Abstract
Diabetes mellitus (DM) is a metabolic disease caused by multiple factors such as genetics, environment, and lifestyle. Bisphenol A (BPA), as one of the most common endocrine-disrupting chemicals (EDCs), has been strongly implicated in the development of type 2 diabetes mellitus (T2DM). BPA exposure is associated with target organ damage in DM and may exacerbate the progression of some chronic complications of DM. This paper reviews relevant epidemiological, in vivo, and in vitro studies to better understand BPA's potential risk associations and pathological mechanisms in several chronic diabetic complications.
Keywords: Bisphenol A, Chronic diabetic complications, Harmful effects, Epidemiology, Pathological mechanisms
1. Introduction
Diabetes mellitus (DM) is a chronic disease severely threatening human health. According to the International Diabetes Federation's latest Global Diabetes Map, in 2021, the global number of adults with diabetes was estimated at 537 million (aged 20–79 years). This number will increase to about 643 million by 2030 and 783 million by 2045 [1]. DM is divided into three types based on its etiology: type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), and other specific types. T2DM is the most common type of clinical DM, accounting for 90–95% of diabetic patients [2]. Long-term, uncontrolled blood glucose levels can damage various organ tissues, including the heart, kidneys, brain, feet, and eyes, leading to chronic diabetic complications [3]. The most critical chronic diabetic complications are coronary heart disease, peripheral vascular disease, end-stage renal disease, retinopathy and neuropathy. These chronic diabetic complications are all major burdens for people with DM [4]. Traditionally, obesity, physical inactivity, advanced age, and an unhealthy diet have been considered significant risk factors for DM [[5], [6], [7]]. In recent years, there has been increasing evidence that endocrine-disrupting chemicals (EDCs) are emerging as additional risk factors for the development of diabetes, including T1DM and T2DM [8].
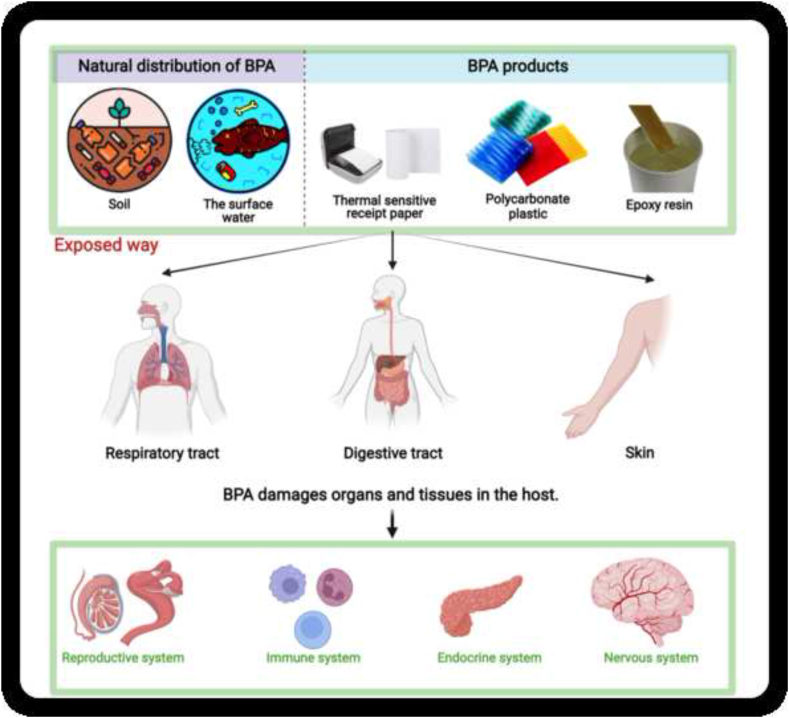
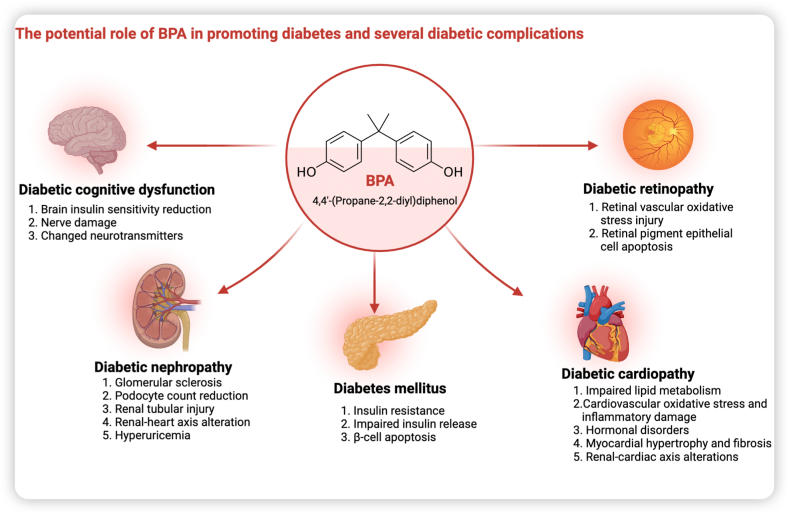
Bisphenol A (BPA) is an organic compound found in a variety of consumer products, including polycarbonate plastics, epoxy resins and thermal receipt paper [9]. Due to its widespread use, BPA has been found in soil and surface water [10]. External BPA can enter the body through the gastrointestinal, respiratory and dermal tracts. Due to its endocrine-disrupting effects, it can cause damage to the reproductive, immune and neuroendocrine systems (Fig. 1) [11]. Recently, increasing evidence suggests that BPA exposure is an independent environmental risk factor for DM that is separate from traditional risk factors [12]. BPA promotes the key pathogenesis of T2DM, including insulin resistance, impaired insulin and glucagon secretion, and pancreatic β-cell dysfunction and injury [[13], [14], [15], [16]]. BPA increases the metabolic stress of high glucose, accelerating cellular senescence and apoptosis, which in turn promotes the progression of DM [17]. BPA exposure is positively associated with obesity, another significant risk factor for the progression of diabetes [18]. By promoting obesity-related disturbances in lipid metabolism and insulin resistance, BPA indirectly exacerbates the progression of DM [19,20]. Furthermore, several studies have identified a possible link between the mechanisms of BPA-induced organ damage and the pathogenesis of diabetic complications in these organs [21,22]. BPA exposure may promote the development and progression of some chronic diabetic complications. However, to our knowledge, no comprehensive review summarises the potential effects and mechanisms of BPA on some common chronic diabetic complications. Therefore, after a brief overview of the sources and hazards of BPA, we review the risk associations and potential mechanisms of action of BPA on diabetic nephropathy, diabetic cognitive dysfunction, diabetic retinopathy, and diabetic cardiopathy (Fig. 2).
Fig. 1.
Major sources of BPA, routes of human exposure and health effects.
Fig. 2.
Potential harmful effects of BPA on DM and various diabetic complications.
