Abstract
Background
Periodontitis is an inflammatory disease of the teeth-supporting tissues caused by microorganisms. Robusta coffee bean extract has antibacterial properties due to its caffeine, flavonoids, trigonelline, and chlorogenic acid contents. The robusta coffee bean extract also regulates alveolar bone healing through bone remodelling.
Aim
The study aimed to investigate robusta coffee bean extract to inhibit bacterial growth and accelerate bone repair in vitro and in vivo.
Methods
This study used the paper disc diffusion method with the research group of robusta coffee bean extract with concentrations of 50%, 25%, 12.5%, 6.25%, and negative control, as much as 20 and dripped onto the disc paper then placed on the surface of the agar media that had been inoculated with bacteria. The diameter of the inhibition zone was measured. Twenty periodontitis rat models were given 0.05 ml of the robusta coffee bean extract on the molars and put in a periodontal pocket for seven days. Rats were decapitated, and alveolar bone tissues were stained with HE and IHC staining. The number of osteoclasts, osteoblasts and BMP-2 was counted using a microscope. Statistical test with Kruskal Wallis followed by Mann Whitney showed a p-value of <0.05.
Results
The average diameter of the inhibitory zone of robusta coffee bean extract showed that the P. gingivalis group of bacteria was higher than that of A. actinomycetemscomitans and S. viridans (p < 0.05) with a concentration of 50%. The average number of osteoblast cells increased, and the average number of osteoclast cells decreased in the 50% concentration group compared to the other groups (p < 0.05). BMP-2 expression in the robusta coffee bean extract group was 50% higher than in the other groups.
Conclusion
Robusta coffee bean extract has a periopathogenic antibacterial and accelerates alveolar bone repair.
Keywords: Periodontitis, Remodelling, Coffee, Osteoblast, Osteoclast
1. Introduction
Periodontitis is an inflammatory disease of the teeth-supporting tissues caused by P. gingivalis, A. actinomycetemcomitans, a periopathogenic bacterium and S. viridans that cause pulp-periapical abnormalities. Among the major periopathogenic bacteria, P. gingivalis is one of the main etiological bacteria in the pathogenesis and development of inflammation in periodontal disease because of its ability to adhere to the oral epithelium, invade the oral epithelium, and possess virulence factors (Shewale et al., 2016, Wahyukundari et al., 2017).
Periodontitis resulting in progressive destruction of the periodontal ligament and alveolar bone resorption shows an imbalance between osteoclasts and osteoblasts (Park et al., 2017). These cellular activities include the resorption of old bone by osteoclasts and the formation of new bone by osteoblasts (Fakhry et al., 2013, Florencio-Silva et al., 2015, Koga et al., 2016).
Treatment of periodontitis can be surgical or non-surgical. Scaling and root planing (SRP) is a non-surgical treatment which is the gold standard in treating periodontal disease. However, this treatment also has limitations. Therefore, additional antimicrobial therapy is used to eliminate or reduce the number of bacterial pathogens. Other antimicrobial treatment can be given locally as it does not cause side effects. People are now turning to use herbs for treatment (Gigi et al., 2022, Panduwawala et al., 2017).
Coffee is a popular plant consumed by Indonesian people, including those living in Jember Regency. Jember Regency has abundant Robusta coffee. The part of coffee plants commonly consumed is the coffee beans. Coffee beans are naturally rich in caffeine, flavonoids, trigonelline and chlorogenic acid, which have an antibacterial properties. The active compounds contained in robusta coffee beans are antibacterial, which destroy amino acids, building blocks of cell walls and DNA, causing changes in bacterial genesis and lysis and disrupting the stability of the bacterial cytoplasmic membrane (Anand et al., 2015, Ojeh et al., 2016).
This study aimed to investigate the effect of coffee extract in inhibiting the growth of periopathogenic bacteria and in accelerating bone repair processes in vitro and in vivo.
2. Materials and methods
This study was divided into 2, in vitro to see the inhibition of periopathogenic bacteria and in vivo to see the accelerating bone growth.
