Abstract
Background
Today, uterine cancer is one of the most important causes of death in the world and is one of the major problems in human health. There have been numerous reports of the effect of Streptococcus agalactiae peptide and capsular products against cancer cell lines. Objective: This study aimed to research recombinant peptide CPSA-CPSC-L-ACAN and investigate its apoptotic effect against the HeLa cell line by Real-Time-RT PCR.
Design
In this study confirmation of the recombinant fusion peptide was performed by Western blotting. The effect of cytotoxicity of different concentrations of recombinant fusion peptide against the HeLa cell line was investigated by the MTT technique. The expression of apoptotic genes including BAX, BCL-2, and Caspase-3 in comparison with the GAPDH reference gene before and after exposure to recombinant fusion peptide was measured by Real-Time RT-PCR.
Results
Recombinant fusion peptide at a concentration of 63 μg/ml destroyed 50% of the HeLa cell line in 24 h and cell treatment with this concentration increased gene expression of Caspase-3 genes by 16 times, bax by 6 times and decreased the expression of bcl-2 by 0.176 times.
Conclusions
The results showed that treatment of the HeLa cell line with recombinant fusion peptide induced an apoptotic effect. The recombinant fusion peptide could probably help the medical community as a prophylactic or therapeutic treatment for cervical cancer.
Keywords: HeLa cell line, Recombinant fusion peptide, Streptococcus agalactiae, Real-time RT-PCR, Cps A, Cps C, Bax, Bcl-2, Caspase3
Highlights
-
•
This research is done for the first time in the world and it is an innovation of researching the effect of CpsA-CpsC-Ligand-ACAN fusion protein against inhibition of bcl2 and caspase3 genes of Hela cells by Real-Time-RT-PCR method.
-
•
Streptococcus agalactiae capsules were used to prevent and inhibit cancer cells.
-
•
This study is to obtain a recombinant fusion protein with anticancer properties against cancer cells or prevention.
-
•
In this research, A and C capsular synthesizing enzymes were used along with anticancer sequences to enhance the apoptosis of Hela tumor cells.
-
•
Introducing CpsA-CpsC-Ligand-ACAN recombinant fusion protein to prevent genital tract cancer in high-risk women with underlying diseases.
Abbreviations
- CpsA
Capsule type A
- CapsC
Capsule type C
- ACAN
Anti-cancer peptide
- L
Ligand
- Bax
Apoptosis activator
- Bcl2
Apoptosis inhibitor
- Caspase3
Cysteine protease and apoptosis activator
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
1. Introduction
Cervical cancer is known as the fourth most common woman's cancer worldwide [[1], [2], [3], [4]]. Due to the lack of proper diagnostic, prophylactic, and treatment methods, the death rate is increasing [5]. Cancer treatment methods include surgery, cryotherapy, chemotherapy, target therapy, radiotherapy, Immunotherapy, and hysterectomy [6]. One of the most common methods of treating cervical cancer is chemotherapy, which has many side effects such as drug resistance [7]. For this reason, the tendency to research new drugs is increasing [[8], [9], [10]].
Many bacteria such as Salmonella spp, Bifidobacterium spp, Streptococcus agalactiae, and Clostridium spp showed anti-cancer properties [11,12]. The anti-cancer property of bacteria is due to the increase in the production of polysaccharides, toxins, or enzymes [13]. Streptococcus agalactiae is part of the normal vaginal flora and due to the protein and polysaccharide production products prevent them from becoming cancerous cancer cells [[14], [15], [16]]. Bacteria contain proteins with a positive charge that reacts with cell membrane phosphatidylserine tumoral cells [17] and induce apoptosis in tumoral cells because of electrostatic force [18]. Therefore, it can probably be used in the treatment of cervical cancer.
Anticancer peptides (ACPs) are one of the emerging methods of cancer treatment that have fewer side effects and toxicity compared to traditional methods such as chemotherapy and antibiotics, although their identification is difficult and their use takes time and is expensive. ACPs destroy cancer cells due to their electrostatic force because ACPs have a positive charge and attract negatively charged cancer cells [19]. ACPs have high selectivity, high penetration, and easy modifications compared to antibodies and small molecules. This has caused ACPs to be used in cancer treatment. The membrane of cancerous and non-cancerous cells is different. ACPs cause lysis or formation of pores and destruction of the cell membrane through apoptosis and necrosis.
