Abstract
In October 1999, H4N6 influenza A viruses were isolated from pigs with pneumonia on a commercial swine farm in Canada. Phylogenetic analyses of the sequences of all eight viral RNA segments demonstrated that these are wholly avian influenza viruses of the North American lineage. To our knowledge, this is the first report of interspecies transmission of an avian H4 influenza virus to domestic pigs under natural conditions.
Waterfowl and seabirds provide a vast global reservoir for influenza A viruses of all 15 hemagglutinin (HA) and 9 neuraminidase (NA) subtypes (26, 47, 60, 61). In these birds, influenza viruses infect epithelial cells of the gastrointestinal tract, but generally do not produce clinical signs of illness (60, 61). In contrast, influenza viruses are important respiratory pathogens in mammals and can also produce highly fatal systemic disease in domestic poultry (36, 60). Although evidence suggests that the mammalian influenza viruses have all been derived evolutionarily from avian viruses (51, 60), host-range restrictions limit avian-to-mammalian interspecies transmission. In particular, human influenza viruses do not replicate efficiently in birds and vice versa (1, 25, 36, 51, 60), although the 1997 H5N1 virus outbreak in Hong Kong (12, 20, 35, 56, 57, 65) clearly demonstrated that zoonotic avian influenza virus infections can occur.
The basis for influenza virus host-range restriction is likely to be polygenic, and evidence exists for contributions by all viral gene products (36, 60). However, HA is thought to be a major contributor because of its role as the viral receptor binding protein. Receptor specificity studies have demonstrated that a wide range of avian influenza viruses (H1 to H9, H11, and H13 subtypes) and H3 and H7 equine viruses bind preferentially to sialyloligosaccharides with α2,3-N-acetylneuraminic acid-galactose linkages (α2,3NeuAcGal). In contrast, H1 to H3 human viruses bind preferentially to sialyloligosaccharide receptors with α2,6-acetylneuraminic acid-galactose linkages (α2,6NeuAcGal) (13, 28, 44, 45). Consistent with these results, human tracheal epithelial cells possess α2,6NeuAcGal receptors (14), and duck intestinal epithelial cells possess α2,3NeuAcGal receptors (27). Tracheal epithelial cells from pigs, however, express both α2,3 and α2,6 receptors (27), and pigs are thereby uniquely susceptible to infection with both mammalian and avian viruses (6, 9, 10, 33). As such, they have been proposed to serve as intermediate hosts for adaptation of avian influenza viruses to replication in mammals (9) and as the “mixing vessel” hosts in which reassortment between avian and human viruses can produce genetically novel viruses with pandemic potential (50, 52, 60). Finally, zoonotic transmission of influenza viruses from pigs to people is well documented (15, 16, 18, 24, 34, 42, 48, 54, 58, 62, 63), and human-avian reassortant viruses have been isolated from children in The Netherlands subsequent to their detection in pigs (10, 11). For all of these reasons, the appearance of avian influenza viruses among pigs poses concerns for both veterinary and human health.
Clinical presentation.
Respiratory disease was first noted among pigs on the affected farm in the first week of October 1999. Approximately 5% of the 2,600 grower or feeder pigs and young boars on the farm exhibited coughing, labored breathing, and weight loss during the 3-week-long outbreak. Twelve animals died during the first 10 days of the outbreak, but deaths ceased after initiation of antibiotic therapy. Based on serologic monitoring that is conducted every 3 months, the pigs on this farm are considered free of infection with porcine reproductive and respiratory syndrome virus, porcine coronaviruses, and Actinobacillus pleuropneumoniae, and they are vaccinated against Mycoplasma hyopneumoniae and Hemophilus parasuis. Prior to 1999, there had been no evidence of influenza virus infection within the herd, and the pigs were not vaccinated against influenza.
Histopathology and virus isolation.
Lung tissue samples were obtained at postmortem from four sick pigs for diagnostic evaluations. Histologic examination revealed bronchointerstitial pneumonia with necrotizing bronchiolitis and hyperplasia of type II pneumocytes, consistent with a mixed viral and bacterial etiology. (Three common opportunistic bacterial pathogens of pigs were isolated from the lungs: Streptococcus suis, Pasteurella multocida, and Arcanobacterium pyogenes. The involvement of these organisms likely explains the clinical improvement observed with antibiotic therapy.) Homogenates (10% [wt/vol]) of pooled lung tissues (two animals per pool) were prepared and inoculated into Madin-Darby canine kidney (MDCK) cell cultures in borosilicate tubes with 1.5 μg of tolylsulfonyl phenylolanyl chloromethyl ketone (TPCK)-treated trypsin per ml (Worthington Biochemical Corporation, Lakewood, N.J.). Viral agents that hemagglutinated chicken and guinea pig erythrocytes to a titer of 128 were isolated from both lung pools. One of these viruses, A/Swine/Ontario/01911-1/99 (Sw/ONT/99-1), was chosen for complete analysis. The isolate from the second lung tissue pool, A/Swine/Ontario/01911-2/99 (Sw/ONT/99-2), was subjected to partial sequence characterization to confirm that it was of the same overall genotype as Sw/ONT/99-1 and to evaluate the level of genetic heterogeneity between the two isolates in the HA and matrix (M) genes.
