Abstract
Protein hydrolysis is a process used in the food industry to generate bioactive peptides of low molecular weight and with additional health benefits, such as antihypertensive, antidiabetic, and antioxidant properties that are often associated with their content on hydrophobic amino acids. This results in an increased bitterness of the products, making them less desirable for their use in food formulations. This review summarizes the main dietary sources of bitter bioactive peptides, including methods to determine their bitterness, such as the Q-values and electronic tongue; and the main factors and mechanisms underlying the bitterness of these compounds. The main strategies currently used to improve the taste and oral delivery of bioactive peptides are also discussed together with the main advantages and drawbacks of each technique. Debittering and masking techniques are reported in detail, including active carbon treatments, alcohol extraction, isoelectric precipitation, chromatographic methods, and additional hydrolytic processes. Other masking or blocking techniques, including the use of inhibitors, such as modified starch, taurine, glycine, and polyphosphates, as well as chemical modifications, such as amination, deamination, acetylation, or cross-linking were also discussed. The findings of this work highlight encapsulation as a highly effective method for masking the bitter taste and promoting the bioactivity of peptides compared to other traditional debittering and masking processes. In conclusion, the article suggests that advanced encapsulation technologies can serve as an effective means to mitigate the bitterness associated with bioactive peptides, while simultaneously preserving their biological activity, increasing their viability in the development of functional foods and pharmaceuticals.
Subject terms: Peptides, Agriculture
Introduction
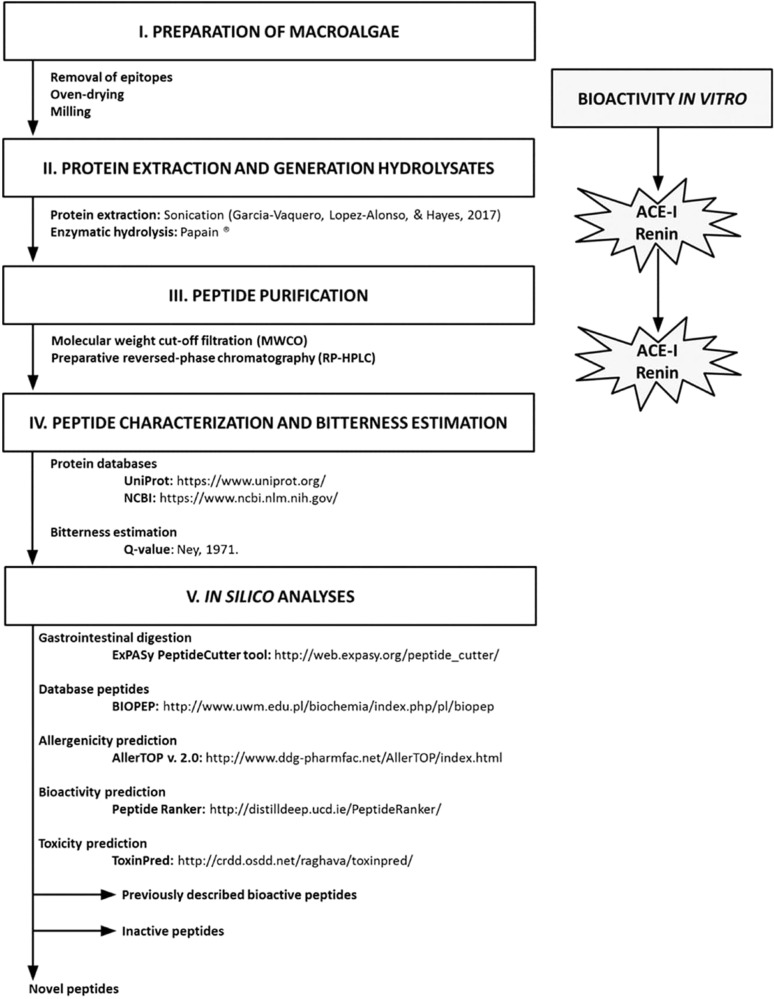
Research has shown that bioactive peptides exhibit a range of potentially beneficial biological activities, which has stimulated interest in their application as therapeutic agents. In the United States, more than 60 peptides exhibiting therapeutic properties have been approved for human consumption since 2018. One of the most significant peptide-based medications licensed and commercialized since the 1920s is insulin1. The World Health Organization (WHO) has stated that “non-communicable illnesses including cancer, diabetes, and hypertension cause 36 million fatalities annually”2. Studies have shown that bioactive peptides may be able to reduce the risk of a range of these chronic illnesses, including diabetes3–6, hypertension7,8, and cancer9–13. Consequently, the development of functional foods, supplements, or drugs containing bioactive peptides may be able to improve the health of the general population and strategies for the generation of these compounds from different protein sources, such as macroalgae, have been proposed (Fig. 1). However, any bioactive formulation intended for oral administration should be appealing to consumers14, which means it should not have an undesirable flavor profile or mouthfeel.
Fig. 1.
Scheme of a proposed strategy for the generation of bioactive peptides from macroalgae21.
The bitter taste of many bioactive peptides is one of the largest hurdles to their widespread use in functional foods, supplements, and drugs intended for oral ingestion15,16. Many animals, including humans, perceive peptides as having an unpleasant flavor due to millions of years of evolution, since peptides are often associated with harmful substances17.
This article reviews the different food sources of bioactive peptides and the various strategies that have been developed to make them more palatable, including debittering, masking, modulation, and encapsulation technologies. In addition, it considers the application of these technologies in the development of functional foods, supplements, and pharmaceuticals intended for oral administration.
Generation of bitter peptides from different protein sources
According to Ney18, the bitterness of peptides is closely linked to their hydrophobicity. The sum of the free energy arising from the transfer of each amino acids’ side chains from ethanol to water divided by the total number of amino acid residues in the peptide is known as the average hydrophobicity of a peptide or Q-value:
| 1 |
Here, ΔG and n are the transfer free energy and number of amino acid residues, respectively. When the Q value of a peptide reaches 1400 cal/mol, it is regarded as being bitter19, whereas a Q value of less than 1300 cal/mol denotes a non-bitter peptide. An intermediate value suggests that the peptide has a slight bitterness. In addition, the Q-value may be used to assess the bitterness of peptides with molecular weights of less than 6 kDa, roughly 55 amino acid residues20. In a study conducted by Garcia-Vaquero et al.21, bioactive peptides from Ulva lactuca were produced using in vitro and in silico methods. Except for the sequences SAGVLPWK, GAAPTPPSPPPATKPSTPPKPPT, IECCLLFALV, PVGCLPK, DAVEIWRVK, DEVIPGAL, PKPPALCN, and PPNPPNPPN, all of the detected peptides had Q-values below the bitterness threshold. The Q-values of these peptides ranged from 1440 to 1743 cal/mol, which suggests that they had a bitter taste. According to Fan et al.22, Alaska pollock frame hydrolysates showed no bitterness when the Q-value was below 5.44 kJ/mol, but did exhibit bitterness when the Q-value was higher than 5.86 kJ/mol. In addition, hydrolysates made from high Q-value proteins like casein (1605 cal/mol), soy protein (1540 cal/mol), and zein (1480 cal/mol) also have a bitter taste23.
The ratio of hydrophilic to hydrophobic amino acids determines how soluble a protein is. In this regard, hydrophobic peptides, which have a bitter taste, are released more by water-insoluble proteins. It has been demonstrated that peptide bitterness is strongly related to the amount of hydrophobic amino acids present, and thus the peptide’s overall hydrophobicity24. Therefore, the release of bitter peptides from protein is related to the amino acid composition and sequence, enzyme active site, as well as degree of hydrolysis25. When hydrolyzing a water-insoluble protein under identical conditions, alcalase and trypsin produce different types of peptides. Trypsin cleaves peptide bonds at the C-terminal with basic residues like arginine and lysine, while alcalase is an endopeptidase that positions hydrophobic residues such as phenylalanine, tyrosine, and tryptophan in the middle of the peptide chain, resulting in hydrophobic residues at the end of the peptide chain. This difference in enzyme specificity leads to the production of bitter and non-bitter peptides. To put it differently, hydrolysis by alcalase leads to the production of peptides with a bitter taste as a result of the hydrophobic residues being present at the end of the protein hydrolysates26,27.
It is possible to adjust the conditions used to hydrolyze proteins to produce bioactive peptides that are not bitter, such as hydrolysis method, time, temperature, or solution conditions. For instance, Fan et al.22 reported that the Q-value of hydrolysates increased during the first 90 min of enzymatic hydrolysis but then decreased at longer times. In the earlier stages of protein hydrolysis, the hydrophobic amino acids generated are relatively large and can interact with the bitterness receptors on the tongue28–30. In contrast, during the later stages of protein hydrolysis, only small peptides or free amino acids are present, which do not strongly interact with the tongue28,31. Table 1 summarizes the taste of each amino acid to establish clear comparisons.
Table 1.
The taste qualities of amino acids and sensing mechanisms by G protein-coupled receptors (GPCRs).
| Amino acid | Human taste perception | Sensing mechanism by GPCRs |
|---|---|---|
| ʟ-Alanine | Sweet | GPRC6A |
| ʟ-Arginine | Bitter | GPRC6A |
| ʟ-Asparagine | Bitter | T1R1/T1R3 |
| ʟ-Aspartic acid | Umami, sour | T1R1/T1R3 |
| ʟ-Cysteine | Sulfurous | T1R1/T1R3 |
| ʟ-Glutamic acid | Umami, salty | T1R1/T1R3, mGluRs |
| ʟ-Glycine | Sweet | GPRC6A |
| ʟ-Histidine | Bitter | CaSR |
| ʟ-Hydroxyproline | Sweet | T1R1/T1R3 |
| ʟ-Isoleucine | Bitter | T1R1/T1R3 |
| ʟ-Leucine | Bitter | T1R1/T1R3 |
| ʟ-Lysine | Bitter, salty, sweet | GPRC6A |
| ʟ-Methionine | Bitter, sulfurous | GPRC6A |
| ʟ-Phenylalanine | Bitter | CaSR |
| ʟ-Proline | Sweet | T1R1/T1R3 |
| ʟ-Serine | Sweet | GPRC6A |
| ʟ-Threonine | Sweet | T1R1/T1R3 |
| ʟ-Tryptophane | Bitter | CaSR |
| ʟ-Tyrosine | Bitter | Not detectable yet |
| ʟ-Valine | Bitter | T1R1/T1R3 |
To a first approximation, the bitterness of many bioactive peptides can be predicted from their Q-value, which mainly depends on their hydrophobicity. However, researchers have shown that the bitterness of peptides also depends on their molecular weight, amino acid sequence, and composition28,32,33.
Cultural and regional differences affect how people perceive bitterness, making it challenging to develop standardized methods to measure bitterness accurately34. The electronic tongue (E-tongue) can be used to overcome this problem and efficiently screen peptides from different sources and hydrolysis methods in a standardized manner. For instance, Nath et al.35 used an E-tongue to evaluate the bitterness of bioactive peptides derived from tryptic and ferment milk protein concentrate (MPC). The MPC was hydrolyzed using the tryptic hydrolysis method followed by hydrolysis using two microbial species utilized in yogurt manufacturing (Lactobacillus bulgaricus and Streptococcus thermophilus). The results of the experiment indicated that there is a direct relationship between the bitterness of the hydrolysates and the concentration of trypsin; and the potential application of the E-tongue was demonstrated by measuring the bitterness of peptides at each hydrolysis step using a standard model solution of quinine. These methods are particularly useful for rapidly screening peptides produced from different sources and different hydrolysis methods. Ideally, researchers would like to use these methods to identify peptides that have a high bioactivity but a low off-flavor. In the remainder of this section, we highlight different sources of food proteins that can be used to produce bioactive peptides.
Milk proteins
The hydrolysates from milk proteins have been shown to exhibit a range of biological activities, which makes them suitable for application in functional food products36. Milk protein hydrolysates are commonly derived from their parent proteins using either enzymatic digestion or fermentation processes37. Hydrolysates have valuable biological activities but are sometimes limited in use due to their bitter taste38, which is influenced by various factors such as the nature of side chains on the peptides produced by hydrolysis, distribution and location of bitter taste residues, hydrophobicity, degree of hydrolysis, amino acid conformation, peptide sequence, and carbon number on the amino acid side chain16,33,39–42. Whey protein hydrolysates have malty, brothy potato, animal, and bitter flavors43, while sodium caseinate generates more bitter peptides during hydrolysis than whey protein44,45.
Researchers have reported that increasing the degree of hydrolysis of proteins by more than 8% leads to the production of bitter-tasting peptides46. Nevertheless, hydrolysis can also reduce the allergenicity of milk proteins, such as casein, β-lactoglobulin, and ɑ-lactalbumin, increase the production of bioactive peptides, and improve the nutritional quality of these products. Consequently, the potential advantages of hydrolysis of dairy proteins should not be ignored.
The type of enzyme used to carry out the hydrolysis of proteins affects the types and amounts of bitter peptides produced. For instance, hydrolyzing whey proteins using alcalase 2.4 L was reported to generate more bitter peptides than hydrolyzing them using prolyve or corolase under the same reaction conditions47. The amino acid sequence of peptides has also been shown to strongly influence their bitterness. For instance, Shinoda et al.42 reported that inverting the peptide sequence of RGPFFIIV (derived from beta-casein) greatly decreased its bitter taste. Interestingly, it has been reported that some animals avoid consuming casein hydrolysates due to the presence of bitter peptides16. Therefore, methods are needed to reduce the bitterness of these hydrolysates if they are going to be utilized as bioactive ingredients in food and feed applications.
Soybean proteins
Soybeans contain relatively high concentrations of good quality proteins. Unfortunately, eight proteins in soybean (Gly m1-Gly m8) have so far been reported to be allergens, which limits their use as a protein source48. The conversion of soybean proteins into hydrolysates and peptides can overcome this limitation. In addition to being used as bioactive ingredients in functional foods, soybean protein hydrolysates are also widely used as techno-functional ingredients, such as thickeners, gelling agents, emulsifiers, and foaming agents49,50. Again, however, one of the most important factors limiting their use in many food and beverage applications is their bitter taste (Table 2). Cho et al.32 reported that the bitterness of soybean protein hydrolysates is associated with their molecular mass, with larger peptides (>4 kDa) being more bitter than smaller ones (<1 kDa). However, it has been reported that the cause of bitterness in alcalase-treated soybean hydrolysates was due to the presence of 1 kDa hydrophobic peptides51. The bitterness of protein hydrolysates has been reported to depend on the type of enzyme used to hydrolyze them, with the bitterness decreasing in the following order: alcalase > neutrase ≈ trypsin > Flavourzyme52. However, in another study, the bitterness of bromelain-treated soybean hydrolysates (4% hydrolysis) was reported to be no different from that of soybean protein isolate53. In contrast, another study showed that soybean hydrolysates generated using bromelain (10–15% hydrolysis) were extremely bitter52. Furthermore, it has also been suggested that hydrophobic amino acids, such as leucine and phenylalanine, do not contribute to the bitter taste of soy hydrolysates54. Dall Aaslyng et al.55 indicated that soybean hydrolysates exhibit a bitter taste when heated, which appeared to be due to pyrazines. Furthermore, bitter peptides were reported from miso (salted and fermented soybean paste), natto (fermented soybean), and soy sauce56–59. Consequently, many factors appear to contribute to the bitterness of soy protein hydrolysates.
Table 2.
