Abstract
Migraine, a common primary headache disorder, is associated with various factors such as stress, hormones in women, fasting, weather, and sleep disturbance as well as odors. We aimed to categorize odors associated with migraine and explore their relationships with clinical characteristics. A total of 101 migraineurs answered a questionnaire to determine the odors associated with migraine attacks. We performed factor analysis to explore the common factors of the odors and the relationship between these factors and the clinical characteristics. The factor analysis estimated six common factors: factor 1, fetid odor; factor 2, cooking products; factor 3, oil derivatives and others; factor 4, shampoo and conditioner; factor 5, cleaning products; factor 6, perfumes, insecticides, and rose. Factor 5 also included hair styling preparations, laundry detergent, and fabric softener, usually those with floral fragrances, and factor 5 was more likely to be associated with migraine attacks in patients with chronic migraine than in those with episodic migraine (P = 0.037). Our study showed that odors associated with migraine attacks could be categorized into six groups and suggested that some chemicals were more likely associated with migraine attacks in patients with chronic migraine than in those with episodic migraine.
Subject terms: Neuroscience, Neurology
Introduction
Migraine is a common primary headache disorder with a prevalence of 8.4% in Japan1. Migraine attacks are believed to be provoked by various triggers such as stress, hormones in women, fasting, weather, and sleep disturbance2,3. Determining causality in headache triggers identified three basic assumptions, including constancy of the sufferer, constancy of the trigger effect, and constancy of the trigger presentation4. In clinical practice, however, these assumptions are very difficult to identify4. The study of agreement between self-reported triggers and early premonitory symptoms showed that some patient-reported triggers, such as light, sound, food, and skipping meals, were consistent with early premonitory symptoms5. This study has suggested that some patient-reported triggers may represent early brain manifestations of the premonitory phase of the migraine attack5. Therefore, we believe that what is considered to be a trigger is more appropriately considered an associated factor.
Odors are also common associated with migraine attacks2,3. Many studies have shown that odor sensitivity is a fairly specific symptom of migraine6–12. Although specific, odor sensitivity does not necessarily add further ability to discriminate patients in comparison to existing diagnostic criteria13. In contrast, few studies have reported the types of odors that are associated with migraine attacks14–16.
There are three anatomical systems for processing odorous stimuli, namely, the olfactory, trigeminal, and pheromone systems15,17,18. The pathway for each system is the olfactory nerve projection to the olfactory bulb, the trigeminal nerve projection to the somatosensory and insular cortex, and projection from the accessory olfactory bulb to the hypothalamus15,17,18. In addition, imaging studies showed that different odors activate different regions of the brain19,20.
We hypothesized that different odors are likely to cause headaches depending on age, sex, and type of migraine, such as episodic (EM) or chronic (CM). Therefore, this study aimed to categorize odors associated with migraine and to explore their relationships with clinical characteristics. We used factor analysis to find common factors in odors associated with migraine and cluster analysis to explore the relationship between odors and migraine onset/severity.
Results
Participants
One hundred two patients were enrolled in this study. However, one patient was excluded because of insufficiently described data. Table 1 presents the demographics of the 101 patients who participated; their ages ranged from 22 to 75 years. Of the 16 patients (15.8%) with CM, 12 (75.0%) had concomitant medication overuse headache. Odors were associated with migraine attacks in 79 (78.2%) patients. There were no significant differences in age, sex, or types of migraine with or without the onset of migraine attacks by odors.
Table 1.
Demographics of the participants.
| All (n = 101) | Migraine attacks associated with odors | ||
|---|---|---|---|
| Yes (n = 79, 78.2%) | No (n = 22, 21.8%) | ||
| Age (years) | 46.2 ± 10.9 | 46.2 ± 10.8 | 46.2 ± 11.9 |
| Sex | |||
| Female | 90 (88.1) | 71 (89.9) | 19 (86.4) |
| Male | 11 (10.9) | 8 (1.01) | 3 (13.6) |
| Migraine type | |||
| EM | 85 (84.2) | 66 (83.5) | 19 (86.4) |
| CM | 16 (15.8) | 13 (16.5) | 3 (13.6) |
Data are presented as mean ± standard deviation or n (%).