2. BPA: sources and hazards
BPA (C15H16O2) is an organic compound that can dissolve in fats and oils. It has a symmetrical chemical structure of two phenolic rings linked by a methyl bridge. BPA is one of the world's most commonly manufactured and used chemicals. It is commonly used to make epoxy resins and polycarbonate (PC) plastics. These materials can be found in everyday products such as water pipes, electronic devices, thermal paper and toys [23]. Due to the high volume of BPA production and use, BPA has been found in air, soil and surface water [10]. In particular, a meta-analysis of 15 studies involving 28,353 participants found BPA in the urine of more than 90% of participants. Despite their differences, the studies highlight the widespread exposure of the population to BPA [24]. People are more likely to get BPA from food and water stored in plastic containers than from BPA in the environment. BPA is found in cups, bottles, packaging, can coatings and other items used as raw materials for food contact materials [25]. Canned food contains higher levels of BPA. The main routes of BPA into the environment and food include migration from PCs, cans and coatings [26]. PC material is commonly used to make cups. Studies have shown that BPA can migrate from PC into water. Increased temperature increases the hydrolysis of the polymer, which accelerates the migration of BPA [27]. As mentioned above, the coatings and paints on canned foods release BPA, so canned foods contain more than fresh foods. When canned foods are exposed to 100 °C, BPA is released up to 18 times faster [28].
As an EDC, BPA could disrupt hormone levels by binding to hormone receptors such as the estrogen receptor, androgen receptor, thyroid hormone receptor, glucocorticoid receptor and peroxisome proliferator-activated receptors, resulting in neuroendocrine disruption. These disruptive neuroendocrine effects cause damage to the reproductive, nervous, immune and metabolic systems [29]. Studies have shown that BPA affects hormones such as oestradiol, progesterone, testosterone, luteinising hormone and cortisol [[30], [31], [32], [33]]. These hormone-altering effects play a role in developing conditions such as polycystic ovary syndrome, recurrent miscarriage and male infertility [34,35]. Moreover, there is increasing epidemiological evidence that BPA exposure is associated with several other human diseases. A longitudinal study found that BPA exposure was independently associated with prediabetes and impaired glucose homeostasis in middle-aged and older women [36]. A cross-sectional study of the National Health and Nutrition Examination Survey (NHANES) found that higher urinary BPA concentrations in the general adult population in the United States were associated with coronary heart disease [37]. BPA exposure was significantly associated with obesity in children and adolescents [38]. BPA may also affect immune function, linked to asthma, T1DM and other autoimmune diseases [[39], [40], [41]].
3. BPA and diabetic nephropathy
Diabetic nephropathy is a significant complication of DM. It is a major cause of chronic and end-stage renal disease worldwide [42]. Diabetic nephropathy accelerates the decline in glomerular filtration rate, shortens the time to start late dialysis treatment, and increases patient mortality [43].
4. Epidemiological evidence linking BPA to diabetic nephropathy
BPA is excreted from the body in the urine. It accumulates in the blood of patients with chronic kidney disease as renal excretion decreases [44]. In a 10-year prospective cohort study of older Chinese women, serum BPA levels were strongly associated with the risk of CKD in all subgroups of women except those with baseline glomerular filtration rates of 60–70 ml/min. The negative association with chronic kidney disease was stronger in women with high serum BPA levels than those with low serum BPA levels [45]. These findings suggest that BPA accumulation is a risk factor for chronic kidney disease in women and that as renal excretion of BPA decreases, more accumulated BPA could worsen chronic kidney disease. In addition, a cross-sectional survey of Chinese adults found that BPA exposure was associated with an increased risk of low-grade albuminuria [46]. Subsequently, a cross-sectional study of US children showed consistent results for an association between BPA exposure and low-grade albuminuria [47]. The association of creatinine excretion and low-grade proteinuria with BPA indirectly suggests a potential renal adverse effect of BPA. Researchers have begun investigating the link between BPA and diabetic nephropathy (Table 1). A 6-year prospective cohort study by Hu et al. found that fasting glucose and serum BPA levels in T2DM patients were significantly and negatively associated with annual and percentage changes in estimated glomerular filtration rate [48]. In addition, people with T2DM who had higher serum BPA levels were about seven times more likely to develop chronic kidney disease than people with T2DM who had lower serum BPA levels [48]. BPA is associated with low-grade albuminuria, and low-grade albuminuria is a feature of early diabetic nephropathy. Therefore, BPA appears to be a risk indicator for the early stages of diabetic nephropathy. However, the relationship between low-grade proteinuria and BPA in DM patients has not been established. There is also a need to clarify the role of BPA in developing diabetic nephropathy.
Table 1.
Summary of epidemiological studies on renal damage caused by BPA exposure.
| Year | Title | Study design, study, country | Number of participants | Follow-up period | Main findings | Reference |
| 2007 | Accumulation of bisphenol A in hemodialysis patients | A cross-sectional study of BPA before and during dialysis in nephropathy, Japan. | 37 | Not available | In patients with nephropathy without hemodialysis, serum BPA concentration increased with worsening renal function, and there was a significant inverse correlation. | [44] |
| 2021 | Associations of serum bisphenol A levels with incident chronic kidney disease risk | A prospective study aimed to evaluate the association between serum BPA levels and CKD in middle-aged and elderly Chinese population, China. | 1370 | 10 years | Serum BPA level is negatively correlated with the risk of CKD. | [45] |
| 2012 | Exposure to bisphenol A is associated with low-grade albuminuria in Chinese adults | A cross-sectional study examined the association between urinary protein and BPA in Shanghai adults over 40 years of age, China. | 3055 | Not available | There is an association between BPA exposure and low-grade albuminuria. | [46] |
| 2013 | Bisphenol A exposure is associated with low-grade urinary albumin excretion in children of the United States | A cross-sectional study examined the association between urinary BPA and low protein levels in children from the 2009–10 NHANES, USA. | 710 | Not available | There is an association between BPA exposure and low-grade albuminuria. | [47] |
| 2015 | Serum bisphenol A and progression of type 2 diabetic nephropathy: a 6-year prospective study | A prospective study investigated whether serum BPA concentration is a predictor of the progression of DN, China. | 121 | 6 years | Serum BPA may be a predictor of CKD in T2D patients. | [48] |
Annotation: BPA: Bisphenol A; CKD: Chronic Kidney Disease; DN: Diabetic Nephropathy; NHANES: National Health And Nutrition Examination Survey; T2D: Type 2 diabetes.
