Abstract
The impact of drug resistance mutations induced by nucleoside reverse transcriptase (RT) inhibitors (NRTI) on cytotoxic T-lymphocyte (CTL) recognition of human immunodeficiency virus type 1 strain LAI (HIV-1LAI) RT was addressed in 35 treated or untreated patients. Two HIV-1LAI RT regions encompassing mutation M41L, L74V, M184V, and T215Y/F were recognized in 75 and 83% mutated and in 33 and 42% unmutated samples, respectively. A total of 41 new CTL epitopes overlapping these mutations were predicted. Mutations enhanced HLA-binding scores of 17 epitopes, decreased scores of 5, and had no effect in 19. Four predicted epitopes containing mutations 41, 74, and 184 were tested and recognized by CD8 cells from mutated or unmutated samples, with frequencies up to 270 gamma interferon spot-forming cells per 106 peripheral blood mononuclear cells. Therefore, RT mutations induced by NRTI can increase the immunogenicity of RT for CTL and might allow a better immune control of resistant viruses in vivo, suggesting that specific immune therapy might help prevent these mutations.
Cytotoxic T lymphocytes (CTL) specific for human immunodeficiency virus (HIV) or simian immunodeficiency virus are considered the most efficient virus-specific immune responses (4, 26, 29, 39). The strength and the diversity of CTL responses (16, 54) have been proposed, together with reverse transcriptase (RT) infidelity (7, 33, 37), as an important factor for virus variability at time of asymptomatic disease and strong immune functions. Some viral mutations can decrease immunogenicity by interfering with the intracellular processing or with the HLA binding of viral peptides, thereby resulting in a lack of CTL recognition (5, 11, 13, 14, 22, 30, 32, 34). In contrast, new HIV variants that do not interfere with such processes can be immunogenic for specific new CTL clones (16), a fact which contributes to some extent to determining HIV variability (54).
The high level of HIV type 1 (HIV-1) RT sequence conservation among different HIV isolates (25) makes RT one of the most frequent targets for CTL recognition; indeed, 80% of HIV-infected individuals have specific RT-specific CTL (17). Prolonged antiviral mono- or bitherapy with nucleoside RT inhibitors (NRTI), however, results in selection of HIV-1 strains containing mutations in the RT gene (36). These mutations often have an impact on the enzymatic activity of RT and on the fitness of the virus (2, 45). These drug-induced mutations are highly standardized and characteristic of the various NRTI used (28, 38). Highly active antiretroviral therapies (HAART) combining various drug regimens have decreased the occurrence of such mutations by reducing levels of virus replication, but they concomitantly decrease the intensity of the HIV-specific CTL responses (10, 15, 29). Currently viral replication is efficiently controlled in only 50% of patients receiving HAART; frequency of treatment failures is increasing and correlates with high levels of drug-induced mutations (56). In industrialized countries, approximately 15% of new cases of HIV primary infection involve strains that show primary drug-induced mutations transmitted by treated individuals (3, 27, 55). The consequences of these mutations for RT recognition by CTL and the ability of the host's RT-specific immune responses to help control growth of resistant variants is not known.
To address this question and to evaluate whether fixed RT mutations induced by nucleoside analogs might alter immune recognition, we performed a prospective analysis of CTL responses directed against RT drug-induced mutations in patients treated by NRTI in mono- or bitherapy between 1991 and 1996, before the advent of protease inhibitors, in order to avoid bias due to decreased CTL frequencies in HAART-treated patients.
A total of 66 samples from 35 patients, either before (n = 29) or during (n = 37) antiretroviral therapy by NRTI, were selected on the basis of positive CTL responses against the whole HIV-1LAI Pol sequence. Polyclonal HIV-specific CTL lines were generated by cocultures of patient peripheral blood mononuclear cells (PBMC) autologous, irradiated phytohemagglutinin (PHA)-stimulated cells, as described elsewhere (16). A standard chromium release assay was performed against autologous B-lymphoblastoid cell lines infected with recombinant vaccinia virus expressing Pol and RT. We also tested recognition of two HIV-1LAI RT truncated regions (RT-1 [1 to 143] and RT-2 [143 to 293]) encompassing the sites of NRTI-induced mutations as described elsewhere (17). CTL responses were considered positive when the specific response exceeded the nonspecific response by 10% or more for at least two successive effector/target ratios. Regions RT-1 and RT-2 were recognized with similar frequencies (59% for each in untreated samples; 49% for RT-1 and 46% for RT-2 in treated samples), independently of their CD4 counts or viral loads (data not shown).
