Abstract
Objective:
Determine whether a novel psychosocial treatment for positive affect improves clinical status and reward sensitivity more than a form of cognitive behavioral therapy that targets negative affect and whether improvements in reward sensitivity correlate with improvements in clinical status.
Method:
In this assessor-blinded, parallel-group, multi-site, 2-arm randomized-controlled clinical superiority trial, 85 treatment-seeking adults with severely low positive affect, moderate-to-severe depression or anxiety, and functional impairment received 15 weekly individual therapy sessions of Positive Affect Treatment (PAT) or Negative Affect Treatment (NAT). Measures were self-reported positive affect, interviewer-rated anhedonia, and self-reported depression and anxiety. Target measures were eleven physiological, behavioral, cognitive, and self-report measures of reward anticipation-motivation, response to reward attainment, and reward learning. All analyses were intent-to-treat.
Results:
Compared to NAT, individuals receiving PAT achieved superior multivariate clinical status measures at post-treatment (b=.37, 95% CI:[.15,.59], t(109)=3.34, p=.001, q=.004, d=.64) Compared to NAT, individuals receiving PAT achieved higher multivariate reward anticipation-motivation (b=.21, 95% CI:[.05, .37], t(268)=2.61, p=.010, q=.020, d=.32) and higher multivariate response to reward attainment (b=.24, 95% CI:[.02,.45], t(266)=2.17, p=.031, q=.041, d=.25) at post-treatment. Measures of reward learning did not differ between the two groups. Improvements in reward anticipation-motivation and response to reward attainment correlated with improvements in self-reported positive affect, interviewer-rated anhedonia, and self-report measures of depression and anxiety.
Conclusions:
Targeting positive affect results in superior improvements in clinical status and reward sensitivity than targeting negative affect. This is the first demonstration of differential target engagement across two psychological interventions for anxious or depressed individuals with low positive affect.
Trial Registration:
Keywords: Low positive affect, reward sensitivity, depression, anxiety, psychotherapy, anhedonia
Low positive affect is increasingly recognized as a critical transdiagnostic feature of many psychiatric disorders (Villanueva et al., 2021). It is also a core feature of transdiagnostic anhedonia, characterized by markedly diminished interest or joy in usual activities. Low positive affect is not only a correlate but also a risk factor for onset of depression and anxiety and is associated with poorer longitudinal course (Morris et al., 2009). Furthermore, low positive affect is associated with suicidal ideation, and anhedonia is a predictor of suicide attempts (Ducasse et al., 2021; Ducasse et al., 2018). Patients with depression view the restoration of positive mood as their primary treatment goal, of more importance than the reduction of depressive symptoms (Demyttenaere et al., 2015). Yet, treatments to date for depression have not reliably improved positive affect (Bompouris, 2016). The goals of the current study were to evaluate the efficacy of a novel treatment for positive affect in depressed or anxious individuals with low positive affect, and to evaluate processes underlying the therapeutic effects.
Even though earlier theoretical models of mood and anxiety disorders linked positive affect almost exclusively to depression (e.g., Brown et al., 1998; Clark & Watson, 1991), evidence has accrued to suggest that low positive affect is relevant to anxiety as well. For example, effect sizes for cross-sectional and longitudinal relationships between positive affect and anxiety are on par with effect sizes for positive affect and depression (Khazanov & Ruscio, 2016; Kotov et al., 2010). Furthermore, hedonic impairments have been observed in social anxiety disorder (Kashdan et al., 2011), posttraumatic stress disorder (Hopper et al., 2008; Litz et al., 2000), and generalized anxiety disorder (Srivastava et al., 2003), including in youth samples (Morris et al., 2015), and reduced positive affect on stressful days was prospectively related to anxiety disorders seven years later (Rackoff & Newman, 2020). Thus, treatments designed to increase positive affect are relevant not only for depressed but also for anxious individuals.
Low positive affect has been relatively unresponsive to treatment. Specifically, meta-analyses of evidence-based psychotherapies for depression (primarily cognitive-behavioral therapy and mindfulness-based cognitive therapy) yield low to moderate effect sizes for change in positive affect, with significant heterogeneity across studies (Boumparis et al., 2016). A re-analysis of a prior randomized-controlled trial (DeRubeis et al., 2005) shows that cognitive therapy and antidepressant medication were less successful in raising positive affect than in decreasing negative affect (Dunn et al., 2020). Even behavioral activation therapy, which aims to increase positive reinforcement from rewarding activities, has limited effects on improving positive affect in the few studies in which such effects have been reported (Dichter et al., 2009; Moore et al., 2013). This is perhaps not surprising since little attention has been given to how to conduct behavioral activation in a manner that maximizes rewarding, positive emotional experiences. Pharmacological treatments show mixed effects and may even worsen positive emotions or responses to rewarding stimuli (McCabe et al., 2010), although newer pharmacological approaches such as kappa-opioid antagonists are showing promising effects (Krystal et al., 2020). The combination of low levels of positive affect in depression and anxiety and limited effectiveness of treatments to date with respect to positive affect, indicates a substantial unmet therapeutic need.
To address this need, newer psychological treatments have emerged to specifically improve positive affect, such as Behavioral Activation for Anhedonia (Cernasov et al., 2021), Amplification of Positivity (Taylor et al., 2017), and Positive Affect Treatment (PAT) (Craske et al., 2019). Drawing from an extensive literature that links anhedonia to reward hyposensitivity (Craske et al., 2016; Wang et al., 2021), PAT aims to increase positive affect by targeting sensitivity to reward. In a prior randomized controlled trial, PAT was compared to a form of cognitive-behavior therapy that focused solely upon decreasing threat sensitivity and negative affect (termed Negative Affect Treatment, NAT) for individuals with moderate to severe depression or anxiety who reported functional impairment. PAT resulted in higher positive affect, lower negative affect and less severe depression, anxiety, and suicidality at six months follow-up (Craske et al., 2019). The first goal of the present study was to evaluate the efficacy of PAT in individuals selected to represent the subset with especially low levels of positive affect in addition to feeling depressed or anxious, and functionally impaired.
Change in reward sensitivity is the purported mechanism of the newer treatments for positive affect. Evidence to date for this treatment mechanism is however sparse and limited to isolated changes in neural circuitries involved in reward-related paradigms (Kryza-Lacombe et al., 2021) and in resting-state connectivity in default mode and frontoparietal networks associated with major depression (Cernasov et al., 2021). By understanding the processes or mechanisms that are responsible for therapeutic outcomes, treatment strategies can be refined to be more precisely targeted and thereby more effective (Holmes et al., 2018). Therefore, the second goal of the present study was to evaluate the degree to which PAT changes reward sensitivity, using a comprehensive set of behavioral, cognitive, physiological and self-report measurements, as recommended by the National Institute of Mental Health (NIMH) Research Domain Criteria Initiative (RDoC). Furthermore, the degree to which changes in reward sensitivity correlated with changes in clinical status was evaluated as an indication of a potential treatment mechanism1.
PAT was explicitly designed to target three core elements of reward hyposensitivity that have been investigated as robust correlates of anhedonia, depression, or low positive affect (Borsini et al., 2020; Craske et al., 2019; Wang et al., 2021). The first component is reward anticipation-motivation, which drives adaptive behaviors towards rewarding stimuli and effort expended to gain reward. Evidence for anhedonic hyposensitivity in reward anticipation-motivation spans across neural and behavioral measures. Specifically, reduced ventral striatal activation during anticipation of reward is particularly related to stronger anhedonic symptoms in depressed samples (Wang et al., 2021). Furthermore, individuals with depression make fewer high effort choices than healthy controls (Allen et al., 2019) and the motivational effort they expend correlates negatively with anhedonia (Treadway et al., 2009). PAT targets reward anticipation-motivation through planning pleasurable activities and imagining positive future outcomes. Measures of reward anticipation-motivation typically include physiological anticipation of monetary/social reward in guessing or cued tasks, behavioral effort to gain reward, and self-report of interest and motivation in rewarding activities (Wang et al., 2021).
The second component is response to reward attainment or the hedonic impact of rewarding stimuli, with evidence of anhedonic hyposensitivity across neural, self-report and cardiac measures. Specifically, striatal hypoactivation and altered orbitofrontal cortex responding to reward attainment in depressed individuals are uniquely associated with anhedonia (Wang et al., 2021). Additionally, individuals with anhedonia report weaker positive emotions to positive stimuli (Liu et al., 2014) and individuals with dysphoria exhibit reduced cardiac acceleration while viewing or imagining pleasant stimuli (Benvenuti et al., 2015; Fiorito & Simons, 1994; Liu et al., 2014). PAT targets reward attainment through engagement in pleasurable activities followed by extensive imaginal savoring of the experience, attending to and imagining the positive, and cultivating and savoring exercises of loving-kindness, generosity, appreciative joy, and gratitude. Measures of reward attainment typically include physiological or experiential response to positive stimuli, and self-report of enjoyment in rewarding activities (Wang et al., 2021).
The third component is learning which actions lead to rewards and which stimuli are rewarding, with anhedonic hyposensitivity evident across neural and behavioral measures. Specifically, blunted ventral striatal responses to instrumental conditioning tasks (Whitton et al., 2015) and impaired acquisition of a response bias to stimuli that are frequently rewarded correlate with anhedonic symptoms (Pizzagalli et al., 2008; Vrieze et al., 2013). PAT targets reward learning through self-attribution of positive outcomes and reinforcement of associations between actions (e.g., pleasant activities) and positive mood state. Measurement of reward learning includes probabilistic reward paradigms and tests of Pavlovian and instrumental learning (Wang et al., 2021).
Consistent with the NIMH experimental therapeutics model for clinical trials, we hypothesized that, at post-treatment, PAT would lead to higher levels of positive affect, lower anhedonia, and less anxious-depressive symptoms than NAT. Second, we hypothesized that PAT would lead to higher levels of reward anticipation-motivation, response to reward attainment, or reward learning than NAT. Finally, we hypothesized that within-person changes in measures of reward targets would correlate with changes in positive affect, anhedonia, and anxious-depressive symptoms over time. Mediational pathways were not hypothesized as they will be investigated in a second, forthcoming trial of this NIMH-sponsored project.
Method
Trial Design
This was a multi-site, assessor-blinded, parallel, 2-arm, stratified (medicated, y/n), randomized (1:1) clinical superiority trial for help-seeking adults with low positive affect and depression or anxiety, and with impairment in functioning, conducted in the greater Los Angeles and Dallas metropolitan areas. Given the focus upon target engagement, assessments were conducted at pre-treatment, throughout treatment, and at post-treatment, without follow-up2.
The institutional review board committees at UCLA and SMU approved the trial, and trial conduct was overseen by a Data and Safety Monitoring Board of four expert researchers from three universities. All participants signed consent. Aside from COVID-19 restrictions, no changes occurred after trial commencement. This study adhered to Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines. No serious adverse events occurred during the trial.
Participants
A brief study description was distributed via lab websites, social media, ClinicalTrials.gov, and campus and local area flyers. Online screening questionnaires were followed by an in-depth phone screen, and then a SCID-5 diagnostic interview (First et al., 2015) conducted by reliability certified interviewers, with consensus diagnosis overseen by experienced clinicians (see Trial Protocol at ClinicalTrials NCT05203861).
Inclusion criteria were (1) 18 to 65 years, (2) English speaking, (3) low positive affect indexed by a score ≤24 (1SD below population mean and <18th percentile relative to a nonclinical reference sample; Crawford & Henry, 2004) on the Positive and Negative Affect Schedule-Positive Affect Subscale (PANAS-P; Watson et al., 1988), (4) Depression, Anxiety and Stress Scales (DASS; Lovibond & Lovibond, 1995) scores of ≥11 for depression, or ≥6 for anxiety, or ≥10 for stress, to represent moderate-to-severe symptom severity (Brown et al., 1998)3, (5) ≥5 on any Sheehan Disability Scale subscale to represent clinically significant impairment (Willams, 2000), and (6) willingness to refrain from starting other psychosocial/pharmacological treatments until study completion.
Exclusion criteria consisted of (1) serious medical conditions (i.e., significant, uncontrolled or unstable cardio-pulmonary disease, organic brain syndrome, seizure disorder, cerebrovascular disease, thyroid dysfunction, and diabetes), (2) active suicidal ideation, (3) lifetime history of bipolar disorder, psychosis, intellectual disability or organic brain damage, (4) substance abuse in last 6 months or dependence in last 12 months (5) ≥11 cigarettes/week or nicotine equivalent (6) marijuana, cocaine, or stimulant use ≥5-7 times/week before 15yrs, (7) pregnancy, (8) bupropion, dopaminergic or neuroleptic medications in last 6 months, consistent with other studies that investigate anhedonia (e.g., Hanuka et al., 2022), given their potential influence upon reward processing (Diego Pizzagalli, study consultant) and (9) refusal of video/audiotaping. Heterocyclics and SSRIs were permitted if stabilized ≥3 months. PRN benzodiazepines and beta-blockers were permitted but discouraged on assessment visits.
Interventions
Consistent with an experimental therapeutics approach and for the purposes of design specificity, we created distinctly different intervention conditions that exclusively targeted positive affect improvement versus negative affect reduction. Each manualized treatment involved 15 weekly, one-on-one sessions (switched to telehealth with COVID-19), delivered over ≤19 weeks (see Supplement and Trial Protocol for details). In brief, PAT began with psychoeducation and positive affect labeling, followed by planning and practice of pleasurable activities with extensive imaginal recounting to savor pleasurable moments. Next were cognitive exercises for attending to positive aspects of experiences, self-attribution for positive outcomes, and imagining future positive events, followed by cultivating and savoring positivity through exercises such as gratitude and generosity. The final session was relapse prevention. PAT was effective in a prior trial for positive affect, negative affect, and symptoms of depression and anxiety (Craske et al., 2019).
