Abstract
Background
Circulatory failure is classified into four types of shock (obstructive, cardiogenic, distributive, and hypovolemic) that must be distinguished as each requires a different treatment. Point-of-care ultrasound (POCUS) is widely used in clinical practice for acute conditions, and several diagnostic protocols using POCUS for shock have been developed. This study aimed to evaluate the diagnostic accuracy of POCUS in identifying the etiology of shock.
Methods
We conducted a systematic literature search of MEDLINE, Cochrane Central Register of Controlled Trials, Embase, Web of Science, Clinicaltrial.gov, European Union Clinical Trials Register, WHO International Clinical Trials Registry Platform, and University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) until June 15, 2022. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and assessed study quality using the Quality Assessment of Diagnostic Accuracy Studies 2 tool. Meta-analysis was conducted to pool the diagnostic accuracy of POCUS for each type of shock. The study protocol was prospectively registered in UMIN-CTR (UMIN 000048025).
Results
Of the 1553 studies identified, 36 studies were full-text reviewed, and 12 studies with 1132 patients were included in the meta-analysis. Pooled sensitivity and specificity were 0.82 [95% confidence interval (CI) 0.68–0.91] and 0.98 [95% CI 0.92–0.99] for obstructive shock, 0.78 [95% CI 0.56–0.91] and 0.96 [95% CI 0.92–0.98] for cardiogenic shock, 0.90 [95% CI 0.84–0.94] and 0.92 [95% CI 0.88–0.95] for hypovolemic shock, and 0.79 [95% CI 0.71–0.85] and 0.96 [95% CI 0.91–0.98] for distributive shock, respectively. The area under the receiver operating characteristic curve for each type of shock was approximately 0.95. The positive likelihood ratios for each type of shock were all greater than 10, especially 40 [95% CI 11–105] for obstructive shock. The negative likelihood ratio for each type of shock was approximately 0.2.
Conclusions
The identification of the etiology for each type of shock using POCUS was characterized by high sensitivity and positive likelihood ratios, especially for obstructive shock.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-023-04495-6.
Keywords: Circulatory failure, Shock, Point-of-care ultrasound, Diagnostic accuracy, Obstructive shock, Early diagnosis, Systematic review
Background
Circulatory failure is a syndrome that should be diagnosed and treated early [1–3]. It is classified into four types of shock: obstructive, cardiogenic, distributive, and hypovolemic, each of which must be treated differently [1]. Therefore, when encountering circulatory failure, it is important to differentiate the type of shock the patient is experiencing. Clinically, shock is differentiated using all available information, including medical history, blood tests, and various imaging studies. Of these, performing an ultrasound, in particular, has the potential to directly delineate and identify the etiology of shock [4–9]. Furthermore, sometimes multiple shocks can overlap, making diagnosis difficult [1]. Therefore, ultrasonography, which allows direct and rapid observation of the pathophysiology with images [7], may be crucial for the management of shock.
The rapid bedside diagnosis of the etiology of an acute condition using ultrasonography is called point-of-care ultrasound (POCUS) [7] and has attracted considerable attention in recent years [10–13]. Several diagnostic protocols have been proposed for POCUS for shock [14–19]. In the standard cardiac view (parasternal long- and short-axis, apical four-chamber, and subcostal four-chamber), qualitative assessment of left and right ventricle size and contractile function, and physiologic assessment of pericardial fluid and tamponade were common to all protocols [14–19]. Their common feature was early bedside goal-directed diagnosis by ultrasound. A systematic review of the diagnostic accuracy of POCUS for shock was reported in 2019 [20]. However, the study was limited to a meta-analysis of only four small observational emergency room studies. Moreover, despite the rapid increase in literature dealing with POCUS in emergency and intensive care settings [10], to the best of our knowledge, no systematic review summarizing the diagnostic accuracy for each type of shock has been reported. Furthermore, although the POCUS protocols for shock have described the findings and rough differentiation steps for shock [20, 21], the specific order and site of ultrasound examination have not been clearly established. These factors should be considered when considering the differences in the diagnostic accuracy for each type of shock.
Therefore, to address these uncertainties, we conducted a systematic review and meta-analysis to assess the accuracy of POCUS for the diagnosis of shock among adult patients with circulatory failure.
Methods
In this study, we adhered to the Cochrane Handbook for Diagnostic Test accuracy [22] and reported according to the Preferred Reporting Items for Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA) guidelines [23, 24]. The study protocol is registered at University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) (UMIN 000048025). In this study, we defined POCUS with echocardiography, which was immediately performed in shock patients for diagnosis of the cause of circulatory failure, as the index test. There were no restrictions regarding where POCUS was conducted. As there is no specific diagnostic method for differentiating the cause of shock [1], we defined the clinical diagnosis based on the medical information available within each study as the reference standard. The location and timing of clinical diagnosis needed to be different from any of those in which POCUS was performed. The target condition of interest was circulatory failure with no identified etiology, and the definition of circulatory failure was based on the definition in each study.
Data sources and searches
A computerized search of the electronic databases of MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, Web of Science, Clinicaltrials.gov, European Union Clinical Trials Register (EU-CTR), WHO International Clinical Trials Registry Platform (ICTRP), and UMIN-CTR was performed from inception of the databases to June 15, 2022. Moreover, we manually searched the reference lists of the relevant articles. Searches involved a combination of free-text words and MeSH (Medical Subject Headings) terms using permutations of the search terms “intensive care,” “critical illness,” “emergencies,” “point of care,” “focus,” “ultrasound,” “echocardiography,” “shock,” “hypotension,” and “circulatory failure” (Additional file 1: Table S1). Methodological search filters were avoided. The results from all languages were included. Furthermore, we also included abstracts presented at national and international conferences if they were published in journal supplements after the conference.
