Abstract
Purpose of Review
Atopic dermatitis (AD) remains a dermatological disease that imposes a significant burden on society. Air pollution has previously been linked to both the onset and severity of atopic dermatitis. As air pollution remains a critical environmental factor impacting human health, this review seeks to provide an overview of the relationship between different air pollutants and AD.
Recent Findings
AD can develop from multiple causes that can be broadly grouped into epidermal barrier dysfunction and immune dysregulation. Air pollution imposes significant health risks and includes a wide variety of pollutant types. AD has been linked to outdoor air pollutants such as particulate matter (PM), volatile organic compounds (VOC), gaseous compounds, and heavy metals. Exposure to indoor pollutants such as tobacco smoke and fungal molds has also been associated with an increased incidence of AD. While different pollutants impact distinct molecular pathways in the cell, they mostly converge on ROS product, DNA damage, and dysregulated T-cell activity and cytokine production.
Summary
The presented review suggests a strengthening tie between air pollution and AD. It points to opportunities for further studies to clarify, as well as potential therapeutic opportunities that leverage the mechanistic relationships between air pollution and AD.
Keywords: Atopic dermatitis, Air pollution, Particulate matter, Eczema
Introduction
Atopic dermatitis (AD) is a common chronic inflammatory skin disease and the leading non-fatal health burden attributable to skin diseases, affecting approximately 31.6 million people in the USA [1, 2]. AD is characterized by pruritus and eczematous lesions that manifest in specific patterns across the body [3]. In addition to the primary symptoms, AD can contribute to a multitude of other health effects such as secondary infection [4], failure to thrive [5], sleep deprivation [6, 7], and impaired psychosocial well-being [8].
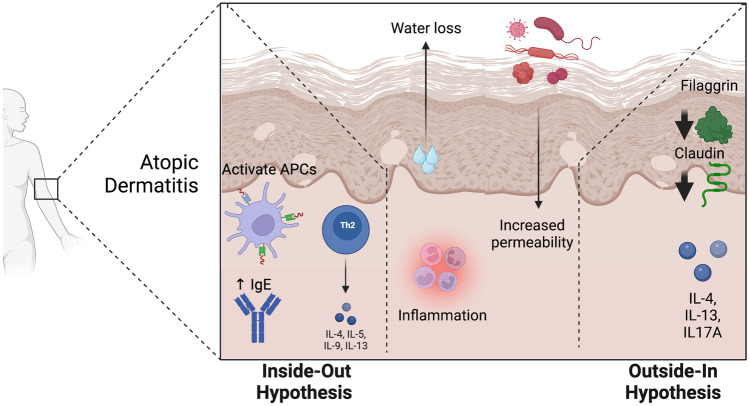
Numerous etiologies of atopic dermatitis have been proposed, including epidermal barrier dysfunction, dysregulation of the immune system, and alterations of the microbiome [9]. These mechanisms of AD pathogenesis have led to the description of two major subtypes of AD: extrinsic and intrinsic. The extrinsic form of the disease, historically described by the “outside-in hypothesis,” is driven by the loss of epidermal barrier integrity (Fig. 1). The intrinsic form, also known as the “inside-out hypothesis,” is driven by the dysregulated activity of certain immune cell types. While there has been a long-standing discussion on the initial cause of AD, researchers have begun to explore other elements that may modify the severity of AD. Over the last two decades, numerous studies have covered the relationship between environmental factors, allergies, and AD. This area of work has not only produced insights into the effect of air pollution on AD but also highlighted crucial questions that remain unanswered. An improved understanding of this relationship may advance public policy and therapeutic development for AD and overall health.
Fig. 1.
Inside-out and outside-in hypotheses of atopic dermatitis. APCs antigen-presenting cells, Th2, T helper 2 cells, IL, interleukin
This review will discuss our evolving understanding of the connection between air pollution exposure and atopic dermatitis. We begin with our current understanding of the pathogenesis of atopic dermatitis. Next, we discuss the body of work on the association and relationship between different pollutants and AD. Finally, we discuss our current mechanistic insights into the role of pollutants and AD. With the framework of molecular mechanisms, we discuss the inside-out and outside-in hypotheses and how they guide future directions in this area of work.
Epidemiology
AD has traditionally been considered to affect mainly children; however, it also affects adults. Smaller epidemiological studies suggest an AD prevalence of about 7% in adults [10], with some studies showing as high as 17% in the elderly [11] and evidence that this may be increasing over time [12]. The overall prevalence of AD in children in the USA is estimated to be around 16% [13–16]. Global studies mirror that of the USA, with approximately 5–20% of children suffering from AD worldwide [17]. Higher rates of sensitization to allergens, including aeroallergens, are seen in children over the age of 5 [18, 19], with the rate further elevated in the AD patient population [20]. While sensitization to allergens and frequency of AD are associated with high socioeconomic status, a lower socioeconomic status is associated with more severe disease [21]. There has been evidence suggesting that air pollution may be linked to increasing incidence seen in the elderly [22].
