Abstract
In critical patients, abdominal perfusion pressure (APP) has been shown to correlate with outcome. However, data from cirrhotic patients is scarce. We aimed to characterize APP in critically ill cirrhotic patients, analyze the prevalence and risk factors of abdominal hypoperfusion (AhP) and outcomes. A prospective cohort study in a general ICU specialized in liver disease at a tertiary hospital center recruited consecutive cirrhotic patients between October 2016 and December 2021. The study included 101 patients, with a mean age of 57.2 (± 10.4) years and a female gender proportion of 23.5%. The most frequent etiology of cirrhosis was alcohol (51.0%), and the precipitant event was infection (37.3%). ACLF grade (1–3) distribution was 8.9%, 26.7% and 52.5%, respectively. A total of 1274 measurements presented a mean APP of 63 (± 15) mmHg. Baseline AhP prevalence was 47%, independently associated with paracentesis (aOR 4.81, CI 95% 1.46–15.8, p = 0.01) and ACLF grade (aOR 2.41, CI 95% 1.20–4.85, p = 0.01). Similarly, AhP during the first week (64%) had baseline ACLF grade (aOR 2.09, CI 95% 1.29–3.39, p = 0.003) as a risk factor. Independent risk factors for 28-day mortality were bilirubin (aOR 1.10, CI 95% 1.04–1.16, p < 0.001) and SAPS II score (aOR 1.07, CI 95% 1.03–1.11, p = 0.001). There was a high prevalence of AhP in critical cirrhotic patients. Abdominal hypoperfusion was independently associated with higher ACLF grade and baseline paracentesis. Risk factors for 28-day mortality included clinical severity and total bilirubin. The prevention and treatment of AhP in the high-risk cirrhotic patient is prudential.
Subject terms: Gastroenterology, Medical research, Risk factors
Introduction
Cirrhosis increases intrahepatic resistance and leads to impairment of hepatosplanchnic blood flow. These changes result in chronic portal vein hypertension, further aggravated, in advanced stages of the disease, by compensatory splanchnic vasodilation, relative hypotension and the development of ascites.
The pathophysiologic aspects of intra-abdominal hypertension (IAH), in the decompensated cirrhotic patient with ascites, have been previously studied, as well as the safety and immediate beneficial effects of therapeutic large volume paracentesis (LVP) on hemodynamic status and regarding renal, respiratory and hepatic functions1–4.
In critically ill patients, abdominal perfusion pressure (APP), resulting from the difference between mean arterial pressure (MAP) and intra-abdominal pressure (IAP), correlates with improved survival10. Furthermore, in the decompensated cirrhotic patient, APP correlates with the clearance of indocyanine green, and may be predictive of organ dysfunction and outcome5,7. Only a few studies have reported various clinical cut-off values for APP, ranging from 50 to 72 mmHg, and potential resuscitation endpoints have been proposed6–10. However, the clinical importance of APP, prevalence, risk factors and outcomes for abdominal hypoperfusion (AhP) require specific research in the area of the critically ill cirrhotic patient.
The objectives of this study were to characterize APP in a population of critically ill cirrhotic patients, to analyze the prevalence and risk factors of AhP, clinical outcomes, including mortality rates at 28 and 90 days, intensive care unit (ICU) and hospital length-of-stay (LOS).
Methods
Design, settings, participants and definitions
This was a single center prospective cohort study of cirrhotic patients admitted to the ICU.
The study was set in a general ICU, with 21 beds, specialized in liver disease, at Hospital de Curry Cabral, Centro Hospitalar Universitário Lisboa Central, Portugal, a tertiary hospital center with a liver transplant program.
Patients were recruited between October 2016 and December 2021 and followed-up to hospital discharge or to the last known date of patient record at the center.
Data was collected at admission and throughout ICU stay, and included demographic and clinical variables, for the calculation of general and liver specific severity scores, as well as liver cirrhosis etiology, precipitating event of acute illness, arterial blood lactate concentration and vital organ support with vasopressors, mechanical ventilation and renal replacement therapy (RRT) during ICU stay.
The study protocol was approved by the Ethics Committee at Centro Hospitalar Universitário Lisboa Central (CES nº397/2017), and waived the need for individual informed consent for this observational study. All study procedures followed the principles of the Declaration of Helsinki11.
All cirrhotic patients admitted in the ICU with a bladder catheter in situ were consecutively screened for eligibility to avoid selection bias and to maximize the number of cases in the study. Patient selection was performed using the following inclusion criteria: (1) age ≥ 18 years, (2) first ICU admission during the index hospital stay, and (3) medical type of admission (no surgery in the 4 weeks preceding the index ICU admission). The exclusion criteria were: (1) any type of surgical ICU admission, (2) contra-indication for intravesical IAP measurements, (3) absence of recorded APP values, (4) patients with ICU stay duration inferior to 24 h and (5) patients with previous LT.
Patient data was retrieved on site or from medical records and collected in an anonymous and protected database.
Cirrhosis was defined as bridging fibrosis on previous liver biopsy or a composite of clinical signs and findings provided by laboratory tests, endoscopy, and radiologic imaging12.
The definition of IAH and abdominal compartment syndrome (ACS), IAP measurement methodology and clinical management of these patients followed the published and updated guidelines by the World Society of Abdominal Compartment Syndrome (WSACS)8,13,14. Accordingly, IAH was classified into grade I-IV (respectively 12–15, 16–20, 21–25 and > 25 mmHg). Abdominal hypoperfusion (AhP) was defined by an APP < 60 mmHg and ACS was defined as IAP > 20 mmHg in this population of critically ill patients.
For this study, “Paracentesis” refers to both diagnostic and LVP, unless otherwise stated. Large-volume paracentesis was defined for a volume ≥ 500 mL of drained ascites. Post-paracentesis circulatory disfunction (PPCD) was actively prevented with 20% albumin (8 g/L of drained ascites) infusion, according to clinical guidelines, and standard-of-care fluid therapy to ensure euvolemic state.
Intra-abdominal pressure monitoring was performed via trans-bladder measurement technique with a maximum of 25 mL of saline solution and zero-pressure reference point was set at the phlebostatic axis in the midaxillary line14.
