Abstract
Vascular alterations induced by a high-fat diet (HFD) are involved in the development of hypertension. Galangin, a flavonoid, is the major active compound isolated from galangal and propolis. The objective of this study was to investigate the effect of galangin on aortic endothelial dysfunction and hypertrophy, and the mechanisms involved in HFD-induced metabolic syndrome (MS) in rats. Male Sprague-Dawley rats (220–240 g) were separated into three groups: control + vehicle, MS + vehicle, and MS + galangin (50 mg/kg). Rats with MS received HFD plus 15% fructose solution for 16 weeks. Galangin or vehicle was orally administered daily for the final four weeks. Galangin reduced body weight and mean arterial pressure in HFD rats (p < 0.05). It also reduced circulating fasting blood glucose, insulin, and total cholesterol levels (p < 0.05). Impaired vascular responses to the exogenous acetylcholine observed in the aortic ring of HFD rats were restored by galangin (p < 0.05). However, the response to sodium nitroprusside did not differ between the groups. Galangin enhanced the expression of the aortic endothelial nitric oxide synthase (eNOS) protein and increased circulating nitric oxide (NO) levels in the MS group (p < 0.05). Aortic hypertrophy in HFD rats was alleviated by galangin (p < 0.05). Increases in tumour necrosis factor-alpha (TNF-α), interleukin (IL)-6 levels, angiotensin-converting enzyme activity and angiotensin II (Ang II) concentrations in rats with MS were suppressed in galangin treated group (p < 0.05). Furthermore, galangin reduced the upregulation of angiotensin II type I receptor (AT1R) and transforming growth factor-beta (TGF-β) expression in rats with MS (p < 0.05). In conclusion, galangin alleviates metabolic disorders and improves aortic endothelial dysfunction and hypertrophy in the MS group. These effects were consistent with increased NO availability, reduced inflammation, and suppressing Ang II/AT1R/TGF-β signalling pathway.
Keywords: Galangin, Metabolic syndrome, Vascular function
1. Introduction
Five major components, namely high blood pressure, impaired glucose metabolism, obesity, insulin resistance, and dyslipidaemia, are classified as signs of MS [1]. The prevalence of MS is high worldwide, approximately from 25 to 35% in the adult population and a 2-fold increase in the risk of cardiovascular disease and a 1.5-fold increase in all-cause mortality [[2], [3], [4]]. Insulin resistance can cause endothelial dysfunction because impairment of the insulin signalling pathway decreases NO production in the vascular endothelium [5,6]. Accumulating evidence indicates that impairment of endothelial function is the major cause of vascular disease in patients with MS [1,7]. In diet-induced rodent models of MS, a blunted endothelial response to vasoactive agents associated with a reduction in nitric oxide production has been observed [8,9]. Furthermore, thickening of the aortic wall and aortic remodelling has been observed in these animal models [10]. The underlying biological mechanisms of the vascular alterations observed in rats with MS are mediated by decreased endothelial nitric oxide synthase (eNOS) protein expression [11]. Recently, hypertrophy of the aorta, carotid artery, renal artery, and mesenteric artery, and dysfunction of aortic rings have been confirmed in rats fed a high-fat/high-fructose diet, which is associated with reduced eNOS protein expression and nitric oxide availability [12].
Chronic low-grade inflammation is an important factor in the pathogenesis of MS. It is also associated with insulin resistance and vascular complications [13]. Rats with MS induced by a high-fat diet (HFD) show an accumulation of visceral adipose tissue that releases high levels of pro-inflammatory cytokines, such as tumour necrosis factor-alpha (TNF-α), C-reactive protein (CRP), and interleukin (IL)-6. These cytokines subsequently activate insulin resistance, vascular dysfunction and hypertrophy [14]. Vascular events present in MS are also linked to the overactivation of the renin-angiotensin system (RAS). Substantial evidence has revealed that the elevation of systemic RAS components in rats with MS [15] is associated with endothelial dysfunction and hypertension [16]. Moreover, the activation of angiotensin-converting enzyme (ACE), angiotensin II (Ang II), and Ang II type I receptor (AT1R) axis-mediated cardiovascular hypertrophy has been established [[17], [18], [19], [20]]. Transforming growth factor-beta (TGF-β) is an essential component of RAS that induces vascular smooth muscle cell hypertrophy [21]. This is supported by the overexpression of protein AT1R/TGF-β in tissues isolated from patients with aortic hypertrophy [22].
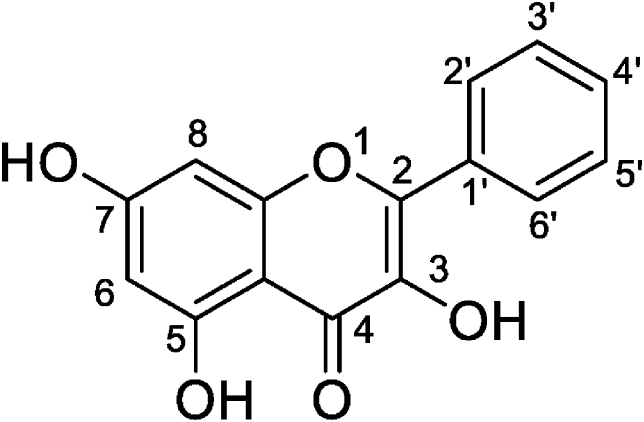
The beneficial effects of natural dietary flavonoids on MS management have been studied extensively. Galangin (an essential flavonoid) is a bioactive compound isolated from Alpinia officinarum (Galangal) and propolis [23,24]. Its chemical structure is 7-trihydroxyflavone, with hydroxyl groups at positions 3 and 5, as shown in Fig. 1 [25]. Galangin has been reported as an antioxidant substance in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay [24]. In addition, galangin exhibits several biological activities, such as anti-inflammatory, neuroprotective, blood glucose reduction, and anti-hypertension [[26], [27], [28]]. The anti-cancer properties of galangin have been observed because it can enhance apoptosis of hepatocellular carcinoma (HCC) cells via regulation of Bcl-2 apoptosis protein [29]. Galangin also attenuated insulin resistance and kidney damage induced by a high-fructose diet in rats [30]. However, little information regarding the effect ganlangin on vascular abnormalities has been reported in MS rats. The objective of this study was to evaluate whether galangin could improve aortic dysfunction and hypertrophy, inflammation, and RAS activation in rats with MS.
Fig. 1.
Chemical structure of galangin [25].
2. Materials and methods
2.1. Chemicals
Galangin was obtained from Aktin Chemicals, Inc. (Mianyang City, Sichuan, China). The remaining chemicals and solvents were of analytical grade and purchased from standard companies.
