Abstract
Immunoproteasome is a variant of proteasome with structural differences in 20S subunits optimizing them for the production of antigenic peptides with higher binding affinity to major histocompatibility complex (MHC)-I molecules. Apart from this primary function in antigen presentation, immunoproteasome is also responsible for the degradation of proteins, both unfolded proteins for the maintenance of protein homeostasis and tumor suppressor proteins contributing to tumor progression. The altered expression of immunoproteasome is frequently observed in cancers; however, its expression levels and effects vary among different cancer types exhibiting antagonistic roles in tumor development. This review focuses on the dichotomous role of immunoproteasome in different cancer types, as well as summarizes the current progression in immunoproteasome activators and inhibitors. Specifically targeting immunoproteasome may be a beneficial therapeutic intervention in cancer treatment and understanding the role of immunoproteasome in cancers will provide a significant therapeutic insight for the prevention and treatment of cancers.
Key words: Immunoproteasome, Ubiquitin–proteasome system, Antigenic peptides, Proteolysis, Cancer, Immunotherapy, Proteasome inhibitor, Targeted therapy
Graphical abstract
This review summarizes the dichotomous role of immunoproteasome in different cancer types and analyses the underlying mechanisms, which provides significant insights for developing immunoproteasome-based targeted therapy in cancer treatment.
1. Introduction
Proteasomes are major components of the ubiquitin–proteasome system (UPS) which is the major pathway for protein degradation in eukaryotic cells. UPS act as a critical mechanism in cells for their dynamic and self-regulating quality control to adapt them to stress conditions and prevent prolonged cell damage1. In this system, proteasomes composed by 19S regulatory particle (19S RP) and 20S core particle (20S CP), exert central function in degradation of polyubiquitylated proteins generated by a cascade of E1, E2, and E3 enzymes that mediate activation, conjugation, and transfer of multiple ubiquitin moieties towards protein substrates ready for degradation2.
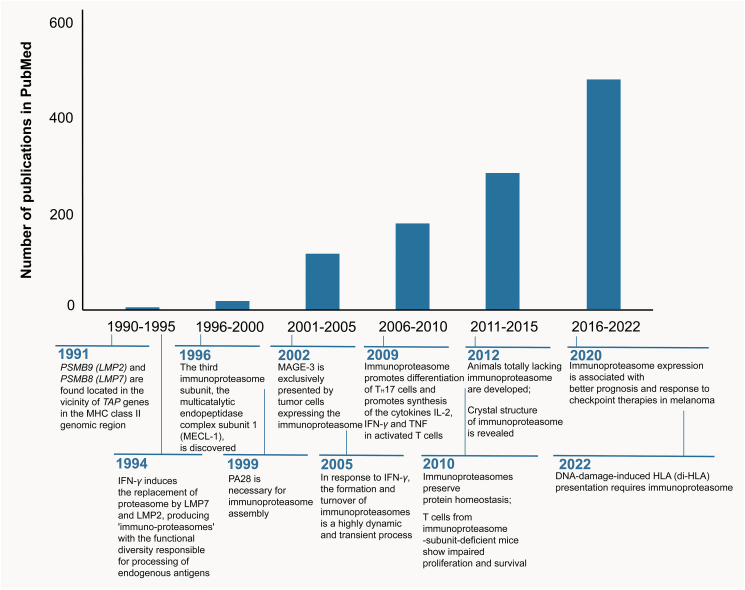
A second isoform of proteasome is immunoproteasome, a term that emphasizes its specialized role in processing intracellular antigens for the presentation by major histocompatibility complex (MHC) class Ⅰ and the subsequent recognition by immune system. In 1990s, the transporter associated with antigen processing (TAP), a pump that translocates peptides degraded by proteasomes from the cytoplasm to the endoplasmic reticulum, was founded in an antigen presentation-related study3. Shortly after, PSMB9 (LMP2) and PSMB8 (LMP7), encoding two alternative subunits of the 20S, β1i and β5i, respectively, were discovered in the proximity of the TAP genes, uncovering a latent role of immunoproteasomes in antigen presentation4. Then the activity of the proteasomes was observed to fluctuate with the treatment of interferon (IFN)-γ, and substitution of proteasome subunits β1, β2, and β5 to their corresponding immune subunits β1i, β2i, and β5i occurred in response to the treatment of this cytokine. This change of subunit structure mediated reduced caspase-like activity and enhanced chymotrypsin and trypsin activity of proteasomes, resulting in more peptides capable of binding to MHC-Ⅰ5 (Fig. 1).
Figure 1.
Key milestones and growth in the literature of immunoproteasome over time. Key events related to immunoproteasome, as well as the number of literatures listed in PubMed are showed.
The increased peptides generated by immunoproteasomes is in positive correlation with antigen presentation mediated by MHC-I essential for the activation of immune system2. Immune system could be applied to recognize and then destroy malignant cancer cells6,7. However, cancer cells often escape from immune system caused by multiple factors, among which, the inability of immune recognition by antitumor lymphocytes arose from defective antigen presentation by MHC-I pathway is a critical one8. Several types of cancers including non-small cell lung cancer (NSCLC)9, melanoma10,11, renal cell carcinoma (RCC)12,13, acute promyelocytic leukemia (APL)14 exhibited reduced antigen repertoire and presentation caused by a lack of immunoproteasome expression, associating with poor therapeutic outcomes. Additionally, the crosstalk between immunoproteasomes and immune checkpoint inhibitors (ICIs) is a newly emerging filed that are under intensive study and has presented a promising future. Overexpression of immunoproteasomes was found to synergize cancer cells to the response of ICIs in NSCLC and melanoma11,15, indicating significant roles of immunoproteasomes in cancer immunotherapy.
Apart from antigen-processing, another major function of immunoproteasomes is maintaining protein homeostasis. Immunoproteasomes were confirmed to facilitate the degradation of polyubiquitylated proteins, efficient in eliminating defective and unfolded proteins, preventing toxic effect of unbalanced protein environment, and preserving cell viability2,16. For instance, cardiomyocyte cells knocking down of β1i, β2i or β5i showed more susceptibility to doxorubicin-induced apoptosis17. Compared to standard proteasomes, immunoproteasomes even manifested enhanced proteasomal peptide-hydrolyzing activity, possibly resulting from the improved accessibility of protein to the active sites due to constructional changes17. Cancer cells rely heavily on the normal function of proteasomes to maintain protein homeostasis and improve their survival, making proteasome inhibitors shifting fine-tuned equilibrium towards cell death a powerful anti-tumor strategy18. Elevated immunoproteasomes were also frequently observed in several hematologic and solid cancer cells, such as multiple myeloma, gastric cancer, glioblastoma, and prostate tumors, making immunoproteasomes emerged as a potential therapeutic target. Intensive studies have focused on immunoproteasome inhibitors, potential to attenuate cytotoxicity and overcome resistance of proteasome inhibitors. Although none of them are currently in clinical trials, promising prospect can be expected in the future19.
Given that immunoproteasomes are closely related to cancer and play a significant role in tumor development but with context-dependent functions, in this review, we concentrate on dichotomous roles of immunoproteasomes based on their structural and functional characters, and discussed current strategies targeting immunoproteasomes in different circumstances by either activating or inhibiting them, in the hope of providing new insights for considering immunoproteasomes as potential targets in cancer treatment.
2. The structure of immunoproteasome
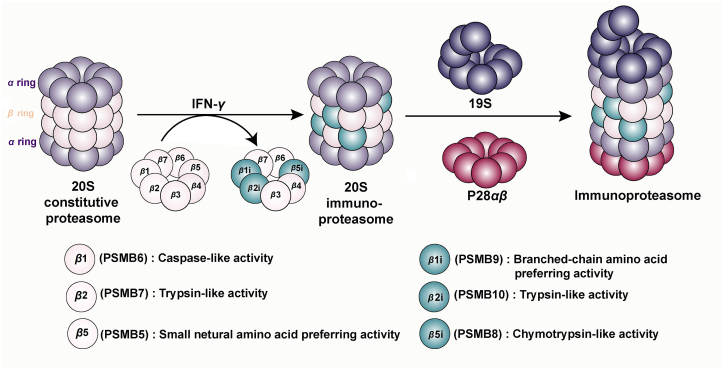
The 26S proteasome is a multifunctional proteolytic complex composing by 20S CP with proteolytic activity and 19S RP assisting substrates to enter the hydrolysis center of the proteasome in ATP-dependent way20. The constitutive CP is a cylindrically packed particle consisted of two outer α-rings and two inner β-rings, each of which is constituted by seven structurally identical α or β subunits. Among them, β1, β2, and β5 possess proteolytic functions and their S1 substrate-binding pockets which interact with amino acid residues, confer them with caspase-like, trypsin-like and small neutral amino acid-preferring activities respectively to cleave peptides21.
Within its biogenesis pathway, immunoproteasomes cooperatively and preferentially incorporate three immune subunits, β1i (LMP2/PSMB9), β2i (MECL-1/PSMΒ10), and β5i (LMP7/PSMB8) to substitute the constitutive catalytic subunits into their β ring22,23. Research have further revealed the structural differences between constitutive subunits and immune subunits, providing an explanation for the efficiency of immunoproteasome in processing and presenting antigens. In the substrate-binding pockets of β1i and β5i, subtle conformational changes were observed by analyzing crystal structure: Compared to β1, the S1 substrate-binding pocket of β1i possesses conserved substitutions such as R45L which replace the positively charged residue R45, making the pocket smaller, more hydrophobic, generating peptides with nonpolar C termini. β5i with chymotrypsin-like activity has a larger S1 pocket than β5, providing enough spaces to accommodate bulky hydrophobic residues. Therefore, immunoproteasomes produce more antigenic peptides with C-terminal hydrophobic residues that are preferential to the cleft of the MHC-I molecule24. Besides, during the de novo formation of the core particle, β5i directly binds to the assembly chaperone proteasome maturation protein (POMP), facilitating the fast assembly of immunoproteasomes and enabling the swift response to immune stimuli25,26. On the contrary, the β2i and β2 are structurally similar, with the identical trypsin-like activity24.
Regulatory particles are another significant component of proteasome, the binding of which to α-subunits promotes the opening of α-gate, enabling the entry of proteins into the catalytic compartment of the proteasome, influencing the repertoire of cleaved peptides27. PA28 which preferentially binds immunoproteasomes is the second most common regulatory particle except for 19S. There are three subunits of PA28, PA28α, β, and γ, among which, PA28αβ are upregulated by proinflammatory cytokines and binds to the end of immunoproteasome, promoting the supply of MHC-I-binding peptides28 (Fig. 2).
