Abstract
Chronic pain is a refractory health disease worldwide causing an enormous economic burden on individuals and society. Accumulating evidence suggests that inflammation in the peripheral nervous system (PNS) and central nervous system (CNS) is the major factor in the pathogenesis of chronic pain. The inflammation in the early- and late phase may have distinctive effects on the initiation and resolution of pain, which can be viewed as friend or foe. On the one hand, painful injuries lead to the activation of glial cells and immune cells in the PNS, releasing pro-inflammatory mediators, which contribute to the sensitization of nociceptors, leading to chronic pain; neuroinflammation in the CNS drives central sensitization and promotes the development of chronic pain. On the other hand, macrophages and glial cells of PNS and CNS promote pain resolution via anti-inflammatory mediators and specialized pro-resolving mediators (SPMs). In this review, we provide an overview of the current understanding of inflammation in the deterioration and resolution of pain. Further, we summarize a number of novel strategies that can be used to prevent and treat chronic pain by controlling inflammation. This comprehensive view of the relationship between inflammation and chronic pain and its specific mechanism will provide novel targets for the treatment of chronic pain.
Keywords: Inflammation, chronic pain, glial cell, pro-inflammatory mediator, specialized pro-resolving mediator
Introduction
Inflammation is associated with both pain and multiple disease outcomes, which is characterized by five typical signs: redness, swelling, heat, pain, and loss of function. Inflammation can be divided into three types according to the progress of pathology underlying the injured tissue: (1) acute inflammation occurs shortly after injury and persists for a few days; (2) chronic inflammation occurs when acute inflammation fails to resolve, which may last for several months or even years; (3) subacute inflammation is a transformational period spanning acute to the chronic stage, which lasts from 2 to 6 weeks. 1 The early stage of inflammation aims to eliminate internal threats and initiate tissue repair. As one of the five cardinal signs of inflammation, pain includes acute pain and chronic pain. Acute pain (also known as nociceptive pain) is considered as a protective reaction involving immune cells, and inflammatory mediators. 2 Chronic pain is known as not only a bothersome symptom but also a disease, which is a major health issue worldwide causing a considerable social and economic burden. 3 An estimated more than 30% of adult worldwide suffer from pain according to reports,4–6 the prevalence of chronic pain from different studies are shown in Table 1. Chronic inflammation is maladaptive and often leads to a considerable amount of chronic pain. 16 Of note, inflammation plays a dual role (protective or detrimental) during different stages (acute or chronic phase). 17
Table 1.
Prevalence of chronic pain in different countries and regions.
| Patients and area | Ages | Participants | Definition of chronic pain | Prevalence, % | Refs |
|---|---|---|---|---|---|
| Patients with chronic low back pain in Brazil (Pelotas) | ≥20 | 2732 | ≥7 weeks in the last 3 months | 9.6 | 7 |
| Patients with chronic low back pain in Brazil (Sao Paulo) | ≥60 | 1271 | Continuous pain ≥6 months | 25.4 | 8 |
| Patients with chronic low back pain in Germany | 65–85 | 3189 | Continuous pain≥ 6 months | M 55.2 | 9 |
| F 41.1 | |||||
| Patients with chronic pain in United States | ≥18 | 33,028 | Continuous pain ≥6 months | 20.4 | 4 |
| Patients with chronic pain in United States | ≥18 | 31,997 | Continuous pain ≥3 months | 20.5 | 10 |
| Patients with breast cancer in United States | ≥18 | 1280 | Continuous pain ≥6 months | 33.3 | 11 |
| Patients with cancer pain in French | 18–82 | 864 | Continuous pain ≥3 months | 32.3 | 12 |
| Patients with cancer pain in United States | ≥18 | 7565 | Continuous pain ≥3 months | 32.5 | 13 |
| Patients with chronic pain in Israel | 18–74 | 1647 | Continuous pain ≥3 months | 31.3 | 14 |
| Patients with chronic pain in China (Hong Kong) | ≥18 | 5001 | Continuous pain ≥3 months | 34.9 | 15 |
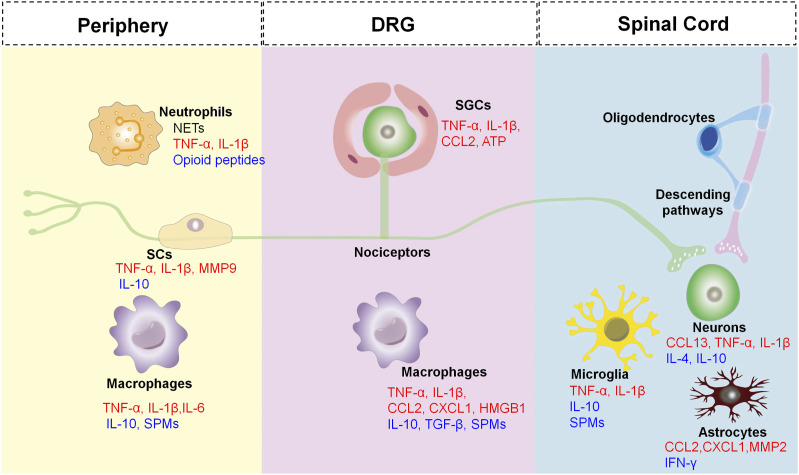
Pain research in the past decades has confirmed that neuronal plasticity is the critical mechanism for the initiation and maintenance of chronic pain. 18 The neuronal plasticity derives not only from the adaptive transformation of neurons themselves, but also from the change of microenvironment composed of immune and glial cells underlying acute and chronic pain. Under inflammatory conditions, there are increased levels of inflammatory mediators in the tissue from immune and glial cells and even neurons, which modulate nociceptors in the PNS and the pathways responsible for pain transmission in the CNS. 1 Neuroinflammation, a special manifestation of tissue inflammation, can occur in the PNS and CNS, the main characteristics of which are the activation of glial cells and the release of neuroinflammatory mediators (Figure 1).19,20
Figure 1.
The cross-talk between neurons and non-neuronal cells leads to deterioration or resolution of pain. Non-neuronal cells [e.g. Schwann cells (SCs), satellite glial cells (SGCs), microglial cells, astrocytes, oligodendrocytes, macrophages, and neutrophils], produce both pro-inflammatory mediators (RED) and anti-inflammatory mediators (BLUE), which can act on their corresponding receptors of the nociceptors causing peripheral and central sensitization marked by a state of hypersensitivity and hyperexcitability of the nociceptor. The central terminals of the nociceptors in the spinal cord form nociceptive synapses with postsynaptic neurons to process pain transmission in the CNS. In addition, descending pathways also exert an inhibitory effect on pain transmission in the spinal cord.
Clinical approaches to treat chronic pain are hampered by various possible causes, such as triggering factors, inflammatory locations, and symptomatic manifestations. In addition, current treatments for persistent or chronic pain are not effective. It usually counteracts the available analgesics, such as opioids and non-steroidal anti-inflammatory drugs, which have serious side effects.21,22 It is therefore imperative to develop new analgesics with a new mechanism of action. Notably, the pro-resolution phase of inflammation is now widely known as an active process in organism, controlled by the specialized pro-resolving mediators (SPMs).23,24 The active process and receptors of SPMs have been confirmed to reduce inflammation, clear microbes, and alleviate pain (Table 2). Thus, SPMs may become promising analgesics for pain relief.
Table 2.
Anti-inflammatory mechanisms of SPMs in chronic pain.
