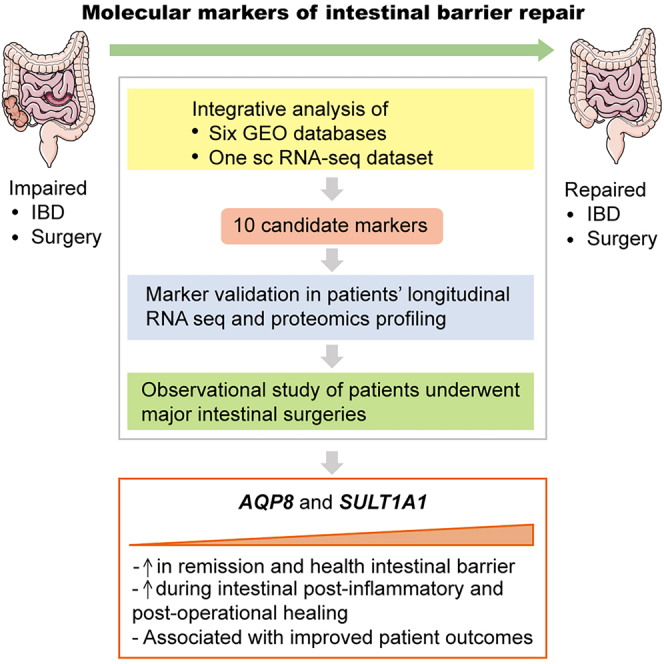
Summary
This study aims to identify biomarkers of intestinal repair and provide potential therapeutic clues for improving functional recovery and prognostic performance after intestinal inflammation or injury. Here, we conducted a large-scale screening of multiple transcriptomic and scRNA-seq datasets of patients with inflammatory bowel disease (IBD), and identified 10 marker genes that potentially contribute to intestinal barrier repairing: AQP8, SULT1A1, HSD17B2, PADI2, SLC26A2, SELENBP1, FAM162A, TNNC2, ACADS, and TST. Analysis of a published scRNA-seq dataset revealed that expression of these healing markers were specific to absorptive cell types in intestinal epithelium. Furthermore, we conducted a clinical study where 11 patients underwent ileum resection demonstrating that upregulation of post-operative AQP8 and SULT1A1 expression were associated with improved recovery of bowel functions after surgery-induced intestinal injury, making them confident biomarkers of intestinal healing as well as potential prognostic markers and therapeutic targets for patients with impaired intestinal barrier functions.
Subject areas: Gastroenterology, Transcriptomics
Graphical abstract
Highlights
-
•
First large-scale screening of public datasets for intestinal healing markers
-
•
10 genes were identified as potential markers of intestinal barrier healing
-
•
The functional role of these 10 marker genes is specific to absorptive cells
-
•
Raising AQP8 and SULT1A1 correlates with improved gut barrier recovery in patients
Gastroenterology; Transcriptomics
Introduction
The small and large intestines are vital organs of the digestive system that transport and absorb nutrients and water. Moreover, they provide a critical physical barrier defending against pathogens that reside in gut microbiota. This barrier function of intestines is conferred by a layer of epithelial cells that is organized into crypts and villi. This barrier structure is essential for maintaining intestinal homeostasis and segregation of intestinal fluids and microbes. The epithelial layer of intestines harbors multiple types of cells with specialized functions, including absorptive cells (enterocytes, also termed colonocytes in the colon), secretory cells (goblet cells, enteroendocrine cells (EECs), and Paneth cells), and intraepithelial immune cells. The homeostasis of the intestinal barrier is maintained through the continuous renewal of epithelial cells derived from intestinal stem cells (ISCs) that reside in the base of crypts.1,2
As a common clinical manifestation, impaired intestinal barrier function has been associated with a broad range of diseases including ischemia-reperfusion (I/R) injury, inflammatory bowel disease (IBD) and postoperative anastomotic leakage. Intestinal barrier dysfunction can be induced by many clinical factors, such as trauma, surgery, infection, and autoimmunity. It is widely hypothesized that when the integrity of the intestinal barrier is compromised, an uncontrolled flux of gut pathogens may enter the bloodstream, leading to secondary infections. In the most severe cases, patients may suffer from shock, multiple organ failures and even death.3 Over the past years, much attention was focused on protecting intestinal barrier integrity in patients with gastrointestinal conditions through clinical interventions including early enteral nutrition, supplementation with glutamine or probiotics, which have been shown to promote intestinal barrier repair in clinical and basic studies.4,5,6 However, the resulting outcomes of these limited interventions were not always satisfactory.7 Therefore, the lack of effective therapeutic manipulation to repair damaged intestinal barrier clearly reflects the fact that the underlying molecular mechanisms of intestinal barrier repair remains relatively unclear.7
Healing of the injured intestinal barrier is an intricate process consisting of inflammation, cell proliferation and tissue remodeling. After the initial inflammatory response, pathogens are removed from the site of injury, following by ISC proliferation and differentiation to remodel and repair the injured tissue.8 Emerging evidence also indicates that, to regenerate the damaged intestinal tissue, intestinal epithelial cells can dedifferentiate into progenitor-like state characterized by expression of fetal gene signatures.9,10 This repairing mechanism is crucial to ensure full recovery of patients from intestinal diseases like I/R and IBD.11 Previous studies have identified several metabolic pathways as the key mediators of intestinal repairing.12,13 One such example is that mitochondrial oxidative phosphorylation (OXPHOS) and pyruvate metabolism pathways cooperatively regulate ISC differentiation.14 In addition, multiple recent studies have confirmed that fatty acid oxidation (FAO) plays a critical role in ISC survival and renewal.15,16,17 Ketone bodies, which are synthesized from the FAO product acetyl-CoA, were also reported to mediate ISC regeneration through NOTCH signaling.18 Furthermore, emerging evidence indicates that dietary nutrients could enhance intestinal tissue repair. For example, enteral nutrients were demonstrated to promote the restoration of the intestinal barrier in patient studies.19,20 Also, administration of fish oil supplements in IBD mouse models has been shown to alleviate inflammation and enhance mucosal healing,21 and a high-fat ketogenic diet has also been shown to boost the regenerative capacity of ISCs in mouse models.18 Despite all these findings, the key regulators and molecular pathways that mediate the repair of intestinal epithelial barrier after injury and inflammation remain largely uncovered.7 Therefore, a further investigation focusing on the key regulators of intestinal barrier repairing would provide a potential scaffold for developing more efficient therapeutic strategies to promote gut epithelial barrier healing in patients with intestinal injury and inflammation.
To this end, we conducted a large-scale analysis of multiple layers of “omics” data retrieved from published studies. Given the resemblance in the underlying mechanisms of epithelial barrier healing of inflamed and injured intestines,22,23 we first analyzed six transcriptomic datasets of inflamed intestine samples from active IBD patients and of non-inflamed/remission intestine samples from control individuals. We established a molecular landscape and identified 10 key regulatory genes of intestinal barrier repairing through a functional transcriptomics approach. We then interrogated their expression pattern in various colonic epithelial cell populations using a published scRNA-seq dataset, showing that the expression of these markers is restricted to the territory of absorptive cell population. Furthermore, we showed that upregulation of two of these markers is associated with improved bowel functional recovery post prophylactic ileostomy through gene and protein expression analyses on intestinal tissues biopsied at the anastomotic wound site. Together, our work provides new insight into the molecular mechanisms of intestinal mucosal repairing at the multiomics level and suggests multiple potential biomarkers that associate with the repairing of the intestinal barrier.
