Abstract
We describe the first meltable iron-based zeolitic imidazolate framework (ZIF), denoted MUV-24. This material, elusive from direct synthesis, is obtained from the thermal treatment of [Fe3(im)6(Him)2], which yields Fe(im)2 upon loss of the neutral imidazole molecules. Different crystalline phase transformations are observed upon further heating, until the material melts at 482 °C. Vitrification upon cooling of the liquid phase gives rise to the first Fe-metal–organic framework glass. X-ray total scattering experiments show that the tetrahedral environment of the crystalline solids is maintained in the glass, whereas nanoindentation measurements reveal an increase in Young’s modulus, in agreement with stiffening upon vitrification.
Introduction
Crystalline metal–organic frameworks (MOFs) are currently one of the most studied classes of materials, although these porous materials typically collapse on heating under irreversible decomposition.1−3 However, the combination of thermal stability with suitable functionalization has led to the appearance in these materials of a molten phase prior to decomposition that was unnoticed until recently.4−7 MOF glasses are generated on cooling these melts.8,9 Both MOF liquids and glasses have recently gained much interest because of their uncommon physical properties such as different mechanical properties,4,10,11 porosity,12,13 ionic conductivity,14,15 or application in devices (e.g., as electrolytes,16 in solar cells,17 as membranes,18 anodes for Li-ion batteries19).
Despite the large number of MOFs already reported (more than 100,000 in the CCDC),20 those that melt prior to decomposition are very scarce. Specifically, these mainly belong to the family of zeolitic imidazolate frameworks (ZIFs),21,22 which are formed by M2+ cations linked by imidazolate derivative anions. Only a handful of Zn2+ and Co2+ based ZIFs have been reported to melt and form hybrid glasses, namely, ZIF-48b and ZIF-62(Zn and Co),10,23,24 all containing imidazolate bridges (im–).
Reducing the melting temperature is very desirable, as it would reduce the energy required for the formation of the glasses and facilitate its interaction with other materials.5,25,26 The most used strategy to achieve this reduction has been the incorporation of bulkier ligands.8a,11,13,27−31 In this sense, incorporating small amounts of bulkier imidazole derivatives, such as benzimidazole, reduces the melting temperature (Tm) from 590 °C (as found in Zn(im)2, also known as ZIF-4),8b to 310 °C (as found in Zn0.8Co0.2(im)1.95(bim)0.025(Clbim)0.025).31 In addition, it is also very important to increase the temperature interval between melting and thermal decomposition, in order to facilitate the preparation of glasses. This has also been achieved using bulky substituents, with the largest reported interval of approximately 200 °C for ZIF-62 derivates.8a
In this work, motivated by the prospect of expanding the family of ZIF glasses, we explore the preparation of a novel Fe-glass based on Fe(im)2, through an indirect manner, as this compound is unachievable by a direct synthetic route. This will also allow the exploration of the effects on Tm of incorporating the more labile iron(II) centers.
Results and Discussion
The nonporous coordination polymer [Fe3(im)6(Him)2] (CCDC code = IMIDFE) was prepared, as previously reported,32 in a solvent-free reaction that also serves to prepare porous Fe-based ZIFs.33,34IMIDFE consists of a 3D coordination polymer with alternating tetrahedral and octahedral centers which are linked via imidazolate bridges. Each iron connects to four other metals, with the octahedral centers having two terminal imidazole molecules in trans-configuration.
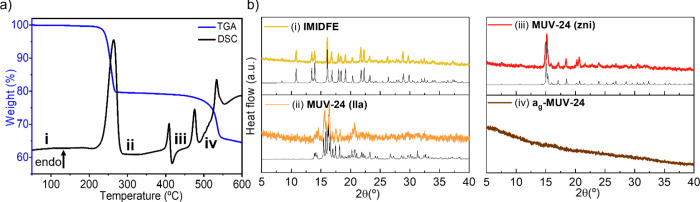
Upon heating this solid, a mass loss of 21.3% is observed in TGA (under a N2 atmosphere) at ca. 283 °C, which is accompanied by an endothermic peak in the DSC measurement (see Figure 1a). This mass loss can be associated with the removal of the terminal imidazole molecules (calc. 19.3%), which is confirmed by TGA coupled to mass spectrometry (see Figure S21). This process is accompanied by a change in the powder X-ray pattern (Figure 1b), revealing a rearrangement in the structure of the solid with formula Fe(im)2. Fortunately, a small single crystal could be isolated upon heating IMIDFE at 300 °C, thus allowing the unequivocal identification of structural changes. The new solid, denoted MUV-24(lla), was solved from single crystal X-ray diffraction and revealed a new polymorph of Fe(im)2 with a hitherto unknown topology. This new phase crystallizes in the space group P21/c (a = 12.358 Å, b = 23.643 Å, c = 19.556 Å, β = 93.237 °) with a 3D structure formed only of tetrahedral Fe(II) centers coordinated via imidazolate ligands.
Figure 1.
(a) Thermogravimetric (in blue) and differential scanning calorimetry (in black) analyses upon heating [Fe3(im)6(Him)2] (IMIDFE), showing the mass loss and the phase transitions; (b) X-ray powder patterns of the different phases obtained upon heating at different temperatures (indicated in the DSC plot), showing the calculated powder patterns as thin black lines at the bottom.
The most prominent feature of MUV-24(lla) is its novel topology with a net point symbol {4.6.273}2{4.6.48}2{6.38.29}{6.47.9}, herein named lla. The novelty of the underlying net topology was assessed using TOPOS35 and has been registered in the personal topology library. The asymmetric unit includes seven crystallographically different Fe atoms (Figure 2 and Figure S1), all of them acting as 4-connected nodes. Four Fe atoms are linked by im– to form a four-membered ring (Figure S1), which is the smallest ring observed in the structure. In addition, 6-, 7-, 8-, and 9-membered rings are also observed (Figure S1), forming a 3D structure (Figure S2).