We then analyzed patients PBMC for the presence of four major standard mutations induced in vivo by nucleoside analogs at positions M41L and L74V located in RT-1 and positions M184V and T215Y/F in RT-2. We used a genotyping line probe assay (LiPA) for HIV-1 RT (Murex Diagnostics S.A., Chatillon, France) according to manufacturer's instructions as described by Descamps et al. (12). This assay simultaneously detects the wild-type and drug-selected variants in codons 41, 69, 70, 74, 184, 214, and 215 (43), with specific oligonucleotide probes immobilized on membrane-based strips. When comparing CTL recognition of RT-1 and RT-2 with frequencies of mutations, we observed RT-1-specific CTL in 5 out of 6 treated samples containing the M41L and/or L74V mutations but in only 5 out of the 12 unmutated samples. Similarly, RT-2 was recognized in six out of eight samples containing mutations M184V and/or T215Y/F but in only three out of nine unmutated samples (data not shown). In half of the cases, the wild-type and mutated strains coexisted, but the LiPA method does not allow quantification of the various clones. These results demonstrate that CTL recognition of these RT regions is twice as frequent in samples containing NRTI-induced mutations as in unmutated samples.
To analyze further the immunogenicity of the M41L, L74V, M184V, and T215Y/F mutations, CTL recognition had to be studied at the epitope level. Only few CTL epitopes surrounding sites of drug-induced mutations have been described up to now: RT 33-41 and 33-43, 179-187, 180-189, and 209-220 (17, 18, 49–51) (Table 1). Therefore, we used first a theoretical analysis to predict new RT epitopes around sites of NRTI-induced mutations based on a computer scoring (http://bimas.dcrt.nih.gov/molbio/hla_bind/) that allows the location and ranking of peptides that contain binding motifs for HLA class I molecules (31). For a given peptide, the best score is obtained when it contains the correct dominant and auxiliary anchor residues for a particular HLA molecule. The median score for 66 known, published HIV CTL epitopes from the Los Alamos HIV molecular immunology database was 15 (25th and 75th percentiles, 3 to 120) (6, 25). For example a known HIV-1LAI RT CTL epitope (309-317) (48) had a predicted binding score to HLA-A0201 of 39. In the present study we used a cutoff of 10 for the HLA-binding scores of our predicted epitopes.
TABLE 1.
Most important RT mutations induced by NRTI and known RT CTL epitopes containing these mutationsa
| Standard mutation position(s) | Most frequent mutation(s) induced by NRTI | Known RT CTL epitope(s) | HLA restriction element(s) | Reference(s) for CTL epitopes |
|---|---|---|---|---|
| 41 | M41L | 33-41, 33-43 | A2, A3 | 17 |
| 65 | K65R | None | None | None |
| 67 | D67N | None | None | None |
| 69/70 | T69S, T69A, T69D, K70R, 69SG70, T or N69D/K70R | None | None | None |
| 74/75 | L74V, V75T, L74V/V75 | None | None | None |
| 115 | Y115F | 107-115, 107-117, 108-118 | B35, A201, A201 | 6, 17, 50, 51 |
| 157 | P157S | 153-165, 156-165, 156-164 | B7, B7, B3501 | 6, 24, 41, 46 |
| 178 | I178M | 175-183 | B3501 | 24 |
| 184 | M184I, M184V | 179-187, 180-189 | A2/A201, A201 | 17, 18, 49 |
| 210 | L210W | 200-211, 203-212, 209-220 | Bw60, B44, A2 | 17, 49, 52 |
| 214/215 | F or L214/T215Y or F, Y215C, T215F, T215Y | 209-220 | A2 | 17 |
| 219 | K219Q | 209-220 | A2 | 17 |
Mutations studied here are underlined; mutations detected by LiPA HIV-1 RT are in bold.
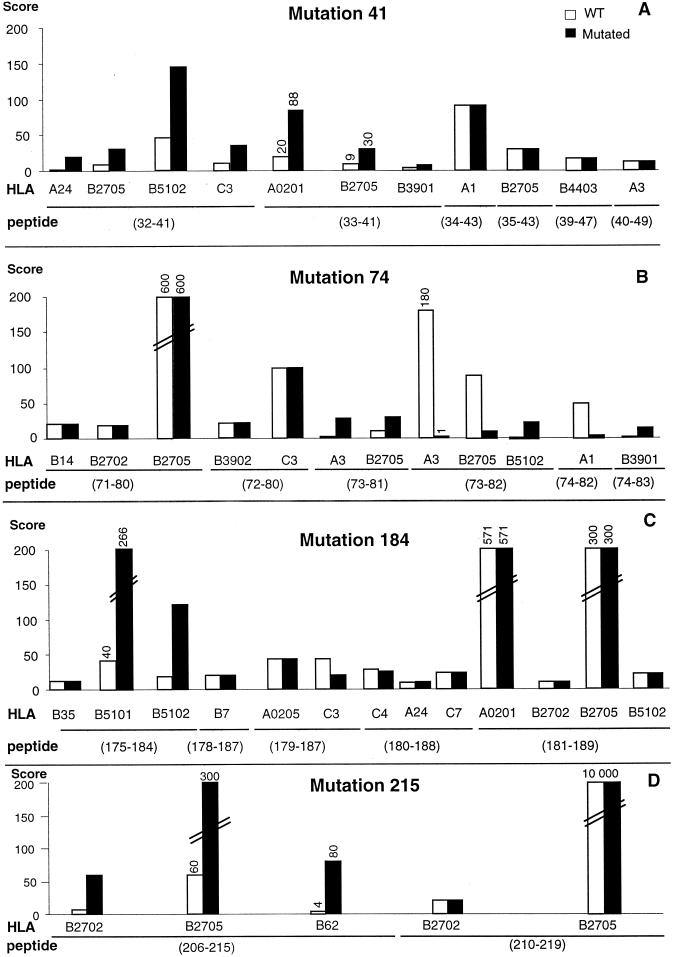
