Abstract
Ketamine, an N-methyl-D-aspartate receptor (NMDAR) antagonist first developed as an anesthetic, has shown significant promise as a medication with rapid antidepressant properties in treatment-resistant depression. However, concerns such as adverse side effects and potential misuse liability have limited its widespread use. Racemic ketamine has two enantiomers—(S)- and (R)-ketamine—that appear to have disparate underlying mechanisms. This brief review summarizes some of the most recent preclinical and clinical research regarding the convergent and divergent prophylactic, immediate, and sustained antidepressant effects of (S)- and (R)-ketamine while addressing potential differences in their side effect and misuse liability profiles. Preclinical research suggests divergent mechanisms underlying (S)- and (R)-ketamine, with (S)-ketamine more directly affecting mechanistic target of rapamycin complex 1 (mTORC1) signaling and (R)-ketamine more directly affecting extracellular signal-related kinase (ERK) signaling. Clinical research suggests that (R)-ketamine has a milder side effect profile than (S)-ketamine and decreases depression rating scale scores, but recent randomized, controlled trials found that it had no significant antidepressant efficacy compared to placebo, suggesting that caution is warranted in interpreting its therapeutic potential. Future preclinical and clinical research is needed to maximize the efficacy of each enantiomer, either by optimizing dose, route of administration, or administration paradigm.
Introduction
The N-methyl-D-aspartate receptor (NMDAR) antagonist ketamine was first developed as a shorter-acting, less psychoactive anesthetic than phencyclidine [1]. Due to its broad safety profile, ketamine was soon approved for human anesthesia and used extensively as a battlefield medication during the Vietnam War. As an anesthetic, ketamine was also found to prevent shock, decrease hyperalgesia, and have neuroprotective effects, including anti-inflammatory actions [2]. However, from the earliest days of its use as an anesthetic, reports emerged of patients experiencing sensations of floating and other dissociative effects after administration, which led to the coining of the term “dissociative anesthetic” [3]. In addition to these psychotomimetic effects, some patients experienced increases in intracranial pressure [4], lowering of seizure thresholds [5], hypertension [6], and tachycardia that led ketamine to be used less frequently [7]. The US Controlled Substances Act subsequently classified ketamine as a Class III substance in parallel with rising social concerns about its potential for misuse [1].
Despite these concerns, researchers in the early 1990s began investigating the potential antidepressant-like actions of another NMDAR antagonist, MK-801 [8]. In parallel, other investigators found that stress increased the probability of glutamate release in specific brain regions [9], sparking a newfound interest in the effects of glutamatergic modulation in depression. Building on this work, Berman and colleagues conducted a small, proof-of-concept clinical study that led to a paradigm shift in psychiatry, finding that subanesthetic-dose ketamine induced rapid and robust antidepressant effects, an outcome previously unheard of with traditional monoaminergic-based therapeutics [10].
These rapid-acting effects were subsequently replicated in participants with treatment-resistant depression (TRD) and bipolar depression, with remission rates significantly higher than those of traditional antidepressants [11,12]. Over the past two decades, multiple randomized, placebo-controlled trials have validated these initial observations [13–15]. In addition to ketamine’s actions as a rapid-acting antidepressant, it appears to uniquely target symptoms that are often resistant to traditional antidepressants, such as anhedonia and suicidal ideation. For instance, multiple meta-analyses have shown that acute intravenous ketamine led to significant short-term reductions in suicidal ideation [16,17], which could be extremely useful in emergency medicine settings. Intranasal esketamine, the (S)-enantiomer of ketamine, has similarly been shown to reduce suicidal ideation in participants with depression [18]. Notably, anhedonia often does not respond to monoaminergic-based antidepressants despite being one of the core symptoms of depression [19,20]; ketamine, however, has proven quite successful at clinically resolving anhedonia symptoms [21]. Ketamine also appears to have broad therapeutic properties, demonstrating treatment efficacy in obsessive compulsive disorder (OCD), social anxiety disorders, and post-traumatic stress disorder (PTSD), which are often comorbid with major depressive disorder (MDD) and impact further treatment resistance [22–26]. However, these results are preliminary, as most of these studies were small and require replication. Notably, one recent, randomized, controlled trial found no significant improvement in PTSD symptoms after ketamine administration [27].
Collectively, this evidence led the Food and Drug Administration (FDA) to approve intranasal esketamine (Spravato)—the (S)-enantiomer of ketamine—for adults with TRD in 2019 and for adults with MDD and acute suicidal ideation or behavior in 2020. The European Union also approved esketamine for the same indications in 2019 and 2021, respectively. In addition, off-label use of intravenous ketamine has demonstrated robust short-term efficacy in TRD and treatment-resistant bipolar disorder, but few studies have explored ketamine’s real-world efficacy or explored optimal dosing and routes of administration [15]. A recent real-world effectiveness meta-analysis found robust efficacy on average, although certain populations—including those with TRD—experienced more variable results [13]. The longer-term effects of ketamine have also been called into question, given that the effectiveness of acute doses generally peaks at 24 hours and lasts up to two weeks after administration [28,29].
The discovery of ketamine’s rapid and potent therapeutic effects led researchers to explore whether other agents could either be developed or repurposed with antidepressant effects similar to those of ketamine but lacking its undesirable side effects; that question seems most likely to be answered by exploring ketamine’s own pharmacology. This review seeks to briefly summarize recent research conducted on ketamine’s enantiomer-specific actions, with a focus on the prophylactic, immediate, and sustained antidepressant effects of ketamine’s enantiomers in clinical and preclinical research.
The Metabolism of Ketamine and its Pharmacokinetics
Ketamine is an aryl-cyclo-alkylamine that is extremely water and lipid soluble, with a pKa of 7.5 and a molecular mass of 238 g/mol [30]. Due to its lipid solubility, ketamine is extensively distributed across the body. At steady-state, its plasma distribution volume is 2.3 L/kg with a high clearance rate that is equivalent to and dependent on liver blood flow (12-20 ml/min/kg), making ketamine’s half-life around two to three hours [31]. In addition, women appear to have a 20% higher clearance rate of ketamine than men [32]. The majority of ketamine (80%) is metabolized rapidly to norketamine through nitrogen-mediated demethylation. This demethylation is mainly catalyzed by cytochrome P450 liver enzymes [33,34]. CYP2B6 and CYP3A4 are thought to be the primary liver enzymes responsible for demethylation to norketamine [35,36], which is subsequently followed by metabolism to dehydronorketamine (DHNK) and hydroxynorketamine (HNK). Although the liver is considered the main site of ketamine metabolism, the kidneys, intestines, and lungs are also potential metabolism sites [37]. Figure 1 depicts the chemical structure of ketamine’s enantiomers and their respective metabolites.
Figure 1. Chemical structure of (S)- and (R)-ketamine and their metabolic pathways.
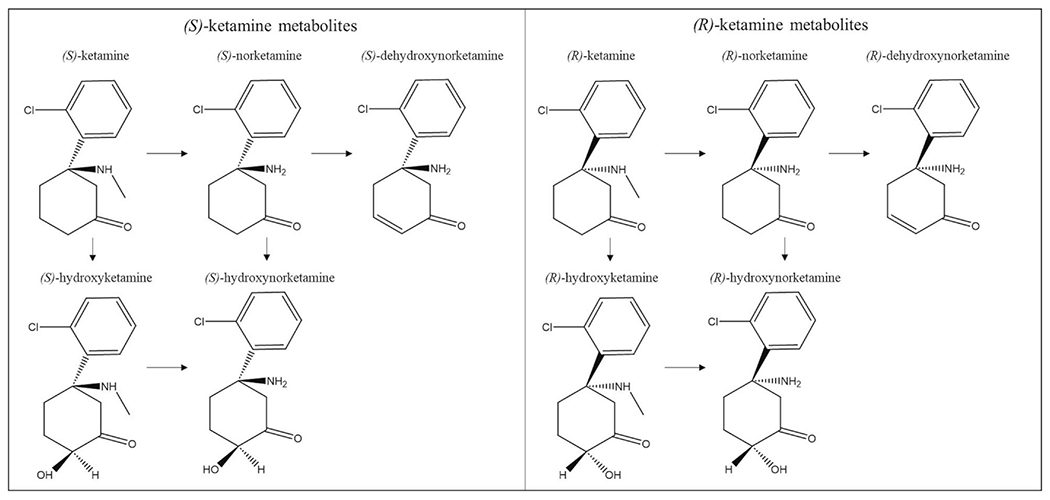
(S)-ketamine and (R)-ketamine are metabolized in a stereoselective manner by P450 liver enzymes. The enantiomers are first metabolized to their norketamine counterparts through nitrogen-mediated demethylation. Subsequent metabolism leads to dehydroxynorketamine (DHNK) or hydroxylation to hydroxynorketamine (HNK). Each enantiomer can also be metabolized to hydroxyketamine, another intermediary step for HNK.
Racemic (R,S)-ketamine (hereafter referred to as ketamine) is made up of two optical enantiomers: (S+) and (R−)-ketamine. These enantiomers are isomers that diverge light in opposing ways, rotated on an asymmetric second carbon of the cyclohexanone radical. Each of ketamine’s enantiomers has a unique pharmacokinetic profile. The demethylation of (S)-ketamine is greater than that of either (R)-ketamine or racemic ketamine [38], with an estimated 22% higher clearance than (R)-ketamine and a wider distribution pattern [39]. The difference in clearance rate between (S)-ketamine and racemic ketamine may be due to (R)-ketamine’s inhibitory actions on the elimination of (S)-ketamine when administered as a racemic mixture [40]. In addition, CYP3A4, one of the main P450 liver enzymes responsible for the metabolism to norketamine, demethylates (S)-ketamine more rapidly [41]. Although HNK was initially thought to be inactive, recent research has explored its potential antidepressant and analgesic effects [42]. Various compartment models have been proposed to best describe the pharmacokinetic profiles of ketamine metabolites [43–45], but significant disagreement exists regarding which model provides the best fit. Recent research suggests that the steady-state concentrations of HNK exceed those of ketamine and other metabolites in a stereoselective manner; for instance, (2R,6R)-HNK levels surpassed those of (R)-ketamine, (R)-norketamine, and (R)-DHNK by 46-fold, four-fold, and eight-fold respectively, and (2S,6S)-HNK exceeded the steady-state concentrations of (S)-ketamine by 14-fold [43]. Sex differences in ketamine metabolism should also be considered. One series of studies found that, in humans, males displayed higher plasma concentrations of ketamine and norketamine, though enantiomer-specific concentrations were not described. Preclinically, plasma levels of ketamine and norketamine were higher in male mice after ketamine administration, whereas levels of (2R,6R;2S,6S)-HNK were lower. In addition, administration of (2R,6R)-HNK in female mice caused greater increases of (2R,6R)-HNK concentrations than in male mice. Ovariectomy had no effect on ketamine metabolism, but orchidectomy in male mice paralleled female pharmacokinetics [46].
