Abstract
T-cell prolymphocytic leukemia (T-PLL) is a rare and aggressive neoplasm that originates from mature post-thymic T-cells. Cutaneous manifestations are a common presentation in T-PLL but rarely are a presentation in the recurrent setting. Here, we describe the case of a 75-year-old female with a history of T-PLL—who at the time of initial diagnosis did not exhibit any rash—presenting with diffuse rash, facial swelling, sore throat, and dysphagia 7 months later and was found to have recurrent T-PLL. She had diffuse lymphadenopathy and diffuse skin lesions. Biopsy of the skin lesions also confirmed infiltration with T-PLL cells. After review of the literature, no previously reported cases of recurrent T-PLL presented as diffuse skin lesions. This case demonstrates that recurrent T-PLL may present with diffuse rash, respiratory distress, and anasarca. It is important to stay vigilant in patients with history of T-PLL to recognize signs of recurrent disease to allow prompt diagnosis and treatment.
Keywords: T-cell prolymphocytic leukemia (T-PLL), erythema, alemtuzumab, Pentostatin, dermatology, hematology oncology
Introduction
T-cell prolymphocytic leukemia (T-PLL) is a rare and aggressive neoplasm originating from mature post-thymic T-cells. It is found in middle-aged males and females with a slight preponderance for males. 1 In a study by Matutes et al, 3 the main clinical features of T-PLL were found to be splenomegaly (73%), lymphadenopathy (53%), hepatomegaly (40%), skin lesions (27%), and leukocytosis (>100 × 109). Although cutaneous manifestations occur in 27% of patients as a presenting symptom, 2 large-scale studies have not been conducted to report the clinical features of recurrence of T-PLL. Shumilov et al 4 noted in a study population of 10 patients that T-PLL relapse occurred in 3 patients with bulky peritoneal disease, pleural effusions, splenomegaly, lymphocytosis, and skin manifestations. Only 1 of these patients relapsed with skin manifestations. To the best of our knowledge, no cases of T-PLL in the manner with which our patient has presented have been reported in the literature—with generalized skin lesions and in the recurrent setting. Therefore, we report a case of a patient with T-PLL who developed diffuse skin rash, anasarca, and respiratory distress requiring intubation and mechanical ventilation in the recurrent setting of T-PLL.
Case History
A 75-year-old female with a past medical history of hypertension and hyperlipidemia was referred to our clinic in 2021 for evaluation of leukocytosis. Her white blood cell (WBC) count from 2019 was 16.3 × 109/L. She stated some weight loss of 6 lbs in 1 year and fatigue for the past 3 months. She did not complain of any fever, chills, or night sweats. Laboratory work showed WBC count of 461 × 109/L and hemoglobin (Hgb) of 6.9 gm/dL. After admission to the hospital, the flow cytometry of the peripheral blood showed T-PLL with hyperleukocytosis. The phenotype was reported as the following: CD2+, CD3+, CD4+, CD5+, CD7+, CD8−, CD56−, CD57−, CD25−, CD26+, TCRab+, CD30−, CD52+, CD34−, and CD45±. HTLV-1 (Human T-lymphotrophic virus 1) was negative. A bone marrow biopsy was done which yielded 90% atypical T-cells. Flow cytometric analysis of the aspirate returned a diagnosis of T-PLL with phenotype CD2+, CD3+, CD4+, CD5+, CD7+, CD30−, CD25−, CD26+, CD34−, TCRab+, and CD45± with 80% T-PLL cells. Cytogenetic analysis was positive for inversion at chromosome 14 and trisomy 8 which have been specifically reported in T-PLL. Her karyotype analysis showed 47, XX, add(4)(q35), +8, inv(14)(q11.2q32), add(20)(P13). 