Abstract
BACKGROUND--Exercise intolerance in patients with cystic fibrosis is commonly attributed to reduced pulmonary and nutritional status. The possible role of diminished efficiency of mitochondrial oxidative phosphorylation in relation to skeletal muscle performance was investigated in patients with cystic fibrosis. METHODS--In vivo synthesis of ATP in skeletal muscle during submaximal exercise was studied in eight patients with cystic fibrosis aged 12-17 years, and in 19 healthy control subjects aged 8-36 years. The intracellular pH and concentrations of phosphate compounds were calculated at four steady states from phosphorus-31 labelled nuclear magnetic resonance spectroscopy measurements in the forearm muscle during bulb squeezing in an exercise protocol. Normalised power output, expressed as percentage maximal voluntary contraction (Y, in %MVC), was related to the energy force of ATP hydrolysis (X = ln [ATP]/[ADP][Pi]). This relationship provides an in vivo measure of efficiency of oxidative work performance of skeletal muscle. RESULTS--During all workloads (but not at rest) intracellular pH was higher in the patients with cystic fibrosis than in the controls. The linear least square fit for Y = a-bX showed high correlations in both groups; the slope b was 19% lower in the patients than in the controls (11.8% v 14.5% MVC/ln M; 95% confidence interval for difference 0.3 to 5.0). CONCLUSIONS--In patients with cystic fibrosis oxidative work performance of skeletal muscle is reduced. This may be related to secondary pathophysiological changes in skeletal muscle in cystic fibrosis.
Full text
PDF
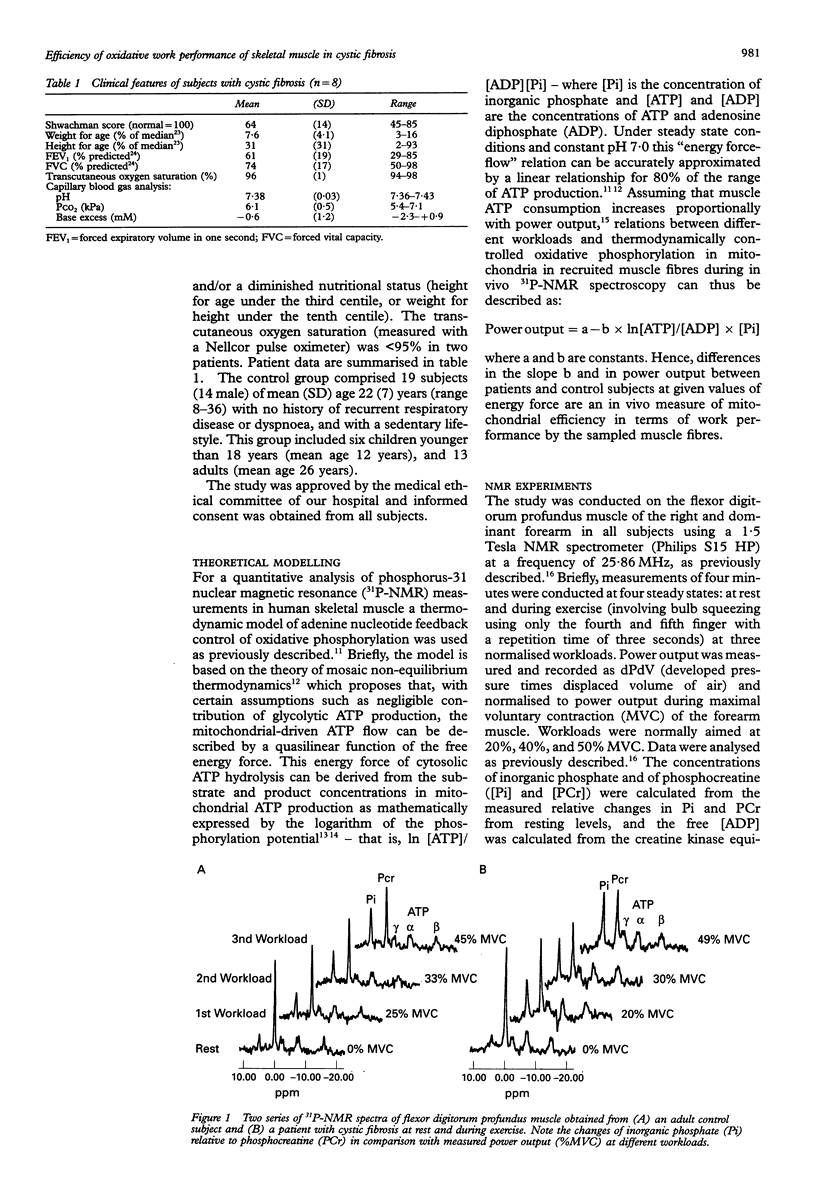
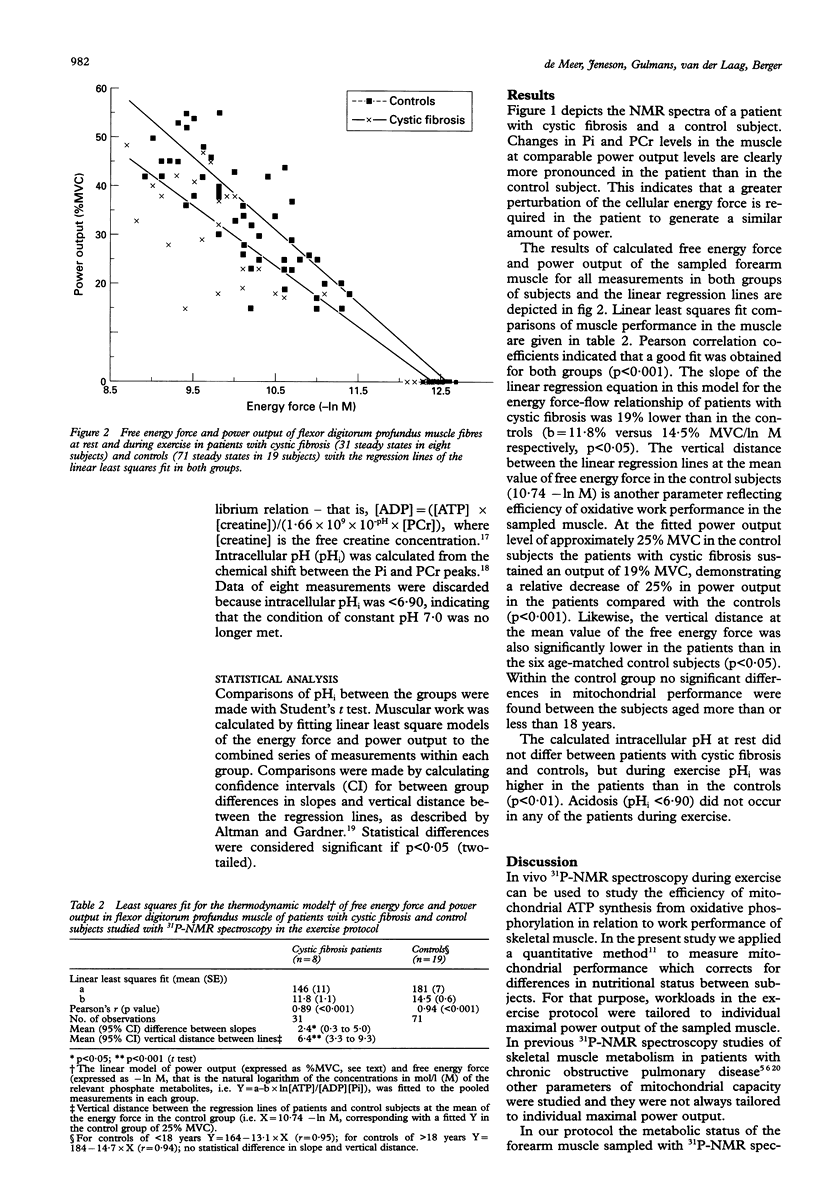

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman D. G., Gardner M. J. Calculating confidence intervals for regression and correlation. Br Med J (Clin Res Ed) 1988 Apr 30;296(6631):1238–1242. doi: 10.1136/bmj.296.6631.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban R. S. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol. 1990 Mar;258(3 Pt 1):C377–C389. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
