Abstract
Extraovarian adult granulosa cell tumor is a very uncommon neoplasm, which probably arises from the ectopic gonadal tissue along the embryonic root of the genital ridge. We report a new and rare case of an extraovarian adult granulosa cell tumor occurring in a 66-year-old woman who was presented with severe abdominal pain focused on the left iliac fossa. The immunohistopathology confirmed the diagnosis of a paratubal adult granulosa cell tumor. This paper illustrates the histogenetic origin of granulosa cell tumor, its clinicopathological and immunohistochemical features.
Keywords: Extraovarian, Adult granulosa cell tumor, Histopathology, Inhibin, Differential diagnosis
Introduction
Granulosa cell tumor (GCT) of the ovary is an uncommon neoplasm accounting for 2–5% of all ovarian cancers [1]. The name “granulosa cell tumor” was proposed by von Werdt in 1914 [2]. The primary extraovarian GCT is an extremely rare tumor. It can develop in the retroperitoneum, omentum, mesentery, broad ligament, liver and adrenals [3]. Histogenetic origin is regarded from the ectopic gonadic stromal tissue of the mesonephros. [3]. In the medical English literature from 1938 till 2022, 30 case reports of primary extraovarian GCT have been reported [4].
The histological features of the ovarian adult granulosa cell tumor (AGCT) are very suggestive. The immunohistochemistry (IHC) confirms the diagnosis of GCT [5]. The differential diagnosis includes undifferentiated carcinoma, endometrial small sarcoma, carcinoïd tumor, and lymphoma [5]. The wide surgical resection is the recommended therapy. Periodic postoperative imaging and long-term follow-up are necessary because of the high rate of local recurrence and metastasis several years after resection [3]. This case report illustrates the histogenetic origin, clinicopathological and immunohistochemical features of this unusual localization of AGCT.
Case report
A 66-year-old female presented to the emergency department of Mohamed VI University Hospital for management of sporadic and unprovoked abdominal pain lasting for 1 month. It involved her right iliac fossa. Her medical history included type 2 diabetes mellitus diagnosed 6 years ago.
The clinical exam revealed a suprapubic scar secondary to caesarian delivery and a right lower quadrant pain without palpable mass. The laboratory assessment was normal apart from normocytic anemia with haemoglobin at 8.8 g/dl. Tumor markers were negative (CA19-9, CA 125, and CEA). No hormonal studies were performed as the diagnosis of GCT was not suspected. Pelvic ultrasound and abdominopelvic computerized tomography scan (CT) revealed a right ovarian tumor with moderately abundant ascites. The patient underwent exploratory laparoscopy which revealed a pelvic involvement. Histologic examination of pelvic involvement biopsy samples demonstrated nonspecific inflammatory changes.
The pelvic MRI objectified an ill-defined, irregular right lateral uterine nodule, measuring 15 × 11 mm with no locoregional invasion (Fig. 1). A laparotomy was carried out. The per operatory exam revealed a nodular lesion attached to the right tube. The patient had undergone an appendectomy with a right salpingo-oophorectomy.
Fig. 1.
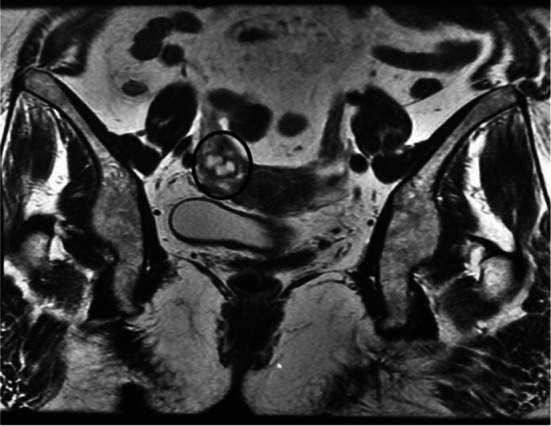
MRI: Right latero-uterine tumoral process without locoregional invasion (circle)
Macroscopic examination demonstrated a brownish, well-circumscribed nodular lesion attached to the right tube and measuring 13 × 10 × 7 mm. The cut surface was predominantly solid, heterogenous, and grayish-brown with some hemorrhagic areas. Ovary, tube and appendix were found to be normal macroscopically.
