Abstract
Previous studies have shown that upregulation of the orphan steroid receptor Nur77 is required for the apoptosis of immature T cells in response to antigen receptor signals. Transcriptional upregulation of Nur77 in response to antigen receptor signaling involves two binding sites for the MEF2 family of transcription factors located in the Nur77 promoter. Calcium signals greatly increase the activity of MEF2D in T cells via a posttranslational mechanism. The mitogen-activated protein (MAP) kinase ERK5 was isolated in a yeast two-hybrid screen using the MADS-MEF2 domain of MEF2D as bait. ERK5 resembles the other MAP kinase family members in its N-terminal half, but it also contains a 400-amino-acid C-terminal domain of previously uncharacterized function. We report here that the C-terminal region of ERK5 contains a MEF2-interacting domain and, surprisingly, also a potent transcriptional activation domain. These domains are both required for coactivation of MEF2D by ERK5. The MEF2-ERK5 interaction was found to be activation dependent in vivo and inhibitable in vitro by the calcium-sensitive MEF2 repressor Cabin 1. The transcriptional activation domain of ERK5 is required for maximal MEF2 activity in response to calcium flux in T cells, and it can activate the endogenous Nur77 gene when constitutively recruited to the Nur77 promoter via MEF2 sites. These studies provide insights into a mechanism whereby MEF2 activity can respond to calcium signaling and suggest a novel, unexpected mechanism of MAP kinase function.
Apoptotic deletion of immature T cells receiving strong antigen receptor signals, termed negative selection, is an important homeostatic mechanism whereby potentially autoreactive and therefore dangerous T cells are deleted from the mature immune repertoire. Nur77, a member of the orphan steroid receptor superfamily (11, 21, 26), is transcriptionally upregulated rapidly in thymocytes and T-cell hybridomas after stimulation through the antigen receptor. Expression of Nur77 or its homologue Nor-1 is both necessary and sufficient for the efficient killing, via apoptosis, of immature T cells. This has been demonstrated both in tissue culture models (17, 34) and in studies with transgenic mice (4). It has been previously shown that two MEF2-binding elements in the region of the promoter of the transcription factor Nur77 from −307 to −242 are required for its upregulation in T-cell hybridomas during activation-induced cell death (35). Although MEF2 proteins are expressed in the nuclei of unstimulated T cells, MEF2 transcriptional activity is induced only after treatment with anti-T-cell receptor antibodies or phorbol ester (phorbol myristate acetate [PMA]) and calcium ionophore (ionomycin) (35).
In mammals, MEF2 (myocyte enhancer-binding factor) is a family of four transcription factors, MEF2A through -D, first identified as a muscle-specific DNA binding activity in the promoters of several muscle-specific genes (3, 8). The four family members were subsequently cloned and characterized by several groups (15, 19, 20, 25, 38). The MEF2 family all contain a MADS box (an acronyme for MCM1, agamous deficiens, and serum response factor) DNA binding domain, which binds to the consensus sequence CTA(A/T)4TAG (29). They also share a conserved sequence termed the MEF2 domain that is important for maximal DNA binding and homodimerization (22, 33). The MADS and MEF2 domains of the four family members are highly conserved, while their transcriptional activation domains, though all are of the proline-rich type, are far more divergent. The MEF2 family members vary in their tissue distributions and physiologic functions. MEF2A is transcribed in many tissues, but the protein is abundant only in skeletal muscle, heart, and brain tissue (1). Gel supershift analysis shows a minimal amount of MEF2A in the DO11.10 T-cell hybridoma (J. Woronicz, unpublished observation). MEF2C seems to play a crucial role in myogenesis (23, 24). MEF2C-deficient mice die during embryonic development, with defects in heart and vascular development (16). MEF2B and MEF2C are not detectable in T cells (Woronicz, unpublished), but MEF2C can be found in B cells (27, 31). MEF2D is ubiquitously expressed and seems to be involved in the expression of c-jun (10). It is the predominant MEF2 family member detectable in T cells (Woronicz, unpublished).
Recently, it has been demonstrated that the response of MEF2 to antigen receptor signaling in T cells can be partly explained by the release of the novel repressor protein Cabin 1 (30, 37). MEF2D, however, contains a weak transactivation domain. In T-cell hybridomas, a fusion of the MEF2D transactivation domain with the yeast Gal4 DNA binding domain exhibits minimal transcriptional activity, about 1,000-fold less than that of a similar construct using the herpes simplex virus (HSV) VP16 activation domain (see below) and only a 2- to 3-fold increase in activity after stimulation with PMA and ionomycin. Furthermore, overexpression of MEF2D alone at levels exceeding the amount of Cabin 1 in the cells activates MEF2 DNA elements very weakly (see below) and has no effect on Nur77 expression (data not shown). Therefore, either interaction with other molecules or posttranslational modification of the MEF2 family members is likely required for their activity. Indeed, the activity of MEF2A and MEF2C is increased via phosphorylation by the p38 mitogen-activated protein (MAP) kinase (9, 40). MEF2B and MEF2D, however, are not substrates for p38 (40). Studies done in our lab using tryptic phospopeptide mapping have revealed no change in the state of phosphorylation of MEF2D that correlates with an increase in its activity in T-cell hybridomas after stimulation (data not shown). We concluded that interaction with some type of coactivator was a likely reason for the large increase in MEF2 activity in response to antigen receptor signaling. To identify such a molecule, we performed a yeast two-hybrid screen using the DNA binding domain of MEF2D as bait. In addition to Cabin 1, we isolated the MAP kinase ERK5. This study details our elucidation of the functional relationship between ERK5 and MEF2D, leading to the novel, unexpected finding that the C-terminal portion of ERK5 contains a MEF2-interacting domain and a potent transcriptional activation domain.
MATERIALS AND METHODS
Plasmids.
