STRUCTURED ABSTRACT
Purpose of review
To review novel modalities for interrogating a kidney allograft biopsy to complement the current Banff schema.
Recent Findings
Newer approaches of Artificial Intelligence (AI), Machine Learning (ML), digital pathology including Ex Vivo Microscopy, evaluation of the biopsy gene expression using bulk, single cell and spatial transcriptomics and spatial proteomics are now available for tissue interrogation.
Summary
Banff Schema of classification of allograft histology has standardized reporting of tissue pathology internationally greatly impacting clinical care and research. Inherent sampling error of biopsies, and lack of automated morphometric analysis with ordinal outputs limit its performance in prognostication of allograft health. Over the last decade, there has been an explosion of newer methods of evaluation of allograft tissue under the microscope. Digital pathology along with the application of AI and ML algorithms could revolutionize histopathological analyses. Novel molecular diagnostics such as spatially resolved single cell transcriptomics are identifying newer mechanisms underlying the pathologic diagnosis to delineate pathways of immunological activation, tissue injury, repair, and regeneration in allograft tissues. While these techniques are the future of tissue analysis, costs and complex logistics currently limit their clinical use.
Keywords: Digital Pathology, Machine Learning, Transcriptomics, Imaging Mass Spectrometry
Introduction
Kidney allograft biopsy is the current gold standard to identify the cause of allograft dysfunction. Histology of biopsy along with clinical data provides a snapshot into organ health. In 1993, Solez et al pioneered the Banff working classification of kidney transplant pathology (1). They compartmentalized kidney tissue lesions and converted injury metrics into ordinal lesion scores, allowing for standardized reporting. This landmark achievement influenced international clinical practice and research. Banff schema is regularly updated to reflect the transplant communities’ current understanding of allograft pathology (elegantly summarized by Loupy et al. (2)).
While Banff-schema helps semi-quantitative grading of immunological injury, recurrent disease, infectious complications and informs cumulative irreversible allograft damage, it is more limited in its prognostic potential. A recent advance in prognostication was the iBOX score, which combined orthogonal clinical data with Banff lesion scores (3). However, Banff scores themselves have shown a large degree of inter-observer variability among reporting pathologists (4), with greater variability across international centers (5). Additional issues are the potential for sampling error inherent to any biopsy along with the lack of automatic application of morphometric quantification – which offers advantages over histology alone (6). Frequent implementation of Banff updates (an admirable approach) may also not readily translate into routine practice. Finally, the Banff schema is a morphologic classification, and novel pathogenetic insights using emerging technologies are limited in their incorporation.
