Abstract
Chronic heart failure (CHF) is a severe public health problem with increasing morbidity and mortality, any treatment targeting a single session is insufficient to tackle this. CHF is characterized by reduced cardiac output resulting from neurohumoral dysregulation and cardiac remodeling, which might be related to oxidative stress, inflammation, endoplasmic reticulum stress, apoptosis, autophagy, mitochondrial function, and angiogenesis. These molecular mechanisms interact with each other through crosstalk. Historically, Chinese medicinal herbs have been widely applied in the treatment of CHF, and therapeutic effects of Chinese medicinal herbs and their ingredients have been scientifically confirmed over the past decades. Traditional Chinese medicine (TCM) with multiple components can confront the different pathogenesis of CHF through multiple targets. This review analyzes commonly used TCM patent drugs and TCM decoctions that are applicable to different stages of CHF based on clinical trials. Diverse bioactive ingredients in Chinese medicinal herbs have been found to treat CHF via multiple molecular mechanisms. This review comprehensively covers the key works on the effects and underlying mechanisms of TCM, herbal ingredients and synergistic effects of constituent compatibility in treating CHF, providing additional ideas to address this threat.
Key words: Natural ingredients, Chronic heart failure, Cardiac remodeling, Chinese medicinal herbs, Traditional Chinese medicine formula, Multiple targets, Constituent compatibility, Synergistic effects
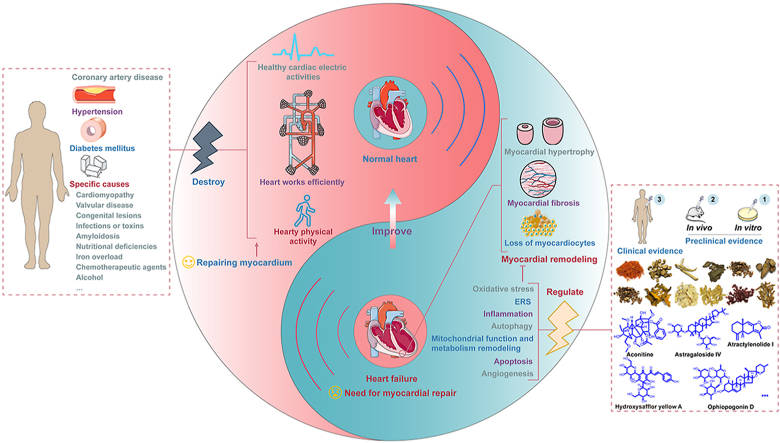
Graphical abstract
This review provides the progress of traditional Chinese herbal medicines and their ingredients against chronic heart failure from the perspective of clinical evidence and preclinical evidence.
1. Introduction
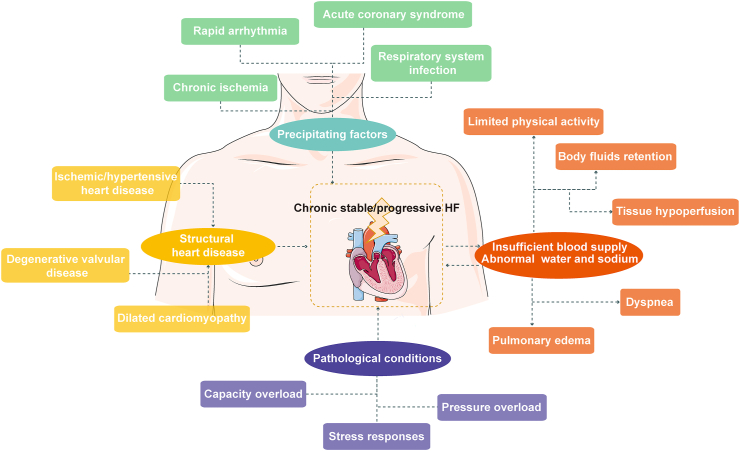
Chronic heart failure (CHF) is a set of complicated clinical syndromes triggered by aberrant changes in the structure and/or function of the heart brought on by a variety of factors. This condition results in compromised ventricular systolic and/or diastolic function when the heart is unable to pump sufficient blood to meet the metabolic needs of the tissues1,2. It is the outcome of numerous end-stage cardiovascular disorders, which present as dyspnea, dyskinesia, and fluid retention (Fig. 1). The Summary of the 2018 Report on Cardiovascular Diseases in China stated that there are 4.5 million people with CHF. There is a 30-day mortality rate of 4.1% for hospitalized CHF patients in China3. CHF has been identified as one of the leading causes of morbidity and mortality, posing a hazard to 1%–2% of adults and tending to affect younger people4. Unfortunately, despite significant worldwide efforts and funding invested in the discovery of anti-CHF drugs each year, and even with optimal treatment available after diagnosis, the overall survival rate after five years is still only 46% so far5,6.
Figure 1.
Chronic heart failure is the end stage of multiple cardiovascular diseases.
Currently, chemical medications that have evolved from “diuretic, cardiotonic, and vasodilator” to the combination of “diuretics, angiotensin-converting enzyme inhibitors, and β-blockers” are used to support conventional therapy for CHF in clinics. These drugs can rapidly improve the hemodynamic index and reduce myocardial oxygen and energy expenditure to help CHF patients relieve symptoms. However, they are unable to entirely address the root cause of CHF and its complications7. The rising prevalence of CHF is a serious issue due to the paucity of drugs that can effectively treat failing hearts.
Traditional Chinese medicine (TCM) remedies are an invaluable source for the creation of new anti-CHF medications. Many Chinese medicinal herbs, including Astragali Radix (Huangqi), Salviae Miltiorrhizae Radix (Danshen), Codonopsis Radix (Dangshen), Notoginseng Radix (Sanqi), etc., have been used successfully in China for thousands of years as CHF treatments. The beneficial effects of TCM formulas made up of Chinese medicinal herbs have recently been further supported by evidence-based medical investigations. Depending on the stages of CHF, different TCM formulas can be selected. At the same time, TCM formulas may be better able to exploit the advantages of multi-targeting and multi-functionality due to the complexity and mixed conditions of CHF patients.
TCM formulas are usually more rapidly adopted into clinical practice, based on thousands of years of rich human experience, whereas chemical agents require a lengthy review process. More profoundly, dozens of TCM formulas, hundreds of Chinese medicinal herbs, and their extracts or constituents have been reported to possess pharmacological research-approved anti-CHF bioactivities, shining a light on the already stagnant development of anti-CHF drugs. For example, cardiac glycosides have been developed as medications to treat CHF and arrhythmias for decades.
Huge efforts have been made over the past few decades to understand how these natural substances impact the pathophysiology of CHF. In this review, we examined the available proprietary TCM formulas from an evidence-based medical standpoint and analyzed their research status as well as potential clinical application situations. Ulteriorly, we summarized the updated progress in the prevention and treatment of CHF with respect to Chinese medicinal herbs and their main ingredients and analyzed their underlying mechanisms.
2. Pathogenesis of CHF
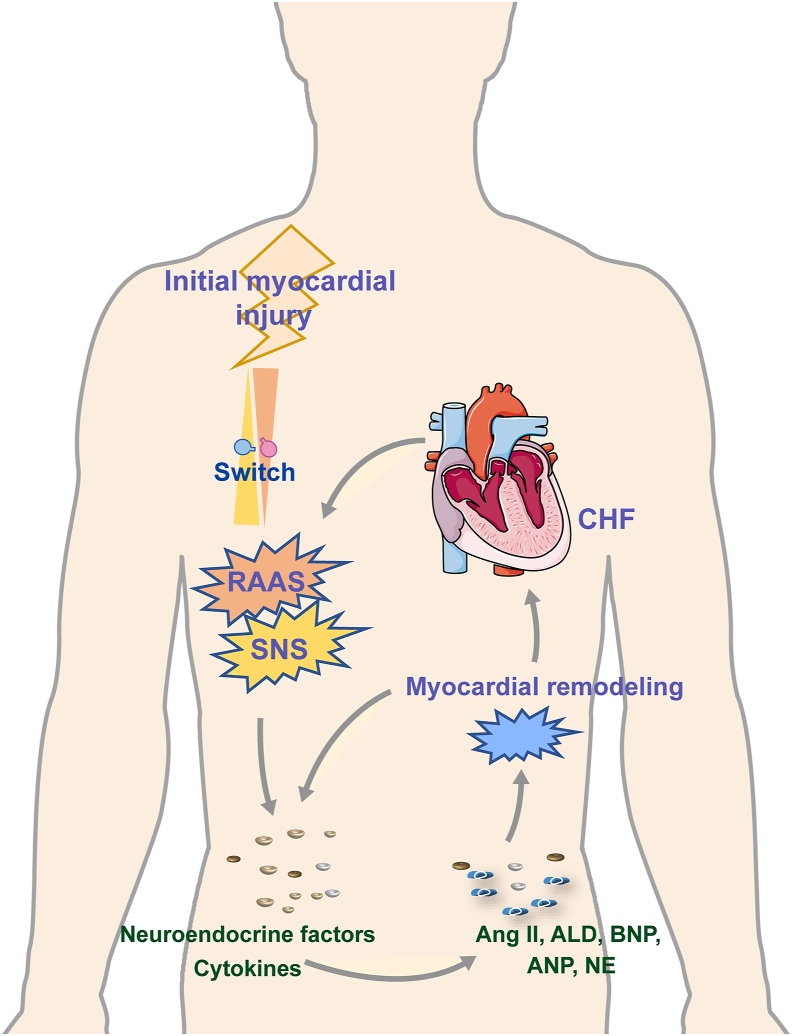
The etiology of CHF is complex and varies with diverse etiologies and phases as a result of the diversity of underlying disorders (Fig. 2). The two main pathophysiological factors that contribute to the formation and progression of CHF are neurohumoral regulation and cardiac remodeling. After myocardial damage, neurohumoral cytokines are initially activated, which causes myocardial structural and phenotypic alterations that emerge as cardiac hypertrophy and overload through a number of signals8, 9, 10. The mechanical burden on the ventricular wall is then increased by high pressure or volume loads, which in turn triggers more neuroendocrine signals that worsen CHF10, 11, 12. The overactivation of the renin–angiotensin–aldosterone system (RAAS), a key hormone system, is a significant factor in the incidence and progression of CHF13. Angiotensin II (Ang II), an effector molecule of RAAS, activates the downstream Ang II type 1 receptor to control cell proliferation and death and impact the deposition of extracellular matrix (ECM)14. Ang II influence the processes of myocardial fibrosis, hypertrophy, and deformation by regulating microRNAs15. Additionally, a variety of released fibrogenic and hypertrophied mediators, including transforming growth factor-β (TGF-β), matrix metalloproteinase (MMP), fibroblast growth factor, vascular endothelial growth factor (VEGF), and several chemokines, are included in the activation of neuroendocrine signals. These signals act on all cell types involved in cardiac fibrotic and hypertrophic responses, leading to cardiac remodeling through oxidative stress, inflammation, endoplasmic reticulum stress (ERS), autophagy, apoptosis, mitochondrial energy metabolism, and angiogenesis16. Taken together, neurohumoral disharmony and cardiac remodeling eventually cause the reduction of myocardial systolic and/or diastolic function, hemodynamic anomalies, and myocardial fibrosis17.
Figure 2.
The neurohumoral regulation mechanism is the key, and ventricular remodeling is the pathophysiological basis of chronic heart failure.
2.1. Pathological manifestations of CHF
2.1.1. Myocardial hypertrophy
Pathological myocardial hypertrophy, in contrast to physiological myocardial hypertrophy brought on by exercise or pregnancy, denotes an increase in cardiac pressure and ventricular wall tension as a result of pressure or volume overload, followed by an increase in cardiomyocyte volume, interstitial fibrosis, and intercellular mass. In cases of volumetric overload, serial sarcomere duplication lengthens cardiomyocytes, resulting in eccentric hypertrophy18; in cases of pressure overload, parallel sarcomere duplication and arrangement lengthen cardiomyocytes, resulting in concentric hypertrophy19. After significant cardiomyocyte loss caused by ischemic or diffuse myocardial disorders, the remaining myocardium may undergo compensatory hypertrophy. Pathological myocardial hypertrophy weakens myocardial contractility and compliance and increases oxygen consumption, culminating in CHF.
Through multiple signaling pathways, pressure, or volume overload and excessive neurohumors control cellular responses such as gene expression, protein synthesis, and cellular metabolism. As a result, capillary thinning, metabolic disorders, Ca2+ overload, inflammatory responses, cell death, and fibrosis occur, causing cardiac hypertrophy. Calcineurin/nuclear factor of activated T cell signaling functions as a master regulator of cardiac hypertrophy20. The activation of phosphatidylinositol 3-kinase (PI3K) signaling may encourage cardiac hypertrophy, interstitial fibrosis, and cardiac dysfunction21.
2.1.2. Myocardial fibrosis and pathological remodeling
Myocardial fibrosis, the process through which cardiac fibroblasts proliferate and transform into the myofibroblast phenotype, is one of the main pathological alterations of CHF. An imbalance in collagen synthesis and degradation can be detected during the process, including an increase in the expression of collagen I, collagen III, and α-smooth muscle actin (α-SMA). It is the primary cause of ventricular remodeling, which results in less myocardial compliance, compromised cardiac systolic and diastolic functions, and chronic volume or pressure overload that eventually progresses to CHF22.
Myocardial fibrosis is characterized by disordered collagen metabolism and excessive diffuse deposition of collagen in the interstitial and perivascular areas23. Initially, ECM deposition is an adaptive and protective mechanism, but excessive and continuous ECM deposition eventually leads to irreversible pathological changes, including ventricular dilation, cardiomyocyte hypertrophy, and apoptosis, decreased tissue compliance, and finally accelerates the progression of CHF24. TGF-β/Smads, WNT/β-Catenin, and PI3K/AKT/mammalian target of rapamycin (mTOR) signaling pathways all play crucial roles in stimulating fibroblast proliferation and ECM remodeling25. Additional factors that contribute to myocardial fibrosis include ATP-dependent chromatin remodeling, apoptosis, autophagy, necrotic cell death, aberrant mitochondrial activity, reactive oxygen species (ROS) generation-stimulated oxidative stress, inflammasome signaling, and the buildup of pro-inflammatory mediators26.
2.2. Molecular mechanisms of CHF
2.2.1. Oxidative stress
Oxidative stress occurs as a result of an excessive amount of free radical production, accumulation, or their oxidant metabolites. These oxidants negatively impact subcellular components like lipid peroxidation, mitochondrial dysfunction, ERS, and DNA fragmentation, which together cause myocardial damage and consequently CHF.
Oxidative stress occurs when the oxidative and antioxidant systems in the body are out of balance and large amounts of ROS and their metabolites build up in cells. These accumulations have toxic effects on cells and activate some signaling pathways, triggering a string of tissue damage27. Increased oxidative stress and excessive ROS production are present in failing hearts, according to clinical investigations and animal trials. Antioxidant systems are classified into non-enzymatic and enzymatic systems. The former mainly contains antioxidants such as glutathione and vitamins C and E, while the latter includes superoxide dismutase, nicotinamide adenine dinucleotide (NAD+/NADH) and nicotinamide adenine dinucleotide phosphate (NADPH)-dependent catalase, glutathione peroxidase, thioredoxin, etc.28. NADPH oxidases (NOX), such as NOX2 and NOX4, regulate oxidative stress and compartmentalize intracellular signaling in endothelial cells, smooth muscle cells, macrophages, cardiomyocytes, and fibroblasts29. There is not only an excess of ROS but also a reduction in the activity of antioxidant enzymes during CHF.
Exposure of cells to elevated levels of ROS provokes an inflammatory cascade and the expression of adhesion molecules, such as nuclear factor-κB (NF-κB) activation30. Increased ROS triggers the activation of metalloproteinases and calpain, which contributes to cell swelling and lysis, leading to mitochondrial permeability transition pores (mPTP) opening and mitochondrial swelling and rupture of the outer mitochondrial membrane, thereby facilitating the deposition of pro-apoptotic factors and intensifying apoptosis31. Therefore, restoring the equilibrium between the oxidative and antioxidant systems necessitates lowering the excess accumulation of ROS as well as activating antioxidant enzymes or administering antioxidants. The prognosis of CHF benefits from the early maintenance of redox homeostasis through exogenous scavenging of excess ROS.
2.2.2. Inflammation
Inflammation is a central mechanism in the emergence of CHF. Cytokines, such as interleukin (IL)-1β, IL-6, and IL-8, have been investigated as potential markers for risk stratification, early diagnosis, and prognostic assessment in CHF32. Initiating, integrating, and maintaining myocardial responses to stress are all determined by proinflammatory cytokines, and all clinical manifestations of CHF show signs of a prolonged inflammatory response33. The short-term prognosis of CHF patients, including 30- and 90-day hospital all-cause mortalities, is adversely affected by higher levels of systemic immune-inflammation indexes34. The early stages of heart healing nonetheless require a moderate inflammatory response. An intense inflammatory response is essential for cardiac repair in the early stages. The protracted, severe inflammation triggers loss of cardiomyocytes and ventricular wall integrity, myocardial rupture, impaired systolic function, and extended fibrotic changes beyond the initial infarction.
The myocardium is mainly composed of cardiomyocytes, fibroblasts, endothelial cells, and leukocytes, all of which are strongly associated with inflammation. Necrotic cardiomyocytes activate innate immune response by releasing a variety of danger signals (high mobility group protein Bl, heat shock protein B1, etc.) to eliminate necrotic cells35,36. Cardiomyocytes in the peri-infarct zone may serve as manufacturing sites for a number of pro-inflammatory substances37. During the healing process, cardiac fibroblasts distributed in the interstitium and perivascular region activate the inflammasome and secrete cytokines, chemokines and lysosomes to promote inflammation38. Proinflammatory cytokines postpone the differentiation of fibroblasts into fibrosis until necrotic cells and stromal components are removed. However, under the volume and pressure overload, fibroblasts in the non-infarct area are activated and transformed into myofibroblasts to promote the formation of interstitial fibrosis. Endothelial cells are the main source of secretion of pro-inflammatory factors, such as monocyte chemoattractant protein 1, CXC motif chemokine 10, and mediate the adhesion and aggregation of circulating leukocytes39. The heart-resident leukocytes, especially macrophages, have inflammatory and protective transcriptional signatures in immune compartment40. A rise in mono-macrophages can be detected in the early injury and late repair stages of CHF41. Macrophages can be classified into M1 and M2 types; the former is thought to have a pro-inflammatory effect [secreting IL-6, IL-1β, and tumor necrosis factor-α (TNF-α)], whereas the latter is involved in resolving of inflammation and reducing collagen production (secreting IL-4, IL-10, and arginase 1)42,43.
Innate immune responses are triggered when inflammatory signals attach to pattern recognition receptors. Activated Toll-like receptor (TLR) interact with myeloid differentiation factor 88 adaptor protein (MYD88) to activate NF-κB and trigger the inflammatory cascade reaction, aggravating inflammation and transducing TGF-β signal to form myocardial fibrosis44, 45, 46. Activated NOD-like receptor 3 (NLRP3) inflammasome signal pathway amplifies the inflammatory response and participates in pyroptosis47. Furthermore, it is important to consider the role of other immune cells such as neutrophils, mast cells, T cells, and natural killer cells in inflammation48.
2.2.3. Endoplasmic reticulum stress
The endoplasmic reticulum is an intracellular organelle responsible for translation of membrane proteins, post-translational modification of proteins, regulation of cellular Ca2+ homeostasis, and lipid synthesis. The collapse of endoplasmic reticulum homeostasis is a trigger for various cardiovascular diseases. When stressors like hypoxia, glucose deprivation, ATP depletion, and calcium overload are present, the accumulation of misfolded and unfolded proteins in the endoplasmic reticulum lumen and disturbance of Ca2+ homeostasis turn on unfolded protein responses and apoptotic signaling pathways, which leads to ERS49.
Moderate ERS is an adaptive response of the cell that facilitates the restoration of endoplasmic reticulum function and guarantees cell survival. Severe or persistent ERS and protein misfolding will induce inflammation and apoptosis, thus accelerating cardiac remodeling50,51. Calcium pools in the endoplasmic reticulum regulate intracellular Ca2+ level; the balance of its concentration maintains the function of the endoplasmic reticulum, membrane transport, and internal environmental homeostasis. Dysregulation of the Ca2+ circulation is one of the key causes of the loss in myocardial contractile capacity in CHF. The imbalance of Ca2+ homeostasis is responsible for the aggregation of misfolded or unfolded proteins in the endoplasmic reticulum, which incites inflammatory responses and oxidative stress, resulting in mitochondrial damage, apoptosis, and myocardial fibrosis52. Impaired myocardium may trigger persistent ERS by activating inositol-requiring enzyme 1 cleaving X-box binding protein 1 to induce the expression of glucose-regulated protein 78, activated transcription factor 4, and CCAAT enhancer binding protein53. The SUMOylation of SERCA2a has been verified to play a critical role in the abnormal Ca2+ cycle of CHF54.
2.2.4. Apoptosis
One of the main causes of cardiac dysfunction is cardiomyocyte loss, which is primarily attributed to apoptosis55. Excessive apoptosis of myocardial cells leads to persistent loss of myocardial contractile units and structural lesions, which impair myocardial perfusion capacity and increase the risk of CHF.
Apoptosis is predominately regulated by two signaling pathways: the endogenous pathway associated with mitochondria and the exogenous pathway associated with death receptors (FAS and other TNFR superfamily members and ligands). The former is regulated by the pro- and anti-apoptotic members of the BCL-2 family proteins. To maintain cell survival, the anti-apoptotic proteins BCL-2, BCL-XL, MCL-1, BCL-W, and A1/BFL1 work in tandem with the cell death effectors BAX and BAK56. The key apoptosis promoter, the BH3-only protein, is upregulated in response to intracellular stress (such as Ca2+ overload, DNA damage, or ERS), and it binds to BCL-2 with a high affinity. This results in the release of BAX and BAK, leading to mitochondrial outer membrane permeabilization (MOMP), which then results in cytochrome c and SMAC/DIABLO57. These apoptotic stimuli encourage the activation of the caspases' cascade reaction, leading to the cleavage of hundreds of proteins, which results in cell destruction. The exogenous apoptotic pathway is initiated by cell surface death receptors that contain death domains triggered by death ligands, forming the death-inducing signaling complex, and activating caspase-8 and downstream caspase-3 and caspase-758. Caspase-8 also cleaves and activates the BH3-only protein BID, causing MOMP59, which links exogenous and endogenous apoptosis.
Limiting the flow of Ca2+ from the outside to the inside of the cell may inhibit cell disintegration caused by calcium overload; stabilizing the mitochondrial structure by restricting the over-opening of MOMP may effectively prevent the caspases-activated cascade reactions to reduce excessive apoptosis. Downregulating FAS receptor and its ligand proteins, TNF-α, TNFR-1, FADD, caspase-8, and caspase-3 may impair exogenous apoptosis directly.
2.2.5. Autophagy
Autophagy is a highly conserved intracellular catabolic process. With the help of lysosomes, autophagy degrades misfolded proteins and damaged organelles, eliminates aging cells, maintains energy homeostasis, and assures cellular survival and function60. Autophagy is mainly classified into macroautophagy, microautophagy, and chaperone-mediated autophagy, each of which is mediated by different regulators61. The mTOR acts as a negative regulator of autophagy, whereas adenosine monophosphate activated protein kinase (AMPK) and glycogen synthase kinase-3β are positive regulators62. In response to a variety of regulatory factors, autophagy plays an integral role in the energy metabolism of cardiomyocytes, the production of collagen, and endothelial–mesenchymal transition (EMT) and thus contributes to cardiac hypertrophy and fibrosis63,64. Abnormal autophagy is reported in the myocardium of patients with CHF due to dilated cardiomyopathy, valvular diseases, and coronary artery diseases65.
An imbalance in autophagy leads to cardiac hypertrophy. In the early stage of CHF, insufficient autophagic activity increases hypertrophic cardiomyocytes and precludes clearance of apoptosis arising from inflammation and oxidative stress; excessive autophagy intensifies of CHF66,67; proper autophagy releases free fatty acids (FFAs) and amino acids to degrade senescent and dysfunctional organelles and proteins68. Under normal physiological settings, autophagy is at a low level. However, ATP depletion, ROS release, and mPTP opening facilitate a rapid increase in autophagic activity, eventually leading to overactive autophagy69. Over-activated autophagy damages important organelles and proteins while eliminating toxic elements, evoking apoptosis and the loss of cardiomyocytes70. The activation of autophagy can induce EMT and cardiac fibrosis, and inhibition of autophagy to EMT is a cardioprotective strategy to ameliorate cardiac fibrosis. Excessive activation of autophagy exacerbates cardiac fibrosis through the upregulation of EMT in transverse aortic constriction (TAC)-induced mice71. However, in certain circumstances, autophagy restriction might lead to EMT and promote cardiac fibrosis. Autophagy disruption established by autophagy related gene 5-specific deletion significantly induces IL6-dependent EMT and exacerbates cardiac fibrosis72, while restoration of TFEB-mediated autophagic flux inhibits TGF-β-mediated EMT73. Myocardial autophagy may be altered differentially by various etiologies of CHF, and the role of autophagy in the progression of CHF may change over time. Further studies are needed to better understand autophagy and obtain cardioprotective effects without maladaptive effects.
2.2.6. Mitochondrial function and metabolism remodeling
There is growing evidence that metabolic remodeling of cardiomyocytes is responsible for cardiac structural remodeling. The apparent alterations in metabolic remodeling are a shift in metabolic substrate usage and the decrease of mitochondrial oxidative capacity in cardiomyocytes. In a healthy heart, cardiomyocytes consume large amounts of FFAs but relatively little glucose to regenerate ATP for contraction. While in a hypertrophic and fibrotic heart, cardiomyocytes switch from using FFAs as a fuel to glucose, which is characterized by decreased metabolism of FFAs and increased glycolysis74. Additionally, the sympathetic-adrenal system in CHF is over-activated, which results in excessive Ca2+ buildup and mitochondrial mPTP opening, resulting in electron leakage during its transfer. The imbalance of mitochondrial quality control exacerbates myocardial energy metabolism disorders75. A diversified analysis of CHF patients proved that the evolution of CHF is linked to the progressive degradation of energy generation and reserve capacity. The endogenous feedback mechanism struggles to balance out the inordinate energy demand after the damage hits the crucial threshold, and CHF worsens76.
Metabolic intermediates and metabolites can regulate the activities of enzymes essential for cardiac hypertrophy and remodeling, including AMPK signal, peroxisome proliferator-activated receptor γ coactivator 1α signal, mTOR signal, PI3K/protein kinase B signaling, and so on77. Alterations in these signals are obvious indicators of energy deficits and energy metabolism disorders, showing a route to a cure for CHF. Disorders of myocardial substrate utilization and metabolism are progressively being recognized as possible causes of cardiac remodeling and dysfunction. Regulatory approaches to cardiac energy metabolism are sensitive and adaptive, allowing the heart to adapt to different states and workloads to maintain its systolic function. Therefore, researchers are looking at myocardial energy metabolism as a new way to try to help CHF patients.
2.2.7. Angiogenesis
Angiogenesis is the process of forming new blood vessels from already existing ones. Myocardial hypertrophy is one of the precursors of myocardial remodeling. In ischemia diseases such as coronary artery disease and myocardial infarction (MI), coronary microvascular dysfunction will accelerate the dysfunction of the myocardium78. One of the prerequisites to cardiac remodeling is myocardial hypertrophy79. The degree of myocardial hypertrophy is closely related to the density of blood vessels. In the early phases of chronic cardiac hypertrophy, myocardial hypoxia stimulates microvascular dilation by inducing the secretion of angiogenic factors. As hypertrophy worsens, the adaptive angiogenic response is suppressed, and thickening of the inner wall and increased wall/lumen ratio lead to capillary thinning and poor coronary artery remodeling, resulting in a mismatch between capillary density and increased oxygen demand that further weakens cardiac function80.
Endothelial cells release a range of angiogenic molecules that participate in endothelial growth, microvascular permeability, and angiogenesis81. VEGF is an angiogenic molecule that both supports vasodilation and promotes neovascularization. In elderly CHF patients, upregulating VEGF expression can promote angiogenesis and endothelial function and improve cardiac ejection82. Endothelin, a powerful endogenous angiogenesis inhibitor, its increase accelerates left ventricular (LV) remodeling in CHF mice due to myocardial ischemia, and its anti-angiogenic activity may be mediated by reducing endothelial neovascularization and plaque growth83. Clinical trials revealed that increased endostatin was significantly associated with worsening ventricular systolic and diastolic function in the elderly community84. Angiogenesis can improve the blood and oxygen supply, thereby inhibiting the apoptosis of myocardial cells, alleviating fibrosis, and improving cardiac function85. Overall, exogenous stimulation of angiogenesis-mediated microvascular recovery by drugs may hold promise for the treatment of CHF.
3. The ameliorating effects of TCM formulas on CHF
TCM, a comprehensive medicinal system, is characterized by holistic theory that emphasizes the regulation of the integrity of the human and the interactions between human individuals and their environments. The diagnostic and therapeutic methods of TCM are based on the differentiation of syndromes (Zheng in Chinese) and the use of herbal formulas (Fang-Ji in Chinese). According to TCM theory, CHF occurs in the heart and also involves the lung, spleen, and kidney, implying that CHF is not only attributed to cardiac conditions. It is characterized by the syndromes of deficiency in origin and excess in superficiality, or the deficiency–excess complex. The deficiency of Qi and Yang of heart is the fundamental cause of CHF, and the blood stasis and phlegm that follow further exacerbate the illness. In the early stages of CHF, both Qi and Yin are deficient; in the middle stages, Qi deficiency, blood stasis, and phlegm are predominant; and in the latter stages, Yang deficiency of the heart and kidney, blood stasis, accumulative phlegm, and sudden Yang collapse are predominant. Overall, Qi deficiency of the heart and blood stasis run through CHF, and Yang deficiency is the main factor. Hence, the therapeutic principles and methods of TCM for CHF concentrate on warming Yang to promote diuresis, supplementing Qi and nourishing Yin, and activating blood circulation to remove blood stasis.
