Abstract
Molecular methods have been used to detect human pathogens in wastewater with sampling typically performed at wastewater treatment plants (WWTP) and upstream locations within the sewer system. A wastewater-based surveillance (WBS) program was established at the University of Miami (UM) in 2020, which included measurements of SARS-CoV-2 levels in wastewater from its hospital and within the regional WWTP. In addition to the development of a SARS-CoV-2 quantitative PCR (qPCR) assay, qPCR assays to detect other human pathogens of interest were also developed at UM. Here we report on the use of a modified set of reagents published by the CDC to detect nucleic acids of Monkeypox virus (MPXV) which emerged during May of 2022 to become a concern worldwide. Samples collected from the University hospital and from the regional WWTP were processed through DNA and RNA workflows and analyzed by qPCR to detect a segment of the MPXV CrmB gene. Results show positive detections of MPXV nucleic acids in the hospital and wastewater treatment plant wastewater which coincided with clinical cases in the community and mirrored the overall trend of nationwide MPXV cases reported to the CDC. We recommend the expansion of current WBS programs' methods to detect a broader range of pathogens of concern in wastewater and present evidence that viral RNA in human cells infected by a DNA virus can be detected in wastewater.
Keywords: COVID-19, Monkeypox virus, Mpox, MPXV, SARS-CoV-2 wastewater-based surveillance, Wastewater
Graphical abstract
1. Introduction
Monkeypox virus (MPXV and also known as Mpox) was first identified in 1958 by examination of pustular lesions appearing in cynomolgus monkeys (von Magnus et al., 1959) with human infection being documented a decade later (Marennikova et al., 1972). As with other viruses in the genus Orthopoxvirus, the clinical presentation can include a localized or disseminated rash often with a prodrome of fever and constitutional symptoms (Breman et al., 1980; Atoui et al., 2023). Prior clusters of MPXV outside of endemic regions of the world have been documented in relation to animal exposure (Huhn et al., 2005). However, in the spring of 2022, the majority of cases were recognized in patients linked to transmission between men who have sex with men, although cases have been documented in other contexts (Noe et al., 2022; Hammerschlag et al., 2022). The clinical presentation differed from prior reports in that the rash included involvement of anogenital areas and association with proctitis (Hammerschlag et al., 2022; Thornhill et al., 2022). Despite recognition and improvement in efforts with regards to expansion of testing, clinicians and patients faced challenges with access, potentially limiting surveillance, prevention, and treatment efforts (Aden et al., 2022). Since its recent outbreak in 2022, social stigmas have also been associated with the presentation of pox and MPXV generally, which limits the ability of human health surveillance (HHS) efforts. If an individual is against getting tested, or does not seek out testing, HHS results may ultimately be skewed. Therefore, other tools such as wastewater-based surveillance (WBS), are needed in tandem with clinical results to provide a more accurate estimate of prevalence within a community (Tiwari et al., 2023; Wolfe et al., 2022a; La Rosa et al., 2022; Wolfe et al., 2023; Gazecka et al., 2023; Marimuthu et al., 2023).
A similar situation early in the COVID-19 pandemic led to the expansion of efforts with sampling of wastewater as an alternative means of clinical testing for community surveillance (Daughton, 2020; Nelson, 2022). Prior to the COVID-19 pandemic, WBS was established as an epidemiological strategy to assess poliovirus outbreaks within communities (Brouwer et al., 2018; Shulman et al., 2000; Shulman et al., 2006), so previous research strategies could be readily adopted for surveilling COVID-19 as a rapid response to assess community trends. To simplify, the COVID-19 pandemic, and the plethora of established mandates which required masking, social distancing, isolation, quarantine, etc. impacted voluntary reporting of results, such that WBS programs were rapidly established to determine SARS-CoV-2 prevalence within communities (Betancourt et al., 2021; Gibas et al., 2021; Rosiles-Gonzalez et al., 2021; Vo et al., 2022; Zulli et al., 2021). Moreover, the hesitancy of the public to receive and report testing, especially as the pandemic progressed and asymptomatic positive cases grew in number, underscored that multiple in-tandem surveillance efforts were necessary for assessing overall spatial and temporal trends of COVID-19. Throughout the pandemic, various lessons regarding the usefulness of WBS accrued including the ability for sampling data to ascertain community infection (Peccia et al., 2020) and variant abundance (Baaijens et al., 2021; Wolfe et al., 2022b), predictions of hospitalization and ICU admission (Galani et al., 2022; Zhan et al., 2022), and fecal shedding and asymptomatic expression relating to HHS methods (Schmitz et al., 2021). In addition, a plethora of methodology comparing laboratory processes and factors impacting the detection ability of WBS to measure SARS-CoV-2 within communities were also added to the literature (Babler et al., 2022; Babler et al., 2023; Bruce et al., 2020; Juel et al., 2021; Khan et al., 2021), allowing for expansion of WBS for alternative pathogen detection.
Recent data from analyzing different body fluids suggests MPXV shedding may occur via multiple body sites as the virus has been detected by quantitative polymerase chain reaction (qPCR) in saliva, semen, blood, urine, and feces (Noe et al., 2022; Tiwari et al., 2023; de Jonge et al., 2022; Peiro-Mestres et al., 2022). All these fluids, to one extent or other, may ultimately be discarded in wastewater. Here we discuss an opportunity to use similar sampling methods, which have been established in many laboratories on a global scale - stemming from the COVID-19 pandemic – for detection of MPXV within samples generated by WBS for protecting public health.
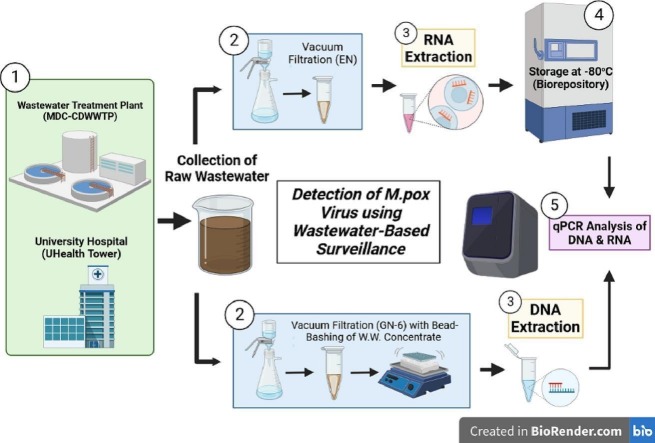
The University of Miami (UM) established a weekly wastewater-based surveillance program for SARS-CoV-2 RNA from September 2020 to September 2022 to proactively surveil the three main campuses of the University, including the University Hospital, UHealth Tower, as well as the Miami-Dade County (MDC) Central District Wastewater Treatment Plant (CDWWTP) (Sharkey et al., 2021). To augment the weekly sample collection schedule, collection of daily samples also from the CDWWTP began in August of 2021, following the emergence of the Delta variant, with the efforts concluding after one year in August of 2022. Due to the focus on quantitation of SARS-CoV-2, an RNA virus, all wastewater concentrate samples here were processed using a Zymo Research kit with selective spin columns that remove DNA during purification retaining RNA only. Routine normalization parameters measured alongside SARS-CoV-2, described by Zhan et al. (2022), included Pepper Mild Mottle Virus (PMMoV) (Roldan-Hernandez et al., 2022), an endogenous RNA plant virus readily detected from wastewater, and Beta-2 Microglobulin (B2M), messenger RNA (mRNA) of a human protein-coding gene detected from cellular waste deposited into wastewater. Both parameters were readily detectable in a majority of wastewater samples collected and were measured to assess possible improvements through normalization purposes (Babler et al., 2022; Sharkey et al., 2021). These qPCR targets were included to gauge integrity and the amplification of the RNA extracted from wastewater samples.