Measures were performed every 6–8 h, and mean APP (APP = MAP − IAP) was calculated on each day for each patient. Values presented for IAP, APP and MAP in this study correspond to daily mean value unless otherwise stated. The expressions "ICU admission" and "baseline" are interchangeable, and refer to the period corresponding to calendar day zero (0) and day one (1) of ICU stay, to assure completeness of 24-h ICU stay data. Whenever a patients underwent emergent liver transplant during the ICU stay, IAP measure and APP calculation were halted due to the change in the type (surgical) of patient. These patients were included in the overall mortality analysis at 28-days.
Outcome measurements included survival data at 28 and 90 days, and length-of-stay in the ICU and hospital.
Statistical analysis
Chi-square test was used to compare the frequency of categorical variables for independent groups. Shapiro–Wilk test was used to assess for normal distribution of continuous variables. T-test was then used to compare the mean between two normally distributed groups and the median test to compare the median of non-normal continuous variables. Multivariate analysis was performed using backward stepwise logistic regression, and included variables based on clinical importance and with p value ≤ 0.10 in univariate analysis, after assessment of statistical assumptions, namely, independence of observations, absence of influential outliers, linearity in the logit for continuous variables and collinearity, using Kendall Tau coefficient to identify and exclude strongly correlated predicting variables (above + 0.35 or less than − 0.35)15. The area under the Receiver operator curve (aROC) was used to determine the ability of a continuous variable to discriminate between a dichotomous outcome and the Younden's J statistic (Younden index) was used to identify the optimal cut-off value. Statistical significance was considered for two-sided p value ≤ 0.05. Statistical software IBM SPSS Statistics for Windows, version 23.0. Armonk, NY was use for analysis.
Results
Overall
This study included 101 cirrhosis patients. A patient flowchart is depicted in Fig. 1.
Figure 1.
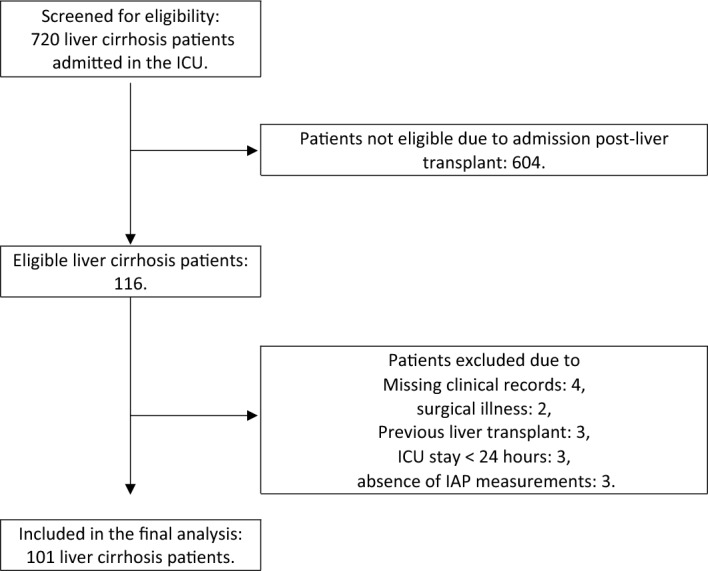
Study patient flowchart.
Patients presented a mean age of 57.2 (± 10.4) years and female gender represented 23.5% of the cohort. Liver disease etiology included alcohol alone (51.0%), alcohol plus hepatitis C virus (HCV) (13.7%), NASH (5.9%), HCV alone and non-C viral hepatitis (both 4.9%). There was a history of past liver disease decompensation in 62.7% of patients, and hepatic related comorbidities included any type of hepatic neoplasm (20.6%), portal vein thrombosis (18.6%) and ascites (88.2%). The most frequent precipitant events leading to index ICU admission were infection (37.3%), bleeding (23.5%), hepatic encephalopathy (7.8%) and acute kidney injury (6.9%). The need for vasopressor therapy was present in 75% of patients at baseline, namely, noradrenaline (30%), or terlipressine (18%), or both in combination in 27% of cases. Clinical severity at ICU admission presented a mean SAPS II of 49 (± 15.0), median MELD-Na of 31 [23, 37], mean CLIF-C of 53 (± 11) and acute-on-chronic liver failure (ACLF) grades (I–III) of 8.9%, 26.7% and 52.5%, respectively. The observed mortality rates were 48.1% in-ICU and 64.4% in-hospital, and 56.4% and 67.6%, at 28 and 90 days, respectively. Median (days) ICU stay was 8 [4, 12] and hospital stay 23 [14, 42]. During the hospital stay liver transplant was performed in 18 (17.8%) cases after index ICU admission (Table 1).
Table 1.
Baseline clinical characteristics in liver cirrhosis patients intensive care and 28-day vital outcome comparison.
