Abstract
Background
Nonalcoholic steatohepatitis (NASH) is a cause of chronic liver disease.
Aim
Model the burden of NASH in the United States according to obesity.
Methods
The discrete-time Markov model comprised adult NASH subjects moving through 9 health states and 3 absorbing death states (liver, cardiac, and other deaths) with 1-year cycles and a 20-year horizon. Given that reliable natural history data for NASH are not available, transition probabilities were estimated from the literature and population-based data. These rates were disaggregated to determine age–obesity group rates by applying estimated age–obesity patterns. The model considers 2019 prevalent NASH cases and new incident NASH cases (2020–2039), assuming that recent trends will continue. Annual per-patient costs by health state were based on published data. Costs were standardized to 2019 US dollars and inflated by 3% annually.
Results
NASH cases in the United States are forecasted to increase by +82.6%, from 11.61 million (2020) to 19.53 million (2039). During the same period, cases of advanced liver disease increased +77.9%, from 1.51 million to 2.67 million, while its proportion remained stable (13.46%–13.05%). Similar patterns were observed in both obese and non-obese NASH. Among NASH, 18.71 million overall deaths, 6.72 million cardiac-specific deaths, and 1.71 million liver-specific deaths were observed by 2039. During this period, the projected cumulative direct healthcare costs were $1208.47 billion (obese NASH) and $453.88 billion (non-obese NASH). By 2039, the projected NASH attributable healthcare cost per patient increased from $3636 to $6968.
Conclusions
There is a substantial and growing clinical and economic burden of NASH in the United States.
Keywords: NAFLD, obesity, age group, NHANES, GBD
The prevalence of obesity is increasing around the world, with data from the World Health Organization suggesting that in 2016 over 650 million people around the world were considered to be obese (39% of all adults).1 Similar data is being reported from the United States with over 50% of the adult Americans estimated to be obese by 2030.2,3 In addition, obesity among children is increasing rapidly, with an estimated 12–13% of preschoolers in the United States currently considered to be obese.4 In this context, overweight or obese children are five times more likely to be obese as adults.4,5
In 2019, obesity was associated with immense economic impact in the United States, which was estimated at about 1.8% of the US gross domestic product.6 This was primarily due to decreased worker productivity (absenteeism and the inability to be as productive at work) and increased obesity-related medical expenses.6 It is also important to recognize that obesity is associated with a number of chronic health conditions such as type 2 diabetes, cardiovascular disease, stroke, various forms of cancer, as well as mental health issues.7, 8, 9, 10, 11
In the context of these chronic diseases, obesity adversely affects a number of organs, such as the liver. In fact, the disruption of lipid metabolism from obesity causes infiltration of the liver with fat, which leads to hepatic steatosis (fatty liver). When fatty liver is present in the absence of excessive alcohol consumption and other causes of fatty liver disease, patients may be diagnosed with non-alcoholic fatty liver disease (NAFLD) and or the more progressive form of non-alcoholic steatohepatitis (NASH).8, 9, 10 Currently, 25–30% of the global adult population has NAFLD.12 Studies on the natural history of NAFLD suggest a heterogeneous pattern of progression with some patients developing hepatic and/or non-hepatic complications.13, 14, 15 In this context, the majority of people with NAFLD have non-progressive fatty liver. In contrast, over 20% can develop NASH, which can progress to hepatic fibrosis, cirrhosis, liver cancer, end-stage liver disease, and death.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 In fact, NASH is now a leading cause of cirrhosis, hepatocellular carcinoma (HCC), and as an indication for liver transplantation in the United States.24, 25, 26 The cause of death in NAFLD is dependent on the stage of liver disease where patients with cirrhosis primarily die from liver related events, while those without cirrhosis die primarily of cardiovascular disease, non-hepatic cancers, and complications from associated co-morbidities such as chronic kidney disease and type 2 diabetes mellitus.27, 28, 29, 30, 31, 32, 33 Regardless of the cause of death, the burden of NAFLD related to morbidity, mortality, health-care costs, and impairment of health-related quality of life can be enormous.16, 17, 18, 19, 20, 21, 22, 23
Despite this substantial and growing burden of NASH, treatments are limited to exercise and diet.34,35 These challenges are compounded by a significant lack of disease awareness among patients, providers, policy makers, and other stakeholders.36,37 Therefore, in an attempt to close this knowledge gap, we assessed the clinical and economic burden associated with NASH in the presence and absence of obesity. The assessment was carried out by developing two models which simulated the probability of moving through the stages of NASH and the associated clinical outcomes and economic costs.
Methods
A Markov model is a computer-based algorithm that assigns each simulated patient to one of a finite number of discrete-health states for a period called a cycle. At the end of each cycle, the patients either travels to a different health state or remains in their current state based on probabilities that are specified for transitioning from one health state to another (hereafter, transition probabilities). We developed a Markov model of the natural history of NASH to assess the long-term clinical and economic burden of NASH, stratified by the presence of obesity in a real-world setting, following the Consolidated Health Economic Evaluation Reporting Standards reporting guideline, and using Microsoft Excel, R, and SAS software.38 In addition, this study was approved by the Inova Health Systems Review Board as exempt due to the use of secondary de-identified data which are publically available.
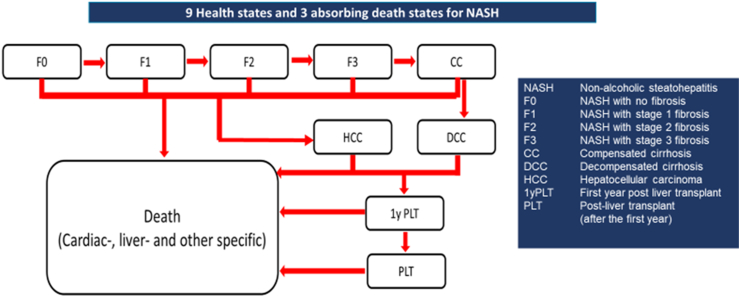
Our Markov model comprised all stages of NASH: NASH without fibrosis (F0), fibrosis stage 1 (F1), fibrosis stage 2 (F2), fibrosis stage 3 (F3), compensated cirrhosis (CC), decompensated cirrhosis (DCC), HCC, first-year post-LT (1yPLT), post-LT after 1 year and onwards (PLT), and 3 absorbing death states (liver, cardiac, and other deaths) (Figure 1). We defined “other causes” of deaths as all remaining deaths not captured under cardiac or liver-specific deaths. Fibrosis regression was permitted from F1 to F0, F2 to F1 and F0, F3 to F2, F1, and F0, and CC to F3. All patients who underwent LT, transitioned to a tunnel state for their first-year PLT stage. The model cycle length was 1-year, and a 20-year horizon was applied.
Figure 1.
9 Health states and 3 absorbing death states for NASH.
The model begins with the prevalent obese NASH and normal/overweight NASH (non-obese NASH) cases in the United States (2019), as well as the annual estimated incident cases of obese and non-obese NASH from 2020 to 2039.