5. Potential pathological mechanisms of BPA-promoted diabetic nephropathy
Diabetes-related glomerular pathology includes 1) diffuse glomerular thylakoid expansion and sclerosis, 2) changes in the endothelial glycocalyx, 3) thickening of the glomerular basement membrane and 4) a decrease in the number of podocytes [49]. In an in vivo test, the offspring of T2DM-prone mouse models exposed to BPA before birth had abnormal glomerular morphology and fewer glomeruli which were more pronounced in female offspring [50]. BPA exposure increased the cell cycle protein-dependent kinase inhibitors p27kip1, TGF-β and collagen IV, which are involved in glomerulosclerosis. In parallel, thylakoid expansion and reduced podocyte numbers were observed in mouse kidneys [51]. Interestingly, although BPA did not cause hyperglycemia in the animals, the renal changes were similar to the structural changes that occur in the early stages of diabetic nephropathy [51]. BPA exposure reduced podocyte density, size and function in mouse glomeruli by inducing downregulation of E-calmodulin, podocin and waveform proteins, resulting in residual podocyte stress hypertrophy and glomerular collapse [52]. The negative correlation between BPA and urinary protein may be explained by BPA-induced podocyte injury. In addition, podocyte injury in diabetic nephropathy has been associated with proteinuria in another study [53]. Based on these two lines of evidence, we hypothesize that BPA-induced podocyte injury may cause diabetic nephropathy. In addition, renal tubular interstitial lesions play a role in the progression of diabetic nephropathy [54]. BPA induces renal tubular injury by promoting autophagy dysregulation and oxidative stress, which may be another potential mechanism by which BPA exacerbates diabetic nephropathy [54]. BPA induces hyperuricemia via the xanthine oxidase pathway [55]. This alteration may also promote the progression of diabetic nephropathy. Furthermore, BPA accelerates renal-cardiac axis alterations in diabetic mouse models by over activating metabolic reconstitution, neuroendocrine disruption, and immune-inflammatory responses via the MAPK pathway [56].
6. BPA and diabetic cognitive dysfunction
Diabetic cognitive dysfunction is an essential complication of DM that progresses insidiously and severely affects the quality of life of older people with DM [57]. Epidemiological evidence suggests that up to 20% of T2DM patients over 60 will develop dementia [58]. As the population ages and the burden of DM increases, the global prevalence of diabetic cognitive dysfunction will continue to rise.
7. Potential pathological mechanisms of BPA-promoted diabetic cognitive dysfunction
There are no evidence-based or mechanistic studies on the relationship between BPA exposure and diabetic cognitive dysfunction in human adults. However, several studies in rodents and non-human primates suggest that BPA exposure impairs learning and memory [59]. BPA, through its effects on brain insulin signaling pathways, neurotoxicity, and neurotransmitter induction, may influence the progression of diabetic cognitive dysfunction. Li and colleagues found that administering a low dose of BPA to adult male mice decreased insulin sensitivity, decreased expression of GLUT1 and GLUT3 in the brain, and hyperactivation of the IR/IRS/AKT/GSK3 axis [60]. Another in vitro study found that the neurotoxic effects of BPA were similar to those of Alzheimer's disease (AD) when tested on SH-SY5Y cells (a tri-clonal subline of the neuroblastoma cell lines SK-N-SH) [61]. This BPA-induced neurotoxicity was associated with disruption of IR, IRS-1, and Akt signaling and activation of downstream GSK3β [61]. In addition, BPA exposure increased the expression of pathological proteins associated with neurotoxicity, such as amyloid precursor protein, beta-site amyloid precursor protein cleavage-1 (BACE1), β-amyloid (Aβ) 1–42 and hyperphosphorylated microtubule-associated protein (p-tau) [58]. Notably, BPA-induced AD-like neurotoxicity implicates pathways consistent with those that induce T2DM [61]. Ni et al. found that BPA-mediated neuroinflammation and blood-brain barrier impairment impaired learning and memory function in male mice. Mechanistically, BPA affects cognitive function in mice by inducing changes in neurotransmitters such as tryptophan, 5-hydroxytryptamine, and 5-hydroxy indole acetic acid via the gut-brain axis [62]. The mechanism of diabetic cognitive dysfunction is not well understood. However, the above studies have shown that brain insulin resistance and impaired insulin signaling pathways are essential in diabetic cognitive dysfunction [63]. Based on the above evidence, BPA-induced brain insulin resistance and disruption of the insulin signaling pathway in the brain may be underlying causes of diabetic cognitive dysfunction.
8. BPA and diabetic retinopathy
Diabetic retinopathy is the most common microvascular complication of DM. It is the leading cause of blindness in adults worldwide. There were reportedly 96 million people with diabetic retinopathy worldwide in 2012 [64]. BPA can cause abnormal retinal development and visual impairment [65]. A cross-sectional study of 100 children with T1DM found an association between BPA and diabetic retinopathy [66]. However, evidence from retrospective studies is limited, and a causal relationship between BPA and diabetic retinopathy has not yet been established.
9. Potential pathological mechanisms of BPA-promoted diabetic retinopathy
The retina is more susceptible to reactive oxygen species (ROS) due to its high concentration of polyunsaturated fatty acids [67]. The reactive oxygen species (ROS) produced by hyperglycemia cause changes in the retinal vasculature, resulting in cellular damage. In an animal study, Ola found that hyperglycemia-induced non-mitochondrial sources may be the primary source of ROS production in diabetic retinopathy rather than hyperglycemia itself [68]. BPA exposure may increase oxidative stress. In vitro, BPA exposure increased ROS, decreased glutathione (GSH) levels, caused lipid peroxidation, and altered the enzymatic activities of superoxide dismutase and catalase [69]. Chronic BPA exposure may be an additional source of oxidative stress in diabetic retinopathy, distinct from hyperglycemia-mediated oxidative stress. In addition, another study found that BPA degrades the antioxidants superoxide dismutase and catalase downstream of nuclear factor erythroid 2-related factor 2 by inhibiting the expression of heme oxygenase-1 and nuclear factor erythroid 2-related factor 2 [70]. Increased oxidative stress via BPA can induce apoptosis of the retinal pigment epithelium (ARPE-19 cell) [70]. Unfortunately, no studies have directly investigated the effects and mechanisms of BPA-induced additional oxidative stress on ex vivo models of diabetic retinopathy. Future research should focus on the potential role of the nuclear factor erythroid 2-related factor 2/oxygenase-1 pathway and downstream components of the oxidative and antioxidant systems in BPA-induced diabetic retinopathy.
10. BPA and diabetic cardiopathy
Diabetic cardiopathy is the leading cause of death in people with DM [71]. Diabetic cardiopathy mainly includes coronary artery disease and diabetic cardiomyopathy. We discuss the epidemiological evidence linking BPA exposure to diabetic coronary artery disease and the possible pathological mechanisms (Table 2). We also summarize the possible pathological mechanisms by which BPA exposure promotes diabetic cardiomyopathy.
Table 2.
