Abstract
Objectives
Polycystic ovary syndrome (PCOS) increases non-alcoholic fatty liver disease (NAFLD) risk and severity in adults, but data in adolescents with diverse backgrounds are limited. We evaluated NAFLD prevalence and characterized NAFLD risk factors in overweight/obese adolescents by PCOS status.
Methods
Retrospective study of overweight (n=52)/obese (n=271) female adolescents (12–18 years old), evaluated clinically 2012–2020, was conducted comparing PCOS patients to age-matched non-PCOS controls. NAFLD was defined as ALT≥44U/L x2 and/or ≥80U/L x1, hepatic steatosis on imaging, or NAFLD on biopsy, in absence of other liver disease. Metabolic comorbidities were captured. Log-binomial regression models estimated prevalence risk ratios (PR).
Results
NAFLD prevalence was 19.1 % in adolescents with PCOS (n=161), similar to those without (n=162) (16.8 %, p=0.6). Adolescents with PCOS were more likely to have insulin resistance, hypercholesterolemia, and higher triglycerides (p<0.05). Those with PCOS and concomitant type 2 diabetes (T2DM) did have increased NAFLD risk (PR 2.5, p=0.04), but those with PCOS without T2DM did not (PR 0.9, p=0.8). Adolescents with PCOS and NAFLD, compared to those with PCOS without NAFLD, had a higher prevalence of metabolic comorbidities including hypercholesterolemia (77 vs. 48 %), T2DM (29 vs. 8 %), and hypertriglyceridemia (65 vs. 37 %) (p<0.01).
Conclusions
Almost 1 in 5 overweight/obese female adolescents had NAFLD, but PCOS did not increase NAFLD risk in this diverse cohort. Among young women with PCOS, concomitant T2DM did increase the risk for NAFLD. Closer monitoring of obesity comorbidities in adolescents with PCOS is essential for optimizing health and merits updating current guidelines.
Keywords: NAFLD, non-alcoholic fatty liver disease, PCOS, pediatric NAFLD, polycystic ovary syndrome, type 2 diabetes
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in children and is present in nearly 20 % of adolescents aged 15–19 years old [1, 2]. Children with NAFLD have increased morbidity and mortality in adulthood. Recently, NAFLD became the fastest growing indication for liver transplantation among young adults and the leading indication for liver transplant in women in the United States [3], [4], [5].
Polycystic ovary syndrome (PCOS), the most common endocrinopathy in women of reproductive age, has been found to increase the risk for NAFLD in adults, independent of other risk factors with advanced hepatic fibrosis found at a younger age in women with PCOS [6]. NAFLD is often reversible in its early stages therefore timely recognition, in young girls in particular, is crucial in preventing further hepatic injury and may greatly improve quality of life.
A 2022 meta-analysis recently published found that women and girls with PCOS have a higher prevalence of NAFLD than those without PCOS however most studies addressing this relationship did not evaluate for risk factors and were not performed in a diverse population [7]. We, therefore, aimed to evaluate NAFLD prevalence in overweight/obese adolescent girls by PCOS status in a racially diverse population and evaluate whether risk factors for NAFLD differed by PCOS status.
Materials and methods
This was a retrospective, single center study of female patients with a previous outpatient encounter that included an ICD-9 or ICD-10 code for overweight or obesity (Supplementary Table 1) at the University of California Benioff Children’s Hospitals between 2012, the beginning of the currently used electronic medical record system, and June 1, 2020. Institutional Review Board approval was obtained. Electronic medical records were reviewed by a single individual trained in pediatrics and pediatric gastroenterology, hepatology, and nutrition, with data extracted into a REDCap (Research Electronic Data Capture) database.