Study selection
We included prospective and retrospective observational studies, and secondary analyses of randomized controlled trial data reporting the diagnostic accuracy of POCUS for the diagnosis of etiology in adult patients (≥ 18 years old) with undifferentiated shock. There were no limitations on the language or publication date for this review. Moreover, we excluded diagnostic case–control studies (two-gate studies) and case studies that lacked DTA data, namely true-positive, false-positive, true-negative, and false-negative values. Two reviewers independently screened the titles and abstracts of all eligible studies to identify candidates for full-text review. The articles selected for full-text review were then independently reviewed to identify those appropriate for inclusion. Disagreements between the reviewers were resolved through discussion or by a third reviewer. If multiple published studies were identified based on the same database, the most recent and complete studies were included in the analysis.
Data extraction and quality assessment
Two authors independently extracted data and assessed study quality and applicability using the QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies 2) tool [25], which includes four risk-of-bias domains (patient selection, index test, reference test, and flow and timing) and three domains of applicability (patient selection, index test, and reference test). Disagreements between the reviewers were resolved through discussion and consensus. The following data were extracted using a pre-defined data extraction form: study characteristics (author, year of publication, country, design, sample size, clinical settings, conflict of interest, and funding source), patient characteristics (inclusion/exclusion criteria and patient clinical and demographic characteristics), index test (timing of diagnosis, protocol of ultrasound, and the person who conducted the test), reference standard (timing of diagnosis, information referred for diagnosis, and the person who conducted the diagnosis), and diagnostic accuracy parameters (the rates of true-positive, true-negative, false-positive, and false-negative results for each pair of index tests and target conditions). If the original manuscript did not contain sufficient relevant data on diagnostic accuracy, we contacted the authors of the paper to request additional data or to incorporate any available data from previous systematic reviews into the analysis. All data were extracted independently and in duplicate, and any differences between the reviewers were resolved by consensus.
Data synthesis and analysis
For each shock category, we calculated the sensitivity and specificity of the index individual studies with corresponding 95% confidence intervals (CIs) and plotted them on forest plots to assess heterogeneity. Synthesis analyses were performed using Reitsma’s bivariate random-effects model [26] for study-specific sensitivities, specificities, positive likelihood ratios, and negative likelihood ratios, considering possible heterogeneities across the studies. We evaluated summary estimates, and calculated their inconsistencies (I2), which described the percentage of total variation across studies due to heterogeneity rather than chance. To visually evaluate the variability in the diagnostic accuracy of POCUS for each shock, we created summary receiver operating characteristic (SROC) curves for each shock [27] based on the estimates of the bivariate random effects model and presented the areas under the curves (AUCs) of the SROC curves as summary measures of the predictive accuracy measures [28]. For statistical inferences, we used the standard restricted maximum likelihood estimation for the Reitsma’s model, and the bootstrap method to calculate the 95% CIs of the AUCs of the SROC curves. In addition, we performed subgroup analyses for each shock based on the following variables that were assumed to influence diagnostic accuracy estimates: suspected disease before POCUS, presence of ultrasound other than echocardiography, settings in which ultrasound was performed, and a training program for point-of-care ultrasound. We also performed sensitivity analysis after excluding studies with a high risk of bias. Publication or reporting bias was not assessed because there is no accepted method that can be used for its evaluation in a meta-analysis of diagnostic test accuracy studies [29–31]. All statistical analyses were performed using R version 4.1.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
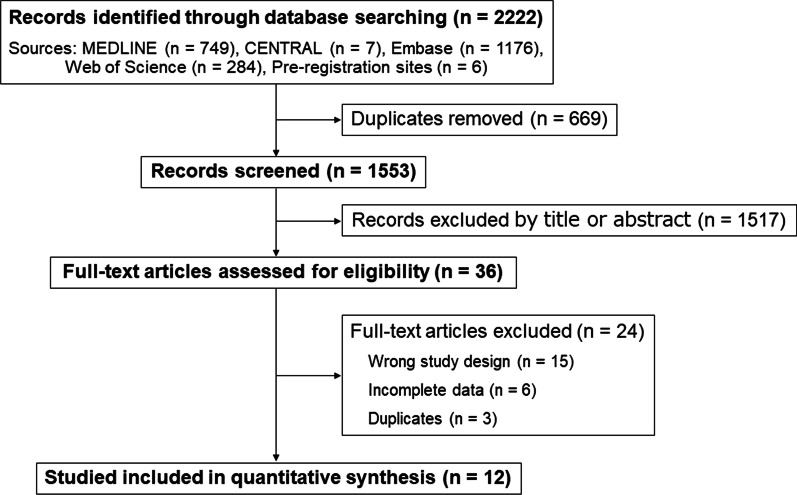
We screened the titles and abstracts of 1553 studies and reviewed 36 full-text articles after excluding studies that were non-diagnostic, clearly unrelated to POCUS, or did not meet the inclusion criteria for this study (Fig. 1). In the full-text review, the number of true positives, true negatives, false positives, or false negatives for 15 studies was unavailable due to the wrong study design. For six studies, the contact information was unavailable, or the authors were contacted, but complete data were unavailable. The list of excluded studies is shown in Additional file 1: Table S2. Finally, twelve studies with 1132 patients with shock [32–43] were identified as eligible for meta-analysis, as well as an additional six that met the inclusion criteria but provided insufficient data. In one study [43], the numbers of true-positive, true-negative, false-positive, and false-negative results reported in a previous systematic review were used because of insufficient data for diagnostic accuracy.
Fig. 1.
Study flow diagram. CENTRAL Cochrane Central Register of Controlled Trials
The baseline characteristics of the eligible studies are presented in Table 1 and Additional file 1: Table S3; eleven of these were prospective cohort studies. Although the detailed definition of shock differed in each study, the occurrence of hypotension was common to all studies. With regard to the index test, all but one study [37] used POCUS, which consists of multiple-organ ultrasound, including echocardiography. In almost all studies, the reference standard was defined as a clinical diagnosis based on medical records. Two observational studies had prior suspected disease in a group of patients with suspected pulmonary embolism [35, 37].
Table 1.
