Abstract
Purpose
The sodium-glucose transporter 2 inhibitor (SGLT2i) empagliflozin may reduce the incidence of obstructive sleep apnea (OSA) in patients with type 2 diabetes (T2D) and cardiovascular (CV) disease. This analysis of VERTIS CV, the CV outcome trial for the SGLT2i ertugliflozin conducted in a similar group of patients, explored the effects of ertugliflozin on reported incident OSA.
Methods
In VERTIS CV, patients ≥ 40 years with T2D and atherosclerotic CV disease (ASCVD) were randomized to ertugliflozin 5 or 15 mg or placebo. The primary endpoint was the composite of major adverse CV events. This exploratory analysis evaluated the impact of ertugliflozin (5 and 15 mg pooled) on incident OSA. Patients with prevalent OSA were excluded. Incident OSA events were based on investigator-reported events using the MedDRA SMQ term “sleep apnea syndrome.” A multivariable Cox proportional hazards regression model was constructed to assess the association between ertugliflozin and incident OSA.
Results
Of 8246 patients enrolled, 7697 (93.3%) were without baseline OSA (placebo, n = 2561; ertugliflozin, n = 5136; mean age 64.4 years; BMI 31.7 kg/m2; HbA1c, 8.2%; 69.2% male; 88.3% White). The OSA incidence rate was 1.44 per 1000 person-years versus 2.61 per 1000 person-years among patients treated with ertugliflozin versus placebo, respectively, corresponding to a 48% relative risk reduction (HR 0.52; 95% CI 0.28–0.96; P = 0.04).
Conclusions
In VERTIS CV, ertugliflozin reduced by nearly half the incidence of OSA in patients with T2D and ASCVD. These data contribute to the literature that SGLT2is may have a significant beneficial impact on OSA.
Trial registration.
ClinicalTrials.gov identifier: NCT01986881.
Keywords: Sodium glucose transporter type 2 inhibitor, SGLT2, Sleep apnea, OSA, Sleep disordered breathing
Introduction
Obstructive sleep apnea (OSA) has been independently associated with important health outcomes, including hypertension, cardiovascular (CV) events, stroke, heart failure, chronic kidney disease (CKD), and metabolic dysfunction (1–3). OSA prevalence in adults ranges between 9 and 36% with higher rates in older men [4]. Among individuals with type 2 diabetes (T2D), concomitant OSA is associated with a higher risk of CV disease (CVD), atrial fibrillation, peripheral neuropathy, diabetic foot disease, CKD, and all-cause mortality [3]. Furthermore, in a randomly selected, nationally representative sample of Medicare beneficiaries, patients with untreated OSA had significantly higher mean total annual healthcare costs [5]. Treatment adherence with continuous positive airway pressure (CPAP), the mainstay intervention for OSA, is poor, ranging from 34 to 60% [6]. While improving CPAP adherence is key to improving OSA outcomes, the discovery of effective medical therapies could transform OSA care.
The sodium-glucose cotransporter 2 inhibitors (SGLT2i) were initially developed and approved to treat hyperglycemia in patients with T2D, but potent CV and kidney benefits were subsequently demonstrated in large-scale randomized trials. Their potential efficacy in OSA is just beginning to be explored. In a recent study, 36 patients with T2D and OSA were randomized to either metformin and glimepiride or metformin and the SGLT2i dapagliflozin and treated for 24 weeks. Home sleep testing was performed at the beginning and end of the study. Dapagliflozin led to significantly improved apnea–hypopnea index (AHI), a small rise in oxygen saturation, and better scores on the Epworth Sleepiness Scale [7]. In another small retrospective study of patients with OSA who had refused CPAP therapy, SGLT2i treatment was associated with significantly lower AHI [8]. Results from analyses exploring the effect of empagliflozin on OSA incidence from the EMPA-REG OUTCOME trial found that empagliflozin versus placebo reduced the incidence of OSA in patients with T2D and atherosclerotic CVD (ASCVD) by more than half (4.6/1000 patient-years [1.13%] vs. 2.2/1000 patient-years [0.57%]; hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.27–0.83; P = 0.009) (9). Notably, this effect was largely independent of weight loss.