We analyzed scores of binding of the HIV-1LAI RT sequences around positions 41, 74, 184, and 215 to 19 patient HLA molecules from our study group for which a prediction motif was available. We could predict 41 new putative CTL epitopes surrounding the major mutations M41L, L74V, M184V, and T215Y. We then compared the HLA-binding capacities of the wild-type (LAI) or mutated RT sequences. Six epitopes were predicted around each of the mutations M41L and L74V in the context of nine distinct HLA molecules (Fig. 1A and B). Mutation M41L occurred at anchor positions (p2 or p9) in three epitopes (32-41, 33-41, and 40-49) and increased binding scores to 6 HLA molecules (Fig. 1A). Mutation L74V affected the p2 anchor position in two RT epitopes (73-81 and 73-82) in the context of three HLA molecules but decreased the binding scores to two of them (Fig. 1B). Mutation at position 184 was also located in a highly immunogenic region since five predicted epitopes surrounding mutation M184V were predicted in the context of 12 HLA molecules (Fig. 1C). Mutation M184V affected anchor motif (p9) in only one predicted epitope (RT 175-184) and induced a strong binding score increase in two out of three HLA molecules tested. The position 184 mutation slightly affected binding scores to only three HLA molecules. In contrast, mutation T215Y occurred within a poorly immunogenic region: only two epitopes could be predicted in the context of three HLA molecules. Mutation T215Y increased the HLA-binding score of peptide 206-215 to three HLA molecules only (Fig. 1D). Therefore, mutations M41L, L74V, and M184V occur in strongly immunogenic regions that contain 11, 12, and 13 predicted CTL epitopes, respectively, while mutation T215Y occurs in a poorly immunogenic region that contains only 5 predicted epitopes.
FIG. 1.
Changes in HLA-binding scores of predicted epitopes surrounding the NRTI-induced mutations 41 (A), 74 (B), 184 (C), and 215 (D) in HIV-1LAI RT wild-type (WT) and mutated sequences. The HLA class I molecules studied were selected from patient haplotypes; computer scoring was done online (see text).
Overall, NRTI-induced mutations increased the HLA-binding scores in 42% of the 41 predicted epitopes. In contrast, HLA binding scores were decreased in only 5 (12%) epitopes. Mutations 41, 74, and 215 affected peptide anchor positions, while mutation 184 affected mostly central amino acids in predicted epitopes. Altogether 21 epitopes had anchor positions affected; for 15 (71%) of these, the HLA-binding scores were increased. Mutation 184, which affected mostly the central amino acid positions of the predicted epitopes, decreased HLA-binding scores in only three combinations. These theoretical results suggest that the major mutations induced by NRTI rarely impair but rather improve at least in half of the cases the capacity of mutated candidate epitopes to bind HLA class I molecules.
We then tested whether such known or predicted epitopes were indeed recognized by CD8 cells when affected by a drug-induced mutation. We used an enzyme-linked spot (ELISPOT) assay for single-cell gamma interferon (IFN-γ) release adapted from Dalod et al. (10), using polyvinylidene difluoride-bottomed well plates (Millipore, Molsheim, France) and two anti-human IFN-γ monoclonal antibodies, the second being biotinylated (Diaclone, Besançon, France). PBMC were added in triplicate wells at 105 and 5 × 104 cells per well in the presence of peptide, PHA as a positive control, or medium alone. A panel of peptides was synthesized according to the HIV-1 epitope sequences available at the Los Alamos HIV molecular immunology database (http://hiv-lanl.gov/immunology/advancedctl.html) or to the predictive analysis described above (Syntem, Nîmes, France). We analyzed recognition of five epitopes affected by mutations M41L, L74V, M184V, and T215Y in the context of the most frequent HLA molecules in nine patients selected according to their treatment and HLA type. As positive controls, known CTL epitopes from the HIV RT, Env, or Nef and from cytomegalovirus (CMV) pp65 were recognized in the context of the same HLA class I molecules, within ranges of frequencies from 0 to 1,545 spot-forming cells (SFC) per 106 PBMC.