Ketamine primarily functions as a non-competitive NMDAR antagonist at concentrations between 2 and 50 μM, decreasing the amplification of channel response to repeat stimulation [47]. At rest, when the membrane is not depolarized, NMDAR channels are blocked by a Mg2+ ion. However, upon membrane depolarization, this Mg2+ block is removed to allow for a calcium influx. The binding site for ketamine is intrachannel, necessitating the removal of the Mg2+ block for ketamine to exert its effects [48]. In addition, evidence suggests that the affinity of (S)-ketamine to the intrachannel binding site is two- to three-fold higher than (R)-ketamine, exerting greater anesthetic and analgesic actions [47]. Ketamine has also been shown to interact with the opioidergic [49], monoaminergic [50–52], and nicotinic [53] systems, among others. A full review of the pharmacology of ketamine and its binding mechanisms is beyond the scope of this review; we refer the interested reader to in-depth reviews that include Ki and KD values [30,54].
The Convergent and Divergent Effects of Ketamine’s Enantiomers
Differences in the pharmacokinetic and pharmacodynamic profiles of ketamine’s enantiomers has spurred significant research into where and how they may differ in their antidepressant actions [42,55]. However, it is important to also index these antidepressant effects by time period, as the molecular mechanisms underlying (S)- or (R)-ketamine’s prophylactic, immediate, and sustained antidepressant effects could be quite disparate. As an important caveat, most of the research presented here—particularly the clinical research—did not directly compare (S)- and (R)-ketamine, so the comparisons made throughout this review are the authors’ discussion of disparate findings rather than direct investigative comparisons. Table 1 provides an enantiomer-specific summary of the behavioral data for prophylactic, immediate, and sustained antidepressant-like and antidepressant effects. Table 2 provides an overview of the preclinical findings for both (S)- and (R)-ketamine. Below, we provide a recent update on the preclinical and clinical evidence for each enantiomer.
Table 1.
Enantiomer-specific behavioral effects of (S)-ketamine and (R)-ketamine.
| (S)-ketamine | (R)-ketamine | |||
|---|---|---|---|---|
| Prophylactic Effects | Preclinical | Despair-like behaviors (FST, TST) | [57]a,b | [57]a,b [61–63] |
| Anhedonic-like behaviors (SPT) | none | [63] | ||
| Other depression-like behaviors | [57]a,b | [57]a,b (fear response) [69] (cognitive impairment) |
||
| Clinical | No enantiomer-specific clinical research prophylactic antidepressant effects | |||
| Immediate | Preclinical | Despair-like behaviors (FST, TST) | [76,79,80,92,93,100] | [76,92,93,100] [80] a,* |
| Anhedonic-like behaviors (SPT) | [76,77,92,100] [80]a [93]* |
[76,77,80,92,93,100] [80] a,* |
||
| Other depression-like behaviors | [77] (self-grooming) [93]* (self-grooming) |
[77,93] | ||
| Clinical | Depression | FDA approval for intranasal esketamine, [102–106,108,109] | [111] [110,112]* |
|
| Sustained | Preclinical | Despair-like behaviors (FST, TST) | [118,119] [117]* |
[117–120] |
| Anhedonic-like behaviors (SPT) | [77,116,119] | [77,119,120] | ||
| Other depression-like behaviors | [116] (OFT) [122]* (cognitive deficits) |
[122] (cognitive deficits) | ||
| Clinical | Depression | FDA approval for intranasal esketamine, [18,104,105,108,125–128] [123,124]* |
No clinical research on the sustained antidepressant effects of (R)-ketamine | |
enantiomer-specific metabolites used (hydroxynorketamine or norketamine)
sex-dependent results
negative results
FDA: US Food and Drug Administration; FST: Forced Swim Test; OFT: Open Field Test; SPT: Sucrose Preference Test; TST: Tail Suspension Test
Table 2.
Preclinical enantiomer-specific biological effects of (S)-ketamine and (R)-ketamine.
| Prophylactic | (S)-ketamine | (2S,6S)-HNK did not prevent changes in AMPAR-mediated bursts in the CA3 [57] |
| (2S,6S)-HNK prevented changes in NMDAR-related currents after chronic stress [57] | ||
| (R)-ketamine | (2R,6R)-HNK prevented changes in AMPAR-mediated bursts in the CA3 [57] | |
| (2R,6R)-HNK prevented changes in NMDAR-related currents after chronic stress [57] | ||
| An association was found between NFATc4s and (R)-ketamine’s behavioral prophylactic effects [61], mediated by changes in miR149 levels [62] | ||
| Clianges in miR-132-5p, BDNF, MeCP2, TGF-β1, GluA1, and PSD-95 after chronic stress were prevented by (R)-ketamine [63] | ||
| (R)-ketamine prevented central and systemic inflammation after LPS insult [69] | ||
| (R)-ketamine prevented changes in gut microbiota composition after LPS insult [62] | ||
| Immediate | (S)-ketamine | mTORC1 inhibition blocked the antidepressant-like effects of (S)-ketamine [92] |
| (S)-ketamine did not decrease inflammation [94–96] | ||
| (S)-ketamine did not affect RANKL expression after chronic stress [100] | ||
| (R)-ketamine | The antidepressant-like effects of (R)-ketamine were not affected by mTORC1 inhibition [92] | |
| MAPK inhibition blocked the immediate antidepressant-like effects of (R)-ketamine [92] | ||
| Administration of (R)-ketamine increased levels of phosphorylated MAPK [92] | ||
| The behavioral effects of (R)-ketamine + mGluR2/3 antagonists were mediated through BDNF-TrkB signaling [93] | ||
| (R)-ketamine decreased inflammation via TrkB signaling [94–96] | ||
| (R)-ketamine decreased RANKL expression after chronic stress [100] | ||
| (R)-ketamine regulated TGF-β1 receptors, MAPKs, RANKL, and SERT differently than (S)-ketamine [101] | ||
| Sustained | (S)-ketamine | Repeated I.P. administration of (S)-ketamine rescued chronic stress-induced deficits in neuronal morphology, hippocampal LTP, GluA1, PSD-95, and Synapsin I through Rac-1-mediated synaptic plasticity [116] |
| The sustained antidepressant-like effects of (S)-ketamine did not depend on TrkB activation [77] | ||
| (R)-ketamine | The prolonged behavioral effects of (R)-ketamine were associated with increased dendritic spine density, synaptogenesis, and BDNF-TrkB signaling in hippocampus and PFC [119] | |
| The sustained behavioral effects of (R)-ketamine were associated with microglial ERK-NRBP1-CREB-BDNF signaling in the PFC [120] | ||
| The sustained antidepressant-like effects of (R)-ketamine depended on TrkB activation [77,122] | ||
| Upregulation of BDNF and AMPAR activity was associated with the sustained antidepressant-like effects of (R)-ketamine + an mGluR2/3 antagonist [77] |
AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; BDNF: brain-derived neurotrophic factor; CREB: cyclic adenosine monophosphate response element-binding protein; ERK: extracellular signal-regulated kinase; HNK: hydroxynorketamine; I.P: Intraperitoneal; LPS: lipopolysaccharide; LTP: long-term potentiation; MAPK: mitogen-activated protein kinase; MeCP2: Methyl CpG binding protein 2; mGluR: metabotropic glutamate receptor; miRNA: microRNA; mTORC1: mechanistic target of rapamycin complex 1; NFATc4: nuclear factor of activated T cells 4; NMDAR: N-methyl-D-aspartate receptor; NRBP1: nuclear receptor binding protein 1; PFC: prefrontal cortex; PSD-95: postsynaptic density protein 95; RANKL: receptor activator of nuclear factor kB ligand; SERT: serotonin transporter; TGF-β1: transforming growth factor β1; TrkB: tropomyosin receptor kinase B.
Prophylactic effects
Recent preclinical evidence suggests that ketamine may have prophylactic effects that prevent the development of stress-induced depressive-like behaviors and biological changes. Nevertheless, while many preclinical models found that ketamine may prevent the development of depressive-like behaviors [56], recent research suggests that ketamine’s enantiomers have some stereoselective effects that should be considered in later applications to clinical work. For instance, in a mouse model of stress, (2S,6S)-HNK attenuated the learned fear response in male mice, whereas (2R,6R)-HNK prevented depressive-like behavior in both male and female mice [57]. The same study found that decreases in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-mediated bursts in the hippocampal CA3 were induced by administration of both racemic ketamine and (2R,6R)-HNK, but not (2S,6S)-HNK. Furthermore, in female mice, ovarian-derived hormones were necessary for the antidepressant-like effects of both racemic ketamine and (2R,6R)-HNK. NMDAR-related currents were attenuated up to one week after the administration of all compounds [57]. In addition to sex differences, age differences have also been noted. Another study found that ketamine prevented stress-induced depressive-like behaviors, including behavioral despair, avoidance, perseverative behavior, and contextual fear discrimination after a contextual fear conditioning stressor in a sex- and age-dependent manner. Specifically, ketamine had prophylactic effects in adolescent (five-week-old) mice but not aged (24-month-old) mice [58]. The same study found that ketamine prevented the development of learned fear and perseverative behavior in female mice but attenuated behavioral despair in male mice.
