Key Points
Question
What is the clinical activity of olvimulogene nanivacirepvec oncolytic immunotherapy and subsequent platinum-doublet chemotherapy with or without bevacizumab in women with platinum-resistant or platinum-refractory ovarian cancer (PRROC)?
Findings
In this phase 2 nonrandomized clinical trial of 27 patients with PRROC and a median of 4 prior lines of therapy, 13 of 24 (54%) evaluable by Response Evaluation Criteria in Solid Tumors version 1.1 experienced an objective response, with a median progression-free survival of 11.0 months and a manageable safety profile.
Meaning
These hypothesis-generating results exceed historically expected outcomes from standard therapies or platinum rechallenge, warranting further clinical evaluation in a larger confirmatory phase 3 clinical trial.
This nonrandomized phase 2 clinical trial examines the clinical activity of Olvi-Vec oncolytic immunotherapy and subsequent platinum-doublet chemotherapy with or without bevacizumab in women with platinum-resistant or -refractory ovarian cancers.
Abstract
Importance
Patients with platinum-resistant or platinum-refractory ovarian cancer (PRROC) have limited therapeutic options, representing a considerable unmet medical need.
Objective
To assess antitumor activity and safety of intraperitoneal (IP) olvimulogene nanivacirepvec (Olvi-Vec) virotherapy and platinum-based chemotherapy with or without bevacizumab in patients with PRROC.
Design, Setting, and Participants
This open-label, nonrandomized multisite phase 2 VIRO-15 clinical trial enrolled patients with PRROC with disease progression following their last prior line of therapy from September 2016 to September 2019. Data cutoff was on March 31, 2022, and data were analyzed between April 2022 and September 2022.
Interventions
Olvi-Vec was administered via a temporary IP dialysis catheter as 2 consecutive daily doses (3 × 109 pfu/d) followed by platinum-doublet chemotherapy with or without bevacizumab.
Main Outcomes and Measures
Primary outcomes were objective response rate (ORR) via Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) and cancer antigen 125 (CA-125) assay, and progression-free survival (PFS). Secondary outcomes included duration of response (DOR), disease control rate (DCR), safety, and overall survival (OS).
Results
Twenty-seven heavily pretreated patients with platinum-resistant (n = 14) or platinum-refractory (n = 13) ovarian cancer were enrolled. The median (range) age was 62 (35-78) years. The median (range) prior lines of therapy were 4 (2-9). All patients completed both Olvi-Vec infusions and chemotherapy. Median follow-up duration was 47.0 months (95% CI, 35.9 months to NA). Overall, ORR by RECIST 1.1 was 54% (95% CI, 33%-74%), with a DOR of 7.6 months (95% CI, 3.7-9.6 months). The DCR was 88% (21/24). The ORR by CA-125 was 85% (95% CI, 65%-96%). Median PFS by RECIST 1.1 was 11.0 months (95% CI, 6.7-13.0 months), and the PFS 6-month rate was 77%. Median PFS was 10.0 months (95% CI, 6.4-NA months) in the platinum-resistant group and 11.4 months (95% CI, 4.3-13.2 months) in the platinum-refractory group. The median OS was 15.7 months (95% CI, 12.3-23.8 months) in all patients, with a median OS of 18.5 months (95% CI, 11.3-23.8 months) in the platinum-resistant group and 14.7 months (95% CI, 10.8-33.6 months) in the platinum-refractory group. Most frequent treatment-related adverse events (TRAEs) (any grade, grade 3) were pyrexia (63.0%, 3.7%, respectively) and abdominal pain (51.9%, 7.4%, respectively). There were no grade 4 TRAEs, and no treatment-related discontinuations or deaths.
Conclusions and Relevance
In this phase 2 nonrandomized clinical trial, Olvi-Vec followed by platinum-based chemotherapy with or without bevacizumab as immunochemotherapy demonstrated promising ORR and PFS with a manageable safety profile in patients with PRROC. These hypothesis-generating results warrant further evaluation in a confirmatory phase 3 trial.
Trial Registration
ClinicalTrials.gov Identifier: NCT02759588
Introduction
Patients with platinum-resistant or platinum-refractory ovarian cancer (PRROC) have limited treatment options.1 Clinical management of PRROC in patients with up to 2 prior lines include the AURELIA regimens of bevacizumab and non–platinum-based single-agent chemotherapies.2 Thereafter, patients typically receive physician’s choice of a nonplatinum chemotherapy or enroll into clinical trials. The latest National Comprehensive Cancer Network Guidelines also include platinum-based regimens for platinum-resistant disease.1 Single-agent therapy response rates, median progression-free survival (PFS), and median overall survival (OS) range from 10% to 15%, 3 to 4 months, and 9 to 12 months, respectively.3 Hence, the development of novel, more effective therapies addresses a critical unmet medical need.