Summary of epidemiological and animal evidence on the potential damage of BPA to diabetic coronary heart disease.
| First author, Year | study design | study object | Main findings | Reference |
|---|---|---|---|---|
| Avinash Soundararajan, | cross-sectional study | human | In patients with T2DM, elevated BPA levels are associated with cellular senescence, proinflammation, poor glycemic control, insulin resistance, and telomere shortening. | [13] |
| Ziwei Chen, 2022 | cross-sectional study | human | BPA was associated with CVD risk in a J-curve relationship. | [72] |
| Fanny Rancière, 2015 | meta-analysis | human | Individuals with higher urinary BPA concentrations are more likely to develop diabetes, general/abdominal obesity, and hypertension than those with lower urinary BPA concentrations. | [73] |
| P Monica Lind, 2011 | cross-sectional study | human | Phthalates and BPA have been linked to plaque echo. | [74] |
| Pei-Lun Chu, 2021 | cross-sectional study | human | In the presence of elevated BPA levels, there is a higher risk of thicker CIMT associated with altered MPs. BPA exposure is associated with endothelial dysfunction and subclinical atherosclerosis in younger populations. | [75] |
| David Melzer, 2012 | prospective study | human | The association between higher BPA exposure, as reflected by higher urinary concentrations, and CHD events over a follow-up period of more than ten years showed a similar trend to the cross-sectional findings reported previously for higher exposure NHANES respondents. | [76] |
| Chunyun Hu, 2019 | nested case-control study | human | BPA exposure is positively associated with diabetes and coronary heart disease. | [77] |
| Alice Marmugi, 2014 | animal experiment | mice | Chronic BPA exposure overexpresses genes critical for cholesterol biosynthesis, resulting in hypercholesterolemia in mice. | [78] |
| Yipeng Sui, 2014 | animal experiment | mice | BPA exposure did not affect plasma lipid levels but increased CD36 expression and lipid accumulation in mouse macrophages. | [79] |
Annotation: BPA: Bisphenol A; CHD: Coronary Heart Disease; CIMT: Carotid artery Intima-Media Thickness; CVD: Cardiovascular Disease; MPs: Microparticles; NHANES: National Health And Nutrition Examination Survey; PXR: Pregnane X Receptor; T2DM: Type 2 diabetes mellitus.
11. Epidemiological evidence for the association of BPA with diabetic coronary artery disease
A cross-sectional study using NHANES data from 2003 to 2012 found a J-shaped association between BPA and the risk of cardiovascular disease, including congestive heart failure, coronary heart disease, and angina pectoris [72]. A meta-analysis on the risk of cardiometabolic disorders and BPA exposure focused on cross-sectional studies. Rancière et al. found that urinary BPA concentrations were associated with an increased risk of developing diabetes, obesity and hypertension [73]. These metabolic disorders are important risk factors for cardiovascular disease. Atherosclerosis-related metabolites are mainly found in the coronary and carotid arteries [73]. Thus, carotid atherosclerosis may indirectly reflect coronary atherosclerosis. Lind et al. found that high serum BPA levels were associated with carotid atheroma in a cross-sectional study of older adults in Uppsala [74]. In another cross-sectional study of a young population in Taiwan, BPA exposure was associated with endothelial dysfunction and subclinical atherosclerosis. There was also an increased risk of carotid intima-media thickness associated with altered extracellular microparticles in elevated BPA levels [75]. A 10.8-year prospective cohort study found that increased urinary BPA was associated with incident coronary heart disease in a healthy population (aged 40–74 years without coronary heart disease, stroke, or diabetes) [76]. This study suggests that the risk of coronary heart disease from BPA exposure is independent of the risk from conventional exposure. Hu et al. found that urinary BPA was significantly associated with myocardial infarction in T2DM patients (OR = 1.97; 95% CI = 1.05–3.70, p = 0.04) in a nested case-control study in two European cohorts [77]. This study found, for the first time, an association between BPA exposure and diabetic coronary heart disease. However, the association needs to be validated by further prospective cohort studies with large samples.
12. Potential pathological mechanisms of BPA-promoted diabetic coronary artery disease
The pathology of DM that promotes the development of atherosclerosis includes dyslipidemia with elevated LDL levels, hyperglycemia, oxidative stress and increased inflammation [80]. Chronic exposure to BPA causes overexpression of genes essential for cholesterol biosynthesis, leading to hypercholesterolemia in mice [78]. Another animal study found that BPA increased the atherosclerotic area in the aorta and cephalic brachial artery of pregnane X receptor (PXR) humanized (ApoE) ApoE-deficient mice by activating human PXR [79]. Interestingly, BPA exposure did not affect plasma lipid concentrations but increased lipid accumulation in mouse macrophages [79]. In addition, BPA was associated with poor glycaemic control and insulin resistance in T2DM patients [13].
BPA is a cardiovascular-independent risk factor, suggesting that there may be other mechanisms by which BPA exposure damages the cardiovascular system and alters glucose and lipid metabolism. BPA may increase the risk of cardiovascular disease through increased stimulation of IκB kinase by estrogen receptors, which promotes the expression of pro-inflammatory genes associated with CRP secretion [81]. In addition, BPA may cause cardiovascular damage through the nuclear factor erythroid 2-related factor 2/NF-κB pathway by inducing oxidative stress and inflammation in the cardiovascular system [82].
13. Potential pathological mechanisms of BPA-promoted diabetic cardiomyopathy
The hormone-disrupting effects of BPA may affect the myocardial structure. A cross-sectional study found that serum BPA levels were significantly higher in the dilated cardiomyopathy group than in the healthy group, with increased total testosterone, sex hormone-binding globulin, and free androgen index [83]. It is reasonable to speculate that the hormonal disruption caused by BPA exposure may act as a non-diabetic pathological mechanism for promoting diabetic cardiomyopathy.
BPA may also contribute to the progression of diabetic cardiomyopathy by participating in diabetes-related metabolic pathological mechanisms. BPA and a high-fat diet caused myocardial hypertrophy and aortic intimal thickening in female mice [84]. These exposures also affected their offspring, increasing cardiomyocyte cross-sectional area and blood pressure in second-generation maternal mice [84]. Prenatal exposure to BPA and a high-fat diet predispose mouse offspring to insulin resistance, obesity, impaired glucose tolerance, hypertension, and other metabolic abnormalities. Myocardial fibrosis is the predominant pathological manifestation of diabetic cardiopathy. In an animal study, El-Haleem et al. found a significant increase in the area percentage of collagen fibers in the myocardium of the BPA intervention group, similar to the pathological changes in diabetic cardiopathy [85]. The exact mechanism by which BPA promotes myocardial fibrosis in diabetic cardiopathy is unknown. It could be related to the role of BPA-induced myocardial insulin resistance and the potential activation of fibrotic pathways. Furthermore, as mentioned above, BPA accelerated renal-cardiac axis alterations in the diabetic mouse model via the MAPK pathway, causing structural remodeling of the heart [86].
14. Conclusion and future direction
The above extensive evidence sheds light on the risk and underlying pathogenic mechanisms linking BPA exposure to several common chronic diabetic complications. By affecting the metabolism and directly damaging specific organs, BPA exposure may contribute to the progression of several chronic diabetic complications, including diabetic nephropathy, diabetic cognitive dysfunction, diabetic retinopathy, and diabetic cardiopathy.
Several cross-sectional and prospective cohort studies have found an association between BPA exposure and diabetic nephropathy. Of course, factors such as individual differences in BPA exposure and metabolism limit the generalisability of the findings. Therefore, more studies should provide more conclusive evidence, especially those with large sample sizes and multicentre prospective cohort studies. In addition, epidemiological evidence linking BPA exposure to diabetic cognitive dysfunction and diabetic retinopathy is currently lacking. Future epidemiological studies are needed to investigate this association. Some mechanistic studies have examined the potential mechanisms by which BPA may promote the diabetic complications mentioned above. Notably, these mechanisms are not limited to the effects of BPA exposure on diabetes; they also include direct damage to target organs from BPA exposure. For example, in animal models, BPA causes renal changes similar to the structural changes seen in the early stages of diabetic nephropathy without causing hyperglycemia [51]. However, few studies have focused directly on the pathogenetic effects of BPA on diabetic complications, while other relevant studies have focused on organ-specific damage caused by BPA. In addition, studies on the effects of BPA on diabetic neuropathy are scarce, although the neurotoxicity of BPA is well established. There is evidence that urinary levels of BPA are associated with the development of diabetic peripheral neuropathy [66]. Relevant epidemiologic and pathogenic studies are warranted as human exposure to BPA remains an ongoing concern.
