Abstract
The hemagglutinin (HA) of H3 human influenza viruses does not support viral replication in duck intestine despite its avian origin. A Leu-to-Gln mutation at position 226 and a Ser-to-Gly mutation at position 228 in the HA of human A/Udorn/307/72 (H3N2) permit a reassortant virus [human Udorn HA, with all other genes from A/mallard/New York/6750/78 (H2N2)] to replicate in ducks. To understand the molecular basis of this change in host range restriction, we investigated the receptor specificity of duck influenza viruses as well as of human-duck virus reassortants. The results indicate that the recognition of a glycoconjugate moiety possessing N-glycolneuramic acid (NeuGc) linked to galactose by the α2,3 linkage (NeuGcα2,3Gal) is associated with viral replication in duck intestine. Immunofluorescence assays with NeuGcα2,3Gal-specific antiserum detected this moiety primarily on the crypt epithelial cells of duck colon. Such recognition, together with biochemical evidence of NeuGc in crypt cells, correlated exactly with the ability of the virus to replicate in duck colon. These results suggest that recognition of the NeuGcα2,3-Gal moiety plays an important role in the enterotropism of avian influenza viruses.
Influenza A viruses have been isolated from a variety of animals, including humans, pigs, horses, sea mammals, and birds (37). Each of the different antigenic subtypes of influenza A viruses (H1 to H15 and N1 to N9) has been isolated from wild aquatic birds (7, 27, 37), which appear to serve as the reservoir for all influenza A viruses that infect mammals (37). Despite their common origin, influenza A viruses do not replicate indiscriminately across animal species but rather show a clear pattern of host range restriction. For example, in experimental infection of nonhuman primates, avian influenza viruses replicate poorly (1, 20, 28), while human viruses replicate well and cause influenza symptoms (20, 28); the titers of avian and human viruses differ by 2 logs in nonhuman primates (19). A notable exception is the direct transmission of an avian influenza virus from birds to humans in Hong Kong in 1997 (2, 31). As one might predict, influenza viruses isolated from ducks replicate well in duck intestine, the major replication site in this host, whereas human viruses do not (14, 36). The importance of the hemagglutinin (HA) and neuraminidase (NA) molecules in the restriction of human virus replication in ducks was demonstrated in studies in which reassortant viruses containing either the HA or NA gene from a human virus and other genes from a duck virus failed to replicate in ducks (8). However, the molecular mechanism of these restrictive effects remains unknown.
Although all influenza viruses recognize oligosaccharide-containing terminal sialic acid, the receptor specificity of the HA differs: most avian influenza viruses preferentially bind to the sialic acid-α2,3-galactose (SAα2,3Gal) linkage, while human influenza viruses favor the SAα2,6Gal linkage on cell surface sialyloligosaccharides (3, 24, 25). Couceiro et al. (4) reported the presence of SAα2,6Gal but not of SAα2,3Gal sialyloligosaccharides on the surface of epithelial cells from human trachea. It was recently shown that the epithelial cells of duck intestine contain SAα2,3Gal but not SAα2,6Gal sialyloligosaccharides (13). Thus, the host range of influenza A viruses may correspond to the presence or absence of certain sialic acid-galactose linkages in host animals, although it is highly likely that this phenomenon is controlled by multiple host and viral genes.
Sialic acid is a generic term for a nine-carbon, acidic amino sugar (5-amino-3,5-dideoxy-d-glycero-d-galacto-nonulosonic acid) whose amino group is replaced with either an N-acetyl or N-glycolyl group, yielding N-acetylneuraminic (NeuAc) or N-glycolylneuraminic (NeuGc) acid, respectively. The hydroxyl groups can be replaced by acetyl, lactoyl, methyl, sulfate, or phosphate residues. The distribution of specific sialic acids differs among animal species. For example, cells from cows, horses, and pigs express both NeuAc and NeuGc (16, 18, 22, 23), but human cells do not express NeuGc (15, 16). Influenza viruses differ in their recognition of NeuAc, NeuGc, and 9-O-Ac-NeuAc (6), suggesting that it is not only the type of sialic acid-galactose linkage but also the sialic acid species that contributes to the host range restrictions of influenza A viruses.