Starting in May of 2022 efforts were expanded to include extraction of DNA from wastewater in-tandem with routine RNA extraction for SARS-CoV-2 monitoring, due to interest in ascertaining other biological microbes in wastewater. Although methods are available for extracting Total Nucleic Acids (TNA) from wastewater samples, our initial protocol was optimized for analyzing SARS-CoV-2 RNA. Given the desire to continue with optimized measurements of SARS-CoV-2 RNA, a separate extraction workflow for DNA was developed. By performing two separate extractions for RNA as well as DNA, we doubled the yield of nucleic acid isolated from wastewater, and ultimately utilized a purer nucleic acid product for downstream molecular application. Therefore, to expand upon microbial targets of interest, experimentation towards modifying routine weekly SARS-CoV-2 methodology for DNA isolation from wastewater has been ongoing at UM since May 2022 and was applied towards the detection of MPXV (Sharkey et al., 2022), a DNA virus, due to the readily available supplies, laboratory accessibility, and interest in detecting this emerging virus in wastewater.
Although MPXV is a double-stranded DNA virus, we tested for the presence of MPXV RNA, in addition, based on past results demonstrating that human B2M RNA is present in wastewater (Zhan et al., 2022; Babler et al., 2022; Sharkey et al., 2021). Substantial amounts of human B2M RNA are detectable in wastewater RNA extracts, which is most likely due to the introduction of human cells into wastewater by excretion and washing. Therefore, we theorized that human cells infected with MPXV, harboring MPXV RNA transcripts, would also be introduced into wastewater and quantitation of these transcripts would permit comparison of the levels of the MPXV nucleic acids in wastewater samples. With this, in addition to analyzing for MPXV in wastewater DNA extracts, we evaluated for MPXV positivity the archival samples that were processed to selectively isolate RNA. We therefore set out to test the hypotheses that 1) MPXV DNA may be detectable in wastewater when MPXV positive individuals are present within the contributing sewershed, and 2) MPXV RNA within infected human cells may be detectable in wastewater due to the contribution of cells excreted from infected individuals.
2. Materials and methods
2.1. Clinical data
Clinical MPXV data were consolidated at the national, state, county, and hospital level, to determine the time frames for wastewater MPXV analysis, and to provide comparisons between outbreak intensity and detection levels of wastewater MPXV. The first case of MPXV in the U.S. was confirmed in the state of Massachusetts during mid-May 2022 and by August 4, 2022 the U.S. Department of Health and Human Services declared MPXV a national public health emergency (CDC, 2023a). U.S. national clinical case data for MPXV was thus available through the CDC (CDC, 2023b) with documentation of cases starting on May 10, 2022, with continued detections, albeit at low levels, through the writing of this manuscript on January 28, 2023. During the period of June to July 2022, clinical testing for MPXV in the jurisdiction of Miami-Dade County (MDC), Florida, USA (population of 2.66 million (Census and QuickFacts, 2023)) began in earnest due to increasing recognition of disease as well as implementation of testing by commercial laboratories. MPXV case counts for Florida and Miami-Dade County were reported through the Florida Department of Health (FDOH, 2022). The University Hospital provides acute care with a bed capacity of 490. Hospital case counts from the UHealth Tower were provided through the electronic health record, Epic® with IRB approval (UM IRB #20210164).
2.2. Wastewater collection & sampling strategy
Three sample sets were collected and analyzed as part of this study. These included weekly samples collected at the University Hospital (referred to as UHealth Tower), weekly samples collected at a regional wastewater treatment plant (referred to as CDWWTP and defined as site WC0Dc), and daily samples collected at the CDWWTP site WC0Dc. Samples from the University's UHealth Tower were collected on a weekly basis (from September 2020 to September 2022) using a grab sampling methodology from two wastewater access points (defined as site WM06 and WM08). Samples from the CDWWTP (weekly and daily) were collected using a refrigerated autosampler (HACH AS950 fitted with an IO9000 for flow proportional sampling) which collected flow-weighted aliquots hourly midnight to midnight the day prior to sample pick up at the plant (Babler et al., 2023). Samples processed daily were focused on SARS-CoV-2 analysis only (RNA workflow only), whereas weekly samples were analyzed for a larger suite of microbes and were thus processed via both RNA and DNA workflows.
2.3. Wastewater primary concentration
Samples analyzed as part of this study were subjected to two workflows, one optimized for viral RNA with chemical lysis (Babler et al., 2022; Sharkey et al., 2021), and another optimized for DNA with physical process lysis (in addition to chemical lysis) due to the stronger integrity of the cell walls of many DNA microbes (Sharkey et al., 2022). Although TNA (includes RNA and DNA) extraction methods are available, we chose to develop a separate DNA workflow given that our original workflow was optimized for the analysis of SARS-CoV-2 RNA. One advantage of adding a second workflow was the increase in overall nucleic acid yield. Moreover, commercially available kits for TNA extractions were found to be more laborious than the kits designed specifically for RNA or DNA. Thus, we chose to incorporate two separate workflows, one optimized for RNA (specifically optimized for SARS-CoV-2) and one optimized for DNA (originally optimized for the recovery of a Mycobacterial DNA).
For extracting RNA from wastewater, the primary concentration methodology utilized for weekly as well as daily-collected samples was electronegative (EN) filtration which utilized mixed cellulose ester (MCE) membranes (47 mm, EMD-Millipore #HAWP04700, negatively charged) and vacuum filtration, within a BSL-2 hood, to create wastewater filter concentrates (Zhan et al., 2022; Babler et al., 2022; Sharkey et al., 2021; Solo-Gabriele et al., 2023). MCE filter membranes capture suspended solids of wastewater, due to size selection of the 0.45 μm pore size in addition to the pretreatment of wastewater samples (500 mL) with magnesium chloride (MgCl2 to a concentration of 50 mM) and 10 % hydrochloric acid (HCl) – reducing the pH to 3.5–4.5, which improves binding affinity of viral particles by altering the chemical charge. Upon completion of EN filtration and saturation of suspended solids, wherein differing volumes of wastewater (30–50 mL) (dependent upon physical/chemical parameters such as turbidity, total suspended solids, etc.) were filtered, whole MCE membranes were folded in on themselves four times and placed within 5 mL centrifuge tubes in 1.5 mL 1× DNA/RNA Shield and kept at 4 °C until subsequent RNA extraction (Zhan et al., 2022; Babler et al., 2022; Sharkey et al., 2021).
