Abstract
Nanopesticides, particularly biosynthesized ones using organic reductants, hold great promise as a cost-effective and eco-friendly alternative to chemical pesticides. However, their efficacy on stored product pests, which can cause damage to dried grains, has not been extensively tested, especially on immature stages. Here, we biosynthesized six types of nanoparticles (NPs) using extracts from the fungus Fusarium solani: silver (AgNPs), selenium (SeNPs), silicon dioxide (SiO2NPs), copper oxide (CuONPs), titanium dioxide (TiO2NPs) and zinc oxide (ZnONPs) ranging in size from 8 to 33 nm. To test their efficacy on stored bean pests, they were applied to the eggs and larvae of pest beetles Callosobruchus chinensis and Callosobruchus maculatus (Coleoptera: Chrysomelidae: Bruchinae), which burrow into seeds as larvae. Susceptibility to the NPs was species-dependent and differed between developmental stages; eggs were more susceptible than larvae inhabiting in seeds. SeNPs and TiO2NPs reduced the hatchability of C. chinensis eggs by 23% and 18% compared to the control, respectively, leading to an 18% reduction in egg-to-adult survival by SeNPs. In C. maculatus, TiO2NPs applied to eggs reduced larva-to-adult survivorship by 11%, resulting in a 15% reduction in egg-to-adult survival. The egg mass of C. chinensis was 23% smaller than that of C. maculatus: the higher surface-area-to-volume ratio of the C. chinensis eggs could explain their higher acute mortality caused by the NPs compared to C. maculatus eggs. The biosynthesized SeNPs and TiO2NPs have potential for controlling major stored bean pests when applied to their eggs. This is the first to show the efficacy of biosynthesized SeNPs and TiO2NPs on stored product pests and the efficacy of Fusarium-synthesized NPs on insects.
Subject terms: Nanobiotechnology, Model invertebrates, Entomology
Introduction
The world population has reached 8 billion in 2022 and is projected to peak at 10.4 billion by the 2080s1. Pulses such as cowpeas (Vigna unguiculata), mung beans (Vigna radiata), and azuki beans (Vigna angularis) are among the most significant protein sources for the human populations of different cultures and vegetarians2. However, storage losses caused by insect pests such as Callosobruchus beetles (Coleoptera: Chrysomelidae: Bruchinae) can have a significant impact on this important food supply. The cowpea beetle (C. maculatus) in tropical areas and azuki bean beetle (C. chinensis) in temperate areas are important stored product pests. These pests have wide host ranges3,4 and can cause severe losses to a majority of dried beans (up to 20% and occasionally higher5,6). Geographical habitat ranges are also expanding7–9, making control of these stored product pests crucial in reducing such losses. Furthermore, Callosobruchus beetles serve as model organisms for population studies10,11.
While chemical insecticides such as fumigants and inert materials such as dusts are effective in controlling bruchine beetles and other stored pests, their use in farmer’s storage facilities, which are often not airtight, can pose risks to human health and the environment12. Therefore, researchers are exploring alternative insecticides to protect both agriculture and ecosystems. One promising approach for stored product protection is the use of nanoparticle formulations13,14. Nanoparticles (NPs) have unique features, such as a high surface-area-to-volume ratio, high reactivity, and enhanced catalytic and biological properties15, making them suitable for a variety of applications, including agriculture16.
Metal and metallic oxide NPs such as silver (Ag), zinc oxide (ZnO), copper oxide (CuO), silica (silicon dioxide, SiO2), titanium dioxide (TiO2), gold (Au), and aluminum oxide (Al2O3) are being developed for pest and disease control. For instance, SiO2NPs have been demonstrated to have physisorption in cuticle lipids of insects, leading to their mortality17. SiO2NPs have also been found to alter volatile emissions from infested plants, which attracts predators18. Selenium nanoparticles (SeNPs) possess antioxidant19, antibacterial20, anticancer21, neuroprotective22, antimicrobial23, and plant-growth-promoting properties24, and can be used in various medical and agricultural treatments25. Recent studies have demonstrated the insecticidal effect of SeNPs on a moth and a beetle14,26. TiO2NPs are used in suncreens and cosmetics to protect from UV and in paint and food coloration. TiO2NPs can affect soil invertebrates as well as control insect pests such as moths, coleopterans and hemipterans27–31.
Biosynthesized NPs are expected to transform the field of integrated pest management (IPM) in the future32,33. Compared to chemical synthesis, the biological synthesis of nanopesticides using plant extracts and microbes is greener, and the produced NPs are stable, environmentally friendly, and cost-effective: They do not require high temperature, high pressure, high energy, or toxic chemicals and do not produce by-products with mammalian toxicity34–38. For example, SeNPs can be synthesized using bacteria23 and fungi (e.g. Mariannaea sp.39). Similarly, TiO2NPs can be synthesized using bacteria40 and plant extracts41. Various species of fungi have also shown potential for use in biogenic synthesis of NPs with different characteristics42. The fungus Fusarium sp. has been used for the extracellular biosynthesis of AgNPs43. However, the efficacy of biosynthesized NPs on stored product pest beetles has been studied on a limited number of species (Sitophilus oryzae, Tribolium castaneum, Tenebrio molitor, and C. maculatus44–47). For example, ZnONPs synthesized with leaf extract or entomopathogens48,49 and NiNPs synthesized using plant extracts44,50 have been tested on adult C. maculatus.