According to the findings of this review, BPA damage to specific organs may contribute to the progression of complications in these organs in DM. In the future, researchers must confirm the adverse effects of BPA in specific in vivo models of diabetic complications. Given that many mechanistic studies have reached their conclusions by studying rodent models, the validity of these mechanisms needs to be verified in vitro in human cells and tissues using alternative techniques. Researchers should pay close attention to the potential role of BPA exposure in the progression of DM and its complications.
Due to the harmful effects of BPA's endocrine-disrupting properties and reproductive toxicity to humans, many countries and regions have imposed restrictions on the use of BPA. For instance, to prevent human exposure to BPA, the E.U., the U.S., and France, among others, have severely restricted the production and sale of containers, utensils, and food packaging containing BPA [87]. With the limited use of BPA in various applications, some of its alternatives, such as BPF (4,4′-methylene diphenyl), BPS (bis(4-hydroxyphenyl)sulfone), and BPAF (2,2-bis(4-hydroxyphenyl)hexafluoropropane), gradually began to be used on a large scale [87]. However, these substitutes may also risk human health, if not less than BPA. Therefore, we urge researchers to conduct more studies to determine the potential risks of BPA and its substitutes for various human diseases, including DM and its major complications. Accordingly, national regulatory agencies and policymakers should promptly adapt and develop relevant policies to reduce human exposure to these compounds.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Additional information
No additional information is available for this paper.
Funding statement
This study was not externally funded.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Professor Huanan Jia of Department of Geriatrics, Hospital of Chengdu University of Traditional Chinese Medicine for critically reviewing the manuscript.
Contributor Information
Wei Jiang, Email: jiangweivv@stu.cdutcm.edu.cn.
Kaixi Ding, Email: jerryding21@gmail.com.
Wenjie Huang, Email: huangwenjie759352@163.com.
Rensong Yue, Email: songrenyue@cdutcm.edu.cn.
References
- 1.Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., Stein C., Basit A., Chan J.C.N., Mbanya J.C., Pavkov M.E., Ramachandaran A., Wild S.H., James S., Herman W.H., Zhang P., Bommer C., Kuo S., Boyko E.J., Magliano D.J. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183 doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaji R., Duraisamy R., Kumar M.P. Drug Invention Today. 2019. Complications of diabetes mellitus: a review. [Google Scholar]
- 4.Harding J.L., Pavkov M.E., Magliano D.J., Shaw J.E., Gregg E.W. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 5.Uloko A.E., Musa B.M., Ramalan M.A., Gezawa I.D., Puepet F.H., Uloko A.T., Borodo M.M., Sada K.B. Prevalence and risk factors for diabetes mellitus in Nigeria: a systematic review and meta-analysis. Diabetes Ther. 2018;9:1307–1316. doi: 10.1007/s13300-018-0441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisman A., Fazli G.S., Johns A., Booth G.L. Evolving trends in the epidemiology, risk factors, and prevention of type 2 diabetes: a review. Can. J. Cardiol. 2018;34:552–564. doi: 10.1016/j.cjca.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Maahs D.M., West N.A., Lawrence J.M., Mayer-Davis E.J. Epidemiology of type 1 diabetes. Endocrinol Metab. Clin. N. Am. 2010;39:481–497. doi: 10.1016/j.ecl.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hectors T.L.M., Vanparys C., van der Ven K., Martens G.A., Jorens P.G., Van Gaal L.F., Covaci A., De Coen W., Blust R. Environmental pollutants and type 2 diabetes: a review of mechanisms that can disrupt beta cell function. Diabetologia. 2011;54:1273–1290. doi: 10.1007/s00125-011-2109-5. [DOI] [PubMed] [Google Scholar]
- 9.Sonavane M., Gassman N.R. Bisphenol A co-exposure effects: a key factor in understanding BPA's complex mechanism and health outcomes. Crit. Rev. Toxicol. 2019;49:371–386. doi: 10.1080/10408444.2019.1621263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao C., Wang L., Zhou Q., Huang X. Hazards of bisphenol A (BPA) exposure: a systematic review of plant toxicology studies. J. Hazard Mater. 2020;384 doi: 10.1016/j.jhazmat.2019.121488. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y., Liu H., Wu J., Yuan L., Wang Y., Du X., Wang R., Marwa P.W., Petlulu P., Chen X., Zhang H. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019;176 doi: 10.1016/j.envres.2019.108575. [DOI] [PubMed] [Google Scholar]
- 12.Rancière F., Botton J., Slama R. Exposure to bisphenol A and Bisphenol S and incident type 2 diabetes: a case-cohort study in the French cohort DESIR. Environ. Health Perspect. 2019;127 doi: 10.1289/EHP5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soundararajan A., Prabu P., Mohan V., Gibert Y., Balasubramanyam M. Novel insights of elevated systemic levels of bisphenol-A (BPA) linked to poor glycemic control, accelerated cellular senescence and insulin resistance in patients with type 2 diabetes. Mol. Cell. Biochem. 2019;458:171–183. doi: 10.1007/s11010-019-03540-9. [DOI] [PubMed] [Google Scholar]
- 14.Gong H., Zhang X., Cheng B., Sun Y., Li C., Li T., Zheng L., Huang K. Bisphenol A accelerates toxic amyloid formation of human islet amyloid polypeptide: a possible link between bisphenol A exposure and type 2 diabetes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J., Zhong L., Wu J., Ke S., Morpurgo B., Golovko A., Ouyang N., Sun Y., Guo S., Tian Y. A Murine pancreatic islet cell-based screening for diabetogenic environmental chemicals. J. Vis. Exp. 2018 doi: 10.3791/57327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrugia F., Aquilina A., Vassallo J., Pace N.P. Bisphenol A and type 2 diabetes mellitus: a review of epidemiologic, functional, and early life factors. Int. J. Environ. Res. Publ. Health. 2021;18:716. doi: 10.3390/ijerph18020716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soundararajan A., Yoganantharajah P., Raghavan S., Mohan V., Balasubramanyam M., Gibert Y. Bisphenol A exposure under metabolic stress induces accelerated cellular senescence in vivo in a p53 independent manner. Sci. Total Environ. 2019;689:1201–1211. doi: 10.1016/j.scitotenv.2019.06.391. Epub 2019 Jun 25. [DOI] [PubMed] [Google Scholar]