The weekly DNA isolation process involved minor modifications of the EN filtration methodology described above. The DNA isolation process omitted the pretreatment with MgCl2 and 10 % HCl and utilized a different membrane-type. For the DNA extraction process, GN-6 Metricel Filter membranes (Pall Corp. #66278) with the same pore size 0.45 μm, designed for microbiological applications to capture bacteria and fungi by size exclusion, were used instead. The pore size of the membrane was kept the same so that the same size suspended particles were captured. The original intention of DNA isolation analysis (as experimentation began prior to the MPXV outbreak) was to expand WBS capacity to include bacterial and fungal targets which would be captured by size. Following vacuum filtration within a BSL-2 hood, GN-6 membranes were first sliced in half with a sterile scalpel within a sterile petri dish, folded in on themselves thrice, and were placed within 2 mL Zymo Research ZR BashingBead Lysis Tubes (0.1 & 0.5 mm) (Cat#S6012-50) in 1 mL 1× DNA/RNA Shield and kept at 4 °C until subsequent DNA extraction.
All personnel handling wastewater samples followed the University's policies of Health and Safety and used personal protective equipment (PPE) in compliance with laboratory safety practices, and disinfected surfaces, and equipment that came into contact with wastewater was cleaned with 99.5 % isopropanol. Laboratory supplies, such as vacuum filtration manifold cups, graduated cylinders, forceps, and beakers necessary for performing primary concentration were rinsed, disinfected with 99.5 % isopropanol, and autoclaved immediately after use.
2.4. Nucleic acid extraction from wastewater concentrates
The RNA extraction process performed at UM for routine monitoring for SARS-CoV-2 utilized a Zymo Research Quick-RNA Viral Kit – modified in-house for the reduction of qPCR inhibitors. This was done by reducing the final elution volume to 10 μL with nuclease-free water (to minimize the physical flushing of inhibitors from the spin column binding matrix) and by increasing the volume of wash buffer/ethanol wash by 35 %. Wastewater concentrates were briefly vortexed (<3 s) and spun down, then repeat-pipetting was utilized to homogenize the DNA/RNA wastewater slurry containing the MCE filter membrane, prior to extraction. Then 250 μL of concentrate was thoroughly mixed with 500 μL of the kit's binding buffer, as per the recommended 2:1 ratio of sample to buffer. RNA extracts were analyzed for MPXV downstream to primarily investigate the second hypothesis.
Sample homogenization, for the DNA extraction process, began with the utilization of an OMNI Bead-Ruptor 12 instrument cycling with a program of 1 min of bashing, 5 min resting (×3) prior to DNA extraction using a ZymoBIOMICS DNA Miniprep Kit and corresponding manufacturers' protocol. One slight modification was made to the corresponding Zymo protocol, in which the initial centrifugation step following bead-bashing was increased from 10,000 ×g to 12,000 ×g to allow for improved pelleting of ruptured membrane pieces and beads. DNA extracts that underwent this process were analyzed for MPXV downstream with qPCR to investigate the first hypothesis of this study.
2.5. Quantitative PCR and amplicon sequencing
MPXV RNA was measured using an in-house developed assay based on the unique activities of a polymerase called Volcano 2nd Generation (V2G) described earlier (Babler et al., 2022; Sharkey et al., 2021). V2G reads both RNA and DNA templates, eliminating the normally required step of converting RNA templates to cDNA by reverse transcription prior to PCR amplification. RNA purified from wastewater concentrates was quantified using primers and a fluorescent reporter probe targeting the CrmB region of the MPXV genome (see Table 1 ). Four of forty microliters of extracted RNA was amplified by qPCR in forty microliter reactions containing 1.1× V2G buffer, 200 nM dNTPs, 2 units V2G, 1 unit anti-Taq antibody, 500 nM of each forward and reverse primer, 250 nM probe and 1× ROX reference dye. qPCR cycling was 95 °C for 1 min followed by 45 cycles of (95 °C for 10 s; 60 °C for 20 s; 72 °C for 15 s). Based on the standard curves generated by the BioRad CFX Connect software, amplification efficiencies were consistently close to 95 % with coefficients of determination (R2) of 1, or very close to 1. All raw data from qPCR reactions were transformed to genomic copies/L of wastewater by correcting for qPCR input, volume of 1× DNA/RNA shield, and volume of wastewater used to prepare concentrates on filters. Numbers are reported based on absolute quantification.
Table 1.
Reagents used to detect MPXV nucleic acids by qPCR and to confirm target specificity by amplicon sequencing. Primers and probes were based on CDC published sequences with some modifications. The forward primer was modified to increase the melting temperature to improve amplification efficiency. Other changes were to account for sequence differences in primers between the CDC set and that of virus isolated in Massachusetts in 2022 (GenBank accession number ON563414) that was used to generate qPCR standards. Primers listed for sequencing were used to generate the amplicon and prime the sequencing in both directions (Centers for Disease Control, and Prevention Poxvirus, and Rabies Branch, C. P, n.d.).
| Assay | Primers/probe sequences | Binding site | |
|---|---|---|---|
| qPCR | Forward | TCTTGCTATCACATAATCTGRAAGCGTA | 2588–2615 |
| Reverse | GATATAGCACCACATGCACCA | 2708–2687 | |
| Probe | 5HEX-AAGCCGTAA/ZEN/TCTATGTTGTCTATCGTGTCC-3IABkFQ | 2655–2626 | |
| Sequencing | Forward | GCTATCACATAATCTGRAAGCGTA | 2592–2615 |
| Reverse | GATATAGCACCACATGCACCA | 2708–2687 | |
Extracted DNA samples were subjected to qPCR analysis utilizing a TaqMan Fast Universal PCR Master Mix (ThermoFisher) and the same MPXV-specific primers and fluorescent probe used for RNA detection. The same BioRad CFX Connect system was also utilized for MPXV DNA qPCR assays, and reactions were set up using appropriate practices to prevent contamination of PCR reactions. Five of one hundred microliters extracted DNA was amplified by qPCR in thirty microliter reactions containing 1× Fast Mix, 500 nM of each forward and reverse primer and 250 nM probe. Cycling parameters were identical to those used for amplification of MPXV RNA with very similar amplification efficiencies and R2 values.
In order to ascertain that products amplifed by qPCR were derived from MPXV RNA, amplification products from two reactions were analyzed by Sanger sequencing. Primers are listed in Table 1 and amplification generated a 117 bp amplicon of the CrmB gene. Products from two sampling days (7/13/22 and 7/20/22) were sequenced in both directions so the complete intervening sequence could be reported.
