13.0%) | |||
| No | 4 (6.0%) | 2 (11.8%) | 1 (5.9%) | |||
| MG | 0.801 | 0.605 | 0.664 | |||
| Yes | 5 (8.3%) | 3 (15.0%) | 1 (5.3%) | |||
| No | 7 (9.6%) | 1 (5.3%) | 6 (13.3%) | |||
| Tumor invasion | 0.002 | 0.037 | 0.001 | |||
| Yes | 10 (20.5%) | 4 (22.2%) | 7 (25.9%) | |||
| No | 2 (3.4%) | 0 (0.0%) | 0 (0.0%) | |||
| Smoking | 0.025 | 0.556 | 0.370 | |||
| Yes | 7 (17.5%) | 0 (0.0%) | 3 (17.6%) | |||
| No | 5 (5.4%) | 4 (13.8%) | 4 (8.5%) |
Abbreviations: MG, myasthenia gravis; MK, Masaoka–Koga stage.
TABLE 4.
Multivariate analyses of the progression‐free survival (PFS).
| Type | Variables | Hazard ratio | PFS 95% confidence interval | p‐value |
|---|---|---|---|---|
| Total | Age (years) | 0.955 | 0.922–0.990 | 0.011 |
| R0 eesection | 14.766 | 5.591–38.998 | <0.001 | |
| MK stage | 10.273 | 2.285–46.183 | 0.002 | |
| B2 | MK stage | 9.209 | 1.954–43.395 | 0.005 |
| R0 resection | 15.795 | 3.565–69.990 | <0.001 | |
| B2 + B3 | R0 resection | 44.951 | 4.649–434.644 | 0.001 |
| B3 | R0 resection | 37.993 | 4.538–318.085 | 0.001 |
Abbreviation: MK, Masaoka–Koga stage.
DISCUSSION
So far as we are aware, this is the first report to examine the clinicopathological characteristics, treatment, and prognosis of patients with type B2 + B3 thymoma.
In this study, we found that the clinical characteristics of type B2 + B3 thymomas were not statistically different from those of type B2 or B3 thymomas. This included patient gender, average age, Masaoka–Koga stage, tumor size, smoking status, tumor invasion, and the proportion of patients with MG (Table 1). Patients with type B2 or B3 thymoma, whose median age ranged from 51 to 54 years old, be not more likely to be male or female in previous large‐scale retrospective studies. 5 , 8 , 9 Patients in this study, however, ranged in age between 47.1–51.3 years old and had a higher proportion of men (skewed male, 57.8%–59.0%).
However, we did find significant differences in some areas. Based on these two classifications of staging system, we classify stages I and II as early‐stage and stages III and IV as an advanced‐stage in the two staging systems. Consistent with the conclusion of Meurgey et al., 10 we found that the percentage of patients with TNM advanced stage B2 (19.5%), B2 + B3 (25.6%), and B3 (35.9%) increased gradually over time (p = 0.045). Similarly, there was a statistically significant difference between the R0 resection rate of the three subgroups (p = 0.005) (i.e., more patients with early‐stage mean higher R0 resection rate) with the B2 group having the highest R0 resection rate (96.2%), and the B3 group having the lowest R0 resection rate (82.8%). The Masaoka–Koga staging system, on the other hand, showed no such variation. Our findings are partially consistent with previous reports that found no correlation between WHO type and stage (either TNM or Masaoka–Koga) in patients with type B thymoma. 8 , 11 However, neither addressed the specific stage at which the patients with type B2 + B3 thymoma presented. Therefore, we can conclude that type B2 + B3 thymoma can be thought of as intermediate between type B2 and type B3 thymomas, and the number of patients with these TNM advanced‐stage thymomas is gradually increasing. In our opinion, R0 resection should be guided more by the TNM stage.
Several studies have found that the MG rate for type B2 thymoma ranged from 19% to 49%, 8 , 11 while for type B3 thymoma it was 30.5% to 40%. 8 , 12 The percentages found in our study, 45.1% and 29.6%, are comparable to those of other large‐scale studies. 8 , 11 , 12 In our research, the MG rate of type B2 + B3 thymoma was the highest of the three subgroups, at 51.2%. This rate was not intermediate between that of type B2 and type B3 thymoma. There was no statistically significant difference in MG incidence between the groups. Thus, we hypothesized that B2, B2 + B3, and B3 thymoma histotypes are unrelated to MG.
