Abstract
Objectives
To examine the circumstances and needs of older adults who were “kinless,” defined as having no living spouse or children, when they developed dementia.
Methods
We conducted a secondary analysis of information from the Adult Changes in Thought study. Among 848 participants diagnosed with dementia between 1994 and 2016, we identified 64 who had no living spouse or child at dementia onset. We then conducted a qualitative analysis of administrative documents pertaining to these participants: handwritten comments recorded after each study visit, and medical history documents containing clinical chart notes from participants’ medical records.
Results
In this community-dwelling cohort of older adults diagnosed with dementia, 8.4% were kinless at dementia onset. Participants in this sample had an average age of 87 years old, half lived alone, and one third lived with unrelated persons. Through inductive content analysis, we identified 4 themes that describe their circumstances and needs: (1) life trajectories, (2) caregiving resources, (3) care needs and gaps, and (4) turning points in caregiving arrangements.
Discussion
Our qualitative analysis reveals that the life trajectories that led members of the analytic cohort to be kinless at dementia onset were quite varied. This research highlights the importance of nonfamily caregivers and participants’ own roles as caregivers. Our findings suggest that clinicians and health systems may need to work with other parties to directly provide dementia caregiving support rather than rely on family, and address factors such as neighborhood affordability that particularly affect older adults who have limited family support.
Keywords: Caregiving, Dementia, Family, Kinless, Qualitative research
Older adults living with dementia who do not have close family are a group who may be particularly vulnerable to adverse events and health outcomes, including unmet needs for care, and about whom little is known (Kolanowski et al., 2018). The care that family and other unpaid caregivers provide is critical to the health and well-being of older adults living with dementia (Bookman & Harrington, 2007; Committee on Family Caregiving for Older Adults et al., 2016). In addition to practical assistance with cooking, cleaning, self-care, and other activities of daily living (ADLs), such caregivers can provide emotional support, ensure a physically safe environment, and offer protection from exploitation or abuse. Many older adults living with dementia also have vision, hearing and/or mobility limitations, or other chronic conditions, and may need help managing medications and interacting with the health system (Griffith et al., 2016). In more broadly defined populations, unmet needs for such unpaid care have been shown to lead to adverse health outcomes (Allen et al., 2014).
Families’ responsibility for dementia care varies from one national context to another, in relation to the availability or lack of dementia-specific government programs (Lillo-Crespo et al., 2018). In the United States, where few such programs exist, the majority of dementia care is unpaid and is provided by family members. Recent research indicates that 63% of caregivers for older adults living with dementia are either spouses or children (National Alliance for Caregiving, 2017). The need to support dementia caregivers is widely recognized (Anderson et al., 2000; Kasper et al., 2015; Wolff et al., 2016) but efforts that target family caregivers may miss older adults who do not have close family. Those with no living spouse or children represent one form of “kinlessness” (Margolis & Verdery, 2017). Being without kin in the two relationship categories that provide the vast majority of unpaid dementia care in the United States, these kinless older adults may be especially vulnerable to unmet care needs and adverse outcomes if they develop dementia.
The challenges faced by kinless older adults with dementia may be expected to largely overlap with those faced by people living alone with dementia (Clare et al., 2020; Duane et al., 2013; Edwards et al., 2020; Gibson & Richardson, 2017; Portacolone et al., 2019, 2022). Older adults with dementia who live alone often “experience unmet needs for care, given that they lack cohabitating family members or others who provide the majority of dementia caregiving in the United States” (Yang et al., 2022), and are less likely to recognize their limitations or to seek the help they need. They are at high risk for self-neglect, malnutrition, accidental injury, medication errors, financial exploitation, social isolation, and unattended wandering (Kolanowski et al., 2018).
Not all older adults with dementia who live alone are kinless, however. One recent study found that 66.4% of older adults living alone with dementia identified their son or daughter as their primary source of support (Gibson & Richardson, 2017). Nor do all kinless older adults with dementia live alone, though research on the subject is limited. Nonetheless, kinlessness entails particular vulnerabilities for older adults with dementia, across a variety of different living situations. As compared with other older adults living in the community, those who are kinless receive less caregiving support and are more likely to die in nursing homes, and less likely to die in their homes or in hospice facilities (Plick et al., 2021). For those who live in nursing homes, staff may try but cannot fully compensate for the absence of family (Jervis, 2006). Kinless older adults in institutional settings may also be more vulnerable to becoming “unrepresented” (i.e., lacking decisional capacity, without close kin, and without a completed advance directive), which can make medical decision-making very difficult (Farrell et al., 2021). In this situation, a court-appointed guardian may be sought, but the process can be very slow and insufficiently responsive to the needs of patients, clinicians, and health systems (Catlin et al., 2021).
Little is known about the lives, relationships, and care of older adults who are kinless and living with dementia. Even in geriatrics research, people living with dementia are often excluded (Taylor et al., 2012), especially those without care partners (de Medeiros et al., 2022). Survey research on kinless older adults, sometimes called “elder orphans” (Montayre et al., 2020), has offered important insights into the dimensions, correlates, and consequences of this growing phenomenon (Carney et al., 2016; Montayre et al., 2019; Plick et al., 2021; Roofeh et al., 2020; Soniat & Pollack, 1994; Verdery et al., 2019). However, survey data provide limited insight into the daily lives of kinless older adults or their interactions with the health system. This creates gaps in knowledge that leave clinicians and health systems ill equipped to understand and address the needs of this vulnerable group.
What can we learn about the circumstances and needs of older adults who do not have a living spouse or children at the time they develop dementia?
Methods
To answer this question, we analyzed existing research and administrative data from the Adult Changes in Thought (ACT) study, a community-based longitudinal cohort study of incident dementia. We identified 64 participants who did not have a living spouse or children at the time the ACT study diagnosed them as having dementia. We used data collected as part of the ACT study to describe the characteristics of these individuals and conducted a qualitative analysis of unstructured textual data from ACT administrative documents, including clinical chart notes abstracted from their health records by ACT study staff.
Study Design, Setting, and Participants
The ACT study is an ongoing population-based prospective cohort study of people aged 65 years and older that is set within Kaiser Permanente Washington (KPWA), an integrated health care delivery system in the northwest of the United States (Kukull et al., 2002; Montine Thomas et al., 2012). ACT randomly selects KPWA members who are at least 65 and living in the community in or near Seattle, WA, and invites them to participate in the study. Those who respond are screened to ensure they do not have dementia at baseline, and then followed at 2-year intervals to identify those who develop dementia. The study has been ongoing since 1994, and continuously enrolls participants to maintain an active cohort of 2,000.
Biennial study visits take place at a research clinic or the participant’s home, and the ACT study continues to follow people who enter nursing homes after enrollment. Each ACT study visit includes an interview, cognitive screening, and physical measurements. Participants who score below 86 on the Cognitive Abilities Screening Instrument (Teng et al., 1994) are referred for a diagnostic evaluation by a study clinician and a neuropsychological testing by a psychometrician. These evaluations and other information are then reviewed by a larger group of clinicians to reach a consensus on whether Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) diagnostic criteria for dementia are met, and if so, whether McKhann et al.’s criteria for probable or possible Alzheimer’s disease are met (American Psychiatric Association Task Force on DSM-IV & American Psychiatric Association, 1994; McKhann G et al., 1984). Dementia onset is assigned by convention as the midpoint between the ACT study visit that triggered a dementia diagnosis and the preceding study visit.