Pathophysiology of Atopic Dermatitis
Multiple mechanisms have been implicated in the development of atopic dermatitis. These mechanisms are delineated into two conceptual categories: those that involve internal dysregulation, the inside-out hypothesis, and those that involve increased external insults, the outside-in hypothesis. The outside-in hypothesis postulates that AD is triggered by epidermal barrier dysfunction and skin inflammation due to environmental insults. Alternatively, the inside-out hypothesis focuses on how immune dysregulation and cascading pathways result in AD.
The core of the inside-out hypothesis is that AD is initiated by immune dysregulation and inflammation. Tissue damage and microorganisms in the skin can activate innate immune receptors on keratinocytes and antigen-presenting cells (APCs). Activation of pattern recognition receptors (PRRs), such as the toll-like receptors (TLRs), can lead to the release of various factors. These include alarmins such as antimicrobial peptides (AMPs); cytokines IL-1A, TSLP, and IL-33; proteases such as kallikreins and cathepsins; and ECM proteins, including periostin [23]. Alarmin-mediated activation of inflammatory dendritic epidermal cells and type-2 immune cells such as Th2 cells drive a local inflammatory response. Activated Th2 cells secrete IL-4 and IL-13 and promote inflammation while also triggering the production of IgE by B cells [24]. Finally, the Th2 cytokines can suppress the expression of terminal keratinocyte differentiation genes (e.g., FLG, loricrin, involucrin), thus promoting epidermal hyperplasia [25].
The immune system also plays a role in mediating chronic itch, one of the cardinal symptoms of AD. The sensation of pruritus is mediated by signal transmission along the peripheral C-nerve fibers, which originate from the pruriceptive sensory neuronal cell bodies in the dorsal root ganglia [26]. These nerve fibers can be activated by endogenous and exogenous pruritogens, which include histamine, cytokines, and proteases. AD skin is characterized by alloknesis, in which hyperinnervation of the skin with C-nerve fibers generates pruritus from even mild mechanical stimulation [27]. Studies utilizing mouse models have identified IL-4, IL-13, and IL-31 receptors on sensory neurons innervating the skin. Interaction of IL-4 with their pruritogenic sensory nerve endings appears to sensitize the neuron to other pruritogens, rather than directly triggering itch [28, 29].
The outside-in hypothesis proposes that AD is initiated by epidermal barrier dysfunction. Filaggrin is critical to the integrity of the stratum corneum, as it is processed from its profilaggrin form to hygroscopic amino acids and their respective derivatives that combine with ions and organic compounds to maintain water retention and hydration in the stratum corneum [30, 31]. Pathologic deficiency of filaggrin results in epidermal barrier dysfunction and disrupts keratinocyte differentiation. A subset of inflammatory cytokines (IL-4, IL-13, IL17A, and others) have been shown to suppress filaggrin expression in the skin, resulting in filaggrin deficiency, thus compromising the integrity of the epidermal barrier [32]. This suggests a point of convergence between the inside-out and outside-in hypotheses, as immune function influences epidermal barrier integrity. In addition to filaggrin, imbalances between proteases and antiproteases that function locally in the stratum corneum can also result in the breakdown of the skin barrier. Within the stratum granulosum, genetic loss of tight junction proteins such as those in the claudin family may lead to impairment of the skin barrier and contribute to AD.
Genetic association studies uncovering loci associated with atopic dermatitis have provided valuable insight into the risk factors for AD, as well as the relative contributions of the different pathogenic mechanisms [33–35]. Across numerous studies, loss-of-function mutations in FLG, encoding profilaggrin, are the strongest genetic risk factor for AD [33, 36, 37]. In addition, GWAS studies have found cytokines clusters, EMSY, the membrane protein, LRRC32, and more recently, candidate genes involved in innate host defense and T-cell function [34]. These add support to the role of autoimmune dysregulation in AD.
AD can also be caused by alteration of the cutaneous microbiome [9]. AD patients have reduced microbiome diversity in the skin [9]. Additionally, the dermal microbiome of AD patients is characterized by the overgrowth of Staphylococcus aureus in the lesional skin, and to a smaller degree, in non-lesional skin [38]. Of note, microbiome diversity is restored after treatment [39]. Various types of secreted bacterial proteins can contribute to pathogenesis. Factors secreted by Staphylococcus include enterotoxin serotypes (SEA, SEB SED, SEE, SEG) that can bind to major histocompatibility complex class II on APCs and T cell receptors on T cells, increasing cytokine production. Superantigens from S. aureus can also increase IgE response and trigger mast cell degranulation [40].