Details of plasmid construction will be provided on request. MEF2 wild-type and mutant reporter constructs (RSRF luc 2wt and RSRF luc 2mut) were as described elsewhere (35). pGal4(5)-luc was a kind gift from Cathy Thut in Robert Tjian's lab. NBRE(3)-wt and mutant vectors were as described elsewhere (34). The mammalian expression constructs containing the GAL4, ERK5, or MEK5 sequence which were used for Fig. 1, 2, 3A, 3C, 3D, 4, and 5C were prepared in the cytomegalovirus promoter-driven expression vector pCI (Promega). All constructs used for Fig. 3B and all of the Gal4 fusion proteins used for Fig. 5A and B, as well as the Renilla reniformis luciferase-encoding vector pEFRL, were made in the EF-1α promoter-driven vector pEF BOS. This vector exhibits no change in activity in response to PMA and ionomycin stimulation in any of the cell lines used. The constructs encoding Gal4 fused to full-length MEF2D, amino acids 93 to 514 of MEF2D, or amino acids 412 to 490 of HSV VP16 were subcloned into pEF BOS using inserts from the corresponding constructs in pCG, which were kindly provided by Ronald Prywes. The expression vectors for constitutively active calcineurin, pBJ5 CNA and pBJ5 CNB, were the kind gift of Gerald Crabtree.
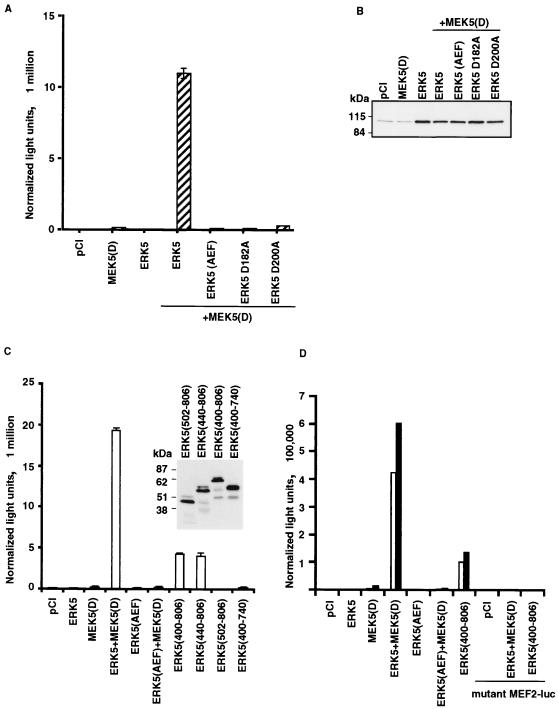
FIG. 1.
ERK5 stimulation of the MEF2-dependent reporter construct in three different cells lines. DO11.10 (A), NIH 3T3 (C), and S194 (D) cells were transfected with a MEF2-luc (35) and full-length wild-type, full-length kinase-deficient [ERK5(AEF), ERK5 D183A, and ERK5 D203A], or truncated ERK5. Activated MEK5 [MEK5(D)] was added where indicated. (D) Mutant MEF2-luc (35) was used to confirm MEF2 specificity. Open bars indicate activity in unstimulated cells. Hatched or filled bars indicate activity in cells stimulated with PMA and ionomycin. Expression of transfected ERK5 constructs in DO11.10 and NIH 3T3 cells was determined by Western blot analysis using anti-ERK5 (B) or anti-HA (C) antibodies.
FIG. 2.
Mapping of the ERK5 transcriptional activation domain. DO11.10 cells were transfected with either GAL4-dependent (GAL4-luc) or MEF2-dependent (MEF2-luc) reporter constructs. Luciferase activity was measured in unstimulated cells (shaded bars in panel A and open bars in panels C and D) or cells treated with PMA and ionomycin (open bars in panel A, filled bars in panel C, and hatched bars in panel D). (A) Activation of Gal4-luc by GAL4-ERK5 fusions. (B) Anti-GAL4 Western blot analysis on nuclear extracts from transfected DO11.10 cells. (C) Activation of MEF2-luc by MEK5(D) plus either full-length ERK5 or ERK5 lacking the C-terminal transactivation domain [ERK5(1-740)]. Expression of full-length and truncated ERK5 were demonstrated by Western blotting using anti-ERK5 antibody (inset). (D) Suppression of activation-dependent MEF2-luc activity by ERK5(1-740) compared to empty vector (pCI) or full-length ERK5.
FIG. 3.
Interaction of ERK5 with MEF2D. (A) HA epitope-tagged deletions of ERK5 were transfected into DO11.10 hybridomas. Cell lysates were incubated with either plain nickel-agarose beads (beads) or beads bearing bacterially expressed MEF2D(1-92) (His-MEF2D). Bead-bound proteins and whole-cell lysates were resolved by SDS-PAGE, blotted, and probed with anti-HA antibody. GST-Cabin 1 (2037-2220) or GST-Cabin 1 (2037-2179) proteins were added to the binding reactions as indicated. (B) Mammalian two-hybrid assay for activation-dependent MEF2-ERK5 interaction. Gal4-luc and either Gal4 DNA binding domain (positions 1 to 142) or Gal4(1-142) fused to the ERK5(400-739) [Gal4-ERK5ΔTAD] were transfected into DO11.10 cells with or without MEF2(1-92)–VP16. Ionomycin (iono) or ionomycin plus cyclosporine (iono/CsA) were added as indicated. The values shown for Gal4-VP16 are 10% of their actual light units. (C) Overexpression of both MEF2D and ERK5(400-806) in DO11.10. Neither molecule alone can overcome the requirement for PMA and ionomycin stimulation for maximal MEF2-luc activity. (D) DO11.10 cells were transfected with a MEF2-dependent reporter construct together with MEF2D, ERK5, and MEK5 expression constructs as indicated. Luciferase activity was measured in untreated cells and cells treated with PMA plus ionomycin (PMA/ionos).