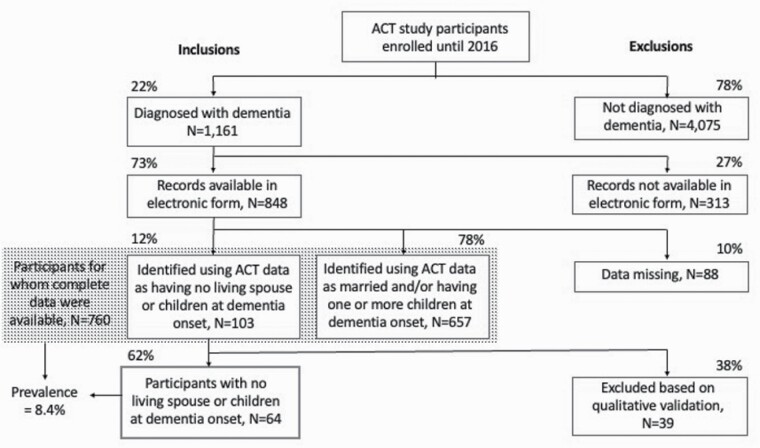
Our team analyzed ACT study data pertaining to 848 participants diagnosed with dementia before 2016 for whom health records were available electronically. We excluded 88 due to missing data on children and/or marital status. Among the remaining 760 participants, we identified 103 recorded as having no children, and who were never married, divorced, or widowed at dementia onset. We excluded 39 of these when qualitative review of documentation within 2 years of dementia onset included mention of a living spouse and/or children. This yielded an analytic sample of 64 older adults who were kinless (i.e., they had no living spouse or children) at dementia onset. See Figure 1.
Figure 1.
Sample selection. ACT = Adult Changes in Thought.
Data Collection
ACT study data
We assembled selected ACT variables for all 64 participants, including information about their gender, race, education level, age at dementia onset, whether they had been diagnosed with probable Alzheimer’s disease, as well as their living situation, marital status, ADLs and instrumental ADLs (IADLs). We also assembled participants’ scores on the Charlson and Klabunde comorbidity indices (Charlson et al., 1994; Klabunde et al., 2000), research tools that quantify the aggregate burden of multiple chronic conditions other than the one under study (in this case, dementia) on survival, using a scale of 1–10, with a higher score indicating a greater impact. For variables collected at biennial study visits, we used data from the visit that prompted the evaluation leading to a dementia diagnosis. We also assembled responses to a survey administered shortly after dementia onset to the participant’s listed contact person.
Unstructured textual data
For the 64 participants in our sample, we collected administrative documents assembled as part of the ACT study’s diagnostic protocol. These included handwritten comments recorded by ACT staff following each biennial visit, and medical history documents containing clinical chart notes abstracted from the medical record by ACT staff (which varied in length from 1 to more than 20 pages). Medical history documents were available for all participants in our sample except one, for whom no documents of any kind were available; ACT study handwritten comments were available for 58 participants out of our sample of 64. We scanned these paper files and used SimpleIndex Optical Character Recognition software (SimpleSoftware) to process scans into digital formats for qualitative analysis.
Data Analysis
Quantitative analysis
We used SAS statistical software version 9.4 (SAS Institute, Cary, NC) to build the analytic cohort, and Stata statistical software version 17 (StataCorp, College Station, TX) to generate descriptive statistics.
Qualitative analysis
Four coauthors (J.S. Taylor, M.S. Figueroa Gray, C. Freitag, and P. Taneja) used inductive content analysis, a method of qualitative inquiry that facilitates discovery of previously unidentified factors, to code all available documents. We coded for some a priori categories (such as “living situation”), and also allowed for emergent codes to describe content that did not fit these predefined categories (Creswell, 2009; Saldaña, Johnny, 2021) Together, we reviewed all codes and corresponding textual passages for discrepancies in interpretation and deliberated to reach consensus. Investigators each independently coded five specific documents using the final coding schema, and compared them to confirm that we were applying codes in a consistent and reliable manner, resolving discrepancies through discussion. All documents were coded by two team members, including at least one PhD-trained researcher (J.S. Taylor or M.S. Figueroa Gray). Atlas.ti qualitative analysis software, version 8.1 (Friese, 2019) was used to assign codes and organize the coded text to facilitate the analysis. Finally, the coded text was reviewed to identify patterns and themes in the circumstances and care of older adults who were kinless when they developed dementia, and representative quotations were selected to illustrate these themes. The research team discussed these themes in meetings and through comments on circulated draft manuscripts, to come to a consensus about the patterns identified in the data.
Results
Description of the Sample
Of 760 participants for whom complete data were available, 64 (8.4%) had no living spouse or children at the time of dementia onset. Their mean age at dementia onset was 87 (standard deviation [SD] 7 years), with a median age of 86 and a range of 71–103. Most members of this cohort had been diagnosed with Alzheimer’s type dementia (88%) and were women (70%). The average number of years of education completed was 14 (SD 4). Using categories employed by the ACT study, most participants in this group (86%) were reported as White, with small percentages reported as each of the other available categories: Black (3%), Asian (6%), or Other/Mixed (5%). None were reported as Native American.
At the ACT study visit that triggered a dementia evaluation, members of the analytic sample reported difficulty with, on average, 1.6 (SD 2.1) ADLs and 1.7 (SD 1.8) IADLs. Comorbidity scores in this group averaged 0.34 (SD 0.12) as measured by the Charlson index, and 0.62 (SD 0.05) as measured by the Klabunde index. The majority of those in the analytic sample (58%) were widowed at the time they developed dementia, nearly one quarter (22%) had never married, and a smaller proportion (14%) were divorced or separated. Nearly half (47%) were living alone at dementia onset, and more than one third (34%) were living with unrelated persons such as hired caregivers, whereas smaller proportions were living in a nursing home (9%) or with relatives or friends (9%) (Table 1).
Table 1.
Characteristics of ACT Participants With Dementia (categorical variables)
| Full sample (N = 848) | Excluded, missing data (N = 88) | Excluded, not kinless (N = 696) | Kinless (N = 64) | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Gender | ||||
| Male | 312 (37) | 23 (26) | 270 (39) | 19 (30) |
| Female | 536 (63) | 65 (74) | 426 (61) | 45 (70) |
| Race | ||||
| White | 764 (90) | 74 (84) | 635 (91) | 55 (86) |
| Black | 28 (3) | 4 (5) | 22 (3) | 2 (3) |
| Asian | 32 (4) | 7 (8) | 21 (3) | 4 (6) |
| Native American | 3 (0.3) | 1 (1) | 2 (0.3) | 0 |
| Other/Mixed | 21 (2) | 2 (2) | 16 (2) | 3 (5) |
| Dementia type | ||||
| Alzheimer’s | 702 (83) | 64 (72) | 582 (84) | 56 (88) |
| Other | 146 (17) | 24 (27) | 114 (17) | 8 (12) |
| Marital status at onset | ||||
| Married or living as married | 262 (31) | 261 (38) | 0 | |
| Never married | 21 (2) | 7 (1) | 14 (22) | |
| Divorced/separated | 93 (11) | 82 (12) | 9 (14) | |
| Widowed | 366 (43) | 327 (47) | 37 (58) | |
| Other | 24 (3) | 19 (3) | 4 (6) | |
| Living situation at onset | ||||
| With spouse only | 188 (22) | 186 (27) | 0 | |
| With spouse and other relatives | 28 (3) | 27 (4) | 0 | |
| With relatives or friends | 97 (11) | 89 (13) | 6 (9) | |
| With unrelated persons (e.g., hired caregiver) | 176 (21) | 152 (22) | 22 (34) | |
| In nursing home | 49 (6) | 41 (6) | 6 (9) | |
| Alone | 232 (27) | 198 (28) | 30 (47) | |
| Refused/do not know | 4 (0.5) | 3 (0.5) |
Note: ACT = Adult Changes in Thought.