3. Results and discussion
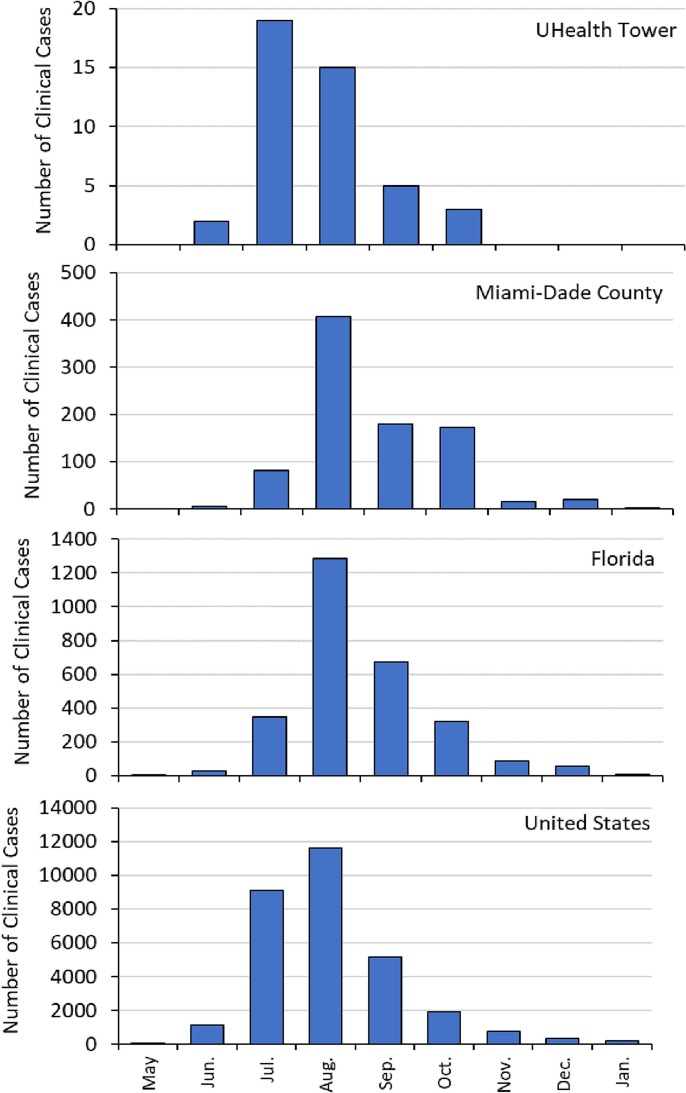
The growing concern of MPXV spreading through communities in MDC over the summer of 2022 guided the timeline for the analysis of wastewater samples. Following the first case of MPXV in the U.S. documented as May 10, 2022, Florida documented its first case on May 22, 2022. The first reported case in Miami-Dade County and at the hospital sampling site, University of Miami UHealth Tower, occurred on June 14, 2022 (Census and QuickFacts, 2023). The vast majority of diagnoses were made in various outpatient clinics or in the emergency room setting. During the time frame of the wastewater data collection, only a total of three diagnosed patients at the UHealth Tower required hospitalization. The hospital length of stay of the patients varied from 3 to 17 days. Another 27 patients were evaluated in the emergency department of the building and 1 patient was evaluated in a clinic in the same building structure as the main hospital. Of the total 31 patients who may have been cared for in the building where the wastewater collection occurred, 41.9 % had reported genital symptoms or lesions while 35.5 % reported rectal symptoms or lesions. Analysis of historical records shows that the monthly distribution of cases (Fig. 1 ) is characterized by a peak in cases at the UHealth Tower during July 2022, with peaks at the national, state, and county scales in August.
Fig. 1.
Number of clinical MPXV cases during May 2022 through January 2023 for UHealth Tower, Miami-Dade County, Florida, and the U.S.
For the hospital wastewater, among the weekly UHealth hospital samples analyzed (n = 16 for site WM06 and n = 16 for site WM08), only one was positive and was detected from a sample collected from one of the two wastewater access points (WM06) on July 13. This detection of MPXV in wastewater (6200 gc/L) coincided with the peak of MPXV positive patients in the hospital and supports the association between patients and wastewater. The contribution of patients towards the hospital wastewater stream would be difficult to assess and sporadic, as it is unknown which patients used the bathroom facilities during their visit. This is particularly true for the clinic and emergency room visits. For the admitted patients, the contribution of MPXV to the wastewater would also be sporadic and possibly associated with patient use of the bathing facilities as well. Although laundering of bedding at this facility is outsourced, it would also be a consideration in other facilities where in house linen laundering occurs in the same building. The fact that MPXV was detected in the hospital wastewater during the peak of positive clinical cases at the hospital shows promise for the ability of WBS to detect pathogens contributed by a small fraction of the population. It also shows promise for detecting pathogens that may be released from patients through pathways not dominated by fecal sources (e.g., skin).
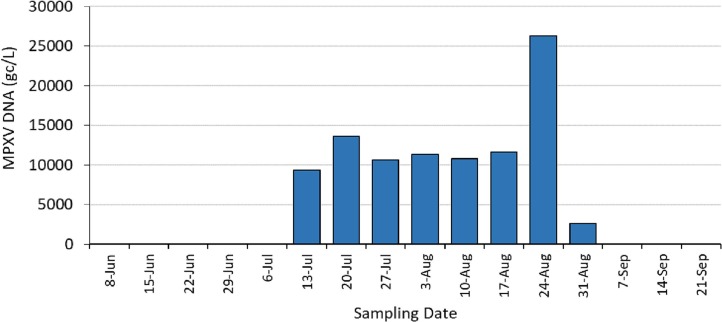
For the weekly CDWWTP samples (Fig. 2 ), MPXV DNA was not detected during the first five weeks of sampling, but qPCR reactions were positive starting at week six (July 13 at 9400 gc/L) which coincided in time when positive cases were also detected in the hospital which is located within the same sewershed. This positivity also coincided with the time frame when overall cases in MDC were on the rise, with MDC documenting a total of 86 clinical cases (sum of cases in June and July) (Census and QuickFacts, 2023). Given that the population of MDC is very large (2.7 million) results suggest the high sensitivity of the detection technology, even if the clinical cases are under reported. Even if under-reporting occurred by two orders of magnitude, results imply that detection of MPXV is possible if the population is infected at rates <0.5 %. If the clinical case numbers were accurate, then wastewater could be used to detect MPXV in the community at rates of 3 per 100,000 residents. Moreover, the peak in wastewater MPXV also coincided with the peak MDC clinical cases. The highest number of new cases (407) was documented in MDC for the month of August (Fig. 2). This coincided with the highest level of MPXV DNA (26,300 gc/L) observed in the county wastewater samples. All subsequent weekly wastewater samples remained positive through August (Fig. 2) with wastewater MPXV DNA levels falling to below detection limits at the beginning of September and remaining below detection for the two wastewater samples analyzed thereafter. This correspondence in trend supports that MPXV virus was not present within the community at detectable levels during the beginning of the summer in 2022 yet was quickly quantified by WBS application once individuals within the community contracted the virus. The non-detection in wastewater following the September timeframe also coincides with the decrease in clinical cases within the MDC community. Overall, results support the first hypothesis of this study, and builds up current studies (La Rosa et al., 2022; de Jonge et al., 2022; Giron-Guzman et al., 2023; Wannigama et al., 2023), that MPXV DNA can be detected in wastewater when MPXV positive individuals are present within the contributing sewershed.