Due to the rarity of thymoma, at present there is no standard treatment protocol. It is typically believed that surgery is the main treatment method, while the selection of supplementary treatment methods is individualized. 13 Currently, radiotherapy is the common choice of adjuvant care, and oftentimes the only option after surgery has failed. Retrospective studies have shown a positive association between postoperative radiotherapy (PORT) and increased overall survival (OS), with the greatest relative benefits seen in patients with Masaoka–Koga stage IIB to IV disease or noncomplete resection. 12 , 14 , 15 Similar to our results (p = 0.237), Kim et al. 16 and Gao et al. 12 observed that the PORT did not affect PFS in patients with type B thymomas, even in the patients with advanced‐stage, p = 0.484 (TNM staging system), p = 0.784 (Masaoka–Koga staging system). We believe that there are two possible hypotheses for explaining this phenomenon. The first thing to note is that as much as 28% of patients in the study by Lim et al. 14 had non‐R0 resections. As a surgical adjunct, PORT can help these patients' conditions be managed more effectively, leading to improved outcomes after surgery. Since the majority of patients in this study underwent R0 resection (92%), the effect of PORT is likely obscured or masked by R0 resection. Furthermore, patients typically require additional treatment after disease progression, so the benefit of OS may come from comprehensive treatment. Although, more systematic large‐scale studies are needed to confirm this hypothesis.
Due to the lack of large‐scale randomized controlled studies, the importance of chemotherapy for thymoma is still uncertain, but it may be proposed for advanced stages or recurrent thymic tumors. 17 , 18 Although there is no standard chemotherapy option, when deciding on first‐line chemotherapy for thymoma, the data suggest that either cisplatin‐anthracycline or cisplatin‐etoposide is the best option, with response rates averaging between 17% and 50%. This finding holds regardless of the treatment line or histological type (thymoma vs. thymic carcinoma). 18 , 19 According to Ma et al., 20 patients with advanced thymoma or thymic carcinoma may have similar long‐term PFS and OS following different first‐line chemotherapy regimens cisplatin, doxorubicin, and cyclophosphamide (CAP); cisplatin and etoposide; and cisplatin and paclitaxel (TP). After chemotherapy, the median PFS was 18.0–34.5 months for patients with Masaoka–Koga stages III and IVA disease, and the 5‐year OS rate was 60.0% to 84.9%; for with Masaoka–Koga stage IVB disease, the median PFS was 8.2–11.6 months, and the 5‐year OS rate was 14.3% to 41.1%. 20
In this series, 34 patients received chemotherapy (both pre‐ and postoperatively), with four patients receiving the cisplatin, doxorubicin, vincristine, and CAP protocol, 14 patients receiving the TP protocol, 12 patients receiving the CAP protocol, and four patients receiving other protocols. The main purpose of patients receiving induction therapy is to increase the possibility of R0 resection. The median PFS of them was 68.0 months; the reason for this difference may be that 32.4% of patients receiving chemotherapy in our study were classified into Masaoka–Koga stage II. Although the chemotherapy group has a lower recurrence rate than the non‐chemotherapy group (22.9% vs. 45.0%), the difference was not statistically significant (p = 0.087). Our result showed that the difference was also absent if R0 resection is selected for advanced patients (11.1% vs. 18.2%, p = 0.517), which was confirmed via previous research. 11 , 19 However, the possibility that resection status plays a function in recurrence is not negated by this result. Our results do not support chemotherapy for type B2, B2 + B3, and B3 thymoma patients based on R0 resection, and it is both unreasonable and inefficient to carry out clinical trials of adjuvant chemotherapy on them. Additionally, immunotherapy and targeted therapy are rarely used, only in a few case reports and small‐scale phase II studies 21 , 22 , 23 , 24 to back them.
