Abstract
We studied the combined anti-human immunodeficiency virus type 1 (HIV-1) effects of a derivative of stroma-derived factor 1β (SDF-1β), Met-SDF-1β, and a modified form of RANTES, aminooxypentane (AOP)-RANTES. The antiviral agents were tested singly or in combination at 95 and 99% virus inhibitory concentrations. Clinical R5 and X4 HIV-1 isolates were used. AOP-RANTES inhibited R5 but not X4 viruses, whereas Met-SDF-1β had the opposite effect. Combinations of these compounds inhibited mixed infections with R5 and X4 viruses (95 to 99%), whereas single drugs were less inhibitory (32 to 61%). Combinations of R5 and X4 inhibitors are promising and deserve further evaluation.
In 1995, Cocchi et al. reported potent in vitro human immunodeficiency virus type 1 (HIV-1) inhibition by three chemokines secreted by CD8+ T lymphocytes (4) and focused attention on this class of molecules with low molecular masses (8 to 12 kDa). The chemokines described, RANTES, macrophage inflammatory protein-1α (MIP-1α), and MIP-1β, belong to the group of C-C chemokines that block HIV-1 entry into cells (5).
Relationships among membrane coreceptors, chemokines, and cellular tropism were further defined in 1996 by Feng et al., who described a novel molecule which acted as a cofactor for T-cell-tropic HIV-1 isolates but not for macrophage-tropic isolates (14). This receptor, which was already known but did not have an identified natural ligand, belongs to the C-X-C chemokine receptor superfamily and was named “fusin,” or CXCR4. The receptor for HIV-1 macrophage-tropic isolates was subsequently identified and named C-C chemokine receptor 5 (CCR5). CCR5 reacts with the chemokines RANTES, MIP-1α, and MIP-1β (9, 12). The natural ligand for CXCR4 is stroma derived factor-1 (SDF-1), an α-chemokine with chemotactic properties for T lymphocytes and a developmental role in B lymphocyte maturation (2, 25).
During the early phases of HIV-1 infection, R5 viral strains usually predominate, whereas X4 strains frequently emerge in the late stages of HIV-1 infection, accompanied by a decline in peripheral blood CD4 lymphocytes and a clinical progression toward AIDS (7, 34). Viral isolates with a dual tropism could represent a transition phase between viral R5 and X4 phenotypes, or they may represent X4 viruses that have maintained an ability to infect macrophages (31, 33).
The aim of our study was to evaluate the interactions between attachment and entry inhibitors of HIV-1 infection. Our experiments suggest that the use of combined inhibitors of R5 and X4 viruses may be useful in inhibiting mixed infections.
(This work was presented in part at the 7th Conference on Retroviruses and Opportunistic Infections, San Francisco, Calif., 30 January to 2 February, 2000 [S. Rusconi, S. La Seta Catamancio, P. Citterio, E. Bulgheroni, F. Croce, S. H. Herrmann, R. E. Offord, M. Galli, and M. S. Hirsch, Abstr. 7th Conf. Retroviruses Opportunistic Infect., abstr. 501, 2000].)
Analysis of cellular tropism of the different viral isolates on transformed cell lines.
The two viral isolates examined in this study, RM and DK, were derived from two patients with primary HIV-1 infection acute syndrome (29), and the isolates were used to infect U87MG-transformed CD4+ cells transfected with CCR5 or CXCR4 coreceptors (8), provided by Dan R. Littman (The Skirball Institute of Biomolecular Medicine, New York University School of Medicine, New York, N.Y.). At day 7 of culture, the production of HIV-1 p24 antigen indicated that RM replicated in CCR5-transfected cells (p24 concentration, 1.9 ng/ml), whereas DK replicated in CXCR4-transfected cells (>10 ng/ml). RM did not replicate in CXCR4 cells and DK did not replicate in CCR5 cells.
Combination experiments with AOP-RANTES and Met-SDF-1β in PBMC.