EM episodic migraine, CM chronic migraine.
Details of odors reported to be associated with migraine attacks
The most frequently reported odors associated with migraine attacks were perfume (55.4%), tobacco (47.5%), fabric softener (32.7%), body odor (32.7%), garbage (24.8%), hairdressing products (22.8%), cars (22.8%), and sweat (19.8%) (Table 2). No patient reported migraine attacks associated with the scent of lemon, tangerine, or apple. The most frequent odors aside from the 35 items of the questionnaire were ikebana (Japanese flower arrangement) (5.9%) and gasoline (3.0%).
Table 2.
Details of odors or odors reported to be associated with migraine attacks.
| Odors | Rate of association (%) |
|---|---|
| Perfumes | 56.4 |
| Tobacco | 47.5 |
| Fabric softener | 32.7 |
| Body odor | 32.7 |
| Garbage | 24.8 |
| Hairdressing products | 22.8 |
| Automobiles | 22.8 |
| Sweat | 19.8 |
| Garlic | 16.8 |
| Rice | 15.8 |
| Grilled fish | 15.8 |
| Alcohol | 14.9 |
| Excrement | 14.9 |
| Machine oil | 13.9 |
| Vomit | 13.9 |
| Chemicals | 11.9 |
| Propane gas | 10.9 |
| Animals | 10.9 |
| Coffee | 9.9 |
| Laundry detergent | 8.9 |
| Grilled meat | 8.9 |
| Shampoo | 7.9 |
| Roses | 7.9 |
| Insect repellent | 7.9 |
| Rinse | 6.9 |
| Mint | 5.9 |
| Socks | 5.0 |
| Soap | 4.0 |
| Curry | 4.0 |
| Cheese | 4.0 |
| Hinoki | 4.0 |
| Black tea | 1.0 |
| Lemon | 0.0 |
| Tangerine | 0.0 |
| Apple | 0.0 |
The most commonly reported places associated with migraine attacks were offices (55.4%), followed by homes (40.6%), restaurants (28.7%), and hospital waiting rooms (5.0%). No patient reported migraine attacks in the consultation or examination room. The most frequent places besides the questionnaire were on the train (7.9%), in town (5.9%), and in department stores (5.0%).
Forty-two percent of the patients used masks, 22.8% used air cleaner, and 21.8% used deodorant spray to prevent odors. In the free-text field, 16.8% of the patients reported moving out of the place, whereas 5.0% reported holding their noses with a handkerchief.
The relationships between odors and age, sex, or types of migraine are described below. Two items showed significant age differences with or without odors. The patients with migraine attacks associated with tobacco or soap were significantly younger than those without (43.0 ± 10.3 vs. 49.0 ± 10.8, P = 0.005; 34.0 ± 8.2 vs. 46.7 ± 10.7, P = 0.022). Only women had migraine attacks associated with body odor (37%) and garbage (28%) (P = 0.014 and P = 0.004, respectively). Fabric softener, sweat, socks, coffee, excrement, vomit, and animals were associated with migraine attacks in significantly more patients with CM than those with EM (55.4% vs. 27.7%, P = 0.028; 43.6% vs. 14.9%, P = 0.009; 24.8% vs. 6.9%, P = 0.028; 49.5% vs. 7.9%, P < 0.001; 30.7% vs. 10.9%, P = 0.028; 30.7% vs. 6.9%, P = 0.004).
Factor analysis
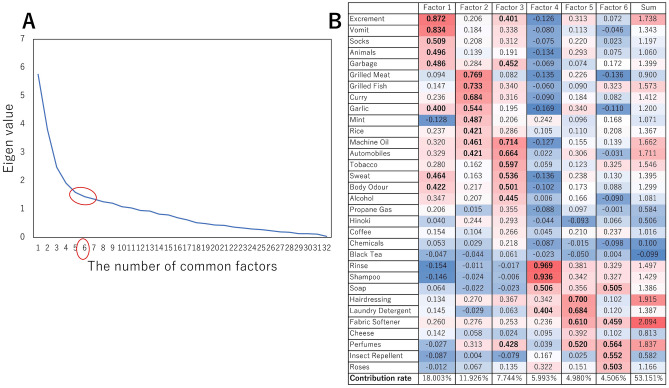
Factor analysis was performed on 35 items of odors, excluding three items that were not associated with migraine attacks. The analysis estimated six common factors with reference to the elbow chart (Fig. 1A).