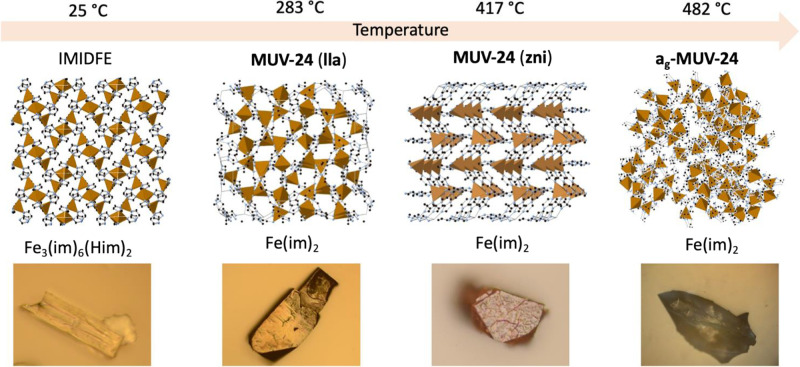
Figure 2.
(From the left to right) Crystalline structures of IMIDFE, MUV-24(lla) and MUV-24(zni), and schematic representation of amorphous ag-MUV-24, all of them accompanied by their respective microscope images (note that ag-MUV-24 was arbitrary created to illustrate the lack of order). The products formed upon melting and quenching at each temperature represent a clear visual change. Furthermore, in the last material, the vitrification is observed.
Further heating of MUV-24(lla) up to 417 °C causes a phase transition, as evidenced by the endothermic peak in the DSC, with no associated mass loss (Figure 1a). Powder X-ray diffraction indicates further structural changes (Figure 1b), showing that the new phase corresponds to the zni topology (thus denoted MUV-24(zni)), previously reported for Zn2+ and Co2+ but elusive for Fe2+.36−38 Significantly, the zni topology is an essential intermediate in the melting process of ZIF-4(Zn).9 The zni topology is considered to be the most thermodynamically stable phase of the Zn-ZIF family,39 although other reports identify the coi topology as the most stable phase at ambient pressure below 360 °C.40 In the case of MUV-24(zni), it transforms to the coi phase (i.e. to MUV-24(coi)) when left at ambient pressure or under vacuum (see Figures S12 and S13), a transformation that is not observed in the Zn2+ analogue. Single crystal X-ray diffraction confirms the structure of MUV-24(coi), also reported for Zn2+,37,38 and Co2+,36 but not for Fe2+.
Upon further heating of MUV-24(zni) to 482 °C, another endothermic peak is observed in the DSC, which is also not associated with any mass loss. This peak corresponds with the melting of the material and a viscous material that corresponds with the liquid phase can be observed after the transition. Powder X-ray diffraction of the material after melt-quenching to room temperature shows the absence of Bragg reflections (see Figure 1b), which clearly proves the vitrification of crystalline MUV-24(zni) into a noncrystalline phase, denoted ag-MUV-24 (amorphous glass MUV-24), which is nonporous. The melting transformation occurs prior to decomposition of the material at about 530 °C (Figure 1).
These phase transitions were also followed via in situ powder X-ray diffraction (see Figure S4), although we can only clearly observe the IMIDFE-to-MUV-24(lla) phase transition. This suggests a high sensitivity of the process to the experimental conditions. In fact, upon modification of the DSC conditions (using a hermetic pan), we can prepare MUV-24(coi) instead of MUV-24(zni). The importance of minor changes in the thermal process has also been recently reported in the formation of the qtz-ZIF-8 phase.41
The sequence of structural phase transformations can also be followed through optical images, in which the morphology of the material changes in each of its phases (see Figure 2). Clear differences can be observed between the crystalline and the amorphous phase. In fact, ag-MUV-24 shows evidence of fusion of the microcrystals into a compact monolithic glass.
Having established the formation of an amorphous phase, we conducted cyclic DSC measurements (two upscans, one downscan) under a N2 atmosphere from room temperature to 500 °C at 10 °C min–1 (Figure 3) in order to establish the melting point (Tm) of the material, defined as the offset temperature of the calorimetric melting point, and the glass transition temperature (Tg).
Figure 3.
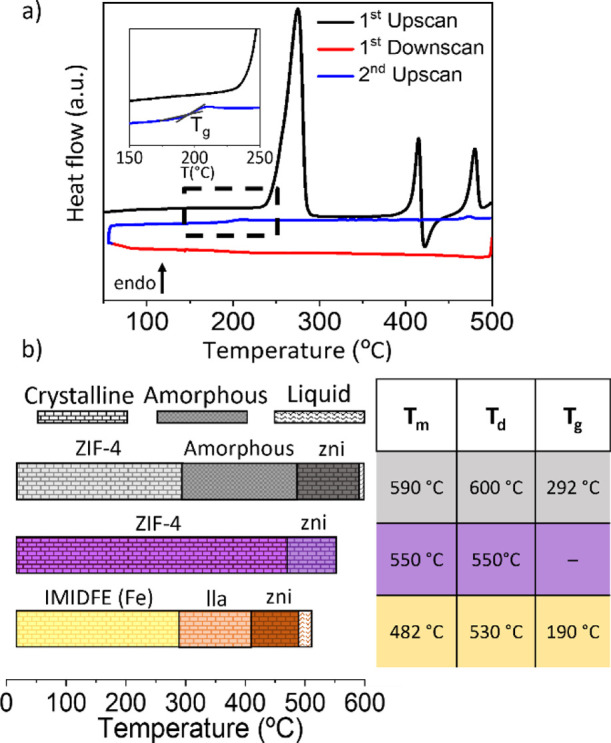
(a) DSC cycles of IMIDFE at 10 °C min–1; the black line corresponds with the first upscan, the red line corresponds with the first downscan and the blue line corresponds with the second upscan. (b) Schematic representation of the different phase changes undergone by ZIF-4(Zn), ZIF-4(Co) and IMIDFE. ZIF-4(Zn) becomes an amorphous phase, then transforms to the crystalline phase zni, which melts and finally decomposes. ZIF-4(Co) is converted to the zni crystal phase and decomposes without melting. IMIDFE becomes the lla crystalline phase, then transforms into the zni phase, which melts and finally decomposes.
