Abstract
Several alphaviruses, including the Sindbis-group viruses, Ross River virus, O'nyong-nyong virus, and Chikungunya virus, are associated with outbreaks of acute and persistent arthralgia and arthritis in humans. Mechanisms underlying alphavirus-induced arthralgia and arthritis are not clearly understood, though direct viral replication within or around the affected joints is thought to contribute to disease. S.A.AR86 is a Sindbis-group alphavirus closely related to the arthralgia-associated Ockelbo and GirdwoodS.A viruses. Following inoculation with S.A.AR86 derived from a molecular clone, infectious virus was isolated from bone and joint tissue 1 to 6 days postinfection. Studies using either in situ hybridization or S.A.AR86-derived double promoter viruses and replicons expressing green fluorescent protein localized sites of viral replication to the periosteum, tendons, and endosteum within the epiphyses of the long bones adjacent to articular joints. These results demonstrate that alphaviruses associated with arthralgia in humans replicate within bone-associated connective tissue adjacent to articular joints in an adult mouse model.
Several Old World alphaviruses, including the Sindbis-group viruses, Ross River virus, O'nyong-nyong virus, and Chikungunya virus, are associated with outbreaks of acute and persistent arthritis and arthralgia in humans (reviewed in reference 10). Chikungunya and O'nyong-nyong viruses have caused massive epidemics of acute, debilitating arthralgia in Africa and Asia (reviewed in reference 10). Ross River virus, the etiologic agent of epidemic polyarthritis, is endemic to Australia (2, 10, 28), and it caused a major epidemic that swept the South Pacific islands in 1979, affecting 50,000 people on the island of Fiji (1). Sindbis-group alphaviruses, including Ockelbo virus, Karelian fever virus, and GirdwoodS.A. virus, are associated with acute and persistent arthralgia in northern Europe and South Africa (14, 26, 29). Ockelbo disease, one of the best characterized of the Sindbis-group alphavirus arthralgias, is often incapacitating (10, 29), and one study found that symptoms lasted for months to years in 31% of patients [(B. Niklasson and Å. Espmark, Letter, Lancet i:1039–1040, 1986). Symptoms include arthralgia in one or more joints, including large joints such as the knee, hip, and elbow (reviewed in references 10 and 29). Pain within or around tendons is also a common trait of Sindbis-group virus infections (reviewed in reference 10). Rubella virus, another member of the family Togaviridae that is distantly related to the alphaviruses, is also associated with acute and persistent arthritis in humans (reviewed in reference 33).
Mechanisms underlying togavirus-induced arthralgia and arthritis are not clearly understood, though direct viral replication within or around the affected joints may contribute to disease (reviewed in reference 29). Ross River virus antigen has been detected in cell aspirates from the joints of acutely infected individuals (6). Furthermore, patients suffering from persistent arthralgia following Ockelbo virus infection often have high levels of Ockelbo virus-specific immunoglobulin M, which suggests that the virus may persistently infect these individuals (19).
Understanding of the mechanisms leading to alphavirus-mediated arthritis and arthralgia in humans has been hampered by the lack of a small-animal model. Sindbis-group alphaviruses, Semliki Forest virus, and Ross River virus replicate in bone-associated connective tissue in neonatal mice, but skin and muscle are also major sites of viral replication (13, 17, 18, 30). Furthermore, infection of neonatal animals with these viruses results in rapidly fatal disease (13, 17, 18, 30). This generalized pattern of replication and lethal outcome in neonatal mice has limited the usefulness of mice as a model of bone and/or joint replication by arthralgia-associated alphaviruses. Since most Sindbis-group viruses do not cause lethal disease in adult mice following peripheral inoculation, experiments were performed to evaluate whether bone and/or joint tissue was a target of Sindbis-group virus infection in adult mice. These experiments were facilitated by the use of S.A.AR86, a Sindbis-group virus isolated in South Africa (32). Sequence comparisons demonstrate that S.A.AR86 is most closely related to GirdwoodS.A. virus, which was isolated from a South African patient suffering from arthralgia (14, 20, 25), and Ockelbo virus, the etiologic agent of Ockelbo disease (24, 26). Full-length infectious cDNA clones of S.A.AR86 are also available (25). This permits maintenance of the viral genome without the introduction of attenuating mutations from repeated passage in cell culture (12), as well as the construction of S.A.AR86-based expression vectors.