Olvimulogene nanivacirepvec (Olvi-Vec; aka GL-ONC1; laboratory name: GLV-1h68) is a modified oncolytic vaccinia virus that selectively infects malignant cells and replicates especially well in ovarian and lung cancers.4 Because ovarian cancer usually metastasizes throughout the peritoneal cavity, it is amenable to intraperitoneal (IP) virotherapy given the large peritoneal surface area of carcinomatosis.5 Olvi-Vec activates both innate immunity via proinflammatory response and adaptive immunity, which modify the tumor microenvironment and promote a condition wherein reversal of platinum resistance is potentially realized.6
This study investigated Olvi-Vec followed by platinum-doublet chemotherapy with or without bevacizumab, evaluating the hypothesis that such sequential therapy produces significant antitumor activity with manageable toxic effects for patients with PRROC.
Methods
Study Design and Participants
The trial protocol is available in Supplement 1 and the analysis plan is in Supplement 2. The primary end points were objective response rate (ORR) by Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) and cancer antigen 125 (CA-125) assay,7 and PFS. The secondary end points comprised safety, duration of response (DOR), disease control rate (DCR), and OS. The baseline for ORR and PFS evaluation was the assessment time point immediately prior to starting subsequent platinum-based therapy to allow direct comparison with historical data. The study was approved by the central Quorum (now Advarra) institutional review board, and all patients provided written informed consent.
Eligible patients included those with primary or acquired histologically confirmed PRROC, Eastern Cooperative Oncology Group performance status of 0 or 1, and documented progressive disease at screening. Patients with mucinous carcinoma, nonepithelial ovarian cancers, and unresolved bowel obstruction were excluded. Participant PRROC status was determined by platinum-free interval, defined as the time from last dose of most recent platinum-based regimen to disease progression, with progression of less than 6 months as platinum-resistant, and lack of response or progression earlier than 1 month as platinum-refractory.
Procedures
All patients received Olvi-Vec at 3 × 109 plaque-forming units (pfu)/d on 2 consecutive days through a temporary IP catheter placed laparoscopically approximately 5 days before. Immediately prior to the first dose of Olvi-Vec, patients underwent an IP instillation and drainage of 1 L lactated Ringer’s to reduce IP complement and facilitate viral replication. Chemotherapy was recommended to start approximately 6 weeks following Olvi-Vec. Platinum doublet was at the discretion of investigators, selected from gemcitabine, taxane, or pegylated liposomal doxorubicin (PLD) coupled with carboplatin or cisplatin. If patients developed undue toxic effects associated with platinum therapy, continued nonplatinum single-agent therapy was encouraged. Bevacizumab was allowed with platinum-doublet or single-agent therapies. Treatment continued until disease progression or unacceptable toxic effects.
Statistical Analysis
The study had 90% power using a 1-sided 5% significance level to detect an improvement in ORR by RECIST 1.1 from 27% (ie, null hypothesis based on the AURELIA study2) to 54% in 28 evaluable patients. This study had a Simon 2-stage design, with stage 1 proceeding to stage 2 after achieving 5 or more responders in up to 15 evaluable participants; and stage 2: the null was rejected if there were 12 or more responders of up to 28 evaluable patients. The DOR, PFS, and OS were summarized using the Kaplan-Meier method. Statistical analyses were conducted using R software (version 4.0.2, R Foundation). Data analyses were conducted between April 2022 and September 2022.
