Abstract
Herein, we report the case of a 73-year-old woman with an occupational history of plaster grinding who developed autoimmune pulmonary alveolar proteinosis (PAP) during the treatment of fibrotic hypersensitivity pneumonitis with steroids and immunosuppressive drugs. Based on the changes in computed tomography imaging findings, poor response to steroid therapy, and markedly elevated KL-6 levels, PAP was suspected and diagnosed by bronchoscopy. Repeated segmental bronchoalveolar lavage under high-flow nasal cannula oxygen therapy resulted in slight improvement. Steroids and immunosuppressive treatments for other interstitial lung diseases may cause PAP or exacerbate latent PAP.
Keywords: Autoimmune pulmonary alveolar proteinosis, Fibrotic hypersensitivity pneumonia, Segmental bronchoalveolar lavage, Anti-GM-CSF antibody
Highlights
-
•
PAP is characterized by accumulation of surfactants and phospholipids in the alveoli.
-
•
A quarter of autoimmune PAP have a history of dust exposure.
-
•
PAP may be revealed or exacerbated by immunosuppressive therapy.
Grants
None.
1. Introduction
Pulmonary alveolar proteinosis (PAP) is a rare disease characterized by alveolar accumulation of surfactant owing to defective surfactant clearance by alveolar macrophages [1]. Secondary PAP due to immunosuppressive has also been reported [2]. This report describes the case of an elderly woman who developed autoimmune PAP during treatment with prednisolone (PSL) and cyclosporine for fibrotic hypersensitivity pneumonia (HP), which was diagnosed by repeated bronchoscopic examination without overlooking changes in imaging findings.
2. Case presentation
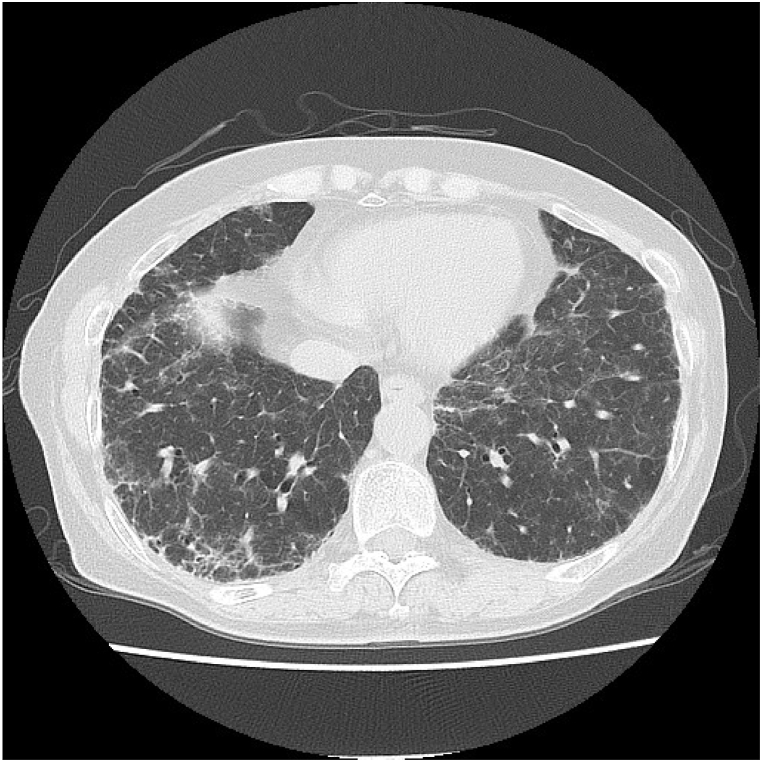
A 73-year-old woman with hypertension and dyslipidemia had persistent cough for 5 years and dyspnea on exertion for 3 years. Two years earlier, chest radiography conducted during a medical checkup revealed abnormal shadows, and she visited a hospital. She was taking angiotensin II receptor blockers and statins, but no other medications such as herbal medicines or health supplements. She had no history of smoking and had been engaged in plaster grinding for 5 years. She lived in a 26-year-old wooden structure with molds in the bathroom. Her respiratory status was not imminent: respiratory rate, 16 breaths/min; and oxygen saturation, 95% in room air. Chest auscultation revealed fine crackles on her back. Chest radiography revealed decreased permeability with a bilateral predominance in the lower lung fields. Chest computed tomography (CT) revealed ground-glass opacity (GGO), traction bronchiectasis, interlobular wall thickening, and bilaterally diffused reticular shadows predominantly in the lower lobes, without centrilobular nodules (Fig. 1). The CT findings in this case were categorized as an alternative diagnosis in the clinical practice guideline for idiopathic pulmonary fibrosis [3] and were compatible with the fibrotic HP pattern in the clinical practice guideline for hypersensitivity pneumonitis [4].