The first upscan of the DSC measurement identifies the Tm of MUV-24 at 482 °C, which is more than 100 °C lower than the Zn analogue (Tm = 593 °C)8 and 70 °C lower than the Co analogue (Tm = 550 °C).23 Not only is the melting temperature reduced but also the temperature range of the liquid is significantly increased. Thus, the working interval of molten MUV-24 ranges from 480 to 530 °C (i.e. 50 °C), whereas that of ZIF-4(Zn) is only 7 °C (from 593 to 600 °C)8 and nonexistent for ZIF-4(Co) as it decomposes as it melts.23
Subsequently, a downscan was performed to obtain the melt-quenched glass and, in a second upscan of ag-MUV-24, a calorimetric signal associated with the glass transition temperature (Tg) is observed. Similar to what is observed for Tm, Tg of ag-MUV-24 is significantly lower than Tg of the ag ZIF-4 (190 °C vs 292 °C)8 and still much lower than the lowest reported Tg (250 °C for Zn(im)1.87(6-Cl-5-Fbim)0.13).27 We believe that the lability of the Fe–im bond is the reason for this clear decrease in Tg.
In order to get further insights into the structural differences and similarities between the different crystalline and amorphous phases of MUV-24, X-ray total scattering experiments were performed on IMIDFE, MUV-24(lla), MUV-24(coi) (as the zni phase was not stable at ambient conditions) and ag-MUV-24. The total scattering structure factors, S(Q), are given in Figure S6 and show that the Bragg peaks at low-Q from the crystalline phases are not present in the ag-MUV-24 data. However, additional Bragg peaks are observed in the ag-MUV-24 data at higher-Q. These can be indexed to crystalline impurities of Al and Fe2N (Figure S7) and are a small proportion of the scattering signal. The corresponding X-ray pair distribution function (PDF or D(r)) data are shown in Figures 4 and S8. The similarity in the short-range correlations up to ∼6 Å (corresponding to the distance between neighboring Fe(II) centers) across all the PDFs clearly shows that the tetrahedral coordination of the Fe(II) centers with imidazolate linkers is preserved in ag-MUV-24.
Figure 4.
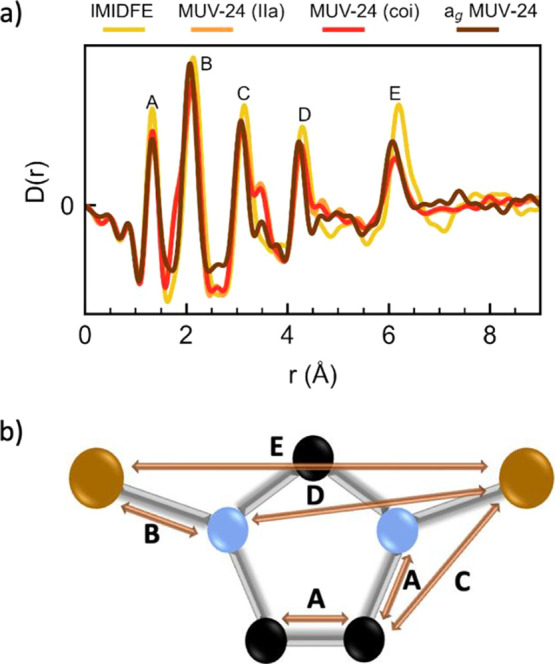
(a) X-ray PDF in the form of D(r) of IMIDFE, MUV-24(lla), MUV-24(coi), and ag-MUV-24. (b) Structural representation of the short-range order matching bonds and pair distances with the peaks shown in a. Fe, C, and N atoms are shown in brown, black, and blue, respectively.