Figure 2.
Structure of immunoproteasome. The 20S proteasome is a cylindrical structure consist of two outer α- and two inner β-rings. Upon stimulation with interferon-γ (IFN-γ), proteasome subunits β1, β2, and β5 are substituted by their corresponding immune subunits β1i, β2i, and β5i, which equip immunoproteasome with catalytic activities that produce more antigenic peptides with higher binding affinity to major histocompatibility complex (MHC) class I molecules. Then 20S immunoproteasome is capped on 19S regulatory particles and PA28αβ on both sides to form immunoproteasome.
3. Physiological function of immunoproteasome
3.1. Antigen processing and presentation
In response to IFN-γ, the production of peptide by the proteasome and antigen presentation by MHC-I is a rate-limiting process, however, de novo generation of immunoproteasomes induced by IFN-γ permits a rapid response of immune system, ensures a quick expansion of peptides repertoire as immune defense. The formation and turnover of immunoproteasomes is a highly dynamic and transient process. In response to IFN-γ, the expression of POMP is upregulated. POMP with greater binding-affinity to β5i than β5 will efficiently recruit β5i subunit, facilitating the swift generation of immunoproteasomes, with the four-time faster formation rate than that of standard proteasomes. Conversely, immunoproteasomes exhibit a shorter half-lifer than standard proteasomes, allowing a rapid return to a normal situation of cells when the function of immunoproteasomes is no more needed29.
Concerning the functional significance of immunoproteasomes in response to inflammatory stimuli, the impairment of immuno-subunits can result in reduced activation of cytotoxic T lymphocytes and the subsequent deficient immune response. Indeed, study has confirmed that mice devoid of three immunoproteasome subunits exhibited severely impaired antigen presentation, with only approximate 50% of the MHC-I-binding peptides generated compared to that of wild-type mice, demonstrating a significant role of the immunoproteasome in antigen processing30. Studies have illustrated that some immunogenic antigens were exclusively generated by immunoproteasomes, such as Melanoma-antigen encoding gene (MAGE)-type antigens, encoded by MAGE family, the prototypes of cancer germline genes which is aberrantly expressed in multiple cancer types31. Recently, it was found that DNA-damage-induced human leukocyte antigen class I presentation was also specifically dependent on immunoproteasomes32. Nevertheless, the function of processing immunogenic peptides is shared between immuno- and standard proteasomes, as cells deficient in β1i and β5i can still express MHC-I and present immunogenic antigens, adding the complexity of the immune response33,34,35. For instance, Human ubiquitous protein RU1, human melanocytic protein Melan-A, GP100 and tyrosinase were observed to be better generated by the standard proteasome36. Further studies have revealed that this different processing pattern was mainly due to the different cleavage efficiency of the two proteasome types: for example, MAGE-C2 could be destructively cleaved by standard proteasome with high caspase-like activity thus they were solely presented by immunoproteasomes37. In addition to affecting repertoire of antigenic peptides, immunoproteasomes also contribute to the quantity and quality of neoantigens, which is irrelevant to proteasomes. In 12T cells (a melanoma cell line) that overexpress immunoproteasome subunits, more neoantigens and tumor-associated antigens (TAAs) with 7.3-fold overall higher reactivity were observed11.
3.2. Beyond antigen processing
In addition to the primary function in antigen processing mentioned above, immunoproteasomes also possess functions beyond antigen processing. Immunoproteasomes have been confirmed to carry out T cell-intrinsic roles in the maintenance and expansion of T cell populations during an immune response22. T cells from mice devoid of immunoproteasome subunits exhibited attenuated proliferation and survival38,39. Mechanistically, it was found that reduced extracellular signal-regulated kinases phosphorylation was accompanied with diminished lymphocyte activation induced by inhibition of immunoproteasome. However, how this attenuated extracellular signal-regulated kinases phosphorylation affects the functionality of T cells is still in need of further elucidation. Another hypothesis is that impaired proteostasis is responsible for the impaired T cell function induced by immunoproteasome inhibition. Since T cells were found to express the immunoproteasome constitutively, T cells are highly susceptible to cellular stress after inhibiting immunoproteasome40. In addition to impaired proliferation of T cells, Muchamuel et al.41 also indicated that the selective block of β5i impeded the differentiation of proinflammatory T helper type 17 (Th17) cells and compromised the generation of IL-23, IFN-γ and IL-2 in peripheral blood mononuclear cell (PBMC), while less so when blocking β5, making immunoproteasome inhibition an ideal strategy in treating inflammatory diseases such as arthritis.
Immunoproteasomes also play a critical role in the maintenance of protein homeostasis. As the basic responsibility of ubiquitin-proteasome system is mediating the degradation of non-functional and misfolded proteins thus maintaining the protein homeostasis and protecting cells from oxidative stress42,43, immunoproteasomes are believed to possess the same property. The expression of immuno-subunits was found to be increased in face of injury or stress, indicating the protective role of immunoproteasomes against unbalanced conditions44. Also, it was reported that under IFN-γ-triggered inflammatory and oxidative conditions, polyubiquitin conjugates were accumulated, and they were preferentially and efficiently degraded by immunoproteasomes2. However, this result is contradictory, and was counteracted by another investigation, stating that standard and immunoproteasomes were similarly capable of degrading polyubiquitinated proteins45. Thus, more efforts are still in need to provide more supportive evidence concerning the capability of immunoproteasomes in the maintenance of protein homeostasis.
4. Double-edged role of immunoproteasome in cancer
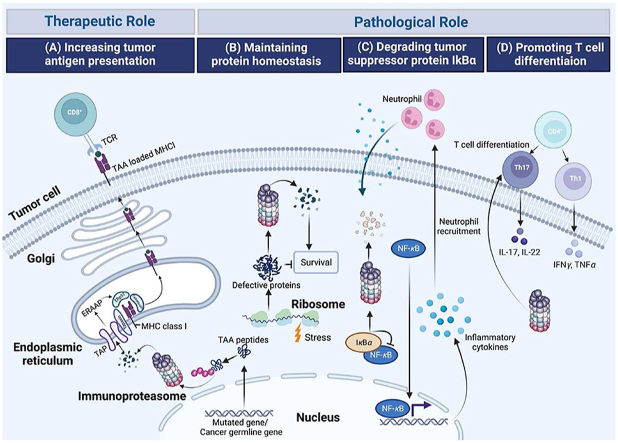
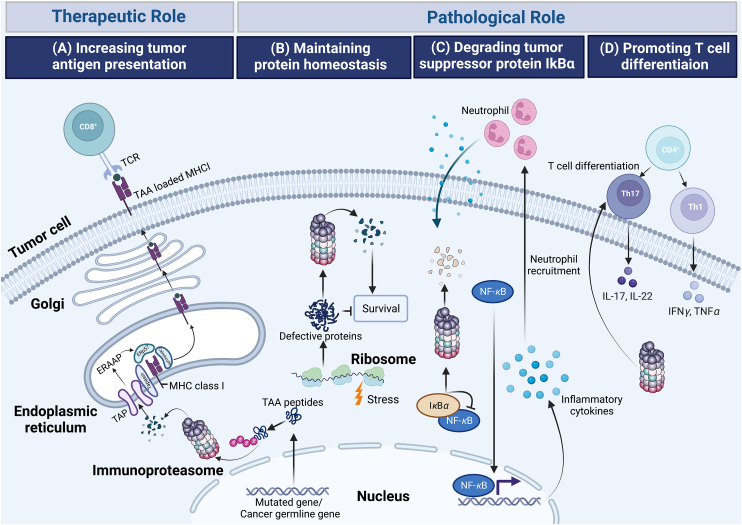
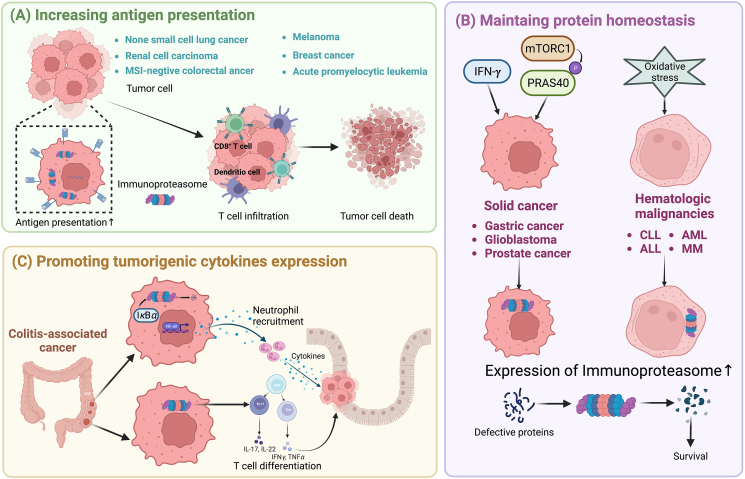
Given the multifunctional properties of immunoproteasomes, alteration in the assembly or expression of immunoproteasomes is diseases relevant. Indeed, immunoproteasome has emerging as a validated target in several different diseases, such as cancer, autoinflammatory syndromes and neurological disorders42. However, unlike the unidimensional role of immunoproteasome in autoimmune disorders, the function of immunoproteasome in cancer is multiple, exhibiting antagonistic roles in different cancer types. The elevated presentation of antigens by immunoproteasome can not only initiate a more efficient cytotoxic T cell response, exerting beneficial effects to immune system-induced eradication of cancer cells, but also enhances the efficacy of the immunotherapies that currently available and potentially enables the development of new therapeutic strategies. However, by maintaining protein homeostasis and promoting cytokine production, immunoproteasome is of help in shaping an ideal tumor microenvironment synergizing cancer development. Thus, immunoproteasomes exhibit a double-edged role in cancer (Fig. 3, Table 1).
Figure 3.