Anticancer drugs are molecularly targeted drugs that have specific targets in cancer cells. Based on the change in the composition of amino acids, sequence length, isoelectric point, molecular weight, net charge, hydrophobic property, amphipathic property, and secondary structure of proteins, anticancer peptides can be used to inhibit the proliferation or eradicate cancer cells. In the production of ACPs, peptide structure, mode of action, selectivity, and efficiency of cancer cells should be considered. The genes capsA and C produce enzymes for biosynthesizing CapsuleA and C of Streptococcus agalactiae [20].
This study aimed to analyze the recombinant fusion peptide CPSA-CPSC-L-Anticancer and determine its apoptotic properties by Real-Time-RT PCR and its statistical analysis.
2. Methods
2.1. Recombinant constructs
Synthesis of the CPSA-CPSC-Linker-ACAN construct was performed according to bioinformatic analyzes. Then it was synthesized by Biomatik Company [20,21].
2.2. Western blotting method
After loading samples in a 10% SDS-PAGE, samples were transferred to a nitrocellulose membrane. The membrane was blocked with 10 ml of blocking solution (BSA 5%) for 2 h and then was washed three times with a TBST buffer (TBS (Tris, 20 mM; NaCl, 150 mM;) + Tween 0.05%) for 15 min. The amount of 8 μl Anti-6XHis Tag antibody was mixed with 4 ml of blocking solution and then poured onto the membrane for 2 h on the shaker, and then the membrane was washed with a TBST buffer three times for another 15 min. The DAP kit developed the membrane [21].
2.3. Bradford assay
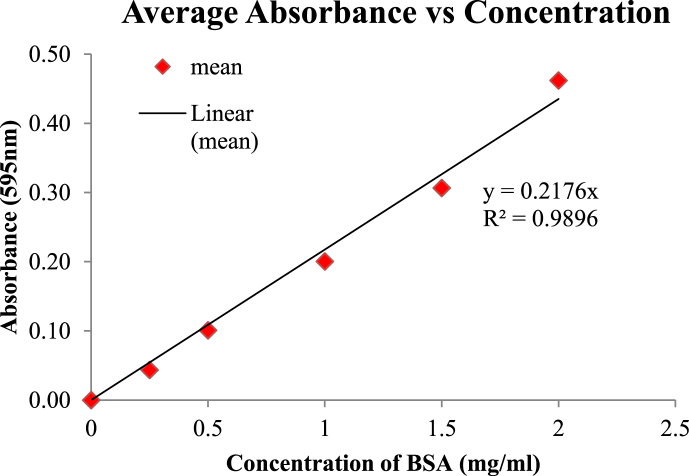
Concentrations of 0.25, 0.5, 1, 1.5, and 2 mg of BSA as standard protein, and an aliquot of each sample was mixed with 40 of Bradford solution, and the total volume was reached 200 μl with PBS after a 5- minute incubation at room temperature. The absorbance of each sample was read at 595 nm. The standard curve was drawn, and recombinant protein concentrations were calculated [21].
2.4. Cancer cell culture protocol
HeLa cell line was cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS) and 10 μg/ml penicillin-streptomycin, and cultivation took place in the incubator at 37 °C temperature and 5% CO2 atmosphere. When cells reached about 80% confluence, cells were harvested using 0.05% Trypsin-EDTA [21].
2.4.1. MTT assay
The MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) tetrazolium reduction assay was used to investigate the potentiation effect of recombinant protein on the HeLa cell line. Many 10000 cells were placed in each well of a 96-well plate and incubated for 24 h. Cells were treated with different concentrations of recombinant fusion peptides 2, 4, 8, 16, 32, 64, 128, and 256 μg/ml and incubated for another 24 h. The whole culture media was removed from the wells, and a 20 μl MTT reagent (5 mg.ml-1) was added to the wells. 100 μl of DMSO was added into wells, the plates were shaken for 20 min, and the absorbance was read at 570 nm [21].
2.5. Real-time RT-PCR
Many 10000 cells were placed in each well of a 24-well plate and incubated for 24 h. Then, a concentration of 64 μg/ml of freshly prepared recombinant protein was added to three plate wells, and three wells were considered as controls that were not treated with recombinant protein. After 24-h incubation, the plates were used for RNA extraction with the Jene Bioscience kit. Then extracted RNA and cDNA were synthesized with a Pars Toos cDNA synthesis kit. Finally, the relative expression of bcl-2, Caspase-3, and Bax genes was investigated. In this study, the GAPDH gene was selected as the reference gene [20,21].