Histological examination of the excised paratubal nodule demonstrated a high cellular monotonous tumor proliferation, composed of small and uniform looking round to oval neoplastic cells (Fig. 2). Tumor cells were arranged predominantly in diffuse and trabecular patterns and had scanty cytoplasm and hyperchromatic nuclei containing granular chromatin (Figs. 2 and 3). Occasional cells showed focally coffee-bean-like nuclear grooves (Fig. 4). Neoplastic cells fit around the vessels realizing pseudorosette figures, but characteristic Call- Exner bodies were focally seen (Fig. 3). Mitotic activity was low and there were no cytonuclear atypia (Figs. 3 and 4). The tumor presented extensive areas of haemorrhage, vascular congestion, and necrosis (Fig. 2). Areas of suppuration, deposits of hemosiderin and foreign body giant cells were observed. These histopathological features proposed the diagnosis of reshaped and probably ruptured extraovarian AGCT. The ovary, uterine tube, and appendix were normal on histologic examination apart from acute suppurated periappendicitis (Fig. 5).
Fig. 2.
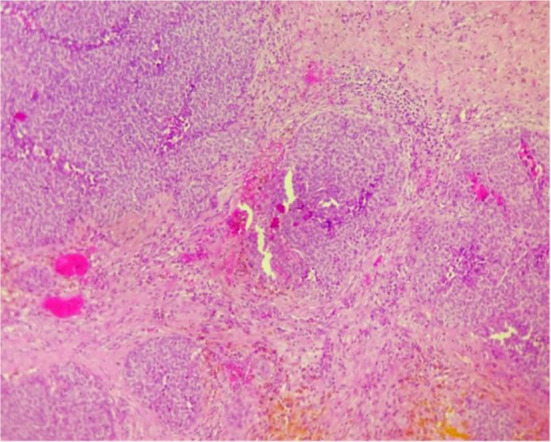
Solid and nested features. A highly vascularised stroma (HEx 100)
Fig. 3.
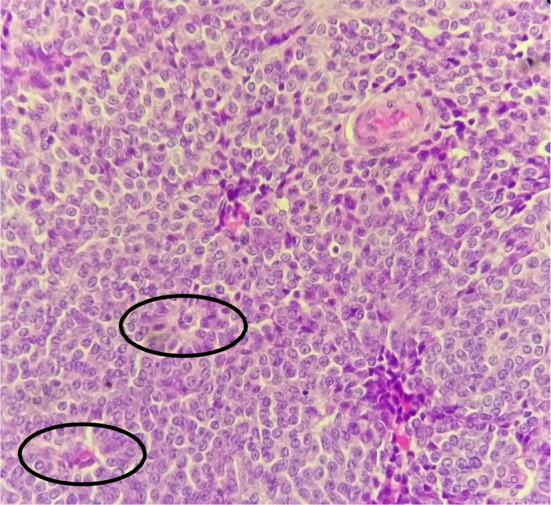
Note some Call-Exner structures (black circles) (HEx 200)
Fig. 4.
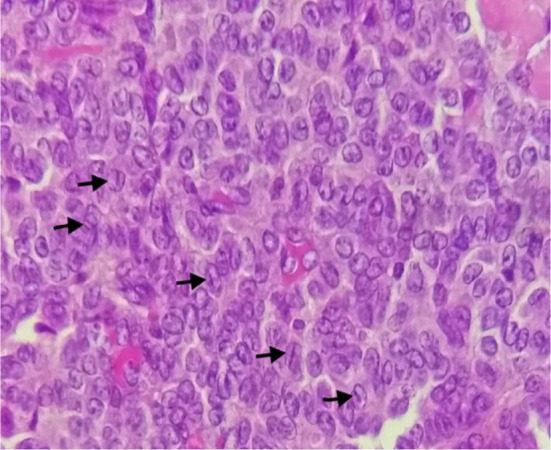
Uniform-looking round to oval neoplastic cells having a scanty cytoplasm and hyperchromatic nuclei with granular chromatin. Some cells had focally coffee-bean-like nuclear grooves (arrows)
Fig. 5.
