Abstract
The glycocalyx is a polysaccharide structure that protrudes from the body of a cell. It is primarily conformed of glycoproteins and proteoglycans, which provide communication, electrostatic charge, ionic buffering, permeability and mechanosensation-mechanotransduction capabilities to cells. In blood vessels, the endothelial glycocalyx that projects into the vascular lumen separates the vascular wall from the circulating blood. Such a physical location allows a number of its components, including sialic acid, glypican-1, heparan sulfate and hyaluronan, to participate in the mechanosensation-mechanotransduction of blood flow-dependent shear stress, which results in the synthesis of nitric oxide and flow-mediated vasodilation. The endothelial glycocalyx also participates in the regulation of vascular permeability and the modulation of inflammatory responses, including the processes of leukocyte rolling and extravasation. Its structural architecture and negative charge work to prevent macromolecules greater than ~70 kDa and cationic molecules from binding and flowing out of the vasculature. This also prevents the extravasation of pathogens such as bacteria and virus, as well as that of tumor cells. Due to its constant exposure to shear and circulating enzymes such as neuraminidase, heparanase, hyaluronidase and matrix metalloproteinases, the endothelial glycocalyx is in a continuous process of degradation and renovation. A balance favoring degradation is associated with a variety of pathologies including atherosclerosis, hypertension, vascular aging, metastatic cancer and diabetic vasculopathies. Consequently, ongoing research efforts are focused on deciphering the mechanisms that promote glycocalyx degradation or limit its syntheses, as well as on therapeutic approaches to improve glycocalyx integrity with the goal of reducing vascular disease.
Introduction
All living cells have extracellular polysaccharide structures attached. These structures fulfill a multiplicity of functions to cells, including but not limited to creating a physical and chemical barrier, allowing for cell-cell communication, buffering extracellular compounds, mechanosensation, etc. Glycocalyx is the universal term that describes this structure, which in different cell types may possess additional more specific names such as “zona pellucida” in mammalian oocytes. Within the vasculature of mammalian organisms, a prominent glycocalyx is present on the luminal surface of the endothelium, which due to its known roles in blood vessel function and pathophysiology has received much attention in the scientific literature. This vascular structure is the focus of this review.
The endothelial glycocalyx is continuously synthesized by the endothelial cells and similarly degraded by both mechanical and biochemical stimuli. It provides multiple functions for blood vessels, including one as a semi-permeable barrier to blood constituents and another as mechanosensor for blood flow generated shear stress forces. In this review we will provide an in-depth examination of the composition of the endothelial glycocalyx. We will also delve into the physiological processes mediated by the endothelial glycocalyx, including the regulation of vascular permeability (35), shear stress mechanosensation and mechanotransduction (330), the generation of nitric oxide (NO) (18), protection against cell adhesion/infiltration, and inflammation (61). In addition, we will provide a summary of the pathophysiological conditions in which glycocalyx degradation has been implicated, including atherosclerosis and inflammation, sepsis, hypertension, aging, cancer, and diabetes. We will also review the surrogate markers of glycocalyx degradation and, where applicable, compare those approaches to direct assessment of glycocalyx length (or thickness, as these terms are used interchangeably to indicate the distance glycocalyx structures protrude from the cell surface). Furthermore, we will highlight current approaches to therapeutically target the glycocalyx for regeneration in the context of these pathologies. Finally, we will include an overview and an assessment of the pros and cons of the approaches used to measure the glycocalyx and assess its function ex vivo, including electron (287) and confocal microscopy (24), as well as alternative approaches using state of the art atomic force microscopy (275) and clinical approaches to measure the glycocalyx in vivo (212, 250).
Historical background
One of the earliest recorded observations of cells was published in Micrographia: or, some Physiological Descriptions of Minute Bodies made by Magnifying Glasses, with Observations and Inquiries thereupon in 1665 (116). There, the author, Robert Hooke, coined the term “cell” to describe the individual units that comprise the honeycomb structure of cork, and set the groundwork for cell theory. The structure Hooke was able to observe through his rudimentary lens/microscope was the plant cell wall that also had a coat mixture of predominantly lignin and cellulose, the latter of which is a polysaccharide comprised of D-glucose subunits. Unbeknownst to Hooke, the presence of an extracellular polysaccharide-rich coating appears to be a universal trait of all cells, regardless of phylogenetic kingdom, and thus includes bacteria, fungi, plants and animals. The course of scientific history would have to wait roughly 300 years from the time of Hooke’s publication to 1961, when the structural cellular coat received an appropriate name and classification.
It was during a manuscript presentation to the Mexican Anatomical Society, when Dr. Stanley Bennet first proposed the term glycocalyx, Latin for “sweet husk”, to describe the polysaccharide-rich cellular components and products external to the plasma membrane of a cell (23, 292). This new name and classification were important, as they differentiated other extracellular structures from the sugar-rich coat of the plasmalemma, which possesses quite distinct physical properties. Glycocalyx is now a universal term that describes the sugary coat of cells and encompasses a variety of names that define the polysaccharide coat specific to a particular cell type, e.g., cell wall, mucous coating, zona pellucida, cuticle, red-cell antigen, etc. In addition, at the time of Bennet’s observations, the plasma membrane was already posited to behave as a fluid mosaic, comprised of a lipid bilayer that functions as a barrier preventing the movement of ions and aqueous-soluble molecules. For a review of the historical development of the fluid mosaic model see (171). The glycocalyx, in contrast to the plasma membrane, allows for the ready passage of aqueous solutes, but acts as a filter for both the size and charge of molecules. Bennet astutely observed “…a cell wall can exhibit the properties of the stationary phase of a chromatography column. If charged groups are present…this coating of a cell can exhibit the properties of an ion exchange column” (23). Thus, in its ability to selectively filter molecules, the glycocalyx is able to modify and influence the biochemical composition at the interface of the plasma membrane and the extracellular environment. This has profound implications in the vasculature, where the endothelial glycocalyx acts as a signaling platform to maintain vascular health and control vascular permeability (329).
The endothelial glycocalyx structure
It is widely accepted that all cells generate a sugar-rich coating that falls under the definition of a glycocalyx. Many glycocalyx characteristics are common to all cells (125). However, herein we will focus on the characteristics of the endothelial glycocalyx and the emerging data implicating its central role in vascular function.
In the mammalian cardiovascular system, blood circulation is continuous and uninterrupted. None-the-less, the vessels that transport blood throughout the body can be segregated into three major distinct compartments, namely, the arterial circulation, the capillary beds, and the venous circulation (282). Each compartment serves a different physiological function. The arteries distribute blood and metabolic/catabolic reactants to the various distinct capillary beds. The capillaries represent the compartment where the majority of metabolites/catabolites are exchanged between the blood and tissues. The venous circulation returns the blood and catabolic/metabolic products to the heart in order to be pumped out for a subsequent cycle. Due to its downflow proximity to the heart, the arterial circulation is subjected to much higher intraluminal pressures and rheological shear forces than the capillaries and veins (94). This is important because shear forces play a preponderant role in both the biosynthesis and degradation of the glycocalyx, as well as in its composition. It is, therefore, predicted that the endothelial glycocalyx in the arterial circulation is not identical, neither functionally nor structurally, to that of the capillary beds or venous circulation (298). Moreover, since shear forces are not equally distributed within the arterial tree (e.g., conduit versus resistance arteries or laminar versus turbulent flow patterns) (272), there are also variances in the form and function of the endothelial glycocalyx within the arterial circulation. However, due in part to the difficulties associated with resolving the characteristics of the glycocalyx, there has not been a systematic examination detailing differences in the endothelial glycocalyx as one moves from the heart through the different circulatory compartments. As an additional caveat, many of the studies focused on increasing our understanding of the endothelial glycocalyx have been performed in cultured cells. This has allowed for the use of techniques that permit a better characterization of the glycocalyx, but have also introduced features that are likely different from its in vivo structural and functional properties.
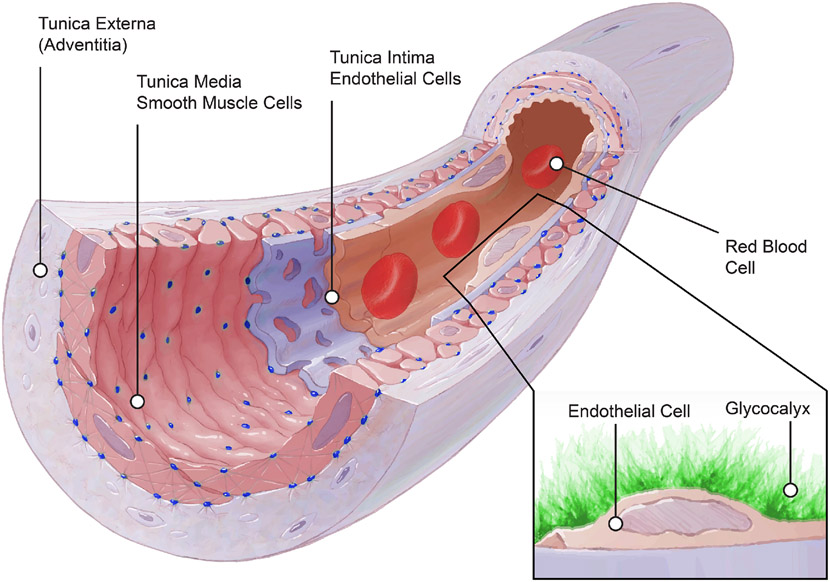
Before proceeding to the structural description of the endothelial glycocalyx, it is important to understand the context in which the glycocalyx is synthesized and functions within the basic architecture of blood vessels. By convention, the wall of arteries and veins consists of three distinct layers (Figure 1). From the lumen proceeding outwardly, they are: the tunica interna (intima), the tunica media and the tunica externa (adventitia). The first layer is next to the lumen and blood flow. It contains the endothelium and its basement membrane. It is also the only layer that conforms the capillary wall. This thin monolayer of endothelial cells in all vessels is oriented in the direction of blood flow and synthesizes the components of the glycocalyx. It also produces a plethora of signaling molecules that provide messages to the underlying smooth muscle media layer in arteries and veins. Such messages cause acute and chronic vascular wall responses that are responsible for vasoconstriction, vasodilation, remodeling and inflammation among many other. For a comprehensive review on the vascular endothelium see (2). Proceeding outwardly from the tunica interna, the media is comprised of vascular smooth muscle cells and some extracellular matrix components. In a number of vascular beds, there is an elastic lamina that separates the endothelial cells from the smooth muscle cells, while allowing for the presence of myoendothelial junctions via structural openings in the lamina (114, 255). The media is primarily responsible for the acute regulation of arterial diameter. This is accomplished via the contraction and relaxation of smooth muscle cells. The sum-total of vasodilatory and vasoconstrictor signaling components in blood, as well as those generated by the smooth muscle itself, the endothelium and the nervous system, determine the local contractile state of the smooth muscle cells and the degree of vascular constriction or relaxation. For a review focused on vascular smooth muscle see (148). Finally, the adventitia is the outermost vascular layer. It is comprised of collagen-rich connective tissue containing fibroblasts and perivascular nerves. Its function is to support growth and repair of the arterial wall. Additional evidence suggests it plays an important role in immune surveillance and inflammatory cell trafficking, while also allowing for communication between the local tissue environment and the more luminally located smooth muscle and endothelial cells (178, 179).
Figure 1.
Schematic illustration of the structural components of a small artery. The vascular wall consists of three distinct layers: the tunica externa (adventitia), the tunica media and the tunica intima. The adventitia is composed of collagen-rich connective tissue containing fibroblasts and perivascular nerves. The tunica media contains mainly vascular smooth muscle cells and extracellular matrix components including elastic laminas between smooth muscle layers as well as an internal elastic lamina that separates the tunica media and from the intima. The tunica intima contains a basement membrane and a monolayer of endothelial cells (endothelium) that synthesizes and anchors all components of the endothelial glycocalyx. The figure was modified from (184).
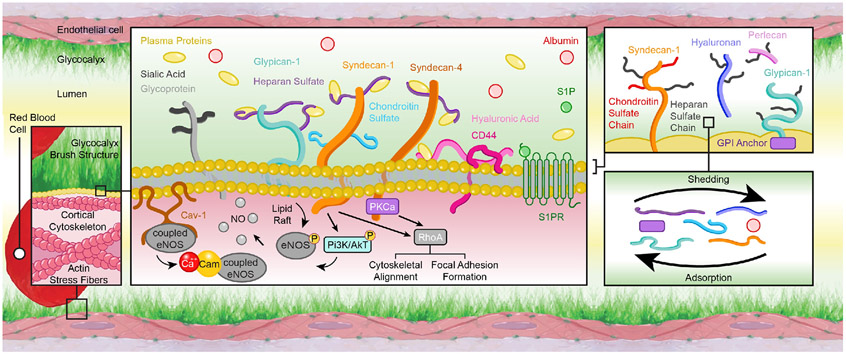
The endothelial glycocalyx is prominently present on the apical side of the endothelium. Its basic components include glycosylated proteins (glycoproteins) and proteoglycans, that is, glycoproteins with attached glycosaminoglycan chains (Figure 2). Some of these components of the glycocalyx are anchored to the cell membrane via either transmembrane domains or covalent links to molecules that associate with the outer leaflet of the plasmalemma. Other components are attached indirectly to the cell via receptor molecules such as the integrins and the hyaluronic acid receptor, CD44. Both glycoproteins and proteoglycans provide structural support to the glycocalyx. However, due primarily to their association with glycosaminoglycans, the proteoglycans are considered the main pillars of the endothelial glycocalyx. Many different proteoglycans are present in the glycocalyx including biglycan, decorins, mimecans and perlecans, but the two most prominent ones found in the endothelial glycocalyx are the syndecans and glypicans.
Figure 2.
Schematic representation of the endothelial glycocalyx components. The endothelial glycocalyx is prominently present on the apical surface of the vascular endothelium. It has a brush-like structure conformed of glycoproteins (e.g., CD44), some of which have attached glycosaminoglycan chains (e.g., heparan sulfate) that together form proteoglycan components (e.g., syndecan-1). The glycocalyx components can be directly anchored to the cell membrane via transmembrane domains or via covalent links to molecules that associate with the endothelial plasmalemma. These anchoring associations include those with caveolin (Cav), protein kinase C (PKC), and other plasma membrane and intracellular molecules. They allow the glycocalyx to participate in the mechanotransduction of physical forces and the subsequent activation of intracellular pathways. Such pathways include the formation of calcium-calmodulin (Ca-Cam) complexes, the phosphorylation of endothelial nitric oxide (NO) synthase (eNOS), the modulation of cytoskeletal structures, and the formation of endothelial focal adhesions. Overall the characteristics of the vascular endothelium are modulated by shedding and adsorption processes that change the abundance of each glycocalyx component present on the cell surface.
The syndecans are proteoglycans that attach to the cell via membrane-spanning domains. They also have cytoplasmic tails that undergo oligomerization and interact with protein kinase C. There are four syndecan family members, namely, syndecan-1, -2, -3 and -4, of which syndecan-1 is the most studied. This is likely due to the role that syndecan-1 plays in modulating cell phenotype and inflammation. In addition, syndecan-1, as other syndecans, is capable of allowing for the transmission of extracellular mechanical forces to the whole cell via its cytoplasmic anchoring associations with protein members of the cortical cytoskeleton such as α-actinin, dynamin, ezrin, syntenin, syndesmos, tubulin, and consequently to all actin stress fibers within the cell (307, 329).
In comparison to the syndecan family of proteoglycans, the glypicans attach to the cell membrane via glycosylphosphatidylinositol molecules within the outer plasmalemma (40, 86, 92, 242). These anchoring moieties are sorted into discrete membrane locales, termed lipid rafts, which contain high concentrations of caveolae, cholesterol, sphingolipids and other signaling molecules (44, 75, 92, 307). The glypican family of proteoglycans has six members (glypican-1 to -6). Of these, glypican-1 is asserted as the isoform with a role mediating shear stress mechanotransduction and other signaling pathways, including those associated with cascades that lead to endothelial NO synthase (eNOS) phosphorylation and NO production (75).
The glycosaminoglycan chains are long repeats of disaccharide units. They are highly polar and generally possess multiple sulfonation patterns, which modulate specific protein attachments as well as influence glycocalyx electrical charge and functions. Different glycosaminoglycan chains, namely, heparan sulfate, chondroitin sulfate, keratan sulfate, dermatan sulfate and hyaluronan are present within the endothelial glycocalyx, and form proteoglycans via N- or O-linked protein glycosylation attachments. Of these glycosaminoglycans, heparan sulfate is the most abundant. It comprises between 50-90% of all glycosaminoglycans attached to proteoglycans on the surface of the vascular endothelium. It is also the only glycosaminoglycan attached to the three to four glycosaminoglycan sites present in glypican-1. Heparan sulfate is also linked to syndecan-1, and although syndecan-1 has both heparan sulfate and chondroitin sulfate glycosaminoglycans attached, it is classified as a heparan sulfate proteoglycan because the ratio of heparan sulfate to chondroitin sulfate is three to four-fold greater for syndecan-1 in the endothelial glycocalyx. Of all the glycosaminoglycans present in the endothelial glycocalyx, hyaluronan (or hyaluronic acid) is the only one not covalently attached to a proteoglycan. It is rather anchored to the cell via CD44, its most specific cell membrane receptor. Overall, the glycosaminoglycans are preponderant determinants of the endothelial glycocalyx physical and functional characteristics. This is a consequence of their abundance, as well as their high level of interaction with themselves and with other components of the glycocalyx.
All glycosylated proteins protruding from the endothelial cell and anchored to the cell membrane can be considered components of the glycocalyx. These glycoproteins have carbohydrate chains attached, which are usually short in length and capped with sialic (neuraminic) acid sugar residues (48). A number of the glycoproteins present within the endothelial glycocalyx are cell adhesion molecules that belong to the selectin, immunoglobulin or integrin families of cellular receptors. All these glycoproteins have small cytoplasmic tails, a transmembrane domain and variable extracellular domains. Their extracellular domains are particularly important in affording them specific functions. The selectins have terminal extracellular lectins that provide them with their characteristic capacity to bind carbohydrate groups on other glycoproteins or glycolipids. The immunoglobulin superfamily is characterized by the presence of immunoglobulin-like domains within their extracellular structure. These domains participate in organismal self-recognition. Lastly, the integrins form α and β heterodimers with extracellular domains that serve as receptors for extracellular matrix proteins. Additional cell adhesion molecules considered part of the endothelial glycocalyx include the intercellular adhesion molecules (ICAM), platelet endothelial cell adhesion molecule (PCAM) and vascular cell adhesion molecule (VCAM), which are glycoproteins with important functions associated with inflammatory processes. Multiple other glycoproteins are present on the endothelial luminal surface that participate in processes such as hemostasis, coagulation or fibrinolysis, and are considered components of the endothelial glycocalyx.
In addition to all the glycoproteins, proteoglycans and glycosaminoglycans that are directly or indirectly anchored to the endothelial cell membrane, there are soluble and insoluble components that associate with and form part of the endothelial glycocalyx. Among them are plasma proteins, enzymes, cofactors and enzyme inhibitors including but not limited to albumin, superoxide dismutase, xanthine-oxidoreductase, thrombomodulin and antithrombin III, all of which participate in maintaining glycocalyx homeostasis (32).
With regard to the physical characteristics of the glycocalyx, reports indicate that the size (thickness or length) of the glycocalyx is highly variable. It protrudes from the endothelial cell membrane by a few hundred nanometers to a few micrometers. This difference in glycocalyx size is related to its location within the vascular tree, as well as dependent on the technique used to measure it (187, 299). As a general rule, the thickness of the glycocalyx increases together with vessel diameter in the arterial circulation, and is greatest in the pulmonary circulation (259).
The endothelial glycocalyx mechanosensation and mechanotransduction
Due to its protruding into the lumen of blood vessels, the apical endothelial glycocalyx is well-positioned to serve in the process of mechanosensation and mechanotransduction of blood-flow generated shear stress (Figure 3). Additional characteristics of the glycocalyx that make it well-suited for sensing and transducing shear forces are its direct connections with the cell membrane and the endothelial cytoskeleton. This is further enhanced by the inclusion of molecules such as integrins in the glycocalyx structure and their relationship with signaling molecules such as ion channels, caveolae signalosomes and focal adhesion-associated kinases (7, 14, 43, 203, 321). The primary end result of glycocalyx mechanosensation and mechanotransduction is the activation of eNOS, the production of NO and ultimately vasodilation (71). However, there are additional responses of the endothelium to shear stress generated mechanotransduction that modulate endothelial cell structure, responsiveness, remodeling and ultimately, overall vascular health (84). Evidence that the endothelial glycocalyx functions as a mechanosensor and mechanotransducer of blood-flow shear forces comes from studies in which a number of its specific components were ablated. Indeed, results showing that such ablation reduces or obliterates the endothelial responses to shear provide evidence that the glycocalyx functions as a mechanosensor or mechanotransducer. Numerous additional endothelial molecules distinct from the glycocalyx, including ion channels, G-protein coupled receptors, caveolae, etc. (3, 123, 137, 175, 189, 317), have been shown to partake in sensing and transducing shear forces. However, the mechanism(s) by which the sum total of endothelial mechanosensing and mechanotransducing molecules interact, depend on or duplicate each other’s activities remains to be fully determined (84).
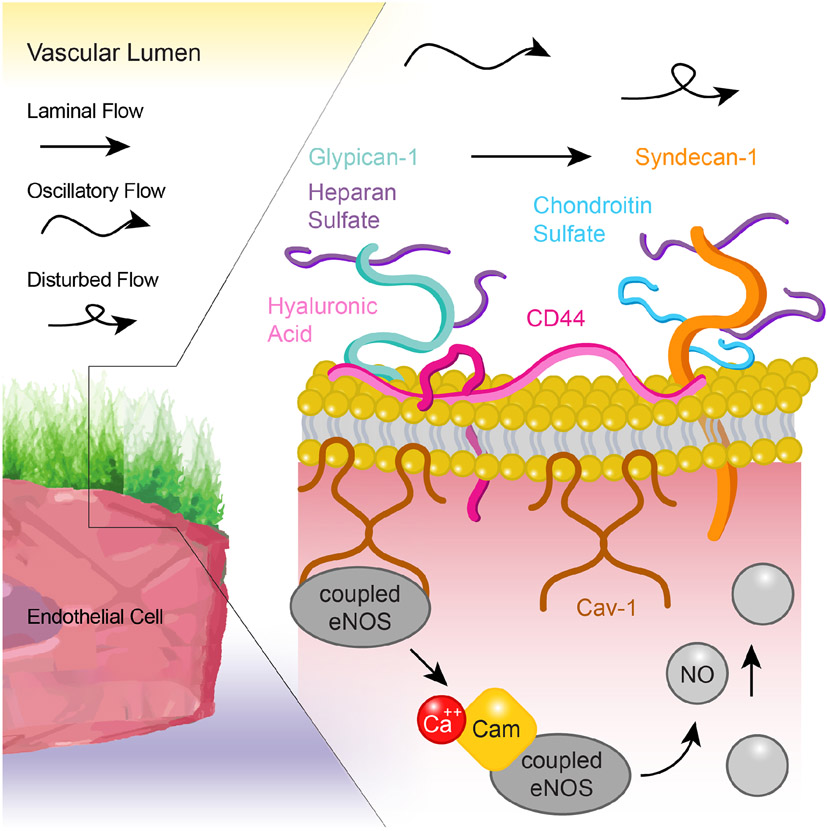
Figure 3.