Compared with the 696 ACT participants who were not kinless, members of the analytic sample were on average 2 years older at the time of dementia onset, with a mean age of 87 (SD 7) years, versus 85 (SD 6) years (p = .04). Among kinless participants, significantly higher proportions were diagnosed with Alzheimer’s type dementia (88% vs 84%, p = .02), were women (70% vs 61%, p = .03), and were living alone (47% vs 28%, p < .001) or in a nursing home (9% vs 6%, p < .001) at the time of dementia onset (Table 1). In other respects, participants in the two groups were similar: most were White and had relatively high levels of educational attainment. At the time of dementia onset, the average number of comorbid conditions, overall comorbid burden, and level of functional impairment were similar between the two groups (Table 2).
Table 2.
Characteristics of ACT Participants With Dementia (quantitative variables)
| Full sample (N = 848) | Missing data (N = 88) | Excluded, not kinless (N = 696) | Kinless (N = 64) | |
|---|---|---|---|---|
| Age | ||||
| Mean | 85 | 84 | 85 | 87 |
| Median | 86 | 85 | 86 | 88 |
| Standard deviation | 6 | 6 | 6 | 7 |
| Range | 63–103 | 68–98 | 67–102 | 71–103 |
| Years of education completed | ||||
| Mean | 14 | 14 | 14 | 14 |
| Median | 13 | 14 | 14 | 13 |
| Standard deviation | 3 | 2 | 3 | 4 |
| Range | 4–21 | 8–19 | 4–21 | 6–21 |
| Number of ADLs difficult at onset | ||||
| Missing | N = 158 (19%) | N = 77 (88%) | N = 72 (10%) | N = 9 (14%) |
| Mean | 1.5 | 1.5 | 1.6 | |
| Median | 1 | 1 | 0 | |
| Standard deviation | 1.8 | 1.8 | 2.1 | |
| Range | 0–6 | 0–6 | 0–6 | |
| Number of IADLs difficult at onset | ||||
| Missing | N = 183 (22%) | N = 77 (88%) | N = 96 (14%) | N = 10 (16%) |
| Mean | 1.5 | 1.6 | 1.7 | |
| Median | 1 | 1 | 1 | |
| Standard deviation | 1.8 | 1.7 | 1.8 | |
| Range | 0–6 | 0–5 | 0–5 | |
| Charlson Comorbidity Score | ||||
| Mean | 0.6 | 0.8 | 0.6 | 0.3 |
| Median | 0 | 0 | 0 | 0 |
| Standard deviation | 1.4 | 1.6 | 1.4 | 1.0 |
| Range | 0–9 | 0–8 | 0–9 | 0–5 |
| Klabunde Comorbidity Score | ||||
| Mean | 1.9 | 2.0 | 1.9 | 1.6 |
| Median | 1.0 | 1.0 | 1.0 | 1.0 |
| Standard deviation | 2.1 | 2.3 | 2.1 | 1.8 |
| Range | 0–11 | 0–9 | 0–11 | 0–8 |
Notes: ACT = Adult Changes in Thought; ADL = activity of daily living; IADL = instrumental activity of daily living.
Description of Contact Persons
Contact persons listed for ACT participants were asked to complete a survey shortly after dementia onset, which included questions about their relationship to the participant, whether the participant needed a caregiver, and whether the respondent was the caregiver. Responses to this survey were available for only one third (N = 21, 33%) of the participants in our analytic sample. Nonetheless, given the overall paucity of information about the social and caregiving networks of older adults living with dementia who are without immediate family, we felt that these data were worth sharing.
Among the 21 survey responses available, most contact persons indicated that the participant did need a caregiver (N = 15, 75%), and that they themselves were the caregivers (N = 18, 86%). The relationships most frequently identified by these respondents were hired caregiver (N = 6, 29%), friend (N = 3, 14%), and nephew or niece (N = 3, 14%). Other relationships listed included grandchild, sister-in-law, son- or daughter-in-law (listed thus on the form), and other in-law. ACT study research coordinators completed surveys for four participants in this group (19% of 21 available responses) for whom no contact person could be located. A high proportion (N = 16, 76%) of respondents were female (Table 3)
Table 3.
Characteristics of Contact Persons of Kinless ACT Participants (from 21 available survey responses)
| N (%) | |
|---|---|
| What is the contact person’s gender? | |
| Male | 5 (24) |
| Female | 16 (76) |
| Data missing | 1 (5) |
| Are you the person who cares for [the participant] or makes certain s/he gets the care s/he needs? | |
| Yes | 18 (86) |
| No | 3 (14) |
| What is your relationship to him/her? | |
| Spouse | 0 |
| Child | 0 |
| Adopted stepchild | 0 |
| Nephew or niece | 3 (14) |
| Brother or sister | 0 |
| Grandchild | 1 (5) |
| Sister-in-law | 1 (5) |
| Friend | 3 (14) |
| Other in-law | 1 (5) |
| Hired caregiver | 6 (29) |
| Son- or daughter-in-law | 1 (5) |
| No relation/ patient coordinator | 4 (19) |
| Data missing | 1 (5) |
Note: ACT = Adult Changes in Thought.
Themes Emerging From Qualitative Analysis
In qualitative analysis of ACT study administrative documents (handwritten comments and medical history documents), we identified four dominant themes relevant to understanding the circumstances and needs of these older adults who were without an immediate family when they developed dementia: (1) life trajectories, (2) caregiving resources, (3) care needs and gaps, and (4) turning points. We provide some excerpts within the text to illustrate these themes; additional example excerpts are presented in Table 4. For each excerpt, we provide the participant’s gender and age at dementia onset; we do not specify race because our sample included so few racialized minorities that the information could be identifying. We also identify the source of each quote, which could be clinical chart notes from the medical history document or the handwritten comments recorded by ACT staff after each research study visit.
Table 4.