In almost all cases, the targeted developmental stage of the studied stored product pests by NPs has been the adult stage, and the comparison of NP efficacy has rarely been made between developmental stages of pests44–46,51. Abdel-Raheem et al.52 tested the efficacy of AgNPs synthesized with entomopathogenic fungi on the egg, larva, and adult stages of the red palm weevil Rhynchophorus ferrugineus. However, it is not yet known whether the result of this comparison can be applied to immature stages of other coleopterans (weevils and beetles) that have the potential to be exposed to pesticides to different extents. Therefore, in this study, we aimed to test the efficacy of biosynthesized NPs of Ag, CuO, Se, SiO2, TiO2, and ZnO by Fusarium solani extract as insecticides against two Callosobruchus beetle species at two immature stages, egg (attached to the surface of seeds) and larva (feeding seeds internally). We hypothesized that the biosynthesized NPs would reduce the survival of both species, regardless of the developmental stage treated. This is the first study to test the control efficacy of Fusarium-synthesized NPs on insects, as well as biosynthesized Se, SiO2, and TiO2 NPs on stored product pests.
Results
Control efficacy on Callosobruchus chinensis
Treatment on eggs of C. chinensis
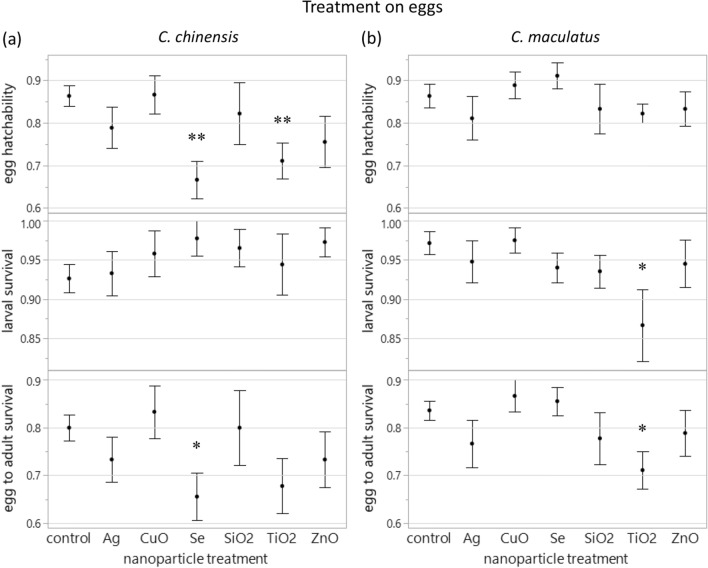
For eggs treated with NPs, there was a significant effect of NP element on hatchability of eggs [LR (likelihood-ratio) χ26 = 19.09, P = 0.004]. Specifically, SeNPs and TiO2NPs reduced the egg hatchability by 22.8% and 17.7%, respectively, compared to the control (posthoc comparison with the control, SeNPs, P < 0.001; TiO2NPs, P = 0.008, Fig. 1a). Larva-to-adult survival was not affected by NP element (LR χ26 = 5.09, P = 0.533, Fig. 1a). However, egg-to-adult survival was affected (LR χ26 = 13.06, P = 0.042): SeNPs reduced egg-to-adult survival by 18.1% compared to the control (P = 0.021).
Figure 1.
Survival (mean ± SE) of (a) Callosobruchus chinensis and (b) Callosobruchus maculatus when eggs were treated with different types of biosynthesized nanoparticles. *P < 0.05, **P < 0.01 compared to the control.
Treatment on larvae of C. chinensis
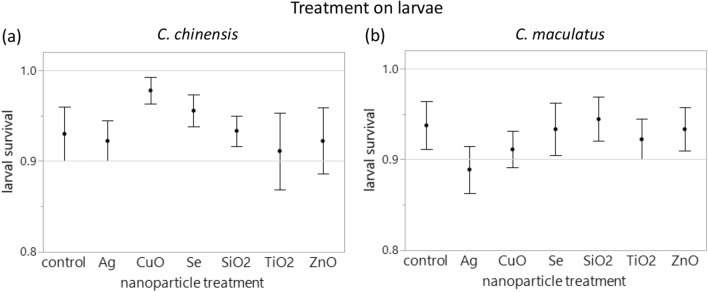
There was no difference among the NP elements and the control in larva-to-adult survival (LR χ26 = 5.53, P = 0.477, Fig. 2a).
Figure 2.
Larva-to-adult survival (mean ± SE) of (a) Callosobruchus chinensis and (b) Callosobruchus maculatus when larvae were treated with different types of biosynthesized nanoparticles. No significant difference compared to the control was found in each species.
Control efficacy on Callosobruchus maculatus
Treatment on eggs of C. maculatus
For eggs treated with NPs, there was no significant effect of NP element on hatchability of eggs (LR χ26 = 6.21, P = 0.400), larva-to-adult survival (LR χ26 = 9.56, P = 0.144), egg-to-adult survival (LR χ26 = 10.56, P = 0.103), or the number of emerged adults (LR χ26 = 9.15, P = 0.165) (Fig. 1b). However, posthoc tests indicated that TiO2NPs reduced larva-to-adult survival and egg-to-adult survival (or the number of emerged adults) by 10.8% and 15.0%, respectively, compared to the control (larva-to-adult survival, P = 0.011; egg-to-adult survival, P = 0.034; emerged adults, P = 0.021, Fig. 1b).
Treatment on larvae of C. maculatus
There was no difference in larva-to-adult survival among the NP elements and the control (LR χ26 = 2.64, P = 0.852, Fig. 2b), with one outlier in the control group excluded from the analysis.
Egg sizes of two Callosobruchus species
Egg mass was different between the two species (F1,90 = 107.7, P < 0.001), with C. chinensis eggs being 22.9% smaller (0.0212 ± 0.00044 mm3, mean ± SE, n = 50) than C. maculatus eggs (0.0275 ± 0.00037 mm3, n = 45). Parental pair ID had a significant effect (F3,90 = 3.8, P = 0.013).