Figure 1.
Elbow chart and factor structure matrix. (A) Six common factors estimated through factor analysis using the elbow chart. (B) Factor structure matrix and factor loading values of the six factors. Factor loading values > 0.400 are in bold. The correlations between the factors were all < 0.388. Factor 1, fetid odor; factor 2, cooking products; factor 3, oil derivatives and others; factor 4, shampoo and conditioner; factor 5, cleaning products; factor 6, perfumes, insecticides, and rose.
The cumulative variance was 53.2%. The factor structure matrix and loading values on the six factors are presented in Fig. 1B. The correlation between the factors was < 0.388. Based on previous reports9,11,12, we defined factor 1 as fetid odor, factor 2 as cooking products, factor 3 as oil derivatives and others, factor 4 as shampoo and conditioner, factor 5 as cleaning products, and factor 6 as perfumes, insecticides, and rose.
The Spearman correlation coefficient test revealed that factors 3 (r = 0.690, P < 0.001), 1 (r = 0.472, P = 0.006), and 2 (r = 0.397, P = 0.025) seemed to be particularly important as hidden factors in headache-inducing odors, in that order. CM was likely induced by factor 5 compared with EM (P = 0.037). Factors 2, 3, 4, 5, and 6 had a stronger influence on headache aggravation (P < 0.001, P < 0.001, P = 0.002, P < 0.001, and P < 0.001, respectively).
Clustering analysis
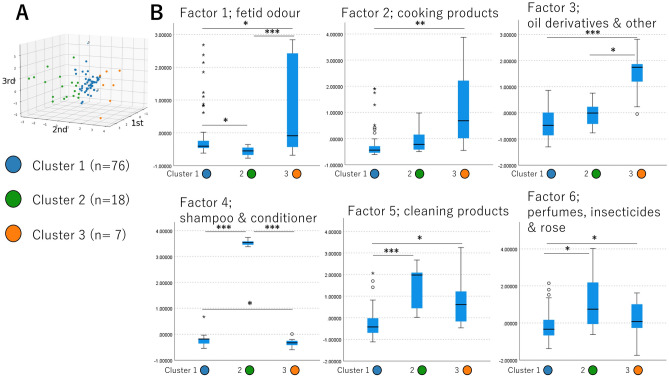
The six common factors were used for k-means ++ clustering. The biggest silhouette score21 was 0.471268, suggesting that 3 is the appropriate number of clusters. The clusters were plotted in the three-dimensional space, with the axes calculated using principal component analysis consisting of the six factors (Fig. 2A and Supplementary Video S1). The barycenter of each cluster is presented in Table 3.
Figure 2.
Clustering results and their barycenters. (A) Clusters plotted in three-dimensional space; axes were calculated using principal component analysis. Cluster 1, 76 individuals (blue); cluster 2, 18 individuals (green); cluster 3, 7 individuals (orange). (B) The sensitivities against the common factors were statistically analyzed. Cluster 1 had a slightly stronger sensitivity than cluster 2 to factor 1 and than cluster 3 to factor 4. Cluster 2 had a strong sensitivity to factors 4, 5, and 6. Cluster 3 had a strong sensitivity to factors 1, 2, 3, 5, and 6. *P < 0.050, **P < 0.010, ***P < 0.001.
Table 3.
Barycenter of each cluster.