The Effect of Air Pollution On Atopic Dermatitis
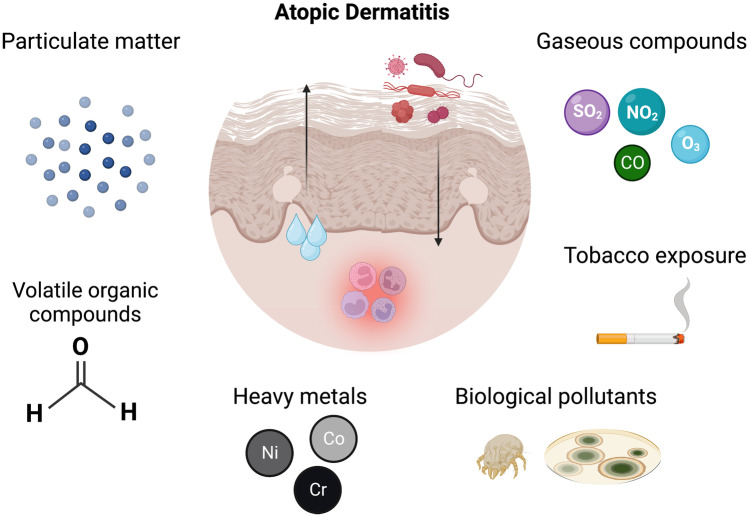
Air pollutants are ubiquitous and can be categorized into outdoor and indoor pollutants. Outdoor pollutants can have both natural and man-made origins. Natural sources of pollution include wildfires, volcanoes, biological decay, and dust storms [41]. The urbanization of society has drastically emphasized the importance of man-made origins, which include motor vehicles, biomass burning, power plants, manufacturing facilities, water incinerators, and others. Humans encounter air pollutants primarily via inhalation, ingestion, and dermal contact. Exposure of pollutants to the skin can result in immune reactivity that drives allergic responses and may contribute to the pathogenesis and/or severity of AD. Air pollutants can refer to a variety of materials, including particulate matter (PM), volatile organic compounds (VOC), traffic-related air pollution (TRAP), tobacco smoke, gaseous compounds, and heavy metals (Fig. 2). Different types of pollutants have been shown to have different levels of association with skin disorders such as atopic dermatitis.
Fig. 2.
Major subtypes of air pollutants associated with atopic dermatitis. Particulate matter includes PM2.5 and PM10. Volatile organic compounds include formaldehyde (shown in the figure). Heavy metals include nickel (Ni), cobalt (Co), and chromium (Cr). Biological pollutants include dust mites, animal dander, and mold spores
Among the air pollutants, particulate matter (PM) is the most detrimental to overall human health, particularly inflammatory lung and cardiovascular diseases [42]. PM is a mixture of particles with a heterogenous chemical composition [41] and can be found in both outdoor and indoor environments, depending on the ventilation of the building [41]. Fine particles (PM2.5) and coarse particles (PM10), referring to particles smaller than 2.5 and 10 µm in diameter, respectively, are most studied in the context of their effects on allergic reactions of the skin and atopic dermatitis. Recent studies have shown a significant positive association between AD and long-term average concentrations of PM2.5 and PM10. A study in South Korea observed an association between increasing levels of PM2.5, PM10, SO2, and CO with increasing monthly patient visits for AD [43••, 44•]. Exposure during infancy and childhood were more harmful [44•].
Volatile organic compounds within air pollution have been shown to result in immune reactivity, allergy, and increase the likelihood of AD. Formaldehyde is a common industrial pollutant that can affect skin health. One particular study found formaldehyde to induce more trans-epidermal water loss (TEWL) in children with AD compared to those without the condition [45]. A shift of skin pH towards neutral pH was also observed. This was the first study that demonstrated the direct link between exposure to chemicals and exacerbation of AD symptoms. Prenatal exposure to tobacco smoke has also been recognized as a significant risk factor for AD. A study of 7030 children aged 6 to 13 years showed a positive correlation between maternal smoking during pregnancy and the development of AD [46].
Specific gaseous compounds have been studied for their roles in different skin conditions. Many of these gaseous compounds are byproducts of combustion, such as in road transport. Key gaseous compounds that have been studied in allergy and AD include sulfur dioxide (SO2), carbon monoxide (CO), nitrogen dioxide (NO2), and ozone (O3). In a study by Park and colleagues, increases in SO2 and CO concentrations were significantly associated with increases in the number of patient visits for AD [43••]. The study found that every 1 parts per billion (ppb) increase in SO2 resulted in a visit increase of 2.26% (95% CI 1.35–3.17; P < 0.001), while every 100 ppb increase in CO resulted in visit increases of 2.86% (95% CI 1.35–4.40; P < 0.001). Furthermore, the increase in the rate of AD correlates with the amount of increase in SO2 and CO levels. However, they found no significant association between O3 and patient visits for AD. NO2 is another toxic gas and common atmospheric pollutant produced by combustion. Common sources of NO2 include fossil fuels, smog, car exhaust, and cigarettes. Studies have reported conflicting observations regarding the role of NO2 in AD. A study in China found that exposure to NO2 had a strong influence on daily outpatient visits of eczema, and an investigation in the Munich metropolitan area observed a modest, positive association between NO2 exposure and doctor-diagnosed eczema, reporting an odds ratio of 1.18 (1.00–1.39) between an increase (per interquartile range) in pollutant and prevalence of eczema diagnosis [47]. Another study in South Korea found no significant association between NO2 and increase in patient visits for AD [43••]. Another single-blind study found that short-term exposure to NO2 through a provocation test resulted in increased trans-epidermal water loss (TWEL) in AD patients [48], suggesting aggravation of AD symptoms.