Patients, aged 12–18 years old at June 1, 2020, were then defined as having PCOS based on ICD codes (ICD-9 256.4/ICD-10 E 28.2) and verified to meet the practice guidelines by the Endocrine Society by chart review [8]. These guidelines state that diagnosis should be made by presence of persistent oligomenorrhea and clinical and/or biochemical evidence of hyperandrogenism. Controls who were overweight or obese but without PCOS were age-matched 2:1 by date of birth within 1 week. Suspected or confirmed NAFLD by PCOS status was the primary outcome, defined using the algorithm found in the North American Society of Pediatric Gastroenterology, Nutrition, and Hepatology guidelines [9]. The definition of suspected NAFLD varies in the literature [10], [11], [12]. Suspected NAFLD in this study was defined using stricter criteria than prior research to strengthen the use of ALT as a surrogate for NAFLD with alanine aminotransferase (ALT) ≥44 U/L (2 times upper limit of normal 22 U/L) checked on two timepoints within 2 years or ≥80 U/L once or hepatic steatosis on imaging. Confirmed NAFLD was defined as biopsy-confirmed NAFLD or hepatic steatosis on computed topography (CT) or magnetic resonance imaging (MRI), in absence of other known chronic liver disease (autoimmune hepatitis, gallstone disease, hepatitis B/C, thyroid disease, or celiac disease). Data collected was limited to a 5-year timespan of 2015–2020 to improve the precision and more accurately reflect prevalence. Laboratory and imaging data were collected within 2 years of most recently documented body mass index (BMI) to time of data collection that met the definition of overweight (BMI≥85 %ile to <95 %ile) or obesity (BMI≥95 %ile), as the American Academy of Pediatrics guidelines on pediatric obesity management recommend repeat testing every 2 years for normal values [13].
Adolescents identified by ICD9/10 codes for overweight/obesity with no available ALT levels were excluded unless they had confirmation of NAFLD by biopsy or cross-sectional imaging. In addition, adolescents with the following were excluded: no documented growth parameters within 6 months of ALT, ALT only taken during episode of acute hepatobiliary disease (cholecystitis, choledocholithiasis, and pancreatitis), self-reported lifetime binge alcohol use (>8 drinks per week), prior or current use of hormonal therapy for gender reassignment, prior or current use of parenteral nutrition, and/or prior or current use of pro-steatotic medications (glucocorticoids, methotrexate, amiodarone, valproic acid, or chemotherapy).
Risk factors for NAFLD, compared by PCOS status, included: body mass index (BMI), BMI percentile, BMI z-score, and metabolic laboratory tests. Laboratory tests were used to define presence of type 2 diabetes (T2DM, diagnosed by an endocrinologist or HbA1c≥6.5 %); hypercholesterolemia (total cholesterol ≥200 mg/dL or LDL≥130 mg/dL) [14]; hypertriglyceridemia (triglycerides ≥130 mg/dL) [14]; and insulin resistance (triglyceride: HDL-C ratio ≥2.16) [15]. Active use of contraception and metformin at the encounter from which BMI and ALT were recorded was also collected, as these are mainstay treatments for PCOS. Hypertension was defined based on an established diagnosis made by a nephrologist or cardiologist found on chart review.
Prevalence risk ratios (PR) and prevalence risk differences (PD) were assessed, avoiding over-estimation of association strength [16]. To perform multivariate analysis to control for confounding variables, adjusted prevalence risk ratios (aPR) were obtained using the log-binomial method [17]. Wilcoxon rank-sum test was used to compare median values of continuous outcomes, and the chi-square test was used to compare categorical outcomes. Statistical analysis was performed on Stata/MP 16.1.
Results
Our study population included 323 overweight or obese adolescent females, 162 with PCOS and 161 age-matched non-PCOS controls. Initially, 180 patients with PCOS and 360 patients without PCOS were enrolled. Then, 6 with PCOS and 112 without PCOS were excluded for not having an ALT available in their electronic medical record. One was excluded from the non-PCOS group for binge alcohol use. The rest were excluded for a history of being on pro-steatotic medications.
The median BMI percentile was 98.3 % (IQR 96.2–99.1), median BMI z-score was 2.1 (IQR 1.77–2.38), and median age was 17.3 years (IQR 15.9 to 18.2) with no significant differences between groups (Table 1). Patients with PCOS had similar percent of overweight patients to those without PCOS (17.4 % (n=28) vs. 14.8 % (n=24), p=0.8) with the rest of patients being obese (82.6 vs. 85.2 %). Patients with PCOS were less likely than the controls to identify their race as Black (8.6 vs. 16.8 %) and more likely to identify as Other (66.1 vs. 52.3 %, p=0.04). In the group that chose Other as their race, 75.4 % chose Latina ethnicity, although no statistical difference was seen comparing the groups by ethnicity. Patients with PCOS were significantly more likely to be on metformin (23.5 vs. 11.8 %, p = <0.01), a mainstay treatment for PCOS, than those without PCOS.