Study characteristics
| Study | Design, no. of patients (location) | Clinical setting | Definition of circulatory failure | US protocol | US Physician | Reference standard |
|---|---|---|---|---|---|---|
| Bagheri-Hariri et al. [40] | Prospective Cohort, one center, 25 patients (Iran) | Emergency department | SBP < 90 mmHg or shock indexa > 1.0 with clinical hypoperfusion symptoms | Multi-organ POCUS (observed in order: heart/IVC, jugular veins, thoracic and abdominal cavities, lungs/deep veins, aorta)b | Emergency physicians with credentials for the emergency department ultrasound | Clinical diagnosis using all medical information |
| Ghane et al. [33] | Prospective Cohort, one center, 77 patients (Iran) | Emergency department | SBP < 100 mmHg or shock indexa > 1.0 | Multi-organ POCUS (observed in order: heart/IVC, jugular veins, thoracic and abdominal cavities, lungs/deep veins, aorta) b | An emergency physician with five years of experience with more than 200 ultrasonographic exams per year | Clinical diagnosis after admission to the medical units (internal medicine, cardiology, or surgery) by board-certified specialists |
| Shokoohi et al. [43] | Prospective Cohort, one center, 118 patients (USA) | Emergency department | SBP < 90 mmHg after an initial fluid resuscitation (> 1L of normal saline) | Multi-organ POCUS (no order specified: heart, IVC, thoracic and abdominal cavities, and lung) | An ultrasound-trained attending physician (including ultrasound fellows) with extensive experience in emergency and critical care ultrasound | Clinical diagnosis by chart review by two board-certified intensivists, blinded to the results of POCUS |
| Agmy et al. [41] | Unknown, one center, 63 patients (Egypt) | Intensive care unit | Circulatory shock patients (definition was unknown) | Multi-organ POCUS (observed in order: heart and lung)c | Unclear | Clinical diagnosis using all medical information |
| Nazerian et al. [35] | Prospective Cohort, two center, 105 patients (Italy) | Emergency department | SBP < 90 mmHg or a drop of SBP > 40 mmHg for more than 15 min, with signs of end-organ hypoperfusion (cold extremities, UO < 30 mL/h, altered mental status, profound asthenia with fatigue and malaise, or respiratory distress), with suspected PE | Multi-organ POCUS (no order specified: heart and deep veins) | Sonographers with more than 2 years’ experience in cardiac and venous US on critically ill patients | Clinical diagnosis by an expert in PE who independently reviewed all the available clinical and imaging data including multidetector computed tomography pulmonary angiography |
| Elbaih et al. [38] | Prospective Cohort, one center, 100 patients (Egypt) | Emergency department | Unstable polytrauma patients (definition of unstable was unknown) | Multi-organ POCUS (observed in order: heart/IVC, jugular veins, thoracic and abdominal cavities, lungs/deep veins, aorta)b | Unclear | Clinical diagnosis using all medical information |
| Tesfaye et al. [42] | Prospective Cohort, one center, 93 patients (Ethiopia) | Emergency department | Hypotension (definition of hypotension was unknown) | Multi-organ POCUS (observed in order: heart/IVC, jugular veins, thoracic and abdominal cavities, lungs/deep veins, aorta)b | Unclear | Clinical diagnosis after full evaluation |
| Daley et al. [37] | Prospective Cohort, six centers, 136 patients (USA) | Emergency department | Tachycardia and/or hypotension with suspected PE (definition of tachycardia and hypotension was unknown) | Heart including the measurement of TAPSEd | Emergency physicians or study investigators (including medical students) trained in FOCUS | Computed tomography angiography |
| Rahulkumar et al. [36] | Prospective Cohort, one center, 97 patients (India) | Emergency department | SBP < 90 mmHg and shock indexa > 1.0 | Multi-organ POCUS (observed in order: heart/IVC, jugular veins, thoracic and abdominal cavities, lungs/deep veins, aorta) b | An emergency physician expert in emergency medicine ultrasound | Clinical diagnosis using all medical information by the consultants of medicine or surgery department |
| Javali et al. [39] | Prospective Cohort, one center, 100 patients (India) | Emergency department | SBP < 90 mmHg and shock index a > 1 with the presence of at least one of the following signs or symptoms of hypoperfusion unresponsiveness, altered mental status, syncope, respiratory distress, generalized fatigue, severe chest pain or abdominal pain | Multi-organ POCUS (no order specified: heart, lung, free fluid in the peritoneal cavity, aorta, IVC, and femoral vein) | A trained emergency physician (unclear regarding ultrasound experience) | Clinical diagnosis after admission to the medical units (internal medicine, cardiology, or surgery) by board-certified specialists, blind to the diagnoses in the emergency department |
| Keefer et al. [32] | Prospective Cohort, six centers, 135 patients (North America and South Africa) | Emergency department | Sustained SBP < 100 mmHg or shock indexa > 1.0 | Multi-organ POCUS (observed in order: heart/IVC, jugular veins, thoracic and abdominal cavities, lungs/deep veins, aorta) b | POCUS-trained emergency physicians | Clinical diagnosis by chart review by two clinicians, blinded to the initial sonographer, and point-of-care ultrasonography findings and diagnosis |
| Zieleskiewicz et al. [34] | Prospective Cohort, one center, 83 patients (France) | General ward | MAP < 65 mmHg or HR < 40 bpm or HR > 120 bpm or UO < 50 ml/4 h | Multi-organ POCUS (no order specified: heart, IVC, lung, thoracic cavity, and the deep veins if required) | ICU physicians trained in ultrasound | Clinical diagnosis by chart review including physical examinations and blood and imaging tests by two physicians blinded of the initial diagnoses made at the bedside |
SBP, systolic blood pressure; MAP, mean arterial pressure; HR, heart rate; bpm, beat per minutes; UO, urine output; PE, pulmonary embolism; US, ultrasound; TAPSE, tricuspid annular plane systolic excursion; and IVC, inferior vena cava
aShock index is defined as the heart rate divided by systolic blood pressure
bDescribed as RUSH (rapid ultrasound for shock and hypotension) exam in this study
cDescribed FALLS (fluid administration limited by lung sonography) protocol in this study
dDescribed FOCUS (focused cardiac ultrasound) in this study
The results of the QUADAS-2 assessment are shown in Table 2. Three studies were judged to have a high risk of bias in the patient selection domain because the inclusion processes were not conducted consecutively or randomly [33, 38, 39]. In one study, POCUS was performed after the implementation of the reference standard and was considered to have a high risk of bias in the domain of the index test because it may have influenced the interpretation of the results of the index test [37]. In the domain of flow and timing in three studies [32, 33, 35], the risk of bias was considered high because of the presence of cases that were excluded from the analysis for unknown reasons. For two studies, for which only conference abstracts were available [41, 42], the detailed definitions of the inclusion criteria were unclear, as were the details of the number of patients involved in the process, from patient selection to analysis. Additionally, the risk of bias with respect to applicability was unknown because the patient populations included were unclear.