PAT was compared to a form of cognitive-behavior therapy that exclusively targeted negative affect (Negative Affect Treatment, NAT). In NAT, psychoeducation was followed by exposure therapy to distressing and avoided situations, cognitive restructuring of overestimates of probability, cost and self-blame for negative events, and respiratory training to reduce hypocapnia and anxiety, with relapse prevention in the final session. NAT was effective in the prior trial for negative affect and symptoms of depression and anxiety (Craske et al., 2019).
Therapists were clinical psychology doctoral students. As is common in treatment comparative studies, therapists were assigned to both treatment conditions to minimize therapist effects (Staines, 2007). Fifteen percent of sessions were randomly selected by reliability-certified independent raters (blinded to group assignment) (see Supplement for details). Ten percent of rated sessions were re-rated for inter-rater reliability.
Clinical Status Measures
Three primary measures of clinical status were registered: (1) The Positive Affect Subscale of the Positive and Negative Affect Schedule (PANAS-P; Watson et al., 1988) measured self-report positive affect at pre-treatment, every therapy session, and post-treatment (one week after session 15). (2) Independent interviewers (blinded to group assignment) rated anhedonia (INTERV-ANH) on ‘loss of interest,’ ‘loss of pleasure,’ and ‘loss of motivation’ during the past month (4-point scale, ‘absent’ to ‘severe’) at pre-treatment, session 5, session 10 and post-treatment (see Supplement for reliability details, kappa=.77). PANAS-P scores were negatively correlated with INTERV-ANH at baseline, r (83) =−.49. (3) Depression, Anxiety and Stress Scale (Lovibond & Lovibond, 1995) measured self-reported depression and anxiety at pre-treatment, every therapy session, and post-treatment. Total DASS (DASS-TOTAL) scores were analyzed to index anxious-depressive symptoms. The DASS was selected given excellent psychometric properties (Antony et al., 1998; Shea et al., 2009), and high correlations with other widely used self-report measures of anxiety and depression (e.g., Gloster et al., 2008; Ng et al., 2007).
Target Measures4
Target measures for each component of reward sensitivity (anticipation-motivation, response to attainment, and learning) were selected based on evidence for aberrations in relation to anhedonia or dysphoria/depression. Consistent with the RDoC initiative, measures were selected across multiple modalities (physiological, behavioral, cognitive, and experiential), with the eventual goal of trimming measures shown to be unrelated to clinical outcomes in our subsequent NIMH trial. Target measures were collected at pre-treatment, session 5, session 10, and post-treatment (see Supplement for target measure details and data quality assurance).
Reward anticipation-motivation was measured using one behavioral, one physiological, and two self-report measures. (1) The Effort for Expenditure for Rewards Task (EEfRT; Treadway et al., 2009) measured effort to work for reward. The dependent variable was the ratio of hard-effort choices to total choices. (2) Anticipatory heart rate acceleration to reward was measured using the Monetary Incentive Task (MIT), a forced reaction time task. The dependent variable was heart rate acceleration calculated by shortening of the cardiac interbeat-interval (IBI) (as the reverse of heart rate) from practice to the initial 30 s of reward trials, where effort and motivation were thought to be most likely maximal. The electrocardiogram was measured with a three-lead configuration (Einthoven II) with active pre-gelled electrodes attached to the sternum and the lower left rib in lateral position. A universal bioamplifier (Biopac MP150; Biopac,Systems, Inc., Goleta, CA) sampled the signal at a rate of 1000 Hz and the digitized raw signal was screened and cleaned offline for artifacts (movement, ectopic beats). The cardiac interbeat-interval (in milliseconds, the reverse of heart rate) was extracted as the time interval between adjacent R-waves. Lower values of these IBI difference scores indicate more cardiac acceleration. We utilized this rather global measure of cardiac activity because the literature has shown robust associations with reward processes (e.g., Fowles et al., 1982; Iaboni et al., 1997; Brinkman & Franzen, 2013) and positive stimuli (Lang al., 1993; Lackner et al., 2013; Vico et al., 2010), and such measurement would be feasible in clinical settings. (3) Self-report measures were the Reward Drive subscale of the Behavioral Inhibition and Activation Scale (BAS-RD), a 4-item index of effort valuation and willingness to work that correlates with measures of positive affect (Carver & White, 1994). (4) A second self-report measure was the Dimensional Anhedonia Rating Scale (DARS; Rizvi et al., 2015), a 26-item questionnaire in which respondents provide their own examples of rewarding experiences across domains of hobbies, social activities, food/drink, and sensory experience and subsequently rate current (“right now”) levels of desire, motivation, effort, and enjoyment for each domain. The 19 items related to desire, motivation, and effort were summed to create an index of reward anticipation-motivation (DARS-A-M) (internal consistency, α=.94).
Response to reward attainment was measured using one cognitive, one physiological, and two self-report measures. (1) A dot probe attentional task (DOTPROBE) was chosen because attention is one facet of stimulus responsivity, and because less attention is directed to positive stimuli and more attention is directed to negative stimuli as a function of low positive affect and depression (Peckham et al., 2010). Response time (ms) indexed biases in disengagement for sad faces and biases in engagement for happy faces, relative to neutral faces.5 (2) Heart rate acceleration was measured to positive relative to neutral images within the International Affective Picture System (IAPS; Lang et al., 1993), a well-established paradigm for studying psychophysiology of affect. The dependent variable was maximum heart rate acceleration during the 6-second positive image presentation relative to the 2 seconds before picture onset compared to acceleration to neutral images, both calculated by maximum shortening of the cardiac interbeat-interval (IBI) (as the reverse of heart rate). (3) Self-reported experiential response to reward was measured using the Temporal Experience of Pleasure Scale-Consummatory Subscale (TEPS-C; Gard et al., 2006) and (4) the DARS scale (Rizvi et al., 2015) from which the seven consummatory items (DARS-C) were summed (internal consistency, α=.87).
Reward learning was measured using two behavioral tasks and one embedded self-report scale. (1) Probabilistic Reward Task (PRT; Pizzagalli et al., 2008) measures propensity to modulate behavior as a function of prior reinforcements. The dependent variable was response bias (measured as accuracy) toward the more frequently rewarded stimulus. (2) The Pavlovian Instrumental Transfer task tests the extent to which a conditional stimulus (CS) paired with reward enhances instrumental responding to gain rewards (Talmi et al., 2008). The dependent variable was handgrip force exerted during CS+ relative to CS− in the test phase (PIT-FORCE), which has been shown to relate to activation in the nucleus accumbens (Talmi et al., 2008). (3) A self-report measure of reward learning embedded within the PIT task was a rating scale for pleasantness/valence for CS+ relative to CS− (PIT-VALENCE).
Randomization and Masking
Participants were randomized 1:1 to PAT vs. NAT via computer-generated allocation using permuted block randomization, stratified by medication status (2 strata; y/n). Randomization was managed by the study statistician and separate randomizations were generated for each site. Assessors were masked to group allocation. Study coordinators enrolled and assigned participants according to the randomization table.
Statistical Methods
Intent-to-treat analyses were performed using multilevel modeling (MLM) via mixed models in SPSS 26.0. As detailed in the Methods section, our measures assessed four constructs: 1) clinical status, 2) reward anticipation-motivation target, 3) response to reward attainment target, and 4) reward learning target. Since each construct was assessed using multiple measures, each construct was analyzed using a single multivariate MLM (MMLM). MMLM can increase power, accommodate measures with different assessment schedules, include all assessments as long as at least one of the dependent variables is measured at that assessment, and minimize inflation of Type I error (for details on MMLM, see Hox et al., 2018). The MMLM models were 3-levels: measures (level-1) were nested within repeated assessments (level-2) which were nested within individuals (level-3). The error covariance matrix at level-1 (the error covariance of the different measures of the construct) was modeled as unstructured, and models included a random intercept for individuals. Each individual measure was z-scored across all assessments as recommended by Hox et al. (2018). Maximum likelihood estimation was used in all analyses. Pre-treatment levels were included as covariates in all analyses to reduce error variance and to correct for any (even slight) group differences. As per hypotheses, we tested differences between PAT and NAT at post-treatment in all analyses. Only when a multivariate analysis indicated a significant difference between PAT and NAT did we conduct separate univariate MLMs on each measure included in the MMLM analysis to determine which univariate measures were significant. These univariate MLMs were conducted using the same model as the corresponding MMLM, but without the multiple dependent variables. The univariate significance tests of PAT vs. NAT were corrected for false discovery rate using the Benjamin-Hochberg approach (Benjamini & Yekutieli, 2001), which corrects for multiple tests while not increasing Type II error rates as much as other corrections for multiple tests (e.g., Bonferroni). The false discovery rate (q-value) is reported for each p-value. A q-value greater than .05 indicates that the corrected false discovery rate is greater than .05.
The assessments for clinical status were conducted at pre-treatment, every therapy session and at post-treatment for PANAS-P and DASS-TOTAL (17 time points) and at four time points for INTERV-ANH (MMLM allows different assessment schedules for different measures). As per our protocol, we selected the growth curve model (linear, quadratic, or LN) that best fit the data (lowest Akaike Information Criteria (AIC) and lowest Bayesian Information criteria [BIC]). Time was centered at post-treatment so that treatment group differences would test the difference between PAT and NAT at post-treatment. A significant MMLM treatment group effect would indicate that PAT and NAT were different on the set of three clinical status measures at post-treatment.
Our three reward targets (anticipation-motivation, response to reward attainment, and reward learning) were each analyzed by a separate MMLM. The individual measures comprising each target were assessed at four timepoints (pre-treatment, session 5, session 10, and post-treatment). As per protocol, each target was analyzed using 2 (treatment group) x 4 (timepoint) ANOVA-type models using MMLM. If the multivariate treatment group effect at post-treatment was significant, it was followed by univariate tests to determine which individual variables were different for PAT and NAT.
Details of the MMLM models, including composite equations for the MMLM models, are in the Supplement under Detailed Description of the MMLM Models). Since four total MMLMs were conducted (one for clinical status measures, and three for the targets), these 4 p-values for treatment group difference at post-assessment were corrected for inflation of the false discovery rate using Benjamini-Hochberg.
To examine the relations between reward targets and clinical status, we used SEM to analyze the within-person, longitudinal, concurrent relations between a latent variable representing the multiple measures of the reward target and a latent variable representing the multiple measures of clinical status (see Figure 6). We used SEM instead of MMLM because MMLM cannot accommodate multivariate (latent) predictors. Since scores on each measure at each timepoint conflate between-person average levels and within-person changes, we disaggregated time-varying predictors (i.e., reward target variables) into between- and within-person components. We only report the results for relations between the within-person components of our latent variables since relations among between-person components can be due to third variable, between-persons differences. Consistent with our protocol, we examined the concurrent relation between the target measures and the clinical status measures (not the cross-lag relations). Data were analyzed using MPlus Two-Level Models (see the Supplement for the syntax).
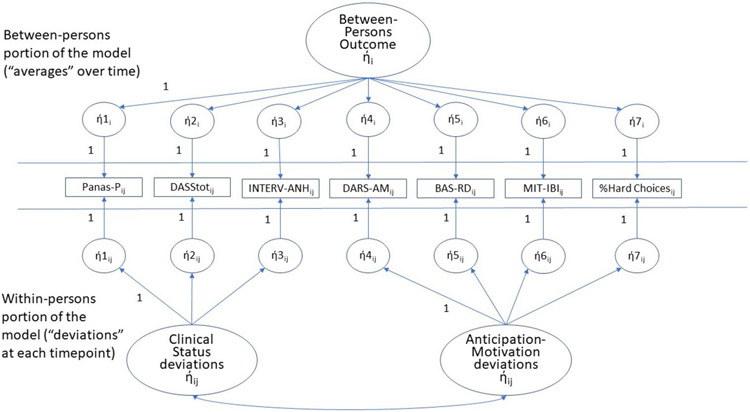
Figure 6.
SEM model for the within-person relation over time between reward anticipation-motivation and clinical status (bottom portion). A second model, with the same design but modeling the relation between response to reward attainment and clinical status, was also calculated.The top portion of the model is the level-2 portion of the model (one value for each parameter per individual). Hence, all parameters in the top portion have a subscript of “i” designating “individual” participants. The bottom portion of the model represents level-1 of the model (one value for each timepoint for each individual). Hence, every parameter in the bottom portion has a subscript of “ij” designating timepoint “j” for individual “i”. As discussed in the text, we focus on the within-person concurrent relation between clinical status and reward anticipation-motivation portion of the model (the bottom portion of the model). The ή1i – ή7i (top portion of model) represent the latent average of variables 1-7 across the assessments for each individual. The ή1ij – ή7ij in the bottom portion of the model represent the latent deviations from the average of variables 1-7 at each timepoint for each individual. The latent Between-Persons Outcome ήi (top portion of the Figure) represents the underlying individual characteristic that drives the average level of each variable across assessments for each individual. In the bottom portion of the model, the latent Clinical Status ήij represents the composite latent deviation score for Clinical Status (at each timepoint for each individual). Similarly, the latent reward anticipation-motivation ήij represents the composite latent deviation score for reward anticipation-motivation at each timepoint for each individual. We report the correlation of these 2 latent variables, a correlation within-persons over time, as our test of whether the target is related to concurrent outcomes.
Note: Abbreviations see Figures 2-5; BAS-RD = Behavioral Activation Scale-Reward Drive Subscale; %Hard Choices = % of hard choices in the Effort Expenditure for Rewards Task.