Table 1:
Characteristics by PCOS status.
| Total | No PCOS (n=161) | PCOS (n=162) | p-Valuea | |
|---|---|---|---|---|
| Age, years, median (IQR) | 17.3 (15.9–18.2) | 17.3 (15.9–18.2) | 17.1 (16.0–18.2) | 0.81 |
| BMI, median (IQR) | 33.5 (29.8–38.2) | 32.6 (29.3–37.7) | 34.4 (30.7–39.4) | 0.40 |
| BMI %, median (IQR) | 98.3 (96.2–99.1) | 98.2 (96.3–99.1) | 98.4 (96.1–99.2) | 0.35 |
| BMI Z-score, median (IQR) | 2.1 (1.8–2.4) | 2.1 (1.8–2.4) | 2.1 (1.8–2.4) | 0.36 |
| Race, % | 0.04 | |||
| White | 20.7 | 21.7 | 19.8 | |
| Black | 12.7 | 16.8 | 8.6 | |
| Asian | 7.4 | 9.3 | 5.6 | |
| Other | 59.1 | 52.3 | 66.1 | |
| Ethnicity, % | 0.26 | |||
| Latina | 48.6 | 44.1 | 53.1 | |
| ALT, U/L, median (IQR) | 24 (17–37) | 23 (16–36) | 25 (18–37) | 0.95 |
| HbA1c (%), median (IQR) | 5.4 (5.2–5.8) | 5.5 (5.2–5.9) | 5.4 (5.2–5.7) | 0.38 |
| Glucoseb (mg/dL), median (IQR) | 90 (84–100.8) | 91 (84–102) | 90 (84–99.5) | 0.52 |
| Total cholesterol (mg/dL), median (IQR) | 161 (141–181) | 158 (141–179) | 163 (141–186) | 0.41 |
| HDL-C (mg/dL), median (IQR) | 45 (38–52) | 46.5(38–53) | 44(38–52) | 0.33 |
| LDL (mg/dL), median (IQR) | 91 (74–114) | 91 (73–108) | 93 (75–118) | 0.07 |
| Triglycerides (mg/dL), median (IQR) | 112.5 (78–162) | 104 (71–151) | 120 (86–166) | 0.04 |
| Insulin resistancec, % | 59.2 | 52.3 | 64.5 | 0.05 |
| Type 2 diabetes, % | 13.9 | 16.1 | 11.7 | 0.25 |
| Hypercholesterolemia, % | 45.8 | 37.9 | 53.7 | 0.004 |
| Hypertriglyceridemia, % | 35.9 | 29.8 | 42.0 | 0.02 |
| Hypertension, % | 3.4 | 2.5 | 4.3 | 0.36 |
Observations performed from 2015 to 2020. ap-Values determined using chi-square test for percentiles and Wilcoxon rank-sum test for median (IQR) values. bGlucose values include both fasting and non-fasting values. c(TG/HDL-c>2.16).
Notably, it was found that nearly 60 % (59.3 %) of PCOS patients had an elevated ALT (>22 U/L, upper limit of normal), similar to 52.2 % of patients without PCOS (p=0.20). Among patients with elevated ALT, those with PCOS were less likely to have serologic chronic hepatitis work-up (11.5 vs. 21.4 %, p=0.07) or liver imaging done (32.3 vs. 37.8 %, p=0.4). Compared to the controls without PCOS, those with PCOS were more likely to have insulin resistance (63.9 vs. 52.2 %, p=0.04), hypercholesterolemia (53.7 vs. 37.9 %, p=0.004), and higher triglycerides (median (IQR) 120 (86–166) vs. 104 (71–151), p=0.04).
Among adolescents with PCOS, the prevalence of NAFLD was 19.1 % (16.0 % suspected NAFLD and 3.1 % confirmed cases) which was similar to adolescents without PCOS (16.8 % (p=0.58), 14.3 % (p=0.56) and 2.5 % (p=0.74) respectively). Likewise, PCOS did not increase NAFLD risk on unadjusted (PR 1.14 (0.71–1.82, p=0.58)) or adjusted models including age and BMI percentile, (aPR 1.12 (0.71–1.77, p=0.62)). When analyzing only suspected NAFLD or confirmed NAFLD risk, the unadjusted and adjusted models similarly did not find statistically significant prevalence risk ratios. Because there were few confirmed cases, subsequent analysis combined all suspected and confirmed NAFLD patients.