Table 2.
QUADAS-2 results
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Bagheri-Hariri et al. [40] | Low | Low | Low | High | Low | Low | Low |
| Ghane et al. [33] | High | Low | Low | High | Low | Low | Low |
| Shokoohi et al. [43] | High | Low | Low | Low | Low | Low | Low |
| Agmy et al. [41] | Unclear | Low | Low | Unclear | Unclear | Low | Low |
| Nazerian et al. [35] | Low | Low | Low | High | Low | Low | Low |
| Elbaih et al. [38] | High | Low | Low | Low | Low | Low | Low |
| Tesfaye et al. [42] | Unclear | Low | Low | Unclear | Unclear | Low | Low |
| Daley et al. [37] | Low | High | Low | Low | Low | Low | Low |
| Rahulkumar et al. [36] | Low | Low | Low | Low | Low | Low | Low |
| Javali et al. [39] | High | Low | Low | Low | Low | Low | Low |
| Keefer et al. [32] | Low | Low | Low | High | Low | Low | Low |
| Zieleskiewicz et al. [34] | Low | Low | Low | Low | Low | Low | Low |
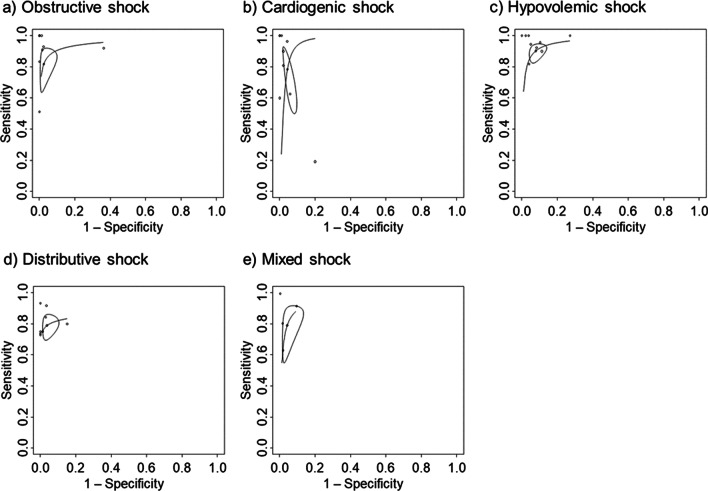
The SROC curves relevant to each shock are shown in Fig. 2, and summary estimates of sensitivity, specificity, AUC of the SROC curve, positive likelihood ratio, and negative likelihood ratio are shown in Table 3. In Fig. 2, the solid curves are the SROC curves integrated into a bivariate random-effects model for diagnosing the cause of each shock using POCUS. The dots represent point estimates of sensitivity and 1-specificity for each included study, and the ellipses represent 95% CIs for sensitivity and 1-specificity. The AUCs of the SROC curves for each shock were approximately 0.95, except for distributive shock. Point estimates of sensitivity for each shock ranged from approximately 0.8 to 0.9 with wide CIs, and point estimates of specificity exceeded 0.9 for all shocks with narrow CIs. For all shocks, the positive likelihood ratios were generally above 20, and the negative likelihood ratios were approximately 0.2. Among these shocks, the specificity and positive likelihood ratio for obstructive shock were particularly high. The I2 value, a measure of heterogeneity, was 15.1% for obstructive shock and 0% for all others. Forest plots for each shock are shown in Additional file 1: Figures S1 to S5.
Fig. 2.
Summary receiver operating characteristic curves. The summary receiver operating characteristic plots of the bivariate meta-analysis for the identification of the cause of shock by point-of-care ultrasound. The ellipse around the point estimates represents a 95% CI. The ROC curves are restricted to the range of specificities for each study
Table 3.
Sensitivities, specificities, AUROCs, and likelihood ratios by shock subtype
| Shock type | No. of patients (study) | Sensitivity | Specificity | Area under the ROC curve | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|
| Obstructive | 810 (9) | 0.82 (0.68–0.91) | 0.98 (0.92–0.99) | 0.95 (0.78–0.97) | 40 (11–105) | 0.20 (0.10–0.33) |
| Cardiogenic | 828 (9) | 0.78 (0.56–0.91) | 0.96 (0.92–0.98) | 0.96 (0.86–0.97) | 19 (7.1–40) | 0.24 (0.09–0.47) |
| Hypovolemic | 688 (9) | 0.90 (0.84–0.94) | 0.92 (0.88–0.95) | 0.96 (0.87–0.96) | 12 (7.3–18) | 0.11 (0.07–0.17) |
| Distributive | 594 (8) | 0.79 (0.71–0.85) | 0.96 (0.91–0.98) | 0.86 (0.75–0.96) | 23 (9.3–49) | 0.22 (0.16–0.30) |
| Mixed | 291 (4) | 0.80 (0.61–0.91) | 0.96 (0.89–0.99) | 0.95 (0.76–0.97) | 20 (7.9–49) | 0.21 (0.10–0.40) |
As a subgroup analysis, we performed a meta-analysis of studies that were only performed in the emergency room [32, 34–40, 42, 43], those that had no suspected disease before POCUS [32–34, 36, 38–43], clearly stated the existence of a POCUS training program [32–34, 37], or that had a POCUS protocol for multi-organ ultrasound [32–36, 38–43]. The results are shown in Additional file 1: Tables S4 to S7. The results of the meta-analysis of the studies performed in the emergency room were similar to those of the main analyses. The results of the meta-analysis in the absence of suspected disease before POCUS and multi-organ ultrasonography showed a noticeably higher specificity and positive likelihood ratio for obstructive shock than the results of the main analysis. Results from studies with POCUS training programs were notable for their high sensitivity, specificity, and likelihood ratio for distributional abnormal shocks; however, the number of studies included in this meta-analysis was small. As a sensitivity analysis, a meta-analysis was performed of the remaining ten studies after excluding two studies for which only conference abstracts were available [41, 42]. These results were similar to those of the main analysis (Additional file 1: Table S8).