Multiple underlying mechanisms have been attributed to the preclinical prophylactic actions of (R)-ketamine, and many have focused on the role of microRNAs (miRNAs), which act as post-translational transcriptional repressors. miRNAs may be important mediators of neuroplasticity and have been shown to be putative blood biomarkers for depression [59,60]. An initial report demonstrated an association between (R)-ketamine’s prophylactic effects after lipopolysaccharide (LPS) insult, an animal model of depression, and the nuclear factor of activated T cells 4 (NFATc4) [61], and a follow-up study found that this effect may be mediated by changes in miR-149 levels [62]. Changes induced by chronic stress in miR-132-5p, a known regulator of Methyl CpG binding protein 2 (MeCP2) and downstream brain-derived neurotrophic factor (BDNF), were prevented by prophylactic administration of (R)-ketamine. In addition, changes in behavioral measures and the expression of BDNF, MeCP2, transforming growth factor β1 (TGF-β1), GluA1, and postsynaptic density protein 95 (PSD-95) by chronic restraint stress were attenuated by pre-treatment with (R)-ketamine seven days beforehand, suggesting that miR-132-5p may have more sustained antidepressant-like effects [63].
In addition to environmental stress-induced depressive-like behaviors, ketamine also appeared to be an effective prophylactic against inflammatory insults in preclinical models [64,65]. Clinically, peripheral inflammation in depression appears to characterize a subset of patients who often have a more severe symptom profile and worse response to treatment [66], making it potentially critical in the treatment of TRD. Interestingly, most recent positron emission tomography (PET) studies have also found consistent increases in translocator protein, a biomarker of neuroinflammation, in participants with MDD [67].
Preclinically, LPS-induced inflammation models some aspects of depressive-like behaviors that can provide insight into some of the inflammatory processes related to depressive-like phenotypes [68]. Using this model, pre-treatment with (R)-ketamine successfully prevented the development of central and systemic inflammation and cognitive impairment [69]. Another study found that pre-treatment with (R)-ketamine also prevented changes in gut microbiota composition after LPS insult [62]. Interestingly, racemic ketamine appears to have sex-specific abilities to prevent inflammation; for instance, one study found that it decreased markers of cyclooxygenase-2 (COX-2) in the hippocampal CA3 region of male, but not female, mice [58]. Ketamine also appears to prevent only a subset of inflammatory insults—as an example, it improved contextual fear conditioning and forced swim test behaviors after LPS injection, but not after poly I:C injection [64]. It should be noted here that most preclinical research has evaluated the efficacy of racemic ketamine or (R)-ketamine for prophylaxis against chronic stress and inflammatory insult, and more research is needed to evaluate the efficacy of (S)-ketamine in preclinical models.
Preliminary clinical research has also assessed the prophylactic effects of ketamine administered after caesarean section to prevent the development of postpartum depression. Two studies found that ketamine significantly reduced the prevalence of postpartum depression and suicidal ideation [70,71]. Similar results were obtained in those administered (S)-ketamine [72], but no clinical studies have examined the use of (R)-ketamine in this context. In contrast, a larger randomized, controlled trial found no differences in the development of postpartum depression after administration of low-dose ketamine (0.25mg/kg), though the different dosing regimens may have impacted results [73]. Though not directly related to depression, a recent randomized clinical trial of intranasal (S)-ketamine found that it had significant preventive effects on postoperative sleep disturbances after gynecological laparoscopic surgery [74].
In healthy volunteers, administration of low-dose ketamine attenuated anxiety induced by the Trier Social Stress Test better than midazolam, though this result was not significant; the same study found that salivary alpha-amylase, considered a proxy of sympathetic-adreno-medullar activity, was significantly reduced in those administered prophylactic ketamine [75]. The existing evidence suggests that ketamine’s combination of anti-inflammatory and synaptogenic effects may make it a uniquely effective prophylactic for stress-related syndromes. Further clinical research is needed to explore the enantiomer-specific prophylactic effects of ketamine in different patient populations.
Immediate antidepressant effects
Recent preclinical research has focused on comparing the immediate antidepressant-like effects of racemic ketamine, (S)-ketamine, and (R)-ketamine to ascertain the potential convergent and divergent mechanisms underlying their antidepressant effects. Preclinical research is essential for determining the molecular mechanisms that underlie behavioral and biological responses to therapeutics and is particularly valuable for investigating comparisons between ketamine enantiomers.
Preclinical behavioral measures have revealed slight differences in the immediate effects between enantiomers. In a chronic stress model, (R)-ketamine attenuated depressive-like behaviors in the tail suspension, forced swim, and sucrose preference tasks to a greater extent than either ketamine or (S)-ketamine [76]. The same study found that (R)-ketamine also had weaker effects on pre-pulse inhibition (PPI) and locomotion, suggesting a lesser side effect profile. Other direct behavioral comparisons between (S)- and (R)-ketamine found that (R)-ketamine had stronger anti-anhedonic and anti-apathetic effects than (S)-ketamine, though both enantiomers had rapid-acting antidepressant-like effects [77]. Interestingly, this result aligns with a study that found decreased habenula activity after (R)-ketamine, but not (S)-ketamine administration, an effect associated with anti-anhedonic efficacy [78]. A recent study found that sex or oestrous cycle did not affect (S)-ketamine’s acute behavioural effects in Flinders sensitive or resistant-line rats [79], though sex differences should be further evaluated in enantiomer-specific research. While preclinical studies have broadly found that (R)-ketamine is potentially more effective at reducing depressive-like behaviors than (S)-ketamine, enantiomer-specific metabolites appear to have contrasting effects. For instance, the two major metabolites of (S)-ketamine—(S)-norketamine and (2S,6S)-HNK—rapidly reduced immobility and anhedonia in a chronic corticosterone model in which (R)-norketamine and (2R,6R)-HNK had no effects [80]. From a translational perspective, preclinical research supports the use of (2R,6R)-HNK as a potential rapid-acting antidepressant with fewer side effects than racemic ketamine [81] but, clinically, high (2R,6R)-HNK levels have been associated with worse antidepressant response and low levels of (2R,6R)-HNK with improved response [82]. These mixed findings could be due to an inverted, U-shaped response curve or to high levels of the metabolite being accompanied by high ketamine levels, blocking the NMDAR activation and long-term potentiation effects of (2R,6R)-HNK [83]. These findings underscore that clinical research will be essential in ascertaining enantiomer-specific effects in depression.
In general, both (S)- and (R)-ketamine appear to increase the probability of glutamate release after administration, which in turn increases AMPAR throughput [42]. This AMPAR throughput leads to downstream mechanisms such as the aforementioned increase in BDNF release and downstream mechanistic target of rapamycin complex 1 (mTORC1) or tropomyosin receptor kinase B (TrkB) signaling [84]. While this is a consistent finding, considerable uncertainty remains regarding how ketamine and its enantiomers facilitate this glutamate surge. One key hypothesis is that ketamine preferentially antagonizes GluN2b-containing NMDARs, which are primarily located on gamma-aminobutyric acid (GABA)-ergic interneurons. This allows the disinhibition of cortical pyramidal neurons, causing an influx of glutamate into the synaptic cleft to bind to AMPARs and activate downstream signaling cascades [85,86]. However, while (S)-ketamine (Ki = 465 nM) has a high affinity for NMDARs, the potency of (R)-ketamine (Ki = 1340 nM) is relatively low. Some preclinical research suggests that (2R,6R)-HNK is unable to antagonize NMDARs at therapeutically-relevant concentrations [81,87], but this finding remains widely debated [88]. Another potential pathway by which ketamine may mediate glutamate release is via blockade of extrasynaptic NMDARs, which dephosphorylates eukaryotic elongation factor 2 in order to disinhibit BDNF release. This desuppression leads to increased GluA1 and GluA2 post-synaptic membrane insertion and the potentiation of synaptic AMPAR signaling [89–91].
Mechanistically, preclinical studies suggest that the actions of (S)-ketamine and racemic ketamine depend on mechanistic target of rapamycin (mTOR) activation but that the antidepressant-like effects of (R)-ketamine are not affected by mTOR inhibition [92]. In contrast, inhibition of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling significantly attenuated (R)-ketamine’s antidepressant-like effects, and administration of (R)-ketamine significantly increased levels of phosphorylated MAPK and ERK in the prefrontal cortex and hippocampus [92]. Interestingly, administering (R)-ketamine in conjunction with other potential rapid-acting antidepressants (such as metabotropic glutamate receptor 2/3 (mGluR2/3) receptor antagonists) had rapid behavioral effects that were mediated through BDNF-TrkB signaling, an upstream mediator of both the mTORC1 and ERK pathways [93]. TrkB signaling has also been repeatedly implicated in the ability of (R)-ketamine to decrease inflammation, an effect that was not found with (S)-ketamine [94–96]. Similar increases in TrkB-BDNF signaling were observed after administration of racemic ketamine [84] but have not yet been confirmed with (S)-ketamine, suggesting that this may be another enantiomer-specific mechanism.
Interestingly, some preclinical evidence suggests that inflammatory bone markers may play a role in depressive pathophysiology and in the antidepressant effects of ketamine, particularly the osteoprotegerin/receptor activator of nuclear factor kB ligand (OPG/RANKL) ratio, an indicator of balance between bone resorption and formation [97]. Bone abnormalities and low bone mineral density have been associated with TRD [98], and ketamine was shown to normalize inflammatory bone markers [99]. Building on this work, another study assessed the disparate effects of (S)- and (R)-ketamine on plasma RANKL expression ligand in a chronic stress animal model and found that (R)-, but not (S)-ketamine, significantly attenuated RANKL expression after chronic stress, an effect associated with sucrose preference, a proxy measure of anhedonia [100]. Similarly, a recent systems biology paper found that (R)-ketamine regulated TGF-β1 receptors, MAPKs (such as ERK), RANKL, and serotonin transporter (SERT) differently than either (S)-ketamine or racemic ketamine [101]. Clearly, more research is needed to ascertain the enantiomer-specific mechanisms that contribute to their antidepressant efficacy. Figure 2 summarizes the hypothesized convergent and divergent mechanisms behind (S)- and (R)-ketamine’s antidepressant effects.
Figure 2. Hypothesized convergent/divergent mechanisms between (S)- and (R)-ketamine.
