Abstract
Background
Saudi Arabia has an overall smoking rate of 15.9%. The link between smoking and periodontal disease has been studied extensively. It is possible for human gingival fibroblasts to accumulate nicotine intracellularly over a period of four hours. Additionally, unmetabolized nicotine is released into the environment. Tobacco presence can impair tissue inflammation, wound healing, and organ development. To counterbalance tobacco toxins, vitamin C has been added to a variety of products.
Aim
This study aims to analyze the RNA expression of antioxidant, anti-inflammatory, and wound healing proteins in human gingival fibroblasts from smokers and nonsmokers using polymerase chain reaction.
Materials and Methods
hGFs were extracted from clinically healthy periodontium sites of adult male subjects. Both heavy cigarette smokers and never-smokers participated as subjects. Cells were cultured and subcultured in supplemented growth medium. Vitamin C was inducted in the medium at the experimental 6th passage. RNA expression analysis (qRT-PCR) was performed to analyze adhesion, proliferation, and extracellular matrix expression.
Results
The results revealed marked expression of a wound healing gene (VEGF-A) in never-smokers (p value = 0.016). GPX3 and SOD3 represent antioxidants that are highly expressed in treated never-smoker cells. SOD2 significantly increased (p value = 0.016) in smokers after vitamin C exposure. The anti-inflammatory markers IL-6 and IL-8 were lower among smokers than among nonsmokers (p < 0.0001).
Conclusion
Tobacco smoking suppressed gingival fibroblasts' abilities to regenerate, heal, combat inflammation, and resist free radicals. Vitamin C at cellular levels was beneficial and should be considered in the treatment component of smokers in the dental clinic.
Keywords: Human gingival fibroblast, Smoker, Vitamin C, Wound healing, Antioxidant, Anti-inflammatory, RNA expression
1. Introduction:
A survey was conducted in Saudi Arabia in 2019 by the Global Adult Tobacco Survey (GATS) to comply with their obligations under the World Health Organization Framework Convention on Tobacco Control (FCTC) to produce comparable data within and across countries. Twelve thousand eight hundred subjects were investigated. Cigarette smokers represent 15.9% of the population in total (24.9% men, 2.5% women). The average smoker consumes 25 cigarettes per day at 18 years old (Health 2019). In addition, smoking exposure has been established as directly related to the prevalence and severity of periodontal disease (Van der Vaart et al., 2004). The combustion of tobacco alters cellular function and increases the likelihood of developing and aggravating periodontitis (Albandar et al., 2000). In the latest multidimensional classification for diagnosing periodontal diseases, smoking is acknowledged as a risk factor in the grading system of periodontitis. It was proposed to determine disease progression, analyze the likelihood of poor treatment outcomes, and evaluate the risk that the disease or its treatment will adversely affect the patient's general health (Papapanou et al., 2018, Tonetti et al., 2018).
As a biological mucosal barrier and an integral part of oral immunity, the gingiva is unique among oral tissues. Healing within the gingiva is characterized by reduced inflammation, rapid re-epithelialization, and scarless healing similar to that of a fetus (Zhang et al., 2009). The dentinal gingiva luminary cells are called “gingival fibroblasts.” These cells have many characteristics in common with mesenchymal stem cells (MSCs) and may represent a more differentiated subpopulation of MSCs or be derived from MSCs under certain conditions. In addition to their presumed role in supporting a scaffold, fibroblasts are directly implicated in self-tolerance, organ development, wound healing, inflammation, and fibrotic tissue development (Haniffa et al., 2009). Periodontal tissues need to remain healthy to achieve optimal wound healing. The same implicates fibroblasts to function normally (Huang et al., 2002).
Many of the underlying effects of tobacco products on periodontal tissues may be due to direct inhibition of normal fibroblast function (Lallier et al., 2017). Tanur and his team studied the effect of nicotine on gingival fibroblast adhesion strength to glass and human root surfaces. The researchers concluded that the substance inhibited fibroblast attachment, affecting the healing process after periodontal therapy (Tanur et al., 2000). Furthermore, gingival fibroblasts could bind and take up nicotine continuously over four hours, resulting in high intracellular nicotine levels (Wyganowska-Swiatkowska and Nohawica 2015). Moreover, they have the ability to release unmetabolized nicotine back into the surrounding environment. These events may disrupt normal cellular functions (Hanes et al., 1991). The toxic substances in cigarettes, such as reactive oxygen species (ROS), can damage DNA and eventually lead to cancerous cell formation in the body (Pryor, 1997, Kovacs et al., 2012). By smoking, smokers generate many free radicals, which cause inflammation in vessels, DNA damage, and premature vessel aging. Notably, cigarette smokers generally experience this imbalance as a side effect (Förstermann et al., 2017). Moreover, chronic smoking aids in maintaining an inflammatory microenvironment that further contributes to the development of body cancer (Pryor, 1997, Kovacs et al., 2012).