Schematic overview showing various activities of immunoproteasome in cancers. (A) Increasing tumor antigen presentation. Tumor-associated antigen (TAA) peptides encoded by mutated genes, or cancer germline genes are degraded by immunoproteasome and are translocated into endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP). TAA peptides, further trimmed by the ER-associated aminopeptidase (ERAAP) are then loaded onto Major histocompatibility complex (MHC) class I complexes with the assistance of peptide loading complex containing tapasin, calreticulim, and ERP57. TAA loaded MHC I then migrate to the cell surface where they are recognized by cytolytic T lymphocytes. (B) Maintaining protein homeostasis. Immunoproteasomes degrade non-functional, misfolded proteins thus maintaining protein homeostasis and protecting cells from proteotoxic stress. (C) Degrading tumor suppressor protein IκBα. Immunoproteasomal mediated degradation of IκBα leads to NF-κB-mediated secretion of cytokines, inducing the recruitment of neutrophils, promoting the initiation of colitis-associated cancers (CAC). (D) Promoting T cells differentiation. Immunoproteasome promotes the differentiation of proinflammatory T helper type 1 (Th1) and type 17 (Th17) cells and the resultant synthesis of the cytokines IL-17, IL-22, TNF and IFN-γ.
Table 1.
Various roles of immunoproteasome in different cancer types.
| Tumor type | Function of immunoproteasome | Outcome | Ref. |
|---|---|---|---|
| Non-small cell lung cancer | Increasing antigen presentation | Activating antigen- presentation machinery and reconstituting immune surveillance for tumor elimination | 10,11 |
| Melanoma | 11 | ||
| Renal cell carcinoma | 12,13 | ||
| MSI-negative colorectal cancer | 50 | ||
| Acute promyelocytic leukemia | 54 | ||
| Breast cancer | Increasing tumor-infiltrating lymphocytes | 56,57 | |
| Hematologic malignancies | Maintaining protein homeostasis | Agitating the survival, migration, and invasion of cancer cells | 69 |
| Gastric cancer | 70 | ||
| Glioblastoma | 71 | ||
| Prostate cancer | 72 | ||
| Colitis-associated cancer | Degradating tumor suppressor proteins IκBα | 76 | |
| Promoting tumorigenic cytokines expression | 79 |
4.1. Therapeutic role: Enhancing tumor antigen presentation and ICI response
The physiological role of immunoproteasomes in antigen presentation confer immunoproteasomes with anti-tumor property as the impairment of antigen presentation exists as the main mechanism contributing to the escape of immune surveillance and the failure of cancer immunotherapies6,46. Indeed, aberrant expression of immunoproteasomes is a predominant character of some malignant cells, which may partially account for the evasion of cytotoxic T lymphocytes-mediated recognition and elimination of these detrimental cells47. Non-small cell lung cancer (NSCLC) with epithelial-to-mesenchymal transition10,11 and renal cell carcinoma (RCC)12,13,48 both exhibited reduced immunoproteasome expression, which was associated with reduced presentation of antigen and poor clinical outcome, and the restoration of immunoproteasomes led to the reconstitution of immune surveillance and better clinical outcomes. In melanoma, the expression of immunoproteasome subunits was variable in different cell lines, with some exhibited overexpressed immunoproteasomes, while others did not49. The Cancer Genome Atlas data revealed that increased PSMB8 and PSMB9 expression is proportional to higher survival rate of patients, which were recently explained to arise from more neo-antigens and TAAs presented on melanoma cells11. In colorectal cancers, around 10%–15% of them showed the loss of MHC surface expression and the resultant immune evasion. Mutations of β2-microglobulin in microsatellite instability (MSI)-positive tumors and downregulation of PSMB8 in MSI-negative tumors appeared to be two major mechanisms accounting for this MHC-I antigen loss50. The decreased expression PSMB8 in colorectal tumor was further clarified to be arose from a polymorphism at amino acid 49 of PSMB8, which affected immunoproteasome assembly51. Acute promyelocytic leukemia (APL) also appeared undetectable HLAs with the impaired of antigen presentation52,53. Recently, the expression of immunoproteasomes was found to be downregulated in APL, accelerating the evasion of APL cells from immune surveillance in tumorigenesis, accounting for the pathogenesis of APL. The restoration of immunoproteasome expression may therefore promote more efficient T-cell-mediated immune actions via activating the antigen-processing/presentation machinery, highlighting a promising strategy in enhancing APL-specific immune responses54. In breast cancer, immunoproteasome expression is correlated with tumor-infiltrating lymphocytes (TILs) which act as a critical component in mediating chemotherapeutic response and improving overall survival rates of patients with breast cancers. Especially in triple negative breast cancers with greater than 50% infiltration of lymphocytics, the greatest survival benefit was observed due to increased TILs55. In each subtype of breast cancer including triple negative breast cancers, PSMB8 expression levels were observed to be proportional to the number of TILs, which might be associated with elevate expression of MHC I on tumor cells and were in positive correlation with better prognosis and longer survival of patients, while standard proteasomes failed to show the same remarkable association56,57 (Figure 3, Figure 4A).
Figure 4.
Schematic overview showing multiple mechanisms of immunoproteasome involvement among different cancer types. Tumorigenesis can be promoted or inhibited by immunoproteasome by various mechanisms. (A) In non-small cell lung cancer, renal cell carcinoma, melanoma, microsatellite instability (MSI)-negative colorectal cancer, acute promyelocytic leukemia and breast cancer, immunoproteasomal mediated increase of tumor antigen presentation leads to increased infiltration of T cell, culminating in the death of tumor cells. (B) In solid cancer, such as gastric cancer, glioblastoma, and prostate cancer, IFN-γ and phosphorylated proline-rich Akt substrate of 40 kDa (PRAS40) induced by hyperactivated mTOR complex 1 (mTORC1) mediates a shift of proteasome population to immunoproteasomes. In hematologic malignancies, such as chronic lymphocytic leukemia (CLL), lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and multiple myeloma (MM), increased oxidative stress leads to enhanced expression of immunoproteasome. Overexpressed immunoproteasome, maintaining protein homeostasis eventually results in increased survival rate of cancer cells. (C) In colitis-associated cancer, immunoproteasome mediates the degradation of IB and differentiation of T cells into inflammatory T helper cells, resulting in the generation of pro-inflammatory cytokines and the subsequent tumorigenesis.
High expression of immunoproteasomes is also found to be related to a favorable response to ICI treatment, a major component of cancer immunotherapy that have substantially extended life span of cancer patients, working by unleashing T cells from inhibitory signal thus activating immune system and the resultant antitumor responses58,59. Despite the success, some patients do not respond to this therapeutic effect, others develop resistance at the late treatment stage. The lacking of neoantigens recognized by circulating T cells were founded to play a significant role in this nonresponse and resistance to ICI, as a high-level exposure of MHC-I on the surface of cancer cells is a prerequisite for effective response to anti-CTLA4 antibodies and pre-existing IFN-γ correlates with the response of cancer cells to anti-PD-1 antibodies54,60. Consist with these findings, the benefits of immunoproteasomes were indicated by a durable benefit of ICI for patients and improved overall survival in both NSCLC and melanoma patients with overexpressed immunoproteasomes11,15.
Moreover, the expression level of immunoproteasome subunits can serve as biomarker for predicting ICI response. Tumor mutation burden (TMB) is a leading marker for predicting therapeutic effect of ICI, conceptually based on the postulation that production of antigenic peptides enhancing immunogenicity is proportional to the numbers of mutant proteins61. However, it has limited usage rage, only available for cancers whose infiltration of CD8+ T cell is in positive correlation with neoantigen load62, thus making additional markers in requirement. Recently, it was found that compared to TMB, PSMB8 and PSMB9 were more potent predictive biomarkers in melanoma patients and independent of TMB, CD8+ T-cell infiltration and IFN-γ. Immunoproteasome subunit expression combined with mutational load exhibited the highest prediction accuracy in evaluating the therapeutic effect of anti-CTLA-4 antibodies. In the evaluation of anti-PD-1 antibodies, clinical efficacy was positively correlated with immunoproteasome subunit expression, but not with mutation load11.
4.2. Pathological role: Exerting agitational effects on malignancies
In addition to the beneficial function of enhancing immune response in cancer treatment, immunoproteasomes also play significant roles in the maintenance of protein homeostasis, degradation of tumor suppressor proteins and modulation of tumorigenic cytokines expression, accelerating the development of cancer.
It is well-established that many cancer cells rely heavily on the normal function of proteasomes to maintain the protein homeostasis, since cancer are characterized by abnormal protein production and proteotoxic stress arising from genome mutations, making the elimination of excessive and defective proteins crucial to the survival and invasion of cancer cells63,64. Along with proteasomes, the overexpression of immunoproteasomes is also frequently observed in many cancer cells, in order to obtain more efficient turnover of defective proteins and cell survival. The expression levels of immunoproteasome are commonly found to be increased in hematopoietic-original cells65. By using ProCISE assay, Francesco et al.66 demonstrated that in cells of hematopoietic origin, such as PBMCs and multiple myeloma CD138+ tumor cells, β5i were predominantly expressed. Although, to our knowledge, no direct research has yet delineated the underling mechanism, we propose that this elevated expression of immunoproteasome in hematopoietic-original cells may be caused by the heavy reliance on UPS system and overwhelming protein burden, as hematopoietic system depends heavily on UPS system to maintain normal function, the loss of which can lead to transformation and leukemogenesis67 and hematologically derived tumor cells such as MM is characterized by the strong need of proteasome to compensate heavy protein burden, caused by the overexpression of immunoglobulin molecules68. In hematologic malignancies of both lymphocytic and myeloid origin such as multiple myeloma (MM), acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), and lymphoblastic leukemia (ALL), immunoproteasomes are found to be upregulated19, among which, ALL showed remarkably higher immunoproteasome-to-proteasome ration than AML, making suppression of immunoproteasomes a rational strategy in treatment of hematologic malignancies69. Apart from hematological cancers, immunoproteasomes are also overexpressed in several solid cancers such as gastric cancer70, glioblastoma71 and prostate tumors72 and are responsible for their migration and invasion. Nevertheless, the underlying mechanisms of these overexpression in solid and hematological cancer are different. In solid cancers, the copious amounts of IFN-γ, released by influx of tumor-infiltrating lymphocytes in the tumor microenvironment64 and the proline-rich Akt substrate of 40 kDa (PRAS40), phosphorylated by hyperactivated mTOR complex 1 induced a shift of proteasome population to immunoproteasomes73. In hematological cancers, however, the overexpression of immunoproteasomes is IFN-γ-independent, which is rather related to the methylation status of immunoproteasome genes in face of oxidative stress57. Additionally, the capacity of immunoproteasome in keeping proteostasis is also regarded as a mechanism of cisplatin resistance in cancer treatment. In cisplatin-resistant lung and gastric cancer cells, increased expression of immunoproteasome subunits was found as a compensatory action to circumvent the cell stress and oxidative stress induced by cisplatin treatment, and inhibition of immunoproteasomes partially restored the therapeutic effect of cisplatin74,75 (Figure 3, Figure 4B).