Results
Antitumor Activity
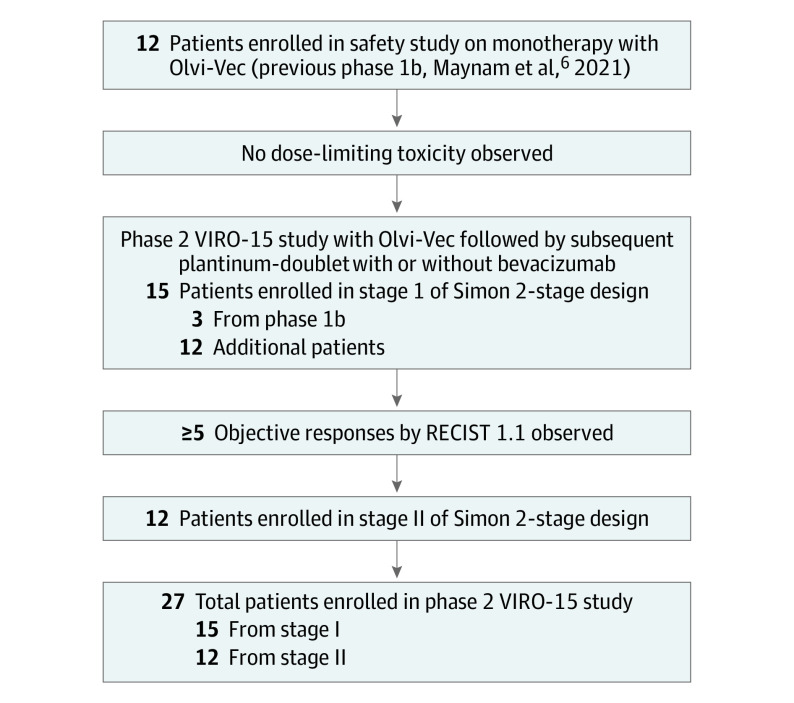
Twenty-seven patients with platinum-resistant (n = 14) or platinum-refractory (n = 13) ovarian cancer (Figure 1; eTable 1 in Supplement 3) were enrolled and received Olvi-Vec. A median 6 cycles (range, 1-17) of platinum-based therapy was given to all patients, which was initiated at a median of 6 weeks following Olvi-Vec. All patients received carboplatin-doublet therapy, except for 1 who had oxaliplatin-doublet therapy. The nonplatinum agents included gemcitabine (44%), docetaxel (26%), paclitaxel (15%), and PLD (15%). Bevacizumab was initiated with platinum-based therapies in 23 of 27 patients; however, the remaining 4 patients also received bevacizumab with delay. Six patients received oral cyclophosphamide with bevacizumab as continued therapy after platinum-based therapy.
Figure 1. Study Flow Diagram.
RECIST 1.1 indicates Response Evaluation Criteria in Solid Tumors (version 1.1); Olvi-Vec, olvimulogene nanivacirepvec.
Twenty-four RECIST-evaluable patients with measurable disease had an ORR of 54% (13/24; 95% CI, 33%-74%; confirmed ORR, 42%; Table). The 13 responders exceeded the Simon stage 2 requirement by rejecting the null when there were 12 or more responders as planned; therefore, enrollment was terminated early. Four patients (16.7%) had 100% reduction in target lesions (2 with confirmed complete response), including 2 patients with platinum-refractory disease (Figure 2A-B). The median time to RECIST response was 4.0 months (95% CI, 2.0-4.9 months). The DOR was 7.6 months (95% CI, 3.7-9.6 months). The DCR was 88% (8 stable disease and 13 responders). Overall, 19 of 22 patients (86%) showed tumor shrinkage.
Table. Outcomes of Antitumor Activity.
| Variable | All | Platinum resistant | Platinum refractory |
|---|---|---|---|
| Response by RECIST 1.1 | |||
| Evaluable patients, No. | 24a | 11 | 13 |
| ORR, % (95% CI) | 54 (33-74)b | 55 (26-84) | 54 (27-81) |
| DOR, median (95% CI), mo | 7.6 (3.7-9.6) | 7.6 (3.7-NA) | 8.0 (3.7-NA) |
| DCR, % (95% CI) | 88 (68-97) | 100 (72-100) | 77 (46-95) |
| Response by CA-125 | |||
| Evaluable patients, No. | 26c | 13 | 13 |
| ORR, % (95% CI) | 85 (65-96) | 85 (55-98) | 85 (55-98) |
| Survival | |||
| Evaluable patients, No. | 27 | 14 | 13 |
| PFS, median (95% CI), mo | 11.0 (6.7-13.0) | 10.0 (6.4-NA) | 11.4 (4.3-13.2) |
| OS, median (95% CI), mo | 15.7 (12.3-23.8) | 18.5 (11.3-23.8) | 14.7 (10.8-33.6) |
Abbreviations: CA-125, cancer antigen 125 assay; DCR, disease control rate; DOR, duration of response; NA, not applicable; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; RECIST 1.1, Response Evaluation Criteria in Solid Tumors (version 1.1).