Discussion
We compared the entomotoxic efficacy of the six types of nanoparticles (NPs) biosynthesized using the fungal extract from F. solani on the immature stages of C. chinensis and C. maculatus. Our results showed that susceptibility to biosynthesized NPs varied by species and developmental stage. The eggs of both species were more susceptible than the last-instar larvae, which were protected by the seed coat. This suggests that direct contact with nanopesticides is crucial for controlling pest populations. When beetle eggs were treated, SeNPs and TiO2NPs reduced egg hatchability in C. chinensis, and larval-to-adult survival in C. maculatus, leading to a reduction in the egg-to-adult survival by SeNPs in C. chinensis and by TiO2NPs in C. maculatus. Since the eggs of C. chinensis were 23% smaller than those of C. maculatus (in line with53), the surface area to volume ratio was higher, resulting in greater exposure of C. chinensis eggs to NPs. This could explain the difference in acute NP efficacy against eggs between the two species. In contrast, when beans containing beetle larvae were treated with NPs, no effect was observed. Since eggs and larvae are similarly more vulnerable than adults when NPs are applied directly52, the apparent resistance of the larvae against the NPs is possibly due to the indirect method of application via the seed coat. The biosynthesized NPs, particularly SeNPs and TiO2NPs, showed the potential to control the major stored bean pests when applied to eggs attached to the surface of seed coat but not when applied to larvae inhabiting in seeds.
This is one of the early demonstrations of the insecticidal effects of SeNPs14,26. Se-based organic molecules can produce reactive oxygen species (ROS) and trigger apoptosis or autophagy of cancer cells21. Sodium selenite induces dose-dependent mortality and dose-dependent accumulation of selenium in the Malpighian tubules of the mealworm beetle Tenebrio molitor but not in the digestive and reproductive organs54, while SeNPs synthesized with plant extracts cause damages on larval cellular components of a mosquito, such as nucleus, lumen, and gut epithelial cells55. However, the mechanism of the effect of SeNPs still remains largely unexplored56. Similarly, TiO2NPs can generate ROSs27. The efficacy of TiO2NPs has been compared to other NPs: the efficacy of TiO2NPs is higher than AgNPs (on Spodoptera litura larvae57) and ZnONPs (on Sitophilus oryzae adults58), in support of our results, regardless of differences in species tested. TiO2NPs synthesized with plant extracts increase the activity of detoxification enzymes and cause histopathological change in the midgut of S. litura and a mosquito59.
Although SeNPs have been synthesized using fungi26,39,60 and plant extracts55, their efficacy has not been tested on stored product pests before. Our study is the first to demonstrate the entomotoxic efficacy of biosynthesized SeNPs and TiO2NPs on stored product pests, and the first to test the efficacy of Fusarium-synthesized NPs on insects. However, the influence of dose dependency remains to be tested (e.g.14,54,59,61,62), as low doses of NPs can enhance insect performance (63, Miksanek et al. under review).
Conclusion
Our results suggest that the direct applications of SeNPs and TiO2NPs to eggs are most effective to control the stored bean pests, C. chinensis (18.1% reduction in egg-to-adult survival compared to the control) and C. maculatus (15.0% reduction in egg-to-adult survival compared to the control), respectively. Quantitative studies regarding impact on optimal dosages for effective control of multiple species of pests with minimum side-effects on crops18,50,64,65, and comparison with their conventional analogues are imperative in the future. Our study cautions that the efficacy of nanopesticides in controlling pests depends on the target developmental stages; direct application of nanopesticides to the highly vulnerable early immature stages of pests is recommended for optimal control.
Materials and methods
Fungal culture
The fungal culture used for synthesizing different NPs in this study was isolated from a soil sample collected from the pots used for the experimental studies at the Laboratory of Insect Natural Enemies, Faculty of Agriculture, Kyushu University, using the direct plating method66. The isolated strain was morphologically differentiated using the classification system by Smith and Onion67. Molecular classification was performed using the method described by Henry et al.68, which is detailed in the following section.
Molecular identification of fungi
The fungal isolate was identified based on the ITS rDNA sequence amplified with the primers ITS1 and ITS468. First, the DNA was extracted by freezing and thawing a small sample of the fungal colony dissolved in TE buffer. The PCR was conducted with an annealing temperature at 53 °C using KOD One (Toyobo, Tokyo, Japan), following the manufacturer’s protocol. The PCR product was purified and subjected to Sanger sequencing. The sequence data were searched for matches in the database nr using BLASTn (NCBI, MD, USA). The fungal isolate was identified with 100% certainty as Fusarium solani (Hypocreales: Nectriaceae) through morphological differentiation and genotypical identification based on the ITS sequence.
Biosynthesis of nanoparticles using fungi
To prepare the biomass for biosynthesis of metal and non-metal NPs, fungal culture was grown aerobically in liquid media consisting of 3.0 g malt extract, 10.0 g glucose, 2.0 g yeast extract, 5.0 g peptone, 20.0 g agar–agar and 1.0 L distilled water, with pH adjusted to 6.2 as per69. The fungal culture was filtered aseptically and incubated in sterilized deionized water for 72 h under aerobic conditions.