Input: Prevalent NASH Cases in the United States 2019
Data sources used in prevalent NASH estimation are available in Supplementary Table 1. The prevalence of NAFLD (24.8%) was taken from the published literature (12), 21.4% in the age group 18–44, 27.0% in the age group 45–64, and 29.0% in age group +65. Based on the estimate that NASH comprises 7–30% of NAFLD prevalence (11), we assumed that 20% of NAFLD cases would be classified as NASH, which corresponded to 11.22 million (4.48%) of the US population in 2019, and was comprised of 3.70 million for age group 19–44, 4.45 million for age group 45–64, and 3.06 million for age group +65, where 7.87 million (70.1%) had obese (BMI ≥ 30 kg/m2).12,39,40
As a second step, the total number of prevalent NASH cases was distributed among the nine non-death health states. From the literature, we obtained the distribution of F0–F4 among the patients with NASH. However, there was no information available on the distribution for the three health states of HCC, 1y-LT, and post LT. Therefore, to overcome this limitation, we obtained data for NASH related HCC cases from the Global Burden of disease (GBD) 2019 US subsample (n = 3965 NASH related HCC cases) and the Organ Procurement Transplant Network/Scientific Registry of Transplant Recipients (OPTN/SRTR) 2019 annual data report for the number of liver transplants for NASH (n = 26,186) and the number of liver transplants due to NASH post 1 year liver transplant (n = 2951).41 The number of fibrosis states (F0–F4) was estimated by multiplying the proportion of fibrosis stages from the literature (31) with the residual number of prevalent NASH cases (the overall number of prevalent NASH cases minus the sum of NASH-related liver cancer, liver transplant, and LT recipients). Furthermore, the number of F4 cases was split into the number of CC and DCC cases by using the ratio of CC and DCC previously published.42
To split the number of health states for each age group from the overall prevalent NASH cases, we used the observed proportion of age groups in NASH with F0-DCC from the SRTR 2002–2020 data,26 1yPLT and PLT from the OPTN/SRTR 2019 annual report data,41 and HCC cases from the GBD 2019 data.43
Subsequently, from NHANES 2017–2018 data, we used the observed proportion of obesity for each age group among those with NASH to further split the number of health states for each age group into those for each age–obesity group, with the assumption that the age pattern for health states remained the same regardless of the obesity status. The estimated prevalent NASH cases in the US population in 2019 are presented in Supplementary Table 2.
Input: Incidental NASH Cases in the United States over the Next Two Decades: 2020–2039
Data sources used in incidental NASH estimation are available in Supplementary Table 3. Due to a lack of data on NASH incidence, we performed a mathematical calculation to provide a NASH incidence estimate for the years 2020–2039, with the assumption that the trends in the past decade continued, and NASH incidence trends were the same as NAFLD's. First, using the GBD 2019 study, we calculated the annual percent change (APC) of NAFLD prevalence (+2.89%) and NAFLD incidence (+1.93%) over the last decade (2010–2019) using Joinpoint Trend Analysis Software, a software package developed by National Cancer Institute for calculating the percent of annual changes.44 Second, using our prevalent model, we estimated the NASH incidence at 4.40 per 1000 which is a +2.89% increase in NASH prevalence in 2020 from 2019. Third, the incidence rates from 4.40 per 1000 in 2020 to 6.45 per 1000 in 2039 were calculated by applying an APC of +1.93%. Our approach using the current trend in NAFLD prevalence is conservative because a recent study reported that high-risk NAFLD defined as a FIB-4 ≥2.67 increased annually by 9.72%.45 Fourth, the number of NASH incident cases was then obtained by applying the incidence rates to the corresponding US projected population for 2020–2039. Fifth, the distribution of age groups in incidental NAFLD cases in 2019 and trends in the proportion of age groups in incidental NAFLD cases over the last decade (APC = −0.464% for 18–44, −0.392% for 45–64, and +1.55% for ≥65 years) were calculated from the GBD 2019 study by splitting the number of NASH incident case into each age group. Finally, trends in the obese prevalence by age group obtained from NHANES (1999–2016) (APC = +2.00% for 18–44, +1.22% for 45–64, and +1.71% for ≥65 years) were applied to further split NASH incident cases in each age group into the age–obesity group. The estimated number of incident NASH cases began their model at F0, F1, or F2, according to age–obesity status. The proportion of the stages of fibrosis (F0, F1, and F2) among incident NASH cases for each age–obesity group is estimated by using transient elastography liver stiffness measurement, with FIB-4 scores among NAFLD defined as a controlled attenuation parameter of ≥285 dB/M (NHANES 2017–2018) (Supplementary Table 3). The annual estimated number of incident NASH cases is available in Supplementary Table 4.
Transition Probabilities
Data sources used in estimating transition probabilities (TPs) in the NASH cohort are available in Supplementary Table 5. Briefly, TPsfor F0, F1, F2, F3, and CC are based on synthesized data from a 2015 meta-analysis46 and the placebo arms from recent clinical trials.47,48 TPs from CC to DCC were obtained from Nyberg's article.42 TPs for HCC and LT were obtained from Estes's article,49 and were adjusted to ensure that the model outputs aligned with the 3965 prevalent NASH-related HCC from GBD data and the number of LT cases from OPTN/SRTR 2020 annual data report (Liver-2906 LT-related NASH in 2020) using our prevalent NASH cases.41
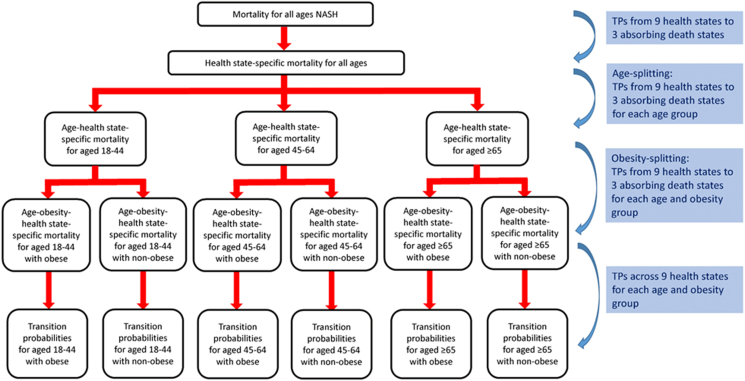
Since the age–obesity pattern can make significant differences in clinical and economic outcomes among NASH, it is essential to have TPs for each age–obesity subgroup. Therefore, we estimated TPs in the overall NASH cases by using the published literature and population data obtained from NHANES III, National Vital Statistics System, GBD, and SRTR. Then, the TPs for the overall NASH cohort were split to build the transition probability for the age–obesity NASH subgroup by using the observed age–obesity distribution of NASH from population-based studies or databases via our developed tool, called the age-splitting method. A detailed description of the development is available in the supplementary information. A methodological summary for TPs is presented in Figure 2. Finally, the TPs for remaining at the same health state were obtained by 1 minus the sum of all other TPs from a state so the total of TP from a state to all other states equaled one (Supplementary Table 6).
Figure 2.
A methodological summary for transition probabilities. TP, Transition probability.
Cost
Annual health state costs for patients with NASH were taken from our previous study (Supplementary Table 7).49 A combination of micro-costing and gross-costing methods was used to estimate the costs associated with each health state in our model. As this study was conducted from a US payer's perspective, we estimated only the direct medical costs associated with NASH health states. Costs included inpatient hospitalization, outpatient visits, emergency room, drugs, laboratory, imaging, and screening tests. Resource use in the first year and subsequent years for F0, F1, F2, and F3 patients in the all-NASH model was based on the input from published data.19,49 Costs for outpatient visits, laboratory, imaging, and screening for hepatitis C and B tests were based on Current Procedural Terminology codes using the Center for Medicare and Medicaid Fee Schedule 2017. Furthermore, costs for F0, F1, F2, and F3 were updated based on a recent study.50 The costs for CC, DCC, and HCC were derived from a study on the costs of advanced NAFLD in the Veterans Administration.24 There are no NASH-specific costs available for patients with advanced liver disease. Nevertheless, we assumed that once patients reach advanced liver disease, the costs of monitoring and management are driven by disease severity rather than the etiology of liver disease. In this context, we assumed that the costs of HCC, LT, and PLT would be the same as the conditions observed in hepatitis C patients, except for the cost of antiviral drugs, which we excluded from the analysis. Therefore, the costs for LT, 1yPLT, and PLT stages were derived from a retrospective study51 that reported incremental all-cause costs for hepatitis C virus patients relative to non-hepatitis C virus patients. We assumed that state costs would not vary among different age groups. Costs were inflated 3% annually for forward years.
Sensitivity Analysis
One-way deterministic sensitivity analysis was performed to test the model output sensitivity to changes in incidental NASH cases and transition probabilities. To calculate the range of trends for the possible number of incident NASH cases for 2020–2039, an APC of 0% (no change) and an APC of +9.72% (rate obtained from a prior study) were applied among those with NAFLD and a FIB-4 of ≥2.67.45 Progression/regression transition probability ranges were based on data obtained via literature review with a range of ±5–10% from the base values, which were used to construct the TPs. For details, please see the supplementary material.