5 Gross cytomorphologic review showed marked lymphocytosis with prominent cytologic atypia (Figure 1(A) and (B)). T-cell percent was 85%. A clonal T-cell gene rearrangement was also detected for the T-cell gamma receptor. A computed tomography chest, abdomen, and pelvis was positive for moderate splenomegaly and negative for any masses or lymphadenopathy. Next-generation sequencing later also detected Tier III: variants of unknown clinical significance in JAK1 p(His374Tyr). It was negative for microsatellite instability and showed a low tumor mutation burden (3.1 mutations/MB). She was diagnosed as T-PLL and was treated with alemtuzumab. She received 3 cycles of 3-mg alemtuzumab subcutaneously initially. Her WBCs were persistently elevated so the dose was increased to 10 mg and pentostatin was added. After 10 cycles of 10-mg alemtuzumab and 2 cycles of pentostatin 4 mg/m2, her WBC was still >500 × 109/L. Alemtuzumab was increased to 30 mg. After cycle 13 for alemtuzumab and cycle 7 for pentostatin, her WBC count started to trend down to around 300 × 109/L. After 18 cycles of alemtuzumab and 7 cycles of pentostatin, she completed her chemotherapy. Postchemotherapy CBC (complete blood count) showed WBC of 2.7 × 109/L and Hgb of 11 gm/dL. Flow cytometry postchemotherapy still showed monoclonal TCR (t-cell receptor) rearrangement of the gamma chain. It did not show significant numbers of circulating blasts or an abnormal lymphoid or myeloid population. The patient was asymptomatic and without complaints until 7 months later when she presented to the emergency department with complaints of rash, facial swelling, sore throat, dysphagia, and conjunctivitis. On physical examination, the rash was noted to involve her entire body including the trunk, extremities, and face, with edema of the bilateral lower extremities (Figure 1(A)-(C)). She was afebrile with a WBC of 41 × 109/L and absolute lymphocyte count of 28 × 109/L. Cytomegalovirus DNA polymerase chain reaction testing was negative, making an infectious cause of her underlying presentation unlikely. Her clinical picture was indicative of T-PLL relapse. It was confirmed with flow cytometry analysis. A peripheral blood smear revealed numerous atypical lymphocytes, normochromic, with round nuclei and prominent nucleoli as shown in Figure 2(A) and (B). Her uric acid (13.70 mg/dL) and creatinine (2.52 mg/dL) were both found to be elevated—likely secondary to tumor lysis syndrome. Rasburicase was given to help manage it. Later during her hospital stay, the patient’s rash continued to worsen. She developed anasarca after her edema progressed. She also developed dyspnea that was partially relieved with steroids, epinephrine, and antihistamines. Her white counts continued to rise. She was restarted on alemtuzumab and pentostatin therapy. She was continued on CMV (cytomegalovirus) and PCP (pneumocystis carinii pneumonia) prophylaxis with valganciclovir and Bactrim. Peripheral blood smear showed the same morphology as Figure 2, and flow cytometry showed the same clone of CD4/CD3+/CD7+ T-cells—findings compatible with persistent disease. Skin biopsy that was taken around her time of admission was positive for atypical perivascular lymphocytic infiltrate, with CD7+, CD2+, CD3+, CD4+, and CD5+, and negative for CD8, CD30, and CD56 (Figure 3(A) and (B)).
Figure 1.
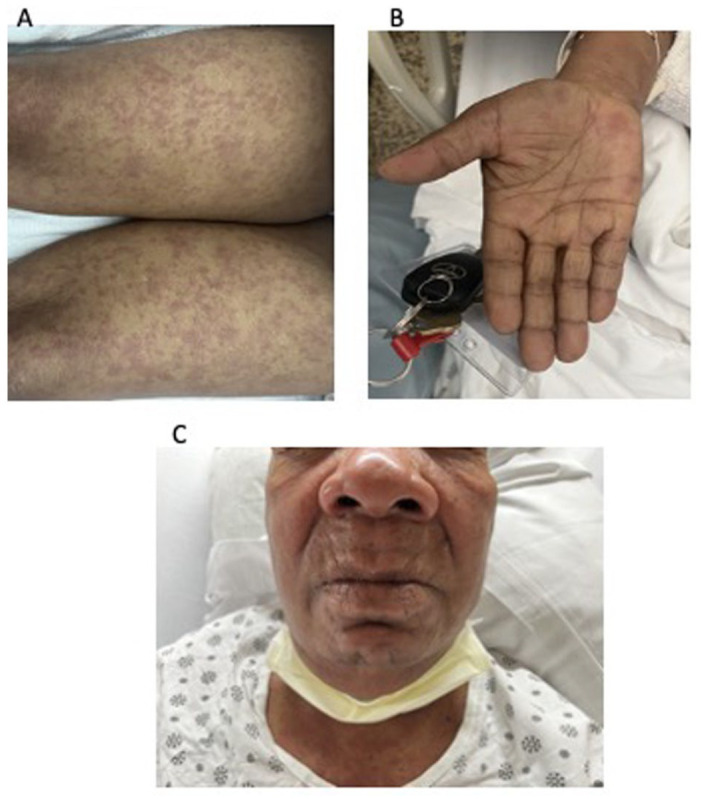
Generalized erythematous maculopapular cutaneous eruption (flat, pink, nontender) observed on both (A) legs, (B) palms, and (C) chest and face, with anasarca.