Antigenic characterization of Sw/ONT/99-1.
Sw/ONT/99-1 was identified as an H4N6 virus by hemagglutination-inhibition (HI) assay (41) and microneuraminidase-inhibition (NI) spot assay (59) by using previously described (3) panels of monospecific chicken antisera. Further investigation demonstrated that Sw/ONT/99-1 reacted in HI assays to approximately equal titers with postinfection chicken antisera to either a North American H4N8 virus (A/Chicken/Alabama/75) or a European H4N6 virus (A/Duck/Czechoslovakia/56) and did not react with antisera to H1 or H3 viruses (Table 1). In NI assays, Sw/ONT/99-1 reacted with N6-monospecific chicken antisera raised against A/Duck/Czechoslovakia/56 (H4N6), A/Duck/England/56 (H11N6), A/Shearwater/Australia/2576/79 (H15N6), and A/Mallard/Gurjev/244/82 (H14N6). It did not react with antisera to A/Turkey/Italy/A141/80 (H6N6) nor with monospecific antisera for NA subtypes 1 to 5 and 7 to 9.
TABLE 1.
HI test results for Sw/ONT/99-1 and additional selected influenza A virus reference strains
| Virus | Subtype | Titer in postinfection chicken serum
|
|||||
|---|---|---|---|---|---|---|---|
| Swine/England/ 117316/86a | Swine/England/ 163266/87b | Swine/England/ 195852/92c | Swine/Texas/ 1/98b | Chicken/Alabama/ 75 | Duck/Czechoslovakia/ 56 | ||
| Swine/England/117316/86 | H1N1 | 640d | <40 | 40 | <40 | <40 | <40 |
| Swine/England/163266/87 | H3N2 | <40 | 640 | <40 | <40 | <40 | <40 |
| Swine/England/195852/92 | H1N1 | 40 | <40 | 320 | <40 | <40 | <40 |
| Swine/Texas/4199-2/98 | H3N2 | <40 | 40 | <40 | 320 | <40 | <40 |
| Chicken/Alabama/75 | H4N8 | <40 | <40 | <40 | <40 | 80 | <40 |
| Duck/Czechoslovakia/56 | H4N6 | <40 | <40 | <40 | <40 | <40 | 160 |
| Duck/Alberta/119/79 | H4N6 | <40 | <40 | <40 | <40 | <40 | 160 |
| Duck/England/96/80 | H4N1 | <40 | <40 | <40 | <40 | <40 | 160 |
| Chicken/Belgium/909/85 | H4N6 | <40 | <40 | <40 | <40 | <40 | 80 |
| Duck/England/1086/85 | H4N6 | <40 | <40 | <40 | <40 | <40 | 160 |
| Swine/Ontario/01911-1/99 | H4N6 | <40 | <40 | <40 | <40 | 320 | 640 |
Classical swine H1 HA.
Human-like H3 HA.
Avian-like H1 HA.
HI titers are expressed as the reciprocal of the dilution of antisera inhibiting 4 hemagglutinating units of virus.
Genetic characterization and phylogenetic analyses of Sw/ONT/99-1 and -2.
The full-length protein-coding regions of all eight viral RNA segments of Sw/ONT/99-1 were amplified by reverse transcription-PCR (RT-PCR) with avian myeloblastosis virus reverse transcriptase (Promega Corporation, Madison, Wis.) and Pfu polymerase (Stratagene, La Jolla, Calif.) as previously described (31). Amplifications of the HA, NA, M, nucleoprotein (NP), and nonstructural (NS) genes were accomplished by multiplex RT-PCR using the SZANA+/− primers developed by Zou (66). The PB1 gene was amplified using the SZAPB1+/− primers developed by Zou (66), and the PB2 and PA polymerase genes were amplified with primers that we developed and described previously (31). The sequences of the amplified genes were determined from the PCR products by cycle sequencing (ABI Big Dye; PE Applied Biosystems, Foster City, Calif.).
The genotype of Sw/ONT/99-1 was determined initially by pairwise comparisons of the nucleotide sequences of each gene segment to the sequences of reference influenza viruses available in GenBank by using DNASTAR software (version 4.0 for Win32). Table 2 lists the reference viruses from GenBank with the highest level of sequence identity to Sw/ONT/99-1 for each gene segment. These results clearly demonstrate that Sw/ONT/99-1 was derived by in toto transmission of an avian influenza virus to pigs. In this regard, it is of interest to note that the farm of origin of Sw/ONT/99-1 is located near a lake on which large numbers of waterfowl congregate each fall. The farm operates a biosecurity program that includes control programs to minimize rodent and bird entry into the barn, as well as requirements for personnel to shower and change clothes before entering the barn. However, as is typical of most commercial swine barns, air entering the facility is not filtered, and water used on the farm was sometimes drawn from the nearby lake. Thus, conditions were favorable for transmission of an avian virus from the adjacent waterfowl population to the pigs on this farm. In contrast, it is unlikely that the H4N6 virus was introduced to this farm through the movement of infected pigs, since this farm did not import animals from unrelated herds. (Note that neither the Animal Health Laboratory of the University of Guelph, where the isolations were made, nor the University of Wisconsin laboratory, where the genetic analyses were conducted, has worked previously with H4 or N6 influenza viruses. Therefore, there is no possibility that the isolations or gene amplifications resulted from laboratory contamination.)