The inhibitory activities of aminooxypentane (AOP)-RANTES and Met-SDF-1β were evaluated singly or in combination against infections with a single HIV-1 isolate or a mixture of the two isolates at a 50:50 ratio. Met-SDF-1β (lot no. 4488-208) was provided by Genetics Institute (Cambridge, Mass.) and had the sequence MKPV at the amino terminus (35). AOP-RANTES was provided by Gryphon Sciences, South San Francisco, Calif. (32). Susceptibilities to Met-SDF-1β and AOP-RANTES were determined in phytohemagglutinin-P-stimulated peripheral blood mononuclear cells (PBMC) by using a fixed amount of infectious virus (1,000 50% tissue culture infective doses [TCID50s]) and a multiplicity of infection of 0.01 TCID50/cell. Cell cultures were either drug free (control wells) or pretreated with four different Met-SDF-1β or AOP-RANTES concentrations in duplicate wells. Met-SDF-1β was used at concentrations ranging from 0.35 to 2.80 μg/ml, and AOP-RANTES was used from 5 to 40 ng/ml. Antiviral effects were tested at the 95 and 99% inhibitory concentrations (IC95 and IC99) for each drug. IC95s were 0.41 μg/ml for Met-SDF-1β and 38 ng/ml for AOP-RANTES, whereas IC99s were 1.75 μg/ml for Met-SDF-1β and 67.68 ng/ml for AOP-RANTES. Each combination experiment was conducted once with compounds at their IC95s and twice with compounds at their IC99s. HIV-1 inhibition was achieved when a single viral isolate was targeted by its specific single attachment inhibitor and when both antiviral agents were combined for the treatment of infections by mixtures of the two isolates, RM and DK (Table 1). Suppression of individual viral replication was 75 to 99% during combination experiments using the IC95 or IC99 of individual agents. In combination experiments using two inhibitors against both viruses, the inhibition varied between 95 and 99%. When a single agent was used in the presence of both X4 and R5 isolates, viral inhibition was less effective (32 to 61% for AOP-RANTES and 45 to 49% for Met-SDF-1β).
TABLE 1.
Analysis of therapeutic combinations of AOP-RANTES and Met-SDF-1β at IC95 and IC99 in PBMC
| Well no. | Drug condition | Viral isolate(s) | IC95 drug exposure
|
IC99 drug exposure
|
||
|---|---|---|---|---|---|---|
| HIV-1 p24 production (ng/ml) | % Inhibition | HIV-1 p24 production (ng/ml) | % Inhibition | |||
| 1 | Infected control | DK | 60.2 | 87.9 | ||
| 2 | 60.5 | 102.0 | ||||
| 3 | Infected control | RM | 70 | 63.4 | ||
| 4 | 60 | 51.3 | ||||
| 5 | Infected control | DK + RM | 136 | 120.4 | ||
| 6 | 130.4 | 115.1 | ||||
| 7 | AOP-RANTES | RM | 6 | 91 | 15.5 | 75 |
| 8 | 7 | 89 | 12.2 | 82 | ||
| 9 | Met-SDF-1β | DK | 0.4 | 99 | 3.7 | 99 |
| 10 | 0.4 | 99 | 6.3 | 99 | ||
| 11 | AOP-RANTES + Met-SDF-1β | DK + RM | 6.2 | 95 | 8.7 | 99 |
| 12 | 6 | 95 | 8.6 | 99 | ||
| 13 | AOP-RANTES | DK + RM | 83.3 | 37 | 66.5 | 46 |
| 14 | 97.7 | 27 | 23.9 | 85 | ||
| 15 | Met-SDF-1β | DK + RM | 69.4 | 48 | 63.2 | 49 |
| 16 | 75.5 | 43 | 30.6 | 79 | ||
Passage of PBMC supernatant fluids onto CCR5- and CXCR4-transformed cells.
The supernatant fluids from PBMC cultures infected with either DK or RM viruses with or without their inhibitors, as described above, were passaged in astroglioma U87MG-transformed CD4+ cells expressing either CXCR4 or CCR5, with 0.5 ml of culture supernatant fluids. Subcultures were maintained until day 7, and cytopathic effects were observed by day 4. Similar replication kinetics were observed for the two viruses in cells bearing the appropriate receptor. The expected patterns of virus inhibition by individual receptor inhibitors, or their combination, were observed, with the most complete inhibition of mixed infection of both cell types being observed when dual inhibitor IC99s had been used in PBMC. In that situation, viral cytopathic effects were not observed and HIV-1 p24 antigen production was minimal at day 7 (<0.1 to 3.1 ng/ml).