Discussion
This systematic review included 12 studies with 1132 patients with shock and evaluated the diagnostic accuracy of POCUS in diagnosing the etiology. Compared to the previous systematic review that addressed the same topic [20], this review, which was updated with an expanded population, was able to incorporate more studies and thus present a narrower confidence interval for each diagnostic accuracy. In addition, the various subgroup analyses confirmed the characteristics of the diagnostic accuracy of POCUS for shock patients. The meta-analysis we conducted showed pooled sensitivity for each type of shock ranged from 0.77 (distributive) to 0.93 (hypovolemic) and specificity ranged from 0.92 (hypovolemic) to 0.97 (obstructive), and the area under the ROC for each type was approximately 0.95. Positive likelihood ratios exceeded 10 for all types of shocks, especially obstructive, and negative likelihood ratios were about 0.2 for each.
Several systematic reviews have been conducted on the diagnostic accuracy of POCUS in emergency conditions. A systematic review of POCUS for respiratory failure reported a sensitivity of 0.92 (95% CI 0.85–0.96) and a specificity of 0.98 (95% CI 0.94–0.99) [44]. Another systematic review for the detection of signs of significant injury in thoracoabdominal trauma (thoracoabdominal fluid retention, large vessel injury, pneumothorax, etc.), including 34 studies and 8635 patients published by Cochrane in 2018, found a sensitivity of 0.74 (95% Cl: 0.65–0.81) and a specificity of 0.96 (95% Cl: 0.94–0.98) [45]. Furthermore, with regard to POCUS in shock differentiation, the previous systematic review showed that the diagnostic accuracies for each type of shock ranged from 0.64–0.93 for sensitivity, 0.80–0.98 for specificity, 8–40 for positive likelihood ratio, and 0.13–0.32 for negative likelihood ratio [20]. Compared to these studies, the characteristics of diagnostic accuracy for each type of shock in our systematic review were similar in terms of high specificity. Therefore, when POCUS detects a finding that could be the cause of shock, clinicians should also recognize it as a probable cause.
Clinically, the difficulty in identifying the cause of shock using POCUS may vary depending on the etiology. In a previous systematic review, a comparison between each type of shock showed high specificity and positive likelihood ratios, especially in obstructive shock (specificity 0.98 (95% CI 0.96–0.99) and positive likelihood ratio 40.54 (95% CI 12.06–136.28)) [20]. Another narrative review examining the diagnostic accuracies for acute diseases also showed particularly high specificity for pericardial effusion, right heart failure, and pneumothorax [1, 6, 46]. In our study, the specificity and positive likelihood ratios were the highest for obstructive shock. In addition, this trend was similar in the subgroup analyses. Clinically, obstructive shock often shows disease-specific findings on POCUS as the cause of shock. However, the echocardiographic findings of distributive and hypovolemic shock are identical, and even if cardiogenic shock is suspected due to apparent cardiac dysfunction, it is not known whether the cardiac dysfunction is new or contributes to shock. Therefore, the differential diagnosis of shock using POCUS is particularly useful for confirming obstructive shock. Furthermore, our subgroup meta-analysis, limited to studies using POCUS protocols for multiple organs, not only the heart, showed high diagnostic accuracy for obstructive shock, especially in terms of specificity and negative likelihood ratio. This may be because ultrasound findings other than echocardiography can rule out the causes of obstructive shock, such as lung sliding to rule out pneumothorax. It should be emphasized that POCUS should be performed on multiple organs in shock patients.
Among the diagnostic protocols for ultrasound in diagnosing the cause of shock, the Rapid Ultrasound for Shock and Hypotension (RUSH) examination is well known and has been used in many of the studies included in our meta-analysis [14]. The RUSH examination comprehensively described the ultrasound findings in multiple organs that were observed in each type of shock. However, to implement POCUS in actual clinical practice, it is necessary to provide more specific instructions on the method of performing it, such as the type of shocks to differentiate first and the view to start with in echocardiography. In our study, we found that the specificity and positive likelihood ratio of POCUS in identifying the cause of shock were high for all types of shock, particularly obstructive shock. Clinically, obstructive shock is a group of diseases that can be treated by eliminating confirmed abnormal findings. Therefore, when using POCUS to identify the cause of shock, it is reasonable to first confirm the diagnosis of obstructive shock, which may improve patient outcomes.
Although the basic level of POCUS in echocardiography requires the acquisition of parasternal long- and short-axis, apical four-chamber, subcostal four-chamber, and inferior vena cava (IVC) views [18, 19, 47, 48], the view with which it is initiated depends on the clinician’s discretion. Typical diseases that cause obstructive shock include tension pneumothorax, severe pulmonary embolism, and cardiac tamponade. Common echocardiographic findings in these diseases are dilation of the IVC and decreased respiratory variability in the IVC [14, 21, 49–53], which can be easily visualized from the subcostal four-chamber and IVC views. In addition, this view allows for quick assessment of cardiac tamponade by confirming the presence of pericardial fluid while viewing the IVC. Therefore, when differentiating shock using POCUS, it is appropriate to start with obstructive shock, given its diagnostic accuracy, and with the subcostal four-chamber view. This should be considered in future POCUS diagnostic protocols.