Heavy metals can also be an irritant that drives symptoms of AD. A common test done to evaluate sensitivity to heavy metals is a patch test using nickel, cobalt, and chromium. Those with positive patch-test to these three metal species have increased odds of a history of atopic eczema [49, 50]. Studies utilizing genetically modified mice models suggest that deficiency in suprabasin (SBSN), a secreted protein in the stratum corneum that is often reduced in AD patients, can cause nickel allergy [51, 52]. Taken as a whole, a recent meta-analysis showed significant effects of outdoor air pollution on the development, severity, and symptoms of adult atopic dermatitis [53•].
In addition, indoor pollutants are associated with both development and aggravation of AD symptoms. Indoor pollutants can include tobacco smoke, as well as biological pollutants such as dust mites, animal dander, and mold spores. In addition, pollutants commonly recognized to have outdoor origins, such as VOCs, PM, and combustion pollutants such as sulfur dioxide, carbon monoxide, and NO2 [41], are also present indoors and can influence AD in this setting. Beyond the effects of VOCs, PM, and combustion byproducts on AD mentioned above, tobacco smoke has been associated with AD development. Prenatal exposure to environmental tobacco smoke has been associated with reduced regulatory T-cell activity and increased AD development after birth [41, 54, 55]. A study from South Korea found that prenatal exposure, but not postnatal exposure to mold is associated with AD (Adjusted odds ratio, 1.36; 95% confidence interval, 1.01–1.83) [56]. These studies demonstrate the varying effects that indoor pollutants have on AD onset and development.
Possible Mechanisms of Pollutants and AD
Studies elucidating the effects of different pollutants relied more on a mixture of clinical studies, animal models, and in vitro studies. Higher levels of PM2.5, PM10, and NO2 result in increased production of ROS, causing higher levels of oxidation in the stratum corneum. Polycyclic aromatic hydrocarbons (PAHs), a significant component of PM, can diffuse through the stratum corneum and bind to aryl hydrocarbon receptors (AhRs) [57]. Activated AhRs translocate to the nucleus and promote transcription of target genes, including CYP1A1, which in turn increases ROS production, DNA damage, and inflammatory cytokines [58]. Increased ROS-mediated damage in the stratum corneum has been associated with AD [59].
An airborne chemical that has been more extensively studied for its mechanistic basis of influencing AD is formaldehyde. While the entirety of the mechanism remain elusive, it has been shown that formaldehyde induces cell death [60], increases Th2 activity and mRNA expression by these cells [61, 62], and increased pro-inflammatory cytokine/IL-4 expression/IL mRNA expression [63].
Heavy metals species have also been shown to influence local immune activity in the skin. Exposure of the skin to nickel, cobalt, chromium, and palladium led to mixed Th1 and Th2-type cytokine response in in vitro models [64, 65].
Additionally, as air pollution and atmospheric humidity influence each other, studies have investigated both the correlation between humidity and levels of different air pollutants, as well as the association of humidity with AD. Levels of PM2.5 and PM10 are negatively associated with humidity. Prolonged periods of humidity have been shown to accelerate trans-epidermal water loss (TEWL) in the skin of AD patients, which amplifies barrier defects and increases cytokine signaling [66].
Indoor air pollutants have varying effects on the development of exacerbation of AD. Prenatal exposure to tobacco smoke has been shown to associate with increased AD development, with some insights into the mechanistic basis for its effects. A study from Germany analyzed the expression of different T-reg cell numbers and miRNA in maternal blood and cord blood during pregnancy. They found that maternal tobacco consumption during pregnancy was associated with a higher expression of miR-233 and fewer regulatory T-cells in the maternal and cord blood. Children with lower T-reg numbers are more likely to develop AD during the first 3 years of life. These associative relationships posit a potential model in which prenatal exposure to VOCs in tobacco smoke predisposes to AD by dysregulated T-reg activity, potentially due to altered miRNA levels [54].
Our understanding of the mechanisms underlying mold exposure and AD remains unclear. The study in South Korea reporting the positive association between prenatal exposure to Ascomycota and AD observed that infants with AD who were previously exposed to mold during pregnancy tended to have higher total serum IgE [56]. More work is required to elucidate the mechanism by which mold exposure promotes the development of AD symptoms.
Future Directions
As urbanization continues, it is important to further clarify the relationship between air pollution and atopic dermatitis. Designing studies that establish causal relationships between different air pollutants and AD remains a challenge in the field. The unusual and abrupt environmental changes that the COVID-19 pandemic caused globally in recent years provide a rare opportunity to investigate how these disruptions have impacted AD disease progression and severity across populations. This may provide further insight into the impact of airborne pollution, particularly TRAP in AD.