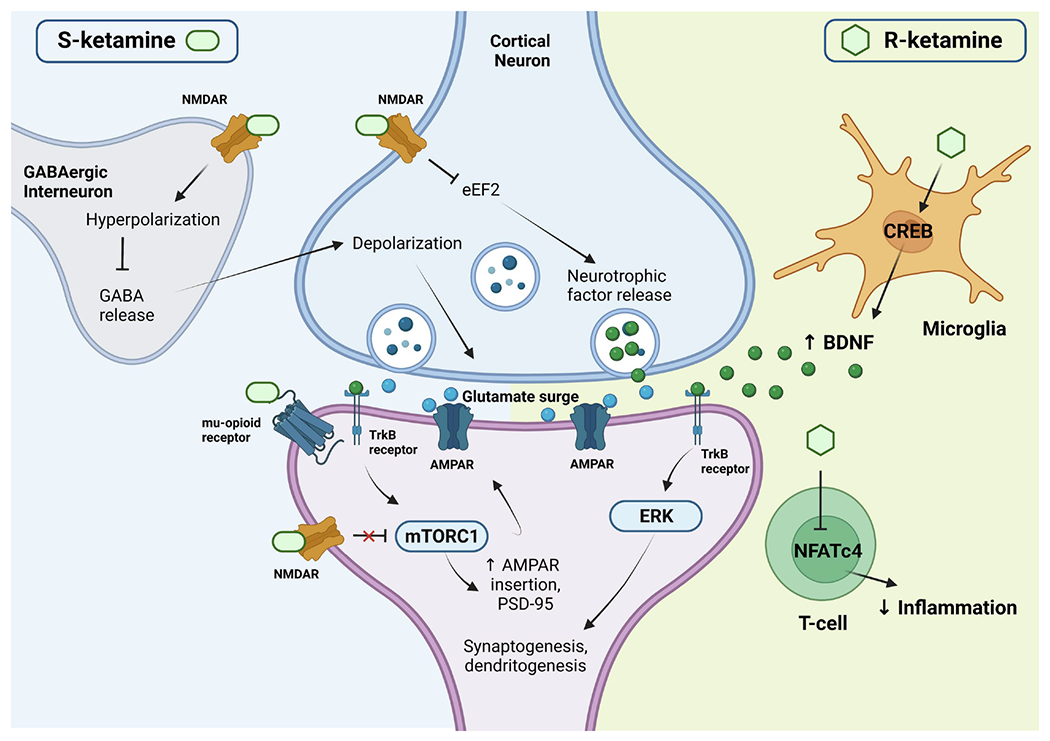
Both (S)- and (R)-ketamine increase the probability of glutamate release into the synaptic cleft, increasing AMPAR throughput and activating downstream cellular signaling mechanisms, as well as increasing synaptic protein translation of AMPAR subunits and PSD-95, contributing to further synaptogenesis and dendritogenesis. The actions of (S)-ketamine appear to be primarily facilitated through preferential binding to NMDARs expressed in GABAergic interneurons, leading to a depolarization of cortical excitatory neurons. This depolarization causes the observed glutamate release, as well as a release of neurotrophic factors such as BDNF, which binds to TrkB receptors. This, in turn, activates the mTORC1 signaling pathway, leading to the upregulation of synaptogenesis and dendritogenesis discussed earlier. In addition, (S)-ketamine binds to extrasynaptic NMDARs, disinhibiting mTORC1 signaling by deactivating eEFK2 (not pictured here). Binding to mu-opioid receptors may facilitate antidepressant effects but may also contribute to increased adverse events. In contrast, (R)-ketamine seems to primarily facilitate immune modulation by affecting microglial signaling and increasing BDNF release. This BDNF release binds to TrkB and activates the ERK signaling pathway, upregulating synaptogenesis and dendritogenesis. While displayed as distinct mechanisms here, please note that there may be overlap and other potential mechanisms not noted in this figure. Figure is approximate for illustrative purposes. Abbreviations: AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; BDNF: brain-derived neurotrophic factor; CREB: cyclic adenosine monophosphate response element-binding protein; eEFK2: eukaryotic elongation factor-2 kinase; ERK: extracellular signal-related kinase; GABA: gamma aminobutyric acid; mTORC1: mechanistic target of rapamycin complex 1; NFATc4: nuclear factor of activated T cells 4; NMDAR: N-methyl-D-aspartate receptor; PSD-95: postsynaptic density protein 95; TrkB: tropomyosin receptor kinase B.
As noted above, considerable clinical research supports racemic ketamine’s rapid antidepressant effects (for a review, see [13–15]). In addition, the FDA and EU approval of esketamine intranasal spray created an influx of reports on (S)-ketamine’s actions in TRD. It should be noted here that clinical studies have examined both intravenous (S)-ketamine and intranasal esketamine in this context. Though not FDA-approved, intravenous (S)-ketamine has also been found to rapidly reduce depressive symptoms within two hours after administration, though it also caused transient, dose-dependent adverse effects [102].
Initial randomized, controlled trials found that intranasal (S)-ketamine was effective and safe in TRD [103,104], a finding confirmed through multiple real-world effectiveness trials [105,106]. In addition, it appears that those who do not respond to intranasal (S)-ketamine may respond to intravenous racemic ketamine, further underscoring the notion that ketamine’s enantiomers may exert their therapeutic effects via different mechanisms [107]. The first randomized controlled trial to directly compare intravenous racemic and intravenous (S)-ketamine for participants with TRD found no significant differences in 24-hour remission rates or side effects, with both treatments being effective and well-tolerated [108]. However, this study may have been underpowered to detect significant differences, and response and remission rates were numerically higher after racemic ketamine administration. A comparison between intravenous racemic ketamine and intranasal (S)-ketamine obtained similar results, finding no significant differences in remission rates between groups; however, more treatments of intranasal (S)-ketamine were needed to achieve remission [109].
In contrast, only limited clinical research exists regarding (R)-ketamine’s antidepressant effects. An initial open-label pilot study found that an acute infusion of (R)-ketamine (0.5 mg/kg) had rapid-acting antidepressant effects similar to those of racemic ketamine and both intravenous and intranasal (S)-ketamine but caused almost no dissociative effects [110]. However, a follow-up randomized, controlled trial in five participants found that (R)-ketamine had no significant antidepressant effects though it also had no dissociative effects [111]. Furthermore, a recent, large, Phase 2a clinical trial reported no significant differences between (R)-ketamine (PCN-101) (30 mg and 60 mg) and placebo at the 24-hour primary endpoint [112]. It should be noted that 60 mg of PCN-101 did improve response and remission rates, but this finding was not significant. Dissociation and sedation were comparable between placebo and both doses of (R)-ketamine, supporting earlier clinical and preclinical work. Despite the negative results, further work is exploring whether alternate dosing and administration routes may improve the antidepressant efficacy of (R)-ketamine.
Sustained antidepressant effects
As previously discussed, ketamine’s rapid-acting therapeutic effects have been well-characterized in both TRD and bipolar disorder. In contrast, the long-term effects of both acute and repeated ketamine administration, particularly as regards the stereoselectivity of its enantiomers, seems less well known. Many different cellular signaling pathways have been implicated in ketamine’s sustained antidepressant effects, including TrkB-mediated hippocampal progenitor differentiation [113], regulation of Kcnq2 [114], and various neurotrophic and growth factors [115]. Preclinically, repeated intraperitoneal (S)-ketamine administration was also shown to rescue stress-induced deficits in behavior, neuronal morphology, and hippocampal long-term potentiation through Rac1-mediated synaptic plasticity, upregulating the expression of GluA1, PSD-95, and Synapsin I [116].
Early preclinical research comparing ketamine’s enantiomers found that (R)-ketamine, but not (S)-ketamine, decreased depressive-like behavior seven days post-administration [117]. In an LPS-insult model and a model of chronic stress, (R)-ketamine also had more potent and longer-lasting effects than its metabolite (2R,6R)-HNK [118]. Further preclinical research found that (R)-ketamine’s longer-lasting behavioral effects were associated with greater increases in dendritic spine density, synaptogenesis, and BDNF-TrkB signaling in the hippocampus and prefrontal cortex [119]. Microglial ERK-nuclear receptor binding protein 1 (NRBP1)-cyclic adenosine monophosphate response element-binding protein (CREB)-BDNF signaling in the prefrontal cortex was also recently implicated in the sustained effects of (R)-ketamine, as suppression of CREB, BDNF, or microglial signaling blocked the antidepressant-like effects of (R)-ketamine three days post-infusion [120]. Other immune activators have been linked to the antidepressant-like actions of (R)-ketamine, which increased the expression of microglial TGF-β1 and its receptors after chronic stress, an effect not observed after administration of (S)-ketamine [121].
Repeated administration of ketamine also induces sustained antidepressant-like effects. Preclinically, PCP-induced cognitive deficits were rescued after repeated administration of (R)-, but not (S)-ketamine, an effect that was blocked with a TrkB inhibitor [122]. (R)-ketamine also sustained anti-anhedonic and anti-apathetic phenotypes up to seven days after administration, in contrast to the effects of (S)-ketamine, which persisted for three days. The sustained antidepressant-like effects of (R)-ketamine, but not (S)-ketamine, appeared to depend on TrkB receptor activation [77]. Interestingly, (R)-ketamine had no effect on ERK activation in that study, in contrast with previous research. As noted above, co-administration of (R)-ketamine and an mGluR2/3 receptor antagonist led to significant sustained antidepressant-like effects thought to be mediated via BDNF and AMPAR activity [77].
Clinically, preliminary research found that repeated ketamine administration (six infusions of 0.5 mg/kg administered 3x/week over 12 days) had more sustained effects than an acute dose; the median time to relapse was found to be 18 days in a sample of 24 participants [28]. Notably, response within four hours of the first infusion strongly predicted response at the end of the study (~80 days after the last infusion). This suggests that repeated administration of either (R)- or (S)-ketamine could similarly prolong clinical response.
Repeated intranasal (S)-ketamine administration (2x/week over four weeks), in conjunction with traditional antidepressant treatment, significantly decreased depressive symptoms and suicidal ideation at four and 24 hours, though no differences were observed at Day 25 [18]. Similarly, another Phase 2b randomized, controlled trial of intranasal (S)-ketamine administered in conjunction with oral antidepressants found no significant differences between (S)-ketamine and placebo at Day 28 [123]. In addition, a recent Phase 3 study found no significant differences between intranasal (S)-ketamine administered in conjunction with standard antidepressant treatment compared to standard antidepressant treatment alone at 28 days [124]. As noted above, the first randomized, controlled trial to directly compare racemic and (S)-ketamine found no significant differences at their primary endpoint of 24 hours [108]; however, that study also analyzed remission rates at 72 hours and seven days after the initial infusion and, while no significant differences were found, mean Montgomery-Asberg Depression Rating Scale (MADRS) scores at 72 hours were slightly higher in the (S)-ketamine group, suggesting that racemic ketamine may be more effective at prolonging antidepressant effects, a finding that needs to be empirically tested. A meta-analysis of over 24 trials and 1877 participants supports this conclusion, demonstrating that intravenous racemic ketamine was more effective than intranasal (S)-ketamine [125]. Another direct comparison of data from the Yale Interventional Psychiatry service, which administers both intravenous racemic ketamine and intranasal (S)-ketamine, found no significant differences in response and remission rates, though once again there was a slight trajectory in favor of racemic ketamine [126].