Fig. 1.
Computed tomography image showing ground-glass opacity, traction bronchiectasis, interlobular wall thickening, and bilateral and diffuse reticular shadows compatible with fibrotic HP.
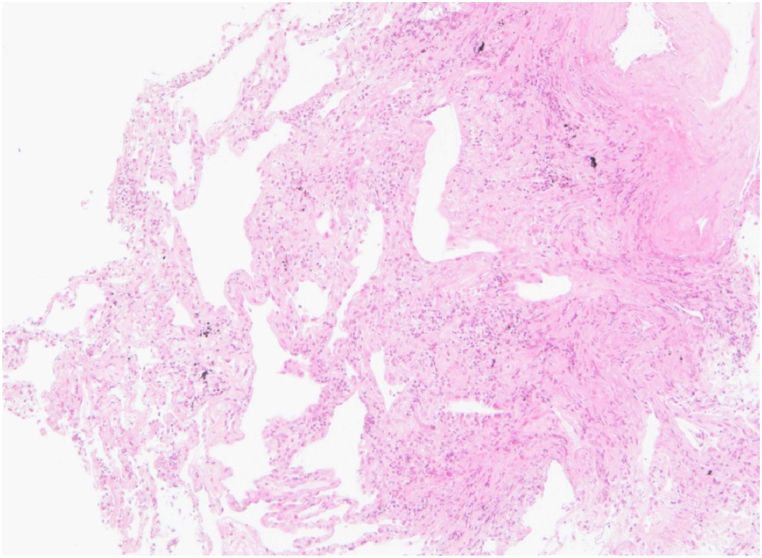
The laboratory findings were as follows: white blood cell count, 8750/μL; hemoglobin, 13.9 g/dL; platelet count, 23.9 × 104/μL; lactate dehydrogenase, 330 U/L; C-reactive protein, 0.07 mg/dL; and Krebs von den Lungen 6 (KL-6) level, 8758 U/mL. The test for anti-Trichosporon asahii antibodies gave positive results. The respiratory function tests results were as follows: forced vital capacity (FVC), 2.1L; %FVC, 89.7%; and, % diffusion capacity for carbon monoxide (DLco), 68.4%. The patient underwent bronchoalveolar lavage (BAL) and transbronchial lung biopsy (TBLB). The color of the BAL fluid (BALF) was normal and the cellular pattern comprised 54.4% lymphocytes, 40.3% macrophages, and 5% neutrophils. The CD4/8 ratio was 6.9. TBLB revealed lymphocytic infiltration, mild thickening due to fibrosis, and hyperplasia of type II pneumocytes in the alveolar wall (Fig. 2). No obvious granulomatous lesions or airway-centered fibrosis was observed.
Fig. 2.
Hematoxylin and eosin stain of TBLB showing lymphocytic infiltration, mild thickening due to fibrosis and hyperplasia of type II pneumocytes in the alveolar wall.