Three of 27 patients were not evaluable as defined by RECIST 1.1 criteria due to no measurable disease. However, these 3 patients were evaluable by the Gynecological Cancer InterGroup (GCIG) CA-125 criteria, showing 2 partial responses and 1 complete response as best response.
Including 3 unconfirmed; 2 in resistant and 1 in refractory groups.
One of 27 patents was not evaluable by GCIG CA-125 criteria. However, this patient was evaluable by RECIST 1.1, showing stable disease as best response.
Figure 2. Evaluations of Antitumor Activity.
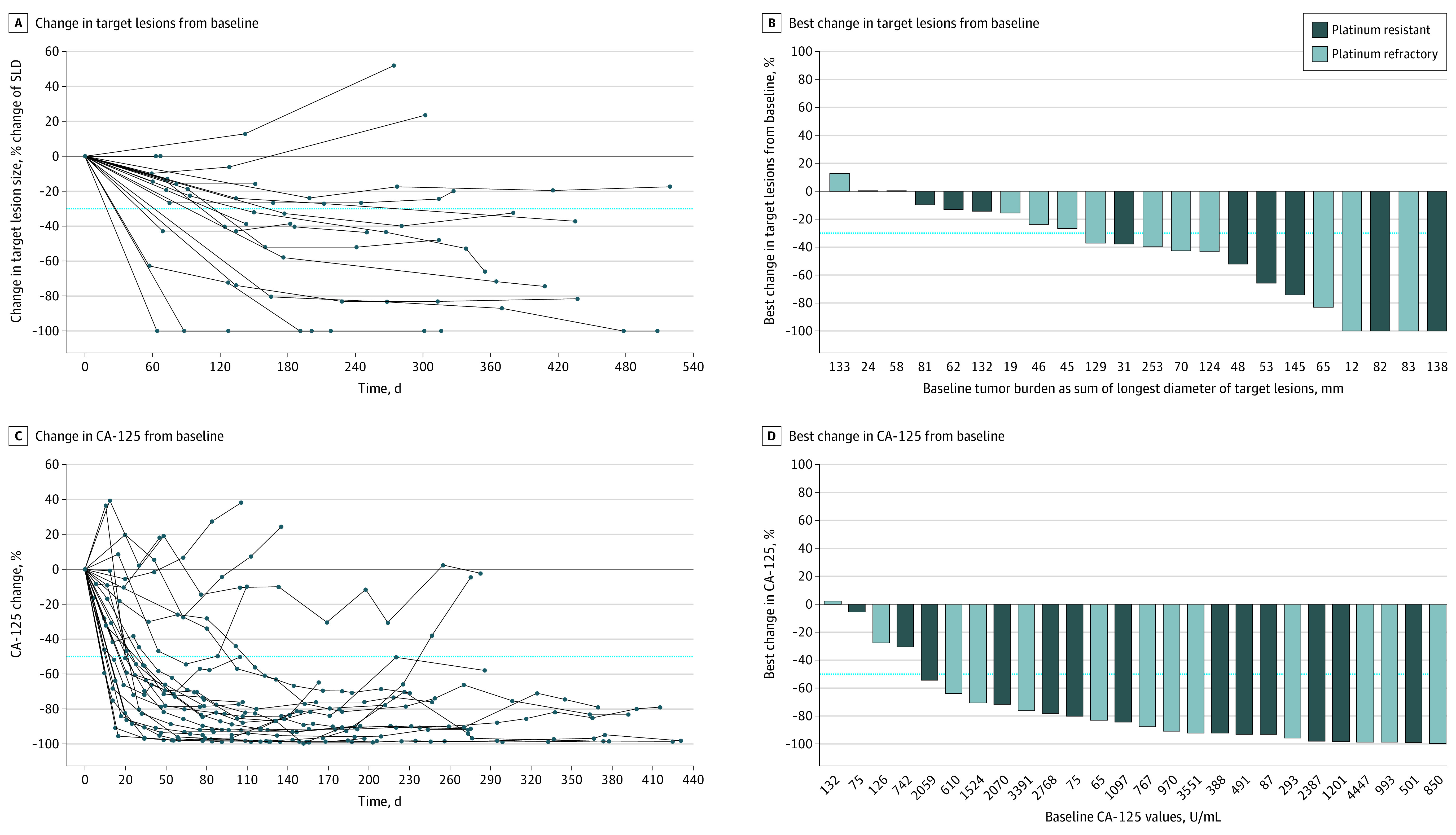
A, Assessment by the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), showing a spider plot of radiographic change over time in target lesions (the dotted line at −30% indicates partial response). B, Waterfall plot of best change in target lesions from baseline. C, Assessment by the cancer antigen 125 (CA-125) assay, showing a spider plot of CA-125 change over time (the dotted line at −50% indicates partial response). D, Waterfall plot of best change in CA-125 from baseline.