Silver (Ag) NPs were synthesized by adding 500 mg L−1 of AgNO3 solution to the cell-free water extract of the fungal isolate. The reduction of Ag ions to AgNPs was confirmed by the color transformation of the mixture to brown70 (Supplementary Fig. S1a). Copper oxide (CuO) NPs were synthesized by adding 500 mg L−1 of CuIISO4 solution to the cell-free water extract of the fungal isolate. The reduction of Cu ions to CuONPs was confirmed by the color transformation of the mixture to blue-green (Fig. S1b). Selenium (Se) NPs were synthesized by adding 500 mg L−1 of Na2SeO3 solution to the cell-free water extract of the fungal isolate. The reduction of Se ions to SeNPs was confirmed by the color transformation of the mixture to red (Fig. S1c). Silicon dioxide or silica (SiO2) NPs were synthesized by adding 500 mg L−1 of SiO2 solution to the cell-free water extract of the fungal isolate. No color transformation of the mixture was observed (Fig. S1d). Titanium dioxide (TiO2) NPs were synthesized by adding 500 mg L−1 of TiO2 solution to the cell-free water extract of the fungal isolate. The reduction of Ti ions to TiO2NPs was confirmed by the color transformation of the mixture to a deep white colloidal solution (Fig. S1e). Zinc oxide (ZnO) NPs were synthesized by adding 500 mg L−1 of ZnSO4.7H2O solution to the cell-free water extract of the fungal isolate. No color transformation of the mixture was observed (Fig. S1f). The characterization of the resulting NPs was carried out using transmission electron microscopy (TEM) and energy-dispersive X-ray spectroscopy (EDX) as described below.
Characterization of nanoparticles
The size and shape of the different NPs synthesized using the fungal isolate were determined using TEM (Philips Tecnai-G2 20, Japan). To prepare TEM samples, a drop of well-dispersed NP solution was placed onto conventional carbon-coated copper TEM grids (150 μm meshes, Plano GmbH, Germany), and the drop was allowed to dry overnight in a desiccator before imaging. Three TEM images of each sample were obtained for morphological analysis and particle size using an accelerating voltage of 200 kV. To analyze the elemental chemical composition of the NPs, the EDX spectra were examined coupled with the TEM (Tecnai-G2 20).
The six types of NPs produced by the F. solani isolate were characterized using TEM and EDX as follows (EDX: Supplementary Fig. S2): The spherical AgNPs produced by this fungal extract had a diameter of 15.3 ± 0.2 nm (mean ± SE). The spherical CuONPs produced had a diameter of 11.7 ± 0.3 nm and the spherical SeNPs produced had a diameter of 20.0 ± 0.1 nm. The size of the amorphous SiO2NPs produced was 32.9 ± 2.6 × 75.1 ± 8.9 nm. Finally, the spherical TiO2NPs had a diameter of 15.4 ± 0.2 nm and the ZnONPs had a diameter of 8.1 ± 0.5 nm.
Efficacy test on pest bean beetles
To test the efficacy of the above-mentioned fungus-synthesized NPs against immature stages, egg (attached to the surface of seeds) and larva (feeding seeds internally) of stored product pests, we used two species of stored bean pest beetles: Callosobruchus chinensis (Coleoptera: Chrysomelidae: Bruchinae) strain jC, which has been maintained on dried azuki beans [Vigna angularis var. angularis (Fabaceae), purchased from Daiwa grain, Obihiro, Japan] under a laboratory condition at 30 °C for over 70 years10,71. The other species Callosobruchus maculatus strain tQ has also been maintained on azuki beans under the same laboratory condition as C. chinensis for over 30 years72. Each of the biosynthesized NPs was directly applied to the seed coat of azuki beans that were either with beetle eggs on the surface or infested by beetle larvae. Each treatment was replicated for 9 times, except for the controls for C. chinensis (11 times for egg treatment and 10 times for larval treatment) and for the control for C. maculatus (11 times for egg treatment) at 30 °C, 60% r.h. and 16L:8D.
Direct application of nanoparticles to eggs
Eggs were deposited for 2 h on azuki beans by females that emerged within 24 h. Beans with 1–2 eggs were chosen. Seven to eight beans with a total of 10 eggs of 24 h old were introduced to a petri dish (6 cm diameter) and 20 μL (10 μg) of the biosynthesized NP solution or distilled deionized water was applied with a micropipette, and the dish was gently agitated to coat the bean and egg surface with the NPs. After eight days from application, hatched eggs were counted. Emerged adults were counted after 37 days from egg deposition to rear the treated eggs into adults, via larvae and pupae. A total of 650 eggs for C. chinensis and 650 eggs for C. maculatus were used for this experiment.
Application of nanoparticles to larvae
Twenty μl (10 μg) of the biosynthesized NP solution or distilled deionized water was applied to seven to eight azuki beans infested by a total of 10 fourth instar larvae (14 days old) at a density of 1–2 larvae/bean in a petri dish (6 cm diameter). The dishes were gently agitated. After 23 days of rearing the treated larvae into adults under the same environmental conditions (i.e., 37 days from egg deposition), emerged adults were counted. A total of 640 larvae for C. chinensis and 620 larvae for C. maculatus were used for this experiment.
Egg sizes of two Callosobruchus species
To explain the possible efficacy difference on eggs between the two species, we estimated the egg mass of the two species based on the length and width of eggs, using the equation by Yanagi and Tuda73. The length and width of hatched eggs laid by each female of two (C. chinensis) or three (C. maculatus) pairs on 20 untreated azuki beans in petri dishes (6 cm in diameter) were measured to the precision of 0.001 mm with a microscope (H-5500, Keyence, Osaka, Japan).
All methods were carried out in accordance with relevant institutional, national, and international guidelines and legislation.
Statistics
We tested the effect of NP element on the life history traits of each species studied: Logistic regression analyses were performed on the survival of eggs (that is, egg hatchability), larva to adult, and egg to adult of each beetle species, with NPs or water as an explanatory variable, followed by posthoc comparisons with the control. Egg mass was tested with a general linear model, with NPs or water treatment and parental pair ID nested within treatment as explanatory variables, confirming the normality of the residual errors. All statistical tests were performed using JMP 14.2.0.