Result
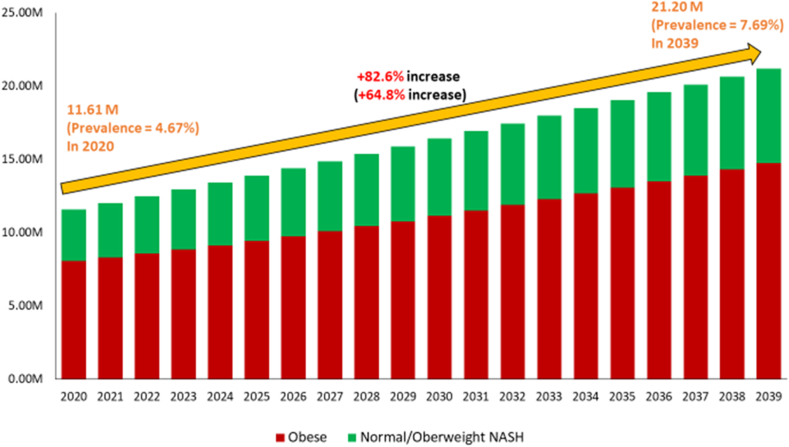
With the assumption that the trends of obesity and NAFLD over the past decade will continue, NASH cases in the United States are forecasted to increase +82.6%, from 11.61 million (prevalence = 4.64%) in 2020 to 19.53 million (prevalence = 7.69%) in 2039. Of patients with NASH, obese NASH will increase by +83.0%, from 8.08 million (prevalence = 3.25%) to 14.78 million (prevalence = 5.36%), while non-obese NASH will increase by +81.8%, from 3.53 million (prevalence = 1.42%) to 6.42 million (prevalence = 2.33%) (Figure 3 and Supplementary Table 8). Among obese NASH, the fastest growth in prevalent NASH cases will occur among those aged 18–44 (+100.7% increase), followed by those aged 45–64 (+84.5%), and then those aged ≥65 years (+62.0%), whereas among non-obese, the fastest growth will occur in those aged 45–65 years (+104.3%), followed by those aged ≥65 years (+91.0%), and then those aged 18–44 years (+56.7%).
Figure 3.
Projected prevalent NASH cases by the presence of obesity in the United States (2020–2039).
From 2020 to 2039, the number of cases of advanced liver disease (combining F3, F4, DCC, and HCC) will increase by 77.9%, from 1.51 million to 2.67 million, while the proportion of advanced liver disease remains relatively stable (13.46%–13.05%). The proportion of advanced liver disease will increase by +18.2, from 6.08% to 7.18%, among younger patients (18–44 years). Similar patterns occur in both obese and non-obese NASH (Table 1). During the same period of time, prevalent cases of NASH-related DCC, HCC, and LT will increase to 219,212 (+118.4% increase from 2020), 13,838 (+237.1%), and 5655 (+107.4%) among obese NASH cases, respectively. Also, 42,627 (+15.1%), 2861 (+165.1%) and 1126 (+1.19%) cases were noted among non-obese NASH, respectively (Supplementary Tables 9–11). It is estimated that obesity will account for 81.58% of cumulative liver transplants (n = 98,171) among NASH patients for the next two decades.
Table 1.
Projected Advanced Liver Diseases Among NASH Cohort, Stratified by the Presence of Obesity in the United States, 2020–2039.
| Year | Total | 18–44 y | 45–64 y | ≥65 y |
|---|---|---|---|---|
| Obese NASH | ||||
| 2020 | 1,086,905 (13.46) | 152,683 (6.09) | 501,629 (15.56) | 432,593 (18.45) |
| 2039 | 1,929,134 (13.05) | 372,503 (7.40) | 914,057 (15.37) | 642,573 (16.91) |
| % Change from 2020 to 2039a | 77.49 (−3.02) | 143.97 (21.54) | 82.22 (−1.24) | 48.54 (−8.32) |
| Normal/Overweight NASH | ||||
| 2020 | 418,202 (11.84) | 88,846 (6.06) | 199,470 (15.01) | 129,886 (17.66) |
| 2039 | 745,202 (11.61) | 154,064 (6.70) | 377,085 (13.89) | 214,054 (15.23) |
| % Change from 2020 to 2039a | 78.19 (−1.96) | 73.41 (10.65) | 89.04 (−7.46) | 64.80 (−13.72) |
| All NASH | ||||
| 2020 | 1,505,107 (12.97) | 241,529 (6.08) | 701,099 (15.40) | 562,479 (18.26) |
| 2039 | 2,674,336 (12.62) | 526,567 (7.18) | 1,291,142 (14.91) | 856,627 (16.46) |
| % Change from 2020 to 2039a | 77.68 (−2.71) | 118.01 (18.17) | 84.16 (−3.22) | 52.29 (−9.86) |
Data displayed as count (proportion, %).
NASH, non-alcoholic steatohepatitis.
Data displayed as percent change in counts (percent change in proportion).
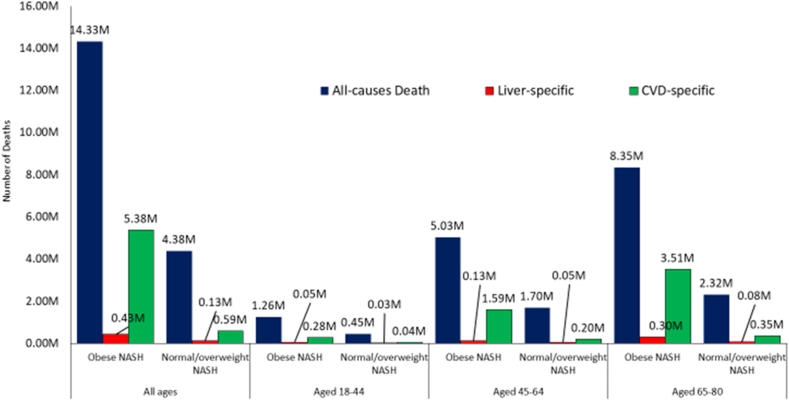
In regards to mortality, from 2020 to 2039, we project 18.71 million deaths (from all causes) among individuals with NASH. Among these, 6.72 million deaths are expected to be cardiac-specific deaths and 1.71 million liver-specific deaths (Table 2, Supplementary Tables 12–14, Figure 4). In this context, cardiac- and liver-specific death rates (per 100,000) will increase by +60.6% and +61.6%, from 88.90 per 100,000 to 144.37 per 100,000 and 8.60 per 100,000 to 13.89 per 100,000, respectively. The fastest increase in the cardiac- and liver-specific death rates is expected in the younger patients (18–44 y) as compared to the middle-aged and older patients (increase +91.6% and +70.3%, respectively, vs. +74.4% and +61.5% in patients aged 45–64; +34.5% and +42.4% in patients aged +65 years). Furthermore, obese NASH accounted for 76.6% of the all-cause deaths, 90.1% of the cardiac specific deaths and 76.5% of the liver specific deaths. The fastest growth in cardiac- and liver-specific deaths are projected to occur among those aged 18–44. Similar patterns are expected to occur in both obese and non-obese NASH.
Table 2.