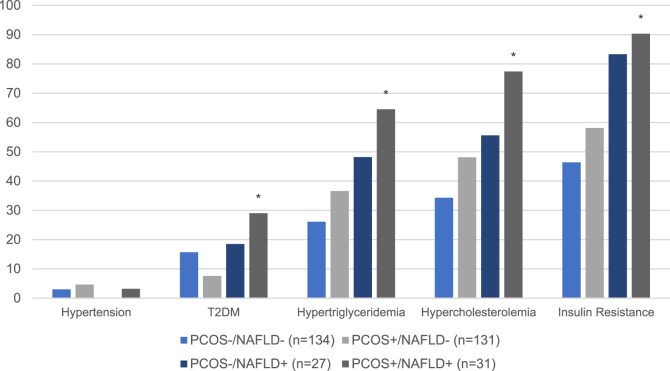
T2DM was found to be an effect modifier of NAFLD risk (Table 2). Among all adolescents with T2DM (n=45), those with PCOS were 2.5 times more likely to have NAFLD than those without PCOS (p=0.04). NAFLD risk among adolescents with hypercholesterolemia, hypertriglyceridemia, or insulin resistance did not differ by PCOS status. Yet in adolescents with PCOS, those found to have NAFLD were significantly more likely to have metabolic co-morbidities, including insulin resistance, T2DM, hypercholesterolemia, and hypertriglyceridemia than those without NAFLD (p≤0.005) (Figure 1). A majority of adolescents with both PCOS and NAFLD had insulin resistance (90.3 %), hypercholesterolemia (77.4 %), as well as hypertriglyceridemia (64.5 %); prevalence of each comorbidity was lower in those with PCOS but without NAFLD: 58.1, 48.1, and 36.6 % respectively.
Table 2:
Prevalence of NAFLD by PCOS status, stratified by type 2 diabetes.
| PCOS absent, % | PCOS present, % | PR (CI) | PD (%) (CI) | p-Value | |
|---|---|---|---|---|---|
| Without type 2 diabetes mellitus | 16.3 | 15.4 | 0.9 (0.5–1.6) | 0.9 (−9.5- 7.6) | 0.84 |
| With type 2 diabetes mellitus | 19.2 | 47.4 | 2.5 (1.0–6.2) | 28.1 (1.1–55.2) | 0.04 |
Figure 1:
Metabolic co-morbidities by NAFLD and PCOS status. *=p-value <0.01 compared to PCOS+/NAFLD- and PCOS+/NAFLD+.
Latina patients with PCOS had a similar prevalence of NAFLD compared to Latina patients without PCOS (24.4 vs. 25.4 %, p=0.89). However, among those who identified as “Not Hispanic or Latina,” patients with PCOS were 2.4 times more likely to have NAFLD than those without PCOS (15.6 vs. 6.5 %, p=0.08)), with a significant difference observed in those who identified as Asian (37.5 vs. 0.0 %, p=0.02). A 4-fold increase of NAFLD prevalence was seen in those identified as White only (16.7 vs. 4.2 %, p=0.17), though not statistically significant. When including ethnicity in an adjusted multivariate model, NAFLD risk remained unchanged (aPR 1.12 (0.71–1.77, p=0.62)).
There were no statistically significant differences in NAFLD risk by PCOS status comparing patients in early (12–14 years old), middle (14–16 years old), or late adolescence (17–18 years old) or comparing patients who were overweight, obese, or severely obese (BMI %ile ≥99 or BMI z-score>3.5). There was also no statistically significant difference in NAFLD risk by PCOS status based on PCOS medication use (metformin or contraceptives).
