Abstract
Autoimmunity associated with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been well-described as the mechanism of development of thyroid dysfunction following Coronavirus Disease 19 (COVID-19) infection and SARS-CoV-2 vaccination. However, the occurrence of thyroid eye disease (TED) after SARS-CoV-2 vaccination is scarcely described. The postulated mechanisms include immune reactivation, molecular mimicry and the autoimmune/inflammatory syndrome induced by adjuvants (ASIA). We report a case of new-onset TED after receiving the SARS-CoV-2 vaccine.
Keywords: thyroid eye disease, SARS-CoV-2 vaccine, radioactive iodine therapy, autoimmune/inflammatory syndrome induced by adjuvants, molecular mimicry
INTRODUCTION
Since COVID-19 struck the world, as of December 12, 2022, there have been 645,084,824 people affected, and it has claimed more than 6 million lives.1 SARS-CoV-2, the virus responsible for the disease, has been shown to cause a multitude of systemic disorders including immune dysregulation, such as autoimmune thyroiditis or Graves’ disease (GD).2 To address the COVID-19 pandemic, vaccination against COVID-19 was started in December 2020, and an estimated total of 13 billion doses have been administered by the end of 2022.1 While COVID-19 vaccination has successfully reduced the number of cases and the severity of the disease, there have been many cases of new-onset or relapse of GD and subacute thyroiditis following COVID-19 vaccination reported.3-11 However, there are limited reports of thyroid eye disease (TED) after COVID-19 vaccination. We describe a patient with underlying GD who developed TED three weeks after injection of BNT162b2 (Pfizer-BioNTech) mRNA COVID-19 vaccine.
CASE
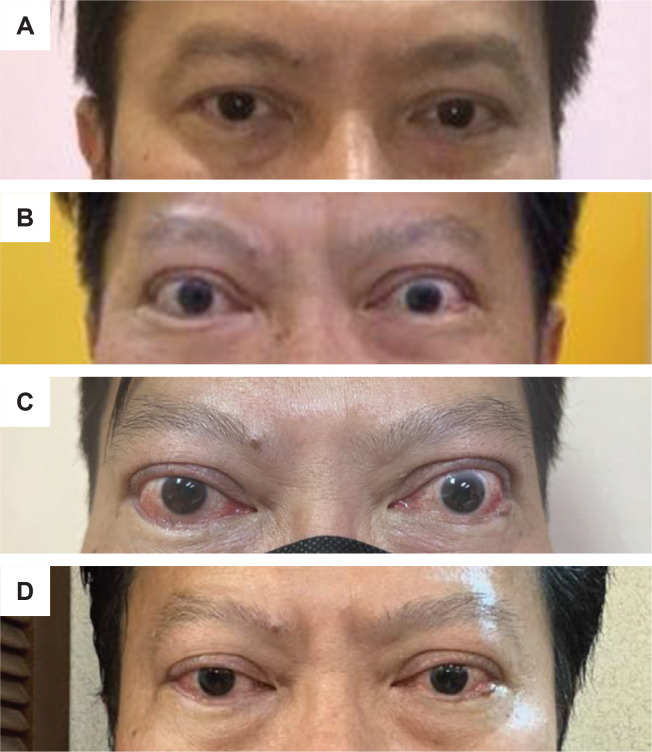
A 54-year-old, non-smoking, Chinese male with underlying Kallman Syndrome and Type 2 Diabetes Mellitus (T2DM) was diagnosed with GD without TED in 2003. He was given carbimazole for three years and achieved remission in 2006. He had relapsed with subclinical hyperthyroidism after 11 years in April 2017 with a thyroid stimulating hormone (TSH) level of <0.01 mIU/L (0.35-4.94) and a free T4 (fT4) level of 18.8 pmol/L (9-19.05). As a result, carbimazole was restarted but discontinued a year later. He remained clinically and biochemically euthyroid until June 2019 when he became overtly hyperthyroid again with a suppressed TSH of <0.01 mIU/L and elevated fT4 of 29.73 pmol/L. He subsequently underwent radioactive iodine (RAI) therapy in September 2020. Two months later, he developed hypothyroidism and was started on levothyroxine replacement. He achieved euthyroidism with levothyroxine 150 mcg daily in June 2021 (7 months after levothyroxine replacement therapy initiation) with TSH of 0.36 mIU/L (0.35-4.94) and fT4 of 11.29 pmol/L (9-19.05). His baseline photograph prior to the vaccine is seen in Figure 1A.