The pro-tumor role of immunoproteasomes also reflects in the proteasomal degradation of tumor suppressor genes. Proteasomes can target and degrade tumor suppressor protein involving in carcinogenesis and cancer survival such as p53, cyclins, cyclin-dependent kinase inhibitor, pro-apoptotic proteins, and NF-κB inhibitor IκBα76. Immunoproteasomes were found to be responsible for the degradation of some of them. Transcription factor NF-κB, regulating the cytokines secreted by inflammatory cells appear to be the key mechanism in chronic inflammation, and is one of the most frequent risk factors of the development of intestinal tumor and progression to malignancy77. IκBα is the suppressor of NF-κB, which leads to the suppression of canonical NF-κB pathways78. In colitis-associated cancers (CAC), immunoproteasomes mediated IκBα degradation, leading to NF-κB-induced secretion of IL-17A which involved in the generation of pro-inflammatory signals and the recruitment of neutrophils into the colon thus promoting carcinogenesis. PSMB8 knock out mice were resistant to this chronic inflammation, shown by the decreased expression of chemokines Cxcl1, Cxcl2, and Cxcl3 and the subsequent CAC development79 (Figure 3, Figure 4C). Additionally, immunoproteasomes also degrade important components in the pathways of cell proliferation and apoptosis. For instance, in cardiac hypertrophy model, upregulated immunoproteasomes promoted degradation of PTEN thus leading to the activation of Akt/mTOR and suppression of AMPK signals80. Overexpressed β1i, β2i or β5i were also found to degrade pro-apoptotic protein ASK1 and p53, resulting in attenuated cardiomyocyte apoptosis17. As sustained proliferative signaling and resistant cell death are hallmarks of cancer81, immunoproteasomes are conceivable to participate in tumor cell growth, however in short of evidence, it remains to be explored in the future.
Additionally, concerning their role in the regulation of proinflammatory immune cells, immunoproteasomes are capable of shaping tumor microenvironment by promoting immune cells-dependent cytokine production fundamental for the initiation of inflammation-related cancers. By promoting recruitment and activation of T cells, immunoproteasomes increased cytokine and chemokine production that perpetuate inflammatory reactions resulting in the development of CAC. Mice lacking all three immunoproteasomes presented with an impaired recruitment of IFN-γ producing Th1 cells and IL-17 producing Th17 cells as well as a lower frequency of infiltrating neutrophils along with reduced tumor numbers10 (Figure 3, Figure 4C).
5. Immunoproteasome as an emerging target in cancer
As mentioned above, immunoproteasomes play critical and context-dependent roles in tumor progression, making them potential target in cancer treatment. Different strategies are adopted as either activating or inhibiting immunoproteasomes based on their dichotomous roles. Here, we summarize these strategies.
5.1. Immunoproteasome activator
As mentioned previously, the elevated expression of immunoproteasomes has beneficial effect in several cancer types by enhancing anti-cancer immune, making activation immunoproteasome as a rational therapeutic strategy. The generation of immunoproteasomes is cytokine-dependent, making cytokines potential candidate of immunoproteasomes activator82. Synthesis and integration of immunoproteasome subunits into core particle are mediated by proinflammatory cytokines such as IFN-γ, IFN-α or IFN-β, and tumor necrosis factor, among which, IFN-γ is the most potent counterpart, making cytokine treatment a feasible strategy in activating immunoproteasomes5,22,83. IFN-γ treatment was observed to upregulate the expression of PSMB9, enabling the enhanced recognition of renal carcinoma cells by the immune system84. IFN-γ also restored the depleted repertoire of HLA class I-bound peptides in mesenchymal cells lacking immunoproteasome thus overcoming immune evasion of NSCL cells9.
Apart from cytokines, immunoproteasomes can also be activated by some chemical agents. DNA methyltransferase inhibitors (DNMTis) are one of them. DNA methylation, mediated by DNA methyltransferases acts as an epigenetic silencing marker that is responsible for transcriptional repression85. The expression of immunoproteasomes was observed to be regulated by DNA methylation: PSMB8 and PSMB9 expression were inversely correlated to the intensity of DNA methylation on several cytosines located in their coding regions57, which can be reactivated by 5-aza-2′-deoxycytidine (5-aza-dC), a typical DNMTis86. Treatment of 5-aza-dC were found to upregulate the expression of PSMB8 and PSMB9 at both RNA and protein levels in colon87 and NSCL9 cells and restored their antitumor ability. All-trans retinoic acid (ATRA) is another chemical agent widely used in cancer treatment, which is reported to activate immunoproteasomes88. ATRA is currently the standard treatment for APL, which is characterized by a unique chromosomal translocation t (15;17) that leads to the formation of the promyelocytic leukemia (PML)–retinoic acid receptor α (RARα) fusion protein89. ATRA can specifically act on PML–RARα by converting them from a transcriptional repressor to a transcriptional activator or by inducing their degradation14. Recently, PML–RARα was found to interact with transcription factor PU.1 which bind to and coordinately transactivated the promoters of PSMBs through protein–protein interaction, thus repressing PU.1-dependent activation of immunoproteasomes, facilitating immune evasion of APL cells. ATRA treatment could abolish this PML/RARα-mediated suppression of immunoproteasomes and thus restoring their expression, leading to reconstituted immune surveillance in APL54 (Table 2).
Table 2.
Compounds targeting immunoproteasome in cancer treatment.
| Type | Name | Target | Structure | Effective against | IC50 | Clinical status | Ref. |
|---|---|---|---|---|---|---|---|
| Activator | IFN-γ | PSMB9 | / | Renal carcinoma, NSCLC | / | Preclinical | 9,84 |
| 5-aza-dC | PSMB8, PSMB9 | 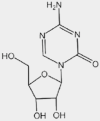 |
Colon carcinoma, NSCLC | ns | On sales | 9,87 | |
| ATRA | PML/RARα-PU.1-PSMBs | 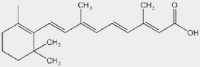 |
APL | ns | On sales | 54 | |
| Inhibitor | ONX-0914 | β1i, β5i | 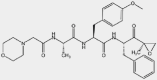 |
MLL-rearranged AML, ALL, MM, colorectal carcinoma | 5.7 nmol/L | Preclinical | 95,101,102 |
| UK-101 | β1i | 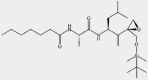 |
MM, Prostate cancer | 0.104 μmol/L | Preclinical | 72,107 | |
| IPSI-001 | β1i | 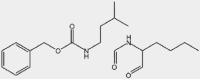 |
MM, CLL, AML, NHL | 1.45 μmol/L | Preclinical | 110 | |
| PR-924 | β5i | 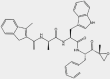 |
AML, ALL, MM | 2.5 nmol/L | Preclinical | 113 | |
| LU-035i | β5i | 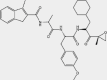 |
MM | 0.11 μmol/L | Preclinical | 116. | |
| M3258 | β5i | 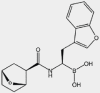 |
MM | 4.1 nmol/L | Phase I trial terminated IND: NCT04075721 |
117 |
Of note, there is a potential crosstalk between immunoproteasome activators and ICIs, as immunoproteasomes are indicated to play a positive role in improving the outcome of ICIs. However, although it was reported that the generation of IFN-γ by infiltrating T lymphocytes was the major driver of clinical response to ICIs in melanoma90, there is no direct evidence showing that the dosage of IFN-γ or other immunoproteasome activators with ICIs could achieve better therapeutic effect. Nevertheless, it remains an interesting therapeutic strategy that worth exploring in the future.
5.2. Immunoproteasome inhibitor
Given the frequent existence of upregulated immunoproteasomes in hematologic and several solid cancer cells, targeting immunoproteasomes appear to be an ideal therapeutic strategy against cancers. Compared to solid cancers, proteasome or immunoproteasome inhibitors are more used in hematologic malignancies and exhibits better sensitivity64. Research has found that in hematological cells, expression of proteasomes was decreased during differentiation, leading to excessive proteotoxic stress which correlates with increased sensitivity to proteasome inhibitors91. In solid cancers, inhibitors failed to achieve the same benefit probably due to the insufficient potency. For maximum proteasome inhibition in solid tumors, multiple subunits should be targeted simultaneously. It was found that apart from β5 inhibition, additional β2 or β2i suppression was required to aggregate Nrf1, which impedes the upregulation of proteasome genes and inhibits the recovery of proteasome activity thus leading to cell death in solid tumors92. Additionally, it was proposed that antiapoptotic and mitogenic signaling pathway such as epidermal growth factor receptor, extracellular signal-regulated kinase and phosphoinositide-3-kinase protein/Akt pathway activated by proteasome inhibitors might involve in their lack of efficacy against solid cancer cells93.
Bortezomib, carfilzomib, and ixazomib, indiscriminately target both proteasomes and immunoproteasomes are first-line proteasome inhibitors that have been approved by food and drug administration for clinical use94. However, this lack of discrimination has result in severe side effects such as cardiac toxicities95, since the required proteasomal function for normal cell survival96. Thus, selective inhibitors of immunoproteasomes, summarized in Table 2 are developed to overcome side effects while maintaining anti-tumor efficacy and as alternatives to deal with drug resistance.
5.2.1. Pan β1i and β5i inhibitor
ONX-0914 (PR-957) is the first developed immunoproteasome-selective inhibitor that contains a ketoepoxide pharmacophore which contributes to the modification of proteasomal N-terminal threonine active site and presents improved inhibition activity to β5i than to β5c subunit41,97. Although initially regarded as β5i-selective, it has been re-categorized as a pan-immunoproteasome inhibitor targeting both β1i and β5i subunits recently98. This compound was found to block the presentation of β1i-specific, MHC-I-restricted antigens99 and reduced the production of IL-23 by inducing the apoptosis of CD14+ monocytes100 and has been widely applied in diseases with elevated immunoproteasomes levels such as chronic inflammatory syndromes, some types of cancers. In hematologic malignancies, treatment of ONX-0914 led to accumulation of polyubiquitinylated proteins and decreased cell viability in MLL-rearranged AML57 and also ameliorated proliferation of ALL95 and MM cells101.There are also some combination strategies reported concerning ONX-0914 in the treatment of MM. ONX-0914 combined with bortezomib achieved elevated survival in mice of MM model. Besides, β2 inhibitor LU-102 were found synergized with ONX-0914, causing reduced viability of MM cells101. In solid cancer, ONX-0914 was found to block the tumor initiation and progression of colorectal carcinoma102.