Q-values of peptides produced during the hydrolysis of various proteins using different hydrolysis conditions.
| Protein source | Identified peptide sequences (Q value)a | Hydrolysis conditionsb | Bitterness evaluation method | Factors influencing bitterness | Bioactivities | References |
|---|---|---|---|---|---|---|
| Milk | VLPVPQK (1876), EIVESLSSSEESITRINK (972), FLLY (2189), PFPIIV (2810), SDISLLDA (1171), TTMPL (1324), ESISSSEEIV (983), FPQY (2088), PPFL (2707), IESPPEIN (1660), DERFFSDK (1233), MMSFV (1591), PTPEG (1272), TTMPLW (1739), MPFPK (2383), VLSR (1185), SDISLLDA (1171), LLFCME (1814), NLPPLTA (1551), SFLY (1918), LCVLH (1683), KHQGL (890), KEGI (1341), KKNQDKTEI (1001), DAQSAP (825) | Bromelain (50 °C, pH = 7), proteinase K (37 °C, pH = 7), papain (65 °C, pH = 7), and ficin (50 °C, pH = 6.5); E/S: 1/100 (w/w); time: 3 h | BIOPEP-UWM database prediction | Hydrophobicity and enzyme active site | Antioxidative, opioid, immunomodulating, ACE inhibitory | 200 |
| LLYQEPVLGPVRGPFPIIV (1933), LYQEPVLGPVRGPFPI (1848), LYQEPVLGPVRGPFP (1760), LYQEPVLGPVRGPFPIIV (1921), LLYQEPVLGPVRGPFP (1784), FFVAPFPEVFGK (2000) | Flavourzyme (50–55 °C, pH = 5.5–7.5), protamex (50 °C, pH = 7–8), promod 523MDPTM (45–55 °C, pH = 5–7), and flavorpro 937MDPTM (50 °C, pH = 5–7); time: 9 h | Taste panelists | Degree of hydrolysis | – | 201 | |
| – | Trypsin (40 °C); E/S: 0.008–0.032 g/L; time: 10 min | Taste panelists | Hydrophobicity | – | 35 | |
| VEELKPTPEGDLEIL (1452), VEE (1009), LKPTPE (1685), GDLE (831), IL (2660) | Alcalase 2.4 L, prolyve 1000 and corolase 7089; 50 °C; pH = 7, E/S: 0.25% | Taste panelists | Hydrophobicity | – | 47 | |
| VLVLDTDYK (1487), GLDIQK (1234), IDALNEK (1269), TPEVDDEALEK (1109), VGINYWLAHK (1684), HIRLS (1374), KTKIPAVF (1845), MAASDISLL (1247), VRTPEVDDE (1075), LVRTPEVDDE (1183), VRTPEVDDEALE (1102), LVRTPEVDDEALE (1182), VLPVPQ (1922), KAVPYPQ (1802), RDMPIQAF (1580), SQSKVLPVPQ (1297), PEGDLEI (1426), VEELKPT (1378), VEELKPTPE (1460), AVPYPQ (1835) | Alcalase (65 °C, pH = 8.5, E/S: 1/100, time: 5 h), corolase PP (45 °C, pH = 7.5, E/S: 1/50, time: 7 h), flavourzyme (50 °C, pH = 6.5, E/s: 1/100, time: 8 h) | Taste panelists | Enzyme active site | Antioxidative | 202 | |
| – | Alcalase (55 °C, pH = 8.5), protamex (50 °C, pH = 6.5), flavourzyme (50 °C, pH = 7); E/S: 1/50; time: 30–180 min | E-tongue measurement | Enzyme active site | – | 26 | |
| – | Commercial protein hydrolysates | Taste panelists | Degree of hydrolysis and hydrophobicity | – | 43,203 | |
| Soybean | FLS (1666), LLPH (2000), INGY (1458), IYIG (2253), VYDV (1734), SVIY (1913), VYFV (2309), ISIY (2245), VVLY (2126), DIF (2211), GYPVV (1851), YVVL (2126), SGFTL (993), SNLNFL (1188) | Alcalase (50 °C, pH = 8, E/S: 12 AU/Kg) | Taste panelists | Hydrophobicity | – | 204 |
| LSVISPK (1657), DVLVIPLG (1829), LIVILNG (1781), NPFLFG (1800), ISSTIV (1348), PQMIIV (2102), PFPSIL (2328), DDFFL (1815), FFEITPEK (1823) |
Bromelain (50 °C, pH = 7), proteinase K (37 °C, pH = 7), papain (65 °C, pH = 7), and ficin (50 °C, pH =6.5); E/S: 1/100 (w/w); time: 3 h |
BIOPEP-UWM database prediction | Hydrophobicity and enzyme active site | Antioxidative, opioid, immunomodulating, ACE inhibitory | 205 | |
| AFPGSAKDIENLIK (1443), YVVNPDNNENLRLITL (1297), RRPSYTNGPQEIY (1253), SLENQLDQMPRRF (1092), VVNPDNNENLRLITL (1205), RIDPELEKEIGAK (1425), ENQLDQMPRRF (1099), SLLNALPEEVIQHTFNLK (1311), NQLDQMPRRF (1149), RAELSEQDIFVIPA (1437), LVPPQESQRR (1171), IIDTNSLENQLDQMPRRFYLA (1271), GINAENNQRNFLA (848), KLHENIARPS (1275), IVRNLQGENEEEDSGAIVTVK (950), LSIVDMNEGALLLPHFNSK (1332), AGANSLLNALPEEVIQH (1094), RVSDDEFNNY (988), NALEPDHRVESE (966), LAGNPDIEHPETM (1237), GKHQQEEENEGGSIL (656), EQGGEQGLEYVVF (997) | Protex 6 L (55 °C, pH = 8), protease A 2 SD (50 °C, pH = 7); E/S: 0.5%; time: 1–5 h | Taste panelists | Hydrophobicity and enzyme active site | Antioxidative | 24 | |
| Corn | – | Flavourzyme (50 °C, pH = 7), alcalase (50 °C, pH = 8), neutrase (50 °C, pH = 7), papain (55 °C, Ph = 7), and trypsin (40 °C, pH = 7.5); E/S: 3%; time: 10–100 min | Taste panelists | Degree of hydrolysis and hydrophobicity | ACE inhibitory | 65 |
| Fish | KAEPAPAPAPAPAPAPAPAP (1775), DEKSPAMPVPGPM (1558), GPAGPRGPSGERGEVGPA (986), LDKNKDPLNDSVVQ (1120), FAGDDAPRA (1125), SGPPVPGPIGPM (1759), LGAGDTDGDGKIGAD (721), DEKSPAMPVPGPMGPM (1549), IEDPFDQDDWEH (1415), LEQQVDDLEGSLE (846), APDPGPGPMG (1461), NWDDMEKIW (1754), GPPVPGPIGPM (1922), ERLEDEEEIN (963), AVIDQDKSGFIEEDELKLF (1349), RDLTDYLMK (1346), MPVPGPMGPM (1834), GPPVPGPIGPMG (1762), LGEQIDNLQRVK (1054), DPFDQDDWEHWAK (1495), GVDNPGHPF (1321), EDDIHPRNPPKFD (1558), MDEPVVVPGKPY (1775), GWLDKNKDPLNDSVVQ (1218), FIEEDELKLF (1719), SVDDEFPDLTK (1241), AGDDAPRAVFPS (1236), DRPGPPDGPLE (1480), SPAMPVPGPM (1747), GPSGKDGPRGPRGD (928), GEEGKRGPTGEIG (784), LDLPGPPIGPIN (1901), DLPGPPIGPIN (1878), GPAGPRGPSGA (1004), PPVPGPIGPM (2114), DRPGPPDGPLEVK (1517) | Neutrase (50 °C, pH = 7, E/S: 0.4–0.8%), bromelain (50 °C, pH = 7, E/S: 0.6–0.8%), flavourzyme (50 °C, pH = 7, E/S: 0.8%), protamex (50 °C, pH =7, E/S: 0.4–0.6%), papain (55 °C, pH = 7, E/S: 0.6–0.8%), and alcalase (55 °C, pH = 9, E/S: 0.6–0.8%); time: 2–10 h | Taste panelists | Hydrophobicity and enzyme active site | – | 25 |
| – | FoodPro PNL enzyme, 55 °C, E/S: 0.17–0.22 w/w, time: 50 min | Taste panelists | Hydrophobicity and enzyme active site | – | 206 | |
| – | Papain (pH = 7), trypsin (pH = 8), pepsin (pH = 2), alkaline (pH = 10) and flavourzyme (pH = 6); 50 °C; E/S: 100 U/g; time: 150 min | Taste panelists | Enzyme active site | Leucocytes and lysozyme activity | 207 | |
| – | Protamex (40 °C, pH = 6), alcalase (60 °C, pH = 8), trypsin (38 °C, pH = 8), flavourzyme (50 °C, pH = 6) and neutrase (45 °C, pH = 7); E/S: 2%; time: 5 h | Taste panelists | Hydrophobicity and enzyme active site | – | 208 | |
| Seaweed | – | Protease A ‘Amano’ G (50 °C, pH = 7), protease M ‘Amano’ (50 °C, pH = 4.5), protease N ‘Amano’ (55 °C, pH = 7), protease P ‘Amano’ 3G (45 °C, pH = 8), protease S ‘Amano’ (70 °C, pH = 8), blomelain F (60 °C, pH = 9), proleather FG-F (60 °C, pH = 10), peptidase R (45 °C, pH = 7), umamizyme (50 °C, pH = 7), newlase F (45 °C, pH = 3), papain W-40 (65 °C, pH = 7), pancreatine F (45 °C, pH = 9), pepsin (45 °C, pH = 2), denazyme AP (50 °C, pH = 7), denapsin 10P (50 °C, pH = 3), XP-415 (55 °C, pH = 3), alcalase 2.4 L FG (60 °C, pH = 7), E/S: 1%; time: 18 h | – | Hydrophobicity | ACE inhibitory | 209 |
| Synthetic peptides | YGLF (1926), YPFPGPIPN (2257), IPAVF (2318), LLF (2393) | – | Taste panelists | Hydrophobicity | – | 121 |
| MPFPKYPVEPF (2336), GPVRGPFPIIV (2034), MAPKHKEMPFPKYPVEPF (1979), APHGKEMPFPKYPVEPF (1905) | – | Taste panelists | Hydrophobicity | – | 210 | |
| Bovine muscle | – | Alcalase (50 °C, pH = 7.5), flavourzyme (50 °C, pH = 7), protamex (55 °C, pH = 7.5), neutrase (50 °C, pH = 7), proteAX (50 °C, pH = 7), protease P 6 SD (40 °C, pH = 7), protease A 2 SD (50 °C, pH = 7), papain P1 (55 °C, pH = 7), bromelain (40 °C, pH = 7) and sumizyme BNP-L (50 °C, pH = 7); E/S: 0.5%; time: 1–5 h | Taste panelists | Degree of hydrolysis and enzyme active site | – | 150 |
| Porcine plasma | – | Taste panelists | ||||
| Bovine hemoglobin | VVYPWTQR (1715), VVYPW (2614) | Pepsin (37 °C, pH = 3) | Taste panelists | Hydrophobicity | – | 211 |
| Pea | – | Alcalase (50 °C, pH = 8), flavourzyme (50 °C, pH = 7), papain (40 °C, pH = 6.5), trypsin and α-chymotrypsin (37 °C, pH = 8); E/S: 4%; time: 4 h | Taste panelists | Hydrophobicity and enzyme active site | Antioxidative, ACE inhibitory | 84 |
| – | Alcalase (55 °C, pH = 8.5, E/S: 3%, time: 2.5 h) followed by protemax (50 °C, pH = 7, E/S: 1%, time: 1 h) | Taste panelists | Hydrophobicity | ACE and DPP-IV inhibitory | 87 | |
| Peanut | – | Multifect PR 6 L and flavorzyme (pH = 9.5), protamex, neutrase, and Papain (pH = 7.5); 45 °C | E-tongue measurement | Degree of hydrolysis | – | 212 |
| Soy, pea, and canola | – | Flavourzyme 1000 L, protease P “Amano” 6 SD, deltazymAPS-M-FG, promod278, proteAX-K, and peptidase R; pH = 7.3; 50 °C; E/S: 2%; time: 2 h | Taste panelists | Hydrophobicity and enzyme active site | – | 213 |
| Chickpea | LR (1465), PLLVE (1926), SPKAGAGK (959), HATGGGSGR (257), PHPATSGGGL (949), TPKASATAAL (976), TLTTGTGGLL (632), YVDGSGTPLT (1005), TKTPGAGTSAGL (677), KEGGGTGTGAAR (376), STGPNAGGGAGGY (546), TLLFTELLF (1654), KNGAAGPSTVAR (787), LASEGASAATGAF (733), VLTSGAGSGAAALT (658), KNGLGAGAGAGSAR (549), LSAHAGGTGATLW (863), LDLARAGGCPTKN (1015), SPQSPPFATPLW (1754), LLSASMGSQLLSF (1053), GKGSGAF (750), TRGTGGR (214), KMTAGSGVT (642), KSGGGGGGTAVT (345), GKAAPGSGGGTKA (649), RASAAGGGGGGVSSR (380), GKGSSGTGAGGASVSGVT (372), NKKSGAGGGSGAGKGGVA (498), LLGELCGSGNTVVEL (991), QNPLSSAAPTGAGKPY (1082), GLTQGASLAGSGAPSPLF (961), AMMELGWSTSGEFLL (1220) | Pepsin/pancreatin (E/S: 1/50), bromelain (55 °C, pH = 5.5–6.5, E/S: 1/50, time: 30 min) | BIOPEP database prediction | Hydrophobicity | DPP-IV, DPP-III, ACE, renin, α-glucosidae and α-amylase inhibitory, Antiamnestic, antithrombotic, antioxidative, hypolipidemic, HMG-CoA reductase | 214 |
| Flaxseed | – | Alcalase (3000 U/g, 60 °C, pH = 8), flavourzyme (120 U/g, 50 °C, pH = 6.5); time: 2 h | Taste panelists | Degree of hydrolysis, hydrophobicity, and enzyme active site | – | 215 |
| Cricket and mealworm | – | Flavourzyme 1000 L, protease P “Amano” 6 SD; pH = 7.3; 50 °C; E/S: 2%; time: 2 h | Taste panelists | Enzyme active site | – | 216 |
aValues in brackets are Q values calculated following the formula as described in Eq. 1.
bAbbreviation E/S indicates the ratio of enzyme to substrate.
Corn proteins (Zein)
Zein is one of the most important proteins in corn, accounting for around 65% of the total corn gluten60. One of the biggest limitations to the use of this protein in the food industry is its poor solubility in water due to the presence of high amounts of non-polar amino acids, such as alanine, leucine, and proline61. For this reason, enzymatic hydrolysis has been investigated as a means of increasing the solubility and functionality of zein62–64. Hydrolysis of corn gluten results in the production of bioactive peptides (Table 2) containing hydrophobic residues, such as leucine, isoleucine, valine, tyrosine, phenylalanine, and tryptophan, which make them bitter65. The addition of salts due to the adjustment of pH during the hydrolysis of corn proteins exposes amino acid hydrophobic groups, contributing to the generation of bitter bioactive peptides. In contrast, the production of corn hydrolysates and peptides using microbial fermentation decreases the production of bitter peptides64. Consequently, the hydrolysis conditions for zein should be optimized to generate bioactive peptides that have a low bitterness.
Fish proteins
Fish is another major source of proteins in the human diet66. The fishing industry generates many by-products that are typically converted into low-value commodity products, such as fish meal, fertilizers, and animal feed67. These protein-rich by-products typically make up about 60–70% of the weight of live fish68. Hydrolyzing these by-products can be used to produce bioactive peptides, thereby converting a low-value product into a high-value one68. Similar to the other proteins described above, the hydrolysis of fish proteins is accompanied by the production of bitter peptides (Table 2). The hydrophobicity of the peptides produced from fish has been shown to be a key factor influencing their bitterness69 and it is generally described well by the Q-rule introduced by Ney23 discussed earlier. Degree of hydrolysis is a critical factor for bitterness of fish protein hydrolysates. Increasing it generates more hydrophobic peptides and increases bitterness, as observed in salmon skeletal protein hydrolysis by alcalase70.
Another factor that affects the bitterness of fish hydrolysates is the type of enzyme used. For instance, the hydrolysis of Alaska Pollock frame using 10 different enzymes resulted in the production of hydrolysates with molar masses below 1355 Da71. The hydrolysates with the lowest bitterness were generated using MEAP, a mixture of animal proteases with both endo- and exopeptidase activities71. Lalasidis et al.72 indicated that the hydrolysis of fish proteins using alcalase followed by pancreatine did not produce bitter hydrolysates. Amino acid sequences can also affect the bitterness of fish hydrolysates. For instance, reversing the sequence of a peptide has been shown to reduce its bitterness42. Furthermore, the molecular weight distribution of fish protein hydrolysates can affect their bitter taste. Atlantic salmon proteins from by-products were hydrolyzed using five enzymes, which resulted in hydrolysates with acceptable bitterness at degrees of hydrolysis above 25%, which contained peptides below 2 kDa73. Mackie74 reported no bitter taste in fish hydrolysates containing peptides larger than 6 kDa, even for peptides with a Q-value exceeding 1300. However, there are several reports on the presence of bitter peptides in fish sauce and dried fish flakes (katsuobushi)75,76.