2.1. Ethical clearance
The ethical clearance for the procedure for treating animals in this study was approved Ethics Committee of the Faculty of Dentistry, the University of Jember, with letter number No.1274/UN25.8/KEPK/DL/2021.
2.2. Robusta coffee beans extract preparation
Five hundred grams of Robusta coffee beans were extracted into powder using the maceration method. The powder was put into a closed container and then soaked using 96% ethanol in a ratio of 1:5 for 72 h and stirred twice a day. The immersion results were filtered using filter paper to produce filtrate and residue. The filtrate was evaporated using a rotary evaporator to obtain viscous extract brown robusta coffee bean extract. The extract with a concentration was diluted using aqua dest with the serial dilution method with a concentration of 50%, 25%, 12.5%, 6.25%, and aqua dest (Macedo et al., 2015).
2.3. Antibacterial sensitivity
The antibacterial test treatment stage was carried out according to the modified NCCLS (National Committee for Clinical Laboratory Standards) standard protocol. Bacterial inoculation was prepared on agar media with a sterile cotton swab. 1 ml of bacterial suspension was taken using a syringe. Inoculate with streaking motion (zig-zag) on the entire media surface. 20 L of robusta coffee bean extract was dropped onto the paper disc with concentrations of 50%, 25%, 12.5%, 6.25%, and negative control (equates) using a micropipette. The Petri dish was closed and then put in an inverted position into a desiccator to create anaerobic conditions. Then, the desiccator was put into the incubator to be incubated for 24 h at 37 °C (Alcayaga-Miranda et al., 2017, Rurenga et al., 2013).
2.4. Inhibition zone diameter
After incubation for 24 h, the Petri dish was removed from the desiccator, and a zone of inhibition was seen, indicated by a clear area around the paper disc. The diameter of the inhibition zone was measured by turning the Petri dish. Observation and measurement of the diameter of the inhibition zone around the paper disc in each research group were conducted using a digital calliper (Magnani et al., 2014).
2.5. Periodontitis rat model
Twenty male Wistar rats aged 2–3 months with a body weight of ±200–250 g were modelled for periodontitis by inducing P. gingivalis in the buccal gingival sulcus of the lower molars as much as 0.05 ml and given once every three days for 14 days using a tuberculin syringe with a needle size of 30 gauge. Periodontitis in rats can be seen by several examinations, such as through clinical and radiographic features. Clinically, there was swelling at the induction site, a redder colour of the gingiva and spontaneous bleeding. (Hienz et al., 2015, Sugihartini et al., 2017).
2.6. The administration of robusta coffee bean
Robusta coffee bean extract was rinsed in the periodontal pocket of the lower molars with as much as 0.05 ml using a syringe. Group I was made a periodontitis model and given robusta coffee bean extract with a concentration of 6.25%, Group II was made a periodontitis model and given robusta coffee bean extract with a concentration of 25%, Group III was made a periodontitis model and was offered robusta coffee bean extract with a concentration of 12.5%, Group III IV was made a periodontitis model and given robusta coffee bean extract with a concentration of 50%. The administration was carried out for seven days, after which the rats were euthanized.
2.7. Sample preparation
First, the experimental animals (2–3 rats) were put into a closed jar (16L size) which contained 5 ml of cotton/tissue moistened with ether. After the rat was unconscious, it was decapitated to take the mandibular bone; then, the mandible was put into a 10% formalin buffer solution for ± 8 h. The goal was to prevent the bone specimens from being damaged and to fix the bone specimens.
2.8. Hematoxylin-Eosin staining
The tissue was cut using a microtome. After that, the cutting thickness setting of the incision was changed to a size of 6 µm, and then the paraffin block containing the tissue was cut. The cut was transferred with a brush to the surface of the water bath with a temperature of 37 °C–40 °C so that the incision expands well. The incision results were then transferred to an object Tissue staining was carried out using Mayer's Hematoxylin-Eosin method with the phases of defaranation, dehydration, core staining, cytoplasmic staining, dehydration, clearing, and mounting (Sugihartini et al., 2017).