2.2. Induction of metabolic syndrome and study protocols
Six-week-old male Sprague-Dawley rats (220–260 g), were supplied by Nomura Siam International Co., Ltd. (Bangkok, Thailand) and were accommodated in the animal house (temperature 23 ± 2 °C, 12 h dark-light cycle). All animal procedures were performed in accordance with the ethical guidelines for the Care and Use of Laboratory Animals, approved by the Animal Ethics Committee of Khon Kaen University (IACUC-KKU-74/62), Thailand. The high-fat diet was prepared by a mixture of standard chow diet, lard, condensed milk, fructose and mixed salt. The composition of a HFD were 24.29 g fat/100 g, 13.25 g protein/100 g, and 46.3 g carbohydrates/100 g, which was verified by the Central Lab Thai (Central Laboratory (Thailand) Company Limited, Khon Kaen, Thailand). The MS in rats was induced by feeding them HFD. Rats in the MS group also received a 15% fructose solution while control rats received a standard chow diet (5.72 g fat/100 g, 22.9 g protein/100 g, and 57.81 g carbohydrates/100 g) and tap water. At week 12 of the experimental period, rats fed with a HFD were subdivided into the MS group which received propylene glycol as a vehicle (n = 8/group) and MS-treated group which received galanin at a dose of 50 mg/kg by oral gavage for the final four weeks of the experiment. Control rats received a standard chow were also received propylene glycol (0.15 mL/100 mg) as the vehicle. Therefore, there are three experimental groups in this study as follows;
| Group I Control + vehicle (propylene glycol, 0.15 mL/100 mg) |
| Group II MS + vehicle (propylene glycol, 0.15 mL/100 mg) |
| Group III MS + GL 50 (galangin, a dose 50 mg/kg) |
2.3. Mean arterial pressure and metabolic parameter measurements
The rats' body weights (BW) and blood pressures were recorded at the end of the experimental day. The rats were anaesthetized by intraperitoneal injection of thiopental sodium (50 mg/kg). A polyethylene tube connected to a pressure transducer was inserted into the left femoral artery. The baseline parameters of blood pressure were continuously monitored for 30 min and mean arterial blood pressure (MAP) was recorded using Acknowledge Data Acquisition software (Biopac Systems Ins., Santa Barbara, CA, USA). Blood samples were collected for biochemical analysis, which included measurement of the levels of triglycerides (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-c), as well as fasting blood glucose (FBG), insulin, and other lipid profile parameters. FBG levels were measured using a glucometer (Roche Diagnostics GmbH, Mannheim, Germany). A commercial enzyme-linked immunosorbent assay (ELISA) kit (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) was used to measure fasting serum insulin levels according to the recommended protocols. Analytical kits (Human Gesellschaft fuer Biochemica and Diagnostica mbH, Wiesbaden, Germany) were used to examine the lipid profiles according to the manufacturer's instructions.
2.4. Assessment of vascular responses to vasoactive agents in mesenteric vascular beds and aortic rings
After exsanguination, the mesenteric vascular beds and aortas were quickly removed from the rats. The physiological Krebs' solution was infused into the mesenteric beds at a flow rate of 5 mL/min, while they were placed in a humid chamber (37 °C). Different doses of acetylcholine (ACh, 1 nM-0.01 mM) or sodium nitroprusside (SNP, 1 nM-0.01 mM) were injected into the preparations to assess the vascular function of the small arteries after raising the tone with methoxamine. The vascular responses to vasoactive agents of the preparations were detected as change in mean perfusion pressure (mmHg) by using a pressure transducer, and recorded via the BIOPAC System (BIOPAC Systems Inc., California, USA). Another set of experiments involved cleaning, slicing, and incubating the thoracic aorta in a 15 mL bath of physiological Krebs' solution. To measure vascular function in conduit arteries, the ring was then toned up with phenylephrine (10 μM) before adding ACh (0.01–3 μM) or SNP (0.01–3 μM), respectively [8].
2.5. Assays of angiotensin II level and angiotensin converting enzyme activity
An Ang II Enzyme Immunoassay (EIA) kit was used to measure the concentration of plasma Ang II (RAB0010-1 KT, St. Louis, MO, USA). Using a fluorescence assay, a previously described technique [31] was modified to measure the serum ACE activity. Hippuryl-L-histidyl-l-leucine (HHL) was briefly mixed with serum in assay buffer (20 mM sodium borate and 0.3 M NaCl, pH 8.3). After 30 min at 37 °C, NaOH was added to halt the reaction. O-Phthaldialdehyde (OPA) was used to label the reaction product, which was then read using a Varioskan LUX multimode microplate reader (Thermo Fisher Scientific Inc., Waltham, MA, USA) at the wavelength 450. ACE activity was expressed as mU/mL.
2.6. Plasma nitric oxide metabolites (NOx) measurements
Using the Griess reaction and enzymatic conversion method, the plasma NOx concentration was measured [32]. Briefly, the samples were deproteinised, and the supernatant was treated with converting enzymes before reacting with Griess solution. The absorbance of the samples was measured using an ELISA plate reader with a filter wavelength of 540 nm (Tecan GmbH., Grodig, Australia).
2.7. Immunohistochemical staining of aortic rings
The expression of TNF-α and IL-6 in aortic sections was observed in all groups of rats using an immunohistochemical technique, as previously described [33]. Mouse anti-TNF-α IgG (1:500), or mouse anti-IL-6 IgG (1:500) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) followed by goat anti-mouse IgG (HRP) (Ab8436, 1:1000, Abcam Plc, Cam-bridge, U.K) were used as primary and secondary antibodies, respectively. The tissues were counterstained with haematoxylin, and 3,3′-diaminobenzidine (DAB) served as a positive control. Using Image-Pro Plus 6 software (Media Cybernetics, Inc., Rockville, MD, USA), the stained sections were photographed, and the levels of TNF- and IL-6 expression were counted and reported as a percentage of the relative stained areas. Morphometric analysis of aortic rings was performed using ImageJ morphometric software (National Institutes of Health, Bethesda, MD, USA). Eight different fields in each sample were quantified to determine wall thickness, media/lumen ratio, and vascular smooth muscle cells (VSMCs). The thickness of the arterial wall was determined from the distance between endothelium and the outer layer of tunica media. Arterial wall thickness was measured every 15 intervals around the vessel circumference. The wall cross-sectional area (CSA) was calculated using the difference between the value of the external circumferential area of the vessel and the lumen. The lumen diameter was calculated by this equation;
| equation (1) |
| equation (2) |
Where 2r is the luminal diameter
In addition, the vascular smooth muscle cells (VSMCs) number was counted and calculated by the ImageJ-Pro plus 6 software (Media Cybernetics, Inc., Rockville, MD, USA), using the following formula. The number of nucleuses per area were obtained from 20X image whereas aortic CSA was obtained from 4X image.
2.8. Angiotensin II type 1 receptor (AT1R), transforming growth factor beta (TGF-β) and eNOS protein expression measurements
Western blotting was used to assess the protein expression of AT1R, TGF-, and eNOS in aortic tissue. Homogenised aortic tissue was electrophoresed on Sodium Dodecyl Sulfate polyacrylamide gel electrophoresis. Proteins were electrotransferred onto a polyvinylidene difluoride membrane and blocked for 2 h at room temperature (25 °C) with 5% bovine serum albumin in Tris-buffered saline with 0.1% Tween 20. The next step was overnight incubation at 4 °C with mouse monoclonal antibodies to AT1R, TGF-, or eNOS [(610296), 1:250, BD Transduction Laboratories, CA, USA] (sc-515884, 1:500, sc-52893, 1:1000, Santa Cruz Biotechnology, Inc., Dallas, Texas, USA). Following the incubation period, the membrane was washed three times with Tris-Buffered Saline Tween-20and incubated for 1–2 h at room temperature (25 °C) with a horseradish peroxidase-conjugated secondary antibody at the appropriate concentration. The signals were developed using Immobilon Forte Western HRP Substrate (EMD Millipore Corp., Burlington, MA, USA) and detected using Amersham Imager 600 (GE Healthcare Life Science, Uppsala, Sweden). The intensity of the protein bands was normalised to that of β-actin. Bands are expressed as a percentage of the values compared to the control group from the same gel [8].