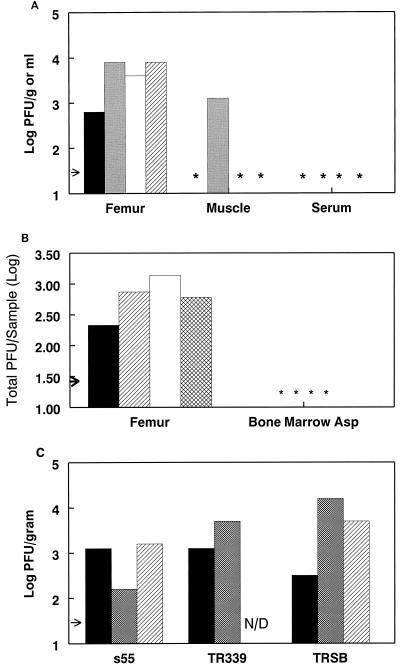
Six-week-old female CD-1 mice (Charles River Breeding Laboratories, Raleigh, N.C.) were infected intravenously (i.v.) with 103 PFU of virus derived from the wild-type S.A.AR86 molecular clone ps55 (25). Three days postinfection mice were sacrificed and serum was harvested, while the quadriceps muscles and the femurs (including the knee and hip joints) were removed and placed in phosphate-buffered saline (PBS) supplemented with 1% donor calf serum (Gibco BRL, Grand Island, N.Y.). Muscle tissue was frozen and thawed before homogenization using a mortar and pestle (Kontes Glass Company, Vineland, N.J.), while calcified tissue was crushed using sterile pliers before freeze-thaw. The tissue homogenate was then clarified by centrifugation and assayed for infectious virus by plaque assay on BHK-21 cells (ATCC CRL 8544) as previously described (23). Virus was consistently isolated from the femurs of infected animals (Fig. 1A). Infectious virus could be isolated from bone and/or joint tissue as early as 24 h and up to 6 days postinoculation (data not shown). Infectious virus was also detectable in femurs with similar kinetics following intraperitoneal and subcutaneous inoculation (data not shown). The possibility that the virus detected within the bone and/or joint tissue was actually due to contamination from serum or surrounding muscle was addressed by measuring virus levels in these tissues. Serum viremia peaked by 24 to 48 h after i.v. S.A.AR86 infection (data not shown) and fell below the limit of detection by 72 h postinoculation (Fig. 1A). Furthermore, comparison of viral titers from perfused and nonperfused animals found no difference in virus levels within the femur on day 3 postinfection (data not shown). Since muscle is a site of Sindbis-group virus replication in suckling mice (13, 30), the level of viral replication within the quadriceps was measured. Unlike with neonatal mice, infectious virus was not consistently isolated from adult mouse muscle tissue (Fig. 1A). This suggests that the infectious virus isolated from the femur was due to direct viral replication within bone and/or joint tissue rather than contamination by virally infected muscle tissue.
FIG. 1.
S.A.AR86, as well as other Sindbis-group alphaviruses, replicates in bone- and joint-associated tissues. (A) Six-week-old female CD-1 mice were infected with 103 PFU of s55 i.v. Three days postinfection mice were sacrificed, exsanguinated, and perfused with PBS, and their femurs and quadriceps muscles were removed by dissection and titrated for infectious virus on BHK-21 cells. Data are shown as log PFU per gram of tissue for femur and muscle and as PFU per milliliter for serum. Each bar represents a single animal. The arrow indicates the limit of detection, and asterisks denote samples below the limit of detection. Data shown are from one of four experiments. (B) Six-week-old female CD-1 mice were infected i.v. with 103 PFU of s51 and sacrificed 3 days postinfection, and right femurs were removed for virus titration. Bone marrow was aspirated from the diaphyses of the femurs using 0.4 ml of PBS–1% donor calf serum per femur. Aspirates were freeze-thawed and titrated for infectious virus by plaque assay. Following marrow aspiration, the remaining femoral tissue was processed as for panel A and titrated for infectious virus by plaque assay. Titer is shown as total PFU per marrow aspirate (Asp) or femur (without aspirated marrow), with each bar representing a single animal. The arrow indicates the limit of detection, and asterisks denote samples with titers below the limit of detection. Shown is one of three comparable experiments. (C) Six-week-old female CD-1 mice were infected i.v. with 103 PFU of the virus s55, TR339, or TRSB. Three days postinfection mice were sacrificed and both femurs were removed for virus titration. Femurs were processed and titrated for infectious virus as for panel A. Each bar represents results from a single animal. The arrow indicates the limit of detection. N/D, not done. Shown is one of two comparable experiments.