2.9. Cell count stage
Cells were counted using a light microscope (Olympus) with 1000x magnification. The counting area consisted of three visual fields in the section of the rat alveolar bone for each sample and was calculated using the computer program Adobe Photoshop CC 2021.
2.10. Immunohistochemical staining (IHC)
The immunohistochemical staining used in this study was for the examination of BMP-2. The method for colouring BMP-2 was by slicing the tissue with a microtome placed on a glass object and then deparaffinized, i.e. pulling/removing the paraffin present in the tissue.
2.11. Statistical analysis
The results of the test show that the data were not normally distributed and not homogeneous. The Kruskal-Wallis nonparametric test was carried out, and then the Mann-Whitney test with a 95% confidence level (α = 0.05; SPSS Version 23).
3. Results
The results of observations on the inhibitory ability of robusta coffee bean extract on the growth of P. gingivalis, A. actinomycetemscomitans and S. viridans are presented in Table. 1.
Table 1.
Average of Inhibitory zone and standard deviation of robusta coffee bean extract on periopathogenic bacteria.
| Num | Group | N | Mean (mm) ± Standart Deviation |
||
|---|---|---|---|---|---|
| P.gingivalis | A. actinomycetemcomitans | S. viridans | |||
| 1 | Negative control | 4 | 0,00 ± 0,00* | 0,00 ± 0,00* | 0,00 ± 0,00* |
| 2 | Concentration 50% | 4 | 19,18 ± 0,18* | 16,15+0,12* | 18,15 ± 0,19* |
| 3 | Concentration 25% | 4 | 16,59 ± 0,17 | 12,20 ± 0,10 | 14,95 ± 0,10 |
| 4 | Concentration 12,5% | 4 | 13,14 ± 0,24 | 8,40 ± 0,22 | 12,15 ± 0,25 |
| 5 | Concentration 6,25% | 4 | 0,00 ± 0,00ns | 0,00 ± 0,00ns | 0,00 ± 0,00ns |
Description: * significant, ns non-significant.
The results showed that all bacteria had an inhibitory effect on robusta coffee bean extract. The growth inhibition of P. gingivalis bacteria was highest at 50% concentration of robusta coffee bean extract, which was 19.18 + 0.18 mm compared to other bacteria. The lowest bacterial growth inhibition with a concentration of 12.5% was 8.40 + 0.22 in A. actinomycetemcomitans bacteria compared to other bacteria (see Table 2).
Table 2.
The number of Osteoblast cells and Osteoclast cells.
| No | Groups | N | Mean ± Standart Deviation |
|
|---|---|---|---|---|
| Osteoclast cells | Osteoblast cells | |||
| 1 | Negative control | 4 | 3.1 ± 8,1* | 99,4 ± 5,9* |
| 2 | Concentration 50% | 4 | 4,5 ± 5,9* | 133 ± 5,1* |
| 3 | Concentration 25% | 4 | 4 ± 7,5ns | 101 ± 9,7ns |
| 4 | Concentration 12,5% | 4 | 4,8 ± 6,7ns | 94,5 ± 8,2ns |
| 5 | Concentration 6,25% | 4 | 4,1 ± 9,1ns | 93 ± 7,8ns |
Description: * significant, ns non-significant.
The negative control had no inhibition with Robusta coffee bean extract, with a concentration of 6.25% in all bacterial groups. The results of the Mann-Whitney test showed that all treatment groups were significantly different from the negative control, with a concentration of 6.25% robusta coffee extract. The results showed an inhibition zone (clear zone) seen around the wells that had been given Robusta coffee extract 50%, 25%, 12.5%, 6.25%, and negative control (see Fig. 1).
