This cohort study investigates factors associated with patient nonadherence to lung cancer screening recommendations across multiple time points.
Key Points
Question
What factors and longitudinal patterns are associated with patient nonadherence to lung cancer screening across multiple screening time points?
Findings
In this cohort study of 1979 patients, 6 factors, including baseline Lung Computed Tomography Screening Reporting & Data System (Lung-RADS) score, were found to be associated with patient nonadherence to recommended follow-up examination. Nonadherence increased over time for patients who received consecutive Lung-RADS scores of 1 or 2.
Meaning
The findings suggest that patients with consecutive negative screening results (Lung-RADS score, 1 or 2) are more likely to become nonadherent to screening over time and may benefit from outreach and education.
Abstract
Importance
Screening with low-dose computed tomography (CT) has been shown to reduce mortality from lung cancer in randomized clinical trials in which the rate of adherence to follow-up recommendations was over 90%; however, adherence to Lung Computed Tomography Screening Reporting & Data System (Lung-RADS) recommendations has been low in practice. Identifying patients who are at risk of being nonadherent to screening recommendations may enable personalized outreach to improve overall screening adherence.
Objective
To identify factors associated with patient nonadherence to Lung-RADS recommendations across multiple screening time points.
Design, Setting, and Participants
This cohort study was conducted at a single US academic medical center across 10 geographically distributed sites where lung cancer screening is offered. The study enrolled individuals who underwent low-dose CT screening for lung cancer between July 31, 2013, and November 30, 2021.
Exposures
Low-dose CT screening for lung cancer.
Main Outcomes and Measures
The main outcome was nonadherence to follow-up recommendations for lung cancer screening, defined as failing to complete a recommended or more invasive follow-up examination (ie, diagnostic dose CT, positron emission tomography–CT, or tissue sampling vs low-dose CT) within 15 months (Lung-RADS score, 1 or 2), 9 months (Lung-RADS score, 3), 5 months (Lung-RADS score, 4A), or 3 months (Lung-RADS score, 4B/X). Multivariable logistic regression was used to identify factors associated with patient nonadherence to baseline Lung-RADS recommendations. A generalized estimating equations model was used to assess whether the pattern of longitudinal Lung-RADS scores was associated with patient nonadherence over time.
Results
Among 1979 included patients, 1111 (56.1%) were aged 65 years or older at baseline screening (mean [SD] age, 65.3 [6.6] years), and 1176 (59.4%) were male. The odds of being nonadherent were lower among patients with a baseline Lung-RADS score of 1 or 2 vs 3 (adjusted odds ratio [AOR], 0.35; 95% CI, 0.25-0.50), 4A (AOR, 0.21; 95% CI, 0.13-0.33), or 4B/X, (AOR, 0.10; 95% CI, 0.05-0.19); with a postgraduate vs college degree (AOR, 0.70; 95% CI, 0.53-0.92); with a family history of lung cancer vs no family history (AOR, 0.74; 95% CI, 0.59-0.93); with a high age-adjusted Charlson Comorbidity Index score (≥4) vs a low score (0 or 1) (AOR, 0.67; 95% CI, 0.46-0.98); in the high vs low income category (AOR, 0.79; 95% CI, 0.65-0.98); and referred by physicians from pulmonary or thoracic-related departments vs another department (AOR, 0.56; 95% CI, 0.44-0.73). Among 830 eligible patients who had completed at least 2 screening examinations, the adjusted odds of being nonadherent to Lung-RADS recommendations at the following screening were increased in patients with consecutive Lung-RADS scores of 1 to 2 (AOR, 1.38; 95% CI, 1.12-1.69).
Conclusions and Relevance
In this retrospective cohort study, patients with consecutive negative lung cancer screening results were more likely to be nonadherent with follow-up recommendations. These individuals are potential candidates for tailored outreach to improve adherence to recommended annual lung cancer screening.
Introduction
Screening with low-dose computed tomography (LDCT) effectively reduced mortality from lung cancer by at least 20% in large randomized clinical trials in which adherence rates were over 90%.1,2 The Lung CT Screening Reporting & Data System (Lung-RADS), released in 2014, has become a nationally accepted standard for lung cancer screening (LCS) CT reporting and management recommendations.3 The follow-up recommendations are to continue annual LDCT screening in patients with Lung-RADS scores of 1 or 2; in patients with Lung-RADS scores of 3 or 4, early or more aggressive follow-up is advised, which may entail LDCT in 6 months, LDCT in 3 months, chest CT, positron emission tomography–CT (PET-CT), or tissue sampling.4 Notably, patient adherence to LCS in clinical practice is substantially lower than the over 90% adherence rates in clinical trials. A systematic review and meta-analysis5 by some of us found that patient adherence to baseline Lung-RADS recommendations was 57% to 65% in clinical LCS programs, with a significantly lower annual adherence rate among patients with Lung-RADS scores of 1 or 2 (45%-49%) compared with early follow-up adherence among those with Lung-RADS scores of 3 or 4 (74%-78%). Similarly, a recent study reported that rates of adherence to recommendations from baseline and the first annual screening were 48% and 44%, respectively, among patients with Lung-RADS scores of 1 or 2 in a large national cohort (N = 30 166).6 Failing to maintain annual adherence to LCS recommendations may diminish the ability of clinical screening programs to achieve the same mortality benefits found in large clinical trials. Interval lung cancers, diagnosed between screening episodes following a preceding negative screening result (Lung-RADS score, 1 or 2), are more likely to be aggressive, emphasizing the importance of regular screening intervals.7
Lung cancer screening is nascent as a preventive measure in the US; as such, barriers to LCS have been incompletely investigated. Patient-level barriers include unawareness of screening benefits and risks; perceptual barriers, such as fear of cancer diagnosis and perceived stigma; screening-related cost concerns; and challenges in accessing imaging sites.8 Identifying factors that affect patient adherence to Lung-RADS recommendations can help clinicians better understand who would benefit from outreach strategies to improve adherence.9 These factors may be used to identify patients who are at risk for nonadherence. Given that patient characteristics in clinical LCS programs vary across institutions, clinical risk stratification models that aid in the identification of potentially nonadherent patients may result in more aggressive, tailored approaches and thus improve the mortality benefit of screening. To our knowledge, no studies have investigated factors associated with patient nonadherence to Lung-RADS recommendations over multiple screening intervals. Specifically, Lung-RADS scores may vary over time. Previous work has shown that the Lung-RADS score was a significant factor associated with nonadherence to baseline Lung-RADS recommendations5; however, evidence on whether longitudinal patterns of Lung-RADS scores affect the risk of nonadherence to screening in the future is lacking.