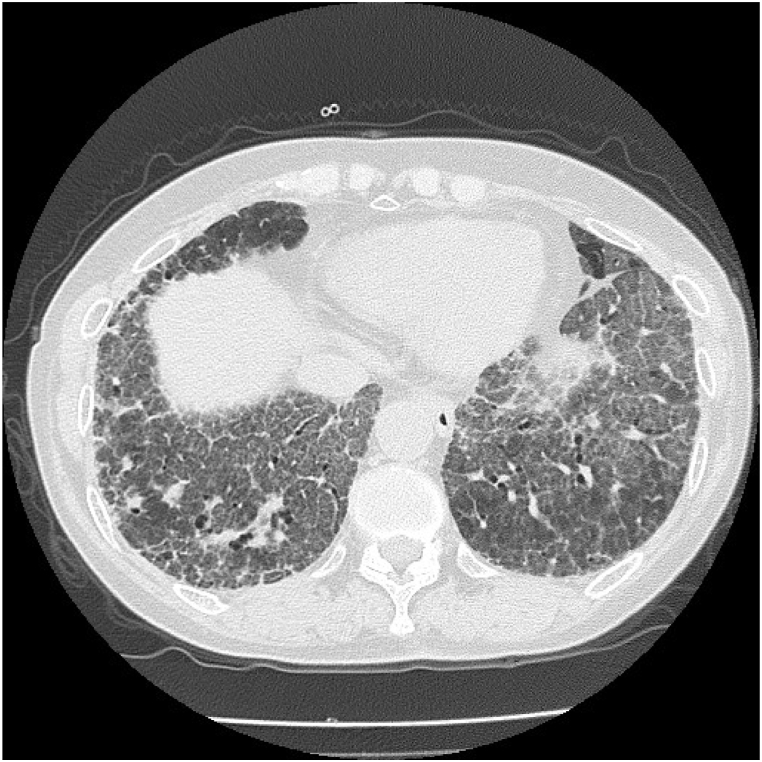
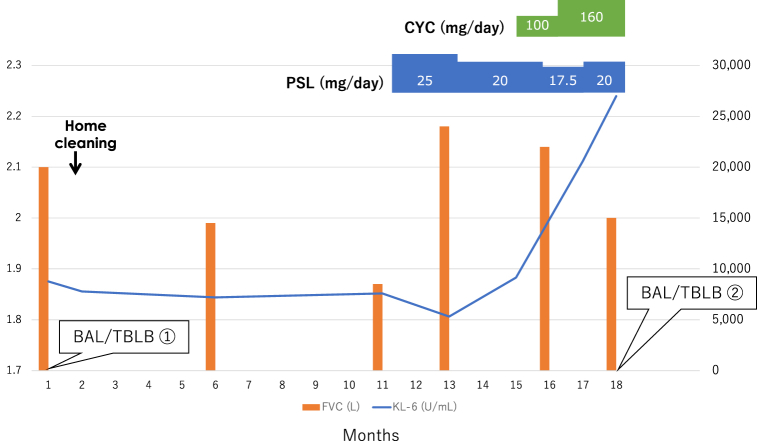
The moderate-confidence diagnosis of HP was based on CT findings, BALF findings, positive results for anti-Trichosporon asahii antibodies, and histopathology in the clinical practice guideline of hypersensitivity pneumonitis [4]. The patient was asked to clean her home; however, 9 months later, a respiratory function test showed a decreased FVC at 1.87 L and her chest CT showed worsening GGO despite a slight decline in the level to 7594 U/mL. The patient was diagnosed with HP exacerbation and treatment was started with oral prednisolone (PSL) at 0.5mg/kg/day (25mg/day). Two months later, when FVC improved to 2.18 L and KL-6 dropped to 5317 U/mL, FVC did not change considerably, but the KL-6 level increased again to 9141 U/mL. Considering it to be an exacerbation of HP that could be controlled by PSL, cyclosporine was additionally administered, and the PSL dosage was gradually decreased. Three months later, dyspnea on exertion worsened, the chest CT showed GGO spreading throughout the lung field with marked thickening of the interlobular septum, resulting in a so-called crazy-paving appearance (Fig. 3), and the KL-6 level increased to 26,974 U/mL (Fig. 4).
Fig. 3.
Computed tomography showing ground-glass opacity spreading throughout the lung field, with marked thickening of the interlobular septum, resulting in a so-called crazy-paving appearance.
Fig. 4.
Patient's clinical course. After initiating prednisolone, FVC temporarily increased, and the KL-6 level decreased. Subsequently, FVC decreased and the KL-6 level increased markedly. PSL; prednisolone, CYC; cyclosporine.