Projected All-cause and Cause-specific Death Among NASH Cohort, Stratified by the Presence of Obesity in the United States, 2020–2039.
| Year | Total | 18–44 y | 45–64 y | ≥65 y |
|---|---|---|---|---|
| Obese NASH | ||||
| All-cause death | ||||
| 2020 | 549,203 (220.86) | 40,609 (33.82) | 190,922 (227.66) | 328,471 (734.35) |
| 2025 | 608,409 (235.32) | 53,126 (42.79) | 211,092 (256.69) | 357,048 (684.76) |
| 2030 | 709,322 (266.92) | 65,593 (51.82) | 244,338 (296.41) | 415,024 (731.65) |
| 2039 | 957,118 (347.27) | 82,270 (64.09) | 348,218 (386.79) | 547,032 (956.13) |
| Cumulative total 2020–2039 | 14,331,178 | 1,260,950 | 5,025,988 | 8,349,118 |
| % Change from 2020 to 2039a | 74.27 (57.23) | 102.59 (89.50) | 82.39 (69.89) | 66.54 (30.20) |
| Cardiac-specific death | ||||
| 2020 | 203,814 (81.96) | 8372 (6.97) | 59,673 (71.16) | 135,768 (303.53) |
| 2025 | 228,477 (88.37) | 11,641 (9.38) | 66,963 (81.43) | 149,874 (287.43) |
| 2030 | 266,140 (100.15) | 14,398 (11.37) | 77,426 (93.93) | 174,315 (307.30) |
| 2039 | 358,983 (130.25) | 17,795 (13.86) | 110,694 (122.95) | 230,493 (402.87) |
| Cumulative total 2020–2039 | 5,375,719 | 275,070 | 1,593,971 | 3,506,677 |
| % Change from 2020 to 2039a | 76.13 (58.91) | 112.54 (98.80) | 85.50 (72.79) | 69.77 (32.73) |
| Liver-specific death | ||||
| 2020 | 15,587 (6.27) | 10,799 (8.99) | 4789 (5.71) | 10,799 (12.88) |
| 2025 | 18,151 (7.02) | 12,857 (10.36) | 5294 (6.44) | 12,857 (15.63) |
| 2030 | 21,877 (8.23) | 15,633 (12.35) | 6244 (7.57) | 15,633 (18.96) |
| 2039 | 29,416 (10.67) | 20,402 (15.89) | 9014 (10.01) | 20,402 (22.66) |
| Cumulative total 2020–2039 | 432,156 | 304,878 | 127,278 | 304,878 |
| % Change from 2020 to 2039a | 88.72 (70.27) | 146.75 (76.72) | 88.24 (75.35) | 88.93 (75.99) |
| Normal/Overweight NASH | ||||
| All-cause death | ||||
| 2020 | 152,552 (61.35) | 16,624 (13.84) | 60,892 (72.61) | 78,285 (175.02) |
| 2025 | 184,517 (71.37) | 20,066 (16.16) | 70,154 (85.31) | 97,527 (187.04) |
| 2030 | 222,892 (83.87) | 23,331 (18.43) | 83,636 (101.46) | 119,865 (211.31) |
| 2039 | 287,909 (104.46) | 25,566 (19.92) | 117,798 (130.85) | 149,722 (261.69) |
| Cumulative total 2020–2039 | 4,379,028 | 445,213 | 1,696,692 | 2,315,892 |
| % Change from 2020 to 2039a | 88.73 (70.27) | 53.79 (43.85) | 93.45 (80.20) | 91.25 (49.52) |
| Cardiac-specific death | ||||
| 2020 | 19,735 (7.94) | 1374 (1.14) | 6811 (8.12) | 11,550 (25.82) |
| 2025 | 24,644 (9.53) | 1776 (1.43) | 8077 (9.82) | 14,790 (28.36) |
| 2030 | 30,058 (11.31) | 2061 (1.63) | 9691 (11.76) | 18,306 (32.27) |
| 2039 | 38,910 (14.12) | 2171 (1.69) | 13,756 (15.28) | 22,984 (40.17) |
| Cumulative total 2020–2039 | 587,824 | 38,795 | 196,246 | 352,784 |
| % Change from 2020 to 2039a | 97.16 (77.88) | 58.00 (47.79) | 101.97 (88.13) | 98.99 (55.57) |
| Liver-specific death | ||||
| 2020 | 5796 (2.33) | 3248 (2.71) | 2548 (3.04) | 3248 (3.87) |
| 2025 | 5448 (2.11) | 3230 (2.60) | 2219 (2.70) | 3230 (3.93) |
| 2030 | 6462 (2.43) | 3940 (3.11) | 2522 (3.06) | 3940 (4.78) |
| 2039 | 8880 (3.22) | 5177 (4.03) | 3703 (4.11) | 5177 (5.75) |
| Cumulative total 2020–2039 | 132,571 | 78,770 | 53,802 | 78,770 |
| % Change from 2020 to 2039a | 53.20 (38.22) | 39.49 (49.07) | 45.33 (35.37) | 59.37 (48.45) |
| All NASH | ||||
| All-cause death | ||||
| 2020 | 701,755 (282.21) | 57,232 (47.66) | 251,814 (300.27) | 406,755 (909.37) |
| 2025 | 792,926 (306.69) | 73,192 (58.95) | 281,246 (342.00) | 454,575 (871.81) |
| 2030 | 932,214 (350.79) | 88,924 (70.25) | 327,974 (397.86) | 534,889 (942.97) |
| 2039 | 1,245,027 (451.73) | 107,836 (84.00) | 466,016 (517.63) | 696,753 (1217.83) |
| Cumulative total 2020–2039 | 18,710,206 | 1,706,163 | 6,722,680 | 10,665,010 |
| % Change from 2020 to 2039a | 77.42 (60.07) | 88.42 (76.24) | 85.06 (72.39) | 71.30 (33.92) |
| Cardiac-specific death | ||||
| 2020 | 223,549 (89.90) | 9746 (8.12) | 66,484 (79.28) | 147,318 (329.35) |
| 2025 | 253,121 (97.90) | 13,417 (10.81) | 75,040 (91.25) | 164,664 (315.80) |
| 2030 | 296,197 (111.46) | 16,459 (13.00) | 87,117 (105.68) | 192,621 (339.58) |
| 2039 | 397,893 (144.37) | 19,966 (15.55) | 124,450 (138.23) | 253,477 (443.04) |
| Cumulative total 2020–2039 | 5,963,543 | 313,865 | 1,790,217 | 3,859,461 |
| % Change from 2020 to 2039a | 77.99 (60.59) | 104.86 (91.61) | 87.19 (74.36) | 72.06 (34.52) |
| Liver-specific death | ||||
| 2020 | 21,383 (8.60) | 14,047 (11.70) | 7336 (8.75) | 14,047 (31.40) |
| 2025 | 23,599 (9.13) | 16,087 (12.96) | 7513 (9.14) | 16,087 (30.85) |
| 2030 | 28,339 (10.66) | 19,573 (15.46) | 8766 (10.63) | 19,573 (34.51) |
| 2039 | 38,296 (13.89) | 25,579 (19.93) | 12,717 (14.13) | 25,579 (44.71) |
| Cumulative total 2020–2039 | 564,727 | 383,648 | 181,079 | 383,648 |
| % Change from 2020 to 2039a | 79.09 (61.58) | 82.10 (70.32) | 73.34 (61.46) | 82.10 (42.36) |
Data displayed as count (age-specific rate per 100,000).
NASH, non-alcoholic steatohepatitis.
Data displayed as percent change in counts (percent change in rate).
Figure 4.
Cumulative projected deaths among NASH population in the United States for the next two decades.
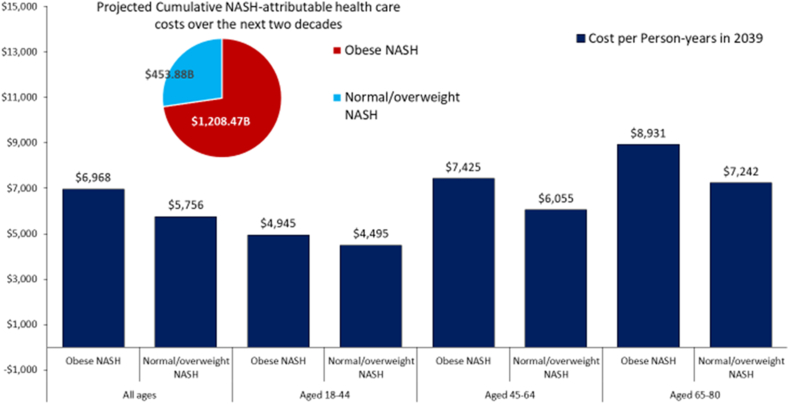
The projected cumulative direct healthcare costs associated with NASH (obese and non-obese) are estimated to reach $893.5 billion ($686.9 billion obese; $206.6 billion for non-obese) (Figure 5, Table 3, and Supplementary Table 15). The highest costs ($275.2 billion) are estimated for the obese NASH cohort who are 65 years or older. This is almost 4 times greater than the cost for the non-obese NASH cohort for the same age group ($70.6 billion). The individual costs are also highest for the obese NASH and are projected to increase 46% by 2039 from $1865 to $4013. Again, the obese NASH group who are 65 years or older are projected to have the highest costs in 2030 ($6105) (see Table 3).