In light of this context, vitamin C, also known as ascorbic acid (vit.c), was shown to modify the epigenetic/gene expression profile and extracellular matrix (microenvironment) of cells (D'Aniello et al., 2017). Antioxidants, including vit.c, are added to foods, toothpaste, or mouthwash to counteract tobacco's harmful effects. A cross-sectional study was performed to measure the ascorbic acid level in smokers compared to nonsmokers and to evaluate its antioxidant salivary effect, revealing that the salivary defense mechanism against oxidative stress was markedly reduced among smokers (Falsafi et al., 2016). Researchers report that when vit.c is administered either systemically, locally or combined it reverses nicotine and cotinine's adverse effects on human gingival fibroblasts; therefore, smokers are encouraged to take antioxidant supplements to combat the adverse effects (Møller et al., 2004, Falsafi et al., 2016, Tatsumi et al., 2021). These results were determined by real-time polymerase chain reaction and quantitative analysis of the expression of apoptosis-related genes. Additionally, vit.c-containing biomaterials (i.e., microspheres, scaffolds, tissue adhesives, and hydrogels) may be helpful in the engineering and regeneration of soft and hard tissues in the mouth (Torshabi et al., 2017).
Considering the limited number of studies conducted on gingival fibroblasts extracted from cigarette smokers, the majority of them were focused merely on provoking some, but not all, of the chemical elements of cigarettes in healthy gingival fibroblasts (Lallier et al., 2017, Torshabi et al., 2017, Tatsumi et al., 2021). An in vitro study of smoker cells will further explore the genetic and functional modifications that the cells express to survive the long-term toxic environment of tobacco use. Additionally, to our limited knowledge, none of the smokers reported experiencing the vit.c effect on their hGFs in vitro. A polymerase chain reaction analysis was used in the current study to investigate the antioxidant, anti-inflammatory, and wound healing effects on RNA expression in human gingival fibroblasts extracted from cigarette smokers and never-smokers before and after vit.c treatment.
2. Materials and methods:
2.1. Ethical guidelines
This study was conducted in accordance with the standards and protocols of King Saud University. Approval of the Ethical Committee was obtained from the Institutional Review Board at the King Saud University and College of Dentistry Research Center (CDRC). Informed consent was obtained from all participants. The work protocol of the study was conducted using the facility and support of the Stem Cell Unit laboratory at the Department of Anatomy, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
2.2. Human gingival fibroblast (hGF) excision
During this study, gingival connective tissue samples were collected from two males aged ≥ 20 years old with unknown systemic diseases or treated with antibiotics within the last month. Clinically, their gingivae were relatively healthy without a clinical appearance of inflammation signs (erythematous, edematous, or bleeding). Gingival samples were obtained as remnant or discarded tissues following dental procedures based on the treatment plan for each subject requiring excision of gingival tissues, such as a third molar extraction procedure or periodontal crown lengthening surgery, at the College of Dentistry, King Saud University (KSU) in Riyadh, Saudi Arabia. Human participants were further divided into two main groups. Among the first group (N-N), there were never (cigarette, hookah, vape) smokers, and among the second group (S-N), there were heavy cigarette users, defined by Kaldahl et al. as those who smoked twenty cigarettes or more per day for two years(Kaldahl et al., 1996). Aseptically treated gingival tissue samples were transferred to the laboratory in 15 mL centrifuge tubes containing fibroblast culture media for direct explant culture under a laminar flow hood (Saczko et al., 2008).
2.3. Human gingival fibroblast (hGF) culture
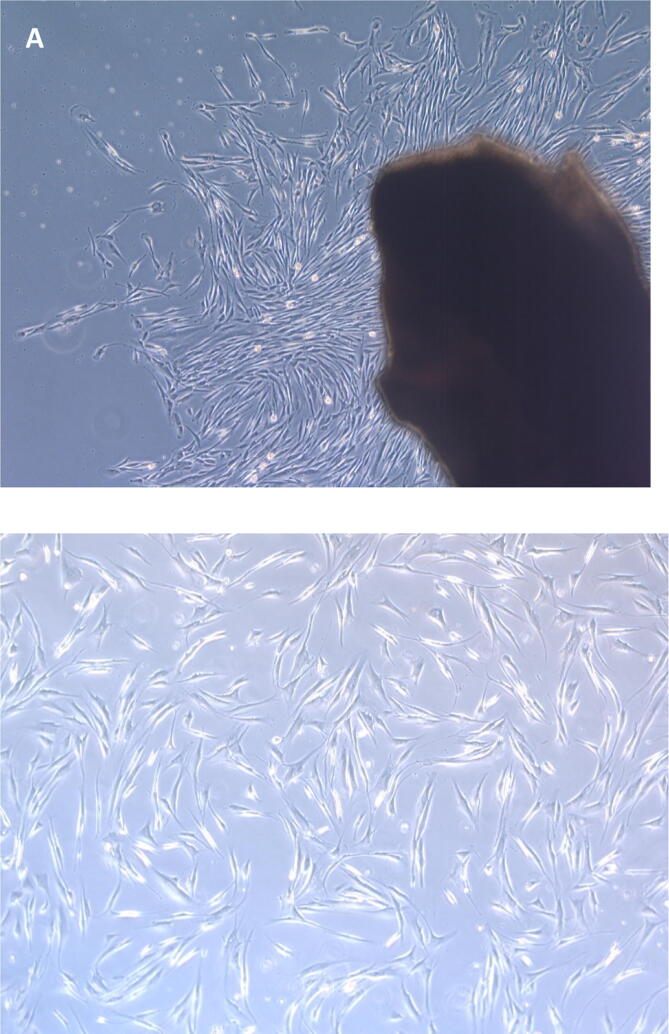
The excised tissues were washed three times using phosphate buffered saline (PBS, Gibco® Life Technologies, Roskilde, Denmark) supplemented with 1% Pen-Strep (penicillin–streptomycin (10,000 IU/mL), Gibco®). Specimens were minced into approximately 2–3 mm pieces and placed in the center of each well of six-well tissue culture plates (Corning®, Tewksbury, MA, USA). Explants were then covered with a minimal amount of fibroblast culturing media (∼0.5 mL) containing Dulbecco's Modified Eagle Medium-high glucose (DMEM; Gibco®, BRL, New York, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco®, Grand Island, NY, USA), 1% Pen-Strep (Gibco®, Carlsbad, CA, USA), and 1% MEM nonessential amino acids (Invitrogen Gibco®, Grand Island, NY, USA) to facilitate the attachment of tissues to the culture plate surface. Tissues were incubated at 37 °C for 30 min in a humidified atmosphere of 5% CO2 and 95% oxygen before adding 1 mL of a new medium. Every 72 h, fresh medium was replaced until the migration of cells from the tissue explant was evident, as shown in Fig. 1-A (Saczko et al., 2008).