Analysis of the V3 region amino acid sequences of RM and DK.
RM and DK viruses were amplified by a two-step PCR. The first step of the PCR was carried out with the primers PSA (5′-TACAATGTACACATGGAATT-3′) and PSD (5′-ATTACAGTAGAAAAATTCC-3′), and the second step was carried out with the primers PSB (5′-TGGCAGTCTAGCAGAAGAAG-3′) and PSC (5′-TCTGGGTCCCCTCCTGAGGA-5′). The V3 loop was sequenced using an ABI 377A automatic sequencer (Perkin-Elmer, Applied Biosystem Inc., Foster City, Calif.). The sequences were identified and analyzed by the Navigator and Factura DNA analysis software package (Perkin-Elmer). The results were compared to the sequences of the prototypic strains Ba-L, MN, and 89.6, with known macrophage-tropic, T-cell-tropic, and dually tropic phenotypes, respectively (6, 16, 17).
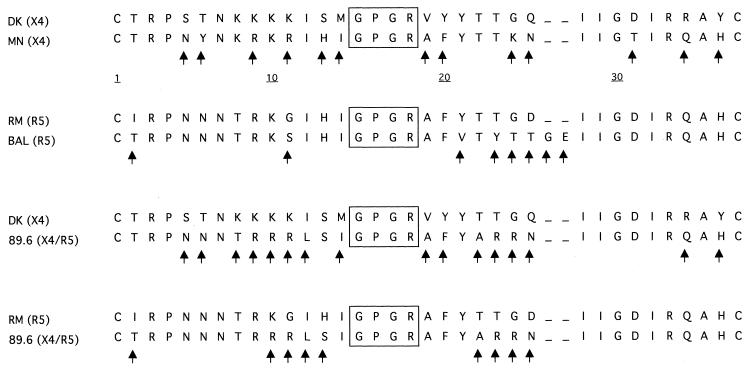
The RM virus had a sequence similar to the prototypic R5 strain Ba-L (identity for 27 of 35 amino acids), whereas the DK isolate resembled the prototypic X4 virus MN (identity for 22 of 35 amino acids). Data are shown in Fig. 1. Both viruses, RM and DK, had conserved regions in the central portion of the V3 domain, namely the GPGR region (15, 26), and were analogous to the other strains used in the comparison.
FIG. 1.
Alignment between amino acid sequences in the V3 loop region of parental HIV-1 isolates. Arrows indicate discordance between the two sequences. Conserved regions are boxed. BAL virus shows an insertion of two amino acids in position 25. The receptor used is indicated in parentheses.
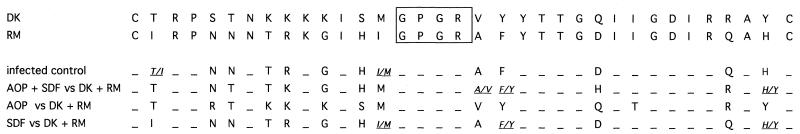
The proviral DNA of PBMC infected with both HIV-1 isolates RM and DK for up to 7 days in the presence or absence of AOP-RANTES and Met-SDF-1β was amplified, and V3 loops were sequenced. Figure 2 shows that in the absence of drug pressure, RM viral sequences predominated. In the presence of AOP-RANTES alone, DK sequences were exclusively observed, whereas when Met-SDF-1β was used alone, RM sequences predominated. When both drugs were used together, sequence mixtures were observed.
FIG. 2.
Direct sequencing of competitive growth experiments. The amino acid mixtures are indicated by italic characters. The amino acids inside the box correspond to a conserved region at the center of the V3 loop. AOP, AOP-RANTES; SDF, Met-SDF-1β.
Molecular clones derived from experiments in the presence of AOP-RANTES and Met-SDF-1β.
To delineate the role of individual strains in mixed viral infections, PCR products corresponding to the V3 loop sequence were also cloned and single clones were sequenced. PCR fragments of the V3 loop from proviral DNA were cloned in the pGEM-T Easy Vector (Promega). JM109 cells were transformed with ligation mixtures and plated onto Luria-Bertani agar with ampicillin and 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (X-Gal). Colonies with the insert were reamplified with the primers PSB and PSC and were used for DNA sequencing after PCR purification. The consensus sequence derived from the single clones was identical to the corresponding proviral sequence.