| Cluster 1 (blue) (n = 76) | Cluster 2 (green) (n = 18) | Cluster 3 (orange) (n = 7) | P value† | P value‡ | |
|---|---|---|---|---|---|
| Factor 1: fetid odor | − 0.56205 | − 0.85752 | − 0.15133 | 0.002 | 1 > 2: P = 0.020 |
| 3 > 2: P < 0.001 | |||||
| 3 > 1: P = 0.019 | |||||
| Factor 2: cooking products | − 0.26273 | − 0.04106 | 1.12527 | < 0.001 | 3 > 1: P = 0.001 |
| Factor 3: oil derivatives and other | − 0.35991 | − 0.06059 | 1.54317 | < 0.001 | 3 > 2: P = 0.027 |
| 3 > 1: P < 0.001 | |||||
| Factor 4: shampoo and conditioner | − 0.24726 | 3.53210 | − 0.32960 | < 0.001 | 3 > 2: P < 0.001 |
| 2 > 1: P < 0.001 | |||||
| 3 > 1: P = 0.040 | |||||
| Factor 5: cleaning products | − 0.28847 | 1.38852 | 0.67802 | < 0.001 | 2 > 1: P < 0.001 |
| 3 > 1: P = 0.040 | |||||
| Factor 6: perfumes, insecticides, and rose | − 0.16231 | 1.20098 | 0.21872 | 0.008 | 2 > 1: P < 0.011 |
| 3 > 1: P = 0.034 |
†Tested using Kruskal–Wallis test.
‡Tested using Mann‒Whitney U test after Kruskal‒Wallis test.
The clustering results are presented in Fig. 2A. Sensitivity to common factors was statistically analyzed, and the results are presented in Fig. 2B.
Discussion
This study analyzed 101 migraineurs to identify odors associated with migraine attacks. The study used factor analysis to group the odorants into six categories, including fetid odor; cooking products; oil derivatives; shampoo and conditioner; cleaning products; and perfumes, insecticides, and rose scent. The study found that factor 5, which included floral fragrances in hair styling preparations, laundry detergent, and fabric softener, usually those with floral fragrances, was more likely to be associated with migraine attacks in patients with CM than in those with EM. Overall, the study suggests that some chemicals are more likely to be associated with migraine attacks in patients with CM (Fig. 3).
Figure 3.
Summary of this study.
Olfaction occurs when an odorant substance binds to a receptor within the nasal cavity, transmitting a signal through the olfactory system. Odors can stimulate nasal trigeminal receptors14,17. Since most odors are bimodal or activate the trigeminal and olfactory nerves, there are two possible mechanisms for inducing migraine attacks. Odor threshold, discrimination, and identification abilities are lower in migraineurs with and without osmophobia than in healthy controls, and discrimination ability was especially lower in patients with osmophobia11.
Factor analysis was used to determine common factors in the odor associated with migraines. If the results indicate that a particular odorant substance is associated with migraine, it may be possible to prevent migraine attacks by avoiding the odorant substance. In addition, some patients are more sensitive to an odorant substance than others, and we observe this in daily clinical practice. Clustering was performed to group patients’ sensitivity to an odorant substance, which may allow us to tentatively consider the relationship between the odorant substance and the onset/severity of migraine headaches. The results of the factor analysis were included in the clustering analysis, and not all of the original odorant substance variables were included in the calculation. This was done to avoid the disadvantages of dimensions such as distance concentration and data sparsity due to the large number of variables. Our factor analysis showed that odor-associated migraine attacks could be estimated among the six clusters. Each cluster may involve different olfactory, trigeminal, and pheromone system rates.
The odors of factors 4 and 5 are predominantly flora-based and overlapping. The reason they were split into different groups in the factor analysis may have to do with the different situations in which they smelled the odorant. Factor 4 is used in the bath or shower, whereas factor 5 is used in the general environment. The possibility that differences in environmental conditions (such as temperature and humidity) and mental state, i.e., people are generally more relaxed while bathing, were mathematically calculated separately was considered.
Compared with EM, CM was likely to be associated with factor 5. Factor 5 included hairdressing products, laundry detergent, and fabric softener; thus, factor 5 was defined as cleaning products. Odors in cleaning products arise due to the use of odorant substances such as floral fragrances (rose oxide)22. Chronification of migraine may increase sensitivity to certain chemicals.
Non-parametric analysis showed that body odor or garbage was associated with migraine attacks in women only. Savic et al. reported the sex-specific effects of pheromones18. The hypothalamus is activated in women and men by the smell of an androgen-like and estrogen-like substance, respectively. Body odor is mainly induced by axillary odor, foot odor, and diacetyl decomposed by lactic acid, occurring in the occiput and around the neck23. Axillary odor is caused by a combination of sweat gland secretions. The exact composition of human sweat contains a mixture of different compounds, including androgen-like androstenone, androstanol, and androstadienone24,25. The reason why body odor was associated with migraine attacks in women only is presumed to be related to the sex-specific effects of pheromones. The main component of garbage is methyl mercaptan, which is not a pheromone23. In Japan, women usually take out the garbage and have more contact with it. This social context could be the reason that garbage was associated with migraine attacks only in women.