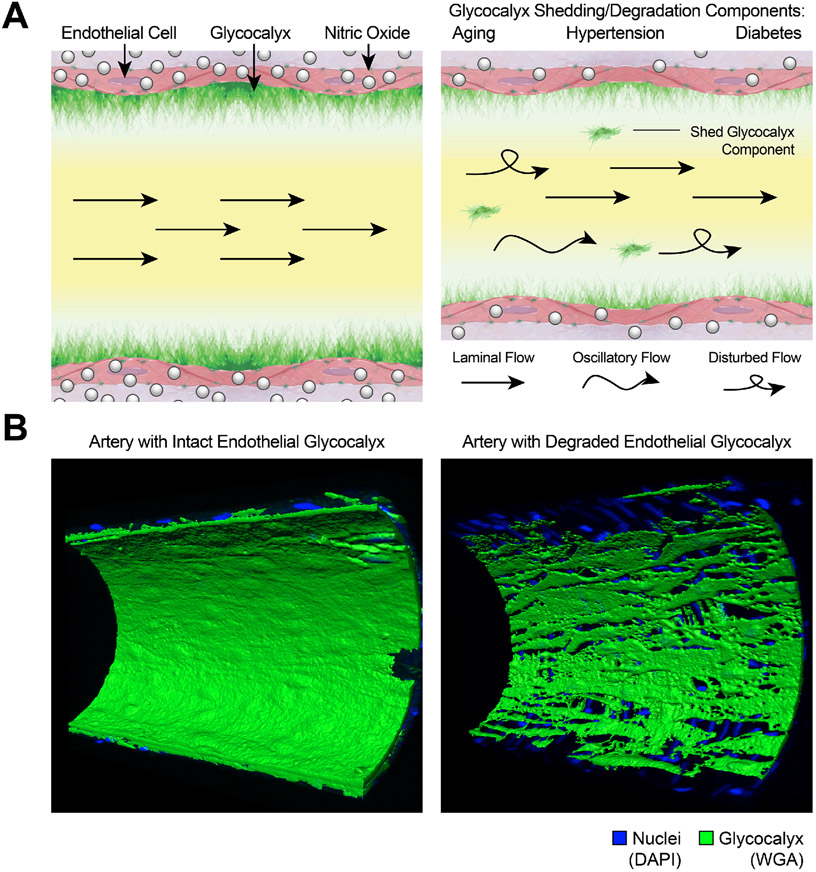
Schematic representation of the endothelial glycocalyx exposed to blood flow-generated shear stress and the signaling cascade that results in shear stress-generated nitric oxide (NO). The endothelial glycocalyx is exposed to laminar, oscillatory or disturbed blood flow patterns that deform, stimulate or shed glycocalyx components resulting in diverse outcomes, including the activation of endothelial NO synthase (eNOS), the production of NO and the subsequent induction of vasodilation.
Core and associated components of the endothelial glycocalyx that have been shown to take part in the mechanosensation and mechanotransduction of shear forces include heparan sulfate, glypican-1, hyaluronan, sialic acids and integrins (88, 172, 177, 226, 235). The specific level of contribution from each of these components to the mechanotransduction of shear into endothelial biochemical signals varies from study to study and may depend on the specific endothelial cells investigated, the vascular bed analyzed and the degree of molecular knockdown of the glycocalyx component. It is undeniable, however, that multiple components of the glycocalyx, if not its whole three-dimensional structure, are needed for the vascular endothelium to appropriately respond to shear forces.
Mechanically, the endothelium is continuously exposed to pressure- and shear stress-induced deformations. Pressure induces a compression force on the whole cell, whereas shear represents the tangential force exerted on the cell surface by the friction of blood as it flows adjacent to the endothelium. Under physiological conditions, endothelial cells are exposed to pressures that range from 0 to 210 mmHg and shear stresses from 1 to 40 dyne/cm2 (84, 264). These forces evoke constant adjustments in vascular tone (active diameter) that allow the circulatory system to rapidly provide adequate blood flow to the tissues. Prolonged responses allow for more permanent structural changes in the form of vascular remodeling (183, 276).
Blood flow induced shear stress is the main mechanical stimulus for glycocalyx-mediated mechanotransduction and is usually described using Poiseuille flow considerations via the Navier-Stokes equations (57). However, the Brinkman equation has been shown to be a better model to determine the interaction between the glycocalyx and flow. In particular, Brinkman’s model has been able to show that the interstitial shear stresses induced by flow on the glycocalyx are of a magnitude capable of allowing for mechanotransduction, while those calculated to occur at the plasma membrane solely by fluid shear stress are not. This is because the Brinkman model considers the effects of both, solid and fluid mechanical stresses developed by flow within the glycocalyx and how they are transmitted to the membrane of a cell that is embedded in and anchored to an extracellular matrix (274). In this manner, the Brinkman equation allows for describing the interaction between flow and the endothelium in two different but complementary ways. In an intact structure, the core components of the glycocalyx serve as lever arms (bush-like formations) that function to amplify torque and subsequently convert shear forces into deformations at glycocalyx–cytoskeleton interfaces. This torque is then transmitted into the cytoplasm and nucleus of the endothelial cell to allow for signal mechanotransduction. Alternatively, when the glycocalyx is compromised, as is the case in multiple disease states, shear mechanotransduction relies on the direct deformation of the cell, its cytoskeleton and its basement membrane anchoring structures (172).
The key role of the glycocalyx in mechanosensing hemodynamic changes has been particularly confirmed in studies focused on flow-mediated vasodilation (226, 318). Experiments in these studies assessed endothelium-dependent NO production/vasodilation in response to different levels of shear stress in glycocalyx-intact or -denuded endothelial cells or isolated vessels (226, 318). The current model of an intact glycocalyx involvement in flow-mediated vasodilation posits that a progressive increase in shear stress disturbs glycocalyx components and this coincides with increases in membrane tension within the endothelial lipid bilayer. It should be noted that the mechanism(s) linking shear mediated alterations to the glycocalyx with increases in membrane tension are not clearly established. It has been demonstrated that modifications to both the structure and concentration of the glycocalyx protein, mucin, can generate entropic bending forces that affect both membrane tension and shape (153, 268). Whether similar alterations in intermolecular interactions occur within the glycocalyx in response to shear-stresses that subsequently alters membrane tension remains to be determined. However, it is clear that additional mechanosensitive components are activated in conjunction to the shear stress effect on the glycocalyx and/or membrane tension. Notably, mechanosensitive ion channels are activated and followed by intracellular cascades that ultimately increase eNOS activation and NO production (71). Studies implicating the glycocalyx in the direct activation of ion channels in response to shear stresses are limited. In the microvasculature, Fancher et al. demonstrated that both the length of the glycocalyx and the presence of heparan sulfate/hyaluronan moieties are critical regulators for the flow activation of inwardly rectifying K+ channels, which activation is implicated in flow-induced vasodilation of human mesenteric adipose arteries (83). Epithelial Na+ channels (ENaC) present in the endothelium are also shear force responsive and play a pivotal role in blood pressure regulation. Similar to the glycocalyx shedding effect on inwardly rectifying K+ channels, removal of hyaluronan blunts shear force induced ENaC activity and reduces current flow in human pulmonary microvascular endothelial cells. The mechanical activation of ENaC is, in part, predicated on N-glycosylation of asparagines, which are thought to act as anchoring points for extracellular tethers that interact with the extracellular matrix and/or the glycocalyx (16, 137). This hypothesis, termed the tethered model, posits that the transmission of mechanical forces occurs via the linkage or tethering of ion channel proteins to intracellular cytoskeletal structures and extracellular elements that interact with the ECM/glycocalyx (326). For a thorough review of the different mechanistic models of the mechanically activated ion channel model see (134). Together these studies implicate the glycocalyx in the activation of both K+ and Na+ channels. The mechanism underlying this activation, whether other ion channels are similarly affected by shear induced alterations to the glycocalyx and how ion channel activation ultimately leads to NO production remains to be determined. For a comprehensive review on the ion channels involved in endothelial cell shear stress responses and subsequent eNOS activation see (98). With regard to the intracellular components of the pathway, additional models suggest that shear stress induces the release of acetylcholine from endothelial cells. Activation of muscarinic receptors by acetylcholine, in turn, promote calcium release from intracellular stores, which activates eNOS (314). In glycocalyx-denuded cells or blood vessels, shear induced NO production is mostly absent, but there is a relatively minor contribution to flow-mediated responses from pathways such as those associated with the production of prostacyclin (226). These data clearly indicate that the glycocalyx is a prominent component of shear-induced mechanosensation and mechanotransduction in endothelial cells.
In addition to studies using flow-mediated vasodilation as the main outcome of shear-induced mechanotransduction, there is complementary supportive evidence on the role of the endothelial glycocalyx as a mechanosensor from experiments using mechanical deformations of cells under no flow conditions. For example, studies using atomic force microscopy have reported increased NO production in isolated endothelial cells or arterial explants in response to glycocalyx (glypican-1 or heparan sulfate) pulling using functionalized cantilevers. Notably, pulling of glypican-1 induced approximately 50% greater NO production than pulling heparan sulfate (18, 75). This suggests that a glycocalyx (glypican, heparan sulfate)-caveolae-eNOS pathway plays a major role in the mechanosensation and mechanotransduction response to physical deformation in endothelial cells (57). It has also been shown that different structural and mechanical characteristics of the glycocalyx (e.g., its length and stiffness) also play a role in glycocalyx-dependent mechanotransduction. In support of this, reductions in glycocalyx length have been positively correlated with diminished endothelial function, including eNOS activation and NO production (275). With regard to glycocalyx stiffness, it is posited that an excessively soft glycocalyx would not be able to sense small mechanical perturbations. Comparatively, an exceedingly rigid glycocalyx could be too sensitive to force perturbations, which would likely disrupt the underlying cytoskeleton (217). Yet a complete understanding on how all the complex mechanical and structural characteristics of the endothelial glycocalyx at different locations of the vascular tree affect shear-mediated mechanotransduction remains to be fully determined.
The endothelial glycocalyx and vascular permeability
The physical architecture of the vasculature functions as a conduit to deliver oxygenated blood to meet the metabolic demands of the body’s tissues. Inherent to its design, a healthy vasculature continually and passively leaks small molecules/solutes into the surrounding tissue. In contrast, larger molecules (>70 KDa) are prevented from passive movement out of the vasculature under non-pathological conditions. The two main physical parameters regulating passive extravasation of molecules out of the vasculature are size and electrostatic charge. The size barrier is predominantly a function of the presence or absence of cell-cell adherens junctions (60) and tight junctions (117) at the interface of adjacent endothelial cells (paracellular) that line the lumen of the vasculature. Additional factors that contribute to the specific filtration size of the barrier are the composition of basement membranes extracellular matrices including the glycocalyx and the presence or absence of endothelial fenestrae as well as fenestral diaphragms (224). The macrovasculature is posited to possess limited permeability, due in part to its continuous endothelium. In contrast, the microvascular endothelium can either be continuous or noncontinuous, with or without fenestrae, depending upon the vascular bed. The presence of fenestra facilitates the exchange of small molecules/solutes (245), thus a continuous fenestrated endothelium displays greater permeability that one without fenestrae (2). In addition to the physical architecture of the vasculature, specific cells (e.g., pericytes (9), mast cells (143, 156) and other immune cells (68)) can further modulate the size barrier and affect permeability.
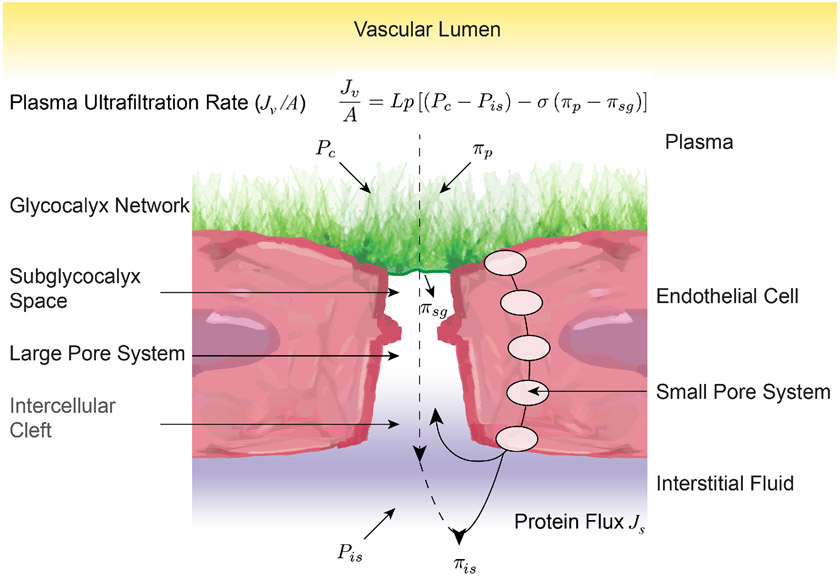
Our understanding of fluid movement across the vasculature has been evolving ever since the Starling Principle of transvascular fluid dynamics was first proposed over 100 years ago in 1896. In this hypothesis, it is the balance of the sum of pressures acting to push fluid out of the vessel versus those forces acting to push fluid into the vessel that determines net fluid movement. Starling proposed that the forces acting to push fluid out the vessel are luminal hydrostatic and tissue oncotic pressures, and those opposing that movement are luminal oncotic and tissue hydrostatic pressures. In this model, the net forces on the arteriolar side of the capillary bed push fluid out, while fluid is forced back into the vasculature, as those forces decrease in the venous side. Subsequent experiments have demonstrated shortcomings in Starling’s hypothesis. A review of these shortcomings is detailed by Alphonsus et al. (6), who in brief state that sustained venous reabsorption does not occur in all tissues. Though Starling was able to measure capillary hydrostatic and oncotic pressures, he was not able to accurately determine interstitial pressures. Subsequent assessments have shown that tissue hydrostatic and oncotic pressures are significantly lower than intracapillary pressures. For example, Bates et al. demonstrated that in the human arm, the net pressure opposing extravasation is less than the antecubital venous pressure (19). A second critique is that the rate of capillary filtration/extravasation is much lower than that predicted by the Starling equation. This arises from the observation that the lymphatics are the only alternative to reabsorption for fluid to return to the circulation, coupled with the assessment that lymphatic flow is much less than the capillary extravasation predicted by the Starling equation. A third critique is that capillary reabsorption is much lower than that predicted from the Starling equation, as well. Notably, a revised Starling equation that accounts for the glycocalyx restricting extravasation/filtration resolves these conundrums. A depiction of this revised Starling equation by Levick et al. is shown in Figure 4, where: Jv/A is volume filtered per unit area; Lp, hydraulic conductance; Pc, capillary hydrostatic pressure; Pis, interstitial hydrostatic pressure; σ, osmotic reflection co-efficient; πp, oncotic pressure on the plasma-side of endothelial surface layer; and πsg, oncotic pressure in the subglycocalyx space (159). In support for this revised version of the Starling principle, experiments examining the barrier function of the mammalian glycocalyx suggest that it excludes macromolecules greater than approximately 70 kDa in size. For example, in mouse mesenteric arteries, 148 kDa fluorescent probes are excluded from the glycocalyx, whereas 50.7 kDa probes are able to cross through the glycocalyx in approximately 30 minutes (291). In hamster cremasteric preparations, white blood cells, which readily pass through the glycocalyx can encompass the full anatomical width of the cremasteric capillaries. In contrast, red blood cells, as well as a 70 kDa fluorescent probe, occupy a significantly smaller column width of the capillary, indicating their exclusion from the glycocalyx (299).
Figure 4.
Illustration of the forces that drive vascular permeability and their relationship with the endothelial glycocalyx. Under non-pathological conditions, the intact endothelial glycocalyx contributes significantly to the vascular permeability process as a physical and electrostatic barrier. Although the filtering of molecules is predominantly a function of cell-cell adherens junctions and paracellular tight junctions, the negatively charged bush-like structure of the glycocalyx, prevents the extravasation of macromolecules greater than approximately 70 kDa, as well as that of cationic molecules.
In addition to size exclusion, there is also an electrostatic barrier impeding the flow of charged molecules out of the vasculature. Here the glycocalyx, as a negatively charged gel, plays a central role in regulating vascular permeability. As previously mentioned, there are five classes of glycosaminoglycans that comprise the glycocalyx and they are heavily sulfonated, leading to a net overall negative charge. This negatively charged barrier of the glycocalyx impedes the flow of cationic molecules out of the vasculature. It must be mentioned that although the glycocalyx is comprised in part of anionic oligosaccharides, its negative charge from sulfonated glycosaminoglycans predominates and plays a central role in regulating vascular permeability. For example, heparan sulfate, which comprises 50-90% of all the glycosaminoglycans present in the endothelial glycocalyx is a sulfonated glycosaminoglycan. Other sulfonated glycosaminoglycans present in the glycocalyx are chondroitin sulfate, dermatan sulfate and keratin sulfate. Therefore, glycosaminoglycan sulfonation is a major contributor to the overall negative charge of the glycocalyx and also provides scaffolding for binding to other proteins both from the circulation as well as those statically embedded in the extracellular matrix. For example, hyaluronan is not covalently attached to proteoglycans. Instead it interacts noncovalently with glycocalyx structures via hyaluronan binding motifs (79). The carboxyl groups on its glucuronic acid residues are negatively charged at physiological pH and thus the presence of hyaluronan in the glycocalyx further contributes to its anionic nature (294).
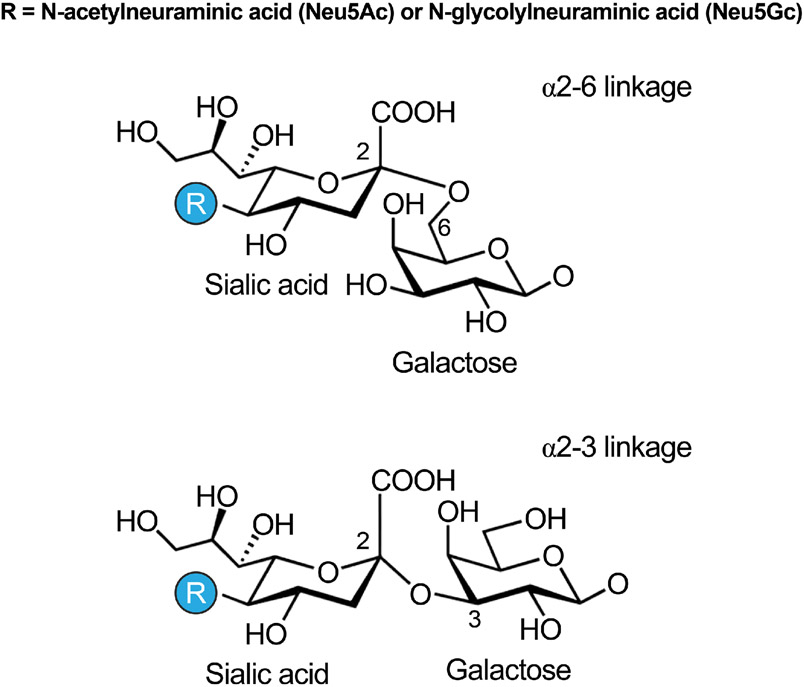
An additional characteristic of the glycocalyx affecting its charge is the presence of sialic acids. Sialic acids are the acylated derivative of the nine-carbon sugar neuraminic acid, and are generally the terminal sugar of glycan chains attached to glycolipids and glycoproteins. In humans the predominant sialic acids are N-acetylneuraminic acid (Figure 5) and its hydroxylated derivative, Neu5Gc, the latter of which is solely derived from dietary sources (253). Sialic acids are a subset of nonulosonic acids, and all nonulosonic acids carry a negatively charged carboxylate (C-1) and a 3-carbon exocyclic side-chain (C-7 to C-9). Early research on erythrocytes determined that sialic acids confer anionic properties to the molecules harboring them. Consequently, erythrocytes from many different animal species display a negative electromobility, which is indicative of a net negative charge. This anionic property is primarily due to the carboxyl group of sialic acids present on their surface. Indeed, it was found that removal of sialic acids, with the enzyme neuraminidase, reduces erythrocyte electrophoretic mobility in proportion to the amount of sialic acids removed (80). Subsequent studies on the glycocalyx have demonstrated that the presence of sialic acids in the endothelial glycocalyx similarly contributes to its polyanionic nature. Therefore, consistent with the role of sialic acids as a contributors to electrostatic status, their removal by neuraminidase significantly reduces the negative charge of the glycocalyx (136). Indeed, in pathologies that are associated with increased plasma neuraminidase activity and reduced sialic acid content, there is also a reduction in the negative surface charge of the vascular endothelium (209). Unfortunately, there have not been systematic studies performed to assess the glycocalyx surface charge. Furthermore, it seems reasonable to assume that the localized negative charge of the glycocalyx also varies throughout the vasculature, depending upon the integrity, robustness and composition of the glycocalyx at specific locations. A factor contributing to this variation is that synthesis of glycocalyx components occurs not just within the endoplasmic reticulum and Golgi compartments, but at a number of different cellular locations. Moreover, their synthesis is contingent upon overlapping interdependent biochemical pathways that are responsive and affected by the metabolic state of the cell (152). For example, hyaluronan synthase isoforms act at the plasma membrane to assemble hyaluronic acid (197), and are activated by phosphorylation (296), ubiquitination (130) or O-GlcNAcylation (297), and are further regulated by the formation of intermolecular disulfide bonds (99). It is therefore reasonable to assume that there is a high degree of heterogeneity in both the composition, electrostatic status and concentration of glycocalyx components within the circulatory system.
Figure 5.
Two of the major sialic acid residues present in the endothelial glycocalyx are N-acetylneuraminic acid and N-glycolylneuraminic acid, which vary on their linkage to sugar moieties present in glycoprotein members of the glycocalyx.