Over the past few years, numerous investigations have been carried out to find evidence-based anti-CHF TCM formulas. This section covered TCM anti-CHF formulas that have been accepted for use in clinical settings by the general population and small bodies of supporting research (Table 1).
Table 1.
Category of effects, composition, patterns of syndrome and origin of TCM formulas.
| Category of effects | TCM formula | Composition | Pattern of syndrome | Origin |
|---|---|---|---|---|
| Tonifying Qi | HQI | Astragali Radix (Huangqi) | Deficiency of heart Qi and stagnation of vessel by blood stasis | Approved by CFDA (Z19993151) |
| SQFZ | Astragali Radix (Huangqi), Codonopsis Radix (Dangshen) | Deficiency of lung-spleen Qi | Approved by CFDA (Z19990065) | |
| SBPs | Moschus (Shexiang), Ginseng Radix (Renshen), Bovis Calculus (Niuhuang), Borneolum (Bingpian), CinnamomI Cortex (Rougui), Styrax (Suhexiang), Bufonis Venenum (Chansu) | Qi stagnation and blood stasis | Approved by CFDA (Z31020068) | |
| Tonifying Qi and activating blood | QLQX | Astragali Radix (Huangqi), Ginseng Radix (Renshen), Salviae Miltiorrhizae Radix (Danshen), Descurainiae Semen Lepidii Semen (Tinglizi), Alismatis Rhizoma (Zexie), Polygonati Odorati Rhizoma (Yuzhu), Cinnamomi Ramulus (Guizhi), Carthami Flos (Honghua), Periplocae Cortex (Xiangjiapi), Citri Reticulatae Pericarpium (Chenpi) | Deficiency of Yang Qi and blood stasis | Approved by CFDA (Z20040141) |
| QSYQ | Astragali Radix (Huangqi), Codonopsis Radix (Dangshen), Notoginseng Radix (Sanqi), Dalbergiae Odoriferae Lignum (Jiangxiang) | Qi deficiency and blood stasis | Approved by CFDA (Z20030139) | |
| TXL | Ginseng Radix (Renshen), Eupolyphaga Steleophaga (Tubiechong), Dalbergia Odorifera Lignum (Jiangxiang), Ziziphi Spinosae Semen (Suanzaoren), Paeoniae Radix Rubra (Chishao), Hirudo (Shuizhi), Scorpio (Quanxie), Cicada Periostracum (Chantui), Scolopendra (Wugong), SantaLi Albi Lignum (Tanxiang), Olibanum (Ruxiang), Borneolum (Bingpian) | Deficiency of heart Qi and blood stasis | Approved by CFDA (Z19980015) | |
| XML | Roach | Deficiency of Qi and Yang, blood stasis | Approved by CFDA (Z20060443) | |
| Tonifying Qi and Yin | SM | Ophiopogonis Radix (Maidong), Ginseng Radix Rubra (Hongshen) | Deficiency of Qi and Yin | Approved by CFDA (Z51021921) |
| SI | Ginseng Radix (Renshen), Ophiopogonis Radix (Maidong), Schisandra Chinensis Fructus (Wuweizi) | Deficiency of Qi and Yin | Approved by CFDA (Z51021264) | |
| YQFM | Ginseng Radix Rubra (Hongshen), Ophiopogonis Radix (Maidong), Schisandra Chinensis Fructus (Wuweizi) | Deficiency of Qi and Yin | Approved by CFDA (Z20060463) | |
| SSYX | Ginseng Radix (Hongshen), Ophiopogonis Radix (Maidong), Corni Fructus (Shanzhuyu), Salviae Miltiorrhizae Radix (Danshen), Nardostachyos Radix (Gansong), Ziziphi Spinosae Semen (Suanzaoren), Taxilli Herba (Sangjisheng), Paeoniae Radix Rubra (Chishao), Eupolyphaga Steleophaga (Tubiechong), Coptidis Rhizoma (Huanglian), Schisandra Chinensis Fructus (Wuweizi), Dragonsbones (Longgu) | Deficiency of Qi and Yin, blood stasis | Approved by CFDA (Z20103032) | |
| Activating blood to remove blood stasis | DSDP | Salviae Miltiorrhizae Radix (Danshen), Notoginseng Radix (Sanqi), Borneolum (Bingpian) | Qi stagnation and blood stasis | Approved by CFDA (Z20080078) |
| DHI | Salviae Miltiorrhizae Radix (Danshen), Carthami Flos (Honghua) | Blood stasis | Approved by CFDA (Z20026866) | |
| TSD | Chuanxiong Rhizoma (Chuanxiong), Carthami Flos (Honghua), Angelica Sinensis Radix (Danggui), Persicae Semen (Taoren), Paeoniae Radix Alba (Baishao), Rehmanniae Radix (Dihuang) |
Blood stasis and blood deficiency | Fu Ke Bing Jian | |
| Warming Yang | ZWD | Poria (Fuling), Atractylodes Macrocephala Rhizoma (Baizhu), Aconiti Lateralis Radix Praeparata (Fuzi), Zingiberis Rhizoma Recens (Shengjiang), Paeoniae Radix Alba (Baishao) |
Edema due to Yang deficiency | Treatise on Febrile Diseases (Shang Han Lun) |
| LZD | Poria (Fuling), Cinnamomi Ramulus (Guizhi), Atractylodes Macrocephala Rhizoma (Baizhu), Glycyrrhizae Radix (Gancao) |
Dampness due to Yang deficiency | Treatise on Febrile Diseases (Shang Han Lun) | |
| Removing phlegm and resolving stasis | GXBD | Trichosanthis Semen (Gualouzi), Alli Macrostemonis Bulbus (Xiebai), Pinelliae Rhizoma (Banxia) |
Phlegm-accumulation stasis | Treatise on Febrile Diseases (Shang Han Lun) |
| Restoring Yang and tonifying Qi | SFI | Ginseng Radix Rubra (Hongshen), Aconiti Lateralis Radix Praeparata (Fuzi) | Sudden collapse of Yang | Approved by SFDA (Z51020664) |
3.1. Patent capsules or pills
3.1.1. Shensong Yangxin capsule (SSYX)
Ginseng Radix Rubra (Hongshen), Ophiopogonis Radix (Maidong), Corni Fructus (Shanzhuyu), Salviae Miltiorrhizae Radix (Danshen), Nardostachyos Radix (Gansong), Ziziphi Spinosae Semen (Suanzaoren), Taxilli Herba (Sangjisheng), Paeoniae Radix Rubra (Chishao), Eupolyphaga Steleophaga (Tubiechong), Coptidis Rhizoma (Huanglian), Schisandra Chinensis Fructus (Wuweizi), and Dragonsbones (Longgu) make up SSYX. The special effect of SSYX is to supplement Qi and nourish Yin, activate blood circulation, and remove blood stasis to treat CHF caused by deficiency of Qi and Yin syndrome86.
SSYX is a Chinese patent medicine recommended by guidelines for the treatment of ventricular premature beats and is preferred to ameliorate arrhythmia symptoms in patients with CHF86. The autonomic imbalance may be an important mechanism for the deterioration of CHF, and heart rate variability is a sensitive indicator commonly used to quantify autonomic function87. In a clinical trial enrolled 120 CHF patients, the control group was given conventional therapy, and the experimental group was given the SSYX and conventional therapies. The electrocardiography results demonstrated that the root mean square of the difference value of an adjacent RR interval and the percentage of differences exceeding 50 ms between an adjacent standard number of interval values in the experimental group were higher than those in the control group, indicating that SSYX improved the LV function and normalization of heart rate variability in CHF patients, thus improving therapeutic effects88. The congestive CHF patients with frequent ventricular premature complexes given SSYX showed a significantly greater decline in the total number of VPCs and N-terminal pro-B-type natriuretic peptide (NT-proBNP) level than the placebo group did89. In addition, the Minnesota Living with Heart Failure Questionnaire score was markedly elevated after SSYX treatment, showing the meliority of SSYX for the quality of life of CHF patients90.
3.1.2. Qishen Yiqi dropping pills (QSYQ)
QSYQ is composed of Astragali Radix (Huangqi), Salviae Miltiorrhizae Radix (Danshen), Notoginseng Radix (Sanqi), Dalbergiae Odoriferae Lignum (Jiangxiang). It can invigorate Qi and promote blood circulation to relieve pain, so it can be applied to CHF patients with Qi deficiency and blood stasis syndrome91.
QSYQ is recommended by TCM treatment guidelines for coronary heart disease (CHD) before or after percutaneous coronary intervention (PCI) for CHD patients91. A clinic trial enrolled 640 CHF patients triggered by MI indicated that 6-min walking distance (6-MWD) and the Minnesota Living with Heart Failure Questionnaire score are significantly improved, while secondary outcomes in composite clinical events (all-cause mortality and emergency treatment or hospitalization due to CHF) and brain natriuretic peptide (BNP) changes were non-significantly greater compared with the placebo group after 6-month QSYQ treatment. Furthermore, a meta-analysis showed that, compared with conventional western medicine alone, such as sacubitril valsartan sodium, trimetazidine, and levosimendan, QSYQ combined with conventional western medicine obviously improved LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), LV ejection fraction (LVEF), BNP, 6-MWD, and weakened adverse reactions92. The effect of conventional western medicine combined with QSYQ is better than conventional western medicine alone93.
3.1.3. Compound Danshen dripping pills (CDDP)
CDDP is a widely used TCM medication in China for treating ischemic angina pectoris and consists of Salviae Miltiorrhizae Radix (Danshen), Notoginseng Radix (Sanqi), and Borneolum (Bingpian). A majority in treating CHF is implied by CDDP's prominence in promoting blood circulation to reduce blood stasis. With quick dissolution, strong bioavailability, and low adverse reactions, CDDP is the first proprietary Chinese medicine to pass Phase II and is undergoing phase III clinical trials for the prevention and treatment of ischemic cardiovascular disease by the U.S. Food and Drug Administration.
CDDP was shown to improve the cardiac index, LVEF, LVEDD, and LVESD, and reduce C-reactive protein, IL-8, and amino-terminal brain natriuretic peptide precursor (NT-proBNP) levels in aged CHF patients94. In CHF patients, an elevated BNP level is a high-risk factor for subsequent readmission; patients with higher BNP level require intensive treatments95. The NT-proBNP level in plasma was not significantly decreased after one month of CDDP treatment but apparently mitigated after three and six months of treatment, suggesting better long-term stabilization96, implying that CDDP could play a role in reducing subsequent readmission rates. CDDP in combination with conventional antihypertensive drugs significantly improved the clinical efficacy in hypertensive myocardial hypertrophy97. In addition, CDDP combined with conventional therapy alleviated hemorheology and blood lipid parameters and inflammatory mediators, thus prominently improving vascular endothelial function and cardiac function98.
3.1.4. Tongxinluo capsule (TXL)
TXL was developed under the “collateral disease theory” founded by Professor Wu Yiling99. It is composed of Ginseng Radix (Renshen), Eupolyphaga Steleophaga (Tubiechong), Dalbergiae Odoriferae Lignum (Jiangxiang), Ziziphi Spinosae Semen (Suanzaoren), Paeoniae Radix Rubra (Chishao), Hirudo (Shuizhi), Scorpio (Quanxie), Cicada Periostracum (Chantui), Scolopendra (Wugong), SantaLi Albi Lignum (Tanxiang), Olibanum (Ruxiang), and Borneolum (Bingpian). It is adept at supplementing Qi and activating blood circulation. CHF caused by Qi deficiency of heart and blood stasis falls under TXL treatment.
Although coronary revascularization decreases mortality and improves prognosis, CHF remains the most frequent and dangerous complication in MI patients. TXL has the potential to reduce myocardial no-reflow and myocardial ischemia/reperfusion (MI/R) injury. In CHF patients after PCI for MI, TXL notably ameliorated symptoms such as chest tightness and shortness of breath and reduced NT-proBNP expression100,101. In MI patients with delayed PCI, TXL not only prevented coronary embolism, attenuated vascular endothelial injury but also enhanced blood flow and strengthened cardiac systolic function to prevent CHF102. However, in another study, although TXL reduced primary cardiovascular events (cardiac death, recurrent MI, arrhythmia, and recurrent angina pectoris), it could not lower the incidence of recurrent CHF, which is even more likely to result in gastrointestinal discomfort103. Therefore, a prospective, randomized, double-blind, placebo-controlled, multicenter clinical study to evaluate the clinical efficacy and safety of TXL is essential to achieving a tough clinical endpoint.
3.1.5. Qiliqiangxin capsule (QLQX)
QLQX is the first Chinese patent drug with evidence-based medical evidence to positively affect CHF in China, and it is composed of Astragali Radix (Huangqi), Ginseng Radix (Renshen), Salviae Miltiorrhizae Radix (Danshen), Descurainiae Semen Lepidii Semen (Tinglizi), Alismatis Rhizoma (Zexie), Polygonati Odorati Rhizoma (Yuzhu), Cinnamomi Ramulus (Guizhi), Carthami Flos (Honghua), Periplocae Cortex (Xiangjiapi), and Citri Reticulatae Pericarpium (Chenpi). QLQX can supplement Qi and warm Yang, activate blood circulation, and induce diuresis (to eliminate edema) is superior in treating mild and moderate congestive CHF specialized by deficiency of Yang, blood stasis, and excessive phlegm. Based on the multi-dimensional “radar chart” mode, songorin, calycosin-7-O-β-d-glucopyranoside, astragaloside, tanshinone IIA (tan IIA), ginsenoside Re, hesperidin, and alisol A are screened out to take the best pharmacological effects on CHF104.
Data from randomized controlled trials (RCTs) suggested QLQX significantly improved quality of life and reduced cardiovascular events, re-hospitalization rates, and mortality in CHF patients105. NT-proBNP, as a diagnostic and prognostic criterion, has been incorporated into the original American Heart Association/American College of Cardiology Heart Failure Diagnostic Criteria and Guidelines, and studies have shown that NT-proBNP level is positively correlated with CHF severity and the New York Heart Association (NYHA) classification of cardiac function and negatively correlated with EF and cardiac output106. In a multicenter, randomized, double-blind, parallel-group, placebo-controlled study enrolled 512 CHF patients, QLQX for 12 weeks reduced the NT-proBNP level, and demonstrated superior performance in the NYHA classification of cardiac function, LVEF, and 6-MWD107.
3.1.6. Shexiang Baoxin Pills (SBPs)
SBPs, a classic patent medicine composed of Moschus (Shexiang), Ginseng Radix (Renshen), Bovis Calculus (Niuhuang), Borneolum (Bingpian), CinnamomI Cortex (Rougui), Styrax (Suhexiang), and Bufonis Venenum (Chansu) originating from the Suhexiang Pills of the Song Dynasty in China, has been extensively used to prevent and treat cardiovascular diseases. SBPs may treat CHF characterized by Qi stagnation and blood stasis syndrome by supplementing Qi firmly and rapidly108.
In a study on therapies in elderly patients with CHF secondary to ischemic cardiomyopathy, compared with trimetazidine alone, SBPs combined with trimetazidine apparently improved the clinical efficacy and indices of cardiac function (increasing LVEF and 6-MWD, decreasing LVEDD and LVESD) without serious adverse reactions108. In CHD-related CHF, the combination of SBPs and conventional drugs exerted preferable effects than conventional medications alone, as evidenced by the normalization of indicators reflecting cardiac function, including LVEF, LVEDD, LVESD, and cardiac output, and the reduction of NT-proBNP109. Furthermore, SBPs decreased endothelin and nitric oxide (NO) to improve cardiac function in CHF patients without changes in laboratory indicators, including blood count and renal and liver function tests, presenting pleasurable safety110.
3.2. Patent injections
3.2.1. Xinmailong injection (XML)
XML is a Periplaneta americana extract with various effective bioactive components, including inosine, adenosine, pyroglutamic acid, and saccharin, approved by the China State Food and Drug Administration (CFDA) for treating CHF in 2006. For CHF caused by deficiency of Qi and Yang and blood stasis, XML can improve cardiac function by supplementing Qi and activating blood circulation, warming Yang to promote diuresis, to be an adjuvant therapy for congestive CHF111.
In a multicenter double-blind RCT, the patients were given XML or a placebo (XML mimetics) in addition to receiving standard treatment111. XML (5 mg/kg) was injected intravenously (200 mL of 0.9% NaCl or 5% glucose at a drip rate 20–40 mL/min) twice a day for 5 days. Tests of the NYHA function showed that patients in the XML group had a greater overall efficacy rate than those in the placebo group. The 6-MWD distance was significantly longer (from 364.23 to 435.65 m) in patients taking XML compared to placebo. XML relieved symptoms and improved cardiac function and exercise tolerance in CHF patients, supported by a series of meta-analyses112,113. Moreover, age is a potential factor affecting efficacy and early use of XML contributes to improving the prognosis.
3.2.2. Shengmai injection (SI)
SI is illuminated by “Shengmai Powder” from “Medicine Origin (Yi Xue Qi Yuan)”, also known as “Shengmai Yin” in the Jin Dynasty (AD 1115–1234), consisting of Ginseng Radix (Renshen), Ophiopogonis Radix (Maidong), and Schisandra Chinensis Fructus (Wuweizi). SI could supplement Qi and nourish Yin to restore Qi and Yin and the feeble pulse in CHF. Shengmai Yin has been verified to control or slow the progression of CHF by inhibiting pathological changes in CHF rat models114.
SI was revealed to decrease high-sensitivity C-reactive protein (hs-CRP) levels, enhance cardiac pumping function, biphasically regulate arterial blood pressure, reduce myocardial oxygen consumption, and improve vascular compliance115. A “real-world study” refers to a study that systematically collects and analyzes real-world data routinely generated from clinical trials116. Based on real-world data from the Hospital Information System, a study intensely explored the clinical characteristics of SI, demonstrating that it increased LVEF and cardiac output and improved cardiac function in CHF patients. Moreover, the combination of SI with chemical medicine such as spironolactone, furosemide, acetylsalicylic acid, and spironolactone can further prevent complications such as thromboembolism and respiratory infections and reduce the risk of adverse cardiovascular events117. A meta-analysis enrolled 20 RCTs demonstrated that adjuvant treatment with SI significantly improved cardiac function, including LVEF, cardiac output, and index, compared with conventional medicine alone118. Interestingly, patients receiving adjuvant therapy with SI showed a higher response to treatment119.
3.2.3. Shenmai injection (SMI)
SMI is extracted from Ophiopogonis Radix (Maidong) and Ginseng Radix Rubra (Hongshen), which supplement Qi and Yin. It can improve the immune competence of tumor patients, increase the efficacy, and reduce the cardiotoxicity of chemotherapy drugs, for instance, anthracyclines120.
In a multicenter, double-blind RCT, patients received SMI or a placebo except standard medication for CHF. After one week, the SMI group demonstrated a remarkable improvement in NYHA cardiac function classification, 6-MWD, and TCM syndrome score, compared with the placebo group121. Increasing evidence indicates that a disruption in myocardial energy metabolism in patients with CHF leads to the progression and deterioration of the disease122. SMI regulated energy metabolism as evidenced by changing FFAs, lactic acid, pyroracemic acid, and branched-chain amino acid levels in serum123. Besides, SMI could relieve respiratory dysfunction and LV systolic disorder in patients with pulmonary CHF124. SMI seeks to capture the attention of patients with pulmonary CHF. But another study found no evidence to support SMI as adjuvant therapy for pulmonary CHF125. This may be due to the low methodological quality and tiny sample size.
3.2.4. Yiqi Fumai injection (YQFM)
YQFM consists of Ginseng Radix Rubra (Hongshen), Ophiopogonis Radix (Maidong), and Schisandra Chinensis Fructus (Wuweizi), which can supplement Qi and nourish Yin to improve CHF caused by the deficiency of Qi and Yin syndrome caused by CHD in particular. By using lyophilized powder injection to make YQFM, the instability of TCM injection, is taken care of, which makes it easier to store126.
During the process of CHF, excessive inflammatory factors may lead to elevated expression of soluble CD146 on endothelial cells, inducing neovascularization and rupture. Soluble CD40 may exacerbate the inflammatory response by binding to sCD40 receptors on the surface of smooth muscle cells and endothelial cells127. In the treatment of CHF induced by CHD, YQFM in combination with atorvastatin is effective. Their combination effectively reduced the levels of soluble CD146 and CD40 and pregnancy-associated plasma protein A, restored cardiac function, and ameliorated ventricular remodeling, and hence CHF patients may reap huge fruits in terms of prognosis128. YQFM can be easily dissolved in normal saline for an intravenous drip, which implies the advantages of rapid action, a high concentration of active ingredients, and accurate drug delivery129. However, it should be properly prepared when used to adequately ensure clinical application safety. In a study comparing the clinical efficacy of YQFM and SI on CHF with Qi and Yin deficiency syndrome, SI was superior to YQFM in improving TCM symptoms, while YQFM exhibited better effects on regulating BNP values and cardiac function130. Current evidence suggests that YQFM, as a complementary therapy, significantly improves cardiac function and indicators in CHF patients.
3.2.5. Huangqi injection (HI)
HI is extracted from Astragali Radix (Huangqi), containing the total flavonoids of Astragalus, Astragalus polysaccharide, astragaloside, and astragalosaponin131. In the case of CHF caused by Qi deficiency of heart and blood stasis, HI contributes to supplementing Qi and invigorating the spleen in order to expel dampness and eliminate pathogens.
A study showed that the combination of HI based on standard treatment improved cardiac function and hemodynamic indexes better in CHF patients compared with standard treatment alone132. Additionally, HI was revealed to decrease malondialdehyde (MDA) and reduce NO and heme oxygenase-1 (HO-1) in CHF patients133, implying that its effect may be associated with the up-regulation of endogenous antioxidation stress. In CHF patients with Qi deficiency syndrome, HI significantly improved 6-MWD, and the therapeutic effect exhibited a positive correlation with the duration and frequency of HI treatment134. However, the safety and efficacy of HI remain controversial135. More high-quality, evidence-based medical work is pressingly needed to investigate further.
3.2.6. Shenfu injection (SFI)
SFI is made up of Ginseng Radix Rubra (Hongshen), and Aconiti Lateralis Radix Praeparata (Fuzi) and originated from Shenfu Decoction in the Song Dynasty. It is irreplaceable in supplementing Qi to solidify and rescuing from collapse by restoring Yang. Thus, it has been widely used to rectify the syndrome of sudden Yang collapse. Since its commercial release in 1987, SFI has demonstrated a remarkable therapeutic effect in CHF patients during the acute phase136.
In patients with acute aggravation of CHF caused by Yang and Qi deficiency syndrome, an RCT revealed that SFI effectively alleviated the respiratory dyspnea and inappetence and improved cardiac tolerance without adverse reactions136. In CHF patients with Yang deficiency of heart and kidney syndrome, SFI could down-regulate NT-proBNP level and main symptom scores137, and SFI is superior to SMI in improving LVEF, BNP level and TCM syndrome curative effect of CHF138. Notably, from the health care system's perspective, a one-year decision tree model was used to estimate the costs and quality-adjusted life years of hospitalized CHF patients in grades III and IV treated with SFI combined with conventional therapy. The incremental cost-effectiveness ratio of SFI (a 14-day course of treatment) was 9016 CNY, far lower than the cost of conventional treatment alone and Chinese GDP per capita. The 7-day course treatment of SFI combined with conventional therapy is a dominant strategy, while the 14-day course of treatment of SFI is a cost-effective strategy139.
3.2.7. Shenqi Fuzheng injection (SQFZ)
SQFZ is extracted from Astragali Radix (Huangqi) and Codonopsis Radix (Dangshen), which can supplement Qi and strengthen body resistance to improve CHF in the deficiency lung-spleen Qi syndrome140. Network pharmacology predicts that the Astragali Radix (Huangqi)–Codonopsis Radix (Dangshen) herbal pair targets PI3K/protein kinase B, mitogen-activated protein kinase (MAPK), and NF-κB pathways141. Calcitonin gene-related peptide (CGRP), a potent endogenous vasodilator, can improve endothelial cell function, promote vascular repair, reduce blood viscosity, and increase coronary blood flow142. It is confirmed that the decrease in CGRP level is highly correlated with myocardial injury in the early stages of CHF, reflecting the degree of cardiac dysfunction in CHF, and can act as an early marker143. Meanwhile, NF-κB mediated inflammatory responses were positively correlated with the severity of ventricular remodeling in CHF patients144. SQFZ was found to significantly reduce NF-κB level and increase CGRP level to suppress the inflammatory response, improving cardiac function145.
MMP-9 and tissue inhibitor of MMP-1 regulate ECM alteration, which is a critical pathological factor of ventricular remodeling in CHF146. SQFZ was depicted to regulate the dynamic balance of MMP-1 and MMP-9, inhibiting ECM degradation to treat CHF147. Moreover, SQFZ could apparently increase heart rate, respiratory rate, BNP, and PaCO2; meanwhile, it elevated the ratio of forced expiratory volume in 1 s148, implying the potential of SQFZ to improve cardiopulmonary function in CHF patients with chronic obstructive pulmonary disease.
3.2.8. Danhong injection (DHI)
DHI contains Salviae Miltiorrhizae Radix (Danshen) and Carthami Flos (Honghua). Since its successful development in 2005, it has been widely used in the treatment of MI and cerebral infarction and is also being gradually applied to CHD and CHF nowadays149,150.
DHI improved LVEF and reperfusion after MI, thereby significantly reducing the risk of mortality, recurrent angina, arrhythmia, and CHF151. Additionally, DHI could increase the 6-MWD and decrease the NT-proBNP level in CHD complicated by CHF152. The microcirculation is a complex network of small arteries, veins, and capillaries responsible for providing a dynamic response of tissue perfusion to local tissues in order to meet their oxygen requirements and to participate in nutrient transport and metabolite removal153. The microcirculatory dysfunction is correlated with systemic endothelial dysfunction and CHF severity154. DHI may reduce microcirculatory resistance to relieve myocardial injury155. CHF could activate platelets through the neuroendocrine system, hemodynamics, cytokines, and NO156. In turn, the activation of platelets participates in thrombosis and the inflammatory responses, which further play a role in the occurrence and development of CHF157. The liquid chromatography-mass spectrometry results demonstrated DHI anti-platelet aggregation in a dose- and concentration-dependent manner, mainly through the nicotinic acid-niacinamide metabolic pathway and the purine metabolic pathway158.
3.3. Common decoctions prescribed by physicians
3.3.1. Zhenwu decoction (ZWD)
ZWD is recorded in “Treatise on Febrile Diseases (Shanghanlun)” by Zhang Zhongjing in the Han Dynasty, containing Poria (Fuling), Atractylodes Macrocephala Rhizoma (Baizhu), Aconiti Lateralis Radix Praeparata (Fuzi), Zingiberis Rhizoma Recens (Shengjiang), and Paeoniae Radix Alba (Baishao). It warms Yang to eliminate diuresis, which is conducive to holding back edema due to Yang deficiency159.
ZWD could boost LVEF, cardiac index, and stroke volume160. Aldosterone (ALD), an indicator of the RAAS, is positively correlated with CHF mortality. Prolonged high levels of ALD can exacerbate CHF caused by water-sodium retention, electrolyte disturbances, collagen deposition, and fibrosis in the myocardium and interstitial vessels, thus exacerbating CHF. However, ZWD was revealed to antagonize the RAAS and down-regulate ALD level by reducing Ang II in a dose-dependent manner159, indicating ZWD can prevent the progression of CHF and decrease mortality partially. ZWD could induce the activity of the cytochrome P450 proteins (CYP), including CYP3A1, CYP2C6, and CYP2C11161, which indicates that whether ZWD can be co-administered with these enzyme-mediated drugs and the potential herbal interactions are noteworthy. In short, ZWD may be appropriate for CHF patients complicated by or paired with renal diseases.
3.3.2. Linggui zhugan decoction (LZD)
During the Han Dynasty, Zhang Zhongjing wrote “Treatise on Febrile Diseases (Shanghanlun)”, which states that LZD is made up of Poria (Fuling), Cinnamomi Ramulus (Guizhi), Atractylodes Macrocephala Rhizoma (Baizhu), Glycyrrhiza Radix (Gancao). LZD is unique in that it warms Yang to eliminate diuresis and strengthens the spleen to get rid of dampness162.
In contrast to, which is better at warming Yang, LZD emphasizes spleen strengthening, which reduces digestive system difficulties in CHF patients. In CHF patients, especially in the elderly who have gastrointestinal clinical manifestations of impaired gastric motility caused by gastrointestinal stasis, such as abdominal distension, belching, a sense of fullness, and poor appetite, are frequent163. In addition to lowering BNP levels and improving LVEF values, LZD had further benefits in lowering the Gastrointestinal Symptom Rating Scale scores and shortening gastric emptying time164. Patients exhibit an improvement in appetite and quality of life, with a positive effect on CHF. For CHF patients with type 2 diabetes, LZD markedly decreased blood glucose, glycosylated hemoglobin, fasting insulin, and indices of triglyceride and total cholesterol. By enhancing glycometabolism, LZD, as a therapy for CHF with diabetes and obesity, has favorable elimination effects on risk factors165.
3.3.3. Taohong Siwu decoction (TSD)
It is documented that TSD from “Fu Ke Bing Jian” in the Qing era activates blood circulation to remove blood stasis and enrich blood. It is composed of Chuanxiong Rhizoma (Chuanxiong), Carthami Flos (Honghua), Angelica Sinensis Radix (Danggui), Persicae Semen (Taoren), Paeoniae Radix Alba (Baishao), and Rehmanniae Radix (Dihuang). The prominent active ingredients in TSD are flavonoids, aromatic organic acids, and benzoquinones (from Carthami Flos, Chuanxiong Rhizoma and Angelica Sinensis Radix, respectively)166.