FIG. 4.
Effect of ERK5, calcineurin, or MEF2 on expression of endogenous Nur77 family members in DO11.10 cells. (A) Either the wild-type or mutant NBRE-luc reporter construct (5) was transfected into DO11.10 cells along with MEF2, calcineurin, or ERK5 expression vectors as indicated. PMA and ionomycin were added to only one set of samples (+PMA iono), showing stimulus-dependent upregulation of Nur77 family members by endogenous factors. (B) Cells were transfected as described above, except a CD8 expression plasmid was used instead of an NBRE-luc plasmid to mark transfected cells, which were then magnetically isolated using anti-CD8 antibodies. Whole-cell extracts were made and Western blot analysis with anti-Nur77 monoclonal antibody was performed.
FIG. 5.
Dependence of ERK5 coactivation of MEF2D on amino acids 1 to 92 of MEF2D and amino acids 440 to 806 of ERK5. (A) Constructs containing fusions of either full-length MEF2D or amino acids 93 to 514 of MEF2D to the Gal4 DNA binding domain were transfected into DO11.10 cells together with a Gal4-dependent reporter construct and either empty vector, ERK5 (440-806), or a combination of full-length ERK5 and MEK5(D) expression constructs. Activity was measured in untreated cells or cells treated with PMA plus ionomycin (PMA/iono). (B) Transfection and measurement were performed as for panel A, except that a fusion of the HSV VP16 transcriptional activation domain to the Gal4 DNA binding domain was used. (C) DO11.10 cells were transfected with a MEF2-dependent reporter construct together with ERK5 and MEK5 expression constructs as indicated. Luciferase activity was measured in untreated cells and cells treated with PMA plus ionomycin (PMA/ionos).
Yeast two-hybrid screen.
Constructs used for the yeast two-hybrid screen, pAS1/CYH2, Y190, and the library described below, were the kind gift of Stephen J. Elledge. The yeast strain Y190 was transformed with pAS1/CYH2 encoding the first 92 amino acids of MEF2D. Expression of the Gal4(1-147)–MEF2D(1-92) fusion in the resulting transformants was verified by Western blotting. A single colony of the bait strain was then grown and transfected with a cDNA library from activated murine peripheral T cells in pACT. Approximately 2 × 106 transformants were screened for LacZ activity. Twenty putative positive clones were transferred to Escherichia coli and sequenced.
ERK5 and MEK5 cloning.
To isolate the full-length mouse ERK5, a murine thymus cDNA library in bacteriophage λZAP (Stratagene) was probed with the partial ERK5 cDNA recovered from the yeast two-hybrid screen using standard techniques. The longest clone recovered spanned the region from the codon for amino acid 109 of ERK5 to the 3′ end of the cDNA. The remainder of the cDNA was obtained by 5′ rapid amplification of cDNA ends using a commercial kit (Clontech). To isolate the mouse MEK5 clone, primers flanking the coding sequence of murine MEK5 were designed based on available mouse and rat sequence information and were used to amplify the MEK5 coding region from a murine spleen cDNA preparation (Clontech). An isoform nearly identical to the published rat MEK5α-1 sequence (7) was used to construct the MEK5 expression vector.
Transient transfections and reporter assay.
DO11.10 and S194 cells were transfected by the DEAE–dextran-chloroquine method. All points not containing any or all of the expression constructs in an experiment were normalized to the same DNA concentration using the corresponding empty vectors. In some cases, 16 h after transfection cells were treated with PMA (10 ng/ml), calcium ionophore A23187 (0.5 μM), cyclosporine (50 ng/ml), or the corresponding diluents for 4 h and then harvested for either reporter assay or Western blotting. NIH 3T3 cells were transfected using Lipofectamine Plus (Gibco/BRL). All transfections were performed in triplicate. Cells were harvested for assay 20 h after transfection. Luciferase reporter assays were done using the dual-luciferase reporter assay system (Promega) using an EF-1α promoter-driven vector encoding the R. reniformis luciferase gene (pEF RL) as an internal control for transfection efficiency. All depicted firefly luciferase activities were normalized to the mean activity of this construct within the experiment. Firefly luciferase activity values given for DO11.10 and S194 cells represent the activity of approximately 106 cells. Values given for NIH 3T3 cells are representative of the activity of approximately 105 cells.
In vitro MEF2-ERK5 interaction assay.
DO11.10 cells were transiently transfected with various hemagglutinin (HA) epitope-tagged ERK5 expression constructs. Eighteen hours after transfection the cells were treated for 1 h with PMA and ionomycin and then washed twice in Tris-buffered saline (pH 7.4) and lysed in 1 ml of 1% NP-40–25 mM Tris (pH 7.4)–150 mM NaCl–0.5% protease inhibitor cocktail (Sigma catalog no. P8340). After 15 min on ice, the lysates were cleared by centrifugation for 5 min at 14,000 × g and transferred to fresh tubes, to which was added 5 μl of Ni-nitrilotriacetic beads (Qiagen), either unconjugated or bearing ∼5 μg of six-His-tagged MEF2D(1-92) per μl (see below). The bead suspensions were tumbled at 4°C for 2.5 h, after which the beads were washed extensively in lysis buffer, taken up in Laemmli sample buffer, denatured, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) along with 10 μl of each of the lysates, blotted, and probed with anti-HA antibody. Competitor and noncompetitor Cabin 1 fragments, positions 2037 to 2220 and 2037 to 2179, respectively, were prepared in E. coli as glutathione S-transferase (GST) N-terminal fusions using the expression vector pGEX3X (Pharmacia). Cabin 1 fragments were eluted from glutathione-agarose beads using phosphate-buffered saline with 0.2% Triton X-100 and 50 mM reduced glutathione and were prepared at a concentration of approximately 100 μg of the full-length fragment per ml in elution buffer. In the competition experiment, 30, 90, or 300 μl of these preparations was added to the MEF2D-His beads together with 500 μl of lysis buffer containing 1% bovine serum albumin and was incubated at 4°C for 1 h prior to the addition of ERK5-containing cell lysates.