Our study included the largest number of studies on the diagnostic accuracy of POCUS for identifying the cause of shock. However, it has several limitations. First, of the 12 studies included, 11 used clinical diagnosis as the reference standard. Out of these studies, only four had blinding to the POCUS results during clinical diagnosis. Although there were no qualitative differences in diagnostic accuracy depending on whether the POCUS results were blinded, this may have led to an overestimation of the diagnostic accuracy of POCUS for shock patients. Second, there was no uniform definition of the reference standard among the included studies in terms of details beyond clinical diagnosis. However, there is no consistent diagnostic method for a definitive diagnosis of the cause of shock. In clinical practice, a definitive diagnosis is made by considering all medical information available on site. In general, the definition comprehensively defined as a clinical diagnosis in each study was considered to be in line with clinical practice. Third, in the index test, the details of POCUS (skill level of the performer and ultrasound protocol) were not consistently defined across all of the studies. However, the results of the meta-analysis of subgroup analyses were similar to those of the main analysis. Fourth, the studies included in our meta-analysis were conducted almost exclusively in emergency rooms. In other clinical settings, diagnostic accuracy may be altered by varying disease severity. For example, diagnostic accuracy in the intensive care unit, where more severely ill patients may be present, may differ from that of our study. However, further studies are required to address this issue. Finally, our study only examined the diagnostic accuracy, and it is unclear whether the use of POCUS truly improves patient outcomes. It is also unclear whether a better protocol would improve diagnostic accuracy or change actual practice. To resolve these uncertainties, further studies are needed to develop more clinically useful diagnostic protocols based on diagnostic accuracy and to examine the impact of protocol implementation on patient outcomes.
Conclusions
In this study, the identification of the etiology of shock by POCUS was characterized by high sensitivity and a positive likelihood ratio, especially for obstructive shock. Hence, these findings should be considered in future diagnostic protocols for shock using POCUS. However, since this study only examined the diagnostic aspect, further interventional studies are necessary to assess the true impact of POCUS on shock patients.
Supplementary Information
Additional file 1: Table S1. The full search strategy. Table S2. The list of excluded studies. Table S3. Additional study characteristics. Table S4. Diagnostic accuracies for studies conducted in the emergency department. Table S5. Diagnostic accuracies for studies with no prior suspected disease. Table S6. Diagnostic accuracies for studies in which the existence of a point-of-care ultrasound training program was explicitly mentioned. Table S7. Diagnostic accuracies for studies in which ultrasound other than transthoracic echocardiography was used in combination. Table S8. Diagnostic accuracies for studies without high risk of bias. Figure S1: Obstructive shock. CI, confidence interval. Figure S2: Cardiogenic shock. CI, confidence interval. Figure S3: Hypovolemic shock. CI, confidence interval. Figure S4: Distributive shock. CI, confidence interval. Figure S5: Mixed shock. CI, confidence interval.
Acknowledgements
Not applicable.
Abbreviations
- POCUS
Point-of-care ultrasound
- CENTRAL
Cochrane Central Register of Controlled Trials
- EU-CTR
European Union Clinical Trials Register
- ICTRP
International Clinical Trials Registry Platform
- UMIN-CTR
University Hospital Medical Information Network Clinical Trials Registry
- MeSH
Medical Subject Headings
- QUADAS-2
Quality Assessment of Diagnostic Accuracy Studies 2
- SROC
Summary receiver operating characteristic
- AUCs
Area under the curve
- CI
Confidence interval
- IVC
Inferior vena cava
Author contributions
This study was designed by Takuo Y and TM. Takuo Y, Takuya Y, and TM screened the articles and extracted data. Takuo Y and Takuya Y assessed the risk of bias. Takuo Y analyzed and interpreted the data under the supervision of HN and TM. The tables and figures were produced by Takuo Y. Takuo Y was primarily responsible for writing the manuscript. All the authors critically revised the manuscript for important intellectual content and approved the final draft. All the authors have read and approved the final version of the manuscript.
Funding
Not applicable.
Availability of data and materials
Data supporting the findings of this study are available from the corresponding author, Takuo Y, upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vincent J-L, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 2.Wacker DA, Winters ME. Shock. Emerg Med Clin North Am. 2014;32(4):747–758. doi: 10.1016/j.emc.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Jones AE, Aborn LS, Kline JA. Severity of emergency department hypotension predicts adverse hospital outcome. Shock. 2004;22(5):410–414. doi: 10.1097/01.shk.0000142186.95718.82. [DOI] [PubMed] [Google Scholar]
- 4.Marbach JA, Almufleh A, Di Santo P, Simard T, Jung R, Diemer G, et al. A shifting paradigm: the role of focused cardiac ultrasound in bedside patient assessment. Chest. 2020;158(5):2107–2118. doi: 10.1016/j.chest.2020.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Vieillard-Baron A, Millington SJ, Sanfilippo F, Chew M, Diaz-Gomez J, McLean A, et al. A decade of progress in critical care echocardiography: a narrative review. Intensive Care Med. 2019;45(6):770–788. doi: 10.1007/s00134-019-05604-2. [DOI] [PubMed] [Google Scholar]