The ORR by CA-125 was 85% (95% CI, 65%-96%) in all patients, and 25 of 26 patients (96%) exhibited decreased CA-125 levels (Figure 2C-D). The median PFS was 11.0 (95% CI, 6.7-13.0) months, with 77% achieving a 6-month PFS, and the median OS was 15.7 (95% CI, 12.3-23.8) months (Table; eFigure in Supplement 3).
Safety
Most frequent TRAEs (any grade) included pyrexia (n = 18; 63%), abdominal pain (n = 14; 51.9%), and nausea (n = 13; 48.1%) (eTable 2A in Supplement 3). Toxic effects were manageable; however, nonsteroidal anti-inflammatory agents were avoided for up to 3 weeks following Olvi-Vec to minimize inhibition of viral activities. Flu-like symptoms, including fever, chills, and myalgia, were primarily transient in hours or overnight. Hydration was administered prophylactically or as needed during and immediately following treatment with Olvi-Vec to reduce symptoms.
Discussion
In this nonrandomized phase 2 clinical trial, we documented 54% ORR by RECIST in all evaluable patients with a median of 4 prior lines of therapy (22 of 27 [81%] had >2 prior lines). The results exceed the 27.3% ORR in the AURELIA study,2 which excluded patients with more than 2 prior lines of therapy and platinum-refractory disease. Of note, only 7% (vs 81% in our study) of patients in the AURELIA study had prior treatment with bevacizumab. Furthermore, only 1 of 13 (8%) patients with platinum-refractory disease had transient response to prior platinum-based therapy, yet the same patients achieved 54% RECIST response and a median DOR of 8.0 months in this study, suggesting reversal of platinum refractoriness by treatment with Olvi-Vec.
Prior studies have shown that BRCA1/2 variants are associated with increased sensitivity to platinum-based therapy.8 However, in this study, ORR by RECIST was 29% (2/7) in patients with BRCA1/2 variants vs 65% (11/17) in those with wild-type BRCA, indicating response was not driven by BRCA1/2 variation. Prior poly(ADP-ribose) polymerase (PARP) inhibitor exposure may induce platinum resistance.9 However, in this study, 11 of 20 patients (55%) who received previous PARP inhibitors achieved RECIST response, further suggesting virus-induced reversal of platinum resistance and/or immune response.
Previous data support the staggered approach of viral immunotherapy and chemotherapy.10,11,12 Virus-mediated tumor cell lysis may induce immune priming by increasing T-cell response through cross-presentation of the tumor (neo)antigens, and being further boosted by subsequent cytotoxic chemotherapy.13 In this population, Olvi-Vec was associated with an influx of CD4-positive and CD8-positive tumor-infiltrating lymphocytes in paired tumor biopsy samples,14 which was a positive prognostic factor in patients with ovarian cancer.15 Overall, Olvi-Vec may convert immunologically “cold” to “hot” tumors by modifying the tumor microenvironment and abrogate platinum resistance.16,17
Limitations
One limitation of this single-arm nonrandomized clinical trial is the small number of participants.
Conclusions
The findings of this nonrandomized clinical trial suggest that Olvi-Vec and platinum-based chemotherapy with or without bevacizumab demonstrated promising ORR and PFS, and clinical reversal of platinum resistance and refractoriness, with manageable toxic effects among patients with PRROC. These hypothesis-generating results warrant further evaluation in a larger confirmatory study. The prospective phase 3 OnPrime/GOG-3076 study is currently enrolling (NCT05281471).