2.6. Statistical analysis
In this study, tests were repeated three times, and a significance level of P < 0.05 was considered. The T-Test was used for significant review between groups in Real time-RT-PCR test, and a one-way ANOVA test was used for flow cytometry.
3. Results
3.1. Western blotting
Western blotting method was used in the presence of an Anti-His tag monoclonal antibody to confirm recombinant protein with a molecular weight of 29 KD (Fig. 1).
Fig. 1.

Western blot results of the recombinant fusion peptide.
Lan2 1 is an induced sample, Lane 2 is a non-induced sample, Lane 3 in BL-21+Pet22b sample.
The results show that the anti-His tag was able to identify the induced recombinant protein.
3.2. Bradford method
Fusion protein concentration using the standard Bradford curve at 595 nm, 2.5 μg/ml was calculated (Fig. 2).
Fig. 2.
Bradford test results.
The vertical axis shows absorption and the horizontal axis shows the protein BSA standard concentration. Results Bradford test showed that the concentration means of recombinant protein was calculated to be 2.5 μg/ml.
3.2.1. MTT assay
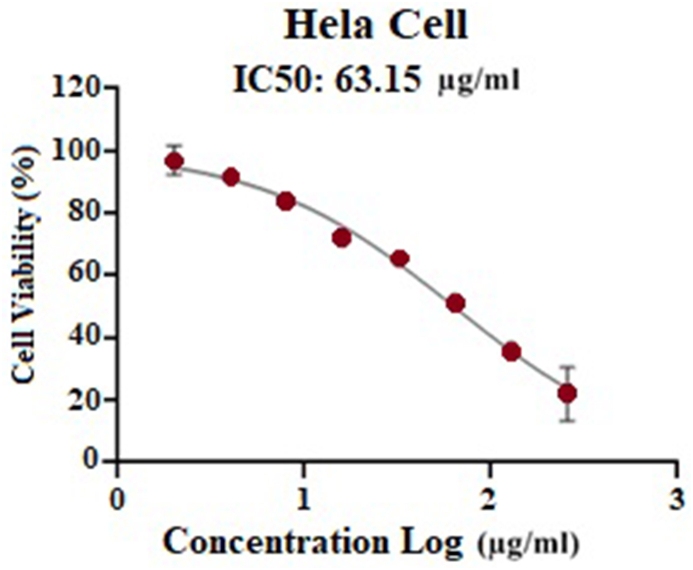
According to Fig. 3, based on the survival percentage, the concentration of recombinant fusion peptide with at least 50% lethality was reported IC50 with a concentration of 63 μg/ml.
Fig. 3.
Results of the MTT test against HeLa cell lines.
Increasing the concentration to 64 μg/ml augment the efficacy of the recombinant fusion peptide CPSA-CPSC-L-Anticancer (Fig. 3).
The results showed that the concentration of 63 μg/ml was able to kill 50% of the cell lines.
3.3. Real-time RT-PCR
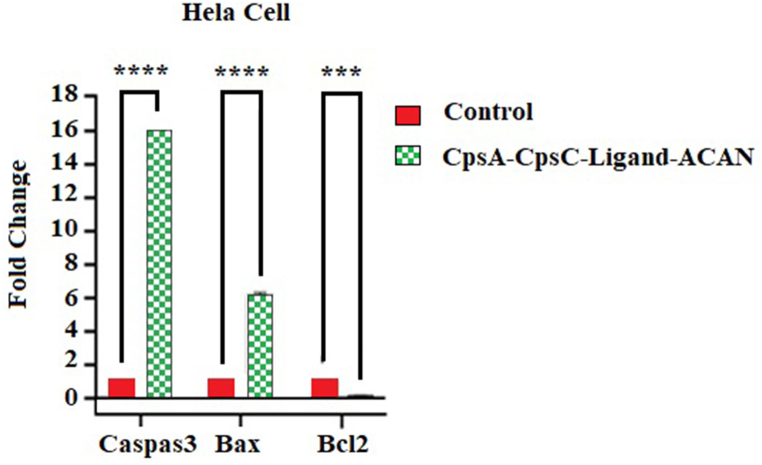
The results of the comparison between the expression of bcl2, Bax, and caspase 3 genes with the GAPDH reference gene were examined by Real-time RT-PCR. The relative expression of the bcl2 gene as an anti-apoptotic protein in the sample affected by the recombinant fusion peptide decreased 0.17 times compared to the control sample. The relative expression of the bax gene belonging to the pro-apoptotic protein family in the sample affected by the recombinant fusion peptide increased 6.27 times compared to the control sample. The relative expression of the caspase-3 gene, which was involved in the path of cell apoptosis, increased 16 times in the sample affected by the recombinant fusion peptide compared to the control sample (Fig. 4).