| Baseline variables | Overall | Non-survivor at day 28 | Survivor at day 28 | p |
|---|---|---|---|---|
| N | 101 | 57 | 44 | |
| Age (years) | 57.1 (10.4) | 57.0 (11.3) | 57.4 (9.4) | 0.8 |
| Male gender, n (%) | 77 (76.2) | 45 (78.9) | 32 (72.7) | 0.6 |
| Liver disease etiology, n (%) | 0.14 | |||
| Alcohol | 52 (51.5) | 29 (50.9) | 23 (52.3) | |
| Alcohol + HCV | 14 (13.9) | 5 (8.8) | 9 (20.5) | |
| Precipitant, n (%) | 0.6 | |||
| AKIa | 7 (6.9) | 4 (7.0) | 3 (6.8) | |
| Bleeding | 24 (23.8) | 13 (22.8) | 11 (25.0) | |
| Encephalopathy | 8 (7.9) | 6 (10.5) | 2 (4.5) | |
| Infection | 38 (37.6) | 23 (40.4) | 15 (34.1) | |
| CRP (mg/L) | 51 [18, 93] | 50 [18, 93] | 56 [18, 84] | 0.9 |
| WBC count (10^3/mL) | 11.9 [6.4, 18.3] | 13.5 [8.4, 19.1] | 8.5 [5.4, 15.7] | 0.02 |
| Hematocrit (%) | 23.9 (5.7) | 23.8 (5.9) | 24.0 (5.6) | 0.9 |
| INR | 2.2 [1.7, 3.1] | 2.40 [1.78, 3.60] | 1.85 [1.60, 2.42] | 0.004 |
| Platelets (10^3/mL) | 67 [42, 121] | 67 [46, 146] | 62 [38, 94.25] | 0.3 |
| Urea (mg/dL) | 90 [55, 131] | 97 [62, 146] | 70.50 [45.75, 105] | 0.03 |
| Creatinine (mg/dL) | 1.8 [0.9, 3.0] | 2.1 [1.3, 3.1] | 1.3 [0.8, 2.6] | 0.04 |
| Urine output (mL/24h) | 1090 [498, 1823] | 1005 [418, 1695] | 1133 [805, 1924] | 0.5 |
| Bilirubin (total, mg/dL) | 6.0 [2.3, 17.6] | 11.1 [4.9, 24.5] | 3.5 [1.9, 6.4] | < 0.001 |
| Ammonia (ug/dL) | 240 [159, 314] | 243 [177, 230] | 189 [137, 306] | 0.2 |
| West-Haven score | 1 [0, 3] | 3 [1, 3] | 1 [0, 2] | 0.001 |
| PaO2/FiO2 ratio | 257 [170, 356] | 257 [166, 356] | 249 [178, 354] | 0.9 |
| Arterial blood pH (minimum) | 7.38 [7.30, 7.43] | 7.35 [7.28, 7.42] | 7.41 [7.35, 7.45] | 0.03 |
| Lactate (mmol/L) | 2.6 [1.5, 4.3] | 2.9 [1.9, 5.3] | 2.2 [1.4, 3.5] | 0.01 |
| Fluid balance (mL) | 1618 [433, 3623] | 1699 [− 410, 3744] | 1563 [715, 2908] | 0.9 |
| Ascites, n (%) (n = 98) | 87 (86.1) | 4885.7) | 38 (90.5) | 0.7 |
| Paracentesis, n (%) | 38 (37.6) | 23 (40.4) | 15 (34.1) | 0.7 |
| Paracentesis volume (mL)b (n = 21) | 3000 [1800, 4500] | 4000 [1825, 5575] | 2300 [1640, 2900] | 0.2 |
| SAPS II score | 49 (15) | 53 (16) | 43 (11) | < 0.001 |
| MELD Na score | 31 [23, 37] | 34 [28, 40] | 26 [17, 32] | < 0.001 |
| ACLF grade | 3 [2, 3] | 3 [2, 3] | 2 [1, 3] | < 0.001 |
| AKIa, n (%) | 66 (65.3) | 44 (77.2) | 22 (50.0) | 0.008 |
| RRT, n (%) | 24 (23.8) | 16 (28.1) | 8 (18.2) | 0.4 |
| IMV, n (%) | 56 (55.4) | 36 (63.2) | 20 (45.5) | 0.12 |
| Vasopressors, n (%) | 72 (71.3) | 41 (71.9) | 31 (70.5) | 1 |
| IAP (mmHg) | 12 [8, 15] | 13 [9, 15] | 11 [8, 14] | 0.2 |
| MAP (mmHg) | 72 [66, 81] | 72 [63, 77] | 75 [68, 90] | 0.2 |
| APP (mmHg) | 63 (15) | 60 (14) | 67 (15) | 0.050 |
| Mortality at day 28, n (%) | 57 (56.4) | |||
| ICU LOS (days) | 8 [4, 12] | 7 [4, 10] | 9 [6, 16] | 0.06 |
| Hosp stay (days) | 24 [14, 42] | 19 [8, 25] | 39 [24, 65] | < 0.001 |
IAP intra-abdominal pressure, APP abdominal perfusion pressure, BMI body mass index, HCV Hepatitis C virus, ACLF acute-on-chronic liver failure, INR international normalization ratio, CRP C-reactive protein, PaFiO2 arterial oxygen partial pressure to fractional inspired oxygen ratio, IMV invasive mechanical ventilation, AKI acute kidney injury, RRT renal replacement therapy, SAPS II simplified acute physiology score II, CLIF-C Chronic Liver Failure Consortium, MELD-Na Model For End-Stage Liver Disease—sodium, MAP mean arterial pressure, ICU intensive care unit, LOS length-of-stay, WBC white blood cell.
aDiagnosis of AKI as indicated in clinical records.
bParacentesis volume (mL) includes only large-volume paracentesis (≥ 500 mL), and excludes diagnostic paracentesis.
Normally distributed continuous variables are presented as mean (SD) and non-normal continuous variables as median [IQR]. IAP and MAP values are drawn from pooled APP data components.
A total of 1274 APP measurements were recorded throughout the ICU stay, approximately, corresponding to a mean of 13 per patient. Measured pressures (mmHg) presented a median IAP of 12.4 [9.6, 13.9], a mean MAP of 78.2 (± 11.0) and a median APP of 65.9 [58.6, 72.0]. The distribution of IAH grades (I–IV) during the ICU stay and associated mortality rates are depicted in Supplementary Fig. 1.
Paracentesis
Paracentesis was performed in 38% of patients at ICU admission, and LVP (≥ 500 mL) (n = 21) presented a median volume of 3000 mL [1800, 4500]. Additionally, the frequency of paracentesis performed prior to ICU admission was 37%, after D1 of ICU stay was 51%. In 27% of cases there was no record of paracentesis during the entire hospital stay and this was justified due to absent/minimal ascites (n = 16) or waived based on a confirmed clinical diagnosis (n = 9, including pneumonia, hydrothorax and ruptured esophageal varices).
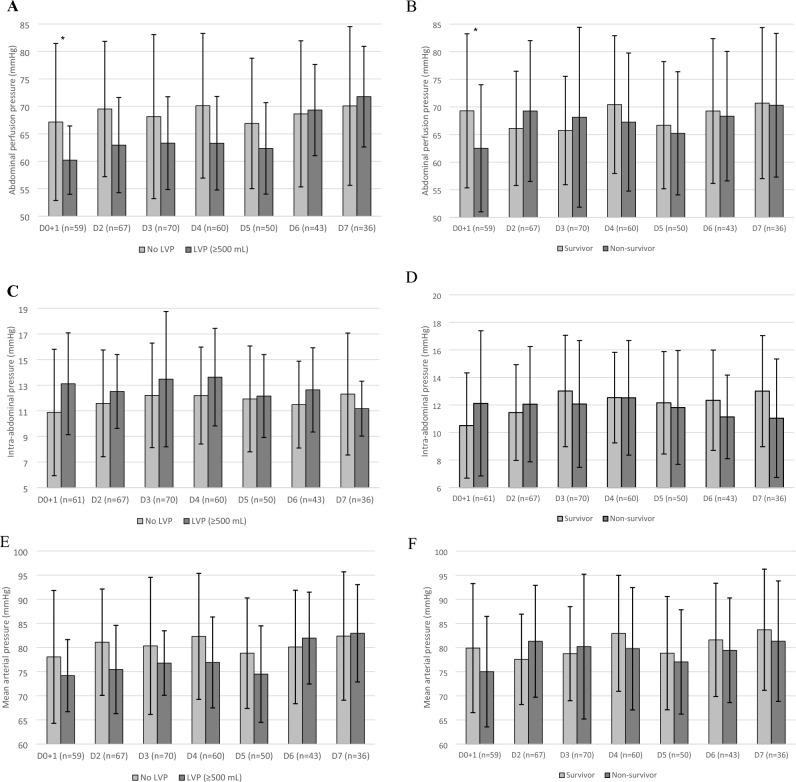
At baseline, APP was significantly lower in patients submitted to LVP (60 ± 6.2 vs. 67 ± 14, p = 0.01) when compared to the rest of the patients and did not significantly differ throughout the rest of the study period days (Fig. 2). The variation in APP from baseline to D2 was not significantly different between patients with/without baseline LVP at ICU admission (p = 0.9).