Trial Protocol
Analysis Plan
eFigure. Kaplan-Meier Estimate of Overall Survival
eTable 1. Patient Demographic and Clinical Characteristics
eTable 2. Adverse Events
Data Sharing Statement
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Ovarian Cancer Continue Including Fallopian Tube Cancer and Primary Peritoneal Cancer. Version 1. 2023. Accessed December 22, 2023. https://www.nccn.org/guidelines/category_1
- 2.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. [published correction appears in J Clin Oncol. 2014;32(35):4025]. J Clin Oncol. 2014;32(13):1302-1308. doi: 10.1200/JCO.2013.51.4489 [DOI] [PubMed] [Google Scholar]
- 3.Davis A, Tinker AV, Friedlander M. “Platinum resistant” ovarian cancer: what is it, who to treat and how to measure benefit? Gynecol Oncol. 2014;133(3):624-631. doi: 10.1016/j.ygyno.2014.02.038 [DOI] [PubMed] [Google Scholar]
- 4.Ascierto ML, Worschech A, Yu Z, et al. Permissivity of the NCI-60 cancer cell lines to oncolytic vaccinia virus GLV-1h68. BMC Cancer. 2011;11:451. doi: 10.1186/1471-2407-11-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauer UM, Schell M, Beil J, et al. Phase I study of oncolytic vaccinia virus GL-ONC1 in patients with peritoneal carcinomatosis. Clin Cancer Res. 2018;24(18):4388-4398. doi: 10.1158/1078-0432.CCR-18-0244 [DOI] [PubMed] [Google Scholar]
- 6.Manyam M, Stephens AJ, Kennard JA, et al. A phase 1b study of intraperitoneal oncolytic viral immunotherapy in platinum-resistant or refractory ovarian cancer. [published correction appears in Gynecol Oncol. 2022;165(2):401]. Gynecol Oncol. 2021;163(3):481-489. doi: 10.1016/j.ygyno.2021.10.069 [DOI] [PubMed] [Google Scholar]
- 7.Rustin GJ, Vergote I, Eisenhauer E, et al. ; Gynecological Cancer Intergroup . Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer. 2011;21(2):419-423. doi: 10.1097/IGC.0b013e3182070f17 [DOI] [PubMed] [Google Scholar]
- 8.Mylavarapu S, Das A, Roy M. Role of BRCA mutations in the modulation of response to platinum therapy. Front Oncol. 2018;8:16. doi: 10.3389/fonc.2018.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose PG. Ovarian cancer recurrence: is the definition of platinum sensitivity modified by PARPi, bevacizumab or other intervening treatments?: a clinical perspective. Cancer Drug Resist. 2022;5(2):415-423. doi: 10.20517/cdr.2022.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song CK, Han HD, Noh KH, et al. Chemotherapy enhances CD8(+) T cell-mediated antitumor immunity induced by vaccination with vaccinia virus. Mol Ther. 2007;15(8):1558-1563. doi: 10.1038/sj.mt.6300221 [DOI] [PubMed] [Google Scholar]
- 11.Wennier ST, Liu J, McFadden G. Bugs and drugs: oncolytic virotherapy in combination with chemotherapy. Curr Pharm Biotechnol. 2012;13(9):1817-1833. doi: 10.2174/138920112800958850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell L, Peng KW, Russell SJ, Diaz RM. Oncolytic viruses: Priming time for cancer immunotherapy. BioDrugs. 2019;33(5):485-501. doi: 10.1007/s40259-019-00367-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436-443. doi: 10.1158/2326-6066.CIR-15-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holloway R, Mendivil A, Kendrick J, et al. Oncolytic vaccinia (Olvi-Vec) primed immunochemotherapy in platinum-resistant/refractory ovarian cancer. Int J Gynecol Cancer. 2020;30:A9-A10. doi: 10.1136/ijgc-2020-IGCS.12 [DOI] [Google Scholar]
- 15.Pinto MP, Balmaceda C, Bravo ML, et al. Patient inflammatory status and CD4+/CD8+ intraepithelial tumor lymphocyte infiltration are predictors of outcomes in high-grade serous ovarian cancer. Gynecol Oncol. 2018;151(1):10-17. doi: 10.1016/j.ygyno.2018.07.025 [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Kryczek I, Dostál L, et al. Effector T-cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell. 2016;165(5):1092-1105. doi: 10.1016/j.cell.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochoa de Olza M, Navarro Rodrigo B, Zimmermann S, Coukos G. Turning up the heat on non-immunoreactive tumours: opportunities for clinical development. Lancet Oncol. 2020;21(9):e419-e430. doi: 10.1016/S1470-2045(20)30234-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Analysis Plan
eFigure. Kaplan-Meier Estimate of Overall Survival
eTable 1. Patient Demographic and Clinical Characteristics
eTable 2. Adverse Events
Data Sharing Statement