Additional experiments were performed to determine whether bone marrow or calcified and fibroblast connective tissue was the site of virus replication within the hind limb. Adult female CD-1 mice were infected i.v. with 103 PFU of virus derived from the S.A.AR86 molecular clone ps51, which differs from the wild-type ps55 clone by a single coding change (nsP1 Thr 538 to Ile). Three days postinfection the femurs, including the hip and knee joints, were removed and the bone marrow was aspirated and titrated for infectious virus by plaque assay. The remaining tissue, including the diaphysis (barrel) of the femur and the knee and hip joints, was also titrated. Viral titers were at or below the limit of detection in bone marrow aspirates, while the bone and joint tissues had significant levels of virus (Fig. 1B). Comparable results were obtained following infection with virus derived from clone ps55, and no difference in replication within the femur was observed between these two viruses (data not shown). These results suggest that cells tightly associated with calcified or fibroblast connective tissue were the main targets of virus replication.
Sequence and serologic comparisons have grouped S.A.AR86 with GirdwoodS.A. virus and Ockelbo virus, both of which are associated with arthralgia in humans (14, 20, 24, 25, 26). Other Sindbis-group alphaviruses, such as the viruses derived from the AR339 isolate, which are not associated with human disease, are more distantly related (reviewed in reference 10). Therefore, experiments were performed to evaluate whether replication within bone and joint tissues was specific for the S.A.AR86/GirdwoodS.A./Ockelbo virus subgroup or whether it was a general characteristic of Sindbis-group alphaviruses. Six-week-old female CD-1 mice were infected i.v. with 103 PFU of the virus derived from clone ps55 or the cloned AR339-like Sindbis-group viruses TR339 (12, 16) and TRSB (16). Three days postinfection hind limbs were removed and femurs, including the knee and hip joints, were evaluated for the presence of infectious virus by plaque assay. s55, TR339, and TRSB replicated to similar levels within bone and joint tissues (Fig. 1C), suggesting that replication within bone and joint tissues is a general characteristic of Sindbis-group alphaviruses rather than a specific attribute of S.A.AR86.
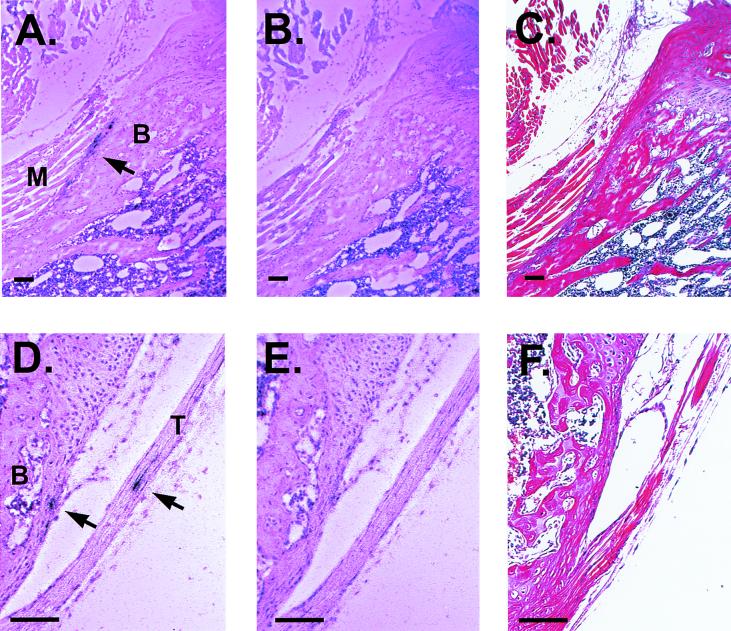
Crude fractionation studies demonstrated that the majority of infectious S.A.AR86 was within calcified or fibroblast connective tissue of the femur or joints, while little virus was in the bone marrow aspirates (Fig. 1B). In order to confirm that bone and/or joint tissue was a target of S.A.AR86 infection and to identify the specific sites of replication within these tissues, in situ hybridization using 35S-labeled riboprobes specific for S.A.AR86 was performed. Mice were infected i.v. with either 103 or 2.5 × 105 PFU of s51, the virus derived from clone ps51, or mock infected with diluent alone and sacrificed at 24 or 48 h postinfection. At the time of sacrifice, mice were perfused with 4% paraformaldehyde in PBS (pH 7.4) prior to decalcification and in situ hybridization. Paraffin-embedded limb sections were probed using either a riboprobe complementary for S.A.AR86 nucleotides 7371 to 7816 or a control riboprobe specific for the influenza virus strain PR/8 hemagglutinin (HA) as described previously (9). S.A.AR86-specific in situ signal was observed in the periosteum and tendons of the long bones adjacent to the joints in mice infected with either dose of virus. Consistent with virus titration results (Fig. 1A), S.A.AR86-specific in situ signal was rarely found in muscle tissue. Replication was also restricted to the epiphyses (ends) of the long bones adjacent to the joints, while in situ signal was not observed in the diaphyses of the long bones. Specific signal was not observed in periosteum or tendons from mock-infected mice probed with the S.A.AR86-specific riboprobe or in tissues from infected mice probed with the influenza virus HA-specific riboprobe (Fig. 2 and data not shown). Similar results were obtained for in situ hybridization of limb sections from mice infected with s51 by the intracranial route (data not shown). Although in situ signal was not observed within the synovial membrane, signal was observed in periosteum and tendons immediately adjacent to the synovial cavity (Fig. 2D). In addition to the periosteum and tendons, in situ signal was observed in the endosteum within the epiphyses of the long bones (data not shown). However, since this signal involved single infected cells, and since the smaller spaces within the marrow cavities might lead to nonspecific probe binding, we were interested in confirming that the endosteum was truly a site of viral replicon.