Seaweed proteins
Seaweed consumption has been increasing over the past two decades, with a market growth of 20% during this period. Seaweeds are a good source of high quality protein compared to traditional terrestrial crops77. According to a study by Fleurence78, the protein content of red seaweeds (35 to 47% of dry weight) is higher than those of green seaweeds (10–26% of dry weight) followed by brown seaweeds (3–15% of dry weight). Therefore, red seaweeds may be particularly useful for the generation of peptide ingredients. The protein fraction used for this purpose is often a by-product of traditional seaweed processing operations, such as the isolation of functional polysaccharide ingredients (like carrageenan or alginate).
Seaweed protein hydrolysates exhibit a wide range of functional activities79. Hydrolyzed seaweed has been reported to have a desirable umami taste, as well as an undesirable bitter taste (Table 2). Laohakunjit et al.80 reported that the bitter taste of seaweed hydrolysates depends on the chain length and hydrophobicity of the peptides, which depends on the hydrolysis conditions used. Leucine is one of the most abundant amino acids in enzymatic bromelain seaweed hydrolysates, which contributes to their bitter taste80.
Other protein sources
In addition to the protein sources mentioned above, there are other protein sources that are also used in the food industry. These protein sources include plant-based foods (like fruits, vegetables, grains, nuts, and seeds) and animal-based foods (like meat, poultry, and eggs). Researchers have also investigated the bioactivity and bitterness of the hydrolysates produced from these proteins.
Legumes have high protein content, low levels of methionine, cysteine, and tryptophan, and high levels of lysine compared to cereals81. The bitterness intensity of lentil protein hydrolysates produced using chymotrypsin is unaffected by the degree of hydrolysis82. The selection of enzyme used to hydrolyze legume proteins impacts the bitterness of the peptides produced, with alcalase producing bitter peptides and flavourzyme producing sweet peptides from lentil proteins83. Other studies have also reported that alcalase produces more bitter peptides than other enzymes during the hydrolysis of pea proteins84–87. In contrast, Koo et al.88 hydrolyzed wheat gluten protein using various enzymes and reported that alcalase-derived hydrolysates exhibited the lowest bitterness, while flavourzyme- and protamex-derived ones exhibited the highest bitterness after extensive hydrolysis. In another study, Taghizadeh et al.12 showed that the hydrolysis of proteins from medicinal plants using pancreatin generates bitter peptides, with Ziziphora clinopodioides producing the bitterest ones. Mushroom protein hydrolysates derived from papain and neutrase have also been shown to have a bitter taste89. Kheeree et al.90 hydrolyzed lemon basil seed proteins using alcalase and identified two ACE inhibitory peptides, which also exhibited a bitter taste. Overall, these studies suggest that the amino acid number, type, and sequence influence the bitterness of the hydrolysates produced from different plant protein sources using different hydrolysis methods.
Animal by-products rich in high-quality proteins can be used to produce high-value functional ingredients91. Singh et al.92 showed that the hydrolysates produced from squid fin proteins using alcalase (40% hydrolysis degree) were rich in aspartic acid/asparagine and glutamic acid/glutamine, which made them bitter, especially at higher concentrations. Also, bitter peptides have been produced from the hydrolysis of the protein-rich by-products of cephalopods93 and shrimp94,95. The utilization of egg protein hydrolysates in the production of cottage cheese has been reported to increase the bitterness in the final product96. Hydrolysates of porcine and bovine hemoglobin have also been reported to exhibit a bitter taste, with the bitterness increasing with increasing molecular weight of the peptides produced97,98. Recently, Chen et al.99 reported bitter peptides from chicken protein hydrolysates generated using flavourzyme. In general, however, there is still a relatively poor understanding of the formation and properties of the bitter peptides generated by hydrolysis of animal by-products100. Synthetic bitter peptides
The bioactivity and functionality of food-derived peptides depend on their structural features, including their amino acid sequence, molecular weight, hydrophobicity, and net charge101. Hydrophobicity plays a vital role in this regard, as the number and location of non-polar functional groups is known to impact peptide properties102. The hydrophobicity of peptides can be increased to improve their bioactivity and functionality, but this can also lead to an increase in their bitterness, which is usually undesirable. Synthetic peptides have been produced to better understand the relationship between the molecular characteristics of peptides and their bioactivities, functionalities, and taste properties. For instance, Pripp and Ardö103 studied the relationship between angiotensin-(I)-converting enzyme (ACE) inhibition and the bitter taste of peptides and showed that peptides with high ACE inhibition activity are more bitter. Moreover, it has also been reported that trans-positioning two cationic residues in an amphiphilic fragment makes the peptides more hydrophobic and bactericidal104. In the case of the antioxidant activities, the presence of hydrophobic amino acids enhances the accessibility of the antioxidant peptides to hit their hydrophobic cellular targets105. Furthermore, the amino acid sequence and distance between the two bitter-taste-determinant sites (binding unit and stimulating unit) have been shown to affect the bitter taste of synthetic peptides16,42.
Mechanism of human perception of peptides’ bitter taste
Peptide bitterness is perceived by humans via 25 recognized bitter taste receptors (T2Rs), which are triggered by various forms of bitter peptides100. As shown in Fig. 2, the generation of bitterness necessitates not just the hydrophobic characteristics, but also the crucial spatial arrangement of the peptide33. Peptides with a bitter taste have two functional units: a hydrophobic group with at least a backbone with three carbons called the binding unit (BU); and a basic group with α-amino group or a hydrophobic group called the stimulation unit (SU). Only when the average distance between the two sites is of approximately 4.1 Å (as in Fig. 2), the bitterness receptors detect the hydrophobicity of the bitter peptide via the hydrophobicity detection zone by starting a brain signaling cascade106. The bitterness intensity is related to the properties of the two determinant sites as well as the space between them. The bitter taste of peptide becomes stronger, when there are more hydrophobic groups in the hydrophobic detection zone of the bitter taste receptor. For example, Liu et al.107 demonstrated that the bitterness intensity of di-, tri-, and tetra-leucine was 8, 15, and 30 times greater than that of mono-leucine, respectively. This observation has been reported for tyrosine and phenylalanine as well107.
Fig. 2.
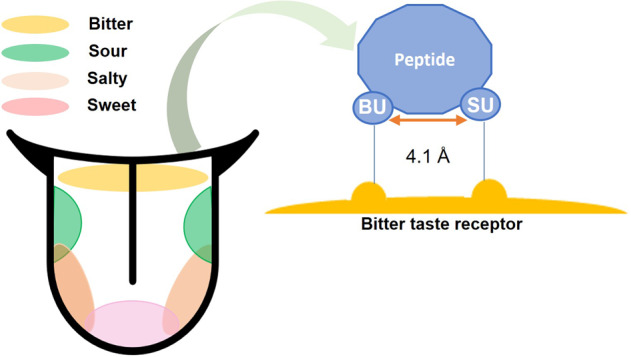
Mechanism of human perception of peptides’ bitter taste.
Strategies to improve the palatability of bioactive peptides
To make bioactive peptides palatable there are three major strategies including debittering, taste modulating, and taste masking. It should be noted that each technique has its own benefits and drawbacks (Table 3) and may not completely remove the bitter taste.
Table 3.
The advantages and drawbacks of various strategies for improving the palatability of bioactive peptides27,217–221.
| Strategies | Advantages | Drawbacks | |
|---|---|---|---|
| Debittering | Good debittering effects, low costs | Decrease nutritional value and biological activity, high osmolality caused by more hydrolysis | |
| Taste masking | Does not decrease nutritional value and biological activity, prevent the dissolution of bitter substances, inhibit the binding of bitter substances with taste receptors, screening method is rapid and simple | Need a large amount of masking agent to reduce bitterness, which affects the taste and texture of the final products and increases the production costs | |
| Encapsulation | Spray drying | Single step, continuous, high speed, high productibility, low processing costs, short processing time, good storage stability, quick and easy process, high process efficiency, microencapsulation of temperature-sensitive substances, possibility to obtain sterile products | Process at high temperature, not suitable for low water-soluble compounds, the loss of product in the walls of the drying chamber, the production of particles at the nanometer scale is limited |
| Freeze-drying | Process at low temperatures and under vacuum conditions, suitable for heat-sensitive compounds, improve stability of peptides, prevent the peptide oxidation, long preservation period, minimal change in the properties, loading quantity accurate and content uniform | Time-consuming, high costs, high energy consumption | |
| Liposome | Entrap hydrophilic and hydrophobic peptides | The leak of trapped peptides, low physical stability, inapplicable upscaling | |
| Extrusion | Fast and inexpensive process | Formation of large-size capsules | |
| Complex coacervation | The process is simple, fast, and reproducible, the possibility of using low concentrations of surfactants, the process can be run in aqueous and anhydrous environments | Agglomeration of droplets under the influence of solvent removal, the toxicity of the solvents used | |
| Emulsification | Ability to encapsulate hydrophilic and hydrophobic substances, high process efficiency | Agglomeration of droplets under the influence of solvent removal, the toxicity of the solvents used | |
| Solid dispersion | Decrease agglomeration and release in a supersaturation state, improve wettability and increase the surface area, increase solubility and dissolution rate | Physical instability, changes in crystallinity, sensitive to temperature and humidity during storage, expensive, low reproducibility of physicochemical characteristics | |
Debittering strategies
Treatment with active carbon, alcohol extraction, and isoelectric precipitation
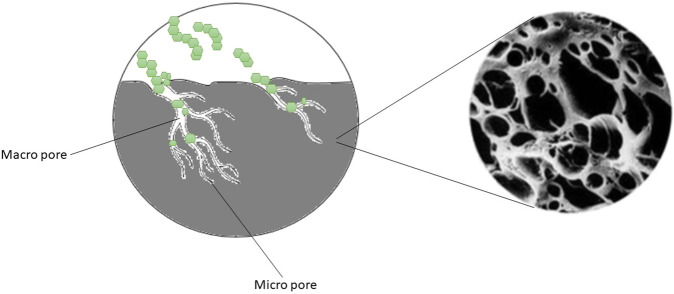
Activated carbon is a good adsorbent material with a high surface area and microporous structure that can be utilized for the removal of bitter tastes (Fig. 3). However, its application does result in the loss of some desirable compounds from the hydrolysates. For instance, Cogan et al.108 reported that the amount of arginine (29.8%), tryptophan (63.1%), histidine (11.2%), tyrosine (24.4%), and phenylalanine (36.1%) in casein hydrolysates was significantly reduced after treatment with activated carbon. It has also been reported that activated carbon can decrease the bitterness of corn gluten hydrolysates, but their ACE inhibitory activity was also reduced109. In general, activated carbon is a relatively non-selective method that absorbs both non-bitter and bitter peptides. Therefore, active carbon treatments have been reported to decrease the total protein content, as well as the essential amino acid and bioactive peptide contents, thereby leading to a decrease in biological activity.
Fig. 3.
Microporous structure of activated carbon trapping bitter bioactive peptides.
While activated carbon is generally recognized as safe110, there are some potential safety concerns that should be noted. One concern is that activated carbon can adsorb not only impurities, but also beneficial nutrients108. In addition, some types of activated carbon can contain trace amounts of impurities, such as polycyclic aromatic hydrocarbons, which could potentially pose a risk to human health111. Therefore, it is important to use activated carbon that is specifically intended for food use and to ensure that it meets appropriate safety standards. Overall, when used appropriately and with all safety measures in place, activated carbon can be considered as a safe and effective way to achieve the debittering of protein hydrolysates.
Some alcohols can be used as solvents to selectively extract bitter peptides from complex mixtures. The most common alcohols used for this purpose include 2-butanol, isopropyl, and ethanol112. Bitter peptides from Setaria italica protein hydrolysates have been extracted using ethanol, which improved the functional performance of the final product113. Liu et al.114 proposed that the amino acid composition and molecular weight distribution were more important factors influencing the bitterness intensity than the amino acid sequence. These authors produced wheat gluten protein hydrolysates using proteax and then debittered them using isopropyl alcohol. Their results showed that different isopropyl-extracted peptide fractions (180–500, 500–1000, and 1000–3000 Da) had different bitter tastes, with the bitterness increasing with molecular weight. In another study, it was shown that a 2-butanol solvent extraction reduced the bitterness of fish protein hydrolysates115. Recently, Sinthusamran et al.116 hydrolyzed salmon frame proteins using alcalase and flavourzyme and then debittered them using 2-butanol and isopropanol. Of the two solvents used, the hydrolysates treated with 2-butanol had the lowest bitterness. But these hydrolysates also had a reduced antioxidant activity. Salmon frame hydrolysates have also been obtained using alcalase and then debittered using 2-butanol followed by β-cyclodextrin treatment117,118. This combined treatment led to hydrolysates that exhibited no bitter taste. The debittered hydrolysates were then used to fortify biscuits without introducing a strong bitter taste.
Isoelectric precipitation can also be applied to reduce the bitterness of protein hydrolysates. In this case, the bitter peptides are selectively precipitated by adjusting the pH to around their isoelectric points. Adler‐Nissen119 showed that the bitterness of soy protein hydrolysates was reduced after precipitation of bitter hydrophobic peptides around their isoelectric pH.
In summary, these debittering methods are usually relatively simple and inexpensive to apply. However, they may remove some desirable peptides from the hydrolysates, and sometimes involve the utilization of organic solvents, which is often undesirable from a cost and sustainability perspective.
Chromatographic separation
Depending on the nature of the resins used, hydrophobic bitter peptides can be separated from complex mixtures using chromatographic methods. The most commonly used resins for debittering are dextran gel, hexyl-gel, and microporous adsorption-resins114. Kim et al.120 isolated bitter peptides from tryptic hydrolysates of 11S glycinin using RP-C18 HPLC, with the final hydrolysates showing a hydrophobicity below 1400 cal/mol. In another study, Liu et al.121 isolated four bitter peptides from whey protein hydrolysates using ultrafiltration (3 kDa cut off) and RP-C18 column chromatography. Initially, the authors showed that the peptide fraction less than 3 kDa had a bitter taste, while the peptide fraction more than 3 kDa was tasteless. Afterward, the lower molecular weight fraction was removed using a C18 column, and 4 peptides were identified as having the strongest bitter taste. A Sephadex G-25 column has been successfully used to remove bitter peptides from skim milk protein hydrolysates by Helbig et al.122. Silica gel chromatography using propyl alcohol as a solvent has also been used to eliminate bitter peptides from casein and cheese hydrolysates123. Ultrafiltration can also be used to remove bitter peptides based on differences in the molecular weights of bitter and non-bitter peptides. For instance, Aubes-Dafau et al.97 debittered hemoglobin hydrolysates using ultrafiltration. These authors showed that peptides ranging from 500 to 5000 Da were very bitter. Dauksas et al.115 used cholestyramine resin, a strong anion exchange resin, to isolate bitter peptides from fish protein hydrolysates. The authors indicated that the bitter intensity was reduced more than with the use of 2-butanol treatment. Cheison et al.124 used a macroporous adsorption resin for simultaneous desalting and debittering of whey protein hydrolysates. The authors hydrolyzed whey protein isolate using protease N ‘Amano’ G (IUB 3.4.24.28) and after loading the hydrolysates on the resin, the compounds were eluted using 20%, 40%, and 75% alcohol solutions. The results showed that the fraction eluted using 75% alcohol was highly bitter, exhibited strong ACE inhibition, had the highest content of hydrophobic amino acids, and was comprised of approximately 71% peptides below 600 Da. Imai et al.125 created a new type of silica resin without chemical modifications for adsorption of non-polar and cationic peptides through hydrophobic and electrical interactions. Imai et al.126 then showed that this resin was effective at removing bitter peptides.
Additional hydrolysis
Considerable efforts have been focused on using peptidases and proteases for reducing the bitter taste of protein hydrolysates. It has been reported that the simultaneous or sequential incubation of hydrolysates with various enzymes can selectively release hydrophobic residues from bitter peptides. The most commonly used enzymes for debittering purposes are exopeptidases, endopeptidases, and proteases19. Since the bitter taste of peptides arises from the existence of branched or hydrophobic residues that may be located at the N- or C-terminal positions, further hydrolysis can be performed using an aminopeptidase or carboxypeptidase, respectively127. However, it is important that this further hydrolysis does not adversely affect the bioactivity and functional properties of the peptides formed128. Moreover, the additional costs and time involved may limit the large-scale utilization of this approach for the industrial production of debittered peptides129.