As shown in Table 2, we observed a predominance of RM over DK (9 RM clones out of 10 total clones) without drug pressure; in the presence of both drugs, 3 clones showed a sequence identical to RM, whereas 7 were identical to DK. Under the other experimental conditions, i.e., both viruses in the presence of one of the inhibitors, the results were as expected: when AOP-RANTES was added to the cells, the obtained virus was only DK (10 of 10 clones), whereas when Met-SDF-1β was used, we observed only RM virus (10 of 10 clones).
TABLE 2.
Molecular clones derived from experiments of competitive growtha
| Well no.b | Drug(s) used | No. of clones obtained from IC99 exposure exptsc |
|---|---|---|
| 5 and 6 | None (infected control) | 9 RM + 1 DK |
| 11 and 12 | AOP-RANTES + Met-SDF-1β | 3 RM + 7 DK |
| 13 and 14 | AOP-RANTES | 10 DK |
| 15 and 16 | Met-SDF-1β | 10 RM |
PCR fragments of V3 loop from proviral DNA were cloned, reamplified, and used for DNA sequencing.
For each drug or drug combination, duplicate wells with mixed DK-RM infection were used.
Of 10 total clones.
Need for new targets and combinations.
Although current antiretroviral regimens have resulted in a dramatic reduction of HIV-1-associated mortality and morbidity, drug resistance is an emerging problem that will complicate these efforts (19). New and more potent regimens are needed, particularly those that target untapped HIV-1 replication sites. Chemokine receptors play important roles as coreceptors for HIV-1 entry into host cells (2, 14, 25). The discovery that chemokine receptors are coreceptors for HIV-1 has allowed the development of novel antiviral approaches, including specific chemokine-receptor antagonists (reviewed in references 3 and 27).
The process of HIV-1 attachment and entry involves several sequential steps involving gp120 binding to CD4 and the chemokine receptors CXCR4 or CCR5, followed by fusion of viral gp41 to the cell membrane. Early infection is often mediated by R5 viruses, and there is often an R5-to-X4 receptor shift during clinical deterioration to AIDS (7, 34). The archive of viruses carried by an HIV-1-infected individual may include both R5 and X4 viruses, suggesting that both cellular targets should be considered for strategic antiviral interventions.
Among C-C chemokines, RANTES was demonstrated to use different cellular receptors, including CCR5 (18). Modification of the amino terminus of RANTES led to the identification of two compounds, Met-RANTES and AOP-RANTES, both of which exhibit a potent and selective receptor antagonism (28, 32). AOP-RANTES activity results in downregulation of the coreceptor due to the inhibition of recycling from the cytoplasm to the cell membrane (21). With regard to CXCR4 ligands, SDF-1α differs from SDF-1β in that SDF-1α lacks the last four amino acid residues of the latter (2, 25, 30). These molecules probably inhibit cell entry by X4 HIV-1 strains through coreceptor competition. Some studies have focused on modified analogues of SDF-1 as receptor antagonists and as inhibitors of viral entry (22, 35).
Given the broad repertoire of viral isolates harbored by an infected individual, single agents directed at a unique coreceptor may select for virus with an alternative receptor tropism (13, 23; D. Schols, G. Bridger, G. Henson, and E. De Clercq, Abstr. 7th Conf. Retroviruses Opportunistic Infect., abstr. S18, 2000). Hence, there is ample rationale for dual attack against both CXCR4 and CCR5 receptors.
Our study demonstrates that both coreceptor blockers are necessary in order to attain profound HIV-1 inhibition when viruses with mixed tropism are present. We conducted experiments utilizing single or combined drugs against infection with R5 or X4 viruses or against mixed infection with these viruses. AOP-RANTES inhibited R5 but not X4 viruses, whereas Met-SDF-1β inhibited X4 but not R5 viruses. The combination of AOP-RANTES and Met-SDF-1β inhibited dual infections with R5 and X4 viruses (95 to 99%), whereas single drugs suppressed dual infections less well (32 to 61%). Sequencing and cloning studies showed the presence of both viral genomes in supernatant fluids from mixed virus cultures in the absence of drug pressure. In the presence of specific inhibitors, viruses with alternative tropisms were cloned and sequenced from culture supernatant fluids.