The goal of this review is to discuss the application of novel technologies to the interrogation of tissue that may potentially be applied to allograft biopsy [Fig-1].
Figure 1 :
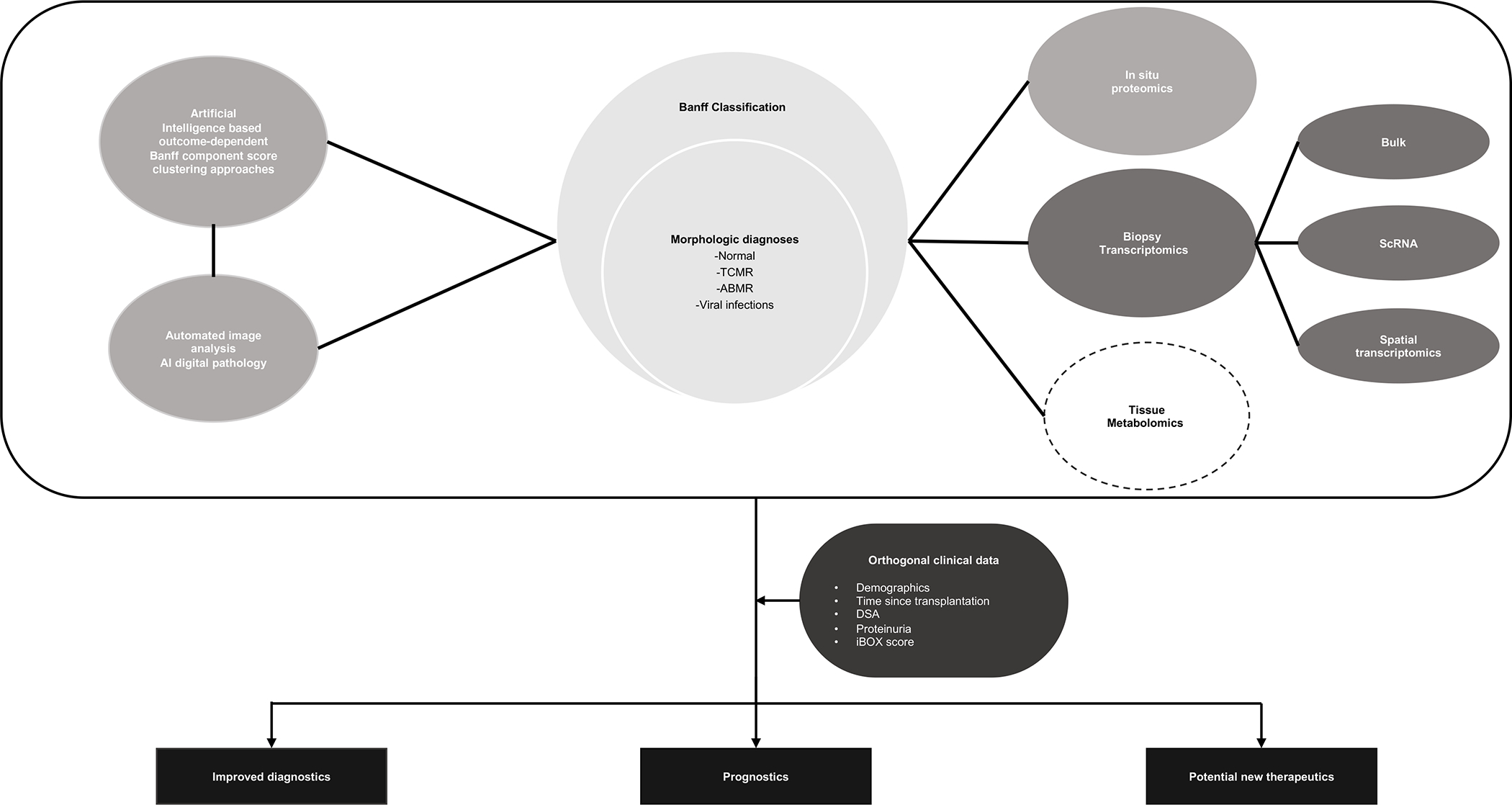
Available histopathologic information from allograft biopsies is interpreted using Banff classification which is limited by sampling error, interobserver variability, and inability to prognosticate future graft function. Described in the image are tissue interrogation techniques (including AI, automated image analysis, in situ proteomics, biopsy transcriptomics, tissue metabolomics) being studied to improve upon our current limitations and in combination with orthogonal clinical data, better inform diagnostic, prognostic, and therapeutic options for the future.
( ABMR: Antibody mediated rejection, AI: Artificial intelligence, DSA: Donor specific antibodies, ScRNA: Single cell RNA, TCMR: T-cell mediated rejection)
Artificial Intelligence-based reporting and Digital Pathology
Digital pathology refers to the combination of automated techniques applied to anatomic pathology, including algorithms for imaging and analysis that use artificial intelligence (AI) / machine learning (ML).
Machine Learning applied to Banff scores
The Banff schema evolved iteratively with expert consensus, using studies that mapped intercorrelated component lesion scores to known diagnoses to classify a diagnostic cluster – for instance T cell–mediated rejection. However, there exists clear prognostic heterogeneity within these ‘supervised’ Banff diagnostic clusters (3), and individual lesion scores are not specific to diagnostic clusters. Recent work has overlaid pathologist-reported Banff lesion scores with graft survival data using ML methods (7, 8). ML can analyze large datasets using algorithms to learn relationship patterns among variables, with ability to make predictions on new data, and potential to improve with each iteration (8).
By weighting acute lesion scores based on their individual associations with graft survival during modelling, Vaulet et al, identified six novel clusters within the same Banff histologic rejection diagnosis using ~7000 biopsies on 936 recipients from multiple centers (9). This work applied K-means clustering to Banff scores – an unsupervised ML algorithm – in a data-driven but semi-supervised approach to extract clinically meaningful clusters within traditional rejection diagnoses. They extended this work and applied semi-supervised approach to chronic Banff lesion scores (cg, ci, ct, cv, mm, and ah), weighting these for time from transplant as well as association with graft loss, to identify 4 prognostically distinct clusters(10). Unsurprisingly, high Cg score cluster-4 representing advanced transplant glomerulopathy, associated with the worst graft survival, and increased cumulative chronicity scores were proportional to graft loss risk. Combining acute- and chronic- lesion score data, in a prediction model suggested both lesion scores bring incremental value to ascertainment of graft loss. Together, these datasets highlight insights revealed by AI, complementing natural intelligence-based Banff-score clustering.
