Abstract
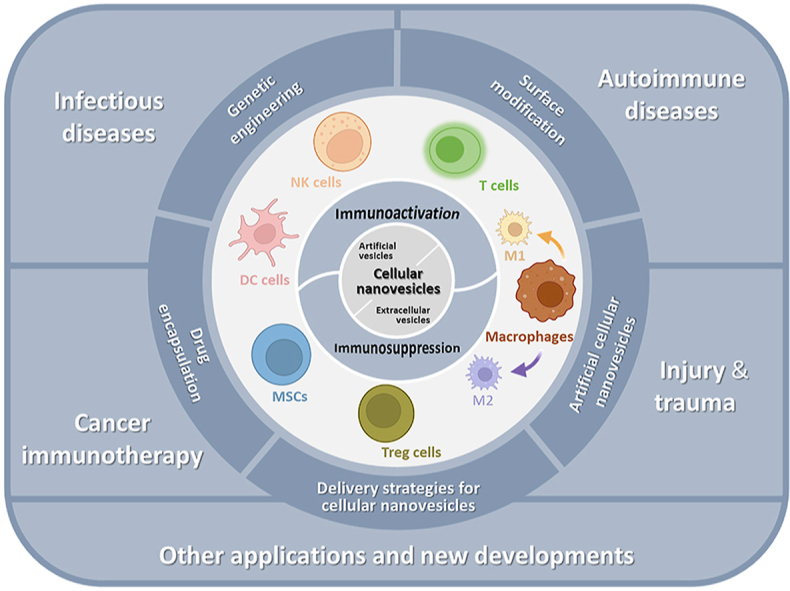
Cellular nanovesicles which are referred to as cell-derived, nanosized lipid bilayer structures, have emerged as a promising platform for regulating immune responses. Owing to their outstanding advantages such as high biocompatibility, prominent structural stability, and high loading capacity, cellular nanovesicles are suitable for delivering various immunomodulatory molecules, such as small molecules, nucleic acids, peptides, and proteins. Immunomodulation induced by cellular nanovesicles has been exploited to modulate immune cell behaviors, which is considered as a novel cell-free immunotherapeutic strategy for the prevention and treatment of diverse diseases. Here we review emerging concepts and new advances in leveraging cellular nanovesicles to activate or suppress immune responses, with the aim to explicate their applications for immunomodulation. We overview the general considerations and principles for the design of engineered cellular nanovesicles with tailored immunomodulatory activities. We also discuss new advances in engineering cellular nanovesicles as immunotherapies for treating major diseases.
Key words: Immunomodulation, Cellular nanovesicle, Extracellular vesicle, Exosome, Infectious disease, Autoimmune disease, Immunotherapy, Immune cell
Graphical abstract
Cellular nanovesicles have emerged as a promising tool to modulate immune responses. This review discusses the biological basis, engineering strategies, and recent advances in leveraging cellular nanovesicles for therapeutic immunomodulation.
1. Introduction
Cellular nanovesicles are cell-derived nano-sized heterogeneous structures of lipid bilayers from natural or engineered cells1. As derivatives of cells, cellular nanovesicles contain components from the parent cells such as signaling molecules consisting of proteins, nucleic acids and lipids. These molecules, together with the nanosized structures, can be conveyed for intercellular communication2, 3, 4. In this review, “cellular nanovesicles” refer to cell-derived nanovesicles with a size of 30–1000 nm including extracellular vesicles (EVs) and synthetic/semi-synthetic cell membrane-derived nanostructures. Despite cellular nanovesicles being intensely exploited for targeted drug delivery, their critical roles as a novel immunotherapeutic approach for treating diseases have not been comprehensively discussed5, 6, 7. Originally, researchers identified the larger EVs by electron microscopy, which allowed the discovery of their ability to transfer cytoplasmic RNAs between cells8. Until the late twentieth century, dapper exosomes (a type of EVs) were investigated from the secreta of B lymphocytes which have immunomodulatory functions to carry major histocompatibility complexes (MHC)9. However, exosomes were initially regarded as an externalized nanovesicle with metabolic waste from sheep reticulocytes by invagination of cell membrane. Along with comprehension of the structure and capabilities of cellular nanovesicles, their generation and secretion process therewith functions are close to the lucidity10, 11, 12, 13. Owing to the stability and tolerance of the host cells, cellular nanovesicles with feasible cavities and engineered envelopes can be isolated as an effective drug carrier for immunomodulatory therapy.
The immune system provides an excellent screen for protecting individuals from external pathogens14. With non-specific recognition, foreign pathogens can be endocytosed by congenital leukocytes. Antigen-presenting cells (APCs) such as macrophages and dendritic cells (DCs) present foreign antigens to T cells15, whereas individual differences lead to the diversity in immune system efficiency15,16. On occasion pathogens can escape from the capture by the immune cells17. Cancer cells can express transmembrane proteins such as PD-L1, which can effectively bind to PD-1 resulting in the suppression of T cells18. Moreover, compared with the adiaphoria of the immune system, hypersensitivity causes an over-attack on intrinsic or transplanted organs by innocuous antigens leading to tissue damage19. Immunomodulatory drugs can partially regulate the immune system20,21, however, their short-term and insufficient therapeutic effects limit their utility in clinical settings22. Owing to their low toxicity, high structural stability, high loading capacity and tunability, cellular nanovesicles have emerged as a promising platform for delivering intrinsic or exogenous immunomodulatory molecules to regulate immune responses23, 24, 25, 26. Cellular nanovesicles from different cell sources have diverse immunoregulatory functions, such as inhibiting the invasion and metastasis of cancer cells, promoting vascularization, and stimulating antigen presentation27, 28, 29, 30, 31. By specifically interacting with recipient cells, delivery of immunomodulatory molecules can be realized using cellular nanovesicles32. For the exploration of cellular nanovesicles besides their unique characteristics, it has been confirmed that synthetic/semi-synthetic nanovesicles generated by fusing natural nanovesicles with functional lipids can compensate for their drawbacks33. Additionally, this works by engineering cellular nanovesicles to specialize with novel functions and load with disease-relevant substrates for cell-specific targeting and effect on local immune responses34.
In this review, we outline the emerging concepts and key advances in harnessing cellular nanovesicles as a delivery approach for therapeutic immunomodulation (Fig. 1). We focus on detailing the immunomodulatory functions of cellular nanovesicles from different cell types for either immunoactivation or immunosuppression. Further, we critically discuss the key principles and strategies for engineering cellular nanovesicles as immunomodulatory therapies. The applications of such cellular nanovesicles in managing major diseases are also discussed.
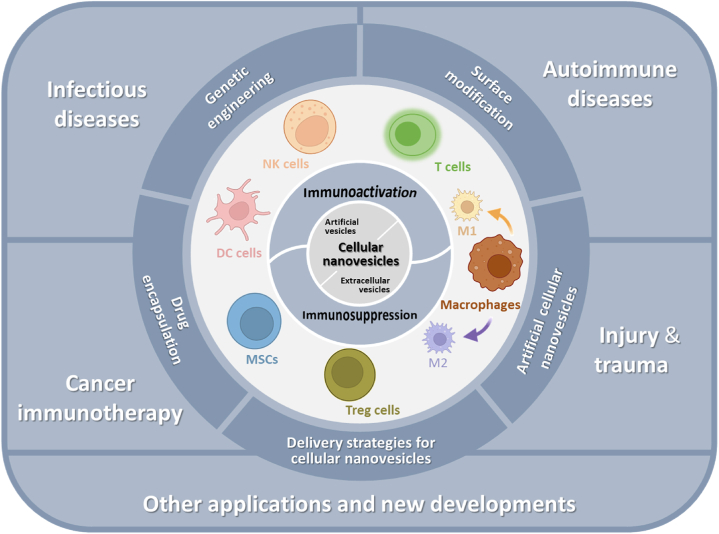
Figure 1.
Overview of cellular nanovesicles derived from various cell types with different modification strategies for disease treatment via immunomodulation. Schematic was created using templates from BioRender.
2. Biological basis for cellular nanovesicle-mediated immunomodulation
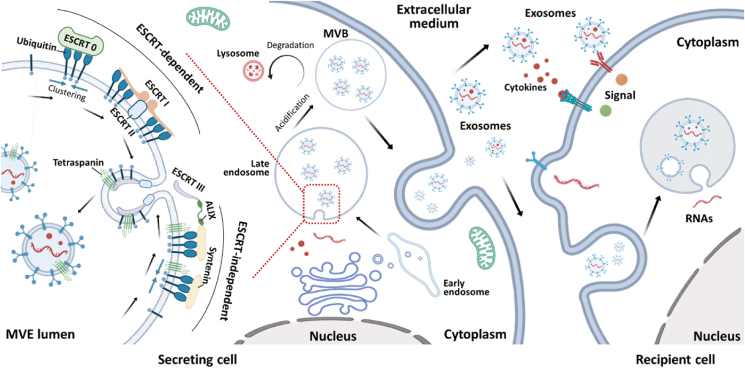
Natural cellular nanovesicles (e.g., EVs) are actively released by mammalian cells with the endomembrane system for secretion of factors, intercellular communication, transmission of signaling molecules, and cell metabolism regulation35. Artificial synthetic/semi-synthetic cellular nanovesicles, which are cell membrane-derived nanoparticles, can be obtained in a rapid and effective process with modification of various desired functions. For natural EVs, their secretions are deemed by constitutive secretory pathways with the stimulation by other cellular biomarker proteins, secreted factors, or chemicals such as calcium ionophores36, 37, 38. Generally, exosomes are high-profile EVs that are produced by invagination of the membrane to form intraluminal vesicles. Multi-vesicle bodies (MVBs) with subcellular structure are generated from the late endosome. The mechanisms for MVB formation fall into two categories; one is the endosomal sorting complex required for transport (ESCRT)-dependent pathway and the other is the ESCRT-independent pathway39,40 (Fig. 2). The ESCRT-dependent pathway is activated by a set of cytoplasmic proteins (four ESCRT complexes) that initiates with the ubiquitination of the cargo protein. The clathrin molecules cluster with the ubiquitinated cargos which is recognized by the ESCRT-0 complex. Then the ubiquitinated cargoes sequentially bind to recruited ESCRT-I and ESCRT-II to form a microdomain, triggering membrane involution. Ultimately, membrane invagination facilitates the release of MVBs via recognition of the disulfide bond by the tumor susceptibility gene 101 (TSG101) in ESCRT-I and the circular filamentous ESCRT-III39,41. However, MVBs can also be formed in the absence of the ESCRT complexes. The apoptosis-linked-gene 2 interacting protein X (ALIX) can directly bind to the intracellular adaptor protein Syntenin which bypasses the ESCRT-0, -I and -II, bridging with the vacuolar protein sorting-associated protein 32 (VPS32) in the ESCRT-III complex to be involved in exosome formation32,42,43. Notably, cytoplasmic sorting of MVBs produced via the ESCRT-independent pathway is regulated by tetraspanin6,42,44. Alternatively, MVBs generated from late endosomes can be fused with lysosomes for degradation and recycling. The other part of MVBs is fused with the plasma membrane, resulting in exosome secretion. Also, microvesicles can directly originate from the plasma membrane blebbing. After concentrating ubiquitinated cargo proteins on the membrane, the reflux of calcium ions promotes proteolytic activities and lipid translocation. Adenosine di-phosphate ribosylation factor 6 (ARF6) is activated to facilitate the abscission of actin for the release and formation of microvesicles from outward membrane blebbing45.
Figure 2.
Schematic showing the biogenesis of extracellular vesicles (EVs) that follow the ESCRT-dependent and ESCRT-independent pathways. After being secreted from parental cells, EVs interfere with the immunological behavior of recipient cells via fusing with recipient cells, endocytosis by recipient cells, and releasing signaling molecules into recipient cells. Schematic was created using templates from BioRender.
Released EVs transport biological information to peripheral target cells. EVs can be generated by immune and nonimmune cells, and then encapsulate and stabilize signaling molecules, such as cytokines, which are crucial in immunomodulation46, 47, 48. An interwoven network is established among immune cells for pathogens or cancer cells via EVs49,50. Generally, there are three different ways for EVs to interact with recipient cells for immunomodulation. First, biomarker proteins or RNAs on the surface of EVs directly interact with signaling molecules on the membrane of the recipient cells through ligand–receptor interactions. For example, EVs loaded with antigens can be recognized by APCs, and this is followed by antigen presentation to CD8+ T cells for immunoactivation41. In addition, APC-derived EVs enriched with MHC class I, MHC class II and other regulatory factors can modulate the activities of other immune cells51. Second, EVs can fuse with target cells to mediate immunomodulation. Many studies have shown that cellular nanovesicles from virus-infected cells can carry viral microRNA (miRNA) and fuse with recipient cells to regulate the expression of immunostimulatory genes52. They have also been found to contain viral proteins that induce an immune response41. The fusion event begins with the recognition and interaction between the surface proteins on EVs and target cells, such as syncytin and major facilitator superfamily domain 2a (MFSD2a)53. However, it still remains a challenge to directly observe the fusion process by current imaging technologies37. Third, cellular nanovesicles can be endocytosed by target cells, which also results in immunomodulation.
When EVs are released from their parent cells, they carry a range of molecules inducing lipids, mRNAs, miRNAs, and proteins54. Of these molecules, over 40 types of proteins are essential components that are relevant to the immunomodulatory function of EVs55. For example, the cluster of differentiation protein (CD) has shown interaction with other proteins on the targeting cells for activation of the downstream immune pathway; MHC I and II assist in the presentation of antigens to T cells; heat shock proteins (Hsp), such as Hsp 70 and Hsp 90, promote the binding of antigenic peptides to MHC, which is considered as an indispensable component for antigen presentation56,57. Besides of proteins, miRNA is one type of vital genetic information which is carried by cellular nanovesicles. Vast literature evidence has shown that miRNA can regulate gene expression and function of recipient cells58. miRNA is considered as an immunoactive or immunosuppressive effector on immune cells which affects the production of cytokines59.
EVs induce different immunomodulatory activities depending on their various disparate components. On one hand, EVs containing immunomodulatory molecules stimulate immune cells to produce proinflammatory factors showing immunoactive effects. For example, EVs secreted from bacteria-infected macrophages can stimulate neutrophils to produce tumor necrosis factor-α (TNF-α) and RANTES60. Mature DC-derived EVs regulate peripheral epithelial cells to generate monocyte chemoattractant protein (MCP), interleukin 8 (IL-8), TNF-α and RANTES as pro-inflammatory factors. As another example, the phagocytic capacity of macrophages can be regulated by the signal regulatory protein α (SIRPα) on EVs61; EVs with SIRPα interfere with the interaction between the SIRPα on bone marrow-derived macrophages (BMMs) and the CD47 on cancer cells, blocking the “do not eat signal” to elicit an immunotherapeutic efficacy62. On the other hand, immunosuppression can be critically initiated by EVs. For example, EVs with the MICA∗008 ligand downregulate the expression of natural killer group 2D (NKG2D) receptor, leading to a reduction in natural killer (NK) cell cytotoxicity63. EVs containing the MICA∗008 ligand have also been shown to regulate NKG2D in T cells with similar immunosuppressive effect64. Release of transforming growth factor beta (TGF-β) from EVs promotes differentiation of myeloid-derived suppressor cells (MDSCs) from bone marrow precursors and inhibits T-cell proliferation and function65.
3. Strategies to isolate cellular nanovesicles
Natural cellular nanovesicles (e.g., EVs) are usually secreted at a low quantity66; this is a major challenge hindering preclinical and clinical studies which require a scalable production of nanovesicles. Hence, there is a need to develop robust methods to produce and isolate nanovesicles. For the production of cellular nanovesicles, methods such as chemical-based (e.g., thrombin and calcium ionophore) and mechanical-based (e.g., shear stress) methods can stimulate parent cells to secret nanovesicles37. Additionally, several separation technologies including density-based67, 68, 69, size-based70,71, charged-based72, 73, 74, affinity-based methods75,76 and their combinations77, 78, 79 have been developed for efficient isolation of cellular nanovesicles.
Ultracentrifugation is one of the most widely used methods to isolate cellular nanovesicles; it is a density-based method by using a speed of >100,000×g80. The density of small cellular nanovesicles such as exosomes are approximately 1.2 g/mL which enables their separation from other larger vesicles81. Cellular nanovesicles isolated using this method can contain impurities, such as lipoprotein particles and protein complexes, which have a similar density to cellular nanovesicles81. Notably, the purity can be further improved by an additional density gradient step using sucrose gradients or commercial OptiPrep density gradients82,83. Size-exclusion chromatography is another method for nanovesicle isolation that is based on the difference in the hydrodynamic size between nanovesicles and other impurities84. In this method, samples, such as blood85, urine86, tears87, and other physiological fluids88, can be loaded into the size-exclusion column for separation. However, the separated nanovesicles are usually eluted into dilution. It is worth mentioning that some impurities may remain in the cellular nanovesicle sample using the size-exclusion chromatographic method; interestingly, extension of the column length can be beneficial to reduce impurities by 90% as reported89. Filtration is another size-based nanovesicle separation method that utilizes cellulose, polyethersulfone, or polyvinylidene difluoride membranes with specific pore sizes90,91. Unlike conventional filtration methods, ultrafiltration is often performed with centrifugation in combination with membrane filtration. Ultrafiltration has been widely used for the purification and concentration of cellular nanovesicles which is less time-consuming than ultracentrifugation92. Notably, novel filtration methods such as tangential flow filtration93 and hydrostatic filtration dialysis94 have been explored, however, it is still challenging to efficiently separate cellular nanovesicles from high-abundance impurities in the complex physiological fluid79. In addition, the integrity of cellular nanovesicles may be damaged because of the membrane filtration24. In contrast, affinity-based isolation strategies such as the nanospring system95, beads system (magnetic beads and agarose beads)96, and polymeric column system97 can better maintain the nanovesicle integrity. Affinity-based methods use the specific interactions between the proteins on cellular nanovesicles and ligands in the separation column, such as antibodies98, peptides85, aptamer99, and other affinity molecules95. For example, many devices have been developed for the detection, isolation, and separation of cellular nanovesicles by conjugation of antibodies100, aptamers101 or heparin102 to the separation column. These novel isolation strategies can reduce the separation time and increase the purity; however, the complex matrices containing impurities may block the affinity binding sites resulting in a low yield.
Of note, despite extensive investigations, the lack of a standardized isolation method to achieve optimal integrity, purity, cost-efficiency, and yield of cellular nanovesicles remains a challenge. Nevertheless, new methods such as asymmetric flow field-flow fractionation103, nano-flow cytometry104, microfluidic devices105 are emerging with the potential to address this challenge. Moreover, commercial reagents/kits such as ExoQuick106, miRCURY107, Total Exosome Isolation Reagent108, PureExo109 and MagCapture110 are also available as options for efficient separation of cellular nanovesicles.
4. Immunomodulatory cellular nanovesicles
Natural cellular nanovesicles are involved in dynamically maintaining the balance of immune activation and suppression24,35. Once disturbed, the immune system loses the equilibrium state which results in the inability to properly balance the immunological homeostasis. Introduction of immunomodulatory cellular nanovesicles can perform either immunoactive or immunosuppressive activities depending on their source and has emerged as an effective strategy to restore the immune equilibrium. Cellular nanovesicles can be engineered to trigger the downstream signaling pathways, elicit immune activation or tolerance, and change the phenotype of immune cells. Here, we discuss major cellular nanovesicles from different cell types that have been leveraged for therapeutic immunomodulation (Fig. 3). Our discussion is based on two broad categories of immunomodulatory cellular nanovesicles including immunoactive and immunosuppressive nanovesicles.
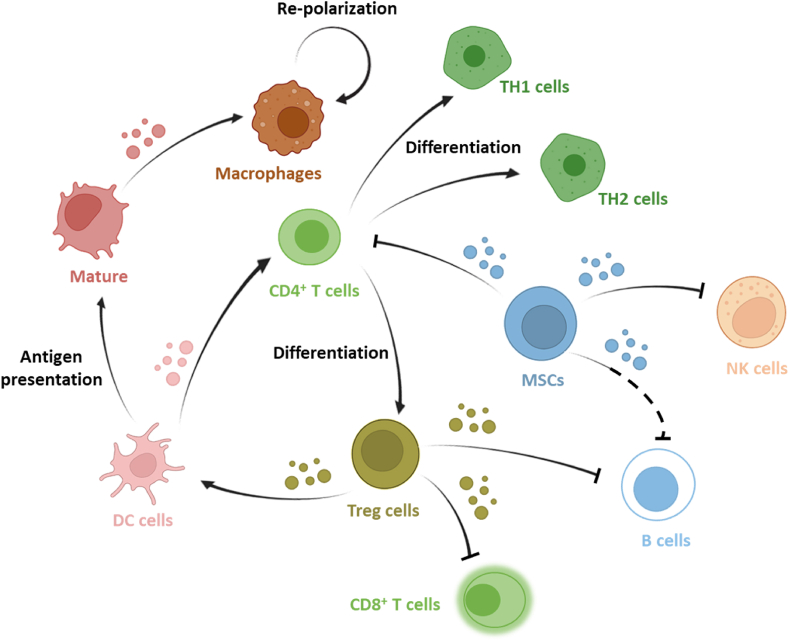
Figure 3.
Extracellular vesicles secreted from different immune cells are involved in dynamically maintaining the balance of immune activation and suppression. EVs from T cells, M1-like macrophages, dendritic cells, and natural killer cells can perform immunoactive effect on other immune cells. EVs from mesenchymal stem cells, regulatory T cells, and M2-like macrophages among others can exhibit an immunosuppressive effect. Schematic was created using templates from BioRender with information from Ref. 32.
4.1. Immunoactive nanovesicles
4.1.1. T cell-derived nanovesicles
T cells play indispensable roles in interweaving the adaptive cellular and humoral immune responses among different immune cell types111. They are generally divided into two subtypes: CD4+ T cells (termed helper T cells, including regulatory T cells), which secrete factors such as cytokines to modulate immune activities, and CD8+ T cells (termed cytotoxic T cells), which express the T-cell receptor (TCR) on cell surface for neutralization of intracellular pathogens112,113. Also, genetically engineered T cells, especially chimeric antigen receptor T (CAR-T) cells, are currently considered a breakthrough in cancer therapy114.
T cell-derived EVs (TEVs) inherit components and functions from the parent T-cell subtypes and exhibit hallmark effects on regulating immune responses. EVs secreted from CD3+ T cells activated with IL-2 were found to be involved in the proliferation of CD8+ T cells via an autocrine signaling mechanism115. Hence, they may prove to be a potent tool for antiviral and antitumor immunoregulation. TEVs derived from CD8+ T cells usually have an immunoactive effect. EVs generated from activated CD8+ T cells were found to shuttle granzyme B to mesenchymal tumoral stromal cells and attenuate the invasion and metastasis of lung tumors116. TEVs were observed to be engulfed by bone marrow-derived mesenchymal stem cells (BMSCs) within 2 h. Because of the lack of basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF), transmigration of malignant tumors with mesenchymal-like features was blocked. The study demonstrated a remarkable inhibition of tumor progression by activated TEVs.
TEVs carry cargo proteins on their surface and play crucial roles in immunomodulation. Pathogenic and cancer cells could imperceptibly recognize and bind to these proteins on T cells, leading to immunotolerance or immune escape117. Kim et al.118 reported that TEVs extruded from activated CD8+ T cells but not resting T cells effectively blocked the programmed death-ligand 1 (PD-L1) and TGF-β, which indirectly enhanced the anti-tumor immune responses by attenuating the exhaustion of T cells. Nanovesicles containing PD-1 and TGF-β receptors infiltrated into solid tumors and bound to the immune checkpoint PD-L1, which led to T-cell activation and protection from immunosuppression, allowing production of interleukins and interferons in the tumor microenvironment (TME)118.
However, it seems that the functions of TEVs are not completely consistent with those of their secreting cells. Despite the expression of CD40L and inducible co-stimulatory molecule (ICOS) in activated CD4+ T cells, EVs secreted from activated CD4+ T cells inhibited the proliferation of ovalbumin (OVA)-pulsed DCs and suppressed CD8+ T cell activation119. In addition, EVs generated by both CD4+ T cells and CD8+ T cells can bind to surface receptors on DCs, such as MHC I, Fas, and intercellular adhesion molecule 1 (ICAM-1), causing DC apoptosis120. Hence, the immunomodulatory effect of TEVs may be dependent on the target cell type. Further, these differences between the functions of the parent T cells and those of the TEVs facilitate immune system balance, rather than over-activation or over-suppression. It has also been reported that CD47-containing EVs from Jurkat T cells, which express low levels of CD4 but not CD8, regulated the activity of T cells and endothelial cells (ECs)121. The phosphorylation of VEGF-VEGFR2 was affected by the uptake of TEVs that controlled CD47-dependent vascular formation and target gene expression in ECs. This work further confirmed the effect of intercellular communication between T cells and other cells, such as ECs121.
EVs from engineered T cells have also been studied114,122,123. EVs from CAR-T cells were found to contain cytotoxic molecules that inhibited tumor growth124. However, since the EVs from CAR-T cells did not contain PD-1 on their surface, the tumor suppressive effect could not be attenuated in a PD-L1-dependent manner. It remains unclear whether the PD-1 signaling pathway was activated, as this was not investigated. This study did, however, provided a novel perspective of the immunomodulatory potential of EVs from CAR-T cells.
4.1.2. Dendritic cell (DC)-derived nanovesicles
DC-derived EVs (DC-EVs) bearing antigen-loaded MHC class I and class II and co-stimulatory molecules (CD86, CD80, and CD40) can directly activate CD4+ and CD8+ T cells for an antigen-specific immune response125, 126, 127. In addition, DC-EVs can transport internal adhesion molecules (e.g., integrins and DC-specific ICAM-3-grabbing non-integrin) and modulation molecules (e.g., TNF-α, interleukin-15 receptor, and NKG2D ligand) to peripheral immune cells to trigger the activation of NK cells126. DC-EVs secreted from both immature and mature bone marrow-derived DCs were found to include surface ligands for immunomodulation, although only DC-EVs from mature DCs displayed an immunoactive effect compared to those from immature DCs128. This was caused by the different co-stimulatory molecules present in the DC-EVs. In contrast, there was no difference in the MHC class I and class II molecules on the surfaces of DC-EVs of different sizes and intracellular origins, and they all had the ability to prime naive CD8+ T cells129. DC-EVs from several different DC subtypes reportedly stimulated IFN-γ secretion from T helper lymphocytes, whereas different transmembrane receptors were found on small DC-EVs (e.g., CD40 and DC-SIGN) and large DC-EVs (e.g., CD80) that were necessary to activate T cells130. In addition, small DC-EVs augmented the production of Th1 cytokine IFN-γ, and large DC-EVs induced the secretion of the Th2 cytokines IL-4, IL-5, and IL-13. Notably, differences in the immunomodulatory activity can be found in EVs derived from syngeneic and allogeneic DCs131. Although both syngeneic- and allogeneic-derived DC-EVs had immunomodulatory effects on CD8+ T cells and germinal center B (GC-B) cells, the allogeneic-derived EVs showed a superior long-term memory effect on T follicular helper (Tfh) cells and antigen-specific antibodies, which led to an antitumor response. Indeed, the equally efficient antitumor activities of the syngeneic- and allogeneic-derived EVs were against the progression of B16mOVA melanoma tumors in vivo. Due to the diverse and complex constituents of cellular nanovesicles, even minor changes to their intrinsic components give rise to tremendous differences in their immunomodulatory effects. In a study, small noncoding RNAs were included as a cargo in DC-EVs, namely miRNA, small nucleolar RNA (snoRNA), and small non-coding RNA (Y-RNA). When compared with the similar loading amount of tRNA and snRNA, the small noncoding RNAs were found to confer the genetic information required for the immunostimulatory activity132.
A previous study reported that DC-EVs could reduce the growth of a tolerogenic tumor by stimulating CD3+ T cells previously primed with SK-BR-3133. The level of IFN-γ secreted by tumor-sensitive T cells was enhanced by the DC-EVs. In another study, mature human DC-EVs (mh-DC-EVs) were mobilized and found to induce the differentiation of peripheral monocytes into various phenotypes128. The expression of biomarkers on the mature human DCs, such as 6-sulfo LacNAc (slan), Zbtb46, CD64, and CD14, was also reported. After injection into the hypodermis, the mh-DC-EVs led to the efficient regression of chronic inflammation via T-cell activation128. In yet another study, DC-EVs generated from pre-active DCs loaded with antigen peptides promoted the cross-priming of T cells, which were regarded as the patient's blood-sourced vaccine that could serve as a cancer immunotherapy134. The peptide-loaded DC-EVs promoted the expression of co-stimulatory markers, such as CD25 on T cells and CD86 on DCs. These findings allow us to envision the possibility of developing a platform for anti-cancer immunotherapeutic vaccines using DC-EVs.
4.1.3. Natural killer (NK) cell-derived nanovesicles
NK cells express the neural cell adhesion molecule CD56 and are divided into two different subgroups based on this expression: CD56dim NK cells, which show a cytotoxic effect in the circulation, and CD56bright NK cells, which exist in secondary lymphoid tissues and produce cytokines for immunomodulation135. Theoretically, all NK cells have the ability to secrete EVs for intercellular communication in specific conditions. Lugini et al.136 first found that NK cell-derived EVs (NK-EVs) were generated by NK cells with multifunctional receptors on their surface for immunoregulation. It has been shown that NK-EVs containing immunomodulatory molecules, such as Fas ligands and perforin molecules, can be internalized by diverse cancer cell lines, but not by peripheral blood mononuclear cells (PBMCs). Also, uptake of NK-EVs was found to increase the population of astrocytes, which diminished the level of cytokines137. NK-EVs have also exhibited tumor-homing and immune cell activation activity. In addition, NK-EVs have been shown to stimulate the immune response by promoting the human leukocyte antigen DR (HLA-DR) isotype and CD80-86 on monocytes via interaction with PBMCs138. Likewise, the expression of CD25 on T cells was induced by NK-EVs. Therefore, CD56+ NK cells, their subgroups (CD56dim and CD56bright), and NK-EVs are potential tools for cancer immunotherapy. These promising findings have been followed by the development of an immune monitoring system (NKExoELISA) that captures the biomarkers, receptors, and other molecules present on the membrane of NK-EVs138. This helps to identify the NK-EV molecules responsible for the anticancer activity. Notably, this monitoring system has been tested using NK-EVs harvested from the plasma of patients with melanoma and of healthy donors138.
Interestingly, literature evidence suggests that natural (NK cell-derived EVs) and artificial (nanovesicles produced by extruding NK cells) nanovesicles show similarity yet differences in their biological functions139. For example, artificial nanovesicles carrying CD63 and Alix exosomal proteins, which downregulate the cell proliferation proteins p-ERK and p-AKT, have shown stronger anticancer activity than natural NK cell-derived EVs in vitro and in vivo139. However, these artificial nanovesicles are less effective in crossing the blood–brain barrier (BBB), which may limit their use in brain delivery applications. In contrast, surprisingly, it has been found that natural NK-EVs can cross the BBB, target glioblastoma, and induce antitumor effects140. While both natural and artificial nanovesicles contain proteins or receptors that decrease p-AKT and p-ERK expression (resulting in cancer cell apoptosis), only the natural NK-EVs contain TNF-α and granzyme B and can cross the BBB to kill brain glioma cells.