Furthermore, small differences to the peak at ∼6 Å, reflect differences in the crystal structures (see Figure S9). The broader, more-structured ∼6 Å peak from the IMIDFE sample is a consequence of the presence of different Fe centers, in this case, tetrahedral and octahedral. The shorter (tetrahedral) distance remains in the amorphous structure, thus confirming the similar environment in all MUV-24 phases. There are also two small peaks in the MUV-24(lla) and MUV-24(coi) PDFs that are not present in the calculated PDFs: a shoulder to peak B at ∼1.8 Å and a peak at ∼3.5 Å next to peak C (Figures 4a and S9). It is difficult to attribute these features to a specific origin. There is no evidence for crystalline impurity in these samples but there could be an amorphous component given that the Bragg peaks from these two phases are much less intense than those from IMIDFE (Figure S6). Also, the PDF data from a laboratory-based X-ray diffractometer are not of the highest quality so experimental artifacts, especially at the lowest r-values, cannot be ruled out. In addition, the region around r ∼ 3.5 Å corresponds to N···N distances within the FeN4 tetrahedra and also to distances between (C,N)···(C,N) atom pairs from different imidazole ions that are not linked to a common Fe ion, both of which will be strongly influenced by disorder and distortion within the structures. Finally, we note that the limited Q-range of the total scattering data means that the PDF data are of relatively low resolution. This makes a discussion of the detail of the Fe(im)4 tetrahedral arrangement difficult, contrary to the recent work based on 67Zn NMR measurements.42
The formation of glass is normally accompanied by changes in the mechanical properties of the solids, observed as an increase in Young’s modulus (E). Different MOF glass specimens have been studied with nanoindentation, typically using a nanoindenter with a diamond tip.11,43 Here, we have used, for the first time, an atomic force microscopy (AFM) instrument in PeakForce quantitative nanomechanical property mapping mode (PF-QNM) to measure the Young’s modulus of a monolithic glass. This technique does not destroy or damage the tested material, does not require large bulk glass samples for mechanical testing, nor any kind of treatment of the sample. Moreover, it provides a Young’s modulus mapping of the scanned surface with nanometer resolution, which could be very interesting for identifying the existence of local inhomogeneities. This technique was used to measure both the IMIDFE crystalline phase and ag-MUV-24. We also measured the previously reported ag-ZIF-62 in order to verify the validity of the methodology, confirming that the system gives reliable values (Figure 5). In this case, different random faces of IMIDFE were measured giving a mean value of E = 2.2 ± 1.7 GPa. Upon vitrification to ag-MUV-24, significant stiffening is observed (E = 9.9 ± 1.2 GPa) which represents enhancement of the hardness of this material (Figure 5). This trend is quite similar to that observed for ZIF-4(Zn) and ag-ZIF-4(Zn).8a
Figure 5.
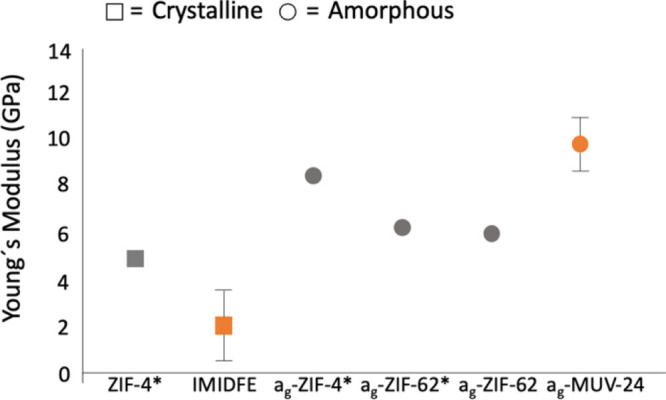
Comparison of Young’s modulus between crystalline (square) and glassy (circle) ZIFs. The gray color represents the ZIFs with Zn centers, while the orange color refers to iron centers. Values marked with an asterisk correspond to previously reported data.8a
Conclusions
We have successfully synthesized and characterized the first Fe-ZIF glass, denoted ag-MUV-24, which is obtained via a three-step structural rearrangement from the known coordination polymer [Fe3(im)6(Him)2], in which octahedral and tetrahedral Fe(II) centers alternate. In the first step, a release of the terminal protonated imidazole ligand occurs, yielding a dense 3D solid with a new topology, denoted MUV-24(lla), with exclusively tetrahedral Fe(II) centers. The second structural transformation causes this solid to rearrange into the well-known zni topology, already reported for Zn(II) and Co(II), but not for Fe(II). Finally, upon further heating MUV-24(zni) to 482 °C, the solid melts. This melting temperature for Fe(im)2 is significantly below the melting temperature of the Zn analogue, thus potentially improving the applicability of the material. This arises from the incorporation of a more labile metal center such as Fe(II), which is contrary to previous studies of mixing ligands in order to increase the working interval and avoid decomposition. Further tuning of the system with bulkier ligands is currently under exploration and could lead to the formation of very low temperature melts.
Experimental Section
Synthesis of IMIDFE
Ferrocene (55.8 mg, 0.3 mmol) and imidazole (40.8 mg, 0.6 mmol) were combined and sealed under vacuum in a layering tube (4 mm diameter). The mixture was heated at 150 °C for 4 days to obtain yellow crystals suitable for X-ray single-crystal diffraction. The product was allowed to cool to room temperature, and the layering tube was then opened. The unreacted precursors were extracted with acetonitrile. IMIDFE was isolated as yellow crystals. Phase purity was established by X-ray powder diffraction.
Synthesis of MUV-24(lla)
Approximately 15 mg of IMIDFE was treated with the following thermal process: Tinitial = 40 °C (15 min) → 300 °C → 25 °C. Heating/cooling rate = 10 °C s–1.
Synthesis of MUV-24(zni)
Approximately 15 mg of IMIDFE was treated with the following thermal process: Tinitial = 40 °C (15 min) → 430 °C → 25 °C. Heating/cooling rate = 10 °C s–1.
Synthesis of MUV-24(coi)
Approximately 15 mg of MUV-24(zni) was sealed under vacuum in a layering tube (4 mm diameter). A progressive transformation of the zni phase into the coi phase takes place, which is completed in approximately 4 h.
Synthesis of ag-MUV-24
Approximately 15 mg of IMIDFE was treated with the following thermal process: Tinitial = 40 °C (15 min) → 500 °C → 25 °C. Heating/cooling rate = 10 °C s–1.