Quote Excerpts Illustrating Themes Identified in Qualitative Analysis
| Quote excerpt | Gender | Age at onset | Source | |
|---|---|---|---|---|
| Q1 | “Retired electrical engineer, never married...” | Male | 80 | ACT study handwritten comments |
| Q2 | “Her husband of 8 months died in WWII and she never remarried…Lives alone (for now) since her sister’s death last Nov. Gets tearful when she talks about her – they lived together for 43 years….” | Female | 89 | ACT study handwritten comments |
| Q3 | “TC [telephone call] - patient’s stepson calls to report that care givers at home where patient lives state that patient is becoming more confused and they worry about his degree of cognitive awareness- they suggested cognitive evaluation” | Male | 91 | Clinical chart notes |
| Q4 | “Over the last one month pt has been feeling fatigued very easily, goes to sleep at the drop of the hat, and is also depressed that her nephew and his family have moved permanently to [town several hours drive from Seattle]. She does have a niece that lives in San Diego and the pt herself does not like San Diego.” | Female | 86 | Clinical chart notes |
| Q5 | “One of her neighbors come[s] in the am & helps her get up, others take her to MD appts, cook, etc. She’s getting by.” | Female | 88 | ACT study handwritten comments |
| Q6 | “TC from food bank director where pt volunteers. States that several employees have come to her over the past 2 mos expressing concern about pt. Pt has attended ‘super regularly for years’ but recently, co-workers have had to go to her house to check on her and she has been asleep and forgotten to come in.” | Female | 79 | Clinical chart notes |
| Q7 | “Pt lives in senior housing and has mild dementia. Home health social workers addressed transportation issues and helped make patient aware of [publicly subsidized buses for seniors], [name of company] shuttle, volunteer transportation. Pt continues to attend her church in [name of suburb] and is active with classic car club.” | Female | 73 | Clinical chart notes |
| Q8 | “pt is here for follow-up, accompanied by her POA for healthcare, from [name of agency] Guardians - pt is 40 minutes late to appt [appointment] -Depression. Pt is doing a bit better now that she has the support of guardian. Still overwhelmed by her living condition but is getting help…” | Female | 76 | Clinical chart notes |
| Q9 | “Disheveled. Has difficulty with bathing, doing household chores, shopping, per questionnaire.” | Male | 85 | Clinical chart notes |
| Q10 | “Spoke with patient by phone. He states that he understands why his medical team is recommending that he hire assistance with medication management but he does not want to do this. He states he is having trouble managing his blood sugar testing and insulin but does not want to hire anyone to assist.” | Male | 86 | Clinical chart notes |
| Q11 | “The patient lives alone in her home but has a friend who helps her with some of her affairs such as keeping her book work, etc. …She admits to having some difficulty with her memory. … She also has had some financial problems in that some plumbers recently took advantage of her, charging her $11,000 for a plumbing bill.” | Female | 88 | Clinical chart notes |
| Q12 | “Consult w/Social Worker: … M [male] widower who lives alone in his own apartment… His only relative is a nephew, who he reports lives 45 minutes away… He said he would call nephew to ask about transporting him to and from hospital...” | Male | 86 | Clinical chart notes |
| Q13 | “TC - One of pt’s friends is calling to let the clinic know that pt is having a very hard time remembering things. She forgot an appointment last week and does not have the assistance of a family member or a legal POA. Friend is not available today, however, she wanted to let the MD know that pt is not aware of an appointment and that she needs some help” | Female | 86 | Clinical chart notes |
| Q14 | “OV Right knee injured when she tripped over a small box in [grocery store] a few days ago. Fell directly onto the kneecap. Says she seems to have a tendency to falls.… Has thought of selling house, move to senior housing- [name of facility] -but that’s quite expensive.” | Female | 83 | ACT study handwritten comments |
| Q15 | [#1, recorded 4 years before ACT diagnosis]: “She & spouse moved to AFH [adult family home] because he needed more care & it was easier for her to move w/him rather than going back & forth “ [#2, recorded year of ACT diagnosis]: “…pt currently lives in AFH… Pt’s husband passed away a few years ago – pt has no children - has designated AFH owner as her DPOA” |
Female | 96 | #1 ACT study handwritten comments #2 Clinical chart notes |
Notes: ACT = Adult Changes in Thought; DPOA = durable power of attorney; POA = power of attorney; pt = patient.
Theme 1: life trajectories
Although all older adults in our sample were kinless at the time they developed dementia, we found that a variety of different life trajectories had led them to this point. Some participants never married or had children (Q1, i.e., quote excerpt #1 in Table 4). Others had been married and then divorced, though ACT data provide no details on timing. For example, after a study visit with one man who was 87 at dementia onset, the ACT study staff noted that he was:
Rather negative, probably depressed, divorced and doesn’t want anything to do w/ women anymore… claims he was cheated out of kids and that’s why he’s in such a mess right now.
More than half the participants in our sample had outlived a spouse, and some had also outlived children and other close family. For example, one entry in the clinical chart notes of a participant who was 88 years old at dementia onset noted that:
She was widowed 8 years ago… her son was seriously ill and has since died, 6 years ago… Pt’s [patient’s] dtr [daughter] died in 1980. Pt is one of many offspring and they have all since expired. Pt notes that she feels isolated, lonely, and still working through grief and loss issues.
Some of the losses described in these materials had happened long ago, others more recently (Q2).
Theme 2: caregiving resources
Though they did not have a living spouse or children at dementia onset, many of those included in the analytic cohort did have other important and supportive relationships.
Among other relatives who provided support, most frequently mentioned in the data we examined were nieces, nephews, and sisters; others mentioned were grandchildren, step-children, god-children, cousins, brothers, and sisters-in-law. Sometimes, the first mention of memory problems came from such relatives (Q3). The nature and degree of involvement of these other relatives varied, however, with some providing extensive support, while others appeared to have a more distant or circumscribed role (Q4).
For some participants, friends and neighbors provided significant assistance, including daily hands-on caregiving support (Q5). Friends sometimes also took on legal responsibilities. For example, the clinical chart notes for one participant who was 71 years old at dementia onset mention that:
pt’s [patient’s] roommate is DPOA [durable power of attorney] - roommate is finding out that pt has a huge financial mess - pt had paid long-term care insurance for 12 years, but stopped payments and is now trying to get reinstated - needs MD letter documenting cognitive impairment.
In many instances, however, neighbors and other community members appeared to have been pulled into participants’ care at the moments of crisis, as a form of rescue, with no evidence of ongoing involvement (Q6). For example, the chart comment recorded after an ACT study visit for one female participant who was 87 years old at the time of dementia onset mentioned that she “fell December 31… was alone and unconscious for 2 days when a neighbor came in and found her. Broken hip….”
Other formal community resources (Q7) were also mentioned in the records we examined, including public programs providing transportation assistance, senior centers, organized activities in retirement homes, and fitness classes. In several instances, we saw mention of the need for a legal guardian or plans to appoint one, but as far as we could determine, only one person in this sample had a legal guardian in place at the time of dementia onset (Q8).
Theme 3: care needs and gaps
The unmet needs experienced by members of the analytic sample likely far exceed what was visible in the materials we examined, but we did identify several kinds of care needs and gaps.
Some participants remained highly functional after the onset of dementia, but many others needed significant help with bathing and dressing (Q9). Difficulty with medication management often appeared as a reason for seeking additional caregiving help or being urged to do so (Q10). For example, this documentation appeared in the clinical chart notes of one participant who was 87 years old when she developed dementia:
Sleep disorder-depression w/failure to thrive. Forgot to refill paxil - caretaker strongly urged to make sure pt takes her paxil every day, as her negativity and energy level do improve when she is on medication.
Managing finances was also noted as an area of difficulty for a number of participants in the analytic sample. The documents we examined revealed instances of caregivers taking over financial management (Q11), or clinical providers recommending the appointment of a durable power of attorney.
From our data, mobility limitations and falls were a common reason for emergency room visits and skilled nursing facility stays, and often precipitated moving to an institutional setting or into a higher level of care. For example, in a note recorded after an Emergency Room visit by one participant who was 87 years old at dementia onset, a clinician stated that:
I specifically asked the patient what he would do if he went home and he said, ‘probably fall.’ Pt … does not appear safe for home discharge at this time- pt is fairly unsteady with his gait and has no help at home.
