Abstract
Objective:
The study’s objectives were to examine rates of severe maternal morbidity (SMM) over a 10-year period and assess racial/ethnic disparities in SMM among insured women in a large, integrated health care system in Southern California.
Methods:
We included Kaiser Permanente Southern California (KPSC) health plan members who gave birth at ≥20 weeks’ gestation in a KPSC-owned hospital during 2008–2017. An SMM case was defined as presence of one or more indicators of an SMM event during a birth hospitalization, identified using maternal electronic health records. Crude SMM rates/10,000 births were calculated by year and maternal race/ethnicity. Modified Poisson regression models were used to assess the association between race/ethnicity and SMM adjusted for other maternal demographics, pregnancy characteristics, and preexisting conditions.
Results:
We identified 5,915 SMM cases among 335,310 births. Crude SMM rates increased from 94.7 per 10,000 in 2008 to 192.6 in 2015 and 249.5 in 2017. Non-Hispanic Black (adjusted risk ratio [aRR] 1.52; 95% confidence interval [CI] 1.37–1.69), Asian/Pacific Islander (aRR 1.29, 95% CI 1.18–1.41), and Hispanic (aRR 1.18, 95% CI 1.10–1.27) women had greater likelihood of SMM than non-Hispanic White women. After further adjusting for preexisting health conditions, differences in SMM by race/ethnicity remained.
Conclusions:
SMM rates increased during 2008–2017 and women of racial and ethnic minority groups, particularly non-Hispanic Black women, were more likely to experience an SMM event than non-Hispanic White women. Multilevel approaches to understanding structural and social factors that may be associated with racial and ethnic disparities in SMM are needed to develop and test effective interventions to reduce SMM.
Severe maternal morbidity (SMM) comprises serious, unexpected consequences of labor and delivery (birth) that can result in both short- and long-term adverse outcomes, including mortality (American College of Obstetrics and Gynecologists and the Society for Maternal-Fetal Medicine et al., 2016; Centers for Disease Control and Prevention, 2018a, 2018b; Creanga et al., 2014; Soma-Pillay et al., 2018; Zanardi et al., 2019). Rates of SMM in the United States have increased steadily over the past 15 years, resulting in increased medical needs, costs, risk for SMM during subsequent births, and longer hospitalizations (Callaghan et al., 2012; Centers for Disease Control and Prevention, 2018a; Fingar et al., 2018; Leonard, Main, Scott, et al., 2019). Studies have attributed increased rates of SMM to increased rates of obstetric risk factors, including maternal age (Fingar et al., 2018; Martin et al., 2017), pre-pregnancy obesity (Fisher et al., 2013; Masters et al., 2018), preexisting medical conditions (Campbell et al., 2013), and cesarean birth (Barber et al., 2011; Leonard, Main, & Carmichael, 2019; Martin et al., 2017; Reid & Creanga, 2018).
SMMs disproportionately affect women of racial and ethnic minority groups. Although, on average, non-Hispanic Black women are younger when giving birth than their non-Hispanic White counterparts, their rates of complications during the labor and birth processes, including SMM, are significantly higher (Booker et al., 2018; Brown et al., 2021; Fingar et al., 2018; Reid & Creanga, 2018; Shen et al., 2005; Wang et al., 2020). Higher risk of SMM for women of color has been attributed to differences in prevalence of chronic conditions and social determinants of health (Wang et al., 2020), such as income, lower neighborhood socioeconomic status (SES), and insurance status (Creanga et al., 2014; Lindquist et al., 2015; Reid & Creanga, 2018); structural issues such as hospital quality and structural racism (Attanasio & Hardeman, 2019; Howell et al., 2016; Reid & Creanga, 2018); and individual experiences of racism including clinician bias and medical mistrust (Armstrong et al., 2008; Attanasio & Hardeman, 2019; Davis et al., 2012; Hoffman et al., 2016; Landrine & Klonoff, 2001; LaVeist et al., 2009; Oakley et al., 2018). As such, women of color may have less access to care, or less access to quality care, resulting in worse pre-pregnancy health and higher rates of SMM (Admon et al., 2017; Fingar et al., 2018; Howell et al., 2020; Reid & Creanga, 2018; Wang et al., 2020).
We conducted this study among persons giving birth in a large integrated health care system to reduce some of the potential health care system variability in contributors to SMM, such as differences in type and quality of care, access to care, and insurance source. In an integrated system, care is based on common clinical practice guidelines and policies and is coordinated through an electronic health record (EHR) that helps to align care in primary and specialty care settings to facilitate both consistency and continuity of care, including comprehensive information about pregnancy and birth. This is different from a fee-for-service system in which primary care, obstetrical care, and specialty care may be performed by clinicians in different practices that do not share a common EHR; thus, patient health records, including laboratory test results and medication lists, cannot be reviewed in real time outside each discrete system. In addition, members of the integrated health care system receive care from the same health care providers at the same facilities, regardless of insurance payer and without pre-approvals. Although some differences among hospitals and health care providers exist across this large system, the health care system aims to provide the same quality of care to all members. In addition, because care is standardized throughout the system, new treatment and practice recommendations can be quickly adopted. To date, SMM rates and racial/ethnic disparities have not been examined in integrated health care systems. Therefore, we examined rates of SMM over a 10-year period to assess trends and racial/ethnic disparities among women giving birth in a large, integrated health care system in Southern California.
Methods
Kaiser Permanente Southern California (KPSC) is a large, integrated health care system providing comprehensive medical and preventive care to 4.4 million members (in 2017) across eight counties. The study cohort included women with one or more births at ≥20 weeks’ gestation (live or stillborn) during January 1, 2008, to December 31, 2017, in any of 12 KPSC-owned hospitals. Although we have used the term “women” throughout this paper to identify individuals in our birth cohort, we recognize that this cohort is diverse and may include individuals who do not identify as women. The unit of analysis is a birth hospitalization, allowing for multiple birth hospitalizations to one woman throughout the study period.
Clinical data were from maternal EHRs; 334,082 (99.6%) of these records were linked with infant birth certificate(s) associated with the birth hospitalization. Maternal demographic data included age at birth (calculated from date of birth and date of birth), race/ethnicity, education, and country of birth from the infant birth certificate, and pre-pregnancy body mass index (BMI) from measured height and weight in the EHR (Centers for Disease Control and Prevention, 2020). The insurance payer (Medicaid or Medicare, employer, or private payer) was included as a proxy for social disadvantage. In addition, we used median neighborhood incomes for the census block of the maternal address at time of birth hospitalization, divided into tertiles, as an indicator of SES. Maternal race/ethnicity was obtained from infant birth certificates to increase the likelihood of self-reported racial/ethnic identity (Klinger et al., 2015). Race/ethnicity categories included non-Hispanic Black, non-Hispanic White, Asian/Pacific Islander, and Hispanic, and we combined the multiple race, other race, and unknown race/ethnicity categories into one. Pregnancy factors included previous births, multiple-gestation birth, previous cesarean birth, gestational weight gain (Moore Simas et al., 2013), preterm birth, and timeliness (prenatal care initiation in the first trimester) and adequacy (number of prenatal visits relative to prenatal care initiation) of prenatal care (Kotelchuck, 1994). Birth information included year, hospital, and birth method. An SMM case was defined as the presence of one or more of the 21 Centers for Disease Control and Prevention (CDC)-defined SMM indicators classified by the International Classification of Diseases (ICD), Ninth Revision (ICD-9) and 10th Revision (ICD-10) diagnosis and procedure codes (Centers for Disease Control and Prevention, 2018b) and Current Procedural Terminology and Health care Common Procedure Coding System codes during a birth hospitalization (Appendix A).