Single-Crystal Diffraction
Single crystals of MUV-24(lla) and MUV-24(coi) were mounted on glass fibers using a viscous hydrocarbon oil to coat the crystals and then transferred directly to the cold nitrogen stream for data collection. X-ray data were collected at 100 K on a DW rotating anode synergy R diffractometer with the (Cu-Kα) X-ray source (λ = 1.5406 Å). Data were measured using the CrysAlisPro suite of programs. The program CrysAlisPro, Rigaku, was used for unit cell determinations and data reduction. Empirical absorption correction was performed using spherical harmonics, implemented in the SCALE3 ABSPACK scaling algorithm, based upon symmetry-equivalent reflections combined with measurements at different azimuthal angles. The crystal structures were solved and refined against all F2 values using the SHELXL and Olex2 suite of programs.44,45 Atomic displacement parameters of all non-hydrogen atoms were refined anisotropically, except those within a disordered imidazolate ring in each structure, which were refined isotropically. Hydrogen atoms were placed in calculated positions, refined using idealized geometries (riding model), and assigned fixed isotropic atomic displacement parameters. CCDC 2238548–2238549 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, UK; fax: (+44)1223-336-033; or deposit@ccdc.cam.ac.uk).
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) measurements were conducted on a TRIOS DSC 250 instrument. The activated sample (10–15 mg) was loaded into an aluminum crucible (30 μL) with a pierced lid. An empty aluminum crucible was used as a reference. Under N2 gas, the sample was heated to a temperature of 40 °C and an isotherm was performed for 15 min to stabilize the sample. Then, the sample was heated to 300, 430, and 500 °C at a rate of 10 °C min–1 for MUV-24(lla), MUV-24(zni), and ag-MUV-24, respectively. Upon reaching the temperature, an isotherm of 10 min was performed to ensure a complete phase change. This was followed by cooling back to 40 °C at 10 °C min–1.
X-ray Total Scattering
X-ray total scattering data were collected at room temperature using the PANalytical Empyrean laboratory diffractometer equipped with an Ag-Kα source and focusing mirrors. The data were collected with the sample loaded in a 1 mm diameter quartz glass capillary. For each sample, multiple scans were measured with a total collection time of over 24 h per sample. Similar measurements were made of an empty capillary and the diffractometer background. The resulting X-ray total scattering patterns were processed in the GudrunX program46 to produce a normalized PDF optimized such that (for example) the low-r portion of g(r) oscillates around −1. A Qmin of 0.6 Å–1 and Qmax of 18.5 Å–1 were used to obtain the PDF.
Atomic Force Microscopy
We performed PeakForce Quantitative Nanoscale Mechanical characterization (PF-QNM) in PeakForce Tapping mode, in a Bruker Dimension Icon AFM (Bruker Corporation, CA, USA) to map the topography and the Young’s modulus of different materials. IMIDFE, ag-MUV-24, and ZIF-62 (as reference material) were drop-casted on silicon substrates and imaged, in air under ambient conditions, with RTESPA-150 probes (spring constant 5 N/m, Bruker). The force applied by the tip was fixed to ∼1 nN in all experiments. The automatic analysis of these curves generates maps of mechanical property distribution and topography simultaneously.
Acknowledgments
The work has been supported by the European Union (ERC-2016-CoG 724681-S-CAGE), grant PID2020-117177GB-I00, María de Maeztu and Severo Ochoa Centre of Excellence Programmes CEX2019-000919-M and CEX2021-001230-S, funded by MCIN/AEI/10.13039/501100011033, and the Generalitat Valenciana (PROMETEO programme). L.L.-A. and I.B.-A. thank MICINN for pre-doctoral fellowships (PRE2019-089295 and FPU 18/0042), respectively. This study forms part of the Advanced Materials program and was supported by MCIN with funding from European Union NextGenerationEU (PRTR-C17.I1) and by Generalitat Valenciana (project MAF/2022/31).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c01455.
General methods; materials; and crystallographic data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Maurin G.; Serre C.; Cooper A.; Férey G. The New Age of MOFs and of Their Porous-Related Solids. Chem. Soc. Rev. 2017, 46, 3104–3107. 10.1039/C7CS90049J. [DOI] [PubMed] [Google Scholar]
- Dincǎ M.; Long J. R. Introduction: Porous Framework Chemistry. Chem. Rev. 2020, 120, 8037–8038. 10.1021/acs.chemrev.0c00836. [DOI] [PubMed] [Google Scholar]