Despite the potentially lower antidepressant efficacy of (S)-ketamine, another study found that continued treatment with intranasal (S)-ketamine alongside a traditional oral antidepressant significantly delayed the time to relapse to 16 weeks after initial (S)-ketamine treatment [127]. Assessment of the real-world effectiveness of intranasal (S)-ketamine also found a significant increase in clinical response (64.2%) and remission (40.6%) rates at three-month follow-up, with extremely low study drop-out rates (2.6%) [105]. Multi-center analyses also confirmed that repeated intranasal (S)-ketamine administered in conjunction with a traditional antidepressant significantly improved depressive symptoms from baseline to 28 days in comparison to traditional antidepressants alone in both men and women diagnosed with TRD [104,128]. However, differences in route of administration could make it more challenging to interpret these results and should be taken into account, especially given the differences in accessibility associated with intravenous versus intranasal administration.
As the research presented above underscores, it is presently unclear whether (R)-ketamine will display acute or sustained antidepressant effects. Significantly more research is needed to draw accurate comparisons between racemic ketamine and its two enantiomers.
Dissociation and adverse side effects
In contrast to research regarding serotonergic psychedelics [129], ketamine’s dissociative and psychotomimetic effects do not appear to be associated with its antidepressant effects [130], a finding that has also been confirmed with repeated subcutaneous and intranasal (S)-ketamine administration [131]. This suggests that dissociation is not necessary for ketamine’s antidepressant actions and that research should prioritize minimizing the adverse side effects that limit its widespread use. While adverse side effects associated with ketamine and intranasal (S)-ketamine administration are transient and fairly well-tolerated at therapeutic doses, lessening these adverse effects would increase treatment adherence and efficacy in a variety of patient populations. The most common adverse events associated with ketamine and (S)-ketamine include dissociation, nausea, vertigo, dizziness, and a metallic taste in the mouth, most of which are associated with NMDAR antagonism [103,104,132]. As noted above, (S)-ketamine is a more potent NMDAR antagonist than racemic or (R)-ketamine and is often associated with more severe psychotomimetic effects [110,111,133,134].
Generally, preclinical research suggests that (S)-ketamine produces more adverse effects than either racemic or (R)-ketamine, most likely due to its higher NMDAR potency [76]. In preclinical research, PPI, a suppression of the startle reflex, and changes in locomotion are commonly used to assess adverse effects in animals. In a chronic stress model, PPI and locomotion deficits were worse after administration of (S)-ketamine and racemic ketamine than (R)-ketamine [76]. However, another preclinical study found that ketamine produced more serious cognitive deficits than either enantiomer, as ascertained via an attentional set-shifting task. In addition, (R)-ketamine did not fully substitute for rats trained with (S)-ketamine, suggesting potential disparate underlying mechanisms of cognitive deficits between enantiomers [135].
Clinical studies of racemic and (S)-ketamine commonly report transient adverse events, particularly dissociation, throughout the administration period. Despite the commonality of these adverse events, drop-out rates have been relatively low and can be managed through patient education and psychiatric support throughout administration [133]. Initial clinical studies with (R)-ketamine found that participants reported fewer adverse events, suggesting that (R)-ketamine might be better tolerated than (S)-ketamine or racemic ketamine, though it does not always appear to have comparable antidepressant efficacy [110,112]. However, current trials may be underdosing (R)-ketamine in order to reduce dissociative effects, and antidepressant effects may emerge with higher doses. This balance between adverse effects and antidepressant efficacy is particularly important in any assessment of ketamine’s enantiomers for the treatment of depression, given the current debate surrounding how important NMDARs are to ketamine’s antidepressant effects. Because so little clinical research on (R)-ketamine currently exists, future studies with different dosing and administration paradigms may reveal previously undocumented adverse events.
Misuse liability
The misuse liability of racemic ketamine and its enantiomers has been heavily debated. While ketamine and other dissociative agents are used recreationally, it remains uncertain whether or not antidepressant doses of ketamine could lead to later misuse. However, the clinical evidence to date suggests extremely low rates of ketamine dependence in participants with depression, though little information has been reported, particularly in naturalistic settings where ketamine misuse may be more likely to occur [136]. Nevertheless, concerns about misuse liability and increasing cases of ketamine-induced urological toxicity have led to restrictions on the therapeutic use of ketamine [137,138]. For instance, in the United States and Canada, intranasal esketamine is classified under the Risk Evaluation and Mitigation Strategy (REMS) and the Janssen Journey™ Program, which require supervision and monitoring by certified providers. Such risks limit the widespread use of (S)-ketamine to participants who are able to dedicate a significant amount of time and resources to treatment. Although additional studies are clearly needed, most of the initial clinical trials focused on the efficacy of acute ketamine administration; in contrast, studies of repeat-dose ketamine or the use of ketamine in outpatient settings has often been conducted with poorly defined endpoints, given that the optimal dose and frequency of ketamine administration remain unknown [139,140].
Even less evidence has been published regarding the stereoselective misuse liability of ketamine’s enantiomers. However, early preclinical and clinical research suggests that (R)-ketamine may mitigate some of the substance misuse liability risks. Preclinically, (S)-ketamine has been shown to increase behaviors associated with misuse liability such as conditioned place preference, hyperlocomotion, self-administration, and deficits in PPI, but all of these metrics remained unchanged with (R)-ketamine administration [78]. In addition, other studies have suggested that (S)-ketamine may preferentially activate mu-opioid and kappa-opioid receptors more than (R)-ketamine, which may contribute to its potential misuse liability [78]. On the other hand, (R)-ketamine increased dopamine release in the nucleus accumbens more than (S)-ketamine [78]. Because dopamine levels in the nucleus accumbens are strongly associated with misuse liability, further investigation into the stereoselective mechanisms of (R)-ketamine’s misuse potential is needed.
It is important to note that this remains a very active area of investigation, especially issues surrounding the dose necessary to exert (S)-ketamine’s versus (R)-ketamine’s antidepressant effects [141]. In particular, early clinical research suggests that misuse liability for (S)-ketamine and (R)-ketamine is highly dose-dependent; when ketamine’s enantiomers are administered at equipotent NMDAR antagonism levels, misuse liability is similar [142]. Thus, much remains unknown regarding the potential misuse liability of ketamine and its enantiomers; future research in this area is needed to shed light on this area of investigation.
Conclusions
In the last few years, a great deal of novel research has explored the enantiomer-specific antidepressant effects of ketamine. In general, it appears that (R)-ketamine and (S)-ketamine may differentially regulate antidepressant effects via downstream signaling pathways such as mTORC1 ((S)-ketamine) and ERK ((R)-ketamine). (R)-ketamine also appears to better target inflammatory processes such as RANKL [101] and microglial signaling [120], which could contribute to the more sustained effects observed with its administration in preclinical animal models. However, while preliminary clinical research found that (R)-ketamine was associated with fewer adverse effects and lower misuse liability [78,110], its lack of antidepressant effects in a recent, large, randomized clinical trial [112] suggests that caution is warranted when assessing the therapeutic potential of this agent. A clinical trial is currently underway to assess the effects of (2R,6R)-HNK, and future clinical trials are necessary to compare and contrast the antidepressant efficacy of ketamine’s separate enantiomers and metabolites in participants with TRD.
Given that much of this research is still in its infancy, future studies should seek to explore how varied administration and dosing paradigms may have affected the results reviewed here. Another important caveat is that most of the preclinical and clinical studies reviewed above did not directly compare (R)-ketamine and (S)-ketamine—or necessarily even compare either of these enantiomers to racemic ketamine—which limits the conclusions that can be drawn. For instance, the molecular changes observed after enantiomer-specific administration could be changed in parallel by the other enantiomer or have completely disparate effects; without a direct comparison, this remains unknown. Given the relative novelty of enantiomer-specific research and the varied results in preclinical and clinical research, more information is needed before concrete conclusions can be drawn.
Acknowledgements
The authors thank the 7SE research unit and staff for their support.
Funding and Role of Funding Source
Funding for this work was provided by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002857). The work was completed as part of the authors’ official duties as Government employees. The views expressed do not necessarily reflect the views of the NIH, the Department of Health and Human Services, or the United States Government.