Fig. 1.
(A) Fibroblast cell migration from the explant human gingival connective tissue to the culture. (B) Confluency, morphology and adhesion of hGF cells under a phase-contrast inverted microscope (X5).
2.4. Human gingival fibroblast (hGF) subculture and passaging
Next, nonadherent cells were removed, and fresh media was added until migrated cells reached 80–90% confluency, displaying a consistent spindle-shaped appearance. The desired confluency, morphology and adhesion of hGF cells were determined by using a phase-contrast inverted microscope (Carl Zeiss Axiovert 40C Imaging Microscope). Plastic-adherent confluent cells were intermittently passaged with 0.25% trypsin-EDTA (Gibco) for 2–3 min at 37 °C in gently rocking flasks. Trypsin neutralization was achieved using complete DMEM (twice the volume used for the trypsin-EDTA reagent). Cell suspensions were then centrifuged at 1500 rpm for 5 min. The next step consisted of supernatant aspiration and discarding. The cell pellets were then resuspended in a 5 mL complete medium volume to increase cell numbers. After diluting the cell pellet and dividing them into two T-75 flasks, fresh growth medium was added to expand the cells. Observations were carried out routinely under an inverted phase-contrast microscope (Fig. 1-B). The experiments were conducted with cells from the sixth passage (Saczko et al., 2008, de Abreu et al., 2019).
2.5. RNA expression analysis: Reverse transcription polymerase chain reaction (qRT-PCR)
A six-well plate was seeded with hGF cells (Falcon™ Polystyrene Microplates 6-well; Nontreated; Flat-bottom; Growth area: 9.6 cm2; Well volume: 15.5 mL). Vit.c at a 200 μM concentration was prepared as a solution by diluting 14.45 g in sterile 1 L H2O to obtain a 50 mM solution, filtered, and stored at 4 °C. Then, this solution was added to the culture medium as described previously by Van Pham et al. (Van Pham et al. 2016). Vit.C solution was then added based on the group assembly: (1) never smoke fibroblasts without the addition of vit.c (N-N Group), (2) never smoke fibroblasts with vit.c addition (N-C Group), (3) smoker fibroblasts with no addition of vit.c (S-N Group), and (4) smoker fibroblasts with vit.c addition (S-C Group). Total RNA was isolated after three days of incubation following the manufacturer's instructions for the RNA extraction kit (Analytik Jena AG). After removing the residual medium, the lysis solution from the kit was added at 133 µL/well of a 6-well culture plate and gently washed with cold, sterile PBS. The lysed cells were incubated for 2–3 min at room temperature, and the final yield of total RNA was obtained. Complete disruption and lysis of the cells was achieved with the aid of a tissue scrubber, which was used to scrub the base of the wells gently. The lysed cells were collected in an Eppendorf microcentrifuge tube and preserved at −80 °C until sample collection. RNA extraction was performed using an RNA extraction kit (Analytik Jena AG). The concentration of total RNA was measured using a NanoDrop™ 2000 spectrophotometer (Thermo Fisher Scientific, DE, USA).
Complementary DNA (cDNA) was synthesized using the Thermo Fisher Scientific High-Capacity cDNA transcription kit and the ProFlex PCR system. Gene expression levels were determined using real-time PCR (Thermo Fisher Scientific, VIA 7 system) with a qRT-PCR kit (Applied Biosystems, Warrington, UK). PCRs were performed using Fast SYBR Green PCR Master Mix. All reactions were performed in triplicate. All values were normalized to the expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the qRT-PCR products were quantified using the previously described 2-ΔΔCt method (Spicer et al., 2012). The panel of genes studied included vascular endothelial growth factor-A (VEGF-A), glutathione peroxidase (GPX3), superoxide dismutase (SOD-2 and SOD-3), and interleukin (IL-6 and IL-8). The sequences of the genes are shown in Table 1.