5.2.2. β1i inhibitor
UK-101 is the first reported β1i-selective inhibitor. The upregulation of β1i were found to be more frequent in malignant cells than normal cells by analyses of clinical samples103, indicating a promising prospect of UK-101. Structurally speaking, UK-101 is derivative of dihydroeponemycin with N-terminal hydrophobic heptanoic tail that points to the interface of β1i subunits and C-terminal tert-butyldimethylsilyl group that inhibits β5i modification104,105. UK-101 covalently target on β1i with an IC50 value of 0.104 μmol/L, which is 144-fold selectivity over β1i106. The growth of MM cells and those with developed bortezomib resistance were reduced when dosed with UK-10107. Moreover, UK-101 has also been shown to inhibit proliferation of prostate cancer cells both in vitro and in vivo without obvious side-effect72. However, the off-target effect to β5 subunits have been observed, which was proposed to arise from the progressive loss of tert-butyldimethylsilyl group108. IPSI-001 is another immunoproteasomes inhibitor with peptidyl-aldehyde backbone that specifically targets β1i subunits109. IPSI-001 showed antiproliferative and apoptotic effects through both intrinsic and extrinsic pathways in MM, CLL, AML and B-cell non-Hodgkin lymphoma cell lines. Moreover, IPSI-001 were found to able to overcome drug resistance to both chemotherapeutics and bortezomib using drug-resistance models. Additionally, IPSI-001 synthesized with dexamethasone, exhibited more remarkable antiproliferative action in MM cells110. However, the defective potency of IPSI-001 has precluded in vivo testing110. Moreover, as peptide aldehyde, its aldehyde head group is inclined to oxidative inactivation and could co-inhibit serine and cysteine proteases, which may bring unwanted side effects111. Either way, concerning its selectivity and activity in vitro, IPSI-001 remains to be a promising leading compound and are potential to be used as a backbone that can be added with other functional groups to further increase its potency.
5.2.3. β5i inhibitor
PR-924 is a selective β5i inhibitor mediating modification of N-terminal threonine sites with covalent action112. This compound was confirmed to prevent proliferation and initiate apoptosis in ALL, AML and MM cell lines, including those resistant to bortezomib without affecting normal cells113. In vivo, PR-924 also induced decreased MM growth and extended survival112. However, PR-924 were found to inhibit both β5i and β5 at the applied concentrations, suggesting that the sole β5i inhibition may not enough to induce anti-cancer effect113. Moreover, PR-924-treated cells were observed to acquire drug resistance due to mutations of PSMB5 gene and no synergistic actions of PR-924 in combination of chemotherapeutic agents were observed114. Thus, more research is in requirement to evaluate whether PR-924 is potential enough for further development. LU-035i, derived from PR-924 is one of the most selective inhibitors for the β5i subunit with IC50 value of 0.11, 500 μmol/L to β5i and β5 respectively115. Recently a peptide epoxyketone conjugating LU-035i to doxorubicin, a well-studied chemical agent in hematological cancers treatment were yield, exerting both toxicity and immunoproteasome inhibition actions to MM cells, resulting more death of them116. M3258 is a newly developed, highly β5i-selective inhibitor with approximately 500-fold greater specificity to the β5i subunit than other sites. This optimized potency and selectivity are achieved by α-aminoboronic acid scaffold interacted with immunoproteasome subunits64. This compound has preclinically shown great potential. After M3258 treatment, the viability of MM, leukemia and lymphoma cells were found to reduce 50%107,117. Treatment of this inhibitor also demonstrated ideal anti-MM effects in vivo64. Clinically, although as a single agent or in combination with dexamethasone, M3258 entered a phase I clinical trial for the treatment of MM in 2019, it has been recently terminated due to altered therapeutic landscape and lack of recruitment and supportive data.
6. Conclusion and perspective
The aberrant expression of immunoproteasome in cancer is a universally acknowledged phenomenon, and the underlying molecular mechanisms have been elucidated to be diverse and context-dependent, explaining why activation and inhibition of immunoproteasomes have contradictory roles in different cancers27,118,119. On the one hand, immunoproteasomes promote the generation of neoantigen peptides which is necessary for the initiation of an efficient cytotoxic T cell response, exerting beneficial effects to eradicate cancer cells escaping from the monitor of immune system22; on the other hand, cancer cells rely more on an appropriate function of ubiquitin-proteasome system than normal cells due to the swift proliferation rate, elevated metabolic activity, and constant exposure to a variety of extrinsic perturbations27,120. By maintaining homeostasis of tumor microenvironment and promoting cytokine production, immunoproteasomes synergize cancer development. These two opposite functions even intertwined in inflammation-related cancers such as colorectal cancer exhibiting both immune evasion due to inadequate antigen processing pathway50 and cancer cells invasion due to undesired pro-inflammatory T cell response79. However, fitting in the adequate tumoral and clinical context, the immunoproteasome remains an attractive target. Thus, the key question for optimizing this strategy is to delineate the various functionality of the immunoproteasome under different circumstances to develop suitable therapeutic approach by either activating or inhibiting them.
Small molecule therapies targeting immunoproteasomes have thus attracted attention as a novel class of anti-cancer drugs. The discovery and application of proteasome inhibitors in clinical practice have therapeutically revolutionized the treatment of MM, significantly improving the survival rate of patients with refractory forms of this abhorrent cancer. Despite the beneficial effects, the lack of specificity as well as potency, the initiation of side effects, and the development of drug resistance are the remarkable disadvantages that limit the therapeutic use of proteasome inhibitor26. By contrast, selective inhibitors of immunoproteasomes have mitigated effect on nonneoplastic cells, making immunoproteasomes a promising drug target. While hematological cancers are at the forefront of this application, treatment of solid cancer just entering the stage, requiring further investigation. Compared to myeloma cells, the threshold to mediate apoptosis by inhibiting proteasome in solid tumors is probably higher, making blockage of more proteasome sites for prolonged periods a reasonable approach to reach a similar degree of actions as which in myeloma cells76. And of note, the development of immunoproteasome inhibitors is quiet lagging behind, with no compounds for cancer treatment entering the clinical trials. We propose that the lack of tools analyzing the composition and activity of immunoproteasome is probably responsible for this slowed development. There are two types of probs available, that are fluorogenic probe, composed by amino acids and 7-amino-4-methylcoumarin, which can determine the β subunits that inhibitors target on and distinguish immunoproteasome from standard proteasome121, and activity-based probes, which was developed on the basis of selective immunoproteasome inhibitors and was designed by attaching a fluorophore to covalent inhibitors of the β subunits122. However, there are some drawbacks that heavily limit their use. For 7-amino-4-methylcoumarin-based substrate probes, the poor cell permeability has confined their usage range only in cell lysates. For the inhibitor-based probes, they failed to monitor activity continuously as they bind to the β subunits irreversibly. Thus, improved probs and assay techniques are still needed as they are crucial link in developing immunoproteasome inhibitors. Since the process of drug discovery is expensive and time-consuming, in order to bring the immunoproteasome inhibitors to clinical stage as soon as possible, an ideal solution may be to identify new uses for existing drugs123. As β5i is characterized by chymotryptic-like catalytic activity, chymotrypsin inhibitors may become potential candidates as selective immunoproteasome inhibitors.
In contrast to the intensive investigation and utilization of immunoproteasome inhibitors, there are still limited exploration of immunoproteasome activators with only handful of them with restricted utility range having been reported, providing a potential filed worth exploring. Since proteasomes and immunoproteasomes have different inclinations for cleavage sites, it is also of interesting to investigate these different repertoires of displayed antigens. Some epitopes may exist that are better processed by immunoproteasomes in certain cancers benefit from overexpressed immunoproteasomes, which can be applied into design of adoptive immunotherapies and vaccines, providing a unique direction to find tumor neoantigens as well as optimize the effectiveness of cancer immunotherapies, showing a prospering prospect.
In conclusion, immunoproteasomes are becoming a potential target in cancer treatment. Since the functionality of the immunoproteasome is various in different cancer types, the critical aspect of the development of immunoproteasomes-based target therapies is to elucidate their functions under different circumstances. In recent years, remarkable progress has been made to understand the immunoproteasomes. However, there is still much left to be explored. The important aspects of understanding a protein lie in the elucidation of its activities and identification of pathway it participates in, but the lack of standardized and sensitive probs has confined the further understanding of immunoproteasome and the development of immunoproteasome regulators. Either way, we expect that, with further research, the exact roles of immunoproteasomes in cancer will be elaborated and the clinical utility of immunoproteasome regulators will be expanded in the future in general.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (No. 81930102 to Bo Yang), Zhejiang Provincial Natural Science Foundation (No. LR22H310002 to Ji Cao, China) and Zhejiang University K.P. Chao's High Technology Development Foundation (China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Author contributions
Concept design: Ji Cao and Boya Chen; Boya Chen, Haiying Zhu, Bo Yang, and Ji Cao wrote the manuscript; Ji Cao and Bo Yang directed the study.
Conflicts of interest
The authors declare no competing interests.