Fig. 2.
MPXV genomic DNA was detected in concentrated wastewater collected weekly at the Miami-Dade Central District Wastewater Treatment Plant. MPXV target was first detected by quantitative PCR in mid-July of 2022 and remained detectable until testing negative in September. All weekly samples analyzed from June 8 through July 6, plus September 7 to September 21 were below detection limits of 180 gc/L.
When comparing the hospital data to the county level data, the more consistent detection of MPXV in the CDWWTP samples is likely because access to hospital facilities may be more sporadic (the number of MPXV positive patients was small and non-resident patients may or may not use the facilities). At the county scale, however, the majority of the wastewater contributions from MPXV positive individuals should be captured as long as the individuals remained within the larger geographic area of the county during the period of infection. The finding of a more stable signal at the county scale, is also consistent with prior studies that evaluated the influence of watershed scale on infectious disease signals in the context of SARS-CoV-2 in wastewater and COVID-19 cases in the community. At larger sewershed scales, the signal of infectious disease agents varies more gradually in comparison to the signals at very small sewershed scales (e.g., individual buildings) which tend to show more sporadic levels of quantification (Babler et al., 2023).
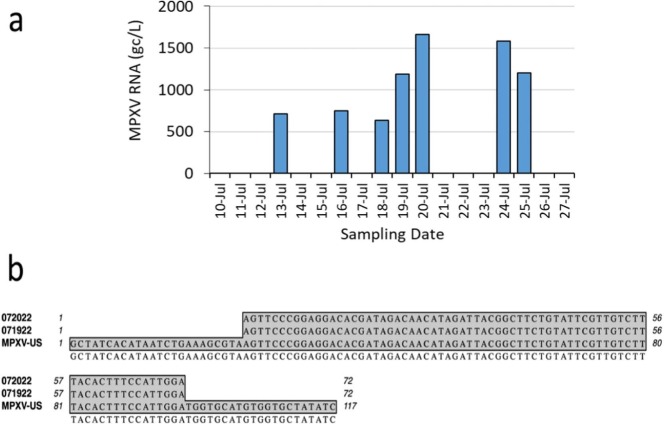
For the daily wastewater samples processed through the RNA workflow, the detection of MPXV supports the second hypothesis of this study, that MPXV RNA within infected human cells may be detectable in wastewater due to the contribution of cells excreted from infected individuals. In other words, the RNA is not from the virus but from the human cells used by the virus for replication. Such results are similar to prior studies that showed human B2M RNA detection in wastewater samples. Thus, the V2G-qPCR results demonstrate that MPXV RNA transcripts within infected human cells can also be quantified using total RNA extracted from wastewater. The presence of MPXV in RNA extracts from wastewater samples from human cells is further supported by the results from Sanger sequencing which showed identical alignment to the MPXV genome (Fig. 3b) confirming the detection of MPXV from wastewater. These results strongly support that MPXV RNA was found in the wastewater samples. Results also suggest that workflows designed for the detection of RNA may be suitable for detecting DNA viruses in wastewater, a finding that is rarely implemented but can find utility in future WBS by simplifying the extraction processes to focus on RNA.
Fig. 3.
MPXV RNA was detected in concentrated wastewater collected daily at the Miami-Dade Central District Wastewater Treatment Plant. All daily samples analyzed for MPXV from June 1 to July 9, 2022 (data not shown below) were below detection limits of 180 gc/L. The MPXV target was first detected by quantitative PCR in mid-July of this year and increased over the sampling period (a). Amplified RNA products from two dates were sequenced and aligned with an MPXV genomic sequence submitted to GenBank (ON563414) in May 2022 (b) for confirmation. Flanking MPXV-US sequences are the PCR primer binding sites.
As observed above for weekly samples, the daily wastewater samples (which were subjected to the RNA workflow) were also consistent with the time frame in which positive clinical MPXV cases were documented within MDC. Positives in wastewater were observed during the July time frame which coincided with the timing of positive MPXV cases in the community. The levels of MPXV RNA detected were lower, however, by about an order-of-magnitude relative to the levels measured using DNA extracts. Three sampling days coincided between the daily RNA and weekly DNA workflows, with positive detections in wastewater consistent for two of the coincident three days. The weekly DNA samples were all positive. Two of the three coincident RNA samples were positive. For these two samples that coincided, the ratios of the RNA to the DNA results were 8 % and 12 %, respectively.
4. Conclusions
Results support both study hypotheses. Results support that MPXV DNA is detectable in wastewater and positivity is correlated with documented clinical cases within the sewershed. Results also support that MPXV RNA within infected human cells is detectable in wastewater due to the contribution of cells excreted from infected individuals.
This study shows that the temporal detection of MPXV by RNA and DNA qPCR coincided with case levels reported in the community. Both extracts tested positive for MPXV nucleic acid, indicating the presence of viral particles or infected human cells in wastewater. In this study, DNA detection of the MPXV was about 10 times more sensitive than the detection of RNA. However, the fact that a virus harboring a DNA genome was detected using RNA protocols suggests that future WBS efforts could potentially expand to target both nucleic acids in efforts to detect pathogens of interest.
Overall, results suggest that WBS approaches can be used to assess the presence of human pathogens beyond SARS-CoV-2 within communities. With illnesses such as MPXV and associated social stigmas which may further limit the ability of documenting cases within the public health system, WBS can serve as a powerful tool to assess and mitigate disease outbreaks. This study supports that WBS can be expanded beyond SARS-CoV-2 surveillance to provide impactful information about pathogens that are key indicators of the prevalence of disease within a regional or local community.
CRediT authorship contribution statement
Mark Sharkey: Conceptualization, Methodology, Visualization, Formal Analysis, Writing – Original Draft, Writing-Review. Kristina M. Babler: Conceptualization, Methodology, Writing – Review. Bhavarth Shukla: Conceptualization, Methodology, Writing – Original Draft, Supervision. Samantha M. Abelson: Methodology. Bader Alsuliman: Methodology. Ayaaz Amirali: Methodology. Samuel Comerford: Methodology. George Grills: Conceptualization, Methodology, Writing – Review. Naresh Kumar: Conceptualization, Methodology. Jennifer Laine: Methodology. Jisue Lee: Methodology. Walter Lamar: Methodology. Christopher Mason: Conceptualization, Methodology, Supervision, Funding Acquisition. Johnathan Penso: Methodology. Brian D. Reding: Methodology. Stephan Schürer: Data Methodology, Funding Acquisition. Mario Stevenson: Resources. Dušica Vidović: Data Methodology. Helena Solo-Gabriele: Conceptualization, Methodology, Writing – Review, Supervision, Project Administration, Funding Acquisition.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgments
The research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health (NIH) under Award Number U01DA053941 and by an NIH award (P30A1073961) to establish the University of Miami Center for AIDS Research (CFAR) which provided supplemental CFAR pilot funding to support a study of Monkeypox / HIV-1 co-infections in Miami-Dade County. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We are thankful to Miami-Dade Water and Sewer Department for provision of wastewater samples from the CDWWTP.