2.9. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM), n = 8. One-way analysis of variance (ANOVA) followed by Tukey's test was used to analyse differences among the groups. In addition, the vascular responses to vasoactive agents were analyzed using two-way ANOVA with Tukey's post-hoc test. A p-value of less than 0.05 indicates that the results are statistically significant.
3. Results
3.1. Effect of galangin on components of MS in rats with MS
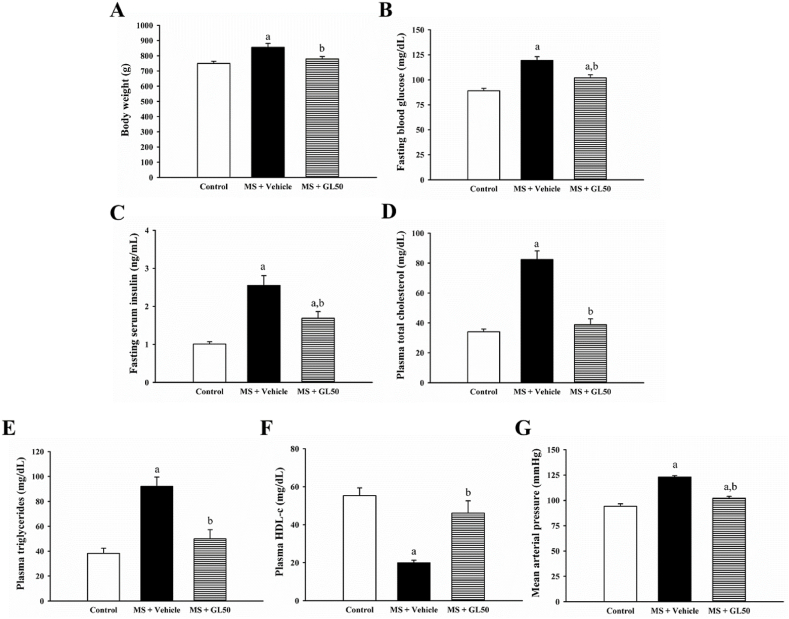
All components of MS, including hyperglycaemia, dyslipidaemia, obesity, and high blood pressure, were statistical significantly in rats fed the HF diet compared to control rats (Fig. 2, p < 0.05). Supplementation with galangin significantly reduced BW, FBG, serum insulin, TC, triglycerides, and mean arterial pressure, and increased HDL-c levels in MS + GL50 compared to MS + vehicle (p < 0.05).
Fig. 2.
Effect of galangin on body weight (A) fasting blood glucose (B) serum insulin (C), total cholesterol (D), triglyceride (E) HDL-c (F) and mean arterial pressure (G) in all groups of rats. Data are expressed as mean ± SEM. ap < 0.05 vs control, bp < 0.05 vs MS (n = 8/group). HDL-c, high density lipoprotein-cholesterol; MS + vehicle, metabolic syndrome rats received vehicle; MS + GL50, metabolic syndrome rats treated with galangin at a dose of 50 mg/kg.
3.2. Galangin improved vascular function in rats with MS
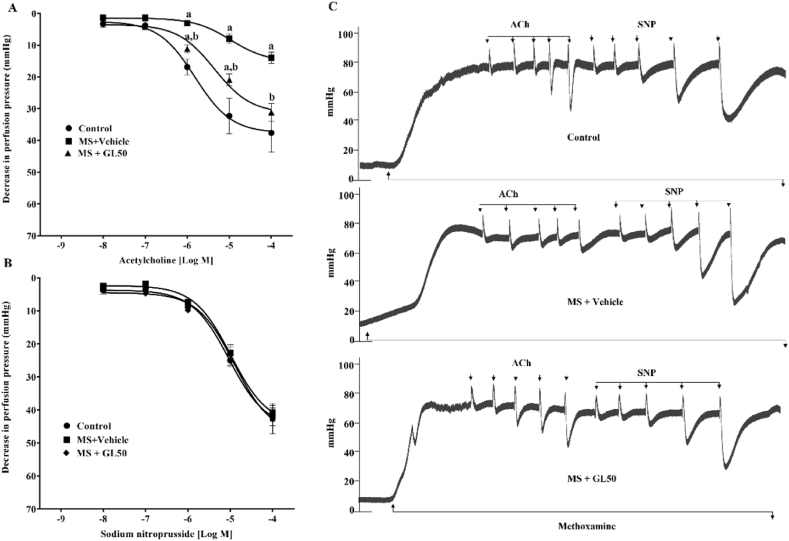
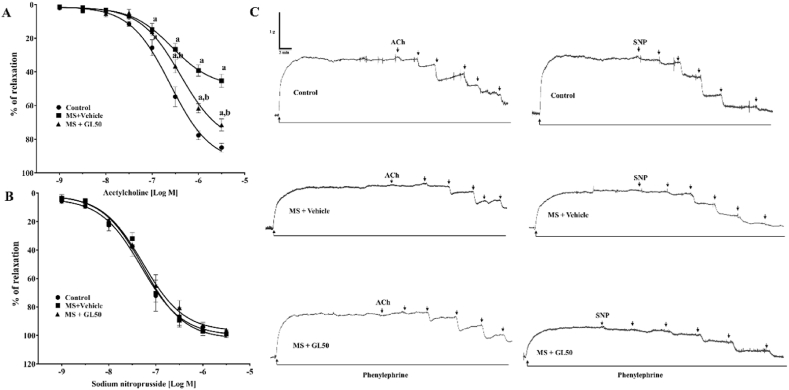
A reduction in endothelium-dependent vasorelaxation was observed in rats with MS since the response to ACh was blunted in mesenteric vascular beds (Fig. 3A) and aortic rings (Fig. 4 A) isolated from the MS + vehicle rats compared to those of the control rats (p < 0.05). Treatment with galangin significantly improved endothelial function by restoring vasodilation in response to ACh (p < 0.05) in the MS + GL50 rats. However, there was no significant difference in the vasorelaxation responses to SNP among the groups (Fig. 3, Fig. 4B). The representative tracing of isolated mesenteric vascular beds (Fig. 3C) and aortic rings (Fig. 4C) are shown.
Fig. 3.
Effect of galangin on vascular responses to exogenous ACh (A) and SNP (B) in mesenteric vascular bed of all groups of rats. Representative tracing of isolated mesenteric vascular beds (C). Data are expressed as mean ± SEM. ap < 0.05 vs control, bp < 0.05 vs MS + vehicle (n = 8/group). ACh, acetylcholine; SNP, sodium nitroprusside; MS + vehicle, metabolic syndrome rats received vehicle; MS + GL50, metabolic syndrome rats treated with galangin at a dose of 50 mg/kg.
Fig. 4.