Influenza viruses isolated from various animal species differentially agglutinate erythrocytes from different animals (11). For example, although all influenza viruses agglutinate human and chicken erythrocytes, duck but not human influenza viruses agglutinate horse erythrocytes (12). Chicken and human erythrocytes both possess SAα2,6Gal, SAα2,3Gal, and only NeuAc, while horse erythrocytes contain primarily NeuGc and SAα2,3Gal (12). These findings suggest that duck viruses recognize NeuGcα2,3Gal molecules, but experimental support for such an association is lacking.
Human influenza A viruses do not replicate in ducks (14, 36). This host restriction appears to reside in the receptor specificity of the HA: a reassortant virus containing only the HA gene from a human A/Udorn/307/72 (Udorn) (H3N2) virus and all remaining genes from A/mallard/New York/6750/78 (Mal/NY) (H2N2) and its horse α2-macroglobulin-resistant R3 variant (with a Leu-to-Gln mutation at position 226 in the HA) did not replicate in duck intestine. A single additional mutation at position 228 (Ser to Gly) endowed a nonreplicating human (HA with a Leu-to-Gln mutation at position 226)-avian (all other genes) reassortant virus with enterotropism in ducks (21, 35). However, it was not known how these mutations convert the human virus HA to one supporting viral replication in duck intestine. We therefore analyzed the receptor specificities of duck and human influenza viruses and determined the distribution of oligosaccharide molecules in duck intestine.
Comparison of receptor specificity between duck and human influenza A viruses.
Two mutations of the human Udorn virus HA at positions 226 (Leu to Gln) and 228 (Ser to Gly) enable a reassortant virus possessing this HA in the context of Mal/NY viral genes to grow in duck intestine. Since both mutations are located in the receptor binding pocket, we analyzed the receptor specificity of the virus by comparing the binding characteristics of the wild-type human Udorn HA, its mutant with a Leu-to-Gln substitution at position 226 (L226Q; R3 HA), and another mutant possessing both the preceding changes and a Ser-to-Gly substitution at position 228 (L228G; R2 HA), using thin-layer chromatography (TLC) virus binding assays with gangliosides (Fig. 1A). Because gangliosides containing NeuGcα2,6Gal have not been found in nature, we did not examine the HA specificity for this molecule. The human Udorn virus preferentially recognized NeuAcα2,6Gal, as previously shown (3), whereas the Mal/NY virus bound to all three gangliosides tested, II3(NeuGc)LacCer, II3(NeuAc)LacCer, and II6(NeuAc)LacCer, thus recognizing NeuAcα2,6Gal, NeuAcα2,3Gal, and NeuGcα2,3Gal (Fig. 1A). A mutation from Leu to Gln at position 226 (R3 virus) shifted the specificity of the human Udorn virus HA from NeuAcα2,6Gal to NeuAcα2,3Gal, as previously reported (26). However, the R3 virus preferentially recognized NeuAcα2,3Gal but recognized NeuGcα2,3Gal only marginally. Interestingly, an additional mutation from Ser to Gly at position 228 (R2) made the virus recognize NeuGcα2,3Gal equally well. Because the mutation converted R3 virus to one that grows in duck intestine (i.e., R2 virus) (21), these findings suggested the importance of the recognition of NeuGc, in addition to the α2,3 linkage, for the replication of influenza virus in duck intestine.
FIG. 1.