Our observations establish the proof of principle for a dual-receptor attack against HIV-1 in vitro and suggest the future clinical development of such a strategy. However, it is only through carefully designed and conducted therapeutic trials that the clinical validity of these in vitro studies can be determined. The potential pharmacological problems with this class of compounds, as well as their possible systemic toxicities, must also be considered in further clinical development.
Although our study utilized specific peptide antagonists of chemokine receptors, there is a need for the development of small-molecule, orally bioavailable inhibitors directed against either X4 or R5 viruses. Some of these have already been developed (1, 10, 11, 24; B. M. Baroudy, Abstr. 7th Conf. Retroviruses Opportunistic Infect., abstr. S17, 2000). One recent report (C. Tremblay, C. Kollman, F. Giguel, T. C. Chou, and M. S. Hirsch, Abstr. 7th Conf. Retroviruses Opportunistic Infect., abstr. 500, 2000) described strong antiviral synergy between one small X4-inhibitory molecule, AMD3100 (10), and the gp41-specific inhibitor T-20 (20), providing further support for the concept of combination therapy targeting HIV-1 attachment and entry.
Nucleotide sequence accession numbers.
The V3 loop sequences identified in this study were submitted to GenBank and assigned accession numbers AF234190 through AF234233, AF234234 (RM), and AF234235 (DK).
Acknowledgments
We thank Bianca M. Ghisi for editorial assistance and Elizabeth L. Kaplan for continuous support.
We acknowledge the financial support of Progetto Terapia Antiretrovirale, Istituto Superiore di Sanità (Rome, Italy), grant 1997 no. 30A.0.43, and NIH grant CA 12464. S.R. was supported by a NATO-CNR senior fellowship (218.1861).
Footnotes
This paper is dedicated to the memory of Alessandro Caporali.
REFERENCES
- 1.Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, Shiraishi M, Aramaki Y, Okonogi K, Ogawa Y, Meguro K, Fujino M. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 3.Cammack N. Human immunodeficiency virus type 1 entry and chemokine receptors: a new therapeutic target. Antivir Chem Chemother. 1999;10:53–62. doi: 10.1177/095632029901000201. [DOI] [PubMed] [Google Scholar]
- 4.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-1-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 5.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 6.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connors R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis C B, Dikic I, Unutmaz D, Hill C M, Arthos J, Siani M A, Thompson D A, Schlessinger J, Littman D R. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;318:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 10.Donzella G A, Schols D, Lin S W, Esté J A, Nagashima K A, Maddon P J, Allaway G P, Sakmar T P, Henson G, De Clercq E, Moore J P. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 11.Doranz B, Grovit-Ferbas K, Sharron M, Mao S, Goetz M, Daar E, Doms R, O'Brien W. A small molecule inhibitor directed against chemokine receptor CXCR4 prevents its use as an HIV-1 coreceptor. J Exp Med. 1997;186:1395–1400. doi: 10.1084/jem.186.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 13.Esté J A, Cabrera C, Blanco J, Gutierrez A, Bridger G, Henson G, Clotet B, De Clercq E. Shift of clinical human immunodeficiency virus type 1 isolates from X4 to R5 and prevention of emergence of the syncytium-inducing phenotype by blockade of CXCR4. J Virol. 1999;73:5577–5585. doi: 10.1128/jvi.73.7.5577-5585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 15.Fouchier R A M, Groenink M, Kootstra N A, Tersmette M, Huisman H G, Miedema F, Schuitemaker H. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J Virol. 1992;66:3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 17.Gurgo C, Guo H-G, Franchini G, Aldovini A, Collalti E, Farrell K, Wong-Staal F, Gallo R C, Reitz M S., Jr Envelope sequences of two new United States HIV-1 isolates. Virology. 1988;164:531–536. doi: 10.1016/0042-6822(88)90568-5. [DOI] [PubMed] [Google Scholar]