Blood stasis destroyed the structure and proliferative activity of endothelial cells, with increasing levels of endothelin, NO, and TGF-β1. Endothelial cell structure and proliferative activity were both restored by TSD, and the levels of ET, NO, and TGF-1 were brought back to normal167. This proved that TSD is capable of removing blood stasis and attenuating endothelial cell damage caused by blood stasis. TSD also had the ability to dispel acute blood stasis168. By enhancing the local ischemia milieu and controlling mitochondrial dynamics, TSD has positive effects on cardiac function in ischemic cardiomyopathies including MI and CHF169, suggesting TSD may be a promising adjunctive strategy for the treatment of ischemic cardiomyopathies. TSD paired with ZWD may partially reverse myocardial remodeling in congestive CHF with Yang deficiency and blood stasis syndrome by raising LVEF and MMP-1 values while lowering LVEDD, LVESD, BNP, and MMP-9 levels170.
3.3.4. Gualou Xiebai Banxia decoction (GXBD)
Originally stated in “Jin Gui Yao Lue” in the Han era, GXBD is a common TCM formula composed of Trichosanthis Semen (Gualouzi), Alli Macrostemonis Bulbus (Xiebai), and Pinelliae Rhizoma (Banxia)171. It is able to treat CHF by activating Yang, removing obstructions, and regulating Qi to dissipate phlegm.
The versatility and effectiveness of GXBD in the treatment of cardiac disorders are backed by a lot of evidence. In individuals with stable angina, GXBD alone or in combination with standard medication dramatically reduced angina symptoms and improved the encephalogram and the blood lipid conditions171. Patients with excess phlegm are frequently plagued by lipid metabolism disorders, and GXBD presented eye-catching effects on CHD patients with phlegm stasis syndrome172. A number of elementary studies have revealed that GXBD may be a prospective TCM formula for therapy of CHF. GXBD could ameliorate the cardiac dysfunction due to myocardial fibrosis by inhibiting the expression of NF-κB pathway-related inflammatory mediators173. GXBD extract significantly alleviated infarct injury in MI rats, and the water-insoluble fraction of GXBD is an effective site of cardioprotective properties174.
To create synergistic benefits and reduce toxicity, TCM formulas typically mix a number of Chinese medicinal herbs in varying amounts and methods. According to recent research, TCM offers CHF patients with various medical states or consequences more individualized and accurate therapy alternatives than chemical medications. In order to expand the window of on how TCM formulas can treat CHF and benefit more CHF patients, high-quality clinical trials are required.
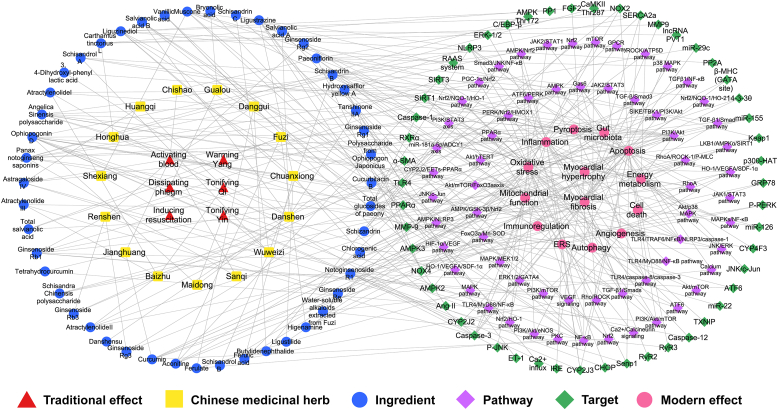
4. Chinese medicinal herbs and their main ingredients with potential implication for CHF and the underlying mechanism
Chinese herbal compatibility lies at the heart of TCM formulas. The function of TCM formulas is based on diverse actions of natural herbs. A number of Chinese medicinal herbs with implications for the cardiovascular system can be applied to treat cardiovascular diseases. We summarized 15 Chinese medicinal herbs available for CHF from TCM formulas listed above and the rules of TCM prescription in the treatment of CHF that have been reported175 (Table 2). Furthermore, we categorized and analyzed their main bioactive ingredients (Table 3 and Fig. 3).
Table 2.
Category, main bioactive ingredients of Chinese medicinal herbs and their structure.
| Category | Chinese medicinal herb | Ingredient | Structure |
|---|---|---|---|
| Tonifying Qi | Astragali Radix (Huangqi) | Astragaloside IV | 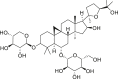 |
| Astragalus polysaccharide |  |
||
| Cycloastragenol | 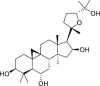 |
||
| Ginseng Radix (Renshen) | Ginsenoside Rb1 | 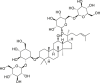 |
|
| Ginsenoside Rb3 | 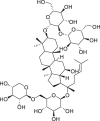 |
||
| Ginsenoside Re | 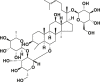 |
||
| Ginsenoside Rg1 | 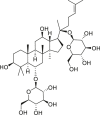 |
||
| Ginsenoside Rg2 | 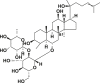 |
||
| Ginsenoside Rg3 |  |
||
| Atractylodes Macrocephala Rhizoma (Baizhu) | Atractylenolide I | 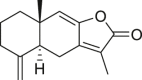 |
|
| Atractylenolide II |  |
||
| Atractylenolide III | 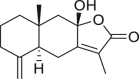 |
||
| Atractylon | 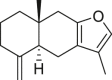 |
||
| Notoginseng Radix (Sanqi) | Panax notoginseng saponins |  |
|
| Notoginsenoside R1 |  |
||
| Ginsenoside Rh2 |  |
||
| Ginsenoside Rg5 |  |
||
| Quercetin |  |
||
| Dencichin | 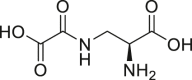 |
||
| Activating blood | Salviae Miltiorrhizae Radix (Danshen) | Tanshinone IIA | 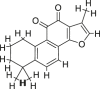 |
| Tanshinone I | 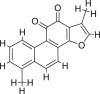 |
||
| Cryptotanshinone | 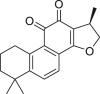 |
||
| Salvianolic acid A | 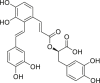 |
||
| Salvianolic acid B | 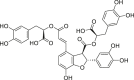 |
||
| Danshensu | 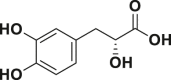 |
||
| Chuanxiong Rhizoma (Chuanxiong) | Ligustrazine | 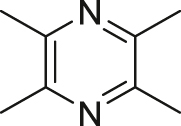 |
|
| Liguzinediol | 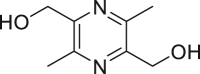 |
||
| Ferulic acid | 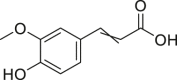 |
||
| Chlorogenic acid | 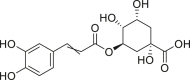 |
||
| Caffeic acid | 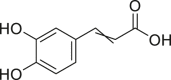 |
||
| Senkyunolide A | 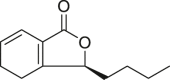 |
||
| Senkyunolide H |  |
||
| Senkyunolide I | 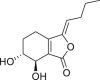 |
||
| Angelica Sinensis Radix (Danggui) | Butenyl phthalide | 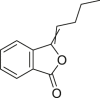 |
|
| α-Angelica lactone | 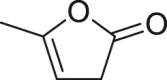 |
||
| Ferulic acid | 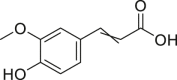 |
||
| Ligustilide |  |
||
| Vanillic acid | 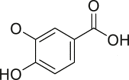 |
||
| Paeoniae Radix Rubra (Chishao) | Paeoniflorin | 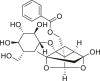 |
|
| Paeoniflorin B |  |
||
| Gallic acid | 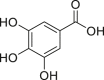 |
||
| Galloylpaeoniflorin | 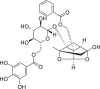 |
||
| Paeonilactone A | 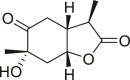 |
||
| Carthami Flos (Honghua) | Safflower yellow | 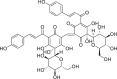 |
|
| Hydroxysafflor yellow A | 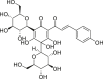 |
||
| Carthamidin | 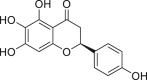 |
||
| Curcumae Longae Rhizoma (Jianghuang) | Curcumin |  |
|
| Tetrahydrocurcumin | 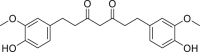 |
||
| Tonifying Yin | Ophiopogonis Radix (Maidong) | Ophiopogonin D | 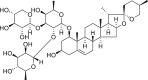 |
| Ophiopogonin C | 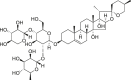 |
||
| Ophiopogonin B |  |
||
| Ruscogenin | 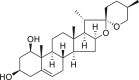 |
||
| Schisandra Chinensis Fructus (Wuweizi) | Schisandrol A | 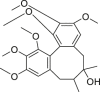 |
|
| Schisandrol B | 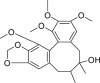 |
||
| Schisandrin A | 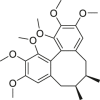 |
||
| Schisandrin B | 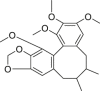 |
||
| Schisandrin C | 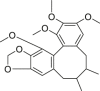 |
||
| Warming Yang | Aconiti Lateralis Radix Praeparata (Fuzi) | Aconitine | 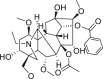 |
| Higenamine | 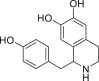 |
||
| Fuziline |  |
||
| Dissipating phlegm | Trichosanthis Fructus (Gualou) | Cucurbitacin B |  |
| Bryonolic acid |  |
||
| Inducing resuscitation | Moschus (Shexiang) | Muscone |  |
Table 3.
Targets/pathways and effects of ingredients of Chinese medicinal herbs on CHF.
| Ingredients | Targets/Pathways | Effects | Ref. |
|---|---|---|---|
| Astragaloside IV | Senp1 JAK2/STAT3 pathway TLR4/MyD88/NF-κB pathway SIKE/TBK1/PI3K/Akt pathway |
Oxidative stress Apoptosis Inflammation Energy metabolism Angiogenesis Myocardial fibrosis |
185, 186, 187, 188, 189, 190 |
| Ginsenoside Rg1 | RhoA signaling pathway AMPK/mTOR pathway MMP-9 α-SMA |
Energy metabolism Apoptosis Myocardial fibrosis Autophagy |
197,198,201,202 |
| Ginsenoside Rg2 | Akt/mTOR pathway | Autophagy | 203 |
| Ginsenoside Rg3 | AMPK pathway NLRP3 pathway |
Autophagy Inflammation Apoptosis |
204 |
| Ginsenoside Rb1 | RhoA signaling pathway Rho/ROCK pathway PI3K/mTOR pathway MAPK/MEK1/2 signaling pathway miR-155 Gas6 pathway |
Apoptosis Autophagy Inflammation Energy metabolism Oxidative stress Apoptosis |
197, 198, 199,200,205,206 |
| Ginsenoside Rb3 | PPARα pathway PERK/Nrf2/HMOX1 pathway |
Mitochondrial function Energy metabolism Oxidative stress Apoptosis Myocardial fibrosis |
207, 208, 209 |
| Ginsenoside Rc | SIRT1 | Energy metabolism | 210 |
| Ginsenoside Re | TGF-β/Smad3 pathway | Myocardial fibrosis | 211 |
| Atractylenolide I | Caspase-3 signaling pathway AMPK pathway PI3K/Akt pathway |
Apoptosis Mitochondrial function Energy metabolism |
214,220 |
| Atractylenolide II | Akt/p38 MAPK pathway AMPK pathway PI3K/Akt pathway |
Energy metabolism | 217,220 |
| Atractylenolide III | Caspase-3 Akt/p38 MAPK pathway |
Apoptosis Mitochondrial function Energy metabolism |
215,217 |
| Panax notoginseng saponins | PPARα, RXRα FoxO3a/Mn-SOD signaling pathway PI3K/Akt signaling pathway AMPK signaling pathway HIF-1α/BNIP3 pathway miR-29c CaMKII Thr287 |
Energy metabolism Mitochondrial function Oxidative stress Apoptosis Autophagy Myocardial fibrosis |
222,225, 226, 227, 228 |
| Notoginsenoside R1 | ROCK/ATP5D GRP78, P-PERK, ATF6, IRE CHOP, Caspase-12, P-JNK RhoA/ROCK-1/P-MLC pathway |
Energy metabolism Oxidative stress ERS Myocardial fibrosis |
223,224,377 |
| Tanshinone IIA | NOX4 TLR4 C/EBP-β |
Oxidative stress Myocardial fibrosis Inflammation |
230, 231, 232, 233, 234 |
| Total salvianolic acid | SIRT1 SIRT3 |
Oxidative stress Mitochondrial function |
235 |
| Salvianolic acid A | LncRNA PVT1 NF-κB pathway |
Apoptosis | 238 |
| Salvianolic acid B | MMP9 α-SMA ERK1/2/GATA4 signaling pathway VEGF signaling |
Autophagy Myocardial fibrosis Angiogenesis |
236,237 |
| 3, 4-dihydroxyl-phenyl lactic acid | SIRT1 RhoA/ROCK-1/P-MLC pathway |
Mitochondrial function Oxidative stress Apoptosis Myocardial fibrosis Energy metabolism |
239,377 |
| Ferulic acid | VEGF signaling Nrf2 signaling pathway |
Apoptosis Oxidative stress Angiogenesis Gut microbiota |
241,243,244 |
| Chlorogenic acid | Oxidative stress Apoptosis Autophagy Gut microbiota |
245 | |
| Ligustilide | AMPK/GSK-3β/Nrf2 pathway | Oxidative stress Inflammation Myocardial fibrosis |
248 |
| Ligustrazine | Ca2+ influx ET-1 Ang II PI3K/Akt pathway TLR4/TRAF6/NF-κB/NLRP3/caspase-1 pathway TLR4/caspase-8/caspase-3 pathway |
Apoptosis ERS Pyroptosis Inflammation |
249, 250, 251, 252 |
| Liguzinediol | RAAS system PP1 and PP2A SERCA2a TGF-β1/Smads pathway MMPs |
Oxidative stress Inflammation Myocardial fibrosis Apoptosis |
253, 254, 255,386 |
| Angelica sinensis polysaccharide | Akt/hTERT pathway. PI3K/Akt signal pathway mTOR signal pathway miR-126 PI3K/Akt pathway JAK1/STAT3 pathway miR-22 ATF6 pathway |
Apoptosis ERS Oxidative stress Myocardial fibrosis |
257, 258, 259, 260, 261, 262 |
| Ferulate | PKC and MAPK signaling pathways | Myocardial hypertrophy | 263 |
| Vanillic acid | AMPK2 AMPK3 |
Apoptosis Oxidative stress |
264 |
| Butylidenephthalide | PI3K/STAT3 axis | Inflammation Myocardial fibrosis |
265 |
| Total glucosides of paeony | miR-181a-5p/ADCY1 axis NF-κB pathway Caspase-1 NLRP3 NOX-2 |
Oxidative stress Pyroptosis Myocardial fibrosis Inflammation Apoptosis |
267, 268, 269, 270, 271, 272,274 |
| Paeoniflorin | NOX-2 NOX-4 p38 MAPK pathway TGF-β1/Smad pathway GPCR pathway MAPKs/NF-κB patway PI3K/Akt/mTOR pathway JAK2/STAT3 pathway |
Oxidative stress Apoptosis Myocardial fibrosis Inflammation Immunoregulation |
271, 272, 273, 274 |
| Hydroxysafflor yellow A | Mitochondrial permeability transition pores HO-1/VEGFA/SDF-1α NLRP3 AMPK/NLRP3 pathway JAK2/STAT1 pathway Nrf2/NQO-1/HO-1 signaling pathway PGC-1α/Nrf2 pathway |
Mitochondrial function Myocardial fibrosis Angiogenesis Autophagy Inflammation Oxidative stress Apoptosis Myocardial hypertrophy |
279, 280, 281, 282, 283, 284, 285, 286, 287 |
| Curcumin |
β-MHC (GATA site) p300-HAT Nrf2 pathway |
Apoptosis Cell death Myocardial hypertrophy Oxidative stress Myocardial fibrosis Mitochondrial function |
289, 290, 291, 292, 293, 294,297, 298, 299, 300 |
| Tetrahydrocurcumin | PI3K/Akt/mTOR pathway | Apoptosis Autophagy |
299 |
| Ophiopogonin D | CYP4F3 JNK/ERK pathway Ca2+/Calcineurin signaling CYP2J3 SERCA2a PI3K/Akt/eNOS pathway CYP2J2 JNK/c-Jun CYP2J2/EETs-PPARα pathway NF-κB pathway |
Energy metabolism Oxidative stress ERS Mitochondrial function Autophagy Inflammation Apoptosis Gut microbiota |
305, 306, 307, 308, 309,311,313, 314, 315, 316 |
| Polysaccharide from Ophiopogon Japonicus | Immunoregulation Gut microbiota |
317, 318, 319 | |
| Schizandrin | Smad3/JNK/NF-κB pathway JAK2/STAT3 pathway |
Apoptosis Myocardial hypertrophy Inflammation |
322,323 |
| Schisandrin B | AMPK/Nrf2 pathway PI3K/Akt pathway ATF6 pathway PERK pathway TGFβ1/NF-κB pathway |
Oxidative stress ERS Apoptosis Inflammation Mitochondrial function Myocardial fibrosis |
324, 325, 326,328,333,334 |
| Schisandrin C | Keap1 Nrf2 pathway |
Oxidative stress | 327 |
| Schisandrol A | 14-3-3θ PI3K/Akt pathway |
Oxidative stress Apoptosis Energy metabolism |
330, 331, 332 |
| Schisandrol B | TGFβ1/NF-κB pathway | Inflammation Myocardial fibrosis |
330,333 |
| Schisandra chinensis polysaccharide | TXNIP and Trx-1 | Oxidative stress Myocardial hypertrophy |
337 |
| Aconitine | SIRT3 Mitochondrial permeability transition pores β-Adrenergic receptor agonist |
Energy metabolism Myocardial hypertrophy Apoptosis Inflammation Mitochondrial function |
341, 342, 343, 344 |
| Higenamine | LKB1/AMPKα/SIRT1 signaling pathway PI3K/Akt Pathway |
Mitochondrial function Energy metabolism Oxidative Stress Apoptosis |
350,351 |
| Water-soluble alkaloids extracted from Aconiti Lateralis Radix Praeparata | Calcium signaling pathway RyR2 RyR3 SERCA2a |
Apoptosis Myocardial fibrosis |
352 |
| Cucurbitacin B | Akt/mTOR/FoxO3a signal axis | Autophagy Pyroptosis Inflammation Myocardial hypertrophy Myocardial fibrosis |
357 |
| Bryonolic acid | Nrf2/HO-1 pathway | Inflammation Oxidative stress Pyroptosis |
358 |
| Muscone | SIRT3 NF-κB and NLRP3 inflammasome HIF-1α/VEGF pathway |
Oxidative stress Apoptosis Inflammation Angiogenesis |
361, 362, 363, 364, 365 |
Figure 3.
The summary of targets and pathways of natural herbal components against chronic heart failure.
4.1. Astragali Radix (Huangqi)
Huangqi is the root of Astragalus membranaceus (Fisch.) Bge.var.mongholicus (Bge.) Hsiao or A. membranaceus (Fisch.) Bge., which mainly contains flavonoids, saponins, and alkaloids176. Flavonoids have antibacterial and antioxidant properties as well as the ability to promote glucose consumption and inhibit α-glucosidase177. Several alkaloids and phenolic components have been proved to possess biological activities such as antioxidation, anti-inflammation, and regulating metabolism178, 179, 180. Astragalus polysaccharide, mainly composed of glucan (water-soluble glucan and water-insoluble glucan) and heteropolysaccharides (mostly water-soluble acidic heteropolysaccharides), can be used as an immune promoter or regulator with the functions of enhancing immunity181, anti-inflammation182, antioxidation183, and remodeling the gut microenvironment184, act on multiple systems of the human body. Saponins, which include astragalosides I–VIII and isoastragalosides I–III, are important active components of Huangqi because they can stimulate the β-oxidation of FFAs, improve mitochondrial function, and increase the expression of VEGF and basic fibroblast growth factor to promote angiogenesis185.
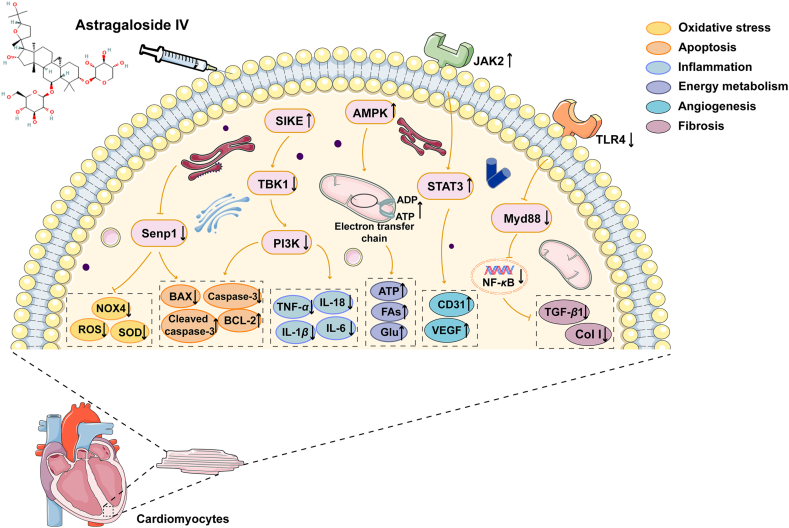
Astragaloside is the main active ingredient of saponins in Huangqi and can be used as a qualitative and quantitative index. The antioxidant activity and anti-apoptosis effects of astragaloside IV (AS-IV) on cardiomyocytes, are primarily achieved by reversing overexpressed small ubiquitin-like modifier-specific protease 1186. Hypercholesterolemia is a risk factor for the development of cardiac hypertrophy. It is reported that AS-IV could inhibit oxidative stress, regulate cardiac homeostasis, prevent hypercholesterolemia-induced cardiac remodeling, and restore ventricular function187. Signal transducer and activator of transcription 3 (STAT3) is a promising molecule for angiogenesis-mediated therapy. AS-IV could activate Janus kinase 2 (JAK2)/STAT3 pathway to increase vascular density to alleviate CHF by inducing the expression of CD31 and VEGF188. The molecular docking prediction showed that AS-IV might target MYD88 and subsequently the downstream TLR4 signaling pathway. AS-IV suppressed collagen I, III to ameliorate myocardial fibrosis by reducing inflammation and blocking TLR4/MYD88/NF-κB and suppressor of IKKε/TANK-binding kinase 1/PI3K/AKT pathways189,190. Moreover, AS-IV prevented against cardiac malfunction induced by MI/R that initiates from the phase of ischemia, via regulating energy metabolism to maintain the integrity of myocardial structure191.
4.2. Ginseng Radix et Rhizoma (Renshen)
Renshen is the dried root and rhizome of Panax ginseng C. A. Mey., a medicinal plant of the Araliaceae family. Renshen has extensive pharmacological effects that work on multiple systems in the human body192. Research on Renshen focuses on Ginsenosides and ginseng polysaccharides, which have pharmacological effects such as inhibiting lipid accumulation193, improving insulin sensitivity194, selectively inhibiting apoptosis195, repairing the blood–retinal barrier196, and regulating energy metabolism197. Ginsenosides are the vital active components of Renshen that act on the central nervous system, cardiovascular system, immunological system and endocrine system. They are classified into three types: type A (panoxadiol type), type B (panaxatriol type), and type C (oleanolic acid type). Based on the tight binding affinity of Ras homolog gene subfamily member A (RhoA) to ginsenoside Rg1 (GRg1) or ginsenoside Rb1 (GRb1), both of these inhibited RhoA signaling pathway to attenuate energy metabolism disorder and apoptosis to recover myocardial injury198,199. GRb1 also could regulate Rho/Rho-associated kinase (ROCK) and PI3K/mTOR pathways against CHF by inhibiting autophagy of cardiomyocytes200. GRb1 can act as a natural agonist of glutathione reductase to protect H9c2 cells from oxidative stress-induced apoptosis201. By dramatically suppressing AMPK and activating the mTOR pathway, GRg1 improved cardiac hypertrophy by preventing the formation of intracellular autophagosomes202. GRg1 also could regulate the interaction between Beclin 1 and BCL-2 and inhibit apoptosis while promoting autophagy203. Ginsenoside Rg2 could activate autophagy to protect human cardiomyocytes against trastuzumab-induced cardiotoxicity via upregulating Beclin 1, LC3, and ATG5204. Ginsenoside Rg3 protects the heart against isoproterenol (ISO)-induced myocardial injury by activating AMPK mediated autophagy and NLRP3 inflammasome205. In addition, GRb1 may reduce IL-1β, IL-6 and TNF-α production and mitigate cardiac inflammation to ease cardiac hypertrophy through MAPK 1/2 signaling pathway206. GRb1 could also reduce calcium and collagen deposition in aged mice, suggesting that GRb1 may be a potentially anti-aging-related vascular injury medication207. In addition to regulating the oxidation of FFAs by targeting the peroxidase proliferator activated receptor α (PPARα) pathway, Ginsenoside Rb3 (GRb3) could protect mitochondrial membrane integrity and exert anti-apoptotic effects208. In order to prevent hypoxia/reoxygenation (H/R)-induced oxidative stress and apoptosis GRb3 could phosphorylate extracellular regulated protein kinases (ERK), induce nuclear translocation of NF-E2-related factor 2 (Nrf2), and stimulate the production of HO-1209. Besides, nanoparticle conjugation of GRb3 corrected the low oral bioavailability to inhibit myocardial fibrosis210, suggesting nano-drug carriers may be a potential solution for the delivery of natural drugs. Recent research found that ginsenoside Rc, as a silent information regulator (SIRT)-1 activator, promotes energy metabolism to improve cardioprotective functions under MI/R injury211. Orally administrated ginsenoside Re for four weeks to ISO-induced CHF rats significantly reduced collagen I by regulating the TGF-β/Smad3 pathway to alleviate myocardial fibrosis, thereby enhancing LV function212. In short, Renshen may be a fantastic agent for the treatment and management of CHF.
4.3. Atractylodes Macrocephala Rhizoma (Baizhu)
Baizhu, a commonly used tonifying Qi herb in TCM, is the rhizome of Atractylodes macrocephala Koidz. of the Asteraceae family. Lactones, polysaccharides, and volatile oil make up its primary chemical constituents. Atractylon is the main active component of volatile oil, and its content is a crucial quality evaluation criterion for Baizhu. Volatile oil from Baizhu is volatile, thus easily oxidized and decomposed into atractylenolides (ATL) I, II, and III213. Their content is arranged as follows: ATL III > ATL I > ATL II > ATL IV, and ATL II and ATL III are the main active components among them214. However, there have been few studies on the effects of A. macrocephala polysaccharides on the cardiovascular system.
ATL I pretreatment suppressed myocardial apoptosis by inactivating the caspase-3 signaling pathway to preserve mitochondrial function, thus improving myocardial morphology215. In rat models of MI, ATL III could also inhibit BAX and caspase-3 activity while up-regulate BCL-2 expression to reduce maladaptive apoptosis of cardiomyocytes and reverse mitochondrial malfunction216.
Platelets are crucial in the development of atherosclerosis-thrombosis and therefore antiplatelet agents are widely used in the treatment of CHD217. ATL II and ATL III have been reported to reduce agonist-induced platelet aggregation and ATP release from dense granules, delay the contraction of clots in platelet-containing platelet-depleted plasma, and prolong the time to the first occlusion of iron chloride-induced carotid thrombosis in mice218. These results suggest ATL II and ATL III may be potential therapeutic candidates for the prevention of thrombosis.
Diabetes mellitus is a common co-morbidity and is recognized as a risk factor for a worse prognosis in CHF patients219,220. Recently, the SGLT2 inhibitors (dapagliflozin and empagliflozin) have been included as Class IA indication in the 2021 ESC guidelines on HF with LVEF ≤40% to reduce the risk of hospitalization and death in CHF patients. Similar to how SGLT2 inhibitor function, ATL I and ATL II were revealed to significantly increase GLUT4 expression and promote GLUT4 translocation to the plasma membrane through the activation of AMPK and PI3K/AKT pathways221. These compounds will have novel therapeutic applications for cardiovascular diseases accompanied by dysglycemia.
4.4. Notoginseng Radix et Rhizoma (Sanqi)
Sanqi is the dried root or rhizome of Panax notoginseng (Burk.) F. H. Chen. Using OB ≥ 30% and DL ≥ 0.15 as the screening conditions, nine main active components of Sanqi were obtained: octadecadiene, lipoxin, diisooctyl phthalate β-sitosterol, stigmasterol, ginsenoside Rh2, enoic acid ester, enoic acid, and quercetin222. P. notoginseng saponins (PNS) are the major cardioprotective substances of Sanqi, in which the contents of GRg1, GRb1, GRd, GRe, and notoginsenoside R1 (NGR1) are rich. PNS exerts a cardiovascular protective role through multiple pathways.
PNS specializes in regulating energy metabolism and mitochondrial function. Transcriptome analysis shows that PNS effectively increased the expression of PPARα and RXRα, which help control the downstream energy metabolism-related proteins to exert a cardioprotective effect223. While NGR1 was demonstrated to inhibit ROCK and enhance mitochondrial ATP synthase δ-subunits to prevent MI/R-induced energy metabolism disorder224. NGR1 pretreatment prevented ERS and apoptosis by decreasing the levels of ERS-responsive proteins GRP78, P-PERK, activated transcription factor 6, and IRE and inhibiting the expression of pro-apoptotic proteins CCAAT enhancer binding protein, caspase-12, and p-JNK225. Mitochondrial dysfunction leads to an increase in oxidative damage, which plays a key role in apoptosis in cardiomyocytes. PNS was reported to ameliorate aging-related mitochondrial dysfunction in a dose-dependent manner to attenuate oxidative stress damage and exert anti-apoptotic effects, thereby significantly improving myocardial morphological changes in aging rats226. PNS also enhanced glucose deprivation-induced mitochondrial autophagy through phosphorylation of AMPK Thr172 and CaMKII Thr287 in cardiomyocytes227.