This study aimed to identify factors associated with risk for patient nonadherence to Lung-RADS recommendations at baseline and across multiple time points. In experiment 1, we investigated whether patient demographics, socioeconomic status, and health status were associated with nonadherence to baseline Lung-RADS recommendations. Experiment 2 adjusted for significant factors from experiment 1 to evaluate the hypothesis that adherence would increase or decrease as Lung-RADS scores were upgraded or downgraded, respectively, and adherence would be stable when Lung-RADS scores remained unchanged.
Methods
Patient Enrollment
Institutional review board approval was obtained from the University of California, Los Angeles (UCLA), to conduct this retrospective cohort study, and informed consent was waived because the risk to participants was minimal. We included patients who underwent at least 1 LDCT screening examination at our institution from July 31, 2013, to November 30, 2021 (last follow-up, December 8, 2021), within 10 geographically distributed sites where LCS is offered. Lung-RADS scores were retrospectively assigned to LDCT screenings performed prior to the release date of Lung-RADS version 1.010 by a board-certified thoracic radiologist (D.R.A.). Patient exclusion criteria are summarized in the Figure. Annual screenings or early follow-up LDCT screenings were excluded if patients were older than 80 years at the time of screening, the patient had a Lung-RADS score of 0, or the screening was incorrectly ordered for nonscreening purposes. Additional details are reported in eAppendix 1 in Supplement 1, such as identification of screening-eligible patients and intervention for adherence. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Figure. Flow Diagram of Patient Enrollment.
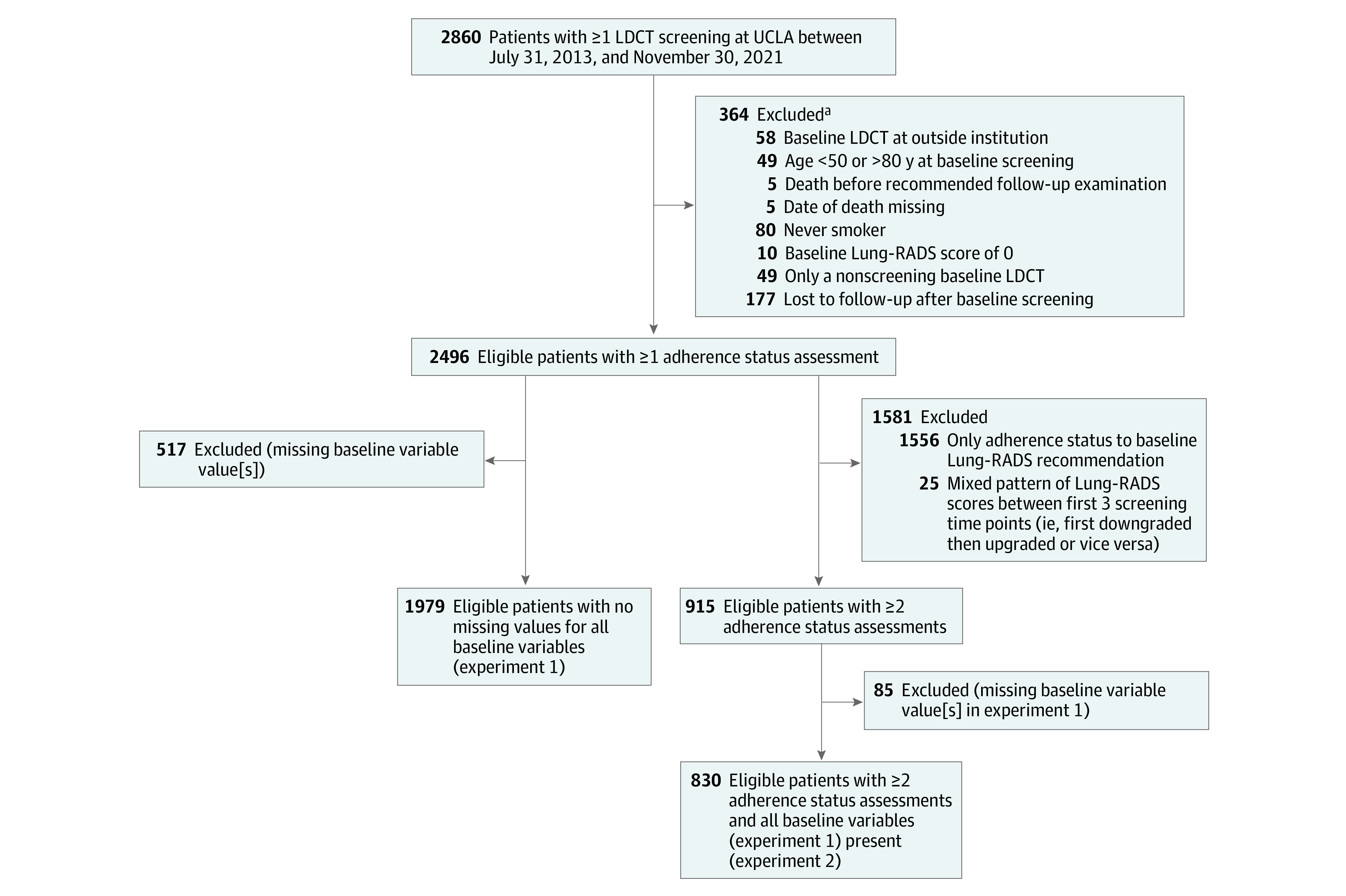
The last follow-up was December 8, 2021. CT indicates computed tomography; LDCT, low-dose CT; Lung-RADS, Lung CT Screening Reporting & Data System; UCLA, University of California, Los Angeles.
aA patient could have more than 1 exclusion criterion.