Effect of galangin on vascular responses to exogenous ACh (A) and SNP (B) in the thoracic aorta of all groups of rats. The representative tracing of isolated aortic rings (C). Data are expressed as mean ± SEM. ap < 0.05 vs control, bp < 0.05 vs MS + vehicle (n = 8/group). ACh, acetylcholine; SNP, sodium nitroprusside; MS + vehicle, metabolic syndrome rats received vehicle; MS + GL50, metabolic syndrome rats treated with galangin at a dose of 50 mg/kg.
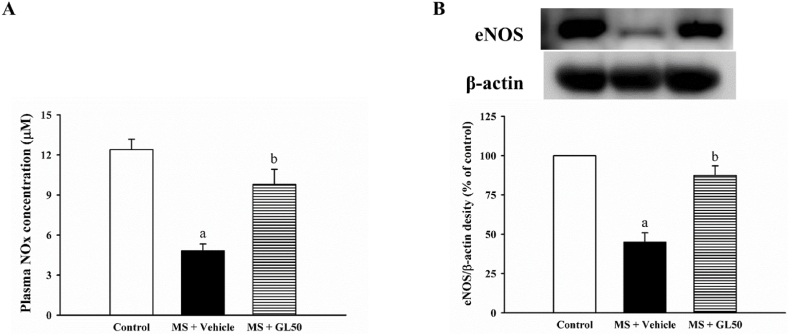
3.3. Galangin restored plasma nitric oxide metabolites and eNOS protein expression in MS rats
Levels of plasma NOx accompanied by the downregulation of eNOS protein expression in the aortic tissue was significantly low in the MS + vehicle group compared to that in the control group (p < 0.05, Fig. 5A and B). Administration of galangin significantly elevated both plasma NOx levels and eNOS protein expression compared to those in MS + vehicle (p < 0.05).
Fig. 5.
Effect of galangin on plasma NOx level (A) and eNOS protein expression in the aorta (B) of MS rats. ap < 0.05 vs control, bp < 0.05 vs MS (n = 7/group). NOx; nitric oxide metabolites; eNOS, endothelial nitric oxide synthase; MS + vehicle, metabolic syndrome rats received vehicle; MS + GL50, metabolic syndrome rats treated with galangin at a dose of 50 mg/kg.
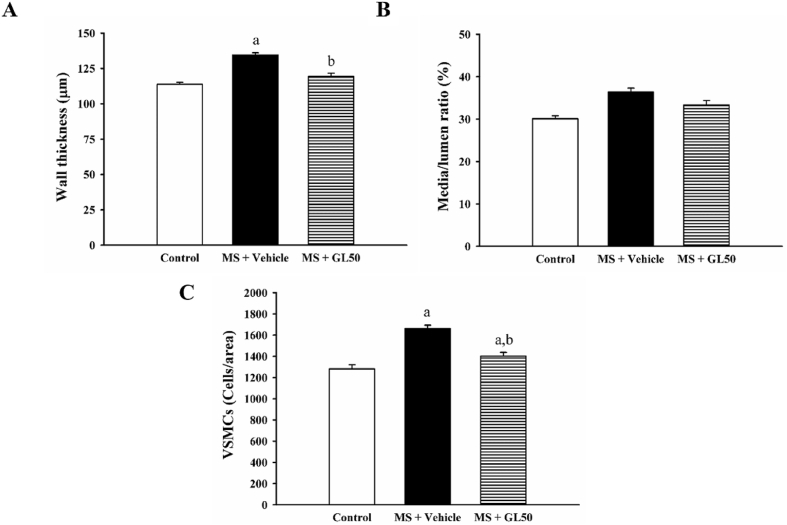
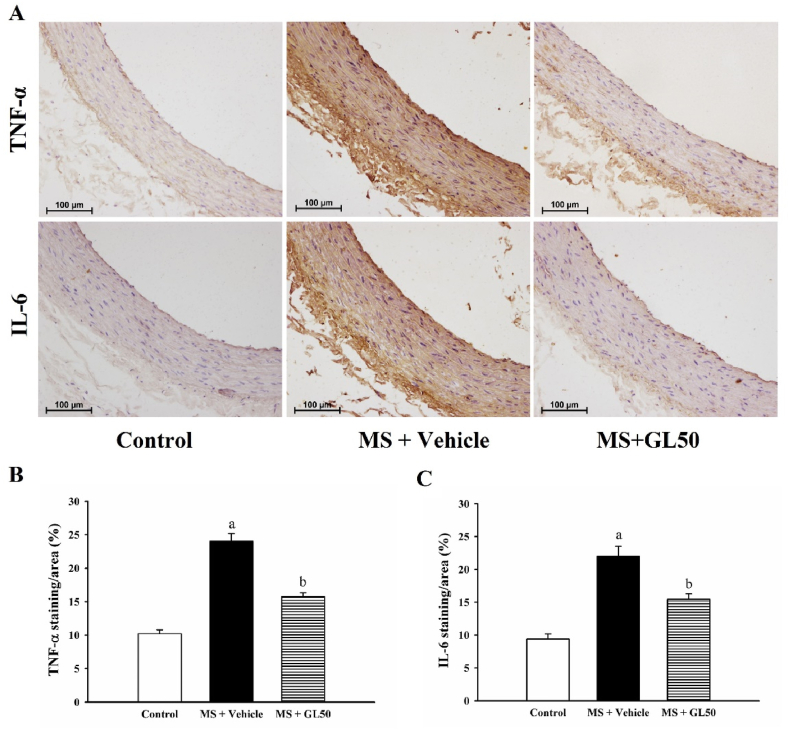
3.4. Galangin alleviated vascular remodelling and inflammation in MS rats
Increases in aortic wall thickness and VSMCs number were observed in the aortic tissue collected from MS + vehicle rats (Fig. 6A, B, and C, p < 0.05). Media/lumen ratio did not differ among groups (Fig. 6B). Galangin supplementation for four weeks alleviated aortic hypertrophy, the increased aortic wall thickness and VSMCs number, compared to that in the MS + vehicle group (p < 0.05). In addition, the expression of proinflammatory cytokines, including TNF-α and IL-6, was increased in the aortic tissue of MS + vehicle rats compared with that in control rats (Fig. 6, p < 0.05). However, all these abnormalities were alleviated by treatment with galangin when compared to MS + vehicle, as shown in Fig. 7A and B.
Fig. 6.
Morphometric data including aortic wall thickness (A), Media/lumen ratio (B) and vascular smooth muscle cell number (VSMC, C) of aorta in all the experimental groups. Data are expressed as mean ± SEM. ap < 0.05 vs control, bp < 0.05 vs MS (n = 7/group). VSMCs, vascular smooth muscle cells; MS + vehicle, metabolic syndrome rats received vehicle; MS + GL50, metabolic syndrome rats treated with galangin at a dose of 50 mg/kg.
Fig. 7.
Representative images of TNF-α (A, upper row) and IL-6 (A, lower row) protein expression in aortic tissue of all groups of rats. Quantitative data of TNF-α (B) and IL-6 (C) in aortic ring in HF diet-induced MS rats. Data are expressed as mean ± SEM. ap < 0.05 vs control, bp < 0.05 vs MS (n = 7/group). TNF-α, tumour necrosis factor alpha; IL-6, interleukin 6; MS + vehicle, metabolic syndrome rats received vehicle; MS + GL50, metabolic syndrome rats treated with galangin at a dose of 50 mg/kg.