References
- 1.Pohl C., Dikic I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science. 2019;366:818–822. doi: 10.1126/science.aax3769. [DOI] [PubMed] [Google Scholar]
- 2.Seifert U., Bialy L.P., Ebstein F., Bech-Otschir D., Voigt A., Schröter F., et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell. 2010;142:613–624. doi: 10.1016/j.cell.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 3.Blum J.S., Wearsch P.A., Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez C.K., Monaco J.J. Homology of proteasome subunits to a major histocompatibility complex-linked LMP gene. Nature. 1991;353:664–667. doi: 10.1038/353664a0. [DOI] [PubMed] [Google Scholar]
- 5.Aki M., Shimbara N., Takashina M., Akiyama K., Kagawa S., Tamura T., et al. Interferon-γ induces different subunit organizations and functional diversity of proteasomes. J Biol Chem. 1994;115:257–269. doi: 10.1093/oxfordjournals.jbchem.a124327. [DOI] [PubMed] [Google Scholar]
- 6.Vigneron N., Abi Habib J., Van den Eynde B.J. Learning from the proteasome how to fine-tune cancer immunotherapy. Trends Cancer. 2017;3:726–741. doi: 10.1016/j.trecan.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y., Yu X., Thamphiwatana S.D., Zheng Y., Pang Z. Nanomedicines modulating tumor immunosuppressive cells to enhance cancer immunotherapy. Acta Pharm Sin B. 2020;10:2054–2074. doi: 10.1016/j.apsb.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinay D.S., Ryan E.P., Pawelec G., Talib W.H., Stagg J., Elkord E., et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35:185–198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Tripathi S.C., Peters H.L., Taguchi A., Katayama H., Wang H., Momin A., et al. Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Proc Natl Acad Sci U S A. 2016;113:E1555–E1564. doi: 10.1073/pnas.1521812113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leister H., Luu M., Staudenraus D., Mollenkopf H., Sharma A., Schmerer N., et al. Pro-and antitumorigenic capacity of immunoproteasomes in shaping the tumor microenvironment. Cancer Immunol Res. 2021;9:682–692. doi: 10.1158/2326-6066.CIR-20-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalaora S., Lee J.S., Barnea E., Levy R., Greenberg P., Alon M., et al. Immunoproteasome expression is associated with better prognosis and response to checkpoint therapies in melanoma. Nat Commun. 2020;11:1–12. doi: 10.1038/s41467-020-14639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkins D., Ferrone S., Schmahl G.E., Störkel S., Seliger B. Down-regulation of HLA class I antigen processing molecules: an immune escape mechanism of renal cell carcinoma?. J Urol. 2004;171:885–889. doi: 10.1097/01.ju.0000094807.95420.fe. [DOI] [PubMed] [Google Scholar]
- 13.Seliger B., Hammers S., Höhne A., Zeidler R., Knuth A., Gerharz C.D., et al. IFN-gamma-mediated coordinated transcriptional regulation of the human TAP-1 and LMP-2 genes in human renal cell carcinoma. Clin Cancer Res. 1997;3:573–578. [PubMed] [Google Scholar]
- 14.Ablain J., de Thé H. Retinoic acid signaling in cancer: the parable of acute promyelocytic leukemia. Int J Cancer. 2014;135:2262–2272. doi: 10.1002/ijc.29081. [DOI] [PubMed] [Google Scholar]
- 15.Thompson J.C., Davis C., Deshpande C., Hwang W.T., Jeffries S., Huang A., et al. Gene signature of antigen processing and presentation machinery predicts response to checkpoint blockade in non-small cell lung cancer (NSCLC) and melanoma. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basler M., Kirk C.J., Groettrup M. The immunoproteasome in antigen processing and other immunological functions. Curr Opin Immunol. 2013;25:74–80. doi: 10.1016/j.coi.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Zhao W.J., Wei S.N., Zeng X.J., Xia Y.L., Du J., Li H.H. Gene expression profiling identifies the novel role of immunoproteasome in doxorubicin-induced cardiotoxicity. Toxicology. 2015;333:76–88. doi: 10.1016/j.tox.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Manasanch E.E., Orlowski R.Z. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol. 2017;14:417–433. doi: 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Besse A., Kraus M., Mendez-Lopez M., Maurits E., Overkleeft H.S., Driessen C., et al. Immunoproteasome activity in chronic lymphocytic leukemia as a target of the immunoproteasome—selective inhibitors. Cells. 2022;11:838. doi: 10.3390/cells11050838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudriaeva A., Kuzina E.S., Zubenko O., Smirnov I.V., Belogurov A., Jr. Charge-mediated proteasome targeting. FASEB J. 2019;33:6852–6866. doi: 10.1096/fj.201802237R. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K. The proteasome: overview of structure and functions. Proc Jpn Acad Ser B. 2009;85:12–36. doi: 10.2183/pjab.85.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murata S., Takahama Y., Kasahara M., Tanaka K. The immunoproteasome and thymoproteasome: functions, evolution and human disease. Nat Immunol. 2018;19:923–931. doi: 10.1038/s41590-018-0186-z. [DOI] [PubMed] [Google Scholar]
- 23.Groettrup M., Standera S., Stohwasser R., Kloetzel P.M. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc Natl Acad Sci U S A. 1997;94:8970–8975. doi: 10.1073/pnas.94.17.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber E.M., Basler M., Schwab R., Heinemeyer W., Kirk C.J., Groettrup M., et al. Immuno- and constitutive proteasome crystal structures reveal differences in substrate and inhibitor specificity. Cell. 2012;148:727–738. doi: 10.1016/j.cell.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Griffin T.A., Nandi D., Cruz M., Fehling H.J., Kaer L.V., Monaco J.J., et al. Immunoproteasome assembly: cooperative incorporation of interferon γ (IFN-γ)-inducible subunits. J Exp Med. 1998;187:97–104. doi: 10.1084/jem.187.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tundo G., Sbardella D., Santoro A., Coletta A., Oddone F., Grasso G., et al. The proteasome as a druggable target with multiple therapeutic potentialities: cutting and non-cutting edges. Pharmacol Ther. 2020;213 doi: 10.1016/j.pharmthera.2020.107579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tundo G.R., Sbardella D., Oddone F., Kudriaeva A.A., Lacal P.M., Belogurov A.A., et al. At the cutting edge against cancer: a perspective on immunoproteasome and immune checkpoints modulation as a potential therapeutic intervention. Cancers. 2021;13:4852. doi: 10.3390/cancers13194852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cascio P. PA28αβ: the enigmatic magic ring of the proteasome?. Biomolecules. 2014;4:566–584. doi: 10.3390/biom4020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heink S., Ludwig D., Kloetzel P.M., Krüger E. IFN-γ-induced immune adaptation of the proteasome system is an accelerated and transient response. Proc Natl Acad Sci U S A. 2005;102:9241–9246. doi: 10.1073/pnas.0501711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kincaid E.Z., Che J.W., York I., Escobar H., Reyes-Vargas E., Delgado J.C., et al. Mice completely lacking immunoproteasomes show major changes in antigen presentation. Nat Immunol. 2012;13:129–135. doi: 10.1038/ni.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma W., Vigneron N., Chapiro J., Stroobant V., Germeau C., Boon T., et al. A MAGE-C2 antigenic peptide processed by the immunoproteasome is recognized by cytolytic T cells isolated from a melanoma patient after successful immunotherapy. Int J Cancer. 2011;129:2427–2434. doi: 10.1002/ijc.25911. [DOI] [PubMed] [Google Scholar]
- 32.Uchihara Y., Permata T.B.M., Sato H., Kawabata-Iwakawa R., Katada S., Gu W., et al. DNA damage promotes HLA class I presentation by stimulating a pioneer round of translation-associated antigen production. Mol Cell. 2022;82:2557–2570. doi: 10.1016/j.molcel.2022.04.030. [DOI] [PubMed] [Google Scholar]
- 33.Ferrington D.A., Gregerson D.S. Immunoproteasomes: structure, function, and antigen presentation. Prog Mol Biol Transl Sci. 2012;109:75–112. doi: 10.1016/B978-0-12-397863-9.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yewdell J., Lapham C., Bacik I., Spies T., Bennink J. MHC-encoded proteasome subunits LMP2 and LMP7 are not required for efficient antigen presentation. J Immunol. 1994;152:1163–1170. [PubMed] [Google Scholar]
- 35.Arnold D., Driscoll J., Androlewicz M., Hughes E., Cresswell P., Spies T. Proteasome subunits encoded in the MHC are not generally required for the processing of peptides bound by MHC class I molecules. Nature. 1992;360:171–174. doi: 10.1038/360171a0. [DOI] [PubMed] [Google Scholar]
- 36.Chapiro J., Claverol S., Piette F., Ma W., Stroobant V., Guillaume B., et al. Destructive cleavage of antigenic peptides either by the immunoproteasome or by the standard proteasome results in differential antigen presentation. J Immunol. 2006;176:1053–1061. doi: 10.4049/jimmunol.176.2.1053. [DOI] [PubMed] [Google Scholar]
- 37.Chapatte L., Ayyoub M., Morel S., Peitrequin A.L., Lévy N., Servis C., et al. Processing of tumor-associated antigen by the proteasomes of dendritic cells controls in vivo T-cell responses. Cancer Res. 2006;66:5461–5468. doi: 10.1158/0008-5472.CAN-05-4310. [DOI] [PubMed] [Google Scholar]
- 38.Zaiss D.M., de Graaf N., Sijts A.J. The proteasome immunosubunit multicatalytic endopeptidase complex-like 1 is a T-cell-intrinsic factor influencing homeostatic expansion. Infect Immun. 2008;76:1207–1213. doi: 10.1128/IAI.01134-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moebius J., van den Broek M., Groettrup M., Basler M. Immunoproteasomes are essential for survival and expansion of T cells in virus-infected mice. Eur J Immunol. 2010;40:3439–3449. doi: 10.1002/eji.201040620. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt C., Berger T., Groettrup M., Basler M. Immunoproteasome inhibition impairs T and B cell activation by restraining ERK signaling and proteostasis. Front Immunol. 2018;9:2386. doi: 10.3389/fimmu.2018.02386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muchamuel T., Basler M., Aujay M.A., Suzuki E., Kalim K.W., Lauer C., et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat Med. 2009;15:781–787. doi: 10.1038/nm.1978. [DOI] [PubMed] [Google Scholar]