Figure 1.
(A) Patient’s photography (taken with the patient’s cellphone) in April 2021 before BNT162b2 mRNA COVID-19 vaccine indicating no obvious sign of clinical thyroid eye disease. (B) Patient’s photograph 3 weeks after the second dose of the vaccine indicating exophthalmos, eyelid swelling and upper eyelid retraction. (C) Clinical photograph of the patient at presentation in March 2022, demonstrating bilateral exophthalmos, chemosis, conjunctival injection, upper eyelid retraction, swollen eyelids and caruncles. (D) Patient’s photography after third dose of IV methylprednisolone indicating marked improvement of the eye signs with no eyelid and caruncles swelling and chemosis, albeit with mild conjunctival injection.
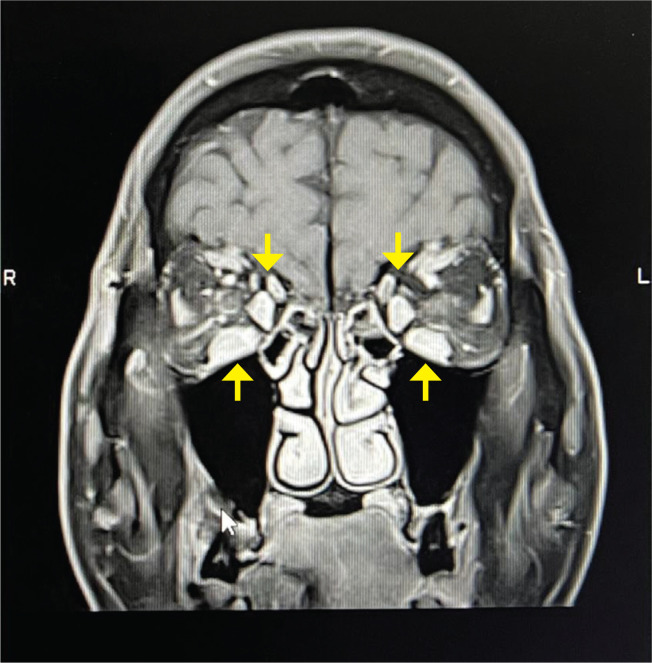
On the months of July and August 2021, he received his first and second doses of BNT162b2 (Pfizer-BioNtech) mRNA COVID-19 vaccine, respectively. Three weeks after receiving the second dose of the vaccine, he experienced new-onset bilateral eyes redness, dryness, proptosis and diplopia, which were gradually worsening (Figure 1B). He has never contracted COVID-19 before the reactivation of the hyperthyroidism to the time of RAI therapy and before the onset of TED. There were also no other acute infections or recent surgery. He sought treatment at a private ophthalmology clinic in November 2021 when his eye condition became worse. He underwent magnetic resonance imaging (MRI) of the orbits which showed bilateral extraocular muscle enlargement, especially of the inferior and medial rectus muscle (Figure 2) and proptosis. He was managed as TED for which he was given weekly intravenous (IV) methylprednisolone (MTP) 500 mg for 4 weeks, followed by 750 mg weekly for 2 weeks. His TED improved after the treatment. Unfortunately, he defaulted to subsequent follow-up.
Figure 2.
Coronal MRI of the orbits demonstrating bilateral extraocular muscle enlargement especially the inferior and medial rectus muscles (yellow arrows) consistent with thyroid eye disease.