Comparison of receptor specificity among duck, human, and reassortant influenza viruses. To determine the receptor specificity of the viruses, we relied on a TLC-virus binding assay using lacto-series gangliosides containing the type I sugar chain. i.e., NeuAcα2,6 lactotetraosyl ceramide [II6(NeuAc)LacCer](NeuAcα2,6), NeuAcα2,3, lactotetraosyl ceramide [II3(NeuAc)LacCer](NeuAcα2,3), and NeuGcα2,3 lactotetraosyl ceramide [II3(NeuGc)LacCer](NeuGcα2,3), as described previously (33). Each ganglioside (1 nmol) was applied to a silica gel (Polygram Sil G plate; Nagel) that was developed in chloroform–methanol–12 mM MgCl2 (5/4/1, vol/vol/vol) and dried. After being blocked by phosphate-buffered saline supplemented with 1% egg albumin and 1% polyvinylpyrrolidone (solution A) at room temperature for 2 h, the plate was incubated with purified virus (28 hemagglutinating units) suspended in phospate-buffered saline for 12 h at 4°C. After being washed with phosphate-buffered saline, the plate was blocked with solution A and incubated with a pool of 11 monoclonal antibodies at 4°C for 2 h. After being washed with phosphate-buffered saline and blocked again with solution A, the plate was incubated at 4°C for 2 h with horseradish peroxidase-conjugated protein A and then incubated with the substrate solution (0.1 M citrate buffer [pH 6.0]–3% 4-chloro-1-naphthol in methanol– 3% aqueous H2O2 [5/1/0.01, vol/vol/vol]) at room temperature for 20 min. The binding activity of the virus was determined by scanning the stained chromatogram at 629 nm with a TLC scanner (CSR-9000; Shimazu, Kyoto, Japan). The results were recorded as the relative binding reactivity of virus with gangliosides, with the highest reactivity set at 100. (A) Receptor specificities of Udorn, Mal/NY, and reassortant viruses possessing a mutation(s) in the Udorn HA; (B) receptor specificities of other avian viruses.
We therefore examined whether duck viruses universally recognize NeuGcα2,3Gal. All seven duck viruses in this study, representing a variety of HA subtypes, recognized NeuGcα2,3Gal, though less readily than NeuAcα2,3Gal in most cases (Fig. 1B). The ability to recognize NeuGcα2,3Gal was marginal for A/duck/Ukraine/1/63 (H3N8), as shown before (6). Since this virus has been passaged extensively in chicken eggs, which lack NeuGc (16), the lack of NeuGc recognition might be due to egg adaptation and the virus may no longer replicate in ducks. We therefore tested its replicative potential in duck intestine. One-day-old mallards (Anas platyrhynchos platyrhynchos) were purchased from Ridgeway Hatcheries Inc., La Rue, Ohio) and used at 6 to 12 weeks of age. The ducks were orally inoculated with 0.5 ml of allantoic fluid containing 107.0 50% egg infective doses (EID50) of virus. They were sacrificed for the isolation of virus from organs at 3 days postinfection. The viruses in colon were titrated in eggs as described previously (14). A/duck/Ukraine/1/63 (H3N8) virus was recovered from only one of six ducks, and in the virus-positive duck, the virus titer in colon was 101.3 EID50/g. By contrast, other duck viruses with substantial ability to recognize NeuGcα2,3Gal [e.g., A/duck/Hokkaido/8/80 (H3N8) and Mal/NY (H2N2)] replicated well in duck intestine, with titers of 104 EID50/g. Thus, NeuGcα2,3Gal recognition does in fact seem to be associated with the efficient intestinal replication of influenza virus in ducks. Some influenza A viruses also recognize NeuAcα2,6Gal [A/teal/Alberta/69/87 (H1N4) in the present study; also see references 24 and 25], but the biologic significance of this capacity is unclear.
Sialyloligosaccharide analysis of the epithelial cells of duck intestine.