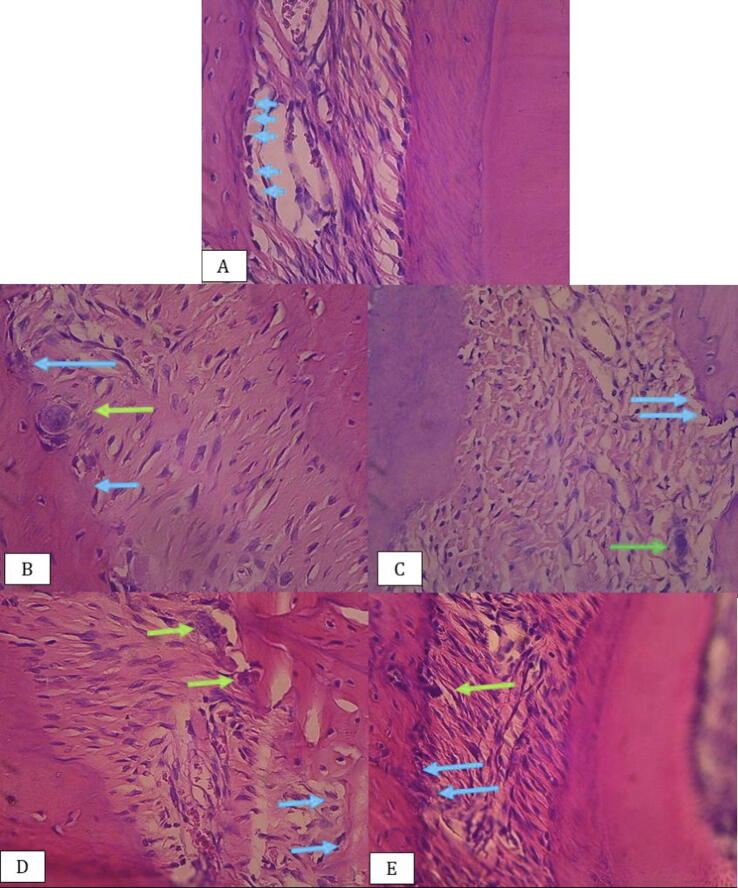
Fig. 1.
Rat alveolar bone tissue: (A) Negative control, (B) Concentration 50%, (C) Concentration 25%, (D) Concentration 12,5%, (E) Concentration 6.25% osteoblast cells (blue arrow), osteoclast cells (green arrow) (Hematoxylin-Eosin staining 400× magnitude).
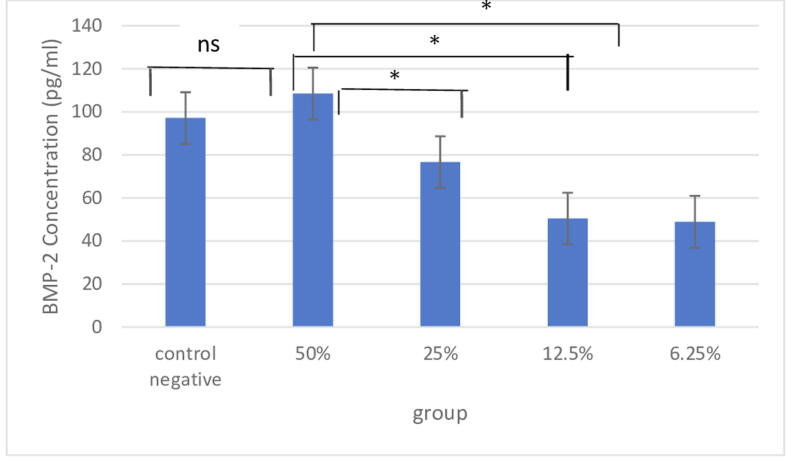
Fig 2 shows the expression of BMP-2 in the 50% concentrated Robusta coffee bean group and the control group. There was no significant difference (p > 0.05), but in the 25%, 12.5%, and 6.25% treatment groups there were significant differences (p < 0.05) (see Fig. 3).
Fig. 2.
Expression of bone morphogenetic protein 2 (BMP-2) in rat alveolar bone: Description: * significant, ns non-significant.