In March 2022, his TED worsened, presenting with bilateral exophthalmos, chemosis, conjunctival injection, swollen eyelids and caruncles (Figure 1C). He was assessed to have active moderate-to-severe TED with a clinical activity score (CAS) of 4 out of 7. On further ophthalmologic assessment, his vision was intact, but there was diplopia on the upward gaze and secondary ocular hypertension. He had normal TSH, fT4 and fT3 levels (0.46 mIU/L [0.35-4.94], 16.47 pmol/L [9-19.05] and 4.5 pmol/L [2.6-5.7], respectively). However, the TSH-receptor antibodies (TRAb) level was elevated at 3.60 IU/L (<1.75) and anti-thyroid peroxidase (TPO) antibodies of >600 IU/ml (0-34). Unfortunately, there were no baseline auto-antibody tests for comparison. Intravenous MTP 500 mg was restarted and given weekly for 6 doses, followed by 200 mg weekly for another 6 doses. Azathioprine was started simultaneously. Congestive eye symptoms and diplopia significantly improved after the third dose of MTP (Figure 1D), with a CAS of 1 out of 10 attributable to mild conjunctival injection.
DISCUSSION
Thyroid eye disease (TED) is one of the extrathyroidal manifestations of autoimmune thyroid disease resulting from an autoimmune and inflammatory process. It is relatively rare, with females more commonly affected than males, and moderate-to-severe forms accounting 5 to 6% of cases.12 Risk factors for developing TED include smoking, thyroid dysfunction, high serum level of thyrotropin receptor antibodies, RAI treatment, and hypercholesterolemia.12 While there are limited data comparing prevalence rates of GD and TED among different ethnic groups within populations, a meta-analysis and systematic review by Chin et al., reported that the pooled prevalence for thyroid eye disease was 44% in Asia, 38% in Europe and 27% in North America.13
Since the start of the COVID-19 pandemic, SARS-CoV-2-related thyroiditis has been increasingly recognized. Lui et al., reported that 15% of patients with mild to moderate COVID-19 had thyroid dysfunction, and SARS-CoV-2 could potentially exacerbate pre-existing autoimmune thyroid disease.14 There were also numerous reported cases of thyroid dysfunction due to new-onset or relapse of GD or subacute thyroiditis following the SARS-CoV-2 vaccination described in recent literature.3-11
In contrast, TED after SARS-CoV-2 vaccination is rare. To date, there have been 16 cases of reactivation or new-onset TED after SARS-CoV-2 vaccination reported.10,15-20 A summary table (Table 1) is presented comparing the patient characteristics, clinical presentation and treatments of other similar post-vaccination TED patients together with the index patient of this report.
Table 1.
Reported cases of thyroid eye disease after COVID-19 vaccination
| Country | Age / Sex | Smoking status | Type of vaccine/dose | Post-vaccination symptoms onset | History of GD / TED | Pre-vaccination thyroid status | History of RAI | History of total thyroidectomy | Laboratory test results at diagnosis | CAS | Severity of TED | Rx for TED | Symptoms improvement | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSH (RR) | fT4 (RR) | fT3 (RR) | TRAb (RR) | TSI (RR) | ||||||||||||||
| Our case | Malaysia | 54 / male | N | mRNA Pfizer / 2nd | 3 weeks | Y / N | Euthyroid with LT | Y (1 year ago) | N | 0.46 (0.35-4.94 mIU/L) |
16.47 (9-19.05 pmol/L) |
4.5 (2.6-5.7 pmol/L) |
3.6 (<1.75 IU/L) |