Table 1.
List of targeted primers used for PCR analysis.
| Cellular Function | Name | Sequence (5′–3′) |
|---|---|---|
| Wound Healing Markers |
VEGF-A - FP VEGF-A - RP |
ATG AGG ACA CCG GCT CTG ACC A AGG CTC CTG AAT CTT CCA GGC A |
| Oxidative Stress Markers |
GPX3 - FP GPX3 - RP |
TGTAATGGAGACCGCTGTGTCT GGGTGCATTGCACTGAGCG |
|
SOD2 - FP SOD2 - RP |
CCTTTCCCATGGAAACTCAGTGAA TTGTACACTTCATTGCAACTTGAAATT |
|
|
SOD3 - FP SOD3 - RP |
GGAATCCTTGGCCCGAAAACC AGATCCACAGGAGGAACGGTTTCTT |
|
| Anti-inflammatory Markers |
IL-6 – FP IL-6 - RP |
AAGCCAGAGCTGTGCAGATGAGTA TGTCCTGCAGCCACTGGTTC |
|
Il-8 – FP Il-8 - RP |
ACACTGCGCCAACACAGAAATTA TTTGCTTGAAGTTTCACTGGCATC |
|
| Reference Marker |
GAPDH-FP GAPDH-RP |
TGA AGG TCG GAG TCA ACG GAT TCA CAC CCA TGA CGA ACA TGG |
FP: forward primer; RP: reverse primer.
3. Statistical analysis:
The experiment was conducted in triplicate for all tested genes. Statistical analysis was performed using SPSS v.21 (IBM, New York, NY, USA). Kruskal-Wallis and post hoc Tukey tests were used at a 0.05 level of significance. A further pairwise comparison test was carried out using the Mann-Whitney U test and Wilcoxon sign rank test. The data were presented in terms of means, medians, and standard deviations (S.D).
4. Results:
Based on the molecular analysis performed, descriptive statistics (mean, standard deviation, median, and interquartile range) of the outcome variable (human gingival fibroblasts) for the four study groups (S-N, S-C, N-N, and N-C) were performed based on the primers used for three different functional expressions.
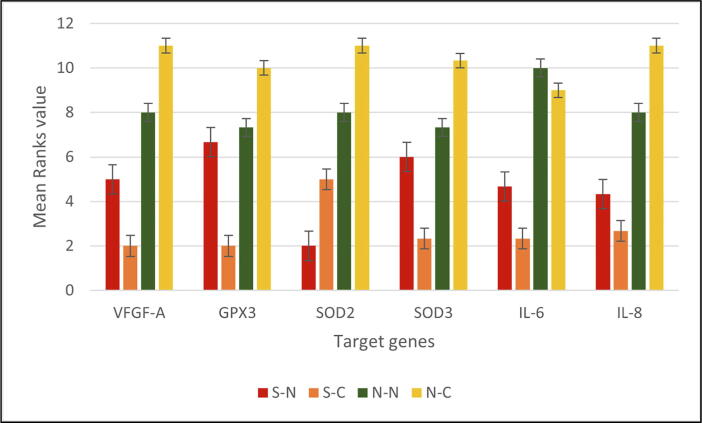
The expression of the wound healing gene (VEGF-A) in the N-N and N-C groups was significantly higher than that in the S-N and S-C groups (P value = 0.016). Human gingival fibroblasts after vit.c treatment showed higher VEGF-A expression in the never-smoker group (N-C) than in the smoker group (S-C) (Fig. 2). In addition, fold change quantification using the 2-ΔΔCt method showed VEGF-A upregulation in the never-smoker groups after the addition of vit.c compared to the smoker groups, as shown in Table 2. Despite this, the Wilcoxon sign rank test statistics value for the never-smoker groups before and after the induction of vit.c (N-N and N-C) inference did not show any statistically significant relationship (-1.604, p value = 0.109) (Table 2).
Fig. 2.
Mean ranks of human gingival fibroblasts in target gene expression.
Table 2.
Comparison of mean rank values and fold change of human gingival fibroblast samples among individual targeted genes.
| Target genes |
Study groups*—Mean ranks |
Kruskal-Wallis -test statistic | p value | |||
|---|---|---|---|---|---|---|
| S-N | S-C | N-N | N-C | |||
| VFGF-A | 5.00 | 2.00 | 8.00 | 11.00 | 10.385 | 0.016* |
| Fold change | 1.00 | 1.07 | 1.55 | 2.60 | ||
| GPX3 | 6.67 | 2.00 | 7.33 | 10.00 | 7.667 | 0.053 |
| Fold change | 1.00 | 0.88 | 1.00 | 1.03 | ||
| SOD2 | 2.00 | 5.00 | 8.00 | 11.00 | 10.385 | 0.016* |
| Fold change | 0.55 | 0.55 | 0.90 | 0.96 | ||
| SOD3 | 6.00 | 2.33 | 7.33 | 10.33 | 7.615 | 0.055 |
| Fold change | 1.00 | 0.82 | 1.01 | 1.14 | ||
| IL-6 | 4.67 | 2.33 | 10.00 | 9.00 | 9.051 | 0.029* |
| Fold change | 0.81 | 0.66 | 12.83 | 12.50 | ||
| IL-8 | 4.33 | 2.67 | 8.00 | 11.00 | 9.667 | 0.022* |
| Fold change | 0.06 | 0.06 | 0.81 | 1.17 | ||
S--N = Smoker without mediation; S-C = Smoker with vitamin C; N-N = Never-smoker without medication; N-C = Never-smoker with vitamin C.