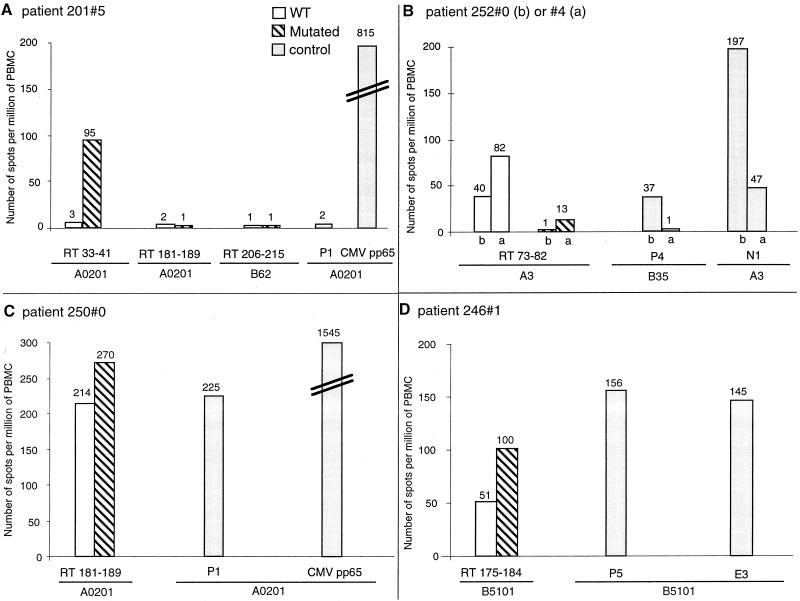
The effect of mutation M41L was evaluated in one HLA-B27 and four HLA-A2 patients before and/or after receiving 3 zidovudine. The patient 201#5 sample harbored mutation M41L, which increased the HLA-A2-binding score of this previously described epitope (33-41) (Fig. 1A). The mutated but not the wild-type peptide was recognized after treatment in this patient, with a frequency of 95 SFC per 106 PBMC (Fig. 2A). We evaluated the effect of mutation L74V on recognition of the predicted epitope 73-82 in two HLA-A3 patients before and after treatment and found that neither of these patients harbored mutations. The wild-type RT 73-82 peptide was recognized both before and after treatment in patients 252#0 and 252#4, with frequencies of 40 and 82 SFC per 106 PBMC, respectively (Fig. 2B). The mutated peptide was not recognized. In that case, the mutation theoretically decreased this epitope's binding score to HLA-A3 (Fig. 1B). Two predicted epitopes surrounding mutation M184V were tested in three patients. Both wild-type and mutated 175-184 peptides were recognized in the context of HLA-B51 in patient 246#1 before treatment, with frequencies of 51 and 100 SFC per 106 PBMC, respectively (Fig. 2D). Another peptide, RT 181-189, was tested in two HLA-A2 patients. Mutation 184 did not affect significantly recognition of peptide 181-189 mutated or wild type (Fig. 2C), the calculated binding score for which was similarly unaffected (Fig. 1C). In patient 250#0, both wild-type and mutated peptides were recognized with high frequencies of 214 to 270 SFC/106 PBMC. In a second HLA-A2 patient (201#5), no CTL could be detected against either wild-type or mutated peptide. Peptide 206-215, encompassing mutation T215Y, was tested in three patients before or after receiving AZT. Only the patient 201#5 sample harbored the mutation T215Y/F. Neither wild-type nor mutated peptides were recognized, however (Fig. 2A).
FIG. 2.
CD8 T cell recognition of mutations 41, 74, and 184. IFN-γ production by PBMC was detected by ELISPOT assay. PBMC from patient 201#5 after treatment (A), patient 252#0 before treatment (b) or 252#4 after treatment (a) (B), and patients 250#0 (C), and 246#1 (D) before treatment were added to 96-well plates in triplicate wells at 105 and 5 × 104 cells per well in the presence of 5 μg of peptide per ml or 0.5 μg of PHA or medium per ml in a 40-h ELISPOT assay. Peptides for HIV proteins tested: RT 33-41 wild type (WT) (ALVEICTEM) and mutated (ALVEICTEL); RT 73-82 wild type (KLVDFRELNK) and mutated (KVVDFRELNK); RT 175-184 wild type (NPDIVIYQYM) and mutated (NPDIVIYQYV); RT 181-189 wild type (YQYMDDLYV) and mutated (YQYVDDLYV); RT 206-215 wild type (RQHLLRWGLT) and mutated (RQHLLRWGLY); P1, RT 309-317 (ILKEPVHGV) (47, 50); P4, RT 311-319 (SPAIFQSSMT) (6); P5, RT 295-302 (TAFTIPSI) (42); E3, gp41 557-565 (RAIEAQQHL) (42); N1, Nef 73-82 (QVPLRPMTYK) (9, 23). Peptide for CMV pp65: 495-503 (NLVPMVATV) (53). The number of specific T cells was calculated after subtracting negative control values. Mean values from triplicate wells are shown.
Therefore, all predicted epitopes that could be tested were recognized at least once, except around mutation T215Y. These new candidate epitopes surrounding NRTI mutations were recognized with frequencies of SFC ranging between 40 and 270 per 106 PBMC, similar to those observed for known HIV or even RT epitopes, except when surrounding the T215Y mutation, which appears to be located in a poorly immunogenic region.