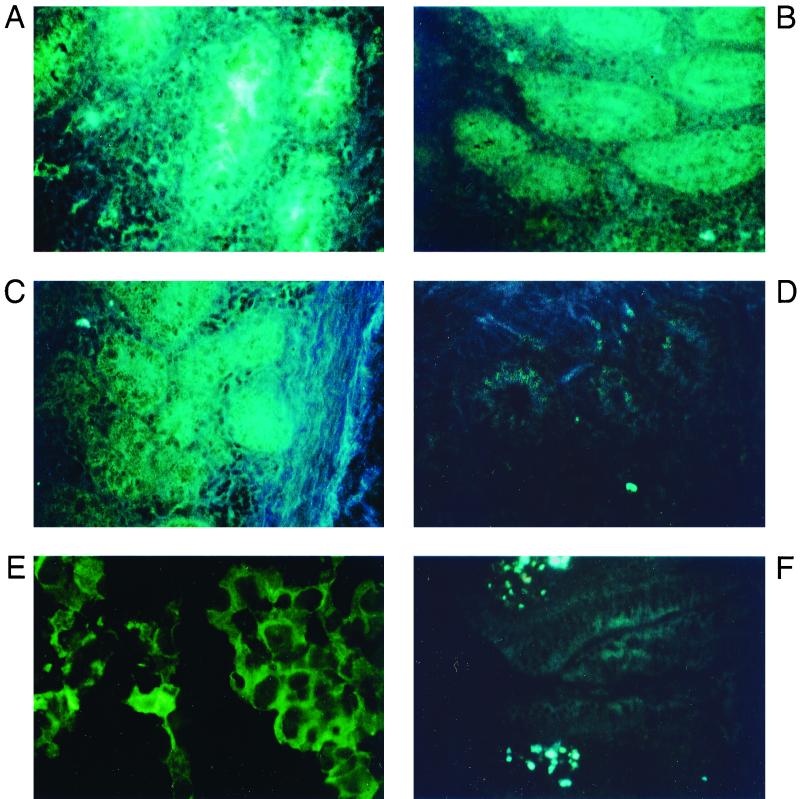
The above observations demonstrating preferential recognition of the NeuGcα2,3Gal linkage by viruses able to replicate in duck intestine prompted us to identify the glycoconjugate that possesses the NeuGcα2,3Gal linkage in this organ. We recently showed that the epithelial cells of duck intestine contain SAα2,3Gal but not SAα2,6Gal, using SA-Gal linkage-specific lectins (13), but these lectins did not allow us to distinguish between NeuAc and NeuGc. Thus, we incubated frozen sections of duck and chicken (negative control) intestine or MDCK cells (positive control) with chicken antihematoside antibody (which specifically recognizes NeuGcα2,3Gal but not NeuAcα2,3Gal [9]) and then with fluorescein-conjugated anti-chicken immunoglobulin antibody. Although it failed to react with chicken intestine (Fig. 2F), which lacks NeuGc (16), the antiserum did react with NeuGc-containing MDCK cells (Fig. 2E), confirming its specificity. The crypt cells of duck colon (Fig. 2A) epithelium reacted with the antiserum, demonstrating the presence of NeuGc. The epithelial cells of duck jejunum (Fig. 2C) and cecum (Fig. 2B) were also positive (though to a lesser extent), while those of duodenum (Fig. 2D) were not, suggesting that much higher concentrations of NeuGc are present in the lower intestine of ducks.
FIG. 2.
Immunodetection of the NeuGcα2,3Gal moiety in duck intestine. The duodenum, jejunum, cecum, and colon of 4-week-old ducks of an F1 cross between Peking ducks (Anas platyrhynchos domesticos) and mallards (A. platyrhynchos platyrhynchos) were collected immediately after exsanguination by cardiac puncture and rinsed with phosphate-buffered saline (pH 7.2) before being cut into 3-mm3 cubes. Tissue blocks were embedded in OCT compound (Miles Inc., Ind.) and frozen in liquid nitrogen. Six-micrometer sections of each tissue were cut with a microtome cryostat, air dried, and fixed for 15 min with cold acetone before immunostaining. The sections were incubated for 1 h with 50 μl of an antiserum (1:100) raised against hematoside [GM3 (NeuGc); II3(NeuGc)LacCer], which is known to lack NeuGc, in chickens (9, 16). After three washes with cold phosphate-buffered saline, the sections were incubated with fluorescein-conjugated anti-chicken immunoglobulin G antibody (Bethyl Laboratories, Montgomery, Tex.) for 1 h at room temperature and after three additional washes with cold phosphate-buffered saline and buffered glycerol (pH 9.0) were mounted for observation. Control slides were incubated with phosphate-buffered saline instead of antiserum. The sections were examined with a fluorescence microscope (BH-RFL; Olympus Optics, Tokyo, Japan) equipped with a dark-field condenser and UV excitation. Antihematoside antibody bound to both duck colon (A) and MDCK cells (E) and bound slightly to cecum (B) and jejunum (C) of duck, whereas no binding was observed in duck duodenum (D) and chicken colon (F). Magnification, ×300.