Discussion
Given limited previous study of the association between PCOS and NAFLD in adolescents, we characterized the prevalence of NAFLD and other metabolic risk factors in overweight/obese adolescents by PCOS status. We found a striking ∼20 % of overweight/obese adolescents with PCOS to have suspected NAFLD, consistent with prior published prevalence data. NAFLD prevalence was found to be similar in overweight/obese adolescents with and without PCOS, though adolescents with both NAFLD and PCOS were more likely to have other metabolic comorbidities. Our data specifically identified T2DM as an effect modifier of NAFLD risk; adolescents with T2DM and PCOS were 2.5 times more likely to have NAFLD as compared to those with T2DM alone. Providers taking care of patients with PCOS and T2DM should be particularly suspicious for NAFLD as both PCOS and T2DM, separately, are known to accelerate the progression of NAFLD to non-alcoholic steatohepatitis and advanced fibrosis in adult women at a younger age [6, 18], [19], [20], [21]. Thus, risk factors for early onset disease may be traced to adolescence, where metabolic comorbidities may set the stage for advanced disease in young adulthood. Severity of metabolic syndrome (including obesity, diabetes, and hypertension) is also associated with risk for advanced hepatic fibrosis and increased morbidity post liver-transplantation [22].
PCOS is the most common endocrinopathy among women of reproductive age and is a set of symptoms that may bring adolescent females to seek medical care. Evaluation for PCOS can also create an opportunity to diagnose NAFLD, a silent disease. A review of clinical practice guidelines of PCOS published from 2007 to 2018 found that only 2 of 13 recommended screening for NAFLD in women with insulin resistance and metabolic syndrome [23]. A positive screen should prompt counselling on lifestyle modification and referral to a dietician and pediatric hepatologist. Reduction of sugar-sweetened beverages and aerobic exercise intervention resulted in hepatic fat reduction and improvement in total and visceral fat in children [9]. Though NAFLD was found to be quite prevalent, we found that patients with PCOS with an elevated ALT rarely had further hepatitis work-up (11.5 %). Our findings underscore the need to consider standardized guidelines for screening and care for NAFLD, an asymptomatic disease, in patients with PCOS, particularly among adolescents with concurrent T2DM, in line with the AAP guidelines for adolescents who are obese/overweight and NASPGHAN guidelines for NAFLD [9, 13].
Racial/ethnic differences in NAFLD prevalence are well described, including the higher risk among those of Latina ethnicity also seen in our study, and lower risk among Black adolescents [9, 24]. Interestingly, we found that among non-Latina patients PCOS diagnosis conferred a 2.4 times higher prevalence of NAFLD while amongst Latinas NAFLD prevalence did not differ by PCOS status. Though the difference in risk ratios was not statistically significant with a p-value of 0.08, this is clinically relevant. Our study population drew from clinics at a tertiary care center in California, likely reflecting the referral patterns of our institution, and may be more diverse and not be reflective of the adolescent population in the United States. This may be the reason we did not appreciate the previously documented increased risk of suspected NAFLD in patients with PCOS as seen in the often cited Raine cohort study in Australia where the race and ethnic distribution differed and was predominantly (∼85 %) Caucasian [25].
Although we were able to report on a large sample of overweight/obese adolescents with detailed characterization of metabolic comorbidities, we did rely on ICD codes for PCOS diagnosis, which may have underestimated or overestimated PCOS diagnosis. Misclassification of PCOS could have decreased our ability to detect differences in NAFLD prevalence by PCOS status. However, notably, there is only 1 ICD code for PCOS and all charts were checked to see if code was added by a medical provider.
Further limitations to our study stemmed from the fact that our data collection was retrospective. The difference in patient exclusion based on ALT availability in the electronic medical record may have biased our controls towards being less representative of the racial and ethnic distribution of obesity seen among adolescent females in population studies [26]. Although the majority of laboratory data was collected within 6 months of most recent BMI available, some data spanned to 2 years prior which may reflect periods of notable fluctuations for metabolic profiles in adolescents. We felt comfortable including data within the 2 years timespan since that is the frequency with which the AAP guidelines for obesity management in children/adolescents recommends for screening for NAFLD [13].
Our NAFLD diagnosis also relied mostly on ALT values. Only 1.9 % of the study population had a liver biopsy performed. Although liver biopsy remains the gold standard for NAFLD/NASH diagnosis, it is an invasive procedure and not always pursued in strongly suspected NAFLD cases without a competing diagnosis. More specific and sensitive non-invasive tests are urgently needed to improve diagnosis and staging of NAFLD in all children. As a retrospective study, the timing of liver enzyme collection was variable based on provider and patient choices; therefore, using date of ALT was not acceptable. Additional risk factors such as age of menarche and hyperandrogenism could not be analyzed due to data unavailability in the non-PCOS population. A prospective cohort study performed in a diverse group of adolescents to further elucidate the risk of PCOS on NAFLD and its progression would be the next step.