- 18.Hadida F, Vieillard V, Autran B, Clark-Lewis I, Baggiolini M, Debre P. HIV-specific T cell cytotoxicity mediated by RANTES via the chemokine receptor CCR3. J Exp Med. 1999;188:609–614. doi: 10.1084/jem.188.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch M S, Brun-Vezinet F, D'Aquila R T, Hammer S M, Johnson V A, Kuritzkes D R, Loveday C, Mellors J W, Clotet B, Conway B, Demeter L M, Vella S, Jacobsen D M, Richman D D. Antiretroviral drug resistance testing in adult HIV-1 infection. Recommendations of an International AIDS Society-USA panel. JAMA. 2000;283:2417–2426. doi: 10.1001/jama.283.18.2417. [DOI] [PubMed] [Google Scholar]
- 20.Kilby J M, Hopkins S, Venetta T M, DiMassimo B, Cloud G A, Lee J Y, Alldredge L, Hunter E, Lambert D, Bolognesi D, Matthews T, Johnson M R, Nowak M A, Shaw G M, Saag M S. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 21.Mack M, Lucknow B, Nelson P J, Cihak J, Simmons G, Clapham P R, Signoret N, Marsh M, Stangassinger M, Borlat F, Wells T N C, Schlondorff D, Proudfoot A E I. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maréchal V, Arenzana-Seisdedos F, Heard J-M, Schwartz O. Opposite effects of SDF-1 on human immunodeficiency virus type 1 replication. J Virol. 1999;73:3608–3615. doi: 10.1128/jvi.73.5.3608-3615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosier D E, Picchio G R, Gulizia R J, Sabbe R, Poignard P, Picard L, Offord R E, Thompson D A, Wilken J. Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants. J Virol. 1999;73:3544–3550. doi: 10.1128/jvi.73.5.3544-3550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami T, Nakajima T, Koyanagi Y, Tachibana K, Fujii N, Tamamura H, Yoshida N, Waki M, Matsumoto A, Yoshie O, Kishimoto T, Yamamoto N, Nagasawa T. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J Exp Med. 1997;186:1389–1393. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J-L, Arenzana-Seisdedos F, Schwartz O, Heard J-M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 26.Page K A, Stearns S M, Littman D R. Analysis of mutations in the V3 domain of gp160 that affect fusion and infectivity. J Virol. 1992;66:524–533. doi: 10.1128/jvi.66.1.524-533.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Proudfoot A E, Wells T N, Clapham P R. Chemokine receptors: future therapeutic targets for HIV? Biochem Pharmacol. 1999;57:451–463. doi: 10.1016/s0006-2952(98)00339-6. [DOI] [PubMed] [Google Scholar]
- 28.Proudfoot A E I, Power C A, Hoogewerf A J, Montjovent M-O, Borlat F, Offord R E, Wells T N C. Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J Biol Chem. 1996;271:2599–2603. doi: 10.1074/jbc.271.5.2599. [DOI] [PubMed] [Google Scholar]
- 29.Rusconi S, Merrill D P, La Seta-Catamancio S, Citterio P, Offord R E, Hirsch M S. Effective inhibition of HIV-1 isolated from patients with acute primary infection by aminooxypentane (AOP)-RANTES. AIDS. 1999;13:1144–1145. doi: 10.1097/00002030-199906180-00022. [DOI] [PubMed] [Google Scholar]
- 30.Signoret N, Oldridge J, Pelchen-Matthews A, Klasse P J, Tran T, Brass L F, Rosenkilde M M, Schwartz T W, Holmes W, Dallas W, Luther M A, Wells T N, Hoxie J A, Marsh M. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139:651–664. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E I. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 33.Valentin A, Albert J, Fenyö E M, Åsjö B. Dual tropism for macrophages and lymphocytes is a common feature of primary human immunodeficiency virus type 1 and 2 isolates. J Virol. 1994;68:6684–6689. doi: 10.1128/jvi.68.10.6684-6689.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao L, Rudolph D L, Owen S M, Spira T J, Lal R B. Adaptation to promiscuous usage of CC- and CXC-chemokine coreceptors in vivo correlates with HIV-1 disease progression. AIDS. 1998;12:F137–F143. doi: 10.1097/00002030-199813000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Yang O O, Swanberg S L, Lu Z, Dziejman M, McCoy J, Luster A D, Walker B D, Herrmann S H. Enhanced inhibition of human immunodeficiency virus type 1 by Met-stromal-derived factor 1β correlates with down-modulation of CXCR4. J Virol. 1999;73:4582–4589. doi: 10.1128/jvi.73.6.4582-4589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]