Additional experiments sought to establish the presence of NeuGc in duck intestine chemically. Epithelial cell fractions obtained by EDTA treatment (10) were hydrolyzed with sulfuric acid and analyzed by liquid chromatography (5, 32). The presence of NeuAc and NeuGc in this sample was determined with authentic sialic acid standards. The molar ratio of NeuAc and NeuGc in this sample was 98:2 (data not shown). These findings suggest that epithelial cells in duck intestine contain NeuGcα2,3Gal, albeit as a minor species. This limited amount of NeuGc among the total sialic acid content in duck intestine seems reasonable, since only crypt cells reacted with antiserum to NeuGcα2,3Gal.
Preferential replication of avian influenza virus in the crypt cells of duck intestine.
To determine whether the presence of NeuGc in crypt cells of duck intestine is important for viral replication, we performed immunofluorescence assays of thin sections of duck colon infected with A/duck/Hokkaido/5/77 (H3N2) virus (Fig. 3). The pooled monoclonal antibodies to the NS1 protein detected viral antigens mainly in the crypt cells, in accord with a previous finding (14). Thus, NeuGc localization is associated with the efficient replication of duck viruses.
FIG. 3.
Presence of viral antigen in epithelial cells in the colon crypts of a duck infected with A/duck/Hokkaido/5/77 (H3N2), 4 days after inoculation. One-day-old mallards (A. platyrhynchos platyrhynchos) were purchased from Ridgeway Hatcheries Inc., and used at 6 to 12 weeks of age. Ducks were orally inoculated with 0.5 ml of allantoic fluid containing 107.0 EID50 of virus. NS1 protein in virus-infected cells was detected with a pool of monoclonal antibodies to this protein. Magnification, ×300.
In the present study, we found that recognition of NeuGcα2,3Gal is associated with the efficient replication of influenza viruses in duck intestine. The HAs of all duck viruses tested (with the exception of A/duck/Ukraine/1/63) recognized this moiety, while those of human viruses did not. The R3 virus, which efficiently recognized NeuAcα2,3Gal but not NeuGcα2,3Gal, failed to replicate in duck intestine, whereas the R2 mutant, which efficiently recognized both NeuAcα2,3Gal and NeuGcα2,3Gal, replicated well (8). These findings indicate that the recognition of NeuAc linked to galactose by the α2,3 linkage is not sufficient to support influenza A virus replication in duck intestine. Rather, NeuGc recognition also appears to be essential. In accord with the receptor specificity of the HA and viral replication in ducks, SAα2,3Gal but not SAα2,6Gal is predominant (13) and NeuGcα2,3Gal was found in the crypt cells of duck colon epithelium (Fig. 2), the exclusive site of viral replication (Fig. 3). However, duck viruses can recognize NeuAcα2,3Gal, which is also present in duck intestine. Why do other intestinal cells likely possessing NeuAcα2,3Gal fail to support viral replication? One possibility is that molecules containing NeuGcα2,3Gal serve as functional receptors, while those possessing NeuAcα2,3Gal do not. Collectively, our data indicate the importance of both the sialic acid species and the type of linkage between sialic acid and galactose in establishing the host range of influenza viruses. Additional support for this hypothesis comes from a recent study in which we show that the recognition of NeuGc is also critical for influenza virus replication in horses (unpublished data).
H3 human viruses isolated in 1968 recognize only NeuAc, whereas those isolated after 1972 recognize both NeuAc and NeuGc (6). The biologic significance of this change is unknown. Since human cells lack NeuGc (15, 16), the acquisition of NeuGc specificity cannot be attributed to the adaptation of the virus in humans. In light of the present finding that recognition of NeuGcα2,3Gal is associated with viral replication in ducks and that the HA of human H3 viruses was introduced from wild birds, we suggest that the human virus isolated in 1968 lost its ability to recognize NeuGc to accommodate its change in linkage specificity from α2,3 (avian type) to α2,6 (human type) or perhaps because of other (unknown) requirements for adaptation to a new environment (i.e., human cells). During subsequent replication in humans, the virus may have undergone further amino acid changes to escape immunologic pressures, rendering it capable of NeuGc recognition.