Antibodies and Western blots.
Monoclonal antibody to the influenza virus HA epitope was obtained from BabCo. Monoclonal antibody to the Gal4 DNA binding domain was obtained from Santa Cruz Biotechnology. Affinity-purified polyclonal antibodies to murine ERK5 were generated in rabbits by standard techniques using a bacterially expressed N-terminal fusion of GST to amino acids 399 to 804 of murine ERK5 as antigen. The murine monoclonal antibody to Nur77 was the kind gift of Jeffrey Millbrandt. Western blotting on whole-cell lysates was performed as follows. Approximately 3 × 106 transiently transfected cells were harvested and taken up in 75 μl of lysis buffer (1% NP-40, 50 mM Tris [pH 7.4], and 150 mM NaCl, with protease inhibitors). After 20 min of incubation on ice, the lysates were cleared by centrifugation at 14,000 × g for 5 min. The lysates were denatured in Laemmli sample buffer. A total of 50 μg of protein per lane was resolved by SDS-PAGE, transferred to nitrocellulose, and probed by standard techniques. Nuclear extracts for the detection of Gal4-ERK5 fusion proteins were prepared from 6 × 106 transfected cells as described previously (28). The extracts were precipitated with 10% trichloroacetic acid and taken up in Laemmli SDS sample buffer. Approximately 3 × 106 cell equivalents per lane were resolved by SDS-PAGE, blotted, and probed as described above. For the Nur77 expression Western blots, since the transfection efficiency by the DEAE-chloroquine method in DO11.10 cells was only ∼5%, it was necessary to isolate the transfected cells from the bulk population. Cells were transfected with ERK5, calcineurin, and/or MEF2 expression vectors as indicated below, plus murine CD8-alpha (pSV CD8). Sixteen hours after transfection, the cells were stained with fluorescein isothiocyanate-conjugated antibody to murine CD8-alpha (Caltag Laboratories), followed by magnetic beads coupled to anti-fluorescein isothiocyanate (Miltenyi Biotech). The CD8-positive cells were then isolated using the Auto-MACS (Miltenyi Biotech) magnetic cell sorting system. From 1 × 108 input cells, approximately 2 × 106 transfected cells were recovered. These were then lysed with radioimmunoprecipitation assay lysis buffer, resolved by SDS-PAGE, blotted, and probed with anti-Nur77.
Mutagenesis.
Mutagenesis of MEK5 and ERK5 was carried out using a PCR-based technique. The mutagenic oligonucleotide used for ERK5(AEF) generated the changes T219A and Y221F. For MEK5(D) the changes were S311D and T315D. ERK5 D182A and ERK5 D200A were made by PCR mutagenesis as for ERK5(AEF). The catalytic residue D182 and the Mg2+-chelating residue D200 were identified by sequence comparison to the published structural analysis of the highly homologous ERK2 (39) and correlated to structure-function analysis of the homologous kinase CAPK (32).
RESULTS
ERK5 is a coactivator of MEF2D.
As stated above, in our yeast two-hybrid screen of murine T-cell cDNA for MADS or MEF2 domain-interacting proteins, we isolated ERK5 as well as the repressor molecule Cabin 1. ERK5 has since been found by others to interact with MEF2D in vitro and in vivo (36). The N-terminal region of ERK5 is highly homologous to those of the other MAP kinase family members, spanning approximately 400 amino acids. However, ERK5 has a large, unique C-terminal domain not found in other MAP kinase family members. The function of this domain has not been previously characterized. To assess the functional significance of the ERK5-MEF2D interaction, we transfected full-length wild-type or mutant ERK5 into the T-cell hybridoma DO11.10 together with a MEF2-dependent reporter construct (35) (Fig. 1A and B). As shown previously (35), the activity of this reporter construct (MEF2-luc) increases approximately 100-fold after treatment with PMA and ionomycin. A further 50-fold increase is observed when wild-type ERK5 is cotransfected with a constitutively active mutant form of its activating kinase, MEK5(D) (12, 41). Addition of full-length ERK5 alone has no or a minimal effect. Addition of MEK5(D) alone typically produces a two- to three-fold increase in stimulated MEF2-luc activity. The effect of activated ERK5 on MEF2-dependent transcription requires ERK5 kinase activity. Mutants of ERK5 which lack the canonical MAP kinase activation motif TEY [ERK5(AEF)] have no catalytic residue (ERK5 D182A), or are unable to bind ATP (ERK5 D200A) have no effect on MEF2-luc activity, even in the presence of MEK5(D). A similar ERK5-dependent activation of the reporter construct MEF2-luc can be observed in the non-T-cell lines NIH 3T3 and S194 (Fig. 1C and D), but the activation is without dependence on PMA and ionomycin treatment. In all cell lines tested, ERK5 plus MEK5(D) had no effect on the activity of a mutant version of MEF2-luc lacking MEF2 sites (Fig. 1D). In conducting a deletional analysis of ERK5, we found that the ERK5 C-terminal region spanning amino acids 400 to 806 is by itself able to increase MEF2-luc activity (Fig. 1C). This suggests that the inactive ERK5 kinase domain may act as a negative regulator of its C-terminal region. Further deletion of the C-terminal portion of ERK5 revealed that residues 440 to 806 constitute the minimal fragment exhibiting the effect.
ERK5 contains a transcriptional activation domain.