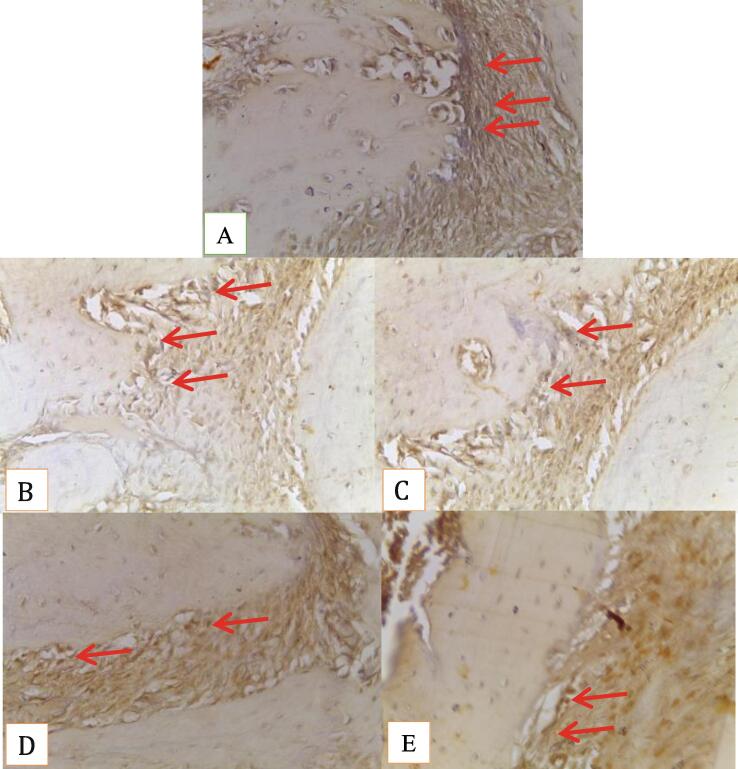
Fig. 3.
Rat alveolar bone tissue: expression BMP-2 (red arrow) (A) Negative control, (B) Concentration 50%, (C) Concentration 25%, (D) Concentration 12,5%, (E) Concentration 6.25% (Immunohistochemistry Staining 400x magnitude).
4. Discussion
The results of this study indicate that not all concentrations of robusta coffee bean extract have inhibitory power against periopathogenic bacteria. A concentration of 6.25% did not show any inhibition result, which was indicated by the absence of a clear zone around the wellbore (inhibition zone = 0). It was possible because the active compound of the antibacterial substance of robusta coffee bean extract in toothpaste was too small, so it was unable to inhibit bacterial growth. (Sukohar et al., 2011).
The ability of robusta coffee bean extract to inhibit bacteria is due to the presence of antibacterial compounds such as caffeine, flavonoids, trigonelline, and chlorogenic acid. These ingredients have antibacterial activity with different mechanisms of inhibiting bacterial growth (Eldin et al., 2016, Macedo et al., 2015).
Caffeine in robusta coffee beans works because it significantly influences the ability of alkaloid compounds; when in contact with bacteria, it will react with amino acid compounds that make up cell walls and bacterial DNA. This reaction will cause changes in the structure and arrangement of amino acids, which will change the performance of the DNA chain so that DNA damage will occur in the bacterial cell nucleus and support the lysis of the bacterial cell nucleus. Thus the bacteria will become inactive and destroyed (Kim et al., 2016, Li et al., 2016).
The above statement is supported by previous research that the caffeine and trigonelline content in the robusta coffee bean extract at concentrations of 1%, 1.25%, 1.5%, and 3% had antibacterial effects against P. gingivalis. The mechanism of phenol compounds in killing bacteria is by denaturing cell proteins. Flavonoids play a role in inhibiting the function of bacterial cell membranes by forming complex compounds against extracellular and dissolved proteins that damage the bacterial cell membrane and are followed by the release of intracellular compounds that result in cell death. The antibacterial activity of chlorogenic acid increases the permeability of the outer membrane and plasma membrane, thereby reducing the defence function, nucleotide leakage and cytoplasmic contents. These compounds also reduce levels of Reactive Oxygen Species (ROS). The decrease in ROS levels results in the disruption of intracellular signalling in bacteria and accumulates Ca2 +levels as a proapoptotic agent, causing bacterial cell death due to apoptotic signals (Martin et al., 2022, Shushtari and Froushani, 2017).