| SPMs | Endogenous sources | Exogenous sources | Anti-inflammatory mechanisms | Refs |
|---|---|---|---|---|
| Resolvin D1 (RvD1) | Neutrophils, macrophages, and platelets | Docosahexaenoic (DHA) | ♦ Promotes resolution of inflammation | 25–29 |
| ♦ Decreases inflammatory response and apoptosis | ||||
| ♦ Protects the endothelial integrity and barrier function | ||||
| ♦ Inhibits the activity of TRPA1, TRPV3, and TRPV4 | ||||
| Resolvin D2 (RvD2) | Macrophages, epithelial cells, and platelets | ♦ Decreases inflammatory response | 30–33 | |
| ♦ Inhibits the activity of TRPV1 and TRPA1 | ||||
| ♦ Increases the production of anti-inflammatory mediator TGF-β1 | ||||
| ♦ Transforms the macrophages from inflammatory to anti-inflammatory phenotype | ||||
| Resolvin D3 (RvD3) | Macrophages and neutrophils | ♦ Inhibits the activity of TRPV1 and CGRP release via a G-protein coupled receptor, such as ALX/FPR2 | 34,35 | |
| ♦ Decreases the inflammatory cytokines and chemokines, such as TNF-α, IL-6, IL-1β, CCL2, and CCL3 | ||||
| Resolvin D4 (RvD4) | Neutrophils, macrophages, and dendritic cells | ♦ Decreases the inflammatory response | 36 | |
| Resolvin D5 (RvD5) | Neutrophils, macrophages, and platelets | ♦ Decreases the expression of IL-6 in male mice with chronic pain | 37 | |
| Resolvin D6 (RvD6) | Neutrophils and macrophages | ♦ Increases the expression of rictor and hepatocyte growth factor (hgf) and rictor genes | 38 | |
| Resolvin E1 (RvE1) | Neutrophils, eosinophils, platelets, and epithelial cells | Eicosapentaenoic (EPA) | ♦ Inhibits the migration of neutrophils to inflammatory site | 39,40 |
| ♦ Decreases the release of TNF-α, IL-1β, and IL-6 | ||||
| Resolvin E2 (RvE2) | Macrophages and dendritic cells | ♦ Inhibits neutrophil infiltration | 40 | |
| ♦ Decreases the expression of pro-inflammatory cytokines | ||||
| Resolvin E3 (RvE3) | Neutrophils and endothelial cells | ♦ Inhibits inflammatory activity | 41 | |
| Maresin 1 (MaR-1) | Macrophages | DHA | ♦ Blocks NLRP3 inflammasome | 42,43 |
| ♦ Shifts from M1 macrophages to M2 phenotype | ||||
| Maresin 2 (MaR-2) | ♦ Blocks neutrophil and monocyte migration | 44 | ||
| ♦ Inhibits the activity of TRPA1, and the release of CGRP | ||||
| Protectins D1 | Neutrophils | DHA | ♦ Inhibits T cell infiltration | 45–47 |
| ♦ Reduces neutrophil recruitment | ||||
| Neuroprotection D1 | Epithelial cells | ♦ Decreases inflammatory response | ||
| Lipoxins | Leukocytes, platelets, endothelial cells, and fibroblasts | Arachidonic acid (AA) | ♦ Inhibits pro-inflammatory mediator production | 48,49 |
| ♦ Blocks neutrophils infiltration |
Peripheral inflammation contributes to the pathogenesis of chronic pain
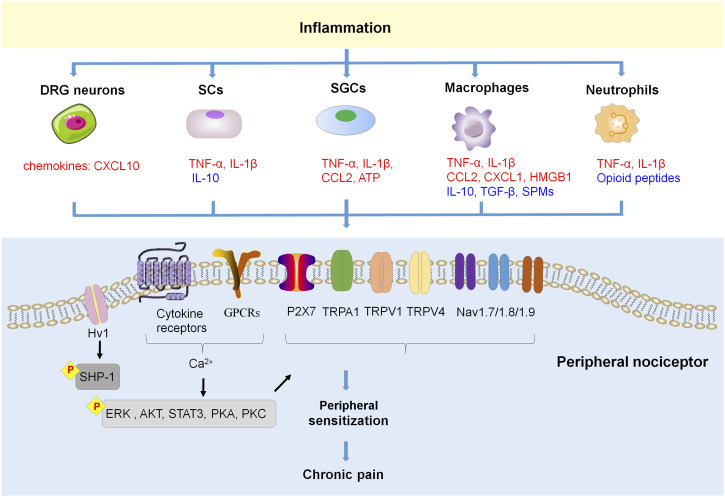
Over the past few decades, accumulating evidence has revealed inflammation in the physiological and pathophysiological processes of chronic pain. Key participants involved are neutrophils, macrophages, and glial cells. In addition, inflammatory mediators (pro-inflammatory cytokines, chemokines), and SPMs also play a significant role in initiation and resolution of chronic pain (Figure 2). The neuroinflammation generated by peripheral glial cells in chronic pain conditions is more persistent, thereby contributing to the maintenance of chronic pain. 50
Figure 2.
Inflammation and peripheral sensitization in chronic pain. Inflammation manifests the releasing of inflammatory mediators from various types of cells, these inflammatory mediators including proinflammatory cytokines, chemokines, HMGB1 and ATP, which can bind with their respective receptors on the nociceptive neurons in peripheral, modulate their sensitivity and excitability, and then lead to chronic pain. In addition, SCs and macrophages also produce anti-inflammatory cytokines and pro-resolving mediators SPMs, which promote pain resolution.
Pain modulation by various cells in PNS
An increase in peripheral sensitization is characterized by the hyperexcitability and hypersensitivity of nociceptors caused by tissue injury and inflammation. Increasing evidence suggests that peripheral sensitization is the cardinal contributor to chronic pain in the peripheral, but not an independent factor. There are a variety of non-neuronal cells, such as immune cells and glial cells, are activated along the pain circuitry by painful injuries, causing a localized inflammation in the PNS and CNS (neuroinflammation). The activation of these cells, in turn, causes bi-directional interactions with nociceptors, which are crucial in the development and maintenance of chronic pain. 2 In addition, inflammatory mediators, such as cytokines and chemokines, produced by these cells also contribute to the initiation and maintenance of pain.51,52 Comparatively, SPMs have been proven to promote the resolution of inflammation, alleviate pain, and promote tissue regeneration via specific cellular and molecular mechanisms. Therefore, SPMs exhibit potent anti-inflammatory actions and act as promising analgesics during the resolution phase of inflammation.53–55
Neurons
A variety of noxious/painful stimuli are detected by neurons, which represent a heterogeneous neuron population that can be further classified in accordance with their characteristics, such as size of the soma, degree of myelination, expression of genes, cell surface markers, electrophysiological characteristics.56–58 Afferents that respond to tissue injury include myelinated C-fibers and unmyelinated C-fibers that terminate in skin, muscle, joints, and visceral organs, with their neuronal bodies located in the dorsal root ganglia (DRGs) and trigeminal ganglia (TGs).
These neurons send centrally-projecting fibers to the dorsal horn (for DRG neurons) and spinal trigeminal nuclei of the medulla (for TG neurons), where they convey information to secondary nociceptive neurons via various neurotransmitters and neuropeptides.18,58
Nociceptors are sensitized by inflammatory mediators (e.g. pro-inflammatory cytokines, chemokines, nerve growth factor, bradykinin, prostaglandins)18,59–61 via their corresponding receptors [e.g. G-protein coupled receptors (GPCRs), tyrosine kinase receptors, and ionotropic receptors] that expressed on the terminals and/or cell bodies of nociceptors.18,62 Peripheral receptor activation under pathological conditions cause hyperexcitability and hypersensitivity of nociceptive neurons (peripheral sensitization), via modulation of various ion channels, including transient receptor potential ion channels (TRPs, e.g. TRPA1, TRPV1, and TRPV4),63–65 sodium channels (e.g. Nav1.7/1.8/1.9),66–68 and mechanosensitive piezo ion channels.69,70 The activation of protein kinases [e.g. protein kinase C (PKC) and protein kinase A (PKA), MAP kinases] in primary sensory neurons also participates in peripheral sensitization. For instance, during the pathogenesis of chronic pain, the activation of p38 in sensory neurons initiates and maintains peripheral sensitization by increasing TRPV1 activity in response to tumor necrosis factor-α (TNF-α)71,72 and also by increasing the activity of Nav1.8 as a consequence of interleukin (IL)-1β.73,74 A recent study indicated that TLR8 in TG neurons facilitates trigeminal neuropathic pain (TNP) via increasing intracellular Ca2+, activating ERK and p38, and further increasing the pro-inflammatory cytokines (e.g. TNF-α, IL-6, and IL-1β) in the TG after infraorbital nerve injury. 75 The CXCR3 activation by C-X-C motif chemokine ligand (CXCL) 10 in DRG neurons enhances neuronal excitability via activating p38 and ERK, which contributes to the maintenance of neuropathic pain. 52
A great deal of evidence suggests that peripheral sensory neurons not only respond to painful stimuli, but also play a role in inflammation and immunity as well. 76 Recent research has shown that peripheral sensory neurons express the proton-selective ion channel Hv1 that was previously known to be selectively expressed in microglia. Neuronal Hv1 is upregulated by peripheral inflammation, which in turn enhances inflammation and nociception. Inhibition of neuronal Hv1 by gene knockout or a selective blocker YHV98-4 reduces intracellular ROS production and alkalization in chronic inflammatory pain, restores downstream SHP-1-pAKT signaling, diminishes the release of chemokines, and finally reduces the effects of morphine-induced hyperalgesia and tolerance. 76
Peripheral glial cells: Schwann cells and satellite glial cells
Glial cells in the PNS consist of Schwann cells (SCs) in the peripheral nerve trunk and satellite glial cells (SGCs) in the DRGs and TGs. As a result of noxious stimuli, glial cells release multiple inflammatory mediators, sensitizing nociceptors at axons and somata which in turn participate in pain processing. 2
SCs are the main glial cells of the PNS, which form myelin sheaths to provide nutrients for nerve fibers and maintain the homeostasis of the neuronal microenvironment. 77 After peripheral nerve injury, the activated SCs release pro-inflammatory cytokines, such as TNF-α and IL-1β, which contribute to nerve degeneration and the development of neuropathic pain.78,79 For instance, experimental studies of chronic constriction injury (CCI) rat models have shown that SCs are activated and release the IL-1β at the injury site.80,81 An increase of IL-1β then promotes SCs de-differentiation in Wallerian degeneration via the c-Jun/AP-1 signal axis. 82 Activated SCs can destruct the blood-nerve barrier through the secretion of MMP-9, which recruits immune cells from the blood vessels into the injured nerve tissue and then releases more pro-nociceptive mediators. 83 Consequently, the increased pro-inflammatory mediators and activated SCs are implicated in pathological pain via glial-neuronal and neuro-immune interactions.