NA | 4/7 | Moderate-to-severe | MTP | Yes (after 3rd infusion) |
| Case 110 | United States | 51 / female | N | mRNA Pfizer / 2nd | 4 days | N / N | Euthyroid | N | N | <0.01 (0.27-4.2 mIU/L) |
3.72 (0.93-1.7 ng/dL) |
12.6 (2-4.4 ng/dL) |
5.04 (<1.5 IU/L) |
NA | 3/7 | Mild | Thyroidectomy | Yes |
| Case 215 | United States | 50 / female | N | mRNA Pfizer / 2nd | 3 days | Y / N | Euthyroid with LT | Y (12 years ago) | N | Normal | Normal | Normal | NA | 2.29 (<0.55 IU/L) |
5/7 | Moderate-to-severe | Teprotumumab | Yes (after 2nd infusion) |
| Case 316 | United States | 66 / female | N | mRNA Moderna / 2nd | 3 weeks | Y / Y* | Euthyroid with LT | Y (15 years ago) | N | 0.04 (0.3-5.0 uIU/mL |
1.7 (0.7-1.7 ng/dL) |
NA | 5.51 (<1.5 IU/L) |
3.91 (<0.55 IU/L) |
6/10 | Moderate-to-severe | Teprotumumab | Yes (at 5 months) |
| Case 416 | United States | 53 / female | N | mRNA Pfizer / 1st | 1 day | N / N | Euthyroid | N | N | 0.99 uIU/mL (0.3-5.0 uIU/mL |
0.9 (0.7-1.7 ng/dL) |
NA | NA | 3.21 (<0.55 IU/L) |
NA | Moderate-to-severe | Teprotumumab | Yes (at 8 months) |
| Case 516 | United States | 45 / female | N | mRNA Moderna / 1st | 3 weeks | Hashimoto / Y* | Euthyroid with LT | N | N | Abnormal | Abnormal | NA | NA | NA | NA | Mild-to-moderate | No Rx | Yes |
| Case 617 | Italy | 58 / female | NA | mRNA Pfizer / 2nd | 3 days | Y / Y* | Euthyroid with LT | Y (2 years ago) | N | 1.17 (0.4-4.00 mIU/L) |
1.26 (0.7-1.7 ng/dL) |
3.54 (2.7-5.7 ng/dL) |
6.82 (<1.5 IU/L) |
NA | 6/10 | Moderate-to-severe | Teprotumumab | NA |
| Case 717 | Italy | 43 / male | NA | mRNA Pfizer / 1st | 2 weeks | Y / Y* | Euthyroid with MMI | N | N | 2.316 (0.4-4.00 mIU/L) |
0.96 (0.7-1.7 ng/dL) |
3.4 (2.7-5.7 ng/dL) |
20.7 (<1.5 IU/L) |
NA | NA | Sight-threatening disease | NA | NA |
| Case 818 | United States | 51 / female | Former smoker | mRNA Moderna / 2nd | 2 weeks | Y / Y* | NA | NA | NA | NA | NA | NA | NA | NA | 9/10 | NA | Prednisolone, teprotumumab, orbital decompression | Yes (13 months after teprotumumab, 2 months after surgery) |
| Case 919 | United States | 50 / male | N | mRNA Pfizer / 2nd | 3 weeks | Y / Y* | Euthyroid with LT | N | Y | 2.3 mIU/L | NA | NA | NA | 4.46 (<1.75 IU/L) |
7/10 | Moderate-to-severe | MTP, tocilizumab and teprotumumab | Yes (after 3rd teprotumumab infusion) |
| Case 1019 | United States | 71 / female | N | mRNA Moderna / 2nd | 3 days | Hypothyroidism / N | Euthyroid with LT | N | N | Undetectable | 1.4 (0.93-1.70 ng/dL) |
3.9 (2.3-4.2 ng/dL) |
NA | 5.5 (≤1.3) | 4/7 | Moderate-to-severe, progressed to sight-threatening disease | MTP followed by teprotumumab | Yes (after 3rd teprotumumab infusion) |
| Case 1120 | France | 70 / female | NA | mRNA Pfizer / 2nd | 60 days | Y / Y* | Euthyroid with LT | N | Y | 1.65 mIU/L | 20 pmol/l | NA | >40 IU/L | NA | 4/7 | Moderate-to-severe | Prednisolone, tocilizumab | Yes (after 2 weeks of tocilizumab infusion) |
| Case 1220 | France | 43 / male | NA | mRNA Moderna / 1st | 1 day | Y / Y* | Mild hypothyroid with CBZ | N | N | 4.04 mIU/L | 6.2 pmol/l | NA | Absent | NA | 7/7 | Sight-threatening disease | Tocilizumab | Yes (after 1st infusion) |
| Case 1320 | France | 73 / male | NA | mRNA Pfizer / 1st | 21 days | Y / N | Euthyroid with CBZ | N | N | 2.4 mIU/L | NA | NA | Normal | NA | 3/7 | Mild | Selenium,MTP | Yes (after 1st infusion |