Figure 5.
Economic burden of NASH in the United States for the next two decades.
Table 3.
Projected NASH Attributable Healthcare Costs, Stratified by the Presence of Obesity in the United States, 2020–2039.
| 2020 | 2025 | 2030 | 2039 | 2020–2039a | |
|---|---|---|---|---|---|
| Obese NASH | |||||
| 18–44 y | $6.53B ($2604) | $10.52B ($3132) | $15.35B ($3754) | $24.89B ($4945) | $303.13B |
| 45–64 y | $12.58B ($3903) | $17.54B ($4889) | $23.59B ($5664) | $44.16B ($7425) | $498.67B |
| ≥65 y | $10.25B ($4373) | $14.68B ($5836) | $19.91B ($6864) | $33.93B ($8931) | $406.66B |
| Total | $29.36B ($3636) | $42.74B ($4517) | $58.85B ($5276) | $102.98B ($6968) | $1208.47B |
| Normal/overweight NASH | |||||
| 18–44 y | $3.77B ($2570) | $5.56B ($2928) | $7.50B ($3430) | $10.33B ($4495) | $144.32B |
| 45–64 y | $4.92B ($3700) | $6.43B ($3989) | $8.82B ($4546) | $16.43B ($6055) | $186.53B |
| ≥65 y | $3.00B ($4076) | $4.30B ($4598) | $6.13B ($5367) | $10.18B ($7242) | $123.02B |
| Total | $11.68B ($3309) | $16.29B ($3664) | $22.45B ($4261) | $36.94B ($5756) | $453.88B |
| NASH overall | |||||
| 18–44 y | $10.30B ($2591) | $16.08B ($3058) | $22.86B ($3641) | $35.22B ($4804) | $447.46B |
| 45–64 y | $17.50B ($3844) | $23.97B ($4610) | $32.41B ($5309) | $60.60B ($6996) | $685.21B |
| ≥65 y | $13.25B ($4302) | $18.98B ($5501) | $26.04B ($6441) | $44.11B ($8475) | $529.69B |
| Total | $41.05B ($3537) | $59.02B ($4244) | $81.30B ($4950) | $139.92B ($6601) | $1662.36B |
Data displayed as total cost (cost per patient).
NASH, non-alcoholic steatohepatitis.
Future cost by the inflated rate of 3%.
Cumulative cost.
Specifically, among the obese NASH cohort, the projected cumulative healthcare cost for those aged 18–44, 45–65, and +65 will be $303.13 billion, $498.67 billion, and $406.66 billion, respectively, while $144.32 billion, $186.53 billion, and $123.02 billion for the normal/obese NASH cohort. Furthermore, the projected NASH attributable healthcare cost per patient will increase from $3636 to $6968 among the obese NASH, and from $3309 to $5756 among the non-obese NASH. Of the obese NASH cohorts in 2039, the highest healthcare cost per patient for the obese NASH is expected to occur among 65 years or older ($8931), followed by age groups 45–64 ($7425) and 18–44 ($4945). A similar pattern across age groups will be expected in the non-obese NASH cohort.
We performed One Way Sensitivity Analysis, which is summarized in Table 4 and in the supplementary material.
Table 4.
Sensitivity Analysis for Among NASH Cohort, Stratified by the Presence of Obesity Compared With Base Values.
| Input (Range) |
Base Values | Change (%) in NASH prevalence in 2039 (lower to upper bound) |
Change (%) in Cumulative Liver-specific Deaths (lower to upper bound) | ||||
|---|---|---|---|---|---|---|---|
| Incidence | Obese | Normal | All | Obese | Normal | All | |
| APC, 0%–9.7% | 1.93% | −19.23 to 162.36 | −16.20 to 131.57 | −18.31 to 153.04 | −6.19 to 40.97 | −4.41 to 27.99 | −5.75 to 37.77 |
| Transition Probabilities | |||||||
| Progression | |||||||
| F0 to F1, 0%–10% | 5.78% | 0.74 to −0.32 | 0.86 to −0.38 | 0.77 to −0.33 | −0.22 to −0.44 | −3.69 to −0.05 | −1.07 to −0.35 |
| F1 to F2, 0%–10% | 2.97% | 0.78 to −1.24 | 0.75 to −1.18 | 0.77 to −1.22 | −4.90 to 7.83 | −7.46 to 9.74 | −5.53 to 8.30 |
| F2 to F3, 0%–10% | 3.93% | 0.24 to −0.26 | 0.07 to −0.06 | 0.19 to −0.20 | −4.36 to 4.69 | −4.17 to 2.52 | −4.31 to 4.16 |
| F3 to CC, 0%–15% | 10.55% | 1.12 to −0.33 | 0.52 to −0.18 | 0.94 to −0.29 | −17.02 to 5.16 | −13.46 to 3.68 | −16.14 to 4.80 |
| CC to DCC, 0%–15% | 9.29% | 1.63 to −0.58 | 0.66 to −0.29 | 1.34 to −0.49 | −29.99 to 11.10 | −19.81 to 7.82 | −27.49 to 10.29 |
| Regression | |||||||
| F1 to F0, 0%–10% | 3.23% | −0.19 to 0.38 | −0.24 to 0.41 | −0.21 to 0.39 | −0.58 to −0.61 | −0.77 to −2.70 | −0.63 to −1.12 |
| F2 to F1, 0%–10% | 5.45% | −0.79 to 0.54 | −0.52 to 0.34 | −0.71 to 0.48 | 4.61 to −3.91 | 3.64 to −4.71 | 4.37 to −4.11 |
| F3 to F2, 0%–10% | 8.09% | −0.14 to 0.06 | −0.06 to 0.03 | −0.11 to 0.05 | 2.84 to −1.25 | 2.52 to −2.27 | 2.76 to −1.50 |
| CC to F3, 0%–10% | 7.63% | −0.04 to 0.14 | −0.26 to 0.08 | −0.36 to 0.12 | 7.03 to −2.54 | 5.79 to −3.27 | 6.73 to −2.72 |
| Change (%) in Proportion of Advanced Liver Disease in 2039 (lower to upper bound) | Change (%) in Cumulative Cardiac-specific Deaths (lower to upper bound) |
||||||
| APC, 0%–9.7% | 1.93% | −15.23 to 117.36 | −11.16 to 80.72 | −14.10 to 107.15 | −14.17 to 108.86 | −10.28 to 74.48 | −13.25 to 100.65 |
| Transition Probabilities | |||||||
| Progression | |||||||
| F0 to F1, 0%–10% | 5.78% | −2.02 to −2.23 | −5.59 to −1.95 | −3.01 to −2.15 | −0.13 to −0.69 | −4.99 to −0.01 | −1.29 to −0.53 |
| F1 to F2, 0%–10% | 2.97% | −4.06 to 3.06 | −4.71 to 2.75 | −4.24 to 2.97 | −6.91 to 9.99 | −10.71 to 13.31 | −7.82 to 10.79 |
| F2 to F3, 0%–10% | 3.93% | −24.74 to 24.03 | −20.88 to 18.21 | −23.66 to 22.41 | −6.64 to 6.7 | −6.13 to 4.09 | −6.52 to 6.08 |
| F3 to CC, 0%–15% | 10.55% | −3.79 to 1.07 | −5.46 to 1.96 | −4.25 to 1.32 | −20.07 to 5.65 | −18.75 to 5.29 | −19.76 to 5.56 |
| CC to DCC, 0%–15% | 9.29% | 12.29 to −4.28 | 5.45 to −2.25 | 10.38 to −3.72 | −32.9 to 10.64 | −24.46 to 8.95 | −30.88 to 10.24 |
| Regression | |||||||
| F1 to F0, 0%–10% | 3.23% | −2.90 to −3.64 | −3.28 to −6.25 | −3.01 to −4.36 | −0.94 to −0.64 | −0.86 to −3.42 | −0.92 to −1.30 |
| F2 to F1, 0%–10% | 5.45% | 1.35 to −5.89 | −0.96 to −6.50 | 0.71 to −6.06 | 6.43 to −5.14 | 5.39 to −6.07 | 6.18 to −5.36 |
| F3 to F2, 0%–10% | 8.09% | 13.50 to −6.19 | 20.26 to −8.67 | 15.38 to −6.89 | 3.85 to −1.67 | 4.41 to −3.01 | 3.99 to −1.99 |
| CC to F3, 0%–10% | 7.63% | −1.24 to −3.68 | −0.95 to −5.27 | −1.16 to −4.12 | 8.92 to −3.22 | 9.55 to −4.47 | 9.07 to −3.52 |
| Change (%) in Cumulative NASH attributable Healthcare Cost (lower to upper bound) | Change (%) in NASH attributable Healthcare Cost per Patient in 2039 (%) (lower to upper bound) | ||||||
| APC, 0%–9.7% | 1.93% | −8.59 to 59.43 | −7.25 to 48.93 | −8.23 to 56.56 | 3.33 to −11.09 | 2.93 to −10.34 | 4.25 to −13.67 |
| Transition Probabilities | |||||||
| Progression | |||||||
| F0 to F1, 0%–10% | 5.78% | 1.02 to −1.16 | 0.45 to −0.87 | 0.86 to −1.08 | 0.46 to −0.99 | −0.31 to −0.68 | 0.28 to −0.92 |
| F1 to F2, 0%–10% | 2.97% | −1.30 to 1.60 | −0.96 to 1.07 | −1.21 to 1.45 | −2.54 to 3.33 | −2.24 to 2.82 | −2.47 to 3.21 |
| F2 to F3, 0%–10% | 3.93% | −5.53 to 5.86 | −3.68 to 3.68 | −5.02 to 5.27 | −7.32 to 7.45 | −4.86 to 4.59 | −6.71 to 6.75 |