Results
Screening markers of intestinal mucosal healing by consensus DEGs in GEO datasets
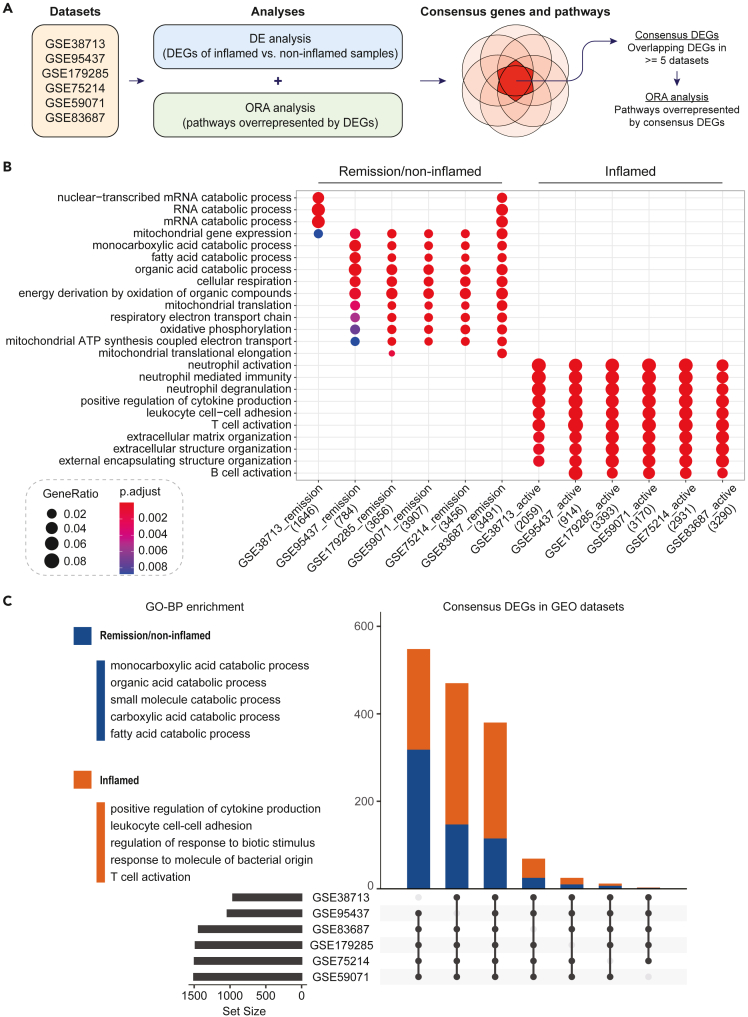
To establish the molecular landscape of the inflamed and post-inflamed (remission)/non-inflamed intestinal barrier, we analyzed six transcriptomic profiling datasets24,25,26,27,28,29 from the Gene-Expression Omnibus (GEO) repository Table 1 as outlined in Figures 1A and S1. Firstly, we performed differential expression analyses to identify a set of Differentially Expressed Genes (DEGs) from each dataset (Figure 1A and Table S1). Consistent with the pathological features of IBD,30 we identified that the most significantly enriched pathways associated with inflamed intestines were immune responses, such as neutrophil, T cell and B-cell activation (Figure 1B).31,32,33 However, for non-inflamed/remission samples, we showed that the top enriched gene sets were associated with metabolic pathways that are known to play key regulatory roles in intestinal wound healing, including fatty acid metabolism, oxidative phosphorylation (OXPHOS)14 and mitochondrial functions13,34 (Figure 1B and Table S2). Then, we carried out Gene Ontology (GO) enrichment analysis to elucidate the functional characteristics of these DEGs (Figure 1C). We compared the DEGs across the six transcriptomic datasets to identify consensus genes. Out of the total of 17,988 DEGs, 1,507 (8.4%) common DEGs were shared by at least five datasets. The GO enrichment analysis of these consensus DEGs further confirmed their relevance to intestinal inflammation- and barrier healing-related biological processes (Figure 1C, Table S2). Intriguingly, the most significantly upregulated DEGs in non-inflamed/remission samples were genes that play critical roles in intestinal regeneration, including the rate-limiting enzyme in fatty acid oxidation, CPT1A,16 and multiple ketogenic enzymes HMGCS2, HMGCL, BDH1, and ACAT118 (Table S1). Taken together, the identification of these consensus DEGs provided us a set of preliminary candidates, which is critical for the screening of key molecular markers for intestinal wound repairing.
Table 1.
GEO datasets
| Dataset | Experiment | Patient samples | Anatomical site | Sample preparation | Country | Year |
|---|---|---|---|---|---|---|
| GSE38713 | Microarray (Affymetrix Human Genome U133 Plus 2.0) |
15 inflamed (active UC) 8 remission (UC in remission) |
colon (n = 23) | Samples were obtained with mucosal pinch biopsies, treated with RNAlater and stored frozen. | Spain | 2012 |
| GSE95437 | CAGE-seq |
45 inflamed (25 active UC, 20 active CD) 20 remission (17 UC in remission, 3 CD in remission) |
colon (n = 65) | Samples were obtained with mucosal pinch biopsies, treated with RNAlater and stored frozen. | Denmark | 2018 |
| GSE179285 | Microarray (Agilent 4 × 44K Whole Human Genome) |
136 inflamed (46 active UC + 90 active CD) 225 uninflamed (48 uninflamed regions in UC patients +177 uninflamed regions in CD patients) |
colon (n = 236), ileum (n = 125) | Samples were obtained with mucosal biopsies, treated with RNAlater and stored frozen. | USA | 2021 |
| GSE75214 | Microarray (Affymetrix Human Gene 1.0 ST) |
125 inflamed (74 active UC + 51 active CD) 39 remission (23 UC in remission, 16 CD in remission) |
colon (n = 97), ileum (n = 67) | Samples were obtained with mucosal biopsies, immediately snap-frozen in liquid nitrogen and stored frozen. | Belgium | 2017 |
| GSE59071 | Microarray (Affymetrix Human Gene 1.0 ST) |
82 inflamed (74 active UC+8 active CD) 23 remission (23 UC in remission) |
colon (n = 105) | Samples were obtained with mucosal biopsies, immediately snap-frozen in liquid nitrogen and stored frozen | Belgium | 2015 |
| GSE83687 | RNA-seq |
74 inflamed (32 active UC + 42 active CD) 60 uninflamed (59 normal control+1 UC in remission) |
ileum (n = 42), colon (n = 86), rectum (n = 6) | Samples were obtained with surgical specimen, immediately snap-frozen in liquid nitrogen and stored frozen | USA | 2017 |
Figure 1.
Transcriptomic profile of the inflamed and post-inflamed (remission)/non-inflamed intestinal barrier
(A) Schematic diagram showing the workflow of analysis.
(B) Dot plot representing the GO-BP enrichment of genes upregulated in active IBD cases and non-inflamed/remission intestinal samples from six GEO datasets. The top three enriched terms in each dataset were selected.
(C) UpSet plot showing the consensus DEGs in at least five out of six GEO datasets. Orange and blue colors represent genes that were upregulated in inflamed and post-inflamed (remission)/non-inflamed patient intestinal samples, respectively. The top five GO-BP terms enriched in the consensus DEGs are listed on the left-hand side.
Identifying central regulatory genes by modular gene Co-expression analysis
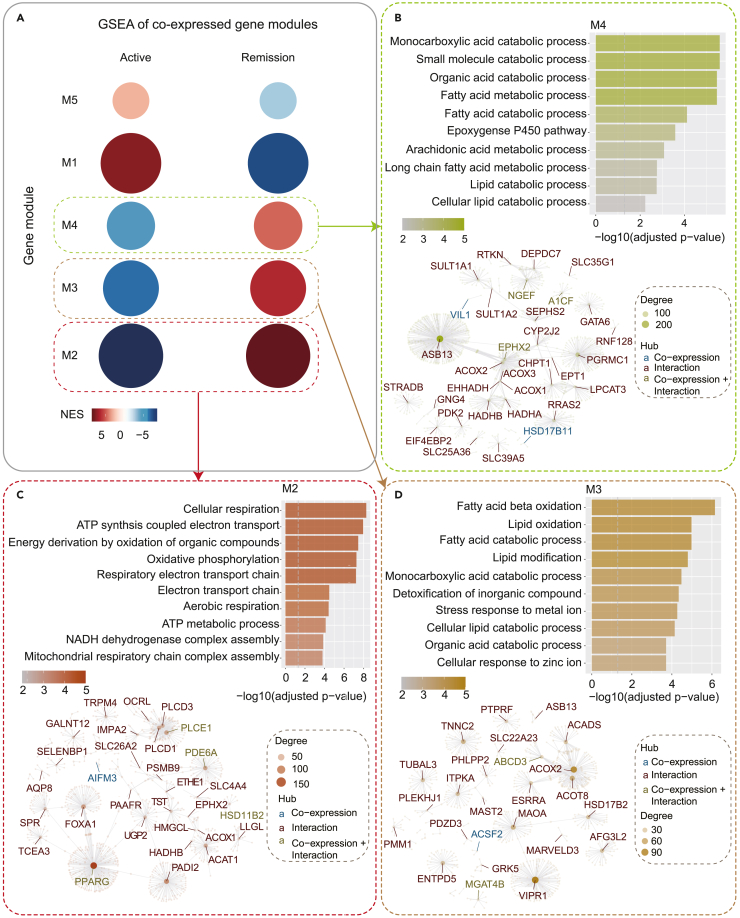
To further uncover the key regulatory genes of post-inflammatory healing of injured intestinal barrier, we firstly sought to identify the key molecular signatures that mark non-inflamed intestinal epithelial barrier by extracting the expression profile of the consensus DEGs and performing a modular gene co-expression analysis using the GEO dataset with the largest sample size (GSE179285, N = 361, including 136 inflamed and 225 non-inflamed intestinal mucosal biopsies). Five co-expressed gene modules were identified, each module corresponds to a cluster of correlated genes that are likely to have similar functions or involved in the same molecular pathways (Figure 2A). A Gene Set Enrichment Analysis (GSEA) was then performed to investigate the module gene enrichment in inflamed and non-inflamed samples (Figure 2A). The functional characteristics of each gene module were explored by GO enrichment analyses and protein‒protein interaction (PPI) network analyses, and all the genes in each module were ranked according to their connectivity with other genes in the co-expression network (Figures 2B–2D and S2). The central hub genes of each module with high connectivity scores were considered to be the potential key regulatory genes of the relevant biological process.
Figure 2.
Co-expressed gene modules
(A) GSEA of five co-expressed gene modules (M1-M5) in IBD active and remission samples. Circle size represents the number of genes in each module, and colour represents the normalized enrichment score (NES).
(B) GSEA of five co-expressed gene modules (M1-M5) in IBD active and remission samples. Circle size represents the number of genes in each module, and color represents the normalized enrichment score (NES).