| Case 1420 | France | 45 / female | NA | mRNA Moderna / 2nd | NR | Y / Y* | Euthyroid with LT | N | Y | 0.76 mIU/L | NA | NA | 151 IU/L | NA | 4/7 | Moderate-to-severe | Lubricants | Yes (at 5 months) |
| Case 1520 | France | 48 / male | NA | mRNA Moderna / 2nd | 30 days | Y / Y* | NR | N | Y | <0.01 mIU/L | 21 pmol/l | NA | 28 IU/L | NA | 5/7 | Sight-threatening disease | MTP, orbital decompression, teprotumumab | Yes (after 1st infusion) |
| Case 1620 | France | 39 / female | NA | mRNA Pfizer / 1st | 7 days | N / N | Euthyroid | N | N | 0.3 mIU/L | NA | NA | 5 IU/L | NA | 2/7 | Mild | Selenium | Unchanged |
GD: Graves’ disease; TED: thyroid eye disease; TSH: thyroid-stimulating hormone; fT4: free thyroxine; fT3: free triiodothyronine; TRAb: TSH receptor antibody; TSI: thyroid stimulating immunoglobulin; CAS: Clinical activity score; MMI: methimazole; CBZ: carbimazole; LT: levothyroxine; MTP: Methylprednisolone; NA: not available; Y: yes; N: no; RR: reference range.
Stable disease after receiving Rx for TED
The mechanisms of developing TED were postulated to be similar to how GD occurs after vaccination. The BNT162b2 (Pfizer-BioNtech) mRNA COVID-19 vaccine induces spike protein-specific neutralizing antibodies associated with protective immunity.21 Vojdani et al., conducted a study in vivo and found that the SARS-CoV-2 spike protein, nucleoprotein, and membrane protein all cross-reacted with TPO, and many TPO peptide sequences shared homology or similarity with sequences in various SARS-CoV-2 proteins.22 These findings suggest that the SARS-CoV-2 vaccine may lead to the onset of autoimmune thyroid disease and TED via molecular mimicry between the SARS-CoV-2 spike proteins and thyroid proteins or TPO peptides.22 The antibodies against these viral targets may also cause thyroid tissue damage, leading to the release of further auto-antigens and the potential development of other auto-antibodies that may trigger TED, such as thyroid stimulating immunoglobulin (TSI), TRAb or anti-TPO.19,23,24 This could explain the mechanism for this patient case, and in the 12 other patients out of the 16 reported cases who did not undergo total thyroidectomy. The four patients who underwent total thyroidectomy might have remnant thyroid tissue that could have served as an immune target. Also, it is important to note that our patient had both elevated TRAb and anti-TPO, whereas most of the reported cases had only either raised TRAb or TSI alone.
Another postulated mechanism was autoimmune/inflammatory syndrome induced by adjuvants (ASIA). ASIA is an entity that incorporates diverse autoimmune conditions induced by exposure to various adjuvants that are found in many vaccines.25 Adjuvants are substances that can trigger autoimmunity via a variety of mechanisms, such as alteration of the host’s immune system, polyclonal activation of B cells, effects on cellular immunity, immunoregulatory cells, viral-induced antibodies and acceleration of molecular mimicry.25 These, in turn, lead to the promotion of inflammation through the activation of macrophages and fibroblasts, as well as the production of Th-1 cells and adipocyte differentiation, which are similar mechanisms seen in TED.26 In mRNA vaccines, polyethylene glycol (PEG) in conjugation with lipid nanoparticles may act as an adjuvant to trigger an autoimmune reaction following SARS-CoV-2 vaccination. Coincidentally, the reported TED cases and our case were given mRNA vaccines.