The finding that the C-terminal moiety of ERK5 can act as a coactivator of MEF2 led us to ask if ERK5 contains a transcriptional activation domain. We therefore constructed vectors encoding fragments of ERK5 fused to the Gal4 DNA binding domain and assayed their effect on the activity of a Gal4-dependent reporter construct in DO11.10 cells (Fig. 2A and B). We found that the Gal4-ERK5(400-806) fusion stimulates a high level of transcription, similar to the activity of a Gal4-HSV VP16 fusion. This activity is independent of stimulation with PMA and/or ionomycin. Deletional analysis localizes the transcriptional activation domain to residues 664 to 789, a region rich in acidic residues. Interestingly, the fusion of Gal4 to full-length ERK5 requires MEK5(D) to stimulate transcription, suggesting again that the inactive kinase domain of ERK5 inhibits the transcriptional activity of its C-terminal portion. To assess the importance of the ERK5 transcriptional activation domain for the coactivation of MEF2, we constructed a truncated ERK5 [ERK5(1-740)] lacking the transcriptional activation domain, but including both the kinase domain and MEF2-interacting regions, and assayed its effect on MEF2-luc activity in DO11.10 cells (Fig. 2C). Unlike full-length ERK5, ERK5(1-740) cotransfected with MEK5(D) does not stimulate MEF2-luc activity in response to PMA and ionomycin treatment. To assess the effect of ERK5 without its transcriptional activation domain on the activation of MEF2-luc by endogenous factors, we transfected either full-length ERK5 or ERK5(1-740) into DO11.10 cells without MEK5(D) (Fig. 2D). Overexpression of ERK5(1-740) alone, but not of full-length ERK5, causes a fourfold reduction in the response of MEF2-luc to stimulation, suggesting that the ERK5 transcriptional activation domain is important for the activation of MEF2 family members in T cells.
Localization of MEF2D-interacting domain of ERK5 and activation dependence of ERK5-MEF2D interaction.
To map the MEF2D-interacting domain of ERK5, we transfected constructs encoding truncated versions of the ERK5 C-terminal region into DO11.10 cells and tested their ability to interact with bacterially expressed MEF2D (Fig. 3A). N-terminal deletion of ERK5 to residue 440 yields a molecule capable of interacting with MEF2D. C-terminal deletion to residue 501 does not affect MEF2 binding activity, leading us to conclude that the MEF2-interacting domain is located between ERK5 amino acids 440 and 501, a region containing a proline-rich tract. The localization of the MEF2-interacting domain and the transactivation domain of ERK5 (Fig. 2A) correlates well with the deletional analysis done in NIH 3T3 cells using MEF2-luc (Fig. 1C), suggesting that both of these regions are required for the ability of ERK5 to coactivate MEF2-dependent transcription. We also show that addition to the binding reactions of a fragment of Cabin 1 (positions 2037 to 2220) which has been shown to interact with MEF2D (37), but not a fragment (2037-2179) which lacks its MEF2-interacting region, can specifically block the interaction of ERK5 with MEF2D(1-92) (Fig. 3A). This suggests that the interaction of activated endogenous ERK5 with MEF2D in T cells would first require the release of Cabin 1 from MEF2D in response to calcium flux. We assessed the activation dependence of the ERK5-MEF2 interaction in DO11.10 cells using a mammalian two-hybrid assay. A fusion of the Gal4 DNA binding domain to a region of ERK5 containing the MEF2-interacting region but lacking the transcriptional activation domain (Gal4-ERK5ΔTAD) was transfected together with a fusion of the MEF2D DNA binding domain to the HSV VP16 transcriptional activation domain. Both constructs were transfected at low levels in order to avoid exceeding the amount of Cabin 1 available to repress the interaction under basal conditions. We found that the Gal4-luc reporter gene exhibited a 20-fold response to ionomycin stimulation when cotransfected with Gal4-ERK5ΔTAD plus MEF2D-VP16 (Fig. 3B) but exhibited only a 2-fold response when cotransfected with a Gal4-VP16 fusion, suggesting that the ERK5-MEF2 interaction is indeed activation dependent. Recruitment of endogenous MEF2D and ERK5 probably accounts for the small activation seen with the Gal4-ERK5ΔTAD bait alone. Cyclosporine is able to inhibit the ionomycin-dependent stimulation of Gal4-luc activity, suggesting that calcineurin may play a role in facilitating the ERK5-MEF2 interaction. Interestingly, Cabin 1 was isolated as a calcineurin-binding protein (30); thus, calcineurin may play an as-yet-undefined role in releasing Cabin 1 or some other repressors from MEF2.
Our findings suggest a model in which ERK5 coactivation of MEF2 is regulated at two levels in T cells: (i) the activation and nuclear localization of ERK5 in response to epidermal growth factor (EGF) (13) or some as-yet-undefined signal in immune cells and (ii) the release of the blocking Cabin 1 molecule in response to calcium flux. A corollary of this model is that if one were to cotransfect both MEF2D and ERK5 which is either activated or lacking its inhibitory kinase domain at a sufficiently high level, it should be possible to titrate any MEF2-bound repressor away from the promoter and thus eliminate the signal dependence of transcriptional activation. When we performed this experiment in DO11.10 cells (Fig. 3C), this is what we observed. Overexpression of MEF2D alone has a minimal effect. The ERK5 C terminus alone produces a more substantial increase in activity which is still stimulus dependent. Cotransfection of both molecules, however, results in a basal level of activity which is greater than the PMA- and ionomycin-stimulated activity of the reporter alone and which increases less than twofold after treatment. The same effect could be observed if we used MEF2D(1-92) instead of the full-length MEF2D construct. Cotransfection of MEF2D(1-92) and ERK5 C-terminal constructs reconstitutes the MEF2 reporter construct (Nur77 promoter) in the absence of PMA and ionomycin (Fig. 3D). Its activity is similar to reconstitution of the Nur77 promoter by the full-length MEF2D and activated ERK5 constructs (Fig. 3D), suggesting that the MEF2 region from amino acids 1 to 92 is the crucial domain mediating coactivation by ERK5 in our system.