To further investigate the effect of SGLT2 inhibition on OSA incidence, we performed a post hoc analysis of VERTIS CV, a double-blind, placebo-controlled trial of the efficacy and safety of the SGLT2i ertugliflozin in patients with T2D and ASCVD.
Method
VERTIS CV (NCT01986881) consisted of 8246 patients ≥ 40 years of age with T2D and ASCVD randomized to ertugliflozin 5 mg, 15 mg, or placebo once daily, with the primary outcome of composite of major adverse CV events. In these exploratory analyses, we evaluated the impact of ertugliflozin on incident OSA. A pooled analysis of ertugliflozin 5 and 15 mg (measured from the first dose of study medication until treatment end plus an additional 14 days) versus placebo was conducted. Patients with OSA at baseline (549; 6.7%) were excluded. Incident OSA events were based on investigator-reported adverse events during the trial using the MedDRA term sleep apnea syndrome (synonyms at: https://bioportal.bioontology.org/ontologies/MEDDRA?p=classes&conceptid=10040979). Kaplan–Meier estimates for time to first OSA event by treatment assignment for participants without OSA at baseline were plotted. A stratified Cox proportional hazards regression model was constructed to compare treatments with adjustment for age, sex, geographic region, baseline body mass index (BMI; < 35, ≥ 35 kg/m2), baseline glycated hemoglobin (HbA1c; < 9.0, ≥ 9.0%), and baseline estimated glomerular filtration rate (eGFR; < 60, ≥ 60 mL/min/1.73 m2) and with stratification by enrollment cohort (before and after protocol amendment).
Results
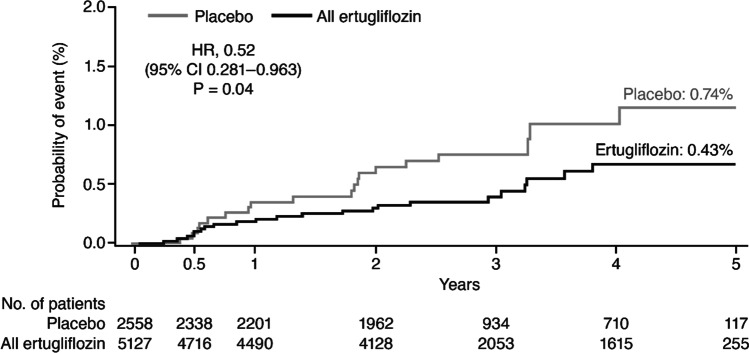
In VERTIS CV, 7697 (93.3%) patients were without baseline OSA. These patients were primarily White (88.3%), male (69.2%), with mean age of 64.3 years, BMI 31.7 kg/m2, HbA1c 8.2% and eGFR 76.2 mL/min/1.73 m2. Among those without baseline OSA, the event rate for incident OSA with ertugliflozin was 1.4 per 1000 patient-years compared with 2.6 per 1000 patient-years with placebo (Table 1), corresponding to a 48% relative risk reduction for incident OSA in patients on ertugliflozin versus placebo (HR 0.52; 95% CI 0.28–0.96; P = 0.04). There was an early differentiation in the incidence of OSA with ertugliflozin versus placebo (Fig. 1).
Table 1.