Figure 2.
Critical pressures during the first 7 days of intensive care. Abdominal perfusion pressure (A, B), intra-abdominal pressure (C, D), mean arterial pressure (E, F). *Statistically significant (p ≤ 0.05) intraday difference between groups. Patients submitted to large-volume paracentesis (≥ 500 mL) at baseline (n = 21) were compared to all other study patients (n = 79). Mortality was assessed at day 28; survivors: n = 43, non-survivors: n = 57. Error bars: ± 1 standard deviation. LVP large-volume paracentesis, D Day.
Large-volume paracentesis (> 500 mL, n = 21) was not associated with baseline AhP (p = 0.3) nor with 28-day mortality (p = 0.4) in multivariate analysis.
Abdominal hypoperfusion
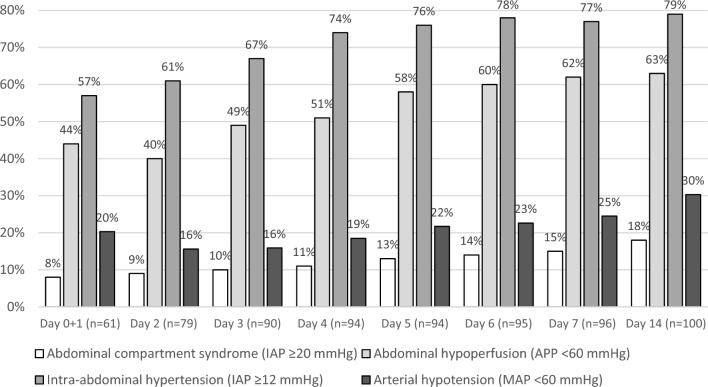
Baseline AhP had a prevalence of 47%, as illustrated in Fig. 3, and was associated with higher serum urea concentration and clinical severity SAPS II score, lower arterial blood pH and the presence of paracentesis in univariate analysis (Table 2). Multivariate analysis (n = 59) revealed independent association of any type of paracentesis (both diagnostic and LVP) (aOR 4.81, CI 95% 1.46–15.8, p = 0.01) and ACLF grade (aOR 2.41, CI 95% 1.20–4.85, p = 0.01) with AhP at baseline (Table 3).
Figure 3.
Cumulative prevalence of critical pressures in the cirrhotic patient in intensive care. The frequencies shown take into account the daily mean values. Abbreviations: D day, IAP intra-abdominal hypertension, APP abdominal perfusion pressure, MAP mean arterial pressure.
Table 2.
Comparison of abdominal perfusion pressure groups in liver cirrhosis patients in intensive care.
| Baseline variables | At ICU admission | During ICU stay | ||||
|---|---|---|---|---|---|---|
| APP < 60 mmHg | APP ≥ 60 mmHg | p | APP < 60 mmHg | APP ≥ 60 mmHg | p | |
| N (%) | 28 (47.5) | 31 (52.5) | 67 (67.7) | 32 (32.3) | ||
| Age (years) | 58 [52, 63] | 58 [52, 63] | 0.9 | 58 (11) | 55 (9) | 0.13 |
| Male gender, n (%) | 22 (78.6) | 25 (80.6) | 1.0 | 53 (79.1) | 23 (71.9) | 0.6 |
| Liver disease etiology, n (%) | 0.8 | 0.3 | ||||
| Alcohol | 15 (53.6) | 15 (48.4) | 39 (58.2) | 12 (37.5) | ||
| Alcohol + HCV | 4 (14.3) | 4 (12.9) | 9 (13.4) | 5 (15.6) | ||
| Precipitant, n (%) | 0.9 | 0.2 | ||||
| AKIa | 2 (7.1) | 4 (12.9) | 3 (4.5) | 4 (12.5) | ||
| Bleeding | 5 (17.9) | 5 (16.1) | 13 (19.4) | 10 (31.2) | ||
| Encephalopathy | 3 (10.7) | 2 (6.5) | 6 (9.0) | 2 (6.2) | ||
| Infection | 12 (42.9) | 11 (35.5) | 26 (38.8) | 12 (37.5) | ||
| CRP (mg/L) | 50 [23, 94] | 27. [13, 66] | 0.2 | 56 [18, 95] | 47 [18, 88] | 0.6 |
| WBC count (10^3/mL) | 13.2 [6.5, 20.0] | 10.8 [6.5, 16.7] | 0.3 | 12 [6.8, 18.4] | 11.3 [6.4, 17.9] | 0.7 |
| Hematocrit (%) | 24.8 (5.9) | 23.4 (6.8) | 0.4 | 24.40 (5.71) | 23.0 (5.8) | 0.2 |
| INR | 2.4 [1.7, 2.8] | 2.2 [1.7, 2.9] | 0.7 | 2.3 [1.7, 3.0] | 1.9 [1.6, 3.5] | 0.4 |
| Platelets (10^3/mL) | 70 [45, 126] | 54 [43, 93] | 0.3 | 69 [41, 99] | 56 [41, 139] | 0.9 |
| Urea (mg/dL) | 115 [78, 178] | 72 [44, 110] | 0.01 | 93 [62, 145] | 66 [41, 98] | 0.02 |
| Creatinine (mg/dL) | 2.3 [1.5, 2.9] | 1.3 [0.8, 2.9] | 0.10 | 2.1 [1.0, 3.2] | 1.2 [0.8, 2.2] | 0.01 |
| Urine output (mL/24h) | 975 [393, 2053] | 1268 [898, 1933] | 0.4 | 970 [409, 1503] | 1330 [930, 1923] | 0.03 |
| Bilirubin (total, mg/dL) | 6.2 [2.4, 12.0] | 6.7 [3.4, 20.2] | 0.5 | 5.70 [2.26, 13.96] | 6.58 [2.52, 19.04] | 0.8 |
| Ammonia (ug/dL) | 238 [170, 286] | 255 [169, 308] | 0.7 | 228 [159, 333] | 255 [163, 294] | 0.7 |
| West-Haven score | 2 [0, 3] | 1 [0, 3] | 0.6 | 1 [0, 3] | 2 [0, 3] | 0.3 |
| PaO2/FiO2 ratio | 253 [157, 354] | 286 [166, 357] | 0.6 | 257 [163, 355] | 257 [197, 359] | 0.7 |
| Arterial blood pH (minimum) | 7.34 [7.24, 7.40] | 7.40 [7.32, 7.44] | 0.03 | 7.36 [7.29, 7.42] | 7.40 [7.36, 7.47] | 0.02 |