| F3 to CC, 0%–15% | 10.55% | −15.22 to 4.60 | −11.14 to 4.01 | −14.11 to 4.44 | −17.51 to 5.13 | −13.93 to 4.90 | −16.7 to 5.12 |
| CC to DCC, 0%–15% | 9.29% | −3.96 to 1.46 | −2.55 to 1.29 | −3.58 to 1.41 | −4.76 to 1.66 | −3.18 to 1.51 | −4.59 to 1.70 |
| Regression | |||||||
| F1 to F0, 0%–10% | 3.23% | −1.06 to −0.18 | −0.90 to −0.47 | −1.02 to −0.26 | −1.12 to −0.53 | −0.96 to −0.95 | −1.09 to −0.63 |
| F2 to F1, 0%–10% | 5.45% | 0.78 to −1.66 | 0.09 to −1.24 | 0.59 to −1.55 | 2.02 to −2.59 | 0.82 to −1.97 | 1.78 to −2.48 |
| F3 to F2, 0%–10% | 8.09% | 3.31 to −1.47 | 3.72 to −1.53 | 3.42 to −1.49 | 4.11 to −1.78 | 4.93 to −1.96 | 4.35 to −1.83 |
| CC to F3, 0%–10% | 7.63% | 5.40 to −2.26 | 5.37 to −2.16 | 5.39 to −2.23 | 6.53 to −2.69 | 7.27 to −2.77 | 6.76 to −2.73 |
APC, annual percent change; NASH, non-alcoholic steatohepatitis.
Data displayed as % change from the outputs of a model using a base value (lower to upper bound).
Lower bound denotes % change from the outputs of a model using a parameter of 0%.
Upper bound denotes % change from outputs of a model using a parameter of the highest value in the specified range.
Discussion
Our economic model forecasts that NASH cases in the United States will increase by +82.6%. In this context, both the obese and non-obese NASH groups will experience substantial increases. Interestingly, the fastest growth in prevalent NASH cases will occur among the younger group who are between 18 and 44 years old. This rapid growth in the younger age group is consistent with the current trend in the growth of obesity in the United States.52 In addition to the prevalent cases, we also project a substantial increase in the number of cases of NASH-related advanced liver disease (F3, F4, DCC, and HCC), liver transplantation, and death (both cardiac- and liver-specific deaths). Again, this increase was greatest among the younger group. Although the increases were noted in both obese and non-obese NASH, the proportion of the increase driven by the obese NASH was substantially higher. In this context, by 2030, 82% of liver transplant subjects with NASH are expected to be obese. These trends are highly concerning not only because of the trajectory of obesity in next decades, but also because it impacts the younger age group in their most productive years of life.
In addition to the adverse clinical outcomes, costs associated with NASH (both obese and the non-obese) will be substantial. As expected, these costs are growing in every age group over the next 2 decades. It is important to recognize that these are direct costs and do not include indirect costs associated with work productivity and impairment of patients' health-related quality of life.
Together, our results show that the clinical and cost burden of NASH is tremendous and should be considered a public health concern, requiring the immediate attention of both policy and clinical decision makers.55 Given that obesity plays a significant role in the development of adverse outcomes of NASH,53, 54, 56 we purposefully provided both age-specific and obesity-specific data to assist and to help clinicians and policy makers target interventions based on age groups with the presence or absence of obesity. In this context, we suggest that attention must be focused towards prevention and early identification of high-risk patients with NAFLD and NASH. As such, a number of recommendations can be considered to address obesity.
First, since the consumption of a diet high in fructose, fats, and sugar, as well as living in an environment that is not conducive to healthy living all play a significant role in the development of NAFLD, improvements in the food environment are imperative. This should include easy access to healthy and reasonably priced food choices and safe places to exercise. Also, schools should provide healthy food choices and safe and age-appropriate areas for exercise and play all year and not just when school is in session.27,35,57,58,59,60 Another important step is early identification of high risk NAFLD patients. In this context, addressing the cardiometabolic risk factors associated with NAFLD will be critical. This risk stratification to identify high risk NAFLD can be done through algorithms and the use of non-invasive tests18 Finally, efforts to raise awareness of this liver disease among the public and healthcare should continue, and be enhanced to ensure success of prevention and treatment interventions.
Our study has several strengths. First, our model structure was designed to comprehensively project obese or normal/overweight NASH burden of disease by simulating movement through incidence, progression, and regression across prevalent NASH patient age bands by interlinking Markov chains in order to generate the most accurate projection in a real-world setting. Given that reliable, prospective, and large scale natural history data for NASH were not available, TPs between states by age–obesity group were estimated. A crucial step in this process was to split aggregated deaths and TPs for all NASH patients into age–obesity specific groups, using the observed age–obesity pattern from national population data. Second, we adjusted data to ensure that the model inputs/outputs aligned with the available reported data from population-based data, such as the GBD and OPTN/SRTR annual data. A unique aspect of the current analysis was that it accounts for all-cause, liver-specific, and cardiac-specific mortality in NASH population, using a sufficient follow-up period for mortality (median of 23 years) and a representative sample of the US population. In addition, we conducted sensitivity analysis and found that our results remained robust, where if the current trends remain, the obese younger NASH patients were most sensitive to an increase in incident NASH cases, which will demonstratively increase all measured outcomes to include costs. On the other hand, if there are no changes in the incidence of NASH, there may actually be a decrease in all measure outcomes. Finally, in this model, mortality was based on population-based data (NHANES), whereas in previous models, mortality was based on data published from tertiary care sources with substantial selection bias.49 In this context, the old models may have overestimated liver related mortality and underestimated cardiovascular mortality among NASH. These are very important differences which will need acknowledgement to help target interventions to provide the most benefit.