Automated Digital Pathology Combined with Deep Learning
Increasing use of convolutional neural networks (CNNs) to identify histological features, with limited guidance from pathologist, may offer an alternative to traditional image processing and manual Banff scoring. CNNs are a type of deep learning neural network that are particularly suited for image analysis which are widely used in medical imaging and oncological pathology (11).
Hermsen et al addressed the applicability of CNNs to kidney allograft by comparing CNN-based quantification of structures to visually scored histological components using Banff. CNNs achieved accurate segmentation of glomeruli, tubuli and interstitium in transplant biopsies with a high degree of correlation to the visual score of three independent pathologists (12). Kers et al used 5844 biopsies, to evaluate CNNs to classify biopsies as normal, rejection, or other diseases. Serial CNNs first classified biopsies as either normal or disease, followed by a second CNN that classified biopsies previously classified into disease further into rejection or other disease (13). Subsequent serial CNNs could be trained to pick up other rare pathological features such as BK polyoma virus nephropathy etc. We recently reported a deep learning-based pipeline using an instance-level object detection algorithm mask Region-based Convolution Neural Network (R-CNN) applied to pre- or post-transplant surveillance biopsies from two independent cohorts GoCAR (Genomics of Chronic Renal Allograft Rejection) and AUSCAD (Australian Chronic Allograft Dysfunction). The digital features from WSI quantified normal tissue compartments, annotated abnormalities, and correlated with Banff scores with enhanced sensitivity for subtle pathological changes below the thresholds of the Banff scores. The Interstitial and Tubular Abnormality Score (ITAS) was predictive of 12-month graft loss in pre-transplant samples and the Composite Damage Score (DMS) applied at the 12-month mark successfully predicted later graft loss. Using deep learning, the ITAS and DMS scores outperformed Banff scores or clinical predictors, with superior graft loss prediction accuracy (14).
An important advantage of using the automated digital pathology is the ability to provide continuous data instead of ordinal lesion scores. This improves the sensitivity to identify graft damage and could empower personalized risk-assessment. The Banff Digital Pathology Working Group’s report remarked that digitalization is not just an end in itself but a precondition for application of AI approaches to improve tissue recognition (15). While WSI is available at near 70% of US centers, the painstaking work involved in annotating lesions for ML-algorithms is a hindrance. An online resource where WSI images can be uploaded to generate automated reports which are then curated by pathologists, may be a future direction (eg https://github.com/SarderLab). Another suggested use of ML is as a prioritization tool to identify biopsies for expedited review for rejection and sorting out completely healthy samples for a later review. The combination of WSI and deep learning ML approaches (CNNs) is a next immediate frontier in allograft histopathological analyses.
Clearing Histology with MultiPhoton Microscopy ( CHiMP )
Clearing Histology with Multiphoton Microscopy ( CHiMP) is a new tissue processing and imaging method that uses ex vivo multiphoton (EVM) microscopy imaging generating optical rather than physical slices for primary diagnosis without the need for paraffin-embedding or microtome cutting, resulting in rapid image processing and result generation. This approach has been validated and discussed by Torres et al in a case series of human prostate biopsies with ongoing work in transplanted kidney allografts (16). Notably, immunohistochemical analysis is not compromised by this method. With a faster turnaround time and direct path to digital imaging, EVM promises rapid global consultations, reduced labor requirements, analysis of more tissue levels and providing 3D histology patterns. Additionally, it helps preserve tissues for further ancillary studies as well. There are ongoing efforts to validate and standardize this process in the kidney transplants specifically (17). We foresee the use of this technique in rapid assessments of WSI and remote expert consultations of procurement biopsies in real time as well.
Interrogating Biopsy Gene Expression
Bulk transcriptomics
Bulk transcriptomics from biopsy tissue, microarray or tissue RNA sequencing (RNA-seq) has been widely adopted in kidney transplant research to offer diagnostic and prognostic insights by comparing transcriptome-wide gene expression profiles (GEP) between different disease states (18) (summarized by prior reviews (19, 20)). Early studies used cDNA micro-arrays and showed that rejection was transcriptionally distinct from “no rejection” (21). In TCMR, subsequent studies have unraveled transcriptomic differences in morphologically similar TCMR-like lesions (22), the disappearance of TCMR signatures after ~10 post-transplant years (23), and helped classify Banff borderline lesions (24). Biopsy transcriptomes have had greatest impact on informing ABMR pathogenesis. A key advance was the identification of intra-graft endothelial cell activation transcripts (ENDATs) that are now recognized to accompany ABMR even when C4d staining was undetectable (25), thus providing a mechanistic link to underlying effector mechanisms in ABMR. The Banff schema subsequently included both histologic as well as transcriptomic endothelial activation signatures in ABMR diagnostic criteria in 2013 update (6, 26).