Data Collection
Patient characteristics at the time of their baseline screening were obtained by medical abstraction from our institution’s electronic health record, including an existing registry of patients who undergo LCS. Baseline factors of interest included Lung-RADS score, age, sex, race and ethnicity, educational level, family history of lung cancer, smoking status, primary insurance status, age-adjusted Charlson Comorbidity Index (CCI) score,11 distance to screening center, median family income, area deprivation index (ADI) state rank,12 and type of referring physician. Race and ethnicity data (included to assess the comparative racial and ethnic proportions of UCLA patients undergoing screening and because differences in risk of lung cancer have been identified across different racial and ethnic groups) were obtained from a self-reported questionnaire administered prior to the LDCT screening examination that was stored as a discrete series of the screening examination in our picture archiving and communication system. When such data were missing from the questionnaire, data in the electronic medical record were extracted. Race and ethnicity categories included Asian, Black, Hispanic or Latino, White, and other (American Indian or Alaska Native, Native Hawaiian or Pacific Islander, more than 1 race, or racial and ethnic group not otherwise stated). Age-adjusted CCI score was grouped into 3 categories: low (0 or 1), intermediate (2 or 3), and high (≥4).11 Median family income was mapped with the 2010 census data using the home zip code. Distance to screening center was estimated between the home zip code and the zip code of the screening center. We dichotomized the variables ADI state rank, median family income, and distance to screening center as low or short (less than or equal to the median) and high or long (greater than the median). Elective imaging examinations, such as LDCT screening examinations, were suspended at the beginning of the COVID-19 pandemic (ie, from March 19 to May 19, 2020) at our institution to conserve health care resources and minimize the risk of viral transmission. To account for the potential association of this pause in elective imaging examinations with patient adherence, we included 1 variable indicating whether or not the date of the expected follow-up examination fell within the 2-month pause period.
Patient Outcomes
The main outcome of the study was patient nonadherence, defined as failing to adhere to follow-up recommendations based on Lung-RADS category, factoring in some time allowance from the recommended period. Adherence was defined for a Lung-RADS score of 1 or 2 as completing the next annual screening within 12 months plus 3 months, for a Lung-RADS score of 3 as completing a recommended repeated LDCT within 6 months plus 3 months, for a Lung-RADS score of 4A as completing an LDCT within 3 months plus 2 months, and for a Lung-RADS score of 4B/X as completing a more aggressive diagnostic workup (ie, diagnostic chest CT, PET-CT, or tissue sampling) within 3 months of abnormal screening findings (eFigure 1 in Supplement 1). Patients were considered adherent if they completed a more invasive follow-up examination (ie, diagnostic chest CT, PET-CT, or tissue sampling vs LDCT) within the defined time intervals.
Statistical Analysis
In experiment 1, we used a multivariable logistic regression model to identify factors significantly associated with nonadherence to baseline Lung-RADS recommendations. Patients who had missing values in some characteristics were excluded from the analysis. A sensitivity analysis was implemented using multiple imputation data (ie, the mice13 package in R, version 3.6.1 [R Project for Statistical Computing]) and found similar results.
In experiment 2, we examined whether the baseline Lung-RADS score and a pattern of subsequent Lung-RADS scores were associated with nonadherence to Lung-RADS recommendations over time. Patients who underwent at least 2 screening examinations were included in this analysis. The Lung-RADS score was binary (1 or 2 vs 3 or 4). A Lung-RADS score of 1 or 2 was defined as a negative screening result, and a Lung-RADS score of 3 or 4 was defined as a positive screening result. Patients were categorized into subgroups based on their longitudinal patterns of Lung-RADS scores (eTable 2 in Supplement 1): unchanged, upgraded (negative to positive), or downgraded (positive to negative). Patients whose Lung-RADS scores were first upgraded and then downgraded or vice versa were excluded. These patients may have had more complex changes in health status (eg, first upgraded, then downgraded: health status worsened, then improved) than those with monotonic or no changes in Lung-RADS scores (eg, downgraded: health status improved). A generalized estimating equations (GEE) model with a logit link and an unstructured working correlation that accounted for repeated measurements within the same patient was used. The fixed effects included in this model were baseline Lung-RADS score (1 or 2 vs 3 or 4), longitudinal patterns of Lung-RADS scores (changed vs unchanged), screening time point (first, second, or third), three 2-way interaction terms, one 3-way interaction term among the 3 variables, and significant baseline factors associated with nonadherence from experiment 1 (ie, z test 2-sided P < .05). Patients who had missing values for some factors were excluded from this analysis. Python, version 3.7.3 (Python Software Foundation) and R, version 3.6.114 were used for data analyses. Two-sided P < .05 was considered significant.
Results
Among the 2496 eligible patients, 1979 had no missing values in all baseline characteristics (Figure). A comparison of the observed baseline characteristics between included and excluded patients is shown in eTable 1 in Supplement 1. No significant differences were found for any variables except family history of lung cancer. The majority of patients (1660 [83.9%]) had a negative baseline screening result and were 65 years of age or older (1111 [56.1%]). Mean (SD) age was 65.3 (6.6) years; 803 patients (40.6%) were female; 1176 (59.4%), male; 169 (8.5%), Asian; 130 (6.6%), Black; 111 (5.6%), Hispanic or Latino; 1526 (77.1%), White; and 43 (2.2%), other race and ethnicity. A total of 1210 patients were former smokers (61.1%). Patient characteristics at the baseline screening are summarized in Table 1. The mean follow-up time was 1.78 years (range, 0.25-3.75 years) (eTable 4 in Supplement 1 shows details). Eighty-one of the 2496 eligible patients (3.2%) were diagnosed with primary lung cancers during follow-up.
Table 1. Baseline Patient Characteristics in Experiment 1.