Supplementary Information
Acknowledgements
We thank O. Nishi (Institute of Biological Control, Kyushu University) for advice on fungal DNA analysis, Y. Fukunaga (Ultra-microscopy Research Center, Kyushu University) for technical support, J. R. Miksanek for insightful comments, and the Ultra-microscopy Research Center.
Author contributions
E.A.M.H. and M.T. conceived the idea and design of the study; Y.Z.Z., P.P.S., E.A.M.H. and M.T. performed the experiment; E.A.M.H. and M.T. performed data analyses and wrote the original draft; M.T. and C.A. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by KAKENHI (19K06840) from JSPS (Japan Society for the Promotion of Science) and Initiative for Realizing Diversity in the Research Environment from MEXT (Ministry of Education, Science and Technology) to MT, JSPS Invitational Fellowship (L22516) to CA, and JDS (Project for Human Resource Development Scholarship by Japanese Grant Aid) to PPS.
Data availability
The datasets associated with the current study are available from the primary corresponding author (M. Tuda: tuda@grt.kyushu-u.ac.jp) upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eman Ahmed Mohamed Helmy, Email: emanhelmo@yahoo.com.
Charles Adarkwah, Email: charles.adarkwah@uenr.edu.gh.
Midori Tuda, Email: tuda@grt.kyushu-u.ac.jp.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-35697-1.
References
- 1.United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results. UN DESA/POP/2022/TR/NO. 3 (2022)
- 2.Kalpna, Hajam YA, Kumar R. Management of stored grain pest with special reference to Callosobruchus maculatus, a major pest of cowpea: A review. Heliyon. 2022;8:e08703. doi: 10.1016/j.heliyon.2021.e08703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuda M, Chou L-Y, Niyomdham C, Buranapanichpan S, Tateishi Y. Ecological factors associated with pest status in Callosobruchus (Coleoptera: Bruchidae): High host specificity of non-pests to Cajaninae (Fabaceae) J. Stored Prod. Res. 2005;41:31–45. doi: 10.1016/j.jspr.2003.09.003. [DOI] [Google Scholar]
- 4.Tuda M. Applied evolutionary ecology of insects of the subfamily Bruchinae (Coleoptera: Chrysomelidae) Appl. Entomol. Zool. 2007;42:337–346. doi: 10.1303/aez.2007.337. [DOI] [Google Scholar]
- 5.Sharma SS. Review of literature of the losses caused by Callosobruchus chinensis (L) (Coleoptera: Bruchidae) during storage of pulses. Bull. Grain Technol. 1984;22(1):62–68. [Google Scholar]
- 6.Phillips TW, Throne JE. Biorational approaches to managing stored-product insects. Annu. Rev. Entomol. 2009;55:375–397. doi: 10.1146/annurev.ento.54.110807.090451. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki A, Ogino R, Onodera K. Field oviposition of adzuki bean beetle, Callosobruchus chinensis, on adzuki bean, Vigna angularis, and estimation of its oviposition period in Hokkaido, Japan. Ann. Rept. Plant Prot. North Japan. 2013;64:171–175. [Google Scholar]
- 8.Tuda M, Kagoshima K, Toquenaga Y, Arnqvist G. Global genetic differentiation in a cosmopolitan pest of stored beans: Effects of geography, host-plant usage and anthropogenic factors. PLoS ONE. 2014;9(9):e106268. doi: 10.1371/journal.pone.0106268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kébé K, Alvarez N, Tuda M, Arnqvist G, Fox CW, Sembène M, Espíndola A. Global phylogeography of the insect pest Callosobruchus maculatus (Coleoptera: Bruchinae) relates to the history of its main host, Vigna unguiculata. J. Biogeogr. 2017;44:2515–2526. doi: 10.1111/jbi.13052. [DOI] [Google Scholar]
- 10.Tuda M, Shimada M. Complexity, evolution and persistence in host-parasitoid experimental systems, with Callosobruchus beetles as the host. Adv. Ecol. Res. 2005;37:37–75. doi: 10.1016/S0065-2504(04)37002-9. [DOI] [Google Scholar]
- 11.Ishii Y, Shimada M. Learning predator promotes coexistence of prey species in host–parasitoid systems. Proc. Natl. Acad. Sci. USA. 2012;109(13):5116–5120. doi: 10.1073/pnas.1115133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bekele AJ, Obeng-Ofori D, Hassanali A. Products derived from the leaves of Ocimum kilimandscharicum (Labiatae) as post-harvest grain protectants against the infestation of three major stored product insect pests. Bull. Entomol. Res. 1995;85:361–367. doi: 10.1017/S0007485300036099. [DOI] [Google Scholar]
- 13.Athanassiou CG, Kavallieratos NG, Benelli G, Losic D, Usha Rani P, Desneux N. Nanoparticles for pest control: Current status and future perspectives. J. Pest. Sci. 2018;91:1–15. doi: 10.1007/s10340-017-0898-0. [DOI] [Google Scholar]
- 14.Miksanek JR, Tuda M. Endosymbiont-mediated resistance to entomotoxic nanoparticles and sex-specific responses in a seed beetle. J. Pest Sci. 2023;96:1257–1270. doi: 10.1007/s10340-023-01596-7. [DOI] [Google Scholar]