- 42.Kaur G., Batra S. Emerging role of immunoproteasomes in pathophysiology. Immunol Cell Biol. 2016;94:812–820. doi: 10.1038/icb.2016.50. [DOI] [PubMed] [Google Scholar]
- 43.Upadhyay A. Natural compounds in the regulation of proteostatic pathways: an invincible artillery against stress, ageing, and diseases. Acta Pharm Sin B. 2021;11:2995–3014. doi: 10.1016/j.apsb.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussong S.A., Kapphahn R.J., Phillips S.L., Maldonado M., Ferrington D.A. Immunoproteasome deficiency alters retinal proteasome’s response to stress. J Neurochem. 2010;113:1481–1490. doi: 10.1111/j.1471-4159.2010.06688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nathan J.A., Spinnenhirn V., Schmidtke G., Basler M., Groettrup M., Goldberg A.L. Immuno-and constitutive proteasomes do not differ in their abilities to degrade ubiquitinated proteins. Cell. 2013;152:1184–1194. doi: 10.1016/j.cell.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aptsiauri N., Cabrera T., Garcia-Lora A., Lopez-Nevot M.A., Ruiz-Cabello F., Garrido F. MHC class I antigens and immune surveillance in transformed cells. Int Rev Cytol. 2007;256:139–189. doi: 10.1016/S0074-7696(07)56005-5. [DOI] [PubMed] [Google Scholar]
- 47.Seliger B., Maeurer M.J., Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–464. doi: 10.1016/s0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 48.Seliger B., Atkins D., Bock M., Ritz U., Ferrone S., Huber C., et al. Characterization of human lymphocyte antigen class I antigen-processing machinery defects in renal cell carcinoma lesions with special emphasis on transporter-associated with antigen-processing down-regulation. Clin Cancer Res. 2003;9:1721–1727. [PubMed] [Google Scholar]
- 49.Woods K., Knights A.J., Anaka M., Schittenhelm R.B., Purcell A.W., Behren A., et al. Mismatch in epitope specificities between IFNγ inflamed and uninflamed conditions leads to escape from T lymphocyte killing in melanoma. J Immunother Cancer. 2016;4:1–14. doi: 10.1186/s40425-016-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cabrera C., Jimenez P., Cabrera T., Esparza C., Ruiz-Cabello F., Garrido F. Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: β2-microglobulin inactivation in MSI-positive tumors and LMP7/TAP2 downregulation in MSI-negative tumors. Tissue Antigens. 2003;61:211–219. doi: 10.1034/j.1399-0039.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 51.Fellerhoff B., Gu S., Laumbacher B., Nerlich A.G., Weiss E.H., Glas J., et al. The LMP7-K allele of the immunoproteasome exhibits reduced transcript stability and predicts high risk of colon cancer. Cancer Res. 2011;71:7145–7154. doi: 10.1158/0008-5472.CAN-10-1883. [DOI] [PubMed] [Google Scholar]
- 52.Zheng P., Guo Y., Niu Q., Levy D.E., Dyck J.A., Lu S., et al. Proto-oncogene PML controls genes devoted to MHC class I antigen presentation. Nature. 1998;396:373–376. doi: 10.1038/24628. [DOI] [PubMed] [Google Scholar]
- 53.Promsuwicha O., Auewarakul C.U. Positive and negative predictive values of HLA-DR and CD34 in the diagnosis of acute promyelocytic leukemia and other types of acute myeloid leukemia with recurrent chromosomal translocations. Asian Pac J Allergy Immunol. 2009;27:209. [PubMed] [Google Scholar]
- 54.Yang X., Wang P., Liu J., Zhang H., Xi W., Jia X., et al. Coordinated regulation of the immunoproteasome subunits by PML/RARα and PU. 1 in acute promyelocytic leukemia. Oncogene. 2014;33:2700–2708. doi: 10.1038/onc.2013.224. [DOI] [PubMed] [Google Scholar]
- 55.Stanton S.E., Disis M.L. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:1–7. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee M., Song I.H., Heo S.H., Kim Y.A., Park I.A., Bang W.S., et al. Expression of immunoproteasome subunit LMP7 in breast cancer and its association with immune-related markers. Cancer Res Treat. 2019;51:80–89. doi: 10.4143/crt.2017.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rouette A., Trofimov A., Haberl D., Boucher G., Lavallée V.P., D'Angelo G., et al. Expression of immunoproteasome genes is regulated by cell-intrinsic and -extrinsic factors in human cancers. Sci Rep. 2016;6 doi: 10.1038/srep34019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bagchi S., Yuan R., Engleman E.G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 59.Sun X., Zhao X., Yang C., Shi M., Zhang B., et al. Combining immune checkpoint. blockade with ATP-based immunogenic cell death amplifier for cancer chemo-immunotherapy. Acta Pharm Sin B. 2022;12:3694–3709. doi: 10.1016/j.apsb.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodig S.J., Gusenleitner D., Jackson D.G., Gjini E., Giobbie-Hurder A., Jin C., et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Transl Med. 2018;10:eaar332. doi: 10.1126/scitranslmed.aar3342. [DOI] [PubMed] [Google Scholar]
- 61.Chan T.A., Yarchoan M., Jaffee E., Swanton C., Quezada S.A., Stenzinger A., et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGrail D., Pilié P., Rashid N., Voorwerk L., Slagter M., Kok M., et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32:661–672. doi: 10.1016/j.annonc.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fosgerau K., Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Zerfas B.L., Maresh M.E., Trader D.J. The immunoproteasome: an emerging target in cancer and autoimmune and neurological disorders. J Med Chem. 2020;63:1841–1858. doi: 10.1021/acs.jmedchem.9b01226. [DOI] [PubMed] [Google Scholar]
- 65.Noda C., Tanahashi N., Shimbara N., Hendil K.B., Tanaka K. Tissue distribution of constitutive proteasomes, immunoproteasomes, and PA28 in rats. Biochem Biophys Res Commun. 2000;277:348–354. doi: 10.1006/bbrc.2000.3676. [DOI] [PubMed] [Google Scholar]
- 66.Parlati F., Lee S.J., Aujay M., Suzuki E., Levitsky K., Lorens J.B., et al. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood. 2009;114:3439–3447. doi: 10.1182/blood-2009-05-223677. [DOI] [PubMed] [Google Scholar]
- 67.Moran-Crusio K., Reavie L.B., Aifantis I. Regulation of hematopoietic stem cell fate by the ubiquitin proteasome system. Trends Immunol. 2012;33:357–363. doi: 10.1016/j.it.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meister S., Schubert U., Neubert K., Herrmann K., Burger R., Gramatzki M., et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67:1783–1792. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 69.Niewerth D., Franke N.E., Jansen G., Assaraf Y.G., van Meerloo J., Kirk C.J., et al. Higher ratio immune versus constitutive proteasome level as novel indicator of sensitivity of pediatric acute leukemia cells to proteasome inhibitors. Haematologica. 2013;98:1896–1904. doi: 10.3324/haematol.2013.092411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwon C.H., Park H.J., Choi Y.R., Kim A., Kim H.W., Choi J.H., et al. PSMB8 and PBK as potential gastric cancer subtype-specific biomarkers associated with prognosis. Oncotarget. 2016;7:21454–21468. doi: 10.18632/oncotarget.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang H.H., Cheng Y.C., Tsai W.C., Chen Y. PSMB8 inhibition decreases tumor angiogenesis in glioblastoma through vascular endothelial growth factor A reduction. Cancer Sci. 2020;111:4142–4153. doi: 10.1111/cas.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wehenkel M., Ban J.O., Ho Y.K., Carmony K.C., Hong J.T., Kim K.B. A selective inhibitor of the immunoproteasome subunit LMP2 induces apoptosis in PC-3 cells and suppresses tumour growth in nude mice. Br J Cancer. 2012;107:53–62. doi: 10.1038/bjc.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yun Y.S., Kim K.H., Tschida B., Sachs Z., Noble-Orcutt K.E., Moriarity B.S., et al. mTORC1 coordinates protein synthesis and immunoproteasome formation via PRAS40 to prevent accumulation of protein stress. Mol Cell. 2016;61:625–639. doi: 10.1016/j.molcel.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shoji T., Kikuchi E., Kikuchi J., Takashima Y., Furuta M., Takahashi H., et al. Evaluating the immunoproteasome as a potential therapeutic target in cisplatin-resistant small cell and non-small cell lung cancer. Cancer Chemother Pharmacol. 2020;85:843–853. doi: 10.1007/s00280-020-04061-9. [DOI] [PubMed] [Google Scholar]
- 75.Matsunaga T., Tsuchimura S., Azuma N., Endo S., Ichihara K., Ikari A. Caffeic acid phenethyl ester potentiates gastric cancer cell sensitivity to doxorubicin and cisplatin by decreasing proteasome function. Anticancer Drugs. 2019;30:251–259. doi: 10.1097/CAD.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 76.Basler M., Groettrup M. Recent insights how combined inhibition of immuno/proteasome subunits enables therapeutic efficacy. Genes Immun. 2020;21:273–287. doi: 10.1038/s41435-020-00109-1. [DOI] [PubMed] [Google Scholar]
- 77.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hayashi T., Faustman D. Essential role of human leukocyte antigen-encoded proteasome subunits in NF-kappaB activation and prevention of tumor necrosis factor-alpha-induced apoptosis. J Biol Chem. 2000;275:5238–5247. doi: 10.1074/jbc.275.7.5238. [DOI] [PubMed] [Google Scholar]
- 79.Vachharajani N., Joeris T., Luu M., Hartmann S., Pautz S., Jenike E., et al. Prevention of colitis-associated cancer by selective targeting of immunoproteasome subunit LMP7. Oncotarget. 2017;8:50447–50459. doi: 10.18632/oncotarget.14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen C., Zou L.X., Lin Q.Y., Yan X., Bi H.L., Xie X., et al. Resveratrol as a new inhibitor of immunoproteasome prevents PTEN degradation and attenuates cardiac hypertrophy after pressure overload. Redox Biol. 2019;20:390–401. doi: 10.1016/j.redox.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 82.Boes B., Hengel H., Ruppert T., Multhaup G., Koszinowski U.H., Kloetzel P.M. Interferon gamma stimulation modulates the proteolytic activity and cleavage site preference of 20S mouse proteasomes. J Exp Med. 1994;179:901–909. doi: 10.1084/jem.179.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kniepert A., Groettrup M. The unique functions of tissue-specific proteasomes. Trends Biochem Sci. 2014;39:17–24. doi: 10.1016/j.tibs.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 84.Seliger B., Hammers S., Höhne A., Zeidler R., Knuth A., Gerharz C.D., et al. IFN-gamma-mediated coordinated transcriptional regulation of the human TAP-1 and LMP-2 genes in human renal cell carcinoma. Clin Cancer Res. 1997;3:573–578. [PubMed] [Google Scholar]