(B–D) Top: GO-BP enrichment of genes in M2-M4. The top 10 terms enriched in each module are listed.
Bottom: Protein‒protein interaction (PPI) and correlation networks of genes in each module. Each dot (hub) represents a gene, and lines connecting the genes indicate gene correlations or PPIs (demonstrated by hub colors). The size of the hubs demonstrates the degree of connectivity.
We found that three modules (M2, M3, and M4), which were positively correlated with the non-inflamed samples as indicated by their normalized enrichment score (NES), demonstrated remarkable functional relevance to known molecular mechanisms that are involved in epithelial barrier maintenance and repairing mechanisms. Genes from M2 were significantly associated with OXPHOS and cellular respiration pathways, which are central mediators of ISC differentiation (Figure 2C).14 The top central hub gene of M2, PPARG, is a master regulator of anti-inflammation processes and wound repair (Figure 2C), the agonists of its encoded receptor protein have been widely used as the first-line treatment for IBD35 and have shown to facilitate tissue healing in multiple diseases.36 M3 consisted of genes involve in FAO, such as ACADS - a key enzyme for butyryl-CoA oxidation (Figure 2D), which is known to regulate colonocyte proliferation and differentiation during wound repair,37 whereas M4 contained cytochrome P450 (CYP) enzymes such as CYP2J2 (Figure 2B). The arachidonic acid epoxidation pathway, which is catalyzed by CYP enzymes, was proposed as a potential target for improving wound healing in diabetic patients.38 Finally, based on the degree of connectivity, we shortlisted the top 30 central hub genes from each module as the potential markers that may play a crucial role in promoting intestinal barrier repair (Table S3).
Next, we thought to further validate the relevance of these 30 markers in the context of remission (post-inflammation). We again applied the same analytical strategy on the dataset with the largest remission cohort - GSE75214, which contains 125 inflamed and 39 remission intestinal mucosal biopsies. Out of the total of six co-expressed gene modules identified, three modules (M2, M3, and M4) exhibited positive correlation with the remission group (Figure S3A). Consistently, the enrichment analysis on these three modules also yielded GO terms that are significantly associated with epithelial barrier homeostasis and wound healing (Figure S3B). Most importantly, the top 30 central hub genes identified from GSE179285 were also seen in M2, M3, and M4 which represent intestinal epithelial barrier at remission stage (Table S4).
Single-cell transcriptomic data revealed celltype-specific expression of wound repair marker genes
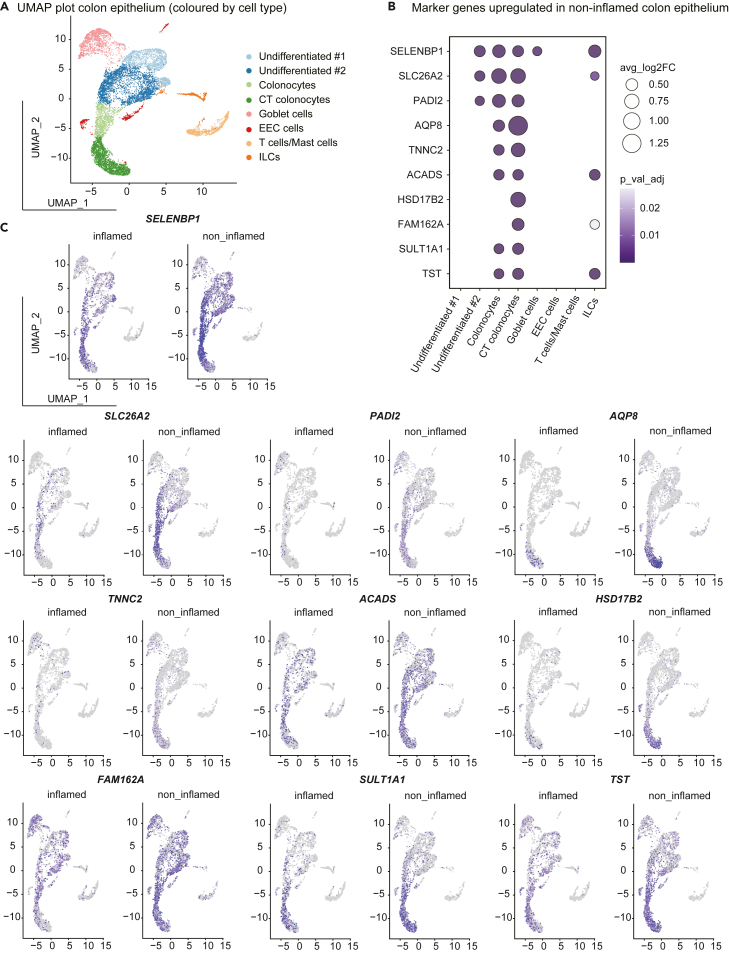
Next, we sought to explore the expression pattern of intestinal barrier repairing genes in different cell types by analysing single-cell transcriptomic data of the inflamed and non-inflamed colonic mucosa from three patients with ulcerative colitis.1 Clustering identified eight colonic epithelial cell types based on their marker genes (Figure S4A, Table S5, including absorptive cells (CT colonocytes and colonocytes), secretory cells (goblet cells and EECs), undifferentiated cells and immune cells (T cells/mast cells and ILCs) (Figure 3A). In addition, comparison between the inflamed and non-inflamed colon epithelium samples determined 195 DEGs (Table S6). Then, to validate our findings from the GSEA analysis, we cross compared the 195 DEGs with the top 30 central hub genes from M2, M3 and M4 (Table S3). Notably, 10 genes were obtained from the intersection: AQP8, SULT1A1, HSD17B2, PADI2, SLC26A2, SELENBP1, FAM162A, TNNC2, ACADS, and TST. Next, we depicted the expression pattern of these 10 genes in inflamed and non-inflamed samples (Figure 3C and Table S7). Of interest, all of the 10 genes were significantly upregulated in absorptive cell types (CT colonocytes and colonocytes) from non-inflamed samples, whereas no difference was observed in secretory or immune cell types (Figures 3B, 3C, and S4B), suggesting that these 10 genes are likely to have celltype specific functions in intestinal absorptive cells. This finding is also consistent with the previous study showing that absorptive colonocytes play a major protective role in intestinal barrier.2
Figure 3.
Cell-specific marker gene expression
(A) UMAP plot of colonic epithelial cells sampled from inflamed and non-inflamed mucosa from patients with ulcerative colitis. Colors indicate the cell types.
(B) The 10 marker genes identified in the current study were upregulated in non-inflamed regions compared with inflamed areas. The dot plot showing the cell-type-specific DE of these marker genes.
(C) UMAP plot comparing the cells in inflamed and non-inflamed regions. Colors represent the expression levels of the marker genes.
Verification of intestinal mucosal healing marker genes in patients’ longitudinal RNA-seq and proteomics datasets
To further verify if the 10 genes obtained through the intersection analysis can be designated as the molecular signatures for intestinal barrier healing, we analyzed two published patient studies which contain longitudinal RNA sequencing (RNA-seq) and proteomic datasets. In the first study, a prospective clinical trial was conducted where active IBD patients were treated with Olamkicept. The patient’s intestinal mucosa was sampled at 0 h (baseline), 4 h, 24 h, 2 weeks, 6 weeks and 14 weeks after the first administration of Olamkicept, and subjected to RNA-seq.39 Therefore, we investigated the expression changes of the 10 genes along this time course in three patients who reached disease remission after treatment. Of note, the expression of PADI2, SLC26A2, SULT1A1 and FAM162A showed an increasing trend during the healing of intestinal mucosa (Figure S5, Table S8).
Next, we validated these 10 genes by analyzing a proteomic study on intestinal mucosa samples from 17 ulcerative colitis patients and 15 healthy volunteers.40 We found that the top enriched pathways in inflamed intestinal mucosa were associated with immune responses whilst in healthy samples were metabolic pathways.40 This data is in agreement with our previous analysis as shown in Figure 1. Moreover, the protein levels of eight of the 10 genes, SLC26A2, SELENBP1, AQP8, PADI2, FAM162A, HSD17B2, SULT1A1 and TST, were significantly upregulated in healthy samples, with expression levels 4.17-, 3.33-, 2.63-, 2.56-, 2.38-, 2.22-, 2.22- and 1.96-fold higher than those in ulcerative colitis samples, respectively (Table 2). Together, these cross comparisons with previous studies further support our notion of the 10 genes as potential molecular markers for intestinal barrier healing.
Table 2.