| Variable | Patients, No. (%) | ||
|---|---|---|---|
| Overall (N = 1979) | Adherent to LCS (n = 693) | Nonadherent to LCS (n = 1286) | |
| Lung-RADS score | |||
| 1 or 2 | 1660 (83.9) | 490 (70.7) | 1170 (91.0) |
| 3 | 154 (7.8) | 83 (12.0) | 71 (5.5) |
| 4A | 99 (5.0) | 67 (9.7) | 32 (2.5) |
| 4B/X | 66 (3.3) | 53 (7.6) | 13 (1.0) |
| Age, y | |||
| <65 | 868 (43.9) | 268 (38.7) | 600 (46.7) |
| ≥65 | 1111 (56.1) | 425 (61.3) | 686 (53.3) |
| Sex | |||
| Female | 803 (40.6) | 276 (39.8) | 527 (41.0) |
| Male | 1176 (59.4) | 417 (60.2) | 759 (59.0) |
| Race and ethnicity | |||
| Asian | 169 (8.5) | 59 (8.5) | 110 (8.6) |
| Black | 130 (6.6) | 49 (7.1) | 81 (6.3) |
| Hispanic or Latino | 111 (5.6) | 35 (5.1) | 76 (5.9) |
| White | 1526 (77.1) | 540 (77.9) | 986 (76.7) |
| Othera | 43 (2.2) | 10 (1.4) | 33 (2.6) |
| Educational level | |||
| Less than college | 958 (48.4) | 337 (48.6) | 621 (48.3) |
| College graduate | 590 (29.8) | 186 (26.8) | 404 (31.4) |
| Postgraduate | 431 (21.8) | 170 (24.5) | 261 (20.3) |
| Family history of lung cancer | |||
| Yes | 466 (23.5) | 187 (27.0) | 279 (21.7) |
| No | 1513 (76.5) | 506 (73.0) | 1007 (78.3) |
| Smoking status | |||
| Current | 769 (38.9) | 246 (35.5) | 523 (40.7) |
| Former | 1210 (61.1) | 447 (64.5) | 763 (59.3) |
| Primary insurance | |||
| Medicare and/or Medicaid | 830 (41.9) | 328 (47.3) | 502 (39.0) |
| Private or commercial | 1121 (56.6) | 358 (51.7) | 763 (59.3) |
| Otherb | 28 (1.4) | 7 (1.0) | 21 (1.6) |
| Age-adjusted CCI score | |||
| 0 or 1 | 287 (14.5) | 72 (10.4) | 215 (16.7) |
| 2 or 3 | 1152 (58.2) | 403 (58.2) | 749 (58.2) |
| ≥4 | 540 (27.3) | 218 (31.5) | 322 (25.0) |
| Distance to screening centerc | |||
| ≤Median | 994 (50.2) | 346 (49.9) | 648 (50.4) |
| >Median | 985 (49.8) | 347 (50.1) | 638 (49.6) |
| Household incomed | |||
| ≤Median | 1029 (52.0) | 340 (49.1) | 689 (53.6) |
| >Median | 950 (48.0) | 353 (50.9) | 597 (46.4) |
| ADI state ranke | |||
| ≤Median | 1072 (54.2) | 387 (55.8) | 685 (53.3) |
| >Median | 907 (45.8) | 306 (44.2) | 601 (46.7) |
| Type of referring physician | |||
| Pulmonology, thoracic oncology, radiology, or surgery | 369 (18.6) | 176 (25.4) | 193 (15.0) |
| Otherf | 1610 (81.4) | 517 (74.6) | 1093 (85.0) |
| Expected follow-up examination | |||
| Before COVID-19 | 1468 (74.2) | 513 (74.0) | 955 (74.3) |
| During COVID-19 pause | 53 (2.7) | 11 (1.6) | 42 (3.3) |
| After COVID-19 pause | 458 (23.1) | 169 (24.4) | 289 (22.5) |
Abbreviations: ADI, Area Deprivation Index; CCI, Charlson Comorbidity Index; LCS, lung cancer screening; Lung-RADS, Lung CT Screening Reporting & Data System.
Subcategories were American Indian or Alaska Native, Native Hawaiian or Pacific Islander, more than 1 race, or racial and ethnic group not otherwise stated.
Subcategories were Veterans Health Administration (n = 1), self-pay (n = 27), and other insurance not specified (n = 1).
Median distance to a screening center was 6.84 miles.
Median household income was $73 478.
Median ADI state rank was 3.
Subcategories were family medicine, general internal medicine, and obstetrics and gynecology.
Lung-RADS Score at Baseline and Nonadherence to Baseline Lung-RADS Recommendations
Among the 1979 patients, the rates of nonadherence to baseline Lung-RADS recommendations were 70.5% (1170 of 1660), 46.1% (71 of 154), 32.3% (32 of 99), and 19.7% (13 of 66) for patients with Lung-RADS scores of 1 or 2, 3, 4A, and 4B/X, respectively. The odds of being nonadherent were lower among patients with a positive baseline Lung-RADS score compared with those with a negative baseline score: for a score of 3, the adjusted odds ratio (AOR) was 0.35 (95% CI, 0.25-0.50); 4A, 0.21 (95% CI, 0.13-0.33); and 4B/X, 0.10 (95% CI, 0.05-0.19) (Table 2). Lower odds of nonadherence were also observed among patients with a postgraduate degree vs a college degree (AOR, 0.70; 95% CI, 0.53-0.92), with family history of lung cancer vs no family history (AOR, 0.74; 95% CI, 0.59-0.93), with a high age-adjusted CCI score (≥4) vs a low score (0 or 1) (AOR, 0.67; 95% CI, 0.46-0.98), in the high vs low income category (AOR, 0.79; 95% CI, 0.65-0.98), and referred by physicians from pulmonary or thoracic-related departments (ie, thoracic oncology, radiology, or surgery) vs another department (AOR, 0.56; 95% CI, 0.44-0.73). These factors were used as inputs into multiple machine learning classifiers to predict patient nonadherence, with the top-performing model achieving a sensitivity of 0.94, specificity of 0.71, and accuracy of 0.72 on the hold-out test data (eAppendix 2, eFigure 2, and eTable 3 in Supplement 1).
Table 2. Multivariable Logistic Regression Analysis of Patient Nonadherence to Baseline Lung-RADS Recommendations Among 1979 Patients in Experiment 1.