Fig. 4.
Gene expression results of bax, caspase3, and bcl2 before and after treatment with recombinant protein against HeLa cell lines.
The results showed the relative changes in the expression of Bax and caspase-3 genes in the samples under treatment compared to the control sample. The bcl-2 gene expression statistically decreased significantly (p < 0.05) and the expression of the bax gene increased statistically significantly (p < 0.05). Also, using the One-way ANOVA test and Tukey's multiple comparisons tests, the groups were compared with each other, and the significance of each is shown in (Fig. 4.).
The cell line treatment with this concentration changed the expression of Caspase-3 genes by 16 times and bax by 6 times and decreased the gene expression of bcl-2 by 0.176 times. Results showed that the changes in gene expression are statistically significant (P < 0.05).
4. Discussion
Today, Cervical tumor is one of the most prevalent cancers among women in the world. Many factors such as early sexual activity, having multiple sexual partners, early pregnancy, HPV infection, weakened immune system, smoking, exposure to abortion drugs, economic status and other factors are among the causes of cervical cancer [22]. Cervical cancer treatment varies depending on the disease stage. Treatment can include surgery, radiation therapy, chemotherapy, or a combination of the aforementioned. Anticancer drugs are small-molecule drugs and are still the most common method of cancer treatment. Anticancer drugs or chemotherapy are chemical drugs that change the structure of cancer cells and stop their growth of cancer cells [23].
Streptococcus agalactiae inhibits the connection of cancer cells to epithelial cells due to its effective factors, and for this reason, it can have anticancer properties [24]. Also, the surface polysaccharides of Streptococcus agalactiae are used as anticancer agents [25]. In the present study, the recombinant peptide CPSA-CPSC- L-ACAN with anticancer properties was surveyed against the HeLa cell line [26].
MTT test indicated this recombinant fusion peptide had a cell-killing effect on the HeLa cell line at a concentration of 63 μg/ml. The level of apoptotic gene expression using the Real-time RT-PCR technique, including Bax, bcl-2 and caspase-3 have changed in the HeLa cell line exposed to the recombinant fusion peptide compared to the reference gene.
In the study of Fathizadeh et al., the recombinant fusion peptide consisting of enterosin A and colicinA1 was investigated, while in the present study, the recombinant fusion peptide included L-ACAN CapsA-CapsC- and its anticancer property was measured.
In the present study, the MTT test was performed to confirm the toxicity of the recombinant fusion peptide on HeLa cells, and according to the obtained results, the IC50 of this fusion peptide was 63 μg/ml. In Yaoxian et al.'s study, the IC50 concentration of the recombinant fusion peptide was50 μM (27). The apoptotic genes examined in Yaoxian et al.'s study included Caspase-3, 8, and 9, while in the present study, they were Caspase-3, bax, and bcl-2 [27].
Similar to Mousavi et al.'s research, they investigated the lethality of various recombinant peptide fusions such as CEPO-Fc on hippocampus brain cells. The study was conducted on brain cells, while the present study was conducted on the HeLa cell line and the types of proteins were different in the two studies [28].
The results of the cell culture indicated that with the concentration of 63 μg/ml lethality effect increased. The expression level of the genes involved in apoptosis using Real Time RT-PCR technique and its normalization with the GAPDH gene as a housekeeping gene and the sample treated with recombinant fusion peptide (bcl-2, bax, and caspase-3) were respectively equal to with 0.176, 276.6 and 16 times [[29], [30], [31], [32], [33]]. One of the limitations of this study was the high cost of the research, which was provided by the researchers. Also, in the case of financing, the researchers were able to investigate the stimulation of cytokine production and TCD8 response. Another limitation is that it can be done in an animal model.
Due to the importance of cancer, efforts are being made to treat it. One of the treatment methods is the use of anti-cancer peptides. Anti-cancer peptides protect their host with different mechanisms. According to the results, the recombinant fusion peptide investigated in this study can be used as an effective compound in anticancer studies This recombinant fusion peptide should be suggested for therapeutic interventions and prevention.
Author contribution statement
Davoud Esmaeili; Taher Mohammadian; Elmira Babakanrad; Payam Behzadi: conceived and designed the experiments; performed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data. Davoud Esmaeili; Elmira Babakanrad: wrote the paper.