Discussion.
We demonstrated herein that mutations induced by mono- or bitherapy with NRTI, M41L, L74V, M184V, and T215Y/F, did not impair CTL recognition of the RT sites of drug-induced mutations in treated patients. On the contrary, the drug-induced mutations were associated with twofold more frequent recognition of the unmutated HIV-1LAI RT regions that encompass those mutations. The limited number of known CTL epitopes that surround these mutations prompted us to use a theoretical analysis to predict new CTL epitopes restricted by patient HLA molecules based on their HLA-binding capacity. Mutations M41L, L74V, and M184V occur in immunogenic regions containing multiple predicted epitopes binding to a number of HLA class I molecules. In contrast, mutation T215Y occurs in a region of poor immunogenicity that contains only five predicted epitopes. Mutations M41L, L74V, and T215Y, but not M184V, frequently involve dominant anchor motifs in these predicted epitopes. When the mutations involved dominant anchor motifs (for 21 predicted epitopes), the HLA-binding scores increased, in 15 cases (15/21 = 71%), correspondng to two-thirds of the cases. Overall, NRTI-induced mutations, whether they affected the anchor positions or not, augmented the HLA-binding scores for 42% of candidate epitopes whereas a decrease was predicted for only five epitopes, suggesting that these NRTI-induced mutations may facilitate CTL recognition of the predicted epitopes. These findings and predictions contrast with observations that for other HIV proteins, some HIV mutations affecting critical amino acid anchor positions result in partial escape of HIV-1 from CTL recognition (4, 14, 32). In addition, mutations sparing anchor motifs in CTL epitopes can also significantly alter peptide processing or CTL recognition (11, 21, 30). The findings presented here are in agreement with our previous report that most natural mutations occurring in HIV-1 Nef are immunogenic and stimulate new variant-specific CTL clones (16).
Here we demonstrated the in vivo CTL recognition of five predicted epitopes affected by mutations M41L, L74V, M184V, and T215Y in nine patients who beared frequent HLA molecules: A2, A3, B27, B51, and B62. All candidate epitopes were shown to be recognized at least once by CD8 T cells, except around mutation T215Y. The ELISPOT assay measures the frequencies of peptide-specific effector CD8 T cells that have been already primed and amplified in vivo and produce IFN-γ (1). The production of IFN-γ detected by ELISPOT assay has also been shown to be associated with cytolytic activity in Epstein-Barr virus infection (44). Our results therefore indicate that the theoretically predicted epitopes that we tested were efficiently processed in vivo and recognized by CD8 T cells in vivo. The frequencies observed ranged between 40 and 270 per 106 PBMC and were similar to those observed for other known RT or HIV epitopes. The epitope-specific CD8 cell frequencies were in accordance with the mutation-induced predicted changes in HLA-binding scores, thus confirming the validity of the predictive model that we used (31). Some discrepancies were observed, but we cannot exclude that the level of mutant expression in vivo might have been insufficient to generate a specific CTL response. Alternatively, the coexistence of other class I loci could have negatively selected the appropriate CD8+ T-lymphocyte response. It is only in the case of mutation T215Y, which is frequently observed within the AZT-induced mutations (35, 56), that predicted epitopes failed to be recognized, suggesting a poor immune control of this region. Thus, both predictive model and ELISPOT analyses confirm the poor immunogenicity of the RT region surrounding mutation 215. In contrast, the high immunogenicity of regions surrounding mutations 41, 74, and 184 found in the context of multiple HLA class I molecules together with the increasing or unchanged HLA-binding scores conferred by most mutations and their ex vivo detection by specific CD8 T cells confirms our hypothesis that these NRTI-induced mutations do not alter and might even improve the immune recognition of HIV-1 RT.
In conclusion, mutations induced by NRTI do not cause loss of CTL recognition of the RT regions harboring the mutations. Moreover, our data strongly suggest that some of these mutations induced by NRTI might increase rather than decrease the immunogenicity of the corresponding CTL epitopes and can be recognized in vivo by CD8 T cells. Therefore, the persistence of these mutations over time might reflect not an immune escape by lack of recognition but rather a strong selective pressure of the drug. These mutations are known to induce replication disadvantage and a loss in viral fitness (2, 8, 19, 40). We propose in addition that the enhanced CTL recognition of the NRTI-induced variants might contribute to the relatively poor growth capacity of mutated viruses in vivo. A larger study is in progress in our laboratory to confirm these findings. At a time when clinical benefits of antiretroviral therapy are clearly shown to depend on the extent and durability of viral suppression (20), the generation of specific immune responses to these mutated regions might improve control of emerging drug-resistant mutations in HIV-1-treated individuals. Similarly, the increasing frequencies of new contaminations with mutated viruses should prompt design of vaccine sequences taking those mutated epitopes into account. We propose that both therapeutic immunization and preventive vaccine programs should incorporate the RT-mutated immunogenic sequences described herein.