Rates of SMM per 10,000 births were calculated by year. Cumulative percentage change in the rate of SMM was calculated from 2008 to 2015 (years using ICD-9 codes) and from 2016 to 2017 (years using ICD-10 codes). Previous research has reported increased rates of blood transfusion as a main driver of increased rates of SMM (Callaghan et al., 2010;Centers for Disease Control and Prevention, 2018a; Fingar et al., 2018; Leonard, Main, Scott, et al., 2019; Reid & Creanga, 2018). Identifying blood transfusions through ICD codes alone, without any information about the number of blood products given, may overestimate SMM based on blood transfusion (Main et al., 2016; Snowden et al., 2021). Therefore, we examined SMM rates with and without the inclusion of the blood transfusion indicator by race/ethnicity and identified pivot points in the slope of the trend line to identify periods in which the trend of the SMM rate changed. We calculated average annual percent change (AAPC) across the entire study period if no point was identified and on either side of the point if a join point was identified. Trend analyses were conducted using JoinPoint software (Statistical Methodology and Applications Branch, 2020).
We assessed the distribution of maternal demographics, pregnancy factors, and birth information for all births in the cohort and stratified by SMM and calculated unadjusted risk ratios (RRs) with 95% confidence intervals (CIs). Most SMM events were rare and some women had multiple births during the study period. Therefore, we assessed the association of race/ethnicity with SMM using unadjusted and adjusted modified Poisson regression models allowing for repeated measures of multiple births during the study period by one woman, or clustering at the patient level. We conducted post hoc pairwise comparisons of all race/ethnicity categories to compare the aRR in each category with all others. In addition, we conducted a sensitivity analysis after excluding women whose insurance was paid for by Medicaid/Medicare to determine if the inclusion of these lower-income women affected the results of the analysis.
To explore whether preexisting medical conditions accounted for racial/ethnic difference in SMM risk, we conducted a sub-analysis including women with health plan membership for at least 6 months before pregnancy to allow opportunities for medical encounters from which preexisting conditions could be ascertained. Preexisting conditions were identified from 6 months before pregnancy through 12 weeks’ gestation and categorized as 0, 1, or ≥2 conditions (Appendix B). Information from the EHR from 2007 were available for women who gave birth in 2008.
For all models, the missing indicator method (Groenwold et al., 2012) was used to avoid listwise deletion of observations because of missing data on covariates. Modeling was performed using SAS statistical software, version 9.4 (SAS Institute, Inc., Cary, North Carolina). This study was reviewed and approved by KPSC Institutional Review Board (IRB) and was determined by Centers for Disease Control and Prevention to not require IRB approval.
Results
This study included 335,310 births from 260,901 women. Among these births, mean maternal age at birth was 29.9 years (SD = 5.8) and the racial/ethnic distribution was 50.7% Hispanic, 25.0% non-Hispanic White, 7.8% non-Hispanic Black, 13.7% Asian/Pacific Islander, and 2.8% multiple/other/unknown race/ethnicity (Table 1). At least one SMM indicator was identified in 1.8% (5,915) of the births. Prevalence of any SMM among these births increased from 94.7 per 10,000 in 2008 to 192.6 in 2015, a 103% increase over 8 years and to 210.7 and 249.5 in 2016 and 2017, respectively (Table 2).
Table 1.
Maternal, Pregnancy Risk, and Birth Characteristics of Women With and Without Indicators of a Severe Maternal Morbidity (SMM) During Their Birth Hospitalization, Kaiser Permanente Southern California, 2008–2017
| Characteristic | All Births |
Births With SMM |
Births Without SMM |
Unadjusted Risk Ratio |
Adjusted Risk Ratio |
|---|---|---|---|---|---|
| n (col %) | n (col %) | n (col %) | (95% CI) | (95% CI) | |
|
| |||||
| Total, row % | 335,310 (100) | 5,915 (1.8) | 329,395 (98.2) | ||
| Maternal characteristics | |||||
| Maternal age, years | |||||
| Mean ± SD | 29.9 ± 5.8 | 30.6 ± 6.2 | 29.8 ± 5.7 | ||
| <25 | 63,122 (18.8) | 1,088 (18.4) | 62,034 (18.8) | 1.08 (1.01–1.16) | 1.00 (0.93–1.08) |
| 25–34 | 198,938 (59.3) | 3,164 (53.5) | 195,774 (59.4) | Reference | Reference |
| 35+ | 73,250 (21.8) | 1663 (28.1) | 71587 (21.7) | 1.43 (1.34–1.51) | 1.32 (1.24–1.40) |
| Race/Ethnicity | |||||
| Non-Hispanic White | 83,902 (25.0) | 1,245 (21.0) | 82,657 (25.1) | Reference | Reference |
| Non-Hispanic Black | 26,080 (7.8) | 657 (11.1) | 25,423 (7.7) | 1.69 (1.54–1.86) | 1.52 (1.37–1.69) |
| Asian/Pacific Islander* | 45,983 (13.7) | 912 (15.4) | 45,071 (13.7) | 1.34 (1.23–1.46) | 1.29 (1.18–1.41) |
| Hispanic | 169,982 (50.7) | 2,893 (48.9) | 167,089 (50.7) | 1.15 (1.07–1.22) | 1.18 (1.10–1.27) |
| Multiple, Other, Unknown† | 9,363 (2.8) | 208 (3.5) | 9,155 (2.8) | 1.50 (1.29–1.73) | 1.26 (1.09–1.47) |
| Maternal Education | |||||
| High school or less | 101,619 (30.3) | 1,659 (28.9) | 99,960 (31.0) | 0.91 (0.86–0.97) | 1.03 (0.95–1.11) |
| Some college | 102,974 (30.7) | 1,862 (32.5) | 101,112 (31.3) | 1.01 (0.95–1.08) | 1.09 (1.02–1.17) |
| College or more | 123,888 (36.9) | 2,210 (38.6) | 121,678 (37.7) | Reference | Reference |