FIG. 2.
S.A.AR86 replication within the bone-associated connective tissue of the epiphyses (ends) of the long bones. Four- to six-week-old female CD-1 mice were infected i.v. with 103 or 2.5 × 105 PFU of s51 or mock infected. Mice were sacrificed at 24 or 48 h postinfection. Following decalcification, 5-μm-thick paraffin-embedded limb sections were probed with 35S-labeled riboprobes specific for S.A.AR86 or influenza virus strain PR/8 HA. (A) S.A.AR86-specific in situ signal in periosteum of the tibia 24 h after infection with 2.5 × 105 PFU of s51 i.v. M, muscle; B, bone. (B) Adjacent section probed with influenza virus-specific riboprobe. (C) Adjacent section, hematoxylin and eosin staining. (D) S.A.AR86-specific in situ signal in the tendon and periosteum of the tibia proximal to the knee joint 24 h after infection with 2.5 × 105 PFU of s51 i.v. T, tendon; B, bone. (E) Adjacent section probed with influenza virus-specific riboprobe. (F) Adjacent section, hematoxylin and eosin staining. Bar = 100 μm in each panel.
To confirm the in situ hybridization results, mice were infected with S.A.AR86-based vectors driving expression of green fluorescent protein (GFP). GFP expression provides a sensitive method of detecting infected cells both in vivo and in vitro (31). GFP is extremely stable, tolerates the decalcification conditions used in making sections from bone and joint tissues (K. Bernard and R. E. Johnston, unpublished data), and provides a sensitive method for detecting virally infected cells in calcified tissues.
The first type of viral vector used was an S.A.AR86-based double promoter vector, which was constructed by placing a second S.A.AR86 subgenomic promoter immediately upstream of the s55 3′ untranslated region, as described previously for other alphaviruses (reviewed in reference 7). This virus, s55-gfp(F), expressed the mut2 GFP gene (kindly provided by Stanley Falkow, Stanford University). The original GFP gene was subsequently replaced with the enhanced GFP (Clontech, Palo Alto, Calif.) to create the clone ps55-gfp. This double promoter virus was placed on the s51 background to create the clone ps51-gfp. Clone ps51-gfp has a single coding change (nsP1 Thr 538 to Ile), which resulted in a 5- to 10-fold increase in GFP expression in BHK-21 cells compared to virus derived from clone ps55-gfp (M. T. Heise, D. A. Simpson, and R. E. Johnston, unpublished data). GFP expression by the viruses was stable in vivo, since 100% of plaques isolated from bone and joint tissues at 3 days postinfection were GFP positive (data not shown).