The negative charge of the glycocalyx also influences the binding characteristics of proteins that interact with its different components. These binding partners provide a complex communication system between endothelial cells and the local chemical environment. Furthermore, they have the ability to significantly affect vascular permeability and intracellular signaling. In general, the electrostatic charge of the glycocalyx functions as a barrier to repel negatively charged molecules, macromolecules and cells including erythrocytes and activated platelets. However, it is the combined size and charge barrier functions of the glycocalyx that prevent the extravasation of molecules greater than approximately 70kDa, and also influences the capacity of molecules with different charges and sizes to bind to glycocalyx components. To maintain tissue homeostasis, larger molecules need to cross the endothelial barrier. Thus, the glycocalyx facilitates the transport of cells, proteins, and nutrients across the endothelial barrier via highly regulated processes. For example, immune cell extravasation is a receptor mediated process that is inhibited by a healthy glycocalyx. In response to localized inflammation, chemokines mediate the degradation of the glycocalyx, exposing receptors for binding to adhesion molecules, such as E-selectin on leukocytes and thus allow for immune cells to bind the endothelium and infiltrate the underlying tissue (241).
In aggregate, data from studies that look at the glycocalyx structure and measure vascular permeability suggest that presence or absence of specific components of the glycocalyx affect endothelial permeability regardless of glycocalyx length. Overall, however, a thinner glycocalyx is generally associated with increased permeability.
The endothelial glycocalyx and immunology
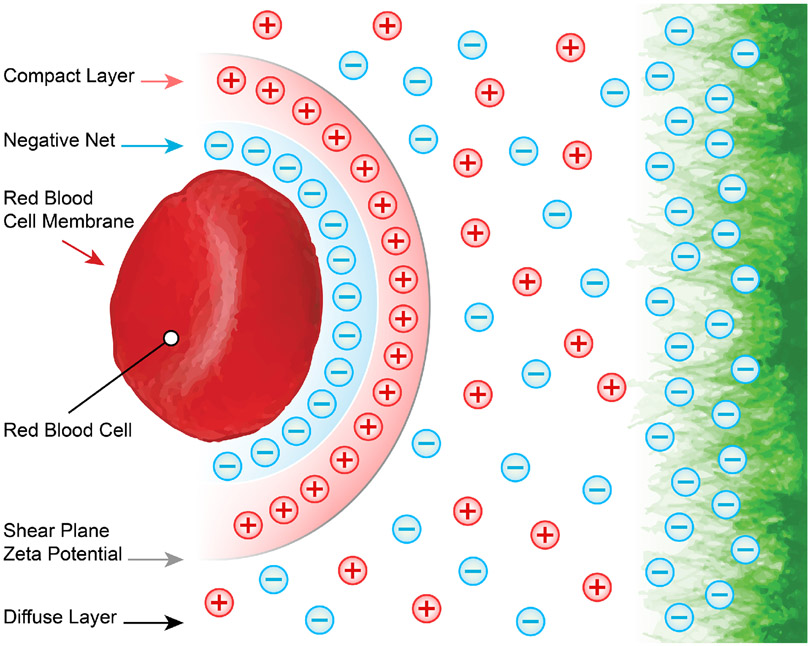
In addition to establishing a filtering sieve to regulate vascular permeability, the glycocalyx plays a central role in protecting the cardiovascular system and blood perfused tissues from pathogens and autologous damaging constituents. The glycocalyx, and by extension the endothelial cells to which it is attached, is subject to exposure to any and all pathogens that enter the blood stream. As already mentioned, the glycocalyx forms a gel-like negatively charged structure that is electrostatically opposed to infiltration by red blood cells (Figure 6). This occurs because the erythrocyte surface carries a negative charge due to the ionization of the carboxyl group of N-acetyl neuraminic acid (80, 233, 248).
Figure 6.
The endothelial glycocalyx is negatively charged. This allows for the formation of a diffuse electrostatic barrier that separates the endothelium from other negatively charged blood components, such as red blood cells.
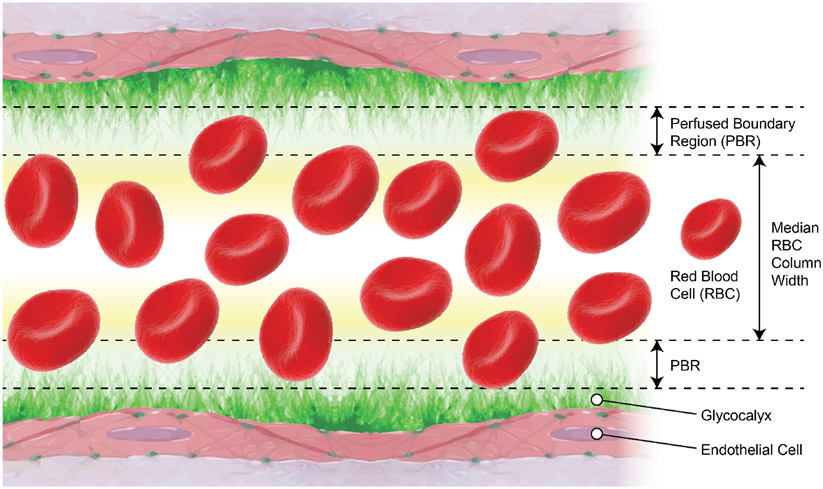
Under nonpathological conditions, the red blood cell column that flows through the vasculature is not physically in contact with the endothelium. Indeed, red blood cells flow alongside endothelial cells, separated by the physical width of the negatively charged glycan structures (glycosaminoglycans and sialic acid) that comprise the glycocalyx. This distance, minus the small space red blood cells are able to penetrate into the glycocalyx, is referred to as the perfused boundary region (PBR) as shown in Figure 7 (56, 69, 299). This PBR represents the in vivo length or thickness of the endothelial glycocalyx, which separates endothelial cells from the flowing blood. Using sidestream darkfield imaging Lee et al. demonstrated that erythrocytes maintained a fixed distance from the endothelium of capillaries in healthy subjects (155). Under conditions in which the glycocalyx is damaged/shortened, the PBR becomes thinner allowing erythrocytes to flow closer to the endothelium. This has the effect of increasing blood volume in capillaries and arterioles. Capillary tube hematocrit is the ratio of the volume of packed red cells to the total blood volume. In conditions in which the glycocalyx is degraded a reduction in glycocalyx thickness results in an increase in capillary hematocrit (46), and decreases the distance between the flow of erythrocytes and the endothelial cell membrane (155).
Figure 7.
Schematic representation of the perfused boundary region (PBR) that allows for assessment of in vivo endothelial glycocalyx thickness. Flowing red blood cells (RBC) form a column as they travel within the vascular lumen. Blood velocity and the electrostatic charges of the endothelial glycocalyx as well as those of RBCs form a PBR, where only a few RBCs occasionally penetrate. The size of the median RBC column width subtracted from the vascular luminal diameter represents a measure of the PBR and the thickness of the endothelial glycocalyx. The figure was modified from (56).
Similarly, one of the first lines of defense against pathogen invasion is the physical distance that separates the red blood cell column, and whatever has infiltrated that column, from the apical membrane of endothelial cells. For example, bacteria that enter the bloodstream use the circulation as a conduit to traverse through the body, and in order to successfully colonize/infect the underlying tissue, they must interact with and penetrate the endothelium. Similar to red blood cells, most bacteria are also enveloped in a negatively charged glycocalyx structure, the characteristics of which vary from strain to strain (315). This causes electrostatic charges in bacteria and endothelium to repel and prevent the pathogen from accessing the surface of endothelial cells. Indeed, most bacteria have negatively charged walls. Both gram-positive and -negative bacteria contain a cell wall, comprised of a disaccharide peptidoglycan, external to the cell membrane. Two glucose derivatives: N-acetylglucosamine and N-acetylmuramic acid comprise the backbone of this disaccharide peptidoglycan molecule. Gram negative bacteria possess an additional endotoxin membrane of lipopolysaccharides distal to the cell wall, which contains negatively charged head groups and the anionic lipid lipoteichoic acid (260). Gram-positive bacteria lack this structure but possess anionic lipids (180). In addition, gram-positive bacteria generally have many more layers of peptidoglycans and phosphate containing anionic teichoic acid, which contribute to an overall net negative surface charge (315). As such, the structure of both the bacterial and endothelial glycocalyx result in a physical distance defense as well as an adhesion defense. This has been corroborated in studies that demonstrate the greatest degree of bacterial adhesion occurs on substrates that are positively charged and hydrophilic, whereas negatively charged substrates are the least amenable to adhesion (219).
With regard to viruses, their surface charge is also mostly negative at a physiological pH of 7.4. This observation is derived from a review of published isoelectric points (the pH at which the net charge is zero) that found the point range was 1.9 - 8.4 with the greatest frequency found within 3.5 - 7 (190). Thus, similar to bacteria, there is an electrostatic charge barrier impeding adsorption onto the endothelial glycocalyx for most viruses, i.e., those with an isoelectric point less than 7.4. Indeed, studies on silica matrices have determined that viral adsorption to negative surfaces occurs only in solutions with a pH less than the isoelectric point (331).
Conceivably, the importance of the structural integrity of the glycocalyx in preventing pathogen infection is best highlighted by the plethora of microbial mechanisms that attempt to circumvent this structural defense. For example, once in circulation, Dengue virus infects target cells such as monocytes/macrophages and triggers the secretion of a protein that activates endothelial sialidases, cathepsin L, and heparinase, which subsequently degrade the endothelial glycocalyx (20, 237). Analogous mechanism(s) involving endothelial glycocalyx disruption have been adopted by other agents, including food borne pathogens such as Salmonella (8) and Listeria (247), air borne pathogens such as betacoronavirus SARS-CoV-2, the virus that causes COVID-19 (279), as well as blood borne pathogens like the malarial parasite Plasmodium falciparum (112).
In addition to the central role electrical charge plays in protecting against pathogen adherence and invasion, other characteristics and components of the endothelial glycocalyx also participate in immunological capacities. For example, sialic acids, the terminal moieties on the branching proteoglycans and gangliosides that comprise the glycocalyx are implicated in a diverse range of immunological functions. These functions include their being antigens in immune reactions and contacts for glycan binding proteins that mediate both cell-cell and cell-pathogen interactions. Sialic acids also mediate cell-cell adhesions between immune cells and the vascular endothelium, in part via the regulation of selectin binding. They further modulate the function of other important endothelial proteins implicated in immune responses, including intercellular adhesion molecule-1, platelet endothelial cell adhesion molecule-1, vascular endothelial growth factor receptor-2 and lymphatic vessel endothelial hyaluronan receptor-1 (51). An additional function of sialic acids in the immune system is to help differentiate native, host cells from foreign pathogens. This is achieved, in part, by their interfering with the complement pathway to prevent the immune system from targeting native cells (27, 258). Other examples of components of the glycocalyx mediating immune responses include the role of heparan sulfate oligosaccharides in the regulation of immune cell adhesion, migration, and activation, in part by its inhibiting proteases of the lectin activated complement pathway (273). Heparan sulfate has also been shown to bind to and modulate cytokines and growth factors, as well as to establish chemical gradients for morphogens, growth factors and cytokines (256). Furthermore, it can function as a co-receptor to stabilize receptor/ligand binding events as well as to mediate immune response signal transduction pathways (230). Yet additional glycocalyx components, namely hyaluronic acid and its fragments are well documented molecules involved in pathological states (41). The lower molecular weight fragments of hyaluronan have been shown to induce inflammatory responses in macrophages and promote angiogenesis (115, 216). In contrast, the higher molecular weight fragments have the opposite effect on angiogenesis (309). Furthermore, the hyaluronan receptor, CD44, when bound to E-selectin, facilitates the process of leukocyte rolling and adhesion to the endothelium (59, 186). Overall, all these examples highlight the plethora of mechanisms by which diverse components and characteristics of the endothelial glycocalyx participate in immunological responses and host defense.
The endothelial glycocalyx and signal transduction molecules
Sphingosine-1-phosphate
The close relationship that exists between glycocalyx structures and the cell membrane provides numerous avenues in which membrane-associated signaling molecules are modulated by the glycocalyx and vice versa. For example, bioactive sphingolipids have been shown to be involved in membrane signal transduction and in a variety of cellular processes (17). Among others, sphingosine-1-phosphate (S1P) is a notable one, as it can modulate several biological functions, such as cell contraction, survival, proliferation and migration (234). S1P is a sphingolipid derived from sphingosine, which is a product of ceramide hydrolysis generated in a process that originates from the degradation of the plasma membrane glycosphingolipids and sphingomyelin (234). Once formed, sphingosine can be reconverted to ceramide by ceramide synthase or further degraded to S1P in a reaction regulated by two sphingosine kinases (SPHK1 and SPHK2) (234). Upon its formation, S1P can be recycled to ceramide through S1P dephosphorylation, further degraded into hexadecenal and phosphoethanolamine, or released to the extracellular environment (234). Although platelets and endothelial cells can produce S1P (295), erythrocytes are the main source of S1P, contributing approximately 70% of the 0.3 to 0.5 μM S1P found in human plasma (28, 220). S1P can be transferred from erythrocytes to endothelial cells by direct contact or via high-density lipoprotein (HDL) and albumin transport (283, 327). In fact, the majority of S1P is bound to these protein carriers, with HDL carrying around 60% and albumin 30% of the circulating S1P (28). The binding of S1P to HDL or albumin prevents its degradation by circulating sphingosine phosphatases, thereby allowing S1P to bind to its receptors on the cell membrane of the targeted tissue (146). So far, five S1P receptors (S1PR) have been identified (S1PR-1-5) with S1PR-1, -2, and -3 being expressed in most organs and tissues, including the vascular endothelium (170). The vascular action of S1P may vary according to the receptors it binds, with S1PR-1 and -3 activation being associated with blood vessel stability and glycocalyx homeostasis (170, 305). As previously mentioned, the endothelial glycocalyx is constantly exposed to conditions that cause glycocalyx degradation and shedding, such as disturbed blood flow, inflammation, hyperglycemia, trauma, and removal of blood proteins (30, 167, 213, 239). This requires continuous glycocalyx repair in order for it to remain functional. In this regard, S1P has been shown to play a crucial role in maintaining blood vessel and glycocalyx homeostasis by inducing NO release and preventing syndecan-1 shedding (39, 327). Previous findings suggest that S1PR activation, inhibits the activity of matrix metalloproteinases (MMPs), specifically that of MMP-9 and MMP-13 (327). When active, these MMPs can cleave syndecan-1 below the chondroitin sulfate attachment site, thereby inducing the shedding of syndecan-1 ectodomains and consequently the loss of chondroitin sulfate and hyaluronic acid. MMP-9 and MMP-13 inhibition has been shown to abolish syndecan-1 shedding in rat-fad-pad endothelial cells, reinforcing the major role of these proteases in glycocalyx shedding (327). Zeng et al. also found that S1PR-1 plays a major role in glycocalyx recovery, mainly via activation of the PI3K pathway (328). They performed a series of experiments in which glycocalyx-depleted endothelial cells were treated with S1P in the presence or absence of the PI3K inhibitor, Y294002 (328). Results showed that presence of Y294002 reduced the content of syndecan-1, chondroitin sulfate and hyaluronic acid on the cell surface. This suggests that S1P signalling via a S1PR-1/PI3K-dependent pathway is an important contributor to glycocalyx recovery.
Notably, evidence also shows that the endothelial glycocalyx may function as a signalling platform connecting hemodynamic forces and S1PR. For example, a study from Jung et al., demonstrated that the mechanosensing properties of the endothelial glycocalyx contributes to S1PR-1 homeostasis by integrating alterations in blood flow and the receptor’s activity. They also found that increases in shear stress promote glycocalyx stabilization via increases in endothelial S1PR-1 activation and that deletion of endothelial S1PR-1 was associated with reductions in the transduction of blood flow into downstream vascular protective cellular chemical signaling (128). It further appears that specific components of the glycocalyx positively regulate S1P signaling. High molecular weight hyaluronan binds to its receptor, CD44, which in turn induces clustering in caveolin-enriched lipid raft microdomains. Evidence suggests that CD44 enriches S1PR1 within these lipid rafts leading to activation of the receptor. This interaction is required to maintain vascular barrier function (271). Additionally, Krüppel-like factor 2 is a transcription factor that regulates expression of hyaluronan synthase 2 (304), which synthesizes hyaluronan and increases surface hyaluronan production and S1PR1 (11). Thus, mechanical stimuli, e.g., shear stress, that activates Krüppel-like factor 2 positively regulates both glycocalyx synthesis and S1P signaling via increases in endothelial cell hyaluronan synthase 2 and S1PR expression.
As for S1PR-2, there is currently no evidence that the endothelial glycocalyx directly affects its expression or activity. Conversely, activation of S1PR-2 has been associated with PI3K pathway suppression through an increase in Rho-dependent/PTEN phosphatase activity (254). Whether there is a direct link between S1PR-2 and shedding of glycocalyx constituents is uncertain, but activation of S1PR-2 has been shown to be detrimental to the endothelium by disrupting adherens junctions, and consequently exacerbating vascular permeability (254). The increased expression of S1PR-2 in vascular tissues exposed to disturbed blood flow, inflammation and hyperglycemia may explain, in part, some controversial findings showing elevated circulating S1P levels in conditions associated with glycocalyx depletion, such as diabetes and insulin resistance (90, 142).
Reactive oxygen species
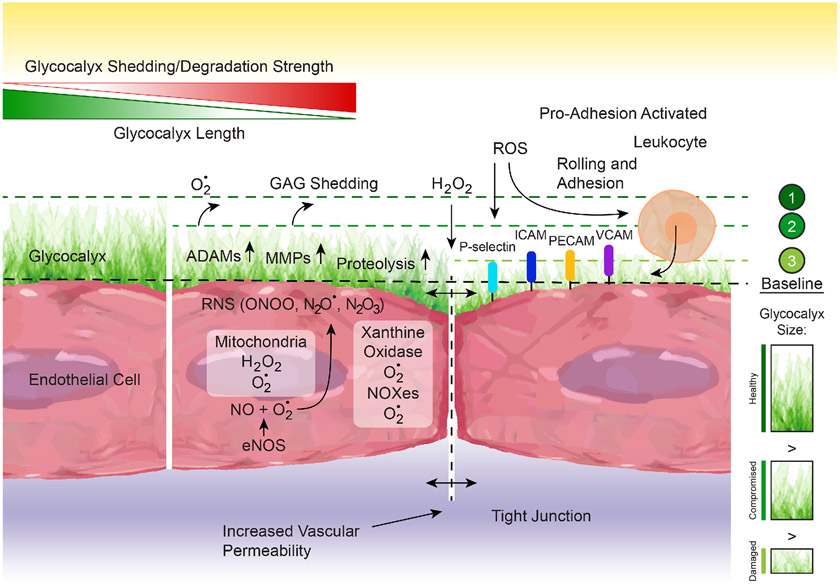
Similar to many other tissues, the vascular wall is in a constant process of molecular oxidation and reduction. Multiple signaling pathways involve production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that are subsequently reduced or inactivated by tissular antioxidant systems, which protect molecular components from oxidative stress. ROS and RNS are derived from balanced physiological redox cellular processes. However, an imbalance in favor of oxidation occurs in several pathological conditions. In the vasculature, oxidative stress is commonly associated with proteolysis, glycocalyx degradation, increased permeability, and endothelial dysfunction due to the scavenging of NO by ROS, which diminishes NO bioavailability, impairs vasodilation and promotes inflammation (Figure 8).
Figure 8.
Schematic representation of the mechanisms that promote endothelial glycocalyx degradation. The endothelial glycocalyx undergoes a constant process of synthesis or adsorption and degradation or shedding. Degradation occurs in response to glycosaminoglycans (GAG) oxidation by reactive oxygen species (ROS) such as nitric oxide (NO), superoxide (O•2), and H2O2, as well as by reactive nitrogen species (RNS) such as peroxynitrite (ONOO), N2O• or N2O3. These reactive molecules are produced by multiple cellular components including mitochondria, endothelial NO synthase (eNOS), xanthine oxidase, and the NADPH oxidases (NOXes). Glycocalyx degradation also occurs by proteolysis or shedding mediated by enzymes such as the matrix metalloprotienases (MMPs) and the a disintegrin and metalloproteinases (ADAMs), amongst others. Glycocalyx degradation in turn increases vascular permeability and allows for exposure of proinflammatory receptors (e.g., P-Selectin, ICAM, PE-CAM, V-CAM) that promote leukocyte rolling and adhesion to the vascular endothelium.
Main sources of ROS in the vascular wall include the NADPH oxidase(s) (NOXes), uncoupled eNOS, xanthine oxidase (XO), and mitochondrial respiratory chain enzymes (73, 77, 97, 126, 149, 151, 251). Under physiological conditions, ROS production by the NOXes prevails. Additional ROS come from NOXes’ interactions with other sources including xanthine oxidase, uncoupled eNOS, mitochondria, cyclooxygenases and lipoxygenases (228, 251, 306). Superoxide anion is the main form of ROS produced by NOX-1 and -2, impaired mitochondria and uncoupled eNOS. This superoxide is readily converted into hydrogen peroxide by superoxide dismutase (SOD) either in the cytosol (SOD1), inside mitochondria (SOD2) or outside the cell (SOD3). Superoxide is also converted to peroxynitrite upon its reaction with NO, and peroxynitrite reacts with proteins, lipids, and nucleic acids to form 3-nitrotyrosine, which is used as an indirect index of oxidative stress in tissues (95, 277, 335). The toxicity of hydrogen peroxide is suppressed by its conversion to oxygen and water by catalase or glutathione peroxidase. In turn, the effectiveness of glutathione peroxidase depends on the availability of reduced glutathione, in conjunction with that of vitamin E, vitamin C and lipoic acid (5, 157, 302). Ultimately, oxidative stress occurs when the amount of ROS produced overwhelms antioxidant mechanisms.
At the level of the endothelial glycocalyx, ROS are either inactivated by antioxidant mechanisms (particularly SOD3) or participate in glycocalyx degradation. Degradation occurs mostly via either direct oxidation or via ROS-dependent activation of enzymes that shed glycocalyx components. Direct oxidation results in fragmentation and depolymerization of the major glycosaminoglycans that comprise the glycocalyx, including chondroitin and dermatan sulfate (202), heparan sulfate (63) and hyaluronan (66); whereas the enzymes that shed glycocalyx components include the neuraminidases, heparanase, hyaluronidases, MMPs, and a disintegrin and metalloproteinases (ADAMs) (1, 127, 168, 199, 201, 290). Additional more intricate signaling cascades, initiated by stimuli that increase ROS production, also culminate with shedding of glycocalyx components. In particular inflammatory signals that induce the expression of cytokines such a tumor necrosis factor (TNF)-α also increase the activity of multiple sheddases including ADAM10 and ADAM17. These proteolytic enzymes have been shown to shed glycocalyx components including endomucin and glypican-1, which when depleted, favor leukocyte-endothelium interactions and impaired mechanosensation, respectively (132, 323). It is posited that oxidative stress and excessive shedding of glycocalyx components initiates a vicious cycle that increases the production of ROS and promotes glycocalyx degradation. This occurs in part because the glycocalyx is protected against oxidation by the presence of SOD3 within its structure (4). Consequently, shedding of glycocalyx components is accompanied by loss of SOD3, a condition that favors oxidative stress, glycosaminoglycan degradation and MMP activation (4). This further increases glycocalyx destruction and the increased production of ROS by the NOXes present in the endothelium (72).