Editor: Warish Ahmed
Data availability
Data will be made available on request.
References
- Aden T.A., Blevins P., York S.W., Rager S., Balachandran D., Hutson C.L., Lowe D., Mangal C.N., Wolford T., Matheny A., Davidson W., Wilkins K., Cook R., Roulo R.M., White M.K., Berman L., Murray J., Laurance J., Francis D., Green N.M., Berumen R.A., 3rd, Gonzalez A., Evans S., Hudziec M., Noel D., Adjei M., Hovan G., Lee P., Tate L., Gose R.B., Voermans R., Crew J., Adam P.R., Haydel D., Lukula S., Matluk N., Shah S., Featherston J., Ware D., Pettit D., McCutchen E., Acheampong E., Buttery E., Gorzalski A., Perry M., Fowler R., Lee R.B., Nickla R., Huard R., Moore A., Jones K., Johnson R., Swaney E., Jaramillo J., Reinoso Webb C., Guin B., Yost J., Atkinson A., Griffin-Thomas L., Chenette J., Gant J., Sterkel A., Ghuman H.K., Lute J., Smole S.C., Arora V., Demontigny C.K., Bielby M., Geeter E., Newman K.A.M., Glazier M., Lutkemeier W., Nelson M., Martinez R., Chaitram J., Honein M.A., Villanueva J.M. Rapid diagnostic testing for response to the monkeypox outbreak - Laboratory Response Network, United States, May 17–June 30, 2022. MMWR Morb. Mortal. Wkly Rep. 2022;71(28):904–907. doi: 10.15585/mmwr.mm7128e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atoui A., Jourdain F., Mouly D., Cordevant C., Chesnot T., Gassilloud B. A review on mpox (monkeypox) virus shedding in wastewater and its presistence evaluation in environmental samples. Case Stud. Chem. Environ. Eng. 2023:7. [Google Scholar]
- Baaijens J.A., Zuuli A., Ott I.M., Petrone M.E., Alpert T., Fauver J.R., Kalinich C.C., Vogels C.B.F., Breban M.I., Duvallet C., McElroy K., Ghaeli N., Imakaev M., Mckenzie-Bennett M., Robison K., Plocik A., Schilling R., Pierson M., Littlefield R., Spencer M., Simen B.B., Initiative Y.S.-C.-G.S., Hanage W.P., Grubaugh N.D., Peccia J., Baym M. Variant abundance estimation for SARS-CoV-2 in wastewater using RNA-Seq quantification. medRxiv. 2021 (preprint) [Google Scholar]
- Babler K.M., Amirali A., Sharkey M.E., Williams S.L., Boone M.M., Cosculluela G.A., Currall B.B., Grills G.S., Laine J., Mason C.E., Reding B.D., Schurer S.C., Stevenson M., Vidovic D., Solo-Gabriele H.M. Comparison of electronegative filtration to magnetic bead-based concentration and V2G-qPCR to RT-qPCR for quantifying viral SARS-CoV-2 RNA from wastewater. ACS ES T Water. 2022;2(11):2004–2013. doi: 10.1021/acsestwater.2c00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babler K.M., Sharkey M.E., Abelson S., Amirali A., Benitez A., Cosculluela G.A., Grills G.S., Kumar N., Laine J., Lamar W., Lamm E.D., Lyu J., Mason C.E., McCabe P.M., Raghavender J., Reding B.D., Roca M.A., Schurer S.C., Stevenson M., Szeto A., Tallon J.J., Jr., Vidovic D., Zarnegarnia Y., Solo-Gabriele H.M. Degradation rates influence the ability of composite samples to represent 24-hourly means of SARS-CoV-2 and other microbiological target measures in wastewater. Sci. Total Environ. 2023;867 doi: 10.1016/j.scitotenv.2023.161423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt W.Q., Schmitz B.W., Innes G.K., Prasek S.M., Pogreba Brown K.M., Stark E.R., Foster A.R., Sprissler R.S., Harris D.T., Sherchan S.P., Gerba C.P., Pepper I.L. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci. Total Environ. 2021;779 doi: 10.1016/j.scitotenv.2021.146408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breman J.G., Kalisa R., Steniowski M.V., Zanotto E., Gromyko A.I., Arita I. Human monkeypox, 1970-79. Bull. World Health Organ. 1980;58(2):165–182. [PMC free article] [PubMed] [Google Scholar]
- Brouwer A.F., Eisenberg J.N.S., Pomeroy C.D., Shulman L.M., Hindiyeh M., Manor Y., Grotto I., Koopman J.S., Eisenberg M.C. Epidemiology of the silent polio outbreak in Rahat, Israel, based on modeling of environmental surveillance data. Proc. Natl. Acad. Sci. U. S. A. 2018;115(45):E10625–E10633. doi: 10.1073/pnas.1808798115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce E.A., Huang M.L., Perchetti G.A., Tighe S., Laaguiby P., Hoffman J.J., Gerrard D.L., Nalla A.K., Wei Y., Greninger A.L., Diehl S.A., Shirley D.J., Leonard D.G.B., Huston C.D., Kirkpatrick B.D., Dragon J.A., Crothers J.W., Jerome K.R., Botten J.W. Direct RT-qPCR detection of SARS-CoV-2 RNA from patient nasopharyngeal swabs without an RNA extraction step. PLoS Biol. 2020;18(10) doi: 10.1371/journal.pbio.3000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC 08/05/22: Lab Advisory: HHS declares Monkeypox a public health emergency. 2023. https://www.cdc.gov/locs/2022/08-05-2022-Lab-Advisory-HHS_Declares_Monkeypox_Public_Health_Emergency.html#:~:text=On%20August%204%2C%202022%2C%20the,to%20the%20current%20monkeypox%20outbreak (January 28)
- CDC U.S. Mpox case trends reported to CDC. 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/mpx-trends.html (January 27)
- Census U., QuickFacts S. Miami-Dade County, Florida. 2023. https://www.census.gov/quickfacts/fact/table/miamidadecountyflorida/POP060210 (January 28)
- Centers for Disease Control & Prevention Poxvirus & Rabies Branch, C. P Test procedure: monkeypox virus generic real-time PCR test. https://www.cdc.gov/poxvirus/monkeypox/pdf/PCR-Diagnostic-Protocol-508.pdf
- Daughton C. The international imperative to rapidly and inexpensively monitor community-wide Covid-19 infection status and trends. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDOH Reportable diseases frequency report. 2022. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=FrequencyMerlin.Frequency&FirstTime=True (August 19)