- 18.Nadal A. Obesity: fat from plastics? Linking bisphenol A exposure and obesity. Nat. Rev. Endocrinol. 2013;9(1):9–10. doi: 10.1038/nrendo.2012.205. Epub 2012 Nov 13. [DOI] [PubMed] [Google Scholar]
- 19.Menale C., Grandone A., Nicolucci C., Cirillo G., Crispi S., Di Sessa A., Marzuillo P., Rossi S., Mita D.G., Perrone L., Diano N., Miraglia Del Giudice E. Bisphenol A is associated with insulin resistance and modulates adiponectin and resistin gene expression in obese children. Pediatr. Obes. 2017;12(5):380–387. doi: 10.1111/ijpo.12154. Epub 2016 May 17. [DOI] [PubMed] [Google Scholar]
- 20.Fang R., Yang S., Gu X., Li C., Bi N., Wang H.L. Early-life exposure to bisphenol A induces dysregulation of lipid homeostasis by the upregulation of SCD1 in male mice. Environ. Pollut. 2022 Jul 1;304 doi: 10.1016/j.envpol.2022.119201. Epub 2022 Mar 25. [DOI] [PubMed] [Google Scholar]
- 21.Sahu C., Jena G. Dietary zinc deficient condition increases the Bisphenol A toxicity in diabetic rat testes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2022;882 doi: 10.1016/j.mrgentox.2022.503547. [DOI] [PubMed] [Google Scholar]
- 22.Moreno-Gómez-Toledano R., Arenas M.I., Muñoz-Moreno C., Olea-Herrero N., Reventun P., Izquierdo-Lahuerta A., Antón-Cornejo A., González-Santander M., Zaragoza C., Saura M., Bosch R.J. Comparison of the renal effects of bisphenol A in mice with and without experimental diabetes. Role of sexual dimorphism. Biochim. Biophys. Acta, Mol. Basis Dis. 2022;1868 doi: 10.1016/j.bbadis.2021.166296. [DOI] [PubMed] [Google Scholar]
- 23.Flint S., Markle T., Thompson S., Wallace E. Bisphenol A exposure, effects, and policy: a wildlife perspective. J. Environ. Manag. 2012;104:19–34. doi: 10.1016/j.jenvman.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 24.Colorado-Yohar S.M., Castillo-González A.C., Sánchez-Meca J., Rubio-Aparicio M., Sánchez-Rodríguez D., Salamanca-Fernández E., Ardanaz E., Amiano P., Fernández M.F., Mendiola J., Navarro-Mateu F., Chirlaque M.D. Concentrations of bisphenol-A in adults from the general population: a systematic review and meta-analysis. Sci. Total Environ. 2021;775 doi: 10.1016/j.scitotenv.2021.145755. [DOI] [PubMed] [Google Scholar]
- 25.Noonan G.O., Ackerman L.K., Begley T.H. Concentration of bisphenol A in highly consumed canned foods on the US market. J. Agric. Food Chem. 2011;59:7178–7185. doi: 10.1021/jf201076f. [DOI] [PubMed] [Google Scholar]
- 26.Michałowicz J. Bisphenol A-sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014;37:738–758. doi: 10.1016/j.etap.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Nam S.-H., Seo Y.-M., Kim M.-G. Bisphenol A migration from polycarbonate baby bottle with repeated use. Chemosphere. 2010;79:949–952. doi: 10.1016/j.chemosphere.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 28.Takao Y., Lee H.C., Kohra S., Arizono K. Release of bisphenol A from food can lining upon heating. J. Health Sci. 2002;48:331–334. doi: 10.1248/jhs.48.331. [DOI] [Google Scholar]
- 29.Ma Y., Liu H., Wu J., Yuan L., Wang Y., Du X., Wang R., Marwa P.W., Petlulu P., Chen X., Zhang H. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019;176 doi: 10.1016/j.envres.2019.108575. [DOI] [PubMed] [Google Scholar]
- 30.Wisniewski P., Romano R.M., Kizys M.M.L., Oliveira K.C., Kasamatsu T., Giannocco G., Chiamolera M.I., Dias-da-Silva M.R., Romano M.A. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis. Toxicology. 2015;329:1–9. doi: 10.1016/j.tox.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Aimuzi R., Nian M., Zhang Y., Luo K., Zhang J. Bisphenol A substitutes and sex hormones in children and adolescents. Chemosphere. 2021;278 doi: 10.1016/j.chemosphere.2021.130396. [DOI] [PubMed] [Google Scholar]
- 32.Mustieles V., Ocón-Hernandez O., Mínguez-Alarcón L., Dávila-Arias C., Pérez-Lobato R., Calvente I., Arrebola J.P., Vela-Soria F., Rubio S., Hauser R., Olea N., Fernández M.F. Bisphenol A and reproductive hormones and cortisol in peripubertal boys: the INMA-Granada cohort. Sci. Total Environ. 2018;618:1046–1053. doi: 10.1016/j.scitotenv.2017.09.093. [DOI] [PubMed] [Google Scholar]
- 33.Šimková M., Vítků J., Kolátorová L., Vrbíková J., Vosátková M., Včelák J., Dušková M. Endocrine disruptors, obesity, and cytokines - how relevant are they to PCOS? Physiol. Res. 2020:S279–S293. doi: 10.33549/physiolres.934521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Y., Zheng Y., Jiang J., Liu Y., Luo X., Shen Z., Chen X., Wang Y., Dai Y., Zhao J., Liang H., Chen A., Yuan W. Higher urinary bisphenol A concentration is associated with unexplained recurrent miscarriage risk: evidence from a case-control study in eastern China. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitku J., Sosvorova L., Chlupacova T., Hampl R., Hill M., Sobotka V., Heracek J., Bicikova M., Starka L. Differences in bisphenol A and estrogen levels in the plasma and seminal plasma of men with different degrees of infertility. Physiol. Res. 2015;64:S303–S311. doi: 10.33549/physiolres.933090. [DOI] [PubMed] [Google Scholar]
- 36.Wang B., Li M., Zhao Z., Lu J., Chen Y., Xu Y., Xu M., Wang W., Wang T., Bi Y., Ning G. Urinary bisphenol A concentration and glucose homeostasis in non-diabetic adults: a repeated-measures, longitudinal study. Diabetologia. 2019;62:1591–1600. doi: 10.1007/s00125-019-4898-x. [DOI] [PubMed] [Google Scholar]
- 37.Melzer D., Rice N.E., Lewis C., Henley W.E., Galloway T.S. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amin M.M., Ebrahim K., Hashemi M., Shoshtari-Yeganeh B., Rafiei N., Mansourian M., Kelishadi R. Association of exposure to Bisphenol A with obesity and cardiometabolic risk factors in children and adolescents. Int. J. Environ. Health Res. 2019;29:94–106. doi: 10.1080/09603123.2018.1515896. [DOI] [PubMed] [Google Scholar]
- 39.Donohue K.M., Miller R.L., Perzanowski M.S., Just A.C., Hoepner L.A., Arunajadai S., Canfield S., Resnick D., Calafat A.M., Perera F.P., Whyatt R.M. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J. Allergy Clin. Immunol. 2013;131:736–742. doi: 10.1016/j.jaci.2012.12.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howard S.G. Exposure to environmental chemicals and type 1 diabetes: an update. J. Epidemiol. Commun. Health. 2019;73:483–488. doi: 10.1136/jech-2018-210627. [DOI] [PubMed] [Google Scholar]
- 41.Aljadeff G., Longhi E., Shoenfeld Y. Bisphenol A: a notorious player in the mosaic of autoimmunity. Autoimmunity. 2018;51:370–377. doi: 10.1080/08916934.2018.1551374. [DOI] [PubMed] [Google Scholar]