Apart from being efficient in transporting immunomodulatory proteins, NK-EVs also contain genetic information in the form of miRNA, which also plays a role in managing the activities of malignant cells. Based on the high expression of miR-3607–3p found in patients with pancreatic cancer, Zhou et al141. Reported that miR-3607–3p was carried by NK-EVs that effectively targeted and curbed the proliferation, migration, and invasion of pancreatic cancer cells in vitro. The authors collected and analyzed the mRNAs from the patients’ pancreatic cancer tissues and found that IL-26 was overexpressed within the tumor. They then created a cell model by transfecting MIA PaCa-2 and PANC-1 cells with the IL-26 gene and a luciferase reporter gene. It was demonstrated that the transfected cells were bound by NK-EVs, leading to reduced luciferase activity compared to that of wild type cells. It was speculated that miR-3607–3p on the surface of NK-EVs specifically targets IL-26 expressed in pancreatic cancer cells. This indicates that this miRNA may be a useful tool for immunotherapy against pancreatic cancer. In addition, NK-EVs containing miRNA-186 induced the expression of MYCN, AURKA, TGFBR1, and TGFBR2 in neuroblastoma cells, leading to growth inhibition142. By regulating the amplification of MYCN, TGFb1-dependent activation of NK cells was restored, as demonstrated by their cytotoxic activity toward malignant cells.
4.1.4. M1 macrophage-derived nanovesicles
Macrophages are widely found in vertebrate tissues. They phagocytose and eliminate foreign matters as well as dead cells. Macrophages exist in different phenotypes; depending on the surrounding environment, M0 macrophages undergo polarization and develop into either the pro-inflammatory M1 phenotype or the anti-inflammatory M2 phenotype143. A range of stimulatory factors such as LPS, IFN-γ, IL-4, and IL-13 can polarize different phenotypes of macrophages, from which the secreted macrophage-derived EVs (M-EVs) have considerable immune activities144, 145, 146, 147. Choo et al.148 reported that injectable M1 macrophage-derived nanovesicles had the ability to repolarize tumor-associated macrophages (TAMs) to the M1 phenotype, which produces cytokines and other pro-inflammatory factors inhibiting tumor growth. In a synergistic treatment, intravenous injection of M1 macrophage-derived nanovesicles and the immune checkpoint inhibitor anti-PD-L1 antibody blocked the suppression of T cells by CT26 colon carcinoma cancer cells in vivo149. Additionally, the nanovesicles have been shown to repolarize M2 macrophages to the M1 phenotype, restraining vascular formation and resulting in receding migration and invasion of endometrial stroma cells (ESCs) in a breast cancer lung metastasis model. While the M1 macrophage-derived nanovesicles affected the ESCs from patients with endometriosis, no other damage was found in peripheral tissues and organs.
In comparison to the M0 and M2 macrophages, M1 macrophages have the tendency to produce pro-inflammatory cytokines. Similarly, M1 macrophage-derived EVs (M1-EVs) have been reported to contain pro-inflammatory miRNA with immunomodulatory effects on surrounding cells150, 151, 152. Li et al.153 reported that M1 macrophage-derived EVs containing miRNA-16–5p acted as a regulator to the immune checkpoint gene that reduced the PD-L1 expression on the surface of gastric carcinoma cells. They blocked the PD-1–PD-L1 interaction, which caused immune escape and reactivated T cells for cancer therapy in tumor-bearing mice. Similarly, M1-EVs containing miRNA-326 were found to downregulate the CD206 expression and mediate the NF-κB signaling pathway in hepatocellular carcinoma cells (HCCs)154. They further suppressed the cell proliferation, colony formation, migration, and invasion of HCCs, providing clear evidence of the potential of these nanovesicles as an immunotherapeutic approach. With a clearer understanding of the composition of M-EVs, more pro-inflammatory miRNAs have been developed that can target a variety of genes. Liu et al.155 reported that miR-155 assisted M1-EVs in targeting ECs and that vasculogenesis declined as a result of deacetylation or phosphorylation being hindered in the ECs, which led to reduced nitric oxide synthase activity. M1-EVs with miR-155 also reduced the expression of the Rac family GTPase 1 (RAC1), P21 (RAC1)-activated kinase 2 (PAK2), Sirtuin 1 (Sirt1), and protein kinase AMP activated catalytic subunit alpha 2 (AMPKα2) in ECs. This activity could be further examined as a treatment for cardiac dysfunction.
To compare the immunomodulatory activities of different M-EVs, Chen et al.156 isolated M-EVs from M0, M1, and M2 macrophages and investigated their effects on the proliferation and differentiation of BMSCs. They found that M1-EVs upregulated the osteogenesis-related genes (e.g., ALP, BMP-2, COL-1, OCN, and Runx 2 genes) and adipogenesis-related genes (i.e., adiponectin and PPAR-γ). This resulted in osteogenic differentiation of BMSCs. However, the same activity was not observed with M0 macrophage-derived EVs or M2 macrophage-derived EVs.
4.2. Immunosuppressive nanovesicles
4.2.1. Regulatory T (Treg) cell-derived nanovesicles
Treg cells are a subpopulation of T cells that have a suppressive effect on other immune cells, such as T cells and DCs. Treg cells express the ectoenzymes CD73 and CD39 that dephosphorylate ATP, ADP, and AMP into adenosine, which then interacts with adenosine receptors (A2aR) on T cells157. This interaction suppresses the immunoactivity of T cells, resulting in reduced production of cytokines, such as IL-2 and IFN-γ. Treg cell-derived EVs (Treg-EVs) from CD4+CD25+Foxp3+ Treg cells were found to have high levels of surface CD73 that is catalytically active, producing adenosine that bound to T cells and negatively regulated their cytotoxicity23. In addition, Zeng et al.158 reported that different concentrations of Treg-EVs have different capabilities for the inhibition of CD8+ T cells. GW4869, an EVs inhibitor, was utilized to prevent the secretion of EVs from Treg cells to verify the specific impact of Treg-EVs on the immunosuppression of T cells. As mentioned in this study, T-cell activity was determined by measuring the IFN-γ and perforin levels, resulting in a downregulated level. Treg-EVs provided an important tool for immunomodulation by manipulating the activities of T cells159.
Interestingly, Treg-EVs from different types of Treg cells have different immunomodulatory effects. For example, a study examined the activities of Treg-EVs from resting Treg cells and active Treg cells160. dnlKK2-Treg-EVs secreted from CD4+CD25− Tregs (dnlKK2- Tregs) were found to have high levels of miRNAs, including miR-503, miR-330, miR-293, miR-297c, miR-207, miR-9, and miR-484; however, these were not detected or at very low levels in Treg-EVs from resting and active Treg cells160. dnlKK2-Treg-EVs were also found to contain iNOS mRNA and proteins, which had a suppressive effect on cell cycle progression in cytotoxic T cells and induced apoptosis160,161. Furthermore, Treg-EVs in both healthy people and disease patients were reported to contain proteins and RNAs that suppress T cells162. The proliferation of T cells was reduced by Treg-EVs that were used to treat multiple sclerosis, which is caused by chronic inflammation in the central nervous system.
Treg-EVs not only have immunomodulatory effects on T cells, but also regulate DCs. Tung et al.163 demonstrated that Treg-EVs deliver miR-150–5p and miR-142–3p which were enriched into DCs. The Treg-EVs regulated the activity of DCs by decreasing IL-6 production and increasing IL-10 production. CD4+FoxP3− T cell-derived EVs were used as controls and had no delivery capacity for miRNA. These findings suggest that there may be a novel Treg cell-DC interaction in terms of immunomodulation that could be exploited for immunosuppressive therapy.
4.2.2. Mesenchymal stem cell (MSC)-derived nanovesicles
Mesenchymal stem cells (MSCs) are present in different tissues such as in the adipose tissue and bone marrow. They exhibit potentiality in several pathways involved in immunomodulation164. Generally, MSCs can lead to an immunosuppressive effect via different mechanisms. For example, they can inhibit T cells, induce their apoptosis, and hold them in the G1/G0 phase of the cell cycle165. MSCs can stimulate the differentiation and proliferation of macrophages, Treg cells, and regulatory B (Breg) cells166,167. They can also attenuate NK cell cytotoxicity by reducing cytokine production168.
MSC-derived EVs (MSC-EVs) have been demonstrated to have similar immunomodulatory effects as the parent MSCs169,170. For example, human umbilical cord MSC-EVs have been found to suppress CD8+IFN-γ+ cytotoxic T cells and CD4+IFN-γ+ Th1 cells by downregulating the pro-inflammatory mediators IFN-γ and TNF-α171. In addition, MSC-EVs have been shown to induce IL-10 production in CD4+CD25+Foxp3+ Treg cells. MSC-EVs containing RAB27B siRNA showed a self-regulating capability172. PI3K-AKT signaling pathway of B cells was negatively modulated by MSC-EVs through targeted binding of miR-155–5 P to B cells173. MSC-EVs exhibited downregulated effect on specific actin cytoskeleton proteins, especially in early-stage B cells, which impeded leukocyte activation174. Moreover, it has been reported that MSC-EVs can impede DC maturation and function175. They can attenuate antigen uptake by immature DCs. The pro-inflammatory cytokines and activation markers, including CD83, CD38, and CD80, were decrease in mature DCs after exposure to MSC-EVs. Regarding their effects on macrophages, MSC-EVs have been shown to regulate the M1/M2-like phenotype balance, shifting it toward the M2-like phenotype176. MSC-EVs containing miRNAs related to healing and anti-inflammation downregulated the inflammatory cytokine IL-6 and upregulated IL-10, leading to the polarization of M2-like macrophages. Notably, after internalization by BMMs, MSC-EVs generated under hypoxic conditions had a higher anti-inflammatory activity than those generated under normal conditions.
In addition, MSC-EVs carrying miRNA can functionally target TLR4, inhibit the NF-κB signaling pathway, and reduce the production of inflammatory cytokines177. It was found that MSC-EVs could render diabetic mice more sensitive to thermal and mechanical stimuli, which was followed by increased nerve conduction velocity, improvement in intraepidermal nerve fibers, and expansion of sciatic nerve myelin sheath thickness and axon diameter. MSC-EVs contributed to the polarization of macrophages from the M1 phenotype to the M2 phenotype and inhibition of pro-inflammatory cytokine production. However, due to off-targeting and deficient productivity, MSC-EVs with low targeting ability are limited in their use as immunotherapeutic tools in clinical applications.
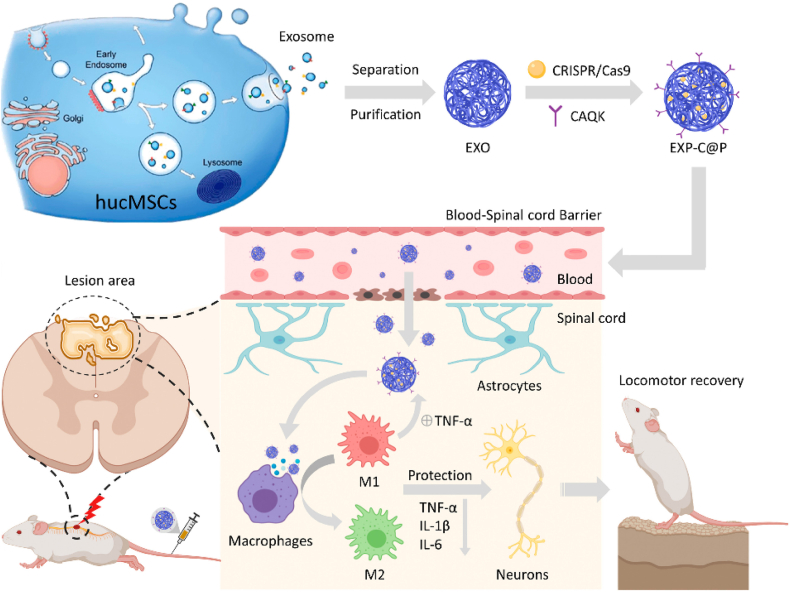
Fortunately, MSC-EVs may have applications when fused with other entities. Blood-derived MSC-EVs were fused with macrophage membrane and the resultant product was tested as an immunosuppressive therapy for spinal cord injury178. The fused nanovesicles accumulated in the spinal cord and promoted anti-inflammatory activity and cell apoptosis. Compared with normal MSC-EVs, the fused nanovesicles enhanced the immigration of human umbilical vein endothelial cells (HUVECs) and the formation of blood vessel.
MSC-EVs contain regulatory molecules, including RNAs, surface protein signals, and cytokines, that mediate the transfer of biological information. A growing number of studies demonstrated that MSC-EVs could modulate immune activity, including the proliferation and differentiation of immune cells via reprogramming of polarization, and regulation of anti- or pro-inflammatory cytokine production, for various disease treatments179, 180, 181. MSC-EVs are thus considered a promising cell-free immunotherapeutic tool for disease treatments, including for graft-versus-host disease 182, 183, 184, organ transplantation-induced immune disease 185, 186, 187, and inflammatory diseases 188, 189, 190.
Preclinical studies have suggested that MSC-EVs may block TLR4 and the NF-κB signaling pathway, showing similar promise as MSCs for treating lung injury caused by intestinal ischemia reperfusion177. MSC-EVs could downregulate the production of IL-17 by iNKT cells and the production of TNF-α and high mobility group box 1 protein (HMGB1) by macrophages. They have also been shown to regulate the polarization of M1-like macrophages to M2-like macrophages, which proved to be beneficial against lung inflammation186. In addition, pro-inflammatory cytokine levels were significantly decreased and accompanied by upregulation of IL-10 and prostaglandin E2 in a lung ischemia–reperfusion mouse model185. In another study focusing on chronic graft-versus-host disease, injection of EVs from bone marrow-derived MSCs resulted in an anti-inflammatory condition. Activation and infiltration of CD4+ and CD8+ T cells were attenuated by the MSC-EVs, supporting the need for further investigation of MSC-EVs as a treatment option for inflammation-associated diseases191,192.
4.2.3. M2 macrophage-derived nanovesicles
M2 macrophage-derived EVs (M2-EVs) play a suppressive role in pathological tissues by mediating intercellular communication143. They inherit some compositional and functional elements from their parental cell types, M2 macrophages. M2-EVs carry various noncoding RNAs and have been shown to be effective in promoting wound healing and against chronic inflammation142,193. M2-EVs were shown to regulate the release of nitric oxide (NO) and pro-inflammatory cytokines from macrophages, such as IL-6 and TNF-α, and pro-inflammatory enzymes were dramatically decreased, which greatly benefited wound healing194. It was found that miR-122–5p and miR-148a–3p were enriched in M2-EVs in concanavalin A-induced hepatitis sites, reducing the level of mRNA related to the mitogen-activated protein kinase, PI3K-AKT, and Rho/Rho-associated coiled-coil containing protein kinase pathways and suppressing inflammatory cytokine production195. Hence, M2-EV-mediated immunotherapy showed therapeutic efficacy to treat acute hepatitis. In another study, Wu et al.196 reported that long noncoding RNA was carried by M2-EVs to treat autoimmune encephalomyelitis. The treatment by M2-EVs led to a decrease in the number of Th17 cells and an increase in Treg cells. This verified that M2-EVs could carry long noncoding RNA to downregulate miR-21–5p in the encephalomyelitis mouse model, leading to phosphorylation of JAK1 and STAT3 in Th17 cells. Treatment by M2-EVs inhibited the expression of TNF-α, IL-17, IL-6, and IL-1β, which was similar to the findings observed in spinal cord cells treated with miR-21–5p.
In addition to transferring proteins, nucleic acids, and other signaling molecules for regulating immune responses, M2-EVs are also associated with tumor cell migration and proliferation, especially TAMs197. Intimate communication has been observed between cancer cells and TAMs via miRNA carried by secreted TAM-derived EVs. In the epithelial ovarian cancer microenvironment, M2-EVs express miR-221–3p, which suppresses the gene encoding the cell cycle regulator (cyclin dependent kinase inhibitor 1 B), and thus promotes the G1/S transition of epithelial ovarian cancer cells. Also, M2-EVs have been shown to accelerate the immune escape of tumor cells in different tumor models such as in hepatocellular carcinoma, glioma, and colon cancers198, 199, 200. The M2-EVs transferred different functional miRNAs to cancer cells, which effectively inhibited the activities of related enzymes. CD8+ T cells were depleted by the M2-EVs, and their cytotoxicity was reduced198,200. Additionally, high expression of IL-6 was detected, which augmented the proliferation of CD3+ T cells199. The capacity of the M2-EVs to exhaust the T cells makes them attractive targets for therapies designed to reduce immune escape and tumor formation.
4.3. Nanovesicle derived from other cell types
Apart from the aforementioned cell types that we have discussed in detail, cellular nanovesicles derived from other cell types such as neutrophils201, 202, 203, 204, 205, platelets206, 207, 208, 209, 210, 211, 212, 213, 214, erythrocytes215, 216, 217, 218, 219, 220, cancer cells221, 222, 223, 224, among others225, 226, 227, also have immunomodulatory activities. The majority of immune cell-derived EVs have immunoactive or immunosuppressive effects like those of their parent cells12,228. EVs secreted from non-immune cells also show immunomodulatory effects229,230. For example, erythrocytes, the most abundant cell type in peripheral blood, secrete EVs that play key roles in immunomodulation during transfusion215. EVs from the supernatant of stored blood were found to elicit a strong pro-inflammatory response by binding to monocytes and increasing the production of TNF-α, IL-6, IL-1, and IL-10216,217. It has also been shown that proliferation of T cells can be enhanced via APCs activated by erythrocyte-derived EVs218. In contrast, erythrocyte-derived EVs bound to different immune cells showed completely opposite immunosuppressive effects; for example, B cell activity could be negatively affected by erythrocyte-derived EVs219. In macrophages, after uptake of erythrocyte-derived EVs, the release of pro-inflammatory TNF-α and IL-8 were reduced by 80% and 76%, respectively220.
Platelet-derived EVs are involved in different immunomodulatory processes231. Platelets and platelet-derived EVs are the biological components that have the best access to ECs232. It was reported that platelet-derived EVs enhanced the adhesion of monocytes to ECs233, and contributed to the activation of pro-inflammation-related genes in monocytes. Moreover, platelet-derived EVs stimulated DCs to mature and enhanced activation of T cells234. Hence, depletion of platelets and platelet-derived EVs is a potential therapeutic option for the treatment of inflammation.
Cancer cells can secret EVs and their impact on regulating immune responses have not been clearly illustrated yet32,235. However, literature evidence suggests that cancer cell-derived EVs exhibit either immunoactive or immunosuppressive effects depending on their source and the target they interact with236, 237, 238. For example, EVs released by metastatic melanoma could express PD-L1 on their surface resulting in depletion of T cells239. With the stimulation of IFN-γ, the level of PD-L1 on these EVs was upregulated which regressed the function of CD8+ T cells and promoted tumor cells growth. In the early treatment state, the expression of PD-L1 on circulating EVs increased by an order of magnitude, which could be considered as an indicator to clinically distinguish responders from non-responders in patients undergoing immunotherapies240,241. This also revealed the potential of EVs with expression of PD-L1 as a predictor to determine which patients can benefit from anti-PD-1 immune checkpoint blockade therapies242, 243, 244. Due to the rapid evolution of cellular nanovesicles in immunomodulation, there are many other examples that are not discussed in detail in this review. We have summarized the immunomodulatory effect of cellular nanovesicles from different cells in Table 1.
Table 1.
Representative examples of cellular nanovesicle-mediated immunoactive and immunosuppressive responses.
| Immunomodulatory effect | Nanovesicle source | Cellular response and function | Ref. |
|---|---|---|---|
| Immunoactivation | CD3+ T cells | Promote proliferation of CD8+ T cell | 115 |
| CD8+ T cells | Depletion of tumor stromal cells for anti-tumor effect | 116 | |
| CD4+ T cells | Inhibit proliferation of OVA-pulsed dendritic cell mediated CD4+ T cell activation; suppress the activation of CD8+ cytotoxic T lymphocytes | 119 | |
| CD4+ T cells CD8+ T cells |
Apoptosis of DCs | 120 | |
| Jurkat T cells | T cell activation | 121 | |
| CAR-T cells | Inhibition of tumor growth | 124 | |
| DCs | Activation of NK cells | 126 | |
| Facilitate secretion of IFN-γ by Th lymphocytes CD4+ and CD8+ T cell activation |
128,130,134 | ||
| Augmentation of Th1 cytokine IFN-γ production (by smaller DC-derived EVs) | 131 | ||
| NK cells | Activation of T cells; proliferation of NK cells; promote human leukocyte antigen DR isotype and expression of CD80-86 on monocytes | 139 | |
| M1-like macrophages | Polarization of M2-like macrophages toward M1 phenotype | 144,155 | |
| Produce pro-inflammatory factors and cytokines in the local microenvironment | 147,148,154 | ||
| Infected macrophages | Carry pathogen-associated molecular patterns to increase cytokine production via Toll-like receptor (TLR) activation and promote immunity | 145,146 | |
| Erythrocytes | Induce strong pro-inflammatory host response; increase the production of TNF-α, IL-6 and IL-1 from monocytes | 217 | |
| Enhance the proliferation of T cells | 218 | ||
| Platelet | Facilitate the aggregation and enhanced adhesion of monocytes to the endothelial cells; activate expression of pro-inflammation related genes in monocytes | 233 | |
| Stimulate DCs into maturation status; enhance activation of T lymphocytes | 234 | ||
| Immunosuppression | CD4+CD25+Foxp3+ Tregs | Reduce cytotoxicity of T cells | 23 |
| dnlKK2-Tregs | Blockage of cell cycle progression of cytotoxic T cells; apoptosis of T cells | 160 | |
| Tregs | Inhibit CD8+ cytotoxic T lymphocytes; attenuate production of IFN- γ and perforin; increase the amount of microRNA; decrease IL-6 and increase IL-10 production | 146 | |
| Inhibit proliferation of CD4+ T cells | 159 | ||
| Promote CD4+CD25+Foxp3+ Tregs; enhance the levels of IL-10; suppress the cytotoxic T cells | 157 | ||
| Suppress the activity of B cells | 158,160 | ||
| Attenuate antigen uptake by immature DCs | 162 | ||
| Polarization of macrophages towards the M2-like phenotype | 163 | ||
| MSCs | Reduce the level of inflammatory cytokines; M2-like macrophage polarization | 164,245 | |
| Downregulate the production of IL-17 by iNKT cells and production of TNF-α and HMGB1 by macrophages. | 171 | ||
| Upregulation of IL-10 | 174 | ||
| Attenuate activation and infiltration of CD4+ and CD8+ T cells | 174,176 | ||
| Reduce the levels of NO and pro-inflammatory cytokines from macrophages, such as IL-6 and TNF; reduce level of pro-inflammatory enzymes | 169,178 | ||
| Decrease Th17 cells accompanied by increasing number of Tregs; inhibit expression of TNF-α, IL-17, IL-6, and IL-1β | 179 | ||
| Suppress the gene of cell cycle regulator cyclin dependent kinase inhibitor 1 B to promote tumor growth | 180 | ||
| Accelerate immune escape of tumor cells; depletion of CD8+ T cells | 170,181,186 | ||
| Induce high expression of IL-6 for decreasing the proliferation of CD3+ T cells and proportion of IFN-γ+ T cells | 170 | ||
| M2-like macrophages | Attenuate the activity of B cells | 197 | |
| Reduce the inflammatory TNF-α and IL-8 from macrophages | 198 | ||
| Cancer cells | Interaction between PD-L1 and PD-1; regress the function of CD8+ T cells and promote tumor cells growth | 240,241 | |
| Macrophage migration inhibitory factor; induce release of TGF-β by Kupffer cells to remodel the extracellular matrix (ECM) in the liver | 236 | ||
| Promote expansion of CD4+CD25+FOXP3+ Tregs and the demise of antitumor CD8+ effector T cells | 237 |
5. Biological and chemical approaches to engineering immunomodulatory cellular nanovesicles
Cellular nanovesicles contain a diverse spectrum of molecular cargoes and are emerging as a prominent platform to regulate immune responses for therapeutic immunomodulation246. However, the use of native cellular nanovesicles face several distinguished challenges34,247, 248, 249. First, isolation of EVs involves serial, complex procedures including removal of intact cells, cell debris, organelle debris, proteins and other inclusions, which usually lead to low production yield and limit their further preclinical and clinical investigations. Second, native cellular nanovesicles derived from a single parent cell type may possess undesirable biological functions that are not compatible with the immunomodulatory requirements in actual disease settings. In addition, native cellular nanovesicles may also carry unwanted or morbigenous cargos such as microRNAs and proteins that are detrimental to their immunomodulatory effects. To solve these challenges, multiple biologically- and chemically-based strategies have been explored to construct engineered cellular nanovesicles with tailored, controllable immunomodulatory activities248. Here, we discuss general considerations and principles for the design of engineered cellular nanovesicles for therapeutic immunomodulation (Fig. 4). In particular, emerging engineering strategies including biological (e.g., genetic engineering) and chemical (e.g., surface modification, drug encapsulation, synthetic or biomimetic nanovesicles) approaches are emphasized. We also discuss emerging formulation and material approaches that enable precision delivery of immunomodulatory cellular nanovesicles.
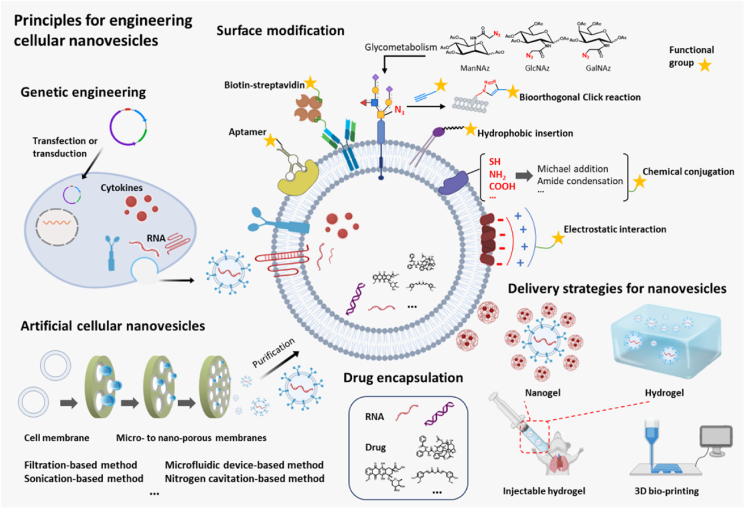
Figure 4.
Emerging biological and chemical strategies to engineer cellular nanovesicles for therapeutic immunomodulation. Cellular nanovesicles can be engineered via diverse strategies to improve their immunomodulatory activity, such as surface modification, drug encapsulation, genetic engineering, and artificial nanovesicle production. Delivery strategies such as hydrogel encapsulation can also be used to enhance their accumulation and retention at target sites. Schematics were created using templates from BioRender.
5.1. Genetic engineering of parent cells
On account of the formation of cellular nanovesicles (especially EVs) through invagination of parent cell membrane that occurred to produce endocytic vesicles which are subsequently excreted out of cells, proteins expressed on the parent cell membrane can be retained on the surface of generated EVs39,40. Toward this end, to express exogenous proteins, for example, receptors and ligands on the surface of cellular nanovesicles via genetic engineering of parent cells has been extensively investigated34. One distinguished example is that EVs can be genetically engineered with specific targeting ligands to improve their targeting to disease sites249,250. For instance, engineered DC-derived EVs were constructed with the expression of rabies viral glycoprotein (RVG) or muscle-specific peptide (MSP)250, both of which were fused with lysosome-associated membrane protein 2 (Lamp2b) that enables the anchoring of RVG or MSP onto EV membrane. RVG-engineered EVs (RVG-EVs) and MSP-engineered EVs (MSP-EVs) were then loaded with GAPDH siRNA by electroporation and exhibited targeting capability to murine neuronal cells (Neuro2A) and muscle cells (C2C12), respectively. Because these EVs were derived from immature engineered DCs which lacked the expression of MHC II and CD86 on their surface251, RVG-EVs and MSP-EVs demonstrated a good immunotolerant property and were devoid for the activation of T cells without overt side effects. The RVG-EVs carrying GAPDH siRNA showed efficient accumulation to the brain and exhibited strong therapeutic efficacy in an Alzheimer's disease model via knockdown of the Bace1 gene. Beyond specific ligands, certain receptors such as chemokine receptors can also be expressed on cellular nanovesicles via the genetic engineering approach for improved targeting252, 253, 254. For example, EV-mimic nanovesicles from M1 macrophages, which were engineered to overexpress C–C chemokine-receptor 2 (CCR2), were exploited for targeting lung metastases to modulate the tumor microenvironment (TME)255. Compared with the native M1-macrophage-derived EV-mimic nanovesicles, the CCR2-engineered counterpart exhibited an enhanced affinity to cancer cells expressing CCL2 (a CCR2 ligand). In vivo study revealed that the engineered nanovesicles showed efficient targeting to the lung metastasis. The accumulation of the engineered nanovesicles reprogrammed the TAMs toward the anti-tumor M1-like phenotype that in turn significantly modulated the tumor immune microenvironment. Moreover, Fe3O4 was loaded into engineered nanovesicles for suppression of cancer cells by the Fenton reaction-induced ferroptosis. The Fe3O4 nanovesicles demonstrated a strong immunotherapeutic efficacy in a 4T1 lung metastasis model255. Besides of the simple targeting abilities, surfaces proteins could be expressed on cellular nanovesicles that serve as an engager to recognize both immune cells and cancer cells to promote immunotherapy. Cheng et al.256 constructed an immune controller synthesized with multivalent antibodies by genetically engineering parent cells to express CD3 antibody and epidermal growth factor receptor (EGFR) antibody (Fig. 5a). EVs secreted from transfected parent cells was demonstrated to contain both anti-CD3 antibody, which binds to the CD3 on T cell surface, and anti-EGFR antibody, which could recognize the membrane protein EGFR overexpressed on MDA-MB-468 breast cancer cells. The smart immune controller induced the cross-linking of T cells and EGFR-expressing MDA-MB-468 breast cancer cells that in turn elicited a potent T-cell-mediated anti-tumor immunity both in vitro and in vivo256.
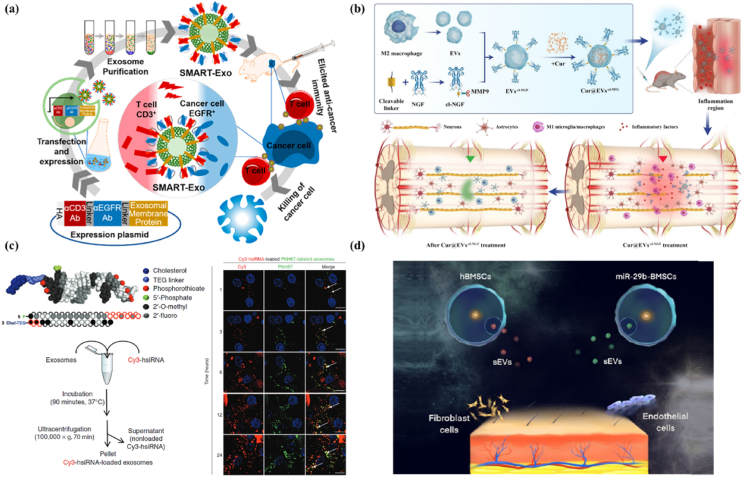
Figure 5.