Acknowledgments
This work was supported by the Agence Nationale de Recherches sur le SIDA (ANRS) and the Fondation pour la Recherche Medicale.
We thank the patients and clinicians of the IMMUNOCO cohort, without whom this work would not be possible, P. Debre for constant support in the study, ANRS for providing peptides and recombinant vaccinia viruses, M. Jung for help with RT sequence analysis, F. Hadida, O. Bonduelle, and J. C. Deschemin for CTL assays, K. Dott for vaccinia virus constructs, G. Jung for peptide synthesis, C. Dehay and M. Geuzoli for HLA typing, N. Profizi for advice in performing LiPA of HIV-1 RT from PBMC, and Dorian McIlroy and Lucile Mollet for reading the manuscript.
REFERENCES
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Back N K, Nijhuis M, Keulen W, Boucher C A, Oude Essink B O, van Kuilenburg A B, van Gennip A H, Berkhout B. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. EMBO J. 1996;15:4040–4049. [PMC free article] [PubMed] [Google Scholar]
- 3.Boden D, Hurley A, Zhang L, Cao Y, Guo Y, Jones E, Tsay J, Ip J, Farthing C, Limoli K, Parkin N, Markowitz M. HIV-1 drug resistance in newly infected individuals. JAMA. 1999;282:1135–1141. doi: 10.1001/jama.282.12.1135. [DOI] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 6.Brander C, Walker B. The HLA-class I restricted CTL response in HIV-1 infection: identification of optimal epitopes. In: Korber B, Brander C, Walker B, Koup R, Haynes B, Moore J, editors. HIV-1 molecular immunology database. Vol. 3. Los Alamos, N.Mex: Theoretical Biology and Biophysics, Los Alamos National Laboratory; 1997. pp. IV50–IV60. [Google Scholar]
- 7.Coffin J M. Genetic diversity and evolution of retroviruses. Curr Top Microbiol Immunol. 1992;176:143–164. doi: 10.1007/978-3-642-77011-1_10. [DOI] [PubMed] [Google Scholar]
- 8.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 9.Culmann B, Gomard E, Kieny M P, Guy B, Dreyfus F, Saimot A G, Sereni D, Sicard D, Levy J P. Six epitopes reacting with human cytotoxic CD8+ T cells in the central region of the HIV-1 NEF protein. J Immunol. 1991;146:1560–1565. [PubMed] [Google Scholar]
- 10.Dalod M, Harzic M, Pellegrin I, Dumon B, Hoen B, Sereni D, Deschemin J C, Levy J P, Venet A, Gomard E. Evolution of cytotoxic T lymphocyte responses to human immunodeficiency virus type 1 in patients with symptomatic primary infection receiving antiretroviral triple therapy. J Infect Dis. 1998;178:61–69. doi: 10.1086/515587. [DOI] [PubMed] [Google Scholar]
- 11.Del Val M, Schlicht H J, Ruppert T, Reddehase M J, Koszinowski U H. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell. 1991;66:1145–1153. doi: 10.1016/0092-8674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- 12.Descamps D, Calvez V, Collin G, Cecille A, Apetrei C, Damond F, Katlama C, Matheron S, Huraux J M, Brun-Vezinet F. Line probe assay for detection of human immunodeficiency virus type 1 mutations conferring resistance to nucleoside inhibitors of reverse transcriptase: comparison with sequence analysis. J Clin Microbiol. 1998;36:2143–2145. doi: 10.1128/jcm.36.7.2143-2145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans D T, O'Connor D H, Jing P, Dzuris J L, Sidney J, da Silva J, Allen T M, Horton H, Venham J E, Rudersdorf R A, Vogel T, Pauza C D, Bontrop R E, DeMars R, Sette A, Hughes A L, Watkins D I. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med. 1999;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- 14.Goulder P J, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland J S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 15.Gray C M, Lawrence J, Schapiro J M, Altman J D, Winters M A, Crompton M, Loi M, Kundu S K, Davis M M, Merigan T C. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART) J Immunol. 1999;162:1780–1789. [PubMed] [Google Scholar]
- 16.Haas G, Plikat U, Debre P, Lucchiari M, Katlama C, Dudoit Y, Bonduelle O, Bauer M, Ihlenfeldt H G, Jung G, Maier B, Meyerhans A, Autran B. Dynamics of viral variants in HIV-1 Nef and specific cytotoxic T lymphocytes in vivo. J Immunol. 1996;157:4212–4221. [PubMed] [Google Scholar]
- 17.Haas G, Samri A, Gomard E, Hosmalin A, Duntze J, Bouley J M, Ihlenfeldt H G, Katlama C, Autran B. Cytotoxic T-cell responses to HIV-1 reverse transcriptase, integrase and protease. AIDS. 1998;12:1427–1436. doi: 10.1097/00002030-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Harrer E, Harrer T, Barbosa P, Feinberg M, Johnson R P, Buchbinder S, Walker B D. Recognition of the highly conserved YMDD region in the human immunodeficiency virus type 1 reverse transcriptase by HLA-A2- restricted cytotoxic T lymphocytes from an asymptomatic long-term nonprogressor. J Infect Dis. 1996;173:476–479. doi: 10.1093/infdis/173.2.476. [DOI] [PubMed] [Google Scholar]