| Variable | AOR (95% CI) | P value |
|---|---|---|
| Intercept | 9.17 (4.14-21.65) | <.001 |
| Lung-RADS score | ||
| 1 or 2 | 1 [Reference] | NA |
| 3 | 0.35 (0.25-0.50) | <.001 |
| 4A | 0.21 (0.13-0.33) | <.001 |
| 4B/X | 0.10 (0.05-0.19) | <.001 |
| Age, y | ||
| <65 | 1 [Reference] | NA |
| ≥65 | 1.00 (0.78-1.28) | .98 |
| Sex | ||
| Female | 1 [Reference] | NA |
| Male | 0.95 (0.77-1.16) | .60 |
| Race and ethnicity | ||
| Asian | 0.98 (0.69-1.41) | .90 |
| Black | 0.84 (0.56-1.25) | .37 |
| Hispanic or Latino | 1.10 (0.71-1.73) | .67 |
| White | 1 [Reference] | NA |
| Othera | 1.55 (0.77-3.39) | .24 |
| Educational level | ||
| Less than college | 0.88 (0.69-1.11) | .28 |
| College graduate | 1 [Reference] | NA |
| Postgraduate | 0.70 (0.53-0.92) | .01 |
| Smoking status | ||
| Current smoker | 1 [Reference] | NA |
| Former smoker | 0.84 (0.68-1.03) | .10 |
| Family history of lung cancer | ||
| Yes | 0.74 (0.59-0.93) | .01 |
| No | 1 [Reference] | NA |
| Primary insurance | ||
| Medicare and/or Medicaid | 1 [Reference] | NA |
| Private or commercial | 1.10 (0.88-1.38) | .41 |
| Otherb | 1.41 (0.60-3.70) | .46 |
| Age-adjusted CCI score | ||
| 0 or 1 | 1 [Reference] | NA |
| 2 or 3 | 0.73 (0.52-1.02) | .07 |
| ≥4 | 0.67 (0.46-0.98) | .04 |
| Distance to screening center | ||
| ≤50th Percentile | 1 [Reference] | NA |
| >50th Percentile | 1.01 (0.81-1.25) | .95 |
| ADI state rank | ||
| ≤50th Percentile | 1 [Reference] | NA |
| >50th Percentile | 1.12 (0.90-1.40) | .30 |
| Median annual income | ||
| ≤50th Percentile | 1 [Reference] | NA |
| >50th Percentile | 0.79 (0.65-0.98) | .03 |
| Type of referring physician | ||
| Pulmonology, thoracic oncology, radiology, or surgery | 0.56 (0.44-0.73) | <.001 |
| Otherc | 1 [Reference] | NA |
| Expected follow-up examination | ||
| Before COVID-19 | 0.56 (0.27-1.08) | .10 |
| During COVID-19 pause | 1 [Reference] | NA |
| After COVID-19 pause | 0.52 (0.24-1.02) | .07 |
Abbreviations: ADI, Area Deprivation Index; AOR, adjusted odds ratio; CCI, Charlson Comorbidity Index; Lung-RADS, Lung CT Screening Reporting & Data System; NA, not applicable.
Subcategories were American Indian or Alaska Native, Native Hawaiian or Pacific Islander, more than 1 race, or racial and ethnic group not otherwise stated.
Subcategories were Veterans Health Administration, self-pay, and other insurance not specified.
Subcategories were family medicine, general internal medicine, and obstetrics and gynecology.
Consecutive Negative Screening Results and Nonadherence at Follow-up Screening
A total of 915 patients had 2 or 3 adherence status assessments and monotonic changes in Lung-RADS scores over time; 830 of them (90.7%) had no missing values in all baseline factors significantly associated with adherence in experiment 1 (Figure). No significant differences in the observed variables were found between the included and excluded patients. Most patients (657 [79.2%]) were in the unchanged category (631 [96.0%] negative, 26 [4.0%] positive); 94 of 830 (11.3%) and 79 of 830 (9.5%) were in the downgraded and upgraded categories, respectively. Patient baseline characteristics stratified by patterns of subsequent Lung-RADS scores are shown in Table 3. In the group with unchanged negative screening results compared with the other 3 groups combined, fewer patients were aged 65 years or older (338 of 631 [53.6%] vs 132 of 199 [66.3%]; P = .002) and were referred by pulmonary medicine or thoracic-related subspecialists (102 of 631 [16.2%] vs 48 of 199 [24.1%]; P = .01).
Table 3. Characteristics at Baseline Among 830 Patients, Stratified by Changes in Lung-RADS Scores Across 3 Screening Time Points in Experiment 2.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| Negative unchanged results (n = 631) | Positive unchanged results (n = 26) | Lung-RADS downgraded (n = 94) | Lung-RADS upgraded (n = 79) | |
| Lung-RADS scorea | ||||
| 1 or 2 | 631 (100) | 0 | 0 | 79 (100) |
| 3 or 4 | 0 | 26 (100) | 94 (100) | 0 |
| Age, yb | ||||
| <65 | 293 (46.4) | 5 (19.2) | 37 (39.4) | 25 (31.6) |
| ≥65 | 338 (53.6) | 21 (80.8) | 57 (60.6) | 54 (68.4) |
| Sex | ||||
| Female | 250 (39.6) | 10 (38.5) | 33 (35.1) | 36 (45.6) |
| Male | 381 (60.4) | 16 (61.5) | 61 (64.9) | 43 (54.4) |
| Race and ethnicity | ||||
| Asian | 56 (8.9) | 2 (7.7) | 10 (10.6) | 5 (6.3) |
| Black | 46 (7.3) | 2 (7.7) | 5 (5.3) | 5 (6.3) |
| Hispanic or Latino | 27 (4.3) | 1 (3.8) | 6 (6.4) | 5 (6.3) |
| White | 472 (74.8) | 20 (76.9) | 69 (73.4) | 61 (77.2) |
| Otherc | 16 (2.5) | 0 | 2 (2.1) | 2 (2.5) |
| Missing | 14 (2.2) | 1 (3.8) | 2 (2.1) | 1 (1.3) |
| Educational levela | ||||
| Less than college | 281 (44.5) | 17 (65.4) | 42 (44.7) | 43 (54.4) |
| College graduate | 196 (31.1) | 5 (19.2) | 34 (36.2) | 19 (24.1) |
| Postgraduate | 154 (24.4) | 4 (15.4) | 18 (19.1) | 17 (21.5) |
| Smoking status | ||||
| Current | 253 (40.1) | 7 (26.9) | 42 (44.7) | 37 (46.8) |
| Former | 364 (57.7) | 19 (73.1) | 52 (55.3) | 41 (51.9) |
| Missing | 14 (2.2) | 0 | 0 | 1 (1.3) |
| Family history of lung cancera | ||||
| Yes | 140 (22.2) | 4 (15.4) | 20 (21.3) | 21 (26.6) |
| No | 491 (77.8) | 22 (84.6) | 74 (78.7) | 58 (73.4) |
| Age-adjusted CCI scorea | ||||
| 0 or 1 | 84 (13.3) | 2 (7.7) | 9 (9.6) | 6 (7.6) |
| 2 or 3 | 407 (64.5) | 16 (61.5) | 58 (61.7) | 48 (60.8) |
| ≥4 | 140 (22.2) | 8 (30.8) | 27 (28.7) | 25 (31.6) |
| Primary insurance | ||||
| Medicare and/or Medicaid | 272 (43.1) | 18 (69.2) | 47 (50.0) | 30 (38.0) |
| Private or commercial | 348 (55.2) | 8 (30.8) | 45 (47.9) | 47 (59.5) |
| Otherd | 9 (1.4) | 0 | 2 (2.1) | 2 (2.5) |
| Missing | 2 (0.3) | 0 | 0 | 0 |
| Distance to screening centere | ||||
| ≤Median | 301 (47.7) | 14 (53.8) | 45 (47.9) | 40 (50.6) |
| >Median | 325 (51.5) | 12 (46.2) | 48 (51.1) | 39 (49.4) |
| Missing | 5 (0.8) | 0 | 1 (1.1) | 0 |
| Median household incomea,f | ||||
| ≤Median | 309 (49.0) | 15 (57.7) | 52 (55.3) | 44 (55.7) |
| >Median | 322 (51.0) | 11 (42.3) | 42 (44.7) | 35 (44.3) |
| ADI state rankg | ||||
| ≤Median | 362 (57.4) | 14 (53.8) | 40 (42.6) | 44 (55.7) |
| >Median | 231 (36.6) | 11 (42.3) | 50 (53.2) | 31 (39.2) |
| Missing | 38 (6.0) | 1 (3.8) | 4 (4.3) | 4 (5.1) |
| Type of referring physiciana,b | ||||
| Pulmonology, thoracic oncology, radiology or surgery | 102 (16.2) | 4 (15.4) | 20 (21.3) | 24 (30.4) |
| Otherh | 529 (83.8) | 22 (84.6) | 74 (78.7) | 55 (69.6) |
| Expected follow-up examination | ||||
| Before COVID-19 | 595 (94.3) | 25 (96.2) | 89 (94.7) | 69 (87.3) |
| During COVID-19 pause | 8 (1.3) | 0 | 1 (1.1) | 2 (2.5) |
| After COVID-19 pause | 28 (4.4) | 1 (3.8) | 4 (4.3) | 8 (10.1) |
Abbreviations: ADI, Area Deprivation Index; CCI, Charlson Comorbidity Index; Lung-RADS, Lung CT Screening Reporting & Data System.