SGCs surround the somata of DRG and TG neurons and are coupled with each other through gap junctions, which are activated and proliferated due to injured nerves and inflammation. The activity of SGCs play a key role by participating in chronic pain through the following ways: (1) upregulation of glial fibrillary acidic protein (GFAP),84,85 (2) enhanced gap junction-mediated SGC-SGC and neuron-SGC coupling,86–88 (3) increased the sensitivity of SGCs to ATP that activates P2X7 receptors leading to TNF-α release. By contrast, it enhanced the P2X3 receptor-mediated responses and increased DRG excitability,89–91 (4) decreased the expression of the Kir4.1,92–94 and increased the release of pro-inflammatory mediators, such as TNF-α, IL-1β, IL-6, and CX3CL1.95–98 A recent study has shown that the upregulation of P2Y14 receptors in TG SGCs may partially trigger orofacial inflammatory pain via activating ERK and p38 signaling, producing cytokines (IL-1β, TNF-α, and CCL2). 99
Neutrophils and macrophages
Neutrophils are considered as the body’s first line of defense against infection and various inflammatory signals, also viewed as important players in tissue repair. In the early stage of inflammation, neutrophils can fight against infections via phagocytosis and/or neutrophil extracellular traps (NETs). Neutrophils may exert analgesic effects by releasing opioid peptides, such as β-endorphin, met-enkephalin, and dynorphin A. 100 However, when the inflammation persists, neutrophils also release NETs to exacerbate tissue inflammation. 101 Parisien et al. reported that transient neutrophil-induced inflammatory responses are protective against the transition from acute to chronic pain in participants with resolved pain within 3 months. A lack of neutrophils delays the resolution of chronic pain, whereas a supplement of neutrophils prevents the development of prolonged pain. 102 Thus, the inhibition of acute inflammation may delay the resolution of chronic pain. In addition, the enhanced phagocytosis of apoptotic neutrophils by macrophages (also termed efferocytosis) has been revealed to reduce inflammation in synovitis. 103
A neuroimmune crosstalk contributes to chronic pathological pain including inflammatory and neuropathic components.104,105 Macrophages play an important role in pain induction and maintenance with different mechanisms in the periphery. 2 The main functions of these cells in the immune system include phagocytosis, antigen presentation, and cytokine production. It is well known that monocytes differentiate into macrophages in tissues where they fight infection and repair wounds under inflammatory conditions. 106 These cells secret multiple mediators [e.g. TNF-α, IL-6, IL-1β] to regulate the excitability of the primary afferents104,107 via modulation of ion channels (e.g. TRPA1, TRPV1, and Nav1.7–1.9),18,50,53 which then enhance pain transduction and conduction.
In addition to pro-inflammatory cytokines, macrophages also release chemokines which contribute to the occurrence and maintenance of chronic pain. 108 Macrophages can produce chemokines CCL2. 17 In a rat model of lumbar disc herniation, CCL2 was increased in DRG neurons, and its receptor CCR2 was increased in DRG macrophages.109,110 Thus, CCL2/CCR2 signaling may be involved in the interaction of neuron-macrophages and contribute to the maintenance of chronic pain.
In the inflammatory microenvironment of local tissue, the macrophages recruited and resident undergo phenotypic and functional changes responding to mediators released by neurons or immune cells.111–113 Pro-inflammatory macrophages are classified as M1 and anti-inflammatory macrophages are classified as M2. Lipopolysaccharide (LPS), granulocyte/macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), and TNF-α polarize macrophage M1 to produce marked effects on inhibiting microbes and tumors, and promoting tissue damage via the production of pro-inflammatory mediators. Transforming growth factor-β (TGF-β), macrophage colony-stimulating factor (M-CSF), IL-4, IL-10, and IL-13 polarize macrophage M2 to phagocytose apoptotic cells and promote tissue repair by secreting anti-proinflammatory mediators IL-10 and TGF-β. 104 After the peripheral nerve and/or adjacent tissues are damaged, macrophages cluster at the injured site, and along primary afferents and its bodies, where M1 macrophages sensitize the nociceptive neurons, which might be subsequently suppressed by M2 macrophages.50,114 M1 macrophages release abundant inflammatory mediators, such as TNF-α, IL-6, IL-1β, nerve growth factor (NGF), insulin-like growth factor 1 (IGF-1), prostaglandin E2 (PGE2), CCL2, and CXCL1, to result in sensitized neurons by activating their specific receptors located in the nociceptive neurons. 104 An increase of high mobility group box 1 (HMGB1) from the activated M1 macrophages stimulates its membrane receptors, such as Toll-like receptor 4 (TLR4), advanced glycation end-product (RAGE), and then accelerates the CXCL12/CXCR4 signal axis in the peripheral nerve, and finally contributes to the pathogenesis of chronic pain.104,115–119
As described above, activated macrophages (mainly M1 macrophages) induce inflammation through releasing pro-inflammatory mediators resulting in neuronal sensitization and then causing pain. Thus, inflammation acts as a role of foe in such situations. On the other hand, M2 macrophages release anti-inflammatory cytokines to suppress inflammation in the resolution phase and resolve pain. As literature reported, the GPR37 in macrophages were activated by neuroprotectin D1 (NPD1) to enhance macrophage phagocytosis and IL-10 production, and then promote the resolution of inflammatory pain. 120 Thus, macrophage activation under these circumstances plays a friend role in pain resolution.
Specialized pro-resolving mediators
SPMs are produced during the resolution phase of inflammation, including various bioactive molecules, such as resolvins (RvD1, RvD2, RvD5, RvE1), 121 maresin (MaR1 and MaR2),55,122,123 protectin (PD1)124,125 or neuroprotectin (NPD1),17,126 and lipoxins,127,128 which derive from polyunsaturated fatty acids (PUFA).44,53–55,129 The synthesis of SPMs plays a crucial role in promoting transformation from a pro-inflammatory to an anti-inflammatory state, boosting a pro-resolution state, and further alleviating inflammatory pain. 123 In addition, SPMs might suppress the excessive sensitization of nociceptors via inhibiting specific channels (e.g. TRPA1 and TRPV1) to achieve the resolution of pain. 126 For example, the peripheral (intraplantar) or spinal (intrathecal) administration of RvE1 and RvD1 has shown a powerful effect on reducing inflammatory pain induced by formalin, carrageenan, or complete Freund’s adjuvant (CFA). Moreover, intrathecal injection of RvE1 inhibits spontaneous pain and heat and mechanical hypersensitivity induced by capsaicin and TNF-α. RvE1 also inhibits TNF-α and TRPV1-induced excitatory postsynaptic current increases via inhibition of ERK signaling pathway. 127 MaR1 and PD1 can suppress the activity of TRPV1 in DRG neurons and reduce neuroinflammatory pain by inhibiting PKA and ERK activity.128,129 MaR2 is a DHA-derived SPM produced by macrophages, which also demonstrates the ability to reduce TRPV1 and TRPA1 activation in DRG neurons exhibited by a reduction in calcium influx and suppressed pain-like behavior in LPS-induced inflammatory pain models, as well as capsaicin and AITC-induced spontaneous pain models of mouse. 44 Moreover, PD1 also decreases hyperexcitability by negatively regulating LTP in the spinal cord evoked by TNF-α. 130 Due to the serious side effects of traditional analgesics, SPMs have become a promising substitute for pain relief, for its potent pro-resolving and anti-inflammatory effects. 123
Central inflammation contributes to the pathogenesis of chronic pain
In the CNS, it is believed that chronic pain is persisted by central sensitization caused by synaptic plasticity in central pain pathways and an increase in neuronal responsiveness as a result of painful insults. Increasing evidence shows that central sensitization is caused by neuroinflammation in the CNS. A classical feature of neuroinflammation is that the glial cells are activated in the CNS, and they release multiple pro-inflammatory mediators to induce hyperalgesia and allodynia (Figure 1).