The ERK5 transcriptional activation domain can activate the endogenous Nur77 promoter.
Finally, we wished to extend our model to the endogenous Nur77 promoter. We constructed a fusion of the MEF2D DNA binding domain to the C terminus of ERK5 to determine if constitutive recruitment of the ERK5 C terminus to the Nur77 promoter was sufficient to drive Nur77 expression. We assayed the expression of Nur77 (or other Nur77 family members) using both a Nur77 family-dependent reporter (NBRE-luc) and Western blot analysis of the transfected cells with an antibody to Nur77. We found that constitutive recruitment of the ERK5 C terminus was, in fact, by itself capable of driving both NBRE-luc activity (Fig. 4A, compare fifth and first columns) and Nur77 expression (Fig. 4B). Expression of activated full-length ERK5 plus MEF2D or constitutively activated calcineurin exhibits a moderate effect on NBRE-luc (Fig. 4A, compare fourth and sixth columns). Expression of either the MEF2D(1-92)–ERK5 fusion or its individual components together with constitutively active calcineurin (6) results in a much stronger stimulation of NBRE-luc activity, equaling or exceeding that observed with PMA and ionomycin treatment (Fig. 4A, compare seventh and eighth columns with second column). This stimulation is also observed when activated calcineurin is coexpressed with full-length ERK5, MEK5(D), and full-length MEF2D (Fig. 4A, ninth column). An NBRE-luc construct containing mutated Nur77 binding sites was not affected by any of the transfected molecules. The synergy between calcineurin and ERK5 in the activation of the Nur77 gene may indicate a novel role of calcineurin in enhancing ERK5 coactivation of MEF2. Alternatively, it may indicate synergy between ERK5 and NFAT1c, which has recently been shown to interact with the C-terminal portion of MEF2D (2).
Recently, another group reported phosphorylation of MEF2D at serine 179 by ERK5 in vitro and in serum-stimulated HeLa cells (14). Introduction of the MEF2D mutant S179A into HeLa cells inhibits EGF-induced c-jun promoter activity, suggesting that ERK5 phosphorylation of MEF2D is important in MEF2-mediated serum induction of the c-jun promoter. However, this is contrary to a previously published report, which found the first 92 amino acids and not the C-terminal region of MEF2D to be responsible for its serum regulation (10). To assess the importance of MEF2D C-terminal phosphorylation in our system, we transfected the Gal4-dependent reporter plasmid along with a construct encoding the Gal4 DNA binding domain fused to the full-length MEF2D (Gal4-MEF2D) or the MEF2D C-terminal region [Gal4-MEF2D(93-514)]. Cotransfection of either the ERK5 C-terminal region [ERK5(440-806)] or full-length ERK5 and MEK5(D) led to a dramatic increase in Gal4-MEF2D activity (Fig. 5A). In contrast, activated ERK5 had a negligible effect on the activity of the Gal4-MEF2D(93-514) construct (Fig. 5A). As an additional control of specificity, we used a Gal4-VP16 construct, which encodes a fusion protein of the Gal4 DNA binding domain and the HSV VP16 transcriptional activation domain. Neither the ERK5 C terminus nor a combination of ERK5 and MEK5(D) had any appreciable effect on the activity of Gal4-VP16 (Fig. 5B). Finally, we found that expression of various ERK5 constructs encoding the intact MAP kinase domain but without the C-terminal transactivation domain [ERK5(1-640) or ERK5(1-430)] had no effect on the MEF2 transcriptional activity (Fig. 5C). We conclude that in our system, MEF2D is predominantly regulated by the ERK5 C-terminal region, which contains a MEF2-interacting region and a powerful transcriptional activation domain.
DISCUSSION
The results described in this study have defined a novel class of signaling molecule, the kinase-coactivator ERK5. This molecule can coactivate MEF2 family transcription factors by providing a strong transcriptional activation domain, the availability of which can be regulated at the levels both of kinase activity and of accessibility of its docking site on MEF2D. The inactivity of the D182A mutant, which has no catalytic residue but should still undergo the conformational changes associated with MAP kinase activation, suggests that autophosphorylation is somehow involved in making the C-terminal coactivator moiety available to interact with MEF2. Elucidation of this mechanism of autoregulation is an active area of investigation in our laboratory.
Our experiments and published data suggest a possible model of MEF2D regulation. In unstimulated T cells, MEF2D is bound with the repressor Cabin 1. An increased calcium level in response to T-cell receptor stimulation leads to a calcium-calmodulin complex which enters the nucleus, binds to Cabin 1, and displaces it from MEF2D. If ERK5 is active in the cells, possibly as the result either of signaling by EGF, some other growth factor or cytokine, or antigen, it also can enter the nucleus and associate with the now-unblocked MEF2D, bringing its potent transcriptional activation domain to the Nur77 promoter. Indeed, ERK5 has been localized to the nucleus (12). In other physiologic contexts (13), ERK5 might also increase MEF2 activity by phosphorylating its transcriptional activation domain. Kato et al. (14) found that phosphorylation of the transcriptional activation domain of MEF2D at serine 179 increases its activity in HeLa cells. We did not observe this effect in our system. This might be because phosphorylation at serine 179 was constitutive in our system, as we did not serum starve the cells. Indeed, a low level of Gal4-MEF2D(93-514) activity is detectable in unstimulated DO11.10 cells. In vivo analysis of MEF2D phosphorylation in DO11.10 cells also showed that MEF2D is extensively phosphorylated under basal conditions, although no phosphorylated peptides correlate with its antigen receptor stimulus-dependent activities (data not shown).