| Lactate (mmol/L) | 2.9 [1.9, 9.1] | 2.5 [1.3, 3.7] | 0.09 | 2.7 [1.7, 4.3] | 2.3 [1.5, 4.1] | 0.2 |
| Fluid balance (mL) | 1565 [115, 4405] | 819 [− 396, 2194] | 0.3 | 1791 [273, 4104] | 1426 [563, 2624] | 0.4 |
| Ascites, n (%) | 27 (100) | 27 (87.1) | 0.2 | 54 (90.0%) | 28 (84.8) | 0.7 |
| Paracentesis, n (%) | 18 (64.3) | 10 (32.3) | 0.03 | 29 (43.2) | 9 (28.1) | 0.11 |
| Paracentesis volume (mL)b (n = 21) | 3500 [1650, 5613] | 3110[2055, 4375] | 0.9 | 3500 [1750, 5538] | 2220[1850, 2600] | 0.4 |
| SAPS II score | 56 (17) | 43 (14) | 0.004 | 50 (16) | 46 (13) | 0.3 |
| MELD Na score | 31 [26, 37] | 29 [20, 38] | 0.5 | 31 [25, 37] | 28 [19, 40] | 0.3 |
| ACLF grade | 3 [2, 3] | 2 [1, 3] | 0.06 | 3 [2, 3] | 2 [1, 3] | 0.03 |
| AKIa, n (%) | 22 (78.6) | 17 (54.8) | 0.10 | 47 (70.1) | 17 (53.1) | 0.2 |
| RRT, n (%) | 6 (21.4) | 8 (25.8) | 0.9 | 18 (26.9) | 6 (18.8) | 0.5 |
| IMV, n (%) | 18 (64.3) | 12 (38.7) | 0.09 | 37 (55.2) | 18 (56.2) | 1.0 |
| Vasopressors, n (%) | 24 (85.7) | 19 (61.3) | 0.07 | 52 (77.6) | 18 (56.2) | 0.05 |
| IAP (mmHg) | 13 [11, 15] | 11 [7, 14] | 0.04 | 13 [9, 15] | 9 [7, 14] | 0.2 |
| MAP (mmHg) | 65 [60, 71] | 81 [72, 92] | < 0.001 | 71 [62, 79] | 81 [74, 97] | 0.002 |
| APP (mmHg) | 51 (7) | 73 (11) | < 0.001 | 59 (13) | 76 (12) | < 0.001 |
| Mortality at day 28, n (%) | 21 (75.0) | 14 (45.2) | 0.04 | 39 (58.2) | 17 (53.1) | 0.8 |
| ICU LOS (days) | 7 [2, 12] | 8 [4, 11] | 0.2 | 9 [5, 14] | 7 [4, 9] | 0.03 |
IAP intra-abdominal pressure, APP abdominal perfusion pressure, BMI body mass index, HCV Hepatitis C virus, ACLF acute-on-chronic liver failure, INR international normalization ratio, CRP C-reactive protein, PaFiO2 arterial oxygen partial pressure to fractional inspired oxygen ratio, IMV invasive mechanical ventilation, AKI acute kidney injury, RRT renal replacement therapy, SAPS II simplified acute physiology score II, CLIF-C Chronic Liver Failure Consortium, MELD-Na Model For End-Stage Liver Disease – sodium, MAP mean arterial pressure, ICU intensive care unit, LOS length-of-stay, WBC white blood cell.
aDiagnosis of AKI as indicated in clinical records.
bParacentesis volume (mL) includes only large-volume paracentesis (≥ 500 mL), and excludes diagnostic paracentesis.
Normally distributed continuous variables are presented as mean (SD) and non-normal continuous variables as median [IQR]. IAP and MAP values are drawn from pooled APP data components.
Table 3.
Multivariate analysis for independent associations with the presence of abdominal hypoperfusion in intensive care.
| Baseline variables | p | Odds ratio | 95% CI | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| AhP at baseline (n = 59)a | Paracentesis | 0.01 | 4.81 | 1.46 | 15.8 |
| ACLF grade | 0.01 | 2.41 | 1.20 | 4.85 | |
| AhP up to day 7 (n = 92)b | Paracentesis | 0.07 | 2.51 | 0.92 | 6.86 |
| ACLF grade | 0.003 | 2.09 | 1.29 | 3.39 | |
Abdominal hypoperfusion corresponds to a daily mean abdominal perfusion pressure < 60 mmHg.
AhP abdominal hypoperfusion, C.I. confidence interval, ACLF acute-on-chronic liver failure.
aMultivariate analysis included: urea, pH, lactate, invasive mechanical ventilation, ACLF grade and paracentesis.
bMultivariate analysis included: creatinine, urine output, pH, paracentesis and ACLF grade.
During the entire ICU stay, AhP presented a cumulative prevalence of 63% (Fig. 3) and was associated with higher creatinine and urea concentrations, urine output, ACLF grade and lower pH at baseline (Table 2). Multivariate analysis (n = 92), with the addition of paracentesis of any type at admission due to clinical relevance, revealed that higher ACLF grade (aOR 2.09, CI 95% 1.29–3.39, p = 0.003) was significantly associated with AhP during the first week of ICU stay, differently from paracentesis (aOR 2.51, CI 95% 0.92–6.86, p = 0.07) (Table 3).