We also restricted our data collection to patients that were overweight and obese to limit confounding, given that the risks of both PCOS and NAFLD are increased in the overweight/obese population. We acknowledge that patients with normal BMI can develop both PCOS and NAFLD and that not including them in our study is a limitation.
Notably, we found that patients with PCOS were significantly more likely to be on metformin, a treatment for PCOS, (23.5 vs. 11.8 %, p=<0.01) which may have also affected the outcomes. Patients on or previously on metformin were not excluded for this study because multiple studies have shown that metformin did not lead to a greater difference in ALT reduction or more histologic improvement compared to placebo in pediatric patients with NAFLD [27, 28]. In contrast, in adults, metformin has been found to have a protective effect against NAFLD in patients with PCOS after 12 months of use [29]. Further research needs to be done to study a possible protective effect of metformin in adolescents with PCOS against NAFLD.
Conclusions
NAFLD was present in nearly 20 % of overweight/obese female adolescents with PCOS, similar to those without PCOS. Type 2 diabetes was a notable risk factor for NAFLD among female adolescents with PCOS. Adolescents with PCOS and NAFLD were more likely to have insulin resistance and dyslipidemia. Clinical guidelines in adolescents with PCOS, particularly those with type 2 diabetes, for screening and counselling of NAFLD should be further developed, similar to the guidelines for all overweight/obese adolescents, to facilitate earlier diagnosis and optimized care – and ideally to optimize long-term liver and overall health of young women.
Supplementary Material
Supplementary Material
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpem-2022-0527).
Footnotes
Research funding: This work was supported in part by NIH grantT32DK007762.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Informed consent: Not applicable.
Ethical approval: Institutional Review Board approval was obtained.
References
- 1.Di Sessa A, Cirillo G, Guarino S, Marzuillo P, Miraglia Del Giudice E. Pediatric non-alcoholic fatty liver disease: current perspectives on diagnosis and management. Pediatr Health Med Ther. 2019;10:89–97. doi: 10.2147/phmt.s188989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 3.Smith SK, Perito ER. Nonalcoholic liver disease in children and adolescents. Clin Liver Dis. 2018;22:723–33. doi: 10.1016/j.cld.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Doycheva I, Issa D, Watt KD, Lopez R, Rifai G, Alkhouri N. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in young adults in the United States. J Clin Gastroenterol. 2018;52:339–46. doi: 10.1097/mcg.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 5.Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113:1649–59. doi: 10.1038/s41395-018-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkar M, Terrault N, Chan W, Cedars MI, Huddleston HG, Duwaerts CC, et al. Polycystic ovary syndrome (PCOS) is associated with NASH severity and advanced fibrosis. Liver Int Off J Int Assoc Study Liver. 2020;40:355–9. doi: 10.1111/liv.14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falzarano C, Lofton T, Osei-Ntansah A, Oliver T, Southward T, Stewart S, et al. Nonalcoholic fatty liver disease in women and girls with polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:258–72. doi: 10.1210/clinem/dgab658. [DOI] [PubMed] [Google Scholar]
- 8.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline [published correction appears in J Clin Endocrinol Metab. 2021 May 13;106(6):e2462] J Clin Endocrinol Metab. 2013;98:4565–92. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the expert committee on NAFLD (ECON) and the North American Society of pediatric gastroenterology, hepatology and nutrition (NASPGHAN) J Pediatr Gastroenterol Nutr. 2017;64:319–34. doi: 10.1097/mpg.0000000000001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezaizi Y, Kabbany MN, Conjeevaram Selvakumar PK, Sarmini MT, Singh A, Lopez R, et al. Comparison between non-alcoholic fatty liver disease screening guidelines in children and adolescents. JHEP Rep. 2019;1:259–64. doi: 10.1016/j.jhepr.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molleston JP, Schwimmer JB, Yates KP, Murray KF, Cummings OW, Lavine JE, et al. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J Pediatr. 2014;164:707–13.e3. doi: 10.1016/j.jpeds.2013.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–5. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 13.Hampl SE, Hassink SG, Skinner AC, Armstrong SC, Barlow SE, Bolling CF, et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. 2023;151:e2022060640. doi: 10.1542/peds.2022-060640. [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Pediatrics National cholesterol education program: report of the expert panel on blood cholesterol levels in children and adolescents. Pediatrics. 1992;89:525–84. [PubMed] [Google Scholar]