The effectiveness of peptide debittering depends on the type of enzyme used. Several aminopeptidases from Thermophilic and Aspergillus fungi have been shown to have a strong specificity for cleaving hydrophobic and aromatic amino acids at the N‐terminal of peptides130–132. Researchers have used enzymes such as thermostable leucine aminopeptidase from Thermomyces lanuginosus and Aspergillus niger131, and aminopeptidase AN-APA from A. niger CICIM F0215 to debitter soy, casein, milk, and cheese hydrolysates by hydrolyzing hydrophobic and hydrophilic residues at the N-terminal of polypeptides132. Aminopeptidases from Aeromonas caviae T-64, Lactococcus lactis subsp. cremoris WG2 or AM2, Lactococcus lactis subsp. Cremoris, Lactobacilli helveticus strain L1, Penicillum caseicolum, Pseudomonas fluorescens ATCC 948, A. niger, and cotyledon of soybean have also been successfully used to debitter milk and cheese hydrolysates19,133–141. Nishiwaki et al.142 indicated that the treatment of bitter soy hydrolysates with an aminopeptidase from Grifola frondosa led to the release of hydrophobic amino acids, such as valine, leucine, phenylalanine, tyrosine, and isoleucine, which decreased the bitterness of the hydrolysates. In addition, Li et al.143 hydrolyzed soybean proteins using alcalase followed by further hydrolysis using an exopeptidase from Actinomucor elegans, to reduce their bitterness. Corolase is a commercial enzyme mixture that contains aminopeptidase, elastase, chymotrypsin, tryptic, and dipeptidase activities. It has been used for hydrolysis of both animal and vegetable proteins as it provides a high degree of hydrolysis without producing bitter peptides127. Accelase is an aminopeptidase from Lactococcus lactis that can be applied to prevent bitterness during cheese ripening. Debitrase is another aminopeptidase from Lactococcus lactis and Aspegillus oryzae that can be used to reduce the bitterness caused by conventional enzymatic hydrolysis127,144. Protease M is an enzyme mix from A. oryzae that has a pepsin-like endopeptidase activity coupled with carboxyl, amino, and leucine exopeptidase activities. Hinnenkamp and Ismail145 recently showed that protease M releases a greater proportion of hydrophilic peptides upon hydrolysis of whey proteins, resulting in a decrease in bitterness. Furthermore, alcalase-treated anchovy hydrolysate was treated with an aminopeptidase derived from the common squid Todarodes pacificus hepatopancreas to reduce bitterness146. Yan et al.147 expressed Streptomyces canus T20 aminopeptidase (ScAPase) in Bacillus subtilis followed by an additional treatment of trypsin-hydrolyzed rice protein isolates previously treated with recombinant ScAPase. Their results showed that hydrolysates could be produced that did not exhibit bitterness but did exhibit high ACE inhibitory and antioxidant activities. Tong et al.24 hydrolyzed soybean proteins using protex 6 L followed by protease A 2 SD, which is an enzyme that cleaves leucine and arginine at the N-terminal of bitter peptides. This combined treatment led to the production of low bitter hydrolysates having high antioxidant activity.
Hydrolysis of corn gluten using a pescalase and protease A mixture was shown to reduce the bitterness of hydrolysates more than a flavourzyme treatment148. In another study, a leucine aminopeptidase Lap1 from Aspergillus sojae GIM3.30 was overexpressed in Pichia pastoris149. This enzyme was then used to produce casein and soy protein hydrolysates with low bitterness. Fu et al.150 hydrolyzed minced beef and porcine plasma using 10 food-grade enzymes, including protease A, protease P, proteAX, flavourzyme, alcalase, papain, bromelain, protamex, neutrase, and sumizyme BNP-L. Among these, the protease A generated hydrolysates with the lowest bitterness.
Several studies have demonstrated the ability of carboxypeptidases to debitter protein hydrolysates. Carboxypeptidases from Actinomucor elegans were able to reduce the bitterness of soybean protein hydrolysates during incubation at 40–95 °C for 6 h151. Kawabata et al.152 further hydrolyzed soybean and gluten hydrolysates derived from pepsin and trypsin using carboxypeptidase from squid liver, leading to decreased bitterness levels. The same goal has been successfully performed on peptic hydrolysates of soybean proteins, peptic hydrolysates of fish protein concentrate, and casein hydrolysates using wheat carboxylases153–155. There are many carboxypeptidases available for debittering protein hydrolysates127. Recently, it was reported that Bacillus subtilis ACCC 01746 was able to produce proteases and carboxypeptidases in the early stage of solid-state fermentation of soybean meal156. These enzymes were shown to hydrolyze soybean proteins and produce peptides with only a mild bitterness.
Meinlschmidt et al.157 fermented soy protein hydrolysates using Lactobacillus perolens, Rhizopus oryzae, and Actinomucor elegans and showed that all strains decreased the bitterness to a minimum level (0.7) in comparison with non-fermented hydrolysates (2.8–8.0) and untreated soy protein isolate (2.8). The bitterness scale used ranged from no perception (0) to strong perception (10).
In addition to peptidases, alkaline/neutral proteases have also been used for debittering protein hydrolysates. Kodera et al.158 hydrolyzed soy proteins using various enzymes, especially protease D3. This enzyme was purified from germinated soy cotyledons and used to generate hydrolysates with low bitterness levels. In another study, tryptic β-casein hydrolysates were hydrolyzed using Xaa-Pro dipeptidyl peptidase, a prolyl aminopeptidase from Lactobacillus casei ssp. casei LLG, resulting in a decrease in the bitterness of proline-rich peptides and other peptides containing proline159. Moreover, the hydrolysis of the by-product of silver carp was also performed using different proteases160. The results indicated that hydrolysis using a combination of neutrase and flavourzyme generated hydrolysates with lower bitterness compared to the other proteases studied. Recently, Zhang et al.161 demonstrated that the proteases isolated from soybean seedlings can completely remove the bitterness of soy protein hydrolysates derived from alcalase 2.4 L, without reducing the antioxidant activity of the final products. In addition, the hydrolysis of soybean proteins using a mixture of tripeptidase and alkaline protease not only decreased the bitterness but also improved the functional properties of the hydrolysates162. Hydrolysis of wheat gluten using proteax for 300 min has been reported to generate hydrolysates ranging from 180 to 500 Da, which had a low bitterness level107. A combination of alkaline protease hydrolysis and transglutaminase cross-linking was able to rearrange the polypeptide structure of soybean protein hydrolysates, resulting in products with reduced bitterness48.
According to these findings, additional hydrolytic processes can be effectively used to reduce the bitterness of protein hydrolysates and to transform them into valuable food ingredients. However, the extent of this additional hydrolysis has to be optimized to produce peptides that maintain their desirable functional attributes. For example, Cheung et al.128 revealed that further hydrolysis of whey protein hydrolysates with exopeptidases could significantly decrease the bitterness, while the ACE inhibitory activity of the resultant hydrolysates dramatically decreased from an IC50 of 0.15 mg/mL to 0.34 mg/mL as well. The authors illustrated that this was due to the fragmentation of active peptides, which resulted in lower ACE-inhibitory activity.
Bitterness inhibitors: masking and blocking strategies
Bitterness inhibitors can mask the bitter taste of peptides. These inhibitors include additives that can mask, block, or modify the taste of bitter peptides. However, the use of these inhibitors is sometimes limited due to their high cost and off flavors129.
The most commonly used masking agents include additives like modified starch, taurine, glycine, polyphosphates, as well as additives that chemically modify peptides through amination, deamination, acetylation, or cross-linking procedures129. A good example of this phenomenon is the Maillard reaction, which involves interactions between the carbonyl groups of reducing sugars and the free amine group of peptides100. Eric et al.163 reported that the taste and antioxidant activity of sunflower protein hydrolysates improved by using the Maillard reaction by heating the peptides in the presence of xylose and cysteine at 120 °C for 2 h. In addition, using transglutaminase to crosslink the Maillard reaction products generated from soybean protein hydrolysates (120 °C for 2 h) has been shown to reduce bitterness and generate savory tastes164. In contrast, de Carvalho et al.165 showed that transglutaminase cross-linking of whey protein hydrolysates did not alter their bitterness. This effect may be explained by the presence of free glutamine and the considerable number of short peptides without glutamine or lysine residues. However, glycation of poultry protein hydrolysates using glucosamine in the presence of microbial transglutaminase resulted in an enhanced salty taste of the hydrolysates166. Deamination of wheat gluten hydrolysates (37 °C for 1.5–3 h) was reported to decrease the bitterness intensity of the hydrolysates167. Moreover, acetylation of lysine in bitter peptides can decrease their bitterness. Won Yeom et al.168 acetylated lysine and tyrosine of soybean hydrolysates using N-acetyl imidazole and then deacetylated the O-acetyl tyrosine at pH 11, resulting in a decrease in the bitterness of the hydrolysates. Recently, Habinshuti et al.169 characterized the taste and antioxidant activity of Maillard reaction products from hydrolysates of sweet potato, potato, soy isolate, egg white, and whey isolate proteins. The authors reported that the Maillard reaction increased the sweetness, sourness, and umami tastes and decreased the bitterness of all the hydrolysates. However, the Maillard reaction products from the whey hydrolysates exhibited the strongest oxygen radical absorbance capacity. Abdelhedi et al.170 hydrolyzed smooth hound viscera protein using neutrase, esperase, and purafect followed by a Maillard reaction of the low molecular peptides produced (<1 kDa) in the presence of sucrose (90 °C for 2 h). The results showed that the glycation degree was considerably enhanced in the esperase-derived peptides/sucrose conjugates, which led to a reduction of the bitter taste and enhancement of the antioxidant activities of the hydrolysates.
The most common blocking agents currently used include sodium salts, phospholipids, neohesperidin, zinc lactate, ferulic acid, γ-aminobutyric acid, β-lactoglobulin, monosodium glutamate, and adenosine monophosphate129,171. Xu et al.172 demonstrated that sodium chloride suppressed the bitter taste of egg white and chicken protein hydrolysates in a concentration-dependent manner. The authors stated that adding sodium chloride at certain concentrations led to a salting-in effect, which buried the hydrophobic groups, decreased the surface hydrophobicity of the peptides, and resulted in a decreased bitterness. Therefore, this strategy is an alternative, effective, and cheap method to suppress the bitterness of protein hydrolysates. Dong et al.173 prepared a complex of neohesperidin dihydrochalcone and glucosyl-β-cyclodextrin to block the bitterness of corn peptides. The complex formed had a better bitterness-masking effect than single neohesperidin dihydrochalcone or glucosyl-β-cyclodextrin, phosphatidic acid, protein-phosphatidic acid complexes, tannin, and neodiosmin. Zhang et al.174 hydrolyzed beef protein using 6 commercial enzymes including alcalase, chymotrypsin, trypsin, pepsin, flavourzyme, and thermoase, and determined the ability of these enzymes to block the T2R4 bitter taste receptor. The results showed that the treatment of the HEK293T cells expressing the T2R4 receptor with hydrolysates effectively reduced calcium mobilization, resulting in a reduction of the bitterness intensity. In addition, Xu et al.175 demonstrated that chicken protein-derived peptides are able to block human bitter taste receptors of T2R4, T2R7, and T2R14.
Other agents, such as salts, sweeteners, and aromatic agents can also be used as taste-correcting agents to reduce bitterness176,177. For instance, Leksrisompong et al.178 added 24 bitter taste inhibitors to whey protein hydrolysates and found that sucralose, fructose, sucrose, adenosine 5’-monophosphate (5’AMP), adenosine 5’-monophosphate disodium (5’AMP Na2), sodium acetate, monosodium glutamate, and sodium gluconate were effective bitter taste inhibitors. The authors also reported that sodium chloride was an effective inhibitor of the bitter taste of hydrolysates containing small peptides, but it was not effective for those containing large peptides. Furthermore, they showed that lysine and arginine could inhibit the bitter taste of quinine, but not the whey hydrolysates.
Encapsulation technologies
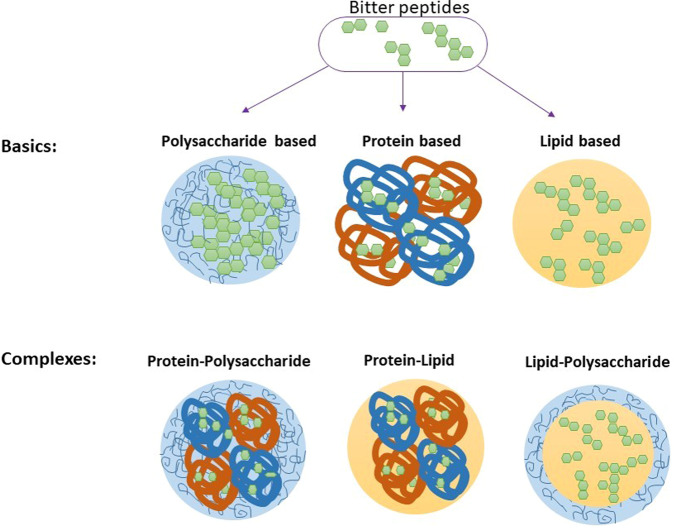
Encapsulation is widely used to improve the dispersibility, stability, and bioactivity of protein hydrolysates, but it can also be used to reduce the bitterness of protein hydrolysates and peptides (Table 4). Encapsulation technologies are often characterized as either micro- or nano-encapsulation depending on the dimensions of the particles used. Nano-encapsulation utilizes particles with dimensions below about 1000 nm, whereas Micro-encapsulation utilizes particles with dimensions above this value. For oral administration applications, nano-encapsulation is often more suitable because it has less impact on the mouthfeel of ingested substances, and leads to products that are more resistant to gravitational separation and aggregation179. The particles used to encapsulate bioactive peptides can be assembled entirely from food-grade ingredients, such as polysaccharides, proteins, and lipids (Fig. 4) using a range of techniques including spray drying, freeze drying, complex coacervation, emulsification, antisolvent precipitation, extrusion, electro-spinning, electro-spraying, liposome, and solid dispersion technologies179. Factors that can affect the encapsulation efficiency include the peptide characteristics (molar mass, polarity, and charge), particle characteristics (composition, size, shape, internal/external polarity, and interfacial properties), and production conditions (method and operating conditions)180.
Table 4.
Summary of the main carriers and technologies used for the encapsulation of protein hydrolysates and peptides for masking their bitter taste.
| Technologies | Carriers | Hydrolysates/peptides | References |
|---|---|---|---|
| Spray drying | Maltodextrin | Casein hydrolysates | 222 |
| Spray drying | Maltodextrin/β-cyclodextrin | Whey protein hydrolysates | 223 |
| Spray drying | Maltodextrin and gum arabic | Chicken meat protein hydrolysate | 224 |
| Spray drying | Gum arabic | Casein hydrolysates | 225 |
| Spray drying | Maltodextrin or maltodextrin/β-cyclodextrin | Whey protein hydrolysates | 223 |
| Spray drying | Gelatin/soy protein isolate | Casein hydrolysates | 44 |
| Spray drying | Chitosan and gelatin | Whey protein hydrolysates | 226 |
| Spray drying | γ-cyclodextrin | Whey protein hydrolysates | 227 |
| Spray drying | Soy protein isolate | Casein hydrolysates | 228 |
| Spray drying | Maltodextrin | Flaxseed protein hydrolysates | 229 |
| Spray drying/freeze drying | Whey protein concentrate/sodium alginate | Whey protein hydrolysates | 181 |
| Spray drying/freeze drying | Maltodextrin | Soybean hydrolysates | 230 |
| Spray drying/freeze drying | Chitosan/lecithin | Oyster protein hydrolysates | 231 |
| Freeze drying | Maltodextrin/gum arabic | Casein hydrolysates | 231 |
| Complex coacervation | Soy protein hydrolysates/pectin | Casein hydrolysates | 232 |
| Complex coacervation | Whey protein/gum Arabic | Bean protein hydrolysates | 233 |
| Complex coacervation | Pectin | Whey protein hydrolysates | 234 |
| Solid dispersion | Chitosan | Rainbow trout skin gelatin hydrolysates | 235 |
| Solid dispersion | Chitosan | Salmon protein hydrolysates | 187 |
| Solid dispersion/spray-drying | Gum arabic and maltodextrin | Pea protein isolate | 236 |
| Emulsification | Polyglycerol polyricinoleate | Soy hydrolysates | 237 |
| Emulsification | Polyglycerol polyricinoleate/alginate | Oyster peptides | 238 |
| Emulsification | Whey protein concentrate/inulin/fucoidan | Fish protein hydrolysate | 239 |
| Extrusion | Alginate | Whey protein hydrolysates | 240 |
| Extrusion | Alginate/collagen, alginate/arabic gum, and alginate/gelatin | Whey protein hydrolysate | 241 |
| Extrusion and emulsification | Sodium alginate-O-carboxymethyl chitosan | ACE inhibitory peptides | 242 |
Fig. 4.