The non-parametric analysis also showed that the patients with migraine attacks associated with tobacco or soap were significantly younger than those with attacks associated with other factors. The chemical substances in tobacco are different from those in soap. Tobacco smoke contains more than 4,000 chemicals, mainly nicotine, tar, carbon monoxide, carbon dioxide, nitrogen oxides, ammonia, hydrogen sulfide, aldehydes, and ketones23. The scent of soap is derived from synthetic fragrances such as floral and aldehyde bouquets24. Therefore, it is unlikely that the chemical substances common to both tobacco and soap were associated with more migraine attacks among younger migraineurs than older migraineurs. We hypothesize that younger people have less exposure to tobacco and soap odors and are, therefore, more sensitive to unfamiliar odors. This can be due to various reasons. First, there is lower exposure to tobacco odor in public places than that in the past, and smoking rates are declining gradually26. Second, the use of solid soap is higher among older people, whereas younger people often use liquid body soaps, which vary from floral to citrus, herbal, and fruit scents. The strength of the odor also varies (from unscented and slightly scented to scented)27,28.
This study has limitations. It was conducted at a single headache clinic with the role of a regional headache center. This setting limits the generalizability of the results and may introduce a selection bias toward patients with more severe outcomes. The main limitations were the retrospective design of the study and the use of a questionnaire to retrospectively identify potential associations in their cohort. This limitation could lead to misattribution or recall bias. Osmophobia is reported in a large number of patients, and early manifestation of osmophobia may be the reason for a large proportion of self-reported odorants as triggers, i.e., an odorant with an associations vs. early manifestation of osmophobia29,30. Prospective confirmation is needed to determine whether or not this is an actual association.
In conclusion, this retrospective study suggests that odors associated with migraine attacks could be categorized into six groups using factor analysis. These results suggest that the chronification of migraine is associated with increased sensitivity to certain chemicals. Further prospective studies are needed to confirm these findings.
Methods
Patients
Patients with migraine who consulted the Department of Neurology, Japanese Red Cross Shizuoka Hospital, were enrolled from April 28 to September 8, 2020. All patients fulfilled the criteria of the International Classification of Headache Disorders, 3rd Edition for migraine without aura, migraine with aura, or CM31, as determined by a headache specialist certified by the International Headache Society. The exclusion criteria were as follows: tension-type headache for > 5 days per month, history of any other primary headache, pregnancy, breastfeeding, cardiovascular or cerebrovascular disease, uncontrolled psychiatric disorder, and drug abuse. The Japanese Red Cross Shizuoka Hospital Ethics Committee approved the study protocol (approval number 2021-05), and the study was conducted in accordance with the Helsinki II Declaration of 1964 with later revisions. Written informed consent was obtained from all participants. The sample size was set at 100 cases based on other studies of odor9,10.
Questionnaire
A questionnaire was used to determine whether odors induced migraine attacks, which odors or location induced migraine attacks, and how the participants cope with the odors. The questionnaire included 35 items of odors selected according to previous studies to determine the types of odors that trigger migraine attacks11,12,14,15. The locations were homes, workplaces, restaurants, and hospitals. The questionnaire items for preventing odors were masks, air cleaners, and deodorant spray. Free-text fields were created for all items so that participants could describe odors, locations, and remedies not included in the items.
Statistical analyses of demographics and relationships between clinical characters and odors
Demographics and relationships between clinical characters and odors were analyzed using t-tests for continuous data with normal distribution, Mann‒Whitney U test or Kruskal‒Wallis test for non-parametric variables, and Fisher’s exact test for classified data using the SPSS Statistics 20.0 (IBM Corp., Armonk, NY, USA). The significance level was defined as P < 0.05. We classified migraine without aura and migraine with aura as EM and compared them with CM.