Time to first OSA event for patients without OSA at baseline
| All ertugliflozin (N = 5126) | Placebo (N = 2557) | HR* (95% CI) | |||
|---|---|---|---|---|---|
| No. (%) | Rate/1000 patient- years | No. (%) | Rate/1000 patient- years | ||
| OSA event | 22 (0.43) | 1.4 | 19 (0.74) | 2.6 |
0.52 (0.28–0.96); P = 0.04 |
OSA events are based on adverse events that occurred between the date of the first dose of study medication until treatment end plus an additional 14 days
*Ertugliflozin (pooled) vs. placebo, based on the stratified Cox proportional hazards model that includes categorical terms for treatment, sex, geographical region, baseline BMI, baseline HbA1c, and baseline eGFR, a continuous term for age, and a stratification factor for enrollment cohort
BMI body mass index, CI confidence interval, eGFR estimated glomerular filtration rate, HbA1c glycated hemoglobin, HR hazard ratio, OSA obstructive sleep apnea
Fig. 1 Kaplan–Meier estimates for time to first OSA event on treatment for patients without OSA at baseline. OSA events are based on adverse events that occurred between the date of the first dose of study medication until treatment end plus an additional 14 days. HR, 95% CI, and P value are based on a stratified Cox proportional hazards model that includes categorical terms for treatment, sex, geographical region, baseline BMI, baseline HbA1c, and baseline eGFR, a continuous term for age, and a stratification factor for enrollment cohort. BMI body mass index; CI confidence interval; eGFR estimated glomerular filtration rate; HbA1c glycated hemoglobin; HR hazard ratio; OSA obstructive sleep apnea
Discussion
Small studies have suggested the potential efficacy of SGLT2i versus placebo in OSA (7, 8). An exploratory analysis of the large EMPA-REG OUTCOME trial suggested that empagliflozin may prevent OSA (9). Based on prior findings (9), we hypothesized that ertugliflozin treatment would reduce the risk of new-onset OSA in the VERTIS CV trial. We found a significant 48% adjusted relative risk reduction that was observed with ertugliflozin versus placebo for incident OSA, similar to the magnitude of effect observed with empagliflozin [9].
Several potential mechanisms may underpin SGLT2i efficacy to improve OSA risk. The first is weight loss associated with SGLT2i, as urinary glucose excretion induced by this mechanism results in a daily net caloric loss. However, weight loss associated with SGLT2i is modest, and, in a mediation analysis from the EMPA-REG OUTCOME trial, weight loss was not significantly associated with the OSA effect [10]. It is also possible that the resultant reduction in plasma volume from osmotic diuresis (and accompanying natriuresis) decreases cardiac preload, resulting in less loop gain and downstream beneficial effects in respiratory physiology relevant to both obstructive and central sleep apnea (9).
There are several limitations to the present study. Patients were identified with OSA by investigator reporting of adverse events, which may have under-reported incident sleep apnea since periodic diagnostic sleep studies were not required in the protocol. Adverse event reporting is known to under-report incident OSA in large trials. Nonetheless, the results were highly consistent between the EMPA-REG OUTCOME trial and VERTIS CV, using two different SGLT2i. Furthermore, any misclassification of the outcome would tend to bias toward the null hypothesis and would not explain our observed results. The AHI was also not measured prospectively in this study; therefore, no comment can be made on the magnitude of AHI reduction or the baseline severity and type of sleep apnea in this population.
Conclusion
In VERTIS CV, the SGLT2i ertugliflozin reduced the incidence of OSA in patients with T2D and ASCVD, an effect similar in magnitude to that previously reported with empagliflozin, contributing to the growing body of literature that SGLT2i may serve to prevent (and possibly treat) sleep apnea syndromes. Accordingly, the SGLT2i class deserves additional exploration for this potential new role, including in patients without diabetes, as well as a further inquiry into the mechanisms involved.
Author contribution
Conception of post hoc analyses: B.S.W., S.E.I., H.K.Y., and U.M. Statistical analyses: J.P.M. Interpretation of results: B.S.W., S.E.I., I.J.N., J.P.M., U.M., R.F., N.B.C., D.K.M., C.P.C., and H.K.Y. Draft manuscript preparation: B.S.W., S.E.I., I.J.N., and H.K.Y. All authors critically reviewed the draft manuscript and approved the final version of the manuscript.