- 42.Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nat. Rev. Nephrol. 2017;13:311–318. doi: 10.1038/nrneph.2017.31. [DOI] [PubMed] [Google Scholar]
- 43.Stanton R.C. Clinical challenges in diagnosis and management of diabetic kidney disease. Am. J. Kidney Dis. 2014;63:S3–S21. doi: 10.1053/j.ajkd.2013.10.050. [DOI] [PubMed] [Google Scholar]
- 44.Murakami K., Ohashi A., Hori H., Shoji Y. Accumulation of bisphenol A in hemodialysis patients. Blood Purif. 2007;25:290–294. doi: 10.1159/000104869. [DOI] [PubMed] [Google Scholar]
- 45.Nie H., Wang F., Zhang Y., Zhang S., Han X., Zhang X., Guo H., He M. Associations of serum bisphenol A levels with incident chronic kidney disease risk. Sci. Total Environ. 2021;771 doi: 10.1016/j.scitotenv.2021.145401. [DOI] [PubMed] [Google Scholar]
- 46.Li M., Bi Y., Qi L., Wang T., Xu M., Huang Y., Xu Y., Chen Y., Lu J., Wang W., Ning G. Exposure to bisphenol A is associated with low-grade albuminuria in Chinese adults. Kidney Int. 2012;81:1131–1139. doi: 10.1038/ki.2012.6. [DOI] [PubMed] [Google Scholar]
- 47.Trasande L., Attina T.M., Trachtman H. Bisphenol A exposure is associated with low-grade urinary albumin excretion in children of the United States. Kidney Int. 2013;83:741–748. doi: 10.1038/ki.2012.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu J., Yang S., Wang Y., Goswami R., Peng C., Gao R., Zhou H., Zhang Y., Cheng Q., Zhen Q., Li Q. Serum bisphenol A and progression of type 2 diabetic nephropathy: a 6-year prospective study. Acta Diabetol. 2015;52:1135–1141. doi: 10.1007/s00592-015-0801-5. [DOI] [PubMed] [Google Scholar]
- 49.Gnudi L., Coward R.J.M., Long D.A. Diabetic nephropathy: perspective on novel molecular mechanisms. Trends Endocrinol. Metabol. 2016;27:820–830. doi: 10.1016/j.tem.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Nuñez P., Fernandez T., García-Arévalo M., Alonso-Magdalena P., Nadal A., Perillan C., Arguelles J. Effects of bisphenol A treatment during pregnancy on kidney development in mice: a stereological and histopathological study. J. Dev. Orig. Health Dis. 2018;9:208–214. doi: 10.1017/S2040174417000939. [DOI] [PubMed] [Google Scholar]
- 51.Moreno-Gómez-Toledano R., Arenas M.I., Sánchez-Esteban S., Cook A., Saura M., Bosch R.J. Hot Topics in Endocrinology and Metabolism. IntechOpen; 2021. Critical analysis of human exposure to bisphenol A and its novel implications on renal, cardiovascular and hypertensive diseases. [Google Scholar]
- 52.Chvojanová Z. 2019. Study of the Variations in the Expression of Different Adhesion and Cytoskeletal Proteins of Podocytes (E-Cadherin, Podocin, Vimentin) Due to Bisphenol A. [Google Scholar]
- 53.Dai H., Liu Q., Liu B. Research progress on mechanism of podocyte depletion in diabetic nephropathy. J. Diabetes Res. 2017;2017:1–10. doi: 10.1155/2017/2615286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen Z., Fang Y., Xing T., Wang F. Diabetic nephropathy: from pathophysiology to treatment. J. Diabetes Res. 2017;2017:1–2. doi: 10.1155/2017/2379432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma L., Hu J., Li J., Yang Y., Zhang L., Zou L., Gao R., Peng C., Wang Y., Luo T., Xiang X., Qing H., Xiao X., Wu C., Wang Z., He J.C., Li Q., Yang S. Bisphenol A promotes hyperuricemia via activating xanthine oxidase. Faseb. J. 2018;32:1007–1016. doi: 10.1096/fj.201700755R. [DOI] [PubMed] [Google Scholar]
- 56.B. Wu, Q. Zhao, Z. Li, Environmental level bisphenol A accelerates alterations of the reno cardiac axis by the MAPK cascades in male diabetic rats: an analysis based on transcriptomic profiling and bioinformatics, Environ. Pollut.. 287 ((n.d.)). [DOI] [PubMed]
- 57.Munshi M.N. Cognitive dysfunction in older adults with diabetes: what a clinician needs to know. Diabetes Care. 2017;40:461–467. doi: 10.2337/dc16-1229. [DOI] [PubMed] [Google Scholar]
- 58.Srikanth V., Sinclair A.J., Hill-Briggs F. Type 2 diabetes and cognitive dysfunction- towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020;8:535–545. doi: 10.1016/S2213-8587(20)30118-2. [DOI] [PubMed] [Google Scholar]
- 59.Mhaouty-Kodja S., Belzunces L.P., Canivenc M.-C., Schroeder H., Chevrier C., Pasquier E. Impairment of learning and memory performances induced by BPA: evidences from the literature of a MoA mediated through an ED. Mol. Cell. Endocrinol. 2018;475:54–73. doi: 10.1016/j.mce.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 60.Li J., Wang Y., Fang F., Chen D., Gao Y., Liu J., Gao R., Wang J., Xiao H. Bisphenol A disrupts glucose transport and neurophysiological role of IR/IRS/AKT/GSK3β axis in the brain of male mice. Environ. Toxicol. Pharmacol. 2016;43:7–12. doi: 10.1016/j.etap.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 61.Wang T., Xie C., Yu P. Involvement of insulin signaling disturbances in bisphenol a induced alzheimer's disease-like neurotoxicity. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-07544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ni Y., Hu L., Yang S., Ni L., Ma L., Zhao Y., Zheng A., Jin Y., Fu Z. Bisphenol A impairs cognitive function and 5-HT metabolism in adult male mice by modulating the microbiota-gut-brain axis. Chemosphere. 2021;282 doi: 10.1016/j.chemosphere.2021.130952. [DOI] [PubMed] [Google Scholar]
- 63.Arnold S.E., Arvanitakis Z., Macauley-Rambach S.L., Koenig A.M., Wang H.-Y., Ahima R.S., Craft S., Gandy S., Buettner C., Stoeckel L.E., Holtzman D.M., Nathan D.M. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat. Rev. Neurol. 2018;14:168–181. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yau J.W.Y., Rogers S.L., Kawasaki R., Lamoureux E.L., Kowalski J.W., Bek T., Chen S.-J., Dekker J.M., Fletcher A., Grauslund J., Haffner S., Hamman R.F., Ikram M.K., Kayama T., Klein B.E.K., Klein R., Krishnaiah S., Mayurasakorn K., O'Hare J.P., Orchard T.J., Porta M., Rema M., Roy M.S., Sharma T., Shaw J., Taylor H., Tielsch J.M., Varma R., Wang J.J., Wang N., West S., Xu L., Yasuda M., Zhang X., Mitchell P., Wong T.Y. Meta-analysis for eye disease (META-EYE) study group, global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li M., Yang T., Gao L., Xu H. An inadvertent issue of human retina exposure to endocrine disrupting chemicals: a safety assessment. Chemosphere. 2021;264 doi: 10.1016/j.chemosphere.2020.128484. [DOI] [PubMed] [Google Scholar]
- 66.A. El-Ghobashy M M R, S.M. Al-Aziz, H. Yahia, Correlation Between Urinary Bisphenol A Levels with Insulin Resistance and Diabetes Complications in Children with Type 1 Diabetes Mellitus, n.d.