Despite the myriad of individuals who received SARS-CoV-2 vaccination, autoimmune thyroid diseases, such as GD and TED, are still relatively rare or underreported. It is hypothesized that these autoimmune conditions may commonly occur in genetically susceptible individuals, where the T lymphocytes are excessively sensitized to the TSH receptor thereby causing GD or TED.20 It is also possible that epigenetic changes or alterations in the patient’s microbiome, such as those demonstrated following mRNA SARS-CoV-2 vaccination, may play a role in TED pathogenesis.19 Lastly, Sriwijitalai and Wiwanitkit suggested that vaccination-induced increase in blood viscosity is another possible pathophysiological process.27 Vaccines can significantly increase blood viscosity, and very high blood viscosity is associated with exophthalmos at a stable stage of hyperthyroidism.27
The time of the onset of TED after mRNA SARS-CoV-2 vaccination in the reported cases ranged from day 1 to day 60 following the first or second dose of vaccination (Table 1), whereas the symptoms onset of thyroid dysfunction ranged from 2 to 37 days after SARS-CoV-2 vaccination.10,11 In our case, the TED symptoms started manifesting after 3 weeks from the second SARS-CoV-2 vaccine and approximately one year after RAI treatment.
The study by Traisk et al., reported that RAI treatment is a significant risk factor for the development of TED in Graves’ hyperthyroidism.28 The proportion of worsening or development of TED after 1 year was 31% in patients who received RAI therapy compared to 16% who received methimazole.28 While the study by Kung et al., reported that the mean time for development or exacerbation of TED after RAI was 6.7 ± 2.2 months (range, l-15 months).29 The temporal relationship suggests that either the SARS-CoV-2 vaccine or RAI could be the triggering event in our patient or the presence of both might have amplified the autoimmune and inflammatory cascades leading to TED. It was also found that hypothyroidism with elevated TSH is an important adverse factor for the development or exacerbation of TED. The adjunctive use of methimazole after RAI was unable to prevent the development or exacerbation of TED.29 In our case, the patient has already achieved a euthyroid state at the time of TED manifestation. Other authors also reported that their patients had received RAI treatment several years before and were already euthyroid before the onset of TED.15-17
Ten of the reported TED cases had a history of being treated for TED and were stable prior to the administration of SARS-CoV-2 vaccine. This contrasts with the other cases which documented new-onset TED. Our patient received a total of 12 doses of IV MTP, which afforded significant improvement of the patient’s eye symptoms after the third dose of the second cycle. Azathioprine was added simultaneously during the second cycle of MTP. Azathioprine has been shown to reduce the relapse rate after glucocorticoid withdrawal.12 Among the other 16 reported cases, the clinical presentations ranged from mild, moderate-to-severe to sight-threatening disease, with the milder disease seen mostly in new-onset TED cases. Generally, most patients with mRNA SARS-CoV-2 vaccine-associated TED had a favorable response to teprotumumab, including patients with the sight-threatening disease.19,20 Three cases were given oral prednisolone, MTP and a combination of MTP and tocilizumab with a limited response, but symptoms improved favorably after teprotumumab treatment.18,19 While teprotumumab has been proven effective in TED treatment, its use is restricted by cost and availability, and long-term efficacy and safety data are still lacking.12
CONCLUSION
In conclusion, to the best of our knowledge, our patient is the first reported case of mRNA SARS-CoV-2 vaccine-associated TED reported in Asia. Although the temporal relationship of developing TED after COVID-19 vaccination might be suggestive, other possible factors may be contributory, such as prior RAI treatment in our case. While there is no cure for COVID-19 yet, the vaccines have been instrumental in its prevention and control. By and large, the benefits of the SARS-CoV-2 vaccine outweigh the risks. Patients with known autoimmune thyroid disease should be monitored closely and periodically after the SARS-CoV-2 vaccination as they might develop TED and require prompt treatment to alleviate the symptoms and signs. Finally, further studies are required to identify the potential mechanisms of new-onset or reactivation of TED following the administration of mRNA SARS-CoV-2 vaccines and to understand the possibility of ethnicity-related predisposition.