Figure 2.
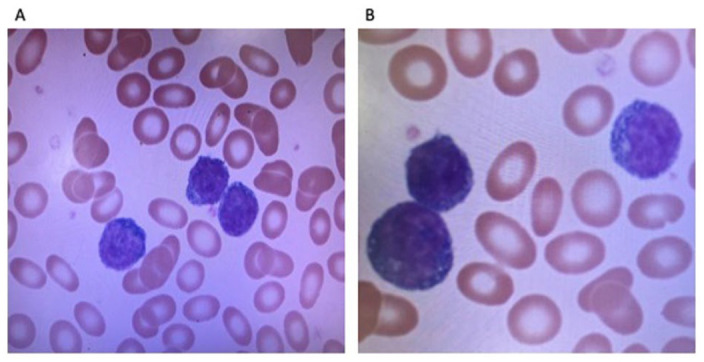
Many medium-sized lymphoid cells with moderately condensed chromatin and prominent nucleolus were seen in the peripheral smear – suggestive of a lymhpoid malignancy.
Figure 3.
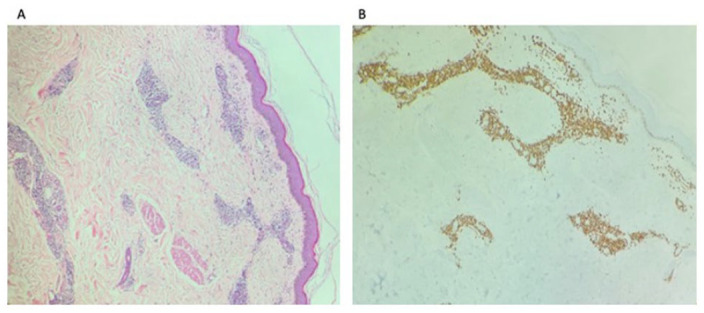
Atypical perivascular T-cell infiltrates (A) and were positive for CD3, CD4, CD5, and CD7 and negative for CD8, CD30, and CD56 (B) consistent with cutaneous involvements by the diagnosis of T-prolymphocytic leukemia (T-PLL).
Computed tomography neck was positive for lymphadenopathy in the mediastinal and cervical chains. Her white counts began to drop after chemotherapy but a few days later, her respiratory status worsened once more. Evaluation by our otolaryngologists led them to attributing the airway edema to lymphocytic infiltration secondary to T-PLL. Later, she was found to be tachypneic, and her oxygen saturation dropped to 82%. Anasarca also worsened. She developed profound hypotension and shock. She was given racemic epinephrine, Benadryl, and methylprednisolone. Her respiratory status did not improve with this treatment so she was emergently intubated, ventilated, and started on intravenous pressor support. Her white cell counts trended back up to 50 × 109/L, lactate increased to 12 mmol/L, and creatinine to 3.28 mg/dL. Infectious workup was done for her as sepsis was suspected. She was transferred to the medical intensive care unit; however, she passed away the next morning.