It should be emphasized that presence of an intact glycocalyx structure favors the production and activity of antioxidant molecules and pathways. This is because, in addition to the presence of SOD3 within the structure of an intact endothelium, stimulation of an intact endothelial glycocalyx with laminar flow has been shown to increase antioxidant signaling. In particular, it has been reported that unidirectional laminar flow increases the amount and phosphorylation of nuclear factor erythroid 2-related factor 2 (Nrf2) which is a well-known positive modulator of antioxidant molecules and pathways (235). This pathway is dependent on the presence of sialic acid residues in the glycocalyx, as removal of these moieties attenuates Nrf2 activation by laminar shear stress. Furthermore, the endogenous neuraminidase enzymes that cleave sialic acid from glycan chains are upregulated in response to hypoxia as well as in pathologies associated with increased ROS in the vasculature including diabetes, atherosclerosis and cancer (232). Thus, loss of glycocalyx components not only reduces the anti-oxidant properties inherent to the glycocalyx structure itself, but also results in dampening of signaling pathways that maintain anti-oxidant enzyme homeostasis.
Lastly, evidence suggests that individual components of the glycocalyx possess intrinsic anti-oxidant properties. For example, sialic acid is an effective scavenger of hydrogen peroxide (119). Similarly, glycosaminoglycans in general also possess intrinsic anti-oxidant properties against both hydrogen peroxide and metal-induced oxidative stress (36, 37). In sum, data indicate that a healthy glycocalyx plays a protective role against oxidative damage via direct anti-oxidant effects, tethering of anti-oxidant enzymes within its structure or via the positive regulation of signaling pathways that dampen ROS. In conditions in which these defenses become compromised, ROS facilitate the direct degradation of the glycocalyx and activate sheddases that strip away its components. Further deterioration of the glycocalyx promotes inflammation and increased generation of ROS through processes thought to play a causative role in the development and progression of multiple cardiovascular pathologies including atherosclerosis (37, 162), diabetes (100, 262) and aging (108).
The endothelial glycocalyx degradation
Hyaluronic acid and hyaluronidase
Hyaluronan (or hyaluronic acid) is an ubiquitous polysaccharide that accounts for 5 to 20% of the total glycosaminoglycans present within the endothelial glycocalyx. Its structure consists of repeating disaccharides composed of N-acetylglucosamine and glucuronic acid. In contrast to the other glycosaminoglycans, hyaluronan preserves its chemical composition across species because it is not further modified by sulfonation or epimerization (111). Its indeterminate and extensive degree of polymerization makes hyaluronan a long, unbranched, negatively charged highly anionic polymer (96). In solution, under physiological conditions, hyaluronan is stabilized by hydrogen bonds. This allows the polymer to assume a stiffened helical configuration and an overall coiled structure that occupies a vast three-dimensional space. Hyaluronan lies on the luminal surface of endothelial cells forming a hexagonal-like network within the glycocalyx structure. Its localization in the same plane as the endothelial cell surface, positions hyaluronan in the inner portion of the glycocalyx and provides for its function as a molecular sieve (82). All these characteristics of hyaluronan contribute to the selective permeability of the endothelial barrier as described before (66).
Three membrane-bound enzymes, named hyaluronan synthase 1-3, make hyaluronan. The enzymes link the cytosolic substrates uridine diphosphate-glucuronic acid and uridine diphosphate-N-acetylglucosamine through glycosidic bonds to form the polymer (124, 304). This synthesis occurs primarily at the inner surface of the plasmalemma, and the growing polymer is extruded through the membrane to form extracellular matrices (111). Once released from the enzyme, hyaluronan integrates into the endothelial glycocalyx via a number of different mechanisms, including binding to the CD44 receptor, associating with chondroitin sulfate and forming a fibrous network by self-assembly (65). In mammals, hyaluronan synthase-2 is the most common enzyme isoform that correlates well with hyaluronan distribution and plays a determinant role in vascular development and endothelial homeostasis (289, 304).
Hyaluronan is synthesized as a high (>500 kDa) or low molecular weight (10-500 kDa) polymer (45, 52). Additionally, the low molecular weight form of hyaluronan can result from the degradation of its higher molecular weight forms (278). High molecular weight hyaluronan prevails under physiological conditions and, as previously stated, provides anti-inflammatory, antiangiogenic and immunosuppressive properties to the endothelial glycocalyx. In contrast, the concentration of low molecular weight hyaluronan increases under pathological conditions, and is highly inflammatory, angiogenic and stimulatory of the immune system. In particular, small hyaluronan fragments are known for their capacity to stimulate proliferation and migration of endothelial cells, and their presence in circulation is associated with disruption of the endothelial barrier and endothelial dysfunction (66).
The tissue half-life of hyaluronan ranges from a few hours to several days (93). Both, enzymatic and non-enzymatic degradation of hyaluronan are responsible for its catabolism and fragmentation. In mammals, hyaluronidase-1 and -2 are the main enzymes responsible for the degradation of this glycosaminoglycan into fragments of 2-6 disaccharides up to 20 kDa, respectively. Mice deficient in these two enzymes present with increased plasma hyaluronan levels in addition to a thicker endothelial glycocalyx structure. Hyaluronidase-1-deficient mice are also protected from the endothelial glycocalyx alterations and endothelial dysfunction associated with the early stages of diabetes mellitus. This underlies the relevance of hyaluronan degradation and this enzyme system in regulating endothelial glycocalyx function and diabetic vascular complications (64, 223). Nonenzymatic degradation of hyaluronan mostly results from oxidation associated with the presence of ROS, including superoxide anion, hydrogen peroxide, NO, and peroxynitrite (66). Once in circulation, 85-90% of hyaluronan fragments are eliminated by the liver and the rest by the kidneys (93). This efficient system can handle the degradation of 10 to 100 mg of hyaluronan fragments per day in the human blood (93). Therefore, the levels of hyaluronan in the bloodstream at any single moment result from the balance between the rapid formation and removal of hyaluronan fragments from the circulation.
Overall, a healthy endothelium is associated with hyaluronan stability. Laminar shear stress imposed on cultured endothelial cells increases hyaluronan synthesis and its incorporation into the endothelial glycocalyx (181, 304). In comparison, disturbed flow patterns are associated with reduced endothelial glycocalyx thickness (106), and low shear stress exposure activates hyaluronidase-2 resulting in shedding of hyaluronan from the endothelial glycocalyx (140). This indicates that hemodynamic forces play a key role in modulating glycocalyx stability and endothelial health.
Sialic acid and neuraminidase
As previously stated, the glycocalyx is constantly undergoing a process of degradation and biosynthesis. It is the sum total of each of those processes that dictates the size or thickness, as well as presumably the overall composition of the glycocalyx structure. Shear forces generated by the flow of blood influence both the synthesis of endothelial glycocalyx components, as well the degradation of the structure. Endothelial cell culture assays have shown that under static (non-flow) conditions, generation of the glycocalyx is highly irregular and discontinuous, indicating a wide degree of heterogenous thickness and coverage. Moreover, expression of individual glycocalyx components is considered to be down-regulated under static conditions. In contrast, flow appears to stimulate expression of glycocalyx components. However, this is not a binary relationship in which flow has a positive effect on glycoprotein and glycosaminoglycan expression and no-flow a negative effect; rather it is a much more complex relationship that reflects a high level of feed-back between biosynthetic processes and the specific type of flow in the local environment imparting shear stress forces on the endothelium. For example, Dai et al. analyzed the flow pattern in atherosclerosis-susceptible and atherosclerosis-resistant regions of the human carotid artery and found two distinctly different shear stress wave forms. They classified them as either athero-protective or athero-prone (53). Notably, when applied to human endothelial cells in cell culture, the two waveforms, which differed in amplitude, had markedly different effects on cell architecture, inflammatory responses and biosynthesis of glycocalyx components (53, 141). In addition, the frequency of the shear force wave also appeared to result in further differences in glycocalyx biosynthesis outcomes. A constant, steady (laminar) shear stress promoted the synthesis of glycocalyx components, whereas a low, oscillatory shear stress was implicated in glycocalyx degradation (107, 304). This emphasizes the complex role that hemodynamics play on glycocalyx synthesis and degradation. As stated before, an additional compounding issue includes that, hyaluronidase, the enzyme that degrades hyaluronic acid, is activated under conditions of low shear stress and leads to glycocalyx degradation (304, 322).
Overall, the process(es) that regulate the differential endothelial responses to laminar vs. disturbed flow have not been fully determined. However, emerging evidence suggests that sialic acids play a role in cellular glycocalyx biosynthesis and degradation responses to shear stresses. In particular, the Nrf2 pathway is activated by laminar flow, resulting in upregulation of Nrf2 and its translocation to the nucleus, where it promotes the expression of a number of cytoprotective proteins, including a suite of antioxidant genes (54). The proteins downstream of Nrf2 activation generate intracellular and extracellular conditions that are conducive to biosynthesis of glycocalyx components. In contrast, oscillatory shear stresses promote glycocalyx degradation, and this is accompanied with shedding of sialic acid residues from glycocalyx structures. Interestingly, exogenously applied neuraminidase, which cleaves sialic acid, attenuates Nrf2 activation in cells exposed to laminar flow and enhances the expression of pro-inflammatory cytokines associated with glycocalyx degradation. In contrast, silencing of endogenous neuraminidase-1, enhances Nrf2 signaling in response to laminar flow degradation (235). These findings have important implications in human health as plasma neuraminidase activity and its product, sialic acid, are elevated in humans with, and animal models of, pathologies associated with reduced NO bioavailability, including aging (243), diet-induced obesity (89) and diabetes (209). Moreover, a number of studies have demonstrated that exogenously applied neuraminidase impairs flow-mediated vasodilation and increases oxidative stress (144).
Another process associated with glycocalyx degradation includes the capacity of cells to dismantle their attached glycan chains once the lifespan of the molecules has been reached. Recycling of sialic acids from cell surface glycoconjugates occurs in endosomes and lysosomes, and is mediated by the neuraminidase family of enzymes. These enzymes are exoglycosidases as they mediate the removal of terminal sialic acids (also referred to as neuraminic acids) from glycoproteins, glycolipids and oligosaccharides. In an attempt to standardize the nomenclature, the preferred name for these enzymes is sialidases, with the term neuraminidase reserved for the viral enzymes due to historical reasons (influenza virus subtypes are identified by an H number (for hemagglutinin) and an N number (for neuraminidase)). There are 16 different HA antigens (H1 to H16) and nine different NA antigens (N1 to N9) for influenza (312). In humans, there are four identified sialidases that despite the preferred nomenclature are known as neuraminidase 1-4, and are differentially expressed within subcellular locations of the cell. Neuraminidase-1 is posited to release sialic acids from glycoconjugates recycled from the plasma membrane in endosomal and lysosomal compartments whereas neuraminidase-2 has been demonstrated to function in the cytoplasm (200, 236). Neuraminidase-3 is associated with the plasma membrane as well as with endosomal membranes and has been shown to exhibit extracellular activity (246). Neuraminidase-4 isoforms are found in both intracellular membranes and the mitochondria (200, 319). At this time, there has not been a systematic evaluation of human sialidase expression in different cell types or tissues. Thus, it is not known how the expression of these isoforms may vary in different organs. Anecdotally, there are a few studies examining the specific isoforms found in human plasma/serum, which suggest that only neuraminidase-1 and neuraminidase-3 are present in plasma at measurable levels, and that plasma activity is predominantly due to neuraminidase-3 (324). In addition to differences in subcellular localization, the enzymes also appear to have varying degrees of specificity towards different glycoconjugates. Moreover, enzymatic activity fluctuates between the different isoforms depending on the type of glycosidic linkage (α 2-3, a 2-6, etc.) of the substrate as well as the local pH (194, 240, 280). Although their direct activity is well characterized, the roles these enzymes play in various cellular processes has not been thoroughly investigated. The membrane-associated neuraminidases are posited to play a role in signaling pathways, apoptosis, and cell–cell interactions, whereas the others are speculated to mediate cell differentiation and proliferation processes. Interestingly, neuraminidase isoforms are found to be upregulated in a number of cardiovascular diseases associated with glycocalyx degradation and endothelial dysfunction. These pathologies include diabetes (42, 257), sepsis (229), hypertension (266), atherosclerosis (101, 104, 311) and obesity (89, 211). In addition, aberrant neuraminidase activity is also observed in a number of diverse malignancies (193).
Heparan sulfate and heparanase
Heparanase is an endoglycosidase that cleaves internal heparan sulfate polymeric chains at sites of reduced sulfonation, and thus facilitates degradation and remodeling of the glycocalyx (231). To date only one heparanase with catalytic activity has been identified in mammals. However, there is a second nonenzymatic protein, heparanase-2 that is closely related, and appears to inhibit heparanase activity (160). The nonenzymatic function(s) of heparanase are mainly linked to pro-angiogenesis pathways that positively modulate vascular endothelial growth factor signaling pathways (138, 300). Conversely, the enzymatic activity of heparanase functions to promote angiogenesis via the release of molecules such as vascular endothelial growth factor and epidermal growth factor that are associated with the heparan sulfate moieties cleaved by the enzyme. In addition to its effect on growth factors, heparanase also mediates the release of chemokines and cytokines that are embedded within the glycocalyx and are also associated with heparan sulfate residues. Additional physiological functions attributed to heparanase include the transcriptional upregulation and expression of the pro-inflammatory cytokines, TNF-α, interleukin (IL)-1, IL-6, as well as the activation of the innate immunity system, exosome formation, and positive regulation of autophagy (300). Pathologically, heparanase upregulation is implicated in the progression of cancer via positive modulation of cellular growth pathways, angiogenesis and metastasis (300). It is also implicated in mediating chronic inflammatory responses and atherosclerosis in diabetic and nondiabetic settings (12, 102, 301). In addition, a specific variant of heparan sulfate that contains a pentasaccharide motif and is synthesized by endothelial cells plays a primary role in anticoagulation pathways, via its high affinity interaction with the protease inhibitor, antithrombin III (269). The latter, negatively regulates coagulation under physiological and pathological settings via inhibition of pro-coagulant pathways (225). It is important to note that the heparins secreted by mast cells, also regulate ant-coagulation pathways. However, albeit similar in structure, they are biochemically distinct from the heparan sulfates generated by endothelial and other cell types (267). With regard to its numerous associations with pathological conditions, degradation of heparan sulfate is particularly implicated in the promotion of thrombosis and pro-coagulant signaling pathways in atherosclerosis and sepsis, due to its positive role in maintaining an anti-coagulant state (13, 208, 313).
Glycocalyx components and other sheddases
In addition, to the three main enzymes that target specific components of the glycocalyx, there are additional sheddases with promiscuous activities towards multiple targets that encompass components of the glycocalyx. These enzymes include the ADAM and MMP families of proteases with the former implicated in shedding of cell surface proteins and receptors and the latter characterized by their ability to digest collagen, fibronectin, elastin, laminin and other extracellular matrices (238). Notably, both families of enzymes are positively regulated by ROS (207), a common by-product of inflammatory pathologies, and are upregulated under pathological conditions associated with inflammation, including hypertension (58, 81, 118), diabetes (154, 281), atherosclerosis (105, 127), sepsis (87), obesity (10, 185) and aging (70). With respect to specific glycocalyx targets, the ADAMs are implicated in shedding syndecan-4, as well as with increasing vascular permeability and inflammation in sepsis (169, 325). As for the MMPs, they are implicated in shedding syndecan-1 and -4 in the setting of diabetes (164). In addition to shedding components of the glycocalyx, these enzymes also release inflammatory cytokines, including TNF-α, soluble Toll-Like Receptor 2, interferon-γ, transforming growth factor-β, IL-4, IL-10, IL-13, IL-6, and fractalkine (26, 58, 150). This sets up a feed forward cascade of events, as inflammatory cytokines have been shown to mediate glycocalyx shedding (113, 227), and upregulate MMPs (50, 161) and ADAMs (91, 303).
The endothelial glycocalyx in vascular health and disease
As studies into the structure and function of the glycocalyx accumulate, it is becoming more apparent that the endothelial glycocalyx plays a central role in maintaining vascular health. Indeed, numerous pathological conditions with concomitant vascular disfunction are also associated with endothelial glycocalyx shedding or degradation (Figure 9). The major pathophysiological effects of an impaired or damaged endothelial glycocalyx are threefold: 1) increased vascular permeability, 2) reduced NO bioavailability and 3) increased inflammation. Due to the difficulty in assessing the glycocalyx integrity and lack of a systematic investigation on the topic, it remains to be determined whether glycocalyx degradation precedes the development of cardiovascular pathologies, is a consequence of alterations in vascular homeostasis that occur in response to various pathologies or results from a combination of the two. Below, we highlight some pathologies in which glycocalyx degradation is implicated in disease progression.
Figure 9.
Homeostasis of the endothelial glycocalyx relies on an appropriate balance between glycocalyx degradation or shedding and biosynthesis or adsorption. However, numerous conditions tilt the balance towards glycocalyx degradation. Such conditions include disturbed blood flow patterns, hypertension, aging and diabetes. Exacerbated glycocalyx degradation exposes the vasculature to infiltration and adhesion of pathogens and pro-inflammatory molecules, thus leading to vascular inflammation, atherosclerosis, and endothelial dysfunction. A, schematic depiction of a healthy endothelial glycocalyx (left panel) and one in which disturbed blood flow, hypertension, aging or diabetes has favored its degradation. B, Confocal microscope generated images of an isolated small artery showing the endothelial glycocalyx (stained with wheat germ agglutinin (WGA), green) and vascular cell nuclei (stained with 4′,6-diamidino-2-phenylindole (DAPI), blue) before (left) and after (right) enzymatic degradation of the glycocalyx.
Atherosclerosis
Atherosclerotic lesions occur via the deposition of cholesterol-rich lipids within the vessel wall, initiating an inflammatory response that leads to the formation of vascular lesions, termed plaques that evolve over time and become susceptible to rupture (122). During disease progression, remodeling of the vasculature in response to plaque formation restricts the flow of blood and mediates arterial stenosis. In the later stages of the disease, thrombosis and myocardial infarction with significant mortality risks may occur subsequent to plaque rupture. One of the first steps posited to play a role in the development of atherosclerosis is degradation of the endothelial glycocalyx. Within the vasculature, areas of disturbed blood flow, such as bends or branch points in the arterial tree, are not conducive to maintenance of a healthy glycocalyx structure (288). It is in these areas (termed athero-prone) of a degraded glycocalyx that plaques are most likely to form (38). Mechanistically, one of the initiating steps in the progression of atherosclerosis involves an inflammatory response mediated by the adhesion of leukocytes to the endothelium. The electrostatic and barrier (steric hindrance) nature of the glycocalyx impedes leukocyte adhesion and functions to dampen inflammatory responses (21). In athero-prone regions of the vasculature, damage to the glycocalyx has been demonstrated to significantly enhance leukocyte adhesion, in the hamster cremaster and post-capillary mesenteric venules in the rat (113, 205). These areas also correlate with an increase in cholesterol-rich lipid uptake (192), which is an important initiating step in triggering inflammatory responses and promoting disease progression (166).
Sepsis
Sepsis is a systemic inflammatory state that results from exposure to an infectious agent and carries a significant mortality risk. Mortality arises either from septic shock and multiple organ failure in response to the direct release of cytokines triggered by the inflammatory response or from the secondary infection caused by immunosuppression in response to the cytokine release. Sepsis is characterized by increased vascular permeability that in turn mediates extravasation of proteins and molecules out of the blood’s fluid phase. The resulting change in solute concentration from within the lumen to the surrounding tissue decreases oncotic (aka colloid osmotic) pressure leading to decreased water uptake within the fluid phase, which is a key contributor to sepsis-induced organ failure. The mechanism of sepsis induced edema is posited to be due to increased vascular permeability via degradation of the endothelial glycocalyx. Potential mediators of glycocalyx degradation in sepsis include oxidative stress, upregulation of TNF-α signaling, and increased sheddase activity by enzymes such as the ADAMs, MMPs, heparanase-1, hyaluronidases and neuraminidase (103, 259, 263).
Hypertension
The American Heart Association defines Stage-1 hypertension as presence of systolic/diastolic blood pressure above 130-139/80-90 (310). These parameters are considered high normal blood pressure by the International Society of Hypertension (284). Though there are a number of independent mechanisms implicated in the development of hypertension, sodium-dependent (high salt sensitivity) hypertension is linked to endothelial glycocalyx degradation (221). The glycocalyx is posited to function as a reservoir for positively charged sodium ions. Thus, in the absence of a healthy glycocalyx, excess sodium ions enter endothelial cells and activate cell signaling pathways that positively regulate production of F-actin cytoskeletal fibers (85). This has the dual effect of increasing cell stiffness and decreasing NO production, which increase vascular resistance and blood pressure (218). Additional observations suggest that the glycocalyx may also play other roles in the development of hypertension. Results from the Framingham study indicate that arterial stiffening precedes the development of hypertension (76, 176, 191), and glycocalyx degradation is posited to play a key role in arterial stiffening. Using an in vivo imaging technique (GlycoCheck) researchers demonstrated an association between reduced glycocalyx thickness and increased central aortic blood pressure, coupled with the observation that a reduced endothelial glycocalyx was also associated with reduced arterial elasticity or increased arterial stiffness (120). It was also demonstrated in a rat model of induced pulmonary arterial hypertension that supplementation with heparin to increase the integrity of the glycocalyx attenuated the development of hypertension (109). Reduced NO bioavailability is also implicated in the development of hypertension (163). Degradation of the glycocalyx is posited to lower eNOS activation by diminishing shear mechanosensation/mechanotransduction. In support of this hypothesis, VanTeeffelen et al. demonstrated that degradation of the glycocalyx in mice with hyaluronidase induced baseline arteriolar vasoconstriction and led to reduced arteriolar diameters at the end of a vascular occlusion intervention and at peak reactive hyperemia (293). Additional studies in canine femoral arteries demonstrated that hyaluronidase treatment significantly decreased flow induced-NO production, but not acetylcholine induced NO production (195). These studies support the premise that a healthy glycocalyx positively regulates blood pressure. More studies are necessary to determine the mechanism(s) by which the glycocalyx protects against the development of hypertension. Specifically, whether protection against hypertension is due to the glycocalyx’s barrier, anti-inflammatory or NO generating properties (or a combination thereof). Interestingly, preeclampsia, a life-threatening elevation in blood pressure that occurs during pregnancy, is associated with a degraded glycocalyx (308). In addition, three pathologies related to glycocalyx degradation are also present in preeclampsia: increased vascular permeability with proteinuria (25), reduced NO bioavailability (173, 261) and an increased inflammatory response or chronic immune activation (47, 198). It is unclear whether these impairments are individually or collectively responsible for the observed elevation in blood pressure, or whether other mechanism(s) are also responsible.