TABLE 2.
Sequence homology of each gene from Sw/ONT/99-1 compared to reference virus sequences available in GenBank
| Gene (nucleotide positions of Sw/ONT/99-1 compared) | Virus with highest degree of sequence identitya | % Nucleotide sequence identity |
|---|---|---|
| PB2 (1–2341) | A/Shorebird/Delaware/9/96 (H9N2) [AF156441] (23) | 96.8 |
| PB1 (1–2341) | A/Turkey/Minnesota/833/80 (H4N2) [M25925] (32) | 96.7 |
| PA (1–2233) | A/Turkey/Minnesota/833/80 (H4N2) [M26085] (40) | 95.0 |
| HA (1–1740) | A/Turkey/Minnesota/833/80 (H4N2) [M25290] (17) | 93.7 |
| NP (34–1565) | A/Ruddy Turnstone/New Jersey/47/85 (H4N6) [M30766] (21) | 97.1 |
| NA (1–277b) | A/Duck/Alberta/28/76 (H4N6) [K01009] (2) | 90.6 |
| M (26–989) | A/Turkey/Colorado/13356/91 (H7N3) [AF073198] (55) | 97.0 |
| NS (1–890) | A/Rhea/North Carolina/39482/93 (H7N1) [AF007036] (55) | 98.2 |
The numbers in brackets are the GenBank accession numbers for the reference virus sequences. The numbers in parentheses are the references for each sequence. Note that all influenza virus lineages were avian.
The full-length protein-coding region of the NA gene of Sw/ONT/99-1 was determined in this study. However, this analysis was restricted to the 5′-most (in mRNA sense) 277 nucleotides, because only partial N6 sequences are available in GenBank.
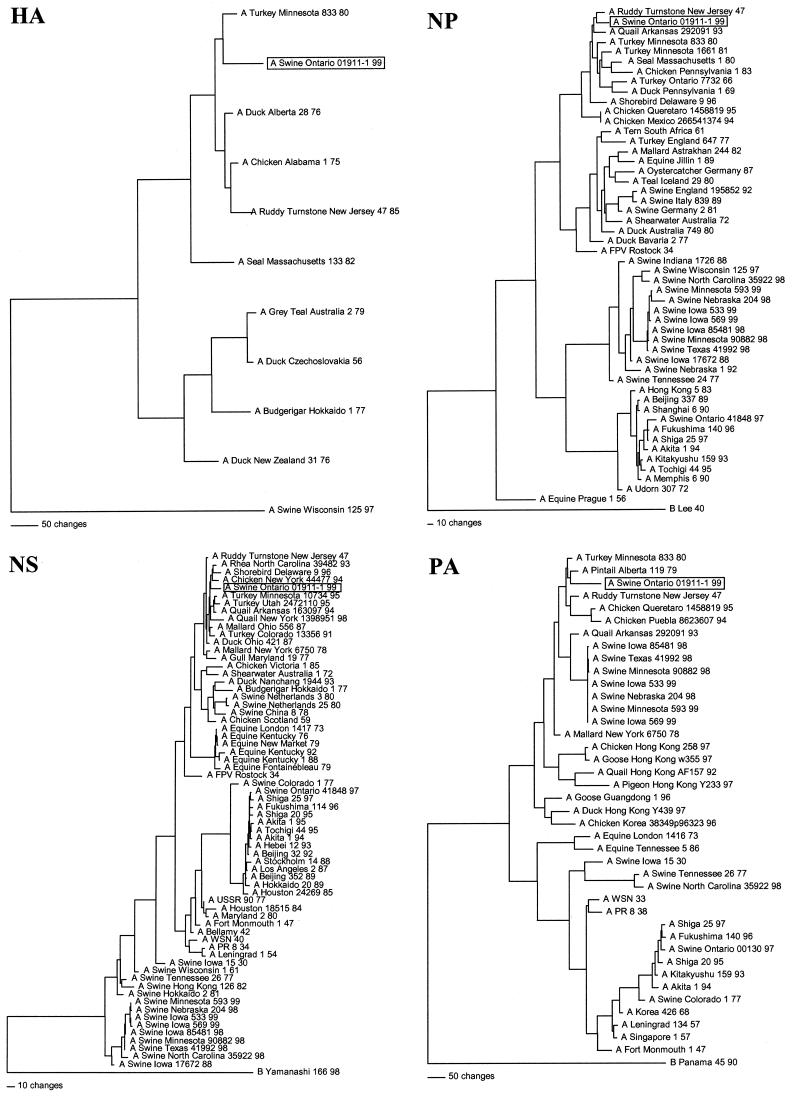
The phylogenetic relationships of Sw/ONT/99-1 to selected reference strains were estimated from the nucleotide sequences of each viral gene (except for the NA gene, for which too few sequences are available in GenBank to create an informative tree). Phylogenies were determined by the method of maximum parsimony (PAUP software v.4.0b2; David Swofford, Smithsonian Institution, Washington, D.C.) by using the tree bisection-reconnection branch-swapping algorithm and with the MULTREES option in effect. For each virus gene, the full-length protein-coding region sequences of Sw/ONT/99-1 were analyzed in relation to the available reference virus sequences in GenBank, with the “gaps treated as missing” PAUP rule in effect. The HA, NP, NS, and PA gene phylograms are shown in Fig. 1. These results, as well as the phylogenetic analyses of the additional internal structural (M) and polymerase (PB1 and PB2) protein genes (data not shown), confirmed the avian genotype of Sw/ONT/99-1 and further demonstrated in each case that Sw/ONT/99-1 is of the North American rather than the Eurasian lineage of avian influenza viruses. Similarly, RT-PCR amplification, sequencing, and phylogenetic analysis of 500 to 600 nucleotides of each gene segment of Sw/ONT/99-2 confirmed that this virus is of the same overall genotype as Sw/ONT/99-1. Furthermore, complete sequence analysis of the M and HA genes of Sw/ONT/99-2 demonstrated that it is highly homologous to Sw/ONT/99-1, with only a single amino acid difference in M (S in Sw/ONT/99-1 to N in Sw/ONT/99-2 at residue 118) and three amino acid differences in HA (N→S at residue 336, P→L at residue 338, and E→G at residue 400 in Sw/ONT/99-1 and Sw/ONT/99-2, respectively).
FIG. 1.