- 19.Harrigan P R, Bloor S, Larder B A. Relative replicative fitness of zidovudine-resistant human immunodeficiency virus type 1 isolates in vitro. J Virol. 1998;72:3773–3778. doi: 10.1128/jvi.72.5.3773-3778.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katzenstein D A, Hammer S M, Hughes M D, Gundacker H, Jackson J B, Fiscus S, Rasheed S, Elbeik T, Reichman R, Japour A, Merigan T C, Hirsch M S. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. AIDS Clinical Trials Group Study 175 Virology Study Team. N Engl J Med. 1996;335:1091–1098. doi: 10.1056/NEJM199610103351502. . (Erratum, 337:1097, 1997.) [DOI] [PubMed] [Google Scholar]
- 21.Klenerman P, Meier U C, Phillips R E, McMichael A J. The effects of natural altered peptide ligands on the whole blood cytotoxic T lymphocyte response to human immunodeficiency virus. Eur J Immunol. 1995;25:1927–1931. doi: 10.1002/eji.1830250720. [DOI] [PubMed] [Google Scholar]
- 22.Klenerman P, Rowland J S, McAdam S, Edwards J, Daenke S, Lalloo D, Koppe B, Rosenberg W, Boyd D, Edwards A, et al. Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 Gag variants. Nature. 1994;369:403–407. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- 23.Koenig S, Fuerst T R, Wood L V, Woods R M, Suzich J A, Jones G M, de la Cruz V F, Davey R J, Venkatesan S, Moss B, et al. Mapping the fine specificity of a cytolytic T cell response to HIV-1 nef protein. J Immunol. 1990;145:127–135. [PubMed] [Google Scholar]
- 24.Korbel B, Koup R, Walker B, Haynes B, Moore J, Myers G E, editors. HIV molecular immunological database. Los Alamos, N.Mex: Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; 1995. [Google Scholar]
- 25.Korber B T, Allen E E, Farmer A D, Myers G L. Heterogeneity of HIV-1 and HIV-2. AIDS. 1995;9(Suppl. A):S5–S18. [PubMed] [Google Scholar]
- 26.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little S J, Daar E S, D'Aquila R T, Keiser P H, Connick E, Whitcomb J M, Hellmann N S, Petropoulos C J, Sutton L, Pitt J A, Rosenberg E S, Koup R A, Walker B D, Richman D D. Reduced antiretroviral drug susceptibility among patients with primary HIV infection. JAMA. 1999;282:1142–1149. doi: 10.1001/jama.282.12.1142. [DOI] [PubMed] [Google Scholar]
- 28.Mellors J, Larder B, Schinazi R. Mutations in HIV-1 reverse transcriptase and protease associated with drug resistance. Int Antiviral News. 1995;3:8–13. [Google Scholar]
- 29.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland J S, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 30.Ossendorp F, Eggers M, Neisig A, Ruppert T, Groettrup M, Sijts A, Mengede E, Kloetzel P M, Neefjes J, Koszinowski U, Melief C. A single residue exchange within a viral CTL epitope alters proteasome-mediated degradation resulting in lack of antigen presentation. Immunity. 1996;5:115–124. doi: 10.1016/s1074-7613(00)80488-4. [DOI] [PubMed] [Google Scholar]
- 31.Parker K C, Bednarek M A, Coligan J E. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 32.Phillips R E, Rowland J S, Nixon D F, Gotch F M, Edwards J P, Ogunlesi A O, Elvin J G, Rothbard J A, Bangham C R, Rizza C R, et al. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991;354:453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- 33.Preston B D, Poiesz B J, Loeb L A. Fidelity of HIV-1 reverse transcriptase. Science. 1988;242:1168–1171. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- 34.Price D A, Goulder P J, Klenerman P, Sewell A K, Easterbrook P J, Troop M, Bangham C R, Phillips R E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puchhammer S E, Schmied B, Mandl C W, Vetter N, Heinz F X. Comparison of line probe assay (LIPA) and sequence analysis for detection of HIV-1 drug resistance. J Med Virol. 1999;57:283–289. doi: 10.1002/(sici)1096-9071(199903)57:3<283::aid-jmv12>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Richman D D. HIV drug resistance. AIDS Res Hum Retroviruses. 1992;8:1065–1071. doi: 10.1089/aid.1992.8.1065. [DOI] [PubMed] [Google Scholar]
- 37.Roberts J D, Bebenek K, Kunkel T A. The accuracy of reverse transcriptase from HIV-1. Science. 1988;242:1171–1173. doi: 10.1126/science.2460925. [DOI] [PubMed] [Google Scholar]
- 38.Schinazi R F, Larder B A, Mellors J W. Mutations in retroviral genes associated with drug resistance: 1999–2000. Int Antiviral News. 1999;7:46–69. [Google Scholar]