Pain modulation by central glial cells
Neuroinflammation in the CNS is caused by the activation of glial cells (such as microglia and astrocytes), which play a critical role in the initiation and maintenance of chronic pain.131–135 Tissue inflammation or nerve injury induces the activation of glial cells, which leads to the release of inflammatory mediators, such as cytokines (e.g. TNF-α, IL-6, and IL-1β), chemokines (e.g. CXCL1, CX3CL1, and CCL2), prostaglandin E2 (PGE2), and inducible nitric oxide synthase (iNOS), to enhance neuronal excitability or synaptic transmission.136–138
Microglia are the resident immune cells in CNS. Microglia become activated (also termed “microgliosis”) in response to disease states or peripheral nerve injury, characterized by significant morphological changes (hypertrophy), functional changes, and proliferation, which is related to transcriptomic changes. 139 The spinal microglia activation is evoked by pro-inflammatory mediators [e.g. IL-1β, caspase-6, colony-stimulating factor 1 (CSF1), and extracellular proteases] derived from sensory neurons.139–143 After nerve injury, activation of microglia in the ipsilateral spinal dorsal horn can be quickly observed, and inhibition of microglia activation is sufficient to mitigate pain development. 144 However, it should be noted that microgliosis induced by nerve injury is transient and self-limiting, which weakens within a few weeks and remains at a reduced level in the later stage, that means in the later stage of microgliosis, microglia inhibitor and inhibition of activated microglia-associated cytokines are no longer effective in pain relief.145,146 Thus, microglia and their pain mediators (cytokines and chemokines) are most actively involved in pain initiation. Of note, SPMs in CNS have been shown to contribute to the resolution of pain. The NPD1 biosynthesized by microglia has a powerful protective effect in pain relief. 147 In addition, microglia express many SPM receptors, such as GPR18 (RvD2 receptor), GPR32 (RvD1 receptor), and ChemR23 (RvE1 and RvE2 receptor).148,149 RvE1 binds to ChemR23 to trigger pro-resolving responses and reduce chronic pain through inhibiting microglial activation in the SC.150,151 Consequently, activation of these microglial receptors down-regulates the production of pro-inflammatory mediators (e.g. TNF-α, IL-1β), and up-regulates the secretion of anti-inflammatory mediators (e.g. IL-10, TGF-β), thus promoting the regression of pathological pain. 139 Besides, ion channels on microglia are involved in regulation of inflammatory pain, such as Hv1, P2X4, P2X7, and TRPV4. Hv1 has been implicated in the pathogenesis of neuropathic pain by regulating ROS production and microglia-astrocyte communication. 152 The microglial P2X4 channel participates in inflammatory pain through activating microglia, and then releasing pro-inflammatory mediators. 153 The ATP-gated ion channel P2X7 on microglia have been revealed to modulate inflammatory pain through regulating NLRP3/Caspase-1 pathway. 154 TRPV4 expression in spinal resident microglia is increased in the mouse model of SNI, which transforms peripheral nerve injury into central sensitization and neuropathic pain through releasing lipocalin-2. 155
Astrocytes are more abundant (approximately 2-4 times) compared to microglia, and play crucial effects on controlling the extracellular microenvironment, repairing the imbalance of potassium, glutamate, and water homeostasis under normal physiological conditions.156,157 Astrocytes are activated in various pathological conditions and convert to reactive states (also termed “astrogliosis”) characterized by obvious morphological changes, a significant increase of GFAP, and a significant cellular proliferation, which is considered to be coupled with a loss of homeostatic functions of astrocytes.58,158 Astrocytes are activated after nerve injury generally following microglial activation (e.g. 1 week), but remain longer time (at least 5 months or more) in a reactive state that contributes to the maintenance of chronic pain. 159 Numerous pieces of evidence show that decreasing activation of astrocyte can attenuate pain behaviors in chronic pain models.160–162
Glia in the CNS are dominated by oligodendrocytes, which account for 46∼75%. 58 There is, however, relatively little research on the role of oligodendrocytes in chronic pain pathogenesis. Some studies suggest that there may be an interaction between oligodendrocyte damage and pain in patients with multiple sclerosis. 163 Moreover, oligodendrocytes play a role in maintaining pain in common conditions. 164 Previous experiments suggest that the role of oligodendrocytes in pain regulation may be connected with primary afferent nerve, microglia, and astrocytes. 165 Oligodendrocyte-derived IL-33 in the dorsal SC plays an important role in neuropathic pain. IL-33 is increased in neuropathic pain model, and its receptor IL-33R (ST2) deficiency has been exhibited analgesic effects. IL-33-induced pain has been significantly alleviated through inhibition of PI3K, MAPKs (p38, ERK, and JNK), and NF-κB. 165
Following peripheral nerve injury (e.g. SNI), microglia are generally thought to activate before astrocytes. Microglia activation in the early stage is necessary for the initial generation of acute pain. Activated microglia secret pro-inflammatory mediators to increase sensitivity of nearby nociceptive neurons and astrocytes, resulting in astrogliosis, which contributes to the maintenance of chronic pain. 58 Understanding the role of inflammation in different stages is helpful for intervention selections for pain relief.
Neurons in the spinal cord
Chemokines combined with their receptors in spinal neurons contribute to the establishment and maintenance of chronic pain. For instance, CXCL1 and CCL2, which are expressed in spinal astrocytes, act respectively on CXCR2 and CCR2 in spinal neurons after spinal nerve ligation (SNL) to enhance excitatory synaptic transmission (astrocyte-to-neuron signal axis). CXCL13 in spinal neurons is also highly up-regulated after SNL and induces spinal astrocyte activation through its receptor CXCR5 (neuron-to-astrocyte signal axis). 166
Descending pathways have been revealed to modulate pain transmission in spinal cord, such as the anterior cingulate cortex (ACC)-spinal cord pathway, noradrenergic descending pathways, and dopaminergic descending pathways.167–169 Descending pathway of the ACC-spinal cord facilitates SNI-induced neuropathic pain through a rostral ventromedial medulla (RVM)-independent manner. 167 Descending noradrenergic pathways also exert an inhibitory effect on pain transmission in chronic pain models.170–172 Peripheral inflammation activates the descending noradrenergic pathways from the locus coeruleus (LC) to the spinal cord, which prevents the hyperalgesia development. 173 As a result of unilateral hind paw inflammation, descending NE-containing neurons in the LC are stimulated, increasing the level of NE in the ipsilateral dorsal horn. 174 A recent study has reported that activation of LC-spinal cord noradrenergic neurons alleviated neuropathic pain in mice via down-regulating the expression of pro-inflammatory cytokines (TNF-α and IL-1β), up-regulating the expression of anti-inflammatory cytokines (IL-4 and IL-10), and inhibiting activation of microglia and astrocytes in SDH. 175
Conclusion and prospects
Given the important role of inflammation (including neuroinflammation) in the induction and maintenance of chronic pain, it may be effective to directly target inflammation through cytokines, chemokines, and MAP kinase inhibitors. There are also possible side effects of these drugs, such as infection after long-term use and an inability to resolve inflammation. Thus, some alternative approaches that can control an excess of inflammation are emerging, including SPMs and cell therapies.
A large number of research suggest that microglia play a vital role in pain generation. When microglia are activated, pro-inflammatory and anti-inflammatory factors are produced in an imbalance. Therefore, discovering original ways to stimulate endogenous anti-inflammatory factors and inhibit pro-inflammatory factors may still be the challenge for researchers. It is generally known that increased production of pro-inflammatory cytokines (e.g. TNFα, IL-6, and IL-1β) mediates allodynia and hyperalgesia. 176 Thus, inhibiting microglial activation by decreasing the production of pro-inflammatory cytokines may have an analgesic effect. TNF-α blocker etanercept has been reported to reduce neuropathic pain in a rodent model of spinal cord injury through intrathecal administration, 177 and administration of etanercept to patients with severe sciatica also showed effective. 178 Administration of anti-IL-1β antibodies also demonstrated a reduce of neuropathic pain. 179
The chemokines not only regulate inflammation in the immune system, but also causes neuroinflammation in PNS and CNS, and promotes the initiation and maintenance of various types of chronic pain. Thus, the chemokine system provides an ideal candidate for treatments of chronic pain. According to the preclinical studies, there are three candidate directions for the therapeutic drugs targeting chemokine and its receptor: (1) blocking or neutralizing antibodies; (2) small-molecule inhibitors; (3) therapeutic small interfering RNA (siRNA). The antibodies of CGRP and its receptors have been used to treat migraine by subcutaneous or intravenous injection. 180 The small molecule CCR5 antagonist maraviroc has been observed to alleviate neuropathic pain in clinical practice.181,182
Many studies have proved that SPMs can control inflammation effectively and hold the potential to suppress pain while promoting pain relief. However, the instability and high production cost of SPMs limits their direct application to patients. 183 Omega-3 fatty acids (such as DHA and EPA) derived from food are precursors of SPMs and have been shown to reduce inflammatory pain in patients, which may be through its own GPCRs, such as GRP120.184,185 A randomized trial has shown that dietary omega-3 fatty acids may alleviate headaches in patients. 186 However, the efficacy of these SPMs precursors for pain relief is much lower compared to their metabolites. Thus, it will be promising to study the synergistic effects of SPM precursors from diet and neuromodulation on chronic pain will be promising, since it targets inflammation and neuroinflammation.
Currently, there has been a rise in gene therapy that is practiced in various inherited diseases in clinics. The recombinant adeno-associated virus (rAAV) is often used as a vector to deliver genes in gene therapy because the rAAV lacks any detrimental viral gene, does not integrate into the genome of humans, and provides persistent gene expression. With respect to the benefits of rAAV, gene therapy will be a promising treatment to tackle the ticklish chronic pain by silencing and increasing the pro-inflammatory and anti-inflammatory mediators, respectively.
Appendix.
Abbreviations
- CNS
Central nervous system
- DRG
Dorsal root ganglion
- ERK
Extracellular signal-regulated kinase
- GFAP
Glial fibrillary acidic protein
- GPCR
G protein-coupled receptor
- IL
Interleukin
- IFN-γ
Interferon-γ
- LPS
Lipopolysaccharide
- Nav
Voltage-gated sodium channel
- NMDA
N-methyl-D-aspartic acid
- NETs
Neutrophil extracellular traps
- PNS
Peripheral nervous system
- SCs
Schwann cells
- SGCs
Satellite glial cells
- SNL
Spinal nerve ligation
- SPMs
Specialized pro-resolving mediators
- TG
Trigeminal ganglion
- TNF-α
Tumor necrosis factor-alpha
- TRP
Transient receptor potential.
Footnotes
Author contributions: Xiao-Xia Fang wrote the manuscript. Meng-Nan Zhai designed the tables. Meixuan Zhu participated in writing and editing the manuscript. Cheng He, Heng Wang, and Juan Wang drew the figures. Zhi-Jun Zhang initiated and supervised the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (NSFC) (31970938, 81571070) and the Natural Science Research Program of Jiangsu Province (BK20191448).