Based on our findings, R0 resection may have an impact on the PFS of patients who have type B2 + B3 thymoma (Table 4). Patients with B2 + B3 thymoma did not have a significantly worse PFS compared to those with B2 or B3 thymoma alone. The most significant factors affecting progression across all patients studied were R0 resection, Masaoka–Koga stage, and age. Previous research has suggested that in order to achieve an 80% test efficiency for the three moderate effect factors, at least 75 patients would need to be tested. 25 Thus, it is important to proceed with caution when interpreting the study's findings regarding the prognostic factors of each pathological subgroup. Although previous studies have shown that pathological subtypes have an impact on the prognosis of patients, it is generally believed that there is no difference in the prognosis between B2 and B3 pathological subtypes. 5 , 8 , 26 Our study's finding that PFS is unaffected by pathological subtypes suggests that the prognosis of patients with these three subtypes can be compared without further subgroup analysis.
There is some debate in the literature about whether or not R0 resection has any prognostic value. Most studies agree that R0 resection is one of the most independent prognostic factors for thymoma, 8 , 11 , 12 , 13 , 26 whether OS or PFS. However, some reports have shown that there was no survival difference for patients who underwent complete resection compared with the patients undergoing incomplete resection. 27 Despite the small sample size, our results favor the former conclusion. In our study, we found that applying some induction therapy before surgery increased the likelihood of R0 resection for four patients who underwent it; of these four, three did not experience a recurrence or metastasis during the follow‐up period.
The Masaoka–Koga stage is another critical prognostic factor in our analysis. PFS was significantly lower in the Masaoka–Koga advanced stage group in both univariate analysis and multivariate analysis (Table 4, Figure 3b). The Masaoka–Koga stage and TNM stage are well‐established as independent prognostic factors for thymomas of all types, 8 , 10 , 28 and this is confirmed by studies on type B2 and B3 thymoma. 11 , 12 , 16 In our study, the Masaoka–Koga stage and TNM stage were both found to have a significant impact on PFS in univariate analysis (Figure 3ab), but only the Masaoka–Koga stage was found to have a prognostic significance in multivariate analysis (Table 4). Therefore, we believe that the Masaoka–Koga staging system is a better prognostic indicator than the TNM staging system in type B2 + B3, type B2, and type B3 thymomas.
Since the tumor diameter is not a part of the Masaoka–Koga or TNM staging system for thymomas, as it is for other solid tumors, 5 , 6 researchers have also evaluated the effect this may have on prognosis. In this context, some studies pointed out smaller tumor size was associated with better survival in thymoma patients, showing its potential as an independent prognostic factor. 13 , 29 Recent meta‐analyses, however, have shown that tumor size is not a reliable prognostic indicator. 5 , 9 , 28 Our findings lend credence to the latter theory, that is, tumor size is irrelevant to the prognosis of patients with thymoma of type B2, B3, and B2 + B3.
However, we do not consider our results to be conclusive, and we still have several major issues to address. In the first place, our study had a major flaw because the patient's care was not coordinated with a prospective institutional treatment protocol. Second, it is limited by its retrospective design. In addition, the sensitivity of fine needle aspiration cytology for thymoma was 87%, and its specificity was 94%. 30 Our study's important conclusion was influenced by the fact that fine needle aspiration cytology was used to establish the pathological diagnosis in nine patients. Further, although patients with advanced thymomas received adjuvant treatment, there was no uniformity in the indications for adjuvant therapy, which may have affected the outcomes. Finally, although the mathematical model of the statistical analysis package could take the short follow‐up time of some patients into consideration, the conclusions may still be biased and should be interpreted with caution. All these concerns should be further investigated in future prospective clinical trials.
AUTHOR CONTRIBUTION
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization: Ke Zhao, Yeye Chen, Lei Liu and Jiaqi Zhang. Data curation: Ke Zhao, Xuehan Gao and Ke Rao. Formal analysis: Ke Zhao, Mengxin Zhou and Libing Yang. Investigation: Ke Zhao and Ke Rao. Visualization: Ke Zhao, Guige Wang and Mengxin Zhou. Writing–original draft preparation: Ke Zhao and Mengxin Zhou. Project administration: Yeye Chen, Hongsheng Liu and Shanqing Li. Resources: Yeye Chen, Chao Guo, Ye Zhang and Cheng Huang. Supervision: Yeye Chen, Hongsheng Liu and Shanqing Li. Writing–review and editing: Yeye Chen. Funding acquisition: Shanqing Li.