Abbreviations
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- BDNF
brain-derived neurotrophic factor
- COX-2
cyclooxygenase-2
- CREB
cyclic adenosine monophosphate response element-binding protein
- DHNK
dehydronorketamine
- eEFK2
eukaryotic elongation factor-2 kinase
- ERK
extracellular signal-regulated kinase
- FDA
Food and Drug Administration
- GABA
gamma-aminobutyric acid
- HNK
hydroxynorketamine
- LPS
lipopolysaccharide
- LTP
long-term potentiation
- MADRS
Montgomery-Asberg Depression Rating Scale
- MAPK
mitogen-activated protein kinase
- MDD
major depressive disorder
- MeCP2
Methyl CpG binding protein 2
- mGluR
metabotropic glutamate receptor
- miRNA
microRNA
- mTOR
mechanistic target of rapamycin
- mTORC1
mechanistic target of rapamycin complex 1
- NFATc4
nuclear factor of activated T cells 4
- NMDAR
N-methyl-D-aspartate receptor
- NRBP1
nuclear receptor binding protein 1
- OCD
obsessive compulsive disorder
- OPG
osteoprotegerin
- PET
positron emission tomography
- PFC
prefrontal cortex
- PPI
pre-pulse inhibition
- PSD-95
postsynaptic density protein 95
- PTSD
post-traumatic stress disorder
- RANKL
receptor activator of nuclear factor kB ligand
- REMS
Risk Evaluation and Mitigation Strategy
- SERT
serotonin transporter
- TGF-β1
transforming growth factor β1
- TRD
treatment-resistant depression
- TrkB
tropomyosin receptor kinase B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydroxylated and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
References
- 1.Mion G. History of anaesthesia: The ketamine story - past, present and future. Eur J Anaesthesiol. 2017;34:571–75. [DOI] [PubMed] [Google Scholar]
- 2.Hirota K, Lambert DG. Ketamine; history and role in anesthetic pharmacology. Neuropharmacology. 2022;216:109171. [DOI] [PubMed] [Google Scholar]
- 3.Domino EF, Warner DS. Classic papers revisited: Taming the ketamine tiger by Edward F. Domino. Anesthesiology 2010;113:678–84. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro HM, Wyte SR, Harris AB. Ketamine anesthesia in patients with intracranial pathology. Br J Anaesth. 1972;44:1200–04. [DOI] [PubMed] [Google Scholar]
- 5.Krystal AD, Weiner RD, Dean MD, Lindahl VH, Tramontozzi LA, Falcone G, et al. Comparison of seizure duration, ictal EEG, and cognitive effects of ketamine and methohexital anesthesia with ECT. J Neuropsychiatry Clin Neurosci. 2003;15:27–34. [DOI] [PubMed] [Google Scholar]
- 6.Broughton Pipkin F, Waldron BA. Ketamine hypertension and the renin-angiotensin system. Clin Exp Hypertens A. 1983;5:875–83. [DOI] [PubMed] [Google Scholar]
- 7.Pai A, Heining M. Ketamine. Continuing Education in Anaesthesia, Critical Care, and Pain. 2007;7:59–63. [Google Scholar]
- 8.Nowak G, Trullas R, Layer RT, Skolnick P, Paul IA. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. J Pharmacol Exp Ther. 1993;265(3):1380–6. [PubMed] [Google Scholar]
- 9.Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–87. [DOI] [PubMed] [Google Scholar]
- 10.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4. [DOI] [PubMed] [Google Scholar]
- 11.Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64. [DOI] [PubMed] [Google Scholar]
- 12.Zarate CA Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alnefeesi Y, Chen-Li D, Krane E, Jawad MY, Rodgrigues NB, Ceban F, et al. Real-world effectiveness of ketamine in treatment-resistant depression: A systematic review & meta-analysis. J Psychiatr Res. 2022;151:693–709. [DOI] [PubMed] [Google Scholar]
- 14.Marcantoni WS, Akoumba BS, Wassef M, Mayrand J, Lai H, Richard-Devantoy S, et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 - January 2019. J Affect Disord. 2020;277:831–41. [DOI] [PubMed] [Google Scholar]
- 15.McIntyre RS, Carvalho IP, Lui LMW, Majeed A, Masand PS, Gill H, et al. The effect of intravenous, intranasal, and oral ketamine in mood disorders: A meta-analysis. J Affect Disord. 2020;276:576–84. [DOI] [PubMed] [Google Scholar]
- 16.Witt K, Potts J, Hubers A, Grunebaum MF, Murrough JW, Loo C, et al. Ketamine for suicidal ideation in adults with psychiatric disorders: a systematic review and meta-analysis of treatment trials. Aust N Z J Psychiatry. 2020;54:29–45. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175:150–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;Apr 16 [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: Potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nogo D, Jasrai AK, Kim H, Nasri F, Ceban F, Lui LMW, et al. The effect of ketamine on anhedonia: improvements in dimensions of anticipatory, consummatory, and motivation-related reward deficits. Psychopharmacology (Berl). 2022;239:2011–39. [DOI] [PubMed] [Google Scholar]
- 22.Whittaker E, Dadabayev AR, Joshi SA, Glue P. Systematic review and meta-analysis of randomized controlled trials of ketamine in the treatment of refractory anxiety spectrum disorders. Ther Adv Psychopharmacol. 2021;11:20451253211056743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belzer K, Schneier FR. Comorbidity of anxiety and depressive disorders: issues in conceptualization, assessment, and treatment. J Psychiatr Pract. 2004;10:296–306. [DOI] [PubMed] [Google Scholar]
- 24.Bandeira ID, Lins-Silva DH, Cavenaghi VB, Dorea-Bandeira I, Faria-Guimarães D, Barouh JL, et al. Ketamine in the treatment of obsessive-compulsive disorder: A systematic review. Harv Rev Psychiatry. 2022;30(2):135–45. [DOI] [PubMed] [Google Scholar]
- 25.Feder A, Costi S, Rutter SB, Collins AB, Govindarajulu U, Jha MK, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178:193–202. [DOI] [PubMed] [Google Scholar]
- 26.Glue P, Neehoff S, Sabadel A, Broughton L, Le Nedelec M, Shadli S, et al. Effects of ketamine in patients with treatment-refractory generalized anxiety and social anxiety disorders: Exploratory double-blind psychoactive-controlled replication study. J Psychopharmacol. 2020;34:267–72. [DOI] [PubMed] [Google Scholar]
- 27.Abdallah CG, Roache JD, Gueorguieva R, Averill LA, Young-McCaughan S, Shiroma PR, et al. Dose-related effects of ketamine for antidepressant-resistant symptoms of posttraumatic stress disorder in veterans and active duty military: a double-blind, randomized, placebo-controlled multi-center clinical trial. Neuropsychopharmacology. 2022;47(8):1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74(4):250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46(7):1459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther. 2013;19:370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schüttler J, Stanski DR, White PF, Trevor AJ, Horai Y, Verotta D, et al. Pharmacodynamic modeling of the EEG effects of ketamine and its enantiomers in man. J Pharmacokinet Biopharm. 1987;15:241–53. [DOI] [PubMed] [Google Scholar]
- 32.Sigtermans M, Dahan A, Mooren R, Bauer M, Kest B, Sarton E, et al. S(+)-ketamine effect on experimental pain and cardiac output: a population pharmacokinetic-pharmacodynamic modeling study in healthy volunteers. Anesthesiology. 2009;111:892–903. [DOI] [PubMed] [Google Scholar]
- 33.Desta Z, Moaddel R, Ogburn ET, Xu C, Ramamoorthy A, Venkata SL, et al. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica. 2012;42:1076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao LK, Flaker AM, Friedel CC, Kharasch ED. Role of cytochrome P4502B6 polymorphisms in ketamine metabolism and clearance. Anesthesiology. 2016;125(6):1103–12. [DOI] [PubMed] [Google Scholar]
- 35.Hijazi Y, Boulieu R. Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos. 2002;30(7):853–8. [DOI] [PubMed] [Google Scholar]
- 36.Yanagihara Y, Kariya S, Ohtani M, Uchino K, Aoyama T, Yamamura Y, et al. Involvement of CYP2B6 in n-demethylation of ketamine in human liver microsomes. Drug Metab Dispos. 2001;29(6):887–90. [PubMed] [Google Scholar]
- 37.Edwards SR, Mather LE. Tissue uptake of ketamine and norketamine enantiomers in the rat: Indirect evidence for extrahepatic metabolic inversion. Life Sci. 2001;69:2051–66. [DOI] [PubMed] [Google Scholar]
- 38.Geisslinger G, Menzel-Soglowek S, Kamp HD, Brune K. Stereoselective high-performance liquid chromatographic determination of the enantiomers of ketamine and norketamine in plasma. J Chromatogr. 1991;568:165–76. [DOI] [PubMed] [Google Scholar]
- 39.Geisslinger G, Hering W, Kamp HD, Vollmers KO. Pharmacokinetics of ketamine enantiomers. Br J Anaesth. 1995;75:506–07. [DOI] [PubMed] [Google Scholar]
- 40.Ihmsen H, Geisslinger G, Schüttler J. Stereoselective pharmacokinetics of ketamine: R(−)-ketamine inhibits the elimination of S(+)-ketamine. Clin Pharmacol Ther. 2001;70:431–38. [DOI] [PubMed] [Google Scholar]
- 41.Portmann S, Kwan HY, Theurillat R, Schmitz A, Mevissen M, Thormann W. Enantioselective capillary electrophoresis for identification and characterization of human cytochrome P450 enzymes which metabolize ketamine and norketamine in vitro. J Chromatogr A. 2010;1217(51):7942–8. [DOI] [PubMed] [Google Scholar]
- 42.Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70:621–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss M, Siegmund W. Pharmacokinetic modeling of ketamine enantiomers and their metabolites after administration of prolonged-release ketamine with emphasis on 2, 6-hydroxynorketamines. Clin Pharmacol Drug Dev. 2022;11:194–206. [DOI] [PubMed] [Google Scholar]
- 44.Kamp J, Jonkman K, van Velzen M, Aarts L, Niesters M, Dahan A, et al. Pharmacokinetics of ketamine and its major metabolites norketamine, hydroxynorketamine, and dehydronorketamine: a model-based analysis. Br J Anaesth. 2020;2020:750–61. [DOI] [PubMed] [Google Scholar]
- 45.Kamp J, Olofsen E, Henthorn TK, van Velzen M, Niesters M, Dahan A, et al. Ketamine pharmacokinetics: a systematic review of the literature, meta-analysis, and population analysis. Anesthesiology. 2020;133:1192–213. [DOI] [PubMed] [Google Scholar]
- 46.Highland JN, Farmer CA, Zanos P, Lovett J, Zarate CA Jr., Moaddel R, et al. Sex-dependent metabolism of ketamine and (2R,6R)-hydroxynorketamine in mice and humans. J Psychopharmacol. 2022;36(2):170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeilhofer HU, Swandulla D, Geisslinger G, Brune K. Differential effects of ketamine enantiomers on NMDA receptor currents in cultured neurons. Eur J Pharmacol. 1992;213:155–58. [DOI] [PubMed] [Google Scholar]
- 48.Yamakura T, Shimoji K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol. 1999;59:279–98. [DOI] [PubMed] [Google Scholar]