ORCID iD
Zhi-Jun Zhang https://orcid.org/0000-0001-6996-8683
References
- 1.Pahwa R, Goyal A, Jialal I. Chronic inflammation. Treasure Island FL: StatPearls, 2022. [PubMed] [Google Scholar]
- 2.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science 2016; 354: 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavandʼhomme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain 2019; 160: 19–27. [DOI] [PubMed] [Google Scholar]
- 4.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep 2018; 67: 1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 2016; 6: e010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet 2021; 397: 2082–2097. [DOI] [PubMed] [Google Scholar]
- 7.Meucci RD, Fassa AG, Paniz VM, Silva MC, Wegman DH. Increase of chronic low back pain prevalence in a medium-sized city of southern Brazil. BMC Musculoskelet Disord 2013; 14: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellaroza MS, Pimenta CA, Duarte YA, Lebrao ML. [Chronic pain among elderly residents in Sao Paulo, Brazil: prevalence, characteristics, and association with functional capacity and mobility (SABE study)]. Cad Saude Publica 2013; 29: 325–334. [DOI] [PubMed] [Google Scholar]
- 9.Scherer M, Hansen H, Gensichen J, Mergenthal K, Riedel-Heller S, Weyerer S, Maier W, Fuchs A, Bickel H, Schon G, Wiese B, Konig HH, van den Bussche H, Schafer I. Association between multimorbidity patterns and chronic pain in elderly primary care patients: a cross-sectional observational study. BMC Fam Pract 2016; 17: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain 2022; 163: e328–e332. [DOI] [PubMed] [Google Scholar]
- 11.Bao T, Seidman A, Li Q, Seluzicki C, Blinder V, Meghani SH, Farrar JT, Mao JJ. Living with chronic pain: perceptions of breast cancer survivors. Breast Cancer Res Treat 2018; 169: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janah A, Bouhnik AD, Touzani R, Bendiane MK, Peretti-Watel P. Underprescription of step III opioids in french cancer survivors with chronic pain: a call for integrated early palliative care in oncology. J Pain Symptom Manage 2020; 59: 836–847. [DOI] [PubMed] [Google Scholar]
- 13.Sanford NN, Sher DJ, Butler SS, Xu X, Ahn C, Aizer AA, Mahal BA. Prevalence of chronic pain among cancer survivors in the United States, 2010–2017. Cancer 2019; 125: 4310–4318. [DOI] [PubMed] [Google Scholar]
- 14.Sharon H, Greener H, Hochberg U, Brill S. The prevalence of chronic pain in the adult population in israel: an internet-based survey. Pain Res Manag 2022; 2022: 3903720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong WS, Fielding R. Prevalence and characteristics of chronic pain in the general population of Hong Kong. J Pain 2011; 12: 236–245. [DOI] [PubMed] [Google Scholar]
- 16.Muley MM, Krustev E, McDougall JJ. Preclinical assessment of inflammatory pain. CNS Neurosci Ther 2016; 22: 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen O, Donnelly CR, Ji RR. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol 2020; 62: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009; 139: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang XX, Wang H, Song HL, Wang J, Zhang ZJ. Neuroinflammation involved in diabetes-related pain and itch. Front Pharmacol 2022; 13: 921612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 2014; 15: 43–53. [DOI] [PubMed] [Google Scholar]
- 21.Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int 2017; 37: 29–42. [DOI] [PubMed] [Google Scholar]
- 22.Han WJ, Ma SB, Wu WB, Wang FD, Cao XL, Wang DH, Wu HN, Xie RG, Li ZZ, Wang F, Wu SX, Zheng MH, Luo C, Han H. Tweety-homolog 1 facilitates pain via enhancement of nociceptor excitability and spinal synaptic transmission. Neurosci Bull 2021; 37: 478–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panigrahy D, Gilligan MM, Serhan CN, Kashfi K. Resolution of inflammation: an organizing principle in biology and medicine. Pharmacol Ther 2021; 227: 107879. [DOI] [PubMed] [Google Scholar]
- 24.Chiang N, Serhan CN. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem 2020; 64: 443–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnardottir H, Thul S, Pawelzik SC, Karadimou G, Artiach G, Gallina AL, Mysdotter V, Carracedo M, Tarnawski L, Caravaca AS, Baumgartner R, Ketelhuth DF, Olofsson PS, Paulsson-Berne G, Hansson GK, Back M. The resolvin D1 receptor GPR32 transduces inflammation resolution and atheroprotection. J Clin Invest 2021; 131: e142883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Y, Tu YH, Wu X, Ji S, Shen JL, Wu HM, Fei GH. ResolvinD1 protects the airway barrier against injury induced by influenza a virus through the Nrf2 pathway. Front Cell Infect Microbiol 2020; 10: 616475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie W, Wang H, Liu Q, Li Y, Wang J, Yao S, Wu Q. ResolvinD1 reduces apoptosis and inflammation in primary human alveolar epithelial type 2 cells. Lab Invest 2016; 96: 526–536. [DOI] [PubMed] [Google Scholar]
- 28.Chattopadhyay R, Raghavan S, Rao GN. Resolvin D1 via prevention of ROS-mediated SHP2 inactivation protects endothelial adherens junction integrity and barrier function. Redox Biol 2017; 12: 438–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br J Pharmacol 2010; 161: 707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009; 461: 1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park CK, Xu ZZ, Liu T, Lu N, Serhan CN, Ji RR. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci 2011; 31: 18433–18438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang LY, Liu ZH, Zhu Q, Wen S, Yang CX, Fu ZJ, Sun T. Resolvin D2 relieving radicular pain is associated with regulation of inflammatory mediators, Akt/GSK-3β signal pathway and GPR18. Neurochem Res 2018; 43: 2384–2392. [DOI] [PubMed] [Google Scholar]
- 33.Dort J, Orfi Z, Fabre P, Molina T, Conte TC, Greffard K, Pellerito O, Bilodeau JF, Dumont NA. Resolvin-D2 targets myogenic cells and improves muscle regeneration in duchenne muscular dystrophy. Nat Commun 2021; 12: 6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SH, Tonello R, Im ST, Jeon H, Park J, Ford Z, Davidson S, Kim YH, Park CK, Berta T. Resolvin D3 controls mouse and human TRPV1-positive neurons and preclinical progression of psoriasis. Theranostics 2020; 10: 12111–12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Joshi HP, Sheen SH, Kim KT, Kyung JW, Choi H, Kim YW, Kwon SY, Roh EJ, Choi UY, Sohn S, Kim YH, Park CK, Kumar H, Han IB. Resolvin D3 promotes inflammatory resolution, neuroprotection, and functional recovery after spinal cord injury. Mol Neurobiol 2021; 58: 424–438. [DOI] [PubMed] [Google Scholar]
- 36.Cherpokova D, Jouvene CC, Libreros S, DeRoo EP, Chu L, de la Rosa X, Norris PC, Wagner DD, Serhan CN. Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood 2019; 134: 1458–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baggio DF, da Luz FMR, Lopes RV, Ferreira LEN, Araya EI, Chichorro JG. Sex dimorphism in resolvin D5-induced analgesia in rat models of trigeminal pain. J Pain 2022; 24(5): 717–729. [DOI] [PubMed] [Google Scholar]
- 38.Pham TL, Kakazu AH, He J, Nshimiyimana R, Petasis NA, Jun B, Bazan NG, Bazan HEP. Elucidating the structure and functions of Resolvin D6 isomers on nerve regeneration with a distinctive trigeminal transcriptome. FASEB J 2021; 35: e21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddiquee A, Patel M, Rajalingam S, Narke D, Kurade M, Ponnoth DS. Effect of omega-3 fatty acid supplementation on resolvin (RvE1)-mediated suppression of inflammation in a mouse model of asthma. Immunopharmacol Immunotoxicol 2019; 41: 250–257. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Xu H, Xia K, Cheng S, Zhang Q. Resolvin E1 accelerates pulp repair by regulating inflammation and stimulating dentin regeneration in dental pulp stem cells. Stem Cell Res Ther 2021; 12: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuda H, Ikeda H, Muromoto R, Hirashima K, Ishimura K, Fujiwara K, Aoki-Saito H, Hisada T, Watanabe M, Ishihara J, Matsuda T, Shuto S. Synthesis of resolvin E3, a proresolving lipid mediator, and its deoxy derivatives: identification of 18-deoxy-resolvin e3 as a potent anti-inflammatory agent. J Org Chem 2020; 85: 14190–14200. [DOI] [PubMed] [Google Scholar]
- 42.Tang S, Gao C, Long Y, Huang W, Chen J, Fan F, Jiang C, Xu Y. Maresin 1 mitigates high glucose-induced mouse glomerular mesangial cell injury by inhibiting inflammation and fibrosis. Mediators Inflamm 2017; 2017: 2438247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, Serhan CN. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J 2013; 27: 2573–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fattori V, Zaninelli TH, Ferraz CR, Brasil-Silva L, Borghi SM, Cunha JM, Chichorro JG, Casagrande R, Verri WA, Jr. Maresin 2 is an analgesic specialized pro-resolution lipid mediator in mice by inhibiting neutrophil and monocyte recruitment, nociceptor neuron TRPV1 and TRPA1 activation, and CGRP release. Neuropharmacology 2022; 216: 109189. [DOI] [PubMed] [Google Scholar]