- 15.Aslan Çin NN, Yardımcı H, Koç N, Uçaktürk SA, Akçil Ok M. Triglycerides/high-density lipoprotein cholesterol is a predictor similar to the triglyceride-glucose index for the diagnosis of metabolic syndrome using International Diabetes Federation criteria of insulin resistance in obese adolescents: a cross-sectional study. J Pediatr Endocrinol Metab. 2020;33:777–84. doi: 10.1515/jpem-2019-0310. [DOI] [PubMed] [Google Scholar]
- 16.Tamhane AR, Westfall AO, Burkholder GA, Cutter GR. Prevalence odds ratio versus prevalence ratio: choice comes with consequences [published correction appears in Stat Med. 2017 Oct 15;36(23 ):3760] Stat Med. 2016;35:5730–5. doi: 10.1002/sim.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastos LS, Oliveira Rde V, Velasque Lde S. Obtaining adjusted prevalence ratios from logistic regression models in cross-sectional studies. Cad Saude Publica. 2015;31:487–95. doi: 10.1590/0102-311x00175413. [DOI] [PubMed] [Google Scholar]
- 18.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Nobili V, Mantovani A, Cianfarani S, Alisi A, Mosca A, Sartorelli MR, et al. Prevalence of prediabetes and diabetes in children and adolescents with biopsy-proven non alcoholic fatty liver disease. J Hepatol. 2019;71:802–10. doi: 10.1016/j.jhep.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Newton KP, Hou J, Crimmins NA, Lavine JE, Barlow SE, Xanthakos SA, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016;170:e161971. doi: 10.1001/jamapediatrics.2016.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardugo A, Bendor CD, Zucker I, Lutski M, Cukierman-Yaffe T, Derazne E, et al. Adolescent nonalcoholic fatty liver disease and type 2 diabetes in young adulthood. J Clin Endocrinol Metab. 2021;106:e34–44. doi: 10.1210/clinem/dgaa753. [DOI] [PubMed] [Google Scholar]
- 22.Golabi P, Otgonsuren M, de Avila L, Sayiner M, Rafiq N, Younossi ZM. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD) Medicine (Baltimore) 2018;97:e0214. doi: 10.1097/md.0000000000010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Wattar BH, Fisher M, Bevington L, Talaulikar V, Davies M, Conway G, et al. Clinical practice guidelines on the diagnosis and management of polycystic ovary syndrome: a systematic review and quality assessment study. J Clin Endocrinol Metab. 2021;106:2436–46. doi: 10.1210/clinem/dgab232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of nonalcoholic fatty liver disease in children: a follow-up study for up to 20-years. Gut. 2009;58:1538–44. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayonrinde OT, Adams LA, Doherty DA, Mori TA, Beilin LJ, Oddy WH, et al. Adverse metabolic phenotype of adolescent girls with non-alcoholic fatty liver disease plus polycystic ovary syndrome compared with other girls and boys. J Gastroenterol Hepatol. 2016;31:980–7. doi: 10.1111/jgh.13241. [DOI] [PubMed] [Google Scholar]
- 26.Andrisse S, Garcia-Reyes Y, Pyle L, Kelsey MM, Nadeau KJ, Cree-Green M. Racial and ethnic differences in metabolic disease in adolescents with obesity and polycystic ovary syndrome. Journal of the Endocrine Society. 2021;5:bvab008. doi: 10.1210/jendso/bvab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jalali M, Rahimlou M, Mahmoodi M, Moosavian SP, Symonds ME, Jalali R, et al. The effects of metformin administration on liver enzymes and body composition in non-diabetic patients with non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis: an up-to date systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2020;159:104799. doi: 10.1016/j.phrs.2020.104799. [DOI] [PubMed] [Google Scholar]
- 28.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Nonalcoholic Steatohepatitis Clinical Research Network Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gangale MF, Miele L, Lanzone A, Sagnella F, Martinez D, Tropea A, et al. Long-term metformin treatment is able to reduce the prevalence of metabolic syndrome and its hepatic involvement in young hyperinsulinaemic overweight patients with polycystic ovarian syndrome. Clin Endocrinol (Oxf) 2011;75:520–7. doi: 10.1111/j.1365-2265.2011.04093.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material