CONFLICT OF INTEREST STATEMENT
The authors declare no interest conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the National High Level Hospital Clinical Research Funding (2022‐PUMCH‐B‐011) and (2022‐PUMCH‐B‐012). The authors would like to thank Yushang Cui MD, Zhijun Han MD, Li Li MD, Naixin Liang MD, Yingzhi Qin MD for contribution of the treatment for patients in this study. The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Zhao K, Chen Y, Liu L, Wang G, Zhang J, Zhou M, et al. Real‐world study of treatment and outcome of type B2 + B3 thymoma: The neglected part of thymoma. Thorac Cancer. 2023;14(15):1339–1347. 10.1111/1759-7714.14875
REFERENCES
- 1. Shin DW, Cho JH, Ha J, Jung KW. Trends in incidence and survival of patients with thymic epithelial tumor in a high‐incidence Asian country: analysis of the Korean central cancer registry 1999 to 2017. J Thorac Oncol. 2022;17(6):827–37. [DOI] [PubMed] [Google Scholar]
- 2. Marx A, Ströbel P, Badve SS, Chalabreysse L, Chan JK, Chen G, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol. 2014;9(5):596–611. [DOI] [PubMed] [Google Scholar]
- 3. Marx A, Chan JKC, Chalabreysse L, Dacic S, Detterbeck F, French CA, et al. The 2021 WHO classification of tumors of the thymus and mediastinum: what is new in thymic epithelial, germ cell, and mesenchymal tumors? J Thorac Oncol. 2022;17(2):200–13. [DOI] [PubMed] [Google Scholar]
- 4. Marx A, Chan JK, Coindre JM, Detterbeck F, Girard N, Harris NL, et al. The 2015 World Health Organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol. 2015;10(10):1383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weissferdt A, Kalhor N, Bishop JA, Jang SJ, Ro J, Petersson F, et al. Thymoma: a clinicopathological correlation of 1470 cases. Hum Pathol. 2018;73:7–15. [DOI] [PubMed] [Google Scholar]
- 6. Detterbeck FC, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, et al. The IASLC/ITMIG Thymic epithelial tumors staging project: proposal for an evidence‐based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9(9 Suppl 2):S65–72. [DOI] [PubMed] [Google Scholar]
- 7. Koga K, Matsuno Y, Noguchi M, Mukai K, Asamura H, Goya T, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non‐invasive thymoma. Pathol Int. 1994;44(5):359–67. [DOI] [PubMed] [Google Scholar]
- 8. Weis CA, Yao X, Deng Y, Detterbeck FC, Marino M, Nicholson AG, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol. 2015;10(2):367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu H, Gu Z, Qiu B, Detterbeck FC, Roden AC, Ruffini E, et al. A recurrence predictive model for Thymic tumors and its implication for postoperative management: a Chinese Alliance for research in Thymomas database study. J Thorac Oncol. 2020;15(3):448–56. [DOI] [PubMed] [Google Scholar]
- 10. Meurgey A, Girard N, Merveilleux du Vignaux C, Maury JM, Tronc F, Thivolet‐Bejui F, et al. Assessment of the ITMIG statement on the WHO histological classification and of the eighth TNM staging of thymic epithelial tumors of a series of 188 Thymic epithelial tumors. J Thorac Oncol. 2017;12(10):1571–81. [DOI] [PubMed] [Google Scholar]
- 11. Song Z, Jin X, Zhang Y. Treatment and prognosis of type B2 thymoma. World J Surg Oncol. 2014;12:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao L, Wang C, Fang W, Zhang J, Lv C, Fu S. Outcome of multimodality treatment for 188 cases of type B3 thymoma. J Thorac Oncol. 2013;8(10):1329–34. [DOI] [PubMed] [Google Scholar]
- 13. Safieddine N, Liu G, Cuningham K, Ming T, Hwang D, Brade A, et al. Prognostic factors for cure, recurrence and long‐term survival after surgical resection of thymoma. J Thorac Oncol. 2014;9(7):1018–22. [DOI] [PubMed] [Google Scholar]