- Dechecchi M. C., Girella E., Cabrini G., Berton G. The Km of NADH dehydrogenase is decreased in mitochondria of cystic fibrosis cells. Enzyme. 1988;40(1):45–50. doi: 10.1159/000469141. [DOI] [PubMed] [Google Scholar]
- Jeneson J. A., van Dobbenburgh J. O., van Echteld C. J., Lekkerkerk C., Janssen W. J., Dorland L., Berger R., Brown T. R. Experimental design of 31P MRS assessment of human forearm muscle function: restrictions imposed by functional anatomy. Magn Reson Med. 1993 Nov;30(5):634–640. doi: 10.1002/mrm.1910300515. [DOI] [PubMed] [Google Scholar]
- Kutsuzawa T., Shioya S., Kurita D., Haida M., Ohta Y., Yamabayashi H. 31P-NMR study of skeletal muscle metabolism in patients with chronic respiratory impairment. Am Rev Respir Dis. 1992 Oct;146(4):1019–1024. doi: 10.1164/ajrccm/146.4.1019. [DOI] [PubMed] [Google Scholar]
- Loke J., Mahler D. A., Man S. F., Wiedemann H. P., Matthay R. A. Exercise impairment in chronic obstructive pulmonary disease. Clin Chest Med. 1984 Mar;5(1):121–143. [PubMed] [Google Scholar]
- Marcotte J. E., Canny G. J., Grisdale R., Desmond K., Corey M., Zinman R., Levison H., Coates A. L. Effects of nutritional status on exercise performance in advanced cystic fibrosis. Chest. 1986 Sep;90(3):375–379. doi: 10.1378/chest.90.3.375. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Kuchmerick M. J., Brown T. R. Application of 31P-NMR spectroscopy to the study of striated muscle metabolism. Am J Physiol. 1982 Jan;242(1):C1–11. doi: 10.1152/ajpcell.1982.242.1.C1. [DOI] [PubMed] [Google Scholar]
- Neijens H. J., Duiverman E. J., Kerrebijn K. F., Sinaasappel M. Influence of respiratory exacerbations on lung function variables and nutritional status in CF patients. Acta Paediatr Scand Suppl. 1985;317:38–41. doi: 10.1111/j.1651-2227.1985.tb14933.x. [DOI] [PubMed] [Google Scholar]
- Payen J. F., Wuyam B., Reutenauer H., Laurent D., Levy P., Le Bas J. F., Benabid A. L. Impairment of muscular metabolism in chronic respiratory failure. A human 31P MRS study. NMR Biomed. 1991 Feb;4(1):41–45. doi: 10.1002/nbm.1940040108. [DOI] [PubMed] [Google Scholar]