Nucleotide phylogenetic trees for the HA, NP, NS, and PA genes of Sw/ONT/99-1. The evolutionary relationships were estimated by the method of maximum parsimony (PAUP software, v.40b2; David Swofford, Smithsonian Institution, Washington, D.C.) by using the tree bisection-reconnection branch-swapping algorithm and with the MULTREES option and “gaps treated as missing” PAUP rule in effect. The trees shown represent the best of multiple rearrangements that were generated. The scores and number of rearrangements for each tree are as follows: HA, score = 1,911 of 684 rearrangements; NP, score = 2,460 of 48,866 rearrangements; NS, score = 1,313 of 2,013,155,259 rearrangements; and, PA, score = 3,286 of 24,377 rearrangements. The horizontal line distances are proportional to the minimum number of nucleotide changes needed to join nodes and gene sequences. The vertical lines are simply for spacing the branches and labels.
Further pairwise analyses of the deduced HA amino acid sequences of Sw/ONT/99-1 and -2 were conducted to more fully characterize these viruses. Consistent with previous studies of H4 viruses (17, 39), the Sw/ONT/99-1 and -2 HA genes encode polypeptides of 564 amino acids. These HAs do not contain any additional basic amino acids at the putative HA1/HA2 cleavage site (KATR/G) compared to other H4 HAs. Furthermore, the N-linked glycosylation sites (four in HA1 and one in HA2) described previously for H4 HAs (17), as well as the amino acids previously defined (39) as comprising the receptor binding site and the right edge of the receptor binding pocket for H4 viruses, are conserved in the Sw/ONT/99 HAs. However, two of the six amino acids making up the left edge of the receptor binding pocket (39) differ in the Sw/ONT/99 HAs compared to previously sequenced avian H4 viruses: amino acid 226 (Q→L) and amino acid 228 (G→S) (using the H3 numbering scheme). These differences are of particular interest because they are the same amino acids that have been suggested to confer preferential binding of influenza viruses to α2,6 rather than α2,3NeuAcGal receptors (13, 29, 37, 46). As such, there is a concern that these viruses may also be infectious for humans. (There were no reports of illness among the farm workers during or immediately after this outbreak, but we are currently attempting to obtain samples for serological assessment of human infection with these viruses.) These specific amino acids would not appear to be responsible more generally for adaptation of avian influenza viruses to replication in other mammals, since similar changes are not present in the HA sequence of A/Seal/Massachusetts/133/82 (17), which is the only other mammalian H4 isolate in GenBank, nor in the HAs of an H10 avian virus isolated from mink (A/Mink/Sweden/84) (19), H7 (A/Seal/Massachusetts/1/80) (38), or H3 (A/Seal/Massachusetts/3911 and 3984/92) (8) avian viruses isolated from seals or H1 avian viruses isolated from pigs in Europe (7). However, passage of the later viruses in eggs may have selected for the α2,3 receptor amino acids at these residues. Since the original lung tissues from which the Sw/ONT/99 viruses were isolated are no longer available, it is not possible to determine whether these amino acid differences were present in the viruses as they existed in pigs or whether they developed during isolation in MDCK cells, which, like pig tracheal cells, also contain both α2,3- and α2,6-linked receptor sialic acids (29).
Serology.