In addition to our own findings and the recent data on Cabin 1, two new calcium-dependent pathways have been identified which regulate the activity of MEF2. Interestingly, they both work via association of coactivators and corepressors. It has recently been reported that the corepressors histone deacetylases 4 and 5 associate constitutively with MEF2D (18). Calcium flux causes them to dissociate via a poorly characterized calcium-calmodulin-dependent protein kinase IV-mediated mechanism. Other recent work has shown that NFAT1c, a well-studied calcium-dependent transcriptional activator in T cells, associates with MEF2D in a calcium-dependent manner (2). This might partly explain the role calcineurin seems to play in MEF2 upregulation by calcium.
This wealth of coactivators and corepressors of MEF2D, acting in a seemingly redundant fashion, may serve a number of purposes. It may be that since Nur77 is such a dangerous gene, it is necessary to regulate it very tightly. Thus, multiple levels of repression and coactivation are applied to MEF2D to increase the gain of signaling so that Nur77 is either completely on or completely off. A rapid response may also necessitate the existence of multiple regulatory molecules acting in concert. If Nur77 is to be upregulated with immediate-early kinetics, there is no time to make multiple transcription factors de novo to bind to the promoter or alter the chromatin structure of the Nur77 locus. In the depicted model of the pathway, everything is regulated posttranslationally via just two DNA binding sites to which the relevant factor is already bound. It may also be the case that these different coactivators and corepressors mediate signal-dependent regulation of MEF2 activity in different physiologic contexts. MEF2 family members seem to have roles in a wide variety of processes, and the broad distribution of ERK5 expression would make it available to act as a coactivator in many of these areas. Clearly, a great deal of further experimentation, in both tissue culture and transgenic mouse models, will be required to sort out these possibilities.
ACKNOWLEDGMENTS
We thank members of the Winoto lab for helpful discussion and Sue Sohn, Bill Sha, Andrea DeYoung, and Arvind Rajpal for critical reading of the manuscript. We also thank Ron Prywes and Eric Olson for the MEF2D constructs, Steven Elledge for the mouse peripheral T-cell cDNA library, Jeff Milbrandt for the Nur77 monoclonal antibody, Gerald Crabtree for the calcineurin constructs, and J. Liu for the Cabin 1 cDNA.
This work is supported by a grant from the National Institutes of Health (CA66236). A.W. is a National Science Foundation Presidential Faculty Fellow.
REFERENCES
- 1.Black B L, Lu J, Olson E N. The MEF2A 3′ untranslated region functions as a cis-acting translational repressor. Mol Cell Biol. 1997;17:2756–2763. doi: 10.1128/mcb.17.5.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaeser F, Ho N, Prywes R, Chatila T A. Ca(2+)-dependent gene expression mediated by MEF2 transcription factors. J Biol Chem. 2000;275:197–209. doi: 10.1074/jbc.275.1.197. [DOI] [PubMed] [Google Scholar]
- 3.Braun T, Tannich E, Buschhausen-Denker G, Arnold H-H. Promoter upstream elements of the chicken cardiac myosin light-chain 2-A gene interact with trans-acting regulatory factors for muscle-specific transcription. Mol Cell Biol. 1989;9:2513–2525. doi: 10.1128/mcb.9.6.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calnan B, Szychowski S, Chan F K M, Cado D, Winoto A. A role of the orphan steroid receptor Nur77 in antigen-induced negative selection. Immunity. 1995;3:273–282. doi: 10.1016/1074-7613(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L E, Chan F K, Cado D, Winoto A. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T cell apoptosis. EMBO J. 1997;16:1865–1875. doi: 10.1093/emboj/16.8.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clipstone N A, Crabtree G R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 7.English J M, Vanderbilt C A, Xu S C, Marcus S, Cobb M H. Isolation of Mek5 and differential expression of alternatively spliced forms. J Biol Chem. 1995;270:28897–28902. doi: 10.1074/jbc.270.48.28897. [DOI] [PubMed] [Google Scholar]
- 8.Gossett L A, Kelvin D J, Sternberg E A, Olson E N. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989;9:5022–5033. doi: 10.1128/mcb.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han J, Jiang Y, Li Z, Kravchenko V V, Ulevitch R J. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296–299. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- 10.Han T-H, Prywes R. Regulatory role of MEF2D in serum induction of the c-jun promoter. Mol Cell Biol. 1995;15:2907–2915. doi: 10.1128/mcb.15.6.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazel T G, Nathans D, Lau L F. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc Natl Acad Sci USA. 1988;85:8444–8448. doi: 10.1073/pnas.85.22.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato Y, Kravchenko V V, Tapping R I, Han J, Ulevitch R J, Lee J-D. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16:7054–7066. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato Y, Tapping R I, Huang S, Watson M H, Ulevitch R J, Lee J D. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–716. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- 14.Kato Y, Zhao M, Morikawa A, Sugiyama T, Chakravortty D, Koide N, Yoshida T, Tapping R I, Yang Y, Yokochi T, Lee J D. Big mitogen-activated kinase regulates multiple members of the MEF2 protein family. J Biol Chem. 2000;275:18534–18540. doi: 10.1074/jbc.M001573200. [DOI] [PubMed] [Google Scholar]