Driving was among the activities that often became problematic or dangerous. For example, we saw this comment in the clinical chart notes of one participant who was 76 years old at dementia onset:
pt reports that her ‘fender-benders’ have involved scraping the passenger door in a parking garage and or scraping another car while backing out of a parking spot - she feels she may have some depth perception issues … and that she may still be getting used to her new car, which is wider than what she’s used to - otherwise, she feels her driving is safe - she is reluctant to give it up
Those who did not drive often relied on others for transportation to medical appointments, grocery shopping, and other events, which could be challenging (Q12).
Older adults in our sample also frequently had difficulty managing appointments. Because the clinical chart notes we examined consisted primarily of records of appointments, we expect that these data will be systematically missing for many who were unable to attend appointments because of such difficulties. Even so, we saw frequent mentions of difficulty managing appointments or getting lost on the way to the clinic (Q13).
Some of the care needs that we identified for this sample are common among older adults more generally (Committee on Family Caregiving for Older Adults et al., 2016), but the challenges and implications may be different when dementia is a factor (Freedman et al., 2022).
Theme 4: turning points in caregiving arrangements
Among older adults living with dementia, frailty and severity of symptoms are associated with high levels of psychological stress and burden among family caregivers (Abreu et al., 2020), which may lead families to seek institutional placement. Among kinless older adults in our sample, we observed several different kinds of turning points that precipitated a change in caregiving arrangements. A health crisis or the worsening of an existing health condition sometimes created needs that exceeded what existing arrangements could support. In such situations, however, costs often presented obstacles to making new arrangements (Q14). For example, in the clinical chart notes of one male participant who was 82 years old at dementia onset, we saw the following notation:
OV [office visit] - consult with social services re: scheduling surgery/ after-surgery care- pt appears to have nobody to assist him after surgery- …Explained skilled nursing facility benefit and non-coverage for custodial care- Pt ruled out temporary assisted living or NHP [nursing home placement] as post-op care plan due to cost.
In some cases, extrinsic changes affecting housing, such as rising rents and landlords’ priorities, negatively affected the existing caregiving arrangements. For example, a clinician recorded in the chart notes of a participant who was 82 years old at dementia onset that:
Pt lives alone, in the same appt [apartment] for 44 years - the rent has recently increased, so pt will be losing two of her neighbors who have provided her with shopping help and transportation assistance - pt has no siblings and no children … Her goal is to remain where she resides bringing in needed resources for as long as she is able. Her concern is the increase in her rent and the upcoming loss of some neighbor support.
We also observed several instances in which an ACT participant’s role as a caregiver for another person affected their own care. Some participants had previously moved into an institutional setting to meet the needs of a spouse, which could mean that they were well situated to access services when they developed dementia (Q15). A participant’s role as a caregiver could also trigger an intervention, if it created a situation that was dangerous for both parties. For example, we saw in the clinical chart notes of a participant who was 72 years old at dementia onset the record of a telephone call noting that she had been referred to social services:
Reason for Referral: Care coordination, unsafe living situation, pt unable to care for her roommate at this time. Pt and her roommate (w/ dementia) were stuck in their garage for an undetermined period of time, possibly up to two days, after roommate had a fall and Pt was unable to rescue her and locked them in the garage. Pt was unable to come up with the idea of opening the garage door from the inside to go out into the world to get them help. Plan: Pt’s friends are hoping to help Pt and roommate move to assisted living at [name of facility].
Notably, only one of these turning points was driven by changes in the health condition of the individual; others had to do with caregiving relationships and the broader community environment.
Discussion
Our secondary analysis of existing ACT study research data and administrative documents affords a rare window into the circumstances of kinless older adults living with dementia, a group that is not well described in the existing geriatrics literature.
We used a rigorous approach to identify our sample of 64 ACT participants who were without a living spouse or children at the time of dementia onset. The prevalence rate of 8.4% that we found for this group is slightly higher than estimates of 6.6% (Margolis & Verdery, 2017) and 7.4% (Plick et al., 2021) reported for the broader U.S. population of older adults (not limited to those living with dementia) in recent research based on data from the Health and Retirement Study.
Participants in this group were advanced in age when they developed dementia, at an average of 87 (SD 7) years old, 2 years older than the average among those who were not kinless. It should be noted that ACT study visits are scheduled on the basis of the calendar, and not due to participant concerns regarding cognition, so differences in age at onset are unlikely to be ascribable to differences in vigilance. Half were living alone at the time of dementia onset, and three quarters were women, consistent with the findings of other research based on national surveys (Margolis & Verdery, 2017). Living alone does not necessarily indicate loneliness or social isolation (Klinenberg, 2016), and some in our sample clearly had extensive social networks, but living alone with dementia entails significant challenges and risks (Gibson & Richardson, 2017; Portacolone et al., 2019).
Our findings suggest that older adults who are kinless when they develop dementia are a group with diverse life trajectories and family histories, defined by a shared predicament to which anyone may be susceptible in late life. The proportion in this group who had never married (22%) was much higher than the 7% rate reported for the broader population of older adults in the United States (Valerio et al., 2021), but far more (58%) had been widowed or divorced, and at least some outlived their children. Most in our sample were women who became kinless late in life and unexpectedly (Verdery & Margolis, 2017). Though not all lived alone and some had good support networks, the partial glimpses that our data afford into the everyday lives of kinless older adults with dementia suggest that like the older adults living alone with cognitive impairment interviewed by sociologist Elena Portacolone, many struggled to manage everyday practical challenges in the context of expectations “that they should be responsible for their own health independently, with little help from others, as well as from institutions” (Portacolone et al., 2019). Our findings thus resonate with other works calling attention to new forms of insecurity affecting the life course, which particularly affect those at the upper extremes of old age (Grenier & Phillipson, 2018).
To our knowledge, our study is the first to offer detailed insight into sources of unpaid care for older adults living with dementia who do not have a living spouse or children, the groups from which unpaid dementia caregivers are most often drawn. Some had important supportive relationships with other relatives, friends, and/or neighbors, who in some cases provided extensive care. These findings suggest that clinicians and health systems should be alert to the possible presence and role of unpaid caregivers in a broad variety of family and nonfamily relationships (Burns et al., 2013). At the same time, however, from the evidence we saw, it seemed that such individuals tended to become involved only in moments of crisis and were unlikely to assume more substantial or ongoing caregiving roles. For 4 (19%) of the 21 participants for whom results of a contact person information survey were available, ACT study research coordinators were unable to locate a contact person, suggesting a concerning lack of social and family support.
We identified several turning points that could trigger changes in caregiving arrangements. Functional decline accompanying health changes, including loss of mobility as well as progression of underlying dementia, could severely strain the established caregiving arrangements that had been working for a time. We also found that some kinless older adults in our cohort were serving (or previously had served) as caregiver for someone else, and this could affect their own access to care. This reveals the complexity of social relations and defies the assumption that a kinless older adult would only be receiving and not providing care. It also suggests that an individual’s role as a caregiver should be treated by clinicians and health systems as relevant to their own health and well-being (Lyons & Lee, 2018).