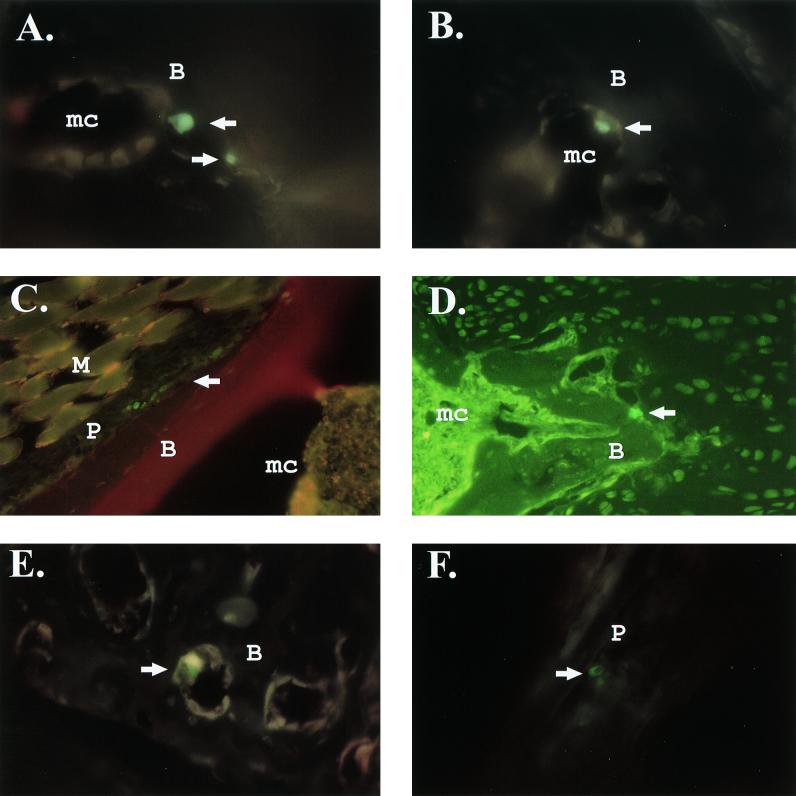
Adult CD-1 mice were infected i.v. with 103 PFU or 1 × 106 to 3 × 106 PFU of GFP-expressing double promoter virus. The 103 dose was chosen for direct comparison to studies with s55 or s51 infection (Fig. 1). However, the double promoter viruses were attenuated for replication in vivo compared to s55 and s51. Following infection with 103 PFU of s51-gfp or s51, the double promoter virus produced titers within the femur and knee joints that were 10-fold lower than those for s51 at 24 h postinfection. When the dose of s51-gfp was increased to 106 PFU, viral titers within the femur were equal to or higher than those observed following infection with 103 PFU of s51 (data not shown). Therefore, additional experiments were performed in which 4-week-old female CD-1 mice were infected with 1 × 106 to 3 × 106 PFU of s51-gfp i.v. Since s55 and s51 replicate equally well in bone and joint tissues (Fig. 1), most in vivo experiments were performed using s51-gfp due to its higher level of GFP expression. However, initial studies using the 103 dose were performed with the clone ps55-gfp(F). Mice were sacrificed at 12 to 14 h or 3 days postinfection, exsanguinated, and perfused with 4% paraformaldehyde in PBS (pH 7.3), and hind limbs were decalcified. Frozen sections were then prepared from the decalcified hind limbs. GFP-positive cells were observed within hind-limb sections from animals that received either low doses of s55-gfp(F) or high doses of s51-gfp, but not in mock-infected animals. GFP-positive cells localized to the same areas with either dose of virus, though more GFP-positive cells were observed with the high dose. Consistent with the in situ hybridization studies, GFP-positive cells localized to bone-associated connective tissue following infection with either low (Fig. 3A and B) or high (Fig. 3C and D) doses of double promoter virus. Furthermore, the GFP-positive cells localized to both the endosteum and periosteum of the epiphyses of the long bones adjacent to the joints. The periosteum was a major site of infection, with clusters of GFP-positive cells readily observable adjacent to bone (Fig. 3C).
FIG. 3.
S.A.AR86-based double promoter viruses and replicons infect cells within the endosteum and periosteum. Four-week-old female CD-1 mice were infected i.v. with 103 PFU of the double promoter virus s55-gfp(F) (A and B), 1.5 × 106 PFU of the double promoter virus s51-gfp (C and D), or 2 × 106 infectious units of the replicon REP91-gfp (E and F). Mock-infected mice received PBS diluent alone. Mice infected with the 103 dose of s55-gfp(F) were sacrificed 3 days postinfection, while mice receiving high doses of s51-gfp or replicon were sacrificed at 12 to 14 h postinfection. Following sacrifice, mice were perfused with 4% paraformaldehyde, and hind limbs were decalcified before preparation of frozen sections. GFP-positive cells were visualized by fluorescent microscopy. (A and B) GFP positive cells within the endosteum of an s55-gfp(F)-infected mouse. Magnification, ×600; triple-pass fluorescein isothiocyanate (FITC)-Texas red filter. (C) GFP-positive cells within the periosteum of a mouse infected with 1.5 × 106 PFU of s51-gfp. Red staining indicates the presence of type I collagen in calcified tissue, which was identified using anti-mouse type I collagen (Rockland). Magnification, ×400; triple-pass FITC-Texas red filter. (D) GFP-positive cell adjacent to calcified tissue in an s51-gfp-infected mouse. Magnification, ×400; FITC filter. (E) GFP-positive cell within the endosteum of a REP91-gfp-infected mouse. Magnification, ×400; triple-pass FITC-Texas red filter. (F) GFP-positive cells within the periosteum of a REP91-gfp-infected mouse. Magnification, ×400; triple-pass FITC-Texas red filter. Abbreviations: B, bone; M, muscle; P, periosteum; mc, marrow cavity.