- 15.Ranjani S, Tamanna K, Hemalatha S. Triphala green nano colloids: Synthesis, characterization and screening biomarkers. Appl. Nanosci. 2020;10(4):1269–1279. doi: 10.1007/s13204-019-01208-w. [DOI] [Google Scholar]
- 16.Deka B, Babu A, Baruah C, Barthakur M. Nanopesticides: A systematic review of their prospects with special reference to tea pest management. Front. Nutr. 2021;8:686131. doi: 10.3389/fnut.2021.686131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barik TK, Sahu B, Swain V. Nanosilica-from medicine to pest control. Parasitol. Res. 2008;103:253–258. doi: 10.1007/s00436-008-0975-7. [DOI] [PubMed] [Google Scholar]
- 18.Thabet AF, Boraei HA, Galal OA, El-Samahy MFM, Mousa KM, Zhang YZ, Tuda M, Helmy EA, Wen J, Nozaki T. Silica nanoparticles as pesticide against insects of different feeding types and their non-target attraction of predators. Sci. Rep. 2021;11:14484. doi: 10.1038/s41598-021-93518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamza AM. Efficacy and safety of non-traditional methods as alternatives for control of Sitophilus oryzae (L.) (Coleoptera: Curculionidae) in rice grains. Egypt. J. Biol. Pest Control. 2012;22(2):103. doi: 10.3923/je.2012.57.67. [DOI] [Google Scholar]
- 20.Vera P, Echegoyen Y, Canellas E, Nerín C, Palomo M, Madrid Y, Cámara C. Nano selenium as antioxidant agent in a multilayer food packaging material. Anal. Bioanal. Chem. 2016;408:6659–6670. doi: 10.1007/s00216-016-9780. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Li X, Huang Z, Zheng W, Fan C, Chen T. Enhancement of cell permeabilization apoptosis-inducing activity of selenium nanoparticles by ATP surface decoration. Nanomedicine. 2013;9:74–84. doi: 10.1016/j.nano.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Turovsky EA, Mal’tseva VN, Sarimov RM, et al. Features of the cytoprotective effect of selenium nanoparticles on primary cortical neurons and astrocytes during oxygen–glucose deprivation and reoxygenation. Sci. Rep. 2022;12:1710. doi: 10.1038/s41598-022-05674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon S, Shrudhi SD, Agarwal H, Shanmugam VK. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid Interface Sci. Commun. 2019;29:1–8. doi: 10.1016/j.colcom.2018.12.004. [DOI] [Google Scholar]
- 24.Nazari MR, Abdossi V, Hargalani FZ, et al. Antioxidant potential and essential oil properties of Hypericum perforatum L. assessed by application of selenite and nano-selenium. Sci. Rep. 2022;12:6156. doi: 10.1038/s41598-022-10109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar A, Prasad KS. Role of nano-selenium in health and environment. J. Biotechnol. 2021;325:152–163. doi: 10.1016/j.jbiotec.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Arunthirumeni M, Veerammal V, Shivakumar MS. Biocontrol efficacy of mycosynthesized selenium nanoparticle using Trichoderma sp. on insect pest Spodoptera litura. J. Clust. Sci. 2021;33:1645–1653. doi: 10.1007/s10876-021-02095-4. [DOI] [Google Scholar]
- 27.Tourinho PS, van Gestel CAM, Lofts S, Svendsen C, Soares AMVM, Loureiro S. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates. Environ. Toxic. Chem. 2012;31:1679–1692. doi: 10.1002/etc.1880. [DOI] [PubMed] [Google Scholar]
- 28.Nazari A, Montazer M, Dehghani-Zahedani M. Nano TiO2 as a new tool for mothproofing of wool: Protection of wool against Anthrenus verbasci. Ind. Eng. Chem. 2013;52:1365–1371. doi: 10.1021/ie302187c. [DOI] [Google Scholar]
- 29.Al-Bartya AM, Hamza RZ. Larvicidal, antioxidant activities and perturbation of transminases activities of titanium dioxide nanoparticles synthesized using Moringa oleifera leaves extract against the red palm weevil (Rhynchophorus ferrugineus) EJPMR. 2015;2:49–54. [Google Scholar]
- 30.Sunderland MR, McNeil SJ. Protecting wool carpets from beetle and moth larvae with nanocidal titanium dioxide desiccant. Clean. Technol. Environ. Policy. 2017;19:1205–1213. doi: 10.1007/s10098-016-1297-6. [DOI] [Google Scholar]
- 31.Gutiérrez-Ramírez JA, Betancourt-Galindo R, Aguirre-Uribe LA, Cerna-Chávez E, Sandoval-Rangel A, Ángel EC-D, Chacón-Hernández JC, García-López JI, Hernández-Juárez A. Insecticidal effect of zinc oxide and titanium dioxide nanoparticles against Bactericera cockerelli Sulc. (Hemiptera: Triozidae) on tomato Solanum lycopersicum. Agronomy. 2021;11:1460. doi: 10.3390/agronomy11081460. [DOI] [Google Scholar]
- 32.Bhattacharya, A., Epidi, T. T., Kannan, M. Nano-technology applications in pest management. In Innovative Pest Management Approaches for the 21st Century (Chakravarthy, A. Ed), 391–401 (Springer, 2020). 10.1007/978-981-15-0794-6_19.