Pollutants and other irritants on the skin activate Th2 cells and downstream upregulation of various cytokines. This cytokine overactivity drives inflammation and IgE production, which contributes to the phenotypes seen in AD. Given the convergence of different environmental factors on cytokine activity, targeting their receptors holds therapeutic potential. Indeed, one of the more promising treatment strategies is dupilumab, a human monoclonal antibody directed at the alpha subunit of IL-4 receptors, which was approved by the Food and Drug Administration in early 2017. In the authors’ experience, this medication has been a major advance in managing atopic dermatitis, though unfortunately, it is not universally efficacious. More recently, tralokinumab, an IL-13 inhibitor, has recently received FDA approval and holds the potential to be another excellent option for the safe and effective management of AD.
Another treatment strategy is to target multiple immune signaling pathways which signal through JAK-STAT molecules. JAK-STAT signaling pathways have been implicated in AD and many other inflammatory diseases, as they regulate multiple immune pathways in AD, including Th2, CCL1, Th22, Th1, and Th17 [67]. The large diversity of downstream effects is driven by a family of four different receptor-associated kinases (JAK1, JAK2, JAK3, and TYK2) and five STAT molecules (STAT1, STAT2, STAT3, STAT5A/B, and STAT6). For instance, JAK1, JAK3, and their interactions with STAT3, STAT5, and STAT6 are necessary for the pathogenic IL-4 signaling in AD [68–70], and STAT6 drives IgE class switching [71, 72]. The JAK-STAT signaling pathway is thus a desirable target for its numerous pathogenic roles in AD, leading to the development of multiple small-molecule Janus kinase inhibitors (JAK-i). Oral (upadacitinib and abrocitinib) and topical (1.5% ruxolitinib) JAK inhibitors were recently approved by the FDA for atopic dermatitis. Both upadacitinib and abrocitinib are oral JAK1 antagonists. As JAK2 is involved in erythropoiesis, myelopoiesis, and platelet activation, selective inhibition of JAK1 over JAK2 and JAK3 may reduce hematologic adverse effects such as anemia and thrombocytopenia. A recent clinical trial found that upadacitinib has higher efficacy compared to dupilumab in moderate-to-severe adult AD patients, with no new safety signals [73]. Similarly, a recent trial found that abrocitinib was well-tolerated and efficacious in adults with moderate-to-severe AD [74]. Safety concerns about tofacitinib, a JAK inhibitor approved for rheumatoid arthritis (RA), have led to a black box warning on all JAK-i for possible increased risk of cancer and thrombotic and cardiovascular events [75]. So far, similar studies in upadacitinib in dermatologic disease have not supported these concerns [76]. While more long-term studies are needed to carefully characterize the safety profiles of different JAK-i in the treatment of AD, data for this class of medications appears to be promising.
Conclusion
The last two decades have seen a significant accumulation of studies exploring the effects of pollution on dermatological diseases, including AD. Here, we presented the current understanding of the pathophysiological mechanisms of AD, the strength of influence of pollutants on the development and progression of AD, and how pollutants fit into or modify the mechanistic understanding of AD pathogenesis. Further studies are needed to more rigorously study the causative role of pollutants such as NO2, while additional efforts in the development of novel therapy should be guided by mechanistic pathways that have been highlighted by the intersection between air pollutants and AD.
Acknowledgements
All figures in the text were created using BioRender.com.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Abuabara K, Magyari A, McCulloch CE, Linos E, Margolis DJ, Langan SM. Prevalence of atopic eczema among patients seen in primary care: data from the health improvement network. Ann Intern Med. 2019;170(5):354–356. doi: 10.7326/M18-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30. doi: 10.1016/j.jid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. The Lancet. 2020;396(10247):345–360. doi: 10.1016/S0140-6736(20)31286-1. [DOI] [PubMed] [Google Scholar]
- 4.Lübbe J. Secondary infections in patients with atopic dermatitis. Am J Clin Dermatol. 2003;4(9):641–654. doi: 10.2165/00128071-200304090-00006. [DOI] [PubMed] [Google Scholar]
- 5.Haskologlu S, Ikinciogulları A. Severe atopic disease and infections. In: Rezaei N, editor. Pediatric immunology: a case-based collection with MCQs, Volume 2 [Internet]. Cham: Springer International Publishing; 2019. p. 237–40. Available from: 10.1007/978-3-030-21262-9_46.