Other turning points reflected extrinsic factors, such as rising rents that erode the continuity of local neighborhood communities, with particularly devastating effects on older adults such as those in our sample, who may rely heavily on networks of neighbors for social support. This suggests to us that efforts to support older adults living with dementia who do not have close family may need to extend beyond the health system to address social determinants of health and include community organizations, policy-makers, and other parties that shape the lived environments of kinless older adults with dementia. For example, a recent study documenting the significant support that neighbors in Berlin, Germany, provide to older adults without family concludes that “careful design of … places of encounter [such as] neighborhood cafés, libraries, urban parks, and allotment sites may contribute to attracting people from different generations and thus, helping older people to develop ties with their neighbors, establish contacts, and access support networks,” and that gentrification presents particular dangers for such individuals (Enßle et al., 2022)
The importance of these extrinsic factors is a finding that also resonates with other research suggesting that older adults, especially those who are disadvantaged and/or those at the upper extremes of old age, may be particularly vulnerable to the effects of changes to public programs and services. As gerontological researchers Amanda Grenier and Christopher Phillipson note, “older people increasingly find themselves confronted by the effects of insecure work (and care) histories, combined with reduced forms of social protection and public safety nets” (2018, p. S16). Thus it follows that policies that address economic instability, like rent control or more robust safety net cash benefits, could have spillover benefits of strengthening and stabilizing community care networks for older adults living with dementia who cannot rely on close family for care.
The care needs and gaps experienced by older adults in our sample were extensive, and likely interacted in ways that would tend to compound them. Although the problems we saw mentioned in clinical chart notes were clearly noticed by the clinicians who recorded them, it was not clear if or how the health system was able to gather and act upon this clinical knowledge. As the population ages and the numbers of people who develop dementia grow, health systems and public agencies may need to provide more direct support for caregiving, rather than assuming that family will provide unpaid care for people with dementia.
This study has limitations, reflecting the limitations of our data sources. Information about social support and caregiving is not systematically collected in ACT study visits nor in clinical encounters, so the kind and amount of information that was captured was limited and uneven. The ACT study historically has collected little information about income and recorded gender as a simple binary. Clinical chart notes, as records of health system interactions, necessarily provide less information about people who have fewer such interactions, whether because they are healthier or because they have less access to care. This documentation also reflects what the clinician considers important, which may not reflect matters of the greatest significance to the patient. The legal, economic, cultural, and social service contexts of the Seattle region likely differ from those found elsewhere, and the ACT study population overall has relatively high education levels, includes few racialized minorities, and includes only people with health insurance, which may limit the generalizability of findings. At the same time, the challenges that we saw among these participants, who were accessing care within an integrated health system, would likely be that much greater in less integrated settings.
These limitations notwithstanding our study is unique in offering a multidimensional portrait of older adults who were kinless when they developed dementia, a difficult-to-research group who may be particularly vulnerable to precarity and unmet needs for care. Though participants are not necessarily representative of the U.S. population more generally, countervailing strengths of the ACT study are that it is a community-based sample of community-dwelling older adults, diagnosed through a rigorous research-based assessment, on whom we have a wealth of high-quality data (health records data and administrative data, as well as ACT study data) collected prospectively over time. The richness of the qualitative analysis was the result of using data that were not deliberately collected to answer questions about social support, but rather happened to be recorded as part of research exploring other questions and as part of the diagnostic process; we were able to learn as much as we did is a testament to how compelling are the human stories of persons living with dementia, and how much they need to rely on others. We believe that ours is the first study to describe this population and the first to examine existing research data and administrative documents in this way. We hope that others too will pursue research into the lives, relationships, needs, views, and experiences of kinless older adults with dementia.
Our findings have implications for clinical practice, health policy, and the design of health systems. Clinicians could be encouraged more systematically to collect and record information about older adults’ own roles as caregivers for others, and about the supportive relationships they rely on, including with neighbors, friends, and other nonkin. When possible, clinicians should keep open lines of communication with such support persons. Health systems should support clinicians in these efforts, and should more systematically collect and act upon the knowledge gained and recorded by clinicians about older adults’ supportive relationships. Actions that the health system could take might include, for example, ensuring that a dementia diagnosis is followed up by a comprehensive needs assessment (Abreu et al., 2019) and that older adults with dementia who have limited caregiving support are referred to social services as early as possible. Further development of home-based primary care services, which have been shown to be an effective way to care for patients with dementia (Zimmer & Yang, 2018), could prove especially beneficial for those who are kinless. Health systems that employ a robust staff of social workers with specific training in dementia care could help kinless older adults access personal care services and other forms of needed support, and “models of care in which social workers are embedded in outpatient clinics may be particularly valuable” (Farrell et al., 2021). Similar models have been implemented, for example, in Denmark, where “older Danes living alone can access state-subsidized home care aides rigorously trained in dementia care—a service essential to them—soon after they receive a diagnosis of cognitive impairment” (Rosenwohl-Mack et al., 2021). In the United States, there has been little investment in the social safety net; such examples speak to what might be possible if as a society we chose to invest in social services and health infrastructure. On a broader social and political level, the predicament of the growing number of kinless older adults demands of all of us that we work to better support the most vulnerable among us.
Acknowledgments
We wish to acknowledge valuable advice and assistance from Cathy Hutchison, Duryah Mohameth, Jennifer Macuiba, and KatieRose Johnson. For helpful comments on earlier versions of this paper, thanks to Uta Poiger, Priti Ramamurthy, Lynn M. Thomas, and audiences at the ACT Symposium, the Institute of Gerontology at Wayne State University, the Center for Studies in Demography and Ecology at the University of Washington, the Institute for Social Ethnology at the University of Hamburg, and the Department of Anthropology at the University of Copenhagen. For their constructive criticism and advice, we are grateful also to the journal’s anonymous reviewers.
Contributor Information
Janelle S Taylor, Department of Anthropology, University of Toronto, Toronto, Ontario, Canada.
Marlaine S Figueroa Gray, Kaiser Permanente Washington Health Research Institute, Seattle, Washington, USA.
Corinne M Mar, International Clinical Research Center, Department of Global Health, University of Washington, Seattle, Washington, USA.
Paul K Crane, Department of Medicine, University of Washington, Seattle, Washington, USA.
Hitomi Kariya, Department of Health Systems and Population Health, University of Washington, Seattle, Washington, USA.
Callie Freitag, Evans School of Public Policy, University of Washington, Seattle, Washington, USA.
Priyanka Taneja, Pacific Northwest University of Health Sciences, Yakima, Washington, USA.
Arvind Ramaprasan, Kaiser Permanente Washington Health Research Institute, Seattle, Washington, USA.
Bettina Shell-Duncan, Department of Anthropology, University of Washington, Seattle, Washington, USA.
Ann M O’Hare, Division of Nephrology, University of Washington, Seattle, Washington, USA.
Clara Berridge, School of Social Work, University of Washington, Seattle, Washington, USA.
Elizabeth K Vig, Department of Medicine, University of Washington, Seattle, Washington, USA; Geriatrics and Extended Care, VA Puget Sound Health Care System, Seattle, Washington, USA.
Stephanie G B Wheeler, Department of Medicine, University of Washington, Seattle, Washington, USA.
Manu Thakral, Manning College of Nursing and Health Sciences, University of Massachusetts Boston, Boston, Massachusetts, USA.
Rene J Hawkes, Kaiser Permanente Washington Health Research Institute, Seattle, Washington, USA.
Eric B Larson, Kaiser Permanente Washington Health Research Institute, Seattle, Washington, USA.
Funding
This work was supported by grant R21AG058056 from the National Institute on Aging at the National Institutes of Health. The Adult Changes in Thought study was supported by U01AG006781. Partial support for this research came from a Eunice Kennedy Shriver National Institute of Child Health and Human Development research infrastructure grant, P2C HD042828, to the Center for Studies in Demography and Ecology (CSDE) at the University of Washington.