Serum samples that had been collected for routine health screening from pigs in the herd were tested by HI assay (41) for antibodies against Sw/ONT/99-1, as well as a recent reassortment swine H3N2 virus (A/Swine/Minnesota/593/99) (31) and a classical H1N1 swine influenza virus, A/Swine/Indiana/1726/88 (53a). Twelve of 12 pigs that were sampled approximately 6 weeks prior to the onset of illness were all seronegative (HI titer, <10) for Sw/ONT/99-1, as well as for the H3N2 virus, while 1 of these 12 pigs was seropositive (HI titer, 40) for the H1N1 virus. In contrast, all 10 of 10 animals sampled approximately 3 months after the outbreak were seropositive for Sw/ONT/99-1 at HI titers of 20 to 80, but seronegative for both the H1N1 and H3N2 viruses. Thus, it is likely that the Sw/ONT/99 viruses spread from pig to pig on the farm of origin. Testing is currently under way to determine whether Sw/ONT/99-like viruses also spread to pigs in additional herds in the area.
In a serologic study of swine influenza in Great Britain in 1991 to 1992, Brown and colleagues were unable to detect evidence of natural infection of pigs with either H4 or H10 avian influenza viruses (5). We suspect that in the present case, the proximity of the affected farm to a lake with waterfowl was a major reason why the pigs on this farm became infected. Viruses with H4 and/or N6 surface glycoproteins have been shown previously to be among the most common influenza viruses in the Canadian duck population (53), and Kida and colleagues demonstrated that pigs can be infected experimentally with H4 avian influenza viruses (33). Furthermore, H1N1 avian influenza viruses infected pigs in northern Europe in 1979 and became the dominant cause of swine influenza in that region thereafter (4, 7, 43, 49, 60), while another avian H1N1 virus was transmitted to pigs in Asia in 1993 (22). To our knowledge, however, this report is the first to document the isolation of a wholly avian influenza virus from pigs in North America and the isolation of an H4 influenza virus from naturally infected pigs. Given the evidence that pigs can support reassortment of human and avian influenza viruses (6, 10, 50, 52, 60), including the recent isolations of human-avian-swine triple reassortant H3N2 and H1N2 viruses from pigs in the United States (30, 31, 64), it is prudent that we enhance surveillance for atypical influenza viruses in pigs as part of overall pandemic preparedness efforts and that we consider the potential for these H4N6 viruses, or H4 reassortant viruses, to enter the human population.
Nucleotide seuqence accession numbers.
The GenBank numbers assigned to the full-length protein-coding region gene sequences of Sw/ONT/99-1 are as follows: HA, AF285885; NA, AF285887; M, AF285886; NP, AF285888; NS, AF285889; PA, AF285890; PB1, AF285891; and PB2, AF285892. The GenBank numbers assigned to the full-length protein-coding region gene sequences for the HA and M gene sequences of Sw/ONT/99-2 are AF285883 (HA) and AF285884 (M). The GenBank accession numbers for all of the reference virus sequences used in the phylogenetic analyses are available upon request.
Acknowledgments
This work was supported in part by a USDA NRICGP grant.
We thank M. Schutten and P. A. Harris for excellent laboratory technical support. We also thank K. Subbarao of the Centers for Disease Control and Prevention and Y. Kawaoka of the University of Wisconsin—Madison for reviewing the manuscript and for many helpful discussions.
REFERENCES
- 1.Beare A S, Webster R G. Replication of avian influenza viruses in humans. Arch Virol. 1991;119:37–42. doi: 10.1007/BF01314321. [DOI] [PubMed] [Google Scholar]
- 2.Blok J, Air G M. Sequence variation at the 3′ end of the neuraminidase gene from 39 influenza type A viruses. Virology. 1982;121:211–229. doi: 10.1016/0042-6822(82)90162-3. [DOI] [PubMed] [Google Scholar]
- 3.Brown I H, Alexander D J, Chakraverty P, Harris P A, Manvell R J. Isolation of an influenza A virus of unusual subtype (H1N7) from pigs in England, and the subsequent experimental transmission from pig to pig. Vet Microbiol. 1994;39:125–134. doi: 10.1016/0378-1135(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 4.Brown I H, Done S H, Spencer Y I, Cooley W A, Harris P A, Alexander D J. Pathogenicity of a swine influenza H1N1 virus antigenically distinguishable from classical and European strains. Vet Rec. 1993;132:598–602. doi: 10.1136/vr.132.24.598. [DOI] [PubMed] [Google Scholar]
- 5.Brown I H, Harris P A, Alexander D J. Serological studies of influenza viruses in pigs in Great Britain. 1991–2. Epidemiol Infect. 1995;114:511–520. doi: 10.1017/s0950268800052225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown I H, Harris P A, McCauley J W, Alexander D J. Multiple genetic reassortment of avian and human influenza A viruses in European pigs, resulting in emergence of an H1N2 virus of novel genotype. J Gen Virol. 1998;79:2947–2955. doi: 10.1099/0022-1317-79-12-2947. [DOI] [PubMed] [Google Scholar]