If the within-person relation between a reward target (e.g., reward anticipation-motivation) and clinical status was significant, we conducted bivariate analyses between each measure of the reward target and each measure of clinical status, using MLM. We used MLM instead of SEM for these univariate analyses because MLM provides unbiased estimates in small sample sizes (in samples as small as N=30; Maas & Hox, 2005) whereas SEM is more appropriate for large sample sizes. Each univariate reward target was disaggregated into a between-persons component (its mean across assessment occasions) and a within-person component (the deviation between the target value at each assessment and the target mean across assessment occasions). Then, these two components were investigated as univariate predictors of each of the three clinical status measures across the four assessments using MLM. These models were 2-level models (assessments nested within individuals), and the covariance matrix of the errors of the repeated measures was modeled as unstructured. All of these univariate tests were corrected for inflation of the false discovery rate using Benjamini-Hochberg.
All analyses were pre-specified in our protocol except those explicitly referred to as “sensitivity”. We used the “t” to “d” conversion to estimate effect sizes for all significant effects.
Our a priori power analysis, based on n=68 participants, indicated that we had greater than .80 power to detect an effect size difference between PAT and NAT as small as d=.35 for the individual clinical status measures that were assessed at 17 timepoints. For our hypotheses regarding target engagement, we had greater than .80 power to detect an effect size difference as small as d=.39. For hypotheses regarding covariation between target measures and clinical status measures, we had .80 power to detect covariation between a target and PANAS-P as small as d=.44. See Supplement for power analyses details.
Results
Participants
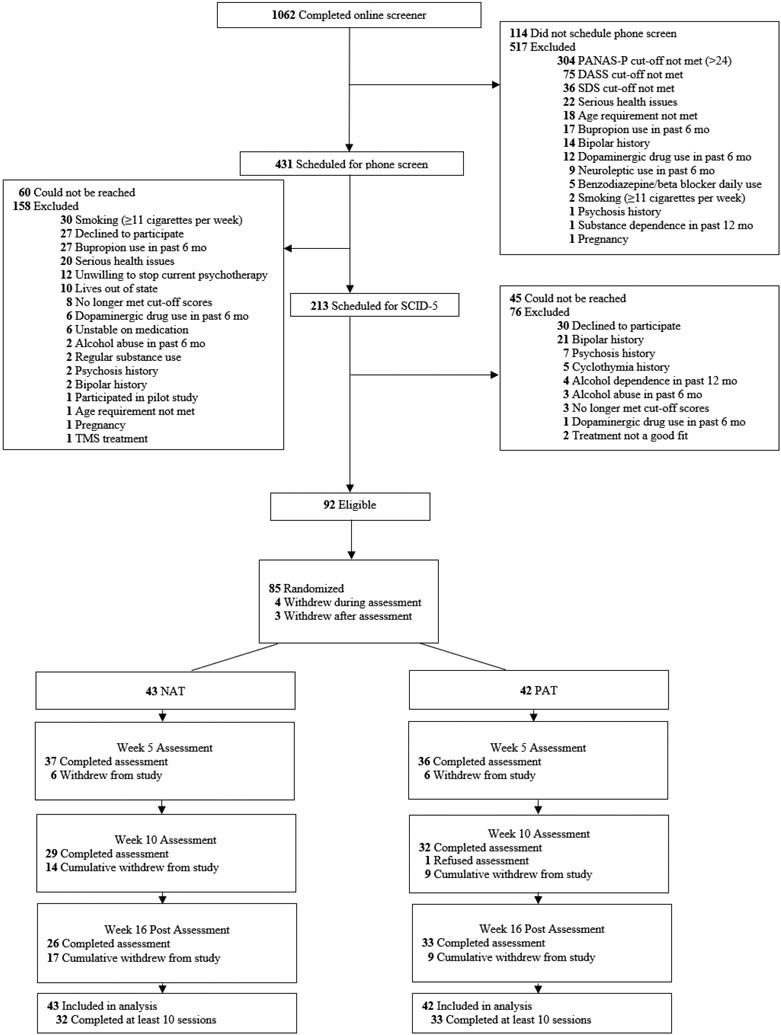
Recruitment began February 23, 2019, ended February 5, 2021, and the final assessment occurred July 19, 2021. Of 1062 individuals initially screened, the majority (694) were deemed ineligible, and 85 were randomly assigned to PAT (n = 42, 49.4%) or NAT (n = 43, 50.6%) (see Figure 1 flowchart). This final N was higher than the original projected N=68 because the COVID-19 lockdown required a temporary halting of the laboratory assessments (e.g., assessment of psychophysiological, cognitive and behavioral target measures), resulting in higher than anticipated rates of missing data specific to those measures (see Supplement). Consequently, additional participants (n = 17) were enrolled beyond original sample projection (n=68) to offset missing assessment data.
Figure 1.
Recruitment Flow Chart. PAT = Positive Affect Treatment, NAT = Negative Affect Treatment
In terms of missing data, n = 69 (69.4%) provided assessments at all 17 timepoints (pre-treatment, 15 sessions, post-treatment). Study attrition rates (at any point during treatment or post-treatment assessment) did not differ between PAT (21.4%) and NAT (39.5%); Fisher’s Exact Test, p = .099 (see Supplement for attrition reasons). Study completers did not differ from non-completers on any pretreatment demographic characteristic (age, sex, ethnicity, race, employment, marital status, use of psychotropic medication) or pre-treatment diagnoses (any anxiety disorder, any depressive disorder, or any diagnosis). 76% (N = 65; NAT = 32, PAT = 33) qualified as treatment completers (≥ 10/15 treatment sessions) and 69% (N = 59, PAT = 32, NAT = 27) completed all 15 treatment sessions.
See Table 1 for pre-treatment demographic and diagnostic characteristics. T-tests and chi-square tests confirmed that there were no significant treatment group differences on any pre-treatment demographic or diagnostic characteristic (ps >.140). Eighty-nine percent met criteria for current DSM-5 diagnoses and 60.0% had two or more diagnoses. 68.2% of participants met diagnostic criteria for a depressive disorder and 58.8% for an anxiety disorder, and 40.0% for both. Eleven (12.9%) participants did not meet diagnostic criteria for either depressive or anxiety disorder. Pre-treatment PANAS-P scores were 8th percentile relative to a nonclinical reference sample (Crawford & Henry, 2004). See Supplement eTablel for all raw means and SDs. See Supplement eTable2 for correlations across all measures at pre-treatment.
Table 1.
Pre-treatment and Demographic Characteristics of Intent-to-Treat Sample Receiving Positive Affect Treatment (PAT) or Negative Affect Treatment (NAT)
| Valuea | |||
|---|---|---|---|
| Characteristics | Total (N = 85) | PAT (n = 42) | NAT (n = 43) |
| Age, M (SD), years | 31.73 (12.3) | 31.95 (11.5) | 31.51 (13.1) |
| Female sex | 67 (78.8) | 31 (73.8) | 36 (83.7) |
| Race/Ethnicity | |||
| White | 53 (62.4) | 28 (66.7) | 25 (58.1) |
| Asian | 17 (20.0) | 8 (19.0) | 9 (20.9) |
| Alaska Native/Native American/Indigenous | 1 (1.2) | 1 (2.4) | 0 (0.0) |
| Pacific Islander/Native Hawaiian | 1 (1.2) | 0 (0.0) | 1 (2.3) |
| Black or African American | 5 (5.9) | 2 (4.8) | 3 (7.0) |
| Hispanic/Latino (White) | 15 (17.6) | 6 (14.3) | 9 (20.9) |
| Hispanic/Latino (non-White) | 8 (9.4) | 4 (9.5) | 4 (9.3) |
| Multiracial | 4 (4.7) | 3 (7.1) | 1 (2.3) |
| Other | 8 (9.4) | 1 (2.4) | 7 (16.3) |
| Marital status | |||
| Married | 19 (22.4) | 9 (21.4) | 10 (23.3) |
| Single, in a relationship | 23 (27.1) | 10 (23.8) | 13 (30.2) |
| Single, not in a relationship | 38 (44.7) | 19 (45.2) | 19 (44.2) |
| Separated | 2 (2.4) | 1 (2.4) | 1 (2.3) |
| Divorced | 3 (3.5) | 3 (7.1) | 0 (0.0) |
| Employed full or part time | 61 (71.8) | 32 (76.2) | 29 (67.4) |
| Education level | |||
| High school diploma/GED | 10 (11.8) | 3 (7.1) | 7 (16.3) |
| Some college or a 2-year degree | 26 (30.6) | 15 (35.7) | 11 (25.6) |
| 4-year college degree | 24 (28.2) | 11 (26.2) | 13 (30.2) |
| Graduate degree | 25 (29.4) | 13 (31.0) | 12 (27.9) |
| Income | |||
| <$10,000 | 14 (16.5) | 7 (16.7) | 7 (16.3) |
| $10,001-30,000 | 11 (12.9) | 5 (11.9) | 6 (14.0) |
| $30,001-50,000 | 16 (18.8) | 11 (26.2) | 5 (11.6) |
| $50,001-70,000 | 11 (12.9) | 6 (14.3) | 5 (11.6) |
| >$70,000 | 33 (38.8) | 13 (31.0) | 20 (46.5) |
| Current psychotropic medication | 24 (28.2) | 12 (28.6) | 12 (27.9) |
| Any diagnosis | 76 (89.4) | 37 (88.1) | 39 (90.7) |
| Depressive diagnoses | 58 (68.2) | 27 (64.3) | 31 (72.1) |
| Major depressive disorder | 46 (54.1) | 19 (45.2) | 27 (62.8) |
| Persistent depressive disorder | 33 (38.8) | 17 (40.5) | 16 (37.2) |
| Anxiety diagnoses | 50 (58.8) | 24 (57.1) | 26 (60.5) |
| Panic disorder | 10 (11.8) | 5 (11.9) | 5 (11.6) |
| Agoraphobia | 3 (3.5) | 1 (2.4) | 2 (4.7) |
| Social anxiety disorder | 34 (40.0) | 17 (40.5) | 17 (39.5) |
| Generalized anxiety disorder | 31 (36.5) | 15 (35.7) | 16 (37.2) |
| Specific phobia | 7 (8.2) | 3 (7.1) | 4 (9.3) |
| Posttraumatic stress disorder | 3 (3.5) | 0 (0.0) | 3 (7.0) |
| Obsessive compulsive disorder | 3 (3.5) | 0 (0.0) | 3 (7.0) |
| Attention deficit hyperactivity | 14 (16.5) | 9 (21.4) | 5 (11.6) |
| Adjustment disorder | 2 (2.4) | 1 (2.4) | 1 (2.3) |
Data are presented as number (percentage) of patients unless otherwise indicated.
Clinical status measures
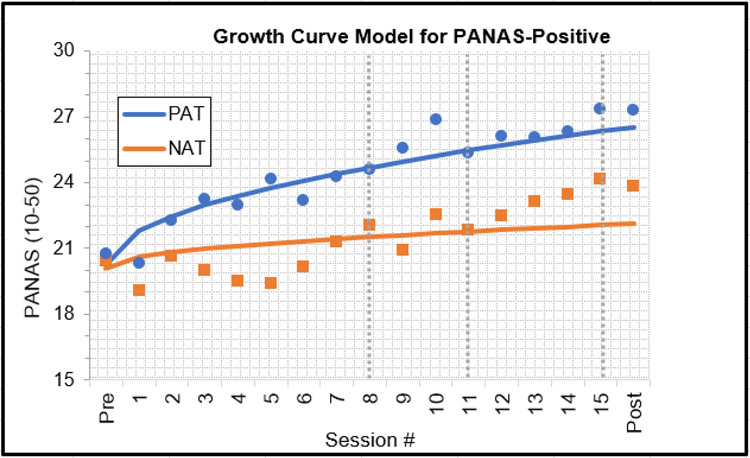
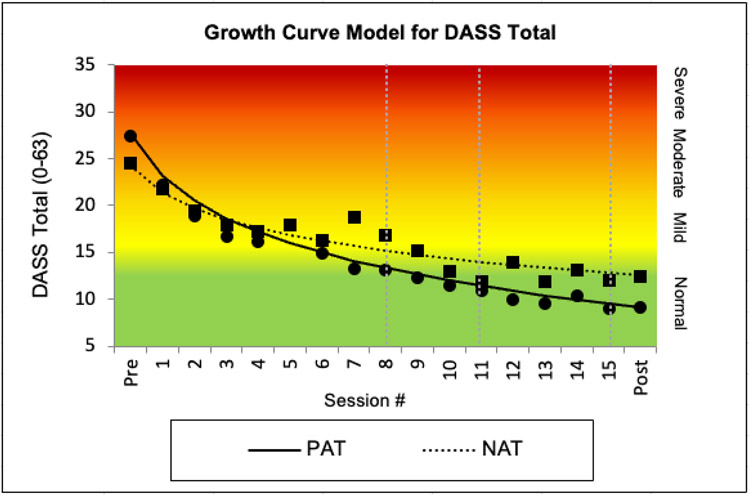
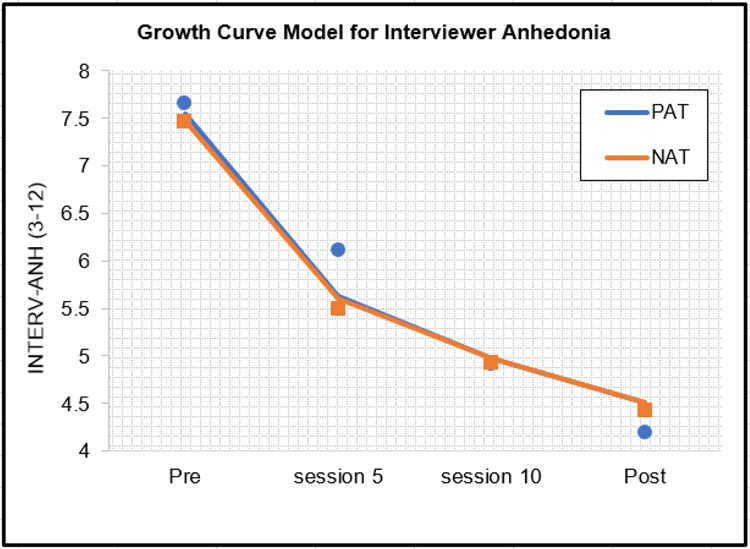
As per protocol, we first determined that the best fitting growth curve model for multivariate clinical status (lowest AIC and BIC), which was LN of Time (AIC = 5713.00, BIC = 5823.23 vs linear time with AIC = 5787.02, BIC = 5869.24 and quadratic time with AIC = 5755.11, BIC = 5849.08). This MMLM analysis showed that PAT participants had significantly higher scores on clinical status measures at post-treatment than NAT participants, b=0.37, 95% CI [0.15, 0.59], t(109) = 3.34, p = .001, q = .004, d = .64. Three univariate MLMs, one for each of the individual dependent variables in this MMLM (PANAS-P, DASS-TOTAL, INTERV-ANH), using scale scores rather than the z-scores used in the MMLM, showed that, at post-treatment, PAT participants had significantly higher PANAS-P scores than NAT participants (Figure 2), b = 4.31, 95% CI [1.67, 6.94], t(169) = 3.23, p = .002, q = .005, d = .47.6 They also had significantly lower DASS-TOTAL scores than NAT participants (Figure 3), b = −5.24, 95% CI [1.83, 8.66], t(193) = −3.03, p= .003, q = .005, d = .44. There were no group differences on INTERV-ANH (Figure 4). See Supplement for analyses for each DASS subscale.