Schematic representation of various polymer-based encapsulation systems.
Spray-drying is currently the most widely used method to encapsulate bitter peptides due to its ease of use, low cost, and scalability179,181. This method typically involves spraying a fluid polymer solution or colloidal dispersion containing the peptides through a nozzle into a high temperature chamber. The water is rapidly evaporated leading to the formation of solid microparticles that contain the peptides trapped within a polymer and/or colloidal matrix, which is usually referred to as the wall material. Proteins and carbohydrates (oligosaccharides or polysaccharides) are often utilized as natural polymers that act as wall materials. For instance, Sarabandi et al.182 used spray drying to encapsulate casein hydrolysates using maltodextrin (DE = 18) as a wall material. Their results showed that the bitterness of the hydrolysates was reduced, while the antioxidant activity was preserved. Murthy et al.183 prepared a vegetable soup enriched with microencapsulated pink perch meat protein hydrolysates using spray drying and maltodextrin/arabic gum as a wall material. Sensory analysis showed that the bitterness of this product was less than that of a soup containing non-encapsulated hydrolysates. Spray-drying has also been used to encapsulate casein and whey hydrolysates using various other kinds of wall material including soy protein isolate, maltodextrin, maltodextrin-β-cyclodextrin, sodium alginate, whey protein concentrate, γ-cyclodextrin, arabic gum, and gelatin184,185.
In some cases, emulsion technology can be used to encapsulate peptides179. For instance, hydrophobic peptides can be dispersed within an oil phase, which is then homogenized to form an oil-in-water (O/W) emulsion or nanoemulsion containing peptide-loaded oil droplets. Alternatively, more hydrophilic peptides can be trapped within the water droplets in water-in-oil (W/O) emulsions. As an example, the encapsulation of bitter peptides in a high internal phase W/O emulsion was reported to greatly reduce the bitterness but increase the gastrointestinal stability of the peptides186. Liposome technology can also be used to encapsulate peptides and reduce their bitterness. The efficacy of liposome encapsulation has been shown to depend on the molecular weight, polarity, and charge of the peptides. For instance, Li et al.187 encapsulated Atlantic salmon protein hydrolysates in chitosan-coated liposomes at a high encapsulation efficiency (71%). Similarly, marine protein hydrolysates (Micropogonias furnieri muscle and by-product) have been incorporated into liposomes at a high encapsulation efficiency (80%)188,189 and whey peptides have been incorporated into soy lecithin liposomes at a high encapsulation efficiency (90%)190. Conversely, other researchers have reported a relatively low encapsulation efficiency (40–60%) for casein peptides in liposomes, which was mainly attributed to an electrostatic repulsion between the anionic casein phosphopeptides and the anionic head groups of the phospholipids used to assemble the liposomes180. Another study reported that even though the encapsulation efficiency (48%) and antioxidant activity of whey peptides in phosphatidylcholine liposomes were relatively low, the antioxidant activity of the encapsulated peptides was higher than that of the non-encapsulated ones after 30 days storage191. The encapsulation of other dairy protein hydrolysates has recently been reviewed by Giroldi et al.185.
Biopolymer-based microgels have also been shown to be suitable for encapsulating bitter peptides. Han et al.192 designed a polysaccharide-based encapsulation system to encapsulate peanut peptides through electrostatic complexation of cationic n-trimethy chitosan and anionic alginate. The authors reported that the antioxidant activity of the peptides increased after encapsulation. They also reported that the peptides were retained within the complexes under simulated gastric conditions but released under simulated small intestinal conditions. Moreover, the authors reported that this complex was biocompatible and nontoxic, which makes it suitable as a delivery system for peptides.
The bitterness of some peptides can also be reduced by forming inclusion complexes with cyclodextrins, which have a hydrophobic cavity and a hydrophilic exterior193. Hydrophobic peptides can be incorporated into the cavity through a guest-host interaction, which reduces their tendency to interact with the bitter receptors in the mouth. For instance, Xia et al.87 compared various methods of reducing the bitterness of pea protein hydrolysates, including flavourzyme treatment, butanol extraction, and encapsulation using β-cyclodextrin. The butanol extraction and β-cyclodextrin encapsulation methods led to the lowest bitterness score. Numerous other studies have shown that the bitterness of hydrophobic and aromatic amino acids can be greatly reduced by forming inclusion complexes with cyclodextrin194,195. As an example, Hou et al.196 reported that β-cyclodextrin interacted with hydrophobic peptides, thereby reducing the bitterness of soybean protein hydrolysates. Sim et al.197 also reported that the bitterness of soybean peptides could be reduced by forming inclusion complexes with β-cyclodextrin.
Conclusions and future perspectives
Many protein hydrolysates and bioactive peptides have been described as functional compounds with one or more biological activities that could potentially improve the health of the population. However, it is often challenging to create functional foods fortified with these bioactive substances because of their bitterness. The perceived bitterness of bioactive peptides depends on the number, type, and sequence of amino acids they contain, which is governed by the protein source, hydrolysis method, and operating conditions. The nature of the side chains on the peptides formed by hydrolysis is mostly responsible for peptide bitterness. The distribution and placement of bitter taste residues, hydrophobicity, the degree of hydrolysis, amino acid conformation, peptide sequence, and the amount of carbon atoms on the side chains of amino acids are other factors that influence the bitterness of protein hydrolysates. Practical approaches are therefore required to reduce the bitterness of protein hydrolysates and peptides, without adversely affecting their beneficial biological activities. Techniques such as the use of active carbon have proven to be beneficial, although they resulted in the loss of some desirable compounds from the hydrolysates and their associated biological activities. Thus, it is necessary to research further and design functional materials that can specifically bind bitter peptides without having a negative impact on the concentration of other peptides or active molecules in order to preserve the biological activities if these compounds.
A variety of strategies have been developed to reduce the bitterness of bioactive peptides, which vary in their efficacy and potential for commercial applications. Debittering and taste masking are two approaches that have been considered as the primary solutions of bitter taste of peptides. However, encapsulation technologies are one of the most promising strategies that have been developed for this purpose. Bitter peptides can be trapped in various kinds of colloidal particles, which can improve their water dispersibility, stability, and bioactivity, as well as reducing their bitterness by preventing them from interacting with bitter receptors in the mouth. At present, however, there is still a relatively poor understanding of the efficacy of different kinds of encapsulation technologies for specific applications, which makes it difficult to select the most appropriate one. Finally, further research is needed in this field in order to create methods that are ideally accessible, scalable, and effective for the oral delivery of biologically active peptides, as well as a standardization of the testing conditions using each technology, to clearly elucidate the efficiency of each method and their industrial scale-up, allowing ultimately the development of palatable and more nutritious food products.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors acknowledge the funding received from AMBROSIA funded by the Department of Agriculture Food and the Marine (DAFM) under the umbrella of the European Joint Programming Initiative “A Healthy Diet for a Healthy Life” (JPI-HDHL) and of the ERA-NET Cofund ERA HDHL (GA No. 696295 of the EU Horizon 2020 Research and Innovation Programme).
Author contributions
A.M.-K. contributed to the conceptualization of the work, reviewed the literature, analyzed the data, and wrote the original draft; M.S.T. contributed to literature review and data analysis and in writing sections of the original draft; A.N. contributed to data analysis and in writing sections of the original draft; D.J.McC. contributed to the conceptualization of the work, revision, and editing of the draft; M.G.-V. conceptualized the work, contributed to writing, revising, editing, and achieved funding for this work.
Data availability
All the data used in this research is described in the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41538-023-00198-y.
References
- 1.Lau JL, Dunn MK. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. medicinal Chem. 2018;26:2700–2707. doi: 10.1016/j.bmc.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 2.Organization, W. H. Global Status Report on Noncommunicable Diseases 2014 (World Health Organization, 2014).
- 3.Mirzapour-Kouhdasht A, Moosavi-Nasab M, Lee CW, Yun H, Eun J-B. Structure–function engineering of novel fish gelatin-derived multifunctional peptides using high-resolution peptidomics and bioinformatics. Sci. Rep. 2021;11:1–15. doi: 10.1038/s41598-021-86808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Admassu H, Gasmalla MAA, Yang R, Zhao W. Bioactive peptides derived from seaweed protein and their health benefits: antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018;83:6–16. doi: 10.1111/1750-3841.14011. [DOI] [PubMed] [Google Scholar]
- 5.Wang R, et al. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α‐glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019;7:1848–1856. doi: 10.1002/fsn3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee C, Gleddie S, Xiao C-W. Soybean bioactive peptides and their functional properties. Nutrients. 2018;10:1211. doi: 10.3390/nu10091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Y, Li Y, Zhang Y, Ruan X, Zhang R. Purification, characterization, synthesis, in vitro ACE inhibition and in vivo antihypertensive activity of bioactive peptides derived from oil palm kernel glutelin-2 hydrolysates. J. Funct. Foods. 2017;28:48–58. [Google Scholar]
- 8.Guerra-Almonacid, C. M., Torruco-Uco, J. G., Murillo-Arango, W., Méndez-Arteaga, J. J. & Rodríguez-Miranda, J. Effect of ultrasound pretreatment on the antioxidant capacity and antihypertensive activity of bioactive peptides obtained from the protein hydrolysates of Erythrina edulis. Emir. J. Food Agric.31, 288–296 (2019).
- 9.Mirzapour-Kouhdasht A, Moosavi-Nasab M, Krishnaswamy K, Khalesi M. Optimization of gelatin production from Barred mackerel by-products: characterization and hydrolysis using native and commercial proteases. Food Hydrocoll. 2020;108:105970. [Google Scholar]
- 10.Díaz-Gómez JL, Castorena-Torres F, Preciado-Ortiz RE, García-Lara S. Anti-cancer activity of maize bioactive peptides. Front. Chem. 2017;5:44. doi: 10.3389/fchem.2017.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karami Z, Peighambardoust SH, Hesari J, Akbari-Adergani B, Andreu D. Antioxidant, anticancer and ACE-inhibitory activities of bioactive peptides from wheat germ protein hydrolysates. Food Biosci. 2019;32:100450. [Google Scholar]
- 12.Taghizadeh MS, Niazi A, Moghadam A, Afsharifar AR. The potential application of the protein hydrolysates of three medicinal plants: cytotoxicity and functional properties. J. Food Sci. 2020;85:3160–3167. doi: 10.1111/1750-3841.15379. [DOI] [PubMed] [Google Scholar]
- 13.Taghizadeh MS, Niazi A, Moghadam A, Afsharifar AR. Novel bioactive peptides of Achillea eriophora show anticancer and antioxidant activities. Bioorg. Chem. 2021;110:104777. doi: 10.1016/j.bioorg.2021.104777. [DOI] [PubMed] [Google Scholar]
- 14.Doostmohammadi M, et al. Hydrogels for peptide hormones delivery: therapeutic and tissue engineering applications. Drug Des., Dev. Ther. 2019;13:3405. doi: 10.2147/DDDT.S217211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bumberger E, Belitz H-D. Bitter taste of enzymic hydrolysates of casein. Z. Lebensm. Unters. Forsch. 1993;197:14–19. doi: 10.1007/BF01202693. [DOI] [PubMed] [Google Scholar]
- 16.Maehashi K, Huang L. Bitter peptides and bitter taste receptors. Cell. Mol. Life Sci. 2009;66:1661–1671. doi: 10.1007/s00018-009-8755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyerhof, W. in Reviews of Physiology, Biochemistry and Pharmacology 37–72 (2005). [DOI] [PubMed]
- 18.Ney, K. H. Bitterness of Peptides: Amino Acid Composition and Chain Length. (ACS Publications, 1979).
- 19.Saha BC, Hayashi K. Debittering of protein hydrolyzates. Biotechnol. Adv. 2001;19:355–370. doi: 10.1016/s0734-9750(01)00070-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhu X, Sun‐Waterhouse D, Chen J, Cui C, Wang W. Bitter‐tasting hydrophobic peptides prepared from soy sauce using aqueous ethanol solutions influence taste sensation. Int. J. Food Sci. Technol. 2020;55:146–156. [Google Scholar]
- 21.Garcia-Vaquero M, Mora L, Hayes M. In vitro and in silico approaches to generating and identifying angiotensin-converting enzyme I inhibitory peptides from green macroalga Ulva lactuca. Mar. Drugs. 2019;17:204. doi: 10.3390/md17040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan W, et al. Relationship between enzyme, peptides, amino acids, ion composition, and bitterness of the hydrolysates of Alaska pollock frame. J. Food Biochem. 2019;43:e12801. doi: 10.1111/jfbc.12801. [DOI] [PubMed] [Google Scholar]
- 23.Ney K. Prediction of bitterness of peptides from their amino acid composition. Z. Lebensm. Unters. Forsch. 1971;147:64–68. [Google Scholar]
- 24.Tong X, et al. An innovative two-step enzyme-assisted aqueous extraction for the production of reduced bitterness soybean protein hydrolysates with high nutritional value. LWT. 2020;134:110151. [Google Scholar]
- 25.Gan R, He Y, Li Y. Structural characteristics of taste active peptides in protein hydrolysates from tilapia by-products. J. Food Meas. Charact. 2022;16:1674–1687. [Google Scholar]
- 26.Cui Q, Sun Y, Zhou Z, Cheng J, Guo M. Effects of enzymatic hydrolysis on physicochemical properties and solubility and bitterness of milk protein hydrolysates. Foods. 2021;10:2462. doi: 10.3390/foods10102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, et al. Review on the release mechanism and debittering technology of bitter peptides from protein hydrolysates. Compr. Rev. Food Sci. Food Saf. 2022;21:5153–5170. doi: 10.1111/1541-4337.13050. [DOI] [PubMed] [Google Scholar]
- 28.Cheung IW, Li-Chan EC. Angiotensin-I-converting enzyme inhibitory activity and bitterness of enzymatically-produced hydrolysates of shrimp (Pandalopsis dispar) processing byproducts investigated by Taguchi design. Food Chem. 2010;122:1003–1012. [Google Scholar]
- 29.Rauh VM, et al. Plasmin activity in UHT milk: relationship between proteolysis, age gelation, and bitterness. J. Agric. Food Chem. 2014;62:6852–6860. doi: 10.1021/jf502088u. [DOI] [PubMed] [Google Scholar]
- 30.Roland WS, et al. Bitter taste receptor activation by flavonoids and isoflavonoids: modeled structural requirements for activation of hTAS2R14 and hTAS2R39. J. Agric. food Chem. 2013;61:10454–10466. doi: 10.1021/jf403387p. [DOI] [PubMed] [Google Scholar]
- 31.Quaglia G, Orban E. Influence of the degree of hydrolysis on the solubility of the protein hydrolysates from sardine (Sardina pilchardus) J. Sci. Food Agric. 1987;38:271–276. [Google Scholar]
- 32.Cho MJ, Unklesbay N, Hsieh F-H, Clarke AD. Hydrophobicity of bitter peptides from soy protein hydrolysates. J. Agric. Food Chem. 2004;52:5895–5901. doi: 10.1021/jf0495035. [DOI] [PubMed] [Google Scholar]
- 33.Ishibashi N, Kouge K, Shinoda I, Kanehisa H, Okai H. A mechanism for bitter taste sensibility in peptides. Agric. Biol. Chem. 1988;52:819–827. [Google Scholar]
- 34.Cavallo C, Cicia G, Del Giudice T, Sacchi R, Vecchio R. Consumers’ perceptions and preferences for bitterness in vegetable foods: The case of extra-virgin olive oil and brassicaceae—a narrative review. Nutrients. 2019;11:1164. doi: 10.3390/nu11051164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nath A, et al. Detecting the bitterness of milk-protein-derived peptides using an electronic tongue. Chemosensors. 2022;10:215. [Google Scholar]
- 36.Mirzapour-Kouhdasht A, Garcia-Vaquero M. Cardioprotective peptides from milk processing and dairy products: from bioactivity to final products including commercialization and legislation. Foods. 2022;11:1270. doi: 10.3390/foods11091270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukhopadhya, A. & Sweeney, T. in Milk Proteins-From Structure to Biological Properties and Health Aspects (IntechOpen, 2016).