Engineering and delivery of cellular nanovesicles for controlled immunomodulation. (a) Genetically engineered EVs to express surface ligands for immunomodulation. Schematic illustration of genetically engineered EVs expressing anti-CD3 antibody (αCD3) and anti-EGFR antibody (αEGFR) as an immune engager to trigger effective anti-tumor immune responses by T cells. Reproduced with permission from Ref. 256. Copyright © 2018, American Chemical Society. (b) Surface modification of EVs for improved targeting and responsive drug release. Schematic illustration of preparation and application of M2 macrophage-derived EVs engineered with enzyme-responsively released NGF for spinal cord injury therapy. Reproduced with permission from Ref. 275. Copyright © 2021, Springer Nature. (c) Loading drug into EVs to extend their function. Schematic illustration of the process of loading of cholesterol-modified siRNA into EVs. Confocal images of the internalization of siRNA-loaded EVs by primary cortical neurons was showed (Red, Cy3 labeled siRNA; Green, PKH67 labeled EVs; Blue, nuclei stained with Hoechst). Reproduced with permission from Ref. 300. Copyright © 2016, Elsevier. (d) Delivery of EVs using hydrogels. Schematic illustration of a bilayer hydrogel loaded with BMSC-derived EVs for promotion of scarless wound healing. Reproduced with permission from Ref. 328. Copyright © 2021, American Chemical Society.
Immunomodulatory ligands can be expressed on the surface of cellular nanovesicles to modulate the behavior of recipient cells in a contact-dependent manner41,249,257. For example, PD-1/PD-L1 as an immune checkpoint ligand was genetically engineered on the surface of EVs which was utilized in many studies for immunomodulation applications258. For instance, engineered HEK293T cell-derived EVs with the expression of PD-L1 were produced and then loaded with a low-dose of an immunosuppressive drug (rapamycin) which could inhibit the proliferation and activation of T cells by blocking the mTOR pathway259. PD-L1 expressed EVs with rapamycin showed a better efficacy in inhibiting the allo-immune response as compared to the PD-L1 nanovesicle or rapamycin alone. The engineered PD-L1 EVs also induced the differentiation of Tregs in the recipient spleens259. Interestingly, accumulating literature evidences suggest that engineered cellular nanovesicles with tailored immunomodulatory ligands could achieve effective immunomodulation without the need of additional exogenous drugs260. For instance, Chen et al.261 developed PD-L1/CTLA-4 dual targeting cellular nanovesicles which specifically bind to PD-1 and CD80 on T cells and DCs respectively, for immunosuppressive therapy in organ transplantation. In this study, HEK 293 T cells were engineered to express PD-L1/CTLA-4 by lentivirus transduction; the secreted cellular nanovesicles were found to inhibit the activity of T cells followed by maintaining immune tolerance on these two immune checkpoints. It was demonstrated that the PD-L1/CTLA-4 cellular nanovesicles inhibited CD8+ T cell proliferation and cytokine production, enriched differentiation of Tregs, and eventually prolonged the survival of mice with skin or heart transplantation261. Based on the activation of T cells by non-self-antigens, cellular nanovesicles addressed with tumor associated antigens were utilized as an immunogenic vaccine for cancer treatment262. The Factor VIII–like C1C2 domain of milk fat globule epidermal growth factor–factor VIII (MFG-E8)/lactadherin was genetically expressed on EVs. These EVs demonstrated a significantly higher immunogenicity than soluble antigens. EVs engineered with MFG-E8 acted as an activator with a potent immunomodulatory ability for cancer treatment. The authors proved that these MFG-E8 EVs promoted the proliferation of CD8+ T cells and stimulated the production of IFN-γ. In a recent study, Liu et al.263 engineered a DC-EV-based antigen self-presentation and immunosuppression reversal platform for cancer immunotherapy. DC-EVs were obtained from mature DCs which were genetically engineered with GFP-labeled antigens on the membrane. Due to the presence of adhesion molecules, engineered DC-EVs could rapidly accumulate in lymph nodes and induce an antigen-specific immune response by activation of CD8+ T cells. Interestingly, unlike conventional vaccine delivery methods, this engineered DC-EV-based vaccine could bypass the process of antigen presentation by APCs and directly deliver antigens to T cells263.
Genetic engineering of parent cells to express signal proteins has also been demonstrated as an efficient approach to produce cellular nanovesicles for avoidance of immune recognition and prolongation of their circulation time264. For example, CD47 that is a “don't eat me” signal and can enable immune escape by binding to the signal regulatory protein α (SIRPα) of mononuclear phagocytes could be engineered on the EVs. Owing to the blockage of the phagocytic signaling pathway, CD47 expressed EVs exhibited a long blood circulation time and high accumulation in tumor tissues265. In addition, these engineered EVs showed minimal internalization by the mononuclear phagocyte system but allowed effective activity on cancer cells when being loaded with a drug.
Cellular nanovesicles could be genetically engineered with not only surface proteins, but also intravesicular cytokines for immunomodulation applications266. For example, EVs were loaded with IL-10 (IL-10+ EVs) by engineering the parent macrophages266. IL-10+ EVs could stabilize IL-10 and protect against acute kidney injury caused by ischemia, cisplatin, or ureteral obstruction. The mechanism was based on the capability of IL-10+ EV to attenuate the production of inflammatory cytokine and to reduce the infiltration of immune cells to the acute kidney injury site. Meanwhile, the adhesion molecules on IL-10+ EVs surface including integrin α4β1, α5β1, αLβ2 and αMβ2 could effectively improve their targeting and accumulation to the injured kidney tissues. The IL-10 carried by IL-10+ EVs inhibited the activity of mTOR, thereby mitophagy was promoted to maintain mitochondrial fitness in tubular epithelial cells. Macrophages were also polarized toward the M2-like phenotype by the IL-10+ EVs that resulted in the inhibition of inflammation in the tubulointerstitium266.
Apart from the abovementioned approaches to engineer parent cells by plasmid transfection or virus transduction, a recent study suggested that parent cells can be irradiated to secret mutant cellular nanovesicles, offering a new strategy to engineer cellular nanovesicles for immunomodulation267. Specifically, Yang et al.267 investigated the immunoregulatory effect of irradiated cancer cell nanovesicles (ICNs) that functioned via a bystander mechanism to induce ferroptosis of peripheral tumor cells. Strikingly, ICNs also activated the Jak-STAT and MAPK signaling pathways that are involved in the control of inflammation responses. Moreover, TAMs were also repolarized to the M1 phenotype by these ICNs. In a different study, it was found that radiation induced cancer cell-derived EVs expressed a tumor associated antigen, CUB domain-containing protein 1 (CDCP), which had a low expression level in para-carcinoma tissues268. These EVs induced effective antigen presentation and T cell activation and inhibited metastasis in various tumor models including lung cancer and colorectal carcinoma.
5.2. Surface modification
Compared with genetic engineering of parent cells, surface modification via chemical engineering approaches represents a more direct means to produce engineered cellular nanovesicles with tailored activities. Fundamentally, membrane components including proteins and lipids on the surface of cellular nanovesicles bear reactive chemical groups, such as hydrosulphonyl-, carboxyl- and amino-groups34,248,249,269. These reactive groups can react with functional groups on exogenous molecules (e.g., nucleic acids, peptides, proteins) to form stable covalent bonds, leading to the anchoring of such exogenous molecules34. This mechanism has enabled surface modification of cellular nanovesicles using different exogenous molecules including small molecules249, nucleic acids270, peptides271, proteins272, and even nanoparticles273. For example, Fan et al.274 functionalized M1-like macrophage-derived EVs with a DNA hinge sequence through a Michael-Addition reaction on the hydrosulphonyl group. Subsequently, quantum dots (QDs) with streptavidin were anchored onto the surface of EVs via reorganization to the biotin of DNA hinge to form quantum dots labeled EVs (EV-DNA-QDs). Upon co-incubation with EV-DNA-QDs, cancer cells were selectively imaged within 3 h by swift engulfing of the EV-DNA-QDs. Tian et al.275 reported that M2-like macrophage-derived EVs, namely Cur@EXs-cl-NGF, were conjugated with nerve growth factor (NGF) through an oligomeric peptide responsive linker which could be cleaved by matrix metalloproteinase 9 (MMP9) in the inflammatory microenvironment (Fig. 5b). The terminal maleimide group on the peptide linker reacted with the cysteine residue of NGF, resulting in the conjugation between transforming growth factor (TGF) and the peptide linker. The NGF-peptide linker was further conjugated to the EVs via the click reaction between the terminal NHS group of the peptide linker and the carboxyl group on EVs membrane. Cur@EXs-cl-NGF loaded with an anti-inflammatory molecule (curcumin) accumulated in the spinal cord inflammation sites and released NGF by MMP9 cleavage. Treatment by Cur@EXs-cl-NGF led to an elevated ratio of M2 to M1 macrophages in the injured spinal cord that resulted in a neuroprotection effect for alleviating spinal cord injury symptoms.
Except for chemical modifications of existing reactive groups on the surface of cellular nanovesicles, exogenous active groups can be introduced for orthogonally grafting with functional elements in a specific condition276, 277, 278, 279. Generally, these active groups can be grafted in a mild condition without significantly interfering the natural biological functions of parent cells or cellular nanovesicles. For example, cancer cellular nanovesicles with azido groups were constructed by the conjugation of Boc-4-azido-l-phenylalanine280. The resultant modified nanovesicles were subsequently anchored onto the surface of PLGA microspheres by the azido-alkyne click reaction, forming nanovesicle-coated PLGA microspheres. The nanovesicles decorated with microspheres exhibited an enhanced internalization ability by APCs and triggered macrophages and DCs to produce IL-6 and TNF-α, respectively. In a different case, Xiong et al.281 developed a strategy for surface modifying cellular nanovesicles with azido groups via a glucometabolic mechanism. Specifically, azido-modified cellular nanovesicles were obtained from parent HEK-293 T cells by treating the cells with azide mannose; the cellular uptake of azide mannose led to the distribution of azido groups on cellular membrane.
In addition to covalent interaction, noncovalent interactions including electrostatic interactions, hydrophobic interactions, and hydrogen-bonding have been explored as another efficient method to surface engineer cellular nanovesicles282, 283, 284. Cheng and co-workers285 synthesized a chimeric nuclear-localizing signaling peptide modified with a long alkane chain and a photosensitizer (porphyrin) which was anchored on the membrane of cancer cell-derived EVs by electrostatic interaction. The guanidine and amino groups of the peptide had a positive charge and could interact with the negatively charged EVs. In addition, the long alkane chain enabled a tight interaction with the cellular nanovesicles by embedding into the membrane. These modified nanovesicles could cross the target cell membranes and penetrate the karyotheca to accumulate in nuclei with the assistance of the nuclear-localizing signal peptide285. Moreover, PEGylated molecules could also be introduced to the EV surface by insertion of lipids. Kooijmans et al.283 designed a nanobody-PEG-lipids conjugate to decorate EVs. The lipid fragment of the nanobody-PEG-lipids was inserted into the lipid bilayer of EVs to form EGFR targeting EVs by taking advantage of the temperature-dependent lipid fluidity. On account of the shielding effect of PEG, PEGylated EVs showed reduced clearance and enhanced specific targeting to EGF-overexpressed tumor cells.
Ligand-receptor mediated binding has emerged as another promising strategy to surface engineer cellular nanovesicles286, 287, 288. For example, synthetic targeting peptides specifically targeting characteristic EV surface proteins have been demonstrated effective in linking functional cargos onto EVs. Based on this strategy, functional molecules could be anchored on the surface of EVs via the linkage of targeting peptide. Gao et al.286 designed a targeting peptide, namely CP05, which was modified with phosphorodiamidate morpholino oligomer (PMO) or a muscle-targeting peptide M12. CP05 could enable the efficient binding of PMO/M12 on the surface of EVs from different sources of cell lines. Mechanistically, the targeting peptide CP05 could recognize and bind to CD63 which was expressed on most EVs that leads to the anchoring of PMO and M12 on EV surfaces. As compared to the naked PMO or free CP05-PMO, modified EVs containing PMO increased the level of dystrophin by 18 times in quadriceps of dystrophin-deficient mdx mice. In addition, EVs modified with CP05-M12 boosted the targeting of M12 to the muscle and improved the functional dystrophin level. In a different study, high mobility group nucleosome-binding protein 1 (HMGN1) was covalently conjugated to the CP05 peptide and was subsequently linked onto tumor cell-derived EVs to activate cytotoxic T cells289. This approach increased the number of DCs and memory T lymphocytes in lymphoid tissues and exhibited a conspicuous immunotherapeutic effect for treating large solid tumors289.
5.3. Drug encapsulation
The cavities of cellular nanovesicles can provide enough capacity to contain various molecular cargos. Cargos such as proteins, nucleic acid and small molecules can be encapsulated into cellular nanovesicles290, 291, 292. The membrane of nanovesicles can provide a stable and functional membranous shell ultimately leading to an extended blood circulation and improving targeting ability. However, loading of exogenous cargos into cellular nanovesicles is challenging owing to the presence of the membrane barrier293. To overcome this challenge, several approaches have been developed to load payloads inside the nanovesicles294,295. For example, due to molecular fluidity of phospholipids on the vesicle membrane, cargos could be directly loaded into the cavity of cellular nanovesicles. Using this strategy, miRNA-150 was used as an immune suppressor encapsulated in CD8+ T cell-derived EV-like nanovesicles to suppress the activity of effector T cells296. The encapsulated miRNA-150 disrupted the function of effector T cells to specifically target antigens and caused T-cell tolerance in an allergic cutaneous contact sensitivity model. Noticeably, hydrophobic molecules could be more easily loaded using a direct incubation method as compared to hydrophilic molecules. This phenomenon has been used to improve the loading of natural or modified hydrophobic molecules into nanovesicles. Naturally hydrophobic molecules such as paclitaxel, doxorubicin, and curcumin indeed exhibit a high encapsulation efficiency into cellular nanovesicles297, 298, 299. Interestingly, hydrophilic molecules can also be modified to become more hydrophobic which allows their direct loading into nanovesicles. Khvorova et al.300 modified the 3′ end of siRNAs with cholesterol (a hydrophobic lipid) to enhance its hydrophobicity (Fig. 5c). It was found that the modified synthetic siRNAs could be directly loaded into U87 glioblastoma cell-derived EVs by simple incubation.
Electroporation is an effective method to load hydrophilic molecules in cellular nanovesicles. Electroporation causes the transient formation of small pores on the nanovesicle membrane301. Cargos could permeate into nanovesicles through the opening pores. Based on this method, Kalluri et al.302 reported the loading of siRNA into clinical grade Evs derived from bone marrow MSCs. In this case, the siRNA enclosed in EVs showed efficient targeting and inhibition of the oncogenic Kras and significantly prolonged the animal survival when being used in combination with chemotherapy in a pancreatic cancer model. Similar to electroporation, sonication is another method to induce transient membrane opening for molecule encapsulation into cellular nanovesicles303, 304, 305. However, aggregation of EVs caused by the electroporation method was 12-fold higher as compared to that caused by the sonication method. Hence, sonication seems to provide a higher efficiency and cause less precipitation or aggregation of cargo molecules303. For example, under ultrasound, TGF-β and IL-10 could cross the membrane through transient gaps and enter the lumen of DC-derived EVs from various DC subtypes including immunoregulatory DCs, immunostimulatory DCs, and immature DCs306. Immunoregulatory DC-derived EVs shuttled these immunoregulatory cytokines to the inflamed tissue which interfered with the activation of DCs in vivo in a chronic bone degenerative disease model. Specifically, recruitment of Tregs to the inflammation site was enhanced while the maturation of recipient DCs and induction of Th17 effector cells were inhibited, following the dosing of immunoregulatory EVs. These eventually led to the inhibition of bone resorptive cytokine production and reduction in osteoclastic bone loss306.
Repeated freeze-thaw cycling represents another approach to load cargos to cellular nanovesicles. As compared to other methods, co-incubating cargos with nanovesicles under cycles of freeze-thawing usually can better preserve the bioactivities of the therapeutic cargos, although the loading efficiency using this method is relatively low307, 308, 309. Diverse types of cargos including hydrophobic drugs and natural enzymes have been successfully encapsulated using this method308,310. For example, catalase was encapsulated into macrophage-derived EVs by freeze-thaw cycles which resulted in a significantly higher loading efficiency (14.7 ± 1.1%) as compared to that achieved by the direct incubation method (4.9 ± 0.5%)310. After catalase loading, macrophage-derived EVs maintained their stable nanoscale EV-like structure which led to the internalization by PC12 cells. The encapsulation by EVs provided a protection from protease degradation. However, interestingly, not many reports have described loading nucleic acids into nanovesicle using this approach, possibly owing to the instability of nucleic acids under repeated freeze-thawing conditions301. Of note, this method may cause aggregation of nanovesicles and/or cargo molecules if not designed appropriately299.
Apart from the aforementioned methods, chemical-induced transient membrane permeabilization has emerged as a novel approach for loading therapeutics into cellular nanovesicles. For example, saponin has been demonstrated to enhance the permeability of EV membranes while not significantly influencing the morphology of EVs. Using this method, catalase was efficiently encapsulated in M-EVs with an efficiency of 18.5 ± 1.3%310, which could be readily internalized by neuronal cells and against oxidative stress/inflammation leading to a neuroprotection effect for Parkinson's disease therapy. Noticeably, in the same study, a direct comparison of different payload loading methods revealed that the saponin-assisted method resulted in a higher efficiency as compare to the direct incubation or freeze-thaw cycling method310. However, saponin may bind to the cholesterol on EV membrane and form a complex which is typically considered as an unintended byproduct. Therefore, the use of saponin may cause the instability issue if not controlled appropriately. In contrast, hypotonic dialysis was utilized to load cargos with a significantly improved loading efficiency while, in the meantime, might avoid EV aggregations and payload inactivation294. Hypotonic dialysis involves treating EVs in a hypotonic solution that would lead vesicles to form pores while not undergoes apparent lysis. For instance, Stevens and Fuhrmann et al.311 reported that EVs derived from various cells could be mixed with a model drug (porphyrin) in a hypotonic solution (10 mmol/L phosphate buffer, pH 7.4) under continuous stirring at room temperature for 4 h to construct drug-loaded EVs. Compared with other methods including the electroporation and saponin-assisted methods, hypotonic dialysis is considered as a straightforward method which has demonstrated higher loading efficiency (>11-fold enhancement over the passive incubation method) with preservation of active cargo activity.
5.4. Strategies to improve the delivery of cellular nanovesicles
Significant progress has been made in the past decade to leverage cellular nanovesicles as a delivery system for immunomodulatory mediators including microRNAs, proteins, and small molecules293. Cellular nanovesicles, especially EVs, possess unique homing ability that are suitable for systemic administration and accumulation in target tissues312. Further, therapeutic cellular nanovesicles have similar functional elements to the parent cells, which can readily fuse with or be engulfed by recipient cells to modulate their immune behavior313. Notably, owing to their nano-size and biological origin, cellular nanovesicles possess outstanding capabilities to navigate biological barriers to reach target tissues314. Usually, cellular nanovesicles can inherit the intrinsic targeting capability of their parent cells, enabling their preferable accumulation in corresponding tissues315. For example, cellular nanovesicles derived from MSCs can naturally target inflammatory sites and macrophage-derived nanovesicles can naturally traffic to tumor tissues316,317. Further, because of their intrinsic function for intercellular communication, cellular nanovesicles may more efficiently overcome the cell membrane barrier for intracellular internalization upon reaching target tissues, as compared to synthetic nanocarriers12. As internalization of cellular nanovesicles by target cells is critical for their biological activities, an adequate therapeutic dose and circulation time are required after their intravenous, intraperitoneal or subcutaneous injections318. However, the majority of cellular nanovesicles are still subject to the rapid clearance by the mononuclear phagocyte system (MES)319. Even though cellular nanovesicles can be applied through non-intravascular routes such as to the skin or ocular surface, they are ultimately swept by body fluids resulting in a short half-life312. Hence, various delivery strategies have been developed to stabilize and functionalize cellular nanovesicles for sustained delivery320. Modification with nanogel on the surface of cellular nanovesicles is one such strategy for efficient EV delivery321. For example, macrophage-derived EVs were self-assembled with an amphiphilic nanogel which offered an effective EV delivery platform312. In this case, the nanogel was considered as a molecule chaperone that were bound on the surface of EVs by electrostatic interactions forming a hybrid delivery system, which was subsequently internalized by target cells via the caveolae-mediated endocytic pathway and exhibited a concentration-dependent delivery efficiency. Based on this method, PC12 cell-derived EVs were introduced into the nanogel-mediated delivery system312. Human adipocyte-derived MSCs were differentiated toward neuron-like cells (hADSCs) through the uptake of PC12 cell-derived EVs via the nanogel-mediated delivery. As compared to the naked EVs, the nanogel-delivered EVs led to an enhanced expression of nerve-specific enolase (a neuronal differentiation marker) in hADSCs312. In another study, PC12 cell-derived EVs were hybridized with a magnetic nanogel and exhibited more precise targeting to the specific cells to promote differentiation from hADSCs into neuron-like cells322. Non-invasive magnetic force facilitated the internalization of magnetic nanogel bound EVs by hADSCs in different orientations.
Although immobilization of nanogels to EVs could improve accurate targeting and efficient uptake, it still remains a challenge to avoid rapid clearance by the MES and to achieve controlled delivery to target tissues323, 324, 325. To solve these challenges, hydrogels have been prepared through the cross-linking of natural or synthetic polymers which provide a three-dimensional (3D) scaffold for controlled delivery and release of cellular nanovesicles320. Hydrogel loaded with functional cellular nanovesicles could be constructed and implanted into lesion sites, such as injured spinal cord and skin wounds, for efficient retention and sustained drug release326,327. For example, Shen et al.328 demonstrated that BMSC-derived EVs were persistently released from a bilayer hydrogel for wound healing (Fig. 5d). Both natural and miR-29 b-3p-enriched BMSC-derived EVs were mixed with the monomer and photo-initiator to fabricate the bilayer hydrogel under transitory UV-irradiation. When the EV-loaded hydrogel was implanted into the full-thickness skin wound, natural BMSC-derived EVs were released from the lower layer to promote angiogenesis and collagen deposition by accelerating fibroblast and endothelial cell proliferation and migration during the early inflammation and proliferation phases; meantime, engineered BMSC-derived EVs were released from the upper layer of the hydrogel to induce polarization of macrophages toward the M2-like phenotype which facilitated wound healing. As another example, a recent study by Wang et al.329 demonstrated that a self-healing hydrogel with dynamic covalent bonds exhibited an outstanding adhesive property and promoted wound healing when being loaded with MSC-EVs. In this study, the hydrogel loaded with MSC-EVs was applied in the skin wound and showed a strong adhesive interaction with the wound site, which provided a depot for sustained release of EVs to facilitate granulation growth329.
Hydrogels can be prepared by mixing with EVs and subsequently implanted to cover the external tissue, such as the skin. However, it is hard to implant bulky hydrogel for delivery of EVs toward intracorporal organs or tissues. Deformability and injectability are indispensable for hydrogels to achieve the stable delivery and sustainable release of EVs in deep tissues. Toward this end, injectable hydrogels with cellular nanovesicles have been developed to adapt to different therapeutic application requirements, such as repair of bone defects330, protection of endometrial microenvironment331, cardiac repair332, and restoration of cornea333. For example, VEGF and transcription factor EB (TFEB) engineered, endothelial cell-derived EVs were immobilized into an injectable methylcellulose hydrogel to promote angiogenesis for rescuing muscle injury and recovering blood flow in limb after critical limb ischemia334. The methylcellulose hydrogel enabled to stabilize endothelial cell-derived EVs at different temperatures. Because of the ability of VEGF and TFEB to inhibit the production of proinflammatory cytokines, engineered EVs released from the hydrogel led to the downregulation of pro-inflammatory cytokines (such as TNF-α, IL-1β, and IL-6) and upregulation of anti-inflammatory cytokine (such as IL-10, TGF-β1, and IL-4) in the muscle tissue after limb ischemia. As another example, dynamic covalent bonds benefited to hydrogel's shear-thinning ability which was utilized as an injectable to boost the healing of full-thickness cutaneous wounds caused by diabetes335. Wang et al.335 reported that oxidized hyaluronic acid with poly-ε-l-lysine was prepared to form a hydrogel for delivery of adipose mesenchymal stem cell-derived EVs (AMSC-EVs) by dynamic covalent crosslinking based on reversible Schiff base reaction. With the combination of thermal-responsive molecules (F127), the hydrogel not only exhibited the self-healing performance and thermal-responsive gelation, but also enabled to provide a pH-responsive condition for long-term release of AMSC-EVs. In addition, the injectable thermosensitive hydrogels enabled to effectively stabilize miRNAs and proteins in MSC-EVs333,336. Thermosensitive chitosan hydrogel was designed as an injectable scaffold for the delivery of human MSC-EVs with higher retention and stability. After injection of the hydrogel containing MSC-EVs into the cornea, miR-432–5p in the MSC-EVs persistently suppressed the expression of translocation-associated membrane protein 2 and accelerated wound healing333.
The aforementioned hydrogel-mediated delivery strategies are based on ex vivo formed hydrogel systems. In addition, cellular nanovesicles can be mixed with individual hydrogel components to form hydrogel in situ at target sites which is suitable for local delivery of cellular nanovesicles. For example, Silva et al.337 developed a Pluronic F127-based in situ gelation strategy to deliver stem cell-derived EVs for colocutaneous fistula therapy. After mixing stem cell-derived EVs with pluronic F127 and subsequently injecting them into the colocutaneous fistula, gelation was implemented in situ at the physiological temperature. In this study, EVs were labelled with a radiotracer, and the biodistribution study demonstrated that EVs delivered by this thermo-responsive hydrogel showed the highest retention at the injection sites as compared to EVs formulated in saline after percutaneous injection. Yang et al.338 reported an in situ hydrogel system formed by gelatin cross-linking with o-nitrobenzyl alcohol moiety modified hyaluronic acids which could be photoinduced to form a hydrogel glue. This hydrogel served as a tissue-like patch and provided a 3D scaffold for controlled release of MSC-derived EVs; this hydrogel-EV system exhibited therapeutic effects in repairing cartilage defects. Based on the plasticity of hydrogels, it conveyed that submucosa mixed mesoporous bioglass in combination with cryogenic 3D bioprinting technology containing nanovesicles contributed to the growth of HUVECs to heal diabetic wounds339.
In addition to the abovementioned delivery strategies, new advances in the area of bio-fabrication have stimulated the development of novel approaches to enable precise control of the spatiotemporal delivery of cellular nanovesicles340, 341, 342. For example, Cheng and co-workers designed a novel EV-eluting stent to immobilize, deliver, and bio-responsively release MSC-EVs at the implantation site for vascular healing after ischemic injury343, based on the rationale that delivery of EVs for vascular healing involves efficient enrichment, stable transformation and long-term release. In this approach, MSC-EVs were functionalized with PEG and immobilized onto EV-eluting stent via a reactive oxygen species (ROS) responsive linker. Compared to the bare metal stents, EV-eluting stent efficiently enriched and ROS-responsively released MSC-EVs at the injured aortas. This system demonstrated effective stimulation of endothelialization, restoration of injury cells, and attenuation of vascular smooth muscle cell migration. Meanwhile, MSC-EVs eluted from the EV-eluting stent promoted polarization of M2-like macrophages and reduced inflammation which benefited to the vascular healing in an atherosclerosis model343.
Administration method is also an important consideration for targeted delivery of cellular nanovesicles. A suitable administration method can be rationally selected depending on the intended target tissues for nanovesicle delivery. Generally, following systemic administration, cellular nanovesicles can be recognized by the MES such as liver macrophages344. Hence, nanovesicles prefer to accumulate in the liver after intravenous injection, followed by the presence in the spleen, gastrointestinal tract, and lung345. However, an over-dose of nanovesicles can result in lower liver accumulation because of the saturation of the phagocytic uptake by liver macrophages345. In addition to intravenous administration346, other methods such as intraperitoneal347, 348, 349, intranasal350, intramuscular351, 352, 353, and subcutaneous injections354, 355, 356 can lead to preferable accumulation of cellular nanovesicles around injection sites and/or in different organs depending on the type of nanovesicles345,357. For example, as for another administration route, MSC-EVs were administered into rats by intratracheal injection and observed to mainly accumulate in the airway of the lung tissue358. MSC-EVs were then internalized by pulmonary cells within 2 h and persisted for at least 72 h. Using this method, MSC-EVs exhibited remarkable anti-inflammatory efficacy in bronchopulmonary dysplasia and allergic airways inflammation359,360. Overall, establishment of a suitable administration route according to the delivery requirements can improve the therapeutic efficacy of cellular nanovesicles.
5.5. Artificial cellular nanovesicles
As described in the previous context, cellular nanovesicles have been brought to the fore by data demonstrating the rapid rise of potential applications in functional immunotherapy32,35,361. However, low yield production in the microgram level and multi-steps required for the isolation and purification process tempered it in the clinical translation33. To this end, artificial or EV-mimetic cellular nanovesicles, which are fabricated from physically breaking the parent cells down to a nano-size, are emerging as a potential alternative to circumvent the drawbacks of natural cellular nanovesicles (EVs). The fabrication strategies to produce artificial cellular nanovesicles can be broadly categorized to bottom-up and top-down approaches. Towards the bottom-up strategy, individual amphiphilic molecules self-assemble into cellular nanovesicles accompanied with addition/modification of functional surface proteins362. The bottom-up strategy has been comprehensively reviewed elsewhere33,363; here we majorly discuss the top-down strategy.
The top-down methodologies are described as a method to construct artificial nanovesicles using additional mechanical forces364. Generally, cell membranes obtained from parent cells are extruded through a serial filter with different pore sizes or are mechanically broken down by nitrogen cavitation or sonication to form nanovesicles with reframed contents. These methods can achieve a >10-fold higher production efficiency as compared to the conventional method of isolating natural EVs by gradient centrifugation365, 366, 367. During the extrusion process, functional elements can be built into extruded cellular nanovesicles368. Meanwhile, endogenous or exogenous therapeutic molecules from parent cells such as microRNAs, proteins, and small molecules can also be carried into extruded cellular nanovesicles369. For example, Jang et al.370 loaded a chemotherapeutic drug doxorubicin (DOX) into monocytes/macrophages and produced DOX-carrying nanovesicles via serial extrusion. They proved that this extrusion method resulted in a 100-fold higher production efficacy as compared to the ultracentrifugation-based natural EV isolation. In addition, they demonstrated that these nanovesicles improved the delivery of DOX to the tumor site and led to a better immune stimulation and antitumor efficacy in a CT26 tumor model as compared to liposomal DOX370. In a different example, ferroptosis-inducing Fe3O4 was incorporated into extruded M1 macrophage-derived nanovesicles for cancer immunotherapy255. In this case, with the addition of hydrogen peroxide, the Fenton reaction was initiated by the hydrophilic Fe3O4 nanoparticles which synergistically regulated the polarization of tumor-associated macrophages toward a M1-like phenotype for cancer therapy. For handy production of cellular nanovesicles with high yield, a device operated under centrifugation was designed by Park et al371. In this device, cellular nanovesicles were generated by forcing cells to go through the micro-sized polycarbonate filter under centrifugation. It was demonstrated that contents from the parent cells could be retained in the cellular nanovesicles produced by this device and could be delivered to recipient cells as efficient as natural cellular nanovesicles (EVs).