Aging
To consider aging a pathology is a bit of a misnomer, as the determination of whether old-age is a disease itself is a topic of much debate. Suffice it to say that as organisms age there is an overall reduction in fitness at all levels of organization i.e., from organs and tissues down to individual cells. The same generalization holds true for the glycocalyx. In older mice, it was observed that mesenteric and skeletal muscle microvessels have a significantly reduced glycocalyx thickness than that of microvessels from young mice. Using in vivo imaging with the GlycoCheck, the same trend was observed in the sublingual arteries of older vs. younger humans (174). It remains to be determined whether the mechanisms mediating this reduction in glycocalyx integrity are due to aberrant biosynthesis of glycocalyx components or an increased activity or concentration of the components and processes that degrade its structure. Alternatively, there are a number of pathophysiological changes associated with aging, including hyperglycemia, dyslipidemia and increased oxidative stress, which have the potential to degrade the glycocalyx. For example, red blood cell sialylation decreases with age in humans via a process associated with increased ROS, specifically lipid peroxidation (188). Presumably, the glycocalyx, which experiences the same insults as red blood cells, is similarly desialylated in old age. ROS are well documented to deleteriously impact glycocalyx structure and function (168, 270). This hypothesis dovetails well with the oxidative stress theory of aging, which posits that the accumulation of oxidative damage to macromolecules mediates functional losses in old age (165).
Cancer
Cancer encompasses a range of malignancies with a multitude of distinct etiologies characterized by a singular deleterious outcome: unchecked cellular growth. The evolution of a cell into a cancerous lesion that threatens the health of the host involves the accumulation of genetic mutations that facilitate proliferation, local invasion within its immediate surroundings, intravasation into the fluid phase of blood, extravasation to secondary sites and the subsequent colonization of new tissue. To varying degrees, the glycocalyx and specific modifications to it by the cancer itself play a role in each one of these steps of cancer progression. The fate of cells, including cancer cells, is significantly influenced by their biochemical interactions with the extracellular matrix including the glycocalyx (110, 129). Herein we will highlight the role of the endothelial glycocalyx in vascular adhesion in cancer biology, but for a more in-depth review of the glycocalyx in cancer see (33). Increased selectin expression is associated with cancer metastasis, and is linked to deleterious health outcomes (210). Selectins are a family of carbohydrate binding proteins, classified as lectins, expressed on the surface of leukocytes (L-Selectin), endothelial cells (E-selectin) and platelets and endothelial cells (P-selectin), which facilitate adhesion processes within the vasculature. This adhesion is thought to be critical in metastasis by facilitating tumor cell adhesion to the vasculature. Studies have demonstrated that tumor cells are able to bind at least one class of selectin, and that tumors in animal models deficient in selectin(s) have attenuated growth profiles and a decreased metastatic phenotype (22). One of the moieties involved in selectin binding are sialylated glycan epitopes (51), which implies that increased sialylation favors adhesion processes and metastasis. Indeed, it has been documented that the tumor cell glycocalyx is characterized by aberrant glycosylation and an increased degree of sialylation (129, 333). Hypersialylation is observed in tumors when compared to normal, nonmalignant tissue of the same kind, and in the serum of patients with a wide range of distinct cancers when compared to plasma from noncancerous subjects (34, 332). In addition, sialic acid is elevated in the plasma of patients with head and neck, breast, endometrial, colorectal, lung and oral cancers (285). Hypersialylation is posited to arise from increases in biosynthetic pathways, such as increased sialyltransferase activity (55). Notably, the neuraminidase enzymes that remove sialic acid are differentially regulated in cancer. For example, the neuraminidase-3 isoform is upregulated in a number of cancers (29, 133) and is posited to positively influence metastatic potential and invasiveness (193, 265). In contrast, neuraminidase-1 has been reported to be down-regulated in a number of cancers, indicating opposing or differential activation of signaling pathways by these distinct neuraminidase isoforms. With respect to neuraminidase-2, studies suggest it has anti-metastatic effects related to apoptotic pathways when transfected into tumor cell lines, but its in vivo role in human cancer pathologies is unclear (194). Similar to neuraminidase-2, neuraminidase-4 has been shown to increase sensitivity to apoptotic pathways, when transfected into human cancer cell lines (320). Neuraminidase-4 mRNA has also been shown to be reduced in some human tumors, which may influence tumor invasiveness (320). This suggests that neuraminidase-4 also has a negative effect on cancer progression, but its complete role in cancer remains to be fully determined. Suffice it to say that alterations in the glycocalyx, specifically in sialic acid residues play critical roles in the development and progression of cancer. Moreover, in addition to mediating adhesion of cancer cells to the vasculature, hypersialylation appears to assist tumor cells in evading immune surveillance by binding siglecs, which signal to the immune system that the tumor is part of the host’s healthy tissue (49, 135). Priming the immune system to recognize tumors is emerging as a powerful therapeutic tool to eliminate cancerous lesions. One of the approaches undertaken to target the tumor for immune recognition is by blocking siglecs specifically or more generally by removing sialic acids from the tumor via inhibition of the sialyltransferases in the biosynthetic pathway (15, 206, 286).
Cardiovascular complications in diabetes mellitus
Diabetes mellitus is associated with severe and progressive cardiovascular disease (CVD). A key mechanism implicated in the pathogenesis of diabetic CVD is endothelial dysfunction, which is characterized by oxidative stress, chronic inflammation, reduced NO bioavailability, and impaired endothelium-dependent vasodilation. As glycocalyx degradation results in endothelial dysfunction, investigators have explored the role of glycocalyx degradation as a central contributor to the development of CVD associated with diabetes.
Indeed, several studies provide evidence of glycocalyx degradation in diabetes. For example, in a preclinical model of diabetes, Szymonski and collaborators showed that db/db male mice exhibit decreased glycocalyx length (assessed by atomic force microscopy) coupled with endothelial dysfunction (275). Similarly, Zuurbier et al. found that hyperglycemia (25mmol/L) is associated with increased glycocalyx permeability with parallel augmentation of vascular permeability (336). In a rodent model of diabetic retinopathy, Kumase and collaborators reported diminished glycocalyx thickness in the retinal vessels of diabetic mice compared to controls (145). Similarly, in a model of nephropathy (streptozotocin-induced DBA2J mice), loss of glomerular glycocalyx length has been documented (222). In support of the role of glycocalyx degradation in the genesis of endothelial dysfunction in diabetes, Dogné et al. showed that deletion of hyaluronidase-1 in male hyperglycemic mice resulted in increased glycocalyx thickness and preservation of its structure, which paralleled with amelioration of the endothelial dysfunction induced by hyperglycemia (67).
Metformin, a first-line antidiabetic agent, has been investigated as a strategy to prevent glycocalyx degradation in a diabetic model (78). In that study, db/db mice treated with metformin in the drinking water for two weeks displayed improvements in glycocalyx barrier properties when compared to control mice. Notably, this short course of metformin did not produce significant changes in glycemia (78).
In humans, similar findings have been reported. Nieuwdorp et al., induced transitory hyperglycemia in healthy men via a hyperglycemic clamp (16mmol/L) and observed a profound reduction in glycocalyx volume that coincided with increased circulating plasma levels of glycocalyx constituents, such as hyaluronan, as well as impaired brachial artery flow-mediated dilation. Importantly, and pointing toward oxidative stress as a mechanism implicated in hyperglycemia-induced glycocalyx degradation, the reduction in glycocalyx volume was largely prevented by treatment with an antioxidant agent (214). It has also been reported that men with type 1 diabetes have reduced endothelial glycocalyx volume when compared to healthy controls (212). Moreover, this diabetic population has also been shown to have elevated circulating markers of glycocalyx degradation (214), and a significant correlation between plasma hyaluronan levels and carotid intima media thickness (215).
Different therapeutic strategies have been used to lessen glycocalyx degradation in diabetes. For example, Vink and collaborators showed that administration of sulodexide for two months is effective at partially restoring the endothelial glycocalyx in well-controlled type 2 diabetic men without history of CVD (31). Sulodexide therapy results in availability of glycocalyx components for adsorption as well as that of precursors needed for glycosaminoglycan synthesis. More recently, a large clinical trial examined the effects of commonly use antidiabetics agents on glycocalyx volume (121). One hundred sixty patients with type 2 diabetes were randomized to receive insulin, liraglutide (glucagon-like peptide-1 receptor analog), empagliflozin (sodium glucose transporter type 2 inhibitor) or a combination of liraglutide + empagliflozin as add-ons to metformin. Subjects underwent assessments at baseline, and at 4 and at 12 months of treatment. At 12 months, all three treatment groups displayed improvements in the PBR of the sublingual arterial microvessels (marker of endothelial glycocalyx integrity using the GlycoCheck) and pulse wave velocity (marker of arterial stiffness). However, and despite similar glycemic control when compared to the insulin group, the liraglutide and empagliflozin-treated subjects demonstrated greater improvements in glycocalyx volume. It is important to note that subjects in the combined treatment also demonstrated greater weight loss during the trial (121). Notably, multiple clinical trials in type 2 diabetic patients have now demonstrated that use of liraglutide and empagliflozin therapy is associated with decreased CVD events and cardiovascular death (182, 334). However, to what extent the cardiovascular protection afforded by liraglutide and empagliflozin is attributed to glycocalyx restoration remains to be determined.
The endothelial glycocalyx integrity assessment
Historically, the importance of the endothelial glycocalyx had not been fully realized as its main function was thought to be limited to a protective cover, encapsulating cells in a sugar coat, and independent of interacting with and regulating biochemical signaling pathways. However, that naïve perspective has been rapidly changing, as the importance of the glycocalyx in maintaining vascular health becomes more apparent. One of the factors impeding the study of the glycocalyx, and why its importance in vascular health has not been fully characterized, is that it is a very difficult structure to study. The difficulty in imaging and assessing the glycocalyx is attributable to a number of technical issues that have prevented a full characterization and understanding of its structure and function in maintaining vascular homeostasis, as well as how its degradation contributes to a myriad of different pathologies including diabetes, hypertension and inflammation. The problems inherent in studying the glycocalyx are well characterized in a review by Mockl et al. (196). In brief, they ascribe three main impediments. First, the depth of the glycocalyx is posited to extend to not much more than the diffraction limit of light ~250nm. Therefore, conventional-light microscopy techniques are unable to adequately approximate the dimensions of the glycocalyx, and are not suitable for identifying fine structures within the glycocalyx. Use of electron microscopy techniques to overcome this diffraction limitation have not adequately addressed this problem. Notably, the oligosaccharides that comprise the glycocalyx are quite labile and are likely affected by the fixation needed in electron microscopy processing. Moreover, this approach is unable to differentiate individual components of the glycocalyx, as the majority of stains used to visualize the glycocalyx are cationic molecules such as lanthanum, Alcian blue and ruthenium red, thus their binding properties are contingent upon charge and not a specific glycan (204). A second impediment is the fact that the oligosaccharides that comprise the glycocalyx are secondary gene products. That is, their genesis is not the simple result of gene expression followed by protein translation. Rather their assembly and attachment to proteoglycans occur within multiple cellular compartments as well as within the extracellular space. This makes them subject to regulation by a multitude of factors, including but not limited to the metabolic and redox state of the cell (62, 139), as well as hemodynamic-associated phenomena such as metabolite concentrations and shear forces occurring within the fluid phase of blood (304, 316). Furthermore, there is a large degree of functional overlap in the enzymes involved in the biosynthesis of all of the glycocalyx components. This intertwined complexity in both machinery and assembly is not conducive to traditional genetic approaches to either label components or utilize knockout technologies to identify regulatory pathways. A final impediment, is that the difference in monosaccharides can be chemically subtle, i.e., the substitution of a single functional group. None-the-less, these small differences can have profound functional consequences. This makes it incredibly difficult to label unique glycan molecules.
Over time, there has been a number of innovative approaches generated to overcome the obstacles that impede glycocalyx assessments, with mixed results. Various microscopic techniques have been used to visually assess the glycocalyx including electron, intravital and confocal microscopy. Van Den berg et al., used a modified electron microscopy approach to generate some incredibly detailed images of a rat left ventricular myocardial capillary that allowed for estimates of glycocalyx length in the range of 200-500 nm. Our own attempts to recapitulate this approach (Figure 10), has led to mixed results, with heterogenous staining across different samples. That there are only a few examples of this approach being used in the literature, highlights the difficulty in using this technique to image the glycocalyx (158, 287). Moreover, as previously mentioned, it is unclear how the glycocalyx is affected by the fixation and dehydration process, and this approach cannot differentiate individual glycan structures. There have been a number of intravital approaches developed to image the glycocalyx in vivo. Fluorescently labelled wheat germ agglutinin lectin, that selectively binds sialic acid, has been used to stain the glycocalyx (131, 147, 244, 252). In our experience, this approach is not very amenable to accurately assess glycocalyx dimensions due to variations in staining intensity between samples. To overcome this limitation, researchers have coupled distinct fluorescent dyes in which one dye labels the fluid phase of blood and the other the surface of endothelial cells. The difference in peak intensity of the two fluorophores is used to infer the width or length of the glycocalyx (24). Salmon et al. used this approach to assess glycocalyx length in exposed mouse mesenteric vessels. One of the shortcomings of this approach, is that the lipophilic dye (R18) used to label the luminal endothelial membrane is not constrained to the luminal (apical) surface, and will eventually label the basal membrane of the endothelial cell as well as the underlying smooth muscle cells. Thus, factors independent of the glycocalyx that affect membrane fluidity can impact the results. Recently, an analogous approach has been employed using light microscopy to measure by proxy the endothelial glycocalyx width in human subjects. In brief, this approach measures the vascular wall as well as the red blood cell column width in sublingual capillaries using the GlycoCheck instrument. An algorithm then calculates the width of the glycocalyx that is excluding the red blood cells from contacting the vessel wall (249). Additional imaging approaches have used super resolution microscopy (196), as well as novel freeze fracture techniques coupled with transmission electron microscopy (74). The limitations of these approaches include that these are usually nonnative investigations of the glycocalyx in cultured cells. Therefore, it is unclear how cell culture models accurately recapitulate the endogenous structure, as they lack the specific hemodynamic as well as metabolic and structural frameworks found in vivo.
Figure 10.
The endothelial glycocalyx is very fragile and difficult to measure. Today two commonly used techniques that provide ex vivo direct measurements of the in situ endothelial glycocalyx characteristics include atomic force microscopy (AFM) and transmitted electron microscopy (TEM). A, AFM allows for the measurement of glycocalyx length and stiffness as assessed by the force curves generated when an AFM cantilever approaches and deforms the glycocalyx structures of en face isolated vascular preparations or cultured endothelial cells. B, TEM allows for visualization of endothelial glycocalyx structures at the highest resolution currently available in microscopy, albeit, requiring complex sample preparation (Bar = 200 nm).
Notably, all the above approaches have yielded different length measurements for the glycocalyx. Standard transmission electron microscopy in cell culture assessed the glycocalyx length at around 40nm. In contrast, the freeze/fracture electron microscopy approach estimated the length of the glycocalyx as being close to 11 μm in bovine aortic endothelial cells (74). The intravital techniques in animal models indicate the average length of the glycocalyx is between 1.5 - 2.5μm (24, 244). All these results highlight the difficulty in determining glycocalyx length using diverse optical microscopy approaches.
In addition to visualizing the glycocalyx, there have been alternative approaches that attempt to measure glycocalyx length using atomic force microscopy that also probe substrate stiffness. In this approach isolated aortae are opened en face and the exposed glycocalyx covered surface is indented with a spherical atomic force microscopy probe (Figure 10). An algorithm, using the Brush model, then analyzes the indentation curves to determine both glycocalyx length as well as the underlying cellular stiffness. This approach has been used to demonstrate a reduction in glycocalyx length in an animal model of diabetes compared with control animals (275). One critique of this approach is that it is not an in vivo measurement, and it is unclear what changes occur in sample preparation. Since fixation and dehydration are not required in the sample preparation, these effects are predicted to be fairly minimal when compared to other ex vivo methods. A clear advantage to this approach, is that it is possible to design experiments that are unbiased (an algorithm determines glycocalyx length) and thus allows for more precise assessments of pathologies that affect the glycocalyx.
Conclusion
The endothelial glycocalyx plays a central role in maintaining vascular health via its regulation of vascular permeability and its mechanical ability to transduce shear stress and facilitate production of NO. Moreover, these processes function in tandem to maintain vascular homeostasis and act as a bulwark to inhibit inflammation, a root cause in the development and progression of cardiovascular pathologies. In this review, we have highlighted the glycocalyx biochemical components that are implicated in mediating these processes and have identified, in a number of pathologies, how these processes go awry, following degradation of the glycocalyx. In addition, we have presented current best practices for assessing glycocalyx integrity and measuring its physical dimensions using an array of approaches amenable to both in vivo and in vitro models. As the realization of the glycocalyx’s primary role in preventing and/or ameliorating cardiovascular pathologies continues to emerge, many therapies targeting either increased biosynthesis (adsorption) or decreased degradation are currently in development to target the structure for protection or restoration.
Didactic Synopsis.
Major teaching points:
All cells have attached a polysaccharide extracellular structure, called the glycocalyx.
The glycocalyx on the apical surface of endothelial cells is primarily conformed of glycoproteins and proteoglycans that provide a physical, chemical and electrostatic boundary between the vascular wall and circulating blood components.
Endothelial glycocalyx components such as glypican-1, heparan sulfate, hyaluronan and sialic acid are involved in shear stress mechanosensation and mechanotransduction.
The endothelial glycocalyx functions as a permeability barrier preventing macromolecules (>70 kDa) and cationic molecules from flowing out of the vascular lumen.
The shedding and reconstruction of glycocalyx structures is regulated by the activity of key regulatory enzymes, such as the neuraminidases and nuclear factor erythroid 2–related factor 2, respectively.
Numerous diseases are associated with endothelial glycocalyx degradation including atherosclerosis, hypertension, vascular aging and diabetic vasculopathies. Consequently, the endothelial glycocalyx is considered a potential target for the diagnosis, prevention and treatment of cardiovascular and metabolic diseases.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (R01 HL088105 to L.A. Martinez-Lemus, R01 HL137769 to J. Padilla, R01 HL153264 to L.A. Martinez-Lemus and J. Padilla, and R01 HL142770 to C. Manrique-Acevedo).