- 33.Duhan JS, Kumar R, Kumar N, Kaur P, Nehra K, Duhan S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017;15:11–23. doi: 10.1016/j.btre.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunalan S, Sivaraj R, Venckatesh R. Aloe barbadensis Miller mediated green synthesis of mono-disperse copper oxide nanoparticles: Optical properties. Spectrochim. Acta A. 2012;97:1140–1144. doi: 10.1016/j.saa.2012.07.096. [DOI] [PubMed] [Google Scholar]
- 35.Devi GD, Murugan K, Panneerselvam C. Green synthesis of silver nanoparticles using Euphorbia hirta (Euphorbiaceae) leaf extract against crop pest of cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae) J. Biopestic. 2014;7:54–66. [Google Scholar]
- 36.Kapinder DK, Verma AK. Efficient and eco-friendly smart nano-pesticides: Emerging prospects for agriculture. Mater. Today Proc. 2021;45:3819–3824. doi: 10.1016/j.matpr.2021.03.211. [DOI] [Google Scholar]
- 37.Selvan SM, Anand KV, Govindaraju K, Tamilselvan S, Kumar VG, Subramanian KS, Kannan M, Raja K. Green synthesis of copper oxide nanoparticles and mosquito larvicidal activity against dengue, zika and chikungunya causing vector Aedes aegypti. IET Nanobiotechnol. 2018;12:1042–1046. doi: 10.1049/iet-nbt.2018.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narayanan M, Vigneshwari P, Natarajan D, Kandasamy S, Alsehli M, Elfasakhany A, Pugazhendhi A. Synthesis and characterization of TiO2 NPs by aqueous leaf extract of Coleus aromaticus and assess their antibacterial, larvicidal, and anticancer potential. Environ. Res. 2021;200:111335. doi: 10.1016/j.envres.2021.111335. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Zhou H, Bai J, Li Y, Yang J, Ma Q, Qu Y. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surf. A. 2019;571:9–16. doi: 10.1016/j.colsurfa.2019.02.070. [DOI] [Google Scholar]
- 40.Khan R, Fulekar MH. Biosynthesis of titanium dioxide nanoparticles using Bacillus amyloliquefaciens culture and enhancement of its photocatalytic activity for the degradation of a sulfonated textile dye Reactive Red 31. J. Colloid Interface Sci. 2016;475:184–191. doi: 10.1016/j.jcis.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Aravind M, Amalanathan M, Mary MSM. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021;3:409. doi: 10.1007/s42452-021-04281-5. [DOI] [Google Scholar]
- 42.Guilger-Casagrande M, de Lima R. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019;7:287. doi: 10.3389/fbioe.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh AK, Rathod V, Singh D, Ninganagouda S, Kulkarni P, Mathew J, Haq M. Bioactive silver nanoparticles from endophytic fungus Fusarium sp. isolated from an ethanomedicinal plant Withania somnifera (Ashwagandha) and its antibacterial activity. Int. J. Nanomater. Biostruct. 2015;5:15–19. [Google Scholar]
- 44.Elango G, Roopan SM, Dhamodaran KI, Elumalai K, Al-Dhabi NA, Arasu MV. Spectroscopic investigation of biosynthesized nickel nanoparticles and its larvicidal, pesticidal activities. J. Photochem. Photobiol. B. 2016;162:162–167. doi: 10.1016/j.jphotobiol.2016.06. [DOI] [PubMed] [Google Scholar]
- 45.Zahir AA, Bagavan A, Kamaraj C, Elango G, Rahuman AA. Efficacy of plant-mediated synthesized silver nanoparticles against Sitophilus oryzae. J. Biopest. 2012;5:95–102. doi: 10.3389/fchem.2020.00341. [DOI] [Google Scholar]
- 46.El-Saadony MT, Abd El-Hack ME, Taha AE, Fouda MMG, Ajarem JSN, Maodaa S, Elshaer N. Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials. 2020;10(3):587. doi: 10.3390/nano10030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vivekanandhan P, Swathy K, Thomas A, Kweka EJ, Rahman A, Pittarate S, Krutmuang P. Insecticidal efficacy of microbial-mediated synthesized copper nano-pesticide against insect pests and non-target organisms. Int. J. Environ. Res. Public Health. 2021;18:10536. doi: 10.3390/ijerph181910536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malaikozhundan B, Vaseeharan B, Vijayakumar S, Thangaraj MP. Bacillus thuringiensis coated zinc oxide nanoparticle and its biopesticidal effects on the pulse beetle, Callosobruchus maculatus. J. Photochem. Photobiol. B. 2017;174:306–314. doi: 10.1016/j.jphotobiol.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Malaikozhundan B, Vinodhini J. Nanopesticidal effects of Pongamia pinnata leaf extract coated zinc oxide nanoparticle against the pulse beetle, Callosobruchus maculatus. Mater. Today Commun. 2018;14:106–115. doi: 10.1016/j.mtcomm.2017.12.015. [DOI] [Google Scholar]
- 50.Rahman MA, Parvin A, Khan MSH, War AR, Lingaraju K, Prasad R, Bhattacharyya A. Efficacy of the green synthesized nickel-oxide nanoparticles against pulse beetle, Callosobruchus maculatus (F.) in black gram (Vigna mungo L.) Int. J. Pest Manag. 2021;67(4):306–314. doi: 10.1080/09670874.2020.1773572. [DOI] [Google Scholar]
- 51.Arumugam G, Velayutham V, Shanmugavel S, Sundaram J. Efficacy of nanostructured silica as a stored pulse protector against the infestation of bruchid beetle, Callosobruchus maculatus (Coleoptera: Bruchidae) Appl. Nanosci. 2015;6(3):445–450. doi: 10.1007/s13204-015-0446-2. [DOI] [Google Scholar]