- 7.Brown I H, Ludwig S, Olsen C W, Hannoun C, Scholtissek C, Hinshaw V S, Harris P A, McCauley J W, Strong I, Alexander D J. Antigenic and genetic analyses of H1N1 influenza A viruses from European pigs. J Gen Virol. 1997;78:553–562. doi: 10.1099/0022-1317-78-3-553. [DOI] [PubMed] [Google Scholar]
- 8.Callan R J, Early G, Kida H, Hinshaw V S. The appearance of H3 influenza viruses in seals. J Gen Virol. 1995;76:199–203. doi: 10.1099/0022-1317-76-1-199. [DOI] [PubMed] [Google Scholar]
- 9.Campitelli L, Donatelli I, Foni E, Castrucci M R, Fabiani C, Kawaoka Y, Krauss S, Webster R G. Continued evolution of H1N1 and H3N2 influenza viruses in pigs in Italy. Virology. 1997;232:310–318. doi: 10.1006/viro.1997.8514. [DOI] [PubMed] [Google Scholar]
- 10.Castrucci M R, Donatelli I, Sidoli L, Barigazzi G, Kawaoka Y, Webster R G. Genetic reassortment between avian and human influenza viruses in Italian pigs. Virology. 1993;193:503–506. doi: 10.1006/viro.1993.1155. [DOI] [PubMed] [Google Scholar]
- 11.Claas E C J, Kawaoka Y, de Jong J C, Masurel N, Webster R G. Infection of children with avian-human reassortant influenza virus from pigs in Europe. Virology. 1994;204:453–457. doi: 10.1006/viro.1994.1553. [DOI] [PubMed] [Google Scholar]
- 12.Claas E C J, Osterhaus A D M E, Van Beek R, de Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 13.Connor R J, Kawaoka Y, Webster R G, Paulson J C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- 14.Couceiro J N S S, Paulson J C, Baum L G. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium: the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 1993;29:155–165. doi: 10.1016/0168-1702(93)90056-s. [DOI] [PubMed] [Google Scholar]
- 15.Dasco C C, Couch R B, Six H R, Young J F, Quarles J M, Kasel J A. Sporadic occurrence of zoonotic swine influenza virus infections. J Clin Microbiol. 1984;20:833–835. doi: 10.1128/jcm.20.4.833-835.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong J C, Paccaud M F, de Ronde-Verloop F M, Huffels N H, Verwei C, Weijers T F, Bangma P J, van Kregten E, Kerckhaert J A M, Wicki F, Wunderli W. Isolation of swine-like influenza A (H1N1) viruses from men in Switzerland and The Netherlands. Annu Inst Pasteur Virol. 1988;139:429–437. doi: 10.1016/s0769-2617(88)80078-9. [DOI] [PubMed] [Google Scholar]
- 17.Donis R O, Bean W J, Kawaoka Y, Webster R G. Distinct lineages of influenza virus H4 hemagglutinin genes in different regions of the world. Virology. 1989;169:408–417. doi: 10.1016/0042-6822(89)90166-9. [DOI] [PubMed] [Google Scholar]
- 18.Eason R J, Sage M D. Deaths from influenza A, subtype H1N1, during the 1979 Auckland epidemic. N Z Med J. 1980;91:129–131. [PubMed] [Google Scholar]
- 19.Feldmann H, Kretzschmar E, Klingeborn B, Rott R, Klenk H-D, Garten W. The structure of serotype H10 hemagglutinin of influenza A virus: comparison of an apathogenic avian and a mammalian strain pathogenic for mink. Virology. 1988;165:428–437. doi: 10.1016/0042-6822(88)90586-7. [DOI] [PubMed] [Google Scholar]
- 20.Gao P, Watanabe S, Ito T, Goto H, Wells K, McGregor M, Cooley A J, Kawaoka Y. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J Virol. 1999;73:3184–3189. doi: 10.1128/jvi.73.4.3184-3189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorman O T, Bean W J, Kawaoka Y, Webster R G. Evolution of the nucleoprotein gene of influenza A virus. J Virol. 1990;64:1487–1497. doi: 10.1128/jvi.64.4.1487-1497.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan Y, Shortridge K F, Krauss S, Li P H, Kawaoka Y, Webster R G. Emergence of avian H1N1 influenza viruses in pigs in China. J Virol. 1996;70:8041–8046. doi: 10.1128/jvi.70.11.8041-8046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan Y, Shortridge K F, Krauss S, Webster R G. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong. Proc Natl Acad Sci USA. 1999;96:9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinshaw V S, Bean W J, Jr, Webster R G, Easterday B C. The prevalence of influenza viruses in swine and the antigenic and genetic relatedness of influenza viruses from man and swine. Virology. 1978;84:51–62. doi: 10.1016/0042-6822(78)90217-9. [DOI] [PubMed] [Google Scholar]
- 25.Hinshaw V S, Webster R G, Naeve C W, Murphy B R. Altered tissue tropism of human-avian reassortant influenza viruses. Virology. 1983;128:260–263. doi: 10.1016/0042-6822(83)90337-9. [DOI] [PubMed] [Google Scholar]
- 26.Hinshaw V S, Webster R G, Turner B. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can J Microbiol. 1980;26:622–629. doi: 10.1139/m80-108. [DOI] [PubMed] [Google Scholar]