Besides extrusion of living cells or cell membrane through filters, nitrogen cavitation was considered as a powerful method for large-scale production of artificial cellular nanovesicles. Wang et al.372 developed a method to disrupt cells in a nitrogen cavitation chamber for the generation of nanovesicles. By using this method, neutrophil nanovesicles were produced and investigated as a delivery system for an anti-inflammatory drug piceatannol373. It was demonstrated that the piceatannol-loaded neutrophil nanovesicles dramatically alleviated acute lung inflammation and sepsis induced by lipopolysaccharide (LPS). Moreover, sonication is another effective method to generate artificial cellular nanovesicles. For example, Go et al.374 employed a sonication method to produce monocyte-derived nanovesicles which were subsequently loaded with dexamethasone using a remote loading method. They demonstrated that these nanovesicles mitigated gram-negative bacterial outer membrane vesicle-induced systemic inflammatory response syndrome374. It should be noted that all the aforementioned methods need to be done manually. Excitingly, several novel technologies are emerging to achieve an automated process for isolating and analyzing cellular nanovesicles. One such approach is the incorporation of the microfluid technology375. This approach could improve production efficiency and avoid the use of high-cost equipment. Moreover, microfluidic devices have also been designed for trapping cellular nanovesicles by immune affinity or sieving376. For example, He and co-workers377 developed an automated microfluidic system capable of integrating real-time cell culture, highly efficient isolation of cellular nanovesicles, and antigenic modification. This microfluidic system offered the potential to engineer cellular nanovesicles as an effective cancer immunotherapy377.
6. Emerging therapeutic immunomodulatory applications
Cellular nanovesicles can be produced from almost all cell types, and it is even possible to induce artificial production from cell membranes378. Recent advances in the area of isolation technology development and functionalization strategies have provided the possibility to design tailored cellular nanovesicles for controllable modulation of the immune responses379. Because of this, cellular nanovesicles have emerged as an immunomodulatory therapeutic approach for treating different diseases, such as cancer, autoimmune disease, infectious diseases, and trauma and injury380.
6.1. Cancer immunotherapy
Cancer cells with abnormal metabolism form the complex tumor microenvironment (TME) that is regarded as a relatively isolated, immunosuppressive space381, 382, 383. Cellular nanovesicles can permeate and accumulate in solid tumors, making them promising candidates as new cancer therapies32,384,385. Along this line, natural or artificial cellular nanovesicles from multiple immune cells (e.g., T cells114,386, NK cells141,387, 388, 389, 390, macrophages147,148,391, DCs392, 393, 394, 395, 396, 397, 398, or cancer cells399, 400, 401) have been proved effective in treating different tumors. Cancer cell-derived EVs have been found to present specific tumor antigens to CD8+ T cells via DCs for enhancement of antitumor immunity402, and Hsp 70 on the surface of cancer cell-derived EVs was shown to activate NK cells and macrophages. However, other surface ligands and intracavitary miRNAs in natural tumor cell-derived EVs exert immunosuppressive effects on surrounding immune cells, such as apoptosis of cytotoxic T cells, reduced cytotoxicity of CD8+ T cells and NK cells, reprogramming of M2-like macrophages, and enhanced proliferation of Treg cells403. In addition, exosomal PD-L1 from melanoma and prostate cancer cells has been reported to bind to PD-1 on the surface of CD8+ T cells to weaken the antitumoral functions and support immune escape404.
The TME is an immunosuppressive environment, whereas cancer immunotherapy often relies on immunoactivation. Therefore, one of the crucial aims of cancer immunotherapy is to activate effector immune cells and to change the immunosuppressive setting of the TME. For example, DC-derived EVs enabled to interact with immune cells, such as CD8+ T cells and NK cells, to stimulate potent immune response for cancer immunotherapy126. Compared with natural DC-derived EVs, Lu et al.405 isolated EVs from DCs transduced with α-fetoprotein by lentivirus. These engineered EVs exhibited a higher expression of MHC I, MHC II and co-stimulatory molecules on their surface. α-Fetoprotein engineered DC-derived EVs enabled to facilitate the production of IL-2 to enhance the proliferation of T cells. TME was modulated by these engineered DC-derived EVs owing to the enhanced production of IFN-γ from CD4+ T cells and CD8+ T cells. The immune response triggered by these engineered EVs inhibited tumor growth and prolonged the survival of mice bearing hepatocellular carcinoma. As another example, Xie et al.406 employed nano-conjugated M1-EVs to modulate the function of macrophages in the TME for cancer treatment. They linked M1-EVs and CD47/SIRPα antibodies using a pH-sensitive covalent linker that, when cleaved, released the CD47 antibodies and SIRPα antibodies to block the CD47-SIRPα signaling pathway. Phagocytosis of tumor cells by macrophages was promoted, and the antibodies blocked the CD47 on tumor cells from interacting with SIRPα on macrophages. TAMs were polarized to the M1 phenotype in the presence of the modified M1-EVs. SIRPα variants were also attached to the fused cellular nanovesicles, creating hybrid M1-EVs with multiple functions that showed antitumor efficacy407 (Fig. 6). These hybrid cellular nanovesicles not only blocked the CD47-SIRPα signaling but improved the activation of CD8+ and CD4+ T cells.
Figure 6.
Hybrid cellular nanovesicles for cancer immunotherapy. (a) Hybrid cellular nanovesicles were prepared by fusion of SIRPα-expressed cancer cell-derived nanovesicles, polarized M1-like macrophage-derived nanovesicles and platelet-derived nanovesicles. (b) Hybrid cellular nanovesicles show accumulation at tumor sites and exert immunomodulation for tumor therapy. Reproduced with permission from Ref. 407. Copyright © 2020, Springer Nature.
In addition to directly activate T cells, engineered cellular nanovesicles expressing PD-1 receptors have also been used to alter the immunosuppressive TME408. PD-1 receptors were overexpressed on engineered HEK293T cell-derived EVs that had a long circulation time to avoid the depletion of CD8+ T cells (via PD-L1 interaction), and the engineered nanovesicles induced the infiltration of lymphocytes into the tumor margin. In another study, M1-EVs were loaded with a pH-responsive fusion protein and siRNA through electroporation for cancer immunotherapy (Fig. 7a)409. The modified M1-EVs could responsively activate the fusion protein to fuse with tumor membranes in the TME so that the anti-PD-L1 siRNA could be internalized by target tumor cells to block the PD-L1-mediated immune escape. After systemic administration, the M1-EVs showed the ability to stimulate the repolarization of M2-like macrophages to the M1 phenotype. CD8+ T cells also showed increased secretion of IFN-γ, demonstrating the antitumor efficacy of the M1-EVs.
Figure 7.
Cellular nanovesicle-based immunomodulatory therapy for treating various disease, including cancer, autoimmune diseases, infectious diseases, and injury and trauma diseases. (a) Schematic illustration of M1-EVs engineered with CD47 and SIRPα antibodies for cancer immunotherapy. Reproduced with permission from Ref. 409. Copyright © 2020, Wiley. (b) Schematic illustration of the immunomodulatory effect of MSC-EVs on the proliferation of Tregs for treating multiple sclerosis. Reproduced with permission from Ref. 454. Copyright © 2019, American Chemical Society. (c) Schematic illustration of EVs expressing ACE2 and CD147 as nano-decoys to prevent SARS-CoV-2 from entering into host cells. Reproduced with permission from Ref. 472. Copyright © 2020, American Chemical Society. (d) Schematic illustration of MSC-EVs encapsulated into hydrogels and injected into the cardiac tissue for prolonged retention to treat heart attack. Reproduced with permission from Ref. 332. Copyright © 2021, Springer Nature.
Notably, cellular nanovesicles can be loaded with different cargos and possibly serve as tumor antigen adjuvants. Morishita et al.410 genetically engineered tumor cell-derived EVs to carry a streptavidin-lactadherin fusion protein on their surface. Biotinylated CpG DNA (an immunostimulant) was then attached to EV surface via the biotin-streptavidin interaction. With the original tumor antigens inside, these engineered EVs were taken up by APCs, which subsequently activated a cytotoxic T cell response. These genetically engineered EVs elicited a potent immune response that was active against tumor cells in vivo. Therefore, this approach could be developed to produce novel vaccines to treat cancer.
6.2. Autoimmune diseases
Autoimmune diseases occur when the immune system mounts a response to a self-antigen due to aberrant immunoregulation, which can lead to tissue damage. Cellular nanovesicles play key roles in the regulation of cellular signaling and inhibition of enzymatic activity by mediating the interactions between various cells. Therefore, cellular nanovesicles can potentially deliver immunosuppressive drugs, cytokines, or other molecules to treat autoimmune diseases411, 412, 413, 414, 415. Indeed, cellular nanovesicles from diverse cell types such as macrophages416, 417, 418, MSCs419, 420, 421, 422, 423, and T cells424 have shown potential in treating a wide spectrum of autoimmune disorders including rheumatoid arthritis425, 426, 427, 428, 429, systemic lupus erythematosus430, 431, 432, 433, 434, multiple sclerosis162,435,436, diabetes437, 438, 439, among others440, 441, 442, 443.
Rheumatoid arthritis is an autoimmune disease caused by chronic inflammation in the joints444. Researchers have developed engineered HEK293T cell-derived EVs that carry cytokines, such as IL-4, to repolarize M1 macrophages to the M2 phenotype, resulting in an immunosuppressive effect in the inflamed joint445. Because of their stable structure and long circulation time, these HEK293T cell-derived EVs showed higher anti-inflammatory efficacy than the soluble IL-4. With biorthogonal modifications, dibenzocyclooctyne-conjugated dextran sulfate (DBCO-DS) reacted with the azide-conjugated mannose on the surface of stem cells, resulting in dextran sulfate-functionalized stem cells. These engineered stem cell-derived EVs showed a greater ability to accumulate in the inflamed joint and acted through a series of anti-inflammatory effects at lower doses than the unmodified EVs (Fig. 8)446. Engineered EVs have carried polarization-related miRNA to affect the repolarization of macrophages in vitro and in vivo. In particular, miR-7b-5p and miR-24–3p were verified as potent miRNAs in EVs for the M1 to M2 phenotype change of macrophages. In addition, modulating macrophages to reduce inflammation in rheumatoid arthritis, efforts have also been made to reduce the number of autoreactive T cells by including OX40 in engineered cellular nanovesicles447. Blocking the OX40–OX40L interaction successfully inhibited tissue infiltration and migration of T cells. Hence, the activation of autoantigen-specific T cells was attenuated by these engineered cellular nanovesicles, which could be utilized as a cell-free immunotherapeutic for rheumatoid arthritis447.
Figure 8.
Surface engineered MSC-EVs for immunotherapy against rheumatoid arthritis (RA). (a) Schematic showing the process of preparing surface engineered MSC-EVs by metabolic glycoengineering-based click chemistry. A targeting ligand, dextran sulfate (DS) which can target the macrophage scavenger receptor class A (SR-A), was conjugated to the engineered EVs for targeting macrophages in the inflamed joint of RA. (b) Mechanism of the engineered MSC-EVs to reprogram macrophages in RA. Systemically administered EVs could accumulate at the inflamed joints because of the presence of the targeting ligand dextran sulfate. Upon reaching the joints, the MSC-EVs reprogram macrophages to the M2 phenotype, resolving inflammation. Reproduced with permission from Ref. 446. Copyright © 2021, American Association for the Advancement of Science.
Systemic lupus erythematosus (SLE) is an autoimmune disease in which the immune system attacks multiple organs in the body448. Studies have identified EVs as potential biomarkers for SLE and as therapeutic agents to inhibit autoimmune-mediated inflammation449. In a study of SLE therapies, keratinocyte-derived EVs were tested for their ability to carry miRNA and anti-inflammatory drugs with the aim of enhancing IFN-α secretion450. It was found that the EVs activated plasmacytoid DCs in a TLR-7-dependent manner in patients with SLE.
Multiple sclerosis is caused by inflammatory demyelination of the white matter in the central nervous system, with the periventricular white matter, optic nerve, spinal cord, brainstem, and cerebellum being potential sites for lesion development411. Immunosuppressive treatment is a therapeutic option for intervening in the activities of T cells and B cells involved in attacking the brain tissues451. Oligodendrocyte-derived EVs (OI-EVs) containing specific myelin self-antigens were found to lead to the suppression of CD4+ T cells452. Intravenous injection of the OI-EVs induced apoptosis of CD4+ T cells in an IL-10-dependent manner and enhanced immune tolerance to reduce the pathophysiology of multiple sclerosis. The self-antigens in the EVs played a crucial role in this outcome. Additionally, circulating EVs isolated from the plasma of patients with multiple sclerosis were found to contain more miRNAs than those from healthy donors, which inhibited the activity of IFN-γ−IL-17 A−Foxp3+CD4+ T cells453. In addition, let-7i miRNA in the EVs was involved in regulating the insulin-like growth factor 1 receptor (IGF1R) and the transforming growth factor beta receptor 1 (TGFBR1) pathways to activate Treg cells for treating inflammatory lesions. Moreover, MSC-derived EVs showed immunosuppressive effect for mitigating multiple sclerosis when being injected into mice bearing encephalomyelitis (Fig. 7b)454. Both of native and IFN-γ-activated MSC-EVs could reduce the demyelinated area in the spinal cord in an experimental autoimmune encephalomyelitis model. The MSC-derived EVs also increased the number of Tregs in vivo. With the treatment by MSC-EVs, the production of pro-inflammatory Th1 and Th17 cytokines, including IL-6, IL-12, P70, IL-17AF, and IL-22, were decreased while anti-inflammatory factors such as indoleamine 2,3-dioxygenase was increased in peripheral blood mononuclear cells.
6.3. Infectious disease
Infectious diseases represent one of the most common disorders that are caused by pathogens such as bacteria, viruses, and fungi. Since 2019, the high pathogenicity and infectivity of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused the coronavirus disease (COVID-19) pandemic455. Strikingly, cellular nanovesicles are similar in composition to viral pathogens in that they contain nucleic acids and proteins packaged within a phospholipid membrane. Cellular nanovesicles differ from viruses in that the immunogenic nucleic acids and proteins that are embedded in the phospholipid membrane form cavities in the nanovesicles. Interestingly, it was found that cellular nanovesicles from virus-infected cells contained viral double-stranded RNA (dsRNA) which mediated immunological memory in arthropods456. The virus-derived complementary DNA was generated, thereby eliciting an antiviral effect in naive animals. Hence, cellular nanovesicles have the potential for the treatment of infectious diseases via immunomodulation457. Indeed, cellular nanovesicles from cells including NK cells458, macrophages195,459,460, among others461,462 have been explored for treating different infections including viral463, 464, 465 and bacterial infections466,467.
EVs could carry various molecules to target sites for infectious disease treatment. Immunomodulatory molecules such as cytokines and miRNAs could be delivered by cellular nanovesicles to mediate the anti-inflammatory response468. EVs loaded with anti-pathogen drugs can treat infectious diseases and be guided by biomarkers on the membrane361. For example, Li et al.469 reported that IFN-α induced the secretion of EVs with antiviral molecules from nonpermissive liver nonparenchymal cells, which migrated to hepatocytes to initiate an immune response against hepatitis virus A59 and adenovirus. Transfer of antiviral molecules, such as miRNA and mRNA, was observed between normal and infected cells for antiviral activity. EVs generated by macrophages treated with IFN-α inhibited the activity of both hepatitis B virus (HBV) and hepatitis C virus (HCV)470.
Regarding SARS-CoV-2 infections, it was found that the virus binds to and infects host cells via the angiotensin-converting enzyme 2 (ACE2) and CD147471. Cellular nanovesicles from different cell types have been engineered to block this mechanism to prevent viral entry472, 473, 474, 475, 476. For example, Zhang and Griffiths472 designed two types of cellular nanovesicles to competitively bind host cells, and this slowed down the infection rate (Fig. 7c). The cellular nanovesicles successfully competed with SARS-CoV-2 for binding to ACE2 and CD147 and specifically blocked the virus from entering host cells. These cellular nanovesicles effectively acted as nano-decoys, trapping and detaining the pathogen by preventing it from entering into cells. Moreover, cellular nanovesicles from MSCs have shown promise in treating SARS-CoV-2 infections owing to their potent anti-inflammatory activities340,477, 478, 479, 480. These nanovesicles are currently being investigated in active clinical trials as a cell-free immunotherapy for managing COVID-19.
6.4. Injury and trauma
Considering the immunomodulatory effects of cellular nanovesicles, they can be explored as cell-free therapeutics for the treatment of injury and trauma351. Cellular nanovesicle-based therapeutics can suppress inflammation at injury and trauma sites, creating an environment for cell recovery and growth481,482. Nanovesicles from different cell types, such as MSCs483, 484, 485, macrophages486,487, and neutrophils488, have been explored for treating diverse injuries including lung injury489, 490, 491, spinal cord injury492, 493, 494, traumatic brain injury495, 496, 497, among others98,498,499. Notably, nanovesicles from MSCs are the most studied because of their demonstrated immunomodulatory capability500, 501, 502, 503, 504, 505, 506. Owing to the differentiation potential and immunosuppressive property of MSCs, they are often loaded with various bioactive cargoes and utilized as immune modulators to treat injury and trauma. They facilitate the healing and repair processes, such as neurogenesis, angiogenesis, osteogenesis, cardiac repair, and lung repair, averting long-term wound recovery and unexpected complications induced by engraftment507. MSC-EVs loaded with cardiac-protective peptides were shown to suppress inflammation in the cardiac tissue508. In another study, MSC-EVs, which were encapsulated into a hydrogel and injected into the cardiac tissue, demonstrated prolonged retention and molecule release at the injury sites (Fig. 7d)332. In addition, intrapericardial injection of MSC-EVs packaged in hydrogels showed a stronger immunomodulatory effect. Compared to the EVs alone, EVs loaded within the hydrogel exhibited a higher efficiency in cardiac repair.
It has also been shown that MSC-EVs can be loaded onto vascular grafts to modulate inflammation509. The vascular grafts were modified with heparin, rendering them susceptible to elimination via thrombosis and calcification. MSC-EVs could be against thrombosis and calcification. With the immunomodulatory effect of the MSC-EVs, macrophages were repolarized to the anti-inflammatory and anti-osteogenic phenotype, which was beneficial to the survival of the vascular grafts. Anti-inflammatory cytokines were increased and pro-inflammatory cytokines were decreased in the presence of the MSC-EVs in vivo510. The macrophage phenotypic switching observed at the injury site caused by the administration of MSC-EVs could also promote myogenesis and angiogenesis during muscle repair511.
In addition to MSC-EVs, other cell-derived nanovesicles have also been investigated as treatments for injury and trauma. It was reported that HEK293T cell-derived EVs were more stable than liposomes and polyethylenimine for the purpose of carrying miR-21 to regulate the expression of PDCD4, an apoptosis protein that mediates heart dysfunction512. EVs loaded with miR-21 alleviated the early apoptosis of cardiomyocytes and ECs, and cardiac function was recovered. Also, it was found that EVs from non-traumatic femoral head necrosis tissues possessed enriched CD41, which not only directed osteogenic differentiation, but also facilitated the migration of MSCs to reduce osteonecrosis and femoral head damage513.
6.5. Other applications and new developments
Cellular nanovesicles have been utilized for treating several other diseases, including bronchopulmonary dysplasia514, spasmolytic polypeptide-expressing metaplasia515, kidney stone516, among others517, 518, 519. A plenty of studies reported that cellular nanovesicle-mediated RNA transfer is necessary for the regulation of diverse physiological and pathological processes520. For example, previous studies suggested that EVs from immune cells have the ability to cross BBB and induce autophagy to repair debilitating neurodegeneration caused by Parkinson's disease143. As another example, pulmonary hypertension (PAH) is induced by the long-term exposure under the hypoxic condition which leads to high blood pressure in the pulmonary artery521. Currently, vascular remodeling is considered an efficient therapeutic strategy for PAH34,521. Immunomodulatory cellular nanovesicles have emerged as a new strategy for vascular remodeling in PAH522. Cellular nanovesicles isolated from MSCs could reduce the expression of proinflammatory factors including MCP-1, IL-6, galectin-3, and HIMF. In addition, MSC-derived nanovesicles could suppress the activation of macrophages523. Because of these effects, MSC-derived EVs exhibited therapeutic effect for treating hypoxia-induced PAH.
In addition, cellular nanovesicles have emerged as an approach for the delivery of gene editing tools for immunomodulation. Due to their outstanding structural stability and loading capacity, de Jong et al.524 designed cellular nanovesicles containing small noncoding RNA molecules for gene editing via CRISPR-Cas9. RNA-loaded cellular nanovesicles secreted from donor cells regulated the recipient cells to express reporter proteins with high sensitivity. Recently, MSC-EVs were modified with a targeting polypeptide for the delivery of CRISPR/Cas9 to program macrophages which showed a prominent anti-inflammatory efficacy in the injured spinal cord (Fig. 9)525. In this approach, CRISPR/Cas9 were encapsulated into MSC-EVs by electroporation and the targeting polypeptide (CAQK) was modified on EV surface, which led to the targeting of EVs to the injured spinal cord. These CRISPR/Cas9-loaded EVs achieved efficient editing of macrophages to regulate the expression of TNF receptor-1, repolarized macrophages to the M2-like phenotype, and downregulated the production of inflammatory cytokines. Overall, the inflammation condition was alleviated by these MSC-EVs loaded with CRISPR/Cas9 which promoted the neuroprotection after spinal cord injury. For comprehensive understanding, we summarized new advances in these major disease areas with a focus on their respective therapeutic mechanism (Table 2).
Figure 9.
Schematic illustration of the delivery of CRISPR-Cas9 by MSC-derived EVs with immunosuppressive effects for neuroprotection. CRISPR/Cas9 loaded MSC EVs were modified with a targeting peptide, CAQK, to target activated macrophages in the spinal cord. Delivery of the CRISPR/Cas9 to these macrophages programed them to the M2 phenotype leading to neuroprotection effect. Reproduced with permission from Ref. 525. Copyright © 2022, Elsevier.
Table 2.
Representative examples of major diseases that immunomodulatory cellular nanovesicles can treat.
| Diseases | Nanovesicle source | Immunomodulatory effect | Ref. | |
|---|---|---|---|---|
| Cancer | Lung metastasis | T cells | Induce apoptosis of MSCs to inhibit tumor growth | 116 |
| CT26 tumor | Engineered M1 macrophages | Knock down PD-L1 related genes; activate CD8+ T cells; repolarize macrophages to the M1 phenotype | 409 | |
| Neuroblastoma | NK cells | Active NK cells to produce cytokines | 390 | |
| Hepatocellular carcinoma | DCs | Increase IFN-γ-producing CD8+ T cells; increase the level of IL-2; reduced number of Tregs | 405 | |
| 4T1 breast cancer | M1 macrophage | Promote phagocytosis of macrophages; block CD47-SIRPα signaling pathway on macrophages; repolarize TAM to the M1 phenotype | 406 | |
| B16F10 and 4T1 metastasis | M1 macrophages (Platelet and engineered cancer cell) | Block CD47-SIRPα signaling pathway on macrophages; repolarize TAM towards the M1 phenotype; activate CD8+ T cells and CD4+ T cells | 407 | |
| B16F10 melanoma | HEK293 T cells | Competitively inhibit PD-L1; avoid exhaustion of CD8+ T cells | 408 | |
| Autoimmune diseases | Rheumatoid arthritis | Mature DCs | Inhibit pro-inflammatory cytokines and promote anti-inflammatory cytokines | 412 |
| Mature bone marrow-derived DCs | Suppress inflammatory and autoimmune responses | 413 | ||
| BMSCs | Delay inflammatory responses | 429 | ||
| Stem cells | Release of cytokines; repolarization of macrophages | 446 | ||
| Neutrophil | Carry anti-inflammatory proteins and annexin A; increase production of TGF-β1 by chondrocytes | 428 | ||
| Engineered HEK293 T cells | Carry IL-4 for repolarization of macrophages | 445 | ||
| Reduce inflammatory levels and the population of auto-reactive T cells; Block the OX40–OX40L interaction | 447 | |||
| Systemic lupus erythematosus | Serum | Regulate MSCs via inhibition of TRAF6/NF-κB signal pathway | 449 | |
| Keratinocytes | Carry microRNA and anti-inflammatory drugs to enhance the secretion of IFN-α | 450 | ||
| Placenta | Downregulation of CD3 and inhibition of CD8+ T cells | 434 | ||
| Platelet | Promote activation of T cells and B cells | 433 | ||
| Multiple sclerosis | MSCs | Upregulate the number of CD4+CD25+FOXP3+ Tregs; reduce the number of total macrophages/microglia and pro-inflammatory T cells in the spinal cords; inhibit proliferation of PBMCs; downregulate levels of pro-inflammatory Th1 and Th17 cytokines; increase levels of immunosuppressive cytokines | 454 | |
| BMSCs | Attenuate inflammation and regulate the polarization of microglia | 423 | ||
| Oligodendrocyte | Induce apoptosis of CD4+ T cells in an IL-10-dependent manner | 452 | ||
| Plasma | Inhibit IFN-γ-IL-17 A−Foxp3+CD4+ T cells; suppress Tregs via regulation of IGF1R and TGFBR1 pathway | 453 | ||
| BV-2 microglia cells | IL-4 is expressed by genetical engineering for repolarization of macrophages. | 436 | ||
| Experimental autoimmune encephalomyelitis | Immature bone marrow-derived DCs | Reduce proliferation of AChR-reactive lymphocyte, level of AChR antibody and pro-inflammatory cytokines | 414 | |
| Atorvastatin-modified bone marrow-derived DCs | Up-regulate indoleamine 2,3-dioxygenase and Tregs | 415 | ||
| Autoimmune hepatitis | MSCs | Delivery of dexamethasone for synergistic treatment to the liver. | 442 | |
| BMSCs | Attenuation of NLRP3 and caspase-1. Inhibition of pro-inflammatory cytokines |
443 | ||
| Infectious diseases | Hepatitis | Nonpermissive liver nonparenchymal cells | Transfer IFN-α | 469 |
| Macrophages | Regulate macrophages for disturbing production of HBV pgRNA and expressions of antigen | 470 | ||
| SARS-CoV-2 | Human lung epithelial type II cell and human macrophage | Bind to protein receptors, angiotensin-converting enzyme 2 and CD147, for blocking cellular entry of virus | 472 | |
| Injury and trauma | Cardiac protection | MSCs | Suppress cardiac tissue surrounding inflammation. | 508 |
| Slightly change pericardial inflamed condition | 332 | |||
| Modulate inflammation level; repolarize ambient macrophages to the anti-inflammatory and anti-osteogenic phenotype | 509 | |||
| Incisional hernia | MSCs | Decrease production of pro-inflammatory cytokines | 510 | |
| Volumetric muscle loss | MSCs | Polarization of macrophages | 511 | |
| Lung fibrosis | MSCs | Inhibit activation of macrophages | 514 | |
| Myocardial infarction | HEK293T | Downregulate the expression of PDCD4 | 512 | |
| Bone defect | Non-traumatic femoral head necrosis tissues | Facilitate the migration of MSCs | 513 |
7. Clinical advances of immunomodulatory cellular nanovesicles for disease management
Extensive preclinical investigations have led to some immunomodulatory cellular nanovesicles reaching clinical studies for disease treatment. Comprehensive overview of the clinical advances of cellular nanovesicles can be found in other reviews32,361,526. Some representative ongoing clinical trials of therapeutic immunomodulatory cellular nanovesicles are shown in Table 3. Evidently, most of the investigated ongoing trials are in early stages (phase I and II) and cover different disease indications including cancer, infectious diseases, among others. For example, EVs from diffuse large B-cell lymphomas are being studied as CD20 and PD-L1 decoys for immunotherapy against B-cell Non-Hodgkin lymphoma. Interestingly, several cellular nanovesicles from different cell sources are studied for treating different infectious diseases including bacterial infections and COVID-19. For instance, owing to their intrinsic anti-inflammatory activity, EVs from MSCs are in several ongoing clinical trials for treating hyperinflammation and acute respiratory distress syndrome caused by bacterial and/or SARS-CoV-2 infections. Further, CD24-overexpressing EVs isolated from engineered T-REX™-293 cells are also being studied for treating COVID-19 in two ongoing trials. However, these EVs employ a different mechanism of action as compared to MSC-derived EVs, which involves the inhibition of T cell proliferation by CD24. Notably, successful clinical translation of immunomodulatory cellular nanovesicles needs to overcome pressing challenges including scale-up and consistency of production, standardized characterization and quality control, suitable storage and stability, and enhanced biological activities527, 528, 529. Although no immunomodulatory cellular nanovesicle products have been approved by regulatory agencies, it is expected that with the continuous, extensive preclinical and clinical studies, more cellular nanovesicles will reach clinical studies and even clinical approvals.
Table 3.
Representative ongoing clinical trial of cellular nanovesicles for therapeutic immunomodulation.
| Nanovesicle source | Indication | Aim of study/function of EVs | Phase | Identifier | Status |
|---|---|---|---|---|---|
| MSCs | Hyper-inflammation caused by COVID-19 | Function as an adjuvant to reduce hyper-inflammation in moderate COVID-19 patients. | II | NCT05216562 | Recruiting |
| MSCs | COVID-19 | Attenuate inflammation and support anti-fibrotic pathways to treat COVID-19. | I | NCT05191381 | Recruiting |
| Mesenchymal progenitor cell | Pulmonary infection | Treat pulmonary infection caused by gram-negative bacilli resistant to carbapenems. | I/II | NCT04544215 | Recruiting |
| CD24 overexpressed exosomes from T-REXTM-293 cells | Moderate or severe COVID-19 infection | Negatively regulate inflammation by controlling the homeostatic proliferation of T cells. | I | NCT04747574 | Recruiting |
| CD24 overexpressed exosomes from T-REXTM-293 cells | Moderate or severe COVID-19 infection | Improvement of COVID19 status. | II | NCT04902183 | Recruiting |
| Diffuse large B-cell lymphomas | B-cell Non-Hodgkin Lymphoma | Immunotherapy by carrying CD20 and PD-L1 as decoy receptors. | – | NCT03985696 | Recruiting |
‒, Not applicable.
8. Conclusions and future perspectives
Cellular nanovesicles inherit the internal and surface molecules from their parent cells. They have multiple functions one being immunomodulation. Due to the differences in parent cell types, tissues, and physiological conditions, the composition of cellular nanovesicles is multifarious, which leads to different regulatory effects on physiological and pathological processes. Immunoactivation and immunosuppression are balanced and modulated by cellular nanovesicles via the cargo they carry and the cells they interact with. Due to their unique plasticity, cellular nanovesicles can be modified and enriched with functional ingredients that could extend their immunoregulatory applications. Given their low toxicity, high structural stability, remarkable loading capacity, and multi-engineered functions, cellular nanovesicles have shown promise as cell-free immunotherapies for the treatment of diverse diseases, such as cancer, autoimmune diseases, infectious diseases, and tissue injuries. Notably, compared with immunomodulator-loaded synthetic nanoparticles such as liposomes and polymer particles, cellular nanovesicles express innate proteins or receptors from parent cells which may result in intrinsic, efficient targeting to specific tissues and cells530,531. In addition, the intrinsic immunomodulatory activity of cellular nanovesicles can bypass the need of exogenous drugs. However, the challenge for large-scale production is one of the key downsides of cellular nanovesicles as compared to synthetic nanoparticles.