We conducted a factor analysis using the maximum-likelihood method with the promax rotation using SPSS Statistics 28.0 (IBM Corp.) to explore common factors of odors that cause migraine. The number of common factors was decided with reference to the elbow chart. The relationship between the common factors and migraine occurrences was tested using Spearman’s correlation coefficient. Furthermore, to investigate the migraineurs’ sensitivities against the common factors calculated by the factor analysis, we performed non-stratified clustering using k-means ++ using Python 3.9.0, scikit-learn 0.24.1, and Matplotlib 3.4.3. The clusters were plotted in the three-dimensional space, the axes of which were calculated using principal component analysis32,33.
Supplementary Information
Author contributions
N.I. contributed to the study design. N.I. and A.O. screened titles and abstracts for relevance and retrieved full texts for eligibility. N.I. and A.O. extracted data. N.I. and M.K. performed statistical analysis. N.I. wrote the manuscript. A.O., A.M., M.K., and E.K. critically reviewed the article. All authors interpreted the data, reviewed the manuscript, and approved the final version.
Data availability
All data generated or analyzed during this study are included in this published article [and its Supplementary Information files].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-35211-7.
References
- 1.Sakai F, Igarashi H. Prevalence of migraine in Japan: A nationwide survey. Cephalalgia. 1997;17:15–22. doi: 10.1046/j.1468-2982.1997.1701015.x. [DOI] [PubMed] [Google Scholar]
- 2.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 3.Marmura MJ. Triggers, protectors, and predictors in episodic migraine. Curr. Pain Headache Rep. 2018;22:81. doi: 10.1007/s11916-018-0734-0. [DOI] [PubMed] [Google Scholar]
- 4.Turner DP, Smitherman TA, Martin VT, Penzien DB, Houle TT. Causality and headache triggers. Headache. 2013;53:628–635. doi: 10.1111/head.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karsan N, Bose P, Newman J, Goadsby PJ. Are some patient-perceived migraine triggers simply early manifestations of the attack? J. Neurol. 2021;268:1885–1893. doi: 10.1007/s00415-020-10344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelman L. The place of osmophobia and taste abnormalities in migraine classification: A tertiary care study of 1237 patients. Cephalalgia. 2004;24:940–946. doi: 10.1111/j.1468-2982.2004.00766.x. [DOI] [PubMed] [Google Scholar]
- 7.Zanchin G, et al. Osmophobia in migraine and tension-type headache and its clinical features in patients with migraine. Cephalalgia. 2007;27:1061–1068. doi: 10.1111/j.1468-2982.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 8.De Carlo D, et al. Osmophobia in migraine classification: A multicentre study in juvenile patients. Cephalalgia. 2010;30:1486–1494. doi: 10.1177/0333102410362928. [DOI] [PubMed] [Google Scholar]
- 9.Saisu A, Tatsumoto M, Hoshiyama E, Aiba S, Hirata K. Evaluation of olfaction in patients with migraine using an odour stick identification test. Cephalalgia. 2011;31:1023–1028. doi: 10.1177/0333102411410612. [DOI] [PubMed] [Google Scholar]
- 10.Silva-Néto RP, et al. May headache triggered by odors be regarded as a differentiating factor between migraine and other primary headaches? Cephalalgia. 2017;37:20–28. doi: 10.1177/0333102416636098. [DOI] [PubMed] [Google Scholar]
- 11.Kayabaşoglu G, Altundag A, Kotan D, Dizdar D, Kaymaz R. Osmophobia and olfactory functions in patients with migraine. Eur. Arch. Otorhinolaryngol. 2017;274:817–821. doi: 10.1007/s00405-016-4283-z. [DOI] [PubMed] [Google Scholar]
- 12.Terrin A, et al. A prospective study on osmophobia in migraine versus tension-type headache in a large series of attacks. Cephalalgia. 2020;40:337–346. doi: 10.1177/0333102419877661. [DOI] [PubMed] [Google Scholar]