Besides, PNS inhibited cardiomyocyte apoptosis and death in the oxygen-glucose deprived-H9c2 cells and reversed hypertrophy through cell cycle arrest, DNA double-strand breakage repair process, and chromosome segregation228. Global gene expression analysis revealed that PNS could protect cardiomyocytes by elevating the expression of several non-coding RNAs, such as miRNAs and lncRNAs229. The anti-fibrotic microRNA miR-29c is involved in the cardioprotective effects of PNS. PNS promoted the expression of miR-29c directly and down-regulated fibrogenesis genes (collagen 1a1, collagen 1a2, collagen 3a1, and collagen 5a1), fibrillin 1 and TGFβ1 increased by ISO229.
4.5. Salviae Miltiorrhizae Radix et Rhizoma (Danshen)
Danshen is the dried root and rhizome of Salvia miltiorrhiza Bge. in the Labiatae family. More than 60 lipid-soluble components and 50 water-soluble components have been isolated. Most of the lipid-soluble components are phenylanthraquinone derivatives, including tanshinone, tanshinone lactone, and tanshinol. Tanshinone, as a research hotspot, is mainly distributed in the root bark. Tanshinones contain o-quinone or p-quinone structures and are classified into tanshinone I, tan IIA, tan IIB, cryptotanshinone, isocryptotanshinone, 15,16-dihydrotanshinone, hydroxytanshinone, methyl tanshinonate, etc230. Among them, tan IIA has the most prominent activity. Most of water-soluble components are phenolic acids, such as salvianolic acid A, salvianolic acid B (Sal B), rosmarinic acid, protocatechualdehyde, and alkannic acid.
Tan IIA treatment prevented myocardial fibrosis to restore LVEF and LV fraction shortening in MI-induced CHF model via suppressing oxidative stress in cardiac fibroblasts231. Tan IIA injection can effectively improve microcirculation and ventricular remodeling, improve cardiac function, and reduce the occurrence of major adverse cardiac events232. In MI mice, the combination of tan IIA and puerarin at a ratio of 1:1 reduced M1 type macrophages, improved M2 type macrophages in the early stages, and reduced collagen synthesis and fibroblast release in the later stages, thereby inhibiting myocardial fibrosis and cardiac remodeling233. Sodium TSA sulfonate, the sulfonated product of tan IIA, has been clinically tested to be effective in treating angina pectoris234. A clinical trial on MI found that short-term treatment of sodium TSA sulfonate reversed progressive LV remodeling and resulted in better clinical outcomes, which may be relevant to the inhibition of eventual injury of the infarcted myocardium by infiltrating neutrophils235.
Total salvianolic acid (TSA) is the main water-soluble component of SM, and TSA injection was approved by CFDA (Z20110011) in 2011 for ischemic stroke. TSA could attenuate oxidative stress injury induced by MI/R through upregulating SIRT1 and SIRT3 to restore the activity of mitochondrial respiratory chain complexes236. Sal B is the most abundant active ingredient in TSA. Elevated MMP-9 after MI may exacerbate ischemia-induced CHF. Increased MMP-9 inhibited autophagy, whereas Sal B inhibited MMP-9 activity and then increased autophagic flux in the peri-infarct myocardium to mitigate cardiac ECM deposition237. Inhibition of ERK1/2/GATA4 signaling pathway is a key mechanism of the anti-CHF effect of Sal B in CHF mice established by TAC-induced pressure overload238. Salvianolic acid A may down-regulate lncRNA plasmacytoma variant translocation 1 to prevent doxorubicin (DOX)-induced H9c2 cell injury and apoptosis by inhibiting NF-κB pathway239. 3,4-Dihydroxyl-phenyl lactic acid (DLA) is a water-soluble compound and regulates mitochondrial function mediated by SIRT1, a core target of DLA activities. DLA improved myocardial structure and cardiac function after MI/R via restoring SIRT1-interceding complex I activity and mitochondrial function240.
4.6. Chuanxiong Rhizoma (Chuanxiong)
Chuanxiong is the dried rhizome of the plant Ligusticum chuanxiong Hort. in the Umbelliferae family. The bioactive ingredients in Chuanxiong can be categorized into phenols and organic acids, phthalates, and alkaloids. Phenols and organic acids are distinct bioactive components in Chuanxiong.
Ferulic acid (FA), chlorogenic acid (CHA), and caffeic acid (CAA), the main phenols and organic aids in Chuanxiong, show the prospect for clinical application. Diet or drugs may interact with the host by altering the intestinal flora, thus affecting the prognosis and progression of cardiovascular diseases. CHA and CAA can be derived into FA via a range of metabolic pathways. Gut microbiota affects the production of FA241, and FA itself could regulate the gut microbiota. A daily supplement of 50 mg/kg FA for eight weeks improved LVEF and cardiac remodeling by increasing abundance of Lactobacillus and Parabacteroides in TAC mice, suggesting FA may be a nutraceutical and dietary option for prevention and treatment of CHF236,242. In both human umbilical vein endothelial cells and zebrafish models, FA enhanced the pro-angiogenic effect of Sal B partly through activating VEGF receptors243. FA ameliorated CHF by inhibiting apoptosis via decreasing Nrf2 signaling pathway-mediated oxidative stress244. CHA is metabolized mainly by the gut microbiota through hydrogenation, dehydroxylation, and hydrolysis, exhibiting multiple modulations of pathological cascade responses that reduce oxidative stress-induced apoptosis through the activation of autophagy245.
The phthalides in Chuanxiong are a large group of substances with the parent nucleus structure of phthalides, for instance, ligustilide, senkyunolide A, senkyunolide H, senkyunolide I are abundant in content and have biological activity. Ligustilide, senkyunolide A, senkyunolide I may aid in the passage of medications across the blood–brain barrier. Therefore, it may be key for Chuanxiong to act as an ‘envoy drug’ to and trigger the upward movement of medications246,247. Ligustilide, the most abundant phthalide in Chuanxiong, could modulate oxidative injury, inflammation, and fibrosis through the AMPK/glycogen synthase kinase-3β/Nrf2 pathway, thereby attenuating glycolipotoxicity-induced cardiomyocyte dysfunction248. Ligustilide may represent a potential therapeutic agent against CHF with diabetes mellitus. Ligustrazine is considered the characteristic alkaloid of Chuanxiong and is supported as a metric for assessing the product quality. Ligustrazine inhibited Ca2+ influx249, endothelin 1/Ang II released from endothelial cells250, excessive apoptosis251 and ERS252. Liguzinediol, 2,5-dimethyl-3,6-dimethylpyrazine, as a lead compound, is a structurally modified derivative of Chuanxiong. For one thing, liguzinediol reduced oxidative stress by inhibiting RAAS activation and the secretion of pro-inflammatory factors. For another, liguzinediol relieved myocardial fibrosis by reducing cardiomyocyte necrosis and collagen deposition253. Liguzinediol enhanced sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) activity via inhibiting of protein phosphatase (PP1 and PP2A) activities, which contributed to positive inotropic effects in CHF rats254. In DOX-induced CHF rats, administration of liguzinediol for two weeks decreased BAX level and increased BCL-2 level, restored cardiac function by suppressing myocardial cell apoptosis. Meanwhile, a decrease in the number of apoptotic bodies and injury to cardiomyocytes could be observed by transmission electron microscope255.
4.7. Angelica Sinensis Radix (Danggui)
Danggui is the dried root of Angelica sinensis (Oliv.) Diels, a plant in the Umbelliferae family. There has always been a saying that “nine out of ten prescriptions have Danggui”. The main bioactive components in Danggui are A. sinensis polysaccharide (ASP), volatile oil, and vanillic acid (VA).
ASP accounts for 15% of the content of Danggui, and its physicochemical properties and biological activities are affected by different drying techniques. Freeze-drying ASP is more suitable for medicinal raw material256. ASP could improve the oxidative damage of diverse cells through various pathways257,258. ASP might protect the heart against MI by ameliorating the detrimental ERS259. ASP pretreatment activated activating transcription factor 6 signaling pathway and thus improved ERS and oxidative stress in hydrogen peroxide treated H9c2 cells260. ASP normalized cardiac functions (increasing the LVEF while decreasing the LVEDD, LVESD) by alleviating oxidative stress and apoptosis261, implying that ASP may be a cutting-edge new natural antioxidant and anti-aging agent.
The volatile oil, another essential practical component, its content in Danggui is about 1%. It can be divided into neutral oil, phenolic oil and acidic oil. Among them, neutral oil accounts for the highest proportion, up to more than 88%, mainly including butylidene phthalide, butenyl phthalide, ligustilide, etc. FA is the representative of organic acids in Danggui. A pharmacokinetic study of FA indicates transdermal administration may be a promising drug delivery route compared with intragastric administration262. Ferulate is the sodium salt of FA, proving that it can inhibit protein kinase C and MAPK signaling pathways to downregulate Ang II and endothelin 1 levels to withstand myocardial hypertrophy263.
VA is another phenolic compound with the high content in Danggui. In H9c2 cells of H/R injury, VA decreased the lactate dehydrogenase activity, accompanied by reduced levels of ROS, alleviated myocardial injury264. Butylidenephthalide is an active compound of VA, which promoted macrophage polarization to M2 type to reduce inflammation and myocardial fibrosis targeting the PI3K/STAT3 axis. Impressively, it even reversed aging-related myofibroblast dysregulation265.
4.8. Paeoniae Radix Rubra (Chishao)
Chishao is the dried root of Paeonia lactiflora Pall., a plant of the Ranunculaceae family. Its active ingredients mainly contain glycosides, flavonoids, and phenolic acids. Glycosides in Chishao are collectively referred to total glucosides of paeony (TGP), mainly including monoterpene glucosides, which are mainly divided into pinane-type and pinane lactone-type glucosides266. The former mainly includes paeoniflorins A–H and desbenzoyl paeoniflorin; the latter includes paeonilactones A–C. These compounds underline the pharmacological effects of Chishao.
TGP can protect cardiomyocytes by regulating the levels of various myocardial enzymes and MDA267. TGP is capable of improving cardiomyocyte dysfunction by protecting antioxidative defenses and bioenergetic systems268. The onset of pyroptosis is dependent on the activation of caspase-1 as a result of the activation of intracellular inflammasome. TGP whittled caspase-1 and NLRP3 activity via miR-181a-5p/adenylate cyclase 1 axis, thus attenuated H/R-induced cardiomyocyte pyroptosis269. TGP reduced collagen fibril production by inhibiting the expression and activity of MMPs through the inhibition of NF-κB pathway270. Paeoniflorin accounts for more than 40% of TGP, which significantly downregulated the expression of NOX2 and NOX4 to fight with DOX-induced apoptosis and oxidative stress in a dose-dependent manner271. In addition, paeoniflorin could relieve myocardial fibrosis to rescue cardiac function via down-regulating the p38 MAPK and TGF-β1/Smad pathways in CHF rats272,273. In brief, TGP and paeoniflorin restore abnormal signaling pathways to exert broad anti-inflammatory effects and thereby treat CHF274.
Gallic acid is the highest content of phenolic acid compounds in Chishao. Wistfully, its therapeutic application is limited by poor bioavailability and toxicity275. Flavonoids isolated from Chishao include kaempferol, dihydroapigenin, etc. These components may be the material basis of Chishao for promoting blood circulation and removing blood stasis276.
4.9. Carthami Flos (Honghua)
Honghua is the dried tubular flower of Carthamus tinctorius L., mainly comprises flavonoids, alkaloids, and polysaccharides. The primary active ingredient in Honghua is safflower yellow, a quinone chalcone carboglycoside derived from flavonoids, whose structure is characterized by the oxidation of the chalcone ring. Nearly all red and yellow pigments in Honghua are classified as quinone chalcone carboglycosides, of which hydroxysafflor yellow A (HSYA) is a typical representative277.
HSYA is one of the key medicinal substances in TSD278. HSYA penetrated the member of cardiomyocytes and was able to repair myocardial injury by interacting with mPTP279. Through the HO-1/VEGFA/SDF-1α signaling cascade response, HSYA increased the BCL-2/BAX ratio and decreased cleaved-caspase 3, TGF-β and Collagen I in post-MI cardiomyocytes to mobilize endothelial progenitor cells to restore impaired LV function and promote myocardial angiogenesis280. In addition, HSYA may resist oxidative stress, inhibit the inflammatory response, and protect cardiomyocytes and endothelial cells, thus promoting VEGF release to promote angiogenesis280. Nucleolin is a multifunctional DNA–RNA–protein binding protein widely expressed in the nucleolus of eukaryotic cells. It might be a “staging post” for HSYA regulation on angiogenesis281. Inflammation, apoptosis, and autophagy play instrumental roles in the pathogenesis of myocardial injury, while HSYA could directly regulate AMPK/NLRP3 and JAK2/STAT3 pathways to suppress NLRP3 inflammasome and apoptosis as well as promote autophagy to protect hearts against MI/R injury282, 283, 284. Besides, HSYA exerted significant anti-hypertrophic effects285. Fibrosis-related factor 2 is engaged in myocardial hypertrophy and collagen deposition caused by pressure or volume overload. The ethanolic extract of Honghua, could antagonize fibrosis-related factor 2 and MMP9 to protect against lipopolysaccharide (LPS)-induced cardiac fibrosis286. In primary cardiomyocytes and human-induced pluripotent stem cell-derived cardiomyocytes, the cardioprotective effects of HSYA have in-depth corroboration. Patch-clamp experiments revealed that HSYA restored the contractile function of cardiomyocytes and abnormal field potential signaling287.
Overall, further development of Honghua for the treatment of CHF is anticipated, given that Honghua injection has been used clinically in China for the treatment of occlusive cerebrovascular diseases and CHD (Approval number: Z14021790).
4.10. Curcuma Longa Rhizoma (Jianghuang)
Jianghuang is the dried rhizome of Curcuma longa L., a plant of the Zingiberaceae family. The main chemical components of Jianghuang are phenols and terpenoids, with curcumin constituting the majority of the former and volatile oil constituting the majority of the latter288.
Curcumin attenuated norepinephrine-induced cell death and modulated apoptosis in H9c2 cardiomyocytes289. Chronic stress, including hypertension, upregulates the expression of p300 and p300 activation causes the release of BNP, endothelin 1, and atrial natriuretic peptide (ANP), which sharpens LV remodeling290. Inhibition of p300 by curcumin markedly reduced hypertension-induced elevations in posterior wall thickness and LV mass index without affecting systolic function in Dahl salt-sensitive rats291. Additionally, curcumin and its demethoxy derivatives inhibited cardiomyocyte hypertrophy by inactivating p300292. To improve the therapeutic potential of curcumin in CHF, a curcumin analog named GO-Y030 was synthesized. 1 μmol/L GO-Y030 is equivalent to 10 μmol/L curcumin, therefore, it could exhibit stronger curcumin-like effects293. Nanocurcumin also presented exceeding cardioprotective effects against DOX-induced cardiac toxicity294. Bevacizumab is a human monoclonal antibody with promising efficacy for the treatment of metastatic malignancies. Bevacizumab disrupts mitochondria and exacerbates the risk of cardiovascular diseases with age, including CHF295,296. Curcumin in combination with bevacizumab significantly reduced the cardiotoxicity of bevacizumab and avoided deterioration of cardiac function297.
Exercise intolerance is a typical symptom of HFrEF. Curcumin might target Nrf2 in skeletal muscle to increase oxidative defense capacity to delay force loss and fatigue in HFrEF mice298. Nrf2 in skeletal muscle could be an imaginable point for treating severe CHF. Tetrahydrocurcumin (50 mg/kg/day), an essential hydrogenated metabolite of Jianghuang, protected rat hearts from MI/R-induced CHF by increasing LVEF, LV fraction shortening and decreasing LVESD and LVEDD299. Curcumin and some of its analogs could improve cardiac dysfunction caused by cardiac fibrosis300. Moreover, curcumin may be used as an alternative therapy for the treatment of patients with fibrotic CHF who are intolerant to angiotensin-converting enzyme inhibitors301. It is worth noting that adrenergic β-blockers and curcumin may interact and affect each other's pharmacodynamic302. Nonetheless, oral curcumin (80 mg/day) for five days had no positive effect on electrocardiographic parameters and echocardiographic parameters, and no differences were demonstrated in arrhythmias and CHF in patients with unstable angina303. Still, there is an urgent need for more research to move promising drugs from basic experiments to clinical practice.
4.11. Ophiopogonis Radix (Maidong)
Maidong is the dried tuberous root of Ophiopogon japonicus (L. f) Ker-Gawl in the Orchidaceae family. A variety of chemical components were isolated from different parts of Maidong, such as steroid saponins, homoisoflavonoids, polysaccharides, volatile oil, etc., among which steroidal saponins and homoisoflavonoids have a variety of pharmacological activities304.
Steroid saponins are divided into ruscosaponin-type and diosgenin-type structures, of which ruscogenin is dominant and regarded as a landmark component for measuring the quality of Maidong. The steroid saponins belonging to ruscogenin are ophiopogonins A, B, C, and D. In human AC16 cardiomyocytes, ophiopogonin D (OPD) can regulate the metabolic molecules of FFAs to protect the cardiovascular system in a dose-dependent manner305; while it also alter gut microbiota composition and fecal metabolites to prevent atherosclerosis306. The antioxidative activity of OPD can reduce mitochondrial membrane potential damage, improve mitochondrial productivity disorders, and balance myocardial energy homeostasis; meanwhile, it plays a comprehensive myocardial protective role by regulating the autophagic activity of mitochondria and clearing the inflammatory factors caused by injury307. Besides, OPD upregulated fusion proteins mitofusion 1/2 and optic atrophin 1 to manage the dynamic equilibrium of mitochondrial division to allay diabetic myocardial injury308. OPD can maintain the Ca2+ homeostasis of cardiomyocytes by suppressing ERS309. As a member of the cytochrome P450 superfamily, CYP2J3 catalyzes the conversion of arachidonic acid into epoxyeicosatrienoic acids (EETs)310. Elevated expression of CYP2J3 and EETs protects the heart by maintaining cardiomyocyte Ca2+ homeostasis through upregulation of phospholamban and SERCA2a expression311. OPD promoted phospholamban phosphorylation and SERCA2a activity via the modulation of CYP2J3 to sustain Ca2+ homeostasis, thus rescuing cardiac function312. Furthermore, OPD upregulated circulating CYP2J3/EETs, exerting anti-apoptotic, antioxidant, and anti-inflammatory effects to reduce MI/R injury and death of endothelial cells313, 314, 315. Inhibition of the inflammatory response with the help of CYP2J3 upregulated by OPD also attenuated myocardial hypertrophy via NF-κB pathway316.
Polysaccharides from O. japonicus (POJ) are composed of mono- and oligosaccharides, including fructose and glucose. POJ possesses conspicuous immune-enhancing activity and may be a novel immunopotentiator317. In addition to slowing a rapid heart rate, POJ could restore cardiac contraction amplitude and coronary flow faster after MI/R318. POJ promotes the intestinal microbial-induced metabolism of ophiopogonin and acts synergistically with ophiopogonin319.
4.12. Schisandra Chinensis Fructus (Wuweizi)
Wuweizi is the dried and mature fruit of Schisandra chinensis (Turcz.) Baill. principallycomposed of lignans and volatile oil. Volatile oil contains terpenoids, aliphatic compounds, and aromatic compounds, among which sesquiterpenoids are the most abundant, playing the role of anti-tumor, and anti-inflammation320. Lignans account for 8% of Wuweizi, schizandrin, and schisandrol are the primary active components of lignans. The composition of Wuweizi grown in different regions of China varies significantly. Wuweizi from southwest China has the best antioxidant capacity, and the main antioxidants are schisandrol A, schisandrol B, and schisandrin B321.
LPS induced excessive secretion of pro-inflammatory factors in H9c2 cells, whereas schizandrin reduced inflammation and cell loss, which may be partially reversed by overexpression of Smad3322. Schisandrin down-regulated JAK2/STAT3 pathway to decrease the BAX/BCL-2 ratio, increased the ratio of the cell surface area to cardiomyocyte protein/DNA, showing the potential for treating myocardial hypertrophy323.
Nrf2 is involved in the regulation of the antioxidant system, while myocardial injuries could disrupt the transcriptional activity of Nrf2. In H/R H9c2 cells, schizandrin B activated the AMPK/Nrf2 pathway to confront oxidative stress and inflammation324. Also, schisandrin B could reduce oxidative stress and ERS-induced excessive apoptosis caused by MI/R injury325,326. Similarly, schisandrin C targeted the Nrf2 pathway to attenuate oxidative stress in endothelial cells327. Nrf2 is a central molecule in the mechanism of the antioxidant and anti-inflammatory activity of schisandrin. Pirarubicin is one of the anthracycline anti-cancer drugs, and its cardiotoxicity cannot be ignored. In the cardiotoxicity rat models induced by pirarubicin, schizandrin B inhibited mPTP opening and sustained mitochondrial function to antagonize cardiotoxicity328. There is growing evidence that 14-3-3 proteins regulate cell cycle, signal transduction, metabolism, protein transport, necroinflammation, and apoptosis329. With rapid absorption (Tmax = 2.07 h) and a longer duration (t1/2 = 9.48 h), schisandrol A binding to 14-3-3θ proteins could resist oxidative stress and reduce apoptosis to attenuate MI/R injury330,331. Moreover, schisandrol A could regulate various endogenous amino acid metabolic pathways to protect against cardiac diseases332.
It has been reported that schisandrol B and schisandrin B had the strongest inhibitory effect on TGFβ1-mediated NF-κB activation333. Hence, schisandrol B and schisandrin B might be candidates for anti-myocardial fibrosis. And the antifibrotic effect of schisandrin B has been demonstrated in TAC mice334. Although schisandrin B shows a promising future, its poor solubility and low bioavailability may limit its clinical application. Schisandrin B delivery via MMP-sensitive peptide-modified PEGylated lipid nanoparticles may be an alternative option335.
It is noteworthy that S. chinensis polysaccharides are also effective at improving cardiac function. Thioredoxin-1 (TRX-1) is among the most important cellular antioxidative systems, reducing oxidized proteins through thiol-disulfide exchange reactions336. S. chinensis polysaccharides may bind to Arg207, Ser169, Lys166, Lys286, and Ser285 in TRX-interacting protein through hydrogen bonds, which may restrict or interfere with the binding between TXNIP and TRX-1 and its interacting protein, resulting in TRX-1 activation to inhibit oxidative stress to prevent cardiac hypertrophy337.
4.13. Aconiti Lateralis Radix Praeparata (Fuzi)
Fuzi is the processed root of Aconitum carmichael, a plant in the Ranunculaceae family, which is regarded as “the first choice for herbs of invigorating Yang and saving lives”. Alkaloids are the main chemical components, representing substantial cardioprotective effects. The raw Fuzi mainly contains diester alkaloids, which can be hydrolyzed into monoester alkaloids after decoction or concoction, and the latter can be further hydrolyzed into alcohol-amine alkaloids338. The toxicity is based on the number of ester bonds. In order of decreasing toxicity, diester type > monoester type > alcohol-amine type.
Aconitine is the main active ingredient of diester alkaloids as well as the main component causing cardiotoxicity339. Although aconitine has certain cardiotonic effects, it is prone to the occurrence of arrhythmias340. The effectiveness of aconitine in the treatment of CHF is debatable. On the one hand, aconitine attenuated Ang II-induced energy metabolism dysfunction in H9c2 cells by activating SIRT3, thus inhibiting mPTP opening, and improving ATP synthesis341. Aconitine may alleviate Ang II-induced cardiac hypertrophy by inhibiting hypertrophic factors including ANP, BNP, β-myosin heavy chain, α-SMA and F-actin342. Selective activation of β-adrenergic receptor agonist by aconitine inhibits cardiomyocytes apoptosis and prevents MI/R injury343. On the other hand, accumulating evidence has proved that aconitine can readily cause heart damage. Aconitine inhibited cardiomyocyte proliferation in a dose- and time-dependent manner and induced inflammation and mitochondria-mediated apoptosis344. Although some people believe that the lowest possible concentration of aconitine would not cause cardiotoxicity, a recent result indicated that the aconitine level in blood did not correlate with symptoms or electrocardiogram findings caused by aconitine345.
Compatibility with other herbs or therapies offers a breakthrough for the clinical application of aconitine. Mass spectrometry analysis revealed that the content of toxic components decreased while the content of protective components increased by compatibility of Glycyrrhiza Radix (Gancao), rapidly ameliorating the inflammation response in HF mice346. Paeoniflorin could also significantly reduce the cardiotoxicity of aconitine on H9c2 cells347. Combined with electroacupuncture, aconitine can not only improve the systolic function of CHF rats more obviously, but also reduce the toxic reaction of aconitine348,349. For long-term drug treatment of CHF, a combination of non-drug therapies, for instance, acupuncture and massage, can be considered as a measure to increase the effectiveness of drugs and reduce their toxicity.
Higenamine is another water-soluble alkaloid in Fuzi. Higenamine in combination with gingerol significantly increased the mitochondrial oxygen consumption rate and extracellular acidification rate and improved mitochondrial dysfunction to alleviate cardiac injury. In addition, the metabonomic analysis showed that the anti-CHF effect of their combination was mainly related to the regulation of the metabolism of FFAs and energy metabolites350,351. The aqueous extracts of Fuzi could attenuate the expression of SERCA2a to maintain intracellular calcium homeostasis and suppress the expression of α-SMA, collagen I, and III, thus inhibiting ventricular remodeling associated with CHF352.
4.14. Trichosanthis Fructus (Gualou)
Gualou is the dried ripe fruit of Trichosanthes kirilowii Maxim. or T. rosthornii Harms in the Cucurbitaceae family. Gualou is an important herb for the treatment of cardiothoracic obstruction, angina pectoris, MI, CHF, etc. Triterpenoids are the main active substances in Gualou. Cucurbitacin B and bryonolic acid are two characteristic triterpenoids found in Gualou that have a variety of activities, inducing angiogenesis, anti-tumor, triggering pyroptosis, and anti-inflammation353, 354, 355.
The aqueous extract of Gualou protects H9c2 cells from H/R injury by regulating PI3K/Akt/NO pathway to decrease apoptosis356. In mice with TAC-induced myocardial hypertrophy and phenylephrine-stimulated human AC16 cardiomyocytes, cucurbitacin B was depicted to promote autophagy, restrain heart weight and interstitial fibrosis, exhibiting a strong resistance to hypertrophy and fibrosis357. Bryonolic acid suppressed the inflammatory response by inhibiting the expression of inducible NO synthase in LPS-stimulated RAW 264.7 macrophages. In addition, bryonolic acid steadily induced the HO-1 protein, which relies on Nrf2 to fight against oxidative stress in vitro and in vivo358.
4.15. Moschus (Shexiang)
Natural Shexiang is the dried secretion of the scent sac of the mature male of Moschus berezovskli Flerov, M. sifanlcus Pmew alssi. and M. moschifems Linaeu. As natural Shexiang is infrequent, synthetic Shexiang is now widely used, for example, in SBPs. Synthetic Shexiang has the same odor and clinical efficacy as natural Shexiang and can be used as a substitute for musk. Muscone is the main active component of Shexiang, which could activate the mouse muscone receptor MOR215-1 and human musk receptors OR5AN1 and OR1A1 to conduct signal cascades via odorant molecules359,360.
Muscone pretreatment significantly reversed the decrease of MMP and BCL-2, and the increase of lactate dehydrogenase, MDA, creatine kinase, and caspase-3 caused by MI/R injury361. Overactive and persistent chronic inflammation leads to worsening ventricular remodeling and depresses cardiac function362. Muscone pretreatment could inhibit oxidative stress and inflammation, attenuate MI/R-induced cardiac dysfunction and LV remodeling, relying on the upregulation SIRT3363. Muscone also alleviated cardiac macrophage-mediated chronic inflammation by inhibiting NF-κB and NLRP3 inflammasome activation, thereby improving cardiac function in MI mice364. Besides, the scavenging of ROS may be its other mechanism364. Moreover, muscone could up-regulate the expression of VEGFA and HIF-1α, promoting myocardial angiogenesis to repair damaged hearts in CHF mice365. Muscone is a chiral compound that can be further divided into S-muscone and R-muscone, both of which are present in synthetic Shexiang. In a zebrafish model, S-muscone and R-muscone were found to cause cardiotoxicity in zebrafish embryos through myh6 and myh7 mRNA, Trh, thβ, and Dio3 genes, resulting in pericardial edema and slowing of heart rate366. Concerns about safety still need to be raised about the chiral isomer composition and clinical application of synthetic Shexiang.
5. The synergistic effects of constituent compatibility in the treatment of CHF
The purpose of compatibility in TCM formulas is to exert synergistic effects, including enhancing effects and reducing toxicity, through the integration of multiple targets and multiple pathways mediated by specific active substance groups. The compatibility of Chinese medicinal herbs was accurately determined through screening for the compatibility of their effective constituents in order to demonstrate the synergistic effects of TCM formulas.
The compatibility with other Chinese medicinal herbs to increase the efficiency and reduce the toxicity of Fuzi may open a window for its clinical application. By upregulating the expression of CYP3A, Glycyrrhizae Radix (Gancao) can promote the metabolism of toxic components of Fuzi including aconitine, mesaconitine, and hypaconitine; reduce the contents of BNP, Ang II and ALD; improve myocardial energy metabolism, and thus preventing the development of CHF367. Moverover, the compatibility could significantly increase the exposure of active components composed of hypaconitine, benzoylmesaconitine, and songorine in vivo368. Acetyl-11-keto-β-boswellic acid is a major component in Ruxiang. HSYA pretreatment with acetyl-11-keto-boswellic acid reduced the level of mitochondrial ROS while increasing superoxide dismutase activity, effectively attenuating ISO or oxygen-glucose deprivation-induced cardiomyocyte damage369. Active fractions were obtained by means of solvent extraction and chromatography from QLQX, and 11 compounds with their own targets were identified that produce cardiotonic, diuretic, and vasodilator effects through synergistic or complementary effects370. Ginsenosides Rb1, Rg1, Rf, Rh1, Rc, Rb2, Ro, and Rg3, were isolated and confirmed as the major anti-inflammatory constituents of YQFM371.