Although many studies have validated the potential usefulness of cellular nanovesicles for immunomodulation, challenges still remain in the better understanding of their biological foundations and clinical translation. It has been demonstrated that modified cellular nanovesicles can carry proteins, lipids, DNA, noncoding RNA, and other cargoes to recipient cells for the regulation of cellular behaviors. It is also clear that they are involved in intercellular signaling, regulation of inflammation, fibrosis, cell survival and apoptosis, angiogenesis, thrombosis, autophagy, immunosuppression, and immunoactivation, which means that they have therapeutic potential in regulating immune responses in diverse pathologies. However, to date, research has majorly focused on preclinical studies, and clinical research data are still relatively scarce. Large-scale production of cellular nanovesicles is required for clinical studies which is still hard to achieve. Besides, variations between different batches of nanovesicles need to be valued which is essential for reproducible studies. Further, assessment of risk-efficiency is one of the key factors for clinical applications of cellular nanovesicles in the future. Moreover, some functional components of cellular nanovesicles and their molecular mechanisms are still unclear. There is also a need to study their noncoding RNA content, such as lncRNA and circRNA, in addition to their miRNA content. The highly efficient isolation and purification techniques used to study cellular nanovesicles at the molecular level should now be matched by equally potent approaches for the rapid clinical transformation and application of cellular nanovesicles.
Acknowledgments
Zongmin Zhao acknowledges support from the College of Pharmacy at University of Illinois Chicago.
Author contributions
Zongmin Zhao and Endong Zhang conceptualized the work. Endong Zhang wrote the manuscript. Endong Zhang, Philana Phan, and Zongmin Zhao revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Cocozza F., Grisard E., Martin-Jaular L., Mathieu M., Théry C. SnapShot:extracellular vesicles. Cell. 2020;182:262. doi: 10.1016/j.cell.2020.04.054. e1. [DOI] [PubMed] [Google Scholar]
- 2.Xu R., Rai A., Chen M., Suwakulsiri W., Greening D.W., Simpson R.J. Extracellular vesicles in cancer—implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15:617–638. doi: 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- 3.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 4.Glassman P.M., Hood E.D., Ferguson L.T., Zhao Z., Siegel D.L., Mitragotri S., et al. Red blood cells: the metamorphosis of a neglected carrier into the natural mothership for artificial nanocarriers. Adv Drug Deliv Rev. 2021;178 doi: 10.1016/j.addr.2021.113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordanaba-Florit G., Royo F., Kruglik S.G., Falcón-Pérez J.M. Using single-vesicle technologies to unravel the heterogeneity of extracellular vesicles. Nat Protoc. 2021;16:3163–3185. doi: 10.1038/s41596-021-00551-z. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R., LeBleu Valerie S. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiklander Oscar P.B., Brennan Meadhbh Á. Lötvall J., Breakefield Xandra O., Samir E.L.A. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11:eaav8521. doi: 10.1126/scitranslmed.aav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yáñez-Mó M., Siljander P.R.M., Andreu Z., Bedina Zavec A., Borràs F.E., Buzas E.I., et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4 doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan S., Luo Z., Li Z., Wang Y., Tao J., Gong C., et al. Improving cancer immunotherapy outcomes using biomaterials. Angew Chem Int Ed. 2020;59:17332–17343. doi: 10.1002/anie.202002780. [DOI] [PubMed] [Google Scholar]
- 11.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7 doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 13.Göran Ronquist K. Extracellular vesicles and energy metabolism. Clin Chim Acta. 2019;488:116–121. doi: 10.1016/j.cca.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 14.Brodin P., Davis M.M. Human immune system variation. Nat Rev Immunol. 2017;17:21–29. doi: 10.1038/nri.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudino S.J., Kumar P. Cross-talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front Immunol. 2019;10:360. doi: 10.3389/fimmu.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liston A., Humblet-Baron S., Duffy D., Goris A. Human immune diversity: from evolution to modernity. Nat Immunol. 2021;22:1479–1489. doi: 10.1038/s41590-021-01058-1. [DOI] [PubMed] [Google Scholar]
- 17.van Kooyk Y., Geijtenbeek T. DC-SIGN: Escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 18.Lawler S.E., Nowicki M.O., Ricklefs F.L., Chiocca E.A. Immune escape mediated by exosomal PD-L1 in cancer. Adv Biosyst. 2020;4 doi: 10.1002/adbi.202000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pichler W.J. Immune pathomechanism and classification of drug hypersensitivity. Allergy. 2019;74:1457–1471. doi: 10.1111/all.13765. [DOI] [PubMed] [Google Scholar]
- 20.Quach H., Ritchie D., Stewart A.K., Neeson P., Harrison S., Smyth M.J., et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24:22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao S., Wang S., Song Y. Novel immunomodulatory drugs and neo-substrates. Biomark Res. 2020;8:2. doi: 10.1186/s40364-020-0182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linhares Y.P.L., Pavletic S., Gale R.P. Chronic GVHD: where are we? where do we want to be? will immunomodulatory drugs help? Bone Marrow Transplant. 2013;48:203–209. doi: 10.1038/bmt.2012.76. [DOI] [PubMed] [Google Scholar]
- 23.Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann I.K., Wood M.J.A., Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotech. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 25.Ha D., Yang N., Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S., Wu X., Chandra S., Lyon C., Ning B., jiang L., et al. Extracellular vesicles: emerging tools as therapeutic agent carriers. Acta Pharm Sin B. 2022;12:3822–3842. doi: 10.1016/j.apsb.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Möller A., Lobb R.J. The evolving translational potential of small extracellular vesicles in cancer. Nat Rev Cancer. 2020;20:697–709. doi: 10.1038/s41568-020-00299-w. [DOI] [PubMed] [Google Scholar]
- 28.De Jong O.G., Van Balkom B.W.M., Schiffelers R.M., Bouten C.V.C., Verhaar M.C. Extracellular vesicles: potential roles in regenerative medicine. Front Immunol. 2014;5:608. doi: 10.3389/fimmu.2014.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao S.C., Guo S.C. Role of extracellular vesicles in tumour microenvironment. Cell Commun Signal. 2020;18:163. doi: 10.1186/s12964-020-00643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuel P., Fabbri M., Carter D.R.F. Mechanisms of drug resistance in cancer: the role of extracellular vesicles. Proteomics. 2017;17 doi: 10.1002/pmic.201600375. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y., Li X., Zhang W., Yu L., Wang Y., Deng Z., et al. Trends in the biological functions and medical applications of extracellular vesicles and analogues. Acta Pharm Sin B. 2021;11:2114–2135. doi: 10.1016/j.apsb.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marar C., Starich B., Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22:560–570. doi: 10.1038/s41590-021-00899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu M., Huang Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials. 2020;242 doi: 10.1016/j.biomaterials.2020.119925. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong J.P.K., Holme M.N., Stevens M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11:69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang T., Atukorala I., Mathivanan S. In: New frontiers: extracellular vesicles. Mathivanan S., Fonseka P., Nedeva C., Atukorala I., editors. Springer International Publishing: E-Publishing Inc.; Cham: 2021. Biogenesis of extracellular vesicles; pp. 19–43. [Google Scholar]
- 36.Qin M., Du G., Sun X. Biomimetic cell-derived nanocarriers for modulating immune responses. Biomater Sci. 2020;8:530–543. doi: 10.1039/c9bm01444f. [DOI] [PubMed] [Google Scholar]
- 37.Kao C.Y., Papoutsakis E.T. Extracellular vesicles: exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr Opin Biotechnol. 2019;60:89–98. doi: 10.1016/j.copbio.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Tong M., Abrahams V.M., Chamley L.W. Immunological effects of placental extracellular vesicles. Immunol Cell Biol. 2018;96:714–722. doi: 10.1111/imcb.12049. [DOI] [PubMed] [Google Scholar]
- 39.van Niel G., D'Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 40.El Andaloussi S., Mäger I., Breakefield X.O., Wood M.J.A. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 41.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao H., Im H., Castro C.M., Breakefield X., Weissleder R., Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118:1917–1950. doi: 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xing Y., Cheng Z., Wang R., Lv C., James T.D., Yu F. Analysis of extracellular vesicles as emerging theranostic nanoplatforms. Coord Chem Rev. 2020;424 [Google Scholar]
- 44.Nikfarjam S., Rezaie J., Kashanchi F., Jafari R. Dexosomes as a cell-free vaccine for cancer immunotherapy. J Exp Clin Cancer Res. 2020;39:258. doi: 10.1186/s13046-020-01781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clancy J.W., Zhang Y., Sheehan C., D'Souza-Schorey C. An ARF6–Exportin-5 axis delivers pre-miRNA cargo to tumour microvesicles. Nat Cell Biol. 2019;21:856–866. doi: 10.1038/s41556-019-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raposo G., Stahl P.D. Extracellular vesicles: a new communication paradigm? Nat Rev Mol Cell Biol. 2019;20:509–510. doi: 10.1038/s41580-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 47.Jabalee J., Towle R., Garnis C. The role of extracellular vesicles in cancer: cargo, function, and therapeutic implications. Cells. 2018;7:93. doi: 10.3390/cells7080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mir B., Goettsch C. Extracellular vesicles as delivery vehicles of specific cellular cargo. Cells. 2020;9:1601. doi: 10.3390/cells9071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hubbell J.A., Thomas S.N., Swartz M.A. Materials engineering for immunomodulation. Nature. 2009;462:449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 50.Najar M., Raicevic G., Fayyad-Kazan H., Bron D., Toungouz M., Lagneaux L. Mesenchymal stromal cells and immunomodulation: a gathering of regulatory immune cells. Cytotherapy. 2016;18:160–171. doi: 10.1016/j.jcyt.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Nakayama MJFii. Antigen presentation by MHC-dressed cells. Front Immunol. 2015;5:672. doi: 10.3389/fimmu.2014.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim K.M., Abdelmohsen K., Mustapic M., Kapogiannis D., Gorospe M. RNA in extracellular vesicles. WIREs RNA. 2017;8:e1413. doi: 10.1002/wrna.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prada I., Meldolesi J. Binding and fusion of extracellular vesicles to the plasma membrane of their cell targets. Int J Mol Sci. 2016;17:1296. doi: 10.3390/ijms17081296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi T., Hoffman M.P. Exosomal microRNA communication between tissues during organogenesis. RNA Biol. 2017;14:1683–1689. doi: 10.1080/15476286.2017.1361098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho J.A., Yeo Dj, Son H.Y., Kim H.W., Jung D.S., Ko J.K., et al. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114:613–622. doi: 10.1002/ijc.20757. [DOI] [PubMed] [Google Scholar]
- 56.Choi D.S., Kim D.K., Kim Y.K., Gho Y.S. Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom Rev. 2015;34:474–490. doi: 10.1002/mas.21420. [DOI] [PubMed] [Google Scholar]
- 57.Buzás E.I., Tóth E.Á., Sódar B.W., Szabó-Taylor K.É. Molecular interactions at the surface of extracellular vesicles. Semin Immunopathol. 2018;40:453–464. doi: 10.1007/s00281-018-0682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vu L.T., Gong J., Pham T.T., Kim Y., Le M.T.N. microRNA exchange via extracellular vesicles in cancer. Cell Prolif. 2020;53 doi: 10.1111/cpr.12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Penfornis P., Vallabhaneni K.C., Whitt J., Pochampally R. Extracellular vesicles as carriers of microRNA, proteins and lipids in tumor microenvironment. Int J Cancer. 2016;138:14–21. doi: 10.1002/ijc.29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obregon C., Rothen-Rutishauser B., Gerber P., Gehr P., Nicod L.P. Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-α-mediated pathway. Am J Pathol. 2009;175:696–705. doi: 10.2353/ajpath.2009.080716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mao S., Maeno N., Yoshiie K., Matayoshi S., Fujimura T., Oda H. CD14-mediated induction of interleukin-8 and monocyte chemoattractant protein-1 by a heatresistant constituent of porphyromonas gingivalis in endothelial cells. Scand J Immunol. 2002;56:484–491. doi: 10.1046/j.1365-3083.2002.01163.x. [DOI] [PubMed] [Google Scholar]
- 62.Koh E., Lee E.J., Nam G.-H., Hong Y., Cho E., Yang Y., et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–129. doi: 10.1016/j.biomaterials.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Schmiedel D., Mandelboim O. NKG2D ligands–critical targets for cancer immune escape and therapy. Front Immunol. 2018;9:2040. doi: 10.3389/fimmu.2018.02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y., Gu Y., Cao X. The exosomes in tumor immunity. OncoImmunology. 2015;4 doi: 10.1080/2162402X.2015.1027472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian X., Shen H., Li Z., Wang T., Wang S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J Hematol Oncol. 2019;12:84. doi: 10.1186/s13045-019-0772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bazzan E., Tinè M., Casara A., Biondini D., Semenzato U., Cocconcelli E., et al. Critical review of the evolution of extracellular vesicles' knowledge: from 1946 to today. Int J Mol Sci. 2021;22:6417. doi: 10.3390/ijms22126417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mueller S.K., Nocera A.L., Bleier B.S. Exosome function in aerodigestive mucosa. Nanomedicine Nanomed-Nanothechnol. 2018;14:269–277. doi: 10.1016/j.nano.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Linares R., Tan S., Gounou C., Arraud N., Brisson A.R. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles. 2015;4 doi: 10.3402/jev.v4.29509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meng W., He C., Hao Y., Wang L., Li L., Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27:585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hagel L., Östberg M., Andersson T. Apparent pore size distributions of chromatography media. J Chromatogr A. 1996;743:33–42. [Google Scholar]
- 71.Lane R.E., Korbie D., Trau M., Hill M.M. Optimizing size exclusion chromatography for extracellular vesicle enrichment and proteomic analysis from clinically relevant samples. Proteomics. 2019;19 doi: 10.1002/pmic.201800156. [DOI] [PubMed] [Google Scholar]
- 72.Heath N., Grant L., De Oliveira T.M., Rowlinson R., Osteikoetxea X., Dekker N., et al. Rapid isolation and enrichment of extracellular vesicle preparations using anion exchange chromatography. Sci Rep. 2018;8:5730. doi: 10.1038/s41598-018-24163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim D-k, Nishida H., An Su Y., Shetty Ashok K., Bartosh Thomas J., Prockop Darwin J. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. 2016;113:170–175. doi: 10.1073/pnas.1522297113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shigemoto-Kuroda T., Oh J.Y., Kim D.K., Jeong H.J., Park S.Y., Lee H.J., et al. MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep. 2017;8:1214–1225. doi: 10.1016/j.stemcr.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma P., Ludwig S., Muller L., Hong C.S., Kirkwood J.M., Ferrone S., et al. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles. 2018;7 doi: 10.1080/20013078.2018.1435138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tauro B.J., Greening D.W., Mathias R.A., Mathivanan S., Ji H., Simpson R.J. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013;12:587–598. doi: 10.1074/mcp.M112.021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brennan K., Martin K., FitzGerald S.P., O'Sullivan J., Wu Y., Blanco A., et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep. 2020;10:1039. doi: 10.1038/s41598-020-57497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei R., Zhao L., Kong G., Liu X., Zhu S., Zhang S., et al. Combination of size-exclusion chromatography and ultracentrifugation improves the proteomic profiling of plasma-derived small extracellular vesicles. Biol Proced Online. 2020;22:12. doi: 10.1186/s12575-020-00125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gandham S., Su X., Wood J., Nocera A.L., Alli S.C., Milane L., et al. Technologies and standardization in research on extracellular vesicles. Trends Biotechnol. 2020;38:1066–1098. doi: 10.1016/j.tibtech.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Helwa I., Cai J., Drewry M.D., Zimmerman A., Dinkins M.B., Khaled M.L., et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liangsupree T., Multia E., Riekkola M.-L. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. 2021;1636 doi: 10.1016/j.chroma.2020.461773. [DOI] [PubMed] [Google Scholar]
- 82.Nocera A.L., Mueller S.K., Stephan J.R., Hing L., Seifert P., Han X., et al. Exosome swarms eliminate airway pathogens and provide passive epithelial immunoprotection through nitric oxide. J Allergy Clin Immunol. 2019;143:1525–15235.e1. doi: 10.1016/j.jaci.2018.08.046. [DOI] [PubMed] [Google Scholar]
- 83.Tauro B.J., Greening D.W., Mathias R.A., Ji H., Mathivanan S., Scott A.M., et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 84.Morales-Sanfrutos J., Munoz J. Unraveling the complexity of the extracellular vesicle landscape with advanced proteomics. Expert Rev Proteomics. 2022;19:89–101. doi: 10.1080/14789450.2022.2052849. [DOI] [PubMed] [Google Scholar]
- 85.Askeland A., Borup A., Østergaard O., Olsen J.V., Lund S.M., Christiansen G., et al. Mass-spectrometry based proteome comparison of extracellular vesicle isolation methods: comparison of me-kit, size-exclusion chromatography, and high-speed centrifugation. Biomedicines. 2020;8:246. doi: 10.3390/biomedicines8080246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zheng H., Guan S., Wang X., Zhao J., Gao M., Zhang X. Deconstruction of heterogeneity of size-dependent exosome subpopulations from human urine by profiling N-glycoproteomics and phosphoproteomics simultaneously. Anal Chem. 2020;92:9239–9246. doi: 10.1021/acs.analchem.0c01572. [DOI] [PubMed] [Google Scholar]
- 87.Aqrawi L.A., Galtung H.K., Guerreiro E.M., Øvstebø R., Thiede B., Utheim T.P., et al. Proteomic and histopathological characterisation of sicca subjects and primary Sjögren’s syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers. Arthritis Res Ther. 2019;21:181. doi: 10.1186/s13075-019-1961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crotti A., Sait H.R., McAvoy K.M., Estrada K., Ergun A., Szak S., et al. BIN1 favors the spreading of Tau via extracellular vesicles. Sci Rep. 2019;9:9477. doi: 10.1038/s41598-019-45676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arntz O.J., Pieters B.C.H., van Lent P.L.E.M., Koenders M.I., van der Kraan P.M., van de Loo F.A.J. An optimized method for plasma extracellular vesicles isolation to exclude the copresence of biological drugs and plasma proteins which impairs their biological characterization. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taylor D.D., Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 91.Zhang H., Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat Protoc. 2019;14:1027–1053. doi: 10.1038/s41596-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Corso G., Mäger I., Lee Y., Görgens A., Bultema J., Giebel B., et al. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci Rep. 2017;7 doi: 10.1038/s41598-017-10646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arslan F., Lai R.C., Smeets M.B., Akeroyd L., Choo A., Aguor E.N.E., et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 94.Xu X., Barreiro K., Musante L., Kretz O., Lin H., Zou H., et al. Management of Tamm–Horsfall protein for reliable urinary analytics. Proteonomics Clin Appl. 2019;13 doi: 10.1002/prca.201900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ziaei P., Geruntho J.J., Marin-Flores O.G., Berkman C.E., Grant Norton M. Silica nanostructured platform for affinity capture of tumor-derived exosomes. J Mater Sci. 2017;52:6907–6916. [Google Scholar]
- 96.Shao H., Chung J., Lee K., Balaj L., Min C., Carter B.S., et al. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat Commun. 2015;6:6999. doi: 10.1038/ncomms7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang T., Turko I.V. Proteomic toolbox to standardize the separation of extracellular vesicles and lipoprotein particles. J Proteome Res. 2018;17:3104–3113. doi: 10.1021/acs.jproteome.8b00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hosseinkhani B., van den Akker N.M.S., Molin D.G.M., Michiels L. (Sub)populations of extracellular vesicles released by TNF-α-triggered human endothelial cells promote vascular inflammation and monocyte migration. J Extracell Vesicles. 2020;9 doi: 10.1080/20013078.2020.1801153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang K., Yue Y., Wu S., Liu W., Shi J., Zhang Z. Rapid capture and nondestructive release of extracellular vesicles using aptamer-based magnetic isolation. ACS Sens. 2019;4:1245–1251. doi: 10.1021/acssensors.9b00060. [DOI] [PubMed] [Google Scholar]
- 100.Malhotra S., Amin Z.M., Dobhal G., Cottam S., Nann T., Goreham R.V. Novel devices for isolation and detection of bacterial and mammalian extracellular vesicles. Microchim Acta. 2021;188:139. doi: 10.1007/s00604-021-04790-5. [DOI] [PubMed] [Google Scholar]
- 101.Vaz R., Serrano V.M., Castaño-Guerrero Y., Cardoso A.R., Frasco M.F., Sales M.G.F. Breaking the classics: next-generation biosensors for the isolation, profiling and detection of extracellular vesicles. Biosens Bioelectron X. 2022;10 [Google Scholar]
- 102.Balaj L., Atai N.A., Chen W., Mu D., Tannous B.A., Breakefield X.O., et al. Heparin affinity purification of extracellular vesicles. Sci Rep. 2015;5 doi: 10.1038/srep10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Multia E., Liangsupree T., Jussila M., Ruiz-Jimenez J., Kemell M., Riekkola M.-L. Automated on-line isolation and fractionation system for nanosized biomacromolecules from human plasma. Anal Chem. 2020;92:13058–13065. doi: 10.1021/acs.analchem.0c01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tian Y., Gong M., Hu Y., Liu H., Zhang W., Zhang M., et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles. 2020;9 doi: 10.1080/20013078.2019.1697028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reátegui E., van der Vos K.E., Lai C.P., Zeinali M., Atai N.A., Aldikacti B., et al. Engineered nanointerfaces for microfluidic isolation and molecular profiling of tumor-specific extracellular vesicles. Nat Commun. 2018;9:175. doi: 10.1038/s41467-017-02261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gemoll T., Strohkamp S., Rozanova S., Röder C., Hartwig S., Kalthoff H., et al. Protein profiling of serum extracellular vesicles reveals qualitative and quantitative differences after differential ultracentrifugation and Exoquick™ isolation. J Clin Med. 2020;9:227–240. doi: 10.3390/jcm9051429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lekchnov E.A., Zaporozhchenko I.A., Morozkin E.S., Bryzgunova O.E., Vlassov V.V., Laktionov P.P. Protocol for miRNA isolation from biofluids. Anal Biochem. 2016;499:78–84. doi: 10.1016/j.ab.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 108.Nath Neerukonda S., Egan N.A., Patria J., Assakhi I., Tavlarides-Hontz P., Modla S., et al. Comparison of exosomes purified via ultracentrifugation (UC) and Total Exosome Isolation (TEI) reagent from the serum of Marek's disease virus (MDV)-vaccinated and tumor-bearing chickens. J Virol Methods. 2019;263:1–9. doi: 10.1016/j.jviromet.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 109.Lane R.E., Korbie D., Anderson W., Vaidyanathan R., Trau M. Analysis of exosome purification methods using a model liposome system and tunable-resistive pulse sensing. Sci Rep. 2015;5:7639. doi: 10.1038/srep07639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Veerman R.E., Teeuwen L., Czarnewski P., Güclüler Akpinar G., Sandberg A., Cao X., et al. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J Extracell Vesicles. 2021;10 doi: 10.1002/jev2.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu J., Wu J., Tian J., Wang S. Role of T cell-derived exosomes in immunoregulation. Immunol Res. 2018;66:313–322. doi: 10.1007/s12026-018-9000-0. [DOI] [PubMed] [Google Scholar]
- 112.Chang H.-C., Sehra S., Goswami R., Yao W., Yu Q., Stritesky G.L., et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., et al. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 114.Tang X.J., Sun X.Y., Huang K.M., Zhang L., Yang Z.S., Zou D.D., et al. Therapeutic potential of CAR-T cell-derived exosomes: a cell-free modality for targeted cancer therapy. Oncotarget. 2015;6 doi: 10.18632/oncotarget.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wahlgren J., Karlson T.D.L., Glader P., Telemo E., Valadi H. Activated human T cells secrete exosomes that participate in IL-2 mediated immune response signaling. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seo N., Shirakura Y., Tahara Y., Momose F., Harada N., Ikeda H., et al. Activated CD8+ T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat Commun. 2018;9:435. doi: 10.1038/s41467-018-02865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Prendergast G.C. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27:3889–3900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 118.Hong J., Kang M., Jung M., Lee Y.Y., Cho Y., Kim C., et al. T-cell-derived nanovesicles for cancer immunotherapy. Adv Mater. 2021;33 doi: 10.1002/adma.202101110. [DOI] [PubMed] [Google Scholar]
- 119.Zhang H., Xie Y., Li W., Chibbar R., Xiong S., Xiang J. CD4+ T cell-released exosomes inhibit CD8+ cytotoxic T-lymphocyte responses and antitumor immunity. Cell Mol Immunol. 2011;8:23–30. doi: 10.1038/cmi.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Menay F., Herschlik L., De Toro J., Cocozza F., Tsacalian R., Gravisaco M.J., et al. Exosomes isolated from ascites of T-cell lymphoma-bearing mice expressing surface CD24 and HSP-90 induce a tumor-specific immune response. Front Immunol. 2017;8:286. doi: 10.3389/fimmu.2017.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kaur S., Singh S.P., Elkahloun A.G., Wu W., Abu-Asab M.S., Roberts D.D. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells. Matrix Biol. 2014;37:49–59. doi: 10.1016/j.matbio.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Johnson L.R., Lee D.Y., Eacret J.S., Ye D., June C.H., Minn A.J. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell. 2021;184:4981–4995. doi: 10.1016/j.cell.2021.08.004. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aharon A., Horn G., Bar-Lev T.H., Zagagi Yohay E., Waks T., Levin M., et al. Extracellular vesicles derived from chimeric antigen receptor-t cells: a potential therapy for cancer. Hum Gene Ther. 2021;32:1224–1241. doi: 10.1089/hum.2021.192. [DOI] [PubMed] [Google Scholar]
- 124.Fu W., Lei C., Liu S., Cui Y., Wang C., Qian K., et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun. 2019;10:4355. doi: 10.1038/s41467-019-12321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kowal J., Tkach M. In: International review of cell and molecular biology. Lhuillier C., Galluzzi L., editors. Academic Press: E-Publishing Inc; 2019. Chapter Five - dendritic cell extracellular vesicles; pp. 213–249. [DOI] [PubMed] [Google Scholar]
- 126.Fernández-Delgado I., Calzada-Fraile D., Sánchez-Madrid F. Immune regulation by dendritic cell extracellular vesicles in cancer immunotherapy and vaccines. Cancers. 2020;12:3558. doi: 10.3390/cancers12123558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Viaud S., Théry C., Ploix S., Tursz T., Lapierre V., Lantz O., et al. Dendritic cell-derived exosomes for cancer immunotherapy: what's next? Cancer Res. 2010;70:1281–1285. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- 128.Schierer S., Ostalecki C., Zinser E., Lamprecht R., Plosnita B., Stich L., et al. Extracellular vesicles from mature dendritic cells (DC) differentiate monocytes into immature DC. Life Science Alliance. 2018;1 doi: 10.26508/lsa.201800093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kowal J., Arras G., Colombo M., Jouve M., Morath J.P., Primdal-Bengtson B., et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tkach M., Kowal J., Zucchetti A.E., Enserink L., Jouve M., Lankar D., et al. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017;36:3012–3028. doi: 10.15252/embj.201696003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Larssen P., Veerman R.E., Akpinar G.G., Hiltbrunner S., Karlsson M.C.I., Gabrielsson S. Allogenicity boosts extracellular vesicle-induced antigen-specific immunity and mediates tumor protection and long-term memory in vivo. J Immunol. 2019;203:825. doi: 10.4049/jimmunol.1801628. [DOI] [PubMed] [Google Scholar]
- 132.Driedonks T.A.P., van der Grein S.G., Ariyurek Y., Buermans H.P.J., Jekel H., Chow F.W.N., et al. Immune stimuli shape the small non-coding transcriptome of extracellular vesicles released by dendritic cells. Cell Mol Life Sci. 2018;75:3857–3875. doi: 10.1007/s00018-018-2842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Romagnoli G.G., Zelante B.B., Toniolo P.A., Migliori I.K., Barbuto J.A.M. Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front Immunol. 2015;5:692. doi: 10.3389/fimmu.2014.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ochyl L.J., Moon J.J. Dendritic cell membrane vesicles for activation and maintenance of antigen-specific T cells. Adv Healthcare Mater. 2019;8 doi: 10.1002/adhm.201801091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu F., Xie M., Hun M., She Z., Li C., Luo S., et al. Natural killer cell-derived extracellular vesicles: novel players in cancer immunotherapy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.658698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lugini L., Cecchetti S., Huber V., Luciani F., Macchia G., Spadaro F., et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012;189:2833. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- 137.Advanced Drug Delivery ReviewsFais S. NK cell-released exosomes. OncoImmunology. 2013;2 [Google Scholar]
- 138.Federici C., Shahaj E., Cecchetti S., Camerini S., Casella M., Iessi E., et al. Natural-killer-derived extracellular vesicles: immune sensors and interactors. Front Immunol. 2020;11:262. doi: 10.3389/fimmu.2020.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhu L., Gangadaran P., Kalimuthu S., Oh J.M., Baek S.H., Jeong S.Y., et al. Novel alternatives to extracellular vesicle-based immunotherapy-exosome mimetics derived from natural killer cells. Artif Cell Nanomed Biotechnol. 2018;46:S166–S179. doi: 10.1080/21691401.2018.1489824. [DOI] [PubMed] [Google Scholar]