- 6.Chang YS, Chou YT, Lee JH, Lee PL, Dai YS, Sun C, et al. Atopic dermatitis, melatonin, and sleep disturbance. Pediatrics. 2014;134(2):e397–405. doi: 10.1542/peds.2014-0376. [DOI] [PubMed] [Google Scholar]
- 7.Yosipovitch G, Reaney M, Mastey V, Eckert L, Abbé A, Nelson L, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–769. doi: 10.1111/bjd.17744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholas MN, Gooderham MJ. Atopic dermatitis, depression, and suicidality. J Cutan Med Surg. 2017;21(3):237–242. doi: 10.1177/1203475416685078. [DOI] [PubMed] [Google Scholar]
- 9.Bjerre RD, Bandier J, Skov L, Engstrand L, Johansen JD. The role of the skin microbiome in atopic dermatitis: a systematic review. Br J Dermatol. 2017;177(5):1272–1278. doi: 10.1111/bjd.15390. [DOI] [PubMed] [Google Scholar]
- 10.Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI, ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009 Dec;124(6):1251–1258.e23. [DOI] [PubMed]
- 11.Bylund S, Kobyletzki LB, Svalstedt M, Svensson Å. Prevalence and incidence of atopic dermatitis: a systematic review. Acta Derm Venereol. 2020 Jun 9;100(12):adv00160. [DOI] [PMC free article] [PubMed]
- 12.Ha J, Lee SW, Yon DK. Ten-year trends and prevalence of asthma, allergic rhinitis, and atopic dermatitis among the Korean population, 2008–2017. Clin Exp Pediatr. 2020;63(7):278–283. doi: 10.3345/cep.2019.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131(1):67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2019;123(2):173–178.e1. doi: 10.1016/j.anai.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Lavery A, Solman L, Grindlay DJC, Rogers NK, Thomas KS, Harman KE. What’s new in atopic eczema? An analysis of systematic reviews published in 2016. Part 2: Epidemiology, aetiology and risk factors. Clin Exp Dermatol. 2019 Jun;44(4):370–5. [DOI] [PubMed]
- 16.Fu T, Keiser E, Linos E, Rotatori RM, Sainani K, Lingala B, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31(1):21–26. doi: 10.1111/pde.12237. [DOI] [PubMed] [Google Scholar]
- 17.Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 Suppl):S115–S123. [PubMed] [Google Scholar]
- 18.Eller E, Kjaer HF, Høst A, Andersen KE, Bindslev-Jensen C. Food allergy and food sensitization in early childhood: results from the DARC cohort. Allergy. 2009;64(7):1023–1029. doi: 10.1111/j.1398-9995.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- 19.Hon KLE, Leung T fan, Ching G, Chow C mo, Luk V, Ko W san F, et al. Patterns of food and aeroallergen sensitization in childhood eczema. Acta Paediatr. 2008;97(12):1734–7. [DOI] [PubMed]
- 20.Fuiano N, Fusilli S, Incorvaia C. House dust mite-related allergic diseases: role of skin prick test, atopy patch test, and RAST in the diagnosis of different manifestations of allergy. Eur J Pediatr. 2010;169(7):819–824. doi: 10.1007/s00431-009-1118-6. [DOI] [PubMed] [Google Scholar]
- 21.Chung J, Simpson EL. The socioeconomics of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(4):360–366. doi: 10.1016/j.anai.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Hüls A, Abramson MJ, Sugiri D, Fuks K, Krämer U, Krutmann J, et al. Nonatopic eczema in elderly women: effect of air pollution and genes. J Allergy Clin Immunol. 2019;143(1):378–385.e9. doi: 10.1016/j.jaci.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Garcovich S, Maurelli M, Gisondi P, Peris K, Yosipovitch G, Girolomoni G. Pruritus as a distinctive feature of type 2 inflammation. Vaccines. 2021;9(3):303. doi: 10.3390/vaccines9030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis Nat Rev Dis Primer. 2018;4(1):1. doi: 10.1038/s41572-018-0001-z. [DOI] [PubMed] [Google Scholar]
- 25.Leung DYM, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134(4):769–779. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yosipovitch G, Berger T, Fassett M s. Neuroimmune interactions in chronic itch of atopic dermatitis. J Eur Acad Dermatol Venereol. 2020;34(2):239–50. [DOI] [PMC free article] [PubMed]
- 27.Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70(1):3–11. doi: 10.1016/j.jdermsci.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 29.Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. Anti–interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376(9):826–835. doi: 10.1056/NEJMoa1606490. [DOI] [PubMed] [Google Scholar]