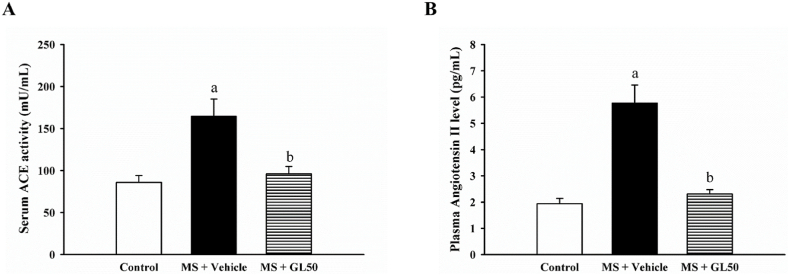
3.5. Galangin alleviated ACEactivity and reduced Ang II level in rats with MS
Elevated serum ACE activity and plasma Ang II levels were observed in MS + vehicle rats compared to those in control rats (p < 0.05). Galangin supplementation significantly reduced ACE activity and Ang II concentration in MS + GL50 rats when compared to MS + vehicle rats, as shown in Fig. 8A and B (p < 0.05).
Fig. 8.
Effect of galangin on serum ACE activity (A) and plasma angiotensin II levels (B) in HF diet-induced MS in rats. Data are expressed as mean ± SEM. ap < 0.05 vs control, bp < 0.05 vs MS (n = 7/group) ACE, angiotensin converting enzyme; MS + vehicle, metabolic syndrome rats received vehicle; MS + GL50, metabolic syndrome rats treated with galangin at a dose of 50 mg/kg.
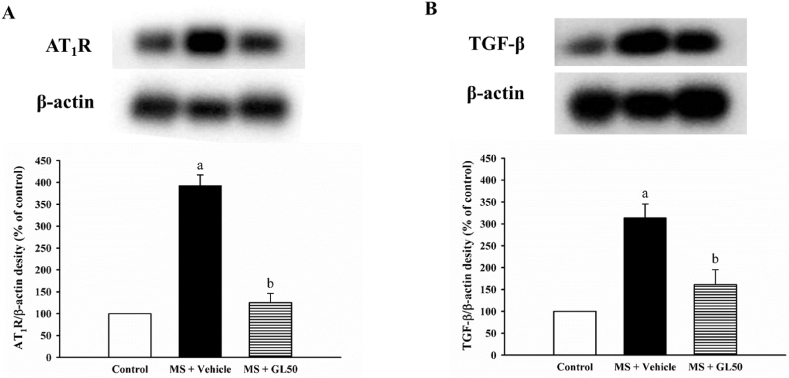
3.6. Galangin suppressed AT1R and TGF-β protein expression in MS rats
There were significant increases in AT1R (Fig. 9A) and TGF-β (Fig. 9B) protein expression levels in the aortic tissue of the MS + vehicle rats when compared with control rats (p < 0.05). This over-expression of AT1R and TGF-β was normalised in MS + vehicle rats that were treated with galangin supplementation (MS + GL50), as shown in Fig. 9A and B (p < 0.05).
Fig. 9.
Effect of galangin on protein expressions of AT1R (A) and TGF-β (B) in aorta in HF diet-induced MS rats. Data are expressed as mean ± SEM. ap < 0.05 vs control, bp < 0.05 vs MS (n = 4/group). AT1R, angiotensin II receptor type I; TGF-β, transforming growth factor beta; MS + vehicle, metabolic syndrome rats received vehicle; MS + GL50, metabolic syndrome rats treated with galangin at a dose of 50 mg/kg.
4. Discussion
The main finding of this study was that galangin alleviated hypertension in rats with MS. Impairment of aortic endothelial function with reduction in eNOS protein expression and plasma NOx were normalised by galangin supplementation. Galangin treatment also suppressed the increase in aortic wall thickness and smooth muscle cell number induced by HFD in rats. The immunohistochemical assay showed overexpression of TNF-α and IL-6 proteins in rats with MS, which were attenuated by galangin treatment. Furthermore, galangin reduced systemic RAS overactivation and upregulation of the AT1R/TGF-β signalling pathway induced by HFD in rats.
In this study, rats that consumed a HFD for 16 weeks had increased body weight, blood pressure, and high systemic concentrations of insulin, glucose, and lipids. Long-term excessive consumption of a HFD has been known to cause metabolic disorders [34]. HFD can increase visceral fat accumulation and free fatty acids, which are responsible for insulin resistance and hyperglycaemia [35,36]. Galangin supplementation ameliorated HFD-induced MS in this study, which may be a consequence of its effect on the reduction of BW and lipid levels. Vascular complications such as aortic endothelial dysfunction and hypertrophy were observed in HFD feeding rats [37]. Under physiological conditions, food intake and energy output can determine the energy balance. The rat food intake in this investigation was unaffected by galangin. Hence, galangin's ability to lower BW in HFD rats may be mediated by an increase in energy output. In a prior study, galangin-containing ginger extract was shown to reduce rat weight gain by boosting energy expenditure [38]. The hypolipidemic effect of galangin was supported the previous report that galangin exhibited an inhibitory effect on pancreatic lipase activity [39]. Galangin reduced lipogenesis by inhibiting SEREP1c mRNA expression [38]. In this study, galangin raised HDL-c levels in HFD rats accompanied by the previous study [28]. They suggested that galangin may increase the lecithin cholesterol acyl transferase enzyme activity [28]. Furthermore, reduced TG concentration may be mediated by the reduction of cholesteryl ester transfer protein activity, which may enhance HDL-c concentration in HFD rats [40]. Galangin alleviated insulin resistance in HepG2 cells through the regulation of the intracellular insulin transduction pathway [41]. Galangin exhibited a dipeptidyl peptidase-4 inhibitory activity responsible for its anti-hyperglycemic effects [42]. The action of galangin on improving insulin sensitivity and FBG in the present study was associated with its anti-inflammatory activity (reduction of TNF-α and IL-6 expression) since inflammation is one of the major causes of insulin resistance in metabolic syndrome [43].
The results showed impairment of vascular reactivity to acetylcholine, but a normal response to SNP in rats in the MS group indicated endothelial dysfunction. These vascular abnormalities are known to increase total peripheral resistance and hypertension. Our findings were consistent with the previous report in rats with MS, the responses to acetylcholine in both conduit and resistance vessels were reduced [8], as supported by evidence in patients with MS who exhibited blunted responses to endothelium-dependent vasodilator, and not to nitric oxide donor. Endothelial dysfunction in these patients is due to the low concentration of circulating NO [44]. Galangin improved aortic function in rats with MS owing to increased circulating NO and aortic eNOS protein expression. Substantial evidence confirms the association between insulin resistance and endothelial dysfunction in rats with MS. Insulin can enhance eNOS expression via PI-3 kinase; thus, insulin resistance may have an opposing effect and reduce endothelial NO production [45,46]. Therefore, this study suggests that galangin improves insulin sensitivity and subsequently enhances eNOS protein expression and NO production in rats fed a HFD.