- 140.Zhu L., Oh J.M., Gangadaran P., Kalimuthu S., Baek S.H., Jeong S.Y., et al. Targeting and therapy of glioblastoma in a mouse model using exosomes ferived from natural killer cells. Front Immunol. 2018;9:824. doi: 10.3389/fimmu.2018.00824. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 141.Sun H., Shi K., Qi K., Kong H., Zhang J., Dai S., et al. Natural killer cell-derived exosomal miR-3607-3p inhibits pancreatic cancer progression by targeting IL-26. Front Immunol. 2019;10:2819. doi: 10.3389/fimmu.2019.02819. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 142.Neviani P., Wise P.M., Murtadha M., Liu C.W., Wu C.H., Jong A.Y., et al. Natural killer–derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res. 2019;79:1151. doi: 10.1158/0008-5472.CAN-18-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shan X., Zhang C., Mai C., Hu X., Cheng N., Chen W., et al. The biogenesis, biological functions, and applications of macrophage-derived exosomes. Front Mol Biosci. 2021;8:702. doi: 10.3389/fmolb.2021.715461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Funes S.C., Rios M., Escobar-Vera J., Kalergis A.M. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154:186–195. doi: 10.1111/imm.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bhatnagar S., Shinagawa K., Castellino F.J., Schorey J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and. in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cronemberger-Andrade A., Aragão-França L., de Araujo C.F., Rocha V.J., Borges-Silva MdC., Figueiras C.P., et al. Extracellular vesicles from Leishmania-infected macrophages confer an anti-infection cytokine-production profile to naïve macrophages. PLoS Neglected Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheng L., Wang Y., Huang L.J.M.T. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol. 2017;25:1665–1675. doi: 10.1016/j.ymthe.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Choo Y.W., Kang M., Kim H.Y., Han J., Kang S., Lee J.R., et al. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018;12:8977–8993. doi: 10.1021/acsnano.8b02446. [DOI] [PubMed] [Google Scholar]
- 149.Ferrari G., Thrasher A.J., Aiuti A. Gene therapy using haematopoietic stem and progenitor cells. Nat Rev Genet. 2021;22:216–234. doi: 10.1038/s41576-020-00298-5. [DOI] [PubMed] [Google Scholar]
- 150.Jiang H., Zhou L., Shen N., Ning X., Wu D., Jiang K., et al. M1 macrophage-derived exosomes and their key molecule lncRNA HOTTIP suppress head and neck squamous cell carcinoma progression by upregulating the TLR5/NF-κB pathway. Cell Death Dis. 2022;13:183. doi: 10.1038/s41419-022-04640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gunassekaran G.R., Poongkavithai Vadevoo S.M., Baek M.C., Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121137. [DOI] [PubMed] [Google Scholar]
- 152.Qi Y., Zhu T., Zhang T., Wang X., Li W., Chen D., et al. M1 macrophage-derived exosomes transfer miR-222 to induce bone marrow mesenchymal stem cell apoptosis. Lab Invest. 2021;101:1318–1326. doi: 10.1038/s41374-021-00622-5. [DOI] [PubMed] [Google Scholar]
- 153.Li Z., Suo B., Long G., Gao Y., Song J., Zhang M., et al. Exosomal miRNA-16-5p derived from M1 macrophages enhances T cell-dependent immune response by regulating PD-L1 in gastric cancer. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.572689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bai Z.Z., Li H.Y., Li C.H., Sheng C.L., Zhao X.N. M1 macrophage-derived exosomal microRNA-326 suppresses hepatocellular carcinoma cell progression via mediating NF-κB signaling pathway. Nanoscale Res Lett. 2020;15:221. doi: 10.1186/s11671-020-03432-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155.Liu S., Chen J., Shi J., Zhou W., Wang L., Fang W., et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res Cardiol. 2020;115:22. doi: 10.1007/s00395-020-0781-7. [DOI] [PubMed] [Google Scholar]
- 156.Xia Y., He X.T., Xu X.Y., Tian B.M., An Y., Chen F.M. Exosomes derived from M0, M1 and M2 macrophages exert distinct influences on the proliferation and differentiation of mesenchymal stem cells. PeerJ. 2020;8 doi: 10.7717/peerj.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Agarwal A., Fanelli G., Letizia M., Tung S.L., Boardman D., Lechler R., et al. Regulatory T cell-derived exosomes: possible therapeutic and diagnostic tools in transplantation. Front Immunol. 2014;5:555. doi: 10.3389/fimmu.2014.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen L., Huang H., Zhang W., Ding F., Fan Z., Zeng Z. Exosomes derived from T regulatory cells suppress CD8+ cytotoxic T lymphocyte proliferation and prolong liver allograft survival. Med Sci Monit. 2019;25:4877–4884. doi: 10.12659/MSM.917058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Torri A., Carpi D., Bulgheroni E., Crosti M.C., Moro M., Gruarin P., et al. Extracellular microrna signature of human helper T cell subsets in health and autoimmunity. J Biol Chem. 2017;292:2903–2915. doi: 10.1074/jbc.M116.769893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aiello S., Rocchetta F., Longaretti L., Faravelli S., Todeschini M., Cassis L., et al. Extracellular vesicles derived from T regulatory cells suppress T cell proliferation and prolong allograft survival. Sci Rep. 2017;7 doi: 10.1038/s41598-017-08617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Giri P.K., Schorey J.S. Exosomes derived from m. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and. in vivo. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Azimi M., Ghabaee M., Moghadasi A.N., Noorbakhsh F., Izad M. Immunomodulatory function of Treg-derived exosomes is impaired in patients with relapsing-remitting multiple sclerosis. Immunol Res. 2018;66:513–520. doi: 10.1007/s12026-018-9008-5. [DOI] [PubMed] [Google Scholar]
- 163.Tung S.L., Boardman D.A., Sen M., Letizia M., Peng Q., Cianci N., et al. Regulatory T cell-derived extracellular vesicles modify dendritic cell function. Sci Rep. 2018;8:6065. doi: 10.1038/s41598-018-24531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gao F., Chiu S.M., Motan D.A.L., Zhang Z., Chen L., Ji H.L., et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Taechangam N., Iyer S.S., Walker N.J., Arzi B., Borjesson D.L. Mechanisms utilized by feline adipose-derived mesenchymal stem cells to inhibit T lymphocyte proliferation. Stem Cell Res Ther. 2019;10:188. doi: 10.1186/s13287-019-1300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu C., Chen Z., Dardalhon V., Xiao S., Thalhamer T., Liao M., et al. The transcription factor musculin promotes the unidirectional development of peripheral Treg cells by suppressing the TH2 transcriptional program. Nat Immunol. 2017;18:344–353. doi: 10.1038/ni.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Franquesa M., Mensah F.K., Huizinga R., Strini T., Boon L., Lombardo E., et al. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cell. 2015;33:880–891. doi: 10.1002/stem.1881. [DOI] [PubMed] [Google Scholar]
- 168.Sotiropoulou P.A., Perez S.A., Gritzapis A.D., Baxevanis C.N., Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cell. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 169.Li D., Zhang P., Yao X., Li H., Shen H., Li X., et al. Exosomes derived from miR-133b-modified mesenchymal stem cells promote recovery after spinal cord injury. Front Neurosci. 2018;12:845. doi: 10.3389/fnins.2018.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu J., Chen T., Lei P., Tang X., Huang P. Exosomes released by bone marrow mesenchymal stem cells attenuate lung injury induced by intestinal ischemia reperfusion via the TLR4/NF-κB pathway. Int J Med Sci. 2019;16:1238–1244. doi: 10.7150/ijms.35369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Guo L., Lai P., Wang Y., Huang T., Chen X., Luo C., et al. Extracellular vesicles from mesenchymal stem cells prevent contact hypersensitivity through the suppression of Tc1 and Th1 cells and expansion of regulatory T cells. Int Immunopharm. 2019;74 doi: 10.1016/j.intimp.2019.05.048. [DOI] [PubMed] [Google Scholar]
- 172.Cheng A., Choi D., Lora M., Shum-Tim D., Rak J., Colmegna I. Human multipotent mesenchymal stromal cells cytokine priming promotes RAB27B-regulated secretion of small extracellular vesicles with immunomodulatory cargo. Stem Cell Res Ther. 2020;11:539. doi: 10.1186/s13287-020-02050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Adamo A., Brandi J., Caligola S., Delfino P., Bazzoni R., Carusone R., et al. Extracellular vesicles mediate mesenchymal stromal cell-dependent regulation of B cell PI3K-AKT signaling pathway and actin cytoskeleton. Front Immunol. 2019;10:446. doi: 10.3389/fimmu.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Batista F.D., Treanor B., Harwood N.E. Visualizing a role for the actin cytoskeleton in the regulation of B-cell activation. Immunol Rev. 2010;237:191–204. doi: 10.1111/j.1600-065X.2010.00943.x. [DOI] [PubMed] [Google Scholar]
- 175.Reis M., Mavin E., Nicholson L., Green K., Dickinson A.M., Wang X.N. Mesenchymal stromal cell-derived extracellular vesicles attenuate dendritic cell maturation and function. Front Immunol. 2018;9:2538. doi: 10.3389/fimmu.2018.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lo Sicco C., Reverberi D., Balbi C., Ulivi V., Principi E., Pascucci L., et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med. 2017;6:1018–1028. doi: 10.1002/sctm.16-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fan B., Li C., Szalad A., Wang L., Pan W., Zhang R., et al. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia. 2020;63:431–443. doi: 10.1007/s00125-019-05043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee J.R., Kyung J.W., Kumar H., Kwon S.P., Song S.Y., Han I.B., et al. Targeted delivery of mesenchymal stem cell-derived nanovesicles for spinal cord injury treatment. Int J Mol Sci. 2020;21:4185. doi: 10.3390/ijms21114185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xie M., Xiong W., She Z., Wen Z., Abdirahman A.S., Wan W., et al. Immunoregulatory effects of stem cell-derived extracellular vesicles on immune cells. Front Immunol. 2020;11:13. doi: 10.3389/fimmu.2020.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mardpour S., Hamidieh A.A., Taleahmad S., Sharifzad F., Taghikhani A., Baharvand H. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. J Cell Physiol. 2019;234:8249–8258. doi: 10.1002/jcp.27669. [DOI] [PubMed] [Google Scholar]
- 181.Harrell C.R., Jovicic N., Djonov V., Arsenijevic N., Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019;8:1605. doi: 10.3390/cells8121605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li K.L., Li J.Y., Xie G.L., Ma X.Y. Exosomes released from human bone marrow–derived mesenchymal stem cell attenuate acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation in mice. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.617589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang L., Gu Z., Zhao X., Yang N., Wang F., Deng A., et al. Extracellular vesicles released from human umbilical cord-derived mesenchymal stromal cells prevent life-threatening acute graft-versus-host disease in a mouse model of allogeneic hematopoietic stem cell transplantation. Stem Cell Dev. 2016;25:1874–1883. doi: 10.1089/scd.2016.0107. [DOI] [PubMed] [Google Scholar]
- 184.Fujii S., Miura Y., Fujishiro A., Shindo T., Shimazu Y., Hirai H., et al. Graft-versus-host disease amelioration by human bone marrow mesenchymal stromal/stem cell-derived extracellular vesicles is associated with peripheral preservation of naive T cell populations. Stem Cell. 2018;36:434–445. doi: 10.1002/stem.2759. [DOI] [PubMed] [Google Scholar]
- 185.Stone M.L., Zhao Y., Robert Smith J., Weiss M.L., Kron I.L., Laubach V.E., et al. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia–reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir Res. 2017;18:212. doi: 10.1186/s12931-017-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhao J., Li X., Hu J., Chen F., Qiao S., Sun X., et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115:1205–1216. doi: 10.1093/cvr/cvz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rigo F., De Stefano N., Navarro-Tableros V., David E., Rizza G., Catalano G., et al. Extracellular vesicles from human liver stem cells reduce injury in an ex vivo normothermic hypoxic rat liver perfusion model. Transplant. 2018;102:e205–210. doi: 10.1097/TP.0000000000002123. [DOI] [PubMed] [Google Scholar]
- 188.Xia C., Zeng Z., Fang B., Tao M., Gu C., Zheng L., et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radical Biol Med. 2019;143:1–15. doi: 10.1016/j.freeradbiomed.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 189.Yang C., Lim W., Park J., Park S., You S., Song G. Anti-inflammatory effects of mesenchymal stem cell-derived exosomal microRNA-146a-5p and microRNA-548e-5p on human trophoblast cells. Mol Hum Reprod. 2019;25:755–771. doi: 10.1093/molehr/gaz054. [DOI] [PubMed] [Google Scholar]
- 190.Teng X., Chen L., Chen W., Yang J., Yang Z., Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem. 2015;37:2415–2424. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 191.Lai P., Chen X., Guo L., Wang Y., Liu X., Liu Y., et al. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD. J Hematol Oncol. 2018;11:135. doi: 10.1186/s13045-018-0680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kordelas L., Rebmann V., Ludwig A.K., Radtke S., Ruesing J., Doeppner T.R., et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 193.Hou Y., Liu Y., Liang S., Ding R., Mo S., Yan D., et al. The novel target: exosoms derived from M2 macrophage. Int Rev Immunol. 2021;40:183–196. doi: 10.1080/08830185.2020.1800687. [DOI] [PubMed] [Google Scholar]
- 194.Li M., Wang T., Tian H., Wei G., Zhao L., Shi Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif Cell Nanomed Biotechnol. 2019;47:3793–3803. doi: 10.1080/21691401.2019.1669617. [DOI] [PubMed] [Google Scholar]
- 195.Kawata R., Oda S., Koya Y., Kajiyama H., Yokoi T. Macrophage-derived extracellular vesicles regulate concanavalin A-induced hepatitis by suppressing macrophage cytokine production. Toxicology. 2020;443 doi: 10.1016/j.tox.2020.152544. [DOI] [PubMed] [Google Scholar]
- 196.Wu L., Xia J., Li D., Kang Y., Fang W., Huang P. Mechanisms of M2 macrophage-derived exosomal long non-coding RNA PVT1 in regulating Th17 cell response in experimental autoimmune encephalomyelitisa. Front Immunol. 2020;11:1934. doi: 10.3389/fimmu.2020.01934. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 197.Yin Z., Ma T., Huang B., Lin L., Zhou Y., Yan J., et al. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway. J Exp Clin Cancer Res. 2019;38:310. doi: 10.1186/s13046-019-1313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang F., Wang T., Du P., Fan H., Dong X., Guo H. M2 bone marrow-derived macrophage-derived exosomes shuffle microRNA-21 to accelerate immune escape of glioma by modulating PEG3. Cancer Cell Int. 2020;20:93. doi: 10.1186/s12935-020-1163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ma Y.S., Wu T.M., Ling C.C., Yu F., Zhang J., Cao P.S., et al. M2 macrophage-derived exosomal microRNA-155-5p promotes the immune escape of colon cancer by downregulating ZC3H12B. Mol Ther - Oncolytics. 2021;20:484–498. doi: 10.1016/j.omto.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pu J., Xu Z., Nian J., Fang Q., Yang M., Huang Y., et al. M2 macrophage-derived extracellular vesicles facilitate CD8+T cell exhaustion in hepatocellular carcinoma via the miR-21-5p/YOD1/YAP/β-catenin pathway. Cell Death Dis. 2021;7:182. doi: 10.1038/s41420-021-00556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Amjadi M.F., Avner B.S., Greenlee-Wacker M.C., Horswill A.R., Nauseef W.M. Neutrophil-derived extracellular vesicles modulate the phenotype of naïve human neutrophils. J Leukoc Biol. 2021;110:917–925. doi: 10.1002/JLB.3AB0520-339RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Youn Y.J., Shrestha S., Lee Y.B., Kim J.K., Lee J.H., Hur K., et al. Neutrophil-derived trail is a proinflammatory subtype of neutrophil-derived extracellular vesicles. Theranostics. 2021;11:2770–2787. doi: 10.7150/thno.51756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rossaint J., Kühne K., Skupski J., Van Aken H., Looney M.R., Hidalgo A., et al. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat Commun. 2016;7 doi: 10.1038/ncomms13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Forrest O.A., Dobosh B., Ingersoll S.A., Rao S., Rojas A., Laval J., et al. Neutrophil-derived extracellular vesicles promote feed-forward inflammasome signaling in cystic fibrosis airways. J Leukoc Biol. 2022:1–10. doi: 10.1002/JLB.3AB0321-149R. [DOI] [PubMed] [Google Scholar]
- 205.Glémain A., Néel M., Néel A., André-Grégoire G., Gavard J., Martinet B., et al. Neutrophil-derived extracellular vesicles induce endothelial inflammation and damage through the transfer of miRNAs. J Autoimmun. 2022;129 doi: 10.1016/j.jaut.2022.102826. [DOI] [PubMed] [Google Scholar]
- 206.French S.L., Butov K.R., Allaeys I., Canas J., Morad G., Davenport P., et al. Platelet-derived extracellular vesicles infiltrate and modify the bone marrow during inflammation. Blood Adv. 2020;4:3011–3023. doi: 10.1182/bloodadvances.2020001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kuravi S.J., Harrison P., Rainger G.E., Nash G.B. Ability of platelet-derived extracellular vesicles to promote neutrophil-endothelial cell interactions. Inflammation. 2019;42:290–305. doi: 10.1007/s10753-018-0893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chimen M., Evryviadou A., Box C.L., Harrison M.J., Hazeldine J., Dib L.H., et al. Appropriation of GPIbα from platelet-derived extracellular vesicles supports monocyte recruitment in systemic inflammation. Haematol. 2020;105:1248–1261. doi: 10.3324/haematol.2018.215145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ma Q., Fan Q., Han X., Dong Z., Xu J., Bai J., et al. Platelet-derived extracellular vesicles to target plaque inflammation for effective anti-atherosclerotic therapy. J Contr Release. 2021;329:445–453. doi: 10.1016/j.jconrel.2020.11.064. [DOI] [PubMed] [Google Scholar]
- 210.Collier M.E.W., Ambrose A.R., Goodall A.H. Does hsa-miR-223-3p from platelet-derived extracellular vesicles regulate tissue factor expression in monocytic cells? Platelets. 2022;8:1–12. doi: 10.1080/09537104.2022.2027903. [DOI] [PubMed] [Google Scholar]
- 211.Ma Q., Fan Q., Xu J., Bai J., Han X., Dong Z., et al. Calming cytokine storm in pneumonia by targeted delivery of TPCA-1 using platelet-derived extracellular vesicles. Matter. 2020;3:287–301. doi: 10.1016/j.matt.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Graça A.L., Gómez-Florit M., Osório H., Rodrigues M.T., Domingues R.M.A., Reis R.L., et al. Controlling the fate of regenerative cells with engineered platelet-derived extracellular vesicles. Nanoscale. 2022;14:6543–6556. doi: 10.1039/d1nr08108j. [DOI] [PubMed] [Google Scholar]
- 213.Vajen T., Benedikter B.J., Heinzmann A.C.A., Vasina E.M., Henskens Y., Parsons M., et al. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J Extracell Vesicles. 2017;6 doi: 10.1080/20013078.2017.1322454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.McVey M.J., Weidenfeld S., Maishan M., Spring C., Kim M., Tabuchi A., et al. Platelet extracellular vesicles mediate transfusion-related acute lung injury by imbalancing the sphingolipid rheostat. Blood. 2021;137:690–701. doi: 10.1182/blood.2020005985. [DOI] [PubMed] [Google Scholar]
- 215.Xu L., Liang Y., Xu X., Xia J., Wen C., Zhang P., et al. Blood cell-derived extracellular vesicles: diagnostic biomarkers and smart delivery systems. Bioengineered. 2021;12:7929–7940. doi: 10.1080/21655979.2021.1982320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Straat M., Böing A.N., Tuip-De Boer A., Nieuwland R., Juffermans N.P. Extracellular vesicles from red blood cell products induce a strong pro-inflammatory host response, dependent on both numbers and storage duration. Transfus Med Hemotherapy. 2016;43:302–305. doi: 10.1159/000442681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fischer D., Büssow J., Meybohm P., Weber C.F., Zacharowski K., Urbschat A., et al. Microparticles from stored red blood cells enhance procoagulant and proinflammatory activity. Transfusion. 2017;57:2701–2711. doi: 10.1111/trf.14268. [DOI] [PubMed] [Google Scholar]
- 218.Danesh A., Inglis H.C., Jackman R.P., Wu S., Deng X., Muench M.O., et al. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses. in vitro. Blood. 2014;123:687–696. doi: 10.1182/blood-2013-10-530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gao Y., Jin H., Tan H., Wang Y., Wu J., Wang Y., et al. The role of extracellular vesicles from stored RBC units in B lymphocyte survival and plasma cell differentiation. J Leukoc Biol. 2020;108:1765–1776. doi: 10.1002/JLB.1A0220-666R. [DOI] [PubMed] [Google Scholar]
- 220.Sadallah S., Eken C., Schifferli J.A. Erythrocyte-derived ectosomes have immunosuppressive properties. J Leukoc Biol. 2008;84:1316–1325. doi: 10.1189/jlb.0108013. [DOI] [PubMed] [Google Scholar]
- 221.Soriani A., Vulpis E., Cuollo L., Santoni A., Zingoni A. Cancer extracellular vesicles as novel regulators of NK cell response. Cytokine Growth Factor Rev. 2020;51:19–26. doi: 10.1016/j.cytogfr.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 222.Han Q., Lv L., Wei J., Lei X., Lin H., Li G., et al. Vps4A mediates the localization and exosome release of β-catenin to inhibit epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2019;457:47–59. doi: 10.1016/j.canlet.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 223.Bardi G.T., Smith M.A., Hood J.L. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine. 2018;105:63–72. doi: 10.1016/j.cyto.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Liu J.Y., Ren L.W., Li S., Li W., Zheng X.J., Yang Y.H., et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11:2783–2797. doi: 10.1016/j.apsb.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Admyre C., Bohle B., Johansson S.M., Focke-Tejkl M., Valenta R., Scheynius A., et al. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol. 2007;120:1418–1424. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 226.Wei M., Gao X., Liu L., Li Z., Wan Z., Dong Y., et al. Visceral adipose tissue derived exosomes exacerbate colitis severity via pro-inflammatory miRNAs in high fat diet fed mice. ACS Nano. 2020;14:5099–5110. doi: 10.1021/acsnano.0c01860. [DOI] [PubMed] [Google Scholar]
- 227.Lv L.L., Feng Y., Wu M., Wang B., Li Z.L., Zhong X., et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 2020;27:210–226. doi: 10.1038/s41418-019-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Le Q.V., Lee J., Lee H., Shim G., Oh Y.K. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm Sin B. 2021;11:2096–2113. doi: 10.1016/j.apsb.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Czernek L., Düchler M. Functions of cancer-derived extracellular vesicles in immunosuppression. Arch Immunol Ther Exp. 2017;65:311–323. doi: 10.1007/s00005-016-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Filipazzi P., Bürdek M., Villa A., Rivoltini L., Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol. 2012;22:342–349. doi: 10.1016/j.semcancer.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 231.Balaphas A., Meyer J., Sadoul K., Fontana P., Morel P., Gonelle-Gispert C., et al. Platelets and platelet-derived extracellular vesicles in liver physiology and disease. Hepatol Commun. 2019;3:855–866. doi: 10.1002/hep4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Barry O.P., Praticò D., Savani R.C., FitzGerald G.A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Investig. 1998;102:136–144. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lin H.C., Chang H.W., Hsiao S.H., Chou M.L., Seghatchian J., Burnouf T. Platelet-derived microparticles trigger THP-1 monocytic cell aggregation and release of pro-coagulant tissue factor-expressing microparticles. in vitro. Transfus Apher Sci. 2015;53:246–252. doi: 10.1016/j.transci.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 234.Badimon L., Suades R., Fuentes E., Palomo I., Padró T. Role of platelet-derived microvesicles as crosstalk mediators in atherothrombosis and future pharmacology targets: a link between inflammation, atherosclerosis, and thrombosis. Front Pharmacol. 2016;7:293. doi: 10.3389/fphar.2016.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chen H., Chengalvala V., Hu H., Sun D. Tumor-derived exosomes: nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm Sin B. 2021;11:2136–2149. doi: 10.1016/j.apsb.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur Basant K., et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Clayton A., Mitchell J.P., Court J., Linnane S., Mason M.D., Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 238.Manning S., Danielson K.M. The immunomodulatory role of tumor-derived extracellular vesicles in colorectal cancer. Immunol Cell Biol. 2018;96:733–741. doi: 10.1111/imcb.12038. [DOI] [PubMed] [Google Scholar]
- 239.Chen G., Huang A.C., Zhang W., Zhang G., Wu M., Xu W., et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ricklefs Franz L., Alayo Q., Krenzlin H., Mahmoud Ahmad B., Speranza Maria C., Nakashima H., et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv. 2018;4:eaar2766. doi: 10.1126/sciadv.aar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Daassi D., Mahoney K.M., Freeman G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020;20:209–215. doi: 10.1038/s41577-019-0264-y. [DOI] [PubMed] [Google Scholar]
- 242.Liu J., Peng X., Yang S., Li X., Huang M., Wei S., et al. Extracellular vesicle PD-L1 in reshaping tumor immune microenvironment: biological function and potential therapy strategies. Cell Commun Signal. 2022;20:14. doi: 10.1186/s12964-021-00816-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fleming V., Hu X., Weller C., Weber R., Groth C., Riester Z., et al. Melanoma extracellular vesicles generate immunosuppressive myeloid cells by upregulating PD-L1 via TLR4 signaling. Cancer Res. 2019;79:4715–4728. doi: 10.1158/0008-5472.CAN-19-0053. [DOI] [PubMed] [Google Scholar]
- 244.Orme J.J., Enninga E.A.L., Lucien-Matteoni F., Dale H., Burgstaler E., Harrington S.M., et al. Therapeutic plasma exchange clears circulating soluble PD-L1 and PD-L1-positive extracellular vesicles. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Adamo A., Brandi J., Caligola S., Delfino P., Bazzoni R., Carusone R., et al. Extracellular vesicles mediate mesenchymal stromal cell-dependent regulation of B cell PI3K-AKT signaling pathway and actin cytoskeleton. Front Immunol. 2019;10:446. doi: 10.3389/fimmu.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.de Abreu R.C., Fernandes H., da Costa Martins P.A., Sahoo S., Emanueli C., Ferreira L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat Rev Cardiol. 2020;17:685–697. doi: 10.1038/s41569-020-0389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bost J.P., Barriga H., Holme M.N., Gallud A., Maugeri M., Gupta D., et al. Delivery of oligonucleotide therapeutics: chemical modifications, lipid nanoparticles, and extracellular vesicles. ACS Nano. 2021;15:13993–14021. doi: 10.1021/acsnano.1c05099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jia X., Tang J., Yao C., Yang D. Recent progress of extracellular vesicle engineering. ACS Biomater Sci Eng. 2021;7:4430–4438. doi: 10.1021/acsbiomaterials.1c00868. [DOI] [PubMed] [Google Scholar]
- 249.Richter M., Vader P., Fuhrmann G. Approaches to surface engineering of extracellular vesicles. Adv Drug Deliv Rev. 2021;173:416–426. doi: 10.1016/j.addr.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 250.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 251.Quah B.J.C., O'Neill H.C. The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol Dis. 2005;35:94–110. doi: 10.1016/j.bcmd.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 252.Liu M., Hu Y., Chen G. The antitumor effect of gene-engineered exosomes in the treatment of brain metastasis of breast cancer. Front Oncol. 2020;10:1453. doi: 10.3389/fonc.2020.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Xu S., Liu B., Fan J., Xue C., Lu Y., Li C., et al. Engineered mesenchymal stem cell-derived exosomes with high CXCR4 levels for targeted siRNA gene therapy against cancer. Nanoscale. 2022;14:4098–4113. doi: 10.1039/d1nr08170e. [DOI] [PubMed] [Google Scholar]
- 254.Yang H.C., Zhang M., Wu R., Zheng H.Q., Zhang L.Y., Luo J., et al. C‒C chemokine receptor type 2-overexpressing exosomes alleviated experimental post-stroke cognitive impairment by enhancing microglia/macrophage M2 polarization. World J Stem Cell. 2020;12:152–167. doi: 10.4252/wjsc.v12.i2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Li P., Gao M., Hu Z., Xu T., Chen J., Ma Y., et al. Synergistic ferroptosis and macrophage re-polarization using engineering exosome-mimic M1 nanovesicles for cancer metastasis suppression. Chem Eng J. 2021;409 [Google Scholar]
- 256.Cheng Q., Shi X., Han M., Smbatyan G., Lenz H.-J., Zhang Y. Reprogramming exosomes as nanoscale controllers of cellular immunity. J Am Chem Soc. 2018;140:16413–16417. doi: 10.1021/jacs.8b10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Li L., Miao Q., Meng F., Li B., Xue T., Fang T., et al. Genetic engineering cellular vesicles expressing CD64 as checkpoint antibody carrier for cancer immunotherapy. Theranostics. 2021;11:6033–6043. doi: 10.7150/thno.48868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Qiu Y., Yang Y., Yang R., Liu C., Hsu J.-M., Jiang Z., et al. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene. 2021;40:4992–5001. doi: 10.1038/s41388-021-01896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yang M., Xu Z., Yan H., Tsai H.I., Su D., Yan F., et al. PD-L1 cellular nanovesicles carrying rapamycin inhibit alloimmune responses in transplantation. Biomater Sci. 2021;9:1246–1255. doi: 10.1039/d0bm01798a. [DOI] [PubMed] [Google Scholar]
- 260.Zhao Y.D., Muhetaerjiang M., An H.W., Fang X., Zhao Y., Wang H. Nanomedicine enables spatiotemporally regulating macrophage-based cancer immunotherapy. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120552. [DOI] [PubMed] [Google Scholar]
- 261.Xu Z., Tsai H.I., Xiao Y., Wu Y., Su D., Yang M., et al. Engineering programmed death ligand-1/cytotoxic T-lymphocyte-associated antigen-4 dual-targeting nanovesicles for immunosuppressive therapy in transplantation. ACS Nano. 2020;14:7959–7969. doi: 10.1021/acsnano.9b09065. [DOI] [PubMed] [Google Scholar]
- 262.Zeelenberg I.S., Ostrowski M., Krumeich S., Bobrie A., Jancic C., Boissonnas A., et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008;68:1228. doi: 10.1158/0008-5472.CAN-07-3163. [DOI] [PubMed] [Google Scholar]
- 263.Liu C., Liu X., Xiang X., Pang X., Chen S., Zhang Y., et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nat Nanotechnol. 2022;17:531–540. doi: 10.1038/s41565-022-01098-0. [DOI] [PubMed] [Google Scholar]
- 264.Du J., Wan Z., Wang C., Lu F., Wei M., Wang D., et al. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics. 2021;11:8185–8196. doi: 10.7150/thno.59121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cheng L., Zhang X., Tang J., Lv Q., Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120964. [DOI] [PubMed] [Google Scholar]
- 266.Tang T.T., Wang B., Wu M., Li Z.L., Feng Y., Cao J.Y., et al. Extracellular vesicle–encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci Adv. 2020;6:eaaz0748. doi: 10.1126/sciadv.aaz0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chen H.Y., Deng J., Wang Y., Wu C.Q., Li X., Dai H.W. Hybrid cell membrane-coated nanoparticles: a multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020;112:1–13. doi: 10.1016/j.actbio.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 268.Lin W., Xu Y., Chen X., Liu J., Weng Y., Zhuang Q., et al. Radiation-induced small extracellular vesicles as “carriages” promote tumor antigen release and trigger antitumor immunity. Theranostics. 2020;10:4871–4884. doi: 10.7150/thno.43539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Richardson J.J., Ejima H. Surface engineering of extracellular vesicles through chemical and biological strategies. Chem Mater. 2019;31:2191–2201. [Google Scholar]