- 85.Baylin S.B., Jones P.A. A decade of exploring the cancer epigenome—biological and translational implications. Nat Rev Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li H., Chiappinelli K.B., Guzzetta A.A., Easwaran H., Yen R.W., Vatapalli R., et al. Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget. 2014;5:587–598. doi: 10.18632/oncotarget.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siebenkäs C., Chiappinelli K.B., Guzzetta A.A., Sharma A., Jeschke J., Vatapalli R., et al. Inhibiting DNA methylation activates cancer testis antigens and expression of the antigen processing and presentation machinery in colon and ovarian cancer cells. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haque A., Banik N.L., Ray S.K. Emerging role of combination of all-trans retinoic acid and interferon-gamma as chemoimmunotherapy in the management of human glioblastoma. Neurochem Res. 2007;32:2203–2209. doi: 10.1007/s11064-007-9420-z. [DOI] [PubMed] [Google Scholar]
- 89.Ni X., Hu G., Cai X. The success and the challenge of all-trans retinoic acid in the treatment of cancer. Crit Rev Food Sci Nutr. 2019;59:71–80. doi: 10.1080/10408398.2018.1509201. [DOI] [PubMed] [Google Scholar]
- 90.Grasso C.S., Tsoi J., Onyshchenko M., Abril-Rodriguez G., Ross-Macdonald P., Wind-Rotolo M., et al. Conserved interferon-γ signaling drives clinical response to immune checkpoint blockade therapy in melanoma. Cancer Cell. 2020;38:500–515. doi: 10.1016/j.ccell.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cenci S., Mezghrani A., Cascio P., Bianchi G., Cerruti F., Fra A., et al. Progressively impaired proteasomal capacity during terminal plasma cell differentiation. EMBO J. 2006;25:1104–1113. doi: 10.1038/sj.emboj.7601009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weyburne E.S., Wilkins O.M., Sha Z., Williams D.A., Pletnev A.A., de Bruin G., et al. Inhibition of the proteasome β2 site sensitizes triple-negative breast cancer cells to β5 inhibitors and suppresses Nrf1 activation. Cell Chem Biol. 2017;24:218–230. doi: 10.1016/j.chembiol.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang Z., Wu Y., Zhou X., Xu J., Zhu W., Shu Y., et al. Efficacy of therapy with bortezomib in solid tumors: a review based on 32 clinical trials. Future Oncol. 2014;10:1795–1807. doi: 10.2217/fon.14.30. [DOI] [PubMed] [Google Scholar]
- 94.Park J.E., Miller Z., Jun Y., Lee W., Kim K.B. Next-generation proteasome inhibitors for cancer therapy. Transl Res. 2018;198:1–16. doi: 10.1016/j.trsl.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jenkins T.W., Downey-Kopyscinski S.L., Fields J.L., Rahme G.J., Colley W.C., Israel M.A., et al. Activity of immunoproteasome inhibitor ONX-0914 in acute lymphoblastic leukemia expressing MLL-AF4 fusion protein. Sci Rep. 2021;11 doi: 10.1038/s41598-021-90451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weathington N.M., Mallampalli R.K. Emerging therapies targeting the ubiquitin proteasome system in cancer. J Clin Invest. 2014;124:6–12. doi: 10.1172/JCI71602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huber E.M., Basler M., Schwab R., Heinemeyer W., Kirk C.J., Groettrup M., et al. Immuno- and constitutive proteasome crystal structures reveal differences in substrate and inhibitor specificity. Cell. 2012;148:727–738. doi: 10.1016/j.cell.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 98.Basler M., Lindstrom M.M., LaStant J.J., Bradshaw J.M., Owens T.D., Schmidt C., et al. Co-inhibition of immunoproteasome subunits LMP2 and LMP7 is required to block autoimmunity. EMBO Rep. 2018;19 doi: 10.15252/embr.201846512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muchamuel T., Basler M., Aujay M.A., Suzuki E., Kalim K.W., Lauer C., et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat Med. 2009;15:781–787. doi: 10.1038/nm.1978. [DOI] [PubMed] [Google Scholar]
- 100.Basler M., Claus M., Klawitter M., Goebel H., Groettrup M. Immunoproteasome inhibition selectively kills human CD14+ monocytes and as a result dampens IL-23 secretion. J Immunol. 2019;203:1776–1785. doi: 10.4049/jimmunol.1900182. [DOI] [PubMed] [Google Scholar]
- 101.Downey-Kopyscinski S., Daily E.W., Gautier M., Bhatt A., Florea B.I., Mitsiades C.S., et al. An inhibitor of proteasome β2 sites sensitizes myeloma cells to immunoproteasome inhibitors. Blood Adv. 2018;2:2443–2451. doi: 10.1182/bloodadvances.2018016360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koerner J., Brunner T., Groettrup M. Inhibition and deficiency of the immunoproteasome subunit LMP7 suppress the development and progression of colorectal carcinoma in mice. Oncotarget. 2017;8:50873–50888. doi: 10.18632/oncotarget.15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miller Z., Ao L., Bo Kim K., Lee W. Inhibitors of the immunoproteasome: current status and future directions. Curr Pharm Des. 2013;19:4140–4151. doi: 10.2174/1381612811319220018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim K.B., Myung J., Sin N., Crews C.M. Proteasome inhibition by the natural products epoxomicin and dihydroeponemycin: insights into specificity and potency. Bioorg Med Chem Lett. 1999;9:3335–3340. doi: 10.1016/s0960-894x(99)00612-5. [DOI] [PubMed] [Google Scholar]
- 105.Lei B., Abdul Hameed M.D.M., Hamza A., Wehenkel M., Muzyka J.L., Yao X.J., et al. Molecular basis of the selectivity of the immunoproteasome catalytic subunit LMP2-specific inhibitor revealed by molecular modeling and dynamics simulations. J Phy Chem B. 2010;114:12333–12339. doi: 10.1021/jp1058098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Bruin G., Huber E.M., Xin B.T., van Rooden E.J., Al-Ayed K., Kim K.B., et al. Structure-based design of β1i or β5i specific inhibitors of human immunoproteasomes. J Med Chem. 2014;57:6197–6209. doi: 10.1021/jm500716s. [DOI] [PubMed] [Google Scholar]
- 107.Kim H.R., Tagirasa R., Yoo E. Covalent small molecule immunomodulators targeting the protease active site. J Med Chem. 2021;64:5291–5322. doi: 10.1021/acs.jmedchem.1c00172. [DOI] [PubMed] [Google Scholar]
- 108.Huber E.M., Groll M. A nut for every bolt: subunit-selective inhibitors of the immunoproteasome and their therapeutic potential. Cells. 2021;10:1929. doi: 10.3390/cells10081929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Borissenko L., Groll M. 20S proteasome and its inhibitors: crystallographic knowledge for drug development. Chem Rev. 2007;107:687–717. doi: 10.1021/cr0502504. [DOI] [PubMed] [Google Scholar]
- 110.Kuhn D.J., Hunsucker S.A., Chen Q., Voorhees P.M., Orlowski M., Orlowski R.Z. Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors. Blood. 2009;113:4667–4676. doi: 10.1182/blood-2008-07-171637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thompson R.C. Peptide aldehydes: potent inhibitors of serine and cysteine proteases. Methods Enzymol. 1977;46:220–225. doi: 10.1016/s0076-6879(77)46023-3. [DOI] [PubMed] [Google Scholar]
- 112.Singh A.V., Bandi M., Aujay M.A., Kirk C.J., Hark D.E., Raje N., et al. PR-924, a selective inhibitor of the immunoproteasome subunit LMP-7, blocks multiple myeloma cell growth both in vitro and in vivo. Br J Haematol. 2011;152:155–163. doi: 10.1111/j.1365-2141.2010.08491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Niewerth D., van Meerloo J., Jansen G., Assaraf Y.G., Hendrickx T.C., Kirk C.J., et al. Anti-leukemic activity and mechanisms underlying resistance to the novel immunoproteasome inhibitor PR-924. Biochem Pharmacol. 2014;89:43–51. doi: 10.1016/j.bcp.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 114.Niewerth D., Meerloo J.V., Assaraf Y., Jansen G., Kaspers G. The novel immunoproteasome inhibitor PR-924: anti-leukemic activity and mechanisms of resistance. Blood. 2013;122:3841. doi: 10.1016/j.bcp.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 115.de Bruin G., Huber E.M., Xin B.T., van Rooden E.J., Al-Ayed K., Kim K.B., et al. Structure-based design of β1i or β5i specific inhibitors of human immunoproteasomes. J Med Chem. 2014;57:6197–6209. doi: 10.1021/jm500716s. [DOI] [PubMed] [Google Scholar]
- 116.Maurits E., van de Graaff M.J., Maiorana S., Wander D.P.A., Dekker P.M., van der Zanden S.Y., et al. Immunoproteasome inhibitor-doxorubicin conjugates target multiple myeloma cells and release doxorubicin upon low-dose photon irradiation. J Am Chem Soc. 2020;142:7250–7253. doi: 10.1021/jacs.9b11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Downey-Kopyscinski S.L., Simoes R.D.M., Bariteau M., Borah M.L., Bender S., Foneska J., et al. Pharmacological perturbation of the immunoproteasome in hematologic neoplasias: therapeutic implications. Blood. 2019;134:1291. [Google Scholar]
- 118.Tripathi S.C., Vedpathak D., Ostrin E.J. The functional and mechanistic roles of immunoproteasome subunits in cancer. Cells. 2021;10:3587. doi: 10.3390/cells10123587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaur G., Batra S. Emerging role of immunoproteasomes in pathophysiology. Immunol Cell Biol. 2016;94:812–820. doi: 10.1038/icb.2016.50. [DOI] [PubMed] [Google Scholar]
- 120.Neubert N.J., Tillé L., Barras D., Soneson C., Baumgaertner P., Rimoldi D., et al. Broad and conserved immune regulation by genetically heterogeneous melanoma cells. Cancer Res. 2017;77:1623–1636. doi: 10.1158/0008-5472.CAN-16-2680. [DOI] [PubMed] [Google Scholar]
- 121.Winter M.B., La Greca F., Arastu-Kapur S., Caiazza F., Cimermancic P., Buchholz T.J., et al. Immunoproteasome functions explained by divergence in cleavage specificity and regulation. Elife. 2017;6 doi: 10.7554/eLife.27364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berkers C.R., Verdoes M., Lichtman E., Fiebiger E., Kessler B.M., Anderson K.C., et al. Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nat Methods. 2005;2:357–362. doi: 10.1038/nmeth759. [DOI] [PubMed] [Google Scholar]
- 123.Chong C.R., Sullivan D.J. New uses for old drugs. Nature. 2007;448:645–646. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]