| Missing | 6,829 (2.0) | 184 | 6,645 | ||
| Country of Birth | — | ||||
| Other country | 95,992 (28.6) | 1,739 (29.8) | 94,253 (28.7) | 1.05 (0.99–1.11) | |
| United States | 237,809 (71.2) | 4,105 (70.2) | 233,704 (71.3) | Reference | |
| Missing | 1,509 (0.5) | 71 | 1,438 | ||
| Pre-Pregnancy body mass index‡ | |||||
| Normal and underweight | 152,859 (45.6) | 2,701 (46.1) | 150,158 (46.0) | Reference | Reference |
| Overweight | 92,959 (27.7) | 1,598 (27.3) | 91,361 (28.0) | 0.97 (0.92–1.04) | 0.96 (0.90–1.02) |
| Obesity | 86,664 (25.8) | 1,561 (26.6) | 85,103 (26.1) | 1.02 (0.96–1.08) | 0.92 (0.86–0.98) |
| Missing | 2,828 (0.8) | 55 | 2,773 | ||
| Insurance type | |||||
| Medicaid and Medicare§ | 17,116 (5.1) | 394 (6.7) | 16,722 (5.1) | 1.33 (1.20–1.47) | 1.26 (1.13–1.39) |
| Other | 318,194 (94.9) | 5,521 (93.3) | 312,673 (94.9) | Reference | Reference |
| Median neighborhood income (yearly) | |||||
| Low (<$48,658) | 110,196 (32.9) | 2,060 (34.9) | 108,136 (32.9) | 1.09 (1.03–1.16) | 1.02 (0.95–1.09) |
| Medium ($48,658–$69,525) | 111,745 (33.3) | 1,922 (32.5) | 109,823 (33.4) | 1.01 (0.94–1.07) | 1.01 (0.94–1.08) |
| High (>$69,525) | 112,636 (33.6) | 1,925 (32.6) | 110,711 (33.7) | Reference | Reference |
| Missing | 733 (0.2) | 8 | 725 | ||
| Pregnancy factors | |||||
| Parity | |||||
| 0 | 139,783 (41.7) | 2,883 (48.9) | 136,900 (41.6) | 1.47 (1.39–1.56) | 1.83 (1.71–1.95) |
| 1 | 113,863 (34.0) | 1,586 (26.9) | 112,277 (34.1) | Reference | Reference |
| 2+ | 81,360 (24.3) | 1,432 (24.3) | 79,928 (24.3) | 1.26 (1.18–1.35) | 1.22 (1.14–1.32) |
| Missing | 304 (0.1) | 14 | 290 | ||
| Multiple-gestation birth | |||||
| No | 329,340 (98.2) | 5,483 (92.7) | 323,857 (98.3) | Reference | Reference |
| Yes | 5,970 (1.8) | 432 (7.3) | 5,538 (1.7) | 4.33 (3.93–4.76) | 3.50 (3.15–3.88) |
| Previous cesarean delivery | |||||
| No | 288,262 (86.0) | 4,729 (80.5) | 283,533 (86.3) | Reference | Reference |
| Yes | 46,333 (13.8) | 1,147 (19.5) | 45,186 (13.7) | 1.49 (1.40–1.59) | 1.86 (1.72–2.00) |
| Missing | 715 (0.2) | 39 | 676 | ||
| Gestational weight gain∥ | |||||
| Inadequate | 88,183 (26.3) | 1,700 (29.0) | 86,483 (26.5) | 1.13 (1.06–1.21) | 1.08 (1.01–1.15) |
| Appropriate | 104,492 (31.2) | 1,780 (30.4) | 102,712 (31.5) | Reference | Reference |
| Excess | 139,714 (41.7) | 2,377 (40.6) | 137,337 (42.1) | 1.00 (0.94–1.06) | 0.99 (0.93–1.05) |
| Missing | 2,921 (0.9) | 58 | 2,863 | ||
| Preterm birth | — | ||||
| Preterm | 29,246 (8.7) | 1,318 (22.3) | 27,928 (8.5) | 2.99 (2.81–3.17) | |
| Term or term+ | 306,064 (91.3) | 4,597 (77.7) | 301,467 (91.5) | Reference | |
| Timeliness of prenatal care | — | ||||
| Yes | 291,045 (86.8) | 5,099 (87.9) | 285,946 (87.8) | Reference | |
| No | 40,458 (12.1) | 705 (12.1) | 39,753 (12.2) | 0.99 (0.92–1.07) | |
| Missing | 3,807 (1.1) | 111 | 3,696 | ||
| Prenatal care adequacy | |||||
| Inadequate | 23,284 (6.9) | 400 (6.9) | 22,884 (7.0) | 1.16 (1.05–1.29) | 1.07 (0.96–1.19) |
| Intermediate | 46,523 (13.9) | 699 (12.1) | 45,824 (14.1) | 1.02 (0.94–1.11) | 1.00 (0.92–1.08) |
| Adequate | 167,518 (50.0) | 2,465 (42.6) | 165,053 (50.7) | Reference | Reference |
| Adequate+ | 93,749 (28.0) | 2,226 (38.4) | 91,523 (28.1) | 1.61 (1.52–1.70) | 1.38 (1.29–1.46) |
| Missing | 4,236 (1.3) | 125 | 4,111 | ||
| Birth information¶ | |||||
| Year of birth hospitalization | |||||
| 2008 | 24,504 (7.3) | 232 (3.9) | 24,272 (7.4) | Reference | Reference |
| 2009 | 30,154 (9.0) | 339 (5.7) | 29,815 (9.1) | 1.19 (1.01–1.41) | 1.19 (1.01–1.40) |
| 2010 | 30,187 (9.0) | 428 (7.2) | 29,759 (9.0) | 1.50 (1.28–1.76) | 1.47 (1.26–1.73) |
| 2011 | 32,137 (9.6) | 525 (8.9) | 31,612 (9.6) | 1.73 (1.49–2.02) | 1.67 (1.43–1.94) |
| 2012 | 34,091 (10.2) | 591 (10.0) | 33,500 (10.2) | 1.84 (1.58–2.14) | 1.77 (1.52–2.06) |
| 2013 | 34,170 (10.2) | 574 (9.7) | 33,596 (10.2) | 1.79 (1.53–2.08) | 1.75 (1.50–2.04) |
| 2014 | 35,270 (10.5) | 722 (12.2) | 34,548 (10.5) | 2.17 (1.87–2.51) | 2.17 (1.87–2.51) |
| 2015 | 36,868 (11.0) | 710 (12.0) | 36,158 (11.0) | 2.05 (1.77–2.37) | 2.07 (1.78–2.40) |
| 2016 | 38,767 (11.6) | 817 (13.8) | 37,950 (11.5) | 2.23 (1.93–2.58) | 2.24 (1.93–2.59) |
| 2017 | 39,162 (11.7) | 977 (16.5) | 38,185 (11.6) | 2.64 (2.29–3.04) | 2.64 (2.29–3.05) |
| Birth method | |||||
| Vaginal | 233,607 (69.7) | 2,476 (42.0) | 231,131 (70.2) | Reference | — |
| Cesarean-primary | 59,869 (17.9) | 2,335 (39.6) | 57,534 (17.5) | 3.66 (3.46–3.87) | |
| Cesarean-repeat | 41,482 (12.4) | 1,085 (18.4) | 40,397 (12.3) | 2.47 (2.30–2.65) | |
| Missing | 352 (0.1) | 19 | 333 | ||
Asian women constitute 97.7% of category; Pacific Islander women constitute 2.3% of category.
For the other/multiple/unknown race/ethnicity category, 352 (0.11%) were American Indian Alaska Native, 118 (0.04%) other race, 6,420 (1.91%) multiple race, and 2,463 (0.73%) were unknown race/ethnicity.
Categorization from Centers for Disease Control and Prevention. About Adult BMI. 2020; https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html#Why, 2020.
0.48% of the women on Medicaid also had Medicare; this category also includes 1.27% who had Medicare only.
Categorization from: Moore Simas T, Waring M, Sullivan G, et al. Institute of Medicine (2009) gestational weight gain guideline knowledge: Survey of Obstetrics/Gynecology and Family Medicine residents of the United States. Birth. 2013; 40(4):237–246.
Specific hospital was also included in the adjusted model as part of birth information but was excluded here for anonymity.
Table 2.