Significant p value (P ≤ 0.05).
GPX3 expression was significantly lower in the S-C (2.00, p < 0.05) group than in the remaining groups (S-N,N-N N and N-C). In contrast, no significant difference was detected among the N-N,N-C, and S--N groups. Similarly, SOD3 expression was significantly lower in the S-C group (2.33, p < 0.05) when compared with N-N and N-C but not different from S-N, while SOD2 antioxidant marker expressed lower in S-N (2.00, p < 0.05) than other samples. Among smoker samples, the expression of SOD2 was significantly higher after induction of vit.c than before (S-C 5.00, p < 0.05) but in total lower value than the never-smoker groups N-N and N-C (8.00 and 11.00, respectively, p < 0.05) (Fig. 1). Anti-inflammatory marker comparison between smoker and never-smoker sample groups performed using the Mann-Whitney U test statistic of mean rank (5.00 and 14.00, respectively) results in significantly lower values among smoker groups when compared to nonsmoker groups (p < 0.0001).
In smoker samples, IL-6 gene expression was significantly lower than that in never-smoker samples, with no differences in expression between the two groups prior to and after vit.c induction. From the perspective of IL-6 expression, it was significantly upregulated in the never-smoker groups, in contrast to smokers, who exhibited downregulation of IL-6 gene expression. In the same pattern, the expression of IL-8 was significantly lower in smoker samples, with the highest expression in the never-smoker sample group after vit.c induction (N-C 11.00, p < 0.05) (Fig. 1).
5. Discussion:
One of the most significant environmental risks associated with lifestyle is smoking. In a recent systematic review, the Kingdom of Saudi Arabia was found to have one of the highest rates of tobacco smoking among Arab university students (42.3%). Significantly, males smoked more frequently than females, a finding that may be explained by social acceptance (Nasser et al., 2020). Smoking has been linked to reduced life expectancy and quality of health in almost all body organs due to multiple diseases. Fortunately, cigarette smoking remains the most preventable death and disease risk factor globally. Cigarette smoke is absorbed into the systemic circulation through the pulmonary alveolar epithelium, whereas direct exposure to inhaled cigarette smoke can cause vasoconstriction of the oral microvasculature and change the ecosystem (Sawhney et al., 2021, Lugg et al., 2022). The hallmark of the current era is the invasion of COVID-19, and cigarette smokers are reported to experience symptoms of the virus 1.4 times more often, while they are 2.4 times more likely to be admitted to an ICU, need mechanical ventilation, or die than nonsmokers (Vardavas and Nikitara 2020).
Conversely, the oral cavity, the body's first point of entry to cigarette heat and toxins, is not without risk. An increase in prevalence, extent, and severity of periodontal disease, tooth loss, and a lack of clinical outcome response to nonsurgical and surgical periodontal therapy are all linked to cigarette smoking (Albandar et al., 2000), as well as the long-term success of implant placement (Sawhney et al., 2021). Additionally, the wound healing process is impaired, complications are more likely to occur, and the quality of the postoperative outcome is affected (Wong and Martins‐Green 2004). In addition to heat, cigarettes contain numerous toxic compounds, such as reactive oxygen species that may damage DNA, increasing the likelihood of all types of cancers (Sawhney et al., 2021).
Vit.c has been used as an antioxidant scavenger in recent decades as a means of preventing the harmful effects of tobacco due to its powerful antioxidant properties and ease of accessibility (Abdullah and Attia 2019). There is substantial evidence in the literature supporting the benefit of vit.c supplementation in smokers (Møller et al., 2004, Falsafi et al., 2016). Furthermore, several in vitro experiments were conducted on body fibroblasts from different origins, including lung, periodontal ligament, mucosal and gingival tissues, and others. They play an essential role in body function and regeneration negatively impacted by exposure to tobacco compounds. To date, however, most studies on the effects of cigarette smoke on gingival fibroblast cells have examined the short-term effects of cigarette components on healthy explanted gingival cells (Tanur et al., 2000, Snyder et al., 2002, Wong and Martins-Green, 2004, Semlali et al., 2011, Silva et al., 2012, Torshabi et al., 2017, Vermehren et al., 2020). In recognition that smoking is a life-long habit that causes damage to cells, we aimed to investigate the cellular effects of smoking on wound healing and the antioxidant and anti-inflammatory properties on human gingival fibroblasts derived from years of heavy smokers compared to never-smokers. Moreover, we compared the characteristics of these cells following vit.c induction. Accordingly, we hypothesized that vit.c induction would provide a more representative sample of in vitro studies with a potential future application of natural products in dentistry.