- 49.Hustveit O, Maurset A, Oye I. Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol Toxicol. 1995;77:355–59. [DOI] [PubMed] [Google Scholar]
- 50.Ago Y, Yokoyama R, Asano S, Hashimoto H. Roles of the monoaminergic system in the antidepressant effects of ketamine and its metabolites. Neuropharmacology. 2023;223:109313. [DOI] [PubMed] [Google Scholar]
- 51.Can A, Zanos P, Moaddel R, Kang HJ, Dossou KSS, Wainer IW, et al. Effects of ketamine and ketamine metabolites on evoked striatal dopamine release, dopamine receptors, and monoamine transporters. J Pharmacol Exp Ther. 2016;359:159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith D, Azzaro A, Zaldivar S, Palmer S, Lee H. Properties of the optical isomers and metabolites of ketamine on the high affinity transport and catabolism of monoamines. Neuropharmacology. 1981;20:391–96. [DOI] [PubMed] [Google Scholar]
- 53.Furuya R, Oka K, Watanabe I, Kamiya Y, Itoh H, Andoh T. The effects of ketamine and propofol on neuronal nicotinic acetylcholine receptors and P2X purinoceptors in PC12 cells. Anesth Analg. 1999;88:174–80. [DOI] [PubMed] [Google Scholar]
- 54.Fourcade EW, Lapidus KAB. The basic and clinical pharmacology of ketamine. In: Mathew SJ, Zarate CAJ, editors. Ketamine for Treatment-Resistant Deprssion: The First Decade of Progress. Cham: Adis; 2016. p. 13–29. [Google Scholar]
- 55.Yang C, Yang J, Luo A, Hashimoto K. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry. 2019;9:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evers AG, Murrough JW, Charney DS, Costi S. Ketamine as a prophylactic resilience-enhancing agent. Front Psychiatry. 2022;13:833259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen BK, Luna VM, LaGamma CT, Xu X, Deng SX, Suckow RF, et al. Sex-specific neurobiological actions of prophylactic (R,S)-ketamine, (2R,6R)-hydroxynorketamine, and (2S,6S)-hydroxynorketamine. Neuropsychopharmacology. 2020;45(9):1545–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mastrodonato A, Pavlova I, Kee NC, Pham VA, McGowan JC, Mann JJ, et al. Prophylactic (R, S)-ketamine is effective against stress-induced behaviors in adolescent but not aged mice. Int J Neuropsychopharmacol. 2022;25:512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao YN, Zhang YQ, Wang YL, Deng NM, Li A. A new player in depression: miRNAs as modulators of altered synaptic plasticity. Int J Mol Sci. 2022;23:4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rasheed M, Asghar R, Firdoos S, Ahmad N, Nazir A, Ullah KM, et al. A systematic review of circulatory microRNAs in major depressive disorder: potential biomarkers for disease prognosis. Int J Mol Sci. 2022;23:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma L, Zhang J, Fujita Y, Qu Y, Shan J, Wan X, et al. Nuclear factor of activated T cells 4 in the prefrontal cortex is required for prophylactic actions of (R)-ketamine. Transl Psychiatry. 2022; 12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma L, Wang L, Chang L, Shan J, Qu Y, Wang X, et al. A role of microRNA-149 in the prefrontal cortex for prophylactic actions of (R)-ketamine in inflammation model. Neuropharmacology. 2022;219:109250. [DOI] [PubMed] [Google Scholar]
- 63.Ma L, Wang L, Chang L, Shan J, Qu Y, Wang X, et al. A key role of miR-132-5p in the prefrontal cortex for persistent prophylactic actions of (R)-ketamine in mice. Transl Psychiatry. 2022;12:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mastrodonato A, Cohensedgh O, LaGamma CT, McGowan JC, Hunsberger HC, Denny CA. Prophylactic (R,S)-ketamine selectively protects against inflammatory stressors. Behav Brain Res. 2020;378:112238. [DOI] [PubMed] [Google Scholar]
- 65.Camargo A, Dalmagro AP, Wolin IAV, Kaster MP, Rodrigues ALS. The resilient phenotype elicited by ketamine against inflammatory stressors-induced depressive-like behavior is associated with NLRP3-driven signaling pathway. J Psychiatr Res. 2021;144:118–28. [DOI] [PubMed] [Google Scholar]
- 66.Johnston JN, Greenwald MS, Henter ID, Kraus C, Mkrtchian A, Clark NG, et al. Inflammation, stress and depression: an exploration of ketamine’s therapeutic profile. Drug Discov Today. 2023;28:103518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyer JH, Cervenka S, Kim M-J, Kreisl WC, Henter ID, Innis RB. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry. 2020;7:1064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao X, Cao F, Liu Q, Li X, Xu G, Liu G, et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav Brain Res. 2019;364:494–502. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Ma L, Wan X, Shan J, Qu Y, Hashimoto K. (R)-Ketamine attenuates LPS-induced endotoxin-derived delirium through inhibition of neuroinflammation. Psychopharmacology. 2021;238:2743–53. [DOI] [PubMed] [Google Scholar]
- 70.Ma J-H, Wang S-Y, Yu H-Y, Li D-Y, Luo S-C, Zheng S-S, et al. Prophylactic use of ketamine reduces postpartum depression in Chinese women undergoing cesarean section. Psychiatry Res. 2019;279:252–58. [DOI] [PubMed] [Google Scholar]
- 71.Alipoor M, Loripoor M, Kazemi M, Farahbakhsh F, Sarkoohi A. The effect of ketamine on preventing postpartum depression. J Med Life. 2021;14:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han Y, Li P, Miao M, Tao Y, Kang X, Zhang J. S-ketamine as an adjuvant in patient-controlled intravenous analgesia for preventing postpartum depression: a randomized controlled trial. BMC Anesthesiol. 2022;22:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Y, Li Y, Huang XP, Chen D, She B, Ma D. Single bolus low-dose of ketamine does not prevent postpartum depression: a randomized, double-blind, placebo-controlled, prospective clinical trial. Arch Gynecol Obstet. 2017;295:1167–74. [DOI] [PubMed] [Google Scholar]
- 74.Qiu D, Wang X-M, Yang J-J. Effect of intraoperative esketamine infusion on postoperative sleep disturbance after gynecological laparoscopy: a randomized clinical trial. JAMA Netw Open. 2022;5:e2244514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Costi S, Evers A, Jha MK, Klein M, Overbey JR, Goosens KA, et al. A randomized pilot study of the prophylactic effect of ketamine on laboratory-induced stress in healthy adults. Neurobiol Stress. 2023;22:100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang LC, Zhang K, Pu Y, Qu Y, Wang S-M, Xiong Z, et al. Comparison of antidepressant and side effects in mice after intranasal administration of (R,S)-ketamine, (R)-ketamine, and (S)-ketamine. Pharmacol Biochem Behav. 2019;181:53–59. [DOI] [PubMed] [Google Scholar]
- 77.Rafało-Ulińska A, Pałucha-Poniewiera A. The effectiveness of (R)-ketamine and its mechanism of action differ from those of (S)-ketamine in a chronic unpredictable mild stress model of depression in C57BL/6J mice. Behav Brain Res. 2022;418:113633. [DOI] [PubMed] [Google Scholar]
- 78.Bonaventura J, Lam S, Carlton M, Boehm MA, Gomez JL, Solis O, et al. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry. 2021;26(11):6704–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arjmand S, Pedersen MV, Silva NR, Landau AM, Joca S, Wegener G. Sex and oestrous cycle are not mediators of S-ketamine’s rapid-antidepressant behavioural effects in a genetic rat model of depression. Int J Neuropsychopharmacol. 2023;April 17 [online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yokoyama R, Higuchi M, Tanabe W, Tsukada S, Naito M, Yamaguchi T, et al. (S)-norketamine and (2S,6S)-hydroxynorketamine exert potent antidepressant-like effects in a chronic corticosterone-induced mouse model of depression. Pharmacol Biochem Behav. 2020;191:172876. [DOI] [PubMed] [Google Scholar]
- 81.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abdallah CG. (2R, 6R)-Hydroxynorketamine (HNK) plasma level predicts poor antidepressant response: is this the end of the HNK pipeline? Neuropsychopharmacology. 2020;45:1245–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zanos P, Brown KA, Georgiou P, Yuan P, Zarate CAJ, Thompson SM, et al. NMDA receptor activation-dependent antidepressant-relevant behavioral and synaptic actions of ketamine. J Neurosci. 2023;43:1038–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin P, Ma ZZ, Mahgoub M, Kavalali E, Monteggia LM. A synaptic locus for TrkB signaling underlying ketamine rapid antidepressant action. Cell Rep. 2021;36:109513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller OH, Moran JT, Hall BJ. Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology. 2016;100:17–26. [DOI] [PubMed] [Google Scholar]
- 86.Widman AJ, McMahon LL. Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci U S A. 2018;115(13):E3007–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lumsden EW, Troppoli TA, Myers SJ, Zanos P, Aracava Y, Kehr J, et al. Antidepressant-relevant concentrations of the ketamine metabolite (2R,6R)-hydroxynorketamine do not block NMDA receptor function. Proc Natl Acad Sci U S A. 2019;116(11):5160–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546:E1–E3. [DOI] [PubMed] [Google Scholar]
- 89.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33:6990–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nosyreva E, Autry AE, Kavalali ET, Monteggia LM. Age dependence of the rapid antidepressant and synaptic effects of acute NMDA receptor blockade. Front Mol Neurosci. 2014;7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang C, Ren Q, Qu Y, Zhang J-C, Ma M, Dong C, et al. Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry. 2018;83:18–28. [DOI] [PubMed] [Google Scholar]
- 93.Rafało-Ulińska A, Brański P, Pałucha-Poniewiera A. Combined administration of (R)-ketamine and the mGlu2/3 receptor antagonist LY341495 induces rapid and sustained effects in the CUMS model of depression via a TrkB/BDNF-dependent mechanism. Pharmaceuticals (Basel). 2022;15:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fujita Y, Hashimoto Y, Hashimoto H, Chang L, Hashimoto K. Dextran sulfate sodium-induced inflammation and colitis in mice are ameliorated by (R)-ketamine, but not (S)-ketamine: A role of TrkB signaling. Eur J Pharmacol. 2021;897:173954. [DOI] [PubMed] [Google Scholar]
- 95.Fujita A, Fujita Y, Pu Y, Chang L, Hashimoto K. MPTP-induced dopaminergic neurotoxicity in mouse brain is attenuated after subsequent intranasal administration of (R)-ketamine: a role of TrkB signaling. Psychopharmacology. 2020;237:83–92. [DOI] [PubMed] [Google Scholar]