Despite these strengths, our study has certain limitations. First, we did not have population-based data on the annual incidence rate of NASH, so it was calculated by maintaining a current trend in NASH prevalence. As such, we suggest that further prospective longitudinal research is required to estimate the current and predicted rise in incidence of NASH more accurately. Second, although we used a nationally representative population-based database (NHANES III) to obtain mortality data for NASH population, the data were collected between 1988 and 1994 when the prevalence of obesity, diabetes, and metabolic abnormalities were most probably much lower than today, which may have resulted in lower mortality for the current NASH population. Third, the inherent assumption of “lack of memory” within a Markov transition state implies that the probability of moving from one state to another is independent of the experiences of previous cycles, which could change the model's outcomes. To minimize the effects of this assumption, we changed the TPs to account for the population aging as they moved through the model. Fourth, although we inflated future costs at a rate of 3% annually, we did not take into account the potential for additional increase in the cost of health care, and the future costs for each state of advanced liver disease will be more expansive.
In conclusion, this study projects a significant increase in the clinical and economic burden of NASH in the United States. In fact, these increases are most prominent in the younger population. As such, policy makers must consider NAFLD as an urgent public health issue, and prioritize interventions that can help reverse the current trajectory of this liver disease. There should also be a comprehensive program to increase awareness of this disease, and the need to use easy-to-use diagnostic tools to identify those most at risk for adverse outcomes. These patients should be the targeted for close clinical monitoring and therapeutic interventions as they develop.
Guarantor of article
Zobair M. Younossi is the guarantor of this work and had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final version of the manuscript.
Data availability
All data are public and submitted with the publication.
Credit authorship contribution statement
Zobair M. Younossi: study design, data interpretation, manuscript writing, and critical review of final manuscript.
James M. Paik: study design, data collection, statistical analysis, data interpretation, manuscript writing, and critical review of final manuscript.
Linda Henry: data interpretation, manuscript writing, and critical review of final manuscript.
Joe Yang: data interpretation andcritical review of final manuscript.
Gail Fernandes: critical review of final manuscript.
Maria Stepanova: statistical analysis and critical review of final manuscript.
Fatema Nader: study coordination and critical review of final manuscript.
Given his role as guest editor, Zobair M. Younossi, MD, was not involved in the peer-review of this article and had no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Ancil Anand, MD.
Conflicts of interest
Dr. Younossi is a consultant to BMS, Gilead, Intercept, NovoNordisk, Novartis, Merck, and Siemens. Joe Yang and Gail Fernandes are employees of Merck & Co., Inc. All other authors have no conflict of interest to disclose.
Funding
Merck and Co.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2022.12.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization (WHO) Facts on obesity. https://www.who.int/health-topics/obesity#tab=tab_1 Obtained from the world wide web at:
- 2.Center for Disease Control (CDC) Facts on obesity. https://www.cdc.gov/obesity/adult/defining.html Obtained from the world wide web at:
- 3.Ward Z.J., Bleich S.N., Cradock A.L., et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019 Dec 19;381:2440–2450. doi: 10.1056/NEJMsa1909301. PMID: 31851800. [DOI] [PubMed] [Google Scholar]
- 4.Center for Disease Control (CDC) Facts on childhood obesity. https://www.cdc.gov/vitalsigns/childhoodobesity/index.html Obtained from the world wide web at:
- 5.Simmonds M., Llewellyn A., Owen C.G., Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. 2016 Feb;17:95–107. doi: 10.1111/obr.12334. Epub 2015 Dec 23. PMID: 26696565. [DOI] [PubMed] [Google Scholar]
- 6.Okunogbe A., Nugent R., Spencer G., et al. Economic impacts of overweight and obesity: current and future estimates for eight countries. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO) World Obesity Day. https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity Obtained from the world wide web at:
- 8.Manne V., Handa P., Kowdley K.V. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin Liver Dis. 2018 Feb;22:23–37. doi: 10.1016/j.cld.2017.08.007. Epub 2017 Oct 18. PMID: 29128059. [DOI] [PubMed] [Google Scholar]
- 9.Powell E.E., Wong V.W., Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021 Jun 5;397:2212–2224. doi: 10.1016/S0140-6736(20)32511-3. Epub 2021 Apr 21. PMID: 33894145. [DOI] [PubMed] [Google Scholar]
- 10.Eslam M., Valenti L., Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol. 2018 Feb;68:268–279. doi: 10.1016/j.jhep.2017.09.003. Epub 2017 Nov 6. PMID: 29122391. [DOI] [PubMed] [Google Scholar]
- 11.Younossi Z., Anstee Q.M., Marietti M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018 Jan;15:11–20. doi: 10.1038/nrgastro.2017.109. Epub 2017 Sep 20. PMID: 28930295. [DOI] [PubMed] [Google Scholar]
- 12.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016 Jul;64:73–84. doi: 10.1002/hep.28431. Epub 2016 Feb 22. PMID: 26707365. [DOI] [PubMed] [Google Scholar]
- 13.Fazel Y., Koenig A.B., Sayiner M., Goodman Z.D., Younossi Z.M. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016 Aug;65:1017–1025. doi: 10.1016/j.metabol.2016.01.012. Epub 2016 Jan 29. PMID: 26997539. [DOI] [PubMed] [Google Scholar]
- 14.Bertot L.C., Adams L.A. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17:774. doi: 10.3390/ijms17050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekstedt M., Nasr P., Kechagias S. Natural history of NAFLD/NASH. Curr Hepatol Rep. 2017;16:391–397. doi: 10.1007/s11901-017-0378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Hara J., Finnegan A., Dhillon H., et al. Cost of non-alcoholic steatohepatitis in Europe and the USA: the GAIN study. JHEP Rep. 2020 Jul 15;2:100142. doi: 10.1016/j.jhepr.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schattenberg J.M., Lazarus J.V., Newsome P.N., et al. Disease burden and economic impact of diagnosed non-alcoholic steatohepatitis in five European countries in 2018: a cost-of-illness analysis. Liver Int. 2021 Jun;41:1227–1242. doi: 10.1111/liv.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Younossi Z.M., Anstee Q.M., Wai-Sun Wong V., et al. The association of histologic and noninvasive tests with adverse clinical and patient-reported outcomes in patients with advanced fibrosis due to nonalcoholic steatohepatitis. Gastroenterology. 2021 Apr;160:1608–1619.e13. doi: 10.1053/j.gastro.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Younossi Z.M., Blissett D., Blissett R., et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016 Nov;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 20.Golabi P., Otgonsuren M., Cable R., et al. Non-alcoholic Fatty Liver Disease (NAFLD) is associated with impairment of Health Related Quality of Life (HRQOL) Health Qual Life Outcomes. 2016 Feb 9;14:18. doi: 10.1186/s12955-016-0420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McSweeney L., Breckons M., Fattakhova G., et al. Health-related quality of life and patient-reported outcome measures in NASH-related cirrhosis. JHEP Rep. 2020 Mar 6;2:100099. doi: 10.1016/j.jhepr.2020.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagström H., Nasr P., Ekstedt M., et al. Health care costs of patients with biopsy-confirmed nonalcoholic fatty liver disease are nearly twice those of matched controls. Clin Gastroenterol Hepatol. 2020 Jun;18:1592–1599.e8. doi: 10.1016/j.cgh.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Gordon S.C., Fraysse J., Li S., Ozbay A.B., Wong R.J. Disease severity is associated with higher healthcare utilization in nonalcoholic steatohepatitis Medicare patients. Am J Gastroenterol. 2020;115:562–574. doi: 10.14309/ajg.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 24.Kanwal F., Kramer J.R., Mapakshi S., et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018 Dec;155:1828–1837.e2. doi: 10.1053/j.gastro.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Younossi Z.M., Marchesini G., Pinto-Cortez H., Petta S. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Transplantation. 2019 Jan;103:22–27. doi: 10.1097/TP.0000000000002484. [DOI] [PubMed] [Google Scholar]