Using indication biopsies with microarray GEPs, the Edmonton group have distilled gene signature “classifiers”, each based on the differential expression of key pathogenesis-based transcripts that quantify different aspects of ongoing allograft injury generating individual scores for ABMR, TCMR, AKI etc. (27). This ‘molecular microscope’ (MMDx) generated automated reports in a multinational cohort with high agreement with pathologic diagnoses, albeit with some residual disagreement (28, 29). Interestingly MMDx signouts were reported to more frequently align with clinician judgement and increase confidence in management.
GEPs have also provided novel insights into immunological pathogenesis, for instance, that of isolated vasculitis (v-lesions) (30). In DSA- and C4D-negative, transplant glomerulopathy (TG), an enrichment of cytotoxic T-cell-associated transcripts was observed, suggesting T cell activation as an unexpected mechanism of injury in these cases, distinct from TG that were DSA-positive (31). While IF/TA without histologic rejection is seen in 30–40% of allografts that fail (32), GEPs analyzed by co-expression network analyses, revealed near 80% overlap with TCMR biopsies, intriguingly suggesting that TCMR activity not detected by histology may underlie IF/TA (33). GEPs from total biopsy RNA, subjected to deconvolution to estimate specific infiltrating cells, have demonstrated the central role of NK cells in ABMR injury (34), therapeutically relevant information. Our group (35), applied microarray GEPs for prognostication using 3-month surveillance biopsies. A 13-gene signature at 3-months was identified that was predictive of subsequent progressive allograft damage and graft loss, superior to Banff histologic scores. Prognostic signatures were identifiable in 6-month surveillance biopsies that correlated with graft loss and progressive damage (36).
While extensive allograft data (>4000 biopsies) have been generated using microarrays, these provide relative rather than absolute quantitation of gene expression, require an amplification step, and do not allow for gene/sequence quantification outside of the probe sets (for instance micro-RNA) (37–41). Another critical issue in biopsy transcriptome studies is the need to dedicate a biopsy core for transcriptomics, which is often not considered in routine clinical use.
B-HOT
The Banff Molecular Diagnostics Working Group created a multiorgan transplant gene panel – the Banff Human Organ Transplant (B-HOT). This panel consists of 770 targeted genes relevant to rejection, tolerance, viral infection, and immune response distilled from prior biopsy microarray studies in solid-organ transplantation, supplemented by pathway- and cell-type specific genes, along with 12 house-keeping genes. B-HOT represents two distinct advantages over prior work. (a) Validated performance in identifying dysregulated pathogenesis-based transcript sets from formalin-fixed paraffin embedded samples (when indirectly compared to microarray gene sets from fresh RNA-stabilized samples), making it possible for use in archived biopsies. (b) Use of the nanostring nCounter system – an automated GEP technology that uses pre-selected barcoded RNA-probes in each assay without necessitating an amplification step to quantify gene expression (42). Validation studies reported thus far with B-HOT are promising (43), but its commercial use is still limited. While standardizing good quality RNA extraction from paraffin samples represents an up-front challenge, the experience with this approach in breast cancer is encouraging (44).
Single Cell Transcriptomics
Bulk transcriptomics in biopsies demonstrated the utility of GEPs. However, biopsy GEPs are a crude reflection of overall transcriptomic perturbation and are influenced by the number and activation state of each of the individual cells. The diversity of infiltrating cells (originating primarily from recipients), and resident kidney cells of donor origin, makes attributing identified injury/response pathways to cell types in allograft GEPs challenging (45). Applying deconvolution approaches (CIBERSORT) may resolve transcriptomes of frequent cell-types (34). However, rarer cell types even with marked gene dysregulation, or new cellular differentiation states cannot be obtained from bulk RNA. Single Cell RNA sequencing (scRNA-seq) of the allograft biopsy offers a powerful advantage providing the ability to examine the transcriptome of individual cells within biopsies (comprehensively reviewed in (46)).