- 6.Labovitz AJ, Noble VE, Bierig M, Goldstein SA, Jones R, Kort S, et al. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23(12):1225–1230. doi: 10.1016/j.echo.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Díaz-Gómez JL, Mayo PH, Koenig SJ. Point-of-care ultrasonography. N Engl J Med. 2021;385(17):1593–1602. doi: 10.1056/NEJMra1916062. [DOI] [PubMed] [Google Scholar]
- 8.Jones AE, Tayal VS, Sullivan DM, Kline JA. Randomized, controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med. 2004;32(8):1703–1708. doi: 10.1097/01.CCM.0000133017.34137.82. [DOI] [PubMed] [Google Scholar]
- 9.Pershad J, Myers S, Plouman C, Rosson C, Elam K, Wan J, et al. Bedside limited echocardiography by the emergency physician is accurate during evaluation of the critically ill patient. Pediatrics. 2004;114(6):e667–e671. doi: 10.1542/peds.2004-0881. [DOI] [PubMed] [Google Scholar]
- 10.Mayo P, Arntfield R, Balik M, Kory P, Mathis G, Schmidt G, et al. The ICM research agenda on critical care ultrasonography. Intensive Care Med. 2017;43(9):1257–1269. doi: 10.1007/s00134-017-4734-z. [DOI] [PubMed] [Google Scholar]
- 11.Whitson MR, Mayo PH. Ultrasonography in the emergency department. Crit Care. 2016;20(1):227. doi: 10.1186/s13054-016-1399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen CA, Holden S, Vela J, Rathleff MS, Jensen MB. Point-of-care ultrasound in general practice: a systematic review. Ann Fam Med. 2019;17(1):61–69. doi: 10.1370/afm.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernier-Jean A, Albert M, Shiloh AL, Eisen LA, Williamson D, Beaulieu Y. The diagnostic and therapeutic impact of point-of-care ultrasonography in the intensive care unit. J Intensive Care Med. 2017;32(3):197–203. doi: 10.1177/0885066615606682. [DOI] [PubMed] [Google Scholar]
- 14.Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically lll. Emerg Med Clin North Am. 2010;28(1):29–56, vii. doi: 10.1016/j.emc.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest. 2015;147(6):1659–1670. doi: 10.1378/chest.14-1313. [DOI] [PubMed] [Google Scholar]
- 16.Sharma V, Fletcher SN. A review of echocardiography in anaesthetic and peri-operative practice. Part 2: training and accreditation. Anaesthesia. 2014;69(8):919–927. doi: 10.1111/anae.12709. [DOI] [PubMed] [Google Scholar]
- 17.Jensen MB, Sloth E, Larsen KM, Schmidt MB. Transthoracic echocardiography for cardiopulmonary monitoring in intensive care. Eur J Anaesthesiol. 2004;21(9):700–707. doi: 10.1097/00003643-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Mayo PH, Beaulieu Y, Doelken P, Feller-Kopman D, Harrod C, Kaplan A, et al. American College of Chest Physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050–1060. doi: 10.1378/chest.08-2305. [DOI] [PubMed] [Google Scholar]
- 19.Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):567–581. doi: 10.1016/j.echo.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Stickles SP, Carpenter CR, Gekle R, Kraus CK, Scoville C, Theodoro D, et al. The diagnostic accuracy of a point-of-care ultrasound protocol for shock etiology: a systematic review and meta-analysis. CJEM. 2019;21(3):406–417. doi: 10.1017/cem.2018.498. [DOI] [PubMed] [Google Scholar]
- 21.Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam 2012: rapid ultrasound in shock in the evaluation of the critically ill patient. Ultrasound Clin. 2012;7(2):255–278. doi: 10.1016/j.cult.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Deeks JJ, Bossuyt PM, Leeflang MM, Takwoingi Y. Cochrane handbook for systematic reviews of diagnostic test accuracy. Version 2.0. The Cochrane Collaboration; 2022. https://training.cochrane.org/handbook-diagnostic-test-accuracy Accessed 9 Apr 2023 [DOI] [PMC free article] [PubMed]
- 23.Salameh JP, Bossuyt PM, McGrath TA, Thombs BD, Hyde CJ, Macaskill P, et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ. 2020;370:m2632. doi: 10.1136/bmj.m2632. [DOI] [PubMed] [Google Scholar]
- 24.McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, PRISMA-DTA Group et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 25.Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 26.Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 27.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865–2884. doi: 10.1002/sim.942. [DOI] [PubMed] [Google Scholar]
- 28.Noma H, Matsushima Y, Ishii R. Confidence interval for the AUC of SROC curve and some related methods using bootstrap for meta-analysis of diagnostic accuracy studies. Commun Stat Case Stud Data Anal Appl. 2021;7(3):344–358. [Google Scholar]
- 29.McInnes MDF, Bossuyt PMM. Pitfalls of systematic reviews and meta-analyses in imaging research. Radiology. 2015;277(1):13–21. doi: 10.1148/radiol.2015142779. [DOI] [PubMed] [Google Scholar]
- 30.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 31.van Enst WA, Ochodo E, Scholten RJPM, Hooft L, Leeflang MM. Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med Res Methodol. 2014;14:70. doi: 10.1186/1471-2288-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keefer S, Atkinson P, Chandra K, Henneberry RJ, Olszynski PA, Peach M, et al. Sonographic findings of left ventricular dysfunction to predict shock type in undifferentiated hypotensive patients: an analysis from the sonography in hypotension and cardiac arrest in the emergency department (SHoC-ED) study. Cureus. 2021;13(7):e16360. doi: 10.7759/cureus.16360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghane MR, Gharib MH, Ebrahimi A, Samimi K, Rezaee M, Rasouli HR, et al. Accuracy of rapid ultrasound in shock (RUSH) exam for diagnosis of shock in critically ill patients. Trauma Mon. 2015;20(1):e20095. doi: 10.5812/traumamon.20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zieleskiewicz L, Lopez A, Hraiech S, Baumstarck K, Pastene B, Di Bisceglie M, et al. Bedside POCUS during ward emergencies is associated with improved diagnosis and outcome: an observational, prospective, controlled study. Crit Care. 2021;25(1):34. doi: 10.1186/s13054-021-03466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nazerian P, Volpicelli G, Gigli C, Lamorte A, Grifoni S, Vanni S. Diagnostic accuracy of focused cardiac and venous ultrasound examinations in patients with shock and suspected pulmonary embolism. Intern Emerg Med. 2018;13(4):567–574. doi: 10.1007/s11739-017-1681-1. [DOI] [PubMed] [Google Scholar]