- 45.Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol 2009; 158: 960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, Zhu M, Winkler JW, Petasis NA. Novel proresolving aspirin-triggered DHA pathway. Chem Biol 2011; 18: 976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butovich IA. On the structure and synthesis of neuroprotectin D1, a novel anti-inflammatory compound of the docosahexaenoic acid family. J Lipid Res 2005; 46: 2311–2314. [DOI] [PubMed] [Google Scholar]
- 48.Minciullo PL, Catalano A, Mandraffino G, Casciaro M, Crucitti A, Maltese G, Morabito N, Lasco A, Gangemi S, Basile G. Inflammaging and anti-inflammaging: the role of cytokines in extreme longevity. Arch Immunol Ther Exp (Warsz) 2016; 64: 111–126. [DOI] [PubMed] [Google Scholar]
- 49.Headland SE, Norling LV. The resolution of inflammation: Principles and challenges. Semin Immunol 2015; 27: 149–160. [DOI] [PubMed] [Google Scholar]
- 50.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014; 13: 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sommer C, Leinders M, Uceyler N. Inflammation in the pathophysiology of neuropathic pain. Pain 2018; 159: 595–602. [DOI] [PubMed] [Google Scholar]
- 52.Kong YF, Sha WL, Wu XB, Zhao LX, Ma LJ, Gao YJ. CXCL10/CXCR3 signaling in the DRG exacerbates neuropathic pain in mice. Neurosci Bull 2021; 37: 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuda M, Huh Y, Ji RR. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth 2019; 33: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chavez-Castillo M, Ortega A, Cudris-Torres L, Duran P, Rojas M, Manzano A, Garrido B, Salazar J, Silva A, Rojas-Gomez DM, De Sanctis JB, Bermudez V. Specialized pro-resolving lipid mediators: the future of chronic pain therapy? Int J Mol Sci 2021; 22: 10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fattori V, Pinho-Ribeiro FA, Staurengo-Ferrari L, Borghi SM, Rossaneis AC, Casagrande R, Verri WA, Jr. The specialised pro-resolving lipid mediator maresin 1 reduces inflammatory pain with a long-lasting analgesic effect. Br J Pharmacol 2019; 176: 1728–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lallemend F, Ernfors P. Molecular interactions underlying the specification of sensory neurons. Trends Neurosci 2012; 35: 373–381. [DOI] [PubMed] [Google Scholar]
- 57.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001; 413: 203–210. [DOI] [PubMed] [Google Scholar]
- 58.Donnelly CR, Andriessen AS, Chen G, Wang K, Jiang C, Maixner W, Ji RR. Central nervous system targets: glial cell mechanisms in chronic pain. Neurotherapeutics 2020; 17: 846–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci 2001; 21: 5027–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dawes JM, Calvo M, Perkins JR, Paterson KJ, Kiesewetter H, Hobbs C, Kaan TK, Orengo C, Bennett DL, McMahon SB. CXCL5 mediates UVB irradiation-induced pain. Sci Transl Med 2011; 3: 90ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018; 129: 343–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med 2010; 16: 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Logu F, Geppetti P. Ion channel pharmacology for pain modulation. Handb Exp Pharmacol 2019; 260: 161–186. [DOI] [PubMed] [Google Scholar]
- 64.Hargreaves KM, Ruparel S. Role of oxidized lipids and trp channels in orofacial pain and inflammation. J Dent Res 2016; 95: 1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shibata M, Tang C. Implications of transient receptor potential cation channels in migraine pathophysiology. Neurosci Bull 2021; 37: 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu XB, Cao DL, Zhang X, Jiang BC, Zhao LX, Qian B, Gao YJ. CXCL13/CXCR5 enhances sodium channel Nav1.8 current density via p38 MAP kinase in primary sensory neurons following inflammatory pain. Sci Rep 2016; 6: 34836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 2007; 55: 365–376. [DOI] [PubMed] [Google Scholar]
- 68.Pan HL, Liu BL, Lin W, Zhang YQ. Modulation of Nav1.8 by lysophosphatidic acid in the induction of bone cancer pain. Neurosci Bull 2016; 32: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of drosophila piezo in mechanical nociception. Nature 2012; 483: 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu XZ. Demystifying mechanosensitive piezo Ion channels. Neurosci Bull 2016; 32: 307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest 2005; 115: 2393–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin X, Gereau RWt. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 2006; 26: 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu XJ, Liu T, Chen G, Wang B, Yu XL, Yin C, Ji RR. TLR signaling adaptor protein MyD88 in primary sensory neurons contributes to persistent inflammatory and neuropathic pain and neuroinflammation. Sci Rep 2016; 6: 28188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang ZJ, Hsu E, Li HC, Rosner AL, Rupert RL, Song XJ. Topical application of compound Ibuprofen suppresses pain by inhibiting sensory neuron hyperexcitability and neuroinflammation in a rat model of intervertebral foramen inflammation. J Pain 2011; 12: 141–152. [DOI] [PubMed] [Google Scholar]
- 75.Zhao LX, Jiang M, Bai XQ, Cao DL, Wu XB, Zhang J, Guo JS, Chen TT, Wang J, Wu H, Gao YJ, Zhang ZJ. TLR8 in the trigeminal ganglion contributes to the maintenance of trigeminal neuropathic pain in mice. Neurosci Bull 2021; 37: 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Q, Ren Y, Mo Y, Guo P, Liao P, Luo Y, Mu J, Chen Z, Zhang Y, Li Y, Yang L, Liao D, Fu J, Shen J, Huang W, Xu X, Guo Y, Mei L, Zuo Y, Liu J, Yang H, Jiang R. Inhibiting Hv1 channel in peripheral sensory neurons attenuates chronic inflammatory pain and opioid side effects. Cell Res 2022; 32: 461–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clements MP, Byrne E, Camarillo Guerrero LF, Cattin AL, Zakka L, Ashraf A, Burden JJ, Khadayate S, Lloyd AC, Marguerat S, Parrinello S. The wound microenvironment reprograms Schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron 2017; 96: 98–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci 2002; 22: 3052–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liedtke W. STING-ing pain: how can pro-inflammatory signaling attenuate pain? Neurosci Bull 2021; 37: 1075–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang X, Ma S, Wu H, Shen X, Xu S, Guo X, Bolick ML, Wu S, Wang F. Macrophage migration inhibitory factor mediates peripheral nerve injury-induced hypersensitivity by curbing dopaminergic descending inhibition. Exp Mol Med 2018; 50: e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yin D, Chen Y, Li Y, Lu R, Wang B, Zhu S, Fan B, Xu Z. Interleukin-1 receptor associated kinase 1 mediates the maintenance of neuropathic pain after chronic constriction injury in rats. Neurochem Res 2019; 44: 1214–1227. [DOI] [PubMed] [Google Scholar]
- 82.Chen G, Luo X, Wang W, Wang Y, Zhu F, Wang W. Interleukin-1β promotes schwann cells de-differentiation in Wallerian degeneration via the c-JUN/AP-1 pathway. Front Cell Neurosci 2019; 13: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calvo M, Dawes JM, Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet Neurol 2012; 11: 629–642. [DOI] [PubMed] [Google Scholar]
- 84.Stephenson JL, Byers MR. GFAP immunoreactivity in trigeminal ganglion satellite cells after tooth injury in rats. Exp Neurol 1995; 131: 11–22. [DOI] [PubMed] [Google Scholar]
- 85.Hanani M, Spray DC. Emerging importance of satellite glia in nervous system function and dysfunction. Nat Rev Neurosci 2020; 21: 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Warwick RA, Hanani M. The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur J Pain 2013; 17: 571–580. [DOI] [PubMed] [Google Scholar]
- 87.Huang TY, Belzer V, Hanani M. Gap junctions in dorsal root ganglia: possible contribution to visceral pain. Eur J Pain 2010; 14: 49.e1–49.e11. [DOI] [PubMed] [Google Scholar]
- 88.Blum E, Procacci P, Conte V, Hanani M. Systemic inflammation alters satellite glial cell function and structure. A possible contribution to pain. Neuroscience 2014; 274: 209–217. [DOI] [PubMed] [Google Scholar]
- 89.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 2007; 87: 659–797. [DOI] [PubMed] [Google Scholar]
- 90.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci 2006; 7: 423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci USA 2007; 104: 9864–9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vit JP, Ohara PT, Bhargava A, Kelley K, Jasmin L. Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. J Neurosci 2008; 28: 4161–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takeda M, Takahashi M, Nasu M, Matsumoto S. Peripheral inflammation suppresses inward rectifying potassium currents of satellite glial cells in the trigeminal ganglia. Pain 2011; 152: 2147–2156. [DOI] [PubMed] [Google Scholar]