- 27.Ito T, Couceiro J N, Kelm S, Baum L G, Krauss S, Castrucci M R, Donatelli I, Kida H, Paulson J C, Webster R G, Kawaoka Y. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol. 1998;72:7367–7373. doi: 10.1128/jvi.72.9.7367-7373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito T, Suzuki Y, Mitnaul L, Vines A, Kida H, Kawaoka Y. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology. 1997;227:493–499. doi: 10.1006/viro.1996.8323. [DOI] [PubMed] [Google Scholar]
- 29.Ito T, Suzuki Y, Takada A, Kawamoto A, Otsuki K, Masudu H, Yamada M, Suzuki T, Kida H, Kawaoka Y. Differences in sialic acid-galactose linkages in the chicken egg amnion and allantois influence human influenza virus receptor specificity and variant selection. J Virol. 1997;71:3357–3362. doi: 10.1128/jvi.71.4.3357-3362.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karasin A I, Olsen C W, Anderson G A. Genetic characterization of an H1N2 influenza virus isolated from a pig in Indiana. J Clin Microbiol. 2000;38:2453–2456. doi: 10.1128/jcm.38.6.2453-2456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karasin A I, Schutten M M, Cooper L A, Smith C B, Subbarao K, Anderson G A, Carman S, Olsen C W. Genetic characterization of H3N2 influenza viruses isolated from pigs in North America, 1977–1999: evidence for wholly human and reassortant virus genotypes. Virus Res. 2000;68:71–85. doi: 10.1016/s0168-1702(00)00154-4. [DOI] [PubMed] [Google Scholar]
- 32.Kawaoka Y, Krauss S, Webster R G. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol. 1989;63:4603–4608. doi: 10.1128/jvi.63.11.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kida H, Ito T, Yasuda J, Shimizu Y, Itakura C, Shortridge K F, Kawaoka Y, Webster R G. Potential for transmission of avian influenza viruses to pigs. J Gen Virol. 1994;75:2183–2188. doi: 10.1099/0022-1317-75-9-2183. [DOI] [PubMed] [Google Scholar]
- 34.Kimura K, Adlakha A, Simon P M. Fatal case of swine influenza virus in an immunocompetent host. Mayo Clin Proc. 1998;73:243–245. doi: 10.4065/73.3.243. [DOI] [PubMed] [Google Scholar]
- 35.Mounts A W, Kwong H, Izurieta H S, Ho Y Y, Au T K, Lee M, Bridges C B, Williams S W, Mak K H, Katz J M, Thompson W W, Cox N J, Fukuda K. Case-control study of risk factors for avian influenza A (H5N1) disease, Hong Kong, 1997. J Infect Dis. 1999;180:505–508. doi: 10.1086/314903. [DOI] [PubMed] [Google Scholar]
- 36.Murphy B R, Webster R G. Orthomyxoviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Field's virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1397–1445. [Google Scholar]
- 37.Naeve C W, Hinshaw V S, Webster R G. Mutations in the hemagglutinin receptor-binding site can change the biological properties of an influenza virus. J Virol. 1984;51:567–569. doi: 10.1128/jvi.51.2.567-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naeve C W, Webster R G. Sequence of the hemagglutinin gene from influenza virus A/Seal/Mass/1/80. Virology. 1983;129:298–308. doi: 10.1016/0042-6822(83)90169-1. [DOI] [PubMed] [Google Scholar]
- 39.Nobusawa E, Aoyama T, Kato H, Suzuki Y, Tateno Y, Nakajima K. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology. 1991;182:475–485. doi: 10.1016/0042-6822(91)90588-3. [DOI] [PubMed] [Google Scholar]
- 40.Okazaki K, Kawaoka Y, Webster R G. Evolutionary pathways of the PA genes of influenza A viruses. Virology. 1989;172:601–608. doi: 10.1016/0042-6822(89)90202-x. [DOI] [PubMed] [Google Scholar]
- 41.Palmer D F, Dowdle W R, Coleman M T, Schild G C. Advanced laboratory techniques for influenza diagnosis. Washington, D.C: U.S. Department of Health, Education, and Welfare Immunology Series; 1975. [Google Scholar]
- 42.Patriarca P A, Kendal A P, Zakowski P C, Cox N J, Trautman M S, Cherry J D, Auervach D M, McCusker J, Belliveau R R, Kappus K D. Lack of significant person-to-person spread of swine influenza-like virus following fatal infection of an immunocompromised child. Am J Epidemiol. 1984;119:152–158. doi: 10.1093/oxfordjournals.aje.a113733. [DOI] [PubMed] [Google Scholar]
- 43.Pensaert M, Ottis K, Vandeputte J, Kaplan M M, Bachmann P A. Evidence for the natural transmission of influenza A virus from wild ducks to swine and its potential importance for man. Bull W H O. 1981;59:75–78. [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers G N, D'Souza B L. Receptor binding properties of human and animal H1 influenza virus isolates. Virology. 1989;173:317–322. doi: 10.1016/0042-6822(89)90249-3. [DOI] [PubMed] [Google Scholar]