- Pybus J., Tregear R. T. The relationship of adenosine triphosphatase activity to tension and power output of insect flight muscle. J Physiol. 1975 May;247(1):71–89. doi: 10.1113/jphysiol.1975.sp010921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosing J., Slater E. C. The value of G degrees for the hydrolysis of ATP. Biochim Biophys Acta. 1972 May 25;267(2):275–290. doi: 10.1016/0005-2728(72)90116-8. [DOI] [PubMed] [Google Scholar]
- SHWACHMAN H., KULCZYCKI L. L. Long-term study of one hundred five patients with cystic fibrosis; studies made over a five- to fourteen-year period. AMA J Dis Child. 1958 Jul;96(1):6–15. doi: 10.1001/archpedi.1958.02060060008002. [DOI] [PubMed] [Google Scholar]
- Shapiro B. L. Evidence for a mitochondrial lesion in cystic fibrosis. Life Sci. 1989;44(19):1327–1334. doi: 10.1016/0024-3205(89)90389-5. [DOI] [PubMed] [Google Scholar]
- Shapiro B. L., Feigal R. J., Lam L. F. Mitrochondrial NADH dehydrogenase in cystic fibrosis. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2979–2983. doi: 10.1073/pnas.76.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. J., Bore P. J., Styles P., Gadian D. G., Radda G. K. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983 Jul;1(1):77–94. [PubMed] [Google Scholar]
- Thompson C. H., Davies R. J., Kemp G. J., Taylor D. J., Radda G. K., Rajagopalan B. Skeletal muscle metabolism during exercise and recovery in patients with respiratory failure. Thorax. 1993 May;48(5):486–490. doi: 10.1136/thx.48.5.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhoff H. V., van Echteld C. J., Jeneson J. A. On the expected relationship between Gibbs energy of ATP hydrolysis and muscle performance. Biophys Chem. 1995 Apr;54(2):137–142. doi: 10.1016/0301-4622(94)00129-8. [DOI] [PubMed] [Google Scholar]
- Young I. H., Woolcock A. J. Arterial blood gas tension changes at the start of exercise in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1979 Feb;119(2):213–221. doi: 10.1164/arrd.1979.119.2.213. [DOI] [PubMed] [Google Scholar]