Acknowledgments
We thank the Dean of Hospital Canselor Tuanku Muhriz, UKMMC, for his permission to publish this article.
Ethical Consideration
Informed consent has been taken before submission of the manuscript.
Statement of Authorship
All authors certified fulfilment of ICMJE authorship criteria.
CRediT Author Statement
JHIT: Conceptualization, Methodology, Software, Validation, Investigation, Resources, Writing – original draft preparation, Writing – review and editing, Visualization, Project administration; NM: Conceptualization, Writing – review and editing, Supervision, Project administration; NW: Conceptualization, Writing – review and editing, Visualization, Supervision, Project administration.
Author Disclosure
The authors have declared no conflict of interest.
Funding Source
None.
References
- 1.World Health Organization . WHO coronavirus disease (COVID-19) dashboard. World Health Organization; 2022. https://covid19.who.int. Accessed December 13, 2022. [Google Scholar]
- 2.Murugan AK, Alzahrani AS. SARS-CoV-2 plays a pivotal role in inducing hyperthyroidism of Graves’ disease. Endocrine. 2021;73(2):243-54. PMID: PMCID: . 10.1007/s12020-021-02770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sánchez Valadez TI, Jara LJ. Two cases of Graves’ disease following SARS-CoV-2 vaccination: An autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021;31(9):1436-9. PMID: . 10.1089/thy.2021.0142. [DOI] [PubMed] [Google Scholar]
- 4.İremli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: Postvaccination ASIA syndrome. J Clin Endocrinol Metab. 2021;106(9):2600-5. PMID: . PMCID: . 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel KR, Cunnane ME, Deschler DG. SARS-CoV-2 vaccine-induced subacute thyroiditis. Am J Otolaryngol. 2021;43(1):103211. PMID: . PMCID: . 10.1016/j.amjoto.2021.103211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soltanpoor P, Norouzi G. Subacute thyroiditis following COVID-19 vaccination. Clin Case Rep. 2021;9(10):e04812. PMID: . PMCID: . 10.1002/ccr3.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lui DTW, Lee KK, Lee CH, Hung IFN, Tan KCB. Development of Graves’ Disease after SARS-CoV-2 mRNA vaccination: A case report and literature review. Front Public Health. 2021;9:778964. PMID: . PMCID: . 10.3389/fpubh.2021.778964.www.asean-endocrinejournal.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chua MWJ. Graves’ disease after COVID-19 vaccination. Ann Acad Med Singap. 2022;51(2):127-8. PMID: . 10.47102/annals-acadmedsg.2021398. [DOI] [PubMed] [Google Scholar]
- 9.Hamouche W, El Soufi Y, Alzaraq A, Okafor BV, Zhang F, Paras C. A case report of new onset graves’ disease induced by SARS-CoV-2 infection or vaccine? J Clin Transl Endocrinol Case Rep. 2022;23:100104. PMID: . PMCID: . 10.1016/j.jecr.2021.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bostan H, Ucan B, Kizilgul M, et al. Relapsed and newly diagnosed Graves’ disease due to immunization against COVID-19: A case series and review of the literature. J Autoimmun. 2022; 128:102809. PMID: . PMCID: . 10.1016/j.jaut.2022.102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jafarzadeh A, Nemati M, Jafarzadeh S, Nozari P, Mortazavi SMJ. Thyroid dysfunction following vaccination with COVID-19 vaccines: A basic review of the preliminary evidence. J Endocrinol Invest. 2022;45(10):1835-63. PMID: . PMCID: . 10.1007/s40618-022-01786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185(4):G43–67. PMID: . 10.1530/EJE-21-0479. [DOI] [PubMed] [Google Scholar]