References
- 1.Agren MS, and Auf dem Keller U. Matrix Metalloproteinases: How Much Can They Do? International journal of molecular sciences 21: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100: 158–173, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Albarran-Juarez J, Iring A, Wang S, Joseph S, Grimm M, Strilic B, Wettschureck N, Althoff TF, and Offermanns S. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med 215: 2655–2672, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali MM, Mahmoud AM, Le Master E, Levitan I, and Phillips SA. Role of matrix metalloproteinases and histone deacetylase in oxidative stress-induced degradation of the endothelial glycocalyx. Am J Physiol Heart Circ Physiol 316: H647–H663, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almenara CCP, Oliveira TF, and Padilha AS. The Role of Antioxidants in the Prevention of Cadmium-Induced Endothelial Dysfunction. Current pharmaceutical design 26: 3667–3675, 2020. [DOI] [PubMed] [Google Scholar]
- 6.Alphonsus CS, and Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia 69: 777–784, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Ando J, and Yamamoto K. Flow detection and calcium signalling in vascular endothelial cells. Cardiovasc Res 99: 260–268, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Arabyan N, Park D, Foutouhi S, Weis AM, Huang BC, Williams CC, Desai P, Shah J, Jeannotte R, Kong N, Lebrilla CB, and Weimer BC. Salmonella Degrades the Host Glycocalyx Leading to Altered Infection and Glycan Remodeling. Sci Rep 6: 29525, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, and Betsholtz C. Pericytes regulate the blood-brain barrier. Nature 468: 557–561, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Badenes M, Amin A, Gonzalez-Garcia I, Felix I, Burbridge E, Cavadas M, Ortega FJ, de Carvalho E, Faisca P, Carobbio S, Seixas E, Pedroso D, Neves-Costa A, Moita LF, Fernandez-Real JM, Vidal-Puig A, Domingos A, Lopez M, and Adrain C. Deletion of iRhom2 protects against diet-induced obesity by increasing thermogenesis. Mol Metab 31: 67–84, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai A, Hu H, Yeung M, and Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol 178: 7632–7639, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Baker AB, Chatzizisis YS, Beigel R, Jonas M, Stone BV, Coskun AU, Maynard C, Rogers C, Koskinas KC, Feldman CL, Stone PH, and Edelman ER. Regulation of heparanase expression in coronary artery disease in diabetic, hyperlipidemic swine. Atherosclerosis 213: 436–442, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker AB, Gibson WJ, Kolachalama VB, Golomb M, Indolfi L, Spruell C, Zcharia E, Vlodavsky I, and Edelman ER. Heparanase regulates thrombosis in vascular injury and stent-induced flow disturbance. Journal of the American College of Cardiology 59: 1551–1560, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baratchi S, Khoshmanesh K, Woodman OL, Potocnik S, Peter K, and McIntyre P. Molecular Sensors of Blood Flow in Endothelial Cells. Trends Mol Med 23: 850–868, 2017. [DOI] [PubMed] [Google Scholar]
- 15.Barenwaldt A, and Laubli H. The sialoglycan-Siglec glyco-immune checkpoint - a target for improving innate and adaptive anti-cancer immunity. Expert Opin Ther Targets 23: 839–853, 2019. [DOI] [PubMed] [Google Scholar]
- 16.Barth D, Knoepp F, and Fronius M. Enhanced Shear Force Responsiveness of Epithelial Na(+) Channel's (ENaC) δ Subunit Following the Insertion of N-Glycosylation Motifs Relies on the Extracellular Matrix. International journal of molecular sciences 22: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartke N, and Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res 50 Suppl: S91–S96, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartosch AMW, Mathews R, and Tarbell JM. Endothelial Glycocalyx-Mediated Nitric Oxide Production in Response to Selective AFM Pulling. Biophys J 113: 101–108, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates DO, Levick JR, and Mortimer PS. Starling pressures in the human arm and their alteration in postmastectomy oedema. J Physiol 477: 355–363, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beatty PR, Puerta-Guardo H, Killingbeck SS, Glasner DR, Hopkins K, and Harris E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Science translational medicine 7: 304ra141, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Becker BF, Chappell D, Bruegger D, Annecke T, and Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res 87: 300–310, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Bendas G, and Borsig L. Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. Int J Cell Biol 2012: 676731, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett HS. Morphological aspects of extracellular polysaccharides. Journal of histochemistry & cytochemistry 11: 14–23, 1963. [Google Scholar]
- 24.Betteridge KB, Arkill KP, Neal CR, Harper SJ, Foster RR, Satchell SC, Bates DO, and Salmon AHJ. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J Physiol 595: 5015–5035, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bills VL, Salmon AH, Harper SJ, Overton TG, Neal CR, Jeffery B, Soothill PW, and Bates DO. Impaired vascular permeability regulation caused by the VEGF(1)(6)(5)b splice variant in pre-eclampsia. BJOG 118: 1253–1261, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Black RA. Tumor necrosis factor-alpha converting enzyme. The international journal of biochemistry & cell biology 34: 1–5, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrin D, and Stehle T. Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat Chem Biol 11: 77–82, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Bode C, Sensken Sc Fau - Peest U, Peest U Fau - Beutel G, Beutel G Fau - Thol F, Thol F Fau - Levkau B, Levkau B Fau - Li Z, Li Z Fau - Bittman R, Bittman R Fau - Huang T, Huang T Fau - Tölle M, Tölle M Fau - van der Giet M, van der Giet M Fau - Gräler MH, and Gräler MH. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. [DOI] [PubMed] [Google Scholar]
- 29.Bovio F, Epistolio S, Mozzi A, Monti E, Fusi P, Forcella M, and Frattini M. Role of NEU3 Overexpression in the Prediction of Efficacy of EGFR-Targeted Therapies in Colon Cancer Cell Lines. International journal of molecular sciences 21: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brands J, Van Teeffelen JW, Van den Berg B, and Vink H. Role for glycocalyx perturbation in atherosclerosis development and associated microvascular dysfunction. Future Lipidology 2: 527–534, 2007. [Google Scholar]
- 31.Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ES, and Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 53: 2646–2655, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brouns SLN, Provenzale I, van Geffen JP, van der Meijden PEJ, and Heemskerk JWM. Localized endothelial-based control of platelet aggregation and coagulation under flow: A proof-of-principle vessel-on-a-chip study. J Thromb Haemost 18: 931–941, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buffone A, and Weaver VM. Don't sugarcoat it: How glycocalyx composition influences cancer progression. J Cell Biol 219: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bull C, Stoel MA, den Brok MH, and Adema GJ. Sialic acids sweeten a tumor's life. Cancer research 74: 3199–3204, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Butler MJ, Down CJ, Foster RR, and Satchell SC. The Pathological Relevance of Increased Endothelial Glycocalyx Permeability. Am J Pathol 190: 742–751, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campo GM, D'Ascola A, Avenoso A, Campo S, Ferlazzo AM, Micali C, Zanghi L, and Calatroni A. Glycosaminoglycans reduce oxidative damage induced by copper (Cu+2), iron (Fe+2) and hydrogen peroxide (H2O2) in human fibroblast cultures. Glycoconj J 20: 133–141, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Campo GM, Avenoso A, Campo S, D'Ascola A, Traina P, Sama D, and Calatroni A. NF-kB and caspases are involved in the hyaluronan and chondroitin-4-sulphate-exerted antioxidant effect in fibroblast cultures exposed to oxidative stress. J Appl Toxicol 28: 509–517, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Cancel LM, Ebong EE, Mensah S, Hirschberg C, and Tarbell JM. Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis 252: 136–146, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantalupo A, Gargiulo A, Dautaj E, Liu C, Zhang Y, Hla T, and Di Lorenzo A. S1PR1 (Sphingosine-1-Phosphate Receptor 1) Signaling Regulates Blood Flow and Pressure. Hypertension (Dallas, Tex: 1979) 70: 426–434, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carey DJ. Syndecans: multifunctional cell-surface co-receptors. Biochem J 327 (Pt 1): 1–16, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chajara A, Raoudi M, Delpech B, Leroy M, Basuyau JP, and Levesque H. Increased hyaluronan and hyaluronidase production and hyaluronan degradation in injured aorta of insulin-resistant rats. Arterioscler Thromb Vasc Biol 20: 1480–1487, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Chari SN, and Nath N. Sialic acid content and sialidase activity of polymorphonuclear leucocytes in diabetes mellitus. Am J Med Sci 288: 18–20, 1984. [DOI] [PubMed] [Google Scholar]
- 43.Chatterjee S, and Fisher AB. Mechanotransduction in the endothelium: role of membrane proteins and reactive oxygen species in sensing, transduction, and transmission of the signal with altered blood flow. Antioxid Redox Signal 20: 899–913, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng F, Mani K, van den Born J, Ding K, Belting M, and Fransson LA. Nitric oxide-dependent processing of heparan sulfate in recycling S-nitrosylated glypican-1 takes place in caveolin-1-containing endosomes. J Biol Chem 277: 44431–44439, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Chistyakov DV, Astakhova AA, Azbukina NV, Goriainov SV, Chistyakov VV, and Sergeeva MG. High and Low Molecular Weight Hyaluronic Acid Differentially Influences Oxylipins Synthesis in Course of Neuroinflammation. International journal of molecular sciences 20: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Constantinescu AA, Vink H, and Spaan JA. Elevated capillary tube hematocrit reflects degradation of endothelial cell glycocalyx by oxidized LDL. Am J Physiol Heart Circ Physiol 280: H1051–1057, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Cornelius DC. Preeclampsia: From Inflammation to Immunoregulation. Clin Med Insights Blood Disord 11: 1179545X17752325, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cosgun ZC, Fels B, and Kusche-Vihrog K. Nanomechanics of the Endothelial Glycocalyx: From Structure to Function. Am J Pathol 190: 732–741, 2020. [DOI] [PubMed] [Google Scholar]
- 49.Crocker PR, Paulson JC, and Varki A. Siglecs and their roles in the immune system. Nature reviews Immunology 7: 255–266, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Cutler SJ, Doecke JD, Ghazawi I, Yang J, Griffiths LR, Spring KJ, Ralph SJ, and Mellick AS. Novel STAT binding elements mediate IL-6 regulation of MMP-1 and MMP-3. Sci Rep 7: 8526, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D'Addio M, Frey J, and Otto VI. The manifold roles of sialic acid for the biological functions of endothelial glycoproteins. Glycobiology 30: 490–499, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Agostino A, Stellavato A, Corsuto L, Diana P, Filosa R, La Gatta A, De Rosa M, and Schiraldi C. Is molecular size a discriminating factor in hyaluronan interaction with human cells? Carbohydr Polym 157: 21–30, 2017. [DOI] [PubMed] [Google Scholar]
- 53.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, and Gimbrone MA Jr. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A 101: 14871–14876, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, and Gimbrone MA Jr. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res 101: 723–733, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Dall'Olio F, Malagolini N, Trinchera M, and Chiricolo M. Sialosignaling: sialyltransferases as engines of self-fueling loops in cancer progression. Biochim Biophys Acta 1840: 2752–2764, 2014. [DOI] [PubMed] [Google Scholar]
- 56.Dane MJ, van den Berg BM, Lee DH, Boels MG, Tiemeier GL, Avramut MC, van Zonneveld AJ, van der Vlag J, Vink H, and Rabelink TJ. A microscopic view on the renal endothelial glycocalyx. Am J Physiol Renal Physiol 308: F956–966, 2015. [DOI] [PubMed] [Google Scholar]
- 57.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Queiroz TM, Lakkappa N, and Lazartigues E. ADAM17-Mediated Shedding of Inflammatory Cytokines in Hypertension. Front Pharmacol 11: 1154, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeGrendele HC, Estess P, Picker LJ, and Siegelman MH. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte-endothelial cell primary adhesion pathway. J Exp Med 183: 1119–1130, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dejana E, Orsenigo F, and Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 121: 2115–2122, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Delgadillo LF, Marsh GA, and Waugh RE. Endothelial Glycocalyx Layer Properties and Its Ability to Limit Leukocyte Adhesion. Biophys J 118: 1564–1575, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dennis JW, Nabi IR, and Demetriou M. Metabolism, cell surface organization, and disease. Cell 139: 1229–1241, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding Z, Wang X, Khaidakov M, Liu S, Dai Y, and Mehta JL. Degradation of heparan sulfate proteoglycans enhances oxidized-LDL-mediated autophagy and apoptosis in human endothelial cells. Biochem Biophys Res Commun 426: 106–111, 2012. [DOI] [PubMed] [Google Scholar]
- 64.Dogné S, Rath G, Jouret F, Caron N, Dessy C, and Flamion B. Hyaluronidase 1 Deficiency Preserves Endothelial Function and Glycocalyx Integrity in Early Streptozotocin-Induced Diabetes. Diabetes 65: 2742–2753, 2016. [DOI] [PubMed] [Google Scholar]
- 65.Dogne S, Flamion B, and Caron N. Endothelial Glycocalyx as a Shield Against Diabetic Vascular Complications: Involvement of Hyaluronan and Hyaluronidases. Arterioscler Thromb Vasc Biol 38: 1427–1439, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dogne S, and Flamion B. Endothelial Glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding. Am J Pathol 190: 768–780, 2020. [DOI] [PubMed] [Google Scholar]
- 67.Dogne S, Rath G, Jouret F, Caron N, Dessy C, and Flamion B. Hyaluronidase 1 Deficiency Preserves Endothelial Function and Glycocalyx Integrity in Early Streptozotocin-Induced Diabetes. Diabetes 65: 2742–2753, 2016. [DOI] [PubMed] [Google Scholar]
- 68.Dolmatova EV, Wang K, Mandavilli R, and Griendling KK. The effects of sepsis on endothelium and clinical implications. Cardiovasc Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donati A, Damiani E, Domizi R, Romano R, Adrario E, Pelaia P, Ince C, and Singer M. Alteration of the sublingual microvascular glycocalyx in critically ill patients. Microvasc Res 90: 86–89, 2013. [DOI] [PubMed] [Google Scholar]
- 70.Dou H, Feher A, Davila AC, Romero MJ, Patel VS, Kamath VM, Gooz MB, Rudic RD, Lucas R, Fulton DJ, Weintraub NL, and Bagi Z. Role of Adipose Tissue Endothelial ADAM17 in Age-Related Coronary Microvascular Dysfunction. Arterioscler Thromb Vasc Biol 37: 1180–1193, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dragovich MA, Chester D, Fu BM, Wu C, Xu Y, Goligorsky MS, and Zhang XF. Mechanotransduction of the endothelial glycocalyx mediates nitric oxide production through activation of TRP channels. Am J Physiol Cell Physiol 311: C846–C853, 2016. [DOI] [PubMed] [Google Scholar]
- 72.Drummond GR, and Sobey CG. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol Metab 25: 452–463, 2014. [DOI] [PubMed] [Google Scholar]
- 73.Duran WN, Breslin JW, and Sanchez FA. The NO cascade, eNOS location, and microvascular permeability. Cardiovasc Res 87: 254–261, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ebong EE, Macaluso FP, Spray DC, and Tarbell JM. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler Thromb Vasc Biol 31: 1908–1915, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ebong EE, Lopez-Quintero SV, Rizzo V, Spray DC, and Tarbell JM. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integrative biology: quantitative biosciences from nano to macro 6: 338–347, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ecobici M, and Stoicescu C. Arterial Stiffness and Hypertension - Which Comes First? Maedica (Bucur) 12: 184–190, 2017. [PMC free article] [PubMed] [Google Scholar]
- 77.Engin A Endothelial Dysfunction in Obesity. Adv Exp Med Biol 960: 345–379, 2017. [DOI] [PubMed] [Google Scholar]
- 78.Eskens BJ, Zuurbier CJ, van Haare J, Vink H, and van Teeffelen JW. Effects of two weeks of metformin treatment on whole-body glycocalyx barrier properties in db/db mice. Cardiovasc Diabetol 12: 175, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Esko JD, and Linhardt RJ. Proteins that Bind Sulfated Glycosaminoglycans. In: Essentials of Glycobiology, edited by nd, Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, and Etzler ME. Cold Spring Harbor (NY): 2009. [PubMed] [Google Scholar]
- 80.Eylar EH, Madoff MA, Brody OV, and Oncley JL. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem 237: 1992–2000, 1962. [PubMed] [Google Scholar]
- 81.Fan D, Takawale A, Shen M, Samokhvalov V, Basu R, Patel V, Wang X, Fernandez-Patron C, Seubert JM, Oudit GY, and Kassiri Z. A Disintegrin and Metalloprotease-17 Regulates Pressure Overload-Induced Myocardial Hypertrophy and Dysfunction Through Proteolytic Processing of Integrin beta1. Hypertension 68: 937–948, 2016. [DOI] [PubMed] [Google Scholar]
- 82.Fan J, Sun Y, Xia Y, Tarbell JM, and Fu BM. Endothelial surface glycocalyx (ESG) components and ultra-structure revealed by stochastic optical reconstruction microscopy (STORM). Biorheology 56: 77–88, 2019. [DOI] [PubMed] [Google Scholar]
- 83.Fancher IS, Le Master E, Ahn SJ, Adamos C, Lee JC, Berdyshev E, Dull RO, Phillips SA, and Levitan I. Impairment of Flow-Sensitive Inwardly Rectifying K(+) Channels via Disruption of Glycocalyx Mediates Obesity-Induced Endothelial Dysfunction. Arterioscler Thromb Vasc Biol ATVBAHA120314935, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fels B, and Kusche-Vihrog K. It takes more than two to tango: mechanosignaling of the endothelial surface. Pflugers Arch 472: 419–433, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fels J, Jeggle P, Liashkovich I, Peters W, and Oberleithner H. Nanomechanics of vascular endothelium. Cell Tissue Res 355: 727–737, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Filmus J, Capurro M, and Rast J. Glypicans. Genome Biol 9: 224, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fingleton B Matrix metalloproteinases as regulators of inflammatory processes. Biochim Biophys Acta Mol Cell Res 1864: 2036–2042, 2017. [DOI] [PubMed] [Google Scholar]
- 88.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, and Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 93: e136–142, 2003. [DOI] [PubMed] [Google Scholar]
- 89.Foote CA, Castorena-Gonzalez JA, Ramirez-Perez FI, Jia G, Hill MA, Reyes-Aldasoro CC, Sowers JR, and Martinez-Lemus LA. Arterial Stiffening in Western Diet-Fed Mice Is Associated with Increased Vascular Elastin, Transforming Growth Factor-beta, and Plasma Neuraminidase. Front Physiol 7: 285, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fox TE, Bewley MC, Unrath KA, Pedersen MM, Anderson RE, Jung DY, Jefferson LS, Kim JK, Bronson SK, and Flanagan JM. Circulating sphingolipid biomarkers in models of type 1 diabetes. J Lipid Res 52: 509–517, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Franchimont N, Lambert C, Huynen P, Ribbens C, Relic B, Chariot A, Bours V, Piette J, Merville MP, and Malaise M. Interleukin-6 receptor shedding is enhanced by interleukin-1beta and tumor necrosis factor alpha and is partially mediated by tumor necrosis factor alpha-converting enzyme in osteoblast-like cells. Arthritis Rheum 52: 84–93, 2005. [DOI] [PubMed] [Google Scholar]
- 92.Fransson LA, Belting M, Cheng F, Jonsson M, Mani K, and Sandgren S. Novel aspects of glypican glycobiology. Cell Mol Life Sci 61: 1016–1024, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fraser JR, Laurent TC, and Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 242: 27–33, 1997. [DOI] [PubMed] [Google Scholar]
- 94.Fronek K, and Zweifach BW. Microvascular pressure distribution in skeletal muscle and the effect of vasodilation. Am J Physiol 228: 791–796, 1975. [DOI] [PubMed] [Google Scholar]
- 95.Fukai T, and Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxidants & redox signaling 15: 1583–1606, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao L, and Lipowsky HH. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res 80: 394–401, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.George J, and Struthers A. The role of urate and xanthine oxidase in vascular oxidative stress: future directions. Therapeutics and clinical risk management 5: 799–803, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gerhold KA, and Schwartz MA. Ion Channels in Endothelial Responses to Fluid Shear Stress. Physiology (Bethesda) 31: 359–369, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghosh A, Kuppusamy H, and Pilarski LM. Aberrant splice variants of HAS1 (Hyaluronan Synthase 1) multimerize with and modulate normally spliced HAS1 protein: a potential mechanism promoting human cancer. J Biol Chem 284: 18840–18850, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giacco F, and Brownlee M. Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Glanz VY, Myasoedova VA, Grechko AV, and Orekhov AN. Sialidase activity in human pathologies. Eur J Pharmacol 842: 345–350, 2019. [DOI] [PubMed] [Google Scholar]
- 102.Goldberg R, Rubinstein AM, Gil N, Hermano E, Li JP, van der Vlag J, Atzmon R, Meirovitz A, and Elkin M. Role of heparanase-driven inflammatory cascade in pathogenesis of diabetic nephropathy. Diabetes 63: 4302–4313, 2014. [DOI] [PubMed] [Google Scholar]
- 103.Goligorsky MS, and Sun D. Glycocalyx in Endotoxemia and Sepsis. Am J Pathol 190: 791–798, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Golovanova NK, Gracheva EV, Il'inskaya OP, Tararak EM, and Prokazova NV. Sialidase activity in normal and atherosclerotic human aortic intima. Biochemistry (Mosc) 67: 1230–1234, 2002. [DOI] [PubMed] [Google Scholar]
- 105.Gooz M ADAM-17: the enzyme that does it all. Critical reviews in biochemistry and molecular biology 45: 146–169, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gouverneur M, Berg B, Nieuwdorp M, Stroes E, and Vink H. Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. J Intern Med 259: 393–400, 2006. [DOI] [PubMed] [Google Scholar]
- 107.Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, and Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 290: H458–452, 2006. [DOI] [PubMed] [Google Scholar]
- 108.Guillaumet-Adkins A, Yanez Y, Peris-Diaz MD, Calabria I, Palanca-Ballester C, and Sandoval J. Epigenetics and Oxidative Stress in Aging. Oxid Med Cell Longev 2017: 9175806, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo J, Yang ZC, and Liu Y. Attenuating Pulmonary Hypertension by Protecting the Integrity of Glycocalyx in Rats Model of Pulmonary Artery Hypertension. Inflammation 42: 1951–1956, 2019. [DOI] [PubMed] [Google Scholar]
- 110.Hamidi H, and Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 18: 533–548, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hascall V, and Esko JD. Hyaluronan. In: Essentials of Glycobiology, edited by rd, Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, and Seeberger PH. Cold Spring Harbor (NY): 2015, p. 197–206. [PubMed] [Google Scholar]
- 112.Hempel C, Pasini EM, and Kurtzhals JAL. Endothelial Glycocalyx: Shedding Light on Malaria Pathogenesis. Trends Mol Med 22: 453–457, 2016. [DOI] [PubMed] [Google Scholar]
- 113.Henry CB, and Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 279: H2815–2823, 2000. [DOI] [PubMed] [Google Scholar]
- 114.Hill MA, Nourian Z, Ho IL, Clifford PS, Martinez-Lemus L, and Meininger GA. Small Artery Elastin Distribution and Architecture - Focus on Three Dimensional Organization. Microcirculation 2016. [DOI] [PubMed] [Google Scholar]
- 115.Hodge-Dufour J, Noble PW, Horton MR, Bao C, Wysoka M, Burdick MD, Strieter RM, Trinchieri G, and Pure E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol 159: 2492–2500, 1997. [PubMed] [Google Scholar]