Funding statement
This review has been funded by all author as part of his PhD studies. No external funding was received.
Data availability statement
Data will be made available on request.
Additional information
No applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal.
Acknowledgments
We thank Baqiyatalla Hospital for the laboratory work.
Footnotes
For the first time, this research has investigated the products of the natural flora of the vagina called Streptococcus agalactia to inhibit the expression of HeLa cancer genes and also induce apoptosis in it.
Contributor Information
Taher Mohammadian, Email: tmohammadian@qodsiau.ac.ir.
Davoud Esmaeili, Email: esm114@gmail.com.
References
- 1.Yabroff K.R., Wu X.C., Negoita S., Stevens J., Coyle L., Zhao J., Mumphrey B.J., Jemal A., Ward K.C. Association of the COVID-19 pandemic with patterns of statewide cancer services. J. Natl. Cancer Inst. 2022;114(6):907–909. doi: 10.1093/jnci/djab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, CA. CANCER. J. Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel L., et al. Global cancer statistics: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA. Cancer. J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Yabroff K.R., Wu X.C., Negoita S., et al. Association of the COVID-19 pandemic with patterns of statewide cancer services. J. Natl. Cancer Inst. 2022;114(6):907–909. doi: 10.1093/jnci/djab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayen A., Jimenez Martinez Y., Boulaiz H. Targeted gene delivery therapies for cervical cancer. Cancers. 2020;12(5):130–131. doi: 10.3390/cancers12051301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burmeister C.A., Khana S.F., Schäfer G., Mbatani N., Adams T., Moodley J., Prince S. Cervical cancer therapies: current challenges and future perspectives. Tumo. Vir. Res. 2022;13:200–238. doi: 10.1016/j.tvr.2022.200238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukowski K., Kciuk M., Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020;21(9):32–33. doi: 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao R., Wang M., Bin Y., Zheng C. DLFF-ACP: prediction of ACPs based on deep learning and multi-view features fusion. PeerJ. 2021;(9) doi: 10.7717/peerj.11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad B., Gamallat Y., Su P., Husain A., Rehman A.U., Zaky M.Y. Alantolactone induces apoptosis in THP-1 cells through STAT3, survivin inhibition, and intrinsic apoptosis pathway. Chem. Biol. Drug Des. 2021;97(2):266–272. doi: 10.1111/cbdd.13778. [DOI] [PubMed] [Google Scholar]
- 10.Greco G., Catanzaro E., Fimognari C. Natural products as inducers of noncanonical cell death: a weapon against cancer. Cancers. 2021;13(2):304. doi: 10.3390/cancers13020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu-LongSieow B., SoonWun K., PengYong W., Hwang I.Y., Wook Chang M.T. Weak to treat: reprograming bacteria for cancer treatment. Trends Cancer. 2021;7(5):447–464. doi: 10.1016/j.trecan.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Kramer M.G., et al. Bacterial therapy of cancer: promises, limitations, and insights for future directions. Front. Microbiol. 2018;23(9):16. doi: 10.3389/fmicb.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair N., Kasai T., Seno M. Bacteria: prospective savior in the battle against cancer. Anticancer Res. 2014;34(11):6289–6296. [PubMed] [Google Scholar]
- 14.Mills H., Acquah R., Tang N., Cheung L., Klenk S., Glassen R. The use of bacteria in cancer treatment: a review from the perspective of cellular microbiology. Emerg. Med. Int. 2022 doi: 10.1155/2022/8127137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzhoseyni Z., Shayestehpour M., Salimian M., Esmaeili D., Saffari M., Fathizadeh H. Designing a novel fusion protein from Streptococcus agalactiae with apoptosis induction effects on cervical cancer cells. Microb. Pathog. 2022;(169):169. doi: 10.1016/j.micpath.2022.105670. [DOI] [PubMed] [Google Scholar]
- 16.Toniolo C., Balducci E., Romano M.R., Proietti D., Ferlenghi I., Grandi G. Streptococcus agalactiae capsule polymer length, and attachment is determined by the proteins CpsABCD. J. Biol. Chem. 2015;290(15):9521–9532. doi: 10.1074/jbc.M114.631499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabernet G., Müller A.T., Schneider J.A.G. Membranolytic anticancer peptides. Med. Chem. Comm. 2016;12:2232–2245. [Google Scholar]