Figure 2.
Clinical improvements in Positive and Negative Affective Schedule (PANAS)-Positive, using estimated means from the growth curve model and superimposed raw means (dots=PAT squares=NAT) for participants randomized to positive affect treatment (PAT) or negative affect treatment (NAT). Gray vertical lines indicate treatment modules (i.e., Session 1–7: behavioral activation plus recounting [positive affect treatment, or PAT] or exposure [negative affect treatment, or NAT]; Session 8–10: cognitive exercises [PAT] or cognitive restructuring [NAT]; Session 11–14: cultivate and savor positive experiences exercises [PAT] or respiratory regulation [NAT]; Session 15: relapse prevention [NAT, PAT])
Figure 3.
Clinical improvements in depression, anxiety, and stress (DASS-TOTAL scores) using estimated means from the growth curve model and superimposed raw means (dots=PAT squares=NAT) for PAT and NAT. Note: Abbreviations and gray vertical lines, see Figure 2
Figure 4.
Clinical improvements in interviewer-rated anhedonia using estimated means from the growth curve model and superimposed raw means (dots=PAT squares=NAT) for PAT and NAT
Note: Abbreviations, see Figure 2
Reward targets
Reward Anticipation-Motivation.
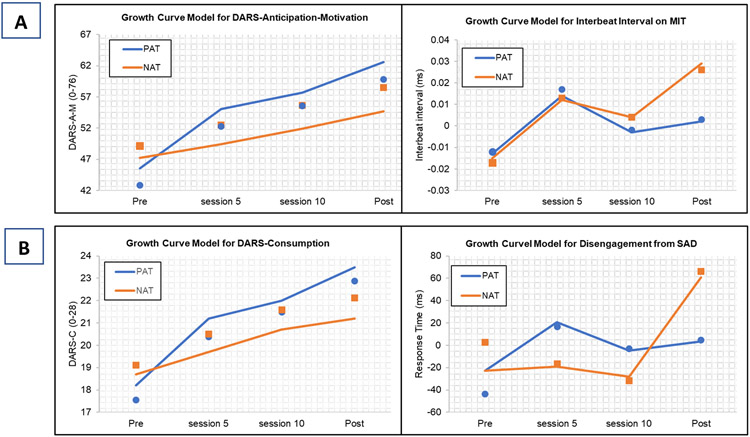
PAT participants showed higher multivariate reward anticipation-motivation than NAT participants at post-treatment on the MMLM, b = 0.21, 95% CI [0.05, 0.37], t(268) = 2.61, p = .010, q = .020, d = .32.7 MLM analyses of the four individual measures showed that PAT participants, compared to NAT, had higher DARS-A-M, b = 7.82, 95% CI [2.51,13.13], t(236) = 2.90, p = .004, q = .012, d = .38, and marginally more stable cardiac responses, compared to a decline in cardiac acceleration in NAT, indicated by lengthening cardiac inter-beat intervals), b = 0.03, 95% CI [0.001, 0.05], t(117) = 1.99, p = .049, q = .074, d = .37 (Figure 5a). PAT and NAT did not differ on the EEfRT task or on BAS-RD at post-treatment.
Figure 5.
Estimated means and superimposed raw means (dots=PAT squares=NAT) for univariate analyses of target measures that were significant or marginally significant with correction for false discovery rate. (A) Reward anticipation-motivation for PAT and NAT (B) Response to reward attainment for PAT and NAT
Note: Abbreviations, see Figure 2; MIT= monetary incentive task; IBI = cardiac inter-beat interval (nb lower interbeat-interval = higher heart rate); DARS-A-M = Dimensional Anhedonia Rating Scale-Anticipation-Motivation Items; DARS-C = Dimensional Anhedonia Rating Scale-Consummatory Items; SAD = sad faces of the dot probe task
Response to Reward Attainment.
The MMLM for response to reward attainment showed that, compared to NAT, PAT participants displayed higher overall response to reward at post-treatment, b = 0.24, 95% CI [0.02, 0.45], t(266) = 2.17, p = .031, q = .041, d = .255. In the four univariate follow-up MLM analyses, PAT participants showed higher self-reported DARS-C, b = 2.27, 95% CI [0.22,4.32], t(232) = 2.18, p = .030, q = .12, d =. 29, and a marginally shorter time to disengage from sad stimuli in the DOTPROBE task, b = −51.20, 95% CI [−109.49, 7.09], t(165) = −1.73, p = .085, q = .17, d = .27 (Figure 5b). PAT and NAT did not differ on TEPS-C scores nor on heart rate acceleration in the IAPS.
Reward Learning.
PAT did not differ from NAT at post-treatment on the MMLM for reward learning. Hence, univariate analyses of the individual components were not computed.
Longitudinal relations between target and clinical status measures
We conducted two SEM analyses to examine the longitudinal relations between our targets and clinical status, following the example of the general multilevel SEM model with latent variables. The first examined the disaggregated within-person relation over time between the latent variable representing the four indicators of reward anticipation-motivation and the latent variable representing the three measures of clinical status. The second examined the disaggregated within-person relation over time between the latent variable representing the four indicators of response to reward attainment and the latent variable representing the three measures of clinical status. We did not examine the relation between reward learning and clinical status since reward learning did not differ between treatment groups. Figure 6 depicts the graphical model for those two analyses. The bottom part of Figure 6 depicts the concurrent covariation over time between latent deviations in clinical status ήij. with the latent deviations in reward anticipation-motivation ήij. Our second model, which is not shown in Figure 6, was conducted with the same structure but used the four measures of response to reward attainment instead of the four measures of reward anticipation-motivation. Thus, these two models examine different aspects of reward functioning separately. The MPlus syntax for the two SEM analyses is reported in the Supplement.
The SEM model examining within-person covariation over time between the latent variable for reward anticipation-motivation with the latent variable for clinical status (Figure 6) showed adequate fit: RMSEA = .047, CFI = .96, TLI = .94, SRMR for the within portion of the model = .04. When participants had higher than their average level of latent reward anticipation-motivation, they also had higher than their average level of latent clinical status, b = 0.10, 95% CI [0.03, 0.17], z = 2.76, p = .006, q = .018. Examining the univariate relations between the four measures of reward anticipation-motivation with the three measures of clinical status using MLM (12 separate univariate analyses), and correcting for false discovery rate (for 12 tests), greater within-person increases in DARS-A-M were related to greater improvements in PANAS-P, INTERV-ANH, and DASS-TOTAL, b = 0.27, 95% CI [0.20, 0.34], t(162) = 7.67, p < .001, q < .001, d = 1.21; b = −0.08, 95% CI [−0.10, −0.06], t(189) = −7.26, p <.001, q <.001, d = 1.07; b = −0.30, 95% CI [−0.40, −0.20], t(128) = −5.87, p < .001, q <.001, d =1.04, respectively. Also, greater within-person increases in BAS-RDD were related to greater increases in PANAS-P, b = 0.92, 95% CI [0.33,1.50], t(202) = 3.10, p = .002, q = .006, d = .44. No other relations were significant.
The SEM model examining within-person covariation between the latent variable for response to reward attainment and the latent variable for clinical status also showed adequate fit: RMSEA = .06, CFI = .94, TLI = .90, SRMR for the within portion of the model = .05. When participants had higher than their average level of latent response to reward attainment they also had higher than their average level of latent clinical clinical status, b = 0.07, 95% CI [0.01, 0.13], z = 2.18, p = .030, q = .045. Examining the univariate relations between the four measures of response to reward attainment and three measures of clinical status, and correcting for false discovery rate (for 12 tests), greater within-person increases in DARS-C were related to greater improvements in PANAS-P, INTERV-ANH, and DASS-TOTAL, b = 0.65, 95% CI [0.46, 0.84], t(160) = 6.62, p <.001, q < .001, d = 1.05; b = −0.20, 95% CI [−0.26, −0.14], t(190) = −6.35, p <.001, q <.001, d = .92; and b = −0.72, 95% CI [−1.00, −0.44], t(138) = −5.04, p <.001, q <.001, d = .86, respectively. Also, greater within-person increases in TEPS-C were related to greater improvements (decreases) in INTERVI-ANH, b = −0.12, 95% CI [−0.19, −0.05], t(154) = 3.38, p =.002, q =.006, d = .54. No other relations were significant.
Sensitivity analyses
The Effect of COVID-19 Protocols.
COVID-19 protocols began during the conduct of this study. All assessments were coded as either before or after COVID-19 protocols. We investigated whether COVID-19 protocols moderated any of the treatment group effects by including the Group x Time x COVID interaction (and all subcomponents of that interaction) in the four MMLMs (one for clinical status and three for the targets). The only significant COVID effect that survived Benjamini-Hochberg correction indicated that improvement in clinical status over time was significantly faster during COVID than before COVID (across both treatment groups), b = 0.22, 95% CI [.012, 0.31], t(946) = 4.37, p <.001, d = .28, for the COVID x Time interaction.
Testing Data Missing at Random.
MLM and SEM assume data is missing at random (MAR). Unfortunately, one cannot directly test whether data are MAR. The analyses reported above showed no pre-treatment differences between those with missing data and those without, are consistent with data being MAR but do not prove that data are MAR. Additional support for the accuracy of estimates from the MAR analyses can come from sensitivity analyses using missing not at random (MNAR) models (MNAR models do not assume that data are MAR). These MNAR sensitivity analyses are reported in the Supplement.
Other Treatment Effects
Treatment Fidelity.
Treatment adherence was rated on 138 (15%) recorded therapy sessions, equally distributed across treatment group and sites. Inter-rater reliability was established on 10% (n=14) and was high (ICC [2,1] = .81). Overall, adherence was high for PAT (91.7% [12.9]) and NAT (96.5% [8.2]) with no significant site differences. NAT sessions were rated as more adherent than PAT sessions (p < .05), due mainly to missing elements (primarily homework review) rather than protocol violations (% non-adherence, p > .05) during PAT.
Treatment Credibility.
Treatment credibility was assessed using the Credibility/Expectancy Questionnaire (CEQ) after the first treatment session. Treatment credibility ratings were high (1–9 scale; 7.28 [SD=1.27]) and did not significantly differ between groups (p = .42) or sites (p = .61).
Patient-Therapist Relationship.
Patient-therapist relationship was assessed using the Working Alliance Inventory, a 12-item short questionnaire at end of final treatment session. Patient-therapist relationship ratings were high for all categories (1–5 scale): affective bond (4.55 [0.58]), agreement on tasks (4.25 [0.77]), and goals (4.59 [0.56]), with no groups (ps = .09–.30) or site differences (ps = .21–.64).
Medication Status.
Change in psychotropic medication occurred infrequently from study entrance through post-treatment (n=1 decreased, and n=3 increased).
Discussion
In this sample of individuals with severely low positive affect, moderate-to-severe depression or anxiety, and functional impairment, of whom close to 90% met diagnostic criteria for either a depressive or an anxiety disorder, we found that a treatment targeted at reward hyposensitivity (PAT) was more effective for positive affect and for symptoms of depression and anxiety than a treatment that targeted negative affect (NAT). PAT was also more effective than NAT at raising reward anticipation-motivation and response to reward attainment. Finally, change in reward anticipation-motivation and reward attainment correlated with improvements in self-reported positive affect, interviewer-rated anhedonia, and symptoms of depression and anxiety.
This set of findings builds upon prior evidence (Craske et al., 2019) for PAT as an effective intervention for individuals with depression or anxiety. Percentile improvements in positive affect at post-treatment were significant and reached ~26th percentile relative to a non-clinical reference sample (Crawford & Henry, 2004), from a pre-treatment level of 8th percentile. Improvements were less than in the prior trial where post-treatment positive affect values approximated the 40th percentile (Craske et al., 2019), perhaps because our current sample was more severely low on positive affect (this trial required low PANAS-P cut-off inclusion criteria whereas the previous trial did not). Positive affect continued to improve over the six-month follow-up after PAT in the prior study (Craske et al., 2019), suggesting that values may have similarly improved in the current study had we included follow-up assessments. Improvements in anxious-depressive symptoms paralleled the prior trial. For example, DASS-depression scores decreased from the extremely severe to normal range and DASS-stress scores decreased from severe to normal in PAT (see Supplement). We should also note that DASS depression and stress scores improved in NAT as well, but to a lesser degree than in PAT.