- 30.Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2020;124(1):36–43. doi: 10.1016/j.anai.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 31.O’Regan GM, Sandilands A, McLean WHI, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122(4):689–693. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009;124(3 Suppl 2):R7–12. doi: 10.1016/j.jaci.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, et al. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol. 2009;123(6):1361–1370.e7. doi: 10.1016/j.jaci.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 34.Paternoster L, Standl M, Waage J, Baurecht H, Hotze M, Strachan DP, et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat Genet. 2015;47(12):1449–1456. doi: 10.1038/ng.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer CNA, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 36.van den Oord RAHM, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ. 2009;9(339):b2433. doi: 10.1136/bmj.b2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baurecht H, Irvine AD, Novak N, Illig T, Bühler B, Ring J, et al. Toward a major risk factor for atopic eczema: meta-analysis of filaggrin polymorphism data. J Allergy Clin Immunol. 2007;120(6):1406–1412. doi: 10.1016/j.jaci.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 38.Totté JEE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pasmans SGMA. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol. 2016;175(4):687–695. doi: 10.1111/bjd.14566. [DOI] [PubMed] [Google Scholar]
- 39.Hrestak D, Matijašić M, Čipčić Paljetak H, Ledić Drvar D, Ljubojević Hadžavdić S, Perić M. Skin microbiota in atopic dermatitis. Int J Mol Sci. 2022;23(7):3503. doi: 10.3390/ijms23073503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geoghegan JA, Irvine AD, Foster TJ. Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship. Trends Microbiol. 2018;26(6):484–497. doi: 10.1016/j.tim.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol. 2014;134(5):993–999. doi: 10.1016/j.jaci.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 42.Wu W, Jin Y, Carlsten C. Inflammatory health effects of indoor and outdoor particulate matter. J Allergy Clin Immunol. 2018;141(3):833–844. doi: 10.1016/j.jaci.2017.12.981. [DOI] [PubMed] [Google Scholar]
- 43.•• Park TH, Park S, Cho MK, Kim S. Associations of particulate matter with atopic dermatitis and chronic inflammatory skin diseases in South Korea. Clin Exp Dermatol. 2022 Feb 1;47(2):325–34. Retrospective study on 23,288,000 individuals in South Korea, showing increases in the levels of PM2.5, PM10, SO2, and CO were associated with significant increases in patient visits for AD and other chronic inflammatory skin diseases. [DOI] [PubMed]
- 44.• Luo P, Wang D, Luo J, Li S, Li MM, Chen H, et al. Relationship between air pollution and childhood atopic dermatitis in Chongqing, China: a time-series analysis. Front Public Health. 2022;10:990464. A 5-year time series study on 214, 747 children in Chongqing, China, showing that increases in PM2.5, PM10, SO2, NO2, and CO were associated with varying extent of increases in AD outpatient visits. [DOI] [PMC free article] [PubMed]
- 45.Kim J, Han Y, Ahn JH, Kim SW, Lee SI, Lee KH, et al. Airborne formaldehyde causes skin barrier dysfunction in atopic dermatitis. Br J Dermatol. 2016;175(2):357–63. [DOI] [PubMed]
- 46.Yi O, Kwon HJ, Kim H, Ha M, Hong SJ, Hong YC, et al. Effect of environmental tobacco smoke on atopic dermatitis among children in Korea. Environ Res. 2012;113:40–45. doi: 10.1016/j.envres.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Krämer U, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008;177(12):1331–1337. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- 48.Eberlein-König B, Przybilla B, Kühnl P, Pechak J, Gebefügi I, Kleinschmidt J, et al. Influence of airborne nitrogen dioxide or formaldehyde on parameters of skin function and cellular activation in patients with atopic eczema and control subjects. J Allergy Clin Immunol. 1998;101(1 Pt 1):141–143. doi: 10.1016/S0091-6749(98)70212-X. [DOI] [PubMed] [Google Scholar]
- 49.Ruff CA, Belsito DV. The impact of various patient factors on contact allergy to nickel, cobalt, and chromate. J Am Acad Dermatol. 2006;55(1):32–39. doi: 10.1016/j.jaad.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 50.Tokura Y, Hayano S. Subtypes of atopic dermatitis: from phenotype to endotype. Allergol Int Off J Jpn Soc Allergol. 2022;71(1):14–24. doi: 10.1016/j.alit.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Aoshima M, Phadungsaksawasdi P, Nakazawa S, Iwasaki M, Sakabe JI, Umayahara T, et al. Decreased expression of suprabasin induces aberrant differentiation and apoptosis of epidermal keratinocytes: Possible role for atopic dermatitis. J Dermatol Sci. 2019;95(3):107–112. doi: 10.1016/j.jdermsci.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Nakazawa S, Shimauchi T, Funakoshi A, Aoshima M, Phadungsaksawasdi P, Sakabe JI, et al. Suprabasin-null mice retain skin barrier function and show high contact hypersensitivity to nickel upon oral nickel loading. Sci Rep. 2020;10(1):14559. doi: 10.1038/s41598-020-71536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.• Hsiao YY, Chen YH, Hung WT, Tang KT. The relationship between outdoor air pollutants and atopic dermatitis of adults: a systematic review and meta-analysis. Asian Pac J Allergy Immunol. 2022 Dec;40(4):295–307. Meta-analysis on 20 relevant studies on air pollution and allergic diseases, showing that long-term exposure to PM2.5 and NO2 associated with higher prevalence of AD. Both short- and long-term exposure to PM10 and SO2 were associated with AD symptoms, while short-term exposure leads to exacerbation of AD. [DOI] [PubMed]