- Galani A., Aalizadeh R., Kostakis M., Markou A., Alygizakis N., Lytras T., Adamopoulos P.G., Peccia J., Thompson D.C., Kontou A., Karagiannidis A., Lianidou E.S., Avgeris M., Paraskevis D., Tsiodras S., Scorilas A., Vasiliou V., Dimopoulos M.A., Thomaidis N.S. SARS-CoV-2 wastewater surveillance data can predict hospitalizations and ICU admissions. Sci. Total Environ. 2022;804 doi: 10.1016/j.scitotenv.2021.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazecka M., Sniezek J., Maciolek K., Kowala-Piaskowska A., Zmora P. Mpox detection in the wastewater and the number of hospitalized patients in Poznan, Poland. Int. J. Infect. Dis. 2023 doi: 10.1016/j.ijid.2023.05.014. (in press) [DOI] [PubMed] [Google Scholar]
- Gibas C., Lambirth K., Mittal N., Juel M.A.I., Barua V.B., Roppolo Brazell L., Hinton K., Lontai J., Stark N., Young I., Quach C., Russ M., Kauer J., Nicolosi B., Chen D., Akella S., Tang W., Schlueter J., Munir M. Implementing building-level SARS-CoV-2 wastewater surveillance on a university campus. Sci. Total Environ. 2021;782 doi: 10.1016/j.scitotenv.2021.146749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron-Guzman I., Diaz-Reolid A., Truchado P., Carcereny A., Garcia-Pedemonte D., Hernaez B., Bosch A., Pinto R.M., Guix S., Allende A., Alcami A., Perez-Cataluna A., Sanchez G. Spanish wastewater reveals the current spread of Monkeypox virus. Water Res. 2023;231 doi: 10.1016/j.watres.2023.119621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag Y., MacLeod G., Papadakis G., Adan Sanchez A., Druce J., Taiaroa G., Savic I., Mumford J., Roberts J., Caly L., Friedman D., Williamson D.A., Cheng A.C., McMahon J.H. Monkeypox infection presenting as genital rash, Australia, May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn G.D., Bauer A.M., Yorita K., Graham M.B., Sejvar J., Likos A., Damon I.K., Reynolds M.G., Kuehnert M.J. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin. Infect. Dis. 2005;41(12):1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- de Jonge E.F., Petersen C.M., Koelewijn J.M., van der Drift A.M.R., van der Beek R.F.H.J., Nagelkerke E., Lodder W.J. The detection of monkeypox virus DNA in wastewater samples in the Netherlands. Sci. Total Environ. 2022;852 doi: 10.1016/j.scitotenv.2022.158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel M.A.I., Stark N., Nicolosi B., Lontai J., Lambirth K., Schlueter J., Gibas C., Munir M. Performance evaluation of virus concentration methods for implementing SARS-CoV-2 wastewater based epidemiology emphasizing quick data turnaround. Sci. Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K., Tighe S.W., Badireddy A.R. Factors influencing recovery of SARS-CoV-2 RNA in raw sewage and wastewater sludge using polyethylene glycol-based concentration method. J. Biomol. Tech. 2021;32(3):172–179. doi: 10.7171/jbt.21-3203-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Mancini P., Veneri C., Ferraro G.B., Lucentini L., Iaconelli M., Suffredini E. Detection of Monkeypox virus DNA in the wastewater of an airport in Rome, Italy: expanding environmental surveillance to emerging threats. Emerg. Infect. Dis. 2022;29(1):193–196. doi: 10.3201/eid2901.221311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Magnus P., Andersen E.K., Petersen K.B., Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 1959;46:156–176. [Google Scholar]
- Marennikova S.S., Seluhina E.M., Mal’ceva N.N., Cimiskjan K.L., Macevic G.R. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull. World Health Organ. 1972;46(5):599–611. [PMC free article] [PubMed] [Google Scholar]
- Marimuthu K., Wong J.C.C., Lim P.L., Octavia S., Huan X., Ng Y.K., Yang J.J., Sutjipto S., Linn K.Z., Setoh Y.X., Ong C.H.C., Griffiths J., Farhanah S., Cheok T.S., Sulaiman N.A.B., Congcong S.B., Neves E.S., Loo L.H., Hakim L., Sim S., Lim M., Nazeem M., Vasoo S., Tham K.W., Ng O.T., Ng L.C. Viable mpox virus in the environment of a patient room. Int. J. Infect. Dis. 2023;131:40–45. doi: 10.1016/j.ijid.2023.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B. What poo tells us: wastewater surveillance comes of age amid covid, monkeypox, and polio. BMJ. 2022;378 doi: 10.1136/bmj.o1869. [DOI] [PubMed] [Google Scholar]
- Noe S., Zange S., Seilmaier M., Antwerpen M.H., Fenzl T., Schneider J., Spinner C.D., Bugert J.J., Wendtner C.M., Wolfel R. Clinical and virological features of first human monkeypox cases in Germany. Infection. 2022:1–6. doi: 10.1007/s15010-022-01874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Arnold W., Omer S.B. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38(10):1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiro-Mestres A., Fuertes I., Camprubi-Ferrer D., Marcos M.A., Vilella A., Navarro M., Rodriguez-Elena L., Riera J., Catala A., Martinez M.J., Blanco J.L., Hospital Clinic de Barcelona Monkeypox Study, G Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022;27(28) doi: 10.2807/1560-7917.ES.2022.27.28.2200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldan-Hernandez L., Graham K.E., Duong D., Boehm A.B. Persistence of endogenous SARS-CoV-2 and pepper mild mottle virus RNA in wastewater-settled solids. ACS ES T Water. 2022;2(11):1944–1952. doi: 10.1021/acsestwater.2c00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosiles-Gonzalez G., Carrillo-Jovel V.H., Alzate-Gaviria L., Betancourt W.Q., Gerba C.P., Moreno-Valenzuela O.A., Tapia-Tussell R., Hernandez-Zepeda C. Environmental surveillance of SARS-CoV-2 RNA in wastewater and groundwater in Quintana Roo, Mexico. Food Environ. Virol. 2021;13(4):457–469. doi: 10.1007/s12560-021-09492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz B.W., Innes G.K., Prasek S.M., Betancourt W.Q., Stark E.R., Foster A.R., Abraham A.G., Gerba C.P., Pepper I.L. Enumerating asymptomatic COVID-19 cases and estimating SARS-CoV-2 fecal shedding rates via wastewater-based epidemiology. Sci. Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey M.E., Kumar N., Mantero A.M.A., Babler K.M., Boone M.M., Cardentey Y., Cortizas E.M., Grills G.S., Herrin J., Kemper J.M., Kenney R., Kobetz E., Laine J., Lamar W.E., Mader C.C., Mason C.E., Quintero A.Z., Reding B.D., Roca M.A., Ryon K., Solle N.S., Schurer S.C., Shukla B., Stevenson M., Stone T., Tallon J.J., Jr., Venkatapuram S.S., Vidovic D., Williams S.L., Young B., Solo-Gabriele H.M. Lessons learned from SARS-CoV-2 measurements in wastewater. Sci. Total Environ. 2021;798 doi: 10.1016/j.scitotenv.2021.149177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey M.E., Babler K.M., Amirali A., Grills G.S., Kumar N., Laine J., Lamar W.E., Mason C.E., Reding B.D., Schurer S.C., Shukla B., Stevenson M., Vidovic D., Solo-Gabriele H.M. 2022. First detection of the Monkeypox virus using wastewater-based surveillance in Miami-Dade County. SSRN (preprint) [Google Scholar]