In addition to double promoter viruses, S.A.AR86-based replicons expressing GFP were used to identify sites of S.A.AR86 infection within bone and/or joint tissue. Alphavirus-derived replicon RNA is packaged with the viral structural proteins provided in trans by helper RNAs. This results in the production of replicon particles that exhibit the same coat proteins as the parental virus, but they are able to undergo only a single round of replication (reviewed in reference 7). Therefore, GFP expression by the replicon can be used to identify the initial cellular targets of viral replication within a given tissue. An S.A.AR86-based replicon containing the s51 nonstructural genes and driving expression of GFP in place of the viral structural genes was constructed and designated REP91-gfp. A glycoprotein helper plasmid was engineered to produce a helper RNA containing the S.A.AR86 26S subgenomic promoter driving expression of a fusion protein consisting of the first 74 amino acids of capsid, a 2-amino-acid linker, 17 amino acids of the foot-and-mouth-disease virus 2A protease (15), and the viral glycoproteins. The capsid RNA sequence permits efficient translation of the fusion protein (8), while the foot-and-mouth-disease virus 2A protease cleaves itself and the capsid fragment from the E3 glycoprotein to allow glycoprotein maturation, as shown previously with Semliki Forest virus-derived replicons (27). The capsid helper was similar to those described for other alphavirus replicon-packaging systems (reviewed in reference 7). In vitro-transcribed RNA from pREP91-gfp, the capsid helper pCAP86, and the glycoprotein helper pHelp102 was prepared using mMessage mMachine SP6 in vitro transcription kits (Ambion, Austin, Tex.) and introduced into BHK-21 cells by electroporation (22). Before the replicon stocks were used in vivo, they were evaluated by serial passage of 10 to 20% of the replicon stock on BHK-21 cells that were examined for cytopathic effect from propagation-competent recombinant virus. If free of cytopathic effect after two passages, replicon particle stocks were considered to be usable for experimental purposes.
Mice were infected i.v. with 1 × 106 to 2 × 106 infectious units of REP91-gfp. Since REP91-gfp is able to undergo only a single round of replication, high doses of replicon particles were required for delivery to bone and/or joint tissue. Mice were sacrificed 12 to 14 h postinfection and frozen sections were prepared as they were after infection with the double promoter viruses. Results with replicons were identical to those seen with the double promoter viruses s55-gfp(F), s55-gfp, and s51-gfp. REP91-gfp-infected cells were localized to the endosteum and periosteum near the ends of the femur and tibia (Fig. 3E and F). Seventy-eight sagittal sections from the fore and hind limbs of replicon-infected mice were screened for GFP-positive cells. These sections included representative areas of the diaphyses, epiphyses, joints, and surrounding muscle tissue. This screen identified 55 cells that were GFP-positive, 46 of which were located in the epiphyses of the long bones. Of these 46 GFP-positive cells, 13 were found in the endosteum and 33 were found in the periosteum. Of the GFP-positive cells not clearly located in the epiphyses, all nine were located in the periosteum or endosteum. Two GFP-positive cells were located in the periosteum of the diaphysis, and it was unclear whether the other seven cells were located in the diaphyses or the epiphyses. No GFP-positive cells were found in muscle tissue, synovial membrane, or bone marrow not intimately associated with calcified tissue. These results, in combination with the in situ hybridization results from Fig. 2, clearly demonstrate that in an adult mouse model, an alphavirus associated with arthralgia in humans exhibits tropism for bone-associated connective tissue and that the predominant site of replication is the epiphyses adjacent to the joints.
Mechanisms which lead to joint pain during togavirus infection of humans have not been clearly defined. Suggested mediators include direct viral replication causing cell death or damage within the tissue (10), the immune response against the virus within the joint tissue causing damage to the joint (10), or immune complex deposition within the joint leading to inflammation (11). Damage by inflammatory cells elicited by the virus may occur; however, Sindbis-group alphavirus infection in humans is associated with a noninflammatory arthralgia rather than with arthritis (29). Studies with rubella virus- and Ross River virus-infected patients saw no correlation between serum immune complexes and/or complement component levels and disease (4, 5). Furthermore, viral antigen has been isolated from the joint tissue of patients suffering from rubella virus- and Ross River virus-mediated arthralgia (4, 6, 21). Therefore, the finding that S.A.AR86 targets tissues that surround the joint is consistent with the idea that arthralgia-associated alphaviruses replicate within or around joint tissue. We did not observe inflammation in the S.A.AR86-infected endosteum or periosteum. However, long-term evaluation of the effects of S.A.AR86 infection on bone-associated connective tissue will be required to fully evaluate this issue.