The 24 ingredients from QSYQ were predicted to be bioactive and named combinatorial bioactive ingredients (CBIs) via UPLC–Q-TOF/MS and the ChEMBL database. CBIs presented greater cardioprotective potency than the individual ingredients in QSYQ, exhibiting similar potency to QSYQ and synergistic effects372. QSYQ without Jiangxiang, on the other hand, significantly weakened myocardial protection. In another study, according to the composition of QSYQ and Jun-Chen-Zuo-Shi theory, supplementing Qi constituents were prepared in the ratio of HQ: Jiangxiang = 148.01:11.97 and activating blood constituents were prepared in the ratio of Danshen:Sanqi:Jiangxiang = 70.35:70.35:11.97. The simultaneous supplementing Qi and activating blood achieved the best therapeutic effect373. DLA and NGR1 are major ingredients in QSYQ. DLA inhibited SIRT1 expression, resulting in a decrease in complex I activity. NGR1 reversed the upregulation of RhoA/ROCK-1 expression and myosin light chain phosphorylation, restored mitochondrial complex V activity and F-actin arrangement, and alleviated myocardial fiber rupture. The additive effect of DLA and NGR1 could exert a stronger protective effect on MI/R injury via inhibiting oxidative stress and recovering energy metabolism374. AS-IV inhibited the abnormal energy metabolic pathway; Danshensu inhibited oxidative stress; NGR1 partially improved myocardial energy metabolism, inhibited oxidative stress injury, and descending aromatic oil up-regulated carnitine palmitoyl transferase lA. Their compatibility supplements Qi and activates blood to reverse myocardial hypertrophy and fibrosis with the best effect375. GRb1 (G), ruscogenin (R), and schisandrin (S) are selected separately from the composition of “Shengmai Yin”, and the three components synergistically exert superimposed therapeutic effects on MI/R injury with best efficacy at the dose of 18.15 mg/kg (G:R:S = 12:0.15:6) to improve myocardial injury376,377. Demethylcoclaurine, benzoylaconine, ATL III, paeoniflorin, 6-gingerol, 8-gingerol, pachymic acid, and dehydrotumulosic acid can be defined as quality markers of ZWD for treating CHF378.
6. Discussions and perspectives
Multiple etiologies and pathophysiological mechanisms that interact with each other make up the complex pathophysiology of CHF. Epidemiological data show a gradual increase in the incidence and prevalence of CHF with aging. According to the majority of earlier research, CHF was driven on by traditional risk factors for cardiovascular disease, including CHD, hypertension, diabetes, and atrial fibrillation, under aging conditions379. However, aging is increasingly becoming recognized as a separate factor that damages myocardial, causing progressive decline of cardiac structure and function that eventually manifests as end-stage heart diseases380. Finding strategies to prevent the early onset of CHF is therefore valuable, and the fact that so many herbal ingredients have exhibited cardioprotective effects is encouraging. With thousands of years of history and a wealth of theories and knowledge, TCM has been used to prevent and treat heart diseases, including CHF.
TCM significantly affects the symptoms and quality of life of CHF patients, according to a growing body of contemporary, evidence-based medical studies381, 382, 383. The cornerstone of TCM theory is treatment based on syndrome differentiation, which necessitates different prescriptions according to individual and syndrome differences. For CHF with deficiency of Qi and Yin, SSYX is superior to nourishing Qi and Yin to improve the syndrome, while CHF with blood stasis and phlegm due to Qi deficiency prefers QSYQ and TXL treatment to supplement Qi and promote blood circulation; QLQX is more suitable for CHF with deficiency of heart and kidney Yang by warming Yang and inducing diuresis; and SBPs are more effective for CHF with Qi stagnation and blood stasis syndrome. Exhilaratingly, the gap between TCM theory and modern medicine theory is narrowing. The Qi deficiency is closely relevant to mitochondrial function and energy disorders. Coincidentally, it has been demonstrated that the impact of Qi-supplementing herbs is concentrated on the regulation of energy metabolism384. The physiology and pathology of phlegm in TCM are related to the imbalance of autophagy385. All of these findings would shed new light on the elaboration of obscure TCM theories and herbal functions.
Even though TCM therapy for CHF worked well, the fact that some key issues remain unresolved has hampered the clinical application of TCM formulas and Chinese herbs in CHF patients. Firstly, a precise connection with objective biological indicators in the diagnosis and treatment system of TCM-based syndrome differentiation has not yet established, which limits the application of TCM in the modern medical system. Hence, it is imperative to incorporate objective indications into TCM theory and establish a unified standard for TCM diagnosis and treatment in order to standardize clinical treatment. The types, quantities, and proportions of the ingredients in Chinese medicinal herbs and TCM formulas are complex, which makes it challenging to grasp their exact mechanisms. It is urgent to put more effort into finding out active constituents and probable action mechanisms of Chinese herbal medications. Besides, the immediate effects of TCM tend to be overlooked, which may squander the finest chance and effectiveness for treating CHF. TCM is worthy of clinic application as early as possible for the sake of the best effect.
As research into synthetic chemical drugs has reached a bottleneck, the development of pharmaceuticals based on Chinese herbal medicines has become a current direction. Numerous natural components control several pathogenic variables by acting on numerous routes and systems. For example, AS-IV could inhibit oxidative stress, reduce inflammation, and regulate energy metabolism to restore cardiac homeostasis187,189, 190, 191 (Fig. 4); liguzinediol may inhibit inflammation and excessive apoptosis, reduce oxidative stress, and alleviate myocardial fibrosis255,386; OPD could maintain mitochondrial function, suppress ERS, and exert anti-apoptotic, antioxidant, and anti-inflammatory effects to treat CHF307, 308, 309,313. These imply the feasibility and advantage of using natural products to improve complications and comorbidities.
Figure 4.
The example showing the superiority of natural ingredients in the treatment of chronic heart failure, attributed to their multi-target potential.
The compatibility of different various components is essential, particularly for medications with large doses and a narrow safety window. Properly formulated drugs can reduce doses, combat drug resistance and mitigate side effects. For example, aconitine combined with liquiritin and glycyrrhetinic acid could regulate the calcium regulation proteins to protect cardiomyocytes from damage, and liquiritin and glycyrrhetinic acid may lessen toxicity and boost the effectiveness of aconitine387. With reference to the Jun-Chen-Zuo-Shi principle, the key components that have isolated and identified from Chinese herbs are formulated to work synergistically to produce therapeutic effects. This preserves the benefits and features of TCM formulas while also shedding light on the standardization, modernization, scientification, internationalization, and marketization of TCM. Some Chinese herbs or components, like Wuweizi, Ginkgo biloba (Yinxing), and curcumin, may have the potential to improve the efficacy and lessen the toxicity of chemical pharmaceuticals, enlightening traditional medicines302.
Although Chinese herbal constituents have shown promise in the treatment of CHF, their actual application in clinical settings is still a long way off. In summary, finding bioactive constituents from TCM and exploring their effects and mechanisms are challenging but intriguing tasks.
Acknowledgments
This work was financially supported by the National Key Research & Development Program of China (Grant Nos. 2017YFC1700400, 2017YFC1700403), National Natural Science Foundation of China (NNSFC; Grant Nos. 82073978, 81725024, and 82104441), Beijing Natural Science Foundation (Grant No. JQ18026, China).
Author contributions
Sheng Lin and Hongcai Shang designed the manuscript. Jie Chen and Xiaohong Wei wrote the manuscript. Guiyang Xia and Huan Xia revised the manuscript. Qian Zhang and Yuzhuo Wu searched the literature. Lingyan Wang aided in the design of the illustrations. All authors approved the manuscript for publication.
Conflicts of interest
The authors have no competing interests to declare.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Hongcai Shang, Email: shanghongcai@126.com.
Sheng Lin, Email: lsznn@bucm.edu.cn.
References
- 1.Virani S., Alonso A., Aparicio H., Benjamin E., Bittencourt M., Callaway C., et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Bertero E., Maack C. Metabolic remodeling in heart failure. Nat Rev Cardiol. 2018;15:457–470. doi: 10.1038/s41569-018-0044-6. [DOI] [PubMed] [Google Scholar]
- 3.Hu S.S., Gao R.L., Liu L.S., Zhu M.L., Wang W., Wang Y.J., et al. Summary of the 2018 report on cardiovascular diseases in China. Chin Circ J. 2019;34:209–220. [Google Scholar]
- 4.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131. doi: 10.1002/ejhf.2333. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich P.A., Albert N.M., Allen L.A., Bluemke D.A., Butler J., Fonarow G.C., et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gudiño-Gomezjurado A., Buitrón-Andrade R. Survival analysis of patients with heart failure in the Ecuadorian Andean population. Medwave. 2021;21 doi: 10.5867/medwave.2021.07.8440. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox C.S., Testani J.M., Pitt B. Pathophysiology of diuretic resistance and its implications for the management of chronic heart failure. Hypertension. 2020;76:1045–1054. doi: 10.1161/HYPERTENSIONAHA.120.15205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin A.Y., Dinatolo E., Metra M., Sbolli M., Dasseni N., Butler J., et al. Thromboembolism in heart failure patients in sinus rhythm: epidemiology, pathophysiology, clinical trials, and future direction. JACC Heart Fail. 2021;9:243–253. doi: 10.1016/j.jchf.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Lee J.R., Park B.W., Park J.H., Lim S., Kwon S.P., Hwang J.W., et al. Local delivery of a senolytic drug in ischemia and reperfusion-injured heart attenuates cardiac remodeling and restores impaired cardiac function. Acta Biomater. 2021;135:520–533. doi: 10.1016/j.actbio.2021.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Miller W.L. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002922. [DOI] [PubMed] [Google Scholar]
- 11.Aimo A., Castiglione V., Borrelli C., Saccaro L.F., Franzini M., Masi S., et al. Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. Eur J Prev Cardiol. 2020;27:494–510. doi: 10.1177/2047487319870344. [DOI] [PubMed] [Google Scholar]
- 12.Bellumkonda L., Tyrrell D., Hummel S.L., Goldstein D.R. Pathophysiology of heart failure and frailty: a common inflammatory origin?. Aging Cell. 2017;16:444–450. doi: 10.1111/acel.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontes M.T., Paula S.M., Lino C.A., Senger N., Couto G.K., Barreto-Chaves M.L.M., et al. Renin–angiotensin system overactivation in perivascular adipose tissue contributes to vascular dysfunction in heart failure. Clin Sci. 2020;134:3195–3211. doi: 10.1042/CS20201099. [DOI] [PubMed] [Google Scholar]
- 14.Oikonomou E., Tsioufis C., Tousoulis D. Angiotensin receptor-neprilisyn inhibitors: are their beneficial effects mediated through diastolic or systolic function improvement?. Hellenic J Cardiol. 2020;61:419–420. doi: 10.1016/j.hjc.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Li H., Xing W., Li J., Du R., Cao D., et al. Vascular smooth muscle cell-specific miRNA-214 knockout inhibits angiotensin II-induced hypertension through upregulation of Smad7. FASEB J. 2021;35 doi: 10.1096/fj.202100766RR. [DOI] [PubMed] [Google Scholar]
- 16.Kong P., Christia P., Frangogiannis N.G. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omar M., Jensen J., Frederiksen P.H., Videbæk L., Poulsen M.K., Brønd J.C., et al. Hemodynamic determinants of activity measured by accelerometer in patients with stable heart failure. JACC Heart Fail. 2021;9:824–835. doi: 10.1016/j.jchf.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Ji S., Guo Y., Li G., Sang N. NO2 exposure contributes to cardiac hypertrophy in male mice through apoptosis signaling pathways. Chemosphere. 2022;309 doi: 10.1016/j.chemosphere.2022.136576. [DOI] [PubMed] [Google Scholar]
- 19.Sato S., Yoshida R., Murakoshi F., Sasaki Y., Yahata K., Kasahara K., et al. Comparison between concentric-only, eccentric-only, and concentric-eccentric resistance training of the elbow flexors for their effects on muscle strength and hypertrophy. Eur J Appl Physiol. 2022;122:2607–2614. doi: 10.1007/s00421-022-05035-w. [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Martínez S., Lozano-Vidal N., López-Maderuelo M.D., Jiménez-Borreguero L.J., Armesilla Á.L., Redondo J.M. Cardiomyocyte calcineurin is required for the onset and progression of cardiac hypertrophy and fibrosis in adult mice. FEBS J. 2019;286:46–65. doi: 10.1111/febs.14718. [DOI] [PubMed] [Google Scholar]
- 21.Ba L., Gao J., Chen Y., Qi H., Dong C., Pan H., et al. Allicin attenuates pathological cardiac hypertrophy by inhibiting autophagy via activation of PI3K/Akt/mTOR and MAPK/ERK/mTOR signaling pathways. Phytomedicine. 2019;58 doi: 10.1016/j.phymed.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Hunkler H.J., Groß S., Thum T., Bär C. Non-coding RNAs: key regulators of reprogramming, pluripotency, and cardiac cell specification with therapeutic perspective for heart regeneration. Cardiovasc Res. 2022;118:3071–3084. doi: 10.1093/cvr/cvab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Q., Liu M., Zhang F., Liu X., Ling S., Chen X., et al. Ubiquitin-specific protease 2 regulates Ang II-induced cardiac fibroblasts activation by up-regulating cyclin D1 and stabilizing β-catenin in vitro. J Cell Mol Med. 2021;25:1001–1011. doi: 10.1111/jcmm.16162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Travers J.G., Kamal F.A., Robbins J., Yutzey K.E., Blaxall B.C. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J., Subbaiah K.C.V., Xie L.H., Jiang F., Khor E.S., Mickelsen D., et al. Glutamyl-prolyl-tRNA synthetase regulates proline-rich pro-fibrotic protein synthesis during cardiac fibrosis. Circ Res. 2020;127:827–846. doi: 10.1161/CIRCRESAHA.119.315999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Pol A., van Gilst W.H., Voors A.A., van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019;21:425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha N., Dabla P.K. Oxidative stress and antioxidants in hypertension—a current review. Curr Hypertens Rev. 2015;11:132–142. doi: 10.2174/1573402111666150529130922. [DOI] [PubMed] [Google Scholar]
- 28.Münzel T., Gori T., Keaney J.F., Jr., Maack C., Daiber A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J. 2015;36:2555–2564. doi: 10.1093/eurheartj/ehv305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teuber J.P., Essandoh K., Hummel S.L., Madamanchi N.R., Brody M.J. NADPH oxidases in diastolic dysfunction and heart failure with preserved ejection fraction. Antioxidants. 2022;11:1822. doi: 10.3390/antiox11091822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundd P., Gladwin M.T., Novelli E.M. Pathophysiology of sickle cell disease. Annu Rev Pathol. 2019;14:263–292. doi: 10.1146/annurev-pathmechdis-012418-012838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao S.G., Sun Y., Sun B., Wang A., Qiu J., Hong L., et al. Sevoflurane postconditioning protects against myocardial ischemia/reperfusion injury by restoring autophagic flux via an NO-dependent mechanism. Acta Pharmacol Sin. 2019;40:35–45. doi: 10.1038/s41401-018-0066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abernethy A., Raza S., Sun J.L., Anstrom K.J., Tracy R., Steiner J., et al. Pro-inflammatory biomarkers in stable versus acutely decompensated heart failure with preserved ejection fraction. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mann D.L. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–1268. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Y., Zeng X., Feng Y., Chen Q., Liu Z., Luo H., et al. Association of systemic immune-inflammation index with short-term mortality of congestive heart failure: a retrospective cohort study. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.753133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y., Liu J., Kong Q., Cheng H., Tu F., Yu P., et al. Cardiomyocyte-specific deficiency of HSPB1 worsens cardiac dysfunction by activating NFκB-mediated leucocyte recruitment after myocardial infarction. Cardiovasc Res. 2019;115:154–167. doi: 10.1093/cvr/cvy163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adamyan S.H., Harutyunyan K.R., Abrahamyan H.T., Khudaverdyan D.N., Mkrtchian S., Ter-Markosyan A.S. Can the calcium-regulating hormones counteract the detrimental impact of pro-inflammatory damage-associated molecular patterns in the development of heart failure?. J Investig Med. 2021;69:1148–1152. doi: 10.1136/jim-2020-001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegel P.M., Schmich J., Barinov G., Bojti I., Vedecnik C., Simanjuntak N.R., et al. Cardiomyocyte microvesicles: proinflammatory mediators after myocardial ischemia?. J Thromb Thrombolysis. 2020;50:533–542. doi: 10.1007/s11239-020-02156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawaguchi M., Takahashi M., Hata T., Kashima Y., Usui F., Morimoto H., et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 39.Bujak M., Dobaczewski M., Gonzalez-Quesada C., Xia Y., Leucker T., Zymek P., et al. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105:973–983. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litviňuková M., Talavera-López C., Maatz H., Reichart D., Worth C., Lindberg E., et al. Cells of the adult human heart. Nature. 2020;588:466–472. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apostolakis S., Lip G.Y., Shantsila E. Monocytes in heart failure: relationship to a deteriorating immune overreaction or a desperate attempt for tissue repair?. Cardiovasc Res. 2010;85:649–660. doi: 10.1093/cvr/cvp327. [DOI] [PubMed] [Google Scholar]
- 42.Lv J., Chen J., Wang M., Yan F. Klotho alleviates indoxyl sulfate-induced heart failure and kidney damage by promoting M2 macrophage polarization. Aging. 2020;12:9139–9150. doi: 10.18632/aging.103183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halade G.V., Norris P.C., Kain V., Serhan C.N., Ingle K.A. Splenic leukocytes define the resolution of inflammation in heart failure. Sci Signal. 2018;11 doi: 10.1126/scisignal.aao1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J., Zhang J., Yu P., Chen M., Peng Q., Wang Z., et al. Remote ischaemic preconditioning and sevoflurane postconditioning synergistically protect rats from myocardial injury induced by ischemia and reperfusion partly via inhibition TLR4/MyD88/NF-κB signaling pathway. Cell Physiol Biochem. 2017;41:22–32. doi: 10.1159/000455815. [DOI] [PubMed] [Google Scholar]
- 45.Sharma S., Garg I., Ashraf M.Z. TLR signalling and association of TLR polymorphism with cardiovascular diseases. Vascul Pharmacol. 2016;87:30–37. doi: 10.1016/j.vph.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Lei R., Li J., Liu F., Li W., Zhang S., Wang Y., et al. HIF-1α promotes the keloid development through the activation of TGF-β/Smad and TLR4/MyD88/NF-κB pathways. Cell Cycle. 2019;18:3239–3250. doi: 10.1080/15384101.2019.1670508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng C., Duan F., Hu J., Luo B., Huang B., Lou X., et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adamo L., Rocha-Resende C., Prabhu S.D., Mann D.L. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17:269–285. doi: 10.1038/s41569-019-0315-x. [DOI] [PubMed] [Google Scholar]
- 49.Xu L., Liu X., Peng F., Zhang W., Zheng L., Ding Y., et al. Protein quality control through endoplasmic reticulum-associated degradation maintains haematopoietic stem cell identity and niche interactions. Nat Cell Biol. 2020;22:1162–1169. doi: 10.1038/s41556-020-00581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Narezkina A., Narayan H.K., Zemljic-Harpf A.E. Molecular mechanisms of anthracycline cardiovascular toxicity. Clin Sci. 2021;135:1311–1332. doi: 10.1042/CS20200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren F.F., Xie Z.Y., Jiang Y.N., Guan X., Chen Q.Y., Lai T.F., et al. Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress. Acta Pharmacol Sin. 2022;43:1721–1732. doi: 10.1038/s41401-021-00805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mao H., Chen W., Chen L., Li L. Potential role of mitochondria-associated endoplasmic reticulum membrane proteins in diseases. Biochem Pharmacol. 2022;199 doi: 10.1016/j.bcp.2022.115011. [DOI] [PubMed] [Google Scholar]
- 53.Pandey S., Kuo C.H., Chen W.S., Yeh Y.L., Kuo W.W., Chen R.J., et al. Perturbed ER homeostasis by IGF–IIRα promotes cardiac damage under stresses. Mol Cell Biochem. 2022;477:143–152. doi: 10.1007/s11010-021-04261-8. [DOI] [PubMed] [Google Scholar]
- 54.Sitsel A., De Raeymaecker J., Drachmann N.D., Derua R., Smaardijk S., Andersen J.L., et al. Structures of the heart specific SERCA2a Ca2+-ATPase. EMBO J. 2019;38 doi: 10.15252/embj.2018100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michalson K.T., Groban L., Howard T.D., Shively C.A., Sophonsritsuk A., Appt S.E., et al. Estradiol treatment initiated early after ovariectomy regulates myocardial gene expression and inhibits diastolic dysfunction in female cynomolgus monkeys: potential roles for calcium homeostasis and extracellular matrix remodeling. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green D.R. The coming decade of cell death research: five riddles. Cell. 2019;177:1094–1107. doi: 10.1016/j.cell.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 58.Moujalled D., Strasser A., Liddell J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021;28:2029–2044. doi: 10.1038/s41418-021-00814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strasser A., Jost P.J., Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu C., Zhou X., Lu J., Zhu L., Li M. Autophagy mediates 2-methoxyestradiol-inhibited scleroderma collagen synthesis and endothelial-to-mesenchymal transition induced by hypoxia. Rheumatology. 2019;58:1966–1975. doi: 10.1093/rheumatology/kez159. [DOI] [PubMed] [Google Scholar]
- 61.Cuervo A.M., Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;24:92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sciarretta S., Forte M., Frati G., Sadoshima J. The complex network of mTOR signalling in the heart. Cardiovasc Res. 2022;118:424–439. doi: 10.1093/cvr/cvab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaaf M.B., Houbaert D., Meçe O., Agostinis P. Autophagy in endothelial cells and tumor angiogenesis. Cell Death Differ. 2019;26:665–679. doi: 10.1038/s41418-019-0287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Z., Klionsky D.J. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kassiotis C., Ballal K., Wellnitz K., Vela D., Gong M., Salazar R., et al. Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation. 2009;120:S191–S197. doi: 10.1161/CIRCULATIONAHA.108.842252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ott C., Jung T., Brix S., John C., Betz I.R., Foryst-Ludwig A., et al. Hypertrophy-reduced autophagy causes cardiac dysfunction by directly impacting cardiomyocyte contractility. Cells. 2021;10:805. doi: 10.3390/cells10040805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oyabu J., Yamaguchi O., Hikoso S., Takeda T., Oka T., Murakawa T., et al. Autophagy-mediated degradation is necessary for regression of cardiac hypertrophy during ventricular unloading. Biochem Biophys Res Commun. 2013;441:787–792. doi: 10.1016/j.bbrc.2013.10.135. [DOI] [PubMed] [Google Scholar]
- 68.Thapalia B.A., Zhou Z., Lin X. Sauchinone augments cardiomyocyte viability by enhancing autophagy proteins—PI3K, ERK(1/2), AMPK and Beclin-1 during early ischemia–reperfusion injury in vitro. Am J Transl Res. 2016;8:3251–3265. [PMC free article] [PubMed] [Google Scholar]
- 69.Shirakabe A., Ikeda Y., Sciarretta S., Zablocki D.K., Sadoshima J. Aging and autophagy in the heart. Circ Res. 2016;118:1563–1576. doi: 10.1161/CIRCRESAHA.116.307474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corsetti G., Chen-Scarabelli C., Romano C., Pasini E., Dioguardi F.S., Onorati F., et al. Autophagy and oncosis/necroptosis are enhanced in cardiomyocytes from heart failure patients. Med Sci Monit Basic Res. 2019;25:33–44. doi: 10.12659/MSMBR.913436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L., He J., Wang J., Liu J., Chen Z., Deng B., et al. Knockout RAGE alleviates cardiac fibrosis through repressing endothelial-to-mesenchymal transition (EndMT) mediated by autophagy. Cell Death Dis. 2021;12:470. doi: 10.1038/s41419-021-03750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takagaki Y., Lee S.M., Dongqing Z., Kitada M., Kanasaki K., Koya D. Endothelial autophagy deficiency induces IL6-dependent endothelial mesenchymal transition and organ fibrosis. Autophagy. 2020;16:1905–1914. doi: 10.1080/15548627.2020.1713641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ke S., Lai Y., Li L., Tu L., Wang Y., Ren L., et al. Molybdenum disulfide quantum dots attenuates endothelial-to-mesenchymal transition by activating TFEB-mediated lysosomal biogenesis. ACS Biomater Sci Eng. 2019;5:1057–1070. doi: 10.1021/acsbiomaterials.8b01253. [DOI] [PubMed] [Google Scholar]
- 74.He Y., Huang W., Zhang C., Chen L., Xu R., Li N., et al. Energy metabolism disorders and potential therapeutic drugs in heart failure. Acta Pharm Sin B. 2021;11:1098–1116. doi: 10.1016/j.apsb.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J., Zhou H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia–reperfusion injury. Acta Pharm Sin B. 2020;10:1866–1879. doi: 10.1016/j.apsb.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abozguia K., Phan T.T., Shivu G.N., Maher A.R., Ahmed I., Wagenmakers A., et al. Reduced in vivo skeletal muscle oxygen consumption in patients with chronic heart failure—a study using near infrared spectrophotometry (NIRS) Eur J Heart Fail. 2008;10:652–657. doi: 10.1016/j.ejheart.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 77.Gibb A.A., Hill B.G. Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res. 2018;123:107–128. doi: 10.1161/CIRCRESAHA.118.312017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cuijpers I., Simmonds S.J., van Bilsen M., Czarnowska E., González Miqueo A., Heymans S., et al. Microvascular and lymphatic dysfunction in HFpEF and its associated comorbidities. Basic Res Cardiol. 2020;115:39. doi: 10.1007/s00395-020-0798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J., Wei X., Zhang Q., Xia H., Xia G., Wu Y., et al. Progress of ubiquitin–proteasome system in the pathophysiology of heart failure and the intervention of traditional Chinese medicine. TMR Mod Herb Med. 2021;4:16. [Google Scholar]
- 80.Oka T., Akazawa H., Naito A.T., Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114:565–571. doi: 10.1161/CIRCRESAHA.114.300507. [DOI] [PubMed] [Google Scholar]
- 81.Erturk I., Saglam K., Elasan S., Aykan M.B., Acar R., Yesildal F., et al. Evaluation of the effects of different treatment modalities on angiogenesis in heart failure patients with reduced/mid-range ejection fraction via VEGF and sVEGFR-1. Saudi Med J. 2018;39:1028–1034. doi: 10.15537/smj.2018.10.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen J., Gu S., Song Y., Ji X., Zeng W., Wang X., et al. The impact of cardiomotor rehabilitation on endothelial function in elderly patients with chronic heart failure. BMC Cardiovasc Disord. 2021;21:524. doi: 10.1186/s12872-021-02327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sugiyama A., Hirano Y., Okada M., Yamawaki H. Endostatin stimulates proliferation and migration of myofibroblasts isolated from myocardial infarction model rats. Int J Mol Sci. 2018;19:741. doi: 10.3390/ijms19030741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruge T., Carlsson A.C., Ingelsson E., Risérus U., Sundström J., Larsson A., et al. Circulating endostatin and the incidence of heart failure. Scand Cardiovasc J. 2018;52:244–249. doi: 10.1080/14017431.2018.1483080. [DOI] [PubMed] [Google Scholar]