Overall Rate of Severe Maternal Morbidity (SMM) During Birth Hospitalizations Among Kaiser Permanente Southern California Members Giving Birth by Year, 2008–2017
| SMM Classification | Rate per 10,000 Births | Cumulative Percent Change in Rate From 2008 to 2015 | Rate per 10,000 Births | Cumulative Percent Change in Rate From 2016 to 2017 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |||
|
| ||||||||||||
| Any SMM | 94.7 | 112.4 | 141.8 | 163.4 | 173.4 | 168 | 204.7 | 192.6 | 103% | 210.7 | 249.5 | 18% |
| Any SMM excluding blood transfusion | 47.3 | 46.4 | 60.6 | 57.3 | 74.8 | 68.5 | 91.3 | 87.3 | 85% | 116.3 | 156.8 | 35% |
A linear model without a pivot point was the best fit for the overall (all race/ethnicities combined) SMM rate and for births among non-Hispanic White, Asian/Pacific Islander, and Hispanic women (Figure 1A). For non-Hispanic Black women, a significant pivot point was identified in 2010 after which the AAPC continued to increase, but less steeply. However, non-Hispanic Black women’s overall rate remained higher than women of other racial/ethnic groups. After excluding blood transfusion (Figure 1B), pivot points were identified for the overall rate and for Hispanic women in 2015, both of which had steeper increases after the 2015 pivot point when the switch to ICD-10 codes occurred. No significant pivot point was found for non-Hispanic Black women. In both models (total SMM and SMM excluding blood transfusion), non-Hispanic Black women experienced the highest rate of SMM for each year.
Figure 1.
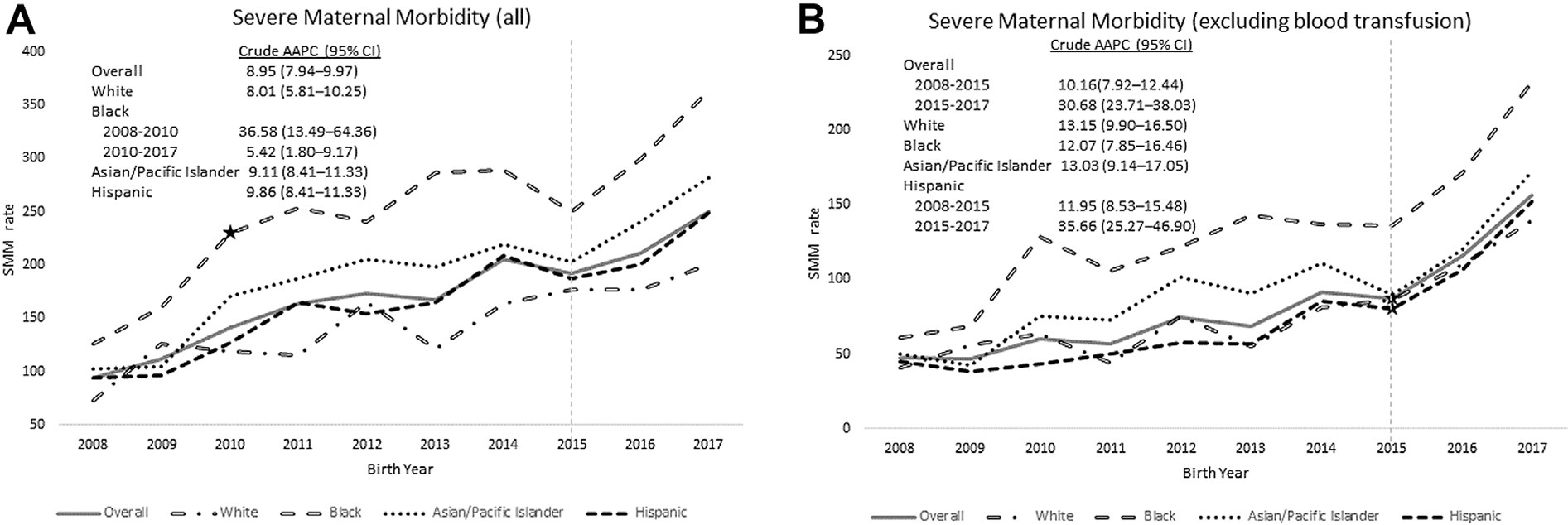
Trends in severe maternal morbidity (SMM) rate per 10,000 births overall and by race/ethnicity for (A) all SMM indicators and (B) all indicators excluding blood transfusion, Kaiser Permanente Southern California, 2008–2017. Note: ★Star indicates significant change point (joinpoint) in the trend line.
For all years combined, births to women who were non-Hispanic Black, Asian/Pacific Islander, Hispanic, or multiple/other/unknown race each had higher SMM rates than births to non-Hispanic White women (148 of 10,000 births). Non-Hispanic Black women (252 of 10,000 births) had a higher rate of SMM than all other racial/ethnic groups (Table 3). Likewise, unadjusted relative risks for women of color were all significantly greater, compared with non-Hispanic White women, and the relative risk was highest for non-Hispanic Black women (RR 1.69; 95% CI 1.54–1.86; Table 1). Because maternal country of birth and timeliness of prenatal care initiation were not significant in bivariate analyses, and preterm birth and birth method could be both a predictor and a consequence of an SMM, these variables were not included in the final multivariable models. After adjustment, all women of color had a significantly higher risk of having an SMM than non-Hispanic White women; non-Hispanic Black women had a 52% greater risk of SMM (aRR 1.52; 95% CI 1.37–1.69), compared with non-Hispanic White women. In addition, in post hoc pairwise comparisons, non-Hispanic Black women had significantly higher risk for SMM compared with all other women (data not shown). Results did not differ after excluding 17,116 births by women who had Medicaid/Medicare-paid insurance (data not shown).
Table 3.
Rates of Severe Maternal Morbidity (SMM) per 10,000 Birth at the Birth Hospitalization by Race/Ethnicity Among Kaiser Permanente Southern California Members, 2008–2017
| Race/Ethnicity | SMM Rate per 10,000 Births |
Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| No. Births With SMM | No. of Total Births | Rate | 95% CI | |||
|
| ||||||
| Main analysis cohort | ||||||
| Non-Hispanic White | 1,245 | 83,902 | 148 | 140–157 | Reference | Reference |
| Non-Hispanic Black | 657 | 26,080 | 252 | 233–271 | 1.69 (1.54,1.86)‡ | 1.52 (1.37,1.69)‡ |
| Asian/Pacific Islander | 912 | 45,983 | 198 | 186–211 | 1.34 (1.23,1.46)‡ | 1.29 (1.18,1.41)‡ |
| Hispanic | 2,893 | 169,982 | 170 | 164–176 | 1.15 (1.07,1.22)‡ | 1.18 (1.10,1.27)‡ |
| Multiple Race, Other Race, Unknown Race/Ethnicity | 208 | 9,363 | 222 | 192–252 | 1.50 (1.29,1.73)‡ | 1.26 (1.09,1.47)† |
| Sub-analysis cohort* | ||||||
| Non-Hispanic White | 924 | 62,146 | 149 | 139–158 | Reference | Reference |
| Non-Hispanic Black | 511 | 19,207 | 266 | 243–289 | 1.79 (1.60,1.99)‡ | 1.57 (1.40,1.77)‡ |
| Asian/Pacific Islander | 681 | 33,458 | 204 | 188–219 | 1.37 (1.24,1.51)‡ | 1.31 (1.18,1.45)‡ |
| Hispanic | 2,134 | 122,960 | 174 | 166–181 | 1.17 (1.08,1.26)‡ | 1.21 (1.11,1.32)‡ |
| Multiple, Other, Unknown | 158 | 6,723 | 235 | 199–271 | 1.58 (1.34,1.87)‡ | 1.29 (1.09,1.54)† |
Sub-analysis cohort was restricted to the subset of women with health plan membership for at least 6 months before pregnancy.
p < .01.
p < .001.