Several important questions remain to be addressed with regard to S.A.AR86 pathogenesis within the infected bone-associated connective tissues. These include the following. (i) What determines S.A.AR86 tropism for bone-associated connective tissues? (ii) Since osteoblasts and osteoclasts are the major cell types in the endosteum and periosteum (3), which cell type or types does the virus infect within these tissues? (iii) What effect does viral infection have on cellular function within the infected periosteum, endosteum, or tendon? (iv) How long does the virus persist within the infected tissue? The ability to use a molecularly cloned virus and virus-based expression systems in a small-animal model should provide valuable insight into these aspects of togavirus pathogenesis.
Acknowledgments
This research was funded by NIH research grant R01 AI22186. M.T.H. was supported by NIH institutional postdoctoral training grant T 32 AI07151 and NIH postdoctoral fellowship F 32 AI10146.
We thank the members of the Johnston laboratory for helpful scientific discussion and Kristen Bernard for assistance in evaluating the location of GFP-positive cells and in situ signal within bone and joint tissues. Cherice Connor, Michael Hawley, Jacqueline Bailey, and Dwayne Muhammed provided excellent technical support with cell culture.
REFERENCES
- 1.Aaskov J G, Mataika J U, Lawrence G W, Rabukawaqa V, Tucker M M, Miles J A, Dalglish D A. An epidemic of Ross River virus infection in Fiji, 1979. Am J Trop Med Hyg. 1981;30:1053–1059. doi: 10.4269/ajtmh.1981.30.1053. [DOI] [PubMed] [Google Scholar]
- 2.Aaskov J G, Ross P V, Harper J J, Donaldson M D. Isolation of Ross River virus from epidemic polyarthritis patients in Australia. Aust J Exp Biol Med Sci. 1985;5:587–597. doi: 10.1038/icb.1985.62. [DOI] [PubMed] [Google Scholar]
- 3.Burkitt H G, Young B, Heath J W. Wheater's functional histology. Edinburgh, United Kingdom: Churchill Livingstone, Ltd.; 1993. [Google Scholar]
- 4.Fraser J R, Cunningham A L, Hayes K, Leach R, Lunt R. Rubella arthritis in adults. Isolation of virus, cytology and other aspects of the synovial reaction. Clin Exp Rheumatol. 1983;1:287–293. [PubMed] [Google Scholar]
- 5.Fraser J R, Cunningham A L, Mathews J D, Riglar A. Immune complexes and Ross River virus disease (epidemic polyarthritis) Rheumatol Int. 1988;8:113–117. doi: 10.1007/BF00272432. [DOI] [PubMed] [Google Scholar]
- 6.Fraser J R E, Ratnamohan V M, Dowling J P, Becker G J, Variogos G A. The exanthem of Ross River virus infection: histology, location of virus antigen and nature of inflammatory infiltrate. J Clin Pathol. 1983;36:1256–1263. doi: 10.1136/jcp.36.11.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frolov I, Hoffman T A, Pragai B M, Dryga S A, Huang H V, Schlesinger S, Rice C M. Alphavirus-based expression vectors: strategies and applications. Proc Natl Acad Sci USA. 1996;93:11371–11377. doi: 10.1073/pnas.93.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frolov I, Schlesinger S. Translation of Sindbis virus mRNA: effects of sequences downstream of the initiating codon. J Virol. 1994;68:8111–8117. doi: 10.1128/jvi.68.12.8111-8117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heise M T, Simpson D A, Johnston R E. A single amino acid change in nsP1 attenuates neurovirulence of the Sindbis-group alphavirus S.A.AR86. J Virol. 2000;74:4207–4213. doi: 10.1128/jvi.74.9.4207-4213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston R E, Peters C J. Alphaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 843–898. [Google Scholar]
- 11.Julkunen I, Brummer Korvenkontio M, Hautanen A, Kuusisto P, Lindstrom P, Wager O, Penttinen K. Elevated serum immune complex levels in Pogosta disease, an acute alphavirus infection with rash and arthritis. J Clin Lab Immunol. 1986;21:77–82. [PubMed] [Google Scholar]
- 12.Klimstra W B, Ryman K D, Johnston R E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J Virol. 1998;72:7357–7366. doi: 10.1128/jvi.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klimstra W B, Ryman K D, Bernard K A, Nguyen K B, Biron C A, Johnston R E. Infection of neonatal mice with Sindbis virus results in a systemic inflammatory response syndrome. J Virol. 1999;73:10387–10398. doi: 10.1128/jvi.73.12.10387-10398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malherbe H, Strickland-Cholmley M, Jackson A L. Sindbis virus infection in man. S Afr Med J. 1963;37:547–552. [PubMed] [Google Scholar]