- 270.Tao S.C., Guo S.C., Zhang C.Q. Modularized extracellular vesicles: the dawn of prospective personalized and precision medicine. Adv Sci. 2018;5 doi: 10.1002/advs.201700449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nakase I. Biofunctional peptide-modified extracellular vesicles enable effective intracellular delivery via the induction of macropinocytosis. Processes. 2021;9:224. [Google Scholar]
- 272.Pham T.C., Jayasinghe M.K., Pham T.T., Yang Y., Wei L., Usman W.M., et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J Extracell Vesicles. 2021;10 doi: 10.1002/jev2.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Park D.J., Yun W.S., Kim W.C., Park J.E., Lee S.H., Ha S., et al. Improvement of stem cell-derived exosome release efficiency by surface-modified nanoparticles. J Nanobiotechnol. 2020;18:178. doi: 10.1186/s12951-020-00739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fan Z., Xiao K., Lin J., Liao Y., Huang X. Functionalized DNA enables programming exosomes/vesicles for tumor imaging and therapy. Small. 2019;15 doi: 10.1002/smll.201903761. [DOI] [PubMed] [Google Scholar]
- 275.Zhang C., Li D., Hu H., Wang Z., An J., Gao Z., et al. Engineered extracellular vesicles derived from primary M2 macrophages with anti-inflammatory and neuroprotective properties for the treatment of spinal cord injury. J Nanobiotechnol. 2021;19:373. doi: 10.1186/s12951-021-01123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wang M., Altinoglu S., Takeda Y.S., Xu Q. Integrating protein engineering and bioorthogonal click conjugation for extracellular vesicle modulation and intracellular delivery. PLoS One. 2015;10 doi: 10.1371/journal.pone.0141860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kwon S., Shin S., Do M., Oh B.H., Song Y., Bui V.D., et al. Engineering approaches for effective therapeutic applications based on extracellular vesicles. J Contr Release. 2021;330:15–30. doi: 10.1016/j.jconrel.2020.11.062. [DOI] [PubMed] [Google Scholar]
- 278.Lim G.T., You D.G., Han H.S., Lee H., Shin S., Oh B.H., et al. Bioorthogonally surface-edited extracellular vesicles based on metabolic glycoengineering for CD44-mediated targeting of inflammatory diseases. J Extracell Vesicles. 2021;10 doi: 10.1002/jev2.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Gai M., Simon J., Lieberwirth I., Mailänder V., Morsbach S., Landfester K. A bio-orthogonal functionalization strategy for site-specific coupling of antibodies on vesicle surfaces after self-assembly. Polym Chem. 2020;11:527–540. [Google Scholar]
- 280.Jung H., Jang H.E., Kang Y.Y., Song J., Mok H. PLGA microspheres coated with cancer cell-derived vesicles for improved internalization into antigen-presenting cells and immune stimulation. Bioconjugate Chem. 2019;30:1690–1701. doi: 10.1021/acs.bioconjchem.9b00240. [DOI] [PubMed] [Google Scholar]
- 281.Xiong J., Wu M., Chen J., Liu Y., Chen Y., Fan G., et al. Cancer-erythrocyte hybrid membrane-camouflaged magnetic nanoparticles with enhanced photothermal-immunotherapy for ovarian cancer. ACS Nano. 2021;15:19756–19770. doi: 10.1021/acsnano.1c07180. [DOI] [PubMed] [Google Scholar]
- 282.Piffoux M., Silva A.K.A., Wilhelm C., Gazeau F., Tareste D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano. 2018;12:6830–6842. doi: 10.1021/acsnano.8b02053. [DOI] [PubMed] [Google Scholar]
- 283.Kooijmans S.A.A., Fliervoet L.A.L., van der Meel R., Fens M.H.A.M., Heijnen H.F.G., van Bergen, Henegouwen P.M.P., et al. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J Contr Release. 2016;224:77–85. doi: 10.1016/j.jconrel.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 284.Tamura R., Uemoto S., Tabata Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017;57:274–284. doi: 10.1016/j.actbio.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 285.Cheng H., Fan J.H., Zhao L.P., Fan G.L., Zheng R.R., Qiu X.Z., et al. Chimeric peptide engineered exosomes for dual-stage light guided plasma membrane and nucleus targeted photodynamic therapy. Biomaterials. 2019;211:14–24. doi: 10.1016/j.biomaterials.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 286.Gao X., Ran N., Dong X., Zuo B., Yang R., Zhou Q., et al. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aat0195. [DOI] [PubMed] [Google Scholar]
- 287.Antes T.J., Middleton R.C., Luther K.M., Ijichi T., Peck K.A., Liu W.J., et al. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J Nanobiotechnol. 2018;16:61. doi: 10.1186/s12951-018-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Chen L., Mou S., Li F., Zeng Y., Sun Y., Horch R.E., et al. Self-assembled human adipose-derived stem cell-derived extracellular vesicle-functionalized biotin-doped polypyrrole titanium with long-term stability and potential osteoinductive ability. ACS Appl Mater Interfaces. 2019;11:46183–46196. doi: 10.1021/acsami.9b17015. [DOI] [PubMed] [Google Scholar]
- 289.Zuo B., Qi H., Lu Z., Chen L., Sun B., Yang R., et al. Alarmin-painted exosomes elicit persistent antitumor immunity in large established tumors in mice. Nat Commun. 2020;11:1790. doi: 10.1038/s41467-020-15569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Schulz-Siegmund M., Aigner A. Nucleic acid delivery with extracellular vesicles. Adv Drug Deliv Rev. 2021;173:89–111. doi: 10.1016/j.addr.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 291.Oskouie M.N., Aghili Moghaddam N.S., Butler A.E., Zamani P., Sahebkar A. Therapeutic use of curcumin-encapsulated and curcumin-primed exosomes. J Cell Physiol. 2019;234:8182–8191. doi: 10.1002/jcp.27615. [DOI] [PubMed] [Google Scholar]
- 292.Gutierrez-Millan C., Calvo Díaz C., Lanao J.M., Colino C.I. Advances in exosomes-based drug delivery systems. Macromol Biosci. 2021;21 doi: 10.1002/mabi.202000269. [DOI] [PubMed] [Google Scholar]
- 293.Liang Y., Duan L., Lu J., Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Jiang L., Vader P., Schiffelers R.M. Extracellular vesicles for nucleic acid delivery: progress and prospects for safe RNA-based gene therapy. Gene Ther. 2017;24:157–166. doi: 10.1038/gt.2017.8. [DOI] [PubMed] [Google Scholar]
- 295.Deshmukh S.K., Khan M.A., Singh S., Singh A.P. Extracellular nanovesicles: from intercellular messengers to efficient drug delivery systems. ACS Omega. 2021;6:1773–1779. doi: 10.1021/acsomega.0c05539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bryniarski K., Ptak W., Jayakumar A., Püllmann K., Caplan M.J., Chairoungdua A., et al. Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J Allergy Clin Immunol. 2013;132:170–181. doi: 10.1016/j.jaci.2013.04.048. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Peng H., Ji W., Zhao R., Yang J., Lu Z., Li Y., et al. Exosome: a significant nano-scale drug delivery carrier. J Mater Chem B. 2020;8:7591–7608. doi: 10.1039/d0tb01499k. [DOI] [PubMed] [Google Scholar]
- 298.Batrakova E.V., Kim M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Contr Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Luan X., Sansanaphongpricha K., Myers I., Chen H., Yuan H., Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Didiot M.C., Hall L.M., Coles A.H., Haraszti R.A., Godinho B.M.D.C., Chase K., et al. Exosome-mediated delivery of hydrophobically modified siRNA for huntingtin mRNA silencing. Mol Ther. 2016;24:1836–1847. doi: 10.1038/mt.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Xi X.M., Cheng M., Xia S.J., Lu R. Drug loading techniques for exosome-based drug delivery systems. Die Pharmazie - An International Journal of Pharmaceutical Sciences. 2021;76:61–67. doi: 10.1691/ph.2021.0128. [DOI] [PubMed] [Google Scholar]
- 302.Mendt M., Kamerkar S., Sugimoto H., McAndrews K.M., Wu C.C., Gagea M., et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3 doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lamichhane T.N., Jeyaram A., Patel D.B., Parajuli B., Livingston N.K., Arumugasaamy N., et al. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell Mol Bioeng. 2016;9:315–324. doi: 10.1007/s12195-016-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hettich B.F., Bader J.J., Leroux J.C. Encapsulation of hydrophilic compounds in small extracellular vesicles: loading capacity and impact on vesicle functions. Adv Healthcare Mater. 2022;11 doi: 10.1002/adhm.202100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Han Y., Jones T.W., Dutta S., Zhu Y., Wang X., Narayanan S.P., et al. Overview and update on methods for cargo loading into extracellular vesicles. Processes. 2021;9:356. doi: 10.3390/pr9020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Elashiry M., Elashiry M.M., Elsayed R., Rajendran M., Auersvald C., Zeitoun R., et al. Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease. in vivo. J Extracell Vesicles. 2020;9 doi: 10.1080/20013078.2020.1795362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Nasiri Kenari A., Cheng L., Hill A.F. Methods for loading therapeutics into extracellular vesicles and generating extracellular vesicles mimetic-nanovesicles. Methods. 2020;177:103–113. doi: 10.1016/j.ymeth.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 308.Wang J., Chen D., Ho E.A. Challenges in the development and establishment of exosome-based drug delivery systems. J Contr Release. 2021;329:894–906. doi: 10.1016/j.jconrel.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 309.Fitts C.A., Ji N., Li Y., Tan C. Exploiting exosomes in cancer liquid biopsies and drug delivery. Adv Healthcare Mater. 2019;8 doi: 10.1002/adhm.201801268. [DOI] [PubMed] [Google Scholar]
- 310.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Contr Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Fuhrmann G., Serio A., Mazo M., Nair R., Stevens M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Contr Release. 2015;205:35–44. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 312.Riau A.K., Ong H.S., Yam G.H.F., Mehta J.S. Sustained delivery system for stem cell-derived exosomes. Front Pharmacol. 2019;10:1368. doi: 10.3389/fphar.2019.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Verweij F.J., Revenu C., Arras G., Dingli F., Loew D., Pegtel D.M., et al. Live tracking of inter-organ communication by endogenous exosomes. in vivo. Dev Cell. 2019;48:573–589.e4. doi: 10.1016/j.devcel.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 314.Wu R., Gao W., Yao K., Ge JJFiI. Roles of exosomes derived from immune cells in cardiovascular diseases. Front Immunol. 2019;10:648. doi: 10.3389/fimmu.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Comino I., Suligoj T., Al-Hassi H., Lee G., Sousa C., Landy J., et al. Constitutive gut-homing capacity on circulating myeloid dendritic cells in coeliac disease. Rev Esp Enferm Dig. 2014;106:64–65. doi: 10.4321/s1130-01082014000100013. [DOI] [PubMed] [Google Scholar]
- 316.Tang T.T., Lv L.L., Wang B., Cao J.Y., Feng Y., Li Z.L., et al. Employing macrophage-derived microvesicle for kidney-targeted delivery of dexamethasone: an efficient therapeutic strategy against renal inflammation and fibrosis. Theranostics. 2019;9:4740–4755. doi: 10.7150/thno.33520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Xin H., Li Y., Liu Z., Wang X., Shang X., Cui Y., et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cell. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Charoenviriyakul C., Takahashi Y., Morishita M., Matsumoto A., Nishikawa M., Takakura Y. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: yield, physicochemical properties, and pharmacokinetics. Eur J Pharm Sci. 2017;96:316–322. doi: 10.1016/j.ejps.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 319.Annabi N., Tamayol A., Uquillas J.A., Akbari M., Bertassoni L.E., Cha C., et al. 25th Anniversary article: rational design and applications of hydrogels in regenerative medicine. Adv Mater. 2014;26:85–124. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Elkhoury K., Koçak P., Kang A., Arab-Tehrany E., Ellis Ward J., Shin S.R. Engineering smart targeting nanovesicles and their combination with hydrogels for controlled drug delivery. Pharmaceutics. 2020;12:849. doi: 10.3390/pharmaceutics12090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sawada S.I., Sato Y.T., Kawasaki R., Yasuoka J.I., Mizuta R., Sasaki Y., et al. Nanogel hybrid assembly for exosome intracellular delivery: effects on endocytosis and fusion by exosome surface polymer engineering. Biomater Sci. 2020;8:619–630. doi: 10.1039/c9bm01232j. [DOI] [PubMed] [Google Scholar]
- 322.Mizuta R., Sasaki Y., Kawasaki R., Katagiri K., Sawada S.I., Mukai S.A., et al. Magnetically navigated intracellular delivery of extracellular vesicles using amphiphilic nanogels. Bioconjugate Chem. 2019;30:2150–2155. doi: 10.1021/acs.bioconjchem.9b00369. [DOI] [PubMed] [Google Scholar]
- 323.Mol E.A., Lei Z., Roefs M.T., Bakker M.H., Goumans M.J., Doevendans P.A., et al. Injectable supramolecular ureidopyrimidinone hydrogels provide sustained release of extracellular vesicle therapeutics. Adv Healthcare Mater. 2019;8 doi: 10.1002/adhm.201900847. [DOI] [PubMed] [Google Scholar]
- 324.Chen C.W., Wang L.L., Zaman S., Gordon J., Arisi M.F., Venkataraman C.M., et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc Res. 2018;114:1029–1040. doi: 10.1093/cvr/cvy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Chen Y., Huang J., Chen R., Yang L., Wang J., Liu B., et al. Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth. Theranostics. 2020;10:1454–1478. doi: 10.7150/thno.39566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Shafei S., Khanmohammadi M., Heidari R., Ghanbari H., Taghdiri Nooshabadi V., Farzamfar S., et al. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: an in vivo study. J Biomed Mater Res. 2020;108:545–556. doi: 10.1002/jbm.a.36835. [DOI] [PubMed] [Google Scholar]
- 327.Li L., Zhang Y., Mu J., Chen J., Zhang C., Cao H., et al. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020;20:4298–4305. doi: 10.1021/acs.nanolett.0c00929. [DOI] [PubMed] [Google Scholar]
- 328.Shen Y., Xu G., Huang H., Wang K., Wang H., Lang M., et al. Sequential release of small extracellular vesicles from bilayered thiolated alginate/polyethylene glycol diacrylate hydrogels for scarless wound healing. ACS Nano. 2021;15:6352–6368. doi: 10.1021/acsnano.0c07714. [DOI] [PubMed] [Google Scholar]
- 329.Wang C., Liang C., Wang R., Yao X., Guo P., Yuan W., et al. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater Sci. 2020;8:313–324. doi: 10.1039/c9bm01207a. [DOI] [PubMed] [Google Scholar]
- 330.Liu X., Yang Y., Li Y., Niu X., Zhao B., Wang Y., et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9:4430–4438. doi: 10.1039/c7nr00352h. [DOI] [PubMed] [Google Scholar]
- 331.Lin J., Wang Z., Huang J., Tang S., Saiding Q., Zhu Q., et al. Microenvironment-protected exosome-hydrogel for facilitating endometrial regeneration, fertility restoration, and live birth of offspring. Small. 2021;17 doi: 10.1002/smll.202007235. [DOI] [PubMed] [Google Scholar]
- 332.Zhu D., Li Z., Huang K., Caranasos T.G., Rossi J.S., Cheng K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat Commun. 2021;12:1412. doi: 10.1038/s41467-021-21682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Tang Q., Lu B., He J., Chen X., Fu Q., Han H., et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials. 2022;280 doi: 10.1016/j.biomaterials.2021.121320. [DOI] [PubMed] [Google Scholar]
- 334.Xing Z., Zhao C., Wu S., Yang D., Zhang C., Wei X., et al. Hydrogel loaded with VEGF/TFEB-engineered extracellular vesicles for rescuing critical limb ischemia by a dual-pathway activation strategy. Adv Healthcare Mater. 2021;11 doi: 10.1002/adhm.202100334. [DOI] [PubMed] [Google Scholar]
- 335.Wang C., Wang M., Xu T., Zhang X., Lin C., Gao W., et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9:65–76. doi: 10.7150/thno.29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhang K., Zhao X., Chen X., Wei Y., Du W., Wang Y., et al. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl Mater Interfaces. 2018;10:30081–30091. doi: 10.1021/acsami.8b08449. [DOI] [PubMed] [Google Scholar]
- 337.Berger A., Araújo-Filho I., Piffoux M., Nicolás-Boluda A., Grangier A., Boucenna I., et al. Local administration of stem cell-derived extracellular vesicles in a thermoresponsive hydrogel promotes a pro-healing effect in a rat model of colo-cutaneous post-surgical fistula. Nanoscale. 2021;13:218–232. doi: 10.1039/d0nr07349k. [DOI] [PubMed] [Google Scholar]
- 338.Yang S., Zhu B., Yin P., Zhao L., Wang Y., Fu Z., et al. Integration of human umbilical cord mesenchymal stem cells-derived exosomes with hydroxyapatite-embedded hyaluronic acid-alginate hydrogel for bone regeneration. ACS Biomater Sci Eng. 2020;6:1590–1602. doi: 10.1021/acsbiomaterials.9b01363. [DOI] [PubMed] [Google Scholar]
- 339.Hu Y., Wu B., Xiong Y., Tao R., Panayi A.C., Chen L., et al. Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing. Chem Eng J. 2021;426 [Google Scholar]
- 340.Moradinasab S., Pourbagheri-Sigaroodi A., Zafari P., Ghaffari S.H., Bashash D. Mesenchymal stromal/stem cells (MSCs) and MSC-derived extracellular vesicles in COVID-19-induced ARDS: mechanisms of action, research progress, challenges, and opportunities. Int Immunopharm. 2021;97 doi: 10.1016/j.intimp.2021.107694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Lee H., Park H., Noh G.J., Lee E.S. pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydr Polym. 2018;202:323–333. doi: 10.1016/j.carbpol.2018.08.141. [DOI] [PubMed] [Google Scholar]
- 342.Xu Z., Huang H., Xiong X., Wei X., Guo X., Zhao J., et al. A near-infrared light-responsive extracellular vesicle as a “Trojan horse” for tumor deep penetration and imaging-guided therapy. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120647. [DOI] [PubMed] [Google Scholar]
- 343.Hu S., Li Z., Shen D., Zhu D., Huang K., Su T., et al. Exosome-eluting stents for vascular healing after ischaemic injury. Nat Biomed Eng. 2021;5:1174–1188. doi: 10.1038/s41551-021-00705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yong T., Wang D., Li X., Yan Y., Hu J., Gan L., et al. Extracellular vesicles for tumor targeting delivery based on five features principle. J Contr Release. 2020;322:555–565. doi: 10.1016/j.jconrel.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 345.Wiklander O.P.B., Nordin J.Z., O'Loughlin A., Gustafsson Y., Corso G., Mäger I., et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4 doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Worthington E.N., Hagood J.S. Therapeutic use of extracellular vesicles for acute and chronic lung disease. Int J Mol Sci. 2020;21:2318. doi: 10.3390/ijms21072318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Pinto A., Marangon I., Méreaux J., Nicolás-Boluda A., Lavieu G., Wilhelm C., et al. Immune reprogramming Precision photodynamic therapy of peritoneal metastasis by scalable stem-cell-derived extracellular vesicles. ACS Nano. 2021;15:3251–3263. doi: 10.1021/acsnano.0c09938. [DOI] [PubMed] [Google Scholar]
- 348.Nojehdehi S., Soudi S., Hesampour A., Rasouli S., Soleimani M., Hashemi S.M. Immunomodulatory effects of mesenchymal stem cell–derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. 2018;119:9433–9443. doi: 10.1002/jcb.27260. [DOI] [PubMed] [Google Scholar]
- 349.Haga H., Yan I.K., Borrelli D.A., Matsuda A., Parasramka M., Shukla N., et al. Extracellular vesicles from bone marrow–derived mesenchymal stem cells protect against murine hepatic ischemia/reperfusion injury. Liver Transplant. 2017;23:791–803. doi: 10.1002/lt.24770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Li Y., Wu H., Jiang X., Dong Y., Zheng J., Gao J. New idea to promote the clinical applications of stem cells or their extracellular vesicles in central nervous system disorders: combining with intranasal delivery. Acta Pharm Sin B. 2022;12:3215–3232. doi: 10.1016/j.apsb.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Brennan M.Á., Layrolle P., Mooney D.J. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater. 2020;30 doi: 10.1002/adfm.201909125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Gomzikova M.O., Aimaletdinov A.M., Bondar O.V., Starostina I.G., Gorshkova N.V., Neustroeva O.A., et al. Immunosuppressive properties of cytochalasin B-induced membrane vesicles of mesenchymal stem cells: comparing with extracellular vesicles derived from mesenchymal stem cells. Sci Rep. 2020;10 doi: 10.1038/s41598-020-67563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Figliolini F., Ranghino A., Grange C., Cedrino M., Tapparo M., Cavallari C., et al. Extracellular vesicles from adipose stem cells prevent muscle damage and inflammation in a mouse model of hind limb ischemia. Arterioscler Thromb Vasc Biol. 2020;40:239–254. doi: 10.1161/ATVBAHA.119.313506. [DOI] [PubMed] [Google Scholar]
- 354.Mou S., Zhou M., Li Y., Wang J., Yuan Q., Xiao P., et al. Extracellular vesicles from human adipose-derived stem cells for the improvement of angiogenesis and fat-grafting application. Plast Reconstr Surg. 2019;144:869–880. doi: 10.1097/PRS.0000000000006046. [DOI] [PubMed] [Google Scholar]
- 355.Zhao X., Liu Y., Jia P., Cheng H., Wang C., Chen S., et al. Chitosan hydrogel-loaded MSC-derived extracellular vesicles promote skin rejuvenation by ameliorating the senescence of dermal fibroblasts. Stem Cell Res Ther. 2021;12:196. doi: 10.1186/s13287-021-02262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Zhu X., Badawi M., Pomeroy S., Sutaria D.S., Xie Z., Baek A., et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017;6 doi: 10.1080/20013078.2017.1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Sun D., Zhuang X., Xiang X., Liu Y., Zhang S., Liu C., et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.You J., Zhou O., Liu J., Zou W., Zhang L., Tian D., et al. Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles alleviate lung injury in rat model of bronchopulmonary dysplasia by affecting cell survival and angiogenesis. Stem Cell Dev. 2020;29:1520–1532. doi: 10.1089/scd.2020.0156. [DOI] [PubMed] [Google Scholar]
- 359.Porzionato A., Zaramella P., Dedja A., Guidolin D., Bonadies L., Macchi V., et al. Intratracheal administration of mesenchymal stem cell-derived extracellular vesicles reduces lung injuries in a chronic rat model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2021;320:L688–L704. doi: 10.1152/ajplung.00148.2020. [DOI] [PubMed] [Google Scholar]
- 360.Fragoso F.Y.I., Michelotto P.V., Angulski A.B.B., Leite L.M.B., Senegaglia A.C., Olandoski M., et al. Management of airway remodeling in a mouse model of allergic airways inflammation using extracellular vesicles from human bone marrow-derived mesenchymal stromal cells. Braz Arch Biol Technol. 2022;65:1–11. [Google Scholar]
- 361.Herrmann I.K., Wood M.J.A., Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotech. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 362.Li Y.J., Wu J.Y., Liu J., Xu W., Qiu X., Huang S., et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnol. 2021;19:242. doi: 10.1186/s12951-021-00986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Minardi S., Shah S., Luo X. Biomimetic nanoparticles for transplantation tolerance. Curr Opin Organ Transplant. 2018;23 doi: 10.1097/MOT.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 364.Aarsberetning. NORCONS-RVYang B. Chen Y., Shi J. Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Adv Mater. 2019;31 doi: 10.1002/adma.201802896. [DOI] [PubMed] [Google Scholar]
- 365.Lee H., Cha H., Park J.H. Derivation of cell-engineered nanovesicles from human induced pluripotent stem cells and their protective effect on the senescence of dermal fibroblasts. Int J Mol Sci. 2020;21:343. doi: 10.3390/ijms21010343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Goh W.J., Zou S., Ong W.Y., Torta F., Alexandra A.F., Schiffelers R.M., et al. Bioinspired cell-derived nanovesicles versus exosomes as drug delivery systems: a cost-effective alternative. Sci Rep. 2017;7 doi: 10.1038/s41598-017-14725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Kim H.Y., Kumar H., Jo M.J., Kim J., Yoon J.K., Lee J.R., et al. Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment. Nano Lett. 2018;18:4965–4975. doi: 10.1021/acs.nanolett.8b01816. [DOI] [PubMed] [Google Scholar]
- 368.Wu J.Y., Li Y.J., Hu X.B., Huang S., Luo S., Tang T., et al. Exosomes and biomimetic nanovesicles-mediated anti-glioblastoma therapy: a head-to-head comparison. J Contr Release. 2021;336:510–521. doi: 10.1016/j.jconrel.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 369.Nasiri Kenari A., Kastaniegaard K., Greening D.W., Shambrook M., Stensballe A., Cheng L., et al. Proteomic and post-translational modification profiling of exosome-mimetic nanovesicles compared to exosomes. Proteomics. 2019;19 doi: 10.1002/pmic.201800161. [DOI] [PubMed] [Google Scholar]
- 370.Jang S.C., Kim O.Y., Yoon C.M., Choi D.-S., Roh T.-Y., Park J., et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 371.Jo W., Kim J., Yoon J., Jeong D., Cho S., Jeong H., et al. Large-scale generation of cell-derived nanovesicles. Nanoscale. 2014;6:12056–12064. doi: 10.1039/c4nr02391a. [DOI] [PubMed] [Google Scholar]
- 372.Gao J., Chu D., Wang Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J Contr Release. 2016;224:208–216. doi: 10.1016/j.jconrel.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Gao J., Wang S., Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials. 2017;135:62–73. doi: 10.1016/j.biomaterials.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Go G., Lee J., Choi D.S., Kim S.S., Gho Y.S. Extracellular vesicle–mimetic ghost nanovesicles for delivering anti-inflammatory drugs to mitigate gram-negative bacterial outer membrane vesicle–induced systemic inflammatory response syndrome. Adv Healthcare Mater. 2019;8 doi: 10.1002/adhm.201801082. [DOI] [PubMed] [Google Scholar]
- 375.Tayebi M., Zhou Y., Tripathi P., Chandramohanadas R., Ai Y. Exosome purification and analysis using a facile microfluidic hydrodynamic trapping device. Anal Chem. 2020;92:10733–10742. doi: 10.1021/acs.analchem.0c02006. [DOI] [PubMed] [Google Scholar]
- 376.Liga A., Vliegenthart A.D.B., Oosthuyzen W., Dear J.W., Kersaudy-Kerhoas M. Exosome isolation: a microfluidic road-map. Lab Chip. 2015;15:2388–2394. doi: 10.1039/c5lc00240k. [DOI] [PubMed] [Google Scholar]
- 377.Zhao Z., McGill J., Gamero-Kubota P., He M. Microfluidic on-demand engineering of exosomes towards cancer immunotherapy. Lab Chip. 2019;19:1877–1886. doi: 10.1039/c8lc01279b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Lv Q., Cheng L., Lu Y., Zhang X., Wang Y., Deng J., et al. Thermosensitive exosome–liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer. Adv Sci. 2020;7 doi: 10.1002/advs.202000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Li P., Kaslan M., Lee S.H., Yao J., Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Chen Z., Wang Z., Gu Z. Bioinspired and biomimetic nanomedicines. Acc Chem Res. 2019;52:1255–1264. doi: 10.1021/acs.accounts.9b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Eng J.W.L., Kokolus K.M., Reed C.B., Hylander B.L., Ma W.W., Repasky E.A. A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother. 2014;63:1115–1128. doi: 10.1007/s00262-014-1617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Tormoen G.W., Crittenden M.R., Gough M.J. Role of the immunosuppressive microenvironment in immunotherapy. Adv Radiat Oncol. 2018;3:520–526. doi: 10.1016/j.adro.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Jiang Z., Hsu J.L., Li Y., Hortobagyi G.N., Hung M.C. Cancer cell metabolism bolsters immunotherapy resistance by promoting an immunosuppressive tumor microenvironment. Frontiers in oncology. 2020;10:1197. doi: 10.3389/fonc.2020.01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Parfejevs V., Sagini K., Buss A., Sobolevska K., Llorente A., Riekstina U., et al. Adult stem cell-derived extracellular vesicles in cancer treatment: opportunities and challenges. Cells. 2020;9:1171. doi: 10.3390/cells9051171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Ruan S., Greenberg Z., Pan X., Zhuang P., Erwin N., He M. Extracellular vesicles as an advanced delivery biomaterial for precision cancer immunotherapy. Adv Healthcare Mater. 2022;11 doi: 10.1002/adhm.202100650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Calvo V., Izquierdo M. T lymphocyte and CAR-T cell-derived extracellular vesicles and their applications in cancer therapy. Cells. 2022;11:790. doi: 10.3390/cells11050790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Di Pace A.L., Tumino N., Besi F., Alicata C., Conti L.A., Munari E., et al. Characterization of human NK cell-derived exosomes: role of dnam1 receptor in exosome-mediated cytotoxicity against tumor. Cancers. 2020;12:661. doi: 10.3390/cancers12030661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Jong A.Y., Wu C.H., Li J., Sun J., Fabbri M., Wayne A.S., et al. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J Extracell Vesicles. 2017;6 doi: 10.1080/20013078.2017.1294368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Neviani P., Wise P.M., Murtadha M., Liu C.W., Wu C.H., Jong A.Y., et al. Natural killer–derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res. 2019;79:1151–1164. doi: 10.1158/0008-5472.CAN-18-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Shoae-Hassani A., Hamidieh A.A., Behfar M., Mohseni R., Mortazavi-Tabatabaei S.A., Asgharzadeh S. NK cell-derived exosomes from NK cells previously exposed to neuroblastoma cells augment the antitumor activity of cytokine-activated NK cells. J Immunother. 2017;40:265. doi: 10.1097/CJI.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Wang P., Wang H., Huang Q., Peng C., Yao L., Chen H., et al. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9:1714–1727. doi: 10.7150/thno.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Pitt J.M., Charrier M., Viaud S., André F., Besse B., Chaput N., et al. Dendritic cell–derived exosomes as immunotherapies in the fight against cancer. J Immunol. 2014;193:1006. doi: 10.4049/jimmunol.1400703. [DOI] [PubMed] [Google Scholar]
- 393.Delcayre A., Shu H., Le Pecq J.B. Dendritic cell-derived exosomes in cancer immunotherapy: exploiting nature's antigen delivery pathway. Expert Rev Anticancer Ther. 2005;5:537–547. doi: 10.1586/14737140.5.3.537. [DOI] [PubMed] [Google Scholar]
- 394.Tian H., Li W. Dendritic cell-derived exosomes for cancer immunotherapy: hope and challenges. Ann Transl Med. 2017;5:221. doi: 10.21037/atm.2017.02.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Viaud S., Terme M., Flament C., Taieb J., André F., Novault S., et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15R α. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Qureshi M., Xiang J.J.C., Immunology M. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol Immunol. 2006;3:205–211. [PubMed] [Google Scholar]
- 397.Amigorena S. Cancer immunotherapy using dendritic cell-derived exosomes. Medicina. 2000;60:51–54. [PubMed] [Google Scholar]
- 398.Phung C.D., Pham T.T., Nguyen H.T., Nguyen T.T., Ou W., Jeong J.-H., et al. Anti-CTLA-4 antibody-functionalized dendritic cell-derived exosomes targeting tumor-draining lymph nodes for effective induction of antitumor T-cell responses. Acta Biomater. 2020;115:371–382. doi: 10.1016/j.actbio.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 399.Pitt J.M., André F., Amigorena S., Soria J.C., Eggermont A., Kroemer G., et al. Dendritic cell–derived exosomes for cancer therapy. J Clin Investig. 2016;126:1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Andre F., Schartz N.E.C., Movassagh M., Flament C., Pautier P., Morice P., et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 401.Wolfers J., Lozier A., Raposo G., Regnault A., Théry C., Masurier C., et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 402.Xie F., Zhou X., Fang M., Li H., Su P., Tu Y., et al. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv Sci. 2019;6 doi: 10.1002/advs.201901779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Phuengkham H., Ren L., Shin I.W., Lim Y.T. Nanoengineered immune niches for reprogramming the immunosuppressive tumor microenvironment and enhancing cancer immunotherapy. Adv Mater. 2019;31 doi: 10.1002/adma.201803322. [DOI] [PubMed] [Google Scholar]