- 54.Herberth G, Bauer M, Gasch M, Hinz D, Röder S, Olek S, et al. Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. J Allergy Clin Immunol. 2014;133(2):543–550.e4. doi: 10.1016/j.jaci.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 55.Hinz D, Bauer M, Röder S, Olek S, Huehn J, Sack U, et al. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy. 2012;67(3):380–389. doi: 10.1111/j.1398-9995.2011.02767.x. [DOI] [PubMed] [Google Scholar]
- 56.Lee E, Choi KY, Kang MJ, Lee SY, Yoon J, Cho HJ, et al. Prenatal mold exposure is associated with development of atopic dermatitis in infants through allergic inflammation. J Pediatr (Rio J) 2018;96(1):125–131. doi: 10.1016/j.jped.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Totlandsdal AI, Herseth JI, Bølling AK, Kubátová A, Braun A, Cochran RE, et al. Differential effects of the particle core and organic extract of diesel exhaust particles. Toxicol Lett. 2012;208(3):262–268. doi: 10.1016/j.toxlet.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 58.Furue M, Tsuji G, Mitoma C, Nakahara T, Chiba T, Morino-Koga S, et al. Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J Dermatol Sci. 2015;80(2):83–88. doi: 10.1016/j.jdermsci.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 59.Niwa Y, Sumi H, Kawahira K, Terashima T, Nakamura T, Akamatsu H. Protein oxidative damage in the stratum corneum: evidence for a link between environmental oxidants and the changing prevalence and nature of atopic dermatitis in Japan. Br J Dermatol. 2003;149(2):248–254. doi: 10.1046/j.1365-2133.2003.05417.x. [DOI] [PubMed] [Google Scholar]
- 60.Kastner PE, Casset A, Pons F. Formaldehyde interferes with airway epithelium integrity and functions in a dose- and time-dependent manner. Toxicol Lett. 2011;200(1–2):109–116. doi: 10.1016/j.toxlet.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Saito A, Tanaka H, Usuda H, Shibata T, Higashi S, Yamashita H, et al. Characterization of skin inflammation induced by repeated exposure of toluene, xylene, and formaldehyde in mice. Environ Toxicol. 2011;26(3):224–232. doi: 10.1002/tox.20547. [DOI] [PubMed] [Google Scholar]
- 62.Xu B, Aoyama K, Takeuchi M, Matsushita T, Takeuchi T. Expression of cytokine mRNAs in mice cutaneously exposed to formaldehyde. Immunol Lett. 2002;84(1):49–55. doi: 10.1016/S0165-2478(02)00126-8. [DOI] [PubMed] [Google Scholar]
- 63.Kastner PE, Le Calvé S, Zheng W, Casset A, Pons F. A dynamic system for single and repeated exposure of airway epithelial cells to gaseous pollutants. Toxicol Vitro Int J Publ Assoc BIBRA. 2013;27(2):632–640. doi: 10.1016/j.tiv.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Borg L, Christensen JM, Kristiansen J, Nielsen NH, Menné T, Poulsen LK. Nickel-induced cytokine production from mononuclear cells in nickel-sensitive individuals and controls. Cytokine profiles in nickel-sensitive individuals with nickel allergy-related hand eczema before and after nickel challenge. Arch Dermatol Res. 2000 Jun;292(6):285–91. [DOI] [PubMed]
- 65.Minang JT, Areström I, Zuber B, Jönsson G, Troye-Blomberg M, Ahlborg N. Nickel-induced IL-10 down-regulates Th1- but not Th2-type cytokine responses to the contact allergen nickel. Clin Exp Immunol. 2006;143(3):494–502. doi: 10.1111/j.1365-2249.2006.03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato J, Denda M, Chang S, Elias PM, Feingold KR. Abrupt decreases in environmental humidity induce abnormalities in permeability barrier homeostasis. J Invest Dermatol. 2002;119(4):900–904. doi: 10.1046/j.1523-1747.2002.00589.x. [DOI] [PubMed] [Google Scholar]
- 67.He H, Guttman-Yassky E. JAK Inhibitors for atopic dermatitis: an update. Am J Clin Dermatol. 2019;20(2):181–192. doi: 10.1007/s40257-018-0413-2. [DOI] [PubMed] [Google Scholar]
- 68.Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAK-STAT. 2013;2(3):e24137. doi: 10.4161/jkst.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380(6575):627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 70.Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, et al. The transcription factor STAT3 is required for T helper 2 cell development. Immunity. 2011;34(1):39–49. doi: 10.1016/j.immuni.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res. 2011;50(1):87–96. doi: 10.1007/s12026-011-8205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380(6575):630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 73.Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055. doi: 10.1001/jamadermatol.2021.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Lond Engl. 2020;396(10246):255–266. doi: 10.1016/S0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]
- 75.Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 76.Simpson EL, Papp KA, Blauvelt A, Chu CY, Hong HC ho, Katoh N, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the measure up 1 and measure Up 2 randomized clinical trials. JAMA Dermatol. 2022;158(4):404–13. [DOI] [PMC free article] [PubMed]