Conflict of Interest
None declared.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Abreu, W., Tolson, D., Jackson, G. A., & Costa, N. (2020). A cross-sectional study of family caregiver burden and psychological distress linked to frailty and functional dependency of a relative with advanced dementia. Dementia, 19(2), 301–318. doi: 10.1177/1471301218773842 [DOI] [PubMed] [Google Scholar]
- Abreu, W., Tolson, D., Jackson, G. A., Staines, H., & Costa, N. (2019). The relationship between frailty, functional dependence, and healthcare needs among community-dwelling people with moderate to severe dementia. Health & Social Care in the Community, 27(3), 642–653. doi: 10.1111/hsc.12678 [DOI] [PubMed] [Google Scholar]
- Allen, S. M., Piette, E. R., & Mor, V. (2014). The adverse consequences of unmet need among older persons living in the community: Dual-eligible versus Medicare-only beneficiaries. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69(Suppl 1), S51–S58. doi: 10.1093/geronb/gbu124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association Task Force on DSM-IV & American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders: DSM-IV (4th ed.). American Psychiatric Association. [Google Scholar]
- Anderson, R. T., Bradham, D. D., Jackson, S., Heuser, M. D., Wofford, J. L., & Colombo, K. A. (2000). Caregivers’ unmet needs for support in caring for functionally impaired elderly persons: A community sample. Journal of Health Care for the Poor and Underserved, 11(4), 412–429. doi: 10.1353/hpu.2010.0776 [DOI] [PubMed] [Google Scholar]
- Bookman, A., & Harrington, M. (2007). Family caregivers: A shadow workforce in the geriatric health care system? Journal of Health Politics, Policy and Law, 32(6), 1005–1041. doi: 10.1215/03616878-2007-040 [DOI] [PubMed] [Google Scholar]
- Burns, C. M., Abernethy, A. P., Dal Grande, E., & Currow, D. C. (2013). Uncovering an invisible network of direct caregivers at the end of life: A population study. Palliative Medicine, 27(7), 608–615. doi: 10.1177/0269216313483664 [DOI] [PubMed] [Google Scholar]
- Carney, M. T., Fujiwara, J., Emmert, B. E., Liberman, T. A., & Paris, B. (2016). Elder orphans hiding in plain sight: A growing vulnerable population. Current Gerontology and Geriatrics Research, 2016, 1–11. doi: 10.1155/2016/4723250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin, C. C., Connors, H. L., Teaster, P. B., Wood, E., Sager, Z. S., & Moye, J. (2021). Unrepresented adults face adverse healthcare consequences: The role of guardians, public guardianship reform, and alternative policy solutions. Journal of Aging & Social Policy, 34(3),–418–437.. doi: 10.1080/08959420.2020.1851433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson, M., Szatrowski, T. P., Peterson, J., & Gold, J. (1994). Validation of a combined comorbidity index. Journal of Clinical Epidemiology, 47(11), 1245–1251. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- Clare, L., Martyr, A., Henderson, C., Gamble, L., Matthews, F. E., Quinn, C., Nelis, S. M., Rusted, J., Thom, J., Knapp, M., Hart, N., & Victor, C. (2020). Living alone with mild-to-moderate dementia: Findings from the IDEAL cohort. Journal of Alzheimer’s Disease, 78(3), 1207–1216. doi: 10.3233/JAD-200638 [DOI] [PubMed] [Google Scholar]
- Committee on Family Caregiving for Older Adults, Board on Health Care Services, Health and Medicine Division, & National Academies of Sciences, Engineering, and Medicine. (2016). In Schulz R. & Eden J. (Eds.), Families caring for an aging America National Academies Press Chapter 2, Older Adults Who Need Caregiving and the Family Caregivers Who Help Them. doi: 10.17226/23606 [DOI] [PubMed] [Google Scholar]
- Creswell, J. (2009). Research design; qualitative, quantitative, and mixed methods approaches (3rd ed.). Sage Publications. [Google Scholar]
- de Medeiros, K., Girling, L. M., & Berlinger, N. (2022). Inclusion of people living with Alzheimer’s disease or related dementias who lack a study partner in social research: Ethical considerations from a qualitative evidence synthesis. Dementia, 14713012211072500. doi: 10.1177/14713012211072501 [DOI] [PubMed] [Google Scholar]
- Duane, F., Brasher, K., & Koch, S. (2013). Living alone with dementia. Dementia—International Journal of Social Research and Practice, 12(1), 123–136. doi: 10.1177/1471301211420331 [DOI] [PubMed] [Google Scholar]
- Edwards, R. D., Brenowitz, W. D., Portacolone, E., Covinsky, K. E., Bindman, A., Glymour, M. M., & Torres, J. M. (2020). Difficulty and help with activities of daily living among older adults living alone with cognitive impairment. Alzheimer’s & Dementia, 16(8), 1125–1133. doi: 10.1002/alz.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enßle, F., Dirksmeier, P., & Helbrecht, I. (2022). Does spatial proximity supplant family ties? Exploring the role of neighborly support for older people in diverse, aging cities. Urban Geography, 43(3), 344–363. doi: 10.1080/02723638.2020.1857991 [DOI] [Google Scholar]
- Farrell, T. W., Catlin, C., Chodos, A. H., Naik, A. D., Widera, E., & Moye, J. (2021). Caring for unbefriended older adults and adult orphans: A clinician survey. Clinical Gerontologist, 44(4), 494–503. doi: 10.1080/07317115.2019.1640332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, V. A., Bandeen-Roche, K., Cornman, J. C., Spillman, B. C., Kasper, J. D., & Wolff, J. L. (2022). Incident care trajectories for older adults with and without dementia. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 77(Suppl_1), S21–S30. doi: 10.1093/geronb/gbab185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese, S. (2019). Qualitative data analysis with Atlas.ti (3rd edition). Sage Publications. [Google Scholar]
- Gibson, A. K., & Richardson, V. E. (2017). Living alone with cognitive impairment: Findings from the National Health and Aging Trends Study. American Journal of Alzheimer’s Disease & Other Dementias, 32(1), 56–62. doi: 10.1177/1533317516673154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier, A., & Phillipson, C. (2018). Precarious aging: Insecurity and risk in late life. Hastings Center Report, 48, S15–S18. doi: 10.1002/hast.907 [DOI] [PubMed] [Google Scholar]
- Griffith, L. E., Gruneir, A., Fisher, K., Panjwani, D., Gandhi, S., Sheng, L., Gafni, A., Patterson, C., Markle-Reid, M., & Ploeg, J. (2016). Patterns of health service use in community living older adults with dementia and comorbid conditions: A population-based retrospective cohort study in Ontario, Canada. BMC Geriatrics, 16(1), 177. doi: 10.1186/s12877-016-0351-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jervis, L. L. (2006). The missing family: Staff perspectives on and responses to familial noninvolvement in two diverse nursing homes. Journal of Aging Studies, 20(1), 55–66. doi: 10.1016/j.jaging.2005.02.001 [DOI] [Google Scholar]
- Kasper, J. D., Freedman, V. A., Spillman, B. C., & Wolff, J. L. (2015). The disproportionate impact of dementia on family and unpaid caregiving to older adults. Health Affairs, 34(10), 1642–1649. doi: 10.1377/hlthaff.2015.0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde, C. N., Potosky, A. L., Legler, J. M., & Warren, J. L. (2000). Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology, 53(12), 1258–67. doi: 10.1016/s0895-4356(00)00256-0 [DOI] [PubMed] [Google Scholar]