- 14. Lim YJ, Kim HJ, Wu HG. Role of postoperative radiotherapy in nonlocalized Thymoma: propensity‐matched analysis of surveillance, epidemiology, and end results database. J Thorac Oncol. 2015;10(9):1357–63. [DOI] [PubMed] [Google Scholar]
- 15. Jackson MW, Palma DA, Camidge DR, Jones BL, Robin TP, Sher DJ, et al. The impact of postoperative radiotherapy for Thymoma and Thymic carcinoma. J Thorac Oncol. 2017;12(4):734–44. [DOI] [PubMed] [Google Scholar]
- 16. Kim HK, Choi YS, Kim J, Shim YM, Han J, Kim K. Type B thymoma: is prognosis predicted only by World Health Organization classification? J Thorac Cardiovasc Surg. 2010;139(6):1431–1435.e1. [DOI] [PubMed] [Google Scholar]
- 17. Bluthgen MV, Boutros C, Fayard F, Remon J, Planchard D, Besse B. Activity and safety of oral etoposide in pretreated patients with metastatic or recurrent thymic epithelial tumors (TET): a single‐institution experience. Lung Cancer. 2016;99:111–6. [DOI] [PubMed] [Google Scholar]
- 18. Hirai F, Toyozawa R, Nosaki K, Seto T. Are Anthracycline‐based regimens truly indicated to Be the standard chemotherapy regimen for Thymic carcinoma? J Thorac Oncol. 2016;11(1):115–21. [DOI] [PubMed] [Google Scholar]
- 19. Berghmans T, Durieux V, Holbrechts S, Jungels C, Lafitte JJ, Meert AP, et al. Systemic treatments for thymoma and thymic carcinoma: a systematic review. Lung Cancer. 2018;126:25–31. [DOI] [PubMed] [Google Scholar]
- 20. Ma WL, Lin CC, Hsu FM, Lee JM, Chen JS, Huang YL, et al. Clinical outcomes for patients with thymoma and thymic carcinoma after undergoing different front‐line chemotherapy regimens. Cancer Med. 2022;11(18):3445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sato J, Satouchi M, Itoh S, Okuma Y, Niho S, Mizugaki H, et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol. 2020;21(6):843–50. [DOI] [PubMed] [Google Scholar]
- 22. Rajan A, Carter CA, Berman A, Cao L, Kelly RJ, Thomas A, et al. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: a multicentre, open‐label, phase 2 trial. Lancet Oncol. 2014;15(2):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Remon J, Girard N, Novello S, de Castro J, Bigay‐Game L, Bernabé R, et al. PECATI: a multicentric, open‐label, single‐arm phase II study to evaluate the efficacy and safety of Pembrolizumab and Lenvatinib in pretreated B3‐Thymoma and Thymic carcinoma patients. Clin Lung Cancer. 2022;23(3):e243–6. [DOI] [PubMed] [Google Scholar]
- 24. Shen L, Chen H, Wei Q. Immune‐therapy‐related toxicity events and dramatic remission after a single dose of Pembrolizumab treatment in metastatic Thymoma: a case report. Front Immunol. 2021;12:621858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang J, Detterbeck FC, Wang Z, Loehrer PJ Sr. Standard outcome measures for thymic malignancies. J Thorac Oncol. 2010;5(12):2017–23. [DOI] [PubMed] [Google Scholar]
- 26. Ströbel P, Bauer A, Puppe B, Kraushaar T, Krein A, Toyka K, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol. 2004;22(8):1501–9. [DOI] [PubMed] [Google Scholar]
- 27. Shen S, Ai X, Lu S. Long‐term survival in thymic epithelial tumors: a single‐center experience from China. J Surg Oncol. 2013;107(2):167–72. [DOI] [PubMed] [Google Scholar]
- 28. Tseng YC, Hsu HS, Lin YH, Tseng YH, Shu CW, Goan YG, et al. Does size affect the prognosis of resectable thymoma beyond the eighth edition TNM? Thoracic Cancer. 2022;13(3):346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cangir AK, Yenigün BM, Direk T, Kocaman G, Yücemen U, Kahya Y, et al. Different view on tumor size dilemma in tumor‐node‐metastasis staging system for thymoma. Thorac Cardiovasc Surg. 2021;69(2):148–56. [DOI] [PubMed] [Google Scholar]
- 30. Marcus A, Narula N, Kamel MK, Koizumi J, Port JL, Stiles B, et al. Sensitivity and specificity of fine needle aspiration for the diagnosis of mediastinal lesions. Ann Diagn Pathol. 2019;39:69–73. [DOI] [PubMed] [Google Scholar]