- 96.Qu Y, Shan J, Wang S, Chang L, Pu Y, Wang X, et al. Rapid-acting and long-lasting antidepressant-like action of (R)-ketamine in Nrf2 knock-out mice: a role of TrkB signaling. Eur Arch Psychiatr Clin Neurosci. 2021;271:439–46. [DOI] [PubMed] [Google Scholar]
- 97.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schweiger JU, Schweiger U, Hueppe M, Kahl KG, Greggersen W, Fassbinder E. Bone density and depressive disorder: a meta-analysis. Behav Brain Res. 2016;6:e00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kadriu B, Gold PW, Luckenbaugh DA, Lener MS, Ballard ED, Niciu MJ, et al. Acute ketamine administration corrects abnormal inflammatory bone markers in major depressive disorder. Mol Psychiatry. 2018;23(7):1626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang K, Ma M, Dong C, Hashimoto K. Role of inflammatory bone markers in the antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Int J Neuropsychopharmacol. 2018;21:1025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scotton E, Casa PL, de Abreu FP, de Avila ESS, Wilges RLB, Rossetto MV, et al. Differentially regulated targets in the fast-acting antidepressant effect of (R)-ketamine: A systems biology approach. Pharmacol Biochem Behav. 2023;223:173523. [DOI] [PubMed] [Google Scholar]
- 102.Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80:424–31. [DOI] [PubMed] [Google Scholar]
- 103.Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. 2019;22:616–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176(6):428–38. [DOI] [PubMed] [Google Scholar]
- 105.Martinotti G, Vita A, Fagiolini A, Maina G, Bertolino A, Dell’Osso B, et al. Real-world experience of esketamine use to manage treatment-resistant depression: A multicentric study on safety and effectiveness (REAL-ESK study). J Affect Disord. 2022;319:646–54. [DOI] [PubMed] [Google Scholar]
- 106.Williamson DJ, Gogate JP, KS JK, Manera LS, Preskorn SH, Winokur A, et al. Longitudinal course of adverse events with esketamine nasal spray: A post hoc analysis of pooled data from Phase 3 trials in patients with treatment-resistant depression. J Clin Psychiatry. 2022;83:21m14318. [DOI] [PubMed] [Google Scholar]
- 107.Bentley S, Artin H, Mehaffey E, Liu F, Sojourner K, Bismark A, et al. Response to intravenous racemic ketamine after switch from intranasal (S)-ketamine on symptoms of treatment-resistant depression and post-traumatic stress disorder in Veterans: A retrospective case series. Pharmacotherapy. 2022;42(3):272–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Correia-Melo FS, Leal GC, Vieira F, Jesus-Nunes AP, Mello RP, Magnavita G, et al. Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: A randomized, double-blind, non-inferiority study. J Affect Disord. 2020;264:527–34. [DOI] [PubMed] [Google Scholar]
- 109.Singh B, Kung S, Pazdernik V, Schak KM, Geske J, Schulte PJ, et al. Comparative effectiveness of intravenous ketamine and intranasal esketamine in clinical practice among patients with treatment-refractory depression: An observational study. J Clin Psychiatry. 2023;84:22m14548. [DOI] [PubMed] [Google Scholar]
- 110.Leal GC, Bandeira ID, Correia-Melo FS, Telles M, Mello RP, Vieira F, et al. Intravenous arketamine for treatment-resistant depression: open-label pilot study. Eur Arch Psychiatr Clin Neurosci. 2021;271:577–82. [DOI] [PubMed] [Google Scholar]
- 111.Leal GC, Souza-Marques B, Mello RP, Bandeira ID, Caliman-Fontes AT, Carneiro BA, et al. Arketamine as adjunctive therapy for treatment-resistant depression: A placebo-controlled pilot study. J Affect Disord. 2023;330:7–15. [DOI] [PubMed] [Google Scholar]
- 112.Atai Life Sciences. atai Life Sciences announces results from Phase 2a trial of PCN-101 (R-ketamine) for treatment-resistant depression. January 6, 2023. Available at: https://www.globenewswire.com/news-release/2023/01/06/2584334/0/en/atai-Life-Sciences-Announces-Results-from-Phase-2a-Trial-of-PCN-101-R-ketamine-for-Treatment-Resistant-Depression.html. 2023.
- 113.Ma Z, Zang T, Birnbaum SG, Wang Z, Johnson JE, Zhang CL, et al. TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response. Nat Commun. 2017;8:1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lopez JP, Lücken MD, Brivio E, Karamihalev S, Kos A, De Donno C, et al. Ketamine exerts its sustained antidepressant effects via cell-type-specific regulation of Kcnq2. Neuron. 2022;110(14):2283–98.e9. [DOI] [PubMed] [Google Scholar]
- 115.Deyama S, Kaneda K. Role of neurotrophic and growth factors in the rapid and sustained antidepressant actions of ketamine. Neuropharmacology. 2023;224:109335. [DOI] [PubMed] [Google Scholar]
- 116.Zhu X, Zhang F, You Y, Wang H, Yuan S, Wu B, et al. S-Ketamine exerts antidepressant effects by regulating Rac1 GTPase mediated synaptic plasticity in the hippocampus of stressed rats. Cell Mol Neurobiol. 2023;43:299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang JC, Li SX, Hashimoto K. R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–41. [DOI] [PubMed] [Google Scholar]
- 118.Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K. (R)-ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R, 6R)-hydroxynorketamine. Biol Psychiatry. 2017;82:e43–e44. [DOI] [PubMed] [Google Scholar]
- 119.Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yao W, Cao Q, Luo S, He L, Yang C, Chen J, et al. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol Psychiatry. 2022;27:1618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang K, Yang C, Chang L, Sakamoto A, Suzuki T, Fujita Y, et al. Essential role of microglial transforming growth factor-β1 in antidepressant actions of (R)-ketamine and the novel antidepressant TGF-β1. Transl Psychiatry. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan Y, Fujita Y, Qu Y, Chang L, Pu Y, Wang S, et al. Phencyclidine-induced cognitive deficits in mice are ameliorated by subsequent repeated intermittent administration of (R)-ketamine, but not (S)-ketamine: role of BDNF-TrkB signaling. Pharmacol Biochem Behav. 2020;188:172839. [DOI] [PubMed] [Google Scholar]
- 123.Takahashi N, Yamada A, Shiraishi A, Shimizu H, Goto R, Tominaga Y. The basic and clinical pharmacology of ketamine. BMC Psychiatry. 2021;21:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen X, Hou X, Bai D, Lane R, Zhang C, Canuso C, et al. Efficacy and safety of flexibly dosed esketamine nasal spray plus a newly initiated oral antidepressant in adult patients with treatment-resistant depression: a randomized, double-blind, multicenter, active-controlled study conducted in China and USA. Neuropsychiatr Dis Treat. 2023;19:693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bahji A, Vazquez GH. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nikayin S, Rhee TG, Cunningham ME, de Fontnouvelle CA, Ostroff RB, Sanacora G, et al. Evaluation of the trajectory of depression severity with ketamine and esketamine treatment in a clinical setting. JAMA Psychiatry. 2022;79:736–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jones JL, Mateus CF, Malcolm RJ, Brady KT, Back SE. Efficacy of ketamine in the treatment of substance use disorders: a systematic review. Front Psychiatry. 2018;9:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johnston JN, Kadriu B, Allen J, Gilbert JR, Henter ID, Zarate CAJ. Ketamine and serotonergic psychedelics: An update on the mechanisms and biosignatures underlying rapid-acting antidepressant treatment. Neuropharmacology. 2023;226:109422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ballard ED, Zarate CA. The role of dissociation in ketamine’s antidepressant effects. Nat Commun. 2020;11:6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Del Sant LC, Sarin LM, Lucchese AC, Magalhães EJM, Tuena MA, Nakahira C, et al. Impact of repeated doses of subcutaneous esketamine on acute dissociative symptoms in treatment-resistant depression. Pharmaceuticals (Basel). 2022;16(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Acevedo-Diaz EE, Cavanaugh GW, Greenstein D, Kraus C, Kadriu B, Zarate CA Jr., et al. Comprehensive assessment of side effects associated with a single dose of ketamine in treatment-resistant depression. J Affect Disord. 2020;263:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ceban F, Rosenblat JD, Kratiuk K, Lee Y, Rodrigues NB, Gill H, et al. Prevention and management of common adverse effects of ketamine and esketamine in patients with mood disorders. CNS Drugs. 2021;35(9):925–34. [DOI] [PubMed] [Google Scholar]
- 134.Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol. 1997;333:99–104. [DOI] [PubMed] [Google Scholar]
- 135.Popik P, Khoo SY, Kuziak A, Golebiowska J, Potasiewicz A, Hogendorf A, et al. Distinct cognitive and discriminative stimulus effects of ketamine enantiomers in rats. Pharmacol Biochem Behav. 2020;197:173011. [DOI] [PubMed] [Google Scholar]
- 136.Le TT, Cordero IP, Jawad MY, Swainson J, Di Vincenzo JD, Jaberi S, et al. The abuse liability of ketamine: A scoping review of preclinical and clinical studies. J Psychiatr Res. 2022;151:476–96. [DOI] [PubMed] [Google Scholar]
- 137.Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M. A review of ketamine abuse and diversion. Depress Anxiety. 2016;33:718–27. [DOI] [PubMed] [Google Scholar]
- 138.Ng J, Lui LMW, Rosenblat JD, Teopiz KM, Lipsitz O, Cha DS, et al. Ketamine-induced urological toxicity: potential mechanisms and translation for adults with mood disorders receiving ketamine treatment. Psychopharmacology (Berl). 2021;238:917–26. [DOI] [PubMed] [Google Scholar]
- 139.Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry. 2019;176:401–09. [DOI] [PubMed] [Google Scholar]
- 140.Shiroma PR, Thuras P, Wels J, Albott CS, Erbes C, Tye S, et al. A randomized, double-blind, active placebo-controlled study of efficacy, safety, and durability of repeated vs single subanesthetic ketamine for treatment-resistant depression. Transl Psychiatry. 2020;10:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen G, Mannens G, De Boeck M, Daly E, Canuso CM, Teuns G, et al. Comments to pharmacological and behavioral divergence of ketamine enantiomers by Jordi Bonaventura et al. Mol Psychiatry. 2022;27:1860–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1992;260:1209–13. [PubMed] [Google Scholar]