- Monge A.; Gutierrez-Puebla E. Reticular Chemistry: Special Issue in Honor of the 2018 Wolf Prize Laureate in Chemistry, Professor Omar Yaghi. Isr. J. Chem. 2018, 58, 946–948. 10.1002/ijch.201800146. [DOI] [Google Scholar]
- Ma N.; Horike S. Metal–Organic Network-Forming Glasses. Chem. Rev. 2022, 122, 4163–4203. 10.1021/acs.chemrev.1c00826. [DOI] [PubMed] [Google Scholar]
- Tuffnell J. M.; Ashling C. W.; Hou J.; Li S.; Longley L.; Ríos Gómez M. L.; Bennett T. D. Novel Metal-Organic Framework Materials: Blends, Liquids, Glasses and Crystal-Glass Composites. Chem. Commun. 2019, 55, 8705–8715. 10.1039/C9CC01468C. [DOI] [PubMed] [Google Scholar]
- Bennett T. D.; Horike S. Liquid, Glass and Amorphous Solid States of Coordination Polymers and Metal–Organic Frameworks. Nat. Rev. Mater. 2018, 3, 431–440. 10.1038/s41578-018-0054-3. [DOI] [Google Scholar]
- Gaillac R.; Pullumbi P.; Beyer K. A.; Chapman K.; Keen D. A.; Bennett T. D.; Coudert F. X. Liquid Metal–Organic Frameworks. Nat. Mater. 2017, 16, 1149–1154. 10.1038/nmat4998. [DOI] [PubMed] [Google Scholar]
- a Bennett T. D.; Yue Y.; Li P.; Qiao A.; Tao H.; Greaves N. G.; Richards T.; Lampronti G. I.; Redfern S. A. T.; Blanc F.; Farha O. K.; Hupp J. T.; Cheetham A. K.; Keen D. A. Melt-Quenched Glasses of Metal-Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 3484–3492. 10.1021/jacs.5b13220. [DOI] [PubMed] [Google Scholar]; b Bennett T. D.; Tan J. C.; Yue Y.; Baxter E.; Ducati C.; Terrill N. J.; Yeung H. H. M.; Zhou Z.; Chen W.; Henke S.; Cheetham A. K.; Greaves G. N. Hybrid Glasses from Strong and Fragile Metal-Organic Framework Liquids. Nat. Commun. 2015, 6, 8079. 10.1038/ncomms9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Yin Z.; Zhao Y.; Wan S.; Yang J.; Shi Z.; Peng S.-X.; Chen M.-Z.; Xie T.-Y.; Zeng T.-W.; Yamamuro O.; Nirei M.; Akiba H.; Zhang Y.-B.; Yu H.-B.; Zeng M.-H. Synergistic Stimulation of Metal–Organic Frameworks for Stable Super-cooled Liquid and Quenched Glass. J. Am. Chem. Soc. 2022, 144, 13021–13025. 10.1021/jacs.2c04532. [DOI] [PubMed] [Google Scholar]; b Liu M.; McGillicuddy R. D.; Vuong H.; Tao S.; Slavney A. H.; Gonzalez M. I.; Billinge S. J. L.; Mason J. A. Network-Forming Liquids from Metal-Bis(Acetamide) Frameworks with Low Melting Temperatures. J. Am. Chem. Soc. 2021, 143, 2801–2811. 10.1021/jacs.0c11718. [DOI] [PubMed] [Google Scholar]
- To T.; Sørensen S. S.; Stepniewska M.; Qiao A.; Jensen L. R.; Bauchy M.; Yue Y.; Smedskjaer M. M. Fracture Toughness of a Metal–Organic Framework Glass. Nat. Commun. 2020, 11, 2593. 10.1038/s41467-020-16382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Renélimbach R.; Longley L.; Shirzadi A. A.; Walmsley J. C.; Johnstone D. N.; Midgley P. A.; Wondraczek L.; Bennett T. D. Mechanical Properties and Processing Techniques of Metal-Organic Framework Glasses. J. Am. Chem. Soc. 2019, 141, 1027–1034. 10.1021/jacs.8b11357. [DOI] [PubMed] [Google Scholar]
- Frentzel-Beyme L.; Kloß M.; Pallach R.; Salamon S.; Moldenhauer H.; Landers J.; Wende H.; Debus J.; Henke S. Porous Purple Glass-a Cobalt Imidazolate Glass with Accessible Porosity from a Meltable Cobalt Imidazolate Framework. J. Mater. Chem. A 2019, 7, 985–990. 10.1039/C8TA08016J. [DOI] [Google Scholar]
- Zhou C.; Longley L.; Krajnc A.; Smales G. J.; Qiao A.; Erucar I.; Doherty C. M.; Thornton A. W.; Hill A. J.; Ashling C. W.; Qazvini O. T.; Lee S. J.; Chater P. A.; Terrill N. J.; Smith A. J.; Yue Y.; Mali G.; Keen D. A.; Telfer S. G.; Bennett T. D. Metal-Organic Framework Glasses with Permanent Accessible Porosity. Nat. Commun. 2018, 9, 5042. 10.1038/s41467-018-07532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horike S.; Ma N.; Fan Z.; Kosasang S.; Smedskjaer M. M. Mechanics, Ionics, and Optics of Metal-Organic Framework and Coordination Polymer Glasses. Nano Lett. 2021, 21, 6382–6390. 10.1021/acs.nanolett.1c01594. [DOI] [PubMed] [Google Scholar]
- Ogawa T.; Takahashi K.; Nagarkar S. S.; Ohara K.; Hong Y.-L.; Nishiyama Y.; Horike S. Coordination Polymer Glass from a Protic Ionic Liquid: Proton Conductivity and Mechanical Properties as an Electrolyte. Chem. Sci. 2020, 11, 5175–5181. 10.1039/D0SC01737J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R.; Li X.; Krajnc A.; Li Z.; Li M.; Wang W.; Zhuang L.; Smart S.; Zhu Z.; Appadoo D.; Harmer J. R.; Wang Z.; Buzanich A. G.; Beyer S.; Wang L.; Mali G.; Bennett T. D.; Chen V.; Hou J. Mechanochemically Synthesised Flexible Electrodes Based on Bimetallic Metal–Organic Framework Glasses for the Oxygen Evolution Reaction. Angew. Chem., Int. Ed. 2022, 61, e202112880 10.1002/anie.202112880. [DOI] [PubMed] [Google Scholar]