In the multivariable model, women aged ≥35 years (aRR 1.32; 95% CI 1.24–1.40, compared with those 25–34 years), and those with Medicaid insurance (aRR 1.26; 95% CI 1.13–1.39), no previous births (aRR 1.83; 95% CI 1.71–1.95) or ≥2 previous births (aRR 1.22; 95% CI 1.14–1.32), multiple-gestation pregnancy (aRR 3.50; 95% CI 3.15–3.88), and history of cesarean birth (aRR 1.86; 95% CI 1.72–2.00) were more likely to experience an SMM (Table 1). Maternal pre-pregnancy overweight or obesity or excess gestational weight gain were not associated with SMM.
The sub-analysis cohort (health plan membership for at least 6 months before pregnancy) was older than the main cohort, with 24.0% of the cohort older than 35 years compared with 16.1% in the main cohort, but did not significantly differ by SMM rate, race/ethnicity, insurance type, median neighborhood income, or maternal education. Among births in the sub-analysis cohort (n = 244,494), 12.4% had one and 1.8% had two or more preexisting conditions. After adjusting for maternal demographics, pregnancy factors, birth information, and preexisting conditions, non-Hispanic Black women still had a higher rate of SMM (266 of 10,000 births) than all other racial/ethnic groups (Table 3). There were no significant changes in aRRs between the sub-analysis and main cohorts by race/ethnicity (data not shown). Similar to the main cohort, in post hoc pairwise comparisons, non-Hispanic Black women had significantly higher risk for SMM compared with all other women after adjustment for preexisting conditions (data not shown).
Discussion
We found significant differences in rates of SMM at the birth hospitalization among women of color, particularly between non-Hispanic Black women and non-Hispanic White women, in this study of women who were insured and had their birth in a large integrated health care system. Overall, we found increasing rates of SMM events across the study period, similar to increases noted in other studies (Creanga et al., 2014; Fingar et al., 2018). Compared with non-Hispanic White women, rates of SMM ranged from 18% higher for Hispanic women to 52% higher for Black women. Increased rates of SMM for women of color reported in this study are similar to rates found in other studies (Brown et al., 2021; Creanga et al., 2014; Leonard, Main, Scott, et al., 2019). This study contributes to our understanding of racial/ethnic disparities in SMM by demonstrating that these disparities are evident after adjusting for preexisting and pregnancy-related health conditions, health system factors, and self-reported maternal demographics. The findings suggest broad and multilevel influences on maternal health, resulting in racial/ethnic disparities, may be at play.
The complex nature and history of race and racism in the United States affects women’s health via multiple intersecting levels of society. Multilevel influences of individual, interpersonal, organizational, community, and societal/political factors all play a role in individual development and health behaviors and outcomes (Bronfenbrenner, 1986; Centers for Disease Control and Prevention, 2022; Granade et al., 2022; Lin et al., 2022; Wilson et al., 2021). At the individual level, a lifetime of exposure to racism, which is commonly experienced by women of color (Ertel et al., 2012), in addition to the intersectionality of discrimination and marginalization based on both race and gender (Rosenthal & Lobel, 2011), can lead to cumulative stress contributing to poor health (Lewis et al., 2019) and could play a role in racial/ethnic disparities in birth outcomes, including SMM (Lu & Halfon, 2003).
Other individual-level factors (e.g., education, insurance, income, preexisting conditions, obesity) have all been associated with an increased risk of SMM (Campbell et al., 2013; Creanga et al., 2014; Fingar et al., 2018; Fisher et al., 2013; Lindquist et al., 2015; Martin et al., 2017; Masters et al., 2018; Reid & Creanga, 2018; Wang et al., 2020). Unlike previous studies (Campbell et al., 2013; Fisher et al., 2013; Hinkle et al., 2012; Masters et al., 2018), we did not find a significant effect of pre-pregnancy obesity or gestational weight gain on SMM risk. This study used measured height and weight from the EHR instead of relying on diagnostic codes, which likely increased measurement accuracy for these covariates. By using EHR data, we evaluated whether preexisting conditions were drivers of SMM and racial/ethnic disparities, something that may not have been possible in studies that used claims data from birth hospitalizations or data from birth certificates only.
Previous studies have attributed higher rates of SMM among women insured by Medicaid (Fingar et al., 2018) to differences in health care quality for Medicaid-insured women (Brown et al., 2021; Epstein et al., 2012; Gardner & Vishwasrao, 2010; Geissler et al., 2016). However, in a recent study, Brown and colleagues reported that, among Black women, there were no differences in SMM risk for those insured by Medicaid compared with those with other forms of insurance, suggesting care funded by Medicaid was not the most prominent risk factor (Brown et al., 2021) and that the Medicaid payer variable is, instead, measuring the effects of structural racism, socioeconomic factors, and other barriers to high-quality health care.
All women in this study were KPSC members, regardless of payer source, and were treated at the same 12 hospitals, by the physicians from the same medical group. Clinicians do not have access to payer source and are, therefore, less likely to bring Medicaid payer bias into decision making. However, other aspects of stigma and discrimination based on race, ethnicity, age, perceived SES, or having obesity, as examples, may still play a role in patient care and SMM outcomes. We found that women whose births were paid by Medicaid or Medicare had statistically higher SMM rates than those with other payer sources. As suggested by previous research, payer source represents more than the inability to obtain high-quality health care and may, in addition, be a proxy for social disadvantage and represent other social determinants that contribute to psychological and physiological risk factors of SMM (Brown et al., 2021; Howell et al., 2020).
A significant body of research demonstrates the complex and multifaceted factors that contribute to racial/ethnic disparities in health and recognizes the influence of structural racism and experiences of minority stress, perceived discrimination, and bias on medical mistrust (Bogart et al., 2010; Landrine & Klonoff, 2001; Oakley et al., 2018), health outcomes (Carter et al., 2017; Korous et al., 2017; Mouzon et al., 2017), and the utilization of health care and clinical care experiences (Davis et al., 2012; LaVeist et al., 2009; Lu & Halfon, 2003; Weech-Maldonado et al., 2012), specifically in maternal health care (Attanasio & Hardeman, 2019). Negative, stigmatizing interactions with health care providers (Mehra et al., 2020), as well as positive, affirming experiences (Benkert & Peters, 2005) have effects on women’s use of and satisfaction with health care, ultimately influencing their health and health outcomes. It is possible that unmeasured aspects of interpersonal relationships between women and their health care providers influenced their utilization of prenatal care or their experiences during labor and birth, which resulted in racial/ethnic disparities in SMM in this study.
In previous literature, structural influences and facility-level factors such as hospital quality, differences in care between hospitals, and structural racism experienced in different medical settings have contributed to adverse birth outcomes, including racial/ethnic disparities in SMM (Attanasio & Hardeman, 2019; Howell et al., 2016; Reid & Creanga, 2018; Wang et al., 2020; Zanardi et al., 2019). However, a strength of the present study is that all women received care in the same integrated health care system and gave birth in hospitals following the same policies and care management guidelines. This may have reduced some of the differences in health care delivery that may have influence on SMM. In addition, we controlled for hospital of birth in our adjusted analyses.