On day 7, the group of periodontitis rats and the treatment group were given Robusta coffee bean chlorogenic acid gel had differences in the amount of OCN expression. This is in line with the results of research from Yamamoto showing that chlorogenic acid from coffee can increase the synthesis of IL-6 in osteoblasts. It can initiate bone formation (Nurman et al., 2019, Struppek et al., 2022). Other studies have shown that chlorogenic acid can enhance the osteogenic differentiation of human adipose tissue-derived mesenchymal stem cells (MSC) by increasing the mineralization of bone tissue. This indicates that the acid chlorogenic acid can increase the potential for osteogenesis (Maalik et al., 2016, Tian et al., 2019, Yamagata et al., 2018).
BMP can effectively induce mesenchymal cells to differentiate into osteoblasts and mesenchymal cell differentiation and initiate bone formation. Furthermore, BMP-2 plays a crucial role in bone formation, growth, and repair. A study by Sari. (2019) showed that BMP-2 could increase the concentration of Ca2+ and activate osteoblasts to differentiate the enhanced formation and integration of mineralized bone nodules. BMP-2 in vitro and in vivo studies induce bone formation by expressing bone markers (Ernie et al., 2017, Sari et al., 2019, Setiawatie et al., 2022).
5. Conclusion
Robusta coffee bean extract has a periopathogenic antibacterial and alveolar bone repair ability.
Research funding
This research was funded by the University of Jember through Reworking Research Grant 2022 contract No.6332/UN25.3.1/LT/2022 .
Author contributions
All authors have accepted responsibility for the entire content of this manuscript and approved its Submission.
CRediT authorship contribution statement
Desi Sandra Sari: Conceptualization, Data curation, Formal analysis, Supervision, Writing – review & editing. Peni Pujiastuti: Methodology, Writing – original draft. Dwi Warna Aju Fatmawati: Investigation, Supervision. Mega Ayu Mardiyana: Formal analysis. Ayu Tri Wulandari: Investigation, Visualization. Yuliana Mahdiyah Daat Arina: Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The author would like to extend our uttermost gratitute to the Institute of Research and community Services of the University of Jember.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sdentj.2023.03.007.
Contributor Information
Desi Sandra Sari, Email: desi_sari.fkg@unej.ac.id.
Peni Pujiastuti, Email: Peni.pujiastuti@unej.ac.id.
Dwi Warna Aju Fatmawati, Email: dwiwarna.fkg@unej.ac.id.
Mega Ayu Mardiyana, Email: 181610101138@mail.unej.ac.id.
Ayu Tri Wulandari, Email: 181610101068@mail.unej.ac.id.
Yuliana Mahdiyah Daat Arina, Email: yuliana.mda@unej.ac.id.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Alcayaga-Miranda F., Cuenca J., Khoury M. Antimicrobial activity of mesenchymal stem cells: Current status and new perspectives of antimicrobial peptide-based therapies. Front. Immunol. 2017;8(MAR):1–15. doi: 10.3389/fimmu.2017.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand J., Upadhyaya B., Rawat P., Rai N. Biochemical characterization and pharmacognostic evaluation of purified catechins in green tea (Camellia sinensis) cultivars of India. 3 Biotech. 2015;5(3):285–294. doi: 10.1007/s13205-014-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldin I., Elgailani H., Eldin I., Elgailani H. Spectrophotometric and Phytochemical Analysis of Black Tea (Camellia sinensis Leaves) Spectrophotometric and Phytochemical Analysis of Black Tea (Camellia sinensis Leaves) J. Appl. Ind. Sci. 2016;3(March):1–6. [Google Scholar]
- Ernie Maduratna, Ulfah Noer, Wahjuningrum Dian Agustin, Desi Sandra Sari, Rubianto. 2017. Viability Bovine Tooth Hydroxiapatite on Bone Marrow Mesenchymal Stem Cells. In: International Medical Device and Technologt Conference, pp. 100–104.