Reverse transcription polymerase chain reaction (qRT-PCR) RNA expression analysis in our study showed that wound healing gene (VEGF-A) expression was higher in never-smoker gingival fibroblasts than in smokers. The scientific evidence that smoking reduces wounds' ability to heal may not be in dispute (Tatsumi et al., 2021). It appears that vit.c induced VEGF-A expression in never-smokers to a small extent, but the changes were not statistically significant. Additionally, smokers' samples showed lower levels of anti-inflammatory markers. Han and colleagues reported in a microarray analysis of oral fibroblasts that the prominent cytokines upregulated in hGFs were the pro- and anti-inflammatory cytokines IL-6 and IL-8, which are involved in neutrophil activation and T-cell regulation (Han and Amar 2002). Therefore, we examined IL-6 and IL-8 expression levels in the present study, which were significantly reduced in smokers even after vit.c induction, except that IL-8 expression levels were significantly elevated in never-smokers’ samples after treatment. Researchers have demonstrated that cigarette exposure alters the host response protective mechanism by altering inflammatory cytokines and enzymes with consequent healing impairment (Chaffee et al., 2021).
In contrast, recent meta-analyses of healthy periodontium cells conducted in smokers and nonsmokers found no significant difference in IL-8 levels (Pai et al., 2021). Nevertheless, the studies explored the level of biomarkers in gingival crevicular fluid (GCF) collectively, with heterogeneity in methodology, which may have contributed to the substantial discrepancies in the cytokine/chemokine levels. Moreover, although the smokers were not diagnosed with periodontitis, the GCF volume was lower in smokers (Pai et al., 2021). Researchers investigated the expression of IL-6 in hGFs after rinsing with vit.c and found that it was elevated, which may contribute to wound healing (Chaitrakoonthong et al., 2020). Nevertheless, the study was based on nonsmoker samples, which is approximately consistent with the present study, indicating higher expression of this cytokine in never-smokers compared to smokers. In line with a recent study investigating hGFs following long-term exposure to nicotine/or cigarette smoke condensate, they found that IL-6 and IL-8 levels are significantly suppressed with an increase in the inflammatory response and a decreased ability to heal wounds (Tatsumi et al., 2021). These results are also in agreement with previous studies that demonstrated that exposure to cigarette smoke inhibited the secretion of IL-6 and IL-8, suggesting that this may be related to the inhibition of cell growth and the induction of apoptosis as a result of cigarette smoke condensate exposure (Alamri et al., 2015).
An additional finding in our study was the statistically significant expression of antioxidant genes. It has been observed that glutathione peroxidase-3 (GPX3) and superoxide dismutase-3 (SOD3) are lower in smoker samples even after vit.c initiation. On the other hand, SOD2 levels were significantly elevated in smoker samples after vit.c treatment. The expression of all antioxidants was increased in never-smoker samples after using vit.c. As protection against free radicals, superoxide dismutase enzyme-2 (SOD2) and glutathione peroxidase isomer-3 (GPX3) are intracellular enzymes, whereas superoxide dismutase enzyme-3 (SOD3) is extracellular (Rudrakshi et al., 2017). Superoxide dismutase enzymes are mainly found in periodontal ligaments, and they are an essential defense mechanism against superoxide ions in gingival fibroblasts (Rudrakshi et al., 2017). The reduction in antioxidative enzymes in smoker samples compared to those who never smoked is not unexpected.
In contrast, the induction of vit.c to cells showed beneficial effects on gingival fibroblasts derived from smokers and never-smokers but was most prominent in the never-smokers. Although vit.c is an exogenous antioxidant that protects against cellular damage by scavenging free radicals produced by cigarette smoke (Rudrakshi et al., 2017), these components of cigarette smoke are powerful cytotoxic and carcinogenic agents that alter cellular function and induce cellular senescence to adapt and survive (Tatsumi et al., 2021). This fact may contribute to the cellular differences observed in our study in response to vit.c induction. Pai et al., 2021 concluded from their systematic review that smokers have significantly lower levels of SOD than nonsmokers, but interestingly, heavy smokers have much lower concentrations of SOD than light smokers(Pai et al., 2021). A similar comparison did not apply in the present study because all of the samples were extracted from heavy long-term smokers. However, it is an interesting point to explore in future studies. Another factor that might influence the results is the concentration of vit.c and the usage duration since its effect is dose dependent. Taking this into consideration, the previous study was meant to replicate the slow dissolving of oral lozenges by rinsing the cultured cells multiple times per day with vit.c, thereby clarifying that vit.c is safe and can be prescribed to patients after oral surgery and to further investigate the necessary dosage and method of administration for maximum benefit (Chaitrakoonthong et al., 2020).
Tobacco smoke is well known to cause significant damage to the human body, particularly the first tissues in contact, the oral tissues. Therefore, dentists are often the first to detect early signs of smoking. Considering this viewpoint, it seems feasible to use vit.c as a natural, easy-to-apply, and inexpensive material during clinical procedures to enhance postoperative healing with the possibility of extending success. This is a worthy idea to be explored in the future. Furthermore, further research on smokers' human gingival fibroblasts may be worthwhile to observe all the preserved changes in the cells that result from the battle and survival of smoking.