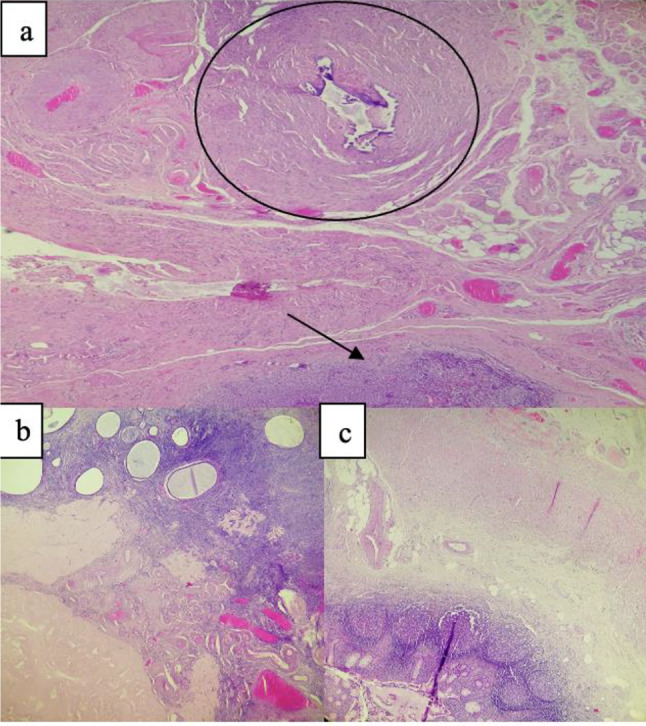
a Normal tube (circle) and tumor in paratubal zone (arrow). b Normal Ovarii. c Normal appendix
The immunohistochemistry revealed that tumor cells positively stained with α-inhibin and calretinin antibodies (Fig. 6a, b). On the contrary, anti-epithelial membrane antigen and anti-synaptophysin were negative. Considering the typical histological morphology, immunohistochemical findings, and the absence of a history of ovarian GCT, the diagnosis of an extraovarian GCT adult type was held.
Fig. 6.
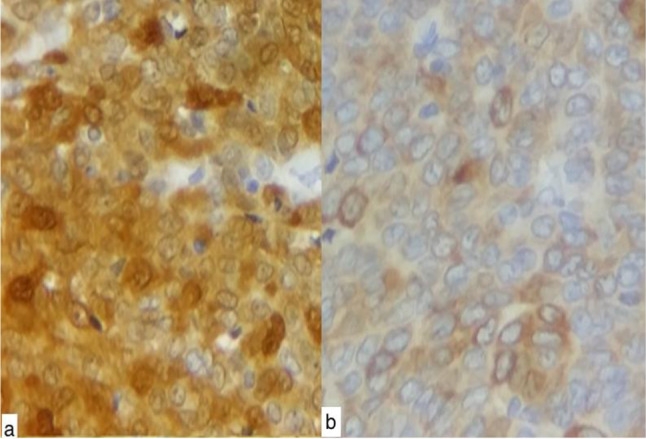
a immunohistochemistry: positive immunostain with anti-calretinine antibody at high power field (Gx400). b positive immunostain with anti-inhibin antibody (Gx400)
Postoperative course was uneventful with complete and immediate resolution of all symptoms.