Discussion
T-cell prolymphocytic leukemia is a very rare and aggressive form of mature T-cell leukemia. The major phenotypes in T-PLL are CD4+/CD8−; however, CD4+/CD8+ and CD4−/ CD8+ phenotypes have also been previously reported. 6 Genetic alterations occur in the form of chromosome 14 rearrangements leading to overexpression of proto-oncogene TCL1. There are recurrent changes on chromosome 14 such as inv (14) (q11q32) and t(14;14) (q11;q32) which are observed in 66% of the cases. TCL1 plays a pivotal role in pathogenesis of T-PLL. Its overexpression leads to activation of protein kinase B which results in increased cell proliferation.6-11 Other genetic abnormalities that have been reported are trisomy for 8q resulting in overexpression of MYC proto-oncogene or rearrangements with 8p12.12,13 Recently, next-generation genomic sequencing/whole-exome sequencing of T-PLL has revealed mutations in IL2RG, JAK1, JAK3, or STAT5B affecting the pathogenesis of T-PLL. Next-generation testing on our patient was positive for JAK-1 p(His374Tyr). Studies have revealed high prevalence of mutations of JAK-STAT pathways with gain of function involving JAK1, JAK3, and STAT5B. These mutations lead to oncogenic transformation of STAT 5.14,15
Cutaneous manifestations in T-PLL include retiform hyperpigmented macules and patches. They also include skin nodules and in rare cases erythroderma. Edema is the most common presenting facial eruption. Conjunctival involvement and periorbital petechiae have also been reported in the literature in cases of T-PLL. 16 Cutaneous findings are present at time of initial diagnosis and are overall relatively common. One study conducted at the Washington University found that nearly 32% of patients presenting with T-PLL exhibited cutaneous manifestations. 13 Another analysis by Matutes et al 3 found splenomegaly as the most common symptom (73%) and skin lesions in 27% of patients in a study of 78 patients with T-PLL. In patients with T-PLL with skin involvement, it is often noted to be a presenting symptom. Therefore, skin involvement is most often a presenting symptom in T-PLL, even if it is not the most common clinical finding. 1 However, our patient had no skin manifestations at initial diagnosis. She manifested a diffuse, generalized skin rash along with anasarca, angioedema, and severe respiratory distress during recurrence of disease. To our knowledge, this is the first report of a patient presenting with this clinical picture in T-PLL. Shumilov et al 4 noted in a study of T-PLL relapse that only 1 of the patients in the study population relapsed with skin manifestations. In T-PLL, punch biopsy of skin lesions shows superficial and deep dermal hemorrhage with perivascular lymphoid infiltration with no evidence of vasculitis and occasional epidermotropism. Immunohistochemistry on skin biopsy is positive for CD3+, CD4+, and CD8+. 17 In our case, skin biopsy was taken at the time of admission for suspected disease recurrence and was positive for atypical perivascular lymphocytic infiltrate, with CD7+, CD2+, CD3+, CD4+, and CD5 positivity. Lymphocytes had high nuclear-to-cytoplasmic ratios. Micrographs of the biopsy specimens are shown in Figure 3(A)-(C).
Patients with T-PLL have an aggressive disease course. The disease is resistant to conventional chemotherapy in most cases. The current recommended treatment approach in active T-PLL remains successful induction by alemtuzumab infusion followed by consolidation with allogeneic hematopoietic stem cell transplantation in eligible patients. Alemtuzumab, previously known as campath (anti-CD52 antibody), remains the main first-line treatment for T-PLL. It is a humanized anti-CD52 antibody that functions by inducing complement activation, apoptosis, and cell lysis. It has shown overall response rates (ORRs) of 90% or higher and complete response of 81% in comparison with traditional chemotherapy.15,18,19 However, despite the good response rate achieved by alemtuzumab, patients who do not receive hematopoietic stem cell transplantation relapse—with median survival of 17 to 33 months. In patients receiving allogeneic stem cell transplantation, the median survival rate is 48 months.3,14,20 Pentostatin, an adenosine deaminase inhibiting purine analog, can be added to the treatment regimen of patients who fail to attain complete remission with alemtuzumab. In a study of 13 patients with resistant T-PLL, alemtuzumab evaluated in combination with pentostatin therapy demonstrated an ORR of 69%. 