- 404.Lindau D., Gielen P., Kroesen M., Wesseling P., Adema G.J. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Lu Z., Zuo B., Jing R., Gao X., Rao Q., Liu Z., et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67:739–748. doi: 10.1016/j.jhep.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 406.Nie W., Wu G., Zhang J., Huang L.L., Ding J., Jiang A., et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed. 2020;59:2018–2022. doi: 10.1002/anie.201912524. [DOI] [PubMed] [Google Scholar]
- 407.Rao L., Wu L., Liu Z., Tian R., Yu G., Zhou Z., et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat Commun. 2020;11:4909. doi: 10.1038/s41467-020-18626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Zhang X., Wang C., Wang J., Hu Q., Langworthy B., Ye Y., et al. PD-1 blockade cellular vesicles for cancer immunotherapy. Adv Mater. 2018;30 doi: 10.1002/adma.201707112. [DOI] [PubMed] [Google Scholar]
- 409.Liu H., Huang L., Mao M., Ding J., Wu G., Fan W., et al. Viral protein-pseudotyped and siRNA-electroporated extracellular vesicles for cancer immunotherapy. Adv Funct Mater. 2020;30 [Google Scholar]
- 410.Morishita M., Takahashi Y., Matsumoto A., Nishikawa M., Takakura Y. Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111:55–65. doi: 10.1016/j.biomaterials.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 411.Turpin D., Truchetet M.-E., Faustin B., Augusto J.F., Contin-Bordes C., Brisson A., et al. Role of extracellular vesicles in autoimmune diseases. Autoimmun Rev. 2016;15:174–183. doi: 10.1016/j.autrev.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 412.Lee E.S., Sul J.H., Shin J.M., Shin S., Lee J.A., Kim H.K., et al. Reactive oxygen species-responsive dendritic cell-derived exosomes for rheumatoid arthritis. Acta Biomater. 2021;128:462–473. doi: 10.1016/j.actbio.2021.04.026. [DOI] [PubMed] [Google Scholar]
- 413.Kim S.-H., Lechman E.R., Bianco N., Menon R., Keravala A., Nash J., et al. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174:6440. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 414.Bu N., Wu H.Q., Zhang G.L., Zhan S.Q., Zhang R., Fan Q.Y., et al. Immature dendritic cell exosomes suppress experimental autoimmune myasthenia gravis. J Neuroimmunol. 2015;285:71–75. doi: 10.1016/j.jneuroim.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 415.Li X.L., Li H., Zhang M., Xu H., Yue L.T., Zhang X.X., et al. Exosomes derived from atorvastatin-modified bone marrow dendritic cells ameliorate experimental autoimmune myasthenia gravis by up-regulated levels of IDO/Treg and partly dependent on FasL/Fas pathway. J Neuroinflammation. 2016;13:8. doi: 10.1186/s12974-016-0475-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Li H., Feng Y., Zheng X., Jia M., Mei Z., Wang Y., et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Contr Release. 2022;341:16–30. doi: 10.1016/j.jconrel.2021.11.019. [DOI] [PubMed] [Google Scholar]
- 417.Ding X., Jing N., Shen A., Guo F., Song Y., Pan M., et al. MiR-21-5p in macrophage-derived extracellular vesicles affects podocyte pyroptosis in diabetic nephropathy by regulating A20. J Endocrinol Invest. 2021;44:1175–1184. doi: 10.1007/s40618-020-01401-7. [DOI] [PubMed] [Google Scholar]
- 418.Smets S. 2021. Macrophage-derived extracellular vesicles impact opc differentiation in multiple sclerosis. [Google Scholar]
- 419.Baharlooi H., Azimi M., Salehi Z., Izad M. Mesenchymal stem cell-derived exosomes: a promising therapeutic ace card to address autoimmune diseases. Int J Stem Cells. 2020;13:13–23. doi: 10.15283/ijsc19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Chen Z., Wang H., Xia Y., Yan F., Lu Y. Therapeutic potential of mesenchymal cell–derived miRNA-150-5p–expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol. 2018;201:2472. doi: 10.4049/jimmunol.1800304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Liu H., Li R., Liu T., Yang L., Yin G., Xie Q. Immunomodulatory effects of mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles in rheumatoid arthritis. Front Immunol. 2020;11:1912. doi: 10.3389/fimmu.2020.01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Tavasolian F., Hosseini A.Z., Soudi S., Naderi M.J.C.G.T. miRNA-146a improves immunomodulatory effects of MSC-derived exosomes in rheumatoid arthritis. Curr Gene Ther. 2020;20:297–312. doi: 10.2174/1566523220666200916120708. [DOI] [PubMed] [Google Scholar]
- 423.Li Z., Liu F., He X., Yang X., Shan F., Feng J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharm. 2019;67:268–280. doi: 10.1016/j.intimp.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 424.Anel A., Gallego-Lleyda A., de Miguel D., Naval J., Martínez-Lostao L. Role of exosomes in the regulation of t-cell mediated immune responses and in autoimmune disease. Cells. 2019;8:154. doi: 10.3390/cells8020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Greisen S.R., Yan Y., Hansen A.S., Venø M.T., Nyengaard J.R., Moestrup S.K., et al. Extracellular vesicles transfer the receptor programmed death-1 in rheumatoid arthritis. Front Immunol. 2017;8:851. doi: 10.3389/fimmu.2017.00851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Wang L., Wang C., Jia X., Yu J. Circulating exosomal miR-17 inhibits the induction of regulatory T cells via suppressing TGFBR II expression in rheumatoid arthritis. Cell Physiol Biochem. 2018;50:1754–1763. doi: 10.1159/000494793. [DOI] [PubMed] [Google Scholar]
- 427.Miao H.-B., Wang F., Lin S., Chen Z. Update on the role of extracellular vesicles in rheumatoid arthritis. Expet Rev Mol Med. 2022;24:e12. doi: 10.1017/erm.2021.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Headland Sarah E., Jones Hefin R., Norling Lucy V., Kim A., Souza Patricia R., Corsiero E., et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med. 2015;7:315ra190. doi: 10.1126/scitranslmed.aac5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Zheng J., Zhu L., Iok In I., Chen Y., Jia N., Zhu W. Bone marrow-derived mesenchymal stem cells-secreted exosomal microRNA-192-5p delays inflammatory response in rheumatoid arthritis. Int Immunopharm. 2020;78 doi: 10.1016/j.intimp.2019.105985. [DOI] [PubMed] [Google Scholar]
- 430.Perez-Hernandez J., Redon J., Cortes R. Extracellular vesicles as therapeutic agents in systemic lupus erythematosus. Int J Mol Sci. 2017;18:717. doi: 10.3390/ijms18040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Dou R., Zhang X., Xu X., Wang P., Yan B. Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization. Mol Immunol. 2021;139:106–114. doi: 10.1016/j.molimm.2021.08.015. [DOI] [PubMed] [Google Scholar]
- 432.Sun W., Yan S., Yang C., Yang J., Wang H., Li C., et al. Mesenchymal stem cells-derived exosomes ameliorate lupus by inducing m2 macrophage polarization and regulatory t cell expansion in MRL/lpr mice. Immunol Invest. 2022;51:1785–1803. doi: 10.1080/08820139.2022.2055478. [DOI] [PubMed] [Google Scholar]
- 433.Burbano C., Villar-Vesga J., Orejuela J., Muñoz C., Vanegas A., Vásquez G., et al. Potential involvement of platelet-derived microparticles and microparticles forming immune complexes during monocyte activation in patients with systemic lupus erythematosus. Front Immunol. 2018;9:322. doi: 10.3389/fimmu.2018.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Sabapatha A., Gercel-Taylor C., Taylor D.D. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences 1. Am J Reprod Immunol. 2006;56:345–355. doi: 10.1111/j.1600-0897.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 435.Pusic A.D., Pusic K.M., Clayton B.L.L., Kraig R.P. IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J Neuroimmunol. 2014;266:12–23. doi: 10.1016/j.jneuroim.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Casella G., Colombo F., Finardi A., Descamps H., Ill-Raga G., Spinelli A., et al. Extracellular vesicles containing IL-4 modulate neuroinflammation in a mouse model of multiple sclerosis. Mol Ther. 2018;26:2107–2118. doi: 10.1016/j.ymthe.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Newton W.C., Kim J.W., Luo J.Z.Q., Luo L. Stem cell-derived exosomes: a novel vector for tissue repair and diabetic therapy. J Mol Endocrinol. 2017;59:R155–R165. doi: 10.1530/JME-17-0080. [DOI] [PubMed] [Google Scholar]
- 438.Xiong J., Hu H., Guo R., Wang H., Jiang H. Mesenchymal stem cell exosomes as a new strategy for the treatment of diabetes complications. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.646233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Cui X., Zhu L., Zhai R., Zhang B., Zhang F. Mesenchymal stem cell-derived exosomes: a promising vector in treatment for diabetes and its microvascular complications. Am J Transl Res. 2021;13:3942–3953. [PMC free article] [PubMed] [Google Scholar]
- 440.Tan L., Wu H., Liu Y., Zhao M., Li D., Lu Q. Recent advances of exosomes in immune modulation and autoimmune diseases. Autoimmunity. 2016;49:357–365. doi: 10.1080/08916934.2016.1191477. [DOI] [PubMed] [Google Scholar]
- 441.Yin W., Ouyang S., Li Y., Xiao B., Yang H. Immature dendritic cell-derived exosomes: a promise subcellular vaccine for autoimmunity. Inflammation. 2013;36:232–240. doi: 10.1007/s10753-012-9539-1. [DOI] [PubMed] [Google Scholar]
- 442.Zhao J., Li Y., Jia R., Wang J., Shi M., Wang Y. Mesenchymal stem cells-derived exosomes as dexamethasone delivery vehicles for autoimmune hepatitis therapy. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.650376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Chen L., Lu F.B., Chen D.Z., Wu J.L., Hu E.D., Xu L.M., et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol. 2018;93:38–46. doi: 10.1016/j.molimm.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 444.Cloutier N., Tan S., Boudreau L.H., Cramb C., Subbaiah R., Lahey L., et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med. 2013;5:235–249. doi: 10.1002/emmm.201201846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Takenaka M., Yabuta A., Takahashi Y., Takakura Y. Interleukin-4-carrying small extracellular vesicles with a high potential as anti-inflammatory therapeutics based on modulation of macrophage function. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121160. [DOI] [PubMed] [Google Scholar]
- 446.You Dong G., Lim Gyeong T., Kwon S., Um W., Oh Byeong H., Song Seok H., et al. Metabolically engineered stem cell–derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci Adv. 2021;7:eabe0083. doi: 10.1126/sciadv.abe0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Fu Y., Wang L., Liu W., Yang L., Li L., Wang L., et al. OX40L blockade cellular nanovesicles for autoimmune diseases therapy. J Contr Release. 2021;337:557–570. doi: 10.1016/j.jconrel.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 448.Sozzani S., Del Prete A., Bosisio D. Dendritic cell recruitment and activation in autoimmunity. J Autoimmun. 2017;85:126–140. doi: 10.1016/j.jaut.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 449.Dong C., Zhou Q., Fu T., Zhao R., Yang J., Kong X., et al. Circulating exosomes derived-miR-146a from systemic lupus erythematosus patients regulates senescence of mesenchymal stem cells. BioMed Res Int. 2019;2019 doi: 10.1155/2019/6071308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Salvi V., Gianello V., Busatto S., Bergese P., Andreoli L., D'Oro U., et al. Exosome-delivered microRNAs promote IFN-α secretion by human plasmacytoid DCs via TLR7. JCI insight. 2018;3 doi: 10.1172/jci.insight.98204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Iversen L.V., Østergaard O., Ullman S., Nielsen C.T., Halberg P., Karlsmark T., et al. Circulating microparticles and plasma levels of soluble E- and P-selectins in patients with systemic sclerosis. Scand J Rheumatol. 2013;42:473–482. doi: 10.3109/03009742.2013.796403. [DOI] [PubMed] [Google Scholar]
- 452.Casella G., Rasouli J., Boehm A., Zhang W., Xiao D., Ishikawa Larissa Lumi W., et al. Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aba0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kimura K., Hohjoh H., Fukuoka M., Sato W., Oki S., Tomi C., et al. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Commun. 2018;9:17. doi: 10.1038/s41467-017-02406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Riazifar M., Mohammadi M.R., Pone E.J., Yeri A., Lässer C., Segaliny A.I., et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13:6670–6688. doi: 10.1021/acsnano.9b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Zhang E., Huang Y., Wang S. Self-luminescent photodynamic therapy and pathogen detection for infectious diseases. Drug Delivery Transl Res. 2021;11:1451–1455. doi: 10.1007/s13346-021-00989-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Tassetto M., Kunitomi M., Andino R. Circulating immune cells mediate a systemic RNAi-based adaptive antiviral response in drosophila. Cell. 2017;169:314–325.e13. doi: 10.1016/j.cell.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Kutchy N.A., Peeples E.S., Sil S., Liao K., Chivero E.T., Hu G., et al. Extracellular vesicles in viral infections of the nervous system. Viruses. 2020;12:700. doi: 10.3390/v12070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Jia R., Cui K., Li Z., Gao Y., Zhang B., Wang Z., et al. NK cell-derived exosomes improved lung injury in mouse model of Pseudomonas aeruginosa lung infection. J Physiol Sci. 2020;70:50. doi: 10.1186/s12576-020-00776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Cai C., Koch B., Akhras S., Hildt E., Zeuzem S., Lange C.M. Macrophage-derived extracellular vesicles mediate a long-lasting innate immune response against hepatitis C virus. Z Gastroenterol. 2016;54:1343–1404. [Google Scholar]
- 460.Cai C., Koch B., Morikawa K., Suda G., Sakamoto N., Rueschenbaum S., et al. Macrophage-derived extracellular vesicles induce long-lasting immunity against hepatitis c virus which is blunted by polyunsaturated fatty acids. Front Immunol. 2018;9:723. doi: 10.3389/fimmu.2018.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Khatri M., Richardson L.A., Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:17. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Abraham A., Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9:28–38. doi: 10.1002/sctm.19-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Wu C., Xu Q., Wang H., Tu B., Zeng J., Zhao P., et al. Neutralization of SARS-CoV-2 pseudovirus using ACE2-engineered extracellular vesicles. Acta Pharm Sin B. 2022;12:1523–1533. doi: 10.1016/j.apsb.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Zhang Y., Dai Y., Wang J., Xu Y., Li Z., Lu J., et al. Mouse circulating extracellular vesicles contain virus-derived siRNAs active in antiviral immunity. EMBO J. 2022;41 doi: 10.15252/embj.2021109902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Scott T.A., Supramaniam A., Idris A., Cardoso A.A., Shrivastava S., Kelly G., et al. Engineered extracellular vesicles directed to the spike protein inhibit SARS-CoV-2. Mol Ther -Methods Clin Dev. 2022;24:355–366. doi: 10.1016/j.omtm.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Bari E., Ferrarotti I., Di Silvestre D., Grisoli P., Barzon V., Balderacchi A., et al. Adipose mesenchymal extracellular vesicles as alpha-1-antitrypsin physiological delivery systems for lung regeneration. Cells. 2019;8:965. doi: 10.3390/cells8090965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Radomski N., Karger A., Franzke K., Liebler-Tenorio E., Jahnke R., Matthiesen S., et al. Chlamydia psittaci-infected dendritic cells communicate with nk cells via exosomes to activate antibacterial immunity. Infect Immun. 2019;88:e00541.19. doi: 10.1128/IAI.00541-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.McNamara R.P., Dittmer D.P. Extracellular vesicles in virus infection and pathogenesis. Curr Opin Virol. 2020;44:129–138. doi: 10.1016/j.coviro.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Li J., Liu K., Liu Y., Xu Y., Zhang F., Yang H., et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat Immunol. 2013;14:793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- 470.Yao Z., Jia X., Megger D.A., Chen J., Liu Y., Li J., et al. Label-free proteomic analysis of exosomes secreted from thp-1-derived macrophages treated with IFN-α identifies antiviral proteins enriched in exosomes. J Proteome Res. 2019;18:855–864. doi: 10.1021/acs.jproteome.8b00514. [DOI] [PubMed] [Google Scholar]
- 471.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Zhang Q., Honko A., Zhou J., Gong H., Downs S.N., Vasquez J.H., et al. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett. 2020;20:5570–5574. doi: 10.1021/acs.nanolett.0c02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.El-Shennawy L., Hoffmann A.D., Dashzeveg N.K., McAndrews K.M., Mehl P.J., Cornish D., et al. Circulating ACE2-expressing extracellular vesicles block broad strains of SARS-CoV-2. Nat Commun. 2022;13:405. doi: 10.1038/s41467-021-27893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Kim H.K., Cho J., Kim E., Kim J., Yang J.S., Kim K.C., et al. Engineered small extracellular vesicles displaying ACE2 variants on the surface protect against SARS-CoV-2 infection. J Extracell Vesicles. 2022;11 doi: 10.1002/jev2.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Tong Y., Ye C., Ren X.S., Qiu Y., Zang Y.H., Xiong X.Q., et al. Exosome-mediated transfer of ACE (Angiotensin-Converting Enzyme) from adventitial fibroblasts of spontaneously hypertensive rats promotes vascular smooth muscle cell migration. Hypertension. 2018;72:881–888. doi: 10.1161/HYPERTENSIONAHA.118.11375. [DOI] [PubMed] [Google Scholar]
- 476.Wu C.H., Xu Q., Wang H.Y., Tu B., Zeng J.X., Zhao P.F., et al. Neutralization of SARS-CoV-2 pseudovirus using ACE2-engineered extracellular vesicles. Acta Pharm Sin B. 2022;12:1523–1533. doi: 10.1016/j.apsb.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Inal Jameel M. Decoy ACE2-expressing extracellular vesicles that competitively bind SARS-CoV-2 as a possible COVID-19 therapy. Clin Sci. 2020;134:1301–1304. doi: 10.1042/CS20200623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Rezakhani L., Kelishadrokhi A.F., Soleimanizadeh A., Rahmati S. Mesenchymal stem cell (MSC)-derived exosomes as a cell-free therapy for patients Infected with COVID-19: real opportunities and range of promises. Chem Phys Lipids. 2021;234 doi: 10.1016/j.chemphyslip.2020.105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Chutipongtanate S., Kongsomros S., Pongsakul N., Panachan J., Khowawisetsut L., Pattanapanyasat K., et al. Anti-SARS-CoV-2 effect of extracellular vesicles released from mesenchymal stem cells. J Extracell Vesicles. 2022;11 doi: 10.1002/jev2.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Cloer C., Roudsari L., Rochelle L., Petrie T., Welch M., Charest J., et al. Mesenchymal stromal cell-derived extracellular vesicles reduce lung inflammation and damage in nonclinical acute lung injury: implications for COVID-19. PLoS One. 2021;16 doi: 10.1371/journal.pone.0259732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Varderidou-Minasian S., Lorenowicz M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10:5979–5997. doi: 10.7150/thno.40122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Hu Q., Lyon C.J., Fletcher J.K., Tang W., Wan M., Hu T.Y. Extracellular vesicle activities regulating macrophage-and tissue-mediated injury and repair responses. Acta Pharm Sin B. 2021;11:1493–1512. doi: 10.1016/j.apsb.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Potter D.R., Miyazawa B.Y., Gibb S.L., Deng X.T., Togaratti P.P., Croze R.H., Srivastava A.K., et al. Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J Trauma Acute Care Surg. 2018;84:245–256. doi: 10.1097/TA.0000000000001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Lankford K.L., Arroyo E.J., Nazimek K., Bryniarski K., Askenase P.W., Kocsis J.A.-O. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Schena F., Gambini C., Gregorio A., Mosconi M., Reverberi D., Gattorno M., et al. Interferon-γ-dependent inhibition of B cell activation by bone marrow–derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheumatol. 2010;62:2776–2786. doi: 10.1002/art.27560. [DOI] [PubMed] [Google Scholar]
- 486.Liu S., Chen J., Shi J., Zhou W., Wang L., Fang W., et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res Cardiol. 2020;115:1–17. doi: 10.1007/s00395-020-0781-7. [DOI] [PubMed] [Google Scholar]
- 487.Wang C., Zhang C., Liu L., Xi A., Chen B., Li Y., et al. Macrophage-derived miR-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol Ther. 2017;25:192–204. doi: 10.1016/j.ymthe.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Kolonics F., Szeifert V., Timár C.I., Ligeti E., Lőrincz Á.M. The functional heterogeneity of neutrophil-derived extracellular vesicles reflects the status of the parent cell. Cells. 2020;9:2718. doi: 10.3390/cells9122718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Ye C., Li H., Bao M., Zhuo R., Jiang G., Wang W.J.A. Alveolar macrophage-derived exosomes modulate severity and outcome of acute lung injury. Aging. 2020;12:6120. doi: 10.18632/aging.103010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Li Z.G., Scott M.J., Brzoska T., Sundd P., Li Y.H., Billiar T.R., et al. Lung epithelial cell-derived IL-25 negatively regulates LPS-induced exosome release from macrophages. Mil Med Res. 2018;5:1–11. doi: 10.1186/s40779-018-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Lee H., Zhang D., Zhu Z., Dela Cruz C.S., Jin YJSr. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep. 2016;6:1–11. doi: 10.1038/srep35250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Ruppert K.A., Nguyen T.T., Prabhakara K.S., Toledano Furman N.E., Srivastava A.K., Harting M.T., et al. Human mesenchymal stromal cell-derived extracellular vesicles modify microglial response and improve clinical outcomes in experimental spinal cord injury. Sci Rep. 2018;8:480. doi: 10.1038/s41598-017-18867-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Rong Y., Liu W., Wang J., Fan J., Luo Y., Li L., et al. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019;10:340. doi: 10.1038/s41419-019-1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Dutta D., Khan N., Wu J., Jay S.M. Extracellular vesicles as an emerging frontier in spinal cord injury pathobiology and therapy. Trends Neurosci. 2021;44:492–506. doi: 10.1016/j.tins.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Chen Y., Li J., Ma B., Li N., Wang S., Sun Z., et al. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging. 2020;12:18274–18296. doi: 10.18632/aging.103692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Ghosh S., Garg S., Ghosh S. Cell-derived exosome therapy: a novel approach to treat post-traumatic brain injury mediated neural injury. ACS Chem Neurosci. 2020;11:2045–2047. doi: 10.1021/acschemneuro.0c00368. [DOI] [PubMed] [Google Scholar]
- 497.Cui L., Saeed Y., Li H., Yang J. Regenerative medicine and traumatic brain injury: from stem cell to cell-free therapeutic strategies. Regen Med. 2021;17:37–53. doi: 10.2217/rme-2021-0069. [DOI] [PubMed] [Google Scholar]
- 498.Li J.K., Yang C., Su Y., Luo J.C., Luo M.H., Huang D.L., et al. Mesenchymal stem cell-derived extracellular vesicles: a potential therapeutic strategy for acute kidney injury. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.684496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Wang S.Y., Hong Q., Zhang C.Y., Yang Y.J., Cai G.Y., Chen X.M. miRNAs in stem cell-derived extracellular vesicles for acute kidney injury treatment: comprehensive review of preclinical studies. Stem Cell Res Ther. 2019;10:281. doi: 10.1186/s13287-019-1371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Zhao X., Zhao Y., Sun X., Xing Y., Wang X., Yang Q. Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Front Bioeng Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.575057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Hao Q., Gudapati V., Monsel A., Park J.H., Hu S., Kato H., et al. Mesenchymal stem cell–derived extracellular vesicles decrease lung injury in mice. J Immunol. 2019;203:1961. doi: 10.4049/jimmunol.1801534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Li J.J., Hosseini-Beheshti E., Grau G.E., Zreiqat H., Little C.B. Stem cell-derived extracellular vesicles for treating joint injury and osteoarthritis. Nanomater. 2019;9:261. doi: 10.3390/nano9020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Drommelschmidt K., Serdar M., Bendix I., Herz J., Bertling F., Prager S., et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun. 2017;60:220–232. doi: 10.1016/j.bbi.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 504.Allan D., Tieu A., Lalu M., Burger D. Mesenchymal stromal cell-derived extracellular vesicles for regenerative therapy and immune modulation: progress and challenges toward clinical application. Stem Cells Transl Med. 2020;9:39–46. doi: 10.1002/sctm.19-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Zheng G., Huang R., Qiu G., Ge M., Wang J., Shu Q., et al. Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. J Cell Tissue Res. 2018;374:1–15. doi: 10.1007/s00441-018-2871-5. [DOI] [PubMed] [Google Scholar]
- 506.Dabrowska S., Andrzejewska A., Strzemecki D., Muraca M., Janowski M., Lukomska B. Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J Neuroinflammation. 2019;16:216. doi: 10.1186/s12974-019-1602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Keshtkar S., Azarpira N., Ghahremani M.H. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Han C., Zhou J., Liang C., Liu B., Pan X., Zhang Y., et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater Sci. 2019;7:2920–2933. doi: 10.1039/c9bm00101h. [DOI] [PubMed] [Google Scholar]
- 509.Wei Y., Wu Y., Zhao R., Zhang K., Midgley A.C., Kong D., et al. MSC-derived sEVs enhance patency and inhibit calcification of synthetic vascular grafts by immunomodulation in a rat model of hyperlipidemia. Biomaterials. 2019;204:13–24. doi: 10.1016/j.biomaterials.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 510.Blázquez R., Sánchez-Margallo F.M., Álvarez V., Usón A., Marinaro F., Casado J.G. Fibrin glue mesh fixation combined with mesenchymal stem cells or exosomes modulates the inflammatory reaction in a murine model of incisional hernia. Acta Biomater. 2018;71:318–329. doi: 10.1016/j.actbio.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 511.Magarotto F., Sgrò A., Dorigo Hochuli A.H., Andreetta M., Grassi M., Saggioro M., et al. Muscle functional recovery is driven by extracellular vesicles combined with muscle extracellular matrix in a volumetric muscle loss murine model. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2021.120653. [DOI] [PubMed] [Google Scholar]
- 512.Song Y., Zhang C., Zhang J., Jiao Z., Dong N., Wang G., et al. Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics. 2019;9:2346–2360. doi: 10.7150/thno.29945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Zhu W., Guo M., Yang W., Tang M., Chen T., Gan D., et al. CD41-deficient exosomes from non-traumatic femoral head necrosis tissues impair osteogenic differentiation and migration of mesenchymal stem cells. Cell Death Dis. 2020;11:293. doi: 10.1038/s41419-020-2496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Willis G.R., Fernandez-Gonzalez A., Anastas J., Vitali S.H., Liu X., Ericsson M., et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Xu X., Cheng J., Luo S., Gong X., Huang D., Xu J., et al. Deoxycholic acid-stimulated macrophage-derived exosomes promote spasmolytic polypeptide-expressing metaplasia in the stomach. Biochem Biophys Res Commun. 2020;524:649–655. doi: 10.1016/j.bbrc.2020.01.159. [DOI] [PubMed] [Google Scholar]
- 516.Singhto N., Kanlaya R., Nilnumkhum A., Thongboonkerd V. Roles of macrophage exosomes in immune response to calcium oxalate monohydrate crystals. Front Immunol. 2018;9:316. doi: 10.3389/fimmu.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Liew L.C., Katsuda T., Gailhouste L., Nakagama H., Ochiya T. Mesenchymal stem cell-derived extracellular vesicles: a glimmer of hope in treating Alzheimer's disease. Int Immunol. 2017;29:11–19. doi: 10.1093/intimm/dxx002. [DOI] [PubMed] [Google Scholar]
- 518.Guo H., Su Y., Deng F. Effects of mesenchymal stromal cell-derived extracellular vesicles in lung diseases: current status and future perspectives. Stem Cell Rev Rep. 2021;17:440–458. doi: 10.1007/s12015-020-10085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Tsuji K., Kitamura S., Wada J. Immunomodulatory and regenerative effects of mesenchymal stem cell-derived extracellular vesicles in renal diseases. Int J Mol Sci. 2020;21:756. doi: 10.3390/ijms21030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Pegtel D.M., Cosmopoulos K., Thorley-Lawson D.A., van Eijndhoven M.A.J., Hopmans E.S., Lindenberg J.L., et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci. 2010;107:6328. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Hassoun P.M. Pulmonary arterial hypertension. N Engl J Med. 2021;385:2361–2376. doi: 10.1056/NEJMra2000348. [DOI] [PubMed] [Google Scholar]
- 522.Lee C., Mitsialis S.A., Aslam M., Vitali S.H., Vergadi E., Konstantinou G., et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Klinger J.R., Pereira M., Del Tatto M., Brodsky A.S., Wu K.Q., Dooner M.S., et al. Mesenchymal stem cell extracellular vesicles reverse sugen/hypoxia pulmonary hypertension in rats. Am J Respir Cell Mol Biol. 2019;62:577–587. doi: 10.1165/rcmb.2019-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.de Jong O.G., Murphy D.E., Mäger I., Willms E., Garcia-Guerra A., Gitz-Francois J.J., et al. A CRISPR-Cas9-based reporter system for single-cell detection of extracellular vesicle-mediated functional transfer of RNA. Nat Commun. 2020;11:1113. doi: 10.1038/s41467-020-14977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Wang B., Chang M., Zhang R., Wo J., Wu B., Zhang H., et al. Spinal cord injury target-immunotherapy with TNF-α autoregulated and feedback-controlled human umbilical cord mesenchymal stem cell derived exosomes remodelled by CRISPR/Cas9 plasmid. Mater Sci Eng C. 2021 doi: 10.1016/j.msec.2021.112624. [DOI] [PubMed] [Google Scholar]
- 526.Srivastava A., Rathore S., Munshi A., Ramesh R. Extracellular vesicles in oncology: from immune suppression to immunotherapy. AAPS J. 2021;23:30. doi: 10.1208/s12248-021-00554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Jeyaram A., Jay S.M. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2017;20:1. doi: 10.1208/s12248-017-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Börger V., Staubach S., Dittrich R., Stambouli O., Giebel B. Scaled isolation of mesenchymal stem/stromal cell-derived extracellular vesicles. Curr Protoc Immunol. 2020;55:e128. doi: 10.1002/cpsc.128. [DOI] [PubMed] [Google Scholar]
- 529.Momen-Heravi F., Balaj L., Alian S., Tigges J., Toxavidis V., Ericsson M., et al. Alternative methods for characterization of extracellular vesicles. Front Physiol. 2012;3:354. doi: 10.3389/fphys.2012.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Comino I., Suligoj T., Al-Hassi H.O., Lee G.H., Sousa C., Landy J., et al. Constitutive gut-homing capacity on circulating myeloid dendritic cells in coeliac disease. Rev Esp Enferm Dig. 2014;106:64–65. doi: 10.4321/s1130-01082014000100013. [DOI] [PubMed] [Google Scholar]
- 531.Wu R., Gao W., Yao K., Ge J. Roles of exosomes derived from immune cells in cardiovascular diseases. Front Immunol. 2019;10:648. doi: 10.3389/fimmu.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]