This study has several potential limitations. The study period covers the transition from ICD-9 to ICD-10 diagnosis and procedure codes. Disruptions in observed rates of SMM related to the coding transition or coding errors (Utter et al., 2019) might have affected our trend analysis. In addition, the change point in 2015 was only significant for the overall sample and Hispanic women, although the trend increased for all women. It is possible that the other racial/ethnic groups were underpowered to identify a significant change in the trend. Recent studies have called for validation of ICD-10 codes for SMM (Metcalfe et al., 2021). Previous research has demonstrated additional SMM incidence and risk for women with stillborn births (Chen et al., 2020; Wall-Wieler et al., 2019); however, we did not have a sufficient number of fetal deaths to analyze them separately. In line with previous research (Campbell et al., 2013; Creanga et al., 2014; Fingar et al., 2018; Howell et al., 2020; Leonard, Main, Scott, et al., 2019; Wall-Wieler et al., 2019), we identified SMM during birth hospitalizations. However, a recent analysis (Chen et al., 2020) found approximately 10% of births in the western region of the United States had SMM present after discharge. Therefore, the true rate of SMM in our cohort may be higher when post-discharge events are included. To take advantage of the breadth of data in the EHRs, we incorporated CPT codes to supplement ICD codes, potentially reducing the comparability of our study to other studies that could not include CPT codes.
Because of the small number of women reporting being Native American, multiple races, or other race, or for whom we had no information to characterize their race or ethnicity, we were unable to separately characterize these women’s SMM experiences in our study. Last, our findings may not be generalizable to all women giving birth.
Strengths of this study include the linkage of infant birth certificates with robust clinical information from EHRs, which allowed us to adjust for information often unavailable in administrative, hospital discharge, or claims datasets. The depth of the EHR data allowed for a more comprehensive and accurate capture of labor and birth information and outcomes as well as important clinical and demographic covariates. The use of maternal race and ethnicity from infant birth certificates rather than from administrative data increased the likelihood that they were self-reported and thus more likely to represent the mother’s identity (Klinger et al., 2015). Availability of data from women giving birth over multiple years among this diverse population enabled us to analyze trends over a 10-year period. In addition, previous work has shown that KPSC membership demographics are generally representative of the Southern California region (Koebnick et al., 2012).
Implications for Policy and/or Practice
Policymakers and all elements of the health care system must act to mitigate SMM occurring during childbirth. Just as racial/ethnic disparities in SMM are driven by complex, multilevel factors, so must be the practical and policy-based solutions. Multilevel approaches addressing individual and structural racism and discrimination in the care of pregnant persons as well as clinical practice recommendations and public policy are all needed to address the multifaceted nature of SMM disparities; future work should be based on these multilevel determinants of health (Ramey et al., 2015).
In part, SMM disparities might be attributable to a lifetime of interpersonal interactions that women have had with clinicians and the health care system. Clinician communication and bias (Hoffman et al., 2016; Wang et al., 2021), women’s medical mistrust (Armstrong et al., 2008; Landrine & Klonoff, 2001; Oakley et al., 2018), and women’s reduced use of health care services based on previous negative experiences (Attanasio & Hardeman, 2019; Davis et al., 2012; Hoffman et al., 2016; LaVeist et al., 2009; Weech-Maldonado et al., 2012) could all affect health care utilization and quality and lead to poor birth outcomes and SMM. Interventions, programs, and training are needed for clinicians and other health care personnel to address known and unknown racial biases and understand how they might contribute to care delivery and utilization. In 2019, legislation was signed in California that mandated training (SB-464) (SB-464 California Dignity in Pregnancy and Childbirth Act, 2019) and continuing education (AB-241) (AB-241 Implicit bias: continuing education: requirements, 2019) on implicit bias for all physicians and health care personnel. Additional mandatory training and continued education throughout health systems, public health jurisdictions, and medical training could contribute to broader policy impacts on stigma and bias in the health care setting. In addition, encouraging clinicians to talk with their patients about life stressors such as employment, transportation, food insecurity, and experiences with discrimination could help build trust and may help health systems connect patients to public resources (American College of Obstetrics and Gynecologists and the Society for Maternal-Fetal Medicine et al., 2016; Kozhimannil et al., 2016).
Health care systems should investigate drivers of SMM within their own system to identify differences in hospital site, quality of care, and poor outcomes. In line with suggestions of other researchers, health care systems can play a role in mediating the effects of social discrimination and stigma. Interventions could include supportive programming for expectant mothers through support groups, midwifery, and connection to community health workers (Brown et al., 2021; Howell et al., 2016; Mehra et al., 2020). At a policy and society level, much more work is needed in addressing structural challenges in communities of color through community-level interventions and policies aimed at mitigating the sociopolitical structures of racism and oppression in the United States. Research that delves further into social determinants of health and builds on findings from epidemiological and clinical studies to deepen our understanding of the drivers of racial and ethnic disparities in SMM is needed.
Conclusions
Despite progress in reducing SMM in California, we found rates of SMM increased during the 10-year study period overall and by racial and ethnic group. Specifically, women of minoritized racial and ethnic groups, particularly non-Hispanic Black women, were more likely to experience an SMM event than non-Hispanic White women. In a large, integrated health care system in which all members, despite payer source, receive care from the same health care providers at the same facilities—and after taking into account maternal demographic characteristics, pregnancy factors, and birth information, and preexisting health conditions—racial and ethnic disparities in SMM remain. These findings suggest the drivers for increased rates and disparate burden of SMM may go beyond individual risk factors to those of the provider, hospital system, and society at large.
Supplementary Material
Acknowledgments
We appreciate Kaiser Permanente Southern California members for the contribution of their electronic health record information to this study. We additionally acknowledge support by Wences Arvelo, Suzanne Beavers, Byron Robinson, and Elena Kuklina for their contributions to this study.
Funding Statement:
This study was supported by Kaiser Permanente Southern California is the funder for the Kaiser Permanente Community Benefit funds. Dr. Jean Lawrence was funded in part through NIH (1R01ES030353-01A1).
The findings and conclusion in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Supplementary Data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.whi.2023.01.001.
References
- AB-241 Implicit bias: continuing education: requirements. (2019). §An act to amend Sections 2190.1 and 3524.5 of, and to add Section 2736.5 to, the Business and Professions Code, relating to healing arts.