14 Consolidation for T-PLL is achieved effectively with hematopoietic stem cell transplantation. Both forms of stem cell transplantation—autologous and allogeneic—prolong overall survival when compared with isolated induction with alemtuzumab.1,5,14,19 In patients who have relapse of T-PLL, the combination of alemtuzumab and pentostatin has been shown to achieve an ORR of 75%. Bendamustine can also be used and has shown an ORR of 53.3. 5 There are other upcoming treatment approaches based on mutations observed in T-PLL. Gain of function mutations in JAK and STAT genes has been described recently to be highly frequent in T-PLL, varying from 36% to 76%. JAK inhibitors (tofacitinib/ruxolitinib) and selective STAT-5 inhibitors like pimozide have proven effective in case reports and are being further studied in clinical trials.21-23 Chimeric antigen receptor (CAR) T-cell therapy is an emerging field that has potential applications in T-cell malignancies. CD30 may be a possible target antigen. In vitro data have shown that malignant T-cells expressing a single beta chain in the TCR beta chain (TRBC1 and TRBC2) were selectively targeted by CAR T-cells—sparing healthy T-cells expressing a different TRBC.17,21 These can be used as targeted treatment. 14
Conclusion
T-cell prolymphocytic leukemia is a rare type of cancer with a very aggressive course. It can manifest with constitutional symptoms, organomegaly, skin lesions, and systemic manifestations. In our case, the patient presented with a diffuse skin rash, anasarca, and respiratory distress in the recurrent setting. The respiratory distress occurred secondary to angioedema and upper airway edema possibly secondary to lymphocytic infiltration of the mucosa. In patients with remission, it is important to stay vigilant to signs of relapse for prompt diagnosis and treatment.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.C.W. received research grants from Kartos.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Ethical approval to report this case was obtained from Brookdale Hospital Institutional Review Board. Our institution does not require ethical approval for reporting individual case reports.
Informed Consent: The patient expired before consent could be taken. Brookdale Hospital Institutional Review Board does not need consent for publishing case reports
ORCID iD: Jen C. Wang  https://orcid.org/0000-0002-9623-6645
https://orcid.org/0000-0002-9623-6645
References
- 1.Dearden CE.T-cell prolymphocytic leukemia. Clin Lymphoma Myeloma. 2009;9(suppl 3):S239-S243. doi: 10.3816/CLM.2009.s.018. [DOI] [PubMed] [Google Scholar]
- 2.Serra A, Estrach MT, Martí R, Villamor N, Rafel M, Montserrat E.Cutaneous involvement as the first manifestation in a case of T-cell prolymphocytic leukaemia. Acta Derm Venereol. 1998; 78(3):198-200. doi: 10.1080/000155598441521. [DOI] [PubMed] [Google Scholar]
- 3.Matutes E, Brito-Babapulle V, Swansbury J, et al. Clinical and laboratory features of 78 cases of T-prolymphocytic leukemia. Blood. 1991;78(12):3269-3274. [PubMed] [Google Scholar]
- 4.Shumilov E, Hasenkamp J, Szuszies CJ, Koch R, Wulf GG.Patterns of late relapse after allogeneic hematopoietic stem cell transplantation in patients with T-cell prolymphocytic leukemia. Acta Haematol. 2021;144(1):105-110. doi: 10.1159/000506302. [DOI] [PubMed] [Google Scholar]