This report appears to be the first to document the presence of NeuGc in avian species. This sialic acid has not been identified in normal human and chicken tissues (15, 16) but is commonly found in cows, horses, and pigs (16, 18, 22, 23). Hence, equine and swine influenza viruses would be expected to recognize both NeuGc and NeuAc, a prediction supported by previous findings (6, 32).
Higa et al. (6) reported that two duck viruses, including A/duck/Ukraine/1/63, failed to recognize NeuGcα2,3Gal on enzymatically modified erythrocytes, while two others reacted. The majority of duck viruses in our study recognized NeuGcα2,3Gal. One exception was the A/duck/Ukraine/1/63 virus, which bound to this glycoconjugate only weakly and failed to replicate well in duck intestine, further emphasizing the essential role of NeuGcα2,3Gal recognition in the successful replication of influenza A viruses in ducks.
Couceiro et al. (4) showed that in addition to the predominance of the NeuAcα2,6Gal linkage in human tracheal epithelial cells, human bronchial mucin, which contains sialic acid primarily with the NeuAcα2,3Gal linkage (17), can potently inhibit the binding of NeuAcα2,3Gal-recognizing viruses to tracheal sections. This finding suggests that a combination of a missing receptor moiety and the presence of receptor analog inhibitors could protect humans from infection by avian influenza viruses. Such strategies may well prove useful in the influenza armamentarium but are not likely to provide universal protection, as demonstrated by the direct transmission of H5N1 avian influenza viruses from birds to humans during the recent outbreak in Hong Kong (2, 31). By contrast, previous studies demonstrate strict restriction of a virus possessing the human virus HA in duck intestine (8). These findings suggest that the extent of host range restriction controlled by the HA appears to depend on the combination of host animal species and virus.
In this study, we focused on the receptor specificity of the HA. However, the genes of influenza A viruses encoding internal proteins in addition to the HA and NA may also play roles in host range restriction. For example, the NP and M genes can attenuate avian influenza virus infection in squirrel monkeys (34), and depending on the human influenza viruses used to prepare reassortants with avian viruses, a combination of polymerase genes may affect viral replication in this host animal (29, 30). Further studies are needed to fully delineate the contribution of these gene products to host range restriction.
Acknowledgments
We thank Martha McGregor for excellent technical assistance, Robert G. Webster for NS1 monoclonal antibodies, and John Gilbert for editing the manuscript. We also thank Mikhail Matrosovich for useful discussion.
Support for this work came from National Institute of Allergy and Infectious Diseases Public Health Service research grants.
REFERENCES
- 1.Beare A S, Webster R G. Replication of avian influenza viruses in humans. Arch Virol. 1991;119:37–42. doi: 10.1007/BF01314321. [DOI] [PubMed] [Google Scholar]
- 2.Claas E C J, Osterhaus A D M E, Van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 3.Connor R J, Kawaoka Y, Webster R G, Paulson J C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- 4.Couceiro J N, Paulson J C, Baum L G. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium: the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 1993;29:155–165. doi: 10.1016/0168-1702(93)90056-s. [DOI] [PubMed] [Google Scholar]
- 5.Hara S, Takemori Y, Yamaguchi M, Nakamura M, Ohkura Y. Fluorometric high-performance liquid chromatography of N-acetyl- and N-glycolylneuraminic acids and its application to their microdetermination in human and animal sera, glycoproteins, and glycolipids. Anal Biochem. 1987;164:138–145. doi: 10.1016/0003-2697(87)90377-0. [DOI] [PubMed] [Google Scholar]