- 94.Tang X, Schmidt TM, Perez-Leighton CE, Kofuji P. Inwardly rectifying potassium channel Kir4.1 is responsible for the native inward potassium conductance of satellite glial cells in sensory ganglia. Neuroscience 2010; 166: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang LY, Gu Y, Chen Y. Communication between neuronal somata and satellite glial cells in sensory ganglia. Glia 2013; 61: 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Souza GR, Talbot J, Lotufo CM, Cunha FQ, Cunha TM, Ferreira SH. Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc Natl Acad Sci USA 2013; 110: 11193–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Afroz S, Arakaki R, Iwasa T, Oshima M, Hosoki M, Inoue M, Baba O, Okayama Y, Matsuka Y. CGRP induces differential regulation of cytokines from satellite glial cells in trigeminal ganglia and orofacial nociception. Int J Mol Sci 2019; 20: 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitterreiter JG, Ouwendijk WJD, van Velzen M, van Nierop GP, Osterhaus A, Verjans G. Satellite glial cells in human trigeminal ganglia have a broad expression of functional toll-like receptors. Eur J Immunol 2017; 47: 1181–1187. [DOI] [PubMed] [Google Scholar]
- 99.Lin J, Zhang YY, Liu F, Fang XY, Liu MK, Huang CL, Wang H, Liao DQ, Zhou C, Shen JF. The P2Y(14) receptor in the trigeminal ganglion contributes to the maintenance of inflammatory pain. Neurochem Int 2019; 131: 104567. [DOI] [PubMed] [Google Scholar]
- 100.Rittner HL, Hackel D, Voigt P, Mousa S, Stolz A, Labuz D, Schafer M, Schaefer M, Stein C, Brack A. Mycobacteria attenuate nociceptive responses by formyl peptide receptor triggered opioid peptide release from neutrophils. PLoS Pathog 2009; 5: e1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019; 133: 2178–2185. [DOI] [PubMed] [Google Scholar]
- 102.Parisien M, Lima LV, Dagostino C, El-Hachem N, Drury GL, Grant AV, Huising J, Verma V, Meloto CB, Silva JR, Dutra GGS, Markova T, Dang H, Tessier PA, Slade GD, Nackley AG, Ghasemlou N, Mogil JS, Allegri M, Diatchenko L. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci Transl Med 2022; 14: eabj9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ouyang J, Zhang B, Kuang L, Yang P, Du X, Qi H, Su N, Jin M, Yang J, Xie Y, Tan Q, Chen H, Chen S, Jiang W, Liu M, Luo X, He M, Ni Z, Chen L. Pulsed electromagnetic field inhibits synovitis via enhancing the efferocytosis of macrophages. Biomed Res Int 2020; 2020: 4307385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Domoto R, Sekiguchi F, Tsubota M, Kawabata A. Macrophage as a Peripheral Pain Regulator. Cells 2021; 10: 1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tan Z, Lin ZJ, Wu LJ, Zhou LJ. The macrophage IL-23/IL-17A pathway: a new neuro-immune mechanism in female mechanical pain. Neurosci Bull 2022; 38: 453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hogg C, Horne AW, Greaves E. Endometriosis-associated macrophages: origin, phenotype, and function. Front Endocrinol (Lausanne) 2020; 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zelenka M, Schafers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain 2005; 116: 257–263. [DOI] [PubMed] [Google Scholar]
- 108.Jiang BC, Liu T, Gao YJ. Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharmacol Ther 2020; 212: 107581. [DOI] [PubMed] [Google Scholar]
- 109.Zhu X, Cao S, Zhu MD, Liu JQ, Chen JJ, Gao YJ. Contribution of chemokine CCL2/CCR2 signaling in the dorsal root ganglion and spinal cord to the maintenance of neuropathic pain in a rat model of lumbar disc herniation. J Pain 2014; 15: 516–526. [DOI] [PubMed] [Google Scholar]
- 110.Wu XB, Zhu Q, Gao YJ. CCL2/CCR2 contributes to the altered excitatory-inhibitory synaptic balance in the nucleus accumbens shell following peripheral nerve injury-induced neuropathic pain. Neurosci Bull 2021; 37: 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol 2013; 14: 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016; 44: 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 2018; 233: 6425–6440. [DOI] [PubMed] [Google Scholar]
- 114.Baral P, Udit S, Chiu IM. Pain and immunity: implications for host defence. Nat Rev Immunol 2019; 19: 433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sekiguchi F, Domoto R, Nakashima K, Yamasoba D, Yamanishi H, Tsubota M, Wake H, Nishibori M, Kawabata A. Paclitaxel-induced HMGB1 release from macrophages and its implication for peripheral neuropathy in mice: evidence for a neuroimmune crosstalk. Neuropharmacology 2018; 141: 201–213. [DOI] [PubMed] [Google Scholar]
- 116.Nishida T, Tsubota M, Kawaishi Y, Yamanishi H, Kamitani N, Sekiguchi F, Ishikura H, Liu K, Nishibori M, Kawabata A. Involvement of high mobility group box 1 in the development and maintenance of chemotherapy-induced peripheral neuropathy in rats. Toxicology 2016; 365: 48–58. [DOI] [PubMed] [Google Scholar]
- 117.Tsujita R, Tsubota M, Sekiguchi F, Kawabata A. Role of high-mobility group box 1 and its modulation by thrombomodulin/thrombin axis in neuropathic and inflammatory pain. Br J Pharmacol 2021; 178: 798–812. [DOI] [PubMed] [Google Scholar]
- 118.Yamasoba D, Tsubota M, Domoto R, Sekiguchi F, Nishikawa H, Liu K, Nishibori M, Ishikura H, Yamamoto T, Taga A, Kawabata A. Peripheral HMGB1-induced hyperalgesia in mice: redox state-dependent distinct roles of RAGE and TLR4. J Pharmacol Sci 2016; 130: 139–142. [DOI] [PubMed] [Google Scholar]
- 119.Irie Y, Tsubota M, Ishikura H, Sekiguchi F, Terada Y, Tsujiuchi T, Liu K, Nishibori M, Kawabata A. Macrophage-derived HMGB1 as a pain mediator in the early stage of acute pancreatitis in mice: targeting RAGE and CXCL12/CXCR4 axis. J Neuroimmune Pharmacol 2017; 12: 693–707. [DOI] [PubMed] [Google Scholar]
- 120.Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji RR. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest 2018; 128: 3568–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ji RR. Specialized pro-resolving mediators as resolution pharmacology for the control of pain and itch. Annu Rev Pharmacol Toxicol 2023; 63: 273–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang YH, Li Y, Wang JN, Zhao QX, Wen S, Wang SC, Sun T. A novel mechanism of specialized proresolving lipid mediators mitigating radicular pain: the negative interaction with NLRP3 inflammasome. Neurochem Res 2020; 45: 1860–1869. [DOI] [PubMed] [Google Scholar]
- 123.Zhang LY, Jia MR, Sun T. The roles of special proresolving mediators in pain relief. Rev Neurosci 2018; 29: 645–660. [DOI] [PubMed] [Google Scholar]
- 124.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol 2015; 27: 200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martini AC, Berta T, Forner S, Chen G, Bento AF, Ji RR, Rae GA. Lipoxin A4 inhibits microglial activation and reduces neuroinflammation and neuropathic pain after spinal cord hemisection. J Neuroinflammation 2016; 13: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fattori V, Zaninelli TH, Rasquel-Oliveira FS, Casagrande R, Verri WA, Jr. Specialized pro-resolving lipid mediators: a new class of non-immunosuppressive and non-opioid analgesic drugs. Pharmacol Res 2020; 151: 104549. [DOI] [PubMed] [Google Scholar]
- 127.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med 2010; 16: 592–597. 1p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lim JY, Park CK, Hwang SW. Biological roles of resolvins and related substances in the resolution of pain. Biomed Res Int 2015; 2015: 830930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J 2012; 26: 1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park CK, Lu N, Xu ZZ, Liu T, Serhan CN, Ji RR. Resolving TRPV1- and TNF-alpha-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci 2011; 31: 15072–15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics 2010; 7: 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiang X, Wang S, Shao F, Fang J, Xu Y, Wang W, Sun H, Liu X, Du J, Fang J. Electroacupuncture stimulation alleviates CFA-induced inflammatory pain via suppressing P2X3 expression. Int J Mol Sci 2019; 20: 3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain 2013; 154(Suppl 1): S10–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist 2010; 16: 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Parusel S, Yi MH, Hunt CL, Wu LJ. Chemogenetic and optogenetic manipulations of microglia in chronic pain. Neurosci Bull 2023; 39: 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Z, Wei H, Piirainen S, Chen Z, Kalso E, Pertovaara A, Tian L. Spinal versus brain microglial and macrophage activation traits determine the differential neuroinflammatory responses and analgesic effect of minocycline in chronic neuropathic pain. Brain Behav Immun 2016; 58: 107–117. [DOI] [PubMed] [Google Scholar]
- 137.Song ZP, Xiong BR, Guan XH, Cao F, Manyande A, Zhou YQ, Zheng H, Tian YK. Minocycline attenuates bone cancer pain in rats by inhibiting NF-κB in spinal astrocytes. Acta Pharmacol Sin 2016; 37: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhu MD, Zhao LX, Wang XT, Gao YJ, Zhang ZJ. Ligustilide inhibits microglia-mediated proinflammatory cytokines production and inflammatory pain. Brain Res Bull 2014; 109: 54–60. [DOI] [PubMed] [Google Scholar]