6. Conclusion:
In this study, reverse transcription polymerase chain reaction was used to verify the harmful effect of cigarettes on gingival fibroblasts in terms of their capacity to regenerate and heal, combat inflammation, and resist reactive free radicals. Moreover, these results shed light on the cellular characteristics of samples taken from Saudis exposed to cigarette smoking. In this study, vit.c administration had beneficial effects on cellular levels. Further investigation is recommended to establish it as a component of treatment for smokers in the dental clinic.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Contributor Information
Ruaa Alyami, Email: Raalyami@ksu.edu.sa.
Reham Al Jasser, Email: Raljasser@ksu.edu.sa.
Fahad Ali Alshehri, Email: Fahalshehri@ksu.edu.sa.
Nouf Alshibani, Email: nalshibani@ksu.edu.sa.
Riham Abdulaziz Alyami, Email: raalyami@kaauh.edu.sa.
Abdurahman A. Niazy, Email: aaniazy@ksu.edu.sa.
References:
- Abdullah, M. and F. N. Attia, 2019. Vitamin C (Ascorbic Acid). StatPearls [Internet], StatPearls Publishing. [PubMed]
- Alamri A., Semlali A., Jacques E., et al. Long-term exposure of human gingival fibroblasts to cigarette smoke condensate reduces cell growth by modulating B ax, c aspase-3 and p 53 expression. J. Periodontal Res. 2015;50:423–433. doi: 10.1111/jre.12223. [DOI] [PubMed] [Google Scholar]
- Albandar J.M., Streckfus C.F., Adesanya M.R., et al. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J. Periodontol. 2000;71:1874–1881. doi: 10.1902/jop.2000.71.12.1874. [DOI] [PubMed] [Google Scholar]
- Chaffee B.W., Couch E.T., Vora M.V., et al. Oral and periodontal implications of tobacco and nicotine products. Periodontology. 2021;2000(87):241–253. doi: 10.1111/prd.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitrakoonthong, T., R. Ampornaramveth and P. Kamolratanakul, 2020. Rinsing with L-ascorbic acid exhibits concentration-dependent effects on human gingival fibroblast in vitro wound healing behavior. International journal of dentistry. 2020, [DOI] [PMC free article] [PubMed]
- D'Aniello, C., F. Cermola, E. J. Patriarca, et al., 2017. Vitamin C in stem cell biology: impact on extracellular matrix homeostasis and epigenetics. Stem cells international. 2017, [DOI] [PMC free article] [PubMed]
- de Abreu F.A.M., Garcia Reis I.D., Borges Silva G.A., et al. Collection and Culture of Human Connective Tissue Cells from Gingival Explant Technique for Oral Tissue Bioengineering. Int. J. Morphol. 2019;37 [Google Scholar]
- Falsafi P., Nasrabadi E.T., Nasrabadi H.T., et al. Comparison of Total Antioxidant Capacity and Vitamin C in smokers and non-smokers. Biomedical and Pharmacology Journal. 2016;9:299–304. [Google Scholar]
- Förstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- Han X., Amar S. Identification of genes differentially expressed in cultured human periodontal ligament fibroblasts vs. human gingival fibroblasts by DNA microarray analysis. J. Dent. Res. 2002;81:399–405. doi: 10.1177/154405910208100609. [DOI] [PubMed] [Google Scholar]
- Hanes P.J., Schuster G.S., Lubas S. Binding, uptake, and release of nicotine by human gingival fibroblasts. J. Periodontol. 1991;62:147–152. doi: 10.1902/jop.1991.62.2.147. [DOI] [PubMed] [Google Scholar]
- Haniffa M.A., Collin M.P., Buckley C.D., et al. Mesenchymal stem cells: the fibroblasts' new clothes? Haematologica. 2009;94:258–263. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health, M. o., 2019. GATS KSA, 2019 Global Adult Tobacco Survey. S. M. o. Health. www.moh.gov.sa, Saudi Ministry of Health.