- 6.Higa H H, Rogers G N, Paulson J C. Influenza virus hemagglutinins differentiate between receptor determinants bearing N-acetyl-, N-glycolyl-, and N,O-diacetylneuraminic acids. Virology. 1985;144:279–282. doi: 10.1016/0042-6822(85)90325-3. [DOI] [PubMed] [Google Scholar]
- 7.Hinshaw V S, Bean W J, Webster R G, Sriram G. Genetic reassortment of influenza A viruses in the intestinal tract of ducks. Virology. 1980;102:412–419. doi: 10.1016/0042-6822(80)90108-7. [DOI] [PubMed] [Google Scholar]
- 8.Hinshaw V S, Webster R G, Naeve C W, Murphy B R. Altered tissue tropism of human-avian reassortant influenza viruses. Virology. 1983;128:260–263. doi: 10.1016/0042-6822(83)90337-9. [DOI] [PubMed] [Google Scholar]
- 9.Hirabayashi Y, Suzuki T, Suzuki Y, Taki T, Matsumoto M, Higashi H, Kato S. A new method for purification of anti-glycosphingolipid antibody. Avian anti-hematoside (NeuGc) antibody. J Biochem (Tokyo) 1983;94:327–330. doi: 10.1093/oxfordjournals.jbchem.a134350. [DOI] [PubMed] [Google Scholar]
- 10.Homaidan F R, Zhao L, Donovan V, Shinowara N L, Burakoff R. Separation of pure populations of epithelial cells from rabbit distal colon. Anal Biochem. 1995;224:134–139. doi: 10.1006/abio.1995.1018. [DOI] [PubMed] [Google Scholar]
- 11.Hoyle L. Influenza viruses. Virol Monogr. 1969;4:88. [Google Scholar]
- 12.Ito T, Suzuki Y, Mitnaul L, Vines A, Kida H, Kawaoka Y. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology. 1997;227:493–499. doi: 10.1006/viro.1996.8323. [DOI] [PubMed] [Google Scholar]
- 13.Ito T, Couceiro J N S S, Kelm S, Baum L G, Krauss S, Castrucci M R, Donatelli I, Kida H, Paulson J C, Webster R G, Kawaoka Y. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol. 1998;72:7367–7373. doi: 10.1128/jvi.72.9.7367-7373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kida H, Yanagawa R, Matsuoka Y. Duck influenza lacking evidence of disease signs and immune response. Infect Immun. 1980;30:547–553. doi: 10.1128/iai.30.2.547-553.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klenk E, Lempfrid H. Uber die Natur der Zellreceptoren fir das Influenzavirus. Hoppe-Seyler's Z Physiol Chem. 1957;307:278–283. [PubMed] [Google Scholar]
- 16.Klenk E, Uhlenbruck G. Uber ein neuraminsaurehaltiges Mucoproteid aus Rindererythrocytenstroma. Hoppe-Seyler's Z Physiol Chem. 1958;311:227–233. [PubMed] [Google Scholar]
- 17.Lamblin G, Lhermitte M, Klein A, Roussel P, Van Halbeek H, Vliegenthart J F. Carbohydrate chains from human bronchial mucus glycoproteins: a wide spectrum of oligosaccharide structures. Biochem Soc Trans. 1984;12:599–600. doi: 10.1042/bst0120599. [DOI] [PubMed] [Google Scholar]
- 18.Martensson E, Raal A, Svennerholm L. Sialic acid in blood serum. Biochim Biophys Acta. 1958;30:124–129. doi: 10.1016/0006-3002(58)90248-8. [DOI] [PubMed] [Google Scholar]
- 19.Murphy B R, Sly D L, Hosier N T, London W T, Chanock R M. Evaluation of three strains of influenza A virus in humans and in owl, cebus, and squirrel monkeys. Infect Immun. 1980;28:688–691. doi: 10.1128/iai.28.3.688-691.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy B R, Hinshaw V S, Sly D L, London W T, Hosier N T, Wood F T, Webster R G, Chanock R M. Virulence of avian influenza A viruses for squirrel monkeys. Infect Immun. 1982;37:1119–1126. doi: 10.1128/iai.37.3.1119-1126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naeve C W, Hinshaw V S, Webster R G. Mutations in the hemagglutinin receptor-binding site can change the biological properties of an influenza virus. J Virol. 1984;51:567–569. doi: 10.1128/jvi.51.2.567-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naiki M. Chemical and immunochemical properties of two classes of globoside from equine organs. Jpn J Exp Med. 1971;41:67–81. [PubMed] [Google Scholar]