Protein validation of key genes contributing to intestinal mucosal barrier recovery using the in-depth proteins
| Ration UC/H | Protein name | Gene names | Majority protein ID | -Log p value |
|---|---|---|---|---|
| Proteins of increased abundance in healthy controls compared to UC | ||||
| 4.17 | Sulfate transporter | SLC26A2 | P50443 | 18.52 |
| 3.33 | Selenium-binding protein 1 | HEL-S-134P; SELENBP1 | V9HWG1 | 15.36 |
| 2.63 | Aquaporin-8 | AQP8 | Q53GF6 | 7.86 |
| 2.56 | Protein-arginine deiminase type-2 | PADI2 | A0A024RA98 | 16.12 |
| 2.38 | Protein FAM162A | FAM162A | F8W7Q4 | 12.91 |
| 2.22 | Estradiol 17-beta-dehydrogenase 2 | HSD17B2 | Q53GD0 | 12.22 |
| 2.22 | Sulfotransferase; Sulfotransferase 1A1 | SULT1A1; hCG_1993905 | A0A024QZB4 | 12.85 |
| 1.96 | Sulfurtransferase; Thiosulfate sulfurtransferase | TST | Q53EW8 | 14.02 |
| 1.00 | Short-chain specific acyl-CoA dehydrogenase, mitochondrial | ACADS | E5KSD5 | −4.34E-08 |
Intestinal mucosal healing markers are associated with improved patients’ post-operational recovery outcomes
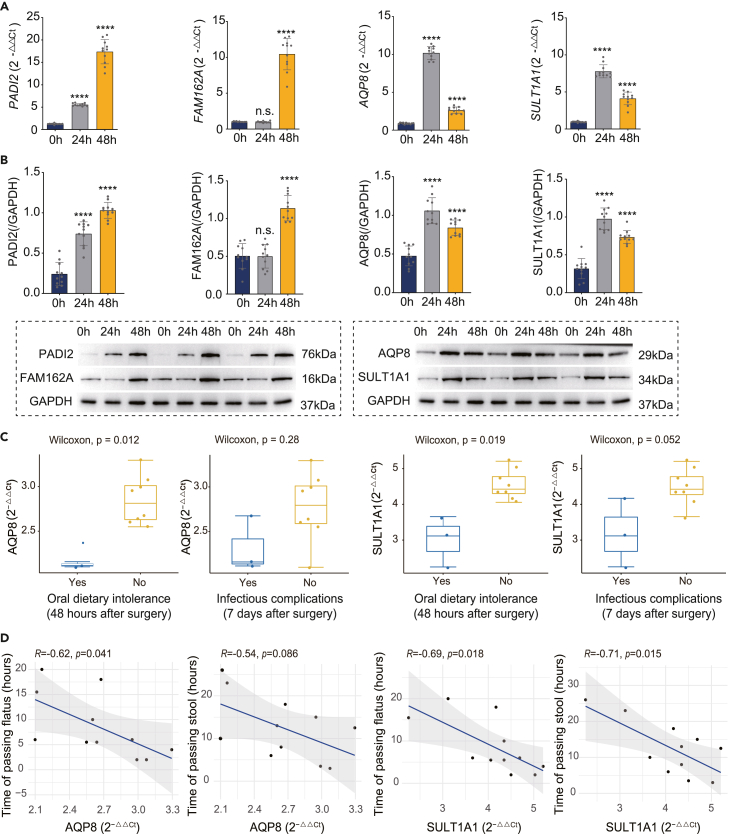
To determine the clinical relevance of the 10 potential intestinal barrier repairing markers identified in the current study, we then conducted a patient study involving 11 patients who underwent ileocecal resection surgery followed by enterostomy. In this study, intestinal mucosal samples were collected at the site of enterostomy during the operation, and at the stoma site at 24 h and 48 h post-operation from each patient. The sampled tissues were then confirmed to be intestinal epithelium using a Hematoxylin and Eosin (H&E) staining (Figure S6C). Next, we measured the gene and protein expression levels of the potential healing markers in each sample using quantitative polymerase chain reaction (q-PCR) and western blot. Among the 10 marker genes, we showed that four of them, namely PADI2, FAM162A, AQP8 and SULT1A1, were significantly upregulated at 24 and/or 48 h post-operation compared with those during the surgery. And most importantly, this upregulation was consistent at both mRNA (q-PCR) and protein (western blot) levels (Figures 4A and 4B). Although a significant downregulation of SLC26A2 expression at both mRNA and protein levels 24 and 48 h following enterostomy was observed, and a mild upregulation of HSD17B2 mRNA expression was detected only at 48 h post-surgery, the gene and protein expression levels of ACADS, SELENBP1 and TNNC2 remained unchanged throughout the study period (Figures S6A and S6B) whereas TST expression was not detected. Together, these findings indicate that the observed expression changes of PADI2, FAM162A, AQP8 and SULT1A1 are not because of a non-specific generalized transcriptional disruption associated with the surgical procedures of ileocecal resection and enterostomy.
Figure 4.
Validation of identified potential biomarkers in clinical patients and correlation analysis on their expression levels and the degree of intestine functional recovery
(A) mRNA expression levels of PADI2, FAM162A, AQP8 and SULT1A1 at different timepoints of intestinal injury repair after surgery in patients.
(B) Protein expression levels of PADI2, FAM162A, AQP8 and SULT1A1 at different timepoints of intestinal injury repair after surgery in patients.
(C) AQP8 and SULT1A1 mRNA levels in patients with and without oral dietary intolerance 48 h after surgery and in patients with and without infectious complications 7 days after surgery.
(D) Correlation analysis of the mRNA levels of AQP8 and SULT1A1 at 48 h after surgery and the time of passing flatus and stool. ∗p ≤0.05, ∗∗p ≤0.01, ∗∗∗p ≤0.001, ∗∗∗∗p ≤0.0001, one-way ANOVA with Tukey’s multiple comparisons test. Data are represented as mean ± standard deviation.
It has been identified that healing of the intestinal barrier after the surgery-induced injury can be reflected by the recovery of patient’s bowel function, which is measured by four parameters (1) incidence of oral dietary intolerance at 48 h post-operation; (2) incidence of infectious complications at seven days post-operation; (3) time to first passage of flatus; (4) time to first passage of stool.41,42 Among the 11 patients in the study, two patients (patient 5 and 9) had both oral intolerance and infectious complications (pneumonia), one patient (patient 1) showed oral intolerance and one patient (patient 8) had only infectious complication (wound infection), whereas the others did not exhibit any post-operational adverse event on record (Table S9). Of interest, we found that despite the relatively small cohort size, the expression levels of SULT1A1 and AQP8 at 48 h after surgery were significantly higher in patients without post-operational oral intolerance or infectious complications compared with those who had these adverse events (Figure 4C). Moreover, SULT1A1 and AQP8 expression levels at 48 h post-operation were negatively correlated with the time to first passage of flatus as well as to first passage of stool after receiving the ileocecal resection surgery (Figure 4D). This finding indicated that higher post-operational expression of SULT1A1 and AQP8 is associated with improved bowel recovery, making them the confident markers of intestinal barrier repair, which can potentially be exploited as the prognostic and therapeutic markers in patients with intestinal barrier injuries.
Discussion
In this study, we identified 10 markers, AQP8, SULT1A1, HSD17B2, PADI2, SLC26A2, SELENBP1, FAM162A, TNNC2, ACADS, and TST, that are potential molecular signatures of intestinal barrier repairing through a series of integrative analyses of multiple transcriptomic and scRNA-seq datasets of IBD patients. In addition, our analysis on the published scRNA-seq data indicated that the functional role of these marker genes was specific to absorptive cells which reside in intestinal epithelium. Finally, we conducted a clinical study on 11 patients who underwent ileum resection surgery and identified that elevated post-operational expression of AQP8 and SULT1A1 were associated with improved bowel function recovery after surgery-induced intestinal injury, making them potential prognostic markers and therapeutic targets of intestinal barrier recovery. To our knowledge, this is the first large-scale screening of public datasets for molecular markers of intestinal barrier healing post-inflammation, providing potential clues for developing new therapeutic strategies to promote the repairing of epithelial barrier in patients with intestinal injuries.
AQP8 encodes aquaporin 8 (AQP8), a cell membrane protein essential for water transfer and absorption that is widely distributed in the gastrointestinal tract.43,44,45 In agreement with our findings, multiple studies have reported significant downregulation of AQP8 in intestinal tissues of ulcerative colitis patients, which is linked to defective absorptive function of colon epithelial cells.27,46,47 In patients with collagenous colitis (CC), reduction of AQP8 expression in colonic mucosa was associated with frequent diarrhea, whereas restoration of AQP8 expression level was observed in CC patients who have reached clinical remission.48 Moreover, the aquaporin protein family has been extensively used as markers to evaluate the efficacy of treatment strategies for diarrhea.49,50,51 Although the functional role of AQP8 in maintaining intestinal fluid-homeostasis has been largely uncovered, AQP8 has not previously been linked to intestinal barrier repairing. Of note, previous studies showed that another member of aquaporin family, AQP3, have a water transport-independent function in regulating intestinal cell proliferation.52,53 Therefore, based on our and other’s findings, it is reasonable to speculate that AQP8 may also play a crucial regulatory role in intestinal epithelial healing and regeneration after injury. The mechanism whereby AQP8 mediates intestinal barrier repairing is an avenue for further research.
HSD17B2 encodes 17β-Hydroxysteroid dehydrogenase 2 (17β-HSD2), a pivotal enzyme that catalyses the intracellular conversion of potent sex hormones estradiol (E2) and testosterone into their weaker precursor form, estrone (E1) and androstenedione, respectively.54 Although some studies showed that E2 and testosterone exert protective effect on injured intestines,55,56,57 other studies indicated that high level of E2 and testosterone could induce colonocytes apoptosis.58,59,60 However, the intestinal barrier-specific functions of E1 and androstenedione, as well as 17β-HSD2 itself, are little-known. In this study, our analysis revealed that 17β-HSD2 may be required for the repairing of intestinal epithelial barrier. Whether it is through catalytic activities of E2 and testosterone conversion or other enzyme-independent functions requires further research.