- 139.Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018; 100: 1292–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S, Basbaum AI. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 2016; 19: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev 2009; 60: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Berta T, Park CK, Xu ZZ, Xie RG, Liu T, Lu N, Liu YC, Ji RR. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J Clin Invest 2014; 124: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang J, Xie S, Zhu S, Xu ZZ. Specialized microglia resolve neuropathic pain in the spinal cord. Neurosci Bull 2023; 39: 173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci USA 2016; 113: E3441–E3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 2003; 306: 624–630. [DOI] [PubMed] [Google Scholar]
- 146.Kohno K, Shirasaka R, Yoshihara K, Mikuriya S, Tanaka K, Takanami K, Inoue K, Sakamoto H, Ohkawa Y, Masuda T, Tsuda M. A spinal microglia population involved in remitting and relapsing neuropathic pain. Science 2022; 376: 86–90. [DOI] [PubMed] [Google Scholar]
- 147.Asatryan A, Bazan NG. Molecular mechanisms of signaling via the docosanoid neuroprotectin D1 for cellular homeostasis and neuroprotection. J Biol Chem 2017; 292: 12390–12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, Coulpier F, Siopi E, David FS, Scholz C, Shihui F, Lum J, Amoyo AA, Larbi A, Poidinger M, Buttgereit A, Lledo PM, Greter M, Chan JKY, Amit I, Beyer M, Schultze JL, Schlitzer A, Pettersson S, Ginhoux F, Garel S. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 2018; 172: 500–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xie YK, Luo H, Qiu XY, Xu ZZ. Resolution of inflammatory pain by endogenous chemerin and g protein-coupled receptor ChemR23. Neurosci Bull 2021; 37: 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu ZZ, Berta T, Ji RR. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J Neuroimmune Pharmacol 2013; 8: 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 2005; 201: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Furutani K, Ji RR. Targeting Hv1 proton channel for pain control. Cell Res 2022; 32: 419–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang WJ, Luo HL, Zhu ZM. The role of P2X4 receptors in chronic pain: a potential pharmacological target. Biomed Pharmacother 2020; 129: 110447. [DOI] [PubMed] [Google Scholar]
- 154.Sun S, Jiang W, Yan X. Ligand-gated ion channel P2X7 regulates NLRP3/caspase-1-mediated inflammatory pain caused by pulpitis in the trigeminal ganglion and medullary dorsal horn. Brain Res Bull 2023; 192: 1–10. [DOI] [PubMed] [Google Scholar]
- 155.Hu X, Du L, Liu S, Lan Z, Zang K, Feng J, Zhao Y, Yang X, Xie Z, Wang PL, Ver Heul AM, Chen L, Samineni VK, Wang YQ, Lavine KJ, Gereau RWt, Wu GF, Hu H. A TRPV4-dependent neuroimmune axis in the spinal cord promotes neuropathic pain. J Clin Invest 2023; 133: e161507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ji RR, Donnelly CR, Nedergaard M. Astrocytes in chronic pain and itch. Nat Rev Neurosci 2019; 20: 667–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu YY, Zhang HL, Lu X, Du H, Li YC, Zhang PA, Xu GY. Targeting GATA1 and p2x7r locus binding in spinal astrocytes suppresses chronic visceral pain by promoting DNA demethylation. Neurosci Bull 2022; 38: 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res 2000; 25: 1439–1451. [DOI] [PubMed] [Google Scholar]
- 159.Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem 2006; 97: 772–783. [DOI] [PubMed] [Google Scholar]
- 160.Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain 1997; 71: 225–235. [DOI] [PubMed] [Google Scholar]
- 161.Okada-Ogawa A, Suzuki I, Sessle BJ, Chiang CY, Salter MW, Dostrovsky JO, Tsuboi Y, Kondo M, Kitagawa J, Kobayashi A, Noma N, Imamura Y, Iwata K. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J Neurosci 2009; 29: 11161–11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lu HJ, Gao YJ. Astrocytes in chronic pain: cellular and molecular mechanisms. Neurosci Bull 2023; 39: 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Urits I, Adamian L, Fiocchi J, Hoyt D, Ernst C, Kaye AD, Viswanath O. Advances in the understanding and management of chronic pain in multiple sclerosis: a comprehensive review. Curr Pain Headache Rep 2019; 23: 59. [DOI] [PubMed] [Google Scholar]
- 164.Gritsch S, Lu J, Thilemann S, Wortge S, Mobius W, Bruttger J, Karram K, Ruhwedel T, Blanfeld M, Vardeh D, Waisman A, Nave KA, Kuner R. Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nat Commun 2014; 5: 5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zarpelon AC, Rodrigues FC, Lopes AH, Souza GR, Carvalho TT, Pinto LG, Xu D, Ferreira SH, Alves-Filho JC, McInnes IB, Ryffel B, Quesniaux VF, Reverchon F, Mortaud S, Menuet A, Liew FY, Cunha FQ, Cunha TM, Verri WA, Jr. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. FASEB J 2016; 30: 54–65. [DOI] [PubMed] [Google Scholar]
- 166.Zhang ZJ, Jiang BC, Gao YJ. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci 2017; 74: 3275–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen T, Taniguchi W, Chen QY, Tozaki-Saitoh H, Song Q, Liu RH, Koga K, Matsuda T, Kaito-Sugimura Y, Wang J, Li ZH, Lu YC, Inoue K, Tsuda M, Li YQ, Nakatsuka T, Zhuo M. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat Commun 2018; 9: 1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li C, Liu S, Lu X, Tao F. Role of descending dopaminergic pathways in pain modulation. Curr Neuropharmacol 2019; 17: 1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Llorca-Torralba M, Borges G, Neto F, Mico JA, Berrocoso E. Noradrenergic locus coeruleus pathways in pain modulation. Neuroscience 2016; 338: 93–113. [DOI] [PubMed] [Google Scholar]
- 170.Caputi FF, Nicora M, Simeone R, Candeletti S, Romualdi P. Tapentadol: an analgesic that differs from classic opioids due to its noradrenergic mechanism of action. Minerva Med 2019; 110: 62–78. [DOI] [PubMed] [Google Scholar]
- 171.Hayashida KI, Obata H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int J Mol Sci 2019; 20: 822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pertovaara A. The noradrenergic pain regulation system: a potential target for pain therapy. Eur J Pharmacol 2013; 716: 2–7. [DOI] [PubMed] [Google Scholar]
- 173.Maeda M, Tsuruoka M, Hayashi B, Nagasawa I, Inoue T. Descending pathways from activated locus coeruleus/subcoeruleus following unilateral hindpaw inflammation in the rat. Brain Res Bull 2009; 78: 170–174. [DOI] [PubMed] [Google Scholar]
- 174.Tsuruoka M, Hitoto T, Hiruma Y, Matsui Y. Neurochemical evidence for inflammation-induced activation of the coeruleospinal modulation system in the rat. Brain Res 1999; 821: 236–240. [DOI] [PubMed] [Google Scholar]
- 175.Li J, Wei Y, Zhou J, Zou H, Ma L, Liu C, Xiao Z, Liu X, Tan X, Yu T, Cao S. Activation of locus coeruleus-spinal cord noradrenergic neurons alleviates neuropathic pain in mice via reducing neuroinflammation from astrocytes and microglia in spinal dorsal horn. J Neuroinflammation 2022; 19: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol 2003; 521: 1–21. [PubMed] [Google Scholar]
- 177.Marchand F, Tsantoulas C, Singh D, Grist J, Clark AK, Bradbury EJ, McMahon SB. Effects of etanercept and minocycline in a rat model of spinal cord injury. Eur J Pain 2009; 13: 673–681. [DOI] [PubMed] [Google Scholar]
- 178.Genevay S, Stingelin S, Gabay C. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann Rheum Dis 2004; 63: 1120–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Z, Wang J, Li X, Yuan Y, Fan G. Interleukin-1 beta of Red nucleus involved in the development of allodynia in spared nerve injury rats. Exp Brain Res 2008; 188: 379–384. [DOI] [PubMed] [Google Scholar]
- 180.Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet 2019; 394: 1765–1774. [DOI] [PubMed] [Google Scholar]
- 181.Piotrowska A, Kwiatkowski K, Rojewska E, Makuch W, Mika J. Maraviroc reduces neuropathic pain through polarization of microglia and astroglia - evidence from in vivo and in vitro studies. Neuropharmacology 2016; 108: 207–219. [DOI] [PubMed] [Google Scholar]
- 182.Matsushita K, Tozaki-Saitoh H, Kojima C, Masuda T, Tsuda M, Inoue K, Hoka S. Chemokine (C-C motif) receptor 5 is an important pathological regulator in the development and maintenance of neuropathic pain. Anesthesiology 2014; 120: 1491–1503. [DOI] [PubMed] [Google Scholar]
- 183.Tao X, Lee MS, Donnelly CR, Ji RR. Neuromodulation, specialized proresolving mediators, and resolution of pain. Neurotherapeutics 2020; 17: 886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 2007; 129: 210–223. [DOI] [PubMed] [Google Scholar]
- 185.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010; 142: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ramsden CE, Faurot KR, Zamora D, Palsson OS, MacIntosh BA, Gaylord S, Taha AY, Rapoport SI, Hibbeln JR, Davis JM, Mann JD. Targeted alterations in dietary n-3 and n-6 fatty acids improve life functioning and reduce psychological distress among patients with chronic headache: a secondary analysis of a randomized trial. Pain 2015; 156: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]