- Admon L, Windleman T, Moniz M, Davis M, Heisler M, & Dalton V (2017). Disparities in chronic conditions among women hospitalized for delivery in the United States, 2005–2014. Obstetrics & Gynecology, 130, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetrics and Gynecologists and the Society for Maternal-Fetal Medicine, Kilpatrick S, & Ecker J (2016). Severe maternal morbidity: Screening and review. American Journal of Obstetrics & Gynecology, 215, B17–B22. [DOI] [PubMed] [Google Scholar]
- Armstrong K, McMurphy S, Dean L, Micco E, Putt M, Halbert C, … Shea J (2008). Differences in patterns of health care system distrust between blacks and whites. Journal of General Internal Medicine, 23, 827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attanasio L, & Hardeman R (2019). Declined care and discrimination during the childbirth hospitalization. Social Science & Medicine, 232, 270–277. [DOI] [PubMed] [Google Scholar]
- Barber E, Lundsberg L, Belanger K, Pettker C, Funai E, & Illuzzi J (2011). Indicators contributing to the increasing cesarean delivery rate. Obstetrics & Gynecology, 118, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkert R, & Peters RM (2005). African American women’s coping with health care prejudice. Western Journal of Nursing Research, 27, 863–889. [DOI] [PubMed] [Google Scholar]
- Bogart L, Wagner G, Galvan F, & Banks D (2010). Conspiracy beliefs about HIV are related to antiretrovial treatment nonadherence among African American men with HIV. Journal of Acquired Immune Deficiency Syndromes, 53, 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker W, Gyamfi-Bennerman C, Sheen J, Wright J, Siddiq Z, D’Alton M, & Friedman A (2018). Maternal outcomes by race for women aged 40 years or older. Obstetrics & Gynecology, 132, 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U (1986). Ecology of the family as a context for human development: Research perspectives. Developmental Psychology, 22, 723–742. [Google Scholar]
- Brown C, Adams C, & Moore J (2021). Race, medicaid coverage, and equity in maternal morbidity. Womens Health Issues, 31, 145–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan W, Creanga A, & Kuklina E (2012). Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstetrics & Gynecology, 120, 1029–1036. [DOI] [PubMed] [Google Scholar]
- Callaghan W, Kuklina E, & Berg C (2010). Trends in postpartum hemorrhage: United States, 1994–2006. American Journal of Obstetrics & Gynecology, 202, 353.e1. [DOI] [PubMed] [Google Scholar]
- Campbell K, Savitz D, Werner E, Pettker C, Goffman D, Chazotte C, & Lipkind HS (2013). Maternal morbidity and risk of death at delivery hospitalization. Obstetrics & Gynecology, 122, 627–633. [DOI] [PubMed] [Google Scholar]
- Carter R, Lau M, Johnson V, & Kirkinis K (2017). Racial discrimination and health outcomes among racial/ethnic minorities: A meta-analytic review. Journal of Multicultural Counseling and Development, 45, 232–259. [Google Scholar]
- Centers for Disease Control and Prevention. (2018a). Severe maternal morbidity in the United States. Available: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html. Accessed: September 9, 2018.
- Centers for Disease Control and Prevention. (2018b). Severe maternal morbidity indicators and corresponding ICD codes during delivery hospitalizations. Available: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm. Accessed: September 9, 2018.
- Centers for Disease Control and Prevention. (2020). About Adult BMI. Centers for Disease Control and Prevention. Available: https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html#Why. Accessed: May 31, 2020. [Google Scholar]
- Centers for Disease Control and Prevention. (2022). The social-ecological model: A framework for prevention. Available: https://www.cdc.gov/violenceprevention/about/social-ecologicalmodel.html. Accessed: August 1, 2022.
- Chen J, Cox S, Kuklina E, Ferre C, Barfield W, & Li R (2020). Assessment of incidence and factors associated with severe maternal morbidity after delivery discharge among women in the US. JAMA Network Open, 4, e2036148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creanga A, Bateman B, Kuklina E, & Callaghan W (2014). Racial and ethnic disparities in severe maternal morbidity: A multistate analysis, 2008–2010. American Journal of Obstetrics & Gynecology, 210, 435.e1. [DOI] [PubMed] [Google Scholar]
- Davis J, Bynum S, Katz R, Buchanan K, & Green B (2012). Sociodemographic differences in fears and mistrust contributing to unwillingness to participate in cancer screenings. Journal of Health Care for the Poor and Underserved, 23, S67–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AJ, Ketcham JD, Rathore SS, & Groeneveld PW (2012). Variations in the use of an innovative technology by payer: the case of drug-eluting stents. Medical Care, 50, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel KA, James-Todd T, Kleinman K, Krieger N, Gillman MW, Wright R, & Rich-Edwards J (2012). Racial discrimination, response to unfair treatment, and depressive symptoms among pregnant Black and African American women in the United States. Annals of Epidemiology, 22, 840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar K, Hambrick M, Heslin K, & Moore J (2018). Trends and disparities in delivery hospitalizations involving severe maternal morbidity, 2006–2015. Agency for Healthcare Research and Quality. Available : https://www.ncbi.nlm.nih.gov/books/NBK532465/. [PubMed] [Google Scholar]
- Fisher S, Kim S, Sharma A, Rochat R, & Morrow R (2013). Is obesity still increasing among pregnant women? Pregnancy obesity trends in 20 states, 2003–2009. Preventive Medicine, 56, 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner L, & Vishwasrao S (2010). Physician quality and health care for the poor and uninsured. Inquiry: The Journal of Health Care Organization, Provision, and Financing, 47, 62–80. [DOI] [PubMed] [Google Scholar]
- Geissler K,H., Lubin B, & Marzilli Ericson KM (2016). Access is not enough: characteristics of physcians who treat Medicaid patients. Medical Care, 54, 350–358. [DOI] [PubMed] [Google Scholar]
- Granade CJ, Lindley MC, Jatlaoui T, Asif AF, & Jones-Jack N (2022). Racial and ethnic disparties in adult vaccination: A review of the state of evidence. Health Equity, 6, 206–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenwold R, White I, Donders A, Carpenter J, Altman D, & Moons K (2012). Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. Canadian Medical Association Journal, 184, 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle S, Sharma A, & Kim S (2012). Pregnancy obesity trends among low-income women, United States, 1999–2008. Maternal and Child Health Journal, 16, 1339–1348. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Trawalter S, Axt J, & Oliver N (2016). Racial bias in pain management and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proceedings of the National Academy of Sciences of the United States of America, 113, 4296–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell E, Egorova N, Balbierz A, Zeitlin J, & Hebert P (2016). Site of delivery contribution to black-white severe maternal morbidity disparity. American Journal of Obstetrics & Gynecology, 215, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell E, Egorova N, Janevic T, Brodman M, Balbierz A, Zeitlin J, & Hebert P (2020). Race and Ethnicity, Medical Insurance, and Within-Hospital Severe Maternal Morbidity Disparities. Obstetrics & Gynecology, 135, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger E, Carlini S, Gonzalez I, St. Hubert S, Linder J, Rigotti N, … Haas J (2015). Accuracy of race, ethnicity, and language preference in an electronic health record. Journal of General Internal Medicine, 30, 719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebnick C, Langer-Dould AM, Gould MK, Chao CR, Iyer RL, & Smith N (2012). Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. The Permanente Journal, 16, 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korous K, Causadia J, & Casper D (2017). Racial discrimination and cortisol output: A meta-analysis. Social Science & Medicine, 193, 90–100. [DOI] [PubMed] [Google Scholar]