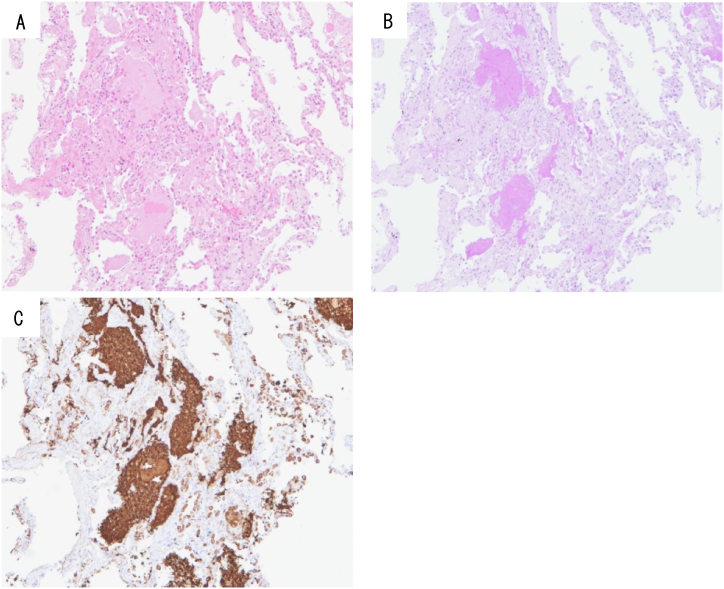
BAL was performed again, cloudy white BALF was collected, and the cellular pattern comprised 8.0% lymphocytes, 50.0% macrophages, and 42.0% neutrophils. The CD4/8 ratio was 0.5. TBLB showed an alveolar space was filled with acidophilic, unstructured material on hematoxylin and eosin staining (Fig. 5A), which tested positive for granules on Periodic acid-Schiff stain (Fig. 5B) and also on Surfactant apoprotein A staining (Fig. 5C).
Fig. 5.
Hematoxylin and eosin stain of TBLB showing the alveolar space filled with acidophilic, unstructured material (A). Periodic acid–Schiff staining (B) and surfactant apoprotein A staining (C) also gave positive results.
AS the test for anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) antibodies returned positive results at 35.5 U/mL (cut-off value, 1.0 μg/mL), autoimmune PAP was diagnosed. The patient was transferred to our hospital for whole-lung lavage. However, as we had no experience with this technique and it was difficult for her to visit a distant hospital during the COVID-19 pandemic, she underwent segmental BAL under high-flow nasal cannula oxygen therapy repeatedly. Subsequently, her respiratory status and chest CT findings showed slight improvements. Subsequently, when the steroids and cyclosporine dosages were reduced and discontinued, the fibrotic changes were exacerbated. She was treated with nintedanib for progressive fibrosing interstitial lung disease, which was thought to be an exacerbation of fibrotic HP.
3. Discussion
PAP has been reported to develop after inhalation of mineral particles, metal particles, or more rarely organic particles [1]. In a Japanese cohort, 26% of patients with anti-granulocyte macrophage-colony stimulation factor (GM-CSF) antibodies autoimmune PAP were considered exposed to dust inhalation [5]. The patient had a history of working in plaster grinding for the past five years, which may have been an indicator of PAP.
HP was diagnosed based on the positive result for anti-Trichosporon asahii antibody with high sensitivity and specificity, consistent chest CT findings, and BAL findings; however, the poor response to steroids, markedly elevated KL-6 levels, and changes in imaging findings led to a suspicion of PAP development. At the onset of PAP, cyclosporine was administered in addition to steroids, which might have aggravated the disease. In a retrospective cohort study of 31 cases of autoimmune PAP, Akasaka et al. reported that corticosteroid therapy may worsen disease severity and increase the risk of infection because corticosteroids suppress alveolar macrophage function [6]. PAP occurred in a renal transplant recipient receiving cyclosporine and mycophenolate [2], and the addition of cyclosporine may have contributed to worsening of the disease in this case as well.