- 38.Pedrosa M, Pascual C, Larco J, Esteban MM. Palatability of hydrolysates and Other substitution formulas for cow’s milk-allergic children: a comparative study of taste, smell, and texture evaluated by healthy volunteers. J. Investig. Allergol. Clin. Immunol. 2006;16:351–356. [PubMed] [Google Scholar]
- 39.Ishibashi N, et al. Role of the hydrophobic amino acid residue in the bitterness of peptides. Agric. Biol. Chem. 1988;52:91–94. [Google Scholar]
- 40.Ishibashi N, et al. Bitterness of phenylalanine-and tyrosine-containing peptides. Agric. Biol. Chem. 1987;51:3309–3313. [Google Scholar]
- 41.Ohyama S, Ishibashi N, Tamura M, Nishizaki H, Okai H. Synthesis of bitter peptides composed of aspartic acid and glutamic acid. Agric. Biol. Chem. 1988;52:871–872. [Google Scholar]
- 42.Shinoda I, Fushimi A, Kato H, Okai H, Fukui S. Bitter taste of synthetic C-terminal tetradecapeptide of bovine β-casein, H-Pro196-Val-Leu-Gly-Pro-Val-Arg-Gly-Pro-Phe-Pro-Ile-Ile-Val209-OH, and its related peptides. Agric. Biol. Chem. 1985;49:2587–2596. [Google Scholar]
- 43.Leksrisompong PP, Miracle RE, Drake M. Characterization of flavor of whey protein hydrolysates. J. Agric. Food Chem. 2010;58:6318–6327. doi: 10.1021/jf100009u. [DOI] [PubMed] [Google Scholar]
- 44.Fávaro-Trindade CS, Santana ADS, Monterrey-Quintero ES, Trindade MA, Netto FM. The use of spray drying technology to reduce bitter taste of casein hydrolysate. Food Hydrocoll. 2010;24:336–340. [Google Scholar]
- 45.Minamiura N, Matsumura Y, Fukumoto J, Yamamoto T. Bitter peptides in cow milk casein digests with bacterial proteinase: part I. Isolation and determination of amino acid sequence of a bitter peptide. Agric. Biol. Chem. 1972;36:588–595. [Google Scholar]
- 46.Rios G, Belleville M, Paolucci D, Sanchez J. Progress in enzymatic membrane reactors—a review. J. Membr. Sci. 2004;242:189–196. [Google Scholar]
- 47.Spellman D, O’cuinn G, FitzGerald R. Bitterness in Bacillus proteinase hydrolysates of whey proteins. Food Chem. 2009;114:440–446. [Google Scholar]
- 48.Zhang Q, et al. Combining Alcalase hydrolysis and transglutaminase-cross-linking improved bitterness and techno-functional properties of hypoallergenic soybean protein hydrolysates through structural modifications. LWT. 2021;151:112096. [Google Scholar]
- 49.Sung M-J, Park Y-S, Chang H-G. Quality characteristics of sponge cake supplemented with soy protein concentrate. Food Sci. Biotechnol. 2006;15:860–865. [Google Scholar]
- 50.Walter J, Greenberg Y, Sriramarao P, Ismail BP. Limited hydrolysis combined with controlled Maillard-induced glycation does not reduce immunoreactivity of soy protein for all sera tested. Food Chem. 2016;213:742–752. doi: 10.1016/j.foodchem.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Lovšin-Kukman I, Zelenik-Blatnik M, Abram V. Bitterness intensity of soybean protein hydrolysates—chemical and organoleptic characterization. Z. Lebensm. Unters. Forsch. 1996;203:272–276. [Google Scholar]
- 52.Seo W, Lee H, Baek H. Evaluation of bitterness in enzymatic hydrolysates of soy protein isolate by taste dilution analysis. J. Food Sci. 2008;73:S41–S46. doi: 10.1111/j.1750-3841.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 53.Lock, S.-L. Flavor Characteristics of Soy Products Modified by Proteases and Alpha-galactosidase (Iowa State University, 2007).
- 54.Geisenhoff, H. Bitterness of Soy Protein Hydrolysates According to Molecular Weight of Peptides (2009).
- 55.Dall Aaslyng M, Poll L, Nielsen PM, Flyge H. Sensory, chemical and sensometric studies of hydrolyzed vegetable protein produced by various processes. Eur. Food Res. Technol. 1999;209:227–236. [Google Scholar]
- 56.Oka S, Nagata K. Isolation and characterization of neutral peptides in soy sauce. Agric. Biol. Chem. 1974;38:1185–1194. [Google Scholar]
- 57.Oka S, Nagata K. Isolation and characterization of acidic peptides in soy sauce. Agric. Biol. Chem. 1974;38:1195–1202. [Google Scholar]
- 58.Takeuchi T. Studies on peptides in miso and soysauce. J. Ferment. Technol. 1969;47:496–501. [Google Scholar]
- 59.Yamasaki Y. The bitter taste in natto. J. Home Econ. Jpn. 1987;38:93–97. [Google Scholar]
- 60.Kim J, Whang J, Kim K, Koh J, Suh HJ. Preparation of corn gluten hydrolysate with angiotensin I converting enzyme inhibitory activity and its solubility and moisture sorption. Process Biochem. 2004;39:989–994. [Google Scholar]
- 61.Tkaczewska J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings-A review. Trends Food Sci. Technol. 2020;106:298–311. [Google Scholar]
- 62.Glusac J, Fishman A. Enzymatic and chemical modification of zein for food application. Trends Food Sci. Technol. 2021;112:507–517. [Google Scholar]
- 63.Casella ML, Whitaker JR. Enzymatically and chemically modified zein for improvement of functional properties. J. Food Biochem. 1990;14:453–475. [Google Scholar]
- 64.Li G, et al. Functions and applications of bioactive peptides from corn gluten meal. Adv. Food Nutr. Res. 2019;87:1–41. doi: 10.1016/bs.afnr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Huang S, et al. Ultrasound-assisted multi-enzymatic system for the preparation of ACE inhibitory peptides with low bitterness from corn gluten meal. Processes. 2021;9:2170. [Google Scholar]
- 66.Klomklao S, Benjakul S. Utilization of tuna processing byproducts: Protein hydrolysate from skipjack tuna (Katsuwonus pelamis) viscera. J. Food Process. Preserv. 2017;41:e12970. [Google Scholar]
- 67.Hsu K-C. Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem. 2010;122:42–48. [Google Scholar]
- 68.Miles, R. D. & Chapman, F. A. The benefits of fish meal in aquaculture diets. EDIS 2006 (2006).
- 69.Benjakul, S., Yarnpakdee, S., Senphan, T., Halldórsdóttir, S. M. & Kristinsson, H. G. in Antioxidants and Functional Components in Aquatic Foods 237–281 (2014).
- 70.Idowu AT, Benjakul S, Sinthusamran S, Sookchoo P, Kishimura H. Protein hydrolysate from salmon frames: production, characteristics and antioxidative activity. J. Food Biochem. 2019;43:e12734. doi: 10.1111/jfbc.12734. [DOI] [PubMed] [Google Scholar]
- 71.Hou H, Li B, Zhao X, Zhang Z, Li P. Optimization of enzymatic hydrolysis of Alaska pollock frame for preparing protein hydrolysates with low-bitterness. LWT-Food Sci. Technol. 2011;44:421–428. [Google Scholar]
- 72.Lalasidis G, Bostrom S, Sjoberg LB. Low molecular weight enzymatic fish protein hydrolysates: chemical composition and nutritive value. J. Agric. Food Chem. 1978;26:751–756. doi: 10.1021/jf60217a045. [DOI] [PubMed] [Google Scholar]
- 73.Aspevik, T. Fish Protein Hydrolysates Based on Atlantic Salmon By-products. Enzyme Cost-efficiency and Characterization of Sensory, Surface-active and Nutritional Properties (2016).
- 74.Mackie IM. General review of fish protein hydrolysates. Anim. Feed Sci. Technol. 1982;7:113–124. [Google Scholar]
- 75.Hosokawa M, Sakakibara H, Yajima I, Hayashi K. Non-volatile flavor components in dorsal, abdominal and red meat parts of dried skipjack (katsuobushi) studies on flavor components of katsuobushi, part II. Nippon Shokuhin Kogyo Gakkaishi. 1990;37:856–861. [Google Scholar]
- 76.Park J-N, et al. Taste effects of oligopeptides in a Vietnamese fish sauce. Fish. Sci. 2002;68:921–928. [Google Scholar]
- 77.Wells ML, et al. Algae as nutritional and functional food sources: revisiting our understanding. J. Appl. Phycol. 2017;29:949–982. doi: 10.1007/s10811-016-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fleurence J. Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999;10:25–28. [Google Scholar]
- 79.Cermeño M, Kleekayai T, Amigo‐Benavent M, Harnedy‐Rothwell P, FitzGerald RJ. Current knowledge on the extraction, purification, identification, and validation of bioactive peptides from seaweed. Electrophoresis. 2020;41:1694–1717. doi: 10.1002/elps.202000153. [DOI] [PubMed] [Google Scholar]
- 80.Laohakunjit N, Selamassakul O, Kerdchoechuen O. Seafood-like flavour obtained from the enzymatic hydrolysis of the protein by-products of seaweed (Gracilaria sp.) Food Chem. 2014;158:162–170. doi: 10.1016/j.foodchem.2014.02.101. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y, et al. Flavor challenges in extruded plant-based meat alternatives: a review. Compr. Rev. Food Sci. Food Saf. 2022;21:2898–2929. doi: 10.1111/1541-4337.12964. [DOI] [PubMed] [Google Scholar]
- 82.Karamac M, Rybarczyk A. Chymotryptic hydrolysis of lentil meal proteins and characteristics of the resulting hydrolysates. Polish J. Food Nutr. Sci. 2008;58:351–357. [Google Scholar]
- 83.Rezvankhah A, Yarmand MS, Ghanbarzadeh B, Mirzaee H. Generation of bioactive peptides from lentil protein: degree of hydrolysis, antioxidant activity, phenol content, ACE-inhibitory activity, molecular weight, sensory, and functional properties. J. Food Meas. Charact. 2021;15:5021–5035. [Google Scholar]
- 84.Humiski L, Aluko R. Physicochemical and bitterness properties of enzymatic pea protein hydrolysates. J. Food Sci. 2007;72:S605–S611. doi: 10.1111/j.1750-3841.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 85.Schlegel K, et al. Enzymatic hydrolysis of lupin protein isolates—Changes in the molecular weight distribution, technofunctional characteristics, and sensory attributes. Food Sci. Nutr. 2019;7:2747–2759. doi: 10.1002/fsn3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arteaga VG, Guardia MA, Muranyi I, Eisner P, Schweiggert-Weisz U. Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov. Food Sci. Emerg. Technol. 2020;65:102449. [Google Scholar]
- 87.Xia Y, et al. Comparative study of various methods used for bitterness reduction from pea (Pisum sativum L.) protein hydrolysates. LWT. 2022;159:113228. [Google Scholar]
- 88.Koo SH, et al. Evaluation of wheat gluten hydrolysates as taste-active compounds with antioxidant activity. J. food Sci. Technol. 2014;51:535–542. doi: 10.1007/s13197-011-0515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao J, et al. Analysis of umami taste substances of morel mushroom (Morchella sextelata) hydrolysates derived from different enzymatic systems. Food Chem. 2021;362:130192. doi: 10.1016/j.foodchem.2021.130192. [DOI] [PubMed] [Google Scholar]
- 90.Kheeree N, et al. ACE inhibitory peptides derived from de-fatted lemon basil seeds: Optimization, purification, identification, structure–activity relationship and molecular docking analysis. Food Funct. 2020;11:8161–8178. doi: 10.1039/d0fo01240h. [DOI] [PubMed] [Google Scholar]
- 91.Toldrá F, Mora L, Reig M. New insights into meat by-product utilization. Meat Sci. 2016;120:54–59. doi: 10.1016/j.meatsci.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 92.Singh, A. et al. Protein hydrolysate from splendid squid (Loligo formosana) fins: antioxidant, functional properties, and flavoring profile. Turkish J. Fish. Aquat. Sci.22 (2022). 10.4194/TRJFAS21005.
- 93.Zulkipli AS, Salleh RM. Flavour improvement of protein hydrolysates derived from cephalopods byproducts using maillard reaction: a short review. ASM Sc. J. 2021;15:1–13. [Google Scholar]
- 94.Synowiecki J, Al-Khateeb NAAQ. The recovery of protein hydrolysate during enzymatic isolation of chitin from shrimp Crangon crangon processing discards. Food Chem. 2000;68:147–152. [Google Scholar]
- 95.Cheung IW, Li-Chan EC. Application of taste sensing system for characterisation of enzymatic hydrolysates from shrimp processing by-products. Food Chem. 2014;145:1076–1085. doi: 10.1016/j.foodchem.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 96.Garcés-Rimón M, Sandoval M, Molina E, López-Fandiño R, Miguel M. Egg protein hydrolysates: New culinary textures. Int. J. Gastron. Food Sci. 2016;3:17–22. [Google Scholar]
- 97.Aubes-Dafau I, Capdevielle J, Seris J, Combes D. Bitter peptide from hemoglobin hydrolysate: isolation and characterization. FEBS Lett. 1995;364:115–119. doi: 10.1016/0014-5793(95)00361-c. [DOI] [PubMed] [Google Scholar]
- 98.Guo SG, Zhao MM, Wang JS, Cui C. Proteolytic degradation and amino acid liberation during extensive hydrolysis of porcine blood hemoglobin by protease admixture. J. Food Process Eng. 2007;30:640–659. [Google Scholar]
- 99.Chen M, et al. Taste characteristics and umami mechanism of novel umami peptides and umami-enhancing peptides isolated from the hydrolysates of Sanhuang Chicken. Eur. Food Res. Technol. 2021;247:1633–1644. [Google Scholar]
- 100.Fu Y, Chen J, Bak KH, Lametsch R. Valorisation of protein hydrolysates from animal by‐products: perspectives on bitter taste and debittering methods: a review. Int. J. Food Sci. Technol. 2019;54:978–986. [Google Scholar]
- 101.Acquah C, Di Stefano E, Udenigwe CC. Role of hydrophobicity in food peptide functionality and bioactivity. J. Food Bioact. 2018;4:88–98. [Google Scholar]
- 102.Rani S, Pooja K, Pal GK. Exploration of rice protein hydrolysates and peptides with special reference to antioxidant potential: Computational derived approaches for bio-activity determination. Trends Food Sci. Technol. 2018;80:61–70. [Google Scholar]
- 103.Pripp AH, Ardö Y. Modelling relationship between angiotensin-(I)-converting enzyme inhibition and the bitter taste of peptides. Food Chem. 2007;102:880–888. [Google Scholar]
- 104.Subbalakshmi C, Nagaraj R, Sitaram N. Biological activities of C-terminal 15-residue synthetic fragment of melittin: design of an analog with improved antibacterial activity. FEBS Lett. 1999;448:62–66. doi: 10.1016/s0014-5793(99)00328-2. [DOI] [PubMed] [Google Scholar]
- 105.Chen H-M, Muramoto K, Yamauchi F, Fujimoto K, Nokihara K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998;46:49–53. doi: 10.1021/jf970649w. [DOI] [PubMed] [Google Scholar]
- 106.Chandrashekar J, et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 107.Liu B-Y, Zhu K-X, Peng W, Guo X-N, Zhou H-M. Effect of sequential hydrolysis with endo-and exo-peptidase on bitterness properties of wheat gluten hydrolysates. RSC Adv. 2016;6:27659–27668. [Google Scholar]
- 108.Cogan U, Moshe M, Mokady S. Debittering and nutritional upgrading of enzymic casein hydrolysates. J. Sci. Food Agric. 1981;32:459–466. [Google Scholar]
- 109.Suh HJ, Bae SH, Noh DO. Debittering of corn gluten hydrolysate with active carbon. J. Sci. Food Agric. 2000;80:614–618. [Google Scholar]
- 110.Akell, R. in Activated Carbon Adsorption for Wastewater Treatment 223–239 (CRC Press, 2018).
- 111.Hilber I, Blum F, Schmidt H-P, Bucheli TD. Current analytical methods to quantify PAHs in activated carbon and vegetable carbon (E153) are not fit for purpose. Environ. Pollut. 2022;309:119599. doi: 10.1016/j.envpol.2022.119599. [DOI] [PubMed] [Google Scholar]
- 112.Wasswa, J., Tang, J. & Gu, X.-H. Desalting Fish Skin Protein Hydrolysates Using Macroporous Adsorption Resin (2007).