- 15.Mattion N M, Harnish E C, Crowley J C, Reilly P A. Foot-and-mouth disease virus 2A protease mediates cleavage in attenuated Sabin 3 poliovirus vectors engineered for delivery of foreign antigens. J Virol. 1996;70:8124–8127. doi: 10.1128/jvi.70.11.8124-8127.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKnight K L, Simpson D A, Lin S-C, Knott T A, Polo J M, Pence D F, Johannsen D B, Heidner H W, Davis N L, Johnston R E. Deduced consensus sequence of Sindbis virus strain AR339: mutations contained in laboratory strains which affect cell culture and in vivo phenotypes. J Virol. 1996;70:1981–1989. doi: 10.1128/jvi.70.3.1981-1989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy F A, Harrison A K, Collins W K. The role of extraneural arbovirus infection in the pathogenesis of encephalitis. Lab Investig. 1970;22:318–328. [PubMed] [Google Scholar]
- 18.Murphy F A, Taylor W P, Mims C A, Marshal I D. The pathogenesis of Ross River virus infection in mice. I. Muscle, heart, and brown fat lesions. J Infect Dis. 1973;127:129–138. doi: 10.1093/infdis/127.2.129. [DOI] [PubMed] [Google Scholar]
- 19.Niklasson B, Espmark Å. Occurrence of arthralgia and specific IgM antibodies three to four years after ockelbo disease. J Infect Dis. 1988;157:832–835. doi: 10.1093/infdis/157.4.832. [DOI] [PubMed] [Google Scholar]
- 20.Norder H, Lundstrom J O, Kozuch O, Magnius L O. Genetic relatedness of Sindbis virus strains from Europe, Middle East, and Africa. Virology. 1996;222:440–445. doi: 10.1006/viro.1996.0441. [DOI] [PubMed] [Google Scholar]
- 21.Ogra P L, Herd J K. Arthritis associated with induced rubella infection. J Immunol. 1971;107:810–813. [PubMed] [Google Scholar]
- 22.Pushko P, Parker M, Ludwig G V, Davis N L, Johnston R E, Smith J F. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 23.Ryman K D, Klimstra W B, Nguyen K B, Biron C A, Johnston R E. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J Virol. 2000;74:3366–3378. doi: 10.1128/jvi.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirako Y, Niklasson B, Dalrymple J M, Strauss E G, Strauss J H. Structure of the Ockelbo virus genome and its relationship to other Sindbis viruses. Virology. 1991;182:753–764. doi: 10.1016/0042-6822(91)90616-j. [DOI] [PubMed] [Google Scholar]
- 25.Simpson D A, Davis N L, Seh-Ching L, Russell D, Johnston R E. Complete nucleotide sequence and full-length cDNA clone of S.A.AR86, a South African alphavirus related to Sindbis. Virology. 1996;222:464–469. doi: 10.1006/viro.1996.0445. [DOI] [PubMed] [Google Scholar]
- 26.Skogh M, Espmark Å. Ockelbo disease: epidemic arthritis-exanthema syndrome in Sweden caused by Sindbis-virus like agent. Lancet. 1982;i:795–796. doi: 10.1016/s0140-6736(82)91834-7. [DOI] [PubMed] [Google Scholar]
- 27.Smerdou C, Liljeström P. Two-helper RNA system for production of recombinant Semliki Forest virus particles. J Virol. 1999;73:1092–1098. doi: 10.1128/jvi.73.2.1092-1098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tai K S, Whelan P I, Patel M S, Currie B. An outbreak of epidemic polyarthritis (Ross River virus disease) in the Northern Territory during the 1990–1991 wet season. Med J Aust. 1993;158:522–525. doi: 10.5694/j.1326-5377.1993.tb121866.x. [DOI] [PubMed] [Google Scholar]
- 29.Tesh R B. Arthritides caused by mosquito-borne viruses. Annu Rev Med. 1982;33:31–40. doi: 10.1146/annurev.me.33.020182.000335. [DOI] [PubMed] [Google Scholar]
- 30.Trgovcich J, Aronson J F, Johnston R E. Fatal Sindbis virus infection of neonatal mice in the absence of encephalitis. Virology. 1996;224:73–83. doi: 10.1006/viro.1996.0508. [DOI] [PubMed] [Google Scholar]
- 31.Valdivia R H, Hromockyj A E, Monack D, Ramakrishnan L, Falkow S. Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene. 1996;173:47–52. doi: 10.1016/0378-1119(95)00706-7. [DOI] [PubMed] [Google Scholar]
- 32.Weinbren M P, Kokernot R H, Smithburn K C. Strains of Sindbis-like virus isolated from Culicine mosquitoes in the Union of South Africa. S Afr Med J. 1956;30:631–636. [PubMed] [Google Scholar]
- 33.Wolinsky J S. Rubella. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippencott-Raven Publishers; 1996. pp. 899–929. [Google Scholar]