- 13.Chalmer MA, Hansen TF, Olesen J. Nosographic analysis of osmophobia and field testing of diagnostic criteria including osmophobia. Cephalalgia. 2019;39:38–43. doi: 10.1177/0333102418771375. [DOI] [PubMed] [Google Scholar]
- 14.Fukui PT, et al. Trigger factors in migraine patients. Arq. Neuro Psiquiatr. 2008;66:494–499. doi: 10.1590/S0004-282X2008000400011. [DOI] [PubMed] [Google Scholar]
- 15.Sjöstrand C, et al. Migraine and olfactory stimuli. Curr. Pain Headache Rep. 2010;14:244–251. doi: 10.1007/s11916-010-0109-7. [DOI] [PubMed] [Google Scholar]
- 16.Silva-Néto RP, Peres MF, Valença MM. Odorant substances that trigger headaches in migraine patients. Cephalalgia. 2014;34:14–21. doi: 10.1177/0333102413495969. [DOI] [PubMed] [Google Scholar]
- 17.Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–745. doi: 10.1016/S0896-6273(00)81209-X. [DOI] [PubMed] [Google Scholar]
- 18.Savic I, Berglund H, Gulyas B, Roland P. Smelling of odorous sex hormone-like compounds causes sex-differentiated hypothalamic activations in humans. Neuron. 2001;31:661–668. doi: 10.1016/S0896-6273(01)00390-7. [DOI] [PubMed] [Google Scholar]
- 19.Savic I, Berglund H. Passive perception of odors and semantic circuits. Hum. Brain Mapp. 2004;21:271–278. doi: 10.1002/hbm.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillert L, Musabasic V, Berglund H, Ciumas C, Savic I. Odor processing in multiple chemical sensitivity. Hum. Brain Mapp. 2007;28:172–182. doi: 10.1002/hbm.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rousseeuw PJ. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987;20:53–65. doi: 10.1016/0377-0427(87)90125-7. [DOI] [Google Scholar]
- 22.Kogami, K. Material Science and Engineering for Perfume and Flavor-technology of Manufacturing and Analysis [Japanese], 105–275 (Chijin Shokan, 1995).
- 23.Mitsuda, M., Iwahashi, T. & Tanamura, T. Fundamentals of Odour and Odour Control, [Japanese], 23–94 (Nikkankougyoushinbunsha, 2018).
- 24.Wyatt TD. The search for human pheromones: The lost decades and the necessity of returning to first principles. Proc. Biol. Sci. 2015;282:20142994. doi: 10.1098/rspb.2014.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornung J, Kogler L, Wolpert S, Freiherr J, Derntl B. The human body odor compound androstadienone leads to anger-dependent effects in an emotional Stroop but not dot-probe task using human faces. PLoS ONE. 2017;12:e0175055. doi: 10.1371/journal.pone.0175055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Japan Health Promotion & Fitness Foundation. Adult Smoking Rates (National Health and Nutrition Survey, Ministry of Health, Labour and Welfare). https://www.health-net.or.jp/tobacco/statistics/kokumin_kenkou_eiyou_report.html (2002).
- 27.Japan Soap Detergent Association. See Import Statistics on Body Soaps and Other Products. https://jsda.org/w/06_clage/4clean_214-1.html. (2002).
- 28.Japan Soap Detergent Association. Liquid Body Soap, A Well-Established Need. https://jsda.org/w/03_shiki/shintaimemo_03.html (2002).
- 29.Hougaard A, Amin FM, Hauge AW, Ashina M, Olesen J. Provocation of migraine with aura using natural trigger factors. Neurology. 2013;80:428–431. doi: 10.1212/WNL.0b013e31827f0f10. [DOI] [PubMed] [Google Scholar]
- 30.Ashina M, et al. Migraine: Integrated approaches to clinical management and emerging treatments. Lancet. 2021;397:1505–1518. doi: 10.1016/S0140-6736(20)32342-4. [DOI] [PubMed] [Google Scholar]
- 31.Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 32.Katsuki M, et al. Questionnaire-based survey on the prevalence of medication-overuse headache in Japanese one city-Itoigawa study. Neurol. Sci. 2022;43:3811–3822. doi: 10.1007/s10072-021-05831-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsuki M, et al. Questionnaire-based survey on the prevalence of elderly's migraine, chronic daily headache, and medication-overuse headache in Japanese one city: Itoigawa Hisui Study. J. Clin. Med. 2022;11:4707. doi: 10.3390/jcm11164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its Supplementary Information files].