The initial chest CT showed GGO, traction bronchiectasis, and reticulation in subpleural areas, which could be defined as a “compatible with HP” pattern in the clinical practice guideline of hypersensitivity pneumonitis [4]. Other differential diagnosis included nonspecific interstitial pneumonia (NSIP), Pneumocystis jirovecii pneumonia, and drug-induced pneumonia; however, PAP was not strongly suspected. Subsequently, GGO became bilaterally more extensive, interlobular septal thickening was noticeable, and PAP was most suspected at this point. A chest CT scan is important for diagnosing PAP and typically shows GGO superimposed over the smooth thickening of the interlobular and intralobular septal lines resulting in a “crazy-paving” appearance [7]. A crazy-paving appearance is not diagnostic for PAP, but it is evocative enough to perform BAL.
The marked elevation of the KL-6 level, which was major indicator of PAP, is considered significant compared to that in other interstitial pneumonias [1]. In a large cohort of Japanese patients with autoimmune PAP, the KL-6 levels were correlated with disease severity [5]. The KL-6 level in the most severe group was remarkably high (median, 15,100 U/mL). The levels were similarly elevated in the present case (26,974 U/mL), but this cannot be directly assumed to be indicative of PAP because the elevation was originally due to HP. The serum KL-6 level is elevated in 70–100% of patients with various ILDs, such as idiopathic interstitial pneumonias, HP, and PAP [8]. Mostafa et al. showed that the median serum KL-6 level in fibrotic HP was 1200 U/mL [9]. The initial value of 8750 U/mL in the present case was relatively high for HP, suggesting that the patient may have had a predisposition to PAP.
The pathological analysis of the first TBLB showed alveolitis with lymphocytic infiltration and thickening of the alveolar septa with fibrosis. No acellular eosinophilic material was observed in the alveolar space. The color of the BALF was not milky, and the onset of PAP was not evident at this point.
There was no pathological evidence of PAP and no characteristic findings on chest CT at the onset of disease, but the high KL-6 level suggests that the patient was originally predisposed to PAP and that steroids and cyclosporine administered for HP may have triggered the onset of PAP.
Several patients who received steroids for some interstitial lung diseases, such as organizing pneumonia or anti-aminoacyl-tRNA synthetase (anti-ARS) antibody-positive interstitial pneumonia, subsequently developed PAP [10,11]. In particular, a case of anti-ARS antibody-positive interstitial pneumonia was misdiagnosed and treated with steroids and immunosuppressive agents, even though the patient had PAP from the beginning. PAP can be misdiagnosed as interstitial lung disease due to its similar presentation [11].
In this case, we hypothesized the following three mechanisms for PAP development: 1) latent PAP became apparent with immunosuppressive treatment; 2) PAP, not HP, was present from the beginning and became apparent with immunosuppressive treatment; and 3) HP was present at the beginning and PAP developed secondary to immunosuppressive treatment. Regarding the first mechanism, initial steroid treatment temporarily improved the disease, but the subsequent exacerbation suggested that latent PAP became apparent; however, the findings of the first BAL and TBLB do not support this. As for the second mechanism, it cannot be ruled out that PAP had developed from the beginning because the initial KL-6 value was very high for HP, and the chest CT showed a non-specific but extensive GGO. However, this was ruled out based on the positive result for anti-Trichosporon asahii antibodies, the lack of CT imaging findings characteristic of PAP (typically a crazy-paving appearance) on the initial images, the temporary but positive effect of steroids, exacerbation of fibrosis after the discontinuation of steroids, and no suggestive findings in BAL and TBLB. Finally, we considered the third mechanism to be the most plausible one based on the obvious changes in the imaging findings, BAL, and TBLB findings, and the marked increased in the KL-6 level.
In conclusion, recognizing findings that are suspicious for PAP, such as changes in imaging findings, exacerbation on steroid administration, and abnormally high KL-6 levels, is critical in diagnosing PAP that develops following other pulmonary diseases.
4. Conclusion
-
•
PAP is characterized by accumulation of surfactants and phospholipids in the alveoli.
-
•
A quarter of autoimmune PAP have a history of dust exposure.
-
•
PAP may be revealed or exacerbated by immunosuppressive therapy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Handling Editor: DR AC Amit Chopra
Contributor Information
Maki Asami-Noyama, Email: noyamama@yamaguchi-u.ac.jp.