Elevation of inflammatory cytokines is characterised by metabolic disorders. It primarily contributes to insulin resistance and vascular changes in HFD-induced MS [14]. The immunohistochemical results showed high expression of TNF-α and IL-6 in the aorta, as well as thickening of the aortic wall in rats with MS. Several studies have reported that inflammation is a major cause of insulin resistance in patients with MS [47,48]. Galangin reduced aortic inflammation and hypertrophy in the MS group. A previous study showed that galangin can alleviate insulin resistance and systemic inflammation in rats with MS [49]. Aortic hypertrophy develops as an adaptive response to increased mechanical stress in the vessel wall due to hypertension [50]. In addition, RAS plays a crucial role in vascular hypertrophic development [17]. Elevation of RAS components, such as AEC activity and Ang II concentrations, induced by a HFD in rats was observed in the present study. Accumulating data indicate that leptin, a hormone released from adipose tissue, can activate RAS in obese mice [51,52]. Ang II binds to its receptor AT1R to mediate vasoconstriction and vascular hypertrophy [53]. Western blot analysis showed upregulation of AT1R/TGF-β protein expression in aortic tissue. This indicates that activation of the Ang II/AT1R/TGF-β signalling pathway was involved in aortic hypertrophy in this study. Furthermore, galangin-normalised aortic hypertrophy associated with suppression of RAS activation was reported in HFD-fed rats in this study. Therefore, at least two main mechanisms can be proposed for the action of galangin in reducing RAS activation: 1) galangin reduced circulating leptin concentration [49] and 2) galangin had an inhibitory effect on ACE activity.
However, this study does have some limitations. For example, the effect of galangin on adipose tissue inflammation and hypertrophy, which could explain the source of inflammation and leptin, has not been investigated. The underlying mechanisms of galangin's effects on dyslipidemia and blood glucose have not been studied. Furthermore, the expression of eNOS phosphorylation to confirm the active form of eNOS protein has not been verified.
In conclusion, galangin mitigated aortic endothelial dysfunction in rats with MS by enhancing eNOS protein expression and NO concentration. It also alleviated aortic hypertrophy by reducing ACE activity and the Ang II/AT1R/TGF-β signalling pathway in rats with MS.
Author contribution statement
Poungrat Pakdeechote: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Anuson Poasakate, Patoomporn Prasatthong, Prapassorn Potue, Juthamas Khamseekaew: Performed the experiments; Contributed reagents, materials, analysis tools or data; Analyzed and interpreted the data.
Putcharawipa Maneesai: Conceived and designed the experiments; Wrote the paper; Contributed reagents, materials, analysis tools or data.
Data availability statement
Data will be made available on request.
Funding statement
This study was supported by the Fundamental Research Fund (65), Research and Graduate Study, Khon Kaen University, provided funding for this study Khon Kaen, Thailand.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Poungrat Pakdeechote, Email: ppoung@kku.ac.th.
Anuson Poasakate, Email: anuson_p@kkumail.com.
Patoomporn Prasatthong, Email: patoomporn.p@nsru.ac.th.
Prapassorn Potue, Email: prappo@kku.ac.th.
Juthamas Khamseekaew, Email: juthakh@kku.ac.th.
Putcharawipa Maneesai, Email: putcma@kku.ac.th.
References
- 1.Tziomalos K., Athyros V.G., Karagiannis A., Mikhailidis D.P. Endothelial dysfunction in metabolic syndrome: prevalence, pathogenesis and management. Nutr. Metabol. Cardiovasc. Dis. 2010;20(2):140–146. doi: 10.1016/j.numecd.2009.08.006. http://10.1016/j.numecd.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 2.Gami A.S., Witt B.J., Howard D.E., Erwin P.J., Gami L.A., Somers V.K., Montori V.M. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007;49(4):403–414. doi: 10.1016/j.jacc.2006.09.032. http://10.1016/j.jacc.2006.09.032 [DOI] [PubMed] [Google Scholar]
- 3.Mottillo S., Filion K.B., Genest J., Joseph L., Pilote L., Poirier P., Rinfret S., Schiffrin E.L., Eisenberg M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. http://10.1016/j.jacc.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 4.Angelico F., Baratta F., Coronati M., Ferro D., Del Ben M. Diet and metabolic syndrome: a narrative review. Intern. Emerg. Med. 2023 doi: 10.1007/s11739-023-03226-7. http://10.1007/s11739-023-03226-7 [DOI] [PubMed] [Google Scholar]
- 5.Vincent M.A., Montagnani M., Quon M.J. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr. Diabetes Rep. 2003;3(4):279–288. doi: 10.1007/s11892-003-0018-9. http://10.1007/s11892-003-0018-9 [DOI] [PubMed] [Google Scholar]
- 6.Zeng G., Quon M.J. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J. Clin. Invest. 1996;98(4):894–898. doi: 10.1172/JCI118871. http://10.1172/jci118871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito K., Ciotola M., Carleo D., Schisano B., Saccomanno F., Sasso F.C., Cozzolino D., Assaloni R., Merante D., Ceriello A., Giugliano D. Effect of rosiglitazone on endothelial function and inflammatory markers in patients with the metabolic syndrome. Diabetes Care. 2006;29(5):1071–1076. doi: 10.2337/diacare.2951071. http://10.2337/diacare.2951071 [DOI] [PubMed] [Google Scholar]
- 8.Maneesai P., Bunbupha S., Kukongviriyapan U., Prachaney P., Tangsucharit P., Kukongviriyapan V., Pakdeechote P. Asiatic acid attenuates renin-angiotensin system activation and improves vascular function in high-carbohydrate, high-fat diet fed rats. BMC Compl. Alternative Med. 2016;16:123. doi: 10.1186/s12906-016-1100-6. http://10.1186/s12906-016-1100-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang N., Wang X.H., Mao S.L., Zhao F. Astragaloside IV improves metabolic syndrome and endothelium dysfunction in fructose-fed rats. Molecules. 2011;16(5):3896–3907. doi: 10.3390/molecules16053896. http://10.3390/molecules16053896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delbosc S., Paizanis E., Magous R., Araiz C., Dimo T., Cristol J.P., Cros G., Azay J. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis. 2005;179(1):43–49. doi: 10.1016/j.atherosclerosis.2004.10.018. http://10.1016/j.atherosclerosis.2004.10.018 [DOI] [PubMed] [Google Scholar]