- 45.Rogers G N, Paulson J C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 46.Rogers G N, Paulson J C, Daniels R S, Skehel J J, Wilson I A, Wiley D C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983;304:76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- 47.Röhm C, Zhou N A, Süss J C, Mackenzie J, Webster R G. Characterization of a novel influenza hemagglutinin, H15: criteria for determination of influenza A subtypes. Virology. 1996;217:508–516. doi: 10.1006/viro.1996.0145. [DOI] [PubMed] [Google Scholar]
- 48.Rota P A, Rocha E P, Harmon M W, Hinshaw V S, Sheerar M G, Kawaoka Y, Cox N J, Smith T F. Laboratory characterization of a swine influenza virus isolated from a fatal case of human influenza. J Clin Microbiol. 1989;27:1413–1416. doi: 10.1128/jcm.27.6.1413-1416.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scholtissek C, Burger H, Bachmann P A, Hannoun C. Genetic relatedness of hemagglutinins of the H1 subtype of influenza A viruses isolated from swine and birds. Virology. 1983;129:521–523. doi: 10.1016/0042-6822(83)90194-0. [DOI] [PubMed] [Google Scholar]
- 50.Scholtissek C, Burger H, Kistner O, Shortridge K. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology. 1985;147:287–294. doi: 10.1016/0042-6822(85)90131-x. [DOI] [PubMed] [Google Scholar]
- 51.Scholtissek C, Hinshaw V S, Olsen C W. Influenza in pigs and their role as the intermediate host. In: Nicholson K G, Webster R G, Hay A, editors. Textbook of influenza. London, United Kingdom: Blackwell Healthcare Communications; 1998. pp. 137–145. [Google Scholar]
- 52.Scholtissek C, Naylor E. Fish farming and influenza pandemics. Nature. 1988;331:215. doi: 10.1038/331215a0. [DOI] [PubMed] [Google Scholar]
- 53.Sharp G B, Kawaoka Y, Wright S M, Turner B, Hinshaw V S, Webster R G. Wild ducks are the reservoir for a limited number of influenza A viruses. Epidemiol Infect. 1993;110:161–176. doi: 10.1017/s0950268800050780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53a.Sheerar M G, Easterday B C, Hinshaw V S. Antigenic conservation of H1N1 swine influenza viruses. J Gen Virol. 1989;70:3297–3303. doi: 10.1099/0022-1317-70-12-3297. [DOI] [PubMed] [Google Scholar]
- 54.Smith T F, Burgert E O, Dowdle W R, Noble G R, Campbell R J, Van Scoy R E. Isolation of swine influenza virus from autopsy lung tissue of man. N Engl J Med. 1976;294:708–710. doi: 10.1056/NEJM197603252941308. [DOI] [PubMed] [Google Scholar]
- 55.Suarez D L, Garcia M, Latimer J, Senne D, Perdue M. Phylogenetic analysis of H7 avian influenza viruses isolated from the live bird markets of the Northeast United States. J Virol. 1999;73:3567–3573. doi: 10.1128/jvi.73.5.3567-3573.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suarez D L, Perdue M L, Cox N, Rowe T, Bender C, Huang J, Swayne D E. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X Y, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 58.Top F H, Russell P K. Swine influenza at Fort Dix, N.J. IV. Summary and speculation. J Infect Dis. 1977;136:S376–S380. doi: 10.1093/infdis/136.supplement_3.s376. [DOI] [PubMed] [Google Scholar]
- 59.Van Deusen R A, Hinshaw V S, Senne D A, Pellacani D. Microneuraminidase-inhibition assay for classification of influenza A virus neuraminidases. Avian Dis. 1983;27:745–750. [PubMed] [Google Scholar]
- 60.Webster R G, Bean W J, Gorman O T, Chambers T M, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Webster R G, Yakhno M, Hinshaw V S, Bean W J, Murti K G. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology. 1978;84:268–278. doi: 10.1016/0042-6822(78)90247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wentworth D E, Thompson B L, Xu X, Regnery H L, Cooley A J, McGregor M W, Cox N J, Hinshaw V S. An influenza A (H1N1) virus closely related to swine influenza virus responsible for a fatal case of human influenza. J Virol. 1994;68:2051–2058. doi: 10.1128/jvi.68.4.2051-2058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wentworth D E, McGregor M W, Macklin M D, Neumann V, Hinshaw V S. Transmission of swine influenza virus to humans after exposure to experimentally infected pigs. J Infect Dis. 1997;175:7–15. doi: 10.1093/infdis/175.1.7. [DOI] [PubMed] [Google Scholar]
- 64.Zhou N N, Senne D A, Landgraf J S, Swenson S L, Erickson G, Rossow K, Liu L, Yoon K-J, Krauss S, Webster R G. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J Virol. 1999;73:8851–8856. doi: 10.1128/jvi.73.10.8851-8856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou N N, Shortridge K F, Claas E C J, Krauss S L, Webster R G. Rapid evolution of H5N1 influenza viruses in chickens in Hong Kong. J Virol. 1999;73:3366–3374. doi: 10.1128/jvi.73.4.3366-3374.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zou S. A practical approach to genetic screening for influenza virus variants. J Clin Microbiol. 1997;35:2623–2627. doi: 10.1128/jcm.35.10.2623-2627.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]