Sulfotransferase 1A1 (SULT1A1) is a member of sulfotransferases enzyme family encoded by SULT1A1. It catalyses sulfonate conjugation, an important metabolic pathway that detoxifies/inactivates a wide range of molecules through the addition of sulfate group.61 Intriguingly, SULT1A1 also plays a critical role in the inactivation of circulating estrogen, particularly the most potent form, E2.62 Our results demonstrated that elevated expression of SULT1A1 is associated with both disease remission of IBD as well as improved post-surgery bowel recovery in patients. Whether this is because of inhibition of estrogen pathway by SULT1A1 in damaged gut barrier is also an avenue for future investigation. The other potential biomarkers of intestinal repair discovered in our study include PADI2, SLC26A2, SELENBP1, FAM162A, TNNC2, ACADS, and TST. Some of them, such as PADI2, already have supporting evidences from previous studies showing its role in regulating wound healing and tissue regeneration in zebrafish.63 Moreover, downregulation of PADI2 has been reported in patients with colorectal cancer and active ulcerative colitis.64 However, the molecular mechanisms by which the majority of these markers influence intestinal barrier repairing remains unclear and needs further studies.
Taken together, our work depicts an analysis strategy for large-scale screening of public datasets to uncover disease-associated biomarkers, and using this approach, we identified 10 potential signature markers for intestinal barrier repairing. These findings bring valuable insights in understanding the fundamental biology of intestinal barrier healing at a molecular level and provide potential clues for the development of new therapeutic strategies aimed at facilitating the repairing of impaired intestinal barrier.
Limitations of the study
Although in the current study, we have identified 10 potential molecular markers of intestinal barrier healing post injury and inflammation by screening public datasets, only two showed clinical relevance in our patient study, suggesting that the rest of eight markers may highly associate with a healthy and functional status of intestine rather than an active healing status. In addition, this may also reflect a potential mechanistical variation in intestinal barrier healing between surgery-induced gut injury and IBD-induced injury given that our clinical study was performed on patients who underwent enterostomy. These highlight the need for a large cohort clinical study where IBD patients are subjected to long-term clinical monitoring to further validate the relevance of the 10 markers. Moreover, the functional roles of these 10 markers can also be explored in various conditions associated with impaired intestinal epithelial barrier like burn, abdominal trauma, enteritis, and other major intestinal surgeries in future studies.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| AQP8 antibody | Bioss | Cat#bs-6786R, RRID:AB_2934320 |
| SULT1A1 antibody | Bioss | Cat# bs-6283R, RRID:AB_11094764 |
| SLC26A2 antibody | Proteintech | Cat# 27759-1-AP, RRID:AB_2880963 |
| PADI2 antibody | Bioss | Cat# bs-11662R, RRID:AB_2934324 |
| HSD17B2 antibody | Bioss | Cat# bs-3856R, RRID:AB_10858090 |
| FAM162A antibody | Abcam | Cat# ab122295, RRID:AB_11129715 |
| ACADS antibody | Abcam | Cat# ab110318, RRID:AB_10860028 |
| SELENBP1 antibody | Bioss | Cat# bs-4200R, RRID:AB_11086827 |
| TNNC2 antibody | Proteintech | Cat# 15875-1-AP, RRID:AB_2878194 |
| GAPDH antibody | Abmart | Cat# P30008M |
| Anti-rabbit IgG, HRP-linked antibody | Cell Signaling Technology | Cat# 7074, RRID:AB_2099233 |
| Anti-mouse IgG, HRP-linked antibody | Cell Signaling Technology | Cat# 7076, RRID:AB_330924 |
| Deposited data | ||
| Gene expression levels of IBD (inflamed VS. remission) RNA-seq data | N. Planell et al., 20134 | GSE38713 |
| Gene expression levels of IBD (inflamed VS. remission) RNA-seq data | M. Boyd et al., 201855 | GSE95437 |
| Gene expression levels of IBD (inflamed VS. uninflamed) RNA-seq data | M. E. Keir et al., 202134 | GSE179285 |
| Gene expression levels of IBD (inflamed VS remission) RNA-seq data | M. Vancamelbeke et al., 201715 | GSE75214 |
| Gene expression levels of IBD (inflamed VS remission) RNA-seq data | W. Vanhove et al., 201518 | GSE59071 |
| Gene expression levels of IBD (inflamed VS uninflamed) RNA-seq data | L. A. Peters et al., 201724 | GSE83687 |
| Gene expression levels of IBD (inflamed VS uninflamed) single-cell RNA-seq data | K. Parikh et al., 201943 | GSE116222 |
| Gene expression levels of IBD restoration after treatment with Olamkicept RNA-seq data | S. Schreiber et al., 202141 | GSE171770 |
| Patients’ proteomics datasets | A. Schniers et al., 201932 | https://clinicalproteomicsjournal.biomedcentral.com/articles/10.1186/s12014-019-9224-6 |
| Oligonucleotides | ||
| q-PCR primers are listed in Table S10 | This paper | N/A |
| Software and algorithms | ||
| GraphPad Prism 8.3.0 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| 3DHISTECH’s Slide Converter | 3DHISTECH Ltd | https://www.3dhistech.com/research/slidecenter/slidemaster/ |
| CaseViewer 2.4 | 3DHISTECH Ltd | https://www.3dhistech.com/solutions/caseviewer/ |
| R 4.1.0 | R Core Team, 202156 | https://cran.r-project.org/bin/windows/base/old/4.1.0/ |
| limma 3.48 | M. E. Ritchie et al., 20155 | https://bioconductor.org/packages/release/bioc/html/limma.html |
| EdgeR 3.34 | Mark D. Robinson et al., 200964 | https://bioconductor.org/packages/release/bioc/html/edgeR.html |
| ClusterProfiler 4.0.0 | G. Yu et al., 20123 | https://bioconductor.org/packages/release/bioc/html/clusterProfiler.html |
| CEMiTool 1.16.0 | Pedro S. T. Russo et al., 201831 | https://www.bioconductor.org/packages/release/bioc/html/CEMiTool.html |
| Seurat 4.0.5 | Yuhan Hao et al., 202158 | https://cran.r-project.org/web/packages/Seurat/index.html |
| Other | ||
| Original code | This paper | https://doi.org/10.5281/zenodo.7879748 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Da-Li Sun (sundali2018@126.com).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Patient selection criteria and ethics statement
A clinical study was designed to verify the identified marker genes. Patients with primary, nonmetastatic colorectal cancer requiring prophylactic enterostomy were recruited between December 2020 to November 2022 from the Department of Gastrointestinal Surgery at The Second Affiliated Hospital of Kunming Medical University.66 The following patient inclusion criteria were applied: 1) patients aged between 40 to 85 years old; 2) patients with colorectal cancer and partial obstruction or patients with ultralow rectal cancer; 3) patients undergoing laparoscopic radical resection for colorectal cancer and requiring prophylactic enterostomy according to intestinal conditions during operation. The exclusion criteria were as follows: 1) current pregnancy; 2) patients with total colorectal obstruction; 3) patients with prior history of major abdominal surgery; 4) patients with severe abdominal infection. Informed consents were provided voluntarily by all patients involved in this study, and all experiments were approved by the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University (PJ-2020-138 and PJ-2021-212) and conducted in accordance with clinical research ethics review guideline (FEY-ZD-5-1.3).
Patient sample collection
A total of 11 patients (6 males, 5 females) were recruited in this study (Table S9). For all participants in this study, an enterostomy in which a 3–4 cm section of ileum was diverted to a stoma created in the abdominal wall, was performed after laparoscopic radical resection for colorectal cancer to prevent anastomotic leakage. The ileum was anchored to the stoma through suture during the enterostomy, the stoma was then reopened at 24 hours postoperation to drain intestinal fluid and after 48 hours of the surgery for stoma bag attachment. Patient intestinal mucosal tissue was sampled at the site of enterostomy during the procedure as well as 24 and 48 hours after the operation through forceps biopsy. Of note, these mucosal samples do not contain tumour tissues. Then, collected mucosal tissue samples were processed for histological and expression analyses. For histology, samples were fixed in 4% paraformaldehyde at least overnight and embedded in paraffin. For q-PCR and western blotting, samples were snap-frozen immediately in liquid nitrogen and stored at -80°C.