- 5.Staber PB, Herling M, Bellido M, et al. Consensus criteria for diagnosis, staging, and treatment response assessment of T-cell prolymphocytic leukemia. Blood. 2019;134(14):1132-1143. doi: 10.1182/blood.2019000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herling M, Patel KA, Teitell MA, et al. High TCL1 expression and intact T-cell receptor signaling define a hyperproliferative subset of T-cell prolymphocytic leukemia. Blood. 2008;111(1): 328-337. doi: 10.1182/blood-2007-07-101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narducci MG, Stoppacciaro A, Imada K, et al. TCL1 is overexpressed in patients affected by adult T-cell leukemias. Cancer Res. 1997;57(24):5452-5456. [PubMed] [Google Scholar]
- 8.Pekarsky Y, Koval A, Hallas C, et al. Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proc Natl Acad Sci U S A. 2000;97(7):3028-3033. doi: 10.1073/pnas.97.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pekarsky Y, Hallas C, Croce CM.Molecular basis of mature T-cell leukemia. JAMA. 2001;286(18):2308-2314. doi: 10.1001/jama.286.18.2308. [DOI] [PubMed] [Google Scholar]
- 10.Schlegelberger B, Himmler A, Bartles H, Kuse R, Sterry W, Grote W.Recurrent chromosome abnormalities in peripheral T-cell lymphomas. Cancer Genet Cytogenet. 1994;78(1):15-22. doi: 10.1016/0165-4608(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 11.Pekarsky Y, Hallas C, Croce CM.The role of TCL1 in human T-cell leukemia. Oncogene. 2001;20(40):5638-5643. doi: 10.1038/sj.onc.1204596. [DOI] [PubMed] [Google Scholar]
- 12.Maljaei SH, Brito-Babapulle V, Hiorns LR, Catovsky D.Abnormalities of chromosomes 8, 11, 14, and X in T-prolymphocytic leukemia studied by fluorescence in situ hybridization. Cancer Genet Cytogenet. 1998;103(2):110-116. doi: 10.1016/s0165-4608(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 13.Hsi AC, Robirds DH, Luo J, Kreisel FH, Frater JL, Nguyen TT.T-cell prolymphocytic leukemia frequently shows cutaneous involvement and is associated with gains of MYC, loss of ATM, and TCL1A rearrangement. Am J Surg Pathol. 2014;38(11): 1468-1483. doi: 10.1097/PAS.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 14.Laribi K, Lemaire P, Sandrini J, de Materre AB.Advances in the understanding and management of T-cell prolymphocytic leukemia. Oncotarget. 2017;8(61):104664-104686. doi: 10.18632/oncotarget.22272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain P, Aoki E, Keating M, et al. Characteristics, outcomes, prognostic factors and treatment of patients with T-cell prolymphocytic leukemia (T-PLL). Ann Oncol. 2017;28(7):1554-1559. doi: 10.1093/annonc/mdx163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventre MO, Bacelieri RE, Lazarchick J, Pollack RB, Metcalf JS. Cutaneous presentation of T-cell prolymphocytic leukemia. Cutis. 2013;91(2):87-91. [PubMed] [Google Scholar]
- 17.Leckey BD, Jr, Kheterpal MK, Selim MA, Al-Rohil RN.Cutaneous involvement by T-cell prolymphocytic leukemia presenting as livedoid vasculopathy. J Cutan Pathol. 2021; 48(7):975-979. doi: 10.1111/cup.14023. [DOI] [PubMed] [Google Scholar]
- 18.Dearden C.How I treat prolymphocytic leukemia. Blood. 2012;120(3):538-551. doi: 10.1182/blood-2012-01-380139. [DOI] [PubMed] [Google Scholar]
- 19.Varadarajan I, Ballen K.Advances in cellular therapy for T-cell prolymphocytic leukemia. Front Oncol. 2022;12:781479. doi: 10.3389/fonc.2022.781479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravandi F, O’Brien S, Jones D, et al. T cell prolymphocytic leukemia: a single-institution experience. Clin Lymphoma Myeloma. 2005;6(3):234-239. doi: 10.3816/CLM.2005.n.051. [DOI] [PubMed] [Google Scholar]
- 21.Braun T, Von Jan J, Wahnschaffe L, et al. Advances perspectives in the treatment of T-PLL. Curr Hematol Malig Rep. 2020;15:113-124. doi: 10.1007/s11899-020-00566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colon Ramos A, Tarekegn K, Aujla A, de de Jesus KG, Gupta S.T-cell prolymphocytic leukemia: an overview of current and future approaches. Cureus. 2021;13(2):e13237. doi: 10.7759/cureus.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbaux C, Poulain S, Roos-Weil D, et al. Preliminary study of ruxolitinib and venetoclax for treatment of patients with T-cell prolymphocytic leukemia refractory to, or ineligible for alemtuzumab [abstract]. Blood. 2021;138(suppl 1):1201. doi: 10.1182/blood-2021-149228. [DOI] [Google Scholar]