Initial comparison of single cell transcriptome of a mixed rejection biopsy vs control identified that classical TCMR-associated transcripts identified from bulk biopsy transcriptomes are over-expressed in T-cells, while most ABMR-enriched transcripts were of endothelial origin, consistent with ENDATs (47). Similarly in a chronic rejection/IF/TA biopsy, both immune cell and fibroblast populations were significantly enriched, with the myofibroblasts demonstrating enhanced canonical profibrotic signaling pathways including PDGF and TGF-beta (48). An exciting study used sc-RNAseq paired with donor and recipient whole-exome DNA sequencing to resolve donor- vs recipient-origin cells within five allograft biopsies. A surprising finding was the detection of donor-origin macrophages (to a smaller extent lymphocytes) months-years after transplantation (49). This study studied clonality of the infiltrating T-cells using V-D-J rearrangements of the T-cell receptors. While no clear enrichment of specific V-D-J sequences were identifiable in the small number of T-cells in this dataset, this demonstrated the feasibility of this approach to study T-cell clonality – a central question in allo-rejection. These and other data (50) show the tremendous potential of Sc-RNA-seq when applied to renal allograft biopsies.
Spatial Transcriptomics
While Sc-RNAseq can resolve cell constitution in a biopsy sample, inform activation state and infer cell state trajectory, it provides no context to the location of a cell within the tissue. Spatial transcriptomics methods map transcripts within spatially intact tissue sections, to overlay gene expression upon cell location and neighborhood. Multiple platforms have been reported in various organs generating excellent insights, for instance, in COVID-related collapsing glomerulopathy (51). Briefly these are based either on in-situ sequencing (ISS) or in-situ hybridization (ISH) (52). While ISH based methods like MERFISH can be performed on paraffin-embedded archived biopsies, ISS methods require dedicated fresh frozen sections. An exciting report evaluated a single rejection biopsy vs two controls (53), using Geo DSP, an ISH-based method using pre-selected photocleavable probes (54). Representative tubulitis vs normal regions-of-interest (ROIs) in rejection/control biopsies were selected using specific antibodies. This was followed by capture of RNA from each ROI, and nanostring quantification. This study confirmed enrichment of TCMR-related genes in tubulitis ROIs, but relayed variability in GEPs within these areas over and above expected from Banff scores. An important advantage of these methods is the ability to generate multiple data points of comparison from a single biopsy i.e., ROIs of tubulitis vs no tubulitis within a biopsy, to study gene dysregulation in specific morphologic areas. Another advantage is the computation of ligand-receptor interactions, which are most meaningful between neighboring cells.
Biopsy proteome: Imaging mass cytometry
The phenotype-determining cellular proteome is often discordant from the transcriptome (55, 56). Bulk or sc-RNA-seq transcriptional analyses do not offer a spatial context for their findings (47) (57). Imaging Mass Cytometry (IMC) is a novel, multiplexed and spatially preserved methodology for immunolabeling of paraffin embedded biopsy sections using metal-tagged antibodies directed against cell-type- or disease-specific proteins. IMC could provide for efficient interrogation of a scarce biopsy sample, permitting concurrent quantitation of more than 40 markers on a single tissue section with resultant reconstructions achieving 1-mm resolution (58) with cell-level proteomic data. Singh et al systematically validated an IMC antibody panel and an analysis pipeline (Kidney-MAPPS, multiplexed antibody-based profiling with preservation of spatial context) to characterize the spatial architecture of normal kidney cells, benchmarking baseline resident and infiltrating cells (59).Their group used IMC in pre-implantation biopsies of high-risk deceased donor kidney transplants to successfully predict delayed graft function (60) identifying tubular cell dropout(59). With validation of IMC panels targeting specific immune-cell subsets, this technique could be a potent addition to understanding allograft rejection and injury.
Conclusion
Based on morphometric analysis of histopathology slides, the current Banff classification system provides an excellent framework for evaluation of allograft dysfunction but, it has intrinsic limitations and possesses certain challenges. With the explosion of novel tissue interrogation techniques described here, there is an expanding opportunity to identify specific molecular mechanisms underlying the pathologic diagnosis, and delineate pathways of immunological activation, tissue injury, repair, and regeneration in each case. High costs and complex logistics currently limit the clinical application of several of these tools, but future work needs to incorporate these new technologies to layer pathogenetic insight onto pathologic features in allograft processes. This will ultimately enable better diagnosis, prognosis, and novel therapeutics enabling transplant professionals to develop a personalized roadmap to prolong allograft longevity in each individual patient.
Key Points.
Intrinsic limitations to allograft histopathology including Banff schema.
Novel complementary technologies for tissue interrogation reveal potential for better pathogenetic insight, diagnoses, and prognosis.