- 85.Fan J., Li H., Nie X., Yin Z., Zhao Y., Zhang X., et al. MiR-665 aggravates heart failure via suppressing CD34-mediated coronary microvessel angiogenesis. Aging. 2018;10:2459–2479. doi: 10.18632/aging.101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J., Ming C. Clinical application guide of Chinese patent medicine in the treatment of ventricular premature beats (2020) Chin J Integr Med. 2021;44:1–6. [Google Scholar]
- 87.Zandstra T.E., Notenboom R.G.E., Wink J., Kiès P., Vliegen H.W., Egorova A.D., et al. Asymmetry and heterogeneity: part and parcel in cardiac autonomic innervation and function. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.665298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu Q. Effects of Shensongyangxin capsule on left ventricular function and heart rate variability in treating heart failure. J Chin Pres Drug. 2021;19:131–132. [Google Scholar]
- 89.Wang X., Hu D., Dang S., Huang H., Huang C.X., Yuan M.J., et al. Effects of traditional Chinese medicine Shensong Yangxin Capsules on heart rhythm and function in congestive heart failure patients with frequent ventricular premature complexes: a randomized, double-blind, multicenter clinical trial. Chin Med J. 2017;130:1639–1647. doi: 10.4103/0366-6999.209906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang W., Xu L.M., He H., Wang W., Lin Y.X., He H., et al. Effect of Shensong Yangxin Capsule combined with amiodarone on quality of life in patients with chronic heart failure complicated with ventricular extrasystole. J New Chin Med. 2012;44:10–12. [Google Scholar]
- 91.Society of Cardiovascular Diseases CAoCM Traditional Chinese medicine treatment guidelines on coronary heart disease before and after percutaneous coronary intervention. Chin J Exp Tradit Med Form. 2018;24:4–6. [Google Scholar]
- 92.Wang M., Shan Y., Wu C., Cao P., Sun W., Han J., et al. Efficacy and safety of Qishen Yiqi Dripping Pill for heart failure with preserved ejection fraction: a systematic review and meta-analysis. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.626375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dai Q.Q., Shi Z.F., Hu J.Y., Han S.J., Zhong C.M., Guan M.K., et al. Meta-analysis of effect of Qishen Yiqi Dripping Pills combined with Western medicine on adverse cardiovascular events and quality of life after percutaneous coronary intervention. Chin J Chin Mater Med. 2021;46:1498–1510. doi: 10.19540/j.cnki.cjcmm.20200618.501. [DOI] [PubMed] [Google Scholar]
- 94.Li H., Pei Q.F. The clinical elderly of Compound Danshen Dripping Pills and milrinone in the treatment of elderly heart failure and its Influence on serum hs-CRP, IL-8 and plasma NT-proBNP levels. Chin J of Integr Med Cardio/Cerebro Dis. 2018;16:1684–1687. [Google Scholar]
- 95.Park S.Y., Lee C.Y., Kim C., Jang S.H., Park Y.B., Park S., et al. One-year prognosis and the role of brain natriuretic peptide levels in patients with chronic cor pulmonale. J Korean Med Sci. 2015;30:442–449. doi: 10.3346/jkms.2015.30.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang N., Tian G., Lei H., Wei T. Clinical effect of compound Danshen dropping pills for the elderly patients with heart failure and its influence on the plasma pro-BNP. J Hunan Norm Univ. 2017;14:42–45. YG. [Google Scholar]
- 97.Chen Z., Peng Y.Y., Yang F.W., Hu H.Y., Liu C.X., Zhang J.H. Meta-analysis of clinical efficacy and safety of Compound Danshen Dripping Pills combined with conventional antihypertensive drugs in treatment of hypertensive left ventricular hypertrophy. Chin J Chin Mater Med. 2021;46:2578–2587. doi: 10.19540/j.cnki.cjcmm.20210222.502. [DOI] [PubMed] [Google Scholar]
- 98.Li C., Li Q., Xu J., Wu W., Wu Y., Xie J., et al. The efficacy and safety of compound Danshen Dripping Pill combined with percutaneous coronary intervention for coronary heart disease. Evid Based Complement Altern Med. 2020;2020 doi: 10.1155/2020/5067137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mi H.Y., Song H.X., Li Y.W., Zhang W., Sun Y.H., Gu H.H., et al. Research progress of Tongxinluo capsule in the treatment of heart failure after myocardial infarction. Tianjin J of Tradit Chin Med. 2020;37:1076–1080. [Google Scholar]
- 100.Ji Y.N., Chen Y.C., Zhao X.H., Xu L., Yang S. Effect of Tongxinluo on heart failure in patients with acute myocardial infarction after PCI. Cardio Dis J Inte Tradit Chin West Med. 2018;6:4–6. [Google Scholar]
- 101.Mao C., Fu X.H., Yuan J.Q., Yang Z.Y., Chung V.C., Qin Y., et al. Tong-xin-luo capsule for patients with coronary heart disease after percutaneous coronary intervention. Cochrane Database Syst Rev. 2015:Cd010237. doi: 10.1002/14651858.CD010237.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Z.Q., Hong L., Wang H., Yin Q.L. Effects of Tongxinluo Capsule on platelet activating factor, vascular endothelial function, blood flow of thrombolysis in myocardial infarction patients after delayed percutaneous coronary intervention. Chin J Integr Tradit West Med. 2016;36:415–420. [PubMed] [Google Scholar]
- 103.Li M., Li C., Chen S., Sun Y., Hu J., Zhao C., et al. Potential effectiveness of Chinese patent medicine Tongxinluo Capsule for secondary prevention after acute myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2018;9:830. doi: 10.3389/fphar.2018.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.He L., Liu Y., Yang K., Zou Z., Fan C., Yao Z., et al. The discovery of Q-markers of Qiliqiangxin Capsule, a traditional Chinese medicine prescription in the treatment of chronic heart failure, based on a novel strategy of multi-dimensional “radar chart” mode evaluation. Phytomedicine. 2021;82 doi: 10.1016/j.phymed.2020.153443. [DOI] [PubMed] [Google Scholar]
- 105.Sun J., Zhang K., Xiong W.J., Yang G.Y., Zhang Y.J., Wang C.C., et al. Clinical effects of a standardized Chinese herbal remedy, Qili Qiangxin, as an adjuvant treatment in heart failure: systematic review and meta-analysis. BMC Complement Altern Med. 2016;16:201. doi: 10.1186/s12906-016-1174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sweeney C., Pharithi R.B., Kerr B., Ryan C., Ryan F., Collins L., et al. NT-proBNP/BNP ratio for prognostication in European Caucasian patients enrolled in a heart failure prevention programme. ESC Heart Fail. 2021;8:5081–5091. doi: 10.1002/ehf2.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li X., Zhang J., Huang J., Ma A., Yang J., Li W., et al. A multicenter, randomized, double-blind, parallel-group, placebo-controlled study of the effects of qili qiangxin capsules in patients with chronic heart failure. J Am Coll Cardiol. 2013;62:1065–1072. doi: 10.1016/j.jacc.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 108.Wen J., Ma X., Zhang L., Lu X., Yang Y., Wang J., et al. Therapeutic efficacy and safety of Shexiang Baoxin Pill combined with trimetazidine in elderly patients with heart failure secondary to ischaemic cardiomyopathy: a systematic review and meta-analysis. Medicine. 2018;97 doi: 10.1097/MD.0000000000013580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li R., Wu T., Zhu C., Huang G., Peng L. Clinical observation on Shexiang Baoxin pill in the treatment of chronic heart failure induced by coronary heart disease. Chin J Integr Med Cardio. 2015;13:1704–1706. [Google Scholar]
- 110.Effects of Shexiang Baoxin Pills combined with trimetazidine on vascular endothelial function and plasma BNP in elderly patients with coronary heart disease and heart failure. J Hubei Univ Chin Med. 2017;19:22–25. Y H. [Google Scholar]
- 111.Xue J., Xu Y., Deng Y., Li F., Liu F., Liu L., et al. The efficacy and safety of Xinmailong Injection in patients with chronic heart failure: a multicenter randomized double-blind placebo-controlled trial. J Altern Complement Med. 2019;25:856–860. doi: 10.1089/acm.2019.0030. [DOI] [PubMed] [Google Scholar]
- 112.Chang X.D., Wei J.J., Hao X.X., Li B., Yu R., Wang Y.X., et al. Meta-analysis of efficacy and safety of Xinmailong Injection in treatment of heart failure after acute myocardial infarction. Chin J Chin Mater Med. 2021;46:1250–1259. doi: 10.19540/j.cnki.cjcmm.20200602.501. [DOI] [PubMed] [Google Scholar]
- 113.Wei J.J., Zhu M.J., Wang Y.X., Li B., Peng G.C., Wang X.L., et al. Systematic review of efficacy and safety of Xinmailong Injection in treatment of coronary heart disease complicated with heart failure. Chin J Chin Mater Med. 2020;45:4756–4765. doi: 10.19540/j.cnki.cjcmm.20200302.505. [DOI] [PubMed] [Google Scholar]
- 114.Zhang K., Zhang J., Wang X., Wang L., Pugliese M., Passantino A., et al. Cardioprotection of Sheng Mai Yin a classic formula on adriamycin induced myocardial injury in Wistar rats. Phytomedicine. 2018;38:1–11. doi: 10.1016/j.phymed.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 115.Wu J.R., Yang S.Y., Zhang X.M., Zhang D., Liu B., Zhang B. Systematic evaluation on Shengmai Injection in treatment of angina pectoris in coronary heart disease. Chin J Exp Tradit Med Form. 2015;21:222–225. [Google Scholar]
- 116.Hurwitz J.T., Brown M., Graff J.S., Peters L., Malone D.C. Is real-world evidence used in P&T monographs and therapeutic class reviews?. J Manag Care Spec Pharm. 2020;26:1604–1611. doi: 10.18553/jmcp.2020.26.12.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang X.Z., Sun C.Q., Liu G.Y., Xie Y.M., Liu F.M., Wei R.L., et al. Discussion on clinical characteristics and rational drug use of Shengmai Injection based on real world. Chin Tradit Herb Drugs. 2021;52:6005–6012. [Google Scholar]
- 118.Wang Y., Zhou X., Chen X., Wang F., Zhu W., Yan D., et al. Efficacy and safety of Shengmai Injection for chronic heart failure: a systematic review of randomized controlled trials. Evid Based Complement Altern Med. 2020;2020 doi: 10.1155/2020/9571627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao P., Wu X., Zhang F.H., Qiao Z.L., Yang L.J. Efficacy of Shenmai Injection for the treatment of chronic heart failure: a protocol of systematic review. Medicine. 2020;99 doi: 10.1097/MD.0000000000020663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu R., Wang J.R.P.G., Wang X.L., Zhao Q.F., Li B., Li X.Y., et al. Systematic evaluation of anthracycline-induced cardiotoxicity in traditional Chinese medicine injection. Chin Tradit Herb Drugs. 2021;52:3051–3060. [Google Scholar]
- 121.Xian S., Yang Z., Lee J., Jiang Z., Ye X., Luo L., et al. A randomized, double-blind, multicenter, placebo-controlled clinical study on the efficacy and safety of Shenmai injection in patients with chronic heart failure. J Ethnopharmacol. 2016;186:136–142. doi: 10.1016/j.jep.2016.03.066. [DOI] [PubMed] [Google Scholar]
- 122.Nabben M., Luiken J., Glatz J.F.C. Metabolic remodelling in heart failure revisited. Nat Rev Cardiol. 2018;15:780. doi: 10.1038/s41569-018-0115-8. [DOI] [PubMed] [Google Scholar]
- 123.Wang S.M., Ye L.F., Wand L.H. Shenmai Injection improves energy metabolism in patients with heart failure: a randomized controlled trial. Front Pharmacol. 2020;11:459. doi: 10.3389/fphar.2020.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Z.T., Zhu H.Q., Xu Q.L., Yin K. Effect of budesonide combined with Shenmai injection on the heart and lung function of patients with acute exacerbations of chronic cor pulmonale complicated with heart failure. Shanghai Med Phar J. 2015;36:31–33. [Google Scholar]
- 125.Shi L., Xie Y., Liao X., Chai Y., Luo Y. Shenmai injection as an adjuvant treatment for chronic cor pulmonale heart failure: a systematic review and meta-analysis of randomized controlled trials. BMC Complement Altern Med. 2015;15:418. doi: 10.1186/s12906-015-0939-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ju A.C., Luo R.Z., Su X.Q., Qin X.P., Li D.K., Zhou D.Z., et al. Research progress on chemical composition and quality control of Yiqi Fumai Lyophilized Injection. Drug Eval Res. 2018;41:365–371. [Google Scholar]
- 127.Heim X., Joshkon A., Bermudez J., Bachelier R., Dubrou C., Boucraut J., et al. CD146/sCD146 in the pathogenesis and monitoring of angiogenic and inflammatory diseases. Biomedicines. 2020;8:592–605. doi: 10.3390/biomedicines8120592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li Y., Jia X.W., Liu S.H., Feng C.N., Feng H.P. Effect of Yiqi Fumai Injection combined with atorvastatin on chronic heart failure patients with coronary heart disease and its effects on sCD40, sCD146 and PAPP-A. Chin Arch Tradit Chin Med. 2019;37:1225–1228. [Google Scholar]
- 129.Liu B. Clinical observation of Yiqi Fumai Injection combined with western medicine for heart failure. J New Chin Med. 2017;49:23–25. [Google Scholar]
- 130.Zhang H.P., Li G.P., Li J.T., Yan J.L. Comparison of the clinical therapeutic effects on chronic heart failure of Qi and Yin deficiency pattern/syndrome between YiqiFumai Injection and Shengmai San. World J Integr Tradit West Med. 2016;11:1735–1737. [Google Scholar]
- 131.Yu L., Zhou C., Luo Z., Zeng W., Lai F., Han G., et al. The lipid-lowering effects of Danhong and Huangqi injections: a meta-analysis of clinical controlled trials. Lipids Health Dis. 2018;17:106. doi: 10.1186/s12944-018-0760-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin W.J., Li S., Han J.D., Tan Y.B., Wang L., Xian S.X. The hemodynamic effects of Huangqi Injection in the treatment of chronic heart failure: a meta-analysis of clinical controlled trials. Chin Med J Res Prac. 2019;33:63–68. [Google Scholar]
- 133.Zhao Y., Yan S.H., Wang H.D., Wang X. Effects of astragalus injection on heart failure patients and MDA, HO-1 and NO. Chin Arch Tradit Chin Med. 2017;35:2055–2057. [Google Scholar]
- 134.Ha Y., Huang P., Yan Y., Xu X., Li B., Guo Y., et al. A systematic review and meta-analysis on a disease in TCM: astragalus injection for gathering Qi depression. Evid Based Complement Altern Med. 2020;2020 doi: 10.1155/2020/2803478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang K., Wu J., Duan X., Wu J., Zhang D., Zhang X., et al. Huangqi injection in the treatment of chronic heart failure: a systematic review and meta-analysis. Medicine. 2017;96 doi: 10.1097/MD.0000000000008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang X., Zhao Z., Mao J., Du T., Chen Y., Xu H., et al. Randomized, double-blinded, multicenter, placebo-controlled trial of Shenfu Injection for treatment of patients with chronic heart failure during the acute phase of symptom aggravation (Yang and Qi deficiency syndrome) Evid Based Complement Altern Med. 2019;2019 doi: 10.1155/2019/9297163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ni H.J., Li W., Wang J., Ren J.X. Efficacy of Shenfu Injection in treating chronic heart failure with syndrome of Yang deficiency of heart and kidney and its effect on serum level of NT-proBNP. J Emer Tradit Chin Med. 2021;30:1434–1437. [Google Scholar]
- 138.Lai J.L., Wang Y., Chen J. Retrospective analysis on Shenfu injection and Shenmai injection in adjuvant treatment of patients with heart failure. J Chin Med Pharma. 2019;9:19–22. [Google Scholar]
- 139.Li Q., Zhou T., Guan J.H., Liu G.E. Cost-effectiveness analysis of Shenfu injection combined with conventional therapy in treating heart failure. Chin J New Drugs. 2017;26:1718–1724. [Google Scholar]
- 140.Li T., Baochen Z., Yue Z., Cheng W., Yali W., Zongxi S., et al. Network pharmacology-based identification of pharmacological mechanism of SQFZ injection in combination with docetaxel on lung cancer. Sci Rep. 2019;9:4533. doi: 10.1038/s41598-019-40954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wen F., Peng S. Network pharmacology study of Huangqi (Astragalus membranaceus)-Dangshen (Codonopsis pilosula) in treatment of gastric cancer. Chin Arch Tradit Chin Med. 2021;39:89–94. [Google Scholar]
- 142.Li W., Zhang Z., Li X., Cai J., Li D., Du J., et al. CGRP derived from cardiac fibroblasts is an endogenous suppressor of cardiac fibrosis. Cardiovasc Res. 2020;116:1335–1348. doi: 10.1093/cvr/cvz234. [DOI] [PubMed] [Google Scholar]
- 143.Mathew P.G., Klein B.C. Getting to the heart of the matter: migraine, triptans, DHE, ditans, CGRP antibodies, first/second-generation gepants, and cardiovascular risk. Headache. 2019;59:1421–1426. doi: 10.1111/head.13601. [DOI] [PubMed] [Google Scholar]
- 144.Remels A.H.V., Derks W.J.A., Cillero-Pastor B., Verhees K.J.P., Kelders M.C., Heggermont W., et al. NF-κB-mediated metabolic remodelling in the inflamed heart in acute viral myocarditis. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2579–2589. doi: 10.1016/j.bbadis.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 145.Xu H.Y., Wang J. Influence of Shenqi Fuzheng Injection on heart function, serum CGRP and NF-κB in patients with chronic heart failure. Chin And Fore Med Res. 2020;18:138–140. [Google Scholar]
- 146.Radosinska J., Barancik M., Vrbjar N. Heart failure and role of circulating MMP-2 and MMP-9. Panminerva Med. 2017;59:241–253. doi: 10.23736/S0031-0808.17.03321-3. [DOI] [PubMed] [Google Scholar]
- 147.Wang J., Chen W. Effect of Shenqi Fuzheng injection on chronic heart failure and the levels of BNP, CRP, MMP-9 and TIMP-1. Shaanxi J Tradit Chin Med. 2018;39:1366–1368. [Google Scholar]
- 148.Hu X.D., Gong W. Effect of Shenqi Fuzheng Injection on COPD complicated with right heart failure. J Emer Tradit Chin Med. 2019;28:1954–1956+60. [Google Scholar]
- 149.Zeng M., Zhou H., He Y., Wang Z., Shao C., Yin J., et al. Danhong injection alleviates cerebral ischemia/reperfusion injury by improving intracellular energy metabolism coupling in the ischemic penumbra. Biomed Pharmacother. 2021;140 doi: 10.1016/j.biopha.2021.111771. [DOI] [PubMed] [Google Scholar]
- 150.Zhang J., Shi X., Gao J., Zhou R., Guo F., Zhang Y., et al. Danhong injection and trimetazidine protect cardiomyocytes and enhance calcium handling after myocardial infarction. Evid Based Complement Altern Med. 2021;2021 doi: 10.1155/2021/2480465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liao P., Wang L., Guo L., Zeng R., Huang J., Zhang M. Danhong injection (a traditional Chinese patent medicine) for acute myocardial infarction: a systematic review and meta-analysis. Evid Based Complement Altern Med. 2015;2015 doi: 10.1155/2015/646530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhu H.L., Huang P.N., Pan J.L., Liu S.L., Yuan T.H., Wu H. Clinical efficacy of Danhong Injection in the adjuvant treatment of coronary heart disease complicated with heart failure: a meta-analysis. J Guangzhou Univ Tradit Chin Med. 2021;38:2029–2035. [Google Scholar]
- 153.Badimon L., Bugiardini R., Cenko E., Cubedo J., Dorobantu M., Duncker D.J., et al. Position paper of the European Society of Cardiology-working group of coronary pathophysiology and microcirculation: obesity and heart disease. Eur Heart J. 2017;38:1951–1958. doi: 10.1093/eurheartj/ehx181. [DOI] [PubMed] [Google Scholar]
- 154.Tavazzi L., Maggioni A.P., Marchioli R., Barlera S., Franzosi M.G., Latini R., et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 155.Xing W.L., Wu Y.J., Liu H.X., Liu Q.R., Zhou Q., Li A.Y., et al. Effects of Danhong Injection on peri-procedural myocardial injury and microcirculatory resistance in patients with unstable angina undergoing elective percutaneous coronary intervention: a pilot randomized study. Chin J Integr Med. 2021;27:846–853. doi: 10.1007/s11655-021-2872-1. [DOI] [PubMed] [Google Scholar]
- 156.Karadağ M.K., Yıldırım E. Relationship of atherogenic index of plasma and mean platelet volume with ejection fraction in ischemic and nonischemic heart failure. Biomark Med. 2019;13:175–183. doi: 10.2217/bmm-2018-0196. [DOI] [PubMed] [Google Scholar]
- 157.Glezeva N., Gilmer J.F., Watson C.J., Ledwidge M. A central role for monocyte-platelet interactions in heart failure. J Cardiovasc Pharmacol Ther. 2016;21:245–261. doi: 10.1177/1074248415609436. [DOI] [PubMed] [Google Scholar]
- 158.Lou T.Y., Ma B.B., Li R.J., Liu J.H., Yu S.Y., Wang T.T., et al. Effect of Danhong Injection on platelet metabolites based on metabonomics technology. Chin J Chin Mater Med. 2021;46:3422–3428. doi: 10.19540/j.cnki.cjcmm.20210317.202. [DOI] [PubMed] [Google Scholar]
- 159.Liu C., Li W.J., Xie W.J. Influence of Zhenwu Decoction on Ang II and ALD in serum of heart failure rats. Chin Arch Tradit Chin Med. 2015;33:1374–1376. [Google Scholar]
- 160.Wang Y.P., Xiong J., Yan X., Li X.H., Li Z. Meta-analysis of supplemented Zhenwu decoction for treating congestive heart failure. Chin J Chin Mater Med. 2016;41:3679–3685. doi: 10.4268/cjcmm20161930. [DOI] [PubMed] [Google Scholar]
- 161.Hong L.L., Wang Q., Zhao Y.T., Zhang S., Zhang K.Q., Chen W.D., et al. Evaluation of Zhenwu Decoction effects on CYP450 enzymes in rats using a cocktail method by UPLC–MS/MS. BioMed Res Int. 2020;2020 doi: 10.1155/2020/4816209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu L.K., Zhao Y., Birling Y., Sun Y.X., Shang Q.H., Hu Z.J., et al. Effectiveness and safety of Linggui Zhugan decoction for the treatment of premature contraction in patients with coronary heart disease: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.1002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kawakami H., Kubota Y., Takeno S., Miyazaki Y., Wada T., Hamada R., et al. Gastrointestinal: severe congestive heart failure and acute gastric mucosal necrosis. J Gastroenterol Hepatol. 2017;32:949. doi: 10.1111/jgh.13739. [DOI] [PubMed] [Google Scholar]
- 164.Hou X., Wang C., Li Y. Clinical efficacy of Linggui Zhugan decoction in the treatment of decreased gastric motility caused by chronic heart failure. Minerva Med. 2021;112:113–117. doi: 10.23736/S0026-4806.21.07597-2. [DOI] [PubMed] [Google Scholar]
- 165.Ke B., Shi L., Zhang J.J., Chen D.S., Meng J., Qin J. Protective effects of modified linggui zhugan decoction combined with short-term very low calorie diets on cardiovascular risk factors in obese patients with impaired glucose tolerance. J Tradit Chin Med. 2012;32:193–198. doi: 10.1016/s0254-6272(13)60010-2. [DOI] [PubMed] [Google Scholar]
- 166.Duan X., Pan L., Peng D., Bao Q., Xiao L., Zhou A., et al. Analysis of the active components and metabolites of Taohong Siwu decoction by using ultra high performance liquid chromatography quadrupole time-of-flight mass spectrometry. J Sep Sci. 2020;43:4131–4147. doi: 10.1002/jssc.202000498. [DOI] [PubMed] [Google Scholar]
- 167.Li X., Li D.Y., Chen W.N., Zhang Y., Liu B.Q., Li S.Z., et al. Effect of ASO blood stasis syndrome serum on vascular endothelial cell injury and regulation of Taohong Siwu Decoction on it. Chin J Integr Tradit West Med. 2015;35:1373–1377. [PubMed] [Google Scholar]
- 168.Ma Q., Li P.L., Hua Y.L., Ji P., Yao W.L., Zhang X.S., et al. Effects of Tao-Hong-Si-Wu decoction on acute blood stasis in rats based on a LC–Q/TOF-MS metabolomics and network approach. Biomed Chromatogr. 2018;32:e4144. doi: 10.1002/bmc.4144. [DOI] [PubMed] [Google Scholar]
- 169.Luo Z.R., Li H., Xiao Z.X., Shao S.J., Zhao T.T., Zhao Y., et al. Taohong Siwu Decoction exerts a beneficial effect on cardiac function by possibly improving the microenvironment and decreasing mitochondrial fission after myocardial infarction. Cardiol Res Pract. 2019;2019 doi: 10.1155/2019/5198278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xiao X., Gao Z.S., Shi Z.Q., Wang J., Huang M.Q. The influence of True Warrior decoction combined with Four Substances decoction on myocardial remodeling of chronic congestive heart failure with syndrome of Yang deficiency and blood stasis. Henan Tradit Chin Med. 2017;37:1746–1748. [Google Scholar]
- 171.Chen M., Men L., Wu H., Zhong G., Ou L., Li T., et al. A systematic review of the effectiveness and safety of Chinese herbal medicine formula Gualou Xiebai Banxia (GLXBBX) decoction for the treatment of stable angina pectoris. Medicine. 2019;98 doi: 10.1097/MD.0000000000018375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lu X.Y., Xu H., Zhao T., Geng L. Study of serum metabonomics and formula-pattern correspondence in coronary heart disease patients diagnosed as phlegm or blood stasis pattern based on ultra performance liquid chromatography mass spectrometry. Chin J Integr Med. 2018;24:905–911. doi: 10.1007/s11655-018-2564-7. [DOI] [PubMed] [Google Scholar]
- 173.Ding Y.F., Peng Y.R., Shen H., Shu L., Wei Y.J. Gualou Xiebai decoction inhibits cardiac dysfunction and inflammation in cardiac fibrosis rats. BMC Complement Altern Med. 2016;16:49. doi: 10.1186/s12906-016-1012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li C., Zhang W.Y., Yu Y., Cheng C.S., Han J.Y., Yao X.S., et al. Discovery of the mechanisms and major bioactive compounds responsible for the protective effects of Gualou Xiebai Decoction on coronary heart disease by network pharmacology analysis. Phytomedicine. 2019;56:261–268. doi: 10.1016/j.phymed.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 175.Li X., Gao Q., Lin Q., Dong F., Long J., Cui X.Y. Rules of traditional Chinese medicine prescription in the treatment of heart failure based on data mining methods. Chin J Integr Med Cardiovasc Dis. 2020;18:3905–3908. [Google Scholar]
- 176.Liu L.J., Li H.F., Xu F., Wang H.Y., Zhang Y.F., Liu G.X., et al. Exploring the in vivo existence forms (23 original constituents and 147 metabolites) of Astragali Radix total flavonoids and their distributions in rats using HPLC–DAD–ESI-IT-TOF-MSn. Molecules. 2020;25:5560–5581. doi: 10.3390/molecules25235560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.El Dib R.A., Soliman H.S., Hussein M.H., Attia H.G. Two new flavonoids and biological activity of Astragalus abyssinicus (Hochst.) Steud. ex A. Rich. Aerial parts. Drug Res. 2015;65:259–265. doi: 10.1055/s-0034-1377003. [DOI] [PubMed] [Google Scholar]
- 178.Zhang C.H., Yang X., Wei J.R., Chen N.M., Xu J.P., Bi Y.Q., et al. Ethnopharmacology, phytochemistry, pharmacology, toxicology and clinical applications of Radix Astragali. Chin J Integr Med. 2021;27:229–240. doi: 10.1007/s11655-019-3032-8. [DOI] [PubMed] [Google Scholar]
- 179.Xiao C.J., Zhang Y., Qiu L., Xu W., Zhao M.Z., Dong X., et al. A new schistosomicidal and antioxidative phenylpropanoid from Astragalus englerianus. J Asian Nat Prod Res. 2015;17:772–777. doi: 10.1080/10286020.2014.977787. [DOI] [PubMed] [Google Scholar]
- 180.Chen W., Zhang Y.Y., Wang Z., Luo X.H., Sun W.C., Wang H.B. Phenolic derivatives from Radix Astragali and their anti-inflammatory activities. Nat Prod Commun. 2014;9:1577–1580. [PubMed] [Google Scholar]
- 181.Zhou Y., Zong Y., Liu Z., Zhao H., Zhao X., Wang J. Astragalus polysaccharides enhance the immune response to OVA antigen in BALB/c mice. BioMed Res Int. 2021;2021 doi: 10.1155/2021/9976079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gong P., Wang D., Cui D., Yang Q., Wang P., Yang W., et al. Anti-aging function and molecular mechanism of Radix Astragali and Radix Astragali preparata via network pharmacology and PI3K/Akt signaling pathway. Phytomedicine. 2021;84 doi: 10.1016/j.phymed.2021.153509. [DOI] [PubMed] [Google Scholar]
- 183.Chen Y., Wang J., Li J., Zhu J., Wang R., Xi Q., et al. Astragalus polysaccharide prevents ferroptosis in a murine model of experimental colitis and human Caco-2 cells via inhibiting NRF2/HO-1 pathway. Eur J Pharmacol. 2021;911 doi: 10.1016/j.ejphar.2021.174518. [DOI] [PubMed] [Google Scholar]