In support of hypotheses, PAT resulted in greater improvements in reward anticipation-motivation and response to reward attainment relative to NAT. This is the first demonstration of differential target engagement across two psychological interventions for anxious or depressed individuals with low positive affect. The target effects extended across units of analyses, from self-report of reward hyposensitivity to marginal (following correction for false discovery rate) univariate effects for physiological (monetary incentive task) and cognitive (attention dot-probe task) measures. As such, the target effects are unlikely to be fully explained by responder biases. Marginal results from the dot-probe attention task were limited to faster disengagement from negative stimuli, and did not extend to longer engagement with positive stimuli, in PAT versus NAT. Whereas more prolonged positive engagement would be expected from a therapy designed to increase attention to positive stimuli, the faster negative disengagement is also relevant, given that more attention is directed to negative stimuli as a function of low positive affect and depression (Peckham et al., 2010). Nonetheless, differences in terms of negative disengagement do not necessarily represent reward sensitivity per se.
PAT did not result in greater improvements in reward learning compared to NAT. This result may indicate the need for stronger reward learning therapeutic strategies (akin to cognitive bias modification training) in order to observe greater changes in reward learning in PAT compared to NAT. It is also possible that the measures of reward learning (Pavlovian Instrumental Transfer and Probabilistic Reward Tasks) were insufficiently sensitive to differences between the two treatments. On the other hand, changes in the Probabilistic Reward Task have been observed as a function of a pharmacological intervention compared to placebo (Pizzagalli et al., 2020). In that case, the task was analyzed with computational modeling, which may produce more sensitive indices for treatment effects. Nevertheless, reaction time tasks (such as the Probabilistic Reward task) are inherently limited and behavioral measures that possess greater ecological validity and capture complex behavioral trajectories across changing conditions (e.g., Mobbs et al., 2021) may offer greater treatment sensitivity in future trials.
As hypothesized, within-person change in reward targets of reward anticipation-motivation and response to reward attainment covaried with within-person change in clinical status. The combination of greater changes in reward targets in PAT compared to NAT and covariation between changes in reward targets and clinical status provides preliminary evidence for reward sensitivity as a possible mediator of PAT. The current study was not powered nor designed for full mediational analyses, which are planned for the next phase of our NIMH-sponsored research. As such, we cannot draw any conclusions on temporal precedence and causality between the reward targets and clinical status, and prior clinical status may have acted as a confounder. Notwithstanding, it is notable that follow-up univariate analyses revealed that only self-report measures (and not physiological, cognitive, or behavioral measures) of the two reward targets covaried significantly with clinical status. Lack of coherence across different modalities of measurement is not uncommon (e.g., Enkavi & Poldrack, 2021; Peng et al., 2021). Such incoherence may represent differential measurement biases (e.g., test-retest reliabilities), differential temporal sensitivities, or differential modulating influences, to name a few, and represents a challenge for multi-modal measurement approaches. Nonetheless, the covariation occurred not just with self-report measures (as in the PANAS-P and DASS-TOTAL) but also with independent interviewer ratings of anhedonia.
Limitations of the study include generalizability from academic settings to community settings, although our sample was diverse in ethnic/racial background, socioeconomic status, and regionality (California and Texas). The majority female distribution is consistent with the epidemiology of depression and anxiety. The large percentage who were ineligible, largely due to insufficiently low positive affect at pre-treatment, necessitated by the mechanistic nature of the study, also limits generalizability. Our sample was selected to have low positive affect, as opposed to anhedonia; hence, the results may not generalize to samples diagnosed with anhedonia. Nonetheless, in the current sample, self-reported positive affect correlated with interviewer-rated anhedonia (r(83) = −.49), and pre-treatment TEPS-consummatory scores closely resembled scores in samples with major depression. Attentional bias was utilized as a measure of reward responsiveness since attention is a facet of stimulus responsivity, but results were limited to disengagement from sad faces rather than engagement with happy faces. Other aspects of reward sensitivity (e.g., reward satiation or devaluation) could be essential therapeutic targets that await future investigation. Although well justified by our focus on target engagement, the lack of follow-up prevented assessment of long-term changes in both target measures and clinical status; such longer-term effects are being assessed in our ongoing mediational trial. Our sample size was powered for moderate effects, and smaller effects may require larger samples. Inherent to the nature of psychological treatment studies, participants were not blinded to condition. On the other hand, treatment credibility ratings were high and did not differ between conditions. Finally, by virtue of the experimental therapeutics approach and in order to limit treatment diffusion, our intervention conditions were designed to specifically target either reward sensitivity and positive affect or threat sensitivity and negative affect. As such, NAT was not fully representative of standard cognitive-behavioral therapy for depression. A third treatment arm of such therapy could be a valuable control comparison in future research.
In conclusion, PAT was more effective for positive affect and symptom reduction than an intervention that targets negative affect for individuals with severely low positive affect who were depressed or anxious and functionally impaired. PAT also led to greater changes in multi-modal measures of reward anticipation-motivation and response to reward attainment. Finally, changes in these two reward targets correlated with changes in positive affect, anhedonia, and depression and anxiety.
Supplementary Material
Public health significance statement.
This study demonstrates that positive affect treatment was significantly more effective than an intervention that targeted negative affect for adults with severely low positive affect, moderate-to-severe depression or anxiety, and functional impairment. The findings of this study suggest that positive affect treatment improves positive affect and aspects of reward hyposensitivity.
NIH Funding:
R61MH115138
Footnotes
The authors are grateful for the excellent work of the independent assessors, therapists, and research coordinators, especially Rebecca Kim, Richard Kim, Emily Wang, and Wendy Huerta. We also express our gratitude for the support of our study consultants, Drs. Diego Pizzagalli and Steven Hollon, and the members of the Data Safety Monitoring Board.
The current trial was designed within the NIMH R61/33 experimental therapeutics framework with the aim of target engagement. By those standards, full mediational modeling is dependent upon initial demonstration of successful “target engagement” (i.e., PAT leading to changes in reward sensitivity measures). This study reports the findings of the initial demonstration of target engagement, laying the groundwork for our ongoing trial (NCT05203861), where full mediational modeling will be performed.
The 2-year funding scheme to demonstrate target engagement was not conducive for conducting follow-up assessments
The DASS-Anxiety subscale assesses autonomic arousal, skeletal muscle effects, situational anxiety, and experience of anxious affect. The DASS-Stress scale assesses difficulty relaxing, nervous arousal, and being easily upset/agitated, irritable/over-reactive and impatient. Thus, both the Anxiety and Stress scales assess features of anxiety.
Note that our clinical trial registration was updated to include target measures alongside clinical status measures. All of the target measures were as specified in the NIMH grant and Trial Protocol and remained unchanged from the start of the trial.
Preliminary analyses indicated no change in engagement bias for happy faces (b = −39.16, 95%CI: [−89.30, 10.98], t(199) = −1.54, p=.125) and thus subsequent analyses were limited to disengagement bias from sad faces.
See exploratory analyses in Supplement for PANAS-Negative Affect Subscale
Note that the results remain the same when using the total DARS score in the MMLM analyses.
References
- Allen TA, Lam RW, Milev R, Rizvi SJ, Frey BN, MacQueen GM, Müller DJ, Uher R, Kennedy SH, & Quilty LC (2019). Early change in reward and punishment sensitivity as a predictor of response to antidepressant treatment for major depressive disorder: a CAN-BIND-1 report. Psychological Medicine, 49(10), 1629–1638. 10.1017/s0033291718002441 [DOI] [PubMed] [Google Scholar]
- Amir N, Taylor CT, & Donohue MC (2011). Predictors of response to an attention modification program in generalized social phobia. Journal of Consulting and Clinical, ology.;79(4):533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony MM, Bieling PJ, Cox BJ, Enns MW, & Swinson RP (1998). Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychological assessment, 10(2), 176. [Google Scholar]
- Armstrong T, & Olatunji BO (2009). What they see is what you get: Eye tracking of attention in the anxiety disorders. Psychological Science Agenda, 23, 3. [Google Scholar]
- Ballard ED, Wills K, Lally N, Richards EM, Luckenbaugh DA, Walls T, Ameli R, Niciu MJ, Brutsche NE, Park L, & Zarate CA Jr. (2017). Anhedonia as a clinical correlate of suicidal thoughts in clinical ketamine trials. Journal of Affective Disorders, 218, 195–200. 10.1016/j.jad.2017.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, & Bakermans-Kranenburg MJ (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin.; 133(1):1–24. [DOI] [PubMed] [Google Scholar]
- Barry TJ, Sze WY, & Raes F (2019). A meta-analysis and systematic review of Memory Specificity Training (MeST) in the treatment of emotional disorders. Behaviour Research and Therapy, 116, 36–51. 10.1016/j.brat.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Beck AT (1972). Cognition, anxiety, and psychophysiological disorders. Anxiety: Current trends in theory and research pp. 343–354. [Google Scholar]
- Benjamini Y, & Yekutieli D (2001). The control of the false discovery rate in multiple testing under dependency. The Annals of Statistics, 29(4), 1165–1188, 1124. 10.1214/aos/1013699998 [DOI] [Google Scholar]
- Benvenuti SM, Mennella R, Buodo G, & Palomba D (2015). Dysphoria is associated with reduced cardiac vagal withdrawal during the imagery of pleasant scripts: Evidence for the positive attenuation hypothesis. Biological psychology, 106, 28–38. [DOI] [PubMed] [Google Scholar]
- Berlin I, Givry-Steiner L, Lecrubier Y, & Puech AJ (1998). Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. European Psychiatry, 13, 303–309. [DOI] [PubMed] [Google Scholar]
- Borsini A, Wallis ASJ, Zunszain P, Pariante CM, & Kempton MJ (2020). Characterizing anhedonia: a systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cognitive, Affective, & Behavioral Neuroscience, 20(4), 816–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumparis N, Karyotaki E, Kleiboer A, Hofmann SG, & Cuijpers P (2016). The effect of psychotherapeutic interventions on positive and negative affect in depression: A systematic review and meta-analysis. Journal of Affective Disorders, 202, 153–162. 10.1016/j.jad.2016.05.019 [DOI] [PubMed] [Google Scholar]
- Bradley MM, & Lang PJ (1994). Measuring emotion: The Self-Assessment Manikin and the Semantic Differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(1), 49–59. [DOI] [PubMed] [Google Scholar]
- Brailean AM, Koster EH, Hoorelbeke K, & De Raedt R (2014). Attentional modulation by reward and punishment cues in relation to depressive symptoms. Journal of Behavior Therapy and Experimental Psychiatry, 45(3), 351–359. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP, Sylvers PD. A comparison of psychophysiological and self-report measures of BAS and BIS activation. Psychophysiology. 2005. Jan;42(1):108–15. doi: 10.1111/j.1469-8986.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP, & Sylvers PD (2005). A comparison of psychophysiological and self-report measures of BAS and BIS activation. Psychophysiology, Jan;42(1):108–15. 10.1111/j.1469-8986.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Brinkmann K, & Franzen J (2013). Not everyone's heart contracts to reward: Insensitivity to varying levels of reward in dysphoria. Biological psychology, 94(2), 263–271. 10.1016/j.biopsycho.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Brinkmann K, Schupbach L, Joye IA, & Gendolla GH (2009). Anhedonia and effort mobilization in dysphoria: Reduced cardiovascular response to reward and punishment. International Journal of Psychophysiology, 74(3), 19808061. [DOI] [PubMed] [Google Scholar]
- Britton JC, Bar-Haim Y, Clementi MA, Sankin LS, Chen G, Shechner T, Norcross MA, Spiro CN, Lindstrom KM, & Pine DS (2013). Training-associated changes and stability of attention bias in youth: Implications for Attention Bias Modification Treatment for pediatric anxiety. Developmental cognitive neuroscience, 4, 52–64. 10.1016/j.dcn.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, & Barlow DH (1998). Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology, 107(2), 179–192. 10.1037//0021-843x.107.2.179 [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, & Rottenberg J (2008). A meta-analysis of emotional reactivity in major depressive disorder. Clinical psychology review, 28(4), 676–691. 10.1016/j.cpr.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Campbell DT, & Fiske DW (1959). Convergent and discriminant validation by the multitrait-multimethod matrix. Psychological bulletin, 56(2), 81–105. [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of personality and social psychology, 67(2), 319–333. 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- Cernasov P, Walsh EC, Kinard JL, Kelley L, Phillips R, Pisoni A, Eisenlohr-Moul TA, Arnold M, Lowery SC, & Ammirato M (2021). Multilevel growth curve analyses of behavioral activation for anhedonia (BATA) and mindfulness-based cognitive therapy effects on anhedonia and resting-state functional connectivity: Interim results of a randomized trial. Journal of Affective Disorders, 292, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, & Raulin ML (1976). Scales for physical and social anhedonia. Journal of Abnormal Psychology, 85, 374–382. [DOI] [PubMed] [Google Scholar]