- Fakhry M., Hamade E., Badran B., Buchet R., Magne D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J. Stem Cells. 2013;5(4):136–148. doi: 10.4252/wjsc.v5.i4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florencio-Silva R., Sasso G.R.D.S., Sasso-Cerri E., Simões M.J., Cerri P.S. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res. Int. 2015;2015 doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigi, M.K., Ramadhani, Y., Rahayu, R., Rahmasari, P., Prajnasari, K.N., Alhakim, M.M., Aljunaid, M., Mohammed, H., 2022. Review article A mucoadhesive gingival patch with Epigallocatechin-3-gallate green tea (Camellia sinensis) as an alternative adjunct therapy for periodontal disease : A narrative review. 114(158), 114–119. 10.20473/j.djmkg.v55.i2.p114. [DOI]
- Hienz, Stefan A., Paliwal, Sweta, Ivanovski, S., 2015. Mechanisms of bone resorption in periodontitis. J. Immunol. Res. 2015, 1–10. 10.1016/8756-3282(85)90328-X. [DOI] [PMC free article] [PubMed]
- Kim H.J., Yoon B.K., Park H., Seok J.W., Choi H., Yu J.H., Choi Y., Song S.J., Kim A., Kim J.W. Caffeine inhibits adipogenesis through modulation of mitotic clonal expansion and the AKT/GSK3 pathway in 3T3-L1 adipocytes. BMB Rep. 2016;49(2):111–115. doi: 10.5483/BMBRep.2016.49.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Minamizato T., Kawai Y., Miura K., Takashi I. Bone regeneration using dentin matrix depends on the degree of demineralization and particle size. PLOS o. 2016;1–12 doi: 10.1371/journal.pone.0147235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A., Wu N., Zou H., Zhu B., Xiong S., Xiao G. Low concentration of caffeine inhibits cell viability, migration and invasion, and induces cell apoptosis of B16F10 melanoma cells. Int. J. Clin. Exp. Path. 2016;9(11):11206–11213. [Google Scholar]
- Maalik A., Bukhari S.M., Zaidi A., Shah K.H., Khan F.A. Chlorogenic acid: A pharmacologically potent molecule. Acta Poloniae Pharmaceutica - Drug Research. 2016;73(4):851–854. [PubMed] [Google Scholar]
- Macedo R.M., Brentegani L.G., de Lacerda S.A. Effects of coffee intake and intraperitoneal caffeine on bone repair process – A histologic and histometric study. Braz. Dent. J. 2015;26(2):175–180. doi: 10.1590/0103-6440201300219. [DOI] [PubMed] [Google Scholar]
- Magnani, C., Galdorfini Chiari, B., Lucia Borges Isaac, V., Correa, M. A., & Nunes Salgado, H. R. 2014. In Vitro Safety Evaluation of Caffeic Acid. Athens J. Health, 1(3), 181–188. 10.30958/ajh.1-3-2. [DOI]
- Martin, M., Sari, D. S., Praharani, D., Ayu, M. roslian. 2022. Combination of Dental Pulp Stem-Cell Secretome and Robusta Coffee Bean Extract (Coffea canephora) in Enhancing Osteocalcin and Alkaline Phosphatase Expression in Periodontitis-Induced Wistar Rats. J. Orofacial Sci. 13(2), 136–141. 10.4103/jofs.jofs. [DOI]
- Nurman S., Yulia R., Irmayanti, Noor E., Sunarti T.C. The optimization of gel preparations using the active compounds of arabica coffee ground nanoparticles. Sci. Pharm. 2019;87(4) doi: 10.3390/scipharm87040032. [DOI] [Google Scholar]
- Ojeh N., Stojadinovic O., Pastar I., Sawaya A., Yin N., Tomic-Canic M. The effects of caffeine on wound healing. Int. Wound J. 2016;13(5):605–613. doi: 10.1111/iwj.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panduwawala C.P., Zhan X., Dissanayaka W.L., Samaranayake L.P., Jin L., Zhang C. In vivo periodontal tissue regeneration by periodontal ligament stem cells and endothelial cells in three-dimensional cell sheet constructs. J. Periodontal Res. 2017;52(3):408–418. doi: 10.1111/jre.12405. [DOI] [PubMed] [Google Scholar]