Mortality
Baseline APP (mmHg) was lower in non-survivors at 28-days when compared to survivors (60 ± 14 vs. 67 ± 15, p = 0.050) (Table 1), and those with AhP had a higher 28-day mortality rate (75.0% vs. 45.2%, p = 0.04) (Table 2). Additionally, mortality was also associated with WBC, bilirubin, urea, SAPS II score, lactate, West-Haven score, paracentesis, INR, creatinine, pH and a clinical diagnosis of AKI at admission. (Table 1) In multivariate analysis (n = 96) we observed that bilirubin (aOR 1.10, CI 95% 1.04–1.16, p < 0.001) and SAPS II score (aOR 1.07, CI 95% 1.03–1.11, p = 0.001) were independently associated with 28-day mortality. Similar results were observed for 90-day mortality. When we included baseline AhP, due to clinical importance, in the multivariate analysis (n = 55) West-Haven hepatic encephalopathy score was the only risk factor for 28-day mortality (Table 4).
Table 4.
Multivariate analysis for 28-day mortality risk factors in liver cirrhosis patients in intensive care.
| Baseline variables | p | Odds ratio | 95% CI | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Mortality at 28 days (n = 96) | Lactate | 0.4 | 1.07 | 0.92 | 1.24 |
| C-reactive protein | 0.07 | 1.01 | 1.00 | 1.02 | |
| SAPS II | 0.048 | 1.04 | 1.00 | 1.09 | |
| Bilirubin | 0.001 | 1.13 | 1.05 | 1.21 | |
| Variables included: white blood cell count, bilirubin, urea, SAPS II score, lactate, West-Haven score, paracentesis at admission | |||||
| Mortality at 28 days (n = 55) | Baseline AhP | 0.09 | 0.31 | 0.08 | 1.20 |
| SAPS II | 0.08 | 1.04 | 1.00 | 1.09 | |
| West-Haven score | 0.02 | 1.88 | 1.11 | 3.21 | |
| Variables included: white blood cell count, bilirubin, urea, SAPS II score, lactate, West-Haven score, paracentesis and AhP at baseline | |||||
Paracentesis corresponds to both diagnostic and large-volume paracentesis.
C.I. confidence interval; SAPS simplified acute physiologic score; AhP abdominal hypoperfusion
Baseline APP presented a poor ability to discriminate between survivors and non-survivors at 28 days (aROC of 0.64, CI 95% 0.50–0.79, p = 0.07) (Supplementary Fig. 2) with an optimal cut-off value of ≥ 59 mmHg (Younden index 0.28).
Finally, patients presenting AhP had a longer ICU LOS (days) (9 [5, 14] vs. 7 [4, 9], p = 0.03) (Table 2), compared to those without AhP.
Discussion
Overall
This is the largest study to address the impact of APP on clinical outcomes in critically ill cirrhotic patients5,6,16. The typical patient in our cohort was a 57-year-old male with alcoholic liver disease and ascites, admitted in intensive care with ACLF grade 3.
The main findings of this study were: (1) a high prevalence of AhP, in approximately half of the population at baseline and in two thirds of patients during the first week of ICU stay, (2) AhP was independently associated with higher clinical severity, (3) these patients were five times more likely to be submitted to paracentesis at baseline, and (4) 28-day mortality risk factors included higher clinical severity, total bilirubin and hepatic encephalopathy at ICU admission.
Chronically increased IAP is present in the physiological state of pregnancy17–20 and in pathological states such as morbid obesity21,22, decompensated heart failure23–25 and liver cirrhosis26,27.
Specific clinical thresholds for IAP and APP in the cirrhotic patient are yet to be defined, particularly, since advanced cirrhosis with portal hypertension leads to multiple compensatory mechanisms. These include a hyperdynamic state (due to splanchnic and systemic arterial vasodilation resulting in reduced effective blood volume) with compensatory vasoconstriction and reduced organ perfusion, cardiomyopathy, microvascular and endothelial dysfunction. Furthermore, systemic inflammation, mitochondrial dysfunction, oxidative stress and metabolic changes can lead to tissue injury and extrahepatic organ failure28. These mechanisms potentially modify pathophysiologic responses to acute critical illness as seen in other types of patients and illnesses (i.e., acute pancreatitis, major burns and abdominal surgery).
In our cohort of critically ill liver cirrhosis patients the overall mean APP baseline value was low when compared to other populations of intensive care patients29,30. In a mixed population of 100 intensive care patients, where 42% of patients had IAH, the overall mean APP value was 74 (± 17) mmHg17. In another study, 50 patients with severe acute pancreatitis had a mean APP of 80 (± 5) mmHg18. Comparatively, our cohort presented lower APP with differences of − 11 and − 17 mmHg, respectively.
Nearly two thirds of our patients had AhP during the ICU stay. Two studies in critically ill cirrhotic patients reported a high prevalence of AhP5,6. In the first study, Al-Dorzi et al. analyzed 61 septic shock patients, reporting a prevalence of AhP of 70% at ICU admission. Interestingly, an APP of 55 mmHg was identified as the best cut-off value to discriminate survivors from non-survivors, and AhP was not significantly associated with any of the studied outcomes in multivariate analysis. This study concluded that IAH was associated with increased ICU morbidity and mortality, although no independent risk factors for IAH were found5. In the second study, Mayr et al. reported a prevalence of AhP between 25 and 50% of cases (inferred from a median APP value of 63 [57, 70] mmHg, n = 22), and was able to quantify hepatosplanchnic blood flow impairment due to IAH6. Our study confirms a high prevalence of AhP among critically ill cirrhotic patients6.
Abdominal hypoperfusion
Acute-on-chronic liver failure severity score was predictive of AhP at baseline and during the first week of ICU stay. This reflected the severity of our typical ACLF grade 3 patient with shock and multiorgan failure, frequently treated with noradrenaline and terlipressine perfusions combined. Whereas, for less severe upper gastrointestinal bleeding and hepato-renal syndrome, terlipressine was the preferred vasoactive agent. Three quarters of our cohort received vasopressor therapy, nonetheless, arterial hypotension persisted in one fifth of patients at baseline. In our view, higher clinical severity with the presence of shock combined with increased IAP was the main reason for the association between higher ACLF grade and AhP.