- 52.Abdel-Raheem MA, Alghamdi HA, Reyad NF. Virulence of fungal spores and silver nanoparticles from entomopathogenic fungi on the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) Egypt J. Biol. Pest Control. 2019;29:97. doi: 10.1186/s41938-019-0200-2. [DOI] [Google Scholar]
- 53.Wightman JA, Southgate BJ. Egg morphology, host, and probable regions of origin of the bruchids (Coleoptera: Bruchidae) that infest stored pulses: An identification aid. N. Zeal. J. Exp. Agric. 1982;10:95–99. [Google Scholar]
- 54.Hogan GR, Razniak HG. Selenium-induced mortality and tissue distribution studies in Tenebrio molitor (Coleoptera: Tenebrionidae) Environ. Entomol. 1991;20:790–794. doi: 10.1093/ee/20.3.790. [DOI] [Google Scholar]
- 55.Cittrarasu V, Kaliannan D, Dharman K, et al. Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa Roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Sci. Rep. 2021;11:1032. doi: 10.1038/s41598-020-80327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang YY, Su EZ, Ren JS, Qu XG. The recent biological applications of selenium-based nanomaterials. Nano Today. 2021;38:101205. doi: 10.1016/j.nantod.2021.101205. [DOI] [Google Scholar]
- 57.Chakravarthy AK, Kandakoor SB, Bhattacharya A, Dhanabala K, Gurunatha K, Ramesh P. Bio efficacy of inorganic nanoparticles CdS, Nano-Ag and Nano-TiO2 against Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) Curr. Biotica. 2012;6:271–281. [Google Scholar]
- 58.Das S, Yadav A, Debnath N. Entomotoxic efficacy of aluminium oxide, titanium dioxide and zinc oxide nanoparticles against Sitophilus oryzae (L.): A comparative analysis. J. Stored Prod. Res. 2019;83:92–96. doi: 10.1016/j.jspr.2019.06.003. [DOI] [Google Scholar]
- 59.Shyam-Sundar N, Karthi S, Senthil-Nathan S, Narayanan KR, Santoshkumar B, Sivanesh H, Chanthini KMP, Stanley-Raja V, Ramasubramanian R, Abdel-Megeed A, Malafaia G. Eco-friendly biosynthesis of TiO2 nanoparticles using Desmostachya bipinnata extract: Larvicidal and pupicidal potential against Aedes aegypti and Spodoptera litura and acute toxicity in non-target organisms. Sci. Total Environ. 2023;858:159512. doi: 10.1016/j.scitotenv.2022.159512. [DOI] [PubMed] [Google Scholar]
- 60.Joshi SM, De Britto S, Jogaiah S, Ito SI. Mycogenic selenium nanoparticles as potential new generation broad spectrum antifungal molecules. Biomolecules. 2019;9(9):419. doi: 10.3390/biom9090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wrobel JK, Power R, Toborek M. Biological activity of selenium: Revisited. IUBMB Life. 2016;68(2):97–105. doi: 10.1002/iub.14669. [DOI] [PubMed] [Google Scholar]
- 62.Mechora S. Selenium as a protective agent against pests: A review. Plants. 2019;8:262. doi: 10.3390/plants8080262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ni M, Li F, Wang B, Xu K, Zhang H, Hu J, Tian J, Shen W, Li B. Effect of TiO2 nanoparticles on the reproduction of silkworm. Biol. Trace Elem. Res. 2015;164(1):106–113. doi: 10.1007/s12011-014-0195-1. [DOI] [PubMed] [Google Scholar]
- 64.Debnath N, Das S, Brahmachary RL, Chandra R, Sudan S, Goswami A. Entomotoxicity assay of silica, zinc oxide, titanium dioxide, aluminium oxide nanoparticlles on Lipaphis pseudobrassicae. AIP Conf. Proc. 2010;1276:307. doi: 10.1063/1.3504316. [DOI] [Google Scholar]
- 65.Thabet AF, Galal OA, El-Samahy MFM, Tuda M. Higher toxicity of nano-scale TiO2 and dose-dependent genotoxicity of nano-scale SiO2 on the cytology and seedling development of broad bean Vicia faba. SN Appl. Sci. 2019;1:956. doi: 10.1007/s42452-019-0960-z. [DOI] [Google Scholar]
- 66.Dhingra OD, Sinclair JB. Basic Plant Pathology Methods. 2. CRC Press; 1995. [Google Scholar]
- 67.Smith D, Onions AH. A comparison of some preservation techniques for fungi. Trans. Br. Mycol. Soc. 1983;81:535–540. doi: 10.1016/S0007-1536(83)80122-3. [DOI] [Google Scholar]
- 68.Henry T, Peter CI, Steven HH. Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. J. Clin. Microbiol. 2000;38:1510–1515. doi: 10.1128/JCM.38.4.1510-1515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Helmy EA, Mekawey AA. Envision of the microbial contact with mycosynthesized silver nanoparticles. Res. J. Pharm. Biol. Chem. Sci. 2014;5:344–354. [Google Scholar]
- 70.El-Naggar NE, Abdelwahed NAM, Darwesh OMM. Fabrication of biogenic antimicrobial silver nanoparticles by Streptomyces aegyptia NEAE 102 as eco-friendly nanofactory. J. Microbiol. Biotechnol. 2014;244:453–464. doi: 10.4014/jmb.1310.10095. [DOI] [PubMed] [Google Scholar]
- 71.Tuda M. Density dependence depends on scale; at larval resource patch and at whole population. Res. Popul. Ecol. 1993;35:261–271. doi: 10.1007/BF02513599. [DOI] [Google Scholar]
- 72.Toquenaga Y, Fujii K. Contest and scramble competition in Callosobruchus maculatus (Coleoptera, Bruchidae). 1. larval competition curves and resource sharing patterns. Res. Popul. Ecol. 1991;33:199–211. doi: 10.1007/BF02513549. [DOI] [Google Scholar]
- 73.Yanagi S, Tuda M. Interaction effect among maternal environment, maternal investment and progeny genotype on life history traits in Callosobruchus chinensis. Funct. Ecol. 2010;24(2):383–391. doi: 10.1111/j.1365-2435.2009.01653.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets associated with the current study are available from the primary corresponding author (M. Tuda: tuda@grt.kyushu-u.ac.jp) upon reasonable request.