- 23.Pettersson S O, Sivertsson R, Sjogren S, Svennerholm L. The sialic acids of hog pancreas. Biochim Biophys Acta. 1958;28:444–445. doi: 10.1016/0006-3002(58)90498-0. [DOI] [PubMed] [Google Scholar]
- 24.Rogers G N, Paulson J C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 25.Rogers G N, D'Souza B L. Receptor binding properties of human and animal H1 influenza virus isolates. Virology. 1989;173:317–322. doi: 10.1016/0042-6822(89)90249-3. [DOI] [PubMed] [Google Scholar]
- 26.Rogers G N, Paulson J C, Daniels R S, Skehel J J, Wilson I A, Wiley D C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983;304:76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- 27.Rohm C, Zhou N, Suss J, Mackenzie J, Webster R G. Characterization of a novel influenza hemagglutinin, H15: criteria for determination of influenza A subtypes. Virology. 1996;217:508–516. doi: 10.1006/viro.1996.0145. [DOI] [PubMed] [Google Scholar]
- 28.Snyder M H, Buckler-White A J, London W T, Tierney E L, Murphy B R. The avian influenza virus nucleoprotein gene and a specific constellation of avian and human virus polymerase genes each specify attenuation of avian-human influenza A/Pintail/79 reassortant viruses for monkeys. J Virol. 1987;61:2857–2863. doi: 10.1128/jvi.61.9.2857-2863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snyder M H, Clements M L, De Borde D, Maassab H F, Murphy B R. Attenuation of wild-type human influenza A virus by acquisition of the PA polymerase and matrix protein genes of influenza A/Ann Arbor/6/60 cold-adapted donor virus. J Clin Microbiol. 1985;22:719–725. doi: 10.1128/jcm.22.5.719-725.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subbarao E K, London W, Murphy B R. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J Virol. 1993;67:1761–1764. doi: 10.1128/jvi.67.4.1761-1764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki T, Horiike G, Yamazaki Y, Kawabe K, Masuda H, Miyamoto D, Matsuda M, Nishimura S I, Yamagata T, Ito T, Kida H, Kawaoka Y, Suzuki Y. Swine influenza virus strains recognize sialylsugar chains containing the molecular species of sialic acid predominantly present in the swine tracheal epithelium. FEBS Lett. 1997;404:192–196. doi: 10.1016/s0014-5793(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki Y, Nakao T, Ito T, Watanabe N, Toda Y, Xu G, Suzuki T, Kobayashi T, Kimura Y, Yamada A, Sugawara K, Nishimura H, Kitame F, Nakamura K, Deya E, Kiso M, Hasegawa A. Structural determination of gangliosides that bind to influenza A, B, and C viruses by an improved binding assay: strain-specific receptor epitopes in sialo-sugar chains. Virology. 1992;189:121–131. doi: 10.1016/0042-6822(92)90687-k. [DOI] [PubMed] [Google Scholar]
- 34.Tian S-F, Buckler-White A J, London W T, Reck L J, Chanock R M, Murphy B R. Nucleoprotein and membrane protein genes are associated with restriction of replication of influenza A/Mallard/NY/78 virus and its reassortants in squirrel monkey respiratory tract. J Virol. 1985;53:771–775. doi: 10.1128/jvi.53.3.771-775.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vines A, Wells K, Matrosovich M, Castrucci M R, Ito T, Kawaoka Y. The role of influenza A virus hemagglutinin residues 226 and 228 in receptor specificity and host range restriction. J Virol. 1998;72:7626–7631. doi: 10.1128/jvi.72.9.7626-7631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webster R G, Yakhno M, Hinshaw V S, Bean W J, Murti K G. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology. 1978;84:268–278. doi: 10.1016/0042-6822(78)90247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webster R G, Bean W J, Gorman O T, Chambers T M, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]