- Klinenberg, E. (2016). Social isolation, loneliness, and living alone: Identifying the risks for public health. American Journal of Public Health, 106(5), 786–7. doi: 10.2105/AJPH.2016.303166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanowski, A., Fortinsky, R. H., Calkins, M., Devanand, D. P., Gould, E., Heller, T., Hodgson, N. A., Kales, H. C., Kaye, J., Lyketsos, C., Resnick, B., Schicker, M., & Zimmerman, S. (2018). Advancing research on care needs and supportive approaches for persons with dementia: Recommendations and rationale. Journal of the American Medical Directors Association, 19(12), 1047–1053. doi: 10.1016/j.jamda.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukull, W. A., Higdon, R., Bowen, J., McCormick, W. C., Teri, L., Schellenberg, G. D., van Belle, G., Jolley, L., & Larson, E. B. (2002). Dementia and Alzheimer disease incidence: A prospective cohort study. Archives of Neurology, 59(11), 1737–1746. doi: 10.1001/archneur.59.11.1737 [DOI] [PubMed] [Google Scholar]
- Lillo-Crespo, M., Riquelme, J., Macrae, R., De Abreu, W., Hanson, E., Holmerova, I., Cabañero, M. J., Ferrer, R., & Tolson, D. (2018). Experiences of advanced dementia care in seven European countries: Implications for educating the workforce. Global Health Action, 11(1), 1478686. doi: 10.1080/16549716.2018.1478686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons, K. S., & Lee, C. S. (2018). The Theory of Dyadic Illness Management. Journal of Family Nursing, 24(1), 8–28. doi: 10.1177/1074840717745669 [DOI] [PubMed] [Google Scholar]
- Margolis, R., & Verdery, A. M. (2017). Older Adults Without Close Kin in the United States. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 72(4), 688–693. doi: 10.1093/geronb/gbx068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., & Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34(7), 939–944. doi: 10.1212/wnl.34.7.939 [DOI] [PubMed] [Google Scholar]
- Montayre, J., Montayre, J., & Thaggard, S. (2019). The elder orphan in healthcare settings: An integrative review. Journal of Population Ageing, 12(4), 515–532. doi: 10.1007/s12062-018-9222-x [DOI] [Google Scholar]
- Montayre, J., Thaggard, S., & Carney, M. (2020). Views on the use of the term “elder orphans”: A qualitative study. Health & Social Care in the Community, 28(2), 341–346. doi: 10.1111/hsc.12865 [DOI] [PubMed] [Google Scholar]
- Montine Thomas, J., Sonnen Joshua, A., Montine Kathleen, S., Crane Paul, K., & Larson Eric, B. (2012). Adult changes in thought study: Dementia is an individually varying convergent syndrome with prevalent clinically silent diseases that may be modified by some commonly used therapeutics. Current Alzheimer Research, 9(6), 718–723. doi: 10.2174/156720512801322555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Alliance for Caregiving. (2017). Dementia caregiving in the U.S.https://www.caregiving.org/wp-content/uploads/2020/05/Dementia-Caregiving-in-the-US_February-2017.pdf
- Plick, N. P., Ankuda, C. K., Mair, C. A., Husain, M., & Ornstein, K. A. (2021). A national profile of kinlessness at the end of life among older adults: Findings from the Health and Retirement Study. Journal of the American Geriatrics Society, 69(8), 2143–2151. doi: 10.1111/jgs.17171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portacolone, E., Rubinstein, R. L., Covinsky, K. E., Halpern, J., & Johnson, J. K. (2019). The precarity of older adults living alone with cognitive impairment. Gerontologist, 59(2), 271–280. doi: 10.1093/geront/gnx193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portacolone, E., Torres, J. M., Johnson, J. K., Benton, D., Rapp, T., Tran, T., Martinez, P., & Graham, C. (2022). The Living Alone with Cognitive Impairment Project’s Policy Advisory Group on Long-Term Services and Supports: Setting a research equity agenda. International Journal of Environmental Research and Public Health, 19(10), 6021. doi: 10.3390/ijerph19106021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roofeh, R., Smith, D. M., & Clouston, S. A. P. (2020). Estimated prevalence of elder orphans using National Health and Aging Trends Study. Journal of Aging and Health, 32(10), 1443–1449. doi: 10.1177/0898264320932382 [DOI] [PubMed] [Google Scholar]
- Rosenwohl-Mack, A., Dubbin, L., Chodos, A., Dulaney, S., Fang, M. -L., Merrilees, J., & Portacolone, E. (2021). Use of Services by people living alone with cognitive impairment: A systematic review. Innovation in Aging, 5(1), igab004. doi: 10.1093/geroni/igab004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldaña, J. (2021). Coding Manual for Qualitative Researchers. Sage Publications. [Google Scholar]
- Soniat, B. A., & Pollack, M. (1994). Elderly orphans with Alzheimer’s disease: Non-traditional support systems. Clinical Gerontologist, 14(1), 33–44. doi: 10.1201/9781315825335-6 [DOI] [Google Scholar]
- Taylor, J. S., DeMers, S. M., Vig, E. K., & Borson, S. (2012). The disappearing subject: Exclusion of people with cognitive impairment and dementia from geriatrics research. Journal of the American Geriatrics Society, 60(3), 413–419. doi: 10.1111/j.1532-5415.2011.03847.x [DOI] [PubMed] [Google Scholar]
- Teng, E. L., Hasegawa, K., Homma, A., Imai, Y., Larson, E., Graves, A., Sugimoto, K., Yamaguchi, T., Sasaki, H., Chiu, D., & White, L. R. (1994). The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia. International Psychogeriatrics, 6(1), 45–58. doi: 10.1017/s1041610294001602 [DOI] [PubMed] [Google Scholar]
- Valerio, T., Knop, B., Kreider, R. M., & He, W. (2021). Childless older Americans: 2018 (No. P70-173; Current Population Reports, p. 25). U.S. Census Bureau. https://www.census.gov/content/dam/Census/library/publications/2021/demo/p70-173.pdf [Google Scholar]
- Verdery, A. M., & Margolis, R. (2017). Projections of white and black older adults without living kin in the United States, 2015 to 2060. Proceedings of the National Academy of Sciences, 114(42), 11109–11114. doi: 10.1073/pnas.1710341114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdery, A. M., Margolis, R., Zhou, Z., Chai, X., & Rittirong, J. (2019). Kinlessness around the world. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 74(8), 1394–1405. doi: 10.1093/geronb/gby138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, J. L., Feder, J., & Schulz, R. (2016). Supporting family caregivers of older Americans. New England Journal of Medicine, 375(26), 2513–2515. doi: 10.1056/nejmp1612351 [DOI] [PubMed] [Google Scholar]
- Yang, Y., Swinnerton, K., Portacolone, E., Allen, I. E., Torres, J. M., & Duchowny, K. (2022). Difficulties with activities of daily living and receipt of care among older adults with cognitive impairment: differences between those living alone and those living with others. Journal of Alzheimer’s Disease, 89(1), 31–37. doi: 10.3233/JAD-220172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer, R., & Yang, M. (2018). Growing role of home-based primary care for individuals with dementia. Journal for Nurse Practitioners, 14(3), 166–171. doi: 10.1016/j.nurpra.2017.11.019 [DOI] [Google Scholar]