- Shulman L.M., Manor Y., Handsher R., Delpeyroux F., McDonough M.J., Halmut T., Silberstein I., Alfandari J., Quay J., Fisher T., Robinov J., Kew O.M., Crainic R., Mendelson E. Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral poliovaccine strain isolated from sewage in Israel. J. Clin. Microbiol. 2000;38(10):3729–3734. doi: 10.1128/jcm.38.10.3729-3734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman L.M., Manor Y., Sofer D., Handsher R., Swartz T., Delpeyroux F., Mendelson E. Neurovirulent vaccine-derived polioviruses in sewage from highly immune populations. PLoS One. 2006;1 doi: 10.1371/journal.pone.0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solo-Gabriele H.M., Kumar S., Abelson S., Penso J., Contreras J., Babler K.M., Sharkey M.E., Mantero A.M.A., Lamar W.E., Tallon J.J., Jr., Kobetz E., Solle N.S., Shukla B.S., Kenney R.J., Mason C.E., Schurer S.C., Vidovic D., Williams S.L., Grills G.S., Jayaweera D.T., Mirsaeidi M., Kumar N. Predicting COVID-19 cases using SARS-CoV-2 RNA in air, surface swab and wastewater samples. Sci. Total Environ. 2023;857(Pt 1) doi: 10.1016/j.scitotenv.2022.159188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill J.P., Barkati S., Walmsley S., Rockstroh J., Antinori A., Harrison L.B., Palich R., Nori A., Reeves I., Habibi M.S., Apea V., Boesecke C., Vandekerckhove L., Yakubovsky M., Sendagorta E., Blanco J.L., Florence E., Moschese D., Maltez F.M., Goorhuis A., Pourcher V., Migaud P., Noe S., Pintado C., Maggi F., Hansen A.E., Hoffmann C., Lezama J.I., Mussini C., Cattelan A., Makofane K., Tan D., Nozza S., Nemeth J., Klein M.B., Orkin C.M., Group S.H.-n.C. Monkeypox virus infection in humans across 16 countries - April-June 2022. N. Engl. J. Med. 2022;387(8):679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- Tiwari A., Adhikari S., Kaya D., Islam M.A., Malla B., Sherchan S.P., Al-Mustapha A.I., Kumar M., Aggarwal S., Bhattacharya P., Bibby K., Halden R.U., Bivins A., Haramoto E., Oikarinen S., Heikinheimo A., Pitkanen T. Monkeypox outbreak: wastewater and environmental surveillance perspective. Sci. Total Environ. 2023;856(Pt 2) doi: 10.1016/j.scitotenv.2022.159166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo V., Tillett R.L., Chang C.L., Gerrity D., Betancourt W.Q., Oh E.C. SARS-CoV-2 variant detection at a university dormitory using wastewater genomic tools. Sci. Total Environ. 2022;805 doi: 10.1016/j.scitotenv.2021.149930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannigama D.L., Amarasiri M., Hongsing P., Hurst C., Modchang C., Chadsuthi S., Anupong S., Phattharapornjaroen P., S M.A., Fernandez S., Huang A.T., Kueakulpattana N., Tanasatitchai C., Vatanaprasan P., Saethang T., Luk-In S., Storer R.J., Ounjai P., Ragupathi N.K.D., Kanthawee P., Sano D., Furukawa T., Sei K., Leelahavanichkul A., Kanjanabuch T., Hirankarn N., Higgins P.G., Kicic A., Chatsuwan T., McLellan A.D., Abe S. Multiple traces of monkeypox detected in non-sewered wastewater with sparse sampling from a densely populated metropolitan area in Asia. Sci. Total Environ. 2023;858(Pt 1) doi: 10.1016/j.scitotenv.2022.159816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe M.K., Duong D., Hughes B., Chan-Herur V., White B.J., Boehm A.B. Detection of monkeypox viral DNA in a routine wastewater monitoring program. medRxiv. 2022 [Google Scholar]
- Wolfe M., Hughes B., Duong D., Chan-Herur V., Wigginton K.R., White B.J., Boehm A.B. Detection of SARS-CoV-2 variants mu, beta, gamma, lambda, delta, alpha, and omicron in wastewater settled solids using mutation-specific assays is associated with regional detection of variants in clinical samples. Appl. Environ. Microbiol. 2022;88(8) doi: 10.1128/aem.00045-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe M.K., Yu A.T., Duong D., Rane M.S., Hughes B., Chan-Herur V., Donnelly M., Chai S., White B.J., Vugia D.J., Boehm A.B. Use of wastewater for Mpox outbreak surveillance in California. N. Engl. J. Med. 2023;388(6):570–572. doi: 10.1056/NEJMc2213882. [DOI] [PubMed] [Google Scholar]
- Zhan Q., Babler K.M., Sharkey M.E., Amirali A., Beaver C.C., Boone M.M., Comerford S., Cooper D., Cortizas E.M., Currall B.B., Foox J., Grills G.S., Kobetz E., Kumar N., Laine J., Lamar W.E., Mantero A.M.A., Mason C.E., Reding B.D., Robertson M., Roca M.A., Ryon K., Schurer S.C., Shukla B.S., Solle N.S., Stevenson M., Tallon J.J., Jr., Thomas C., Thomas T., Vidovic D., Williams S.L., Yin X., Solo-Gabriele H.M. Relationships between SARS-CoV-2 in wastewater and COVID-19 clinical cases and hospitalizations, with and without normalization against indicators of human waste. ACS ES T Water. 2022;2(11):1992–2003. doi: 10.1021/acsestwater.2c00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulli A., Pan A., Bart S.M., Crawford F.W., Kaplan E.H., Cartter M., Ko A.I., Sanchez M., Brown C., Cozens D., Brackney D.E., Peccia J. Predicting daily COVID-19 case rates from SARS-CoV-2 RNA concentrations across a diversity of wastewater catchments. FEMS Microbes. 2021;2 doi: 10.1093/femsmc/xtab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.