- Huang F.-M., Tai K.-W., Chou M.-Y., et al. Resinous perforation-repair materials inhibit the growth, attachment, and proliferation of human gingival fibroblasts. J. Endod. 2002;28:291–294. doi: 10.1097/00004770-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Kaldahl W.B., Johnson G.K., Patil K.D., et al. Levels of cigarette consumption and response to periodontal therapy. J. Periodontol. 1996;67:675–681. doi: 10.1902/jop.1996.67.7.675. [DOI] [PubMed] [Google Scholar]
- Kovacs K., Erdelyi K., Hegedus C., et al. Poly(ADP-ribosyl)ation is a survival mechanism in cigarette smoke-induced and hydrogen peroxide-mediated cell death. Free Radic Biol Med. 2012;53:1680–1688. doi: 10.1016/j.freeradbiomed.2012.08.579. [DOI] [PubMed] [Google Scholar]
- Lallier T.E., Moylan J.T., Maturin E. Greater sensitivity of oral fibroblasts to smoked versus smokeless tobacco. J. Periodontol. 2017;88:1356–1365. doi: 10.1902/jop.2017.170232. [DOI] [PubMed] [Google Scholar]
- Lugg S.T., Scott A., Parekh D., et al. Cigarette smoke exposure and alveolar macrophages: Mechanisms for lung disease. Thorax. 2022;77:94–101. doi: 10.1136/thoraxjnl-2020-216296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller P., Viscovich M., Lykkesfeldt J., et al. Vitamin C supplementation decreases oxidative DNA damage in mononuclear blood cells of smokers. Eur. J. Nutr. 2004;43:267–274. doi: 10.1007/s00394-004-0470-6. [DOI] [PubMed] [Google Scholar]
- Nasser A.M., Geng Y., Al-Wesabi S.A. The prevalence of smoking (cigarette and waterpipe) among university students in some Arab countries: a systematic review. Asian Pac. J. Cancer Prev. 2020;21:583. doi: 10.31557/APJCP.2020.21.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai S., Shenoy N., John D.S. Evaluation of IL-6, vitamin D level in chronic periodontitis patients after non-surgical periodontal therapy. Romanian Journal of Diabetes Nutrition and Metabolic Diseases. 2021;28:376–382. [Google Scholar]
- Papapanou, P. N., Sanz, M., Buduneli, N. et al., 2018. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 89 Suppl 1, S173-S182. https://doi.org/10.1002/JPER.17-0721. [DOI] [PubMed]
- Pryor W.A. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ. Health Perspect. 1997;105:875–882. doi: 10.1289/ehp.97105s4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrakshi C., Prabhuji M., Parween S., et al. Relationship between antioxidants and the development of the periodontal disease. J. Cytol. Tissue Biol. 2017;4:16. [Google Scholar]
- Saczko J., Dominiak M., Kulbacka J., et al. A simple and established method of tissue culture of human gingival fibroblasts for gingival augmentation. Folia Histochem. Cytobiol. 2008;46:117–119. doi: 10.2478/v10042-008-0017-4. [DOI] [PubMed] [Google Scholar]
- Sawhney A., Ralli M., Dhar S., et al. Role of smoking and its impact on periodontium. Journal of the International Clinical Dental Research Organization. 2021;13:3. [Google Scholar]
- Semlali A., Chakir J., Rouabhia M. Effects of whole cigarette smoke on human gingival fibroblast adhesion, growth, and migration. J. Toxic. Environ. Health A. 2011;74:848–862. doi: 10.1080/15287394.2011.570230. [DOI] [PubMed] [Google Scholar]
- Silva D., Cáceres M., Arancibia R., et al. Effects of cigarette smoke and nicotine on cell viability, migration and myofibroblastic differentiation. J. Periodontal Res. 2012;47:599–607. doi: 10.1111/j.1600-0765.2012.01472.x. [DOI] [PubMed] [Google Scholar]
- Snyder H.B., Caughman G., Lewis J., et al. Nicotine modulation of in vitro human gingival fibroblast β1 integrin expression. J. Periodontol. 2002;73:505–510. doi: 10.1902/jop.2002.73.5.505. [DOI] [PubMed] [Google Scholar]
- Spicer P.P., Kretlow J.D., Young S., et al. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012;7:1918–1929. doi: 10.1038/nprot.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanur E., McQuade M.J., McPherson J.C., et al. Effects of nicotine on the strength of attachment of gingival fibroblasts to glass and non-diseased human root surfaces. J. Periodontol. 2000;71:717–722. doi: 10.1902/jop.2000.71.5.717. [DOI] [PubMed] [Google Scholar]
- Tatsumi M., Yanagita M., Yamashita M., et al. Long-term exposure to cigarette smoke influences characteristics in human gingival fibroblasts. J Periodontal Res. 2021;56:951–963. doi: 10.1111/jre.12891. [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S., H. Greenwell and K. S. Kornman, 2018. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol. 89 Suppl 1, S159-S172. https://doi.org/10.1002/JPER.18-0006. [DOI] [PubMed]
- Torshabi M., Rezaei Esfahrood Z., Jamshidi M., et al. Efficacy of vitamins E and C for reversing the cytotoxic effects of nicotine and cotinine. Eur. J. Oral Sci. 2017;125:426–437. doi: 10.1111/eos.12375. [DOI] [PubMed] [Google Scholar]
- Van der Vaart H., Postma D., Timens W., et al. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax. 2004;59:713–721. doi: 10.1136/thx.2003.012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas C.I., Nikitara K. COVID-19 and smoking: A systematic review of the evidence. Tob. Induc. Dis. 2020;18 doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermehren M., Wiesmann N., Deschner J., et al. Comparative analysis of the impact of e-cigarette vapor and cigarette smoke on human gingival fibroblasts. Toxicol. In Vitro. 2020;69 doi: 10.1016/j.tiv.2020.105005. [DOI] [PubMed] [Google Scholar]
- Wong L.S., Martins-Green M. Firsthand cigarette smoke alters fibroblast migration and survival: implications for impaired healing. Wound Repair Regen. 2004;12:471–484. doi: 10.1111/j.1067-1927.2004.12403.x. [DOI] [PubMed] [Google Scholar]
- Wyganowska-Swiatkowska M., Nohawica M. Effect of tobacco smoking on human gingival and periodontal fibroblasts. A systematic review of literature. Przegl. Lek. 2015;72:158–160. [PubMed] [Google Scholar]
- Zhang Q., Shi S., Liu Y., et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]