Patient outcome measurement
All participants involved in the study were surveyed to evaluate the postoperative recovery outcome based on four measurements: 1) incidence of oral dietary intolerance at 48 hours postoperation. Oral intolerance is defined as the inability to tolerate oral nutrition (ON) that supplies 30% full nutritional requirements, with digestive symptoms, including vomiting, diarrhoea, abdominal distension and ileus, at 48 hours after the operation41,42; 2) incidence of infectious complications, including pneumonia, systemic inflammatory reaction syndrome (SIRS), septicaemia, intra-abdominal abscess and wound infection; 3) time of first passage of flatus; 4) time of first passage of stool. (Table S9)
Method details
Transcriptomic data collection
Gene expression and clinical annotation datasets from GSE38713, GSE95437, GSE179285, GSE75214, GSE59071 and GSE83687, which included 477 (56.0%) inflamed and 375 (44.0%) remission/non-inflamed bowel epithelial patient samples, were retrieved from the Gene-Expression Omnibus (GEO) database. All the patient samples involved in these datasets were fresh-frozen tissues collected from the distal ileum (27.5%), colon (71.8%) and rectum (0.7%) in the past 10 years. Among the inflamed samples, 266 were from patients with ulcerative colitis (UC), and 211 were from patients with Crohn’s disease (CD). The remission/non-inflamed samples were tissues collected from patients at disease remission stage (GSE38713, GSE95437, GSE59071, GSE75214), adjacent non-inflamed regions (GSE179285) or healthy controls (GSE83687) (Table 1).
Differential expression analysis and pathway enrichment analysis
Differential expression (DE) analysis of inflamed versus remission/non-inflamed patient intestinal samples was performed on each dataset using limma (v.3.48)67 for microarray datasets or EdgeR (v.3.34)68 for RNA sequencing datasets. The differentially expressed genes (DEGs) were identified with false discovery rate (FDR) < 0.01. Functional enrichment tests were performed using the R package ClusterProfiler (v.4.0.0).69 Over-representation analysis (ORA) of gene ontology biological processes (GO-BPs) was conducted using the R package ClusterProfiler (v.4.0.0). The significantly enriched pathways were identified with the Benjamini‒Hochberg procedure adjusted p-value <0.01.
Gene co-expression module analysis and protein‒protein interaction (PPI) network
The normalised expression profile of the consensus DEGs (DEGs appeared in at least five out of six GEO datasets) was extracted from GSE179285 for gene co-expression modular analysis. The R package CEMiTool (v.1.16.0) was used to identify co-expressed gene modules with the following settings: filter = FALSE, cor_method = pearson, network_type = signed. The soft-thresholding parameter beta was selected as the first value that leads the gene co-expression network to be approximately scale-free (linear regression fit of adherence to scale-free topology R2> 0.8), as recommended by the authors of CEMiTool.70
Gene set enrichment analysis (GSEA) was performed to evaluate the enrichment score of each gene module in inflamed and remission samples. A GO-BP enrichment analysis was performed to interrogate the biological function of the co-expressed gene modules. Protein‒protein interaction (PPI) information was extracted from the STRING database (v.11). Confident protein interactors (combined score >700) of the module genes were extracted from STRING and visualised using CeMiTool.
Single-cell RNA-seq data analysis
Seurat 4.0.571 was used to analyze the published single-cell RNA-seq (scRNA-seq) dataset of colonic epithelial cells from healthy individuals and IBD patients (GSE116222). Sequencing data from three individuals in each group were merged and integrated using the SCTransform and anchored-based integration workflow. Principal component analysis (PCA) was performed, and the top 10 significant principal components (PCs) were used as the input to generate the uniform manifold approximation and projection (UMAP). Eight cell populations were classified using the FindCluster function at a resolution of 0.3. FindAllMarkers was used to identify the marker genes of each cluster, and the cell types were manually assigned according to the top marker genes. DEGs (log2FC > 0.25, adj_pval <0.01) between the inflamed and non-inflamed/healthy samples were then identified in each celltype using FindMarkers function.
q-PCR
For patient ileum biopsies, TRIzol reagent (Lifetech 15596026) was used for total RNA extraction according to the manufacturer’s instructions. The total RNA samples were then reverse transcribed using a FastKing RT Kit (with gDNase, TIANGEN, KR116). Quantitative PCR (q-PCR) was performed with Taq ProUniversal SYBR qPCR Master Mix (Vazyme) on a LightCycle 96 Instrument (Roche). Relative gene expression levels were calculated using the ΔΔCT method by normalising genes of interest to GAPDH analysed using GraphPad Prism 9. The oligonucleotide primers used for q-PCR are listed in Table S10.
Western blotting
To isolate total protein, intestinal mucosa tissue was homogenised in RIPA lysis buffer (Beyotime) containing 10% protease inhibitor cocktail (Roche) using standard methods. Equal amount of protein samples (80 μg) was separated by SDS-PAGE method and transferred onto PVDF membrane. Blots were blocked in 5% BSA in TBST at room temperature for 1 hour and processed for primary antibody incubation at 4°C for overnight. Primary antibodies used for western blotting were anti-PADI2 (Bioss, bs-11662R, 1:1000), anti-FAM162A (Abcam, ab122295, 1:1000), anti-AQP8 (Bioss, bs-6786R, 1:500), anti-SULT1A1 (Bioss, bs-6283R, 1:1000) and anti-GAPDH (Abmart, P30008 M, 1:4000). Then, blots were washed in TBST and incubated with HRP-linked secondary antibodies (CST7074, 1:2000) at room temperature for 2 hours. Bands were developed with 1 mL ECL solution (Applygen) and imaged using a Tanon Chemi-Image System. Antibody information and the greyscale values of western blot images are provided in Table S11.
Histological analysis
The intestinal mucosal biopsies sampled from 11 patients at three timepoints were processed and embedded in paraffin as described above. A total of 33 5-μm transverse sections were obtained and stained with haematoxylin and eosin (H&E). The sections were imaged and evaluated through microscopy (Danjier, China) with 3DHISTECH’s Slide Converter and CaseViewer 2.4 (scale bar = 1mm).
Quantification and statistical analysis
All expression analysis results were expressed as mean ± standard deviation. Statistical analysis was performed using the statistical software GraphPad Prism v8.3.0, and one-way ANOVA was used to test statistical difference among multiple groups, followed by the Tukey post hoc test. Wilcoxon rank sum test (two-group comparisons) or Pearson correlation (correlation tests) were used accordingly. A P-value < 0.05 was considered statistically significant.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NSFC) (No. 81860098) and Yunnan Young Academic and Technical Leaders Reserve Talent Project (No. 202105AC160049) to D.L.S, Yunnan Province Joint Special Project of Science & Technology Department of Yunnan Province and Kunming Medical University to H.Y.H (No. 2019FE001-233) and Y.X.Q (No. 202101AY070001-155) and Postgraduate Education Innovation Fund Project of Kunming Medical University to X.H.Z (NO. 2022S076). The authors would like to thank Yunnan Labreal Biotechnology Co., Ltd for their outstanding technical services.
Author contributions
Conceptualization, Z.X.H., Z.P.N., D.Z.H., and S.D.L.; Methodology, Z.X.H., Z.P.N., D.Z.H., and S.D.L.; Software and Formal Analysis, Z.X.H. and Z.P.N.; Investigation, Z.X.H., Y.T, Q.Y.X., and A.L.Y.; Writing – Original Draft, Z.X.H., Z.P.N., and S.D.L.; Writing – Review and Editing, Z.X.H., Z.P.N., D.Z.H., and H.H.Y.; Visualization, Z.X.H., Z.P.N., and D.Z.H.; Supervision, S.D.L. and H.H.Y.; Project Administration, Z.X.H.; Funding Acquisition, Z.X.H., Q.Y.X., H.H.Y., and S.D.L.; All authors have revised and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: May 6, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106831.
Contributor Information
Da-Li Sun, Email: sundali2018@126.com.
Hai-Yu He, Email: zhaoyu1396@163.com.
Supplemental information
Data and code availability
-
•
This paper analyses existing, publicly available datasets from the GEO repository. These accession numbers for the datasets analysed during the current are listed in the key resources table.
-
•
All dataset analyses were performed based on R 4.1.0.65 The original code has been deposited into Zenodo and is publicly available as of the date of publication. DOI of the deposited original code is listed in the key resources table.
-
•
Any additional information required for re-analysing the datasets reported in this paper is available from the lead contactupon request.