- 18.Tornesello A.L., Borrelli A., Buonaguro L., Buonaguro F.B., Tornesello M.L. Antimicrobial peptides as anticancer agents: functional properties and biological activities. Molecules. 2020;25(12):2850. doi: 10.3390/molecules25122850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kao C., Chandna R., Ghode A., Dsouza C., Chen M., Larsson A., Lim S.H., Wang M., Cao Z., Zhu Y. Proapoptotic cyclic peptide BC71 targets cell-surface GRP78 and functions as an anticancer therapy in mice. EBioMedicine. 2018;33:22–32. doi: 10.1016/j.ebiom.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiangjong W., Chutipongtanate S., Hongeng S. Anticancer peptide: physicochemical property, functional aspect, and trend in clinical application. Int. J. Oncol. 2020;57(3):678–696. doi: 10.3892/ijo.2020.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babakanrad E., Mohammadian T., Esmaeili D., Behzadi P. Efficacy of the apoptotic activity of CpsA-CpsC-L-ACAN fusion peptide against HeLa cell line. Mol. Genet. Microbiol. Virol. 2022;37(3):153–158. [Google Scholar]
- 22.Dillner J., Rebol M., Birembaut P., Petry K., Szarewski A., Munk C., de Sanjose S., Naucler P., Lloveras B., Kjaer S. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;13:337. doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taskin-Tok T., Gowder S. In: Pharmacology and Therapeutics. Gowder S.J.T., editor. Innpot. Open. Publish.; 2014. Anticancer drug-friend or foe; pp. 255–269. [Google Scholar]
- 24.Sawant S.S., Patil S.M., Gupta V., Kunda N.K. Microbes as medicines: harnessing the power of bacteria in advancing cancer treatment, Internation. J. Molec. Sci. 2020;21(20):7575. doi: 10.3390/ijms21207575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berti F., Campisi E., Toniolo C., Morelli L., Crotti S., Rosini R., Romano M.R., Pinto V., Brogioni B., Torricelli G. Structure of the type IX group B Streptococcus capsular polysaccharide and its evolutionary relationship with types V and VII. J. Biol. Chem. 2014;289(34):23437–23448. doi: 10.1074/jbc.M114.567974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gharagozloo Hesari N., Esmaeili D., Mohammadian T., Shahhosseini M.H., Ferdosi A. Bioinformatical analysis of lipase-subtilisin protein fusion. Medic. Lab. J. 2020;14(6):23–27. [Google Scholar]
- 27.Yaoxian W., Hui Y., Yunyan Z., Yanqin L., Xin G., Xiaoke W. Emodin induces apoptosis of human cervical cancer HeLa cells via intrinsic mitochondrial and extrinsic death receptor pathway. Canc. Cell. Int. 2013;13(1):1–8. doi: 10.1186/1475-2867-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mousavi S.M., Gouya M.M., Ramazani R., Davanlou M., Hajsadeghi N., Seddighi Z. Cancer incidence and mortality in Iran. Ann. Oncol. 2009;20(3):556–563. doi: 10.1093/annonc/mdn642. [DOI] [PubMed] [Google Scholar]
- 29.Taheri-Anganeh M., Khatami S.H., Jamali Z., Movahedpour A., Ghasemi Y., Savardashtaki A., Mostafavi-Pour Z. LytU-SH3b fusion protein as a novel and efficient enzybiotic against methicillin-resistant Staphylococcus aureus. Mol. Biol. Res. Commun. 2019;8(4):151. [PMC free article] [PubMed] [Google Scholar]
- 30.Jalalvand N., Esmaeili D., Raiszadeh M., Naeimi S. Evaluation of physicochemical activity of anticancer fusion proteins; enterocin A-R type pyocin-lactocin-ligand against gastric cancer cell line by real-time RT PCR technique, internation. J. Peptid. Res. Ther. 2021;27(2):1167–1175. [Google Scholar]
- 31.Fathizadeh H., Saffari M., Esmaeili D., Moniri R., Salimian M. Evaluation of antibacterial activity of enterocin A-colicin E1 fusion peptide, Iran. J. Basic. Med. Sci. 2020;23(11):1471. doi: 10.22038/ijbms.2020.47826.11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meskini M., Esmaeili D. The study of formulated Zoush ointment against wound infection and gene expression of virulence factors Pseudomonas aeruginosa. BMC Compl. Alternative Med. 2018;18(1):185. doi: 10.1186/s12906-018-2251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esmaeilli D., Mobarez A.M., Salmanian A.H., Hosseini A.Z. Bioactivity and immunological evaluation of LPS from different serotypes of Helicobacter pylori. Irani, J, of Microb. 2013;5(2):142–146. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.