- Hou J.; Chen P.; Shukla A.; Krajnc A.; Wang T.; Li X.; Doasa R.; Tizei L. H. G.; Chan B.; Johnstone D. N.; Lin R.; Schülli T. U.; Martens I.; Appadoo D.; Ari M. S.; Wang Z.; Wei T.; Lo S. C.; Lu M.; Li S.; Namdas E. B.; Mali G.; Cheetham A. K.; Collins S. M.; Chen V.; Wang L.; Bennett T. D. Liquid-Phase Sintering of Lead Halide Perovskites and Metal-Organic Framework Glasses. Science 2021, 374, 621–625. 10.1126/science.abf4460. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Jin H.; Ma Q.; Mo K.; Mao H.; Feldhoff A.; Cao X.; Li Y.; Pan F.; Jiang Z.; Wang Y.; Pan F.; Jiang Z.; Jin H.; Ma Q.; Mo K.; Mao H.; Li Y.; Feldhoff A.; Cao X. A MOF Glass Membrane for Gas Separation. Angew. Chem., Int. Ed. 2020, 59, 4365–4369. 10.1002/anie.201915807. [DOI] [PubMed] [Google Scholar]
- a Yan J.; Gao C.; Qi S.; Jiang Z.; Jensen L. R.; Zhan H.; Zhang Y.; Yue Y. Encapsulation of Nano-Si into MOF Glass to Enhance Lithium-Ion Battery Anode Performances. Nano Energy 2022, 103, 107779 10.1016/j.nanoen.2022.107779. [DOI] [Google Scholar]; b Gao C.; Jiang Z.; Qi S.; Wang P.; Jensen L. R.; Johansen M.; Christensen C. K.; Zhang Y.; Ravnsbæk D. B.; Yue Y. Metal-Organic Framework Glass Anode with an Exceptional Cycling-Induced Capacity Enhancement for Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2110048 10.1002/adma.202110048. [DOI] [PubMed] [Google Scholar]
- Moghadam P. Z.; Li A.; Wiggin S. B.; Tao A.; Maloney A. G. P.; Wood P. A.; Ward S. C.; Fairen-Jimenez D. Development of a Cambridge Structural Database Subset: A Collection of Metal–Organic Frameworks for Past, Present, and Future. Chem. Mater. 2017, 29, 2618–2625. 10.1021/acs.chemmater.7b00441. [DOI] [Google Scholar]
- a Park K. S.; Ni Z.; Côté A. P.; Choi J. Y.; Huang R.; Uribe-Romo F. J.; Chae H. K.; O’Keeffe M.; Yaghi O. M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 10186–10191. 10.1073/pnas.0602439103. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Huang X. C.; Lin Y. Y.; Zhang J. P.; Chen X. M. Ligand-Directed Strategy for Zeolite-Type Metal-Organic Frameworks: Zinc(II) Imidazolates with Unusual Zeolitic Topologies. Angew. Chem., Int. Ed. 2006, 45, 1557–1559. 10.1002/anie.200503778. [DOI] [PubMed] [Google Scholar]; c Tian Y. Q.; Zhao Y. M.; Chen Z. X.; Zhang G. N.; Weng L. H.; Zhao D. Y. Design and Generation of Extended Zeolitic Metal-Organic Frameworks (ZMOFs): Synthesis and Crystal Structures of Zinc(II) Imidazolate Polymers with Zeolitic Topologies. Chem. – Eur. J. 2007, 13, 4146–4154. 10.1002/chem.200700181. [DOI] [PubMed] [Google Scholar]
- Eddaoudi M.; Sava D. F.; Eubank J. F.; Adil K.; Guillerm V. Zeolite-like Metal–Organic Frameworks (ZMOFs): Design, Synthesis, and Properties. Chem. Soc. Rev. 2014, 44, 228–249. 10.1039/C4CS00230J. [DOI] [PubMed] [Google Scholar]
- Frentzel-Beyme L.; Kloß M.; Kolodzeiski P.; Pallach R.; Henke S. Meltable Mixed-Linker Zeolitic Imidazolate Frameworks and Their Microporous Glasses: From Melting Point Engineering to Selective Hydrocarbon Sorption. J. Am. Chem. Soc. 2019, 141, 12362–12371. 10.1021/jacs.9b05558. [DOI] [PubMed] [Google Scholar]
- Qiao A.; Bennett T. D.; Tao H.; Krajnc A.; Mali G.; Doherty C. M.; Thornton A. W.; Mauro J. C.; Greaves G. N.; Yue Y. A Metal-Organic Framework with Ultrahigh Glass-Forming Ability. Sci. Adv. 2018, 4, eaao6827 10.1126/sciadv.aao6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Yu S.; Collins S. M.; Johnstone D. N.; Ashling C. W.; Sapnik A. F.; Chater P. A.; Keeble D. S.; McHugh L. N.; Midgley P. A.; Keen D. A.; Bennett T. D. A New Route to Porous Metal-Organic Framework Crystal-Glass Composites. Chem. Sci. 2020, 11, 9910–9918. 10.1039/D0SC04008H. [DOI] [Google Scholar]
- Chester A. M.; Castillo-Blas C.; Wondraczek L.; Keen D. A.; Bennett T. D. Frontispiece: Materials Formed by Combining Inorganic Glasses and Metal-Organic Frameworks. Chem. – Eur. J. 2022, 28, e202283861 10.1002/chem.202283861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J.; Ríos Gómez M. L.; Krajnc A.; McCaul A.; Li S.; Bumstead A. M.; Sapnik A. F.; Deng Z.; Lin R.; Chater P. A.; Keeble D. S.; Keen D. A.; Appadoo D.; Chan B.; Chen V.; Mali G.; Bennett T. D. Halogenated Metal-Organic Framework Glasses and Liquids. J. Am. Chem. Soc. 2020, 142, 3880–3890. 10.1021/jacs.9b11639. [DOI] [PubMed] [Google Scholar]
- Ali M. A.; Ren J.; Zhao T.; Liu X.; Hua Y.; Yue Y.; Qiu J. Broad Mid-Infrared Luminescence in a Metal-Organic Framework Glass. ACS Omega 2019, 4, 12081–12087. 10.1021/acsomega.9b01559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne M. F.; Gómez M. L. R.; Bumstead A. M.; Li S.; Bennett T. D. Mechanochemical Synthesis of Mixed Metal, Mixed Linker, Glass-Forming Metal-Organic Frameworks. Green Chem. 2020, 22, 2505–2512. 10.1039/D0GC00546K. [DOI] [Google Scholar]