References
- 1.Parikh K., Antanaviciute A., Fawkner-Corbett D., Jagielowicz M., Aulicino A., Lagerholm C., Davis S., Kinchen J., Chen H.H., Alham N.K., et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature. 2019;567:49–55. doi: 10.1038/s41586-019-0992-y. [DOI] [PubMed] [Google Scholar]
- 2.Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 3.Di Tommaso N., Gasbarrini A., Ponziani F.R. Intestinal barrier in human health and disease. Int. J. Environ. Res. Publ. Health. 2021;18 doi: 10.3390/ijerph182312836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buscemi S., Damiano G., Palumbo V.D., Spinelli G., Ficarella S., Lo Monte G., Marrazzo A., Lo Monte A.I. Enteral nutrition in pancreaticoduodenectomy: a literature review. Nutrients. 2015;7:3154–3165. doi: 10.3390/nu7053154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri M. Human intestinal barrier: effects of stressors, diet, prebiotics, and probiotics. Clin. Transl. Gastroenterol. 2021;12 doi: 10.14309/ctg.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokoyama Y., Asahara T., Nomoto K., Nagino M. Effects of synbiotics to prevent postoperative infectious complications in highly invasive abdominal surgery. Ann. Nutr. Metab. 2017;71(Suppl 1):23–30. doi: 10.1159/000479920. [DOI] [PubMed] [Google Scholar]
- 7.Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eming S.A., Murray P.J., Pearce E.J. Metabolic orchestration of the wound healing response. Cell Metabol. 2021;33:1726–1743. doi: 10.1016/j.cmet.2021.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Hageman J.H., Heinz M.C., Kretzschmar K., van der Vaart J., Clevers H., Snippert H.J. Intestinal regeneration: regulation by the microenvironment. Dev. Cell. 2020;54:435–446. doi: 10.1016/j.devcel.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Sprangers J., Zaalberg I.C., Maurice M.M. Organoid-based modeling of intestinal development, regeneration, and repair. Cell Death Differ. 2021;28:95–107. doi: 10.1038/s41418-020-00665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q., Cheng H., Liu Y., Wang X., He F., Tang L. Activation of mTORC1 by LSECtin in macrophages directs intestinal repair in inflammatory bowel disease. Cell Death Dis. 2020;11:918. doi: 10.1038/s41419-020-03114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eming S.A., Wynn T.A., Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356:1026–1030. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- 13.Wang D., Odle J., Liu Y. Metabolic regulation of intestinal stem cell homeostasis. Trends Cell Biol. 2021;31:325–327. doi: 10.1016/j.tcb.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Colman M.J., Schewe M., Meerlo M., Stigter E., Gerrits J., Pras-Raves M., Sacchetti A., Hornsveld M., Oost K.C., Snippert H.J., et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature. 2017;543:424–427. doi: 10.1038/nature21673. [DOI] [PubMed] [Google Scholar]
- 15.Chen L., Vasoya R.P., Toke N.H., Parthasarathy A., Luo S., Chiles E., Flores J., Gao N., Bonder E.M., Su X., Verzi M.P. HNF4 regulates fatty acid oxidation and is required for renewal of intestinal stem cells in mice. Gastroenterology. 2020;158:985–999.e9. doi: 10.1053/j.gastro.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mihaylova M.M., Cheng C.W., Cao A.Q., Tripathi S., Mana M.D., Bauer-Rowe K.E., Abu-Remaileh M., Clavain L., Erdemir A., Lewis C.A., et al. Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell. 2018;22:769–778.e4. doi: 10.1016/j.stem.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stine R.R., Sakers A.P., TeSlaa T., Kissig M., Stine Z.E., Kwon C.W., Cheng L., Lim H.W., Kaestner K.H., Rabinowitz J.D., Seale P. PRDM16 maintains homeostasis of the intestinal epithelium by controlling region-specific metabolism. Cell Stem Cell. 2019;25:830–845.e8. doi: 10.1016/j.stem.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng C.W., Biton M., Haber A.L., Gunduz N., Eng G., Gaynor L.T., Tripathi S., Calibasi-Kocal G., Rickelt S., Butty V.L., et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell. 2019;178:1115–1131.e15. doi: 10.1016/j.cell.2019.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Q., Ren H., Hong Z., Wang C., Zheng T., Ren Y., Chen K., Liu S., Wang G., Gu G., et al. Early enteral nutrition preserves intestinal barrier function through reducing the formation of neutrophil extracellular traps (NETs) in critically ill surgical patients. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/8815655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swaminath A., Feathers A., Ananthakrishnan A.N., Falzon L., Li Ferry S. Systematic review with meta-analysis: enteral nutrition therapy for the induction of remission in paediatric Crohn's disease. Aliment. Pharmacol. Ther. 2017;46:645–656. doi: 10.1111/apt.14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y., Choo J., Kim S.J., Heo G., Pothoulakis C., Kim Y.H., Im E. Analysis of endogenous lipids during intestinal wound healing. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehandru S., Colombel J.F. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat. Rev. Gastroenterol. Hepatol. 2021;18:83–84. doi: 10.1038/s41575-020-00399-w. [DOI] [PubMed] [Google Scholar]
- 23.Rieder F., Karrasch T., Ben-Horin S., Schirbel A., Ehehalt R., Wehkamp J., de Haar C., Velin D., Latella G., Scaldaferri F., et al. Results of the 2nd scientific workshop of the ECCO (III): basic mechanisms of intestinal healing. J. Crohns Colitis. 2012;6:373–385. doi: 10.1016/j.crohns.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Boyd M., Thodberg M., Vitezic M., Bornholdt J., Vitting-Seerup K., Chen Y., Coskun M., Li Y., Lo B.Z.S., Klausen P., et al. Characterization of the enhancer and promoter landscape of inflammatory bowel disease from human colon biopsies. Nat. Commun. 2018;9:1661. doi: 10.1038/s41467-018-03766-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keir M.E., Fuh F., Ichikawa R., Acres M., Hackney J.A., Hulme G., Carey C.D., Palmer J., Jones C.J., Long A.K., et al. Regulation and role of αE integrin and gut homing integrins in migration and retention of intestinal lymphocytes during inflammatory bowel disease. J. Immunol. 2021;207:2245–2254. doi: 10.4049/jimmunol.2100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters L.A., Perrigoue J., Mortha A., Iuga A., Song W.M., Neiman E.M., Llewellyn S.R., Di Narzo A., Kidd B.A., Telesco S.E., et al. A functional genomics predictive network model identifies regulators of inflammatory bowel disease. Nat. Genet. 2017;49:1437–1449. doi: 10.1038/ng.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Planell N., Lozano J.J., Mora-Buch R., Masamunt M.C., Jimeno M., Ordás I., Esteller M., Ricart E., Piqué J.M., Panés J., Salas A. Transcriptional analysis of the intestinal mucosa of patients with ulcerative colitis in remission reveals lasting epithelial cell alterations. Gut. 2013;62:967–976. doi: 10.1136/gutjnl-2012-303333. [DOI] [PubMed] [Google Scholar]
- 28.Vancamelbeke M., Vanuytsel T., Farré R., Verstockt S., Ferrante M., Van Assche G., Rutgeerts P., Schuit F., Vermeire S., Arijs I., Cleynen I. Genetic and transcriptomic bases of intestinal epithelial barrier dysfunction in inflammatory bowel disease. Inflamm. Bowel Dis. 2017;23:1718–1729. doi: 10.1097/MIB.0000000000001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanhove W., Peeters P.M., Staelens D., Schraenen A., Van der Goten J., Cleynen I., De Schepper S., Van Lommel L., Reynaert N.L., Schuit F., et al. Strong upregulation of AIM2 and IFI16 inflammasomes in the mucosa of patients with active inflammatory bowel disease. Inflamm. Bowel Dis. 2015;21:2673–2682. doi: 10.1097/MIB.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 30.Rubin S.J.S., Bai L., Haileselassie Y., Garay G., Yun C., Becker L., Streett S.E., Sinha S.R., Habtezion A. Mass cytometry reveals systemic and local immune signatures that distinguish inflammatory bowel diseases. Nat. Commun. 2019;10:2686. doi: 10.1038/s41467-019-10387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casalegno Garduño R., Däbritz J. New insights on CD8(+) T cells in inflammatory bowel disease and therapeutic approaches. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.738762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro-Dopico T., Colombel J.F., Mehandru S. Targeting B cells for inflammatory bowel disease treatment: back to the future. Curr. Opin. Pharmacol. 2020;55:90–98. doi: 10.1016/j.coph.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wéra O., Lancellotti P., Oury C. The dual role of neutrophils in inflammatory bowel diseases. J. Clin. Med. 2016;5 doi: 10.3390/jcm5120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger E., Rath E., Yuan D., Waldschmitt N., Khaloian S., Allgäuer M., Staszewski O., Lobner E.M., Schöttl T., Giesbertz P., et al. Mitochondrial function controls intestinal epithelial stemness and proliferation. Nat. Commun. 2016;7 doi: 10.1038/ncomms13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rousseaux C., Lefebvre B., Dubuquoy L., Lefebvre P., Romano O., Auwerx J., Metzger D., Wahli W., Desvergne B., Naccari G.C., et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J. Exp. Med. 2005;201:1205–1215. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michalik L., Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J. Clin. Invest. 2006;116:598–606. doi: 10.1172/JCI27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiko G.E., Ryu S.H., Koues O.I., Collins P.L., Solnica-Krezel L., Pearce E.J., Pearce E.L., Oltz E.M., Stappenbeck T.S. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell. 2016;167:1137–1720. doi: 10.1016/j.cell.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 38.Zhao H., Chen J., Chai J., Zhang Y., Yu C., Pan Z., Gao P., Zong C., Guan Q., Fu Y., Liu Y. Cytochrome P450 (CYP) epoxygenases as potential targets in the management of impaired diabetic wound healing. Lab. Invest. 2017;97:782–791. doi: 10.1038/labinvest.2017.21. [DOI] [PubMed] [Google Scholar]