- 67.Inazo-Durán M.D., Gallego-Pinazo R., Garcia-Medina J.J. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging. 2014;9 doi: 10.2147/CIA.S52662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ola M.S. Does hyperglycemia cause oxidative stress in the diabetic rat retina? Cells. 2021;10:794. doi: 10.3390/cells10040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maćczak A., Cyrkler M., Bukowska B., Michałowicz J. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study) Toxicol. In Vitro. 2017;41:143–149. doi: 10.1016/j.tiv.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 70.Chiang Y.W., Su C.H., Sun H.Y. Bisphenol A induced apoptosis via oxidative stress generation involved Nrf2/HO- 1 pathway and mitochondrial dependent pathways in human retinal pigment epithelium (ARPE- 19) cells. Environ. Toxicol. 2022:131–141. doi: 10.1002/tox.23384. n.d. [DOI] [PubMed] [Google Scholar]
- 71.Brown A., Reynolds L.R., Bruemmer D. Intensive glycemic control and cardiovascular disease: an update. Nat. Rev. Cardiol. 2010;7:369–375. doi: 10.1038/nrcardio.2010.35. [DOI] [PubMed] [Google Scholar]
- 72.Chen Z., He J., Shi W. Association between urinary environmental phenols and the prevalence of cardiovascular diseases in US adults. Environ. Sci. Pollut. Res. Int. 2022;29:42947–42954. doi: 10.1007/s11356-021-18323-3. [DOI] [PubMed] [Google Scholar]
- 73.Rancière F., Lyons J.G., Loh V.H.Y., Botton J., Galloway T., Wang T., Shaw J.E., Magliano D.J. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ. Health. 2015;14:46. doi: 10.1186/s12940-015-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lind P.M., Lind L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis. 2011;218:207–213. doi: 10.1016/j.atherosclerosis.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Chu P.-L., Lin C.-Y., Sung F.-C., Su T.-C. Apoptotic microparticles mediate the association between bisphenol A and subclinical atherosclerosis in a young population: a population-based study. Ecotoxicol. Environ. Saf. 2021;224 doi: 10.1016/j.ecoenv.2021.112663. [DOI] [PubMed] [Google Scholar]
- 76.Melzer D., Osborne N.J., Henley W.E., Cipelli R., Young A., Money C., McCormack P., Luben R., Khaw K.-T., Wareham N.J., Galloway T.S. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. 2012;125:1482–1490. doi: 10.1161/CIRCULATIONAHA.111.069153. [DOI] [PubMed] [Google Scholar]
- 77.Hu C., Schöttker B., Venisse N., Limousi F., Saulnier P.J., Albouy-Llaty M., Dupuis A., Brenner H., Migeot V., Hadjadj S. Bisphenol A, chlorinated derivatives of Bisphenol A and occurrence of myocardial infarction in patients with type 2 diabetes: nested case-control studies in two European cohorts. Environ. Sci. Technol. 2019;53:9876–9883. doi: 10.1021/acs.est.9b02963. [DOI] [PubMed] [Google Scholar]
- 78.Marmugi A., Lasserre F., Beuzelin D., Ducheix S., Huc L., Polizzi A., Chetivaux M., Pineau T., Martin P., Guillou H., Mselli-Lakhal L. Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology. 2014;325:133–143. doi: 10.1016/j.tox.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Sui Y., Park S.-H., Helsley R.N., Sunkara M., Gonzalez F.J., Morris A.J., Zhou C. Bisphenol A increases atherosclerosis in pregnane X receptor-humanized ApoE deficient mice. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poznyak A., Grechko A.V., Poggio P., Myasoedova V.A., Alfieri V., Orekhov A.N. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci. 2020;21:1835. doi: 10.3390/ijms21051835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsen C.-M., Liu J.-H., Yang D.-P., Chao H.-R., Chen J.-L., Chou W.-C., Ho Y.-C., Chuang C.-Y. Study on the correlation of bisphenol A exposure, pro-inflammatory gene expression, and C-reactive protein with potential cardiovascular disease symptoms in young adults. Environ. Sci. Pollut. Res. Int. 2021;28:32580–32591. doi: 10.1007/s11356-021-12805-0. [DOI] [PubMed] [Google Scholar]
- 82.Oluranti O.I., Alabi B.A., Michael O.S., Ojo A.O., Fatokun B.P. Rutin prevents cardiac oxidative stress and inflammation induced by bisphenol A and dibutyl phthalate exposure via NRF-2/NF-κB pathway. Life Sci. 2021;284 doi: 10.1016/j.lfs.2021.119878. [DOI] [PubMed] [Google Scholar]
- 83.Xiong Q., Liu X., Shen Y., Yu P., Chen S., Hu J., Yu J., Li J., Wang H.-S., Cheng X., Hong K. Elevated serum Bisphenol A level in patients with dilated cardiomyopathy. Int. J. Environ. Res. Publ. Health. 2015;12:5329–5337. doi: 10.3390/ijerph120505329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J., Liao M., Huang R., You Y., Lin X., Yang H., Fan L., Zhong Y., Li X., Li J., Xiao X. Perinatal combinational exposure to bisphenol A and a high-fat diet contributes to transgenerational dysregulation of cardiovascular and metabolic systems in mice. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.834346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sivashanmugam P., Mullainadhan V., Karundevi B. Dose-dependent effect of Bisphenol-A on insulin signaling molecules in cardiac muscle of adult male rat. Chem. Biol. Interact. 2017;266:10–16. doi: 10.1016/j.cbi.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 86.Wu B., Zhao Q., Li Z., Min Z., Shi M., Nie X., He Q., Gui R. Environmental level bisphenol A accelerates alterations of the reno-cardiac axis by the MAPK cascades in male diabetic rats: an analysis based on transcriptomic profiling and bioinformatics. Environ. Pollut. 2021;287 doi: 10.1016/j.envpol.2021.117671. [DOI] [PubMed] [Google Scholar]
- 87.Liguori F., Moreno-Marrodan C., Barbaro P. Biomass-derived chemical substitutes for bisphenol A: recent advancements in catalytic synthesis. Chem. Soc. Rev. 2020 Sep 1;49(17):6329–6363. doi: 10.1039/d0cs00179a. PMID: 32749443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.