- 113.Kamara M, Amadou I, Kexue Z, Kelfala Foh M, Huiming Z. The influence of debittering and desalting on defatted foxtail millet (Setaria italica L.) protein hydrolysate. Am. J. Biochem. Mol. Biol. 2011;1:39–53. [Google Scholar]
- 114.Liu B-Y, Zhu K-X, Guo X-N, Peng W, Zhou H-M. Changes in the enzyme-induced release of bitter peptides from wheat gluten hydrolysates. RSC Adv. 2016;6:102249–102257. [Google Scholar]
- 115.Dauksas E, Slizyte R, Rustad T, Storro I. Bitterness in fish protein hydrolysates and methods for removal. J. Aquat. Food Prod. Technol. 2004;13:101–114. [Google Scholar]
- 116.Sinthusamran S, et al. Effect of proteases and alcohols used for debittering on characteristics and antioxidative activity of protein hydrolysate from salmon frames. J. Food Sci. Technol. 2020;57:473–483. doi: 10.1007/s13197-019-04075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh A, Benjakul S, Huda N. Characteristics and nutritional value of biscuits fortified with debittered salmon (Salmo salar) frame hydrolysate. Int. J. Food Sci. Technol. 2020;55:3553–3562. [Google Scholar]
- 118.Singh A, et al. Debittering of salmon (Salmo salar) frame protein hydrolysate using 2-butanol in combination with β-cyclodextrin: Impact on some physicochemical characteristics and antioxidant activities. Food Chem. 2020;321:126686. doi: 10.1016/j.foodchem.2020.126686. [DOI] [PubMed] [Google Scholar]
- 119.Adler‐Nissen J. Control of the proteolytic reaction and of the level of bitterness in protein hydrolysis processes. J. Chem. Technol. Biotechnol. Biotechnol. 1984;34:215–222. [Google Scholar]
- 120.Kim IMR, Kawamura Y, Lee CH. Isolation and Identification of Bitter Peptides of Tryptic Hydrolysate of Soybean 11S Glycinin by Reverse‐phase High‐performance Liquid Chromatography. J. Food Sci. 2003;68:2416–2422. [Google Scholar]
- 121.Liu X, Jiang D, Peterson DG. Identification of bitter peptides in whey protein hydrolysate. J. Agric. Food Chem. 2014;62:5719–5725. doi: 10.1021/jf4019728. [DOI] [PubMed] [Google Scholar]
- 122.Helbig N, Ho L, Christy G, Nakai S. Debittering of skim milk hydrolysates by adsorption for into into acidic beverages. J. Food Sci. 1980;45:331–335. [Google Scholar]
- 123.Visser, S., Slangen, K. & Hup, G. Some bitter peptides from rennet-treated casein. A method for their purification, utilizing chromatographic separation on silica gel. Netherlands Milk and Dairy Journal (Netherlands) (1975).
- 124.Cheison SC, Wang Z, Xu SY. Use of macroporous adsorption resin for simultaneous desalting and debittering of whey protein hydrolysates. Int. J. Food Sci. Technol. 2007;42:1228–1239. [Google Scholar]
- 125.Imai K, Shimizu K, Kamimura M, Honda H. Interaction between porous silica gel microcarriers and peptides for oral administration of functional peptides. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-29345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Imai K, Ikeda A, Shimizu K, Honda H. Selective elimination of bitter peptides by adsorption to heat-treated porous silica gel. Food Sci. Technol. Res. 2019;25:179–186. [Google Scholar]
- 127.Raksakulthai, R. & Haard, N. F. Exopeptidases and Their Application to Reduce Bitterness in Food: A Review (2003). [DOI] [PubMed]
- 128.Cheung LK, Aluko RE, Cliff MA, Li-Chan EC. Effects of exopeptidase treatment on antihypertensive activity and taste attributes of enzymatic whey protein hydrolysates. J. Funct. Foods. 2015;13:262–275. [Google Scholar]
- 129.Hu, X. et al. An updated review of functional properties, debittering methods, and applications of soybean functional peptides. Crit. Rev. Food Sci. Nutr. 1–16 (2022). [DOI] [PubMed]
- 130.Fernandez‐Espla MD, Rul F. PepS from Streptococcus thermophilus: a new member of the aminopeptidase T family of thermophilic bacteria. Eur. J. Biochem. 1999;263:502–510. doi: 10.1046/j.1432-1327.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- 131.Lin X, Dong L, Yu D, Wang B, Pan L. High-level expression and characterization of the thermostable leucine aminopeptidase Thelap from the thermophilic fungus Thermomyces lanuginosus in Aspergillus niger and its application in soy protein hydrolysis. Protein Expr. Purif. 2020;167:105544. doi: 10.1016/j.pep.2019.105544. [DOI] [PubMed] [Google Scholar]
- 132.Song P, et al. A novel aminopeptidase with potential debittering properties in casein and soybean protein hydrolysates. Food Sci. Biotechnol. 2020;29:1491–1499. doi: 10.1007/s10068-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Asano, M., Kawai, M., Miwa, T. & Nio, N. Aminopeptidase GX, and a method of hydrolyzing a protein with the same. US Patent 582 4534 (Google Patents, 1998).
- 134.Gobbetti M, Cossignani L, Simonetti M, Damiani P. Effect of the aminopeptidase from Pseudomonas fluorescens ATCC 948 on synthetic bitter peptides, bitter hydrolysate of UHT milk proteins and on the ripening of Italian Caciotta type cheese. Le. Lait. 1995;75:169–179. [Google Scholar]
- 135.Izawa N, Tokuyasu K, Hayashi K. Debittering of protein hydrolysates using Aeromonas caviae aminopeptidase. J. Agric. Food Chem. 1997;45:543–545. [Google Scholar]
- 136.Matsuoka H, Fuke Y, Kaminogawa S, Yamauchi K. Purification and debittering effect of aminopeptidase II from Penicillium caseicolum. J. Agric. Food Chem. 1991;39:1392–1395. [Google Scholar]
- 137.McDonnell M, Fitzgerald R, Fhaoláin IN, Jennings PV, O’CUINN G. Purification and characterization of aminopeptidase P from Lactococcus lactis subsp. cremoris. J. Dairy Res. 1997;64:399–407. doi: 10.1017/s0022029997002318. [DOI] [PubMed] [Google Scholar]
- 138.Prost F, Chamba JF. Effect of aminopeptidase activity of thermophilic lactobacilli on Emmental cheese characteristics. J. Dairy Sci. 1994;77:24–33. [Google Scholar]
- 139.Tan P, et al. Degradation and debittering of a tryptic digest from beta-casein by aminopeptidase N from Lactococcus lactis subsp. cremoris Wg2. Appl. Environ. Microbiol. 1993;59:1430–1436. doi: 10.1128/aem.59.5.1430-1436.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bouchier P, O’cuinn G, Harrington D, FitzGerald R. Debittering and Hydrolysis of a Tryptic Hydrolysate of β‐casein with Purified General and Proline Specific Aminopeptidases from Lactococcus lactis ssp. cremoris AM2. J. Food Sci. 2001;66:816–820. [Google Scholar]
- 141.Edens L, et al. Extracellular prolyl endoprotease from Aspergillus niger and its use in the debittering of protein hydrolysates. J. Agric. Food Chem. 2005;53:7950–7957. doi: 10.1021/jf050652c. [DOI] [PubMed] [Google Scholar]
- 142.Nishiwaki T, Yoshimizu S, Furuta M, Hayashi K. Debittering of enzymatic hydrolysates using an aminopeptidase from the edible basidiomycete Grifola frondosa. J. Biosci. Bioeng. 2002;93:60–63. [PubMed] [Google Scholar]
- 143.Li L, et al. Debittering effect of Actinomucor elegans peptidases on soybean protein hydrolysates. J. Ind. Microbiol. Biotechnol. 2008;35:41–47. doi: 10.1007/s10295-007-0264-y. [DOI] [PubMed] [Google Scholar]
- 144.Gruppi A, Dermiki M, Spigno G, FitzGerald RJ. Impact of enzymatic hydrolysis and heat inactivation on the physicochemical properties of milk protein hydrolysates. Foods. 2022;11:516. doi: 10.3390/foods11040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hinnenkamp C, Ismail BP. A proteomics approach to characterizing limited hydrolysis of whey protein concentrate. Food Chem. 2021;350:129235. doi: 10.1016/j.foodchem.2021.129235. [DOI] [PubMed] [Google Scholar]
- 146.Yoon IS, Kim J-S, Lee JS, Kwon IS, Heu MS. Optimization of reduced bitterness of alcalase-treated anchovy Engrauris japonica hydrolysate by aminopeptidase active fraction from common squid Todarodes pacificus hepatopancreas. Korean J. Fish. Aquat. Sci. 2021;54:724–732. [Google Scholar]
- 147.Yan, Z.-F., Yuan, S., Qin, Q. & Wu, J. Enhancement of rice protein hydrolysate quality using a novel dual enzyme system. LWT158, 113110 (2022).
- 148.Suh HJ, Whang J, Kim YS, Bae S, Noh D. Preparation of angiotensin I converting enzyme inhibitor from corn gluten. Process Biochem. 2003;38:1239–1244. [Google Scholar]
- 149.Huang W-Q, et al. The structure and enzyme characteristics of a recombinant leucine aminopeptidase rlap1 from Aspergillus sojae and its application in debittering. Appl. Biochem. Biotechnol. 2015;177:190–206. doi: 10.1007/s12010-015-1737-5. [DOI] [PubMed] [Google Scholar]
- 150.Fu Y, Liu J, Hansen ET, Bredie WL, Lametsch R. Structural characteristics of low bitter and high umami protein hydrolysates prepared from bovine muscle and porcine plasma. Food Chem. 2018;257:163–171. doi: 10.1016/j.foodchem.2018.02.159. [DOI] [PubMed] [Google Scholar]
- 151.Fu J, Li L, Yang X-Q. Specificity of carboxypeptidases from Actinomucor elegans and their debittering effect on soybean protein hydrolysates. Appl. Biochem. Biotechnol. 2011;165:1201–1210. doi: 10.1007/s12010-011-9338-4. [DOI] [PubMed] [Google Scholar]
- 152.Kawabata, C., Komai, T. & Gocho, S. Elimination of Bitterness of Bitter Peptides by Squid Liver Carboxypeptidase (ACS Publications, 1996). https://pubs.acs.org/doi/abs/10.1021/bk-1996-0637.ch015.
- 153.Umetsu H, Ichishima E. Mechanism of digestion of bitter peptide from a fish protein concentrate by wheat carboxypeptidase. Nippon Shokuhin Kogyo Gakkaishi. 1985;32:281–287. [Google Scholar]
- 154.Umetsu H, Ichishima E. Mechanism of digestion of bitter peptides from soybean protein by wheat carboxypeptidase. Nippon Shokuhin Kogyo Gakkaishi. 1988;35:440–447. [Google Scholar]
- 155.Umetsu H, Matsuoka H, Ichishima E. Debittering mechanism of bitter peptides from milk casein by wheat carboxypeptidase. J. Agric. Food Chem. 1983;31:50–53. [Google Scholar]
- 156.Yin H, Jia F, Huang J. The variation of two extracellular enzymes and soybean meal bitterness during solid-state fermentation of Bacillus subtilis. Grain Oil Sci. Technol. 2019;2:39–43. [Google Scholar]
- 157.Meinlschmidt P, Schweiggert-Weisz U, Eisner P. Soy protein hydrolysates fermentation: Effect of debittering and degradation of major soy allergens. LWT-Food Sci. Technol. 2016;71:202–212. [Google Scholar]
- 158.Kodera T, Asano M, Nio N. Characteristic property of low bitterness in protein hydrolysates by a novel soybean protease D3. J. Food Sci. 2006;71:S609–S614. [Google Scholar]
- 159.Habibi, N. M. & Lee, B. Debittering of tryptic digests from β-casein and enzyme modified cheese by x-prolyl dipeptidylpeptidase from Lactobacillus casei ssp. casei. LLG. Iranian J. Sci. Technol. Transaction A.31, 263-270 (2007).