Patients with AhP were five times more likely to be submitted to paracentesis at baseline, although, we did not observe an improvement in the ensuing daily APP variation when compared to the rest of the patients without paracentesis at baseline, nor did we find and association between LVP and AhP. We speculate these results signaled an increased clinical awareness for diagnostic screening of spontaneous bacterial peritonitis and the treatment and prevention of IAH in these high-risk patients. Particularly, since PPCD preventive measures were standard-of-care, a higher fluid balance at ICU admission was observed in the AhP. Although we did not find an association between LVP and AhP, an increase in APP (with a decrease of IAP and central venous pressure, without change in circulating volume), as well as improved hepatosplanchnic blood flow, has been described after LVP. This was corroborated by ultrasound hepatic artery resistance index, hepatic vein maximum flow velocity, and indocyanine green plasma disappearance rate (positively correlated to APP and inversely correlated to IAP), considered a dynamic surrogate marker of hepatic perfusion and hepatocellular function6,7,31,32. Importantly, patients not submitted to paracentesis were clinically justified, inasmuch as the use of paracentesis has been suggested as a key inpatient quality measure in cirrhosis33,34.
Mortality
Baseline APP was lower in non-survivors than in survivors at 28 days, although it presented an inadequate discriminatory ability. Abdominal hypoperfusion was not a risk factor for mortality in our cohort, probably due to the small sample size and the multifactorial nature of critical illness. Unambiguously, mortality was associated with baseline clinical severity and total bilirubin, reflecting the dual character of the “acute” critical illness and the “chronic” liver disease in this population of cirrhotic patients. Additionally, West-Haven hepatic encephalopathy score was the only independent risk factor for 28-day mortality in the subset of patients with available baseline APP data. This highlights the vital importance of acute neurologic disfunction in critically ill cirrhotic patients, as previously reported16,34,35.
Furthermore, patients with AhP had longer ICU LOS, indicating greater patient comorbidity and higher associated healthcare costs.
We support the rationale for considering APP a critical vital sign and to use it to further assist the clinician in titrating volume repletion, vasopressor use and optimizing IAP, thus preventing deleterious effects of persistent critical pressures9,10,14,36,37. The treatment and prevention of AhP in high-risk cirrhotic patients is prudential.
Limitations
Limitations in this study include a relatively small sample size, due to slow recruitment aggravated by the COVID-19 pandemic onset, and missing baseline APP data due to work load and delayed patient enrolment into the study protocol. Additionally, the lack of longitudinal data on organ dysfunction and the impact of therapies aimed at optimizing APP precluded further results and outcome analysis. The impact of AhP on specific organ failures should be specifically addressed in the future.
Strengths of this study include the fact that it is the largest prospective study addressing APP in consecutive critically ill cirrhotic patients, this way minimizing selection bias, provides data on the impact of baseline paracentesis in the critically ill cirrhotic patient, and opens the field for further research38. Future studies on AhP, IAH and ACS should focus on the first week of ICU admission38,39.
Conclusion
This study confirms a high prevalence of AhP in critically ill cirrhotic patients. Abdominal hypoperfusion was independently associated with higher ACLF grade and paracentesis performed at ICU admission.
Mortality at 28 days was higher among patients with AhP and independent risk factors were higher clinical severity, total bilirubin and hepatic encephalopathy.
Abdominal perfusion pressure can be considered a critical vital sign and prevention and treatment of AhP in the high-risk cirrhotic patient is prudential.
Supplementary Information
Acknowledgements
The authors of this study would like to thank the entire nursing and medical staff of the intensive care unit, Unidade de Cuidados Intensivos Polivalente 7, at Hospital de Curry Cabral, for their fundamental work in this study, and the “Centro de Investigação”, at Centro Hospitalar Universitário Lisboa Central, for their support.
Author contributions
R.P. conceived and designed the analysis, collected data, performed analysis and wrote the manuscript, A.E. collected data and contributed with the analysis and reviewed the manuscript, F.C., R.P., P.M. and F.S. contributed with the analysis and reviewed the manuscript.
Funding
No funding nor financial support was received for this study.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-34367-6.
References
- 1.Levesque E, et al. Respiratory impact of paracentesis in cirrhotic patients with acute lung injury. J. Crit. Care. 2011;26:257–261. doi: 10.1016/j.jcrc.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Mayr, U. et al. Impact of large volume paracentesis on respiratory parameters including transpulmonary pressure and on transpulmonary thermodilution derived hemodynamics: A prospective study. PLoS One. 13(3), e0193654 (2018). [DOI] [PMC free article] [PubMed]
- 3.Umgelter A, et al. Effects of plasma expansion with albumin and paracentesis on haemodynamics and kidney function in critically ill cirrhotic patients with tense ascites and hepatorenal syndrome: A prospective uncontrolled trial. Crit. Care. 2008;12:R4. doi: 10.1186/cc6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillip V, et al. Effects of paracentesis on hemodynamic parameters and respiratory function in critically ill patients. BMC Gastroenterol. 2014;14:18. doi: 10.1186/1471-230X-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayr, U. et al. Large-volume paracentesis effects plasma disappearance rate of indo-cyanine green in critically ill patients with decompensated liver cirrhosis and intraabdominal hypertension. Ann. Intensive Care. 8(1), 78 (2018). [DOI] [PMC free article] [PubMed]
- 6.Al-Dorzi HM, Tamim HM, Rishu AH, Aljumah A, Arabi YM. Intra-abdominal pressure and abdominal perfusion pressure in cirrhotic patients with septic shock. Ann. Intensive Care. 2012;2012:S4. doi: 10.1186/2110-5820-2-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malbrain MLNG, et al. Relationship between intra-abdominal pressure and indocyanine green plasma disappearance rate: Hepatic perfusion may be impaired in critically ill patients with intra-abdominal hypertension. Ann. Intensive Care. 2012;2012:1–11. doi: 10.1186/2110-5820-2-S1-S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheatham ML, et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. II. Recommendations. Intensive Care Med. 2007;33:951–962. doi: 10.1007/s00134-007-0592-4. [DOI] [PubMed] [Google Scholar]