While technically and logistically challenging on precious biopsy tissue, these techniques are the future of tissue interrogation
Acknowledgements
MCM acknowledges Salary support from Natera Inc, and research support from NIH grants R01DK122164, and R01DK132274-01A1. The authors also wish to acknowledge the late Dr Barbara Murphy.
Financial Support and Sponsorship: No specific financial support for this publication.
Footnotes
Conflicts of Interest: None to report
Contributor Information
Sarthak Virmani, Section of Nephrology, Division of Internal Medicine, Yale University School of Medicine, New Haven, CT.
Arundati Rao, Section of Nephrology, Division of Internal Medicine, Yale University School of Medicine, New Haven, CT.
Madhav C Menon, Section of Nephrology, Division of Internal Medicine, Yale University School of Medicine, New Haven, CT.
References
- 1.Solez K, Axelsen RA, Benediktsson H, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993;44(2):411–22. [DOI] [PubMed] [Google Scholar]
- 2. Loupy A, Mengel M, Haas M. Thirty years of the International Banff Classification for Allograft Pathology: the past, present, and future of kidney transplant diagnostics. Kidney Int. 2022;101(4):678–91. *Elegant summary of the evolution of the Banff Classification Schema.
- 3.Loupy A, Aubert O, Orandi BJ, et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: international derivation and validation study. BMJ. 2019;366:l4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furness PN, Taub N, Convergence of European Renal Transplant Pathology Assessment Procedures P. International variation in the interpretation of renal transplant biopsies: report of the CERTPAP Project. Kidney Int. 2001;60(5):1998–2012. [DOI] [PubMed] [Google Scholar]
- 5.Furness PN, Taub N, Assmann KJ, et al. International variation in histologic grading is large, and persistent feedback does not improve reproducibility. Am J Surg Pathol. 2003;27(6):805–10. [DOI] [PubMed] [Google Scholar]
- 6.Solez K, Racusen LC. The Banff classification revisited. Kidney Int. 2013;83(2):201–6. [DOI] [PubMed] [Google Scholar]
- 7.Vasquez-Rios G, Menon MC. Kidney Transplant Rejection Clusters and Graft Outcomes: Revisiting Banff in the Era of “Big Data”. J Am Soc Nephrol. 2021;32(5):1009–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthukumar T, Anglicheau D. The ABCD of Kidney Allograft Pathology-The Beginning of the Beginning. J Am Soc Nephrol. 2022;33(11):1960–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaulet T, Divard G, Thaunat O, et al. Data-driven Derivation and Validation of Novel Phenotypes for Acute Kidney Transplant Rejection using Semi-supervised Clustering. J Am Soc Nephrol. 2021;32(5):1084–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaulet T, Divard G, Thaunat O, et al. Data-Driven Chronic Allograft Phenotypes: A Novel and Validated Complement for Histologic Assessment of Kidney Transplant Biopsies. J Am Soc Nephrol. 2022;33(11):2026–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–44. [DOI] [PubMed] [Google Scholar]
- 12.Hermsen M, de Bel T, den Boer M, et al. Deep Learning-Based Histopathologic Assessment of Kidney Tissue. J Am Soc Nephrol. 2019;30(10):1968–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kers J, Bulow RD, Klinkhammer BM, et al. Deep learning-based classification of kidney transplant pathology: a retrospective, multicentre, proof-of-concept study. Lancet Digit Health. 2022;4(1):e18–e26. [DOI] [PubMed] [Google Scholar]
- 14.Yi Z, Salem F, Menon MC, et al. Deep learning identified pathological abnormalities predictive of graft loss in kidney transplant biopsies. Kidney Int. 2022;101(2):288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farris AB, Moghe I, Wu S, et al. Banff Digital Pathology Working Group: Going digital in transplant pathology. Am J Transplant. 2020;20(9):2392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres R, Olson E, Homer R, et al. Initial Evaluation of Rapid, Direct-to-Digital Prostate Biopsy Pathology. Arch Pathol Lab Med. 2021;145(5):583–91. [DOI] [PubMed] [Google Scholar]
- 17.Perincheri S, Luciano R, Moeckel G, et al. Evaluation of a Direct-to-Digital Histology Method for Rapid Evaluation of Kidney Biopsies ASN Kidney Week 2020 Abstract ASN; 2020.