- 11.Huang P.L. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol. Metabol. 2009;20(6):295–302. doi: 10.1016/j.tem.2009.03.005. http://10.1016/j.tem.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunbupha S., Prasarttong P., Poasakate A., Maneesai P., Pakdeechote P. Imperatorin alleviates metabolic and vascular alterations in high-fat/high-fructose diet-fed rats by modulating adiponectin receptor 1, eNOS, and p47(phox) expression. Eur. J. Pharmacol. 2021;899 doi: 10.1016/j.ejphar.2021.174010. http://10.1016/j.ejphar.2021.174010 [DOI] [PubMed] [Google Scholar]
- 13.Esposito K., Giugliano D. The metabolic syndrome and inflammation: association or causation? Nutr. Metabol. Cardiovasc. Dis. 2004;14(5):228–232. doi: 10.1016/s0939-4753(04)80048-6. http://10.1016/s0939-4753(04)80048-6 [DOI] [PubMed] [Google Scholar]
- 14.Oishi J.C., Castro C.A., Silva K.A., Fabricio V., Cárnio E.C., Phillips S.A., Duarte A., Rodrigues G.J. Endothelial dysfunction and inflammation precedes elevations in blood pressure induced by a high-fat diet. Arq. Bras. Cardiol. 2018;110(6):558–567. doi: 10.5935/abc.20180086. http://10.5935/abc.20180086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boustany C.M., Bharadwaj K., Daugherty A., Brown D.R., Randall D.C., Cassis L.A. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287(4):R943–R949. doi: 10.1152/ajpregu.00265.2004. http://10.1152/ajpregu.00265.2004 [DOI] [PubMed] [Google Scholar]
- 16.Putnam K., Shoemaker R., Yiannikouris F., Cassis L.A. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2012;302(6):H1219–H1230. doi: 10.1152/ajpheart.00796.2011. http://10.1152/ajpheart.00796.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakui H., Dejima T., Tamura K., Uneda K., Azuma K., Maeda A., Ohsawa M., Kanaoka T., Azushima K., Kobayashi R., Matsuda M., Yamashita A., Umemura S. Activation of angiotensin II type 1 receptor-associated protein exerts an inhibitory effect on vascular hypertrophy and oxidative stress in angiotensin II-mediated hypertension. Cardiovasc. Res. 2013;100(3):511–519. doi: 10.1093/cvr/cvt225. http://10.1093/cvr/cvt225 [DOI] [PubMed] [Google Scholar]
- 18.Maneesai P., Bunbupha S., Kukongviriyapan U., Senggunprai L., Kukongviriyapan V., Prachaney P., Pakdeechote P. Effect of asiatic acid on the Ang II-AT(1)R-NADPH oxidase-NF-κB pathway in renovascular hypertensive rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017;390(10):1073–1083. doi: 10.1007/s00210-017-1408-x. http://10.1007/s00210-017-1408-x [DOI] [PubMed] [Google Scholar]
- 19.Maneesai P., Chaihongsa N., Iampanichakul M., Meephat S., Prasatthong P., Bunbupha S., Wunpathe C., Pakdeechote P. Clitoria ternatea (Linn.) flower extract attenuates vascular dysfunction and cardiac hypertrophy via modulation of Ang II/AT(1) R/TGF-β1 cascade in hypertensive rats. J. Sci. Food Agric. 2022;102(6):2253–2261. doi: 10.1002/jsfa.11563. http://10.1002/jsfa.11563 [DOI] [PubMed] [Google Scholar]
- 20.Potue P., Maneesai P., Kukongviriyapan U., Prachaney P., Pakdeechote P. Cratoxylum Formosum extract exhibits antihypertensive effects via suppressing the renin-angiotensin cascade in hypertensive rats. J. Funct.Foods. 2020;73 [Google Scholar]
- 21.Gibbons G.H., Pratt R.E., Dzau V.J. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J. Clin. Invest. 1992;90(2):456–461. doi: 10.1172/JCI115881. http://10.1172/jci115881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poasakate A., Maneesai P., Potue P., Bunbupha S., Tong-Un T., Settheetham-Ishida W., Khamseekaew J., Pakdeechote P. Genistein alleviates renin-angiotensin system mediated vascular and kidney alterations in renovascular hypertensive rats. Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112601. http://10.1016/j.biopha.2021.112601 [DOI] [PubMed] [Google Scholar]
- 23.Basri A.M., Taha H., Ahmad N. A review on the pharmacological activities and phytochemicals of Alpinia officinarum (galangal) extracts derived from bioassay-guided fractionation and isolation. Pharm. Rev. 2017;11(21):43–56. doi: 10.4103/phrev.phrev_55_16. http://10.4103/phrev.phrev_55_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isla M.I., Zampini I.C., Ordóñez R.M., Cuello S., Juárez B.C., Sayago J.E., Moreno M.I., Alberto M.R., Vera N.R., Bedascarrasbure E., Alvarez A., Cioccini F., Maldonado L.M. Effect of seasonal variations and collection form on antioxidant activity of propolis from San Juan, Argentina. J. Med. Food. 2009;12(6):1334–1342. doi: 10.1089/jmf.2008.0286. http://10.1089/jmf.2008.0286 [DOI] [PubMed] [Google Scholar]
- 25.Zhao F., Ma Y., Yin J., Li Y., Cao Y., Zhang L. Analysis of galangin and its in vitro/in vivo metabolites via ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Metabolites. 2022;12(11) doi: 10.3390/metabo12111032. http://10.3390/metabo12111032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaihongsa N., Maneesai P., Sangartit W., Potue P., Bunbupha S., Pakdeechote P. Galangin alleviates vascular dysfunction and remodelling through modulation of the TNF-R1, p-NF-κB and VCAM-1 pathways in hypertensive rats. Life Sci. 2021;285 doi: 10.1016/j.lfs.2021.119965. http://10.1016/j.lfs.2021.119965 [DOI] [PubMed] [Google Scholar]
- 27.Chen Q.X., Zhou L., Long T., Qin D.L., Wang Y.L., Ye Y., Zhou X.G., Wu J.M., Wu A.G. Galangin exhibits neuroprotective effects in 6-OHDA-induced models of Parkinson's disease via the nrf2/keap1 pathway. Pharmaceuticals. 2022;15(8) doi: 10.3390/ph15081014. http://10.3390/ph15081014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aloud A.A., Chinnadurai V., Govindasamy C., Alsaif M.A., Al-Numair K.S. Galangin, a dietary flavonoid, ameliorates hyperglycaemia and lipid abnormalities in rats with streptozotocin-induced hyperglycaemia. Pharm. Biol. 2018;56(1):302–308. doi: 10.1080/13880209.2018.1474931. http://10.1080/13880209.2018.1474931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H.T., Luo H., Wu J., Lan L.B., Fan D.H., Zhu K.D., Chen X.Y., Wen M., Liu H.M. Galangin induces apoptosis of hepatocellular carcinoma cells via the mitochondrial pathway. World J. Gastroenterol. 2010;16(27):3377–3384. doi: 10.3748/wjg.v16.i27.3377. http://10.3748/wjg.v16.i27.3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivakumar A.S., Viswanathan P., Anuradha C.V. Dose-dependent effect of galangin on fructose-mediated insulin resistance and oxidative events in rat kidney. Redox Rep. 2010;15(5):224–232. doi: 10.1179/135100010X12826446921545. http://10.1179/135100010x12826446921545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maneesai P., Bunbupha S., Kukongviriyapan U., Senggunprai L., Kukongviriyapan V., Prachaney P., Pakdeechote P. Effect of asiatic acid on the Ang II-AT1R-NADPH oxidase-NF-κB pathway in renovascular hypertensive rats. N. Schmied. Arch. Pharmacol. 2017;390(10):1073–1083. doi: 10.1007/s00210-017-1408-x. http://10.1007/s00210-017-1408-x [DOI] [PubMed] [Google Scholar]