Funding
This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA in collaboration with Pfizer Inc., New York, NY, USA. The support provided by Engage Scientific Solutions, funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Pfizer, New York, NY, USA, consisted solely of copyediting and formatting for submission; no contribution was made to content.
Data availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Declarations
Competing interests
B.S.W. declares no conflicts of interest. S.E.I. has participated on clinical trial executive/steering/publications committees and/or served as an adviser for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Novo Nordisk, and VTV Therapeutics; has delivered lectures supported by AstraZeneca, Boehringer Ingelheim, and Merck; and has received support for attending meetings from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. I.J.N. has received a grant (directed to his institution) from the National Institutes of Health, consulting fees from Boehringer Ingelheim/Lilly Alliance, and Merck & Co., and has delivered lectures supported by Nestlé Health Sciences. H.K.Y. declares no conflicts of interest. D.K.M. has received consulting fees from AstraZeneca, Boehringer Ingelheim, Lexicon, Merck Sharp & Dohme, and Pfizer Inc. and has received payment for expert testimony from Kirkland & Ellis on behalf of Boehringer Ingelheim. C.P.C. has received research grants from Amgen, Better Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen, Merck, Novo Nordisk, and Pfizer; fees from Aegerion/Amryt, Alnylam, Amarin, Amgen, Applied Therapeutics, Ascendia, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Janssen, Lexicon, Merck, Pfizer, Rhoshan, and Sanofi; and has served on Data and Safety Monitoring Boards for the Veteran’s Administration, Applied Therapeutics, and NovoNordisk. J.P.M., R.F., U.M., and N.B.C. are employees and shareholders of Pfizer Inc.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yaggi H, Concato J, Kernan W, Lichtman J, Brass L, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. NEJM. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 2.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 3.Adderley NJ, Subramanian A, Toulis K, Gokhale K, Taverner T, Hanif W, Haroon S, Thomas GN, Sainsbury C, Tahrani AA, Nirantharakumar K. Obstructive sleep apnea, a risk factor for cardiovascular and microvascular disease in patients with type 2 diabetes: findings from a population-based cohort study. Diabetes Care. 2020;43:1868–1877. doi: 10.2337/dc19-2116. [DOI] [PubMed] [Google Scholar]
- 4.Senaratna C, Perret J, Lodge C, Lowe A, Campbell B, Matheson M, Hamilton G, Dharmage S. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2016;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Wickwire EM, Tom SE, Vadlamani A, Diaz-Abad M, Cooper LM, Johnson AM, Scharf SM, Albrecht JS. Older adult US Medicare beneficiaries with untreated obstructive sleep apnea are heavier users of health care than matched control patients. J Clin Sleep Med. 2020;16:81–89. doi: 10.5664/jcsm.8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45:43. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Y, Sun Q, Bai X-Y, Zhou Y-F, Zhou Q-L, Zhang M. Effect of dapagliflozin on obstructive sleep apnea in patients with type 2 diabetes: a preliminary study. Nutr Diabetes. 2019;9:32. doi: 10.1038/s41387-019-0098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawada K, Karashima S, Kometani M, Oka R, Takeda Y, Sawamura T, Fujimoto A, Demura M, Wakayama A, Usukura M, Yagi K, Takeda Y, Yoneda T. Effect of sodium glucose cotransporter 2 inhibitors on obstructive sleep apnea in patients with type 2 diabetes. Endocr J. 2018;65:461–467. doi: 10.1507/endocrj.EJ17-0440. [DOI] [PubMed] [Google Scholar]
- 9.Neeland IJ, Eliasson B, Kasai T, Marx N, Zinman B, Inzucchi SE, Wanner C, Zwiener I, Wojeck BS, Yaggi HK, Johansen OE. The impact of empagliflozin on obstructive sleep apnea and cardiovascular and renal outcomes: an exploratory analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2020;43:3007–3015. doi: 10.2337/dc20-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591:1179–1193. doi: 10.1113/jphysiol.2012.245159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.