- 18.Huang S, Sheng X, Susztak K. The kidney transcriptome, from single cells to whole organs and back. Curr Opin Nephrol Hypertens. 2019;28(3):219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halloran PF, Famulski KS, Reeve J. Molecular assessment of disease states in kidney transplant biopsy samples. Nat Rev Nephrol. 2016;12(9):534–48. [DOI] [PubMed] [Google Scholar]
- 20. Menon MC, Keung KL, Murphy B, et al. The Use of Genomics and Pathway Analysis in Our Understanding and Prediction of Clinical Renal Transplant Injury. Transplantation. 2016;100(7):1405–14. * Our group’s review of use of GEP in kidney allograft assessment
- 21.Akalin E, Hendrix RC, Polavarapu RG, et al. Gene expression analysis in human renal allograft biopsy samples using high-density oligoarray technology. Transplantation. 2001;72(5):948–53. [DOI] [PubMed] [Google Scholar]
- 22.Reeve J, Sellares J, Mengel M, et al. Molecular diagnosis of T cell-mediated rejection in human kidney transplant biopsies. Am J Transplant. 2013;13(3):645–55. [DOI] [PubMed] [Google Scholar]
- 23.Halloran PF, Chang J, Famulski K, et al. Disappearance of T Cell-Mediated Rejection Despite Continued Antibody-Mediated Rejection in Late Kidney Transplant Recipients. J Am Soc Nephrol. 2015;26(7):1711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halloran PF, Pereira AB, Chang J, et al. Potential impact of microarray diagnosis of T cell-mediated rejection in kidney transplants: The INTERCOM study. Am J Transplant. 2013;13(9):2352–63. [DOI] [PubMed] [Google Scholar]
- 25.Sis B, Jhangri GS, Bunnag S, et al. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9(10):2312–23. [DOI] [PubMed] [Google Scholar]
- 26.Haas M. The relationship between pathologic lesions of active and chronic antibody-mediated rejection in renal allografts. Am J Transplant. 2018;18(12):2849–56. [DOI] [PubMed] [Google Scholar]
- 27.Halloran PF, Bohmig GA, Bromberg J, et al. Archetypal Analysis of Injury in Kidney Transplant Biopsies Identifies Two Classes of Early AKI. Front Med (Lausanne). 2022;9:817324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeve J, Bohmig GA, Eskandary F, et al. Generating automated kidney transplant biopsy reports combining molecular measurements with ensembles of machine learning classifiers. Am J Transplant. 2019;19(10):2719–31. [DOI] [PubMed] [Google Scholar]
- 29.Halloran PF, Reeve J, Akalin E, et al. Real Time Central Assessment of Kidney Transplant Indication Biopsies by Microarrays: The INTERCOMEX Study. Am J Transplant. 2017;17(11):2851–62. [DOI] [PubMed] [Google Scholar]
- 30.Salazar ID, Merino Lopez M, Chang J, et al. Reassessing the Significance of Intimal Arteritis in Kidney Transplant Biopsy Specimens. J Am Soc Nephrol. 2015;26(12):3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayde N, Bao Y, Pullman J, et al. The clinical and genomic significance of donor-specific antibody-positive/C4d-negative and donor-specific antibody-negative/C4d-negative transplant glomerulopathy. Clin J Am Soc Nephrol. 2013;8(12):2141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying Specific Causes of Kidney Allograft Loss. American Journal of Transplantation. 2009;9(3):527–35. [DOI] [PubMed] [Google Scholar]
- 33.Modena BD, Kurian SM, Gaber LW, et al. Gene Expression in Biopsies of Acute Rejection and Interstitial Fibrosis/Tubular Atrophy Reveals Highly Shared Mechanisms That Correlate With Worse Long-Term Outcomes. Am J Transplant. 2016;16(7):1982–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yazdani S, Callemeyn J, Gazut S, et al. Natural killer cell infiltration is discriminative for antibody-mediated rejection and predicts outcome after kidney transplantation. Kidney Int. 2019;95(1):188–98. [DOI] [PubMed] [Google Scholar]
- 35.O’Connell PJ, Zhang W, Menon MC, et al. Biopsy transcriptome expression profiling to identify kidney transplants at risk of chronic injury: a multicentre, prospective study. Lancet. 2016;388(10048):983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naesens M, Khatri P, Li L, et al. Progressive histological damage in renal allografts is associated with expression of innate and adaptive immunity genes. Kidney Int. 2011;80(12):1364–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thareja G, Yang H, Hayat S, et al. Single nucleotide variant counts computed from RNA sequencing and cellular traffic into human kidney allografts. Am J Transplant. 2018;18(10):2429–42. * The article reported Het/Hom ratio, based on single nucleotide polymorphism derived from bulk RNA-sequencing data of human allograft kidney tissue, as a novel measure of immune cell invasion of the kidney allograft.