Variable was adjusted for in experiment 2 (ie, significant baseline factors from experiment 1).
P < .05 from the χ2 test.
Subcategories were American Indian or Alaska Native, Native Hawaiian or Pacific Islander, more than 1 race, or racial and ethnic group not otherwise stated.
Subcategories were Veterans Health Administration, self-pay, and other insurance not specified.
Median distance to screening center was 5.48 miles.
Median household income was $74 011.
Median ADI state rank was 3.
Subcategories were family medicine, general internal medicine, and obstetrics and gynecology.
For patients with a negative screening result at baseline, results from the GEE model suggested that the odds of being nonadherent to the Lung-RADS recommendations at the second screening increased in the group with unchanged negative screening results (AOR, 1.38; 95% CI, 1.12-1.69) but decreased in patients in the upgraded category (AOR, 0.29; 95% CI, 0.14-0.60) (Table 4). For those with a positive baseline screening result, the odds of being nonadherent at the following screening with a negative result increased (AOR, 5.08; 95% CI, 1.28-20.1). There was no significant change in adherence in the group with unchanged positive screening results at the second screening. In addition, no significant difference in adherence at the third screening was found across the 4 subgroups.
Table 4. Summary of Findings From Generalized Estimating Equations Analysis of Nonadherence to Lung-RADS Recommendations Measured Over Time Among 830 Patients in Experiment 2.
| Comparison of interest | Nonadherence to T1 Lung-RADS recommendations | Nonadherence to T2 Lung-RADS recommendations | ||
|---|---|---|---|---|
| AOR (95% CI)a | P value | AOR (95% CI)a | P value | |
| Baseline Lung-RADS score of 1 or 2 | ||||
| Unchanged subsequently vs T0 | 1.38 (1.12-1.69) | .002 | 1.17 (0.90-1.52) | .23 |
| Upgraded subsequently vs T0 | 0.29 (0.14-0.60) | <.001 | 0.44 (0.19-1.01) | .054 |
| Baseline Lung-RADS score of 3 or 4 | ||||
| Unchanged subsequently vs T0 | 1.81 (0.62-5.22) | .28 | 1.34 (0.16-10.9) | .78 |
| Downgraded subsequently vs T0 | 5.08 (1.28-20.1) | .02 | 6.99 (0.66-74.1) | .11 |
Abbreviations: AOR, adjusted odds ratio; Lung-RADS, Lung CT Screening Reporting & Data System; T0, first screening time point; T1, second screening time point; T2, third screening time point.
Adjusted baseline variables included baseline Lung-RADS score, family history of lung cancer, educational level, median household income, age-adjusted Charlson Comorbidity Index score, and type of referring physician.
Discussion
As the volume of patients participating in clinical LCS practices increases, the challenge of addressing low adherence to Lung-RADS recommendations is magnified as observed among patients with negative screening results in this study. Identifying factors associated with nonadherence may help resource-constrained health systems to direct targeted outreach to patients who are at a high risk of nonadherence and thus likely to receive the greatest benefit from targeted interventions. Appointment reminders and/or LCS educational materials sent to patients by mail or via patient health portals in the electronic medical record as well as reinforcement of LCS-related benefits by the screening program are possible interventions to mitigate nonadherence.
Our findings that Lung-RADS scores and the type of referring physician were associated with patient nonadherence to baseline Lung-RADS recommendations aligned with previous studies.15,16,17 The baseline Lung-RADS score was the most important variable when estimating whether a patient would be adherent in returning for their initial follow-up screening examination. Patients with a negative baseline screening result were at high risk for nonadherence. A study by Wildstein et al18 found that a higher educational level (eg, at least a college degree) was associated with annual adherence to LCS, although the study was conducted prior to the release date of the Lung-RADS recommendations. Our study found that a family history of lung cancer, comorbidity (high vs low CCI score), and lower income were factors significantly associated with nonadherence at the first follow-up, a finding that, to our knowledge, has not been previously reported in LCS literature. These factors have been previously studied in colorectal and breast cancer screening19,20,21 but with sometimes conflicting results, as in the case of medical comorbidity.19,20 As such, further investigation on the clinical significance of these factors is necessary.