- Kotelchuck M (1994). The adequacy of prenatal care utilization index: Its US Distribution and association with low birthweight. American Journal of Public Health, 84, 1486–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhimannil KB, Thao V, Hung P, Tilden E, Caughey AB, & Snowden JM (2016). Association between hospital birth volume and maternal morbidity among low-risk pregnancies in rural, urban, and teaching hospitals in the United States. American Journal of Perinatology, 33, 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrine H, & Klonoff E (2001). Cultural diversity and health psychology. In Baum A, Revenson T, & Singer J (Eds.), Handbook of Health Psychology (pp. 851–891). Mahway, NJ: Erlbaum. [Google Scholar]
- LaVeist T, Isaac L, & Williams K (2009). Mistrust of health care organizations is associated with underutilization of health services. Health Services Research, 44, 2093–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Main E, & Carmichael S (2019). The contribution of maternal characteristics and cesarean delivery to an increasing trend of severe maternal morbidity. BMC Pregnancy and Childbirth, 19, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Main E, Scott K, Profit J, & Carmichael S (2019). Racial and ethnic disparities in severe maternal morbidity prevalence and trends. Annals of Epidemiology, 33, 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Lampert R, Charles D, & Katz S (2019). Expectations of racism and carotid itima-media thickness in African American Women. Psychosomatic Medicine, 81, 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Kolak M, Watts B, Anselin L, Pollack H, Schneider J, & Taylor B (2022). Individual, interpersonal, and neighborhood measures associated with opioid use stigma: Evidence from a nationally representative survey. Social Science & Medicine, 305, 115034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist A, Noor N, Sullivan E, & Knight M (2015). The impact of socioeconomic position on severe maternal morbidity outcomes among women in Australia: a national case-control study. British Journal of Obstetrics and Gynaecology, 122, 1601–1609. [DOI] [PubMed] [Google Scholar]
- Lu MC, & Halfon N (2003). Racial and ethnic disparities in birth outcomes: A life-course perspective. Maternal and Child Health Journal, 7, 13–30. [DOI] [PubMed] [Google Scholar]
- Main EK, Abreo A, McNulty J, Gilbert W, McNally C, Poeltler D, … Kilpatrick S (2016). Measuring severe maternal morbidity: validation of potential measures. American Journal of Obstetrics & Gynecology, 214, 643.e1. [DOI] [PubMed] [Google Scholar]
- Martin J, Hamilton B, Osterman M, Driscoll A, & Matthews T (2017). Births: final data for 2015 National vital statistics reports; v. 66, no. 1; DHHS publication; no. (PHS) 2017–1120. Available: https://stacks.cdc.gov/view/cdc/43595. [PubMed] [Google Scholar]
- Masters HR, Housley E, van Hook JW, & Defranco E (2018). Maternal obesity is an independent risk factor for intensive care unit admission during delivery hospitalization. American Journal of Perinatology, 35, 1423–1428. [DOI] [PubMed] [Google Scholar]
- Mehra R, Boyd LM, Magriples U, Kershaw TS, Ickovics JR, & Keene DE (2020). Black pregnant women “get the most judgment”: A qualitative study of the experiences of Black women at the intersection of race, gender, and pregnancy. Womens Health Issues, 30, 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe A, Sheikh M, & Hetherington E (2021). Impact of the ICD-9-CM to ICD-10-CM transition on the incidence of severe maternal morbidity among delivery hospitalizations in the United States. American Journal of Obstetrics & Gynecology, 225, 422.e1. [DOI] [PubMed] [Google Scholar]
- Moore Simas T, Waring M, Sullivan G, Liao X, Rosal M, Hardy J, & Berry R (2013). Institute of Medicine 2009 gestational weight gain guideline knowledge: Survey of Obstetrics/Gynecology and Family Medicine residents of the United States. Birth, 40, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouzon DM, Taylor RJ, Woodward AT, & Chatters LM (2017). Everyday racial discrimination, everyday non-racial discrimination, and physical health among African-Americans. Journal of Ethnic & Cultural Diversity in Social Work, 26, 68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley L, Harvey S, & López-Cevallos D (2018). Racial and ethnic discrimination, medical mistrust, and satisfaction with birth control services among young adult Latinas. Womens Health Issues, 28, 313–320. [DOI] [PubMed] [Google Scholar]
- Ramey SL, Schafer P, DeClerque JL, Lanzi RG, Hobel C, Shalowitz M, … Community Child Newtork (2015). The preconception stress and resiliency pathways model: A multi-level framework on maternal, paternal, and child health disparities derived by community-based participatory research. Maternal and Child Health Journal, 19, 707–719. [DOI] [PubMed] [Google Scholar]
- Reid LD, & Creanga AA (2018). Severe maternal morbidity and related hospital quality measures in Maryland. Journal of Perinatology, 38, 997–1008. [DOI] [PubMed] [Google Scholar]
- Rosenthal L, & Lobel M (2011). Explaining racial disparities in adverse birth outcomes: Unique sources of stress for Black American women. Social Science & Medicine, 72, 977–983. [DOI] [PubMed] [Google Scholar]
- SB-464 California Dignity in Pregnancy and Childbirth Act. (2019). § An act to amend Secitons 1262.6 and 102875 of, and to add Article 4.6 (commencing with Section 123630) to Chapter 2 of Part 2 of Division 106 of, the Health and Safety Code, relating to maternal health. Available: https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB464. Accessed: May 31, 2020.
- Shen J, Tymkow C, & MacMullen N (2005). Disparities in maternal outcomes among four ethnic populations. Ethnicity & Disease, 15, 492–497. [PubMed] [Google Scholar]
- Snowden JM, Lyndon A, Kan P, El Ayadi A, Main E, & Carmichael S (2021). Severe maternal morbidity: A comparison of definitions and data sources. American Journal of Epidemiology, 190, 1890–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma-Pillay P, Makin JD, & Pattinson RC (2018). Quality of life 1 year after a maternal near-miss event. International Journal of Gynaecology & Obstetrics, 141, 133–138. [DOI] [PubMed] [Google Scholar]
- Statistical Methodology and Applications Branch. (2020). JoinPoint Regression Program, version 4.8.01. Available: https://surveillance.cancer.gov/joinpoint/. Accessed: May 31, 2020.
- Utter G, Atolagbe O, & Cooke D (2019). The use of the International Classification of Diseases, Tenth Revision, Clinical Modification and Procedure Classification System in clinical and health services research: the deveil is in the details. JAMA Surgery, 154, 1089–1090. [DOI] [PubMed] [Google Scholar]
- Wall-Wieler E, Carmichael S, Gibbs R, Lyell D, Girsen A, El-Sayed Y, & Butwick A (2019). Severe maternal morbidity among stillbirth and live birth deliveries in California. Obstetrics & Gynecology, 134, 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Glazer K, Howell E, & TM J (2020). Social determinants of pregnancy-related mortality and morbidity in the United States: A systematic review. Obstetrics & Gynecology, 135, 896–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Glazer K, Sofaer S, Balbierz A, & Howell E (2021). Racial and ethnic disparities in severe maternal morbidity: A qualitative study of women’s experiences of peripartum care. Womens Health Issues, 31, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weech-Maldonado R, Hall A, Bryant T, Jenkins K, & Elliott M (2012). The relationship between perceived discrimination and patient experiences with health care. Medical Care, 50, S62–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Philips AK, Iobst SE, Myers ER, Trego L, Allard RJ, & Landoll R (2021). A scoping review of unintended pregnancy in active duty United States Military Women. Womens Health Issues, 31, S66–S80. [DOI] [PubMed] [Google Scholar]
- Zanardi D, Parpinelli M, Haddad S, Costa M, Sousa M, Leite D, … Brazilian Network for Surveillance of Severe Maternal Morbidity Study Group (2019). Adverse perinatal outcomes are associated with severe maternal morbidity and mortality: Evidence from a National Multicentre Cross-Sectional Study. Archives of Gynecology, 299, 645–654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