- 184.Ding G., Gong Q., Ma J., Liu X., Wang Y., Cheng X. Immunosuppressive activity is attenuated by Astragalus polysaccharides through remodeling the gut microenvironment in melanoma mice. Cancer Sci. 2021;112:4050–4063. doi: 10.1111/cas.15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yu J.M., Zhang X.B., Jiang W., Wang H.D., Zhang Y.N. Astragalosides promote angiogenesis via vascular endothelial growth factor and basic fibroblast growth factor in a rat model of myocardial infarction. Mol Med Rep. 2015;12:6718–6726. doi: 10.3892/mmr.2015.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu J., Li Y., Bian X., Xue N., Yu J., Dai S., et al. Astragaloside IV alleviates heart failure by regulating SUMO-specific protease 1. Exp Ther Med. 2021;22:1076. doi: 10.3892/etm.2021.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li X.Z., Ding Y.Z., Wu H.F., Bian Z.P., Xu J.D., Gu C.R., et al. Astragaloside IV prevents cardiac remodeling in the apolipoprotein E-deficient mice by regulating cardiac homeostasis and oxidative stress. Cell Physiol Biochem. 2017;44:2422–2438. doi: 10.1159/000486166. [DOI] [PubMed] [Google Scholar]
- 188.Sui Y.B., Wang Y., Liu L., Liu F., Zhang Y.Q. Astragaloside IV alleviates heart failure by promoting angiogenesis through the JAK–STAT3 pathway. Pharm Biol. 2019;57:48–54. doi: 10.1080/13880209.2019.1569697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shi H., Zhou P., Gao G., Liu P.P., Wang S.S., Song R., et al. Astragaloside IV prevents acute myocardial infarction by inhibiting the TLR4/MyD88/NF-κB signaling pathway. J Food Biochem. 2021;45 doi: 10.1111/jfbc.13757. [DOI] [PubMed] [Google Scholar]
- 190.Liu Z.H., Liu H.B., Wang J. Astragaloside IV protects against the pathological cardiac hypertrophy in mice. Biomed Pharmacother. 2018;97:1468–1478. doi: 10.1016/j.biopha.2017.09.092. [DOI] [PubMed] [Google Scholar]
- 191.Tu L., Pan C.S., Wei X.H., Yan L., Liu Y.Y., Fan J.Y., et al. Astragaloside IV protects heart from ischemia and reperfusion injury via energy regulation mechanisms. Microcirculation. 2013;20:736–747. doi: 10.1111/micc.12074. [DOI] [PubMed] [Google Scholar]
- 192.Kim Y., Cho S.H. The effect of ginsenosides on depression in preclinical studies: a systematic review and meta-analysis. J Ginseng Res. 2021;45:420–432. doi: 10.1016/j.jgr.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jiao L., Li H., Li J., Bo L., Zhang X., Wu W., et al. Study on structure characterization of pectin from the steamed ginseng and the inhibition activity of lipid accumulation in oleic acid-induced HepG2 cells. Int J Biol Macromol. 2020;159:57–65. doi: 10.1016/j.ijbiomac.2020.04.167. [DOI] [PubMed] [Google Scholar]
- 194.Kim G.W., Pyo M.K., Chung S.H. Pectin lyase-modified red ginseng extract improves glucose homeostasis in high fat diet-fed mice. J Ethnopharmacol. 2020;249 doi: 10.1016/j.jep.2019.112384. [DOI] [PubMed] [Google Scholar]
- 195.Xue H., Zhao Z., Lin Z., Geng J., Guan Y., Song C., et al. Selective effects of ginseng pectins on galectin-3-mediated T cell activation and apoptosis. Carbohydr Polym. 2019;219:121–129. doi: 10.1016/j.carbpol.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 196.Jung E., Kim C.S., Jung W., Park S.B., Pyo M.K., Kim J. Ginseng extract modified by pectin lyase inhibits retinal vascular injury and blood–retinal barrier breakage in a rat model of diabetes. J Med Food. 2019;22:337–343. doi: 10.1089/jmf.2018.4256. [DOI] [PubMed] [Google Scholar]
- 197.Chen J., Huang Q., Li J., Yao Y., Sun W., Zhang Z., et al. Panax ginseng against myocardial ischemia/reperfusion injury: a review of preclinical evidence and potential mechanisms. J Ethnopharmacol. 2023;300 doi: 10.1016/j.jep.2022.115715. [DOI] [PubMed] [Google Scholar]
- 198.Li L., Pan C.S., Yan L., Cui Y.C., Liu Y.Y., Mu H.N., et al. Ginsenoside Rg1 ameliorates rat myocardial ischemia–reperfusion injury by modulating energy metabolism pathways. Front Physiol. 2018;9:78. doi: 10.3389/fphys.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cui Y.C., Pan C.S., Yan L., Li L., Hu B.H., Chang X., et al. Ginsenoside Rb1 protects against ischemia/reperfusion-induced myocardial injury via energy metabolism regulation mediated by RhoA signaling pathway. Sci Rep. 2017;7 doi: 10.1038/srep44579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang T., Miao Y., Zhang T., Mu N., Ruan L., Duan J., et al. Ginsenoside Rb1 inhibits autophagy through regulation of Rho/ROCK and PI3K/mTOR pathways in a pressure-overload heart failure rat model. J Pharm Pharmacol. 2018;70:830–838. doi: 10.1111/jphp.12900. [DOI] [PubMed] [Google Scholar]
- 201.Fan H.J., Tan Z.B., Wu Y.T., Feng X.R., Bi Y.M., Xie L.P., et al. The role of ginsenoside Rb1, a potential natural glutathione reductase agonist, in preventing oxidative stress-induced apoptosis of H9C2 cells. J Ginseng Res. 2020;44:258–266. doi: 10.1016/j.jgr.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang Z.L., Fan Y., Liu M.L. Ginsenoside Rg1 inhibits autophagy in H9c2 cardiomyocytes exposed to hypoxia/reoxygenation. Mol Cell Biochem. 2012;365:243–250. doi: 10.1007/s11010-012-1265-3. [DOI] [PubMed] [Google Scholar]
- 203.Li D., Wang J., Hou J., Fu J., Chang D., Bensoussan A., et al. Ginsenoside Rg1 protects starving H9c2 cells by dissociation of Bcl-2-Beclin1 complex. BMC Complement Altern Med. 2016;16:146. doi: 10.1186/s12906-016-1112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu G., Qi X., Li X., Sun F. Ginsenoside Rg2 protects cardiomyocytes against trastuzumab-induced toxicity by inducing autophagy. Exp Ther Med. 2021;21:473. doi: 10.3892/etm.2021.9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sun G.Z., Meng F.J., Cai H.Q., Diao X.B., Zhang B., Bai X.P. Ginsenoside Rg3 protects heart against isoproterenol-induced myocardial infarction by activating AMPK mediated autophagy. Cardiovasc Diagn Ther. 2020;10:153–160. doi: 10.21037/cdt.2020.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang S., Cui Y., Xiong M., Li M., Wang P., Cui J., et al. Dual activity of ginsenoside Rb1 in hypertrophic cardiomyocytes and activated macrophages: implications for the therapeutic intervention of cardiac hypertrophy. J Inflamm Res. 2021;14:1789–1806. doi: 10.2147/JIR.S310633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ke S., Wu L., Wang M., Liu D., Shi G., Zhu J., et al. Ginsenoside Rb1 attenuates age-associated vascular impairment by modulating the Gas6 pathway. Pharm Biol. 2021;59:1369–1377. doi: 10.1080/13880209.2021.1986076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chen X., Wang Q., Shao M., Ma L., Guo D., Wu Y., et al. Ginsenoside Rb3 regulates energy metabolism and apoptosis in cardiomyocytes via activating PPARα pathway. Biomed Pharmacother. 2019;120 doi: 10.1016/j.biopha.2019.109487. [DOI] [PubMed] [Google Scholar]
- 209.Sun J., Yu X., Huangpu H., Yao F. Ginsenoside Rb3 protects cardiomyocytes against hypoxia/reoxygenation injury via activating the antioxidation signaling pathway of PERK/Nrf2/HMOX1. Biomed Pharmacother. 2019;109:254–261. doi: 10.1016/j.biopha.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 210.Zhang Y., Ji H., Qiao O., Li Z., Pecoraro L., Zhang X., et al. Nanoparticle conjugation of ginsenoside Rb3 inhibits myocardial fibrosis by regulating PPARα pathway. Biomed Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111630. [DOI] [PubMed] [Google Scholar]
- 211.Huang Q., Su H., Qi B., Wang Y., Yan K., Wang X., et al. A SIRT1 activator, ginsenoside Rc, promotes energy metabolism in cardiomyocytes and neurons. J Am Chem Soc. 2021;143:1416–1427. doi: 10.1021/jacs.0c10836. [DOI] [PubMed] [Google Scholar]
- 212.Wang Q.W., Yu X.F., Xu H.L., Zhao X.Z., Sui D.Y. Ginsenoside Re improves isoproterenol-induced myocardial fibrosis and heart failure in rats. Evid Based Complement Altern Med. 2019;2019 doi: 10.1155/2019/3714508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rong S.H., Lin H., Gao N. Study on processing technology and processing principles of Atractrylodis Macrocephalae Rhizoma. Chin J Chin Mater Med. 2011;36:1001–1003. [PubMed] [Google Scholar]
- 214.Li M.Z., Wang J.H., Guo H.Z., Wang M.M., Fu X.T., Chen Y.G. Simultaneous determination of multiple components in decoction pieces of Atractylodes macrocephala by HPLC. Chin J Pharm Anal. 2017;37:1585–1592. [Google Scholar]
- 215.Sun C., Zhang X., Yu F., Liu C., Hu F., Liu L., et al. Atractylenolide I alleviates ischemia/reperfusion injury by preserving mitochondrial function and inhibiting caspase-3 activity. J Int Med Res. 2021;49 doi: 10.1177/0300060521993315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cao M., Yu C., Yao Z., Gao X., Wu S. Atractylodesin III maintains mitochondrial function and inhibits caspase-3 activity to reverse apoptosis of cardiomyocytes in AMI rats. Int J Clin Exp Pathol. 2019;12:198–204. [PMC free article] [PubMed] [Google Scholar]
- 217.Elsidege Ali L.A., Mahdi Hassan F. Association of platelet Integrin αIIbβ3 polymorphisms with atherosclerotic coronary heart disease in sudanese patients. Pak J Biol Sci. 2019;22:335–341. doi: 10.3923/pjbs.2019.335.341. [DOI] [PubMed] [Google Scholar]
- 218.Chen Y., Yang W., Guo L., Wu X., Zhang T., Liu J., et al. Atractylodes lactone compounds inhibit platelet activation. Platelets. 2017;28:194–202. doi: 10.1080/09537104.2016.1209477. [DOI] [PubMed] [Google Scholar]
- 219.Bozkurt B., Aguilar D., Deswal A., Dunbar S.B., Francis G.S., Horwich T., et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e535–e578. doi: 10.1161/CIR.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 220.McMurray J.J.V., Solomon S.D., Inzucchi S.E., Køber L., Kosiborod M.N., Martinez F.A., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 221.Chao C.L., Huang H.C., Lin H.C., Chang T.C., Chang W.L. Sesquiterpenes from Baizhu stimulate glucose uptake by activating AMPK and PI3K. Am J Chin Med. 2016;44:963–979. doi: 10.1142/S0192415X16500531. [DOI] [PubMed] [Google Scholar]
- 222.Tian H., Guo L., Wang D., Li S., Wang R. Investigating the main components and mechanism of Panax notoginseng based on pharmacology network. Drug Eval Res. 2019;42:70–75. [Google Scholar]
- 223.Chen X., Ma L., Shao M., Wang Q., Jiang Q., Guo D., et al. Exploring the protective effects of PNS on acute myocardial ischaemia-induced heart failure by transcriptome analysis. J Ethnopharmacol. 2021;271 doi: 10.1016/j.jep.2021.113823. [DOI] [PubMed] [Google Scholar]
- 224.He K., Yan L., Pan C.S., Liu Y.Y., Cui Y.C., Hu B.H., et al. ROCK-dependent ATP5D modulation contributes to the protection of notoginsenoside NR1 against ischemia–reperfusion-induced myocardial injury. Am J Physiol Heart Circ Physiol. 2014;307:H1764–H1776. doi: 10.1152/ajpheart.00259.2014. [DOI] [PubMed] [Google Scholar]
- 225.Yu Y., Sun G., Luo Y., Wang M., Chen R., Zhang J., et al. Cardioprotective effects of notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress-related signaling pathways. Sci Rep. 2016;6 doi: 10.1038/srep21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhou Z., Wang J., Song Y., He Y., Zhang C., Liu C., et al. Panax notoginseng saponins attenuate cardiomyocyte apoptosis through mitochondrial pathway in natural aging rats. Phytother Res. 2018;32:243–250. doi: 10.1002/ptr.5961. [DOI] [PubMed] [Google Scholar]
- 227.Wang D.D., Lv L.Y., Xu Y., Jiang K., Chen F., Qian J., et al. Cardioprotection of Panax notoginseng saponins against acute myocardial infarction and heart failure through inducing autophagy. Biomed Pharmacother. 2021;136 doi: 10.1016/j.biopha.2021.111287. [DOI] [PubMed] [Google Scholar]
- 228.Chen S., Wu Y., Qin X., Wen P., Liu J., Yang M. Global gene expression analysis using RNA-seq reveals the new roles of Panax notoginseng saponins in ischemic cardiomyocytes. J Ethnopharmacol. 2021;268 doi: 10.1016/j.jep.2020.113639. [DOI] [PubMed] [Google Scholar]
- 229.Liu L., Ning B., Cui J., Zhang T., Chen Y. miR-29c is implicated in the cardioprotective activity of Panax notoginseng saponins against isoproterenol-induced myocardial fibrogenesis. J Ethnopharmacol. 2017;198:1–4. doi: 10.1016/j.jep.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 230.Ming Q., Dong X., Wu S., Zhu B., Jia M., Zheng C., et al. UHPLC–HRMSn analysis reveals the dynamic metabonomic responses of Salvia miltiorrhiza hairy roots to polysaccharide fraction from Trichoderma atroviride. Biomolecules. 2019;9:541–555. doi: 10.3390/biom9100541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chen R., Chen W., Huang X., Rui Q. Tanshinone IIA attenuates heart failure via inhibiting oxidative stress in myocardial infarction rats. Mol Med Rep. 2021;23:1–10. doi: 10.3892/mmr.2021.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lu Y., Yan Y., Liu X. Effects of alprostadil combined with tanshinone IIa injection on microcirculation disorder, outcomes, and cardiac function in AMI patients after PCI. Ann Palliat Med. 2021;10:97–103. doi: 10.21037/apm-20-2147. [DOI] [PubMed] [Google Scholar]
- 233.Gao S., Li L., Li L., Ni J., Guo R., Mao J., et al. Effects of the combination of tanshinone IIA and puerarin on cardiac function and inflammatory response in myocardial ischemia mice. J Mol Cell Cardiol. 2019;137:59–70. doi: 10.1016/j.yjmcc.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 234.Yin Z.K., Feng Z.M., Jiang J.S., Zhang X., Zhang P.C., Yang Y.N. Two new tanshinone derivatives from the rhizomes of Salvia miltiorrhiza and their antiviral activities. J Asian Nat Prod Res. 2020;22:24–29. doi: 10.1080/10286020.2019.1645132. [DOI] [PubMed] [Google Scholar]
- 235.Mao S., Taylor S., Chen Q., Zhang M., Hinek A. Sodium tanshinone IIA sulfonate prevents the adverse left ventricular remodelling: focus on polymorphonuclear neutrophil-derived granule components. J Cell Mol Med. 2019;23:4592–4600. doi: 10.1111/jcmm.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Huang D.D., Wei X.H., Mu H.N., Pan C.S., Li Q., Hu B.H., et al. Total salvianolic acid injection prevents ischemia/reperfusion-induced myocardial injury via antioxidant mechanism involving mitochondrial respiratory chain through the upregulation of Sirtuin1 and Sirtuin3. Shock. 2019;51:745–756. doi: 10.1097/SHK.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nandi S.S., Katsurada K., Sharma N.M., Anderson D.R., Mahata S.K., Patel K.P. MMP9 inhibition increases autophagic flux in chronic heart failure. Am J Physiol Heart Circ Physiol. 2020;319:H1414–H1437. doi: 10.1152/ajpheart.00032.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yu J., Chen R., Tan Y., Wu J., Qi J., Zhang M., et al. Salvianolic acid B alleviates heart failure by inactivating ERK1/2/GATA4 signaling pathway after pressure overload in mice. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wu Y., Xiu W., Wu Y. Salvianolic acid A protects H9C2 cardiomyocytes from doxorubicin-induced damage by inhibiting NFKB1 expression thereby downregulating long-noncoding RNA (lncRNA) plasmacytoma variant translocation 1 (PVT1) Med Sci Monit. 2021;27 doi: 10.12659/MSM.929824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yang X.Y., He K., Pan C.S., Li Q., Liu Y.Y., Yan L., et al. 3,4-Dihydroxyl-phenyl lactic acid restores NADH dehydrogenase 1 α subunit 10 to ameliorate cardiac reperfusion injury. Sci Rep. 2015;5 doi: 10.1038/srep10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Makki K., Deehan E.C., Walter J., Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 242.McCarty M.F. Nutraceutical, dietary, and lifestyle options for prevention and treatment of ventricular hypertrophy and heart failure. Int J Mol Sci. 2021;22:3321. doi: 10.3390/ijms22073321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chen J., Wang Y., Wang S., Zhao X., Zhao L., Wang Y. Salvianolic acid B and ferulic acid synergistically promote angiogenesis in HUVECs and zebrafish via regulating VEGF signaling. J Ethnopharmacol. 2022;283 doi: 10.1016/j.jep.2021.114667. [DOI] [PubMed] [Google Scholar]
- 244.Zhang X.J., Cui Z.H., Zhao Y.X., He T.T., Wang L., Liang X.W. Ferulic acid ameliorates isoproterenol-induced heart failure by decreasing oxidative stress and inhibiting cardiocyte apoptosis via activating Nrf2 signaling pathway in rats. Biol Pharm Bull. 2021;44:396–403. doi: 10.1248/bpb.b20-00783. [DOI] [PubMed] [Google Scholar]
- 245.Zada S., Pham T.M., Hwang J.S., Ahmed M., Lai T.H., Elashkar O., et al. Chlorogenic acid protects human chondrocyte C28/I2 cells from oxidative stress-induced cell death through activation of autophagy. Life Sci. 2021;285 doi: 10.1016/j.lfs.2021.119968. [DOI] [PubMed] [Google Scholar]
- 246.Hu P.Y., Liu D., Zheng Q., Wu Q., Tang Y., Yang M. Elucidation of transport mechanism of paeoniflorin and the influence of ligustilide, senkyunolide I and senkyunolide A on paeoniflorin transport through Mdck-Mdr1 Cells as blood–brain barrier in vitro model. Molecules. 2016;21:300. doi: 10.3390/molecules21030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zheng Q., Tang Y., Hu P.Y., Liu D., Zhang D., Yue P., et al. The influence and mechanism of ligustilide, senkyunolide I, and senkyunolide A on echinacoside transport through MDCK-MDR1 cells as blood–brain barrier in vitro model. Phytother Res. 2018;32:426–435. doi: 10.1002/ptr.5985. [DOI] [PubMed] [Google Scholar]
- 248.Cao Y., Dong Z., Yang D., Ma X., Wang X. Alleviation of glucolipotoxicity-incurred cardiomyocyte dysfunction by Z-ligustilide involves in the suppression of oxidative insult, inflammation and fibrosis. Chem Phys Lipids. 2021;241 doi: 10.1016/j.chemphyslip.2021.105138. [DOI] [PubMed] [Google Scholar]
- 249.Yu B., Zhong F.M., Yao Y., Deng S.Q., Xu H.Q., Lu J.F., et al. Synergistic protection of tetramethylpyrazine phosphate and borneol on brain microvascular endothelium cells injured by hypoxia. Am J Transl Res. 2019;11:2168–2180. [PMC free article] [PubMed] [Google Scholar]
- 250.Zhang B., Zhang Y., Deng F., Fang S. Ligustrazine prevents basilar artery remodeling in two-kidney-two-clip renovascular hypertension rats via suppressing PI3K/Akt signaling. Microvasc Res. 2020;128 doi: 10.1016/j.mvr.2019.103938. [DOI] [PubMed] [Google Scholar]
- 251.Jiang R., Xu J., Zhang Y., Zhu X., Liu J., Tan Y. Ligustrazine alleviate acute lung injury through suppressing pyroptosis and apoptosis of alveolar macrophages. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.680512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hou Y., Li M., Jin Y., Xu F., Liang S., Xue C., et al. Protective effects of tetramethylpyrazine on dysfunction of the locus coeruleus in rats exposed to single prolonged stress by anti-ER stress mechanism. Psychopharmacology. 2021;238:2923–2936. doi: 10.1007/s00213-021-05908-6. [DOI] [PubMed] [Google Scholar]
- 253.Chen Q., Zhang D., Bi Y., Zhang W., Zhang Y., Meng Q., et al. The protective effects of liguzinediol on congestive heart failure induced by myocardial infarction and its relative mechanism. Chin Med. 2020;15:63. doi: 10.1186/s13020-020-00345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li S., Huang H., Zhang M., Wang W., Xue S., Gao Y., et al. Liguzinediol enhances the inotropic effect of rat hearts via inhibition of protein phosphatase (PP1 and PP2A) activities. J Cardiovasc Pharmacol. 2017;69:236–244. doi: 10.1097/FJC.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 255.Li Y., Song P., Zhu Q., Yin Q., Ji J., Li W., et al. Liguzinediol improved the heart function and inhibited myocardial cell apoptosis in rats with heart failure. Acta Pharmacol Sin. 2014;35:1257–1264. doi: 10.1038/aps.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wang Y., Li X., Zhao P., Qu Z., Bai D., Gao X., et al. Physicochemical characterizations of polysaccharides from Angelica Sinensis Radix under different drying methods for various applications. Int J Biol Macromol. 2019;121:381–389. doi: 10.1016/j.ijbiomac.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 257.Lai P., Liu Y. Angelica sinensis polysaccharides inhibit endothelial progenitor cell senescence through the reduction of oxidative stress and activation of the Akt/hTERT pathway. Pharm Biol. 2015;53:1842–1849. doi: 10.3109/13880209.2015.1027779. [DOI] [PubMed] [Google Scholar]
- 258.Zhang X., Xue H., Zhou P., Liu L., Yu J., Dai P., et al. Angelica polysaccharide alleviates oxidative response damage in HaCaT cells through up-regulation of miR-126. Exp Mol Pathol. 2019;110 doi: 10.1016/j.yexmp.2019.104281. [DOI] [PubMed] [Google Scholar]
- 259.Niu X., Zhang J., Ni J., Wang R., Zhang W., Sun S., et al. Network pharmacology-based identification of major component of Angelica sinensis and its action mechanism for the treatment of acute myocardial infarction. Biosci Rep. 2018;38 doi: 10.1042/BSR20180519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Niu X., Zhang J., Ling C., Bai M., Peng Y., Sun S., et al. Polysaccharide from Angelica sinensis protects H9c2 cells against oxidative injury and endoplasmic reticulum stress by activating the ATF6 pathway. J Int Med Res. 2018;46:1717–1733. doi: 10.1177/0300060518758863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Song X., Kong J., Song J., Pan R., Wang L. Angelica sinensis polysaccharide alleviates myocardial fibrosis and oxidative stress in the heart of hypertensive rats. Comput Math Methods Med. 2021;2021 doi: 10.1155/2021/6710006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yan N., Tang Z., Xu Y., Li X., Wang Q. Pharmacokinetic study of ferulic acid following transdermal or intragastric administration in rats. AAPS PharmSciTech. 2020;21:169. doi: 10.1208/s12249-020-01709-w. [DOI] [PubMed] [Google Scholar]
- 263.Luo M., Chen P.P., Yang L., Wang P., Lu Y.L., Shi F.G., et al. Sodium ferulate inhibits myocardial hypertrophy induced by abdominal coarctation in rats: involvement of cardiac PKC and MAPK signaling pathways. Biomed Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108735. [DOI] [PubMed] [Google Scholar]
- 264.Yao X., Jiao S., Qin M., Hu W., Yi B., Liu D. Vanillic acid alleviates acute myocardial hypoxia/reoxygenation injury by inhibiting oxidative stress. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/8348035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lin C.C., Chen S.Y., Lien H.Y., Lin S.Z., Lee T.M. Targeting the PI3K/STAT3 axis modulates age-related differences in macrophage phenotype in rats with myocardial infarction. J Cell Mol Med. 2019;23:6378–6392. doi: 10.1111/jcmm.14526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Jiang H., Li J., Wang L., Wang S., Nie X., Chen Y., et al. Total glucosides of paeony: a review of its phytochemistry, role in autoimmune diseases, and mechanisms of action. J Ethnopharmacol. 2020;258 doi: 10.1016/j.jep.2020.112913. [DOI] [PubMed] [Google Scholar]
- 267.Long J., Gao M., Kong Y., Shen X., Du X., Son Y.O., et al. Cardioprotective effect of total paeony glycosides against isoprenaline-induced myocardial ischemia in rats. Phytomedicine. 2012;19:672–676. doi: 10.1016/j.phymed.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 268.Luo C., Wang H., Chen X., Cui Y., Li H., Long J., et al. Protection of H9c2 rat cardiomyoblasts against oxidative insults by total paeony glucosides from Radix Paeoniae Rubrae. Phytomedicine. 2013;21:20–24. doi: 10.1016/j.phymed.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 269.Yan X., Huang Y. Mechanism of total glucosides of paeony in hypoxia/reoxygenation-induced cardiomyocyte pyroptosis. J Bioenerg Biomembr. 2021;53:643–653. doi: 10.1007/s10863-021-09921-4. [DOI] [PubMed] [Google Scholar]
- 270.Naveed M., Han L., Hasnat M., Baig M., Wang W., Mikrani R., et al. Suppression of TGP on myocardial remodeling by regulating the NF-κB pathway. Biomed Pharmacother. 2018;108:1460–1468. doi: 10.1016/j.biopha.2018.09.168. [DOI] [PubMed] [Google Scholar]
- 271.Li J.Z., Yu S.Y., Wu J.H., Shao Q.R., Dong X.M. Paeoniflorin protects myocardial cell from doxorubicin-induced apoptosis through inhibition of NADPH oxidase. Can J Physiol Pharmacol. 2012;90:1569–1575. doi: 10.1139/y2012-140. [DOI] [PubMed] [Google Scholar]
- 272.Liu M., Feng J., Du Q., Ai J., Lv Z. Paeoniflorin attenuates myocardial fibrosis in isoprenaline-induced chronic heart failure rats via inhibiting P38 MAPK pathway. Curr Med Sci. 2020;40:307–312. doi: 10.1007/s11596-020-2178-0. [DOI] [PubMed] [Google Scholar]
- 273.Liu M., Ai J., Feng J., Zheng J., Tang K., Shuai Z., et al. Effect of paeoniflorin on cardiac remodeling in chronic heart failure rats through the transforming growth factor β1/Smad signaling pathway. Cardiovasc Diagn Ther. 2019;9:272–280. doi: 10.21037/cdt.2019.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zhang L., Wei W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol Ther. 2020;207 doi: 10.1016/j.pharmthera.2019.107452. [DOI] [PubMed] [Google Scholar]
- 275.Ashrafizadeh M., Zarrabi A., Mirzaei S., Hashemi F., Samarghandian S., Zabolian A., et al. Gallic acid for cancer therapy: molecular mechanisms and boosting efficacy by nanoscopical delivery. Food Chem Toxicol. 2021;157 doi: 10.1016/j.fct.2021.112576. [DOI] [PubMed] [Google Scholar]
- 276.Wu L.F., Wang Z.M., He K.Q., Li W.Y., Jia F., Wen W.Y., et al. Chemical constituents and pharmacological effects of paeoniae radix rubra: a review. Chin J Exp Tradit Med Form. 2021;27:198–206. [Google Scholar]
- 277.Qiao Y., Shi Y., Wu C., Hou X., Pan X., Deng Z., et al. Rapid screening and identification of anticoagulation component from carthami flos by two-dimensional thrombin affinity chromatography combined with HPLC–MS/MS. J Sep Sci. 2021;44:3061–3069. doi: 10.1002/jssc.202100092. [DOI] [PubMed] [Google Scholar]
- 278.Jiang H., Li M., Du K., Ma C., Cheng Y., Wang S., et al. Traditional Chinese medicine for adjuvant treatment of breast cancer: Taohong Siwu Decoction. Chin Med. 2021;16:129. doi: 10.1186/s13020-021-00539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Huber G., Priest S., Geisbuhler T. Cardioprotective effect of hydroxysafflor yellow A via the cardiac permeability transition pore. Planta Med. 2018;84:507–518. doi: 10.1055/s-0043-122501. [DOI] [PubMed] [Google Scholar]
- 280.Wei G., Yin Y., Duan J., Guo C., Zhu Y., Wang Y., et al. Hydroxysafflor yellow A promotes neovascularization and cardiac function recovery through HO-1/VEGF-A/SDF-1α cascade. Biomed Pharmacother. 2017;88:409–420. doi: 10.1016/j.biopha.2017.01.074. [DOI] [PubMed] [Google Scholar]
- 281.Zou J., Wang N., Liu M., Bai Y., Wang H., Liu K., et al. Nucleolin mediated pro-angiogenic role of hydroxysafflor Yellow A in ischaemic cardiac dysfunction: post-transcriptional regulation of VEGF-A and MMP-9. J Cell Mol Med. 2018;22:2692–2705. doi: 10.1111/jcmm.13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ye J., Lu S., Wang M., Ge W., Liu H., Qi Y., et al. Hydroxysafflor yellow A protects against myocardial ischemia/reperfusion injury via suppressing NLRP3 inflammasome and activating autophagy. Front Pharmacol. 2020;11:1170. doi: 10.3389/fphar.2020.01170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ye J.X., Wang M., Wang R.Y., Liu H.T., Qi Y.D., Fu J.H., et al. Hydroxysafflor yellow A inhibits hypoxia/reoxygenation-induced cardiomyocyte injury via regulating the AMPK/NLRP3 inflammasome pathway. Int Immunopharmacol. 2020;82 doi: 10.1016/j.intimp.2020.106316. [DOI] [PubMed] [Google Scholar]
- 284.Zhou D.L., Ding T.T., Ni B., Jing Y.Y., Liu S.X. Hydroxysafflor yellow A mitigated myocardial ischemia/reperfusion injury by inhibiting the activation of the JAK2/STAT1 pathway. Int J Mol Med. 2019;44:405–416. doi: 10.3892/ijmm.2019.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ni B., Zhou D., Jing Y., Liu S. Hydroxysafflor yellow A protects against angiotensin II-induced hypertrophy. Mol Med Rep. 2018;18:3649–3656. doi: 10.3892/mmr.2018.9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Han C.K., Tien Y.C., Jine-Yuan Hsieh D., Ho T.J., Lai C.H., Yeh Y.L., et al. Attenuation of the LPS-induced, ERK-mediated upregulation of fibrosis-related factors FGF-2, uPA, MMP-2, and MMP-9 by Carthamus tinctorius L. in cardiomyoblasts. Environ Toxicol. 2017;32:754–763. doi: 10.1002/tox.22275. [DOI] [PubMed] [Google Scholar]
- 287.Ye J., Wang R., Wang M., Fu J., Zhang Q., Sun G., et al. Hydroxysafflor yellow A ameliorates myocardial ischemia/reperfusion injury by suppressing calcium overload and apoptosis. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/6643615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Soleimani V., Sahebkar A., Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother Res. 2018;32:985–995. doi: 10.1002/ptr.6054. [DOI] [PubMed] [Google Scholar]