- 38.McNulty MT, Fermin D, Eichinger F, et al. A glomerular transcriptomic landscape of apolipoprotein L1 in Black patients with focal segmental glomerulosclerosis. Kidney Int. 2022;102(1):136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banu K, Lin Q, Basgen JM, et al. AMPK mediates regulation of glomerular volume and podocyte survival. JCI Insight. 2021;6(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller FB, Yang H, Lubetzky M, et al. Landscape of innate immune system transcriptome and acute T cell-mediated rejection of human kidney allografts. JCI Insight. 2019;4(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben-Dov IZ, Muthukumar T, Morozov P, et al. MicroRNA sequence profiles of human kidney allografts with or without tubulointerstitial fibrosis. Transplantation. 2012;94(11):1086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mengel M, Loupy A, Haas M, et al. Banff 2019 Meeting Report: Molecular diagnostics in solid organ transplantation-Consensus for the Banff Human Organ Transplant (B-HOT) gene panel and open source multicenter validation. Am J Transplant. 2020;20(9):2305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith RN, Rosales IA, Tomaszewski KT, et al. Utility of Banff Human Organ Transplant Gene Panel in Human Kidney Transplant Biopsies. Transplantation. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373(21):2005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malone AF, Humphreys BD. Single-cell Transcriptomics and Solid Organ Transplantation. Transplantation. 2019;103(9):1776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Varma E, Luo X, Muthukumar T. Dissecting the human kidney allograft transcriptome: single-cell RNA sequencing. Curr Opin Organ Transplant. 2021;26(1):43–51. * Comprehensive review of how scRNAseq helps examine the transcriptome of individual cells within biopsies
- 47.Wu H, Malone AF, Donnelly EL, et al. Single-Cell Transcriptomics of a Human Kidney Allograft Biopsy Specimen Defines a Diverse Inflammatory Response. J Am Soc Nephrol. 2018;29(8):2069–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Hu J, Liu D, et al. Single-cell analysis reveals immune landscape in kidneys of patients with chronic transplant rejection. Theranostics. 2020;10(19):8851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malone AF, Wu H, Fronick C, et al. Harnessing Expressed Single Nucleotide Variation and Single Cell RNA Sequencing To Define Immune Cell Chimerism in the Rejecting Kidney Transplant. J Am Soc Nephrol. 2020;31(9):1977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suryawanshi H, Yang H, Lubetzky M, et al. Detection of infiltrating fibroblasts by single-cell transcriptomics in human kidney allografts. bioRxiv. 2022:2020.09.03.281733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith KD, Prince DK, Henriksen KJ, et al. Digital spatial profiling of collapsing glomerulopathy. Kidney Int. 2022;101(5):1017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li JSY, Raghubar AM, Matigian NA, et al. The Utility of Spatial Transcriptomics for Solid Organ Transplantation. Transplantation. 9900: 10.1097/TP.0000000000004466. [DOI] [PubMed] [Google Scholar]
- 53.Salem F, Perin L, Sedrakyan S, et al. The spatially resolved transcriptional profile of acute T cell-mediated rejection in a kidney allograft. Kidney Int. 2022;101(1):131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez S, Lazcano R, Serrano A, et al. Challenges and Opportunities for Immunoprofiling Using a Spatial High-Plex Technology: The NanoString GeoMx((R)) Digital Spatial Profiler. Front Oncol. 2022;12:890410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong F, Chen H, Xie Y, et al. Protein S Protects against Podocyte Injury in Diabetic Nephropathy. J Am Soc Nephrol. 2018;29(5):1397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghazalpour A, Bennett B, Petyuk VA, et al. Comparative analysis of proteome and transcriptome variation in mouse. PLoS Genet. 2011;7(6):e1001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu H, Kirita Y, Donnelly EL, et al. Advantages of Single-Nucleus over Single-Cell RNA Sequencing of Adult Kidney: Rare Cell Types and Novel Cell States Revealed in Fibrosis. J Am Soc Nephrol. 2019;30(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giesen C, Wang HA, Schapiro D, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods. 2014;11(4):417–22. [DOI] [PubMed] [Google Scholar]
- 59. Singh N, Avigan ZM, Kliegel JA, et al. Development of a 2-dimensional atlas of the human kidney with imaging mass cytometry. JCI Insight. 2019;4(12). *Use of IMC to develop KIDNEY-MAPPS
- 60.Avigan Z. Development of Imaging Mass Cytometry based Cellular Profiling to Investigate Delayed Graft Function. Yale Medicine Thesis Digital Library: Yale University; 2020. [Google Scholar]