- 116.Hooke R. Micrographia : or some physiological descriptions of minute bodies made by magnifying glasses. With observations and inquiries thereupon. London: : Printed by Martyn Jo., and Allestry Ja. … and are to be sold at their shop …, 1665., 1665. [Google Scholar]
- 117.Hu YJ, Wang YD, Tan FQ, and Yang WX. Regulation of paracellular permeability: factors and mechanisms. Mol Biol Rep 40: 6123–6142, 2013. [DOI] [PubMed] [Google Scholar]
- 118.Hurst LA, Dunmore BJ, Long L, Crosby A, Al-Lamki R, Deighton J, Southwood M, Yang X, Nikolic MZ, Herrera B, Inman GJ, Bradley JR, Rana AA, Upton PD, and Morrell NW. TNFalpha drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nature communications 8: 14079, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Iijima R, Takahashi H, Namme R, Ikegami S, and Yamazaki M. Novel biological function of sialic acid (N-acetylneuraminic acid) as a hydrogen peroxide scavenger. FEBS letters 561: 163–166, 2004. [DOI] [PubMed] [Google Scholar]
- 120.Ikonomidis I, Voumvourakis A, Makavos G, Triantafyllidi H, Pavlidis G, Katogiannis K, Benas D, Vlastos D, Trivilou P, Varoudi M, Parissis J, Iliodromitis E, and Lekakis J. Association of impaired endothelial glycocalyx with arterial stiffness, coronary microcirculatory dysfunction, and abnormal myocardial deformation in untreated hypertensives. J Clin Hypertens (Greenwich) 20: 672–679, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ikonomidis I, Pavlidis G, Thymis J, Birba D, Kalogeris A, Kousathana F, Kountouri A, Balampanis K, Parissis J, Andreadou I, Katogiannis K, Dimitriadis G, Bamias A, Iliodromitis E, and Lambadiari V. Effects of Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Endothelial Glycocalyx, Arterial Function, and Myocardial Work Index in Patients With Type 2 Diabetes Mellitus After 12-Month Treatment. Journal of the American Heart Association 9: e015716, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Insull W Jr. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med 122: S3–S14, 2009. [DOI] [PubMed] [Google Scholar]
- 123.Iring A, Jin YJ, Albarran-Juarez J, Siragusa M, Wang S, Dancs PT, Nakayama A, Tonack S, Chen M, Kunne C, Sokol AM, Gunther S, Martinez A, Fleming I, Wettschureck N, Graumann J, Weinstein LS, and Offermanns S. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J Clin Invest 130: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, and Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 274: 25085–25092, 1999. [DOI] [PubMed] [Google Scholar]
- 125.Ito S. Form and function of the glycocalyx on free cell surfaces. Philos Trans R Soc Lond B Biol Sci 268: 55–66, 1974. [DOI] [PubMed] [Google Scholar]
- 126.Jha JC, Watson AMD, Mathew G, de Vos LC, and Jandeleit-Dahm K. The emerging role of NADPH oxidase NOX5 in vascular disease. Clin Sci (Lond) 131: 981–990, 2017. [DOI] [PubMed] [Google Scholar]
- 127.Johnson JL. Metalloproteinases in atherosclerosis. Eur J Pharmacol 816: 93–106, 2017. [DOI] [PubMed] [Google Scholar]
- 128.Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, Skoura A, Kinzel B, Brinkmann V, Rafii S, Evans T, and Hla T. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell 23: 600–610, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kang H, Wu Q, Sun A, Liu X, Fan Y, and Deng X. Cancer Cell Glycocalyx and Its Significance in Cancer Progression. International journal of molecular sciences 19: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Karousou E, Kamiryo M, Skandalis SS, Ruusala A, Asteriou T, Passi A, Yamashita H, Hellman U, Heldin CH, and Heldin P. The activity of hyaluronan synthase 2 is regulated by dimerization and ubiquitination. J Biol Chem 285: 23647–23654, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kataoka H, Ushiyama A, Kawakami H, Akimoto Y, Matsubara S, and Iijima T. Fluorescent imaging of endothelial glycocalyx layer with wheat germ agglutinin using intravital microscopy. Microsc Res Tech 79: 31–37, 2016. [DOI] [PubMed] [Google Scholar]
- 132.Kawahara R, Granato DC, Yokoo S, Domingues RR, Trindade DM, and Paes Leme AF. Mass spectrometry-based proteomics revealed Glypican-1 as a novel ADAM17 substrate. J Proteomics 151: 53–65, 2017. [DOI] [PubMed] [Google Scholar]
- 133.Kawamura S, Sato I, Wada T, Yamaguchi K, Li Y, Li D, Zhao X, Ueno S, Aoki H, Tochigi T, Kuwahara M, Kitamura T, Takahashi K, Moriya S, and Miyagi T. Plasma membrane-associated sialidase (NEU3) regulates progression of prostate cancer to androgen-independent growth through modulation of androgen receptor signaling. Cell Death Differ 19: 170–179, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kefauver JM, Ward AB, and Patapoutian A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 587: 567–576, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim R, Emi M, and Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology 121: 1–14, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kincses A, Santa-Maria AR, Walter FR, Der L, Horanyi N, Lipka DV, Valkai S, Deli MA, and Der A. A chip device to determine surface charge properties of confluent cell monolayers by measuring streaming potential. Lab Chip 20: 3792–3805, 2020. [DOI] [PubMed] [Google Scholar]
- 137.Knoepp F, Ashley Z, Barth D, Baldin JP, Jennings M, Kazantseva M, Saw EL, Katare R, Alvarez de la Rosa D, Weissmann N, and Fronius M. Shear force sensing of epithelial Na(+) channel (ENaC) relies on N-glycosylated asparagines in the palm and knuckle domains of alphaENaC. Proc Natl Acad Sci U S A 117: 717–726, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Koganti R, Suryawanshi R, and Shukla D. Heparanase, cell signaling, and viral infections. Cell Mol Life Sci 77: 5059–5077, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kolarova H, Ambruzova B, Svihalkova Sindlerova L, Klinke A, and Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators of inflammation 2014: 694312, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kong X, Chen L, Ye P, Wang Z, Zhang J, Ye F, and Chen S. The role of HYAL2 in LSS-induced glycocalyx impairment and the PKA-mediated decrease in eNOS-Ser-633 phosphorylation and nitric oxide production. Mol Biol Cell 27: 3972–3979, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koo A, Dewey CF Jr., and Garcia-Cardena G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am J Physiol Cell Physiol 304: C137–146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kowalski GM, Carey AL, Selathurai A, Kingwell BA, and Bruce CR. Plasma Sphingosine-1-Phosphate Is Elevated in Obesity. PLoS One 8: e72449, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Krystel-Whittemore M, Dileepan KN, and Wood JG. Mast Cell: A Multi-Functional Master Cell. Front Immunol 6: 620, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kumagai R, Lu X, and Kassab GS. Role of glycocalyx in flow-induced production of nitric oxide and reactive oxygen species. Free radical biology & medicine 47: 600–607, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kumase F, Morizane Y, Mohri S, Takasu I, Ohtsuka A, and Ohtsuki H. Glycocalyx degradation in retinal and choroidal capillary endothelium in rats with diabetes and hypertension. Acta Med Okayama 64: 277–283, 2010. [DOI] [PubMed] [Google Scholar]
- 146.Kurano M, and Yatomi Y. Sphingosine 1-Phosphate and Atherosclerosis. Journal of atherosclerosis and thrombosis 25: 16–26, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kutuzov N, Flyvbjerg H, and Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier. Proc Natl Acad Sci U S A 115: E9429–E9438, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lacolley P, Regnault V, Nicoletti A, Li Z, and Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res 95: 194–204, 2012. [DOI] [PubMed] [Google Scholar]
- 149.Landolfo M, and Borghi C. Hyperuricaemia and vascular risk: the debate continues. Curr Opin Cardiol 34: 399–405, 2019. [DOI] [PubMed] [Google Scholar]
- 150.Langjahr P, Diaz-Jimenez D, De la Fuente M, Rubio E, Golenbock D, Bronfman FC, Quera R, Gonzalez MJ, and Hermoso MA. Metalloproteinase-dependent TLR2 ectodomain shedding is involved in soluble toll-like receptor 2 (sTLR2) production. PLoS ONE 9: e104624, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lassegue B, San Martin A, and Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, and Dennis JW. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 129: 123–134, 2007. [DOI] [PubMed] [Google Scholar]
- 153.Le Roux AL, Quiroga X, Walani N, Arroyo M, and Roca-Cusachs P. The plasma membrane as a mechanochemical transducer. Philos Trans R Soc Lond B Biol Sci 374: 20180221, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee AC, Lam JK, Shiu SW, Wong Y, Betteridge DJ, and Tan KC. Serum Level of Soluble Receptor for Advanced Glycation End Products Is Associated with A Disintegrin And Metalloproteinase 10 in Type 1 Diabetes. PLoS ONE 10: e0137330, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee DH, Dane MJ, van den Berg BM, Boels MG, van Teeffelen JW, de Mutsert R, den Heijer M, Rosendaal FR, van der Vlag J, van Zonneveld AJ, Vink H, and Rabelink TJ. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS ONE 9: e96477, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee KS, Kim SR, Park SJ, Min KH, Lee KY, Choe YH, Park SY, Chai OH, Zhang X, Song CH, and Lee YC. Mast cells can mediate vascular permeability through regulation of the PI3K-HIF-1alpha-VEGF axis. Am J Respir Crit Care Med 178: 787–797, 2008. [DOI] [PubMed] [Google Scholar]
- 157.Lee SH, Fujioka S, Takahashi R, and Oe T. Angiotensin II-Induced Oxidative Stress in Human Endothelial Cells: Modification of Cellular Molecules through Lipid Peroxidation. Chem Res Toxicol 32: 1412–1422, 2019. [DOI] [PubMed] [Google Scholar]
- 158.Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J, Cosentino F, Deanfield J, Gallino A, Ikonomidis I, Kremastinos D, Landmesser U, Protogerou A, Stefanadis C, Tousoulis D, Vassalli G, Vink H, Werner N, Wilkinson I, and Vlachopoulos C. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil 18: 775–789, 2011. [DOI] [PubMed] [Google Scholar]
- 159.Levick JR, and Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 87: 198–210, 2010. [DOI] [PubMed] [Google Scholar]
- 160.Levy-Adam F, Feld S, Cohen-Kaplan V, Shteingauz A, Gross M, Arvatz G, Naroditsky I, Ilan N, Doweck I, and Vlodavsky I. Heparanase 2 interacts with heparan sulfate with high affinity and inhibits heparanase activity. J Biol Chem 285: 28010–28019, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li DQ, Shang TY, Kim HS, Solomon A, Lokeshwar BL, and Pflugfelder SC. Regulated expression of collagenases MMP-1, -8, and -13 and stromelysins MMP-3, - 10, and -11 by human corneal epithelial cells. Invest Ophthalmol Vis Sci 44: 2928–2936, 2003. [DOI] [PubMed] [Google Scholar]
- 162.Li H, Horke S, and Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 237: 208–219, 2014. [DOI] [PubMed] [Google Scholar]
- 163.Li Q, Youn JY, and Cai H. Mechanisms and consequences of endothelial nitric oxide synthase dysfunction in hypertension. J Hypertens 33: 1128–1136, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li Z, Wu N, Wang J, and Zhang Q. Roles of Endovascular Calyx Related Enzymes in Endothelial Dysfunction and Diabetic Vascular Complications. Front Pharmacol 11: 590614, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, and Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging 13: 757–772, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Linton MRF, Yancey PG, Davies SS, Jerome WG, Linton EF, Song WL, Doran AC, and Vickers KC. The Role of Lipids and Lipoproteins in Atherosclerosis. In: Endotext, edited by Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, Grossman A, Hershman JM, Hofland J, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Purnell J, Singer F, Stratakis CA, Trence DL, and Wilson DP. South Dartmouth (MA): 2000. [Google Scholar]
- 167.Lipowsky HH. The endothelial glycocalyx as a barrier to leukocyte adhesion and its mediation by extracellular proteases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lipowsky HH, and Lescanic A. The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvasc Res 90: 80–85, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lipphardt M, Song JW, and Goligorsky MS. Sirtuin 1 and endothelial glycocalyx. Pflugers Arch 472: 991–1002, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu W, Liu B, Liu S, Zhang J, and Lin S. Sphingosine-1-phosphate receptor 2 mediates endothelial cells dysfunction by PI3K-Akt pathway under high glucose condition. Eur J Pharmacol 776: 19–25, 2016. [DOI] [PubMed] [Google Scholar]
- 171.Lombard J Once upon a time the cell membranes: 175 years of cell boundary research. Biology direct 9: 32, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Loufrani L, and Henrion D. Role of the cytoskeleton in flow (shear stress)-induced dilation and remodeling in resistance arteries. Medical & biological engineering & computing 46: 451–460, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lowe DT. Nitric oxide dysfunction in the pathophysiology of preeclampsia. Nitric Oxide 4: 441–458, 2000. [DOI] [PubMed] [Google Scholar]
- 174.Machin DR, Bloom SI, Campbell RA, Phuong TTT, Gates PE, Lesniewski LA, Rondina MT, and Donato AJ. Advanced age results in a diminished endothelial glycocalyx. Am J Physiol Heart Circ Physiol 315: H531–H539, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mack JJ, Mosqueiro TS, Archer BJ, Jones WM, Sunshine H, Faas GC, Briot A, Aragon RL, Su T, Romay MC, McDonald AI, Kuo CH, Lizama CO, Lane TF, Zovein AC, Fang Y, Tarling EJ, de Aguiar Vallim TQ, Navab M, Fogelman AM, Bouchard LS, and Iruela-Arispe ML. NOTCH1 is a mechanosensor in adult arteries. Nature communications 8: 1620, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mahmood SS, Levy D, Vasan RS, and Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet 383: 999–1008, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mahmoud M, Mayer M, Cancel LM, Bartosch AM, Mathews R, and Tarbell JM. The Glycocalyx core protein Glypican 1 protects vessel wall endothelial cells from stiffness-mediated dysfunction and disease. Cardiovasc Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Majesky MW, Dong XR, Hoglund V, Mahoney WM Jr., and Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol 31: 1530–1539, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Majesky MW, Dong XR, Regan JN, and Hoglund VJ. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res 108: 365–377, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Malanovic N, and Lohner K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim Biophys Acta 1858: 936–946, 2016. [DOI] [PubMed] [Google Scholar]
- 181.Maroski J, Vorderwulbecke BJ, Fiedorowicz K, Da Silva-Azevedo L, Siegel G, Marki A, Pries AR, and Zakrzewicz A. Shear stress increases endothelial hyaluronan synthase 2 and hyaluronan synthesis especially in regard to an atheroprotective flow profile. Exp Physiol 96: 977–986, 2011. [DOI] [PubMed] [Google Scholar]
- 182.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, and Buse JB. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. New England Journal of Medicine 375: 311–322, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Martinez-Lemus LA, Hill MA, and Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 24: 45–57, 2009. [DOI] [PubMed] [Google Scholar]
- 184.Martinez-Lemus LA. The dynamic structure of arterioles. Basic Clin Pharmacol Toxicol 110: 5–11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mazor R, Friedmann-Morvinski D, Alsaigh T, Kleifeld O, Kistler EB, Rousso-Noori L, Huang C, Li JB, Verma IM, and Schmid-Schonbein GW. Cleavage of the leptin receptor by matrix metalloproteinase-2 promotes leptin resistance and obesity in mice. Science translational medicine 10: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.McDonald B, and Kubes P. Interactions between CD44 and Hyaluronan in Leukocyte Trafficking. Front Immunol 6: 68, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Megens RT, Reitsma S, Schiffers PH, Hilgers RH, De Mey JG, Slaaf DW, oude Egbrink MG, and van Zandvoort MA. Two-photon microscopy of vital murine elastic and muscular arteries. Combined structural and functional imaging with subcellular resolution. J Vasc Res 44: 87–98, 2007. [DOI] [PubMed] [Google Scholar]
- 188.Mehdi MM, Singh P, and Rizvi SI. Erythrocyte sialic acid content during aging in humans: correlation with markers of oxidative stress. Dis Markers 32: 179–186, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mehta V, Pang KL, Rozbesky D, Nather K, Keen A, Lachowski D, Kong Y, Karia D, Ameismeier M, Huang J, Fang Y, Del Rio Hernandez A, Reader JS, Jones EY, and Tzima E. The guidance receptor plexin D1 is a mechanosensor in endothelial cells. Nature 578: 290–295, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Michen B, and Graule T. Isoelectric points of viruses. J Appl Microbiol 109: 388–397, 2010. [DOI] [PubMed] [Google Scholar]
- 191.Mitchell GF. Arterial stiffness: insights from Framingham and Iceland. Current opinion in nephrology and hypertension 24: 1–7, 2015. [DOI] [PubMed] [Google Scholar]
- 192.Mitra R, O'Neil GL, Harding IC, Cheng MJ, Mensah SA, and Ebong EE. Glycocalyx in Atherosclerosis-Relevant Endothelium Function and as a Therapeutic Target. Curr Atheroscler Rep 19: 63, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Miyagi T Aberrant expression of sialidase and cancer progression. Proc Jpn Acad Ser B Phys Biol Sci 84: 407–418, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Miyagi T, and Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology 22: 880–896, 2012. [DOI] [PubMed] [Google Scholar]
- 195.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, and Kajiya F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol 285: H722–726, 2003. [DOI] [PubMed] [Google Scholar]
- 196.Mockl L, Pedram K, Roy AR, Krishnan V, Gustavsson AK, Dorigo O, Bertozzi CR, and Moerner WE. Quantitative Super-Resolution Microscopy of the Mammalian Glycocalyx. Dev Cell 50: 57–72 e56, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mockl L The Emerging Role of the Mammalian Glycocalyx in Functional Membrane Organization and Immune System Regulation. Front Cell Dev Biol 8: 253, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Molvarec A, Szarka A, Walentin S, Beko G, Karadi I, Prohaszka Z, and Rigo J Jr. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod Biol Endocrinol 9: 124, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Montaner J, Ramiro L, Simats A, Hernandez-Guillamon M, Delgado P, Bustamante A, and Rosell A. Matrix metalloproteinases and ADAMs in stroke. Cell Mol Life Sci 76: 3117–3140, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Monti E, Bonten E, D'Azzo A, Bresciani R, Venerando B, Borsani G, Schauer R, and Tettamanti G. Sialidases in vertebrates: a family of enzymes tailored for several cell functions. Adv Carbohydr Chem Biochem 64: 403–479, 2010. [DOI] [PubMed] [Google Scholar]
- 201.Monzon ME, Fregien N, Schmid N, Falcon NS, Campos M, Casalino-Matsuda SM, and Forteza RM. Reactive oxygen species and hyaluronidase 2 regulate airway epithelial hyaluronan fragmentation. J Biol Chem 285: 26126–26134, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Moseley R, Waddington RJ, and Embery G. Degradation of glycosaminoglycans by reactive oxygen species derived from stimulated polymorphonuclear leukocytes. Biochim Biophys Acta 1362: 221–231, 1997. [DOI] [PubMed] [Google Scholar]
- 203.Mui KL, Chen CS, and Assoian RK. The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J Cell Sci 129: 1093–1100, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mukai S, Takaki T, Nagumo T, Sano M, Kang D, Takimoto M, and Honda K. Three-dimensional electron microscopy for endothelial glycocalyx observation using Alcian blue with silver enhancement. Med Mol Morphol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mulivor AW, and Lipowsky HH. Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline. Microcirculation 16: 657–666, 2009. [DOI] [PubMed] [Google Scholar]
- 206.Munkley J, and Scott E. Targeting Aberrant Sialylation to Treat Cancer. Medicines (Basel) 6: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Myers TJ, Brennaman LH, Stevenson M, Higashiyama S, Russell WE, Lee DC, and Sunnarborg SW. Mitochondrial reactive oxygen species mediate GPCR-induced TACE/ADAM17-dependent transforming growth factor-alpha shedding. Mol Biol Cell 20: 5236–5249, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nadir Y, and Brenner B. Heparanase procoagulant effects and inhibition by heparins. Thromb Res 125 Suppl 2: S72–76, 2010. [DOI] [PubMed] [Google Scholar]
- 209.Nassimizadeh M, Ashrafian H, Drury NE, Howell NJ, Digby J, Pagano D, Frenneaux MP, and Born GV. Reduced negative surface charge on arterial endothelium explains accelerated atherosclerosis in type 2 diabetic patients. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease 7: 213–215, 2010. [DOI] [PubMed] [Google Scholar]
- 210.Natoni A, Macauley MS, and O'Dwyer ME. Targeting Selectins and Their Ligands in Cancer. Front Oncol 6: 93, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Natori Y, Ohkura N, Nasui M, Atsumi G, and Kihara-Negishi F. Acidic sialidase activity is aberrant in obese and diabetic mice. Biological & pharmaceutical bulletin 36: 1027–1031, 2013. [DOI] [PubMed] [Google Scholar]
- 212.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes ES, and Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 55: 1127–1132, 2006. [DOI] [PubMed] [Google Scholar]
- 213.Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, and Vink H. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 55: 480–486, 2006. [DOI] [PubMed] [Google Scholar]
- 214.Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, Kastelein JJ, and Stroes ES. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 55: 480–486, 2006. [DOI] [PubMed] [Google Scholar]
- 215.Nieuwdorp M, Holleman F, de Groot E, Vink H, Gort J, Kontush A, Chapman MJ, Hutten BA, Brouwer CB, Hoekstra JB, Kastelein JJ, and Stroes ES. Perturbation of hyaluronan metabolism predisposes patients with type 1 diabetes mellitus to atherosclerosis. Diabetologia 50: 1288–1293, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Noble PW, McKee CM, Cowman M, and Shin HS. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J Exp Med 183: 2373–2378, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.O'Callaghan R, Job KM, Dull RO, and Hlady V. Stiffness and heterogeneity of the pulmonary endothelial glycocalyx measured by atomic force microscopy. Am J Physiol Lung Cell Mol Physiol 301: L353–360, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, and Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A 104: 16281–16286, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Oh JK, Yegin Y, Yang F, Zhang M, Li J, Huang S, Verkhoturov SV, Schweikert EA, Perez-Lewis K, Scholar EA, Taylor TM, Castillo A, Cisneros-Zevallos L, Min Y, and Akbulut M. The influence of surface chemistry on the kinetics and thermodynamics of bacterial adhesion. Sci Rep 8: 17247, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ohkawa R, Nakamura K Fau - Okubo S, Okubo S Fau - Hosogaya S, Hosogaya S Fau - Ozaki Y, Ozaki Y Fau - Tozuka M, Tozuka M Fau - Osima N, Osima N Fau - Yokota H, Yokota H Fau - Ikeda H, Ikeda H Fau - Yatomi Y, and Yatomi Y. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. [DOI] [PubMed] [Google Scholar]
- 221.Olde Engberink RH, Rorije NM, Homan van der Heide JJ, van den Born BJ, and Vogt L. Role of the vascular wall in sodium homeostasis and salt sensitivity. Journal of the American Society of Nephrology: JASN 26: 777–783, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Oltean S, Qiu Y, Ferguson JK, Stevens M, Neal C, Russell A, Kaura A, Arkill KP, Harris K, Symonds C, Lacey K, Wijeyaratne L, Gammons M, Wylie E, Hulse RP, Alsop C, Cope G, Damodaran G, Betteridge KB, Ramnath R, Satchell SC, Foster RR, Ballmer-Hofer K, Donaldson LF, Barratt J, Baelde HJ, Harper SJ, Bates DO, and Salmon AH. Vascular Endothelial Growth Factor-A165b Is Protective and Restores Endothelial Glycocalyx in Diabetic Nephropathy. J Am Soc Nephrol 26: 1889–1904, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Onclinx C, Dogne S, Jadin L, Andris F, Grandfils C, Jouret F, Mullier F, and Flamion B. Deficiency in mouse hyaluronidase 2: a new mechanism of chronic thrombotic microangiopathy. Haematologica 100: 1023–1030, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ono S, Egawa G, and Kabashima K. Regulation of blood vascular permeability in the skin. Inflamm Regen 37: 11, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Opal SM, Kessler CM, Roemisch J, and Knaub S. Antithrombin, heparin, and heparan sulfate. Critical care medicine 30: S325–331, 2002. [DOI] [PubMed] [Google Scholar]