- Bumstead A. M.; Laura M.; Gómez R.; Thorne M. F.; Sapnik A. F.; Longley L.; Tuffnell J. M.; Keeble D. S.; Keen D. A.; Bennett T. D. Investigating the melting behaviour of polymorphic zeolitic imidazolate frameworks. CrystEngComm 2020, 22, 3627–3637. 10.1039/D0CE00408A. [DOI] [Google Scholar]
- Bumstead A. M.; Thorne M. F.; Bennett T. D. Identifying the Liquid and Glassy States of Coordination Polymers and Metal-Organic Frameworks. Faraday Discuss. 2021, 225, 210–225. 10.1039/D0FD00011F. [DOI] [PubMed] [Google Scholar]
- Rettig S. J.; Storr A.; Summers D. A.; Thompson R. C.; Trotter J. Transition Metal Azolates from Metallocenes. 2. Synthesis, X-Ray Structure, and Magnetic Properties of a Three-Dimensional Polymetallic Iron(II) Imidazolate Complex, a Low-Temperature Weak Ferromagnet. J. Am. Chem. Soc. 1997, 119, 8675–8680. 10.1021/ja971558i. [DOI] [Google Scholar]
- López-Cabrelles J.; Miguel-Casañ E.; Esteve-Rochina M.; Andres-Garcia E.; Vitórica-Yrezábal I. J.; Calbo J.; Mínguez Espallargas G. Multivariate Sodalite Zeolitic Imidazolate Frameworks: A Direct Solvent-Free Synthesis. Chem. Sci. 2022, 13, 842–847. 10.1039/D1SC04779E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Cabrelles J.; Romero J.; Abellán G.; Giménez-Marqués M.; Palomino M.; Valencia S.; Rey F.; Mínguez Espallargas G. Solvent-Free Synthesis of ZIFs: A Route toward the Elusive Fe(II) Analogue of ZIF-8. J. Am. Chem. Soc. 2019, 141, 7173–7180. 10.1021/jacs.9b02686. [DOI] [PubMed] [Google Scholar]
- Blatov V. A.; Schevchenko A. P.; Proserpio D. M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. 10.1021/cg500498k. [DOI] [Google Scholar]
- Tian Y. Q.; Cai C. X.; Ren X. M.; Duan C. Y.; Xu Y.; Gao S.; You X. Z. The Silica-Like Extended Polymorphism of Cobalt(II) Imidazolate Three-Dimensional Frameworks: X-Ray Single-Crystal Structures and Magnetic Properties. Chem. – Eur. J. 2003, 9, 5673–5685. 10.1002/chem.200304957. [DOI] [PubMed] [Google Scholar]
- Lehnert R.; Seel F. Darstellung Und Kristallstruktur Des Mangan(II)- Und Zink(II)-Derivates Des Imidazols. J. Inorg. Gen. Chem. 1980, 464, 187–194. 10.1002/zaac.19804640117. [DOI] [Google Scholar]
- Banerjee R.; Phan A.; Wang B.; Knobler C.; Furukawa H.; O’Keeffe M.; Yaghi O. M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. 10.1126/science.1152516. [DOI] [PubMed] [Google Scholar]
- Lewis D. W.; Ruiz-Salvador A. R.; Gómez A.; Rodriguez-Albelo L. M.; Coudert F. X.; Slater B.; Cheetham A. K.; Mellot-Draznieks C. Zeolitic Imidazole Frameworks: Structural and Energetics Trends Compared with Their Zeolite Analogues. CrystEngComm 2009, 11, 2272–2276. 10.1039/b912997a. [DOI] [Google Scholar]
- Schröder C. A.; Baburin I. A.; Van Wüllen L.; Wiebcke M.; Leoni S. Subtle Polymorphism of Zinc Imidazolate Frameworks: Temperature-Dependent Ground States in the Energy Landscape Revealed by Experiment and Theory. CrystEngComm 2013, 15, 4036–4040. 10.1039/C2CE26045J. [DOI] [Google Scholar]
- Thorne M. F.; Castillo-Blas C.; McHugh L. N.; Bumstead A. M.; Robertson G.; Sapnik A. F.; Coates C. S.; Sayed F. N.; Grey C. P.; Keen D. A.; Etter M.; da Silva I.; Užarevič K.; Bennett T. D. Formation of New Crystalline Qtz-[Zn(MIm)2] Polymorph from Amorphous ZIF-8. Chem. Commun. 2022, 58, 11949–11952. 10.1039/D2CC04241J. [DOI] [PubMed] [Google Scholar]
- Madsen R. S. K.; Qiao A.; Sen J.; Hung I.; Chen K.; Gan Z.; Sen S.; Yue Y. Ultrahigh-Field 67Zn NMR Reveals Short-Range Disorder in Zeolitic Imidazolate Framework Glasses. Science 2020, 367, 1473–1476. 10.1126/science.aaz0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondraczek L.; Bouchbinder E.; Ehrlicher A.; Mauro J. C.; Sajzew R.; Smedskjaer M. M. Advancing the Mechanical Performance of Glasses: Perspectives and Challenges. Adv. Mater. 2022, 34, 2109029 10.1002/adma.202109029. [DOI] [PubMed] [Google Scholar]
- Sheldrick G. M. SHELXT – Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolomanov O. V.; Bourhis L. J.; Gildea R. J.; Howard J. A. K.; Puschmann H. J. Appl. Crystallogr. 2009, 42, 339–341. 10.1107/S0021889808042726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper A. K.GudrunN and GudrunX: Programs for Correcting Raw Neutron and X-ray Diffraction Data to Differential Scattering Cross Section, 2011, http://purl.org/net/epubs/work/56240 (accessed on September 29, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.