- Park, S. Min, Kim, D.H., Pang, E.K., 2017. Bone formation of demineralized human dentin block graft with different demineralization time: In vitro and in vivo study. J. Cranio-Maxillofacial Surgery. 10.1016/j.jcms.2017.03.007. [DOI] [PubMed]
- Rurenga P., Raangs E., Singadji Z., Wekema-Mulder G., Veloo A.C.M., van Winkelhoff A.J. Evaluation of three selective media for isolation of Aggregatibacter actinomycetemcomitans. J. Periodontal Res. 2013;48(5):549–552. doi: 10.1111/jre.12037. [DOI] [PubMed] [Google Scholar]
- Sari D.S., Maduratna E., Ferdiansyah, Latief F.D.E., Satuman, Nugraha A.P., Sudiana K., Rantam F.A. Osteogenic Differentiation and Biocompatibility of Bovine Teeth Scaffold with Rat Adipose-derived Mesenchymal Stem Cells. Eur. J. Dentis. 2019;13(2):206–212. doi: 10.1055/s-0039-1694305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawatie E.M., Gani M.A., Rahayu R.P., Ulfah N., Kurnia S., Augustina E.F., Sari D.S. Nigella sativa toothpaste promotes anti-inflammatory and anti-destructive effects in a rat model of periodontitis. Arch. Oral Biol. 2022;137(March) doi: 10.1016/j.archoralbio.2022.105396. [DOI] [PubMed] [Google Scholar]
- Shewale A.H., Gattani D.R., Bhatia N., Mahajan R., Saravanan S.P. Prevalence of periodontal disease in the general population of India-A systematic review. J. Clin. Diagnostic Res. 2016;10(6):ZE04–ZE09. doi: 10.7860/JCDR/2016/17958.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shushtari N., Froushani S.M.A. Caffeine augments the instruction of anti-inflammatory macrophages by the conditioned medium of mesenchymal stem cells. Cell J. 2017;19(3):415–424. doi: 10.22074/cellj.2017.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struppek J., Walther C., Bunte K., Zyriax B.C., Wenzel J.P., Senftinger J., Nikorowitsch J., Heydecke G., Seedorf U., Beikler T., Borof K., Mayer C., Aarabi G. The association between coffee consumption and periodontitis: a cross-sectional study of a northern German population. Clin. Oral Invest. 2022;26(3):2421–2427. doi: 10.1007/s00784-021-04208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihartini, N., Saridewi, R., M, U.R., Rahmawanti, F., Yuliani, S., Sophia, V. 2017. Anti-inflammatory Activity of Camellia sinensis, l. Extract Cream Combined with Vitamin C as Antioxidant on Croton Oil-induced Inflamation in Male Mice Strain BALB/C. Majalah Obat Tradisional, 22(2), 73. 10.22146/tradmedj.27915. [DOI]
- Sukohar A., Setiawan, Wirakusumah F., Sastramihardja H. Isolation and characterization cytotoxic compounds caffeine and chlorogenic acid isolasi dan karakterisasi. Jurnal Medika Planta. 2011;1(4):11. [Google Scholar]
- Tian L., Su C.P., Wang Q., Wu F.J., Bai R., Zhang H.M., Liu J.Y., Lu W.J., Wang W., Lan F., Guo S.Z. Chlorogenic acid: A potent molecule that protects cardiomyocytes from TNF-α–induced injury via inhibiting NF-κB and JNK signals. J. Cell Mol. Med. 2019;23(7):4666–4678. doi: 10.1111/jcmm.14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahyukundari, M.A., Sari, D.S., Pujiastuti, P., Praharani, D., Arina, Y.M.D., Ermawati, T., Latief, F.D.E. 2017. Virulence of periodontopathogens Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis: micro-computed tomography and inflammatory cytokine analysis. In: 11th International Dentistry Scientific Meeting (IDSM 2017), 4, 312–321. 10.2991/idsm-17.2018.42. [DOI]
- Yamagata K., Izawa Y., Onodera D., Tagami M. Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in A549 human lung cancer cells. Mol. Cell. Biochem. 2018;441(1–2):9–19. doi: 10.1007/s11010-017-3171-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.