- 9.Gül F, et al. Abdominal perfusion pressure is superior from intra-abdominal pressure to detect deterioration of renal perfusion in critically Ill patients. Ulus Travma Acil Cerrahi Derg. 2019;25:561–566. doi: 10.14744/tjtes.2019.25263. [DOI] [PubMed] [Google Scholar]
- 10.Cheatham ML, White MW, Sagraves SG, Johnson JL, Block EFJ. Abdominal perfusion pressure: a superior parameter in the assessment of intra-abdominal hypertension. J. Trauma Injury Infect. Crit. Care. 2000;49:621–627. doi: 10.1097/00005373-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association World Medical Association Declaration of Helsinki. JAMA. 2013;310:2191. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 12.Ginès Pere, et al. Liver cirrhosis. Lancet. 2021;398:1359–13-76. doi: 10.1016/S0140-6736(21)01374-X. [DOI] [PubMed] [Google Scholar]
- 13.Malbrain MLNG, et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome I. Definitions. Intensive Care Med. 2006;32:1722–1732. doi: 10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick AW, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akoglu H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018;18:91–93. doi: 10.1016/j.tjem.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira R, et al. Intra-abdominal hypertension and abdominal compartment syndrome in the critically ill liver cirrhotic patient–prevalence and clinical outcomes. A multicentric retrospective cohort study in intensive care. PLoS ONE. 2021;16:e0251498. doi: 10.1371/journal.pone.0251498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozada MJ, et al. Management of peripartum intra-abdominal hypertension and abdominal compartment syndrome. Acta Obstet. Gynecol. Scand. 2019;98:1386–1397. doi: 10.1111/aogs.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyagi A, Singh S, Kumar M, Sethi AK. Intra-abdominal pressure and intra-abdominal hypertension in critically ill obstetric patients: A prospective cohort study. Int. J. Obstet. Anesth. 2017;32:33–40. doi: 10.1016/j.ijoa.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Sawchuck DJ, Wittmann BK. Pre-eclampsia renamed and reframed: Intra-abdominal hypertension in pregnancy. Med. Hypotheses. 2014;83:619–632. doi: 10.1016/j.mehy.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Chun R, Baghirzada L, Tiruta C, Kirkpatrick AW. Measurement of intra-abdominal pressure in term pregnancy: A pilot study. Int. J. Obstet. Anesth. 2012;21:135–139. doi: 10.1016/j.ijoa.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Frezza EE, Shebani KO, Robertson J, Wachtel MS. Morbid obesity causes chronic increase of intraabdominal pressure. Dig. Dis. Sci. 2007;52:1038–1041. doi: 10.1007/s10620-006-9203-4. [DOI] [PubMed] [Google Scholar]
- 22.De Keulenaer BL, De Waele JJ, Powell B, Malbrain MLNG. What is normal intra-abdominal pressure and how is it affected by positioning, body mass and positive end-expiratory pressure? Intensive Care Med. 2009;35:969–976. doi: 10.1007/s00134-009-1445-0. [DOI] [PubMed] [Google Scholar]
- 23.Mullens W, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: A potential contributor to worsening renal function? J. Am. Coll. Cardiol. 2008;51:300–306. doi: 10.1016/j.jacc.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 24.Mullens W, et al. Prompt reduction in intra-abdominal pressure following large-volume mechanical fluid removal improves renal insufficiency in refractory decompensated heart failure. J. Card Fail. 2008;14:508–514. doi: 10.1016/j.cardfail.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Verbrugge FH, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J. Am. Coll. Cardiol. 2013;62:485–495. doi: 10.1016/j.jacc.2013.04.070. [DOI] [PubMed] [Google Scholar]
- 26.Umgelter A, et al. Renal resistive index and renal function before and after paracentesis in patients with hepatorenal syndrome and tense ascites. Intensive Care Med. 2009;35:152–156. doi: 10.1007/s00134-008-1253-y. [DOI] [PubMed] [Google Scholar]
- 27.De Laet IE, Malbrain MLNG, De Waele JJ. A clinician’s guide to management of intra-abdominal hypertension and abdominal compartment syndrome in critically ill patients. Crit. Care. 2020;24:1–9. doi: 10.1186/s13054-020-2782-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J. Hepatol. 2021;75:S49. doi: 10.1016/j.jhep.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim IB, Prowle J, Baldwin I, Bellomo R. Incidence, risk factors and outcome associations of intra-abdominal hypertension in critically ill patients. Anaesth. Intensive Care. 2012;40:79–89. doi: 10.1177/0310057X1204000107. [DOI] [PubMed] [Google Scholar]
- 30.Ke L, et al. Intra-abdominal pressure and abdominal perfusion pressure: Which is a better marker of severity in patients with severe acute pancreatitis. J. Gastrointest. Surg. 2011;15:1426–1432. doi: 10.1007/s11605-011-1553-3. [DOI] [PubMed] [Google Scholar]
- 31.Drazen Z, et al. Doppler ultrasound of hepatic and system hemodynamics in patients with alcoholic liver cirrhosis. Digest. Diseases Sci. 2009;55:458–466. doi: 10.1007/s10620-009-0760-1. [DOI] [PubMed] [Google Scholar]
- 32.Sakka SG. Indocyanine green plasma disappearance rate during relief of increased abdominal pressure [9] Intensive Care Med. 2006;32:2090–2091. doi: 10.1007/s00134-006-0411-3. [DOI] [PubMed] [Google Scholar]
- 33.Gaetano JN, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J. Gastroenterol. Hepatol. 2016;31:1025–1030. doi: 10.1111/jgh.13255. [DOI] [PubMed] [Google Scholar]
- 34.Thabut D, et al. Diagnosis and management of hepatic encephalopathy: The French recommendations. Liver Int. 2023 doi: 10.1111/LIV.15510. [DOI] [PubMed] [Google Scholar]
- 35.Montagnese S, et al. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J. Hepatol. 2022;77:807–824. doi: 10.1016/j.jhep.2022.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Kyoung, K. H. & Hong, S. K. The duration of intra-abdominal hypertension strongly predicts outcomes for the critically ill surgical patients: A prospective observational study. World J. Emerg. Surg. 10, 22 (2015). [DOI] [PMC free article] [PubMed]
- 37.Evans L, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 2021;49:e1063–e1143. doi: 10.1097/CCM.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 38.Pereira, Rui (Hospital Curry Cabral, C. Continuous Passive Paracentesis for Intra-abdominal Hypertension—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04322201.
- 39.Reintam Blaser A, et al. Incidence, risk factors, and outcomes of intra-abdominal hypertension in critically ill patients—A prospective multicenter study (IROI study) Crit. Care Med. 2019;47:535–542. doi: 10.1097/CCM.0000000000003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.