- 226.Pahakis MY, Kosky JR, Dull RO, and Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun 355: 228–233, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pahwa R, Nallasamy P, and Jialal I. Toll-like receptors 2 and 4 mediate hyperglycemia induced macrovascular aortic endothelial cell inflammation and perturbation of the endothelial glycocalyx. J Diabetes Complications 30: 563–572, 2016. [DOI] [PubMed] [Google Scholar]
- 228.Paravicini TM, and Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes care 31 Suppl 2: S170–180, 2008. [DOI] [PubMed] [Google Scholar]
- 229.Paulson JC, and Kawasaki N. Sialidase inhibitors DAMPen sepsis. Nat Biotechnol 29: 406–407, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pessentheiner AR, Ducasa GM, and Gordts P. Proteoglycans in Obesity-Associated Metabolic Dysfunction and Meta-Inflammation. Front Immunol 11: 769, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Peterson S, and Liu J. Deciphering mode of action of heparanase using structurally defined oligosaccharides. J Biol Chem 287: 34836–34843, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Piccoli M, Conforti E, Varrica A, Ghiroldi A, Cirillo F, Resmini G, Pluchinotta F, Tettamanti G, Giamberti A, Frigiola A, and Anastasia L. NEU3 sialidase role in activating HIF-1alpha in response to chronic hypoxia in cyanotic congenital heart patients. Int J Cardiol 230: 6–13, 2017. [DOI] [PubMed] [Google Scholar]
- 233.Pollack W, and Reckel RP. A reappraisal of the forces involved in hemagglutination. Int Arch Allergy Appl Immunol 54: 29–42, 1977. [DOI] [PubMed] [Google Scholar]
- 234.Proia RL, and Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. The Journal of clinical investigation 125: 1379–1387, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Psefteli PM, Kitscha P, Vizcay G, Fleck R, Chapple SJ, Mann GE, Fowler M, and Siow RC. Glycocalyx sialic acids regulate Nrf2-mediated signaling by fluid shear stress in human endothelial cells. Redox biology 38: 101816, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pshezhetsky AV, Richard C, Michaud L, Igdoura S, Wang S, Elsliger MA, Qu J, Leclerc D, Gravel R, Dallaire L, and Potier M. Cloning, expression and chromosomal mapping of human lysosomal sialidase and characterization of mutations in sialidosis. Nat Genet 15: 316–320, 1997. [DOI] [PubMed] [Google Scholar]
- 237.Puerta-Guardo H, Glasner DR, and Harris E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog 12: e1005738, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ra HJ, and Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol 26: 587–596, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rahbar E, Cardenas JC, Baimukanova G, Usadi B, Bruhn R, Pati S, Ostrowski SR, Johansson PI, Holcomb JB, and Wade CE. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med 13: 117, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rahman MM, Hirokawa T, Tsuji D, Tsukimoto J, Hitaoka S, Yoshida T, Chuman H, and Itoh K. Novel pH-dependent regulation of human cytosolic sialidase 2 (NEU2) activities by siastatin B and structural prediction of NEU2/siastatin B complex. Biochem Biophys Rep 4: 234–242, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rai S, Nejadhamzeeigilani Z, Gutowski NJ, and Whatmore JL. Loss of the endothelial glycocalyx is associated with increased E-selectin mediated adhesion of lung tumour cells to the brain microvascular endothelium. J Exp Clin Cancer Res 34: 105, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, and oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 454: 345–359, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Reitsma S, Oude Egbrink MG, Heijnen VV, Megens RT, Engels W, Vink H, Slaaf DW, and van Zandvoort MA. Endothelial glycocalyx thickness and platelet-vessel wall interactions during atherogenesis. Thrombosis and haemostasis 106: 939–946, 2011. [DOI] [PubMed] [Google Scholar]
- 244.Reitsma S, oude Egbrink MG, Vink H, van den Berg BM, Passos VL, Engels W, Slaaf DW, and van Zandvoort MA. Endothelial glycocalyx structure in the intact carotid artery: a two-photon laser scanning microscopy study. J Vasc Res 48: 297–306, 2011. [DOI] [PubMed] [Google Scholar]
- 245.Roberts WG, and Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci 108 (Pt 6): 2369–2379, 1995. [DOI] [PubMed] [Google Scholar]
- 246.Rodriguez-Walker M, and Daniotti JL. Human Sialidase Neu3 is S-Acylated and Behaves Like an Integral Membrane Protein. Sci Rep 7: 4167, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rose F, Zeller SA, Chakraborty T, Domann E, Machleidt T, Kronke M, Seeger W, Grimminger F, and Sibelius U. Human endothelial cell activation and mediator release in response to Listeria monocytogenes virulence factors. Infect Immun 69: 897–905, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rouger P, and Salmon C. Tissue distribution and development of blood group antigens. Rev Fr Transfus Immunohematol 25: 643–656, 1982. [DOI] [PubMed] [Google Scholar]
- 249.Rovas A, Lukasz AH, Vink H, Urban M, Sackarnd J, Pavenstadt H, and Kumpers P. Bedside analysis of the sublingual microvascular glycocalyx in the emergency room and intensive care unit - the GlycoNurse study. Scand J Trauma Resusc Emerg Med 26: 16, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rovas A, Seidel LM, Vink H, Pohlkotter T, Pavenstadt H, Ertmer C, Hessler M, and Kumpers P. Association of sublingual microcirculation parameters and endothelial glycocalyx dimensions in resuscitated sepsis. Crit Care 23: 260, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Salazar G NADPH Oxidases and Mitochondria in Vascular Senescence. International journal of molecular sciences 19: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, Bates DO, and Peti-Peterdi J. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. Journal of the American Society of Nephrology: JASN 23: 1339–1350, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Samraj AN, Laubli H, Varki N, and Varki A. Involvement of a non-human sialic Acid in human cancer. Front Oncol 4: 33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, and Hla T. Induction of Vascular Permeability by the Sphingosine-1-Phosphate Receptor–2 (S1P2R) and its Downstream Effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol 27: 1312–1318, 2007. [DOI] [PubMed] [Google Scholar]
- 255.Sandow SL, Gzik DJ, and Lee RM. Arterial internal elastic lamina holes: relationship to function? Journal of anatomy 214: 258–266, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sarrazin S, Lamanna WC, and Esko JD. Heparan sulfate proteoglycans. Cold Spring Harbor perspectives in biology 3: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sasaki A, Hata K, Suzuki S, Sawada M, Wada T, Yamaguchi K, Obinata M, Tateno H, Suzuki H, and Miyagi T. Overexpression of plasma membrane-associated sialidase attenuates insulin signaling in transgenic mice. J Biol Chem 278: 27896–27902, 2003. [DOI] [PubMed] [Google Scholar]
- 258.Schmidt CQ, Hipgrave Ederveen AL, Harder MJ, Wuhrer M, Stehle T, and Blaum BS. Biophysical analysis of sialic acid recognition by the complement regulator Factor H. Glycobiology 28: 765–773, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, and Tuder RM. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 18: 1217–1223, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schneck E, Schubert T, Konovalov OV, Quinn BE, Gutsmann T, Brandenburg K, Oliveira RG, Pink DA, and Tanaka M. Quantitative determination of ion distributions in bacterial lipopolysaccharide membranes by grazing-incidence X-ray fluorescence. Proc Natl Acad Sci U S A 107: 9147–9151, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Seligman SP, Buyon JP, Clancy RM, Young BK, and Abramson SB. The role of nitric oxide in the pathogenesis of preeclampsia. Am J Obstet Gynecol 171: 944–948, 1994. [DOI] [PubMed] [Google Scholar]
- 262.Sena CM, Pereira AM, and Seica R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta 1832: 2216–2231, 2013. [DOI] [PubMed] [Google Scholar]
- 263.Serroukh Y, Djebara S, Lelubre C, Zouaoui Boudjeltia K, Biston P, and Piagnerelli M. Alterations of the Erythrocyte Membrane during Sepsis. Crit Care Res Pract 2012: 702956, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sharman JE, and LaGerche A. Exercise blood pressure: clinical relevance and correct measurement. J Hum Hypertens 29: 351–358, 2015. [DOI] [PubMed] [Google Scholar]
- 265.Shiga K, Takahashi K, Sato I, Kato K, Saijo S, Moriya S, Hosono M, and Miyagi T. Upregulation of sialidase NEU3 in head and neck squamous cell carcinoma associated with lymph node metastasis. Cancer science 106: 1544–1553, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Shivananda Nayak B, Duncan H, Lalloo S, Maraj K, Matmungal V, Matthews F, Prajapati B, Samuel R, and Sylvester P. Correlation of microalbumin and sialic acid with anthropometric variables in type 2 diabetic patients with and without nephropathy. Vasc Health Risk Manag 4: 243–247, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Shriver Z, Capila I, Venkataraman G, and Sasisekharan R. Heparin and heparan sulfate: analyzing structure and microheterogeneity. Handb Exp Pharmacol 159–176, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shurer CR, Kuo JC, Roberts LM, Gandhi JG, Colville MJ, Enoki TA, Pan H, Su J, Noble JM, Hollander MJ, O'Donnell JP, Yin R, Pedram K, Mockl L, Kourkoutis LF, Moerner WE, Bertozzi CR, Feigenson GW, Reesink HL, and Paszek MJ. Physical Principles of Membrane Shape Regulation by the Glycocalyx. Cell 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Shworak NW, Kobayashi T, de Agostini A, and Smits NC. Anticoagulant heparan sulfate to not clot--or not? Prog Mol Biol Transl Sci 93: 153–178, 2010. [DOI] [PubMed] [Google Scholar]
- 270.Singh A, Ramnath RD, Foster RR, Wylie EC, Friden V, Dasgupta I, Haraldsson B, Welsh GI, Mathieson PW, and Satchell SC. Reactive oxygen species modulate the barrier function of the human glomerular endothelial glycocalyx. PLoS ONE 8: e55852, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Singleton PA, Dudek SM, Ma SF, and Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem 281: 34381–34393, 2006. [DOI] [PubMed] [Google Scholar]
- 272.Stroev PV, Hoskins PR, and Easson WJ. Distribution of wall shear rate throughout the arterial tree: a case study. Atherosclerosis 191: 276–280, 2007. [DOI] [PubMed] [Google Scholar]
- 273.Talsma DT, Poppelaars F, Dam W, Meter-Arkema AH, Vives RR, Gal P, Boons GJ, Chopra P, Naggi A, Seelen MA, Berger SP, Daha MR, Stegeman CA, van den Born J, and Consortium C. MASP-2 Is a Heparin-Binding Protease; Identification of Blocking Oligosaccharides. Front Immunol 11: 732, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tarbell JM, and Shi ZD. Effect of the glycocalyx layer on transmission of interstitial flow shear stress to embedded cells. Biomech Model Mechanobiol 12: 111–121, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Targosz-Korecka M, Jaglarz M, Malek-Zietek KE, Gregorius A, Zakrzewska A, Sitek B, Rajfur Z, Chlopicki S, and Szymonski M. AFM-based detection of glycocalyx degradation and endothelial stiffening in the db/db mouse model of diabetes. Sci Rep 7: 15951, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tarhouni K, Guihot AL, Vessieres E, Toutain B, Procaccio V, Grimaud L, Loufrani L, Lenfant F, Arnal JF, and Henrion D. Determinants of flow-mediated outward remodeling in female rodents: respective roles of age, estrogens, and timing. Arterioscler Thromb Vasc Biol 34: 1281–1289, 2014. [DOI] [PubMed] [Google Scholar]
- 277.Tarpey MM, and Fridovich I. Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ Res 89: 224–236., 2001. [DOI] [PubMed] [Google Scholar]
- 278.Tavianatou AG, Caon I, Franchi M, Piperigkou Z, Galesso D, and Karamanos NK. Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J 286: 2883–2908, 2019. [DOI] [PubMed] [Google Scholar]
- 279.Teuwen LA, Geldhof V, Pasut A, and Carmeliet P. COVID-19: the vasculature unleashed. Nature reviews Immunology 20: 389–391, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tringali C, Fiorilli A, Venerando B, and Tettamanti G. Different behavior of ghost-linked acidic and neutral sialidases during human erythrocyte ageing. Glycoconj J 18: 407–418, 2001. [DOI] [PubMed] [Google Scholar]
- 281.Tsioufis C, Bafakis I, Kasiakogias A, and Stefanadis C. The role of matrix metalloproteinases in diabetes mellitus. Curr Top Med Chem 12: 1159–1165, 2012. [DOI] [PubMed] [Google Scholar]
- 282.Tucker WD, Arora Y, and Mahajan K. Anatomy, Blood Vessels. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC., 2021. [PubMed] [Google Scholar]
- 283.Tukijan F, Chandrakanthan M, and Nguyen LN. The signalling roles of sphingosine-1-phosphate derived from red blood cells and platelets. Br J Pharmacol 175: 3741–3746, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, Wainford RD, Williams B, and Schutte AE. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 75: 1334–1357, 2020. [DOI] [PubMed] [Google Scholar]
- 285.Vajaria BN, Patel KR, Begum R, and Patel PS. Sialylation: an Avenue to Target Cancer Cells. Pathol Oncol Res 22: 443–447, 2016. [DOI] [PubMed] [Google Scholar]
- 286.van de Wall S, Santegoets KCM, van Houtum EJH, Bull C, and Adema GJ. Sialoglycans and Siglecs Can Shape the Tumor Immune Microenvironment. Trends Immunol 41: 274–285, 2020. [DOI] [PubMed] [Google Scholar]
- 287.van den Berg BM, Vink H, and Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res 92: 592–594, 2003. [DOI] [PubMed] [Google Scholar]
- 288.van den Berg BM, Spaan JA, Rolf TM, and Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am J Physiol Heart Circ Physiol 290: H915–920, 2006. [DOI] [PubMed] [Google Scholar]
- 289.van den Berg BM, Wang G, Boels MGS, Avramut MC, Jansen E, Sol W, Lebrin F, van Zonneveld AJ, de Koning EJP, Vink H, Grone HJ, Carmeliet P, van der Vlag J, and Rabelink TJ. Glomerular Function and Structural Integrity Depend on Hyaluronan Synthesis by Glomerular Endothelium. J Am Soc Nephrol 30: 1886–1897, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.van den Hoven MJ, Waanders F, Rops AL, Kramer AB, van Goor H, Berden JH, Navis G, and van der Vlag J. Regulation of glomerular heparanase expression by aldosterone, angiotensin II and reactive oxygen species. Nephrol Dial Transplant 24: 2637–2645, 2009. [DOI] [PubMed] [Google Scholar]
- 291.van Haaren PM, VanBavel E, Vink H, and Spaan JA. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physiol 285: H2848–2856, 2003. [DOI] [PubMed] [Google Scholar]
- 292.Van Teeffelen JW, Brands J, Stroes ES, and Vink H. Endothelial glycocalyx: sweet shield of blood vessels. Trends Cardiovasc Med 17: 101–105, 2007. [DOI] [PubMed] [Google Scholar]
- 293.VanTeeffelen JW, Brands J, Jansen C, Spaan JA, and Vink H. Heparin impairs glycocalyx barrier properties and attenuates shear dependent vasodilation in mice. Hypertension 50: 261–267, 2007. [DOI] [PubMed] [Google Scholar]
- 294.Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, and Seeberger PH. Essentials of Glycobiology. In: Essentials of Glycobiology, edited by rd, Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, and Seeberger PH. Cold Spring Harbor (NY): 2015. [PubMed] [Google Scholar]
- 295.Venkataraman K, Lee Y-M, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, and Hla T. Vascular Endothelium As a Contributor of Plasma Sphingosine 1-Phosphate. Circ Res 102: 669–676, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Vigetti D, Genasetti A, Karousou E, Viola M, Clerici M, Bartolini B, Moretto P, De Luca G, Hascall VC, and Passi A. Modulation of hyaluronan synthase activity in cellular membrane fractions. J Biol Chem 284: 30684–30694, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Vigetti D, Deleonibus S, Moretto P, Karousou E, Viola M, Bartolini B, Hascall VC, Tammi M, De Luca G, and Passi A. Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J Biol Chem 287: 35544–35555, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Villalba N, Baby S, and Yuan SY. The Endothelial Glycocalyx as a Double-Edged Sword in Microvascular Homeostasis and Pathogenesis. Front Cell Dev Biol 9: 711003, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Vink H, and Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 79: 581–589, 1996. [DOI] [PubMed] [Google Scholar]
- 300.Vlodavsky I, Ilan N, Naggi A, and Casu B. Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Current pharmaceutical design 13: 2057–2073, 2007. [DOI] [PubMed] [Google Scholar]
- 301.Vlodavsky I, Blich M, Li JP, Sanderson RD, and Ilan N. Involvement of heparanase in atherosclerosis and other vessel wall pathologies. Matrix Biol 32: 241–251, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Voskoboinik I, Soderholm K, and Cotgreave IA. Ascorbate and glutathione homeostasis in vascular smooth muscle cells: cooperation with endothelial cells. Am J Physiol 275: C1031–1039, 1998. [DOI] [PubMed] [Google Scholar]
- 303.Waheed F, Dan Q, Amoozadeh Y, Zhang Y, Tanimura S, Speight P, Kapus A, and Szaszi K. Central role of the exchange factor GEF-H1 in TNF-alpha-induced sequential activation of Rac, ADAM17/TACE, and RhoA in tubular epithelial cells. Mol Biol Cell 24: 1068–1082, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wang G, Kostidis S, Tiemeier GL, Sol W, de Vries MR, Giera M, Carmeliet P, van den Berg BM, and Rabelink TJ. Shear Stress Regulation of Endothelial Glycocalyx Structure Is Determined by Glucobiosynthesis. Arterioscler Thromb Vasc Biol 40: 350–364, 2020. [DOI] [PubMed] [Google Scholar]
- 305.Wang H, Huang H, and Ding SF. Sphingosine-1-phosphate promotes the proliferation and attenuates apoptosis of Endothelial progenitor cells via S1PR1/S1PR3/PI3K/Akt pathway. [DOI] [PubMed] [Google Scholar]
- 306.Wang MH, Hsiao G, and Al-Shabrawey M. Eicosanoids and Oxidative Stress in Diabetic Retinopathy. Antioxidants (Basel) 9: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Weinbaum S, Tarbell JM, and Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 9: 121–167, 2007. [DOI] [PubMed] [Google Scholar]
- 308.Weissgerber TL, Garcia-Valencia O, Milic NM, Codsi E, Cubro H, Nath MC, White WM, Nath KA, and Garovic VD. Early Onset Preeclampsia Is Associated With Glycocalyx Degradation and Reduced Microvascular Perfusion. J Am Heart Assoc 8: e010647, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.West DC, Hampson IN, Arnold F, and Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science 228: 1324–1326, 1985. [DOI] [PubMed] [Google Scholar]
- 310.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71: 1269–1324, 2018. [DOI] [PubMed] [Google Scholar]
- 311.White EJ, Gyulay G, Lhotak S, Szewczyk MM, Chong T, Fuller MT, Dadoo O, Fox-Robichaud AE, Austin RC, Trigatti BL, and Igdoura SA. Sialidase down-regulation reduces non-HDL cholesterol, inhibits leukocyte transmigration, and attenuates atherosclerosis in ApoE knockout mice. J Biol Chem 293: 14689–14706, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.WHO. A revision of the system of nomenclature for influenza viruses: a WHO memorandum. Bull World Health Organ 58: 585–591, 1980. [PMC free article] [PubMed] [Google Scholar]
- 313.Wiedermann CJ. Clinical review: molecular mechanisms underlying the role of antithrombin in sepsis. Crit Care 10: 209, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Wilson C, Lee MD, and McCarron JG. Acetylcholine released by endothelial cells facilitates flow-mediated dilatation. J Physiol 594: 7267–7307, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wilson WW, Wade MM, Holman SC, and Champlin FR. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J Microbiol Methods 43: 153–164, 2001. [DOI] [PubMed] [Google Scholar]
- 316.Wong M, Xu G, Barboza M, Maezawa I, Jin LW, Zivkovic A, and Lebrilla CB. Metabolic flux analysis of the neural cell glycocalyx reveals differential utilization of monosaccharides. Glycobiology 30: 859–871, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Xu J, Mathur J, Vessieres E, Hammack S, Nonomura K, Favre J, Grimaud L, Petrus M, Francisco A, Li J, Lee V, Xiang FL, Mainquist JK, Cahalan SM, Orth AP, Walker JR, Ma S, Lukacs V, Bordone L, Bandell M, Laffitte B, Xu Y, Chien S, Henrion D, and Patapoutian A. GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 173: 762–775 e716, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Yalcin O, Jani VP, Johnson PC, and Cabrales P. Implications Enzymatic Degradation of the Endothelial Glycocalyx on the Microvascular Hemodynamics and the Arteriolar Red Cell Free Layer of the Rat Cremaster Muscle. Front Physiol 9: 168, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Yamaguchi K, Hata K, Koseki K, Shiozaki K, Akita H, Wada T, Moriya S, and Miyagi T. Evidence for mitochondrial localization of a novel human sialidase (NEU4). Biochem J 390: 85–93, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Yamanami H, Shiozaki K, Wada T, Yamaguchi K, Uemura T, Kakugawa Y, Hujiya T, and Miyagi T. Down-regulation of sialidase NEU4 may contribute to invasive properties of human colon cancers. Cancer science 98: 299–307, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Yang B, and Rizzo V. Shear Stress Activates eNOS at the Endothelial Apical Surface Through β1 Containing Integrins and Caveolae. Cell Mol Bioeng 6: 346–354, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Yang H, Zhu L, Chao Y, Gu Y, Kong X, Chen M, Ye P, Luo J, and Chen S. Hyaluronidase2 (Hyal2) modulates low shear stress-induced glycocalyx impairment via the LKB1/AMPK/NADPH oxidase-dependent pathway. J Cell Physiol 233: 9701–9715, 2018. [DOI] [PubMed] [Google Scholar]
- 323.Yang J, LeBlanc ME, Cano I, Saez-Torres KL, Saint-Geniez M, Ng YS, and D'Amore PA. ADAM10 and ADAM17 proteases mediate proinflammatory cytokine-induced and constitutive cleavage of endomucin from the endothelial surface. J Biol Chem 295: 6641–6651, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yang WH, Aziz PV, Heithoff DM, Mahan MJ, Smith JW, and Marth JD. An intrinsic mechanism of secreted protein aging and turnover. Proc Natl Acad Sci U S A 112: 13657–13662, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Yang X, Meegan JE, Jannaway M, Coleman DC, and Yuan SY. A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation. Cardiovasc Res 114: 1752–1763, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zanini D, and Göpfert MC. Mechanosensation: tethered ion channels. Curr Biol 23: R349–351, 2013. [DOI] [PubMed] [Google Scholar]
- 327.Zeng Y, Adamson RH, Curry F-RE, and Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. American Journal of Physiology-Heart and Circulatory Physiology 306: H363–H372, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zeng Y, Liu X-H, Tarbell J, and Fu B. Sphingosine 1-phosphate induced synthesis of glycocalyx on endothelial cells. Exp Cell Res 339: 90–95, 2015. [DOI] [PubMed] [Google Scholar]
- 329.Zeng Y Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling. J Cell Mol Med 21: 1457–1462, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zeng Y, Zhang XF, Fu BM, and Tarbell JM. The Role of Endothelial Surface Glycocalyx in Mechanosensing and Transduction. Adv Exp Med Biol 1097: 1–27, 2018. [DOI] [PubMed] [Google Scholar]
- 331.Zerda KS, Gerba CP, Hou KC, and Goyal SM. Adsorption of viruses to charge-modified silica. Appl Environ Microbiol 49: 91–95, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhang Z, Wuhrer M, and Holst S. Serum sialylation changes in cancer. Glycoconj J 35: 139–160, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zhou X, Yang G, and Guan F. Biological Functions and Analytical Strategies of Sialic Acids in Tumor. Cells 9: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, and Inzucchi SE. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. New England Journal of Medicine 373: 2117–2128, 2015. [DOI] [PubMed] [Google Scholar]
- 335.Zou MH, Cohen R, and Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium 11: 89–97, 2004. [DOI] [PubMed] [Google Scholar]
- 336.Zuurbier CJ, Demirci C, Koeman A, Vink H, and Ince C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol (1985) 99: 1471–1476, 2005. [DOI] [PubMed] [Google Scholar]