- 289.Manghani C., Gupta A., Tripathi V., Rani V. Cardioprotective potential of curcumin against norepinephrine-induced cell death: a microscopic study. J Microsc. 2017;265:232–244. doi: 10.1111/jmi.12492. [DOI] [PubMed] [Google Scholar]
- 290.Yanazume T., Hasegawa K., Morimoto T., Kawamura T., Wada H., Matsumori A., et al. Cardiac p300 is involved in myocyte growth with decompensated heart failure. Mol Cell Biol. 2003;23:3593–3606. doi: 10.1128/MCB.23.10.3593-3606.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sunagawa Y., Funamoto M., Shimizu K., Shimizu S., Sari N., Katanasaka Y., et al. Curcumin, an Inhibitor of p300-HAT activity, suppresses the development of hypertension-induced left ventricular hypertrophy with preserved ejection fraction in Dahl rats. Nutrients. 2021;13:2608. doi: 10.3390/nu13082608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sunagawa Y., Funamoto M., Sono S., Shimizu K., Shimizu S., Genpei M., et al. Curcumin and its demethoxy derivatives possess p300 HAT inhibitory activity and suppress hypertrophic responses in cardiomyocytes. J Pharmacol Sci. 2018;136:212–217. doi: 10.1016/j.jphs.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 293.Shimizu K., Sunagawa Y., Funamoto M., Wakabayashi H., Genpei M., Miyazaki Y., et al. The synthetic curcumin analogue GO-Y030 effectively suppresses the development of pressure overload-induced heart failure in mice. Sci Rep. 2020;10:7172. doi: 10.1038/s41598-020-64207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Namdari M., Eatemadi A. Cardioprotective effects of curcumin-loaded magnetic hydrogel nanocomposite (nanocurcumin) against doxorubicin-induced cardiac toxicity in rat cardiomyocyte cell lines. Artif Cells Nanomed Biotechnol. 2017;45:731–739. doi: 10.1080/21691401.2016.1261033. [DOI] [PubMed] [Google Scholar]
- 295.Fogarassy G., Vathy-Fogarassy Á., Kenessey I., Kásler M., Forster T. Risk prediction model for long-term heart failure incidence after epirubicin chemotherapy for breast cancer—a real-world data-based, nationwide classification analysis. Int J Cardiol. 2019;285:47–52. doi: 10.1016/j.ijcard.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 296.Nanegrungsunk D., Apaijai N., Yarana C., Sripetchwandee J., Limpastan K., Watcharasaksilp W., et al. Bevacizumab is superior to temozolomide in causing mitochondrial dysfunction in human brain tumors. Neurol Res. 2016;38:285–293. doi: 10.1080/01616412.2015.1114233. [DOI] [PubMed] [Google Scholar]
- 297.Sabet N.S., Atashbar S., Khanlou E.M., Kahrizi F., Salimi A. Curcumin attenuates bevacizumab-induced toxicity via suppressing oxidative stress and preventing mitochondrial dysfunction in heart mitochondria. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1447–1457. doi: 10.1007/s00210-020-01853-x. [DOI] [PubMed] [Google Scholar]
- 298.Wafi A.M., Hong J., Rudebush T.L., Yu L., Hackfort B., Wang H., et al. Curcumin improves exercise performance of mice with coronary artery ligation-induced HFrEF: Nrf2 and antioxidant mechanisms in skeletal muscle. J Appl Physiol. 2019;126:477–486. doi: 10.1152/japplphysiol.00654.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yu Z., Efstathiou N.E., Correa V., Chen X., Ishihara K., Iesato Y., et al. Receptor interacting protein 3 kinase, not 1 kinase, through MLKL-mediated necroptosis is involved in UVA-induced corneal endothelium cell death. Cell Death Discov. 2021;7:366. doi: 10.1038/s41420-021-00757-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Gorabi A.M., Hajighasemi S., Kiaie N., Rosano G.M.C., Sathyapalan T., Al-Rasadi K., et al. Anti-fibrotic effects of curcumin and some of its analogues in the heart. Heart Fail Rev. 2020;25:731–743. doi: 10.1007/s10741-019-09854-6. [DOI] [PubMed] [Google Scholar]
- 301.Pang X.F., Zhang L.H., Bai F., Wang N.P., Garner R.E., McKallip R.J., et al. Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Des Dev Ther. 2015;9:6043–6054. doi: 10.2147/DDDT.S95333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Maideen N.M.P., Rajkapoor B., Muthusamy S., Ramanathan S., Thangadurai S.A., Sughir A.A. A review on pharmacokinetic and pharmacodynamic drug interactions of adrenergic β-blockers with clinically relevant drugs—an overview. Curr Drug Metab. 2021;22:672–682. doi: 10.2174/1389200222666210614112529. [DOI] [PubMed] [Google Scholar]
- 303.Dastani M., Bigdelu L., Hoseinzadeh M., Rahimi H.R., Karimani A., Hooshang Mohammadpour A., et al. The effects of curcumin on the prevention of atrial and ventricular arrhythmias and heart failure in patients with unstable angina: a randomized clinical trial. Avicenna J Phytomed. 2019;9:1–9. [PMC free article] [PubMed] [Google Scholar]
- 304.Tian Y., Chang S., Xu J., Gong P., Yu B., Qi J. Investigation of the effective components inhibited macrophage foam cell formation in Ophiopogonis Radix. J Ethnopharmacol. 2022;283 doi: 10.1016/j.jep.2021.114678. [DOI] [PubMed] [Google Scholar]
- 305.Tang X., Lin Y., Wang Y., Gao Y. Effects of ophiopogonin D on fatty acid metabolic enzymes in cardiomyocytes. Chin J Chin Mater Med. 2021;46:3672–3677. doi: 10.19540/j.cnki.cjcmm.20210311.401. [DOI] [PubMed] [Google Scholar]
- 306.Zhang Y.X., Qu S.S., Zhang L.H., Gu Y.Y., Chen Y.H., Huang Z.Y., et al. The role of ophiopogonin D in atherosclerosis: impact on lipid metabolism and gut microbiota. Am J Chin Med. 2021;49:1449–1471. doi: 10.1142/S0192415X21500683. [DOI] [PubMed] [Google Scholar]
- 307.Zhang Y.Y., Meng C., Zhang X.M., Yuan C.H., Wen M.D., Chen Z., et al. Ophiopogonin D attenuates doxorubicin-induced autophagic cell death by relieving mitochondrial damage in vitro and in vivo. J Pharmacol Exp Ther. 2015;352:166–174. doi: 10.1124/jpet.114.219261. [DOI] [PubMed] [Google Scholar]
- 308.Li W., Ji L., Tian J., Tang W., Shan X., Zhao P., et al. Ophiopogonin D alleviates diabetic myocardial injuries by regulating mitochondrial dynamics. J Ethnopharmacol. 2021;271 doi: 10.1016/j.jep.2021.113853. [DOI] [PubMed] [Google Scholar]
- 309.You W.T., Zhou T., Ma Z.C., Liang Q.D., Xiao C.R., Tang X.L., et al. Ophiopogonin D maintains Ca2+ homeostasis in rat cardiomyocytes in vitro by upregulating CYP2J3/EETs and suppressing ER stress. Acta Pharmacol Sin. 2016;37:368–381. doi: 10.1038/aps.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Xu X., Li R., Chen G., Hoopes S.L., Zeldin D.C., Wang D.W. The role of cytochrome P450 epoxygenases, soluble epoxide hydrolase, and epoxyeicosatrienoic acids in metabolic diseases. Adv Nutr. 2016;7:1122–1128. doi: 10.3945/an.116.012245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wang X., Ni L., Yang L., Duan Q., Chen C., Edin M.L., et al. CYP2J2-derived epoxyeicosatrienoic acids suppress endoplasmic reticulum stress in heart failure. Mol Pharmacol. 2014;85:105–115. doi: 10.1124/mol.113.087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wang J., You W., Wang N., Zhou W., Ge Y., Ma Z., et al. Ophiopogonin D increases SERCA2a interaction with phospholamban by promoting CYP2J3 upregulation. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/8857906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Huang X., Wang Y., Wang Y., Yang L., Wang J., Gao Y. Ophiopogonin D reduces myocardial ischemia–reperfusion injury via upregulating CYP2J3/EETs in rats. Cell Physiol Biochem. 2018;49:1646–1658. doi: 10.1159/000493500. [DOI] [PubMed] [Google Scholar]
- 314.Huang X., Wang Y., Wang Y., Gao Y. Effect of ophiopogonin D in resisting vascular endothelial cell apoptosis induced by Ang II through up-regulating CYP2J2/EETs. Chin J Chin Mater Med. 2018;43:377–384. doi: 10.19540/j.cnki.cjcmm.20171027.019. [DOI] [PubMed] [Google Scholar]
- 315.Huang X., Wang Y., Zhang Z., Wang Y., Chen X., Wang Y., et al. Ophiopogonin D and EETs ameliorate Ang II-induced inflammatory responses via activating PPARα in HUVECs. Biochem Biophys Res Commun. 2017;490:123–133. doi: 10.1016/j.bbrc.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 316.Wang Y., Huang X., Ma Z., Wang Y., Chen X., Gao Y. Ophiopogonin D alleviates cardiac hypertrophy in rat by upregulating CYP2J3 in vitro and suppressing inflammation in vivo. Biochem Biophys Res Commun. 2018;503:1011–1019. doi: 10.1016/j.bbrc.2018.06.110. [DOI] [PubMed] [Google Scholar]
- 317.Zhang J., Chen J., Wang D., Hu Y., Zhang C., Qin T., et al. Immune-enhancing activity comparison of sulfated ophiopogonpolysaccharide and sulfated jujube polysaccharide. Int J Biol Macromol. 2013;52:212–217. doi: 10.1016/j.ijbiomac.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 318.Wang S., Zhang Z., Lin X., Xu D.S., Feng Y., Ding K. A polysaccharide, MDG-1, induces S1P1 and bFGF expression and augments survival and angiogenesis in the ischemic heart. Glycobiology. 2010;20:473–484. doi: 10.1093/glycob/cwp199. [DOI] [PubMed] [Google Scholar]
- 319.Wang H., Guo S., Peng Z., Wang C., Duan R., Dong T., et al. Ophiopogon polysaccharide promotes the in vitro metabolism of ophiopogonins by human gut microbiota. Molecules. 2019;24:2286. doi: 10.3390/molecules24162886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Tsai Y.C., Cheng Y.B., Lo I.W., Cheng H.H., Lin C.J., Hwang T.L., et al. Seven new sesquiterpenoids from the fruits of Schisandra sphenanthera. Chem Biodivers. 2014;11:1053–1068. doi: 10.1002/cbdv.201300259. [DOI] [PubMed] [Google Scholar]
- 321.Wang X., Yu J., Li W., Wang C., Li H., Ju W., et al. Characteristics and antioxidant activity of lignans in Schisandra chinensis and Schisandra sphenanthera from different locations. Chem Biodivers. 2018;15 doi: 10.1002/cbdv.201800030. [DOI] [PubMed] [Google Scholar]
- 322.Zhang X., Zhao Y., Bai D., Yuan X., Cong S. Schizandrin protects H9c2 cells against lipopolysaccharide-induced injury by downregulating Smad3. J Biochem Mol Toxicol. 2019;33 doi: 10.1002/jbt.22301. [DOI] [PubMed] [Google Scholar]
- 323.Yang M., Jiang X.C., Wang L., Cui D.A., Zhang J.Y., Wang X.R., et al. Schisandrin protects against norepinephrine-induced myocardial hypertrophic injury by inhibiting the JAK2/STAT3 signaling pathway. Evid Based Complement Altern Med. 2021;2021 doi: 10.1155/2021/8129512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zhao B., Li G.P., Peng J.J., Ren L.H., Lei L.C., Ye H.M., et al. Schizandrin B attenuates hypoxia/reoxygenation injury in H9c2 cells by activating the AMPK/Nrf2 signaling pathway. Exp Ther Med. 2021;21:220. doi: 10.3892/etm.2021.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhao X., Xiang Y., Cai C., Zhou A., Zhu N., Zeng C. Schisandrin B protects against myocardial ischemia/reperfusion injury via the PI3K/Akt pathway in rats. Mol Med Rep. 2018;17:556–561. doi: 10.3892/mmr.2017.7926. [DOI] [PubMed] [Google Scholar]
- 326.Zhang W., Sun Z., Meng F. Schisandrin B ameliorates myocardial ischemia/reperfusion injury through attenuation of endoplasmic reticulum stress-induced apoptosis. Inflammation. 2017;40:1903–1911. doi: 10.1007/s10753-017-0631-4. [DOI] [PubMed] [Google Scholar]
- 327.Han J., Shi X., Du Y., Shi F., Zhang B., Zheng Z., et al. Schisandrin C targets Keap1 and attenuates oxidative stress by activating Nrf2 pathway in Ang II-challenged vascular endothelium. Phytother Res. 2019;33:779–790. doi: 10.1002/ptr.6271. [DOI] [PubMed] [Google Scholar]
- 328.Shi H., Tang H., Ai W., Zeng Q., Yang H., Zhu F., et al. Schisandrin B antagonizes cardiotoxicity induced by pirarubicin by Inhibiting mitochondrial permeability transition pore (mPTP) opening and decreasing cardiomyocyte apoptosis. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.733805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Martens M.D., Seshadri N., Nguyen L., Chapman D., Henson E.S., Xiang B., et al. Misoprostol treatment prevents hypoxia-induced cardiac dysfunction through a 14-3-3 and PKA regulatory motif on Bnip3. Cell Death Dis. 2021;12:1105. doi: 10.1038/s41419-021-04402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Gong S., Liu J., Wan S., Yang W., Zhang Y., Yu B., et al. Schisandrol A attenuates myocardial ischemia/reperfusion-induced myocardial apoptosis through upregulation of 14-3-3θ. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/5541753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Liu X., Cong L., Wang C., Li H., Zhang C., Guan X., et al. Pharmacokinetics and distribution of schisandrol A and its major metabolites in rats. Xenobiotica. 2019;49:322–331. doi: 10.1080/00498254.2017.1418543. [DOI] [PubMed] [Google Scholar]
- 332.Lai Q., Yuan G.Y., Wang H., Liu Z.L., Kou J.P., Yu B.Y., et al. Exploring the protective effects of schizandrol A in acute myocardial ischemia mice by comprehensive metabolomics profiling integrated with molecular mechanism studies. Acta Pharmacol Sin. 2020;41:1058–1072. doi: 10.1038/s41401-020-0377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Chun J.N., Park S., Lee S., Kim J.K., Park E.J., Kang M., et al. Schisandrol B and schisandrin B inhibit TGFβ1-mediated NF-κB activation via a Smad-independent mechanism. Oncotarget. 2018;9:3121–3130. doi: 10.18632/oncotarget.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Ai F., Guo Q.H., Yu B., Li W., Guo X., Chen Z. Schisandrin B attenuates pressure overload-induced cardiac remodeling in mice by inhibiting the MAPK signaling pathway. Exp Ther Med. 2019;18:4645–4652. doi: 10.3892/etm.2019.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Shao M., Yang W., Han G. Protective effects on myocardial infarction model: delivery of schisandrin B using matrix metalloproteinase-sensitive peptide-modified, PEGylated lipid nanoparticles. Int J Nanomed. 2017;12:7121–7130. doi: 10.2147/IJN.S141549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Stancill J.S., Corbett J.A. The role of thioredoxin/peroxiredoxin in the β-cell defense against oxidative damage. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.718235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Shi X., Han B., Zhang B., Chu Z., Zhang X., Lu Q., et al. Schisandra chinensis polysaccharides prevent cardiac hypertrophy by dissociating thioredoxin-interacting protein/thioredoxin-1 complex and inhibiting oxidative stress. Biomed Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111688. [DOI] [PubMed] [Google Scholar]
- 338.Zhang Y., Yang C., Huang Z., Liu Y., Chen Y., Liu Y., et al. Study on dynamic variation patterns of 13 alkaloids in Aconiti Lateralis Radix Praeparata during decocting process. Chin J Pharm Anal. 2015;35:16–23. [Google Scholar]
- 339.Zhang X.C., Zheng Q.G.Y.J., Rong R., Yang Y. Research progress on structure and activity of C19 diterpeneoid alkaloids from Aconiti Lateralis Radix Praeparata. Chin Tradit Herb Drugs. 2020;51:531–541. [Google Scholar]
- 340.Zhang F., Cai L., Zhang J., Qi X., Lu C. Aconitine-induced cardiac arrhythmia in human induced pluripotent stem cell-derived cardiomyocytes. Exp Ther Med. 2018;16:3497–3503. doi: 10.3892/etm.2018.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Wang M., Wang R., Sun H., Sun G., Sun X. Ginsenoside Rb1 ameliorates cardiotoxicity triggered by aconitine via inhibiting calcium overload and pyroptosis. Phytomedicine. 2021;83 doi: 10.1016/j.phymed.2021.153468. [DOI] [PubMed] [Google Scholar]
- 342.Wang N.N., Wang J., Tan H.L., Wang Y.G., Gao Y., Ma Z.C. Aconitine ameliorates cardiomyocyte hypertrophy induced by angiotensin II. Chin J Chin Mater Med. 2019;44:1642–1647. doi: 10.19540/j.cnki.cjcmm.20190117.002. [DOI] [PubMed] [Google Scholar]
- 343.Wu M.P., Zhang Y.S., Zhou Q.M., Xiong J., Dong Y.R., Yan C. Higenamine protects ischemia/reperfusion induced cardiac injury and myocyte apoptosis through activation of β2-AR/PI3K/AKT signaling pathway. Pharmacol Res. 2016;104:115–123. doi: 10.1016/j.phrs.2015.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Peng F., Zhang N., Wang C., Wang X., Huang W., Peng C., et al. Aconitine induces cardiomyocyte damage by mitigating BNIP3-dependent mitophagy and the TNFα–NLRP3 signalling axis. Cell Prolif. 2020;53 doi: 10.1111/cpr.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Jeon S.Y., Jeong W., Park J.S., You Y., Ahn H.J., Kim S., et al. Clinical relationship between blood concentration and clinical symptoms in aconitine intoxication. Am J Emerg Med. 2021;40:184–187. doi: 10.1016/j.ajem.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 346.Yan P., Mao W., Jin L., Fang M., Liu X., Lang J., et al. Crude radix Aconiti lateralis preparata (Fuzi) with Glycyrrhiza reduces inflammation and ventricular remodeling in mice through the TLR4/NF-κB pathway. Mediators Inflamm. 2020;2020 doi: 10.1155/2020/5270508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Li J., Zhang S.H., He D., Wang J.F., Li J.Q. Paeoniflorin reduced the cardiotoxicity of aconitine in h9c2 cells. J Biol Regul Homeost Agents. 2019;33:1425–1436. doi: 10.23812/19-257A. [DOI] [PubMed] [Google Scholar]
- 348.Liu Q., Zhou C., Xin J.J., Zhao Y.X., Chen A.L., Zhang W.X., et al. Observation on facilitation and attenuation effect of electroacupuncture combined with aconitine for treatment of heart failure in rats. Zhen Ci Yan Jiu. 2021;46:570–574. doi: 10.13702/j.1000-0607.200804. [DOI] [PubMed] [Google Scholar]
- 349.Zhou C., Liu Q., Xin J.J., Zhao Y.X., Chen A.L., Lu F.Y., et al. Preliminary study on mechanism of electroacupuncture with the involvement of SERCA2a/PLB on the synergistic and attenuated effect of aconitine in treatment of heart failure. Zhongguo Zhen Jiu. 2021;41:1029–1035. doi: 10.13703/j.0255-2930.20200729-k0001. [DOI] [PubMed] [Google Scholar]
- 350.Wen J., Zhang L., Wang J., Wang J., Wang L., Wang R., et al. Therapeutic effects of higenamine combined with [6]-gingerol on chronic heart failure induced by doxorubicin via ameliorating mitochondrial function. J Cell Mol Med. 2020;24:4036–4050. doi: 10.1111/jcmm.15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Chen Y.L., Zhuang X.D., Xu Z.W., Lu L.H., Guo H.L., Wu W.K., et al. Higenamine combined with [6]-gingerol suppresses doxorubicin-triggered oxidative stress and apoptosis in cardiomyocytes via upregulation of PI3K/Akt Pathway. Evid Based Complement Altern Med. 2013;2013 doi: 10.1155/2013/970490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Xu X., Xie X., Zhang H., Wang P., Li G., Chen J., et al. Water-soluble alkaloids extracted from Aconiti Radix lateralis praeparata protect against chronic heart failure in rats via a calcium signaling pathway. Biomed Pharmacother. 2021;135 doi: 10.1016/j.biopha.2020.111184. [DOI] [PubMed] [Google Scholar]
- 353.Yuan R., Zhao W., Wang Q.Q., He J., Han S., Gao H., et al. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol Res. 2021;170 doi: 10.1016/j.phrs.2021.105748. [DOI] [PubMed] [Google Scholar]
- 354.Cheng W.X., Liu Y.Z., Meng X.B., Zheng Z.T., Li L.L., Ke L.Q., et al. PLGA/β-TCP composite scaffold incorporating cucurbitacin B promotes bone regeneration by inducing angiogenesis. J Orthop Translat. 2021;31:41–51. doi: 10.1016/j.jot.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Khallouki F., Owen R., Silvente-Poirot S., Poirot M. Bryonolic acid blocks cancer cell clonogenicity and invasiveness through the inhibition of fatty acid: cholesteryl ester formation. Biomedicines. 2018;6:21. doi: 10.3390/biomedicines6010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Chu D., Zhang Z. Trichosanthis pericarpium aqueous extract protects H9c2 cardiomyocytes from hypoxia/reoxygenation injury by regulating PI3K/Akt/NO pathway. Molecules. 2018;23:2409. doi: 10.3390/molecules23102409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Xiao Y., Yang Z., Wu Q.Q., Jiang X.H., Yuan Y., Chang W., et al. Cucurbitacin B protects against pressure overload induced cardiac hypertrophy. J Cell Biochem. 2017;118:3899–3910. doi: 10.1002/jcb.26041. [DOI] [PubMed] [Google Scholar]
- 358.Gatbonton-Schwager T.N., Letterio J.J., Tochtrop G.P. Bryonolic acid transcriptional control of anti-inflammatory and antioxidant genes in macrophages in vitro and in vivo. J Nat Prod. 2012;75:591–598. doi: 10.1021/np200823p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Ahmed L., Zhang Y., Block E., Buehl M., Corr M.J., Cormanich R.A., et al. Molecular mechanism of activation of human musk receptors OR5AN1 and OR1A1 by (R)-muscone and diverse other musk-smelling compounds. Proc Natl Acad Sci U S A. 2018;115:3950–3958. doi: 10.1073/pnas.1713026115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Ben Khemis I., Mechi N., Ben Lamine A. Stereochemical study of mouse muscone receptor MOR215-1 and vibrational theory based on statistical physics formalism. Prog Biophys Mol Biol. 2018;136:54–60. doi: 10.1016/j.pbiomolbio.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 361.Wu Q., Li H., Wu Y., Shen W., Zeng L., Cheng H., et al. Protective effects of muscone on ischemia–reperfusion injury in cardiac myocytes. J Ethnopharmacol. 2011;138:34–39. doi: 10.1016/j.jep.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 362.Saxena A., Russo I., Frangogiannis N.G. Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl Res. 2016;167:152–166. doi: 10.1016/j.trsl.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Wei C.J., Hua F., Chen Y.H., Zhang Z.W., Shen Z.Y. Muscone alleviates myocardial ischemia–reperfusion injury via inhibition of oxidative stress and enhancement of SIRT3. J Biol Regul Homeost Agents. 2021;35:85–96. doi: 10.23812/20-101-A. [DOI] [PubMed] [Google Scholar]
- 364.Du Y., Gu X., Meng H., Aa N., Liu S., Peng C., et al. Muscone improves cardiac function in mice after myocardial infarction by alleviating cardiac macrophage-mediated chronic inflammation through inhibition of NF-κB and NLRP3 inflammasome. Am J Transl Res. 2018;10:4235–4246. [PMC free article] [PubMed] [Google Scholar]
- 365.Du Y., Ge Y., Xu Z., Aa N., Gu X., Meng H., et al. Hypoxia-inducible factor 1 alpha (HIF-1α)/vascular endothelial growth factor (VEGF) pathway participates in angiogenesis of myocardial infarction in muscone-treated mice: preliminary study. Med Sci Monit. 2018;24:8870–8877. doi: 10.12659/MSM.912051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Li M., Yao L., Chen H., Ni X., Xu Y., Dong W., et al. Chiral toxicity of muscone to embryonic zebrafish heart. Aquat Toxicol. 2020;222 doi: 10.1016/j.aquatox.2020.105451. [DOI] [PubMed] [Google Scholar]
- 367.Ni L., Miao P., Jiang J., Wan F., Li J., Ai M., et al. Glycyrrhiza uralensis promote the metabolism of toxic components of Aconitum carmichaeli by CYP3A and alleviate the development of chronic heart failure. PLoS One. 2022;17 doi: 10.1371/journal.pone.0270069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Chen Z.Y., Wei X.Y., Qiu Z.D., Huang Y., Tan T., Feng Y.L., et al. Compatibility of Fuzi and Ginseng significantly increase the exposure of aconitines. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.883898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Chen M., Wang M., Yang Q., Wang M., Wang Z., Zhu Y., et al. Antioxidant effects of hydroxysafflor yellow A and acetyl-11-keto-β-boswellic acid in combination on isoproterenol-induced myocardial injury in rats. Int J Mol Med. 2016;37:1501–1510. doi: 10.3892/ijmm.2016.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Liu Y.X., Yu H.S., Kang L.P., Zou P., Jia J.J., Wang H.T., et al. Chemical components of active fractions from Qili Qiangxin Capsula. Chin Tradit Herb Drugs. 2010;41:1060–1065. [Google Scholar]
- 371.Xing L., Jiang M., Dong L., Gao J., Hou Y., Bai G., et al. Cardioprotective effects of the YiQiFuMai injection and isolated compounds on attenuating chronic heart failure via NF-κB inactivation and cytokine suppression. J Ethnopharmacol. 2013;148:239–245. doi: 10.1016/j.jep.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 372.Zhang Y., Yu J., Zhang W., Wang Y., He Y., Zhou S., et al. An integrated evidence-based targeting strategy for determining combinatorial bioactive ingredients of a compound herbal medicine Qishen Yiqi dripping pills. J Ethnopharmacol. 2018;219:288–298. doi: 10.1016/j.jep.2018.02.041. [DOI] [PubMed] [Google Scholar]
- 373.Wang Y., Liu X., Zhang W., He S., Zhang Y., Orgah J., et al. Synergy of “Yiqi” and “Huoxue” components of QishenYiqi formula in ischemic stroke protection via lysosomal/inflammatory mechanisms. J Ethnopharmacol. 2022;293 doi: 10.1016/j.jep.2022.115301. [DOI] [PubMed] [Google Scholar]
- 374.Yan L., Pan C.S., Liu Y.Y., Cui Y.C., Hu B.H., Chang X., et al. The composite of 3,4-dihydroxyl-phenyl lactic acid and notoginsenoside R1 attenuates myocardial ischemia and reperfusion injury through regulating mitochondrial respiratory chain. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.538962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Han J.Y. Scientific connotation of cardiac Qi deficiency and stasis of blood, and the mechanism of Qishen Yiqi Dropping Pills in invigorating Qi and activating Blood. World Sci Tech Modern Tradit Chin Med. 2019;21:139–147. [Google Scholar]
- 376.Yang W., Lai Q., Zhang L., Zhang Y., Zhang Y., Yu B., et al. Mechanisms dissection of the combination GRS derived from ShengMai preparations for the treatment of myocardial ischemia/reperfusion injury. J Ethnopharmacol. 2021;264 doi: 10.1016/j.jep.2020.113381. [DOI] [PubMed] [Google Scholar]
- 377.Li F., Fan X.X., Chu C., Zhang Y., Kou J.P., Yu B.Y. A strategy for optimizing the combination of active components based on Chinese Medicinal Formula Sheng-Mai-San for myocardial ischemia. Cell Physiol Biochem. 2018;45:1455–1471. doi: 10.1159/000487572. [DOI] [PubMed] [Google Scholar]
- 378.Hong L.L., Yang C.Y., Zhao Y., Wang H.S., Chen W.D., Cheng X.Y. Progress in pharmacodynamic basis, mechanism, and prediction of Q-markers of Zhenwu Decoction in treatment of chronic heart failure. Chin J Chin Mater Med. 2021;46:5512–5521. doi: 10.19540/j.cnki.cjcmm.20210705.203. [DOI] [PubMed] [Google Scholar]
- 379.Ziaeian B., Fonarow G.C. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–378. doi: 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Lin Y., Fu S., Yao Y., Li Y., Zhao Y., Luo L. Heart failure with preserved ejection fraction based on aging and comorbidities. J Transl Med. 2021;19:291. doi: 10.1186/s12967-021-02935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Cao X., Liu H., Zhou M., Chen X., Long D. Comparative efficacy of five Chinese medicine injections for treating dilated cardiomyopathy with heart failure: a Bayesian network meta-analysis. J Ethnopharmacol. 2022;282 doi: 10.1016/j.jep.2021.114604. [DOI] [PubMed] [Google Scholar]
- 382.Cao X., Zhou M., Liu H., Chen X., Li X., Jia S. Clinical efficacy and safety of Shensong Yangxin capsule-amiodarone combination on heart failure complicated by ventricular arrhythmia: a meta-analysis of randomized controlled trials. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.613922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Li X.X., Wu Y., Fan Z.J., Cui J., Li D., Lin Q., et al. Qishen Taohong Granule as adjuvant therapy for improving cardiac function and quality of life in patients with chronic heart failure: a randomized controlled trial. Chin J Integr Med. 2022;28:12–19. doi: 10.1007/s11655-021-2866-z. [DOI] [PubMed] [Google Scholar]
- 384.Han J.Y., Li Q., Ma Z.Z., Fan J.Y. Effects and mechanisms of compound Chinese medicine and major ingredients on microcirculatory dysfunction and organ injury induced by ischemia/reperfusion. Pharmacol Ther. 2017;177:146–173. doi: 10.1016/j.pharmthera.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 385.Fan X.G., Tang N., Ji Y.X., Zhang Y.Z., Jiang L., Huang G.H., et al. Explration of the essence of "endogenous turbidity" in Chinese medicine. Chin J Integr Tradit West Med. 2015;35:1011–1014. [PubMed] [Google Scholar]
- 386.Wu X., Qi X., Lu Y., Lin C., Yuan Y., Zhu Q., et al. Liguzinediol protects against cardiac fibrosis in rats in vivo and in vitro. Biomed Pharmacother. 2016;80:260–267. doi: 10.1016/j.biopha.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 387.Zhang Y., Yu L., Jin W., Fan H., Li M., Zhou T., et al. Reducing toxicity and increasing efficiency: aconitine with liquiritin and glycyrrhetinic acid regulate calcium regulatory proteins in rat myocardial cell. Afr J Tradit Complement Altern Med. 2017;14:69–79. doi: 10.21010/ajtcam.v14i4.9. [DOI] [PMC free article] [PubMed] [Google Scholar]