The major contribution of this study lies in the identification of changes in Lung-RADS scores as an important factor associated with nonadherence to LCS across multiple screening time points. Our analysis provides insights into which groups of patients may be more likely to be nonadherent in subsequent screening examinations. For example, if patients have had consecutive screenings with negative results, their adherence may diminish over time. In this study, individuals in this group tended to be younger at baseline and referred by physicians from nonpulmonary or nonthoracic departments. These observations may help inform which patients are at highest risk of nonadherence to annual screening, which can delay the diagnosis of lung cancer.7,22 Of note, cancers first observed at incidence screenings tend to be faster growing and more aggressive than those identified at prevalence screens,7 a finding indicating the importance of adherence to follow-up recommendations. In the present study, the GEE model results suggest that patients with a positive baseline screening result followed by a negative screening result may also need assistance in maintaining adherence at the first annual screening (ie, nonadherence increased over time). However, further investigation is needed given the wide 95% CI. Our findings regarding changes in adherence as patients underwent subsequent screenings underscore the need for screening programs to provide ongoing patient education and reminders, facilitate adherence by providing screening locations near the patient, and minimize patient inconvenience through timely scheduling and efficient patient throughput.
In the future, the findings of this study may be incorporated into a temporal model that helps evaluate adherence status at each screening time point, adding time-varying variables into the temporal model to achieve better performance by considering the changes in patients’ health status at each screening (eg, age-adjusted CCI score, smoking status, and insurance status). Finally, the use of specific types of outreach intervention (eg, reminders, consultations, and educational materials) to improve adherence will vary based on the underlying reason why an individual may be nonadherent. While reminders and educational outreach have been helpful in other screening contexts,23,24 a greater understanding of the psychological, cognitive, social, and health care practitioner factors associated with screening adherence may be essential to optimize outreach interventions. Further studies that explicitly examine these factors are needed.
Limitations
This study has limitations. Several potential risk factors were not considered in our investigation due to a lack of data. Carter-Harris et al25 proposed additional important factors associated with patient behavior toward LCS, including patient psychological, cognitive, social, and environmental factors and health care practitioner recommendations. These variables were previously shown to be associated with patient behaviors toward screening for lung or other types of cancer.26,27,28,29,30,31,32,33 Unlike immutable factors, such as race and ethnicity, psychological and cognitive factors can change over time, providing opportunities for outreach interventions. Other factors associated with cancer screening rates are social determinants of health.34,35 Moreover, it was not possible to track patients who had permanently moved but continued LCS at outside institutions. The factors that we assessed were limited to data elements that were captured routinely in the medical records. Future work is needed to evaluate other life circumstances (eg, personal, such as health [eg, had other medical issues, so LCS was not a priority], family, and socioeconomic), professional activities (eg, workload), and social and environmental factors (eg, childcare and family responsibilities) that might affect adherence.
The lack of primary care physician involvement may be another major factor associated with patients’ adherence behaviors in LCS. Primary care physicians may be less familiar with LCS, its relative risks and benefits, and eligibility requirements for reimbursement compared with other cancer screening examinations. Although annual review of preventive health measures is inherent to primary care, LCS is nascent in practice, and there are myriad reasons why primary care referrals may be associated with less adherence. Compared with other preventive measures, LCS requires a greater time commitment for shared decision-making, smoking cessation counseling, and formal documentation. Our study only examined a high-level variable to distinguish primary care and subspecialty referrals, which cannot capture nuances of physician awareness or practice constraints.
Conclusions
In this cohort study, we identified factors associated with patient nonadherence to Lung-RADS recommendations across 3 screening time points. We showed that the Lung-RADS score at baseline was the most important factor associated with nonadherence at the initial follow-up screening. Patients with consecutive negative screening results were at the greatest risk of being nonadherent to a subsequent screening. Our study provides evidence that may be used as the basis of a decision-support tool to estimate nonadherence across multiple time points and inform future outreach interventions designed to improve patient adherence to LCS.
eAppendix 1. Supplemental Methods and Results
eAppendix 2. Performance of Machine Learning Classifiers Trained Using Identified Predictors
eFigure 1. Examples of Determining Patient Adherence Statuses to Lung-RADS Recommendations
eFigure 2. Overall Pipeline of the Experiment Described in eAppendix 1
eTable 1. Comparison of Observed Baseline Characteristics Between Included Patients and Excluded Patients for Experiment 1
eTable 2. Possible Scenarios of Longitudinal Patterns in Lung-RADS Scores
eTable 3. Validation Performance of Machine Learning Models Using Repeated (n = 5) 10-fold Cross-Validation
eTable 4. Summary of the Number of Patients Enrolled Each Year and Their Mean Follow-Up Time
Data Sharing Statement
References
- 1.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team . Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi: 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 3.American College of Radiology. Lung CT screening reporting & data system (Lung-RADS). Accessed May 2, 2022. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads
- 4.American College of Radiology. Lung-RADS version 1.1. Accessed May 2, 2022. https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf
- 5.Lin Y, Fu M, Ding R, et al. Patient adherence to Lung CT Screening Reporting & Data System—recommended screening intervals in the United States: a systematic review and meta-analysis. J Thorac Oncol. 2022;17(1):38-55. doi: 10.1016/j.jtho.2021.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith HB, Schneider E, Tanner NT. An evaluation of annual adherence to lung cancer screening in a large national cohort. Am J Prev Med. 2022;63(2):e59-e64. doi: 10.1016/j.amepre.2022.01.016 [DOI] [PubMed] [Google Scholar]