- 26.Younossi Z.M., Stepanova M., Ong J., et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2021 Mar;19:580–589. doi: 10.1016/j.cgh.2020.05.064. e5. [DOI] [PubMed] [Google Scholar]
- 27.Paik J.M., Mir S., Alqahtani S.A., Younossi Y., Ong J.P., Younossi Z.M. Dietary risks for liver mortality in NAFLD: global burden of disease data. Hepatol Commun. 2022 Jan;6:90–100. doi: 10.1002/hep4.1707. Epub 2021 Jul 8. PMID: 34558838; PMCID: PMC8710798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paik J.M., Kabbara K., Eberly K.E., Younossi Y., Henry L., Younossi Z.M. Global burden of NAFLD and chronic liver disease among adolescents and young adults. Hepatology. 2021 Nov 6 doi: 10.1002/hep.32228. Epub ahead of print. PMID: 34741554. [DOI] [PubMed] [Google Scholar]
- 29.Golabi P., Paik J.M., Eberly K., de Avila L., Alqahtani S.A., Younossi Z.M. Causes of death in patients with Non-alcoholic Fatty Liver Disease (NAFLD), alcoholic liver disease and chronic viral Hepatitis B and C. Ann Hepatol. 2022 Jan-Feb;27 doi: 10.1016/j.aohep.2021.100556. Epub 2021 Nov 18. PMID: 34800721. [DOI] [PubMed] [Google Scholar]
- 30.Vilar-Gomez E., Calzadilla-Bertot L., Wai-Sun Wong V., et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018 Aug;155:443–457.e17. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 31.Angulo P., Kleiner D.E., Dam-Larsen S., et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haflidadottir S., Jonasson J.G., Norland H., etal Long-term follow-up and liver-related death rate in patients with non-alcoholic and alcoholic related fatty liver disease. BMC Gastroenterol. 2014 Sep 27;14:166. doi: 10.1186/1471-230X-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golabi P., Paik J.M., Arshad T., Younossi Y., Mishra A., Younossi Z.M. Mortality of NAFLD according to the body composition and presence of metabolic abnormalities. Hepatol Commun. 2020 May 19;4:1136–1148. doi: 10.1002/hep4.1534. PMID: 32766474; PMCID: PMC7395070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalasani N., Younossi Z., Lavine J.E., et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018 Jan;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 35.Younossi Z.M., Corey K.E., Lim J.K. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2021 Feb;160:912–918. doi: 10.1053/j.gastro.2020.11.051. Epub 2020 Dec 9. PMID: 33307021. [DOI] [PubMed] [Google Scholar]
- 36.Alqahtani S.A., Paik J.M., Biswas R., Arshad T., Henry L., Younossi Z.M. Poor awareness of liver disease among adults with NAFLD in the United States. Hepatol Commun. 2021 Nov;5:1833–1847. doi: 10.1002/hep4.1765. Epub 2021 Jul 1. PMID: 34558829; PMCID: PMC8557315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le M.H., Yeo Y.H., Cheung R., Wong V.W., Nguyen M.H. Ethnic influence on nonalcoholic fatty liver disease prevalence and lack of disease awareness in the United States, 2011-2016. J Intern Med. 2020 Jun;287:711–722. doi: 10.1111/joim.13035. Epub 2020 Mar 4. PMID: 32128904. [DOI] [PubMed] [Google Scholar]
- 38.Husereau D., Drummond M., Petrou S., et al. CHEERS Task Force Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health Care. 2013 Apr;29:117–122. doi: 10.1017/S0266462313000160. [DOI] [PubMed] [Google Scholar]
- 39.2017 National Population Projections Datasets- Projections for the United States: 2017 to 2060. https://www.census.gov/data/datasets/2017/demo/popproj/2017-popproj.html Obtained from the world wide web at:
- 40.National Institute of Health Body Mass Index. https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi_tbl.htm Obtained from the world wide web at:
- 41.Kwong A.J., Kim W.R., Lake J.R., et al. OPTN/SRTR 2019 annual data report: liver. Am J Transplant. 2021;21:208–315. doi: 10.1111/ajt.16494. [DOI] [PubMed] [Google Scholar]
- 42.Nyberg L.M., Cheetham T.C., Patton H.M., et al. The natural history of NAFLD, a community-based study at a large health care delivery system in the United States. Hepatol Commun. 2021;5:83–96. doi: 10.1002/hep4.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Global Burden of Disease Collaborative Network . Institute for Health Metrics and Evaluation (IHME); Seattle, United States: 2020. Global Burden of Disease Study 2019 (GBD 2019) Results.http://ghdx.healthdata.org/gbd-results-tool Available from: [Google Scholar]
- 44.Joinpoint Regression Program, Version 4.9.1.0 - April 2022; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute.
- 45.Golabi P., Paik J.M., Herring M., Younossi E., Kabbara K., Younossi Z.M. Prevalence of high and moderate risk nonalcoholic fatty liver disease among adults in the United States, 1999-2016. Clin Gastroenterol Hepatol. 2021 Dec 17 doi: 10.1016/j.cgh.2021.12.015. S1542-3565(21)01339-2. [DOI] [PubMed] [Google Scholar]
- 46.Singh S., Allen A.M., Wang Z., Prokop L.J., Murad M.H., Loomba R. Fibrosis progression in nonalcoholic fatty liver versus nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654. doi: 10.1016/j.cgh.2014.04.014. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newsome P.N., Buchholtz K., Cusi K., et al. NN9931-4296 Investigators A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021 Mar 25;384:1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 48.Harrison S.A., Wong V.W., Okanoue T., et al. STELLAR-3 and STELLAR-4 Investigators Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol. 2020 Jul;73:26–39. doi: 10.1016/j.jhep.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 49.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018 Jan;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Hara J., Finnegan A., Dhillon H., et al. Cost of non-alcoholic steatohepatitis in Europe and the USA: the GAIN study. JHEP Rep. 2020 Jul 15;2 doi: 10.1016/j.jhepr.2020.100142. PMID: 32775976; PMCID: PMC7397699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McAdam-Marx C., McGarry L.J., Hane C.A., Biskupiak J., Deniz B., Brixner D.I. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. JMCP. 2011;17:531–546. doi: 10.18553/jmcp.2011.17.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease Control (CDC) Age Obesity facts. https://nccd.cdc.gov/dnpao_dtm/rdPage.aspx?rdReport=DNPAO_DTM.ExploreByTopic&islClass=OWS&islTopic=&go=GO Accessed form the world wide web at:
- 53.Younossi Z.M., Henry L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 2021 May 11;3 doi: 10.1016/j.jhepr.2021.100305. PMID: 34189448; PMCID: PMC8215299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singal A.G., El-Serag H.B. Rational HCC screening approaches for patients with NAFLD. J Hepatol. 2022 Jan;76:195–201. doi: 10.1016/j.jhep.2021.08.028. Epub 2021 Sep 9. PMID: 34508791; PMCID: PMC8688224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Younossi Z.M. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol. 2019 Mar;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 56.Younossi Z.M., Tampi R., Priyadarshini M., Nader F., Younossi I.M., Racila A. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology. 2019 Feb;69:564–572. doi: 10.1002/hep.30254. [DOI] [PubMed] [Google Scholar]
- 57.Lazarus J.V., Mark H.E., Anstee Q.M., et al. NAFLD Consensus Consortium Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2022 Jan;19:60–78. doi: 10.1038/s41575-021-00523-4. [DOI] [PubMed] [Google Scholar]
- 58.WHO Global Strategy on Diet, Physical Activity and Health- Global action plan on physical activity 2018–2030: more active people for a healthier world. https://www.who.int/publications/i/item/9241592222 Obtained from the world wide web at:
- 59.Healthy People 2030 Objective and Goals. https://health.gov/healthypeople/objectives-and-data Obtained from the world wide web at:
- 60.Healthy People 2030 Social Determinants of Health. https://health.gov/healthypeople/objectives-and-data/social-determinants-health Obtained from the world wide web at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are public and submitted with the publication.