- 32.Pakdeechote P., Bunbupha S., Kukongviriyapan U., Prachaney P., Khrisanapant W., Kukongviriyapan V. Asiatic acid alleviates hemodynamic and metabolic alterations via restoring eNOS/iNOS expression, oxidative stress, and inflammation in diet-induced metabolic syndrome rats. Nutrients. 2014;6(1):355–370. doi: 10.3390/nu6010355. http://10.3390/nu6010355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meephat S., Prasatthong P., Potue P., Bunbupha S., Pakdeechote P., Maneesai P. Diosmetin ameliorates vascular dysfunction and remodeling by modulation of Nrf2/HO-1 and p-JNK/p-NF-kappaB expression in hypertensive rats. Antioxidants. 2021;10(9) doi: 10.3390/antiox10091487. http://10.3390/antiox10091487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lasker S., Rahman M.M., Parvez F., Zamila M., Miah P., Nahar K., Kabir F., Sharmin S.B., Subhan N., Ahsan G.U., Alam M.A. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-56538-0. http://10.1038/s41598-019-56538-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zabielski P., Hady H.R., Chacinska M., Roszczyc K., Gorski J., Blachnio-Zabielska A.U. The effect of high fat diet and metformin treatment on liver lipids accumulation and their impact on insulin action. Sci. Rep. 2018;8(1):7249. doi: 10.1038/s41598-018-25397-6. http://10.1038/s41598-018-25397-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Q., Hu L., Zhu J., Chen J., Wang Z., Yue Z., Qiu M., Shan A. Valine supplementation does not reduce lipid accumulation and improve insulin sensitivity in mice fed high-fat diet. ACS Omega. 2020;5(48):30937–30945. doi: 10.1021/acsomega.0c03707. http://10.1021/acsomega.0c03707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madkhali H.A. Morin attenuates high-fat diet induced-obesity related vascular endothelial dysfunction in Wistar albino rats. Saudi Pharmaceut. J. 2020;28(3):300–307. doi: 10.1016/j.jsps.2020.01.009. http://10.1016/j.jsps.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sayed S., Ahmed M., El-Shehawi A., Alkafafy M., Al-Otaibi S., El-Sawy H., Farouk S., El-Shazly S. Ginger water reduces body weight gain and improves energy expenditure in rats. Foods. 2020;9(1) doi: 10.3390/foods9010038. http://10.3390/foods9010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S., Alagawadi K.R. Anti-obesity effects of galangin, a pancreatic lipase inhibitor in cafeteria diet fed female rats. Pharm. Biol. 2013;51(5):607–613. doi: 10.3109/13880209.2012.757327. http://10.3109/13880209.2012.757327 [DOI] [PubMed] [Google Scholar]
- 40.Shrestha S., Wu B.J., Guiney L., Barter P.J., Rye K.A. Cholesteryl ester transfer protein and its inhibitors. J. Lipid Res. 2018;59(5):772–783. doi: 10.1194/jlr.R082735. http://10.1194/jlr.R082735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y., Liang X., Zhang G., Kong L., Peng W., Zhang H. Galangin and pinocembrin from propolis ameliorate insulin resistance in HepG2 cells via regulating akt/mTOR signaling. Evid. Bas. Comp. Alter. Med. 2018;2018 doi: 10.1155/2018/7971842. http://10.1155/2018/7971842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalhotra P., Chittepu V.C.S.R., Osorio-Revilla G., Gallardo-Velázquez T. Discovery of galangin as a potential DPP-4 inhibitor that improves insulin-stimulated skeletal muscle glucose uptake: a combinational therapy for diabetes. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welty F.K., Alfaddagh A., Elajami T.K. Targeting inflammation in metabolic syndrome. Transl. Res. 2016;167(1):257–280. doi: 10.1016/j.trsl.2015.06.017. http://10.1016/j.trsl.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tesauro M., Schinzari F., Iantorno M., Rizza S., Melina D., Lauro D., Cardillo C. Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation. 2005;112(19):2986–2992. doi: 10.1161/CIRCULATIONAHA.105.553883. http://10.1161/circulationaha.105.553883 [DOI] [PubMed] [Google Scholar]
- 45.Kuboki K., Jiang Z.Y., Takahara N., Ha S.W., Igarashi M., Yamauchi T., Feener E.P., Herbert T.P., Rhodes C.J., King G.L. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation. 2000;101(6):676–681. doi: 10.1161/01.cir.101.6.676. http://10.1161/01.cir.101.6.676 [DOI] [PubMed] [Google Scholar]
- 46.Muniyappa R., Chen H., Montagnani M., Sherman A., Quon M.J. Endothelial dysfunction due to selective insulin resistance in vascular endothelium: insights from mechanistic modeling. Am. J. Physiol. Endocrinol. Metab. 2020;319(3):E629–e646. doi: 10.1152/ajpendo.00247.2020. http://10.1152/ajpendo.00247.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avtanski D., Pavlov V.A., Tracey K.J., Poretsky L. Characterization of inflammation and insulin resistance in high-fat diet-induced male C57BL/6J mouse model of obesity. Ani. Mod. Exp. Med. 2019;2(4):252–258. doi: 10.1002/ame2.12084. http://10.1002/ame2.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J., Sole J., Nichols A., Ross J.S., Tartaglia L.A., Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. http://10.1172/jci19451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prasatthong P., Meephat S., Rattanakanokchai S., Khamseekaew J., Bunbupha S., Prachaney P., Maneesai P., Pakdeechote P. Galangin resolves cardiometabolic disorders through modulation of AdipoR1, COX-2, and NF-κB expression in rats fed a high-fat diet. Antioxidants. 2021;10(5) doi: 10.3390/antiox10050769. http://10.3390/antiox10050769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hassona M.D., Abouelnaga Z.A., Elnakish M.T., Awad M.M., Alhaj M., Goldschmidt-Clermont P.J., Hassanain H. Vascular hypertrophy-associated hypertension of profilin1 transgenic mouse model leads to functional remodeling of peripheral arteries. Am. J. Physiol. Heart Circ. Physiol. 2010;298(6):H2112–H2120. doi: 10.1152/ajpheart.00016.2010. http://10.1152/ajpheart.00016.2010 [DOI] [PubMed] [Google Scholar]
- 51.Hilzendeger A.M., Morais R.L., Todiras M., Plehm R., da Costa Goncalves A., Qadri F., Araujo R.C., Gross V., Nakaie C.R., Casarini D.E., Carmona A.K., Bader M., Pesquero J.B. Leptin regulates ACE activity in mice. J. Mol. Med. 2010;88(9):899–907. doi: 10.1007/s00109-010-0649-7. http://10.1007/s00109-010-0649-7 [DOI] [PubMed] [Google Scholar]
- 52.Schütten M.T., Houben A.J., de Leeuw P.W., Stehouwer C.D. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology. 2017;32(3):197–209. doi: 10.1152/physiol.00037.2016. http://10.1152/physiol.00037.2016 [DOI] [PubMed] [Google Scholar]
- 53.Eguchi S., Kawai T., Scalia R., Rizzo V. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension. 2018;71(5):804–810. doi: 10.1161/HYPERTENSIONAHA.118.10266. http://10.1161/hypertensionaha.118.10266 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.