- 39.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner R K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 40.Sharma P L, Crumpacker C S. Decreased processivity of human immunodeficiency virus type 1 reverse transcriptase (RT) containing didanosine-selected mutation Leu74Val: a comparative analysis of RT variants Leu74Val and lamivudine-selected Met184Val. J Virol. 1999;73:8448–8456. doi: 10.1128/jvi.73.10.8448-8456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiga H, Shioda T, Tomiyama H, Takamiya Y, Oka S, Kimura S, Yamaguchi Y, Gojoubori T, Rammensee H G, Miwa K, Takiguchi M. Identification of multiple HIV-1 cytotoxic T-cell epitopes presented by human leukocyte antigen B35 molecules. AIDS. 1996;10:1075–1083. [PubMed] [Google Scholar]
- 42.Sipsas N V, Kalams S A, Trocha A, He S, Blattner W A, Walker B D, Johnson R P. Identification of type-specific cytotoxic T lymphocyte responses to homologous viral proteins in laboratory workers accidentally infected with HIV-1. J Clin Investig. 1997;99:752–762. doi: 10.1172/JCI119221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stuyver L, Wyseur A, Rombout A, Louwagie J, Scarcez T, Verhofstede C, Rimland D, Schinazi R F, Rossau R. Line probe assay for rapid detection of drug-selected mutations in the human immunodeficiency virus type 1 reverse transcriptase gene. Antimicrob Agents Chemother. 1997;41:284–291. doi: 10.1128/aac.41.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan L C, Gudgeon N, Annels N E, Hansasuta P, O'Callaghan C A, Rowland J S, McMichael A J, Rickinson A B, Callan M F. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 45.Tisdale M, Kemp S D, Parry N R, Larder B A. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci USA. 1993;90:5653–5636. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomiyama H, Miwa K, Shiga H, Moore Y I, Oka S, Iwamoto A, Kaneko Y, Takiguchi M. Evidence of presentation of multiple HIV-1 cytotoxic T lymphocyte epitopes by HLA-B*3501 molecules that are associated with the accelerated progression of AIDS. J Immunol. 1997;158:5026–5034. [PubMed] [Google Scholar]
- 47.Tsomides T J, Aldovini A, Johnson R P, Walker B D, Young R A, Eisen H N. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J Exp Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsomides T J, Walker B D, Eisen H N. An optimal viral peptide recognized by CD8+ T cells binds very tightly to the restricting class I major histocompatibility complex protein on intact cells but not to the purified class I protein. Proc Natl Acad Sci USA. 1991;88:11276–11280. doi: 10.1073/pnas.88.24.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Burg S H, Klein M R, Pontesilli O, Holwerda A M, Drijfhout J W, Kast W M, Miedema F, Melief C J. HIV-1 reverse transcriptase-specific CTL against conserved epitopes do not protect against progression to AIDS. J Immunol. 1997;159:3648–3654. [PubMed] [Google Scholar]
- 50.van der Burg S H, Klein M R, van de Velde C J, Kast W M, Miedema F, Melief C J. Induction of a primary human cytotoxic T-lymphocyte response against a novel conserved epitope in a functional sequence of HIV-1 reverse transcriptase. AIDS. 1995;9:121–127. [PubMed] [Google Scholar]
- 51.van der Burg S H, Visseren M J, Brandt R M, Kast W M, Melief C J. Immunogenicity of peptides bound to MHC class I molecules depends on the MHC-peptide complex stability. J Immunol. 1996;156:3308–3314. [PubMed] [Google Scholar]
- 52.Walker B D, Flexner C, Birch L K, Fisher L, Paradis T J, Aldovini A, Young R, Moss B, Schooley R T. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wills M R, Carmichael A J, Mynard K, Jin X, Weekes M P, Plachter B, Sissons J G. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolinsky S M, Korber B T, Neumann A U, Daniels M, Kunstman K J, Whetsell A J, Furtado M R, Cao Y, Ho D D, Safrit J T. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science. 1996;272:537–542. doi: 10.1126/science.272.5261.537. [DOI] [PubMed] [Google Scholar]
- 55.Yerly S, Kaiser L, Race E, Bru J P, Clavel F, Perrin L. Transmission of antiretroviral-drug-resistant HIV-1 variants. Lancet. 1999;354:729–733. doi: 10.1016/S0140-6736(98)12262-6. [DOI] [PubMed] [Google Scholar]
- 56.Young B, Johnson S, Bahktiari M, Shugarts D, Young R K, Allen M, Ramey R N, Kuritzkes D R. Resistance mutations in protease and reverse transcriptase genes of human immunodeficiency virus type 1 isolates from patients with combination antiretroviral therapy failure. J Infect Dis. 1998;178:1497–1501. doi: 10.1086/314437. [DOI] [PubMed] [Google Scholar]