- 160.Duan ZH, Wang JL, Yi MH, Yin AQ. Recovery of proteins from silver carp by‐products with enzymatic hydrolysis and reduction of bitterness in hydrolysate. J. Food process Eng. 2010;33:962–978. [Google Scholar]
- 161.Zhang M, Xin X, Wu H, Zhang H. Debittering effect of partially purified proteases from soybean seedlings on soybean protein isolate hydrolysate produced by alcalase. Food Chem. 2021;362:130190. doi: 10.1016/j.foodchem.2021.130190. [DOI] [PubMed] [Google Scholar]
- 162.Durand E, Beaubier S, Ilic I, Kapel R, Villeneuve P. Production and antioxidant capacity of bioactive peptides from plant biomass to counteract lipid oxidation. Curr. Res. Food Sci. 2021;4:365–397. doi: 10.1016/j.crfs.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Eric K, et al. Sensory attributes and antioxidant capacity of Maillard reaction products derived from xylose, cysteine and sunflower protein hydrolysate model system. Food Res. Int. 2013;54:1437–1447. [Google Scholar]
- 164.Song N, et al. Transglutaminase cross-linking effect on sensory characteristics and antioxidant activities of Maillard reaction products from soybean protein hydrolysates. Food Chem. 2013;136:144–151. doi: 10.1016/j.foodchem.2012.07.100. [DOI] [PubMed] [Google Scholar]
- 165.de Carvalho NC, et al. Physicochemical changes and bitterness of whey protein hydrolysates after transglutaminase cross-linking. LWT. 2019;113:108291. [Google Scholar]
- 166.Hong PK, Ndagijimana M, Betti M. Glucosamine-induced glycation of hydrolysed meat proteins in the presence or absence of transglutaminase: Chemical modifications and taste-enhancing activity. Food Chem. 2016;197:1143–1152. doi: 10.1016/j.foodchem.2015.11.096. [DOI] [PubMed] [Google Scholar]
- 167.Liu BY, Zhu KX, Guo XN, Peng W, Zhou HM. Effect of deamidation‐induced modification on umami and bitter taste of wheat gluten hydrolysates. J. Sci. Food Agric. 2017;97:3181–3188. doi: 10.1002/jsfa.8162. [DOI] [PubMed] [Google Scholar]
- 168.Won Yeom H, Kim KS, Rhee JS. Soy protein hydrolysate debittering by lysine‐acetylation. J. Food Sci. 1994;59:1123–1126. [Google Scholar]
- 169.Habinshuti I, Zhang M, Sun HN, Mu TH. Comparative study of antioxidant and flavour characteristics of Maillard reaction products from five types of protein hydrolysates. Int. J. Food Sci. Technol. 2022;57:3652–3664. [Google Scholar]
- 170.Abdelhedi O, et al. Effect of ultrasound pretreatment and Maillard reaction on structure and antioxidant properties of ultrafiltrated smooth-hound viscera proteins-sucrose conjugates. Food Chem. 2017;230:507–515. doi: 10.1016/j.foodchem.2017.03.053. [DOI] [PubMed] [Google Scholar]
- 171.Sun XD. Enzymatic hydrolysis of soy proteins and the hydrolysates utilisation. Int. J. Food Sci. Technol. 2011;46:2447–2459. [Google Scholar]
- 172.Xu Q, et al. Sodium chloride suppresses the bitterness of protein hydrolysates by decreasing hydrophobic interactions. J. Food Sci. 2019;84:86–91. doi: 10.1111/1750-3841.14419. [DOI] [PubMed] [Google Scholar]
- 173.Dong Q, et al. Inclusion complex of neohesperidin dihydrochalcone and glucosyl-β-cyclodextrin: Synthesis, characterization, and bitter masking properties in aqueous solutions. J. Mol. Liq. 2017;241:926–933. [Google Scholar]
- 174.Zhang C, et al. Beef protein-derived peptides as bitter taste receptor T2R4 blockers. J. Agric. Food Chem. 2018;66:4902–4912. doi: 10.1021/acs.jafc.8b00830. [DOI] [PubMed] [Google Scholar]
- 175.Xu Q, et al. Hen protein-derived peptides as the blockers of human bitter taste receptors T2R4, T2R7 and T2R14. Food Chem. 2019;283:621–627. doi: 10.1016/j.foodchem.2019.01.059. [DOI] [PubMed] [Google Scholar]
- 176.Zhang Q-W, Lin L-G, Ye W-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018;13:1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zheng X, et al. Developments in taste-masking techniques for traditional Chinese medicines. Pharmaceutics. 2018;10:157. doi: 10.3390/pharmaceutics10030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Leksrisompong P, Gerard P, Lopetcharat K, Drake M. Bitter taste inhibiting agents for whey protein hydrolysate and whey protein hydrolysate beverages. J. Food Sci. 2012;77:S282–S287. doi: 10.1111/j.1750-3841.2012.02800.x. [DOI] [PubMed] [Google Scholar]
- 179.Shishir MRI, Xie L, Sun C, Zheng X, Chen W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018;78:34–60. [Google Scholar]
- 180.Mohan A, Rajendran SR, He QS, Bazinet L, Udenigwe CC. Encapsulation of food protein hydrolysates and peptides: a review. Rsc Adv. 2015;5:79270–79278. [Google Scholar]
- 181.Ma J-J, et al. Effect of spray drying and freeze drying on the immunomodulatory activity, bitter taste and hygroscopicity of hydrolysate derived from whey protein concentrate. LWT-Food Sci. Technol. 2014;56:296–302. [Google Scholar]
- 182.Sarabandi K, Mahoonak AS, Hamishekar H, Ghorbani M, Jafari SM. Microencapsulation of casein hydrolysates: Physicochemical, antioxidant and microstructure properties. J. Food Eng. 2018;237:86–95. [Google Scholar]
- 183.Murthy LN, et al. Characterization of spray-dried hydrolyzed proteins from pink perch meat added with Maltodextrin and gum Arabic. J. Aquat. Food Prod. Technol. 2017;26:913–928. [Google Scholar]
- 184.Sarabandi K, Gharehbeglou P, Jafari SM. Spray-drying encapsulation of protein hydrolysates and bioactive peptides: Opportunities and challenges. Dry. Technol. 2020;38:577–595. [Google Scholar]
- 185.Giroldi M, Grambusch IM, Neutzling Lehn D, Volken de Souza CF. Encapsulation of dairy protein hydrolysates: Recent trends and future prospects. Dry. Technol. 2021;39:1513–1528. [Google Scholar]
- 186.Gao Y, et al. Encapsulation of bitter peptides in water-in-oil high internal phase emulsions reduces their bitterness and improves gastrointestinal stability. Food Chem. 2022;386:132787. doi: 10.1016/j.foodchem.2022.132787. [DOI] [PubMed] [Google Scholar]
- 187.Li Z, Paulson AT, Gill TA. Encapsulation of bioactive salmon protein hydrolysates with chitosan-coated liposomes. J. Funct. Foods. 2015;19:733–743. [Google Scholar]
- 188.da Rosa Zavareze E, et al. Production and characterization of encapsulated antioxidative protein hydrolysates from Whitemouth croaker (Micropogonias furnieri) muscle and byproduct. LWT-Food Sci. Technol. 2014;59:841–848. [Google Scholar]
- 189.Mosquera M, et al. Nanoencapsulation of an active peptidic fraction from sea bream scales collagen. Food Chem. 2014;156:144–150. doi: 10.1016/j.foodchem.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 190.Mohan A, McClements DJ, Udenigwe CC. Encapsulation of bioactive whey peptides in soy lecithin-derived nanoliposomes: Influence of peptide molecular weight. Food Chem. 2016;213:143–148. doi: 10.1016/j.foodchem.2016.06.075. [DOI] [PubMed] [Google Scholar]
- 191.Corrêa APF, et al. Characterization of nanoliposomes containing bioactive peptides obtained from sheep whey hydrolysates. LWT. 2019;101:107–112. [Google Scholar]
- 192.Han, C., Fang, L., Song, S. & Min, W. Polysaccharides-based delivery system for efficient encapsulation and controlled release of food-derived active peptides. Carbohydr. Polym.291, 119580 (2022). [DOI] [PubMed]
- 193.Idowu AT, Benjakul S. Bitterness of fish protein hydrolysate and its debittering prospects. J. Food Biochem. 2019;43:e12978. doi: 10.1111/jfbc.12978. [DOI] [PubMed] [Google Scholar]
- 194.Linde GA, et al. The use of 2D NMR to study β-cyclodextrin complexation and debittering of amino acids and peptides. Food Res. Int. 2010;43:187–192. [Google Scholar]
- 195.Sompornpisut P, Deechalao N, Vongsvivut J. An inclusion complex of β-Cyclodextrin-L-Phenylalanine: 1H NMR and molecular docking studies. ScienceAsia. 2002;28:263–270. [Google Scholar]
- 196.Hou L, Wang J, Zhang D. Optimization of debittering of soybean antioxidant hydrolysates with β-cyclodextrins using response surface methodology. J. Food Sci. Technol. 2013;50:521–527. doi: 10.1007/s13197-011-0358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sim SYJ, Srv A, Chiang JH, Henry CJ. Plant proteins for future foods: a roadmap. Foods. 2021;10:1967. doi: 10.3390/foods10081967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.San Gabriel A, Uneyama H. Amino acid sensing in the gastrointestinal tract. Amino Acids. 2013;45:451–461. doi: 10.1007/s00726-012-1371-2. [DOI] [PubMed] [Google Scholar]
- 199.Wellendorph P, Bräuner‐Osborne H. Molecular basis for amino acid sensing by family CG‐protein‐coupled receptors. Br. J. Pharmacol. 2009;156:869–884. doi: 10.1111/j.1476-5381.2008.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Iwaniak, A., Minkiewicz, P., Hrynkiewicz, M., Bucholska, J. & Darewicz, M. Hybrid approach in the analysis of bovine milk protein hydrolysates as a source of peptides containing di- and tripeptide bitterness indicators. Polish J. Food Nutr. Sci.70, 139–150 (2020).
- 201.Daher D, et al. Sensopeptidomic kinetic approach combined with decision trees and random forests to study the bitterness during enzymatic hydrolysis kinetics of micellar caseins. Foods. 2021;10:1312. doi: 10.3390/foods10061312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mann B, et al. Antioxidant activity of whey protein hydrolysates in milk beverage system. J. Food Sci. Technol. 2015;52:3235–3241. doi: 10.1007/s13197-014-1361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Newman J, Egan T, Harbourne N, Jacquier J. Correlation of sensory bitterness in dairy protein hydrolysates: Comparison of prediction models built using sensory, chromatographic and electronic tongue data. Talanta. 2014;126:46–53. doi: 10.1016/j.talanta.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 204.Kukman ILI, Zelenik-Blatnik M, Abram V. Isolation of low-molecular-mass hydrophobic bitter peptides in soybean protein hydrolysates by reversed-phase high-performance liquid chromatography. J. Chromatogr. A. 1995;704:113–120. [Google Scholar]
- 205.Iwaniak A, Hrynkiewicz M, Minkiewicz P, Bucholska J, Darewicz M. Soybean (Glycine max) protein hydrolysates as sources of peptide bitter-tasting indicators: An analysis based on hybrid and fragmentomic approaches. Appl. Sci. 2020;10:2514. [Google Scholar]
- 206.Aspevik T, Thoresen L, Steinsholm S, Carlehög M, Kousoulaki K. Sensory and chemical properties of protein hydrolysates based on mackerel (Scomber scombrus) and salmon (Salmo salar) side stream materials. J. Aquat. Food Prod. Technol. 2021;30:176–187. [Google Scholar]
- 207.Luo C, et al. Bitter peptides from enzymatically hydrolyzed protein increase the number of leucocytes and lysozyme activity of large yellow croaker (Larimichthys crocea) Fish. Shellfish Immunol. 2018;81:130–134. doi: 10.1016/j.fsi.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 208.Yang Y, Wang W, Liu Y. Umami and bitterness profile of enzymatic protein hydrolysates from cultured Takifugu obscurus by-products. J. Food Meas. Charact. 2020;14:476–484. [Google Scholar]
- 209.Sato M, et al. Antihypertensive effects of hydrolysates of wakame (Undaria pinnatifida) and their angiotensin-I-converting enzyme inhibitory activity. Ann. Nutr. Metab. 2002;46:259–267. doi: 10.1159/000066495. [DOI] [PubMed] [Google Scholar]
- 210.Karametsi K, Kokkinidou S, Ronningen I, Peterson DG. Identification of bitter peptides in aged cheddar cheese. J. Agric. Food Chem. 2014;62:8034–8041. doi: 10.1021/jf5020654. [DOI] [PubMed] [Google Scholar]
- 211.Aubes-Dufau I, Seris J-L, Combes D. Production of peptic hemoglobin hydrolyzates: bitterness demonstration and characterization. J. Agric. Food Chem. 1995;43:1982–1988. [Google Scholar]
- 212.Zhang, L., Song, C., Chang, J., Wang, Z. & Meng, X. Optimization of protein hydrolysates production from defatted peanut meal based on physicochemical characteristics and sensory analysis. LWT.163, 113572 (2022).
- 213.Großmann KK, Merz M, Appel D, Thaler T, Fischer L. Impact of peptidase activities on plant protein hydrolysates regarding bitter and umami taste. J. Agric. Food Chem. 2020;69:368–376. doi: 10.1021/acs.jafc.0c05447. [DOI] [PubMed] [Google Scholar]
- 214.Chandrasekaran S, Luna-Vital D, de Mejia EG. Identification and comparison of peptides from chickpea protein hydrolysates using either bromelain or gastrointestinal enzymes and their relationship with markers of type 2 diabetes and bitterness. Nutrients. 2020;12:3843. doi: 10.3390/nu12123843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wei C-K, Thakur K, Liu D-H, Zhang J-G, Wei Z-J. Enzymatic hydrolysis of flaxseed (Linum usitatissimum L.) protein and sensory characterization of Maillard reaction products. Food Chem. 2018;263:186–193. doi: 10.1016/j.foodchem.2018.04.120. [DOI] [PubMed] [Google Scholar]
- 216.Grossmann KK, Merz M, Appel D, De Araujo MM, Fischer L. New insights into the flavoring potential of cricket (Acheta domesticus) and mealworm (Tenebrio molitor) protein hydrolysates and their Maillard products. Food Chem. 2021;364:130336. doi: 10.1016/j.foodchem.2021.130336. [DOI] [PubMed] [Google Scholar]
- 217.Nasr NEH, ElMeshad AN, Fares AR. Nanocarrier systems in taste masking. Sci. Pharm. 2022;90:20. [Google Scholar]
- 218.Kamalakkannan V, Puratchikody A, Masilamani K, Senthilnathan B. Solubility enhancement of poorly soluble drugs by solid dispersion technique—a review. J. Pharm. Res. 2010;3:2314–2321. [Google Scholar]
- 219.Bhatta S, Stevanovic Janezic T, Ratti C. Freeze-drying of plant-based foods. Foods. 2020;9:87. doi: 10.3390/foods9010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kłosowska A, Wawrzyńczak A, Feliczak-Guzik A. Microencapsulation as a route for obtaining encapsulated flavors and fragrances. Cosmetics. 2023;10:26. [Google Scholar]
- 221.Tran P, et al. Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer drugs. Pharmaceutics. 2019;11:132. doi: 10.3390/pharmaceutics11030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rocha GA, Trindade MA, Netto FM, Fávaro-Trindade CS. Microcapsules of a casein hydrolysate: production, characterization, and application in protein bars. Food Sci. Technol. Int. 2009;15:407–413. [Google Scholar]
- 223.Yang S, et al. The improving effect of spray-drying encapsulation process on the bitter taste and stability of whey protein hydrolysate. Eur. Food Res. Technol. 2012;235:91–97. [Google Scholar]
- 224.Kurozawa LE, Park KJ, Hubinger MD. Effect of carrier agents on the physicochemical properties of a spray dried chicken meat protein hydrolysate. J. Food Eng. 2009;94:326–333. [Google Scholar]
- 225.Subtil SF, et al. Effect of spray drying on the sensory and physical properties of hydrolysed casein using gum arabic as the carrier. J. Food Sci. Technol. 2014;51:2014–2021. doi: 10.1007/s13197-012-0722-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gómez-Mascaraque LG, Miralles B, Recio I, López-Rubio A. Microencapsulation of a whey protein hydrolysate within micro-hydrogels: Impact on gastrointestinal stability and potential for functional yoghurt development. J. Funct. Foods. 2016;26:290–300. [Google Scholar]
- 227.Rukluarh S, Kanjanapongkul K, Panchan N, Niumnuy C. Effect of inclusion conditions on characteristics of spray dried whey protein hydrolysate/γ-cyclodextrin complexes. J. Food Sci. Agric. Technol. 2019;5:5–12. [Google Scholar]
- 228.Ortiz SEM, et al. Production and properties of casein hydrolysate microencapsulated by spray drying with soybean protein isolate. LWT-Food Sci. Technol. 2009;42:919–923. [Google Scholar]
- 229.Akbarbaglu Z, et al. Influence of spray drying encapsulation on the retention of antioxidant properties and microstructure of flaxseed protein hydrolysates. Colloids Surf. B: Biointerfaces. 2019;178:421–429. doi: 10.1016/j.colsurfb.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 230.Wang H, et al. Effect of spray-drying and freeze-drying on the properties of soybean hydrolysates. J. Chem. 2020;2020:1–8. [Google Scholar]
- 231.Rao PS, Bajaj RK, Mann B, Arora S, Tomar S. Encapsulation of antioxidant peptide enriched casein hydrolysate using maltodextrin–gum arabic blend. J. Food Sci. Technol. 2016;53:3834–3843. doi: 10.1007/s13197-016-2376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mendanha DV, et al. Microencapsulation of casein hydrolysate by complex coacervation with SPI/pectin. Food Res. Int. 2009;42:1099–1104. [Google Scholar]
- 233.Free-Manjarrez S, Mojica L, Espinosa-Andrews H, Morales-Hernández N. Sensory and biological potential of encapsulated common bean protein hydrolysates incorporated in a Greek-style yogurt matrix. Polymers. 2022;14:854. doi: 10.3390/polym14050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zeeb B, et al. Modulation of the bitterness of pea and potato proteins by a complex coacervation method. Food Funct. 2018;9:2261–2269. doi: 10.1039/C7FO01849E. [DOI] [PubMed] [Google Scholar]
- 235.Ramezanzade L, Hosseini SF, Nikkhah M. Biopolymer-coated nanoliposomes as carriers of rainbow trout skin-derived antioxidant peptides. Food Chem. 2017;234:220–229. doi: 10.1016/j.foodchem.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 236.Lan Y, Xu M, Ohm J-B, Chen B, Rao J. Solid dispersion-based spray-drying improves solubility and mitigates beany flavour of pea protein isolate. Food Chem. 2019;278:665–673. doi: 10.1016/j.foodchem.2018.11.074. [DOI] [PubMed] [Google Scholar]
- 237.Ying X, et al. Preparation and drying of water-in-oil-in-water (W/O/W) double emulsion to encapsulate soy peptides. Food Res. Int. 2021;141:110148. doi: 10.1016/j.foodres.2021.110148. [DOI] [PubMed] [Google Scholar]
- 238.Yang W, et al. Construction of a water‐in‐oil‐in‐water (W/O/W) double emulsion system based on oyster peptides and characterisation of freeze‐dried products. Int. J. Food Sci. Technol. 2021;56:6635–6648. [Google Scholar]
- 239.Jamshidi A, Shabanpour B, Pourashouri P, Raeisi M. Using WPC-inulin-fucoidan complexes for encapsulation of fish protein hydrolysate and fish oil in W1/O/W2 emulsion: characterization and nutritional quality. Food Res. Int. 2018;114:240–250. doi: 10.1016/j.foodres.2018.07.066. [DOI] [PubMed] [Google Scholar]
- 240.Krunić TŽ, Rakin MB. Enriching alginate matrix used for probiotic encapsulation with whey protein concentrate or its trypsin-derived hydrolysate: impact on antioxidant capacity and stability of fermented whey-based beverages. Food Chem. 2022;370:130931. doi: 10.1016/j.foodchem.2021.130931. [DOI] [PubMed] [Google Scholar]
- 241.Alvarado Y, Muro C, Illescas J, Díaz MDC, Riera F. Encapsulation of antihypertensive peptides from whey proteins and their releasing in gastrointestinal conditions. Biomolecules. 2019;9:164. doi: 10.3390/biom9050164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Huang G-Q, Xiao J-X, Hao L-Q, Yang J. Microencapsulation of an angiotensin I-converting enzyme inhibitory peptide VLPVP by membrane emulsification. Food Bioprocess Technol. 2017;10:2005–2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data used in this research is described in the manuscript.