- 7.Schabath MB, Massion PP, Thompson ZJ, et al. Differences in patient outcomes of prevalence, interval, and screen-detected lung cancers in the CT arm of the National Lung Screening Trial. PLoS One. 2016;11(8):e0159880. doi: 10.1371/journal.pone.0159880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang GX, Baggett TP, Pandharipande PV, et al. Barriers to lung cancer screening engagement from the patient and provider perspective. Radiology. 2019;290(2):278-287. doi: 10.1148/radiol.2018180212 [DOI] [PubMed] [Google Scholar]
- 9.Borondy Kitts AK. The patient perspective on lung cancer screening and health disparities. J Am Coll Radiol. 2019;16(4, pt B):601-606. doi: 10.1016/j.jacr.2018.12.028 [DOI] [PubMed] [Google Scholar]
- 10.American College of Radiology. Lung-RADS version 1.0. Accessed May 2, 2022. https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADS_AssessmentCategories.pdf
- 11.Suidan RS, Leitao MM Jr, Zivanovic O, et al. Predictive value of the Age-Adjusted Charlson Comorbidity Index on perioperative complications and survival in patients undergoing primary debulking surgery for advanced epithelial ovarian cancer. Gynecol Oncol. 2015;138(2):246-251. doi: 10.1016/j.ygyno.2015.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the Neighborhood Atlas. N Engl J Med. 2018;378(26):2456-2458. doi: 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 14.R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2022. Accessed December 19, 2022. https://www.R-project.org/
- 15.Bellinger C, Foley K, Genese F, Lampkin A, Kuperberg S. Factors affecting patient adherence to lung cancer screening. South Med J. 2020;113(11):564-567. doi: 10.14423/SMJ.0000000000001167 [DOI] [PubMed] [Google Scholar]
- 16.Bernstein MA, Gold S, Ronk M, Drysdale L, Krinsley J, Ebright MI. The challenge of achieving appropriate follow-up in a community lung cancer screening program. Am J Respir Crit Care Med. 2019;199:A4482. doi: 10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A4482 [DOI] [Google Scholar]
- 17.Triplette M, Thayer JH, Kross EK, et al. The impact of smoking and screening results on adherence to follow-up in an academic multisite lung cancer screening program. Ann Am Thorac Soc. 2021;18(3):545-547. doi: 10.1513/AnnalsATS.202006-631RL [DOI] [PubMed] [Google Scholar]
- 18.Wildstein KA, Faustini Y, Yip R, Henschke CI, Ostroff JS. Longitudinal predictors of adherence to annual follow-up in a lung cancer screening programme. J Med Screen. 2011;18(3):154-159. doi: 10.1258/jms.2011.010127 [DOI] [PubMed] [Google Scholar]
- 19.Hubbard RA, O’Meara ES, Henderson LM, et al. Multilevel factors associated with long-term adherence to screening mammography in older women in the US. Prev Med. 2016;89:169-177. doi: 10.1016/j.ypmed.2016.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomsen MK, Rasmussen M, Njor SH, Mikkelsen EM. Demographic and comorbidity predictors of adherence to diagnostic colonoscopy in the Danish Colorectal Cancer Screening Program: a nationwide cross-sectional study. Clin Epidemiol. 2018;10:1733-1742. doi: 10.2147/CLEP.S176923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman SMDM, Dignan MB, Shelton BJ. Factors influencing adherence to guidelines for screening mammography among women aged 40 years and older. Ethn Dis. 2003;13(4):477-484. [PMC free article] [PubMed] [Google Scholar]
- 22.Silvestri GA, Goldman L, Tanner NT, et al. Outcomes from more than 1 million people screened for lung cancer with low-dose CT imaging. Chest. Published online February 10, 2023. doi: 10.1016/j.chest.2023.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougherty MKBA, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(12):1645-1658. doi: 10.1001/jamainternmed.2018.4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baron RCRB, Rimer BK, Breslow RA, et al. ; Task Force on Community Preventive Services . Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening a systematic review. Am J Prev Med. 2008;35(1)(suppl):S34-S55. doi: 10.1016/j.amepre.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 25.Carter-Harris L, Davis LL, Rawl SM. Lung cancer screening participation: developing a conceptual model to guide research. Res Theory Nurs Pract. 2016;30(4):333-352. doi: 10.1891/1541-6577.30.4.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonnalagadda S, Bergamo C, Lin JJ, et al. Beliefs and attitudes about lung cancer screening among smokers. Lung Cancer. 2012;77(3):526-531. doi: 10.1016/j.lungcan.2012.05.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel D, Akporobaro A, Chinyanganya N, et al. ; Lung-SEARCH Investigators . Attitudes to participation in a lung cancer screening trial: a qualitative study. Thorax. 2012;67(5):418-425. doi: 10.1136/thoraxjnl-2011-200055 [DOI] [PubMed] [Google Scholar]
- 28.Carter-Harris L, Ceppa DP, Hanna N, Rawl SM. Lung cancer screening: what do long-term smokers know and believe? Health Expect. 2017;20(1):59-68. doi: 10.1111/hex.12433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charkazi A, Samimi A, Razzaghi K, et al. Adherence to recommended breast cancer screening in Iranian Turkmen women: the role of knowledge and beliefs. ISRN Prev Med. 2013;2013:581027. doi: 10.5402/2013/581027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tessaro I, Mangone C, Parkar I, Pawar V. Knowledge, barriers, and predictors of colorectal cancer screening in an Appalachian church population. Prev Chronic Dis. 2006;3(4):A123. [PMC free article] [PubMed] [Google Scholar]
- 31.Ye J, Xu Z, Aladesanmi O. Provider recommendation for colorectal cancer screening: examining the role of patients’ socioeconomic status and health insurance. Cancer Epidemiol. 2009;33(3-4):207-211. doi: 10.1016/j.canep.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 32.Anderson JO, Mullins RM, Siahpush M, Spittal MJ, Wakefield M. Mass media campaign improves cervical screening across all socio-economic groups. Health Educ Res. 2009;24(5):867-875. doi: 10.1093/her/cyp023 [DOI] [PubMed] [Google Scholar]
- 33.Allen JD, Sorensen G, Stoddard AM, Peterson KE, Colditz G. The relationship between social network characteristics and breast cancer screening practices among employed women. Ann Behav Med. 1999;21(3):193-200. doi: 10.1007/BF02884833 [DOI] [PubMed] [Google Scholar]
- 34.Shin D, Fishman MDC, Ngo M, Wang J, LeBedis CA. The impact of social determinants of health on lung cancer screening utilization. J Am Coll Radiol. 2022;19(1, pt B):122-130. doi: 10.1016/j.jacr.2021.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurani SS, McCoy RG, Lampman MA, et al. Association of neighborhood measures of social determinants of health with breast, cervical, and colorectal cancer screening rates in the US Midwest. JAMA Netw Open. 2020;3(3):e200618. doi: 10.1001/jamanetworkopen.2020.0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Supplemental Methods and Results
eAppendix 2. Performance of Machine Learning Classifiers Trained Using Identified Predictors
eFigure 1. Examples of Determining Patient Adherence Statuses to Lung-RADS Recommendations
eFigure 2. Overall Pipeline of the Experiment Described in eAppendix 1
eTable 1. Comparison of Observed Baseline Characteristics Between Included Patients and Excluded Patients for Experiment 1
eTable 2. Possible Scenarios of Longitudinal Patterns in Lung-RADS Scores
eTable 3. Validation Performance of Machine Learning Models Using Repeated (n = 5) 10-fold Cross-Validation
eTable 4. Summary of the Number of Patients Enrolled Each Year and Their Mean Follow-Up Time
Data Sharing Statement