- 36.Rahulkumar HH, Bhavin PR, Shreyas KP, Krunalkumar HP, Atulkumar S, Bansari C. Utility of point-of-care ultrasound in differentiating causes of shock in resource-limited setup. J Emerg Trauma Shock. 2019;12(1):10–17. doi: 10.4103/JETS.JETS_61_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daley JI, Dwyer KH, Grunwald Z, Shaw DL, Stone MB, Schick A, et al. Increased sensitivity of focused cardiac ultrasound for pulmonary embolism in emergency department patients with abnormal vital signs. Acad Emerg Med. 2019;26(11):1211–1220. doi: 10.1111/acem.13774. [DOI] [PubMed] [Google Scholar]
- 38.Elbaih AH, Housseini AM, Khalifa MEM. Accuracy and outcome of rapid ultrasound in shock and hypotension (RUSH) in Egyptian polytrauma patients. Chin J Traumatol. 2018;21(3):156–162. doi: 10.1016/j.cjtee.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Javali RH, Loganathan A, Srinivasarangan M, Patil A, Siddappa GB, Satyanarayana N, et al. Reliability of emergency department diagnosis in identifying the etiology of nontraumatic undifferentiated hypotension. Indian J Crit Care Med. 2020;24(5):313–320. doi: 10.5005/jp-journals-10071-23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bagheri-Hariri S, Yekesadat M, Farahmand S, Arbab M, Sedaghat M, Shahlafar N, et al. The impact of using RUSH protocol for diagnosing the type of unknown shock in the emergency department. Emerg Radiol. 2015;22(5):517–520. doi: 10.1007/s10140-015-1311-z. [DOI] [PubMed] [Google Scholar]
- 41.Agmy G, Ahmed R, Mohamed A, Hamed S, Saad M. Implication of transthoracic sonography in assessment of circulatory failure: Fayoum experience with falls protocol. Chest. 2017;152(4):A618. doi: 10.1016/j.chest.2017.08.650. [DOI] [Google Scholar]
- 42.Tesfaye E, Zewude T. Rapid ultrasonographic assessment of undifferentiated shock in hypotensive patients. Crit Care. 2018;22.
- 43.Shokoohi H, Boniface KS, Pourmand A, Liu YT, Davison DL, Hawkins KD, et al. Bedside ultrasound reduces diagnostic uncertainty and guides resuscitation in patients with undifferentiated hypotension. Crit Care Med. 2015;43(12):2562–2569. doi: 10.1097/CCM.0000000000001285. [DOI] [PubMed] [Google Scholar]
- 44.Yuan X, Liu L, Chang W, Wu Z, Huang L, Chao Y, et al. Diagnosis Accuracy of lung ultrasound for arf in critically ill patients: a systematic review and meta-analysis. Front Med. 2021;8:705960. doi: 10.3389/fmed.2021.705960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stengel D, Leisterer J, Ferrada P, Ekkernkamp A, Mutze S, Hoenning A. Point-of-care ultrasonography for diagnosing thoracoabdominal injuries in patients with blunt trauma. Cochrane Database Syst Rev. 2018;12(12):CD012669. doi: 10.1002/14651858.CD012669.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140(4):859–866. doi: 10.1378/chest.10-2946. [DOI] [PubMed] [Google Scholar]
- 47.Robba C, Wong A, Poole D, Al Tayar A, Arntfield RT, Chew MS, et al. Basic ultrasound head-to-toe skills for intensivists in the general and neuro intensive care unit population: consensus and expert recommendations of the European Society of Intensive Care Medicine. Intensive Care Med. 2021;47(12):1347–1367. doi: 10.1007/s00134-021-06486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blans MJ, Bosch FH, van der Hoeven JG. A practical approach to critical care ultrasound. J Crit Care. 2019;51:156–164. doi: 10.1016/j.jcrc.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Lanks CW, Correa V. Advantages of cardiopulmonary ultrasound in post-cardiopulmonary resuscitation tension pneumothorax. J Ultrasound Med. 2018;37(4):819–822. doi: 10.1002/jum.14437. [DOI] [PubMed] [Google Scholar]
- 50.Haskings EM, Eissa M, Allard RV, MirGhassemi A, McFaul CM, Miller EC. Point-of-care ultrasound use in emergencies: what every anaesthetist should know. Anaesthesia. 2023;78(1):105–118. doi: 10.1111/anae.15910. [DOI] [PubMed] [Google Scholar]
- 51.Volpicelli G, Lamorte A, Tullio M, Cardinale L, Giraudo M, Stefanone V, et al. Point-of-care multiorgan ultrasonography for the evaluation of undifferentiated hypotension in the emergency department. Intensive Care Med. 2013;39(7):1290–1298. doi: 10.1007/s00134-013-2919-7. [DOI] [PubMed] [Google Scholar]
- 52.Inocencio M, Childs J, Chilstrom ML, Berona K. Ultrasound findings in tension pneumothorax: a case report. J Emerg Med. 2017;52(6):e217–e220. doi: 10.1016/j.jemermed.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Kearns MJ, Walley KR. Tamponade: hemodynamic and echocardiographic diagnosis. Chest. 2018;153(5):1266–1275. doi: 10.1016/j.chest.2017.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The full search strategy. Table S2. The list of excluded studies. Table S3. Additional study characteristics. Table S4. Diagnostic accuracies for studies conducted in the emergency department. Table S5. Diagnostic accuracies for studies with no prior suspected disease. Table S6. Diagnostic accuracies for studies in which the existence of a point-of-care ultrasound training program was explicitly mentioned. Table S7. Diagnostic accuracies for studies in which ultrasound other than transthoracic echocardiography was used in combination. Table S8. Diagnostic accuracies for studies without high risk of bias. Figure S1: Obstructive shock. CI, confidence interval. Figure S2: Cardiogenic shock. CI, confidence interval. Figure S3: Hypovolemic shock. CI, confidence interval. Figure S4: Distributive shock. CI, confidence interval. Figure S5: Mixed shock. CI, confidence interval.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author, Takuo Y, upon reasonable request.