- Chentsova-Dutton Y, & Hanley K (2010). The effects of anhedonia and depression on hedonic responses. Psychiatry Research, 179, 176–180. [DOI] [PubMed] [Google Scholar]
- Chung YS, & Barch D (2015). Anhedonia is associated with reduced incentive cue related activation in the basal ganglia. Cognitive, Affective & Behavioral Neuroscience, 15(4), 749–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, & Watson D (1991). Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology, 100(3), 316–336. 10.1037//0021-843x.100.3.316 [DOI] [PubMed] [Google Scholar]
- Clepce M, Gossler A, Reich K, Kornhuber J, & Thuerauf N (2010). The relation between depression, anhedonia and olfactory hedonic estimates--a pilot study in major depression. Neuroscience letters, 471(3), 139–143. 10.1016/j.neulet.2010.01.027 [DOI] [PubMed] [Google Scholar]
- Craske MG, Hermans D, & Vervliet B (2018). State of the art and future directions for extinction as a translational model for fear and anxiety. Philosophical Transactions B: Biological Sciences, 373(1742), 20170025. 10.1098/rstb.2017.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Hermans D, & Vervliet B (2018). State-of-the-art and future directions for extinction as a translational model for fear and anxiety. Philosophical Transactions of the Royal Society B, 373(1742). 10.1098/rstb.2017.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, & Dour HJ (2016). Treatment for anhedonia: A neuroscience driven approach. Depression and anxiety, 33(10), 927–938. [DOI] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour H, & Rosenfield D (2019). Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia. Journal of Consulting and Clinical Psychology, 87(5), 457–471. [DOI] [PubMed] [Google Scholar]
- Craske MG, Treanor M, Zbozinek TD, & Vervliet B (2022). Optimizing exposure therapy with an inhibitory retrieval approach and the OptEx Nexus. Behaviour Research and Therapy, 152, 104069. 10.1016/j.brat.2022.104069 [DOI] [PubMed] [Google Scholar]
- Crawford JR, & Henry JD (2004). The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British journal of clinical psychology, 43(3), 245–265. 10.1348/0144665031752934 [DOI] [PubMed] [Google Scholar]
- Davies CD, & Craske MG (2014). Low baseline pCO2 predicts poorer outcome from cognitive behavioral therapy: Evidence from a mixed anxiety disorders sample. Psychiatry Research, 219, 311–315. 10.1016/j.psychres.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, Donneau AF, Albert A, Ansseau M, Constant E, & van Heeringen K (2015). What is important in being cured from depression? Discordance between physicians and patients (1). Journal of Affective Disorders, 174, 390–396. 10.1016/j.jad.2014.12.004 [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, & Markou A (2012). The neurobiology of anhedonia and other reward-related deficits. Trends in neurosciences, 35(1), 68–77. 10.1016/j.tins.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, O’Reardon JP, Lovett ML, Gladis MM, Brown LL, & Gallop R (2005). Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry, 62(4), 409–416. 10.1001/archpsyc.62.4.409 [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, & Smoski M (2010). The Effects of Brief Behavioral Activation Therapy for Depression on Cognitive Control in Affective Contexts: An fMRI investigation. Journal of Affective Disorders, 126(1–2), 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, & Smoski MJ (2009). The effects of psychotherapy on neural responses to rewards in major depression. Biological Psychiatry, 66(9), 886–897. 10.1016/j.biopsych.2009.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Smith J, & Mirenowicz J (2000). Dissociation of Pavlovian and instrumental incentive learning under dopamine antagonists. Behavioral Neuroscience, 114(3), 468–483. 10.1037/0735-7044.114.3.468 [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, & Beck AT (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience, 12, 467–477. 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- Ducasse D, Dubois J, Jaussent I, Azorin JM, Etain B, Gard S, Henry C, Bougerol T, Kahn JP, Aubin V, Bellivier F, Belzeaux R, Dubertret C, Dubreucq J, Llorca PM, Loftus J, Passerieux C, Polosan M, Samalin L, … Courtet P (2021). Association between anhedonia and suicidal events in patients with mood disorders: A 3-year prospective study. Depression and anxiety, 38(1), 17–27. 10.1002/da.23072 [DOI] [PubMed] [Google Scholar]
- Ducasse D, Loas G, Dassa D, Gramaglia C, Zeppegno P, Guillaume S, Olié E, & Courtet P (2018). Anhedonia is associated with suicidal ideation independently of depression: A meta-analysis. Depression and Anxiety, 35(5), 382–392. 10.1002/da.22709 [DOI] [PubMed] [Google Scholar]
- Dunn BD (2012). Helping depressed clients reconnect to positive emotion experience: current insights and future directions. Clinical psychology & psychotherapy, 19(4), 326–340. 10.1002/cpp.1799 [DOI] [PubMed] [Google Scholar]
- Dunn BD, German RE, Khazanov G, Xu C, Hollon SD, & DeRubeis RJ (2020). Changes in Positive and Negative Affect During Pharmacological Treatment and Cognitive Therapy for Major Depressive Disorder: A Secondary Analysis of Two Randomized Controlled Trials. Clinical Psychological Science, 8(1), 36–51. 10.1177/2167702619863427 [DOI] [Google Scholar]
- Emmons RA, & McCullough ME (2003). Counting blessings versus burdens: An experimental investigation of gratitude and subjective well-being in daily life. Journal of Personality and Social Psychology, 84(2), 377–389. 10.1037//0022-3514.84.2.377 [DOI] [PubMed] [Google Scholar]
- Enders CK (2011). Missing not at random models for latent growth curve analyses. Psychological methods, 16(1), 1–16. 10.1037/a0022640 [DOI] [PubMed] [Google Scholar]
- Enkavi AZ, & Poldrack RA (2021). Implications of the Lacking Relationship Between Cognitive Task and Self-report Measures for Psychiatry. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 6(7), 670–672. 10.1016/j.bpsc.2020.06.010 [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, & Silbersweig DA (2006). Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. The American Journal of Psychiatry, 163(10), 1784–1790. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, & Gibbons RD (1983). Assessing Anhedonia in Psychiatric Patients: The Pleasure Scale. Arch Gen Psychiatry, 40(1), 79–84. doi: 10.1001/archpsyc.1983.01790010081010 [DOI] [PubMed] [Google Scholar]
- Fawcett J, Scheftner WA, Fogg L, Clark DC, Young MA, Hedeker D, & Gibbons R (1990). Time-related predictors of suicide in major affective disorder. The American Journal of Psychiatry, 147(9), 1189–1194. doi: 10.1176/ajp.147.9.1189 [DOI] [PubMed] [Google Scholar]
- Fiorito ER, & Simons RF (1994). Emotional imagery and physical anhedonia. Psychophysiology, 31(5), 513–521. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JB, Karg RS, & Spitzer RL (2015). Structured clinical interview for DSM-5-Research version (SCID-5 for DSM-5, research version; SCID-5-RV) (pp. 1–94). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Fowles DC, Fisher AE, & Tranel DT (1982). The heart beats to reward: the effect of monetary incentive on heart rate. Psychophysiology, 19(5), 506–513. doi: 10.1111/j.1469-8986.1982.tb02577.x [DOI] [PubMed] [Google Scholar]
- Fitzgibbons L, & Simons RF (1992). Affective response to color-slide stimuli in subjects with physical anhedonia: A three-systems analysis. Psychophysiology, 29(6), 613–620. [DOI] [PubMed] [Google Scholar]
- Forbes CN (2020). New directions in behavioral activation: Using findings from basic science and translational neuroscience to inform the exploration of potential mechanisms of change. Clinical Psychology Review, 79(101860), 101860. doi: 10.1016/j.cpr.2020.101860 [DOI] [PubMed] [Google Scholar]
- Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Birmaher B, Axelson DA, & Dahl RE (2006). Reward-related decision-making in pediatric major depressive disorder: an fMRI study. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 47(10), 1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, & Dahl RE (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. The American Journal of Psychiatry, 166(1), 64–73. doi: 10.1176/appi.ajp.2008.07081336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen J, & Brinkmann K (2015). Blunted cardiovascular reactivity in dysphoria during reward and punishment anticipation. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 95(3), 270–277. doi: 10.1016/j.ijpsycho.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Fredrickson BL (2001). The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. The American Psychologist, 56(3), 218–226. doi: 10.1037//0003-066x.56.3.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, & Finkel SM (2008). Open hearts build lives: Positive emotions, induced through loving-kindness meditation, build consequential personal resources. Journal of Personality and Social Psychology, 95(5), 1045–1062. doi: 10.1037/a0013262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsche A, Dahme B, Gotlib IH, Joormann J, Magnussen H, Watz H, … von Leupoldt A (2010). Specificity of cognitive biases in patients with current depression and remitted depression and in patients with asthma. Psychological Medicine, 40(5), 815–826. doi: 10.1017/S0033291709990948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froh JJ, Kashdan TB, Ozimkowski KM, & Miller N (2009). Who benefits the most from a gratitude intervention in children and adolescents? Examining positive affect as a moderator. The Journal of Positive Psychology, 4(5), 408–422. doi: 10.1080/17439760902992464 [DOI] [Google Scholar]
- Gard DE, Gard MG, Kring AM, & John OP (2006). Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality, 40(6), 1086–1102. doi: 10.1016/j.jrp.2005.11.001 [DOI] [Google Scholar]
- Garland EL, Fredrickson B, Kring AM, Johnson DP, Meyer PS, & Penn DL (2010). Upward spirals of positive emotions counter downward spirals of negativity: insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clinical Psychology Review, 30(7), 849–864. doi: 10.1016/j.cpr.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty AWA, Wood AM, & Hyland ME (2010a). Attrition from self-directed interventions: investigating the relationship between psychological predictors, intervention content and dropout from a body dissatisfaction intervention. Social Science & Medicine (1982), 71(1), 30–37. doi: 10.1016/j.socscimed.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Geraghty AWA, Wood AM, & Hyland ME (2010b). Dissociating the facets of hope: Agency and pathways predict dropout from unguided self-help therapy in opposite directions. Journal of Research in Personality, 44(1), 155–158. doi: 10.1016/j.jrp.2009.12.003 [DOI] [Google Scholar]
- Gloster AT, Rhoades HM, Novy D, Klotsche J, Senior A, Kunik M, … Stanley MA (2008). Psychometric properties of the Depression Anxiety and Stress Scale-21 in older primary care patients. Journal of Affective Disorders, 110(3), 248–259. doi: 10.1016/j.jad.2008.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, I R, J H, & Steele JD (2011). Expected value and prediction error abnormalities in depression and schizophrenia. Brain, 134(6), 1751–1764. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Chase HW, Almeida JR, Stiffler R, Zevallos CR, Aslam HA, Deckersbach T, Weyandt S, Cooper C, Toups M, Carmody T, Kurian B, Peltier S, Adams P, McInnis MG, Oquendo MA, McGrath PJ, Fava M, Weissman M, … Phillips ML (2015). Moderation of the relationship between reward expectancy and prediction error-related ventral striatal reactivity by anhedonia in unmedicated major depressive disorder: Findings from the EMBARC study. The American Journal of Psychiatry, 172(9), 881–891. doi: 10.1176/appi.ajp.2015.14050594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ (1998). The emerging field of emotion regulation: An integrative review. Review of General Psychology: Journal of Division 1, of the American Psychological Association, 2(3), 271–299. doi: 10.1037/1089-2680.2.3.271 [DOI] [Google Scholar]
- Hallford DJ, Farrell H, & Lynch E (2022). Increasing anticipated and anticipatory pleasure through episodic thinking. Emotion (Washington, D.C.), 22(4), 690–700. doi: 10.1037/emo0000765 [DOI] [PubMed] [Google Scholar]
- Hallford DJ, Sharma MK, & Austin DW (2020). Increasing anticipatory pleasure in major depression through enhancing episodic future thinking: A randomized single-case series trial. Journal of Psychopathology and Behavioral Assessment, 42(4), 751–764. doi: 10.1007/s10862-020-09820-9 [DOI] [Google Scholar]
- Hamer RM, & Simpson PM (2009). Last observation carried forward versus mixed models in the analysis of longitudinal psychiatric clinical trials. American Journal of Psychiatry, 166, 639–641. [DOI] [PubMed] [Google Scholar]
- Heck RH, Thomas SL, & Tabata LN (2022). Multilevel and longitudinal modeling with IBM SPSS (3rd ed.). London, England: Routledge. [Google Scholar]
- Henriques JB, & Davidson RJ (2000). Decreased responsiveness to reward in depression. Cognition & Emotion, 14(5), 711–724. doi: 10.1080/02699930050117684 [DOI] [Google Scholar]
- Hoerger M, Quirk SW, Chapman BP, & Duberstein PR (2012). Affective forecasting and self-rated symptoms of depression, anxiety, and hypomania: evidence for a dysphoric forecasting bias. Cognition & Emotion, 26(6), 1098–1106. doi: 10.1080/02699931.2011.631985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Grossman P, & Hinton DE (2011). Loving-kindness and compassion meditation: potential for psychological interventions. Clinical Psychology Review, 31(7), 1126–1132. doi: 10.1016/j.cpr.2011.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Petrocchi N, Steinberg J, Lin M, Arimitsu K, Kind S, … Stangier U (2015). Loving-kindness meditation to target affect in mood disorders: A proof-of-concept study. Evidence-Based Complementary and Alternative Medicine: ECAM, 2015, 269126. doi: 10.1155/2015/269126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D, Murray SJ, Perron A, & Rail G (2006). Deconstructing the evidence-based discourse in health sciences: truth, power and fascism. International Journal of Evidence-Based Healthcare, 4(3), 180–186. doi: 10.1111/j.1479-6988.2006.00041.x [DOI] [PubMed] [Google Scholar]