Discussion
GCT, a rare ovarian cancer, may be of juvenile or adult or type depending on different clinical and histological characteristics [6]. It represents 2% of all ovarian cancers [1]. The estimated incidence of GCT in the United States is 0.99/100,000, whereas the reported incidence in other developed countries ranges from 0.4 to 1.7 per 100,000 [7]. The most common adult type of GCT is usually present during perimenopause or early menopause. The median age is about 50–54 years [1]. AGCT of the ovary is often times a stromal cell tumor with hormonal activity distinguished by its ability to express aromatase and secrete sex steroids like estrogen [8].
Extraovarian GCT is rarely developed. Only thirty cases have been reported in the English literature until 2022 [4]. According to the literature, our observation represents the first extraovarian granulosa cell tumor arising in the paratubal wall.
The ectopic gonadal stromal tissue of the mesonephros represent the histogenetic origin of extraovarian sex-cord stromal tumors [3]. A possible two sources from both mesonephros and coelomic epithelium have also been suggested [9]. Mesonephros appears necessary to create the sex cord, which may explain extraovarian stromal sex-cord tumor sites restricted to the retroperitoneum, broad ligament and the adrenal gland [9].
The histological findings of adult GCT are usually highly cellular. The cells are presented in loose aggregates, follicular groupings, and some in a dispersed fashion [10]. They are monomorphic, small, and round to oval with scanty cytoplasm; granular chromatin and longitudinal nuclear grooves named "coffee-bean" nuclei [10]. The characteristic Call-Exner bodies, amorphous globular structures, are present in 30–60% of GCT cases. A variety of histological patterns, e.g., microfollicular, macrofollicular, trabecular, insular, watered-silk, diffuse, or a mixture of these patterns, are often found [10]. Similar histological findings were noted in our case with a predominantly diffuse and trabecular pattern, but with no Call-Exner bodies. In immunohistochemistry, a number of tumor markers, especially the inhibin is useful to confirm the histological diagnosis of GCT [5]. In pregnancy, the placenta secretes inhibin, whereas its only source in nonpregnant women is the ovary. It has been found to be the most reliable marker for GCT than estradiol. Although high levels of inhibin can be observed in some ovarian carcinomas, these may be confirmed by their positive EMA, which is negative for GCT [5]. GCT is positive for calretinin also [5]. In our case, the diagnosis of GCT was confirmed by strong positivity for inhibin with negative EMA [5]. Immunostains should be carried out to differentiate GCTs from other neoplasms, such as undifferentiated carcinoma, small-cell carcinoma, lymphoma, carcinoids, and endometrial stromal sarcoma. These tumors are inhibin negative [5]. Cytokeratins, CD99, EMA, LCA, and Chromogranin are helpful in diagnosing and differentiating these tumors [5].
The treatment of GCT depends on surgery first [11]. Total abdominal hysterectomy with bilateral salpingo-oophorectomy represents the first choice of treatment in postmenopausal women [11]. However, conservative surgery, including unilateral salpingo-oophorectomy, is applied for patients with stage I disease and those of reproductive age [11].
Patients with GCT require long-term follow-up, because 17% of relapses occur more than 10 years after diagnosis [8]. The most common site of recurrence is in the pelvis [12]. Distant metastases are rare, but have been reported from many sites, especially in the intra-abdominal cavity [12].
Recently, Activin B was introduced as a new marker for postoperative follow-up of patients with GCT [13]. Up to now, there is no consensual follow-up. Pelvic examination, tumor marker tests and inhibin levels assay are proposed every 3 months for the first 2 years then every 4–6 months for 3–5 years and annually thereafter.
Conclusion
The primary extraovarian AGCT were confirmed after excluding any history of GCT of the ovary. Because of their rarity, the diagnostic of extraovarian AGCTs on cytological preparations is challenging. However, a good clinical history and cytological examination, histopathological correlation, and a positive immunostaining with inhibin may help us in practice.
The difficulty of our publication stands on the fact of the absence of a visible mass at laparoscopy and laparotomy time and the presence of fistulized appendicitis at surgery time. In our case, the macroscopic examination reported a paratubal nodule of 13 mm, detectable only at MRI exam. Histology and IHC reports confirmed the diagnosis of paratubal AGCT. To our knowledge, it is the first case described on this site. Surgery is the recommended treatment plan for this tumor. A follow-up on the long term should be performed.