- 13.Lui DTW, Lee CH, Chow WS, et al. Thyroid dysfunction in relation to immune profile, disease status and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab. 2021;106(2):e926-35. PMID: . PMCID: . 10.1210/clinem/dgaa813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin YH, Ng CH, Lee MH. Prevalence of thyroid eye disease in Graves’ disease: A meta-analysis and systematic review. Clin Endocrinol (Oxf). 2020;93(4):363-74. PMID: . 10.1111/cen.14296. [DOI] [PubMed] [Google Scholar]
- 15.Rubinstein TJ. Thyroid Eye Disease Following COVID-19 vaccine in a patient with a history Graves’ disease: A case report. Ophthalmic Plast Reconstr Surg. 2021;37(6): e221-3. PMID: . PMCID: . 10.1097/IOP.0000000000002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park KS, Fung SE, Ting M, et al. Thyroid eye disease reactivation associated with COVID-19 vaccination. Taiwan J Ophthalmol. 2022;12(1):93-6. PMID: . PMCID: . 10.4103/tjo.tjo_61_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrizio A, Ferrari SM, Antonelli A, Fallahi P. Worsening of Graves’ ophthalmopathy after SARS-CoV-2 mRNA vaccination. Autoimmun Rev. 2022; 21(7):103096. PMID: . PMCID: . 10.1016/j.autrev.2022.103096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng OT, Schlachter DM. Teprotumumab in advanced reactivated thyroid eye disease. Am. J. Ophthalmol Case Rep. 2022;26:101484. PMID: . PMCID: . 10.1016/j.ajoc.2022.101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed A, Tzoulis P, Kossler AL, Dosiou C. New onset or deterioration of thyroid eye disease after mRNA SARS-CoV-2 vaccines: Report of 2 cases and literature review. J Clin Endocrinol Metab. 2022:dgac606. PMID: . PMCID: . 10.1210/clinem/dgac606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abeillon-du Payrat J, Grunenwald S, Gall E, Ladsous M, Raingeard I, Caron P. Graves' orbitopathy post-SARS-CoV-2 vaccines: Report on six patients. J Endocrinol Invest. 2022:1-11. PMID: . PMCID: . 10.1007/s40618-022-01955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jalkanen P, Kolehmainen P, Häkkinen HK. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 2021;12(1):3991. PMID: . PMCID: . 10.1038/s41467-021-24285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: Implications for autoimmune diseases. Front Immunol. 2021;11:617089. PMID: . PMCID: . 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLachlan SM, Nagayama Y, Pichurin PN, et al. The link between Graves’ disease and Hashimoto’s thyroiditis: A role for regulatory T cells. Endocrinology. 2007;148(12):5724-33. PMID: . 10.1210/en.2007-1024. [DOI] [PubMed] [Google Scholar]
- 24.Fröhlich E, Wahl R. Thyroid autoimmunity: Role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521. PMID: . PMCID: . 10.3389/fimmu.2017.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watad A, David P, Brown S, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front Endocrinol. 2017;7:150. PMID: . PMCID: . 10.3389/fendo.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor PN, Zhang L, Lee RWJ, et al. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat Rev Endocrinol. 2020;16(2):104-16. PMID: . 10.1038/s41574-019-0305-4. [DOI] [PubMed] [Google Scholar]
- 27.Sriwijitalai W, Wiwanitkit V. Re: “Thyroid eye disease following COVID-19 vaccine in a patient with a history Graves’ disease: A case report.” Ophthalmic Plast Reconstr Surg. 2022;38(1):95. PMID: . PMCID: . 10.1097/IOP.0000000000002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Träisk F, Tallstedt L, Abraham-Nording M, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94(10):3700–7. PMID: . 10.1210/jc.2009-0747. [DOI] [PubMed] [Google Scholar]
- 29.Kung AWC, Yau CC, Cheng A. The incidence of ophthalmopathy after radioiodine therapy for Graves’ disease: Prognostic factors and the role of methimazole. J Clin Endocrinol Metab. 1994; 79(2):542-6. PMID: . 10.1210/jcem.79.2.7913934. [DOI] [PubMed] [Google Scholar]