- Holmes EA, Ghaderi A, Harmer CJ, Ramchandani PG, Cuijpers P, Morrison AP, … Craske MG (2018). The Lancet Psychiatry Commission on psychological treatments research in tomorrow’s science. The Lancet. Psychiatry, 5(3), 237–286. doi: 10.1016/S2215-0366(17)30513-8 [DOI] [PubMed] [Google Scholar]
- Holmes EA, Mathews A, Mackintosh B, & Dalgleish T (2008). The causal effect of mental imagery on emotion assessed using picture-word cues. Emotion, 8(3), 395–409. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Pitman RK, Su Z, Heyman GM, Lasko NB, Macklin ML, Orr SP, Lukas SE, & Elman I (2008). Probing reward function in posttraumatic stress disorder: expectancy and satisfaction with monetary gains and losses. Journal of Psychiatric Research, 42(10), 802–807. doi: 10.1016/j.jpsychires.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M, Wilner N, & Alvarez W (1979). Impact of Event Scale: a measure of subjective stress. Psychosomatic Medicine, 41(3), 209–218. doi: 10.1097/00006842-197905000-00004 [DOI] [PubMed] [Google Scholar]
- Hox J (2010). Multilevel analysis: Techniques and applications, second edition (2nd ed.). Routledge. [Google Scholar]
- Hox JJ, Moerbeek M, Van de Schoot R (2018). Multilevel Analysis: Techniques and Applications. New York, NY: Routledge. [Google Scholar]
- Hutcherson CA, Seppala EM, & Gross JJ (2008). Loving-kindness meditation increases social connectedness. Emotion (Washington, D.C.), 8(5), 720–724. doi: 10.1037/a0013237 [DOI] [PubMed] [Google Scholar]
- Iaboni F, Douglas VI, & Ditto B (1997). Psychophysiological response of ADHD children to reward and extinction. Psychophysiology, 34(1), 116–123. doi: 10.1111/j.1469-8986.1997.tb02422.x [DOI] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, & Ernst M (2005). Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biological Psychiatry, 58(8), 632–639. doi: 10.1016/j.biopsych.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, & Gotlib IH (2007). Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology, 116(1), 135–143. doi: 10.1037/0021-843X.116.1.135 [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Weeks JW, & Savostyanova AA (2011). Whether, how, and when social anxiety shapes positive experiences and events: a self-regulatory framework and treatment implications. Clinical Psychology Review, 31(5), 786–799. doi: 10.1016/j.cpr.2011.03.012 [DOI] [PubMed] [Google Scholar]
- Kaz A, Sarapas C, Bishop J, Patel S, & Shankman S (2015). The mediating effect of prefrontal asymmetry on the relationship between the COMT Val(158)Met SNP and trait consummatory positive affect. Cognition and Emotion, 29, 867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney DJ, Malte CA, McManus C, Martinez ME, Felleman B, & Simpson TL (2013). Loving-kindness meditation for posttraumatic stress disorder: a pilot study. Journal of Traumatic Stress, 26(4), 426–434. doi: 10.1002/jts.21832 [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, & Phillips ML (2005). The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry, 58(11), 843–853. doi: 10.1016/j.biopsych.2005.05.019 [DOI] [PubMed] [Google Scholar]
- Kellough JL, Beevers CG, Ellis AJ, & Wells TT (2008). Time course of selective attention in clinically depressed young adults: an eye tracking study. Behaviour Research and Therapy, 46(11), 1238–1243. doi: 10.1016/j.brat.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall AD, Zinbarg RE, Mineka S, Bobova L, Prenoveau JM, Revelle W, & Craske MG (2015). Prospective associations of low positive emotionality with first onsets of depressive and anxiety disorders: Results from a 10-wave latent trait-state modeling study. Journal of Abnormal Psychology, 124(4), 933–943. doi: 10.1037/abn0000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazanov GK, & Ruscio AM (2016). Is low positive emotionality a specific risk factor for depression? A meta-analysis of longitudinal studies. Psychological Bulletin, 142(9), 991–1015. doi: 10.1037/bul0000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, & Gotlib IH (2008). Neural responses to monetary incentives in major depression. Biological Psychiatry, 63(7), 686–692. doi: 10.1016/j.biopsych.2007.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Verschuere B, & De Houwer J (2004). Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behaviour Research and Therapy, 42(10), 1183–1192. doi: 10.1016/j.brat.2003.08.001 [DOI] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, & Watson D (2010). Linking ‘big’ personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychological Bulletin, 136(5), 768–821. doi: 10.1037/a0020327 [DOI] [PubMed] [Google Scholar]
- Krystal AD, Pizzagalli DA, Smoski M, Mathew SJ, Nurnberger J, Lisanby SH, Iosifescu D, Murrough JW, Yang H, Weiner RD, Calabrese JR, Sanacora G, Hermes G, Keefe RSE, Song A, Goodman W, Szabo ST, Whitton AE, Gao K, & Potter WZ (2020). A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nature Medicine, 26(5), 760–768. doi: 10.1038/s41591-020-0806-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryza-Lacombe M, Pearson N, Lyubomirsky S, Stein MB, Wiggins JL, & Taylor CT (2021). Changes in neural reward processing following Amplification of Positivity treatment for depression and anxiety: Preliminary findings from a randomized waitlist controlled trial. Behaviour Research and Therapy, 142(103860), 103860. doi: 10.1016/j.brat.2021.103860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landén M, Högberg P, & Thase ME (2005). Incidence of sexual side effects in refractory depression during treatment with citalopram or paroxetine. The Journal of Clinical Psychiatry, 66(1), 100–106. doi: 10.4088/jcp.v66n0114 [DOI] [PubMed] [Google Scholar]
- Lackner HK, Weiss EM, Schulter G, Hinghofer-Szalkay H, Samson AC, & Papousek I (2013). I got it! Transient cardiovascular response to the perception of humor. Biological Psychology, 93(1), 33–40. doi: 10.1016/j.biopsycho.2013.01.014 [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, & Hamm AO (1993). Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology, 30(3), 261–273. [DOI] [PubMed] [Google Scholar]
- Litz BT, Orsillo SM, Kaloupek D, & Weathers F (2000). Emotional processing in posttraumatic stress disorder. Journal of Abnormal Psychology, 109(1), 26–39. doi: 10.1037/0021-843x.109.1.26 [DOI] [PubMed] [Google Scholar]
- Liu W, Wang L, Shang H, Shen Y, Li Z, Cheung EF, & Chan RC (2014). The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia, 53, 213–220. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, & Lovibond SH (1995). The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy, 33(3), 335–343. doi: 10.1016/0005-7967(94)00075-u [DOI] [PubMed] [Google Scholar]
- Lovibond SH, & Lovibond PF (1995). Manual for the depression anxiety stress scales. Psychology Foundation of Australia. [Google Scholar]
- Maas CJM & Hox JJ (2005). Sufficient sample sizes for multilevel modeling. Methodology, 1, 86–92. [Google Scholar]
- Maltby J, Wood AM, Day L, Kon TWH, Colley A, & Linley PA (2008). Personality predictors of levels of forgiveness two and a half years after the transgression. Journal of Research in Personality, 42(4), 1088–1094. doi: 10.1016/j.jrp.2007.12.008 [DOI] [Google Scholar]
- Martell CR, Dimidjian S, & Herman-Dunn R (2010). Behavioral activation for depression: A clinician’s guide. The Guilford Press. [Google Scholar]
- Matsumoto D, & Ekman P (1988). Japanese and Caucasian facial expressions of emotion (JACFEE) Department of Psychology, San Francisco State University. [Google Scholar]
- Mayhew SL, & Gilbert P (2008). Compassionate mind training with people who hear malevolent voices: A case series report. Clinical Psychology & Psychotherapy, 15(2), 113–138. 10.1002/cpp.566 [DOI] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, & Harmer CJ (2009). Neural representation of reward in recovered depressed patients. Psychopharmacology. 205(4):667–77. 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, & Harmer CJ (2010). Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biological Psychiatry, 67(5), 439–445. 10.1016/j.biopsych.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, & Harmer CJ (2010). Diminished Neural Processing of Aversive and Rewarding Stimuli During Selective Serotonin Reuptake Inhibitor Treatment. Biological Psychiatry, 67, 439–445. 10.1016/j.biopsych.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland BR, & Klein DN (2009). Emotional reactivity in depression: Diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depression and Anxiety, 26(2), 117–122. 10.1002/da.20513 [DOI] [PubMed] [Google Scholar]
- McGuire JT, & Kable JW (2010). Medial prefrontal cortical activity reflects dynamic re-evaluation during voluntary persistence. Nature Neuroscience, 18(5), 760–766. 10.1038/nn.3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Siegle GJ, & Shirk S (2011). Positive Affect Stimulation and Sustainment (PASS) module for depressed mood: A preliminary investigation of treatment-related effects. Cognitive Therapy and Research, 35(3), 217–226. 10.1007/s10608-010-9311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Ritz T, Wilhelm FH, Roth WT, & Rosenfield D (2018). Hypoventilation therapy alleviates panic by repeated induction of dyspnea. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3, 539–545. 10.1016/j.bpsc.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Seidel A, Bhaskara L, & Hofmann SG (2010). Respiratory and cognitive mediators of treatment change in panic disorder: Evidence for intervention specificity. Journal of Consulting and Clinical Psychology, 78, 691–704. 10.1037/a0019552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, & Roth WT (2008). Feedback of end-tidal pCO2 as a therapeutic approach for panic disorder. Journal of Psychiatric Research, 42, 560–568. 10.1016/j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miendlarzewska EA, Bavelier D, & Schwartz S (2016). Influence of reward motivation on human declarative memory. Neuroscience and Biobehavioral Reviews, 61, 156–176. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Wise T, Suthana N, Guzmán N, Kriegeskorte N, & Leibo JZ (2021). Promises and challenges of human computational ethology. Neuron, 109(14), 2224–2238. 10.1016/j.neuron.2021.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Chattillion EA, Ceglowski J, Ho J, Känel R, Mills PJ, Ziegler MG, Patterson TL, Grant I, & Mausbach BT (2013). A randomized clinical trial of Behavioral Activation (BA) therapy for improving psychological and physical health in dementia caregivers: Results of the Pleasant Events Program (PEP. Behaviour Research and Therapy, 51(10), 623–632. 10.1016/j.brat.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris BH, Bylsma LM, & Rottenberg J (2009). Does emotion predict the course of major depressive disorder? A review of prospective studies. British Journal of Clinical Psychology, 48(Pt 3), 255–273. 10.1348/014466508x396549 [DOI] [PubMed] [Google Scholar]
- Morris BH, Bylsma LM, Yaroslavsky I, Kovacs M, & Rottenberg J (2015). Reward learning in pediatric depression and anxiety: preliminary findings in a high-risk sample. Depression and anxiety, 32(5), 373–381. 10.1002/da.22358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naragon-Gainey K, Prenoveau JM, Brown TA, & Zinbarg RE (2016). A comparison and integration of structural models of depression and anxiety in a clinical sample: Support for and validation of the tri-level model. Journal of Abnormal Psychology, 125, 853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SK, Layous K, Cole SW, & Lyubomirsky S (2016). Do unto others or treat yourself? The effects of prosocial and self-focused behavior on psychological flourishing. Emotion, 16(6), 850–861. 10.1037/emo0000178 [DOI] [PubMed] [Google Scholar]
- Ng F, Trauer T, Dodd S, Callaly T, Campbell S, & Berk M (2007). The validity of the 21-item version of the Depression Anxiety Stress Scales as a routine clinical outcome measure. Acta Neuropsychiatrica, 19(5), 304–310. 10.1111/j.1601-5215.2007.00217.x [DOI] [PubMed] [Google Scholar]
- Nieremberg AA, Keefe BR, Leslie VC, Alpert JE, Pava JA, Worthington JJ 3rd, Rosenbaum JF, & Fava M (1999). Residual symptoms in depressed patients who respond acutely to fluoxetine. The Journal of Clinical Psychiatry, 60(4), 221–225. 10.4088/jcp.v60n0403 [DOI] [PubMed] [Google Scholar]
- Nord CL, Lawson RP, Huys QJM, Pilling S, & Roiser JP (2018). Depression is associated with enhanced aversive Pavlovian control over instrumental behaviour. Scientific Reports, 8(1), 12582. 10.1038/s41598-018-30828-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham AD, McHugh RK, & Otto MW (2010). A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety, 27(12), 1135–1142. 10.1002/da.20755 [DOI] [PubMed] [Google Scholar]
- Peng Y, Knotts JD, Taylor CT, Craske MG, Stein MB, Bookheimer S, Young KS, Simmons AN, Yeh HW, Ruiz J, & Paulus MP (2021). Failure to Identify Robust Latent Variables of Positive or Negative Valence Processing Across Units of Analysis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 6(5), 518–526. 10.1016/j.bpsc.2020.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KD, Constans JI, & Mathews A (2011). Experimental modification of attribution processes. Journal of Abnormal Psychology, 120(1), 168–173. 10.1037/a0021899 [DOI] [PubMed] [Google Scholar]