Acknowledgements
We gratefully acknowledge the patient for allowing us to publish this case.
Abbreviations
- AGCT
Granulosa cell tumor
- GCT
Granulosa cell tumor
- CT
Computerized tomography scan
- IHC
Immunohistochemistry
- EMA
Epithelial membrane antigen
- CEA
Carcinoembryonic antigen
Author contributions
OK was responsible for the original manuscript writing. AS was involved in the revision and correction of the manuscript. All authors read and approved the final manuscript.
Funding
None declared.
Availability of data and materials
Not applicable in this section.
Declarations:
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable in this section.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21(6):1180–1189. doi: 10.1200/JCO.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Al-Shraideh Y, Mahfooz AB, Aslam M, Alhazmi A, Alshakweer W. Primary retroperitoneal granulosa cell tumor. UroToday Int J. 2012;5(6):art 61. doi: 10.3834/uij.1944-5784.2012.12.11. [DOI] [Google Scholar]
- 3.Paul PC, Chakraborty J, Chakrabarti S, Chattopadhyay B. Extraovarian granulose cell tumor. Indian J Pathol Microbiol. 2009;52:231–233. doi: 10.4103/0377-4929.48928. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Singh V, Mishra N, Gopinath M, Gupta P. Primary retroperitoneal extraovarian granulosa cell tumor. Autops Case Rep. 2022;12:e2021355. doi: 10.4322/acr.2021.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neeli SI, Malur PR. Primary retroperitoneal extraovarian granulosa cell tumor: a case report. Uro Today Int J. 2010;3:1. [Google Scholar]
- 6.Geetha P, Nair MK. Granulosa cell tumours of the ovary. Aust N Z J Obstet Gynaecol. 2010;50:216–220. doi: 10.1111/j.1479-828X.2010.01154.x. [DOI] [PubMed] [Google Scholar]
- 7.Rifki Jai S, Robleh Hassan F, Boussabir A, Boufettal R, Chehab F. Extraovarian granulosa cell tumor: a case report. Pan Afr Med J. 2016;23:86. doi: 10.11604/pamj.2016.23.86.8048. [DOI] [Google Scholar]
- 8.Kim SH, Park HJ, Linton JA, Shin DH, Yang WI, Chung WY, et al. Extraovarian granulosa cell tumor. Yonsei Med J. 2001;42:360–363. doi: 10.3349/ymj.2001.42.3.360. [DOI] [PubMed] [Google Scholar]
- 9.Motta PM, Makabe S. Germ cells in the ovarian surface during fetal development in humans. J Submicrosc Cytol. 1986;18:271–290. [PubMed] [Google Scholar]
- 10.Charles Z, Brenda WN. The ovary and fallopian tube. In: Silverberg SG, editor. Silverberg's principles and practice of surgical pathology and cyopathology. Mississippi: Churchill Livingstone; 2006. pp. 2015–2017. [Google Scholar]
- 11.Koukourakis GV, Kouloulias VE, Koukourakis MJ, Zacharias GA, Papadimitriou C, Mystakidou K, Pistevou- Gompaki K, Kouvaris J, Gouliamos A. Granulosa cell tumor of the ovary: tumor review. Integr Cancer Ther. 2008;7:204–215. doi: 10.1177/1534735408322845. [DOI] [PubMed] [Google Scholar]
- 12.Pranita M, Swagata D. A rare case of extraovarian granulosa cell tumor presenting as a retroperitoneal mass. Ann Pathol Lab Med [S.l.] 2016;3(3):C153–155. [Google Scholar]
- 13.Vihko KK, Blδuer M, Puistola U, Tuohimaa P. Activin B in patients with granulosa cell tumours: serum levels in comparison to inhibin. Acta Obstet Gynecol Scand. 2003;82:570–574. doi: 10.1034/j.1600-0412.2003.00146.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable in this section.