- 39.Schreiber S., Aden K., Bernardes J.P., Conrad C., Tran F., Höper H., Volk V., Mishra N., Blase J.I., Nikolaus S., et al. Therapeutic interleukin-6 trans-signaling inhibition by Olamkicept (sgp130Fc) in patients with active inflammatory bowel disease. Gastroenterology. 2021;160:2354–2366.e11. doi: 10.1053/j.gastro.2021.02.062. [DOI] [PubMed] [Google Scholar]
- 40.Schniers A., Goll R., Pasing Y., Sørbye S.W., Florholmen J., Hansen T. Ulcerative colitis: functional analysis of the in-depth proteome. Clin. Proteonomics. 2019;16:4. doi: 10.1186/s12014-019-9224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapman S.J., Thorpe G., Vallance A.E., Harji D.P., Lee M.J., Fearnhead N.S., Association of Coloproctology of Great Britain and Ireland Gastrointestinal Recovery Group Systematic review of definitions and outcome measures for return of bowel function after gastrointestinal surgery. BJS open. 2019;3:1–10. doi: 10.1002/bjs5.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun D.L., Li W.M., Li S.M., Cen Y.Y., Xu Q.W., Li Y.J., Sun Y.B., Qi Y.X., Lin Y.Y., Yang T., et al. Comparison of multi-modal early oral nutrition for the tolerance of oral nutrition with conventional care after major abdominal surgery: a prospective, randomized, single-blind trial. Nutr. J. 2017;16:11. doi: 10.1186/s12937-017-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bestetti S., Medraño-Fernandez I., Galli M., Ghitti M., Bienert G.P., Musco G., Orsi A., Rubartelli A., Sitia R. A persulfidation-based mechanism controls aquaporin-8 conductance. Sci. Adv. 2018;4:eaar5770. doi: 10.1126/sciadv.aar5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krüger C., Waldeck-Weiermair M., Kaynert J., Pokrant T., Komaragiri Y., Otto O., Michel T., Elsner M. AQP8 is a crucial H(2)O(2) transporter in insulin-producing RINm5F cells. Redox Biol. 2021;43 doi: 10.1016/j.redox.2021.101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu C., Chen Z., Jiang Z. Expression, distribution and role of aquaporin water channels in human and animal stomach and intestines. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17091399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dotti I., Mora-Buch R., Ferrer-Picón E., Planell N., Jung P., Masamunt M.C., Leal R.F., Martín de Carpi J., Llach J., Ordás I., et al. Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis. Gut. 2017;66:2069–2079. doi: 10.1136/gutjnl-2016-312609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Min M., Peng L.H., Sun G., Guo M.Z., Qiu Z.W., Yang Y.S. Aquaporin 8 expression is reduced and regulated by microRNAs in patients with ulcerative colitis. Chin. Med. J. 2013;126:1532–1537. [PubMed] [Google Scholar]
- 48.Escudero-Hernández C., Münch A., Østvik A.E., Granlund A.V.B., Koch S. The water channel aquaporin 8 is a critical regulator of intestinal fluid homeostasis in collagenous colitis. J. Crohns Colitis. 2020;14:962–973. doi: 10.1093/ecco-jcc/jjaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He L., Huang N., Li H., Tian J., Zhou X., Li T., Yao K., Wu G., Yin Y. AMPK/α-Ketoglutarate Axis regulates intestinal water and ion homeostasis in Young pigs. J. Agric. Food Chem. 2017;65:2287–2298. doi: 10.1021/acs.jafc.7b00324. [DOI] [PubMed] [Google Scholar]
- 50.Lv H., Li Y., Xue C., Dong N., Bi C., Shan A. Aquaporin: targets for dietary nutrients to regulate intestinal health. J. Anim. Physiol. Anim. Nutr. 2022;106:167–180. doi: 10.1111/jpn.13539. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W., Zhu B., Xu J., Liu Y., Qiu E., Li Z., Li Z., He Y., Zhou H., Bai Y., Zhi F. Bacteroides fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses. Front. Immunol. 2018;9:1040. doi: 10.3389/fimmu.2018.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galán-Cobo A., Ramírez-Lorca R., Serna A., Echevarría M. Overexpression of AQP3 modifies the cell cycle and the proliferation rate of mammalian cells in culture. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thiagarajah J.R., Zhao D., Verkman A.S. Impaired enterocyte proliferation in aquaporin-3 deficiency in mouse models of colitis. Gut. 2007;56:1529–1535. doi: 10.1136/gut.2006.104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peltoketo H., Luu-The V., Simard J., Adamski J. 17beta-hydroxysteroid dehydrogenase (HSD)/17-ketosteroid reductase (KSR) family; nomenclature and main characteristics of the 17HSD/KSR enzymes. J. Mol. Endocrinol. 1999;23:1–11. doi: 10.1677/jme.0.0230001. [DOI] [PubMed] [Google Scholar]
- 55.Albayrak Y., Halici Z., Odabasoglu F., Unal D., Keles O.N., Malkoc I., Oral A., Yayla M., Aydin O., Unal B. The effects of testosterone on intestinal ischemia/reperfusion in rats. J. Invest. Surg. 2011;24:283–291. doi: 10.3109/08941939.2011.591894. [DOI] [PubMed] [Google Scholar]
- 56.Chen G., Zeng H., Li X., Liu J., Li Z., Xu R., Ma Y., Liu C., Xue B. Activation of G protein coupled estrogen receptor prevents chemotherapy-induced intestinal mucositis by inhibiting the DNA damage in crypt cell in an extracellular signal-regulated kinase 1- and 2- dependent manner. Cell Death Dis. 2021;12:1034. doi: 10.1038/s41419-021-04325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin Y., Gao X.Y., Zhao J., Tian W.S., Zhang Y.L., Tian E.J., Zhou B.H., Wang H.W. Estrogen deficiency aggravates fluoride-induced small intestinal mucosa damage and junctional complexes proteins expression disorder in rats. Ecotoxicol. Environ. Saf. 2022;246 doi: 10.1016/j.ecoenv.2022.114181. [DOI] [PubMed] [Google Scholar]
- 58.Alkahtani S. Testosterone induced apoptosis in colon cancer cells is regulated by PI3K/Rac1 signaling. Asian J. Androl. 2013;15:831–834. doi: 10.1038/aja.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennelly R., Kavanagh D.O., Hogan A.M., Winter D.C. Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol. 2008;9:385–391. doi: 10.1016/S1470-2045(08)70100-1. [DOI] [PubMed] [Google Scholar]
- 60.Marino M., Galluzzo P., Leone S., Acconcia F., Ascenzi P. Nitric oxide impairs the 17beta-estradiol-induced apoptosis in human colon adenocarcinoma cells. Endocr. Relat. Cancer. 2006;13:559–569. doi: 10.1677/erc.1.01106. [DOI] [PubMed] [Google Scholar]
- 61.Hempel N., Gamage N., Martin J.L., McManus M.E. Human cytosolic sulfotransferase SULT1A1. Int. J. Biochem. Cell Biol. 2007;39:685–689. doi: 10.1016/j.biocel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Gamage N.U., Tsvetanov S., Duggleby R.G., McManus M.E., Martin J.L. The structure of human SULT1A1 crystallized with estradiol. An insight into active site plasticity and substrate inhibition with multi-ring substrates. J. Biol. Chem. 2005;280:41482–41486. doi: 10.1074/jbc.M508289200. [DOI] [PubMed] [Google Scholar]
- 63.Golenberg N., Squirrell J.M., Bennin D.A., Rindy J., Pistono P.E., Eliceiri K.W., Shelef M.A., Kang J., Huttenlocher A. Citrullination regulates wound responses and tissue regeneration in zebrafish. J. Cell Biol. 2020;219 doi: 10.1083/jcb.201908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cantariño N., Musulén E., Valero V., Peinado M.A., Perucho M., Moreno V., Forcales S.V., Douet J., Buschbeck M. Downregulation of the deiminase PADI2 is an early event in colorectal carcinogenesis and indicates poor prognosis. Mol. Cancer Res. 2016;14:841–848. doi: 10.1158/1541-7786.MCR-16-0034. [DOI] [PubMed] [Google Scholar]
- 65.Team R.C. R Foundation for Statistical Computing; Vienna, Austria: 2021. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- 66.Zhao X.H., Yang T., Zheng M.Y., Zhao P., An L.Y., Qi Y.X., Yi K.Q., Zhang P.C., Sun D.L. Cystathionine gamma-lyase (Cth) induces efferocytosis in macrophages via ERK1/2 to modulate intestinal barrier repair. Cell Commun. Signal. 2023;21:17. doi: 10.1186/s12964-022-01030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS A J. Integr. Biol. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russo P.S.T., Ferreira G.R., Cardozo L.E., Bürger M.C., Arias-Carrasco R., Maruyama S.R., Hirata T.D.C., Lima D.S., Passos F.M., Fukutani K.F., et al. CEMiTool: a Bioconductor package for performing comprehensive modular co-expression analyses. BMC Bioinf. 2018;19:56. doi: 10.1186/s12859-018-2053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hao Y., Hao S., Andersen-Nissen E., Mauck W.M., Zheng S., Butler A., Lee M.J., Wilk A.J., Darby C., Zager M., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This paper analyses existing, publicly available datasets from the GEO repository. These accession numbers for the datasets analysed during the current are listed in the key resources table.
-
•
All dataset analyses were performed based on R 4.1.0.65 The original code has been deposited into Zenodo and is publicly available as of the date of publication. DOI of the deposited original code is listed in the key resources table.
-
•
Any additional information required for re-analysing the datasets reported in this paper is available from the lead contactupon request.