- Pictet A, Coughtrey AE, Mathews A, & Holmes EA (2011). Fishing for happiness: The effects of generating positive imagery on mood and behaviour. Behaviour Research and Therapy, 49(12), 885–891. 10.1016/j.brat.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pictet A, Coughtrey A, Mathews A, & Holmes E (2011). Fishing for happiness: The effects of positive imagery on interpretation bias and a behavioral task. Behaviour Research and Therapy, 49(12), 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pictet A, Jermann F, & Ceschi G (2016). When less could be more: Investigating the effects of a brief internet-based imagery cognitive bias modification intervention in depression. Behaviour Research and Therapy, 84, 45–51. 10.1016/j.brat.2016.07.008 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA (2014). Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annual Review of Clinical Psychology, 10(1), 393–423. 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes, Dillon DG, Holmes AJ, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated subjects with Major Depressive Disorder. American Journal of Psychiatry, 166, 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, & Fava M (2008). Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. Journal of Psychiatric Research, 43(1), 76–87. 10.1016/j.jpsychires.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, & Davidson RJ (2005). Frontal Brain Asymmetry and reward responsiveness. Psychological Science, 16(10), 805–813. 10.1111/j.1467-9280.2005.01618.x [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Smoski M, Ang YS, Whitton AE, Sanacora G, Mathew SJ, Nurnberger J Jr., Lisanby SH, Iosifescu DV, Murrough JW, Yang H, Weiner RD, Calabrese JR, Goodman W, Potter WZ, & Krystal AD (2020). Selective kappa-opioid antagonism ameliorates anhedonic behavior: evidence from the Fast-fail Trial in Mood and Anxiety Spectrum Disorders (FAST-MAS). Neuropsychopharmacology, 45(10), 1656–1663. 10.1038/s41386-020-0738-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenoveau JM, Zinbarg RE, Craske MG, Mineka S, Griffith JW, & Epstein AM (2010). Testing a hierarchical model of anxiety and depression in adolescents: A tri-level model. Journal of Anxiety Disorders, 24(3), 334–344. 10.1016/j.janxdis.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Price J, Cole V, & Goodwin GM (2009). Emotional side-effects of selective serotonin reuptake inhibitors: Qualitative study. British Journal of Psychiatry, 195(3), 211–217. 10.1192/bjp.bp.108.051110 [DOI] [PubMed] [Google Scholar]
- Rackoff GN, & Newman MG (2020). Reduced positive affect on days with stress exposure predicts depression, anxiety disorders, and low trait positive affect 7 years later. Journal of abnormal psychology, 129(8), 799–809. 10.1037/abn0000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M, & Gendolla GH (2007). Incentive value, unclear task difficulty, and cardiovascular reactivity in active coping. International Journal of Psychophysiology, Mar;63(3):294–301. 10.1016/j.ijpsycho.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Quilty LC, Sproule BA, Cyriac A, Michael Bagby R, & Kennedy SH (2015). Development and validation of the Dimensional Anhedonia Rating Scale (DARS) in a community sample and individuals with major depression. Psychiatry Research, 229(1-2), 109–119. 10.1016/j.psychres.2015.07.062 [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, & Drevets WC (2012). Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. American Journal of Psychiatry, 169(2), 152–159. 10.1176/appi.ajp.2011.11010137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland L, & Curry OS (2019). A range of kindness activities boost happiness. The Journal of Social Psychology, 159(3), 340–343. 10.1080/00224545.2018.1469461 [DOI] [PubMed] [Google Scholar]
- Rudaizky D, Basanovic J, & MacLeod C (2014). Biased attentional engagement with, and disengagement from, negative information: Independent cognitive pathways to anxiety vulnerability? Cognition & Emotion, 28(2), 245–259. 10.1080/02699931.2013.815154 [DOI] [PubMed] [Google Scholar]
- Schacter HL, & Margolin G (2019). When it feels good to give: Depressive symptoms, daily prosocial behavior, and adolescent mood. Emotion. 10.1037/emo0000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewart AR, Zbozinek TD, Hammen C, Zinbarg RE, Mineka S, & Craske MG (2019). Positive Affect as a Buffer Between Chronic Stress and Symptom Severity of Emotional Disorders. Clinical Psychological Science, 7(5), 914–927. 10.1177/2167702619834576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Barrett MS, Cucchiara AJ, Gooneratne NS, & Thase ME (2017). A Breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: A randomized pilot study. The Journal of Clinical Psychiatry, 78, 59–63. 10.4088/JCP.16m10819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea TL, Tennant A, & Pallant JF (2009). Rasch model analysis of the Depression, Anxiety and Stress Scales (DASS). BMC Psychiatry, 9, 21. 10.1186/1471-244x-9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, & Raj BA (1996). The measurement of disability. International Clinical Psychopharmacology, 11, 89–95. [DOI] [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, & Gotlib IH (2012). Anticipatory pleasure predicts motivation for reward in major depression. Journal of Abnormal Psychology, 121, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonin E, Van Gordon W, Compare A, Zangeneh M, & Griffiths MD (2015). Buddhist-Derived Loving-Kindness and Compassion Meditation for the Treatment of Psychopathology: a Systematic Review. Mindfulness, 6(5), 1161–1180. 10.1007/s12671-014-0368-1 [DOI] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, & Dichter GS (2009). FMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders, 118(1–3), 69–78. 10.1016/j.jad.2009.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, & Trigwell P (1995). A scale for the assessment of hedonic tone the snaith–hamilton pleasure scale. British Journal of Psychiatry, 167(1), 99–103. 10.1192/bjp.167.1.99 [DOI] [PubMed] [Google Scholar]
- Snijders TA, & Bosker RJ (1993). Standard errors and sample sizes for two-level research. Journal of Educational Statistics, 18(3), 237. 10.2307/1165134 [DOI] [Google Scholar]
- Snippe E, Jeronimus BF, Aan Het Rot M, Bos EH, Jonge P, & Wichers M (2018). The Reciprocity of Prosocial Behavior and Positive Affect in Daily Life. Journal of Personality, 86(2), 139–146. 10.1111/jopy.12299 [DOI] [PubMed] [Google Scholar]
- Soltani S, Newman K, Quigley L, Fernandez A, Dobson K, & Sears C (2015). Temporal changes in attention to sad and happy faces distinguish currently and remitted depressed individuals from never depressed individuals. Psychiatry Research, 230(2), 454–463. 10.1016/j.psychres.2015.09.036 [DOI] [PubMed] [Google Scholar]
- Srivastava S, Sharma HO, & Mandal MK (2003). Mood induction with facial expressions of emotion in patients with generalized anxiety disorder. Depression and Anxiety, 18(3), 144–148. 10.1002/da.10128 [DOI] [PubMed] [Google Scholar]
- Staines GL (2007). Comparative outcome evaluations of psychotherapies: Guidelines for addressing eight limitations of the gold standard of causal inference. Psychotherapy (Chic), 44(2), 161–174. 10.1037/0033-3204.44.2.161 [DOI] [PubMed] [Google Scholar]
- Steele JD, Kumar P, & Ebmeier KP (2007). Blunted response to feedback information in depressive illness. Brain, 130(9), 2367–2374. 10.1093/brain/awm150 [DOI] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hägele C, Suchotzki K, Schmack K, Wrase J, Ricken R, Knutson B, Adli M, Bauer M, Heinz A, & A.J S (2012). Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. Journal of Psychopharmacology, 26(5), 677–688. 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Swiecicki L, Zatorski P, Bzinkowska D, Sienkiewicz-Jarosz H, Szyndler J, & Scinska A (2009). Gustatory and olfactory function in patients with unipolar and bipolar depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 33(5), 827–834. 10.1016/j.pnpbp.2009.03.030 [DOI] [PubMed] [Google Scholar]
- Talmi D, Seymour B, Dayan P, & Dolan RJ (2008). Human pavlovian-instrumental transfer. Journal of Neuroscience, 28(2), 360–368. 10.1523/jneurosci.4028-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir M, & Robinson MD (2007). The happy spotlight: Positive mood and selective attention to rewarding information. Personality and Social Psychology Bulletin, 33(8), 1124–1136. 10.1177/0146167207301030 [DOI] [PubMed] [Google Scholar]
- Taylor CT & Amir N. (2010). Attention and emotion regulation. Emotion regulation and psychopathology: A transdiagnostic approach to etiology and treatment. 380–404. [Google Scholar]
- Taylor CT, Bomyea J, & Amir N (2010). Attentional bias away from positive social information mediates the link between social anxiety and anxiety vulnerability to a social stressor. Journal of anxiety disorders, 24(4), 403–408. 10.1016/j.janxdis.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CT, Lyubomirsky S, & Stein MB (2017). Upregulating the positive affect system in anxiety and depression: Outcomes of a positive activity intervention. Depression and Anxiety, 34(3), 267–280. 10.1002/da.22593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RK, Baker G, Lind J, & Dursun S (2018). Rapid effectiveness of intravenous ketamine for ultraresistant depression in a clinical setting and evidence for baseline anhedonia and bipolarity as clinical predictors of effectiveness. Journal of Psychopharmacology, 32, 1110–1117. [DOI] [PubMed] [Google Scholar]
- Thomsen K, Whybrow PC, & Kringelbach ML (2015). Reconceptualizing anhedonia: Novel perspectives on balancing the pleasure networks in the human brain. Frontiers in Behavioral Neuroscience, 9. 10.3389/fnbeh.2015.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel DT, Fisher AE, & Fowles DC (1982). Magnitude of incentive effects on heart rate. Psychophysiology. 10.1111/j.1469-8986.1982.tb02578.x. [DOI] [PubMed] [Google Scholar]
- Treadway MT, & Zald DH (2011). Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience & Biobehavioral Reviews, 35(3), 537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, & Zald DH (2012). Effort-based decision-making in major depressive disorder: A translational model of motivational anhedonia. Journal of Abnormal Psychology. 121, 553–558. 10.1037/a0028813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, & Zald DH (2009). Worth the ‘EEfRT’? The Effort Expenditure for Rewards Task as an Objective Measure of Motivation and Anhedonia. PloS one, 4(8), e6598. 10.1371/journal.pone.0006598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, & Busto UE (2005). Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Archives of General Psychiatry, 62(11), 1228–1236. [DOI] [PubMed] [Google Scholar]
- Trøstheim M, Eikemo M, & Meir R (2020). Assessment of Anhedonia in Adults With and Without Mental Illness: A Systematic Review and Meta-analysis. JAMA Network Open, 3(8). 10.1001/jamanetworkopen.2020.13233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubl B, Kuehner C, Kirsch P, Ruttorf M, Diener C, & Flor H (2015). Altered neural reward and loss processing and prediction error signalling in depression. Social cognitive and affective neuroscience, 10(8), 1102–1112. 10.1093/scan/nsu158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vico C, Guerra P, Robles H, Vila J, & Anllo-Vento L (2010). Affective processing of loved faces: contributions from peripheral and central electrophysiology. Neuropsychologia, 48, 2894–2902. 10.1016/j.neuropsychologia.2010.05.031 [DOI] [PubMed] [Google Scholar]
- Villanueva CM, Silton RL, Heller W, Barch DM, & Gruber J (2021). Change is on the horizon: Call to action for the study of positive emotion and reward in psychopathology. Current Opinion in Behavioral Sciences, 39, 34–40. 10.1016/j.cobeha.2020.11.008 [DOI] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Schmidt M, & Claes S (2013). Reduced reward learning predicts outcome in major depressive disorder. Biological Psychiatry, 73(7), 639–645. 10.1016/j.biopsych.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, & Pizzagalli DA (2009). The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. NeuroImage, 46(1), 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadlinger HA, & Isaacowitz DM (2008). Looking happy: the experimental manipulation of a positive visual attention bias. Emotion, 8(1), 121–126. 10.1037/1528-3542.8.1.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadlinger HA, & Isaacowitz DM (2011). Fixing our focus: training attention to regulate emotion. Personality and Social Psychology Review, 15(1), 75–102. 10.1177/1088868310365565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadlinger HA, & Isaacowitz IDML (2008). The experimental manipulation of a positive visual attention bias. Emotion, 8(1), 121–126. 10.1037/1528-3542.8.1.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, & Maxwell SE (2015). On disaggregating between-person and within-person effects in longitudinal data using multilevel models. Psychological Methods, 20, 63–83. [DOI] [PubMed] [Google Scholar]
- Wang S, Leri F, & Rizvi SJ (2021). Anhedonia as a central factor in depression: neural mechanisms revealed from preclinical to clinical evidence. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 110, 110289. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology, 54(6), 1063. [DOI] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, & Pizzagalli DA (2015). Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry, 28(1), 7–12. 10.1097/yco.0000000000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willams JBW (2000). Mental health status, functioning, and disabilities measures. In American Psychiatric Association (Eds), Handbook of psychiatric measures (pp. 93–100). American Psychiatric Association. [Google Scholar]
- Winer ES, & Salem T (2016). Reward devaluation: Dot-probe meta-analytic evidence of avoidance of positive information in depressed persons. Psychological Bulletin, 142(1), 18–78. 10.1037/bul0000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer ES, Nadorff MR, Ellis TE, Allen JG, Herrera S, & Salem T (2014). Anhedonia predicts suicidal ideation in a large psychiatric inpatient sample. Psychiatry Research, 218(1–2), 124–128. 10.1016/j.psychres.2014.04.016 [DOI] [PubMed] [Google Scholar]
- Wood AM, JJ F, & Geraghty AWA (2010). Gratitude and well-being: A review and theoretical integration. Clinical Psychology Review, 30(7), 890–905. 10.1016/j.cpr.2010.03.005 [DOI] [PubMed] [Google Scholar]
- Wood AM, Joseph S, & Maltby J (2008). Gratitude uniquely predicts satisfaction with life: Incremental validity above the domains and facets of the five factor model. Personality and Individual Differences, 45(1), 49–54. 10.1016/j.paid.2008.02.019 [DOI] [Google Scholar]
- Yang XH, Huang J, Zhu CY, Wang YF, Cheung EF, Chan RC, & Xie GR (2014). Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Research, 220(3), 874–882. [DOI] [PubMed] [Google Scholar]
- Yuan Joyce W. & Kring Ann M. (2009) Dysphoria and the prediction and experience of emotion, Cognition and Emotion, 23:6, 1221–1232, DOI: 10.1080/02699930802416453 [DOI] [Google Scholar]
- Zeng X, Chiu CPK, Wang R, Oei TPS, & Leung FYK (2015). The effect of loving-kindness meditation on positive emotions: a meta-analytic review. Frontiers in Psychology, 6. 10.3389/fpsyg.2015.01693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Wang R, Oei TPS, & Leung FYK (2019). Heart of joy: A randomized controlled trial evaluating the effect of an appreciative joy meditation training on subjective well-being and attitudes. Mindfulness, 10(3), 506–515. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.