- 15.Leifer D, Krainc D, Yu Y T, McDermott J, Breitbart R E, Heng J, Neve R L, Kosofsky B, Nadal-Ginard B, Lipton S A. MEF2C, a MADS/MEF2-family transcription factor expressed in a laminar distribution in cerebral cortex. Proc Natl Acad Sci USA. 1993;90:1546–1550. doi: 10.1073/pnas.90.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Q, Lu J R, Yanagisawa H, Webb R, Lyons G E, Richardson J A, Olson E N. Requirement of the MADS-box transcription factor MEF2C for vascular development. Development. 1998;125:4565–4574. doi: 10.1242/dev.125.22.4565. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z-G, Smith S W, McLaughlin K A, Schwartz L M, Osborne B. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature. 1994;367:281–284. doi: 10.1038/367281a0. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, McKinsey T A, Nicol R L, Olson E N. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc Natl Acad Sci USA. 2000;97:4070–4075. doi: 10.1073/pnas.080064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin J F, Miano J M, Hustad C M, Copeland N G, Jenkins N A, Olson E N. A Mef2 gene that generates a muscle-specific isoform via alternative mRNA splicing. Mol Cell Biol. 1994;14:1647–1656. doi: 10.1128/mcb.14.3.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin J F, Schwarz J J, Olson E N. Myocyte enhancer factor (MEF) 2C: a tissue-restricted member of the MEF-2 family of transcription factors. Proc Natl Acad Sci USA. 1993;90:5282–5286. doi: 10.1073/pnas.90.11.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988;1:183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- 22.Molkentin J D, Black B L, Martin J F, Olson E N. Mutational analysis of the DNA binding, dimerization, and transcriptional activation domains of MEF2C. Mol Cell Biol. 1996;16:2627–2636. doi: 10.1128/mcb.16.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molkentin J D, Olson E N. Combinatorial control of muscle development by basic helix-loop-helix and MADS-box transcription factors. Proc Natl Acad Sci USA. 1996;93:9366–9373. doi: 10.1073/pnas.93.18.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson E N, Perry M, Schulz R A. Regulation of muscle differentiation by the MEF2 family of MADS box transcription factors. Dev Biol. 1995;172:2–14. doi: 10.1006/dbio.1995.0002. [DOI] [PubMed] [Google Scholar]
- 25.Pollock R, Treisman R. Human SRF-related proteins: DNA-binding properties and potential regulatory targets. Genes Dev. 1991;5:2327–2341. doi: 10.1101/gad.5.12a.2327. [DOI] [PubMed] [Google Scholar]
- 26.Ryseck R P, Macdonald B H, Mattei M G, Ruppert S, Bravo R. Structure, mapping and expression of a growth factor inducible gene encoding a putative nuclear hormonal binding receptor. EMBO J. 1989;8:3327–3335. doi: 10.1002/j.1460-2075.1989.tb08494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satyaraj E, Storb U. Mef2 proteins, required for muscle differentiation, bind an essential site in the Ig lambda enhancer. J Immunol. 1998;161:4795–4802. [PubMed] [Google Scholar]
- 28.Schreiber E, Matthias P, Muller M M, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shore P, Sharrocks A D. The MADS-box family of transcription factors. Eur J Biochem. 1995;229:1–13. doi: 10.1111/j.1432-1033.1995.tb20430.x. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, Youn H-D, Loh C, Stolow M, He W, Liu J O. Cabin 1, a negative regulator for calcineurin signaling in T lymphocytes. Immunity. 1998;8:703–711. doi: 10.1016/s1074-7613(00)80575-0. [DOI] [PubMed] [Google Scholar]
- 31.Swanson B J, Jack H-M, Lyons G E. Characterization of myocyte enhancer factor 2 (MEF2) expression in B and T cells: MEF2C is a B-cell-restricted transcription factor in lymphocytes. Mol Immunol. 1998;35:445–458. doi: 10.1016/s0161-5890(98)00058-3. [DOI] [PubMed] [Google Scholar]
- 32.Taylor S S, Sowadski J M, Knighton D R, Zheng J, Gibbs C S, Zoller M J. A template for the protein kinase family. Trends Biochem Sci. 1993;18:84–89. doi: 10.1016/0968-0004(93)80001-r. [DOI] [PubMed] [Google Scholar]
- 33.West A G, Shore P, Sharrocks A D. DNA binding by MADS-box transcription factors: a molecular mechanism for differential DNA bending. Mol Cell Biol. 1997;17:2876–2887. doi: 10.1128/mcb.17.5.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woronicz J D, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- 35.Woronicz J D, Lina A, Calnan B J, Szychowski S, Cheng L, Winoto A. Regulation of the Nur77 orphan steroid receptor in activation-induced apoptosis. Mol Cell Biol. 1995;15:6364–6376. doi: 10.1128/mcb.15.11.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang C, Ornatsky O I, McDermott C J, Cruz F T, Prody C A. Interaction of myocyte enhancer factor (MEF2) with a mitogen-activated protein kinase, ERK5/BMK1. Nucleic Acids Res. 1998;26:4771–4777. doi: 10.1093/nar/26.20.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youn H-D, Sun L, Prywes R, Liu J O. Apoptosis of T cells mediated by Ca2+-induced release of the transcription factor MEF2. Science. 1999;286:790–793. doi: 10.1126/science.286.5440.790. [DOI] [PubMed] [Google Scholar]
- 38.Yu Y-T, Breitbart R E, Smoot L B, Lee Y, Mahdavi V, Nadal-Ginard B. Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev. 1992;6:1783–1798. doi: 10.1101/gad.6.9.1783. [DOI] [PubMed] [Google Scholar]
- 39.Zhang F, Strand A, Robbins D, Cobb M H, Goldsmith E J. Atomic structure of the MAP kinase ERK2 at 2.3 A resolution. Nature. 1994;367:704–711. doi: 10.1038/367704a0. [DOI] [PubMed] [Google Scholar]
- 40.Zhao M, New L, Kravchenko V V, Kato Y, Gram H, di Padova F, Olson E N, Ulevitch R J, Han J. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999;19:21–30. doi: 10.1128/mcb.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou G, Bao A, Guan K, Dixon J E. Specifical interactions between newly identified human kinases, MEK5 and ERK5. FASEB J. 1995;9:A1306. [Google Scholar]