Kosuke Ito, Email: shoji_dayo_ito_chigauyo@yahoo.co.jp.
Misa Harada, Email: harada.1993.misa@gmail.com.
Yukari Hisamoto, Email: yukari727@yamaguchi-u.ac.jp.
Yoshie Kunihiro, Email: kyoshie@yamaguchi-u.ac.jp.
Eiji Ikeda, Email: ikedae@yamaguchi-u.ac.jp.
Tasuku Yamamoto, Email: t.yamamoto.93@gmail.com.
Junki Suizu, Email: relativity.theory135@gmail.com.
Ayumi Fukatsu, Email: chiku05@yamaguchi-u.ac.jp.
Syuichiro Ohata, Email: j015ebponyou@gmail.com.
Yoriyuki Murata, Email: ymurata-ygc@umin.ac.jp.
Keiji Oishi, Email: ohishk@yamaguchi-u.ac.jp.
Yoshikazu Yamaji, Email: yyamaji@yamaguchi-u.ac.jp.
Nobutaka Edakuni, Email: edakuni@yamaguchi-u.ac.jp.
Tomoyuki Kakugawa, Email: kakugawa@yamaguchi-u.ac.jp.
Tsunahiko Hirano, Email: tsuna@yamaguchi-u.ac.jp.
Kazuto Matsunaga, Email: kazmatsu@yamaguchi-u.ac.jp.
References
- 1.Borie R., Danel C., Debray M.P., et al. Pulmonary alveolar proteinosis. Eur. Respir. Rev. 2011;20(120):98–107. doi: 10.1183/09059180.00001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasan A., Ram R., Swamy T. Pulmonary alveolar proteinosis due to mycophenolate and cyclosporine combination therapy in a renal transplant recipient. Lung India. 2014;31(3):282–284. doi: 10.4103/0970-2113.135782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G., Remy-Jardin M., Myers J.L., et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G., Remy-Jardin M., Ryerson C.J., et al. Diagnosis of hypersensitivity pneumonitis in adults. An official ATS/JRS/ALAT clinical practice guideline [published correction appears in. Am. J. Respir. Crit. Care Med. 2021 Jan 1;203(1):150–151. doi: 10.1164/rccm.202005-2032ST. ] [published correction appears in Am J Respir Crit Care Med. 2022 Aug 15;206(4):518]. Am J Respir Crit Care Med. 2020;202(3):e36-e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue Y., Trapnell B.C., Tazawa R., et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am. J. Respir. Crit. Care Med. 2008;177(7):752–762. doi: 10.1164/rccm.200708-1271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akasaka K., Tanaka T., Kitamura N., et al. Outcome of corticosteroid administration in autoimmune pulmonary alveolar proteinosis: a retrospective cohort study. BMC Pulm. Med. 2015;15:88. doi: 10.1186/s12890-015-0085-0. 2015 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jouneau S., Ménard C., Lederlin M. Pulmonary alveolar proteinosis. Respirology. 2020;25(8):816–826. doi: 10.1111/resp.13831. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa N., Hattori N., Yokoyama A., Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig. 2012;50(1):3–13. doi: 10.1016/j.resinv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Mostafa A.I., Salem A.E., Ahmed H.A.M., Bayoumi A.I., Halim R.M.A., Samie R.M.A. Role of Krebs von den Lungen-6 (KL-6) in Assessing Hypersensitivity Pneumonitis. Tuberc. Respir. Dis. 2021;84(3):200–208. doi: 10.4046/trd.2020.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosoda C., Saito K., Fujimoto S., et al. Pulmonary alveolar proteinosis developing during steroid treatment in a patient with organizing pneumonia in association with atypical chronic myeloid leukemia. Clin Case Rep. 2019;7(3):477–481. doi: 10.1002/ccr3.2014. 2019 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishimoto H., Sakamoto N., Yura H., et al. Autoimmune pulmonary alveolar proteinosis exacerbated by steroid therapy due to misdiagnosis as anti-aminoacyl-tRNA synthetase (ARS) antibody positive- interstitial pneumonia: a case report. BMC Pulm. Med. 2022;22